Highlights
-
•
Our meta-analysis included a total of 47 articles.
-
•
The prevalence rates in new TB cases: Any-resistance to first-line drugs: 31 % (95 % CI, 24–38), mono-drug resistance: 15 % (95 % CI, 10–22), and multi-drug resistance to first-line drugs: 6 % (95 % CI, 4–8).
-
•
There was a significant variation in the rate of MDR among new TB cases based on the year of publication, location, and DST methods (P < 0.0001).
-
•
The prevalence rates in retreatment cases: Any resistance: 68 % (95 % CI 58–78), mono-resistance: 19 % (95 % CI 7–34), multi-drug resistance: 28 % (95 % CI 15–43).
Keywords: Tuberculosis, Drug resistance, First- and second-line drugs, Iran, Systematic review and meta-analysis
Abstract
Drug resistance among Mycobacterium tuberculosis (MTB) strains is a growing concern in developing countries. We conducted a comprehensive search for relevant studies in Iran on PubMed, Scopus, and Embase until June 12, 2020. Our study focused on determining the prevalence of antibiotic resistance in MTB isolates, with subgroup analyses based on year, location, and drug susceptibility testing (DST) methods. Statistical analyses were performed using STATA software. Our meta-analysis included a total of 47 articles. Among new TB cases, we found the following prevalence rates: Any-resistance to first-line drugs: 31 % (95 % CI, 24–38), mono-drug resistance: 15 % (95 % CI, 10–22), and multidrug resistance to first-line drugs: 6 % (95 % CI, 4–8). There was a significant variation in the rate of MDR among new TB cases based on the year of publication, location, and DST methods (P < 0.0001). We observed substantial variability in multidrug-resistant TB rates among new cases across the studies. Stratified analyses revealed that publication years and DST methods significantly affected resistance rates. Studies from southern and central Iran reported higher any-drug resistance rates, suggesting regional differences. Among retreatment cases, the prevalence rates were as follows: Any resistance: 68 % (95 % CI 58–78), mono-resistance: 19 % (95 % CI 7–34), multidrug resistance: 28 % (95 % CI 15–43). Our study revealed that the prevalence of drug-resistant TB (DR-TB) among TB cases in Iran is higher than the global average. Particularly, MDR-TB among retreatment TB cases is a significant public health issue.
1. Introduction
Globally, tuberculosis (TB) is the second leading infectious killer after Coronavirus disease (COVID-19) worldwide [1], [2]. The continuing spread of drug-resistant TB is one of the biggest challenges and concerns [3]. Identifying high-risk areas is crucial for each country to achieve the ultimate goal of eliminating TB by 2050. Accurate and timely detection of resistance to second-line drugs is of paramount importance to optimize treatment and direct infection control measures to block the transmission of multidrug-resistant TB (MDR-TB) and minimize the risk of further resistance development. The World Health Organization (WHO) suggests that all presumptive TB patients undergo drug susceptibility testing (DST), although numerous countries still lack the laboratory capacity for this [4]. Thus, in many low- and middle-income countries, there may be a high level of under-diagnosis and misdiagnosis of drug-resistant TB (DR-TB). Both phenotypic and genotypic DST methods are employed for susceptibility testing to anti-TB drugs [5]. The proportion method, a phenotypic DST method, is widely used in developing countries and low-income countries like Iran. Therefore, we have stratified a subgroup based on DST methods (proportion versus proportion methods plus other methods).
TB remains a significant public health concern in developing countries such as Iran. However, TB incidence in Iran showed a decreasing trend in 2020 (13 cases per 100,000 populations), with a treatment success rate of 85 % among new and relapse cases registered in 2019.
Two systematic review and meta-analysis studies [6], [7] on TB drug resistance have already been published in Iran. However, these published studies lack the most detailed data on TB drug resistance patterns in new or retreated TB cases. Therefore, our comprehensive meta-analysis was conducted to evaluate the weighted pooled resistance rate (WPR: proportion of strains resistant to specific antimicrobial agents) in different drug resistance statuses (any drug, mono drug, MDR, pre-extensively drug-resistant (XDR), XDR, and also first-line (isoniazid, rifampicin, streptomycin, ethambutol, pyrazinamide, HRES (isoniazid + rifampicin + streptomycin + ethambutol), HRE (isoniazid + rifampicin + ethambutol), HRS (isoniazid + rifampicin + streptomycin), and HR (isoniazid + rifampicin)) or second-line anti-TB drugs (amikacin, kanamycin, ethionamide, ofloxacin, capreomycin)) in MTB isolates. We also conducted subgroup analyses by year (2000–2015, 2016–2020), location (North, Center, East, West, South), and DST methods (proportion method and proportion method plus other methods) in new and retreated pulmonary TB cases.
The results of our review will provide a more comprehensive understanding of the current epidemiology of drug-resistant pulmonary TB in Iran. This knowledge will promote the development of more effective antimicrobial stewardship programs to combat, control, manage, and limit the development of these drug-resistant organisms.
2. Methods
2.1. Guidelines, data sources and search strategy
This review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [8]. A comprehensive systematic literature search was conducted in three databases: MEDLINE [PubMed], Scopus, Web of Science, and Embase for relevant articles (up to June 12, 2020). The search used the following terms: (“Mycobacterium tuberculosis” OR “M. tuberculosis” OR “tuberculosis” OR “TB”) AND (“drug resistance” OR “drug susceptibility” OR “Anti-tuberculosis resistance” OR “anti-TB resistance”) AND (“Iran”) in the Title/Abstract/Keywords fields.
2.2. Inclusion and exclusion criteria
Studies providing sufficient original data on the prevalence of first (isoniazid, rifampin, ethambutol, streptomycin, pyrazinamide) or second (fluoroquinolones, aminoglycosides, ethionamide, capreomycin) line anti-TB drugs and reporting the status of drug resistance in pulmonary TB in Iran were included. Drug-resistance data for either new or retreated cases, or both were also incuded. If the study was reported in duplicate, the first published version was included.
Animal research, reviews, congress/conference abstracts, meta-analyses, or systematic reviews, duplicate publication of the same study, and articles available only in abstract form were excluded. Moreover, studies reported in languages other than English, articles focused solely on extrapulmonary TB or TB cases coinfected with human immunodeficiency viruses (HIV) or childhood TB, studies that have not performed or reported DST were excluded. To minimize potential bias due to small sample size, articles with < 10 cases were also excluded.
2.3. Data extraction and definitions
Two authors independently extracted data from the included studies. The information extracted from each study included first author, year of publication, study period, province, distribution of age and gender in the population, sample size of cases, number of isolates, and drug resistance status (any drug, mono drug, MDR, pre-XDR, XDR, and also first or second-line anti-TB drugs).
Resistance among new TB cases is defined as a newly registered episode of TB in a patient who has never been treated for TB or has taken anti-TB medicines for less than one month. Resistance among retreatment TB cases refers to patients who have received one month or more of anti-TB medicines in the past [9], [10]. Mono-resistance is defined as resistance to only one first-line anti-TB drug. MDR represents resistance to at least isoniazid and rifampin. Any drug resistance is defined as resistance to any drug regardless of mono-resistance or MDR [9], [10]. Pre-XDR is defined as TB caused by MTB strains that fulfill the definition of MDR and rifampicin-resistant TB (RR-TB) and are also resistant to fluoroquinolone [9], [10]. XDR is defined as TB caused by MTB strains that fulfill the definition of MDR/RR-TB and are also resistant to any fluoroquinolone and at least one additional Group A drug [9], [10].
2.4. Quality assessment
The quality of the included studies was assessed independently by two reviewers using an adapted version of the Newcastle-Ottawa assessment scale adapted for cross-sectional studies [11]. A score ranging from 0 to 7 points was attributed to each study (7 points: high quality, ≤ 6 points: low quality).
