Abstract
Upon uptake of toxins, insects launch a detoxification program. This program is deployed in multiple organs and cells to raise their tolerance against the toxin. The molecular mechanisms of this program inside the insect body have been studied and understood in detail. Here, we report on a yet unexplored extra-corporeal detoxification of insecticides in Drosophila melanogaster. Wild-type D. melanogaster incubated with DDT, a contact insecticide, in a closed environment died as expected. However, incubation of a second cohort in the same environment after removal of the dead flies was not lethal. The effect was significantly lower if the flies of the two cohorts were unrelated. Incubation assays with Chlorpyrifos, another contact insecticide, yielded identical results, while incubation assays with Chlorantraniliprole, again a contact insecticide, was toxic for the second cohort of flies. A cohort of flies incubated in a DDT environment after an initial incubation of a honeybee survived treatment. Together, our data suggest that insects including Apis mellifera and D. melanogaster have the capacity to modify their proximate environment. Consequently, in their ecological niche, following individuals might be saved from intoxication thereby facilitating colonisation of an attractive site.
Keywords: Xenobiotic, Insecticide, Detoxification, Drosophila, Kin selection
1. Introduction
Xenobiotics including plant secondary metabolites and insecticides challenge insects in their daily life as they may perturb cell, tissue and organ physiology at worst causing death. For survival, hence, they have developed elaborate structural and molecular defence mechanisms to prevent or disarm xenobiotic toxicity [[1], [2], [3], [4]]. Many modern insecticides such as imidacloprid and deltamethrin both applied against mosquitoes are taken up through the cuticle at the body surface. In general, the cuticle that covers the body and the endings of the digestive system serves as a structural barrier against xenobiotic penetration into the body. Hydrophobic molecules may nevertheless percolate through the cuticle. This occurs especially at the ends of the legs, the tarsi, that contact the substratum. Consistently, tarsi have been reported already in the 40's of the last century to be site of entry for insecticides such as Dichlorodiphenyltrichloroethane (DDT), for instance Ref. [5]. They are equipped with gustatory sensilla that have cuticular pores needed for sensing the proximal environment [6,7]. Thickening of the tarsal cuticle in response to continuous exposure to insecticides has been demonstrated in mosquitos [8]. The uptake mechanisms of insecticides via the tarsi have not been studied in detail. Tarsi are not simple passive structures in receiving and taking up information from the outside. They also actively produce and deposit material that may be sensed by mates at least in hymenopterans, including ants, honey, and bumble bees. Members of this order possess tarsal or leg tendon glands that secrete waxes and hydrocarbons marking the site of visit [9]. Nestmates or non-nestmates are able to recognize and interpret the secreted footprint material and adjust their behaviour according to the information. Tarsal or leg tendon glands have been also described in other insect orders such as Hemiptera [10]. In this case, the secreted material is probably rather needed for adhesion. The dipteran Calliphora erythrocephala, when walking, produces tracks of secreted material, which is soluble in lipid solvents [11]. The origin and the function of this material is yet unknown. It is not impossible that footprints outside hymenopterans may be processed by mates, as well.
In the present work, we sought to study the dynamics of insecticide uptake through the cuticle in the model insect Drosophila melanogaster. In particular, we were primarily interested in analysing how fly populations responded to exposure to the model contact insecticide DDT. Our finding suggest that an extra-corporeal detoxification mechanism may exist in insects that protects them against their proximal environment. As protection extends to insects visiting the site of the toxic micro-environment after the first visit of their relatives, we consider this behaviour as altruistic.
2. Results
2.1. DDT toxicity is reduced after incubation with flies
To start with, we determined the toxicity of DDT by incubation of two different wild-type fly populations (Tübingen2018, Dijon2000) in a series of DDT amounts (Fig. 1A and Fig. S1). We used two different wild-type lines in order to exclude possible line-specific responses. The effects of DDT were also tested on 91R flies that had been selected for DDT resistance [12]. We scored for paralysis and subsequent death that occurred with an efficiency that depended on the insecticide amounts (see supplementary Movies 1 and 2 for experimental setup). In general, females were more robust than males. In Tübingen2018 flies, the LD50 was around 1 μg/vial for males and 2–5 μg/vial for females. In a vial with 10 or 20 μg of DDT almost all flies of both sexes died after 4 h. The following experiments were therefore performed with these two amounts.
Fig. 1.
