Abstract
Triple negative breast cancer (TNBC) represents the breast cancer subtype with least favorable outcome because of the lack of effective treatment options and its molecular features. Recently, ADCs have dramatically changed the breast cancer treatment landscape; the anti-TROP2 ADC Sacituzumab Govitecan has been approved for treatment of previously treated, metastatic TNBC patients. The novel ADC Datopotecan-deruxtecan (Dato-DXd) has recently shown encouraging results for TNBC. In the current paper, we summarize and discuss available data regarding this TROP-2 directed agent mechanism of action and pharmacologic activity, we describe first results on efficacy and safety of the drug and report characteristics, inclusion criteria and endpoints of the main ongoing clinical trials.
Keywords: Breast cancer, Triple-negative breast cancer, ADC, Antibody-drug conjugates, Dato-DXd, Datopotamab deruxtecan
1. Introduction
Several drugs with novel mechanisms of action – including antibody drug conjugates (ADC) – have been and are currently being tested for advanced and metastatic solid tumors, including breast cancer [1]. ADCs present high selectively and low systemic toxicities, and this definition was firstly proposed by Paul Ehrlich [2], which described the idea of “magic bullets” suggesting the concept that specific targets may directly enter into cells in order to treat diseases [3].
ADCs represent a novel class of drugs which are able to selectively deliver chemotherapeutic compounds into cancer cells, with less toxicity compared with standard chemotherapy [4,5]. Of note, ADCs share some characteristics with monoclonal antibodies and small molecule drugs, with the aim of enabling targeted delivery on non-tumoral cells while boosting the antitumor effect of the cytotoxic compound [6]. Typically, ADCs are composed of three different parts: a small-molecule drug or cytotoxic payload; the monoclonal antibody, which has a selective activity against tumor-associated cell surface antigens; a stable linker, that releases the cytotoxic agent in target cells [7]. According to the selective mechanism of action, ADCs typically target receptors that are over-expressed in tumor cells, but are low in normal tissues including HER2, CD33, CD30, CD22, and TROP-2 [8,9].
ADCs have been approved for the treatment of HER2 overexpressed breast cancer based on the relevant evidence of efficacy of this therapeutical approach. Trastuzumab emtansine (T-DM1) was the first ADC which was approved for treatment of early and advanced HER2 overexpressed breast cancer; according to its structure, T-DM1 contains trastuzumab and the microtubule inhibitor DM1 [[10], [11], [12]]. Moreover, T-DM1 has also reported practice-changing results in the adjuvant setting in high-risk patients according to the results of KATHERINE trial which demonstrated an advantage in Invasive Disease Free Survival (IDFS) compared to trastuzumab alone [13].
An important, recent example of anti-HER2 ADC is trastuzumab deruxtecan (T-DXd), which has shown notable results in breast cancer patients [14]. Similar to T-DM1, T-DXd is composed of a mAb of trastuzumab, but its Drug-Antibody-Ratio (DAR) is considerably higher than T-DM1 (8 vs 3.5); in addition, T-DXd has a topoisomerase I inhibitor, differently to T-DM1 it is membrane permeable, and this makes its capable of exerting a bystander effect [15]. Results of DESTINY-Breast03 showed a significant advantage in term of DFS and OS: at 12 months, the percentage of patients who were alive without disease progression was 75.8% (95% CI, 69.8–80.7) with T-DXd compared with 34.1% (95% CI, 27.7–40.5) of T-DM1; the hazard ratio (HR) for disease progression or death from any cause was 0.28 (95% CI, 0.22–0.37; P < 0.001) [16].
TROP-2 (or tumor-associated calcium signal transducer 2, TACSTD2) has recently emerged as an important therapeutic target in several tumors, including breast cancer, with the development of anti-TROP-2 agents, such as Sacituzumab Govitecan (SG) [[17], [18], [19]]. SG is an ADC composed by a mAb against human trophoblast cell-surface antigen 2 (TROP-2) linked to SN-38, an active derivate of irinotecan that is an inhibitor of topoisomerase 1. Results of ASCENT phase III trial that evaluated the efficacy of SG vs standard chemotherapy in patients affected by metastatic triple negative breast cancer (TNBC) have been recently reported: PFS was 5.6 vs. 1.7 months (HR = 0.41; 95% CI: 0.32–0.52; P < 0.001), OS was of 12.1 vs. 6.7 months (HR = 0.48; 95% CI: 0.38−0.59; P < 0.001) [20].