2.5. Statistical analysis
To analyze and combine the results of different studies, the prevalence of resistance in each study was considered as a binomial distribution, and its standard error was calculated accordingly. Heterogeneity among studies was assessed using Cochran's Q test, I2 index, and interval. Due to the heterogeneity of the studies, a random-effects model was used in the meta-analysis. Sensitivity analysis was performed to evaluate the sources of heterogeneity between studies. The analysis was conducted using Stata/SE software, v.14 (StataCorp, College Station, TX). Publication bias was analyzed using Egger’s linear regression test. All statistical interpretations were reported with a 95 % confidence interval (CI).
2.6. Study outcomes
The primary outcome of the study was the weighted pooled prevalence of drug resistance status (any drug, mono drug, MDR, pre-XDR, XDR, and first or second-line anti-TB drugs) in MTB isolates. Subgroup analyses were performed based on (1) DST method (proportion method vs proportion methods plus other methods [PCR-based sequencing methods or minimal inhibitory concentration (MIC)-based methods or GeneXpert or line-probe assays or sequencing]), (2) year of publication (2000–2015, 2016–2020), and (3) geographical areas (North, Center, East, West, South).
3. Results
3.1. Characteristics of studies
Fig. 1 illustrates the study selection process. Initially, 591 studies were identified during the early literature search. Following the initial screening, 500 articles were excluded due to duplication, irrelevance based on title and abstract. The full texts of the remaining 91 articles were carefully reviewed. Among these, 44 articles were excluded based on predefined exclusion criteria. Finally, 47 eligible articles [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58] were included in the meta-analysis, providing valuable information on first-line anti-TB drug resistance among new and retreatment cases, as well as data on second-line anti-TB drug resistance among new cases.
Fig. 1.
Flow Diagram Showing the Study Selection Process.
The majority of the included studies were conducted in the central region of Iran (n = 16), followed by the southern region (n = 12), western region (n = 6), eastern region (n = 2), northern region (n = 2), and various locations across Iran (n = 9). In terms of drug susceptibility testing (DST) methods, 33 studies exclusively utilized the proportion method, while 13 studies employed a combination of proportion methods and PCR-based sequencing methods, minimal inhibitory concentration (MIC)-based methods, GeneXpert, line-probe assays, or sequencing. Additionally, one study (53) employed a PCR-sequencing assay. Diagnosing drug resistance TB preferably carried out in reference laboratories that are subject to rigorous and standardized quality assurance measures.
3.2. Study population
The analysis encompassed a total of 12,492 TB patients, comprising 11,492 new TB cases and 1,000 previously-treated TB cases.
3.3. First-line Anti-TB drug resistance among new and retreatment cases
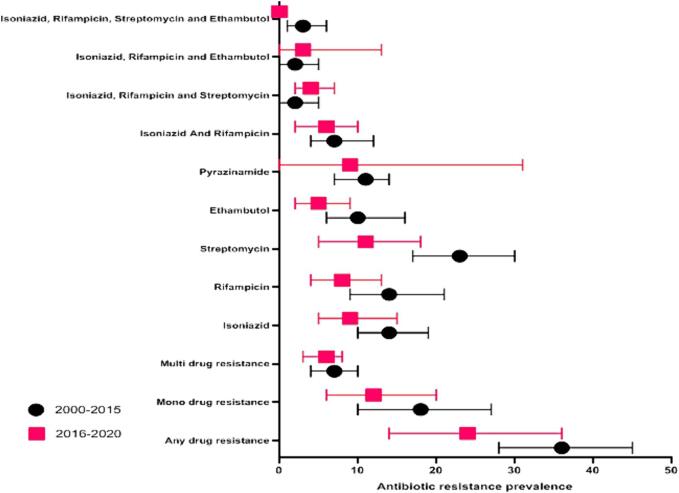
For new cases, resistance was most common against streptomycin (18 %), followed by isoniazid (12 %), rifampin (11 %), pyrazinamide (10 %), and ethambutol (8 %). The prevalence of any-drug resistance, mono-drug resistance, and multidrug-resistant TB (MDR) among new cases was found to be 31 % (95 % CI, 24–38), 15 % (95 % CI, 10–22), and 6 % (95 % CI 4–8), respectively.
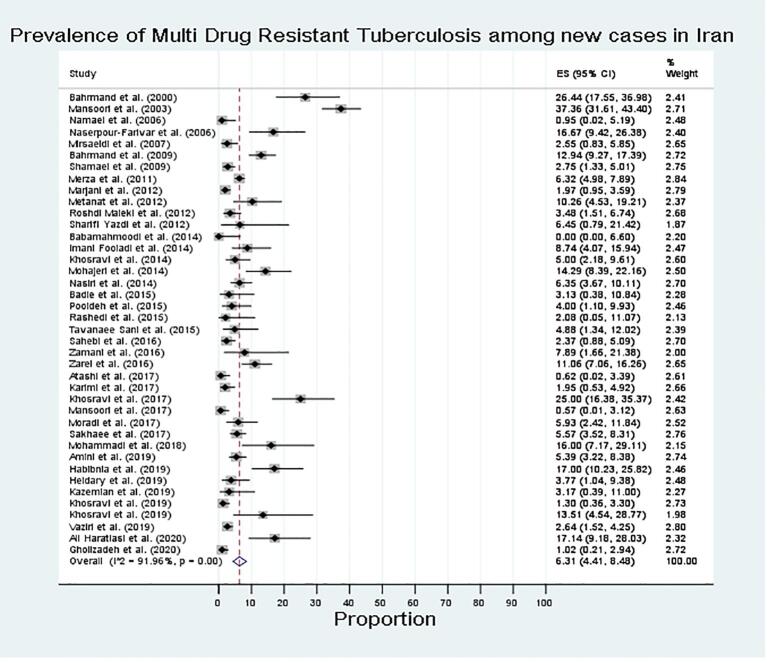
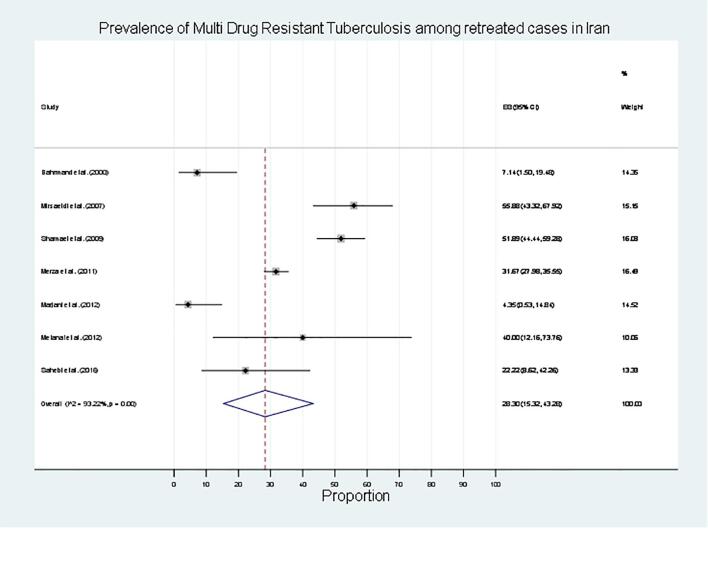
Fig. 2, Fig. 3 show the forest plots of the meta-analysis of MDR in new and retreatment TB cases. There was a significant variation in the rate of MDR among new TB cases among the included studies (P < 0.0001; Fig. 4). Stratified analyses revealed significant differences between published years and DST methods in pooled rates of any-drug resistance, mono-drug resistance, first-line anti-TB drug resistance, and MDR (Table 1; P < 0.0001). Table 1 displays the stratified location of the meta-analysis in new TB cases. Higher rates of any-drug resistance TB were observed for studies from the south and center of Iran.
Fig. 2.
Prevalence of Multi Drug Resistant TB among new cases in Iran.
Fig. 3.