DDT toxicity declined after contact with flies. Wild-type flies (females and males were treated separately) were incubated with different DDT amounts as indicated for 4 h (A). The knockout (KO) rate of these flies was assessed. The p-values after a pairwise Student's T-test were: for males: control (acetone only) vs. 2, p = 0.7151, not significant; control vs. 10, p = 0.0008, ***; control vs. 50, p < 0.0001, ****; control vs. 100, p < 0.0001, ****; control vs. 200, p < 0.0001, ****; control vs. 500, p < 0.0001, ****; control vs. 1000, p < 0.0001, ****; control vs. 2000, p < 0.0001, ****. For females: control vs. 2, p = 0.5604, not significant; control vs. 10, p = 0.0184, *; control vs. 50, p = 0.0018, **; control vs. 100, p < 0.0001, ****; control vs. 200, p < 0.0001, ****; control vs. 500, p < 0.0001, ****; control vs. 1000, p < 0.0001, ****; control vs. 2000, P < 0.0001, ****. First cohort wild-type or 91R flies (females and males were incubated separately) were incubated in DDT-vials (100 μg) for 4 h; after removal of these flies, a second cohort of wild-type or 91R flies was incubated in the same vial for 4 h (B). As a control, flies were incubated in formerly unused DDT-vials for 4 h in parallel to the second cohort flies. The survival rate of these flies was assessed. The p-values after a pairwise Student's T-test were: for Dijon males: first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; for Dijon females: first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; for Tübingen males: first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; for Tübingen females: first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, P < 0.0001, ****; for 91R males: first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, P < 0.0001, ****; for 91R females: first cohort vs. second cohort, p = 0.0005, ***; second cohort vs. control, p = 0.0001, ***.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28392
The following are the Supplementary data related to this article:
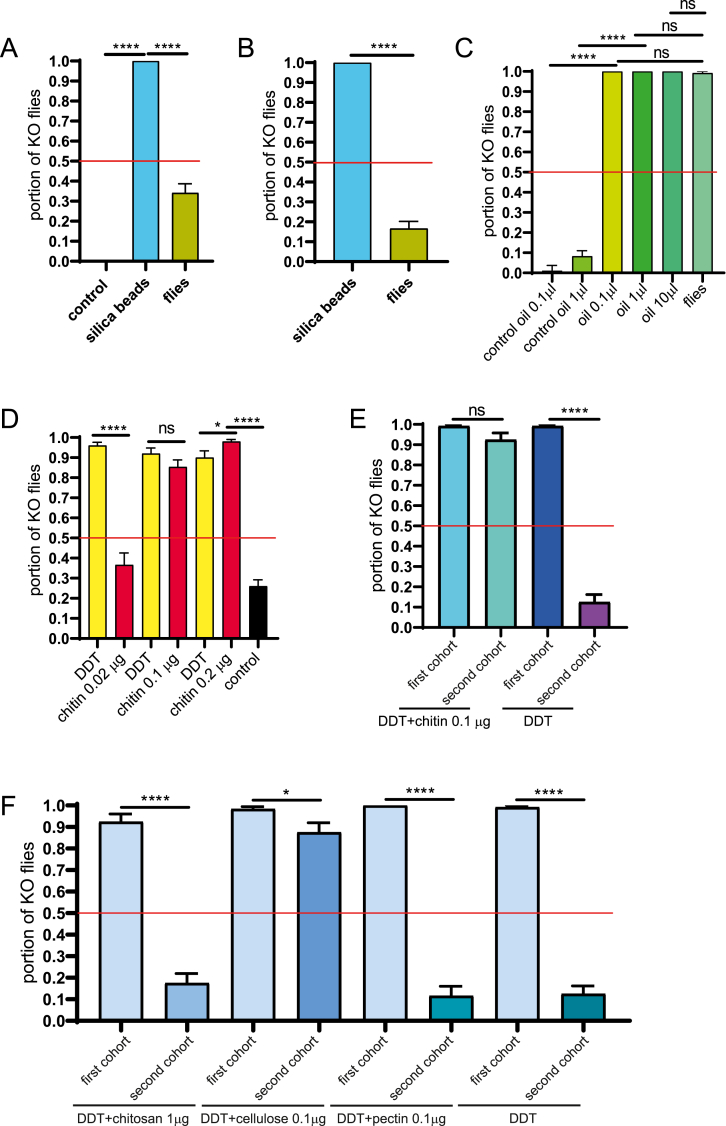
To test whether repeated exposure to insecticides may decline insecticide toxicity, we incubated two populations of different wild-type flies successively in a DDT contaminated vial (Fig. 1B). Flies of the first population, as shown above, died after 4 h of incubation. Flies of the second population survived, by contrast, when incubated in the same vial after removal of the corpses. This effect was observed in all wild-type lines tested. Two scenarios may explain this finding. Either the first cohort flies actively detoxified the DDT contaminated site, or DDT amounts were degraded or depleted by adhesion to the surface of these flies. In both cases, the flies of the second cohort encountered lower amounts of DDT that were no longer toxic. In either case, the first cohort flies play an important role in this assay. As we did not observe fundamental differences between the wild-type lines, most of the following experiments we continued with a single wild-type population (Tübingen2018). We replaced the first cohort flies (Tübingen2018) with silica beads before incubating the second cohort of flies (Tübingen2018) 4 h to challenge both possibilities. First, we reasoned that adsorption of DDT on silica beads mimicking the mere physical presence of fly bodies (with similar sizes, Fig. S3) and, by consequence, depletion of DDT might reduce its toxicity. Second, we argued that the observation that DDT retained its toxicity when second cohort flies were incubated after incubation of silica beads, should suggest that the first cohort flies in the previous experiments actively modified and detoxified their environment. Indeed, flies (equivalent to second cohort flies) died after incubation of silica beads for 4 h (Fig. 2A and B). Adsorption at the surface, thus, seems not to significantly reduce DDT toxicity. Rather, modification and detoxification of DDT by first cohort flies may explain the observation of reduced DDT toxicity.
Fig. 2.