Based on the efficacy of SG, a novel ADC against TROP-2 has been emerging. Datopotamab Deruxtecan (Dato-DXd) has been assessed as monotherapy and is being tested in combination with immune checkpoint inhibitors (ICIs) and other immunotherapeutic approaches, and some promising clinical data are available. In the current manuscript, we provide a summary of the recently presented and published data regarding Dato-DXd in metastatic breast cancer, with a specific focus on mechanism of action, pharmacological properties, and future research avenues.
2. Mechanism of action
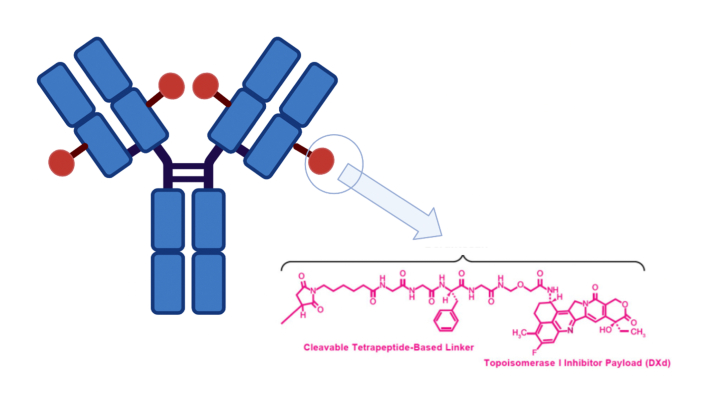
As briefly reported, Dato-DXd is an ADC, whose mechanism of action is based on the delivering of a cytotoxic agent to tumor cells giving to the drug maximum selectivity and minimal toxicities. Dato-DXd is a TROP-2 directed ADC consisting of a humanized anti-TROP-2 IgG1 monoclonal antibody (Mab) linked to a topoisomerase I inhibitor payload by tetrapeptide-based cleavable linker [21] (Fig. 1).
Fig. 1.
Schematic figure representing the structure of the humanized, anti-TROP-2 agent Datopotamab Deruxtecan.
TROP-2 is a trophoblast cell surface antigen involved in calcium signal and the TACSTD2 gene produces this protein; TROP-2 is involved in several cells signaling pathways since it has been reported to play a key role in placenta formation, the development of embryony, and the proliferation of stem cells with a regulatory role in cell-cell adhesion [22]. Several recent studies showed that TROP-2 may stimulate cancer growth and the upregulation of TROP-2 is frequently found in tumor cells [23]. TROP-2 regulatory network is linked to the complex of transcription factors which includes TP63/TP53L, WT1, GLIS2, AIRE, FOXP3, HNF1A, which are associated with tumorigenesis, and in fact, when TROP-2 is overexpressed, it acts as an oncogene promoting tumor cells proliferation [[24], [25]]. All breast cancer subtypes are characterized by overexpression of TROP-2; however, Aslan et al. recently showed that it is most elevated in TNBC rather the in hormone receptor positive or HER 2 positive BC [26]. Ping Zeng and colleagues recently suggested in their meta-analysis evaluating 2569 patients that TROP-2 overexpression in solid tumors may represent a poor prognostic factor, since the authors observed a statistically significant association with shorter OS and DFS [27]. TROP-2 is also expressed in normal epithelial tissues of heart, liver, kidney, lung, skin and esophagus, but expression is lower than tumor cells, something that makes TROP-2 an ideal therapeutic target[28,29].
Dato-DXd uses IgG1 Mab to bind to TROP-2; after the linking, the ADC is internalized into the tumor cells where it is released. In its turn, the internalization leads to formation of endosomes and subsequently of lisosomes where the proteolytic environment causes the releases of payload from Mab [30]. Then, topisomerase I diffuse into cell cytoplasm producing DNA damage and necessarily cellular apoptosis [31].