Prevalence of Multi Drug Resistant TB among retreated cases in Iran.
Fig. 4.
Prevalence of first line drug TB among new cases in Iran based on published year.
Table 1.
Prevalence of Drug-Resistant TB Among New Cases in Iran.
| Variables | Sub-groups | Number of studies | Prevalence of Drug Resistance (95 % CI) |
n, N | Heterogeneity Test (I2) | Heterogeneity Test (P value) |
|---|---|---|---|---|---|---|
| Any Drug Resistance | Overall | 36 | 31 (24, 38) | 2045, 7480 | 97.65 | <0.01 |
| Stratified by Years | ||||||
| 2000–2015 | 21 | 36.44 (28.3–44.99) | 1605/5024 | 97.16 | <0.01 | |
| 2016–2020 | 15 | 24.01 (14.12–35.51) | 440/2456 | 97.37 | <0.01 | |
| Stratified by Location | ||||||
| North | 2 | 12.2 (8.19–16.84) | 29/230 | – | – | |
| Center | 14 | 42.07 (29.82–54.83) | 1124/3554 | 98.05 | <0.01 | |
| East | 2 | 23.79 (17.9–30.21) | 45/187 | – | – | |
| West | 5 | 22.86 (4.46–49.48) | 123/711 | 98.10 | <0.01 | |
| South | 9 | 33.54 (17.34–51.98) | 348/1175 | 97.62 | <0.01 | |
| Different Parts | 4 | 15.6 (6.07–28.42) | 376/1623 | 96.90 | <0.01 | |
| Stratified by DST methods | ||||||
| Proportion Method | 24 | 31.44 (23.6–39.84) | 1634/5931 | 97.69 | <0.01 | |
| Double method | 12 | 30.49 (16.17–47) | 411/1549 | 97.73 | <0.001 | |
| Mono drug Resistance | Overall | 26 | 15 (10, 22) | 940/5357 | 97.33 | <0.001 |
| Stratified by Years | ||||||
| 2000–2015 | 16 | 36.44 (28.30–44.99) | 820/4003 | 97.16 | <0.001 | |
| 2016–2020 | 5 | 24.01 (14.12–35.51) | 120/1354 | 97.37 | <0.001 | |
| Stratified by Location | ||||||
| North | 2 | 9.8 (6.19–14.08) | 23/230 | – | ||
| Center | 11 | 25.39 (13.77–39.08) | 684/2527 | 97.79 | <0.001 | |
| East | 2 | 15.44 (10.54–21.04) | 32/187 | – | ||
| West | 4 | 5.67 (0.53–15.08) | 38/661 | 93.33 | <0.001 | |
| South | 4 | 12.45 (0.60–33.95) | 40/446 | 94.90 | <0.001 | |
| Different Parts | 4 | 8.66 (6.75–10.78) | 123/1370 | <0.001 | ||
| Stratified by DST methods | ||||||
| Proportion Method | 18 | 17.86 (10.52–26.60) | 854/4459 | 97.76 | <0.001 | |
| Double method | 8 | 10.48 (4.05–19.26) | 86/962 | 92.84 | <0.001 | |
| Multi-drug Resistance | Overall | 40 | 6 (4, 8) | 529/7903 | 91.96 | <0.001 |
| Stratified by Years | ||||||
| 2000–2015 | 21 | 6.92 (4.02–10.47) | 192/3429 | 93.44 | <0.001 | |
| 2016–2020 | 19 | 5.53 (3.38–8.12) | 177/3602 | 88.17 | <0.001 | |
| Stratified by Location | ||||||
| North | 2 | 0.25 (0.00–1.69) | 1/230 | – | – | |
| Center | 16 | 7.71 (4.12–12.22) | 310/4239 | 98.08 | <0.001 | |
| East | 2 | 2.34 (0.49–5.20) | 5/187 | – | – | |
| West | 5 | 6.00 (1.67–12.48) | 39/793 | 96.15 | <0.001 | |
| South | 9 | 8.11 (4.19–12.48) | 103/1253 | 95.58 | <0.001 | |
| Different Parts | 7 | 5.35 (2.11–9.82) | 71/1255 | 96.25 | <0.001 | |
| Stratified by DST methods | ||||||
| Proportion Method | 27 | 6.45 (4.08–9.28) | 398/5775 | 92.95 | <0.001 | |
| Double method | 13 | 6.03 (3.06–9.83) | 131/2182 | 89.56 | <0.001 | |
| Isoniazid | Overall | 38 | 12 (8, 16) | 1029/7391 | 95.91 | <0.001 |
| Stratified by Years | ||||||
| 2000–2015 | 21 | 14.49 (10.13–19.45) | 352/4301 | 94.15 | <0.001 | |
| 2016–2020 | 17 | 9.42 (4.97–15.02) | 229/2946 | 95.19 | <0.001 | |
| Stratified by Location | ||||||
| North | 2 | 3.23 (1.17–6.08) | 8/230 | – | – | |
| Center | 14 | 16.67 (6.59–27.23) | 435/2955 | 95.94 | <0.001 | |
| East | 2 | 5.01 (2.20–8.73) | 10/187 | – | – | |
| West | 5 | 4.52 (1.07–9.92) | 41/891 | 89.44 | <0.001 | |
| South | 8 | 15.55 (6.59–27.23) | 123/917 | 94.0.13 | <0.001 | |
| Different Parts | 9 | 12.03 (4.27–22.88) | 412/2211 | 97.48 | <0.001 | |
| Stratified by DST methods | ||||||
| Proportion Method | 27 | 12.16 (7.95–17.08) | 881/6060 | 98.63 | <0.001 | |
| Double method | 7 | 11.94 (5.37–20.53) | 148/1331 | 97.71 | <0.001 | |
| Rifampin | Overall | 34 | 11 (8, 16) | 589/4896 | 95.15 | <0.001 |
| Stratified by Years | ||||||
| 2000–2015 | 19 | 14.14 (8.53–20.82) | 352/4301 | 94.80 | <0.001 | |
| 2016–2020 | 15 | 8.10 (4.22–13.01) | 173/2513 | 93.55 | <0.001 | |
| Stratified by Location | ||||||
| North | 2 | 1.13 (0.04–3.18) | 4/230 | – | – | |
| Center | 11 | 14.75 (7.69–23.52) | 229/1421 | 93.75 | <0.001 | |
| East | 1 | 8.54 (3.5–16.8) | 7/82 | – | – | |
| West | 5 | 2.71 (0.28–7.05) | 30/891 | 88.57 | <0.001 | |
| South | 8 | 21.15 (10.2–34.68) | 225/1165 | 96.38 | <0.001 | |
| Different Parts | 7 | 8.66 (3.73–15.27) | 94/1255 | 91.89 | <0.001 | |
| Stratified by DST methods | ||||||
| Proportion Method | 22 | 13.27 (7.89–19.73) | 419/3427 | 96.08 | <0.001 | |
| Double method | 12 | 8.27 (3.87–14.03) | 170/1617 | 92.35 | <0.001 | |
| Ethambutol | Overall | 26 | 8 (4, 11) | 363/ 34,985 | 94.21 | <0.001 |
| Stratified by Years | ||||||
| 2000–2015 | 14 | 10.38 (5.51–16.48) | 215/1563 | 92.40 | <0.001 | |
| 2016–2020 | 12 | 4.82 (1.91–8.85) | 118/2196 | 93.57 | <0.001 | |
| Stratified by Location | ||||||
| North | 1 | 0.00 (0.00–2.07) | 0/176 | – | – | |
| Center | 9 | 15.51 (7.10–26.30) | 213/1550 | 95.89 | <0.001 | |
| East | 2 | 3.19 (0.99–6.36) | 6/187 | – | – | |
| West | 4 | 4.52 (0.04–13.98) | 25/605 | 93.20 | <0.001 | |
| South | 4 | 10.19 (3.26–20.17) | 49/590 | 91.82 | <0.001 | |