Chitin retains DDT toxicity. Silica beads were incubated in a DDT-vial (100 μg) for 4 h prior to the addition of the second cohort wild-type flies (Tübingen2018, five females and five males, A). As a first control (control), silica beads were incubated with flies for 4 h; they are non-toxic. As a second, standard control (flies), second cohort of flies were incubated in a DDT-vial (100 μg) after a first cohort of flies for 4 h. The p-values after a pairwise Student's T-test were: control vs. silica beads, p < 0.0001, ****, and silica beads vs. flies, p < 0.0001, ****. Tübingen2018 flies (five females and five males) were exposed to DDT-contaminated silica beads or dead fly corpses of the first cohort for 4 h (B). The p-values after a pairwise Student's T-test was: silica beads vs. fly corpses, p < 0.0001, ****. In both experiments, the knockout (KO) rate of flies was assessed. Female and male wild-type flies (Tübingen2018, each five) were exposed to DDT (200 μg) or DDT (200 μg) with various amounts of oil or fly (Tübingen2018) wash solution for 4 h (C). The p-values after a pairwise Student's T-test were: control oil 0.1 vs. oil 0.1, p < 0.0001, ****; control oil 1 vs. oil 1, p < 0.0001, ****; oil 0.1 vs. flies, p = 0.3282, not significant; oil 1 vs. flies, p = 0.3282, not significant; oil 10 vs. flies, p = 0.3282, not significant. After incubation of female wild-type flies (Tübingen2018) for 4 h with DDT (200 μg), various amounts of chitin (0.02, 0.1, 0.2 and 0 mg) were supplemented before the second cohort of flies were added (D). As a control, a second cohort of flies was incubated in a DDT-vial (200 μg) after the first cohort of flies. A similar experiment was conducted with chitin present during incubation of both cohorts (E). The p-values after a pairwise Student's T-test were: DDT vs. 0.02 μg chitin, p < 0.0001, ****; DDT vs. 0.1 μg chitin, p = 0.1478, not significant; DDT vs. 0.2 μg chitin, p < 0.0320, *; 0.2 μg chitin vs. control (0 μg chitin), p < 0.0001, ****. First cohort female wild-type flies (Tübingen2018) were incubated with chitosan, cellulose and pectin together with DDT (100 μg) for 4 h before the addition of the second cohort female wild-type flies (F). The p-values after a pairwise Student's T-test were: DDT+ 1 μg chitosan, first cohort vs. second cohort, p < 0.0001, ****; DDT+ 0.1 μg cellulose, first cohort vs. second cohort, p = 0.0278, *; DDT+ 0.1 μg pectin, first cohort vs. second cohort, p < 0.0001, ****; DDT: first cohort vs. second cohort, p < 0.0001, ****. In all experiments, the knockout (KO) rate of flies was assessed.
2.2. DDT morphology differs before and after incubation with flies
After addition of the DDT solution to a glass vial and evaporation of acetone, an opaque precipitate marked the bottom of the vial. We had a closer look at the appearance of this precipitate in a stereo-microscope wondering whether its morphology might be different after incubation with different wild-type flies. Before addition of flies to a DDT coated vial, numerous drop-like structures of various sizes are found on the bottom of the vial (Fig. S2). After addition of flies, the precipitate was flattened with a distinct crystalline appearance. The texture of the bottom material was similar in vials incubated with silica beads. Despite of this similarity, DDT retains its toxicity after incubation with silica beads; we, therefore, conclude that flies somehow modify the morphology of DDT in our assays.
2.3. Effects of cuticle material to DDT toxicity
How may flies detoxify their environment? This question was addressed using Tübingen2018 flies. Candidate molecules that may interfere with DDT toxicity are cuticular hydrocarbons on the fly body surface. Addition of fly surface wash solutions or vegetable oil (mimicking surface lipids, the so-called cuticular hydrocarbons, CHCs) to the DDT incubation vial did not detoxify DDT exposed to the first cohort flies (Fig. 2C). Thus, lipids are probably not involved in DDT detoxification.
The insect cuticle harbours large amounts of the polysaccharide chitin [13]. Chitin and its derivative chitosan are being used as adsorbents of organic dyes and metals for waste management [14,15]. Potentially, thus, cuticle chitin of the first cohort flies may adsorb DDT and thereby reduce its effective amounts on site. If this is true, we reasoned that second cohort flies should survive at a higher rate in presence of chitin. Chitin was supplemented to the DDT vial after incubation of the first cohort flies (Fig. 2D). In these vials, the second cohort flies died at a higher rate than in vials without chitin. This effect occurred also when chitin was supplemented already to the vial with the first cohort flies (Fig. 2E). Thus, contrary to our expectation, chitin enhances DDT toxicity to the second cohort flies. Of note, chitin alone is not toxic to flies. In summary, DDT retains its toxicity in the presence of chitin. Based on these results, we asked whether other polysaccharides including chitosan (the product of chitin deacetylation), cellulose and pectin have a similar effect on DDT toxicity as chitin. After incubation of first cohort flies with DDT and either of these polysaccharides, we monitored the survival of second cohort flies. While in the presence of cellulose, DDT retained its toxicity, chitosan and pectin did not influence DDT detoxification by the first cohort flies (Fig. 2F) We tentatively conclude that not the physical but the chemical properties of polysaccharides might be important for their interaction with DDT in our assays.
Although the mode of function of chitin on DDT is enigmatic, we can draw an important conclusion from these experiments as they demonstrate that in the initial trials without chitin or cellulose, DDT is present but chemically masked or detoxified when the second cohort flies are incubated in the vial after the first cohort. In other words, the first cohort flies do actively, but reversibly, modify the substratum. Again, chitin and cellulose are obviously able to reconstitute DDT toxicity despite of the detoxification efforts of the flies.