3. Chemistry; pharmacodynamics; pharmacokinetics and metabolism
Among its cytotoxic mechanisms against tumor cells, Dato-DXd induces DNA damage and apoptosis by the release and accumulation of DXd [32]. DXd is an exatecan-derivative that is covalently linked to ADC via a peptidyl spacer (Gly-Gly-Phe-Gly), which is stable in circulation [33]. The effect of DXd was verified by evaluating the induced phosphorylation of H2AX, KAP1 and Chk1. The drug to antibody ratio (DAR) of Dato-DXd is 4:1, and the specific features of the linker makes Dato-DXd highly stable in circulation. Specifically, the payload of Dato-DXd is very potent – more than ten times than sacizutumab govitecan SN-38 – and this ADC has a long half-life. The longer half-life of Dato-DXd allows a Q3 week dosing schedule and Dato-DXd is eliminated mainly via bile/faeces with negligible metabolism, including glucuronidation or oxidation [[21], [34]]. According to Ogitani et al., data on the pharmacokinetic profiles of Dato-DXd in cynomolgus monkeys are comparable to trastuzumab deruxtecan (DS-8201a). Indeed in cynomolgus monkeys pharmacokinetics and safety profiles of DS-8201a were favorable supporting DS-8201a's being well tolerated in humans [35].
4. Clinical trials: preliminary efficacy, safety and tolerability
Preliminary data posted about efficacy and safety profile of Dato-DXd for triple-negative breast cancer (TNBC) are encouraging. TROPION-PanTumor01 is an ongoing, first-in-human, dose-escalation and -expansion, phase 1 study of Dato-DXd in patients with advanced solid tumors, including TNBC. Updated results of TROPION-PanTumor01 (NCT03401385) about Dato-DXd activity in TNBC were presented as Poster Discussion at the San Antonio Breast Cancer Symposium 2022 (Abstract#P6-10-03). Dato-DXd showed highly encouraging and durable efficacy and demonstrated a manageable safety profile with no new safety signals. 44 heavily pretreated patients (median of 3 lines of treatment for metastatic disease including taxanes, anthracyclines, capecitabine, platinum, immunotherapy, PARP-inhibitors) received dato-DXd at 6 mg/kg or 8 mg/kg every 3 weeks. According to the results of this study, 86% of patients discontinued the treatment for disease progression (PD or clinical progression), 4 pts are still in active treatment.
Overall Response Rate (ORR) in all subjects was 32%, Disease Control Rate (DCR) 80%, and clinical benefit rate 34%. In patients who had received a prior treatment with topoisomerase inhibitor-based ADC, ORR was 44%, mPFS 7.3 mo (95% CI, 3.0-NE) and mOS 14.3 mo (95% CI, 10.5-NE) (Table 1).
Table 1.
Summary of available data regarding TROPION-PanTumor01 and BEGONIA.