| Different Parts | 6 | 2.97 (1.15–5.49) | 40/1137 | 73.97 | <0.001 | |
| Stratified by DST methods | ||||||
| Proportion Method | 20 | 6.84 (3.83–10.75) | 263/3465 | 93.13 | <0.001 | |
| Double method | 6 | 10.54 (1.3–26.26) | 70/780 | 96.77 | <0.001 | |
| Streptomycin | Overall | 23 | 18 (12, 24) | 552/3141 | 94.50 | <0.001 |
| Stratified by Years | ||||||
| 2000–2015 | 14 | 23.10 (16.64–3025) | 390/1717 | 90.84 | <0.001 | |
| 2016–2020 | 9 | 10.82 (4.98–18.45) | 162/1424 | 94.35 | <0.001 | |
| Stratified by Location | ||||||
| North | 2 | 8.12 (4.82–12.12) | 19/230 | – | – | |
| Center | 9 | 24.74 (13.58–37.52) | 317/1550 | 96.22 | <0.001 | |
| East | 2 | 20.12 (14.62–26.23) | 39/187 | – | – | |
| West | 2 | 16.66 (12.34–21.48) | 44/260 | – | – | |
| South | 4 | 17.56 (7.17–31.1) | 51/321 | 89.13 | <0.001 | |
| Different Parts | 4 | 10.27 (1.38–25.47) | 82/755 | 96.68 | <0.001 | |
| Stratified by DST methods | ||||||
| Proportion Method | 18 | 18.45 (12.86–24.77) | 470/2814 | 93.80 | <0.001 | |
| Double method | 5 | 15.69 (2.12–37.64) | 82/489 | 96.73 | <0.001 | |
| HRES | overall | 8 | 3 (1, 5) | 39/1022 | 80.18 | <0.001 |
| HRE | overall | 9 | 2 (0, 5) | 29/1116 | 87.97 | <0.001 |
| HRS | overall | 8 | 3 (0, 8) | 33/836 | 91.73 | <0.001 |
| HR | overall | 26 | 6 (4, 9) | 164/4141 | 92.03 | <0.001 |
| HR Stratified by Years | ||||||
| 2000–2015 | 14 | 7.16 (3.67–11.61) | 164/1932 | 91.32 | <0.001 | |
| 2016–2020 | 12 | 5.59 (2.37–9.94) | 12/2209 | 92.39 | <0.001 | |
| HR Stratified by DST methods | ||||||
| Proportion Method | 18 | 6.77 (3.54–10.87) | 199/2823 | 92.75 | <0.001 | |
| Double method | 8 | 5.66 (2.15–10.55) | 71/1514 | 90.40 | <0.001 | |
| Stratified by Location | ||||||
| North | 1 | 0.57 (0.01–3.12) | 1/176 | – | – | |
| Center | 11 | 7.44 (3.41–12.74) | 140/2087 | 93.05 | <0.001 | |
| East | 1 | 4.88 (1.34–12.02) | 4/82 | – | – | |
| West | 4 | 4.52 (0.92–10.34) | 31/743 | 88.99 | <0.001 | |
| South | 5 | 8.49 (0.99–21.38) | 54/699 | 95.08 | <0.001 | |
| Different Parts | 4 | 6.31 (0.42–17.29) | 40/550 | 93.18 | <0.001 | |
| Pyrazinamide | Overall | 8 | 10 (2, 23) | 40/496 | 95.27 | <0.001 |
| HR Stratified by Years | ||||||
| 2000–2015 | 7 | 3.39 (1.40–6.07) | 37/308 | 73.15 | <0.001 | |
| 2016–2020 | 1 | 0 (0–2.25) | 34/188 | 95.27 | <0.001 | |
| Stratified by Location | ||||||
| North | 0 | – | 0/0 | – | – | |
| Center | 2 | 7.27 (4.37–10.8) | 21/266 | |||
| East | 0 | – | 0/0 | – | – | |
| West | 1 | 24.11 (16.53–33.10) | 27/112 | |||
| South | 0 | – | 0/0 | – | – | |
| Different Parts | 2 | 4.3 (2.15–7.08) | 23/280 | – | – | |
| HR Stratified by DST methods | ||||||
| Proportion Method | 7 | 3.39 (1.40–6.07) | 10/196 | 73.15 | <0.001 | |
| Double method | 1 | 0 (0–2.25) | 61/462 | – | – | |
CI, confidence interval; n, number of events (drug resistance); N, total number of new cases and retreatment cases from the included studies; Double method, Proportion methods plus other methods; DST, drug susceptibility testing; HRES, be resistant to isoniazid + rifampicin + streptomycin + ethambutol; HRE, be resistant to isoniazid + rifampicin + ethambutol; HRS, be resistant to isoniazid + rifampicin + streptomycin; HR, be resistant to isoniazid + rifampicin.
For retreatment cases, resistance was most common against isoniazid (19 %), followed by rifampin (12 %), streptomycin (12 %), and ethambutol (0 %) (Table 2). The summarized prevalence of any-drug resistance, mono-drug resistance, and MDR among retreatment cases in the study population were found to be 68 % (95 % CI 58 %–78 %), 19 % (95 % CI 7 %–34 %), and 28 % (95 % CI 15 %–43 %), respectively (Table 2).
Table 2.
Prevalence of Drug-Resistant TB Among Retreated Cases in Iran.
| Variables | Number of studies | n/N | Prevalence of Drug Resistance [95 % CI] |
p-value | Heterogeneity Test (I2) |
|---|---|---|---|---|---|
| Any drug resistance | 6 | 644, 2562 | 68 [58], [78] | <0.01 | 83.53 |
| Mono drug resistance | 3 | 180, 1605 | 19 [7], [34] | <0.01 | 91.03 |
| Multi drug resistance | 7 | 340, 2624 | 28 [15], [43] | <0.01 | 93.22 |
| Isoniazid resistance | 4 | 281, 1643 | 19 [2], [46] | 0.01 | 88.09 |
| Rifampicin resistance | 3 | 6, 504 | 12 [3], [25] | <0.01 | 0.00 |
| Streptomycin resistance | 3 | 6, 504 | 12 [3], [25] | <0.01 | 0.00 |
| Ethambutol resistance | 3 | 1, 504 | 0 [0, 7] | 0.67 | 4.70 |
CI, confidence interval; n, number of events (drug resistance); N, total number of retreatment cases from the included studies.
3.4. Second-line Anti-TB drug resistance
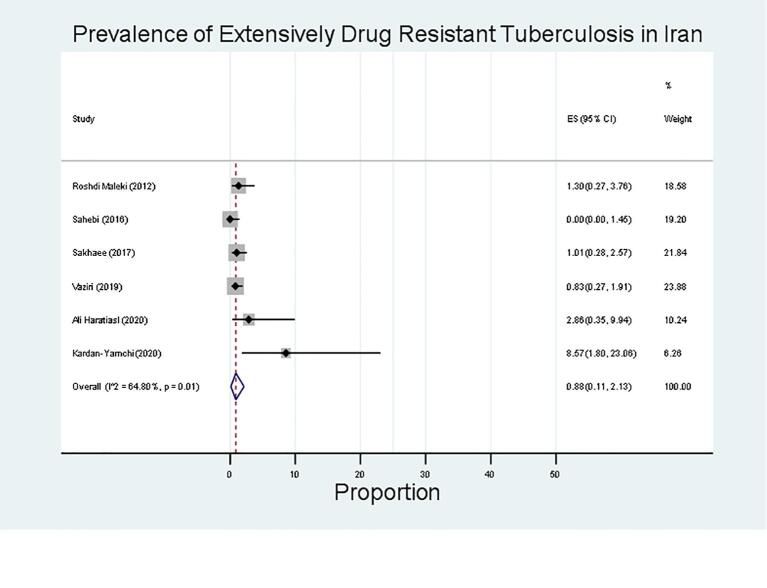
Resistance was most common against ethionamide (50 %; 95 % CI, 18–81; 145/313), followed by kanamycin (12 %; 95 % CI, 3–24; 57/861), capreomycin (4 %; 95 % CI, 2–6; 21/430), amikacin (2 %; 95 % CI, 1–5; 11/265), and ofloxacin (4 %; 95 % CI, 0–11; 24/695) (Table 3). The prevalence of any-drug resistance, MDR, pre-extensively drug-resistant (pre-XDR), and extensively drug-resistant (XDR) among new cases was found to be 60 % (95 % CI, 30–86), 10 % (95 % CI, 4–18), 8 % (95 % CI, 0–5), and 1 % (95 % CI, 0–2), respectively. Fig. 5 shows the forest plot of the meta-analysis of XDR in TB cases.