2.4. Tarsi are involved in extracorporeal detoxification
DDT is a contact insecticide. In our movies (suppl. movies 1 & 2), we observed that flies mostly contact the vial surface with their tarsi. During paralysis and death, they lie on their backs. To identify the body part that is responsible for DDT uptake and detoxification, we removed either the proboscis, the wings (that are held dorsally) or the tarsi of Tübingen2018 flies (first cohort) before exposure to the insecticide and monitored the effect of the site on second cohort Tübingen2018 flies. After successful wound-healing, flies without proboscis, wings or tarsi died upon contact with DDT (Fig. 3). Significantly more second cohort flies were paralysed in these assays compared to the control. Therefore, we conclude that the body surface including the proboscis, the wings and the tarsi contributes to detoxification. This effect was especially strong when the first cohort flies did not have tarsi.
Fig. 3.
Different body parts contribute to DDT detoxification outside the fly body. First cohort females (Tübingen2018) without proboscis (A), legs or wings (B) were exposed to DDT (100 μg) before second cohort flies for 4 h. The knockout (KO) rate of these flies was assessed. As a control, unmutilated first and second cohort flies were used. The p-values after a pairwise Student's T-test were: control vs. no proboscis, P = 0.0106,*; control vs. no legs, p < 0.0001, ****; control vs. no wings, p = 0.0366, *.
2.5. DDT toxicity differs in different wild-type populations
We had noted that on average Dijon flies were more sensitive to DDT than Tübingen flies (Fig. S1). We questioned whether DDT sensitivity may correlate with the efficiency of the first cohort flies to detoxify their environment in our assay. Therefore, after incubation of the first cohort flies with DDT, we incubated a different wild-type population as a second cohort (Fig. 4A). The survival rate of the second cohort was lower when the wild-type populations differed in the experiment than when the same population was incubated in the consecutive vials. This finding suggests that there might be a population-specific effect on detoxification and sensing a detoxified environment.
Fig. 4.
DDT detoxification is more efficient if the first and second cohort flies are related. In this experiment, the first and second cohort female flies derived from different wild-type (Tübingen2018 and Dijon2000) populations (A). First cohort flies were incubated in DDT-vials (200 μg) for 4 h. Thereafter, these flies were removed and second cohort flies were added in the same vial. The p-values after a two-way ANOVA test were: Tübingen-Tübingen (2000) vs. Tübingen-Dijon (2000), p < 0.0001, ****; Tübingen-Tübingen (2000) vs. Dijon-Tübingen (2000), p < 0.0001, ****. Instead of first cohort flies, a honeybee worker was incubated in a DDT-vial (500 μg) before addition of a second cohort of wild-type flies (Tübingen2018; five females and five males, B). As a standard control, second cohort flies were added to a DDT-vial after the first cohort of flies. In all experiments, the knockout (KO) rate of flies was assessed. The p-values after a pairwise Student's T-test were: DDT 500: honey bee vs. flies, p < 0.0001, ****, flies vs. flies, p < 0.0001, ****; DDT 1000: honey bee vs. flies, p < 0.0001, ****; flies vs. flies, p < 0.0001, ****; DDT 2000: honey bee vs. flies, p < 0.0001, ****; flies vs. flies, p < 0.0001, ****; DDT 5000: honey bee vs. flies, p < 0.0001, ****; flies vs. flies, p < 0.0001, ****.
2.6. Honeybees detoxify their environment
Next, we addressed the possibility that other insect species than D. melanogaster might have an similar effect on DDT toxicity. For this purpose, we incubated a honeybee (Apis mellifera) worker in a vial containing different amounts of DDT (Fig. 4B). This incubation was lethal to the honeybee. After removal of the dead honeybee, a cohort of wild-type D. melanogaster was incubated in the same vial. These flies survived this treatment. We conclude that insects, along with their internal detoxification responses, may possess a detoxification mechanism that acts outside their body.
2.7. Chlorpyrifos toxicity is reduced after repeated exposure to flies
We wondered if this extra-corporeal detoxification response may modify the efficiency of other, unrelated xenobiotics. We repeated the two-cohort experiments with the insecticides Chlorpyrifos and Chlorantraniliprole (Fig. 5A and B). While Chlorpyrifos was detoxified by the first cohort flies in these assays, Chlorantraniliprole retained its toxicity. Thus, whereas some chemically different xenobiotics are detoxified by the extra-corporeal detoxification response, some others are not targeted by this process.
Fig. 5.
Flies selectively detoxify external insecticides. Exposure of first and second cohort wild-type flies (Tübingen2018; females and males were incubated separately) to Chlorpyriphos (A) or Chlorantraniliprole (B) for 4 h. In both experiments, the knockout (KO) rate of flies was assessed. As a control experiment, second cohort flies were incubated in a insecticide-vial without prior incubation with the first cohort of flies The p-values after a pairwise Student's T-test were: (A) Dijon males, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; Dijon females, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; Tübingen males, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; Tübingen females, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; 91R males, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; 91R females, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****. (B) Dijon males, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; Dijon females, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; Tübingen male, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; Tübingen female, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; 91R males, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****; 91R females, first cohort vs. second cohort, p < 0.0001, ****; second cohort vs. control, p < 0.0001, ****.