| NCT Number/Trial name | Trial Design/Patient Population | Characteristics | Number of pts | Intervention | Primary endpoint | Clinical Trial Data | Safety |
|---|---|---|---|---|---|---|---|
| NCT03401385, TROPION-PanTumor01 | FIH trial with Datopotamab deruxtecan in refractory metastatic TNBC (N = 44) |
A Phase 1, Two-Part, Multicenter, Open-Label, Multiple-Dose, First-in-Human Study of Dato-DXd in Patients With Advanced/Metastatic Solid Tumors | 770 | Drug: Datopotamab Deruxtecan (Dato-DXd) Drug: Steroid Containing Mouthwash Other: Non-Steroid Containing Mouthwash |
Safety and tolerability | ORR 32% (ORR-44% in Topo I inhibitors-naive patients) | G3 AEs were observed in 52% of pts. Most common TEAEs (any grade, grade ≥3) were stomatitis (73%, 11%), nausea (66%, 2%), and vomiting (39%, 5%) |
| NCT03742102BEGONIA trial | Phase Ib/II platform trial with Datopotamab deruxtecan + durvalumab in first-line metastatic TNBC (N = 29) | Multi-center, Open-Label, Platform Study Evaluating the Efficacy and Safety of Durvalumab in Combination With Novel Oncology Therapies for First-Line Treatment in Patients With Metastatic TNBC | 210 | Drug: Durvalumab Drug: Capivasertib Drug: Oleclumab |
Safety and tolerability | ORR 79% Median DoR not reached Responses were seen regardless of PD-L1 expression |
G3/4 AEs were observed in 36% of pts. Most common TEAEs (any grade, grade ≥3) were gastrointestinal (nausea in 26 patients [55%] and stomatitis in 24 patients [51%]) |
In the intention-to-treat population, median duration of response (mDOR) was not yet reached; mPFS was 4.3 months (95% CI, 3.0–7.3), and mOS 12.9 months (95% CI, 10.1–14.7). Safety profile was manageable: adverse effects treatment emergent (TEAEs) occurred in all patients and half of these events were Grade (G) ≥3. Most common G3 TEAEs were stomatitis, nausea, vomiting, fatigue, and alopecia; G4 events were reported in 20% of subjects, while there were no treatment-related deaths.
BEGONIA trial (NCT03742102) is an ongoing phase 1b/2 study, whose preliminary results were presented at San Antonio Breast Cancer Symposium 2022 (Abstract#PD11-09). The study has the aim to determine the efficacy and safety of durvalumab (D) in combination with novel oncology therapies with or without paclitaxel and durvalumab plus paclitaxel as first-line treatment for treatment-naïve metastatic TNBC. PD-L1 assessment was performed by using VENTANA PD-L1 SP263. Dato-DXd was administered at 6 mg/kg with durvalumab at 1120 mg every 3 weeks until progression or unacceptable toxicity.
Early results of the study are encouraging. 47 patients received the combination of dato-DXd and D, and 33 were included in efficacy analysis. ORR was 79% (95% CI, 61–91), with complete response (CR) observed in 6% of patients and partial response (PR) in 73% of cases. Median DOR was not reached. According to subgroup analyses, no statistically significant association between tumor response and PD-L1 expression was reported. In addition, the combination reported a manageable safety profile. Adverse Events (AEs) occurred in 87% of patients, and 36% of patients experienced G3/4 toxicity. Nausea and stomatitis were the most common AEs observed, and dose reduction was required in 19% of cases. No deaths for treatment-related AEs were detected.
5. Ongoing clinical trials
Safety and efficacy of Dato-DXd has nowadays been evaluated; many trials are ongoing and results, not yet accessible, will be interesting and helpful to better understand pharmaceutical characteristics of this ADC.
There are two ongoing phase III TROPION-Breast trials with dato-DXd versus investigator's choice of chemotherapy in patients with metastatic TNBC [36].
TROPION-Breast01 (NCT05104866) aims to assess Dato-DXd compared with investigator's choice of standard of care single-agent chemotherapy (including agents such as gemcitabine, capecitabine, eribulin, or vinorelbine) in 725 participants with inoperable or metastatic HR-positive, HER2-negative breast cancer previously treated with one or two prior chemotherapies. Patients are randomized 1:1 to DatoDXd 6 mg/kg IV Q3W or single-agent chemotherapy until progression. Primary endpoints are progression-free survival (PFS) and overall survival (OS); secondary endpoints are PFS per investigator, ORR, disease control rate, pt-reported outcomes, and Dato-DXd pharmacokinetics and immunogenicity.
The phase III, open-label, randomized TROPION-Breast02 (NCT05374512) study is evaluating Dato-DXd (6 mg/kg every 3 weeks) compared with investigator's choice of chemotherapy (paclitaxel, nab-paclitaxel, capecitabine, carboplatin or eribulin) in 600 participants with TNBC who are not candidates for PD-1/PD-L1 inhibitor therapy (Table 2). The dual primary endpoints of TROPION-Breast02 are PFS assessed by blinded independent central review and overall survival. Secondary endpoints include PFS assessed by investigator, objective response rate, duration of response, disease control rate, pharmacokinetics and safety.
Table 2.