Table 3.
Prevalence of Second line Drug-Resistant TB in Iran.
| Variables |
N. Studies (n, N) |
|||
|---|---|---|---|---|
| Proportion (95 % CI) | P-value |
Heterogeneity Test (I2) |
||
| Any Drug Resistance | 5 (335, 965) | 60 (30, 86) | <0.01 | 98.73 |
| Multidrug resistance | 9 (110, 1950) | 10 (4, 18) | <0.01 | 95.69 |
| Extensively drug-resistant | 5 (17, 1589) | 1 (0, 2) | 0.01 | 64.80 |
| Pre-Extensively Drug Resistant | 2 (22, 711) | 8 (0, 25) | 0.04 | 93.44 |
| Amikacin | 1 (11, 265) | 2 (1, 5) | <0.01 | 0.00 |
| Kanamycin | 5 (57, 861) | 12 (3, 24) | <0.01 | 94.12 |
| Ethionamide | 3 (145, 313) | 50 (18, 81) | <0.01 | 97.10 |
| Ofloxacin | 2 (24, 695) | 4 (0, 11) | 0.01 | 89.08 |
| Capreomycin | 1 (21, 430) | 4 (2, 6) | <0.01 | 0.00 |
CI, confidence interval; n, number of events (drug resistance); N, total number of retreatment cases from the included studies.
Fig. 5.
Prevalence of Extensively Drug Resistant TB among new cases in Iran.
4. Discussion
Tuberculosis (TB) remains a significant global health challenge, with millions of people infected and hundreds of thousands of deaths each year. Despite international efforts, the reduction targets for TB incidence and mortality set for 2020 were only partially achieved, emphasizing the persistent threat of TB as a public health problem [59]. This study provides an updated review of first- and second-line drug resistance among new and previously treated TB patients in Iran over the past two decades.
Isoniazid is a crucial first-line anti-TB drug, and resistance to it can compromise treatment success and increase the risk of developing multidrug-resistant TB (MDR-TB) [60], [61]. Rifampin, another essential first-line drug, is a potent sterilizing agent for TB treatment. Rifampin resistance is a key indicator for MDR-TB and poses significant challenges in patient management [62]. Cases resistant to both isoniazid and rifampin can lead to treatment failure and the emergence of MDR-TB [63]. This study found that between 2000 and 2020 in Iran, 12 % of new cases were resistant to isoniazid, while 11 % were resistant to rifampin. Among retreatment cases, 19 % were resistant to isoniazid, and 12 % were resistant to rifampin. Globally, the prevalence of isoniazid resistance among new TB patients is 10.7 %, while it is 27.2 % among previously treated cases [64]. The observed isoniazid resistance rate in Iran (12 %) was lower than that reported in some African countries like Benin (27.9 %) [65] and Ethiopia (15.62 %) [66] but similar to China (12.0 %) [67]. Rifampin resistance rates in this study (11 %) align with international data across different regions [68], [69]. Notably, the rate of any drug resistance among new and previously treated TB cases in Iran was 31 % and 68 %, respectively, suggesting a high rate of acquired resistance to anti-TB medications. This phenomenon may be attributed to factors such as inappropriate prescription practices, drug supply issues, poor drug quality, and high treatment failure rates [61], [70], [71], [72].
MDR-TB is a growing concern that poses a significant challenge to the prevention and control of infectious diseases [73]. The prevalence of MDR-TB among new cases in this study was 6 %, higher than the global (3.4 %) and national average (2.84 %) [74]. This indicates that the burden of MDR-TB among new cases may be underestimated, necessitating increased efforts in detection and treatment. While two previous systematic reviews [6], [7] on TB drug resistance in Iran reported similar findings for new patients, the prevalence of MDR-TB among retreated patients decreased from 33.7 % (Nasiri et al., 2014) [6] to 28 % (present study). However, the rate of MDR-TB among retreated cases in this study was higher than reported in several African countries [66], [75], [76].
Streptomycin resistance was the most prevalent among first-line drugs, affecting 18 % of patients in this study. Conversely, Khademi et al. [7] reported that pyrazinamide was the most resistant anti-TB drug. These variations may stem from differences in drug susceptibility testing methodologies, particularly the drug concentrations used [77].
Among second-line drugs, ethionamide exhibited the highest resistance rate (50 %), followed by kanamycin (12 %), capreomycin (4 %), amikacin (2 %), and ofloxacin (4 %). The global surveillance and treatment of XDR-TB is wholly crucial [78]. The prevalence of any drug resistance, MDR, pre-extensively drug-resistant (pre-XDR), and extensively drug-resistant (XDR) among new cases was 60 %, 10 %, 8 %, and 1 %, respectively. The prevalence of XDR-TB in this study aligns with findings from Khademi et al. [7] (0.9 %), but the pre-XDR prevalence (8 %) is a cause for concern, as it suggests the potential development of XDR cases in Iran.
The study revealed geographic disparities in drug resistance rates, with higher rates observed in central and southern Iran. The central region had the highest resistance rates, possibly due to the concentration of published studies, the referral of drug-resistant MTB isolates to Tehran (the capital), and a higher number of immigrants, particularly Afghans, who have a higher rate of resistance [19].
Despite a decreasing trend in drug resistance from 2000 to 2020, the study highlighted a significant gap between confirmed cases and drug resistance testing in TB laboratories in Iran. This suggests missing drug resistance data and underscores the need for better screening and diagnosis of suspected TB cases [79]. Irregular and incomplete TB treatment and inadequate screening for suspected patients are key factors contributing to drug resistance development and distribution [79].
5. Conclusion
In summary, this study reveals that although new and previously treated TB patients in Iran exhibit high levels of resistance to various anti-TB drugs, there has been a promising downward trend in drug resistance rates and TB incidence over the past two decades. However, several critical considerations emerge from these findings.
Firstly, there remains a substantial gap between confirmed TB cases and those who undergo drug resistance testing. This discrepancy highlights the need for improved diagnostic facilities and expanded drug susceptibility testing to cover all confirmed TB cases. Bridging this gap is essential for achieving a sustained decline in TB incidence and drug resistance trends as part of TB control efforts.
Secondly, the study identifies regional variations in drug resistance rates, with some areas exhibiting notably higher rates than others. These disparities emphasize the importance of the lab capacity and staff experience, tailored interventions and resource allocation to address the specific challenges posed by drug-resistant TB in different regions.
Lastly, Iran's proximity to countries with higher TB incidence rates underscores the need for heightened vigilance and cooperation in TB control efforts. Collaborative strategies and information sharing with neighboring nations can contribute to a more comprehensive approach to TB management.
In conclusion, while challenges persist, the overall trajectory of declining TB incidence and drug resistance trends in Iran is encouraging. Continued investment in diagnostic infrastructure, regional-specific interventions, and international collaboration are crucial for sustaining and accelerating progress in TB control and reducing drug resistance rates.