3. Discussion
3.1. DDT is a lethal contact insecticide
In the present work, the LD50DDT for Tübingen2018 males is between 1 and 2 μg per vial, while for females the value lies between 2 and 5 μg per vial. These ranges are in agreement with previously determined LD50 values of around 1 μg per vial for Canton S wild-type flies [16]. We also observed that Dijon2000 flies were more sensitive to DDT than Tübingen2018 flies. In our assays, females of both populations were more robust than males, a trait that had not been investigated in previous works. In general, differences in susceptibility to DDT are common in fruit flies [12]. In principle, these differences partly rely on over-transcription of genes coding for detoxification enzymes such as Cyp6g1 and Cyp12d4 in resistant or tolerant fly populations (e.g. 91R). Selective sweeps in genome regions including other types of loci not related to detoxification may also occur in fly populations exposed to DDT. Genomics and transcriptomics analyses of Tübingen2018 and Dijon2000 flies should shed light on this issue.
3.2. Extra-corporeal detoxification in insects
Based on our finding that a second cohort of flies survives incubation in a DDT or chlorpyrifos contaminated site after incubation of a first cohort of flies, we conclude that flies deploy an extra-corporeal detoxification event on site. Albeit, due to their tolerance, with a lesser amplitude, the DDT resistant 91R flies display a similar response as non-selected wild-type flies. We conclude that the extra-corporeal mode of detoxification is additive to the detoxification mode occurring inside the body.
In any case, the detoxification of the environment is not exhaustibly explained by mere absorption of DDT by the fly body. It rather relies on a reversable biochemical or physical reaction involving a secreted tarsal substance. This interpretation is strongly supported by the finding that addition of polysaccharides (chitin, cellulose) after incubation of the first cohort of flies reconstitutes toxicity of the substratum probably by physical or chemical interaction with the complex consisting of DDT and the deposited fly substance. Chitin and cellulose (but not chitosan or pectin) interact either directly with sequestered DDT molecules or with the possible fly DDT-masking substance to trigger DDT release or reconstitute its toxicity. The underlying molecular mechanisms await characterisation (see below).
The extra-corporeal detoxification process involves different body parts. Wings, the proboscis and the tarsi of the first cohort flies contribute to survival of later visitors of the contaminated site. While the tarsi and the proboscis may evidently participate at this process through repeated contact with the DDT substratum, the role of the wings is probably rather indirect. As obvious in our movies, paralysed flies may for an extended time lie on their backs and thereby directly contact the DDT with their wings. We speculate that grooming may cause a distribution of the detoxifying material produced by the proboscis or legs (see below) on the wings. Alternatively, surface material such as CHCs present on the wings could directly exert a detoxifying effect on DDT and Chlorpyrifos.
Protection of next visitors is lowest when first visitors were tarsa-less. Several evidence suggest that the insect tarsi are active organs involved in interaction with the proximal environment. They are endowed with gustatory and campaniform sensilla that have pores and receive molecular information by contact. This sensing activates the nervous system that ultimately controls the behaviour of the insect. In addition, tarsi have glands or gland-like cells that secrete material needed for adhesion or communication at least in eusocial insects. The content of these glands, at least in some species including the hemipteran Coreus marginatus, can be actively discharged to the substratum [17]. This secreted material consists of lipidic molecules [18]. Moreover, tarsi are colonised by bacteria that may have an impact on tarsal physiology [19]. Together, we think that insect tarsi as the site of contact with xenobiotics may possess an autonomous program to deal with information of the proximal environment. In a working model, a tarsal circuitry launched by uptake of a xenobiotic trough the pores of the sensilla may trigger a local secretion of detoxifying molecules for survival.
3.3. What may be the molecular mechanism of tarsal DDT detoxification?
If xenobiotics overcome the cuticle barrier, potent genetic and molecular programs are elicited for detoxification [20]. The molecular players of the detoxification response have been studied extensively in various insect species. They act in concert in different internal tissues such as the fat body, the Malpighian tubules, and the midgut. Internal DDT detoxification has been shown to involve several P450 monooxygenases including Cyp4p1 and Cyp4p2 or Cyp6g1, for example [21,22]. According to the FlyAtlas anatomy expression data, these enzymes are especially present in the midgut, the fat body and the Malpighian tubules [23]. However, the dynamics of their expression on xenobiotic exposure has not been determined in detail. The tarsal detoxification mechanism may require the activity of these enzymes in the tarsi or, after transmission of the response to the main body, in the fat body and the Malpighian tubules. These alternatives remain to be studied. This inside detoxification, however, that may explain DDT sensitivity differences in different fly strains possibly does not contribute to the outside detoxification. Rather, extra-corporeal detoxification seemingly involves a yet unidentified reversible modification under the control of molecules or enzymes at the tarsal cuticle surface.
The activity of the yet unknown types of enzymes that reversibly detoxify DDT and Chlorpyrifos may target the crystalline structure of these insecticides. Consistently, we observe a change in the morphology of the DDT precipitate in glass vials before and after incubation with flies. DDT is known to occur in two different crystalline structures, named Form I and II [24]. The transition of one form into the other is temperature dependent. Form II toxicity is somewhat higher (by 22%) than Form I toxicity. The smallness of this difference, hence, would not exhaustively explain the observed difference between DDT toxicity to first and second cohort flies. Regarding Chlorpyrifos, two conformations of the molecule were observed in the crystalline unit cell [25]. Differences in toxicity of these two forms was not analysed. Together, the recurrent occurrence of different crystal structures of insecticides [24,26,27] suggests that changes in this property my represent a means to control toxicity by insects. The model insect D. melanogaster is a perfect model system to advance in ecological genetics in this direction as understanding this problem will have a considerable impact on insect ecology and pest science.