Summary of ongoing clinical trials exploring the role of Dato-DXd as monotherapy or in combination with other anticancer agents.
| NCT Number/Trial name | Trial Design/Patient Population | Characteristic | Number of pts | Intervention | Primary endpoint |
|---|---|---|---|---|---|
| NCT05460273, TROPION-PanTumor02 | Phase 1/Phase 2 trial assessing the efficacy and safety of Dato-DXd in inoperable locally advanced or metastatic TNBC in Chinese population (N = 15) | Multicentre, open-label, multiple-cohort study, which is designed to evaluate the efficacy, safety, Pharmacokinetic, and immunogenicity of Dato-DXd in adult Chinese participants compared with ICC in inoperable locally advanced or metastatic TNBC who have received at least 2 prior chemotherapy regimens | 118 | Drug: Datopotamab Deruxtecan (Dato-DXd) | Efficacy and safety |
| NCT05104866, TROPION-Breast01 | Phase 3 evaluate the safety and efficacy of Dato-DXd in participants with inoperable or metastatic TNBC | Open-label, randomized study of Dato-DXd versus investigator's choice of chemotherapy in participants with inoperable or metastatic HR-positive, HER2-negative breast cancer who have been treated with one or two prior lines of systemic chemotherapy | 733 | Drug: Dato-DXd Drug: Capecitabine Drug: Gemcitabine Drug: Eribulin Drug: Vinorelbine | PFS and OS |
| NCT05374512, TROPION-Breast02 | Phase-3 evaluate the safety and efficacy in TNBC Not Candidates for PD-1/PD-L1 Inhibitor Therapy | Randomized, open-label, 2 arm, multicentre, international study assessing the efficacy and safety of Dato-DXd | 600 | Drug: Dato-DXd Drug: Paclitaxel Drug: Nab-paclitaxel Drug: CarboplatinDrug: Capecitabine Drug: Eribulin mesylate | PFS and OS |
| NCT05629585, TROPION-Breast03 | Phase 3, demonstrate superiority of Dato-DXd in combination with durvalumab in stage I to III TNBC | Randomized, open-label, 3-arm, multicenter, international study assessing the efficacy and safety of Dato-DXd with or without durvalumab compared with ICT in participants with stage I to III TNBC with residual invasive disease in the breast and/or axillary lymph nodes at surgical resection following neoadjuvant systemic therapy | 1075 | Drug: Dato-DXd Drug: Durvalumab Drug: Capecitabine Drug: Pembrolizumab | Superiority of Dato-DXd in combination with durvalumab |
The phase III, randomized, open-label, 3-arm, multicenter, international TROPION-Breast 03 (NCT05629585) study is evaluating the efficacy of Dato-DXd with or without combination with durvalumab in the neoadjuvant setting in participants with stage I to III TNBC who have residual invasive disease in the breast and/or axillary lymph nodes at surgical resection. The study design includes 1075 participants, two experimental arms with Dato-DXd in combination and without durvalumab and a control arm with Investigator's choice treatment (capecitabine and/or pembrolizumab). The first patients were enrolled in November 2022.
The TROPION-PanTumor02 is a Phase 1/Phase 2 designed to evaluate the efficacy, safety, Pharmacokinetic, and immunogenicity of Dato-DXd in adult Chinese participants with advanced or metastatic solid tumors. This is the first time Dato-DXd is being studied in Chinese population. The primary objective of the study is to estimate the effectiveness of Dato-DXd by assessment of confirmed ORR by independent central review.
6. Future perspectives and conclusions
Despite notable advances in treatment of metastatic TNBC following the advent of novel therapeutic options, ranging from ICIs to PARP inhibitors and ADCs, several challenges persist in the treatment of this tumor, and the need for more effective therapies still exists, across all lines of treatment. ADCs such as Dato-DXd have represented a key moment in the progress of systemic treatment for metastatic breast cancer. However, the access to these anticancer agents is not possible yet in several countries and thus, further real-world data are needed to better understand the role of Dato-DXd. In addition, since earlier studies have clearly shown the benefits of ADCs as monotherapies or in combination with other anticancer agents, the potential for benefit, especially when used ADCs are combined with ICIs, does exist, and should be explored. Further data and studies are needed in this changing, challenging treatment scenario.