6. Limitations
Several limitations should be considered in this analysis, including the uneven representation of Iranian provinces in the included studies, potential heterogeneity between studies, and the inability to analyze the impact of factors such as age, sex, ethnicity, nationality (Iranian vs. Afghan), socioeconomic status, and lifestyle on drug resistance prevalence.
Ethical statement
The study protocol was approved by the Health Research Ethics Committee of Ilam University of Medical Sciences (reference no. IR.MEDILAM.REC.1400.006).
Funding
Not applicable.
CRediT authorship contribution statement
Sara Abbasian: Writing – original draft, Conceptualization. Hamid Heidari: Writing – review & editing, Writing – original draft, Conceptualization. Danyal Abbasi Tadi: Software, Methodology, Formal analysis. Jalil Kardan-Yamchi: Writing – review & editing, Data curation. Asieh Taji: Investigation, Validation. Atieh Darbandi: Methodology, Data curation. Parisa Asadollahi: Writing – review & editing. Abbas Maleki: Data curation, Writing – review & editing. Hossein Kazemian: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Ilam University of Medial Sciences (Project no: 1226).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2024.100430.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Alene KA, Wangdi K, Clements ACJTm, disease i. Impact of the COVID-19 pandemic on tuberculosis control: an overview. 2020; 5(3): 123. [DOI] [PMC free article] [PubMed]
- 2.Dookie N, Padayatchi N, Naidoo KJCID. Tuberculosis elimination in the era of coronavirus disease 2019 (COVID-19): a moving target. Oxford University Press US; 2022. p. 509-10. [DOI] [PMC free article] [PubMed]
- 3.Marais BJJAddr. The global tuberculosis situation and the inexorable rise of drug-resistant disease. 2016; 102(3-9). [DOI] [PubMed]
- 4.Organization WH. Guidelines for surveillance of drug resistance in tuberculosis: World Health Organization; 2009.
- 5.Faksri K, Kaewprasert O, Ong RT-H, Suriyaphol P, Prammananan T, Teo Y-Y, et al. Comparisons of whole-genome sequencing and phenotypic drug susceptibility testing for Mycobacterium tuberculosis causing MDR-TB and XDR-TB in Thailand. 2019; 54(2): 109-16. [DOI] [PubMed]
- 6.Nasiri M.J., Dabiri H., Darban-Sarokhalil D., Rezadehbashi M., Zamani S. Prevalence of drug-resistant tuberculosis in Iran: systematic review and meta-analysis. Am J Infect Control. 2014;42(11):1212–1218. doi: 10.1016/j.ajic.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Khademi F., Sahebkar A. An updated systematic review and meta-analysis on Mycobacterium tuberculosis antibiotic resistance in Iran (2013–2020) Iran J Basic Med Sci. 2021;24(4):428. doi: 10.22038/IJBMS.2021.48628.11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, med PGJP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed]
- 9.Mirzayev F, Viney K, Linh NN, Gonzalez-Angulo L, Gegia M, Jaramillo E, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. 2021; 57(6). [DOI] [PMC free article] [PubMed]
- 10.World Health Organization %J WHO: Geneva S. WHO announces updated definitions of extensively drug-resistant tuberculosis; 2021.
- 11.Stang AJEjoe. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. 2010; 25(9): 603-5. [DOI] [PubMed]
- 12.Atashi S, Izadi B, Jalilian S, Madani S, Farahani A, Mohajeri P. Evaluation of GeneXpert MTB/RIF for determination of rifampicin resistance among new tuberculosis cases in west and northwest Iran. New microbes and new infections. 2017; 19(117-20). [DOI] [PMC free article] [PubMed]
- 13.Babamahmoodi F., Mahdavi M.R., Jalali H., Talebi B., Roshan P., Mahdavi M. Evaluation of gene mutations involved in drug resistance in Mycobacterium tuberculosis strains derived from tuberculosis patients in Mazandaran, Iran, 2013. Int J Molecul Cellul Med. 2014;3(3):190. [PMC free article] [PubMed] [Google Scholar]
- 14.Badie F., Arshadi M., Mohsenpoor M., Gharibvand S.S. Drug resistance pattern of Mycobacterium tuberculosis isolates from patients referred to TB reference laboratory in Ahvaz. Osong Public Health Res Perspect. 2016;7(1):32–35. doi: 10.1016/j.phrp.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahrmand A., Velayati A., Bakayev V. Treatment monitoring and prevalence of drug resistance in tuberculosis patients in Tehran. Int J Tuberc Lung Dis. 2000;4(6):544–549. [PubMed] [Google Scholar]
- 16.Farazi A., Sofian M., Zarrinfar N., Katebi F., Hoseini S.D., Keshavarz R. Drug resistance pattern and associated risk factors of tuberculosis patients in the central province of Iran. Caspian J Intern Med. 2013;4(4):785–789. [PMC free article] [PubMed] [Google Scholar]
- 17.Haratiasl A.A., Hamzelou G., Amini S., Kardan-Yamchi J., Haeili M., Heidari F., et al. Molecular identification of mutations conferring resistance to rifampin, isoniazid and pyrazinamide among Mycobacterium tuberculosis isolates from Iran. J Chemother. 2020;32(2):75–82. doi: 10.1080/1120009X.2020.1716479. [DOI] [PubMed] [Google Scholar]
- 18.Gholizadeh P, Pourlak T, Asgharzadeh M, Barhagi MHS, Taghizadeh S, Rezaee MA, et al. Gene mutations related to rifampin resistance of tuberculosis in northwest of Iran. Gene Rep 2020; 19(100672).
