Abstract
Fenestration of the septum between the true and false lumen might be necessary after aortic dissection. We report the technical aspects of in situ laser fenestration of the aortic dissection septum. Two illustrative cases are provided: a 56-year-old man with false lumen deployment of a frozen elephant trunk graft, and a 67-year-old man who underwent fenestrated endovascular aortic repair with a target branch vessel off the false lumen. In both cases, the septum was crossed using in situ laser fenestration. This technique is a precise option to enable passage between true and false lumens during endovascular repair of an aortic