CRediT authorship contribution statement
Francesca Matilde Schipilliti: Writing – original draft, Resources, Conceptualization. Denise Drittone: Writing – original draft, Visualization, Validation, Conceptualization. Federica Mazzuca: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration. Daniele La Forgia: Writing – review & editing, Visualization, Validation, Supervision. Deniz Can Guven: Writing – review & editing, Visualization, Validation, Supervision, Data curation. Alessandro Rizzo: Writing – review & editing, Validation, Supervision, Resources, Project administration, Investigation, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Grinda T., Rassy E., Pistilli B. Antibody-drug conjugate revolution in breast cancer: the road ahead. Curr. Treat. Options Oncol. 2023;24(5):442–465. doi: 10.1007/s11864-023-01072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmé F. Antibody-drug conjugates for breast cancer. Oncol. Res. Treat. 2022;45(1–2):26–36. doi: 10.1159/000521499. [DOI] [PubMed] [Google Scholar]
- 3.Strebhardt K., Ullrich A. Paul ehrlich's magic bullet concept: 100 Years of progress. Nat. Rev. Cancer. 2008;8(6):473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 4.Bardia A., Messersmith W.A., Kio E.A., Berlin J.D., Vahdat L., Masters G.A., Moroose R., Santin A.D., Kalinsky K., Picozzi V., O'Shaughnessy J., Gray J.E., Komiya T., Lang J.M., Chang J.C., Starodub A., Goldenberg D.M., Sharkey R.M., Maliakal P., Hong Q., Wegener W.A., Goswami T., Ocean A.J. Sacituzumab govitecan, a trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021;32(6):746–756. doi: 10.1016/j.annonc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Rugo H.S., Tolaney S.M., Loirat D., Punie K., Bardia A., Hurvitz S.A., O'Shaughnessy J., Cortés J., Diéras V., Carey L.A., Gianni L., Piccart M.J., Loibl S., Goldenberg D.M., Hong Q., Olivo M., Itri L.M., Kalinsky K. Safety analyses from the phase 3 ASCENT trial of Sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer. 2022;8(1):98. doi: 10.1038/s41523-022-00467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu B., Ma F., Wang T., Wang S., Tong Z., Li W., Wu X., Wang X., Sun T., Pan Y., Yao H., Wang X., Luo T., Yang J., Zeng X., Zhao W., Cong X.J., Chen J.A. Phase IIb, single arm, multicenter trial of Sacituzumab govitecan in Chinese patients with metastatic triple-negative breast cancer who received at least two prior treatments. Int. J. Cancer. 2023;152(10):2134–2144. doi: 10.1002/ijc.34424. [DOI] [PubMed] [Google Scholar]
- 7.O'Shaughnessy J., Brufsky A., Rugo H.S., Tolaney S.M., Punie K., Sardesai S., Hamilton E., Loirat D., Traina T., Leon-Ferre R., Hurvitz S.A., Kalinsky K., Bardia A., Henry S., Mayer I., Zhu Y., Phan S., Cortés J. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT study of Sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res. Treat. 2022;195(2):127–139. doi: 10.1007/s10549-022-06602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalinsky K., Diamond J.R., Vahdat L.T., Tolaney S.M., Juric D., O'Shaughnessy J., Moroose R.L., Mayer I.A., Abramson V.G., Goldenberg D.M., Sharkey R.M., Maliakal P., Hong Q., Goswami T., Wegener W.A., Bardia A. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020;31(12):1709–1718. doi: 10.1016/j.annonc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Damelin M., Zhong W., Myers J., Sapra P. Evolving strategies for target selection for antibody-drug conjugates. Pharm. Res. (N. Y.) 2015;32(11):3494–3507. doi: 10.1007/s11095-015-1624-3. [DOI] [PubMed] [Google Scholar]