- 19.Kardan-Yamchi J., Kazemian H., Battaglia S., Abtahi H., Rahimi Foroushani A., Hamzelou G., et al. Whole genome sequencing results associated with minimum inhibitory concentrations of 14 anti-tuberculosis drugs among rifampicin-resistant isolates of Mycobacterium tuberculosis from Iran. J Clin Med. 2020;9(2):465. doi: 10.3390/jcm9020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazemian H., Kardan-Yamchi J., Bahador A., Khonsari S., Nasehi M., Hamzehloo G., et al. Efficacy of line probe assay in detection of drug-resistant pulmonary tuberculosis in comparison with GeneXpert and phenotypic methods in Iran and genetic analysis of isolates by MIRU-VNTR. Infect Drug Resist. 2019;12:3585. doi: 10.2147/IDR.S222905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansoori N, Douraghi M, Rajabloo AA, Taziki M, Yaseri M, Vaziri F. Mycobacterium tuberculosis complex drug resistance in a high tuberculosis incidence area from the WHO eastern mediterranean region. Journal of Pharmacy & Pharmaceutical Sciences. 2017; 20 p. 428-34. [DOI] [PubMed]
- 22.Nasiri M.J., Rezaei F., Zamani S., Darban-Sarokhalil D., Fooladi A.A.I., Shojaei H., et al. Drug resistance pattern of Mycobacterium tuberculosis isolates from patients of five provinces of Iran. Asian Pac J Trop Med. 2014;7(3):193–196. doi: 10.1016/S1995-7645(14)60019-5. [DOI] [PubMed] [Google Scholar]
- 23.Mohajeri P., Norozi B., Atashi S., Farahani A. Anti tuberculosis drug resistance in west of Iran. J Glob Infect. 2014;6(3):114. doi: 10.4103/0974-777X.138506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammadi B, Mohajeri P, Rouhi S, Ramazanzadeh R. The relationship between embb306 and embb406 mutations and ethambutol resistant in Mycobacterium tuberculosis isolated from patiens in west of Iran. Med J Islamic Republic Iran. 2018; 32(117). [DOI] [PMC free article] [PubMed]
- 25.Rashedi J., Mahdavi Poor B., Rafi A., Asgharzadeh M., Abdolalizadeh J., Moaddab S.R. Multidrug-resistant tuberculosis in north-west of Iran and republic of Azerbaijan: a major public health concern for iranian people. J Res Health Sci. 2015;15(2):101–103. [PubMed] [Google Scholar]
- 26.Sahebi L., Ansarin K., Mohajeri P., Khalili M., Monfaredan A., Farajnia S., et al. Patterns of drug resistance among tuberculosis patients in west and northwestern Iran. Open Respir Med J. 2016;10(29 doi: 10.2174/1874306401610010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zakerbostanabad S., Titov L.P., Bahrmand A.R. Frequency and molecular characterization of isoniazid resistance in katG region of MDR isolates from tuberculosis patients in southern endemic border of Iran. Infect Genet Evol. 2008;8(1):15–19. doi: 10.1016/j.meegid.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Bahrmand A.R., Titov L.P., Tasbiti A.H., Yari S., Graviss E.A. High-level rifampin resistance correlates with multiple mutations in the rpoB gene of pulmonary tuberculosis isolates from the Afghanistan border of Iran. J Clin Microbiol. 2009;47(9):2744–2750. doi: 10.1128/JCM.r00548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehdi R.M., Reza M.S., Mohammad R. Study prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis in East Azerbaijan province of Iran. HealthMED. 2012;6(9):3091–3094. [Google Scholar]
- 30.Moradi J., Mohajeri P., Alvandi A., Farahani A., Atashi S., Nasseri K. Molecular identification of mutations associated with pyrazinamide-resistance in multidrug-resistant tuberculosis in eight provinces of iran. J Clin Diagnost Res. 2017;11(11):DC9-DC12. [Google Scholar]
- 31.Tavanaee Sani A, Shakiba A, Salehi M, Bahrami Taghanaki HR, Ayati Fard SF, Ghazvini K. Epidemiological characterization of drug resistance among Mycobacterium tuberculosis isolated from patients in northeast of Iran during 2012-2013. BioMed Res Int. 2015; 2015. [DOI] [PMC free article] [PubMed]
- 32.Habibnia S., Zaker S., Nasiri M.J., Doustdar F., Ghalavand Z., Ghalami M., et al. Prevalence of multidrug-resistant tuberculosis: a six-year single-center retrospective study in Tehran. Iran Arch Clin Infect Dis. 2019;14(5):e82828. [Google Scholar]
- 33.Heidary F, Lashgarian HE, Karkhane M, Peerayeh SN. Molecular detection of isoniazid and rifampin resistance in Mycobacterium tuberculosis isolates from lorestan province, Iran from 2014 to 2017. Archives of Clinical Infectious Diseases. 2020; 15(1).
- 34.Varahram M., Nasiri M.J., Farnia P., Mozafari M., Velayati A.A. A retrospective analysis of isoniazid-monoresistant tuberculosis: among iranian pulmonary tuberculosis patients. Open Microbiol J. 2014;8(1) doi: 10.2174/1874285801408010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaziri F., Kohl T.A., Ghajavand H., Kargarpour Kamakoli M., Merker M., Hadifar S., et al. Genetic diversity of multi-and extensively drug-resistant Mycobacterium tuberculosis isolates in the capital of Iran, revealed by whole-genome sequencing. J Clin Microbiol. 2019;57(1):e01477–e01518. doi: 10.1128/JCM.01477-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shamaei M., Marjani M., Chitsaz E., Kazempour M., Esmaeili M., Farnia P., et al. First-line anti-tuberculosis drug resistance patterns and trends at the national TB referral center in Iran—eight years of surveillance. Int J Infect Dis. 2009;13(5):e236–e240. doi: 10.1016/j.ijid.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 37.Sy M.K., Jabbari H. Primary drug resistance patterns in newly diagnosed tuberculosis patients in Yazd, Southern Province of Iran. Afr J Biotechnol. 2012;11(3):702–706. [Google Scholar]
- 38.Mansouri S, Mirabolhasani Z. The pattern of drug resistance among newly diagnosed and old cases of pulmonary tuberculosis in NRITLD. 2003.
- 39.Merza M.A., Farnia P., Tabarsi P., Khazampour M., Masjedi M.R., Velayati A.A. Anti-tuberculosis drug resistance and associated risk factors in a tertiary level TB center in Iran: a retrospective analysis. J Infect Dev Count. 2011;5(07):511–519. doi: 10.3855/jidc.1259. [DOI] [PubMed] [Google Scholar]
- 40.Marjani M., Baghaei P., Tabarsi P., Shamaei M., Mansouri D., Masjedi M., et al. Drug resistance pattern and outcome of treatment in recurrent episodes of tuberculosis. East Mediterr Health J. 2012;18(9) doi: 10.26719/2012.18.9.957. [DOI] [PubMed] [Google Scholar]
- 41.Mirsaeidi M.S., Tabarsi P., Farnia P., Ebrahimi G., Morris M.W., Masjedi M.R., et al. Trends of drug resistant Mycobacterium tuberculosis in a tertiary tuberculosis center in Iran. Saudi Med J. 2007;28(4):544. [PubMed] [Google Scholar]
- 42.Namaei M.H., Sadeghian A., Naderinasab M., Ziaee M. Prevalence of primary drug resistant Mycobacterium tuberculosis in Mashhad, Iran. Indian J Med Res. 2006;124(1):77. [PubMed] [Google Scholar]
- 43.Heidarnejad H, Nagili B. Primary resistance of Mycobacterium tuberculosis to isoniazid, streptomycin, rifampin, and ethambutol in pulmonary tuberculosis; 2001.
- 44.Ramazanzadeh R., Farnia P., Amirmozafari N., Ghazi F., Ghadertotonchi Z., Kamran J., et al. Comparison between molecular epidemiology, geographical regions and drug resistance in Mycobacterium tuberculosis strains isolated from Iranian and Afghan patients. Chemotherapy. 2006;52(6):316–320. doi: 10.1159/000095971. [DOI] [PubMed] [Google Scholar]
- 45.Metanat M., Sharifi-Mood B., Shahreki S., Dawoudi S. Prevalence of multidrug-resistant and extensively drug-resistant tuberculosis in patients with pulmonary tuberculosis in zahedan, southeastern iran. Iran Red Crescent Med J. 2012;14(1):53. [PMC free article] [PubMed] [Google Scholar]
- 46.Farivar T.N. Drug resistance of Mycobacterium tuberculosis strains isolated from patients with Pulmonary tuberculosis in south. J Med Sci. 2006;6(2):275–278. [Google Scholar]
- 47.Amini S., Hoffner S., Torkaman M.R.A., Hamzehloo G., Nasiri M.J., Salehi M., et al. Direct drug susceptibility testing of Mycobacterium tuberculosis using the proportional method: a multicenter study. J Glob Antimicrob Resist. 2019;17:242–244. doi: 10.1016/j.jgar.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Zarei Z., Emami A., Moghadami M., Kashkooli G.S., Pirbonyeh N. Molecular characterization of isoniazid and rifampicin target genes in multi-drug resistant Mycobacterium tuberculosis isolates from southwest of Iran. Gene Rep. 2017;6:19–25. [Google Scholar]
- 49.Honarvar B, Moghadami M, Emami A, Behbahani AB, Taheri M, Roudgari A, et al. Mycobacterium strain and type of resistance in pulmonary tuberculosis patients: a missed link in Iran’s national tuberculosis plan. Shiraz E-Med J. 2015; 16. p. e27748.