3.4. Is the extra-corporeal detoxification an altruistic trait?
According to W. D. Hamilton's inclusive fitness theory (kin selection), a trait or behaviour is altruistic when the fitness cost of the actor is lower than the fitness benefit of the recipient which is directly proportional to the genetic relatedness between actor and recipient (r.b > c; r = relatedness, b = benefit for recipient, c = cost for actor; [28]). In insects, usually eusocial species such as ants, bees and termites are considered to show altruistic behaviour. This extends to the point that “an animal acting on this principle would sacrifice its life if it could thereby save more than two brothers, but not for less” [29]. Here, we report on our observations during exposure of the non-eusocial fruit flies (Drosophila melanogaster) to insecticides arguing that first visitors of a contaminated site are able to detoxify the site to the benefit of the second visitors while, in the extreme case, they die. In conclusion, along with the internal detoxification response, insects have developed an extra-corporeal detoxification mechanism that, in contrast to the former, does not only protect the individual that launches it but the population of insects in the niche (Fig. 6). The altruistic notion comes into play considering that in the field, D. melanogaster flies tend to cluster in their micro-habitat [30].
Fig. 6.
Model. Insects contacting xenobiotics including insecticides or plant secondary metabolites in their proximal environment are able to modify it with their tarsi. In the field, this may be sufficient to ensure survival. Even if they do not survive the contact, this process potentially protects the following visitors.
3.5. Limitations of the study
Admittedly, our data were collected in an artificial environment. Compared to the surfaces of fruits and leaves, which represent the natural habitat of fruit flies, a glass vial is relatively inert. Thus, in the field, physical and chemical interactions between plant- and insect-borne molecule cocktails (even when neglecting present microorganisms) are supposedly more complex than in our laboratory set-up. Moreover, DDT or Chlorpyrifos are not natural compounds; D. melanogaster is usually exposed to plant secondary metabolites and molecules from decaying fruits. Besides, the chemicals including chitin, chitosan, cellulose, pectin and the vegetable oil that were used in this work were applied at arbitrary amounts in the range of the fly weight. Possibly, their amounts and purities are not reflecting those present in nature. Together, the relevance and effectiveness of the postulated tarsal substance to modify the proximal environment of the fly are vague and need to be explored in detail in the field.
4. Materials and methods
4.1. Fly husbandry
Tübingen2018 and Dijon2000 wild-type [31] and 91R [16] flies were kept in vials with standard fly food containing 8.5 g Agar, 76.6 g corn grist, 10 g soy flour, 18 g dry yeast, 21.5 g treacle, 81.6 g malt extract, 15 ml Nipagin (stock solution 140 g in 1 l ethanol) and 4.5 ml propionic acid at 25 °C. Three to five days old flies were collected for experiments. As the wild-type populations in trend yielded identical results, most experiments were conducted using Tübingen2018 flies. 91R flies are considered as a control for DDT resistance.
4.2. Preparation of insecticides
DDT (1,1,1-trichloro-2,2-bischlorophenylethane, Lot: #BCBW0671), chlorpyriphos (Lot: #SZBD343XV), were purchased from Sigma-Aldrich. Chlorantraniliprole (Lot: #CEAG210011) was purchased from 3A Chemicals. Insecticides were dissolved in acetone. Solutions with 2, 10, 50, 100, 200, 500, 1000, 2000 μg of DDT per ml yielding 0.02, 0.1, 0.5, 1, 2, 5, 10 and 20 μg per vial (according to Abdu-Allah et al. [32]), three chlorpyrifos solutions with the concentrations of 1 μg/ml yielding 0.01 μg per vial (according to Wang et al. (31)) and chlorantraniliprole solutions with 5000 μg chlorantraniliprole per ml yielding 50 μg per vial (according to Ma et al. [33]) were prepared.
4.3. Preparation of other chemicals
Silica beads (Lot: #BCBX4624) and chitin (Lot: #59F7265), chitosan, cellulose and pectin were purchased from Sigma-Aldrich. Chitin, chitosan, cellulose and pectin were emulsified in acetone. Emulsions of 2, 10, and 20 mg of chitin per ml were prepared. The concentration of chitosan was 100 mg/ml. Cellulose and pectin concentrations were both 10 mg/ml. Vegetable oil (rapeseed oil) was purchased at Penny (Tübingen). 0.1, 1 and 10 μl of oil were dissolved in 100 μl acetone or in 100 μl of a DDT (2000 μg/ml) solution.
The amounts of applied polysaccharides is roughly in the range of the weight of a couple of flies (appr. 10 mg/fly). Likewise, the applied amounts of vegetable oil are roughly in the range of the amounts of surface lipids in a couple of flies (1–2 μg/fly, Wang, Moussian). Arguing that only a fraction of surface lipids or cuticle material (chitin and chitosan; cellulose and pectin were used as controls) is in contact with the vial surface, these amounts are considered as rather excessive.