- 10.Ferraro E., Drago J.Z., Modi S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions. Breast Cancer Res. 2021;23(1):84. doi: 10.1186/s13058-021-01459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diéras V., Miles D., Verma S., Pegram M., Welslau M., Baselga J., Krop I.E., Blackwell K., Hoersch S., Xu J., Green M., Gianni L. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (emilia): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krop I.E., Kim S.-B., González-Martín A., LoRusso P.M., Ferrero J.-M., Smitt M., Yu R., Leung A.C.F., Wildiers H. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 13.von Minckwitz G., Huang C.-S., Mano M.S., Loibl S., Mamounas E.P., Untch M., Wolmark N., Rastogi P., Schneeweiss A., Redondo A., Fischer H.H., Jacot W., Conlin A.K., Arce-Salinas C., Wapnir I.L., Jackisch C., DiGiovanna M.P., Fasching P.A., Crown J.P., Wülfing P., Shao Z., Rota Caremoli E., Wu H., Lam L.H., Tesarowski D., Smitt M., Douthwaite H., Singel S.M., Geyer C.E., Katherine Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 14.Nicolò E., Repetto M., Boscolo Bielo L., Tarantino P., Curigliano G. Antibody-drug conjugates in breast cancer: what is beyond HER2? Cancer J. Sudbury Mass. 2022;28(6):436–445. doi: 10.1097/PPO.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 15.Giugliano F., Corti C., Tarantino P., Michelini F., Curigliano G. Bystander effect of antibody-drug conjugates: fact or fiction? Curr. Oncol. Rep. 2022;24(7):809–817. doi: 10.1007/s11912-022-01266-4. [DOI] [PubMed] [Google Scholar]
- 16.Cortés J., Kim S.-B., Chung W.-P., Im S.-A., Park Y.H., Hegg R., Kim M.H., Tseng L.-M., Petry V., Chung C.-F., Iwata H., Hamilton E., Curigliano G., Xu B., Huang C.-S., Kim J.H., Chiu J.W.Y., Pedrini J.L., Lee C., Liu Y., Cathcart J., Bako E., Verma S., Hurvitz S.A., DESTINY-Breast03 Trial Investigators Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 17.Furlanetto J., Marmé F., Loibl S. Sacituzumab govitecan: past, present and future of a new antibody-drug conjugate and future horizon. Future Oncol. Lond. Engl. 2022;18(28):3199–3215. doi: 10.2217/fon-2022-0407. [DOI] [PubMed] [Google Scholar]
- 18.Lang Y., Chai Q., Tao W., Liao Y., Liu X., Wu B. Cost-effectiveness of Sacituzumab govitecan versus chemotherapy in advanced or metastatic triple-negative breast cancer. Breast Edinb. Scotl. 2023;68:173–180. doi: 10.1016/j.breast.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarantino P., Carmagnani Pestana R., Corti C., Modi S., Bardia A., Tolaney S.M., Cortes J., Soria J.-C., Curigliano G. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA A Cancer J. Clin. 2022;72(2):165–182. doi: 10.3322/caac.21705. [DOI] [PubMed] [Google Scholar]
- 20.Bardia A., Hurvitz S.A., Tolaney S.M., Loirat D., Punie K., Oliveira M., Brufsky A., Sardesai S.D., Kalinsky K., Zelnak A.B., Weaver R., Traina T., Dalenc F., Aftimos P., Lynce F., Diab S., Cortés J., O'Shaughnessy J., Diéras V., Ferrario C., Schmid P., Carey L.A., Gianni L., Piccart M.J., Loibl S., Goldenberg D.M., Hong Q., Olivo M.S., Itri L.M., Rugo H.S. ASCENT clinical trial investigators. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 2021;384(16):1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 21.Shastry M., Jacob S., Rugo H.S., Hamilton E. Antibody-drug conjugates targeting TROP-2: clinical development in metastatic breast cancer. Breast Edinb. Scotl. 2022;66:169–177. doi: 10.1016/j.breast.2022.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaman S., Jadid H., Denson A.C., Gray J.E. Targeting trop-2 in solid tumors: future prospects. OncoTargets Ther. 2019;12:1781–1790. doi: 10.2147/OTT.S162447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ripani E., Sacchetti A., Corda D., Alberti S. Human trop-2 is a tumor-associated calcium signal transducer. Int. J. Cancer. 1998;76(5):671–676. doi: 10.1002/(sici)1097-0215(19980529)76:5<671::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Fang Y.J., Lu Z.H., Wang G.Q., Pan Z.Z., Zhou Z.W., Yun J.P., Zhang M.F., Wan D.S. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int. J. Colorectal Dis. 2009;24(8):875–884. doi: 10.1007/s00384-009-0725-z. [DOI] [PubMed] [Google Scholar]
- 25.Guerra E., Trerotola M., Aloisi A.L., Tripaldi R., Vacca G., La Sorda R., Lattanzio R., Piantelli M., Alberti S. The trop-2 signalling network in cancer growth. Oncogene. 2013;32(12):1594–1600. doi: 10.1038/onc.2012.151. [DOI] [PubMed] [Google Scholar]
- 26.Aslan M., Hsu E.-C., Garcia-Marques F.J., Bermudez A., Liu S., Shen M., West M., Zhang C.A., Rice M.A., Brooks J.D., West R., Pitteri S.J., Győrffy B., Stoyanova T. Oncogene-mediated metabolic gene signature predicts breast cancer outcome. NPJ Breast Cancer. 2021;7(1):141. doi: 10.1038/s41523-021-00341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng P., Chen M.-B., Zhou L.-N., Tang M., Liu C.-Y., Lu P.-H. Impact of TROP2 expression on prognosis in solid tumors: a systematic review and meta-analysis. Sci. Rep. 2016;6 doi: 10.1038/srep33658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldenberg D.M., Stein R., Sharkey R.M. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9(48):28989–29006. doi: 10.18632/oncotarget.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stepan L.P., Trueblood E.S., Hale K., Babcook J., Borges L., Sutherland C.L. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2011;59(7):701–710. doi: 10.1369/0022155411410430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalim M., Chen J., Wang S., Lin C., Ullah S., Liang K., Ding Q., Chen S., Zhan J. Intracellular trafficking of new anticancer therapeutics: antibody-drug conjugates. Drug Des. Dev. Ther. 2017;11:2265–2276. doi: 10.2147/DDDT.S135571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplon H., Crescioli S., Chenoweth A., Visweswaraiah J., Reichert J.M. Antibodies to watch in 2023. mAbs. 2023;15(1) doi: 10.1080/19420862.2022.2153410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okajima D., Yasuda S., Maejima T., Karibe T., Sakurai K., Aida T., Toki T., Yamaguchi J., Kitamura M., Kamei R., Fujitani T., Honda T., Shibutani T., Muramatsu S., Nakada T., Goto R., Takahashi S., Yamaguchi M., Hamada H., Noguchi Y., Murakami M., Abe Y., Agatsuma T. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol. Cancer Therapeut. 2021;20(12):2329–2340. doi: 10.1158/1535-7163.MCT-21-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakada T., Masuda T., Naito H., Yoshida M., Ashida S., Morita K., Miyazaki H., Kasuya Y., Ogitani Y., Yamaguchi J., Abe Y., Honda T. Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads. Bioorg. Med. Chem. Lett. 2016;26(6):1542–1545. doi: 10.1016/j.bmcl.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Nagai Y., Oitate M., Shiozawa H., Ando O. Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys. Xenobiotica fate foreign compd. Biol. Syst. 2019;49(9):1086–1096. doi: 10.1080/00498254.2018.1531158. [DOI] [PubMed] [Google Scholar]
- 35.Ogitani Y., Aida T., Hagihara K., Yamaguchi J., Ishii C., Harada N., Soma M., Okamoto H., Oitate M., Arakawa S., Hirai T., Atsumi R., Nakada T., Hayakawa I., Abe Y., Agatsuma T. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22(20):5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 36.Sakach E., Sacks R., Kalinsky K. Trop-2 as a therapeutic target in breast cancer. Cancers. 2022;14(23):5936. doi: 10.3390/cancers14235936. [DOI] [PMC free article] [PubMed] [Google Scholar]