- 50.Zamani S, Haeili M, Nasiri MJ, Imani Fooladi AA, Javadpour S, Feizabadi MM. Genotyping of Mycobacterium tuberculosis isolates from Hormozgan province of Iran based on 15-locus MIRU-VNTR and spoligotyping. International journal of bacteriology. 2016; 2016. [DOI] [PMC free article] [PubMed]
- 51.Karimi S., Mirhendi H., Zaniani F.R., Manesh S.E., Salehi M., Esfahani B.N. Rapid detection of streptomycin-resistant Mycobacterium tuberculosis by rpsL-restriction fragment length polymorphism. Adv Biomed Res. 2017;6 doi: 10.4103/abr.abr_240_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khosravi A.D., Sirous M., Abdi M., Ahmadkhosravi N. Characterization of the most common embCAB gene mutations associated with ethambutol resistance in Mycobacterium tuberculosis isolates from Iran. Infect Drug Resist. 2019;12(579) doi: 10.2147/IDR.S196800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khosravi A.D., Sirous M., Absalan Z., Tabandeh M.R., Savari M. Comparison of drrA and drrB efflux pump genes expression in drug-susceptible and-resistant Mycobacterium tuberculosis strains isolated from tuberculosis patients in Iran. Infection and Drug Resistance. 2019;12:3437. doi: 10.2147/IDR.S221823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khosravi A.D., Shahraki A.H., Dezfuli S.K., Hashemzadeh M., Goodarzi H., Mohajeri P. Genetic diversity of multidrug-resistant Mycobacterium tuberculosis strains isolated from tuberculosis patients in Iran using MIRU-VNTR technique. Kaohsiung J Med Sci. 2017;33(11):550–557. doi: 10.1016/j.kjms.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khosravi A.D., Goodarzi H., Alavi S.M., Akhond M.R. Application of deletion-targeted multiplex PCR technique for detection of Mycobacterium tuberculosis Beijing strains in samples from tuberculosis patients. Iran J Microbiol. 2014;6(5):330. [PMC free article] [PubMed] [Google Scholar]
- 56.Sakhaee F., Ghazanfari M., Ebrahimzadeh N., Vaziri F., Jamnani F.R., Davari M., et al. A comparative study of phenotypic and genotypic first-and second-line drug resistance testing of Mycobacterium tuberculosis. Biologicals. 2017;49:33–38. doi: 10.1016/j.biologicals.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Ali I.F.A., Babak F., Fazlollah M.S., Nematollah J.J. Rapid detection of MDR–Mycobacterium tuberculosis using modified PCR-SSCP from clinical specimens. Asian Pac J Trop Biomed. 2014:4. doi: 10.12980/APJTB.4.2014C1186. p. S165–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pooideh M., Jabbarzadeh I., Ranjbar R., Saifi M. Molecular epidemiology of Mycobacterium tuberculosis isolates in 100 patients with tuberculosis using pulsed field gel electrophoresis. Jundishapur J Microbiol. 2015;8(7) doi: 10.5812/jjm.8(5)2015.18274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WHO GJGTR. Global tuberculosis report 2020. 2020.
- 60.Vemula SL, Gupta MTJJoP, Diagnostic, Medicine TSi. Isoniazid nano-drug delivery systems targeting macrophages for the treatment of tuberculosis. 2022; 1(2): 96.
- 61.Xi Y, Zhang W, Qiao R-J, Tang JJPo. Risk factors for multidrug-resistant tuberculosis: A worldwide systematic review and meta-analysis. 2022; 17(6): e0270003. [DOI] [PMC free article] [PubMed]
- 62.Choi H, Mok J, Kang Y, Jeong D, Kang H-Y, Kim HJ, et al. Nationwide Treatment Outcomes of Patients With Multidrug/Rifampin-Resistant Tuberculosis in Korea, 2011–2017: A Retrospective Cohort Study (Korean TB-POST). 2023; 38(5). [DOI] [PMC free article] [PubMed]
- 63.Ahmad S, Mokaddas EJRMC. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. 2010; 3(2): 51-61. [DOI] [PubMed]
- 64.Dean AS, Zignol M, Cabibbe AM, Falzon D, Glaziou P, Cirillo DM, et al. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: a multicountry analysis of cross-sectional data. 2020; 17(1): e1003008. [DOI] [PMC free article] [PubMed]
- 65.Affolabi D, Adjagba O, Tanimomo-Kledjo B, Gninafon M, Anagonou S, Portaels FJTIJoT, et al. Anti-tuberculosis drug resistance among new and previously treated pulmonary tuberculosis patients in Cotonou, Benin. 2007; 11(11): 1221-4. [PubMed]
- 66.Reta MA, Tamene BA, Abate BB, Mensah E, Maningi NE, Fourie PBJTM, et al. Mycobacterium tuberculosis Drug Resistance in Ethiopia: An Updated Systematic Review and Meta-Analysis. 2022; 7(10): 300. [DOI] [PMC free article] [PubMed]
- 67.Duan Q, Chen Z, Chen C, Zhang Z, Lu Z, Yang Y, et al. The prevalence of drug-resistant tuberculosis in mainland China: an updated systematic review and meta-analysis. 2016; 11(2): e0148041. [DOI] [PMC free article] [PubMed]
- 68.Jenkins HE, Zignol M, Cohen TJPO. Quantifying the burden and trends of isoniazid resistant tuberculosis, 1994–2009. 2011; 6(7): e22927. [DOI] [PMC free article] [PubMed]
- 69.Feyisa SG, Abdurahman AA, Jimma W, Chaka EE, Kardan-Yamchi J, Kazemian HJH. Resistance of Mycobacterium tuberculosis strains to Rifampicin: A systematic review and meta-analysis. 2019; 5(1): e01081. [DOI] [PMC free article] [PubMed]
- 70.Alobu I, Oshi SN, Oshi DC, Ukwaja KNJAPJoTM. Risk factors of treatment default and death among tuberculosis patients in a resource-limited setting. 2014; 7(12): 977-84. [DOI] [PubMed]
- 71.Blöndal KJBotWHO. Barriers to reaching the targets for tuberculosis control: multidrug-resistant tuberculosis. 2007; 85: p. 387-90. [DOI] [PMC free article] [PubMed]
- 72.Zhao P, Li X, Zhang S, Wang X, Liu CJJoIMR. Social behaviour risk factors for drug resistant tuberculosis in mainland China: a meta-analysis. 2012; 40(2): 436-45. [DOI] [PubMed]
- 73.Harichander S, Wiafe E, Mensah KB, Bangalee V, Oosthuizen FJSr. The incidence of TB and MDR-TB in pediatrics and therapeutic options: a systematic review. 2022; 11(1): 1-15. [DOI] [PMC free article] [PubMed]
- 74.Shivekar SS, Kaliaperumal V, Brammacharry U, Sakkaravarthy A, Raj C, Alagappan C, et al. Prevalence and factors associated with multidrug-resistant tuberculosis in South India. 2020; 10(1): 1-11. [DOI] [PMC free article] [PubMed]
- 75.Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw MJTd, travel medicine, vaccines. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. 2018; 4(1): 1-12. [DOI] [PMC free article] [PubMed]
- 76.Gudo PS, Cuna Z, Coelho E, Maungate S, Borroni E, Miotto P, et al. Is multidrug-resistant tuberculosis on the rise in Mozambique? Results of a national drug resistance survey. 2011; 38(1): 222-4. [DOI] [PubMed]
- 77.Amini S, Kardan-Yamchi J, Kazemian H, Nasiri MJ, Hamzehloo G, Hoffner S, et al. The 7H11 agar medium supplemented with calf bovine serum for susceptibility testing of Mycobacterium tuberculosis isolates against pyrazinamide. 2021; 27(12): 1652-7. [DOI] [PubMed]
- 78.Migliori GB, Tiberi S, Zumla A, Petersen E, Chakaya JM, Wejse C, et al. MDR/XDR-TB management of patients and contacts: Challenges facing the new decade. The 2020 clinical update by the Global Tuberculosis Network. 2020; 92. P. S15-S25. [DOI] [PubMed]
- 79.Goyal V., Kadam V., Narang P., Singh V. Prevalence of drug-resistant pulmonary tuberculosis in India: systematic review and meta-analysis. BMC Public Health. 2017;17(1):1–21. doi: 10.1186/s12889-017-4779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.