4.4. Insecticides toxicity assays
In general, to apply insecticides to a vial, 100 μl of an insecticide solution was added into 5 ml empty glass vials, which were placed in a fume hood for 4 h to allow complete evaporation of the acetone. Vials treated with acetone alone were used as control. By this treatment, the bottom of the glass vial and about 1 mm of the lateral walls were coated with the substance. 10 females or males (first cohort) were transferred into the vial, which was closed by a plastic cap with holes. The number of knockdown flies was recorded every hour for 4 h. Knockdown occurred when flies showed paralysis, were lying on their backs and were unable to climb on the wall of the vial and eventually died. After incubation of the first cohort flies, the vial was emptied and a second cohort of male or female flies was added to the vial. Again, knockdown was recorded every hour for 4 h. As a control, no first cohort flies were added to the DDT vial before incubation of the second cohort in order to test for time-dependent degradation of DDT. For proboscis or leg removal experiments, flies without proboscis or leg served as the first cohort flies. The body parts were removed using ultra-fin scissors under a binocular, and the wounds were allowed to heal for several hours before the experiment. In the honeybee experiment, a single Apis mellifera worker (Lomersheim, Germany) was incubated in a DDT-vial (500 μg) instead of the first cohort of flies. Second cohort flies (five males and five females) were added to the vial after 4 h of incubation when the honeybee was dead. In the silica beads experiment, 10 silica beads were added to the DDT-vial (500 μg) without flies. Thereafter, after removal of the beads, 10 flies were incubated in the same vial. Also, 10 flies were exposed to the removed silica beads to test for DDT adhesion to the beads. 10 flies were incubated with silica beads only as a control. In all cases, the knockdown phenotype was recorded every hour for 4 h at room temperature.
100 μl of rapeseed oil solution (0, 0.1, 1 and 10 μl of oil, with or without 200 μg of DDT) was pipetted into 5 ml empty glass vials. All glass vials were placed under the fume hood for 4 h to allow complete evaporation of acetone. 10 wild-type (Tübingen2018) females were added to each vial and the number of knockdown flies was recorded every hour for 4 h. After the incubation of 10 flies with 200 μg of DDT in a glass vial, emulsions of chitin (0, 0.02, 0.1 and 0.2 mg in acetone), chitosan (1 mg), cellulose (0.1 mg) or pectin (0.1 mg) were deposited at the bottom of the vial. After complete evaporation of the acetone, a second cohort of 10 wild-type (Tübingen2018) females was incubated in the respective vials. The number of knockdown flies was counted every hour for 4 h. Wild-type flies were incubated with chitin only as a control.
4.5. Microscopy
The bottom of glass vials was taken pictures after adding insecticides solution to evaporation completely and after exposure to flies or honey bee for 4h, respectively. Images were observed by a Nikon AZ100 zoom microscope with a Digital Sight DS-Fi1 camera.
4.6. Statistics
Pairwise Student's T-test was applied to statistically compare data presented in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5A and B and Supplementary Fig. 1. Two-way ANOVA was applied to test the data presented in Fig. 4A. The p-values are given in the respective legends. All experiments were conducted with at least three biological replicates.
Data accessibility statement
Upon acceptance the data's will be deposited in the open repository.
CRediT authorship contribution statement
Jing Yang: Validation, Methodology, Investigation, Formal analysis. Yiwen Wang: Methodology, Investigation, Formal analysis, Conceptualization. Abeer El Wakil: Investigation, Formal analysis. Bernard Moussian: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the participants of the Ringberg Symposium in October 2022 for valuable discussions on this matter. We thank the DFG for financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28392.
Contributor Information
Yiwen Wang, Email: yiwen.wang@tju.edu.cn.
Abeer El Wakil, Email: abeer_elwakil@alexu.edu.eg.
Bernard Moussian, Email: bernard.moussian@unice.fr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gao L., Qiao H., Wei P., Moussian B., Wang Y. Xenobiotic responses in insects. Arch. Insect Biochem. Physiol. 2022;109(3) doi: 10.1002/arch.21869. [DOI] [PubMed] [Google Scholar]
- 2.Nauen R., Bass C., Feyereisen R., Vontas J. The role of Cytochrome P450s in insect toxicology and resistance. Annu. Rev. Entomol. 2022;67:105–124. doi: 10.1146/annurev-ento-070621-061328. [DOI] [PubMed] [Google Scholar]
- 3.Rane R.V., et al. Are feeding preferences and insecticide resistance associated with the size of detoxifying enzyme families in insect herbivores? Curr Opin Insect Sci. 2016;13:70–76. doi: 10.1016/j.cois.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Balabanidou V., Grigoraki L., Vontas J. Insect cuticle: a critical determinant of insecticide resistance. Curr Opin Insect Sci. 2018;27:68–74. doi: 10.1016/j.cois.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Sarkaria D.S., Patton R.L. Histological and morphological factors in the penetration of DDT through the Pulvilli of several insect species. Trans. Am. Entomol. Soc. 1949;75(2):71–82. [Google Scholar]
- 6.Ling F., Dahanukar A., Weiss L.A., Kwon J.Y., Carlson J.R. The molecular and cellular basis of taste coding in the legs of Drosophila. J. Neurosci. 2014;34(21):7148–7164. doi: 10.1523/JNEUROSCI.0649-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinges G.F., et al. Location and arrangement of campaniform sensilla in Drosophila melanogaster. J. Comp. Neurol. 2021;529(4):905–925. doi: 10.1002/cne.24987. [DOI] [PubMed] [Google Scholar]
- 8.Balabanidou V., et al. Mosquitoes cloak their legs to resist insecticides. Proc. Biol. Sci. 1907;286 doi: 10.1098/rspb.2019.1091. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltz T. Tracing pollinator footprints on natural flowers. J. Chem. Ecol. 2006;32(5):907–915. doi: 10.1007/s10886-006-9055-6. [DOI] [PubMed] [Google Scholar]
- 10.Gorb SN & Gorb EV Ontogenesis of the attachment ability in the bug Coreus marginatus (Heteroptera, Insecta) J. Exp. Biol. 2004;207(Pt 17):2917–2924. doi: 10.1242/jeb.01127. [DOI] [PubMed] [Google Scholar]
- 11.Bauchhenss E. Die Pulvillen von Calliphora erythrocephala (Diptera, Brachycera) als Adhäsionsorgane. Zoomorphologie. 1979;93:99–123. [Google Scholar]
- 12.Seong K.M., Mittapalli O., Clark J.M., Pittendrigh B.R. A review of DDT resistance as it pertains to the 91-C and 91-R strains in Drosophila melanogaster. Pestic. Biochem. Physiol. 2019;161:86–94. doi: 10.1016/j.pestbp.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Moussian B. Chitin: structure, Chemistry and Biology. Adv. Exp. Med. Biol. 2019;1142:5–18. doi: 10.1007/978-981-13-7318-3_2. [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar A., Sillanpaa M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater--a short review. Adv. Colloid Interface Sci. 2009;152(1–2):26–38. doi: 10.1016/j.cis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Hassan M.M., Carr C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere. 2018;209:201–219. doi: 10.1016/j.chemosphere.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 16.Strycharz J.P., et al. Resistance in the highly DDT-resistant 91-R strain of Drosophila melanogaster involves decreased penetration, increased metabolism, and direct excretion. Pestic. Biochem. Physiol. 2013;107(2):207–217. [Google Scholar]
- 17.Rebora M., Salerno G., Piesanti S., Gorb E. Gorb SN Attachment devices and the tarsal gland of the bug Coreus marginatus (Hemiptera: Coreidae) Zoomorphology. 2021;140:85–102. doi: 10.1007/s00435-020-00515-z. [DOI] [Google Scholar]
- 18.Gerhardt H., Betz O., Albert K., Lammerhofer M. Insect adhesion secretions: similarities and dissimilarities in hydrocarbon profiles of tarsi and corresponding Tibiae. J. Chem. Ecol. 2016;42(8):725–738. doi: 10.1007/s10886-016-0718-7. [DOI] [PubMed] [Google Scholar]
- 19.Hong S., Sun Y., Sun D., Wang C. Microbiome assembly on Drosophila body surfaces benefits the flies to combat fungal infections. iScience. 2022;25(6) doi: 10.1016/j.isci.2022.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra J.R., Horner M.A., Lam G., Thummel C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011;25(17):1796–1806. doi: 10.1101/gad.17280911. 25/17/1796 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seong K.M., Coates B.S., Pittendrigh B.R. Cytochrome P450s Cyp4p1 and Cyp4p2 associated with the DDT tolerance in the Drosophila melanogaster strain 91-R. Pestic. Biochem. Physiol. 2019;159:136–143. doi: 10.1016/j.pestbp.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Le Goff G., Hilliou F. Resistance evolution in Drosophila: the case of CYP6G1. Pest Manag. Sci. 2017;73(3):493–499. doi: 10.1002/ps.4470. [DOI] [PubMed] [Google Scholar]
- 23.Chintapalli V.R., Wang J. Dow JA Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007;39(6):715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 24.Yang J., et al. DDT Polymorphism and the Lethality of crystal forms. Angew Chem. Int. Ed. Engl. 2017;56(34):10165–10169. doi: 10.1002/anie.201703028. [DOI] [PubMed] [Google Scholar]
- 25.Baughman R.G., Jorgensen S.K., Jacobsen R.A. Crystal and molecular structure of organophosphorus insecticides. 10. chlorpyrifos. J. Agric. Food Chem. 1989;37(6):1505–1507. [Google Scholar]
- 26.Yang J., et al. A deltamethrin crystal polymorph for more effective malaria control. Proc. Natl. Acad. Sci. U. S. A. 2020;117(43):26633–26638. doi: 10.1073/pnas.2013390117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X., et al. Imidacloprid crystal Polymorphs for disease Vector control and pollinator protection. J. Am. Chem. Soc. 2021;143(41):17144–17152. doi: 10.1021/jacs.1c07610. [DOI] [PubMed] [Google Scholar]
- 28.West S.A., Griffin A.S., Gardner A. Evolutionary explanations for cooperation. Curr. Biol. 2007;17(16):R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton W.D. The evolution of altruistic behavior. Am. Nat. 1963;97(896):354–356. [Google Scholar]
- 30.Soto-Yeber L., Soto-Ortiz J., Godoy P., Godoy-Herrera R. The behavior of adult Drosophila in the wild. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0209917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., et al. Transcriptional control of Quality differences in the lipid-Based cuticle barrier in Drosophila suzukii and Drosophila melanogaster. Front. Genet. 2020;11:887. doi: 10.3389/fgene.2020.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdu-Allah G.A.M., et al. Dietary antioxidants impact DDT resistance in Drosophila melanogaster. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma R., et al. Structural basis for diamide modulation of ryanodine receptor. Nat. Chem. Biol. 2020;16(11):1246–1254. doi: 10.1038/s41589-020-0627-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon acceptance the data's will be deposited in the open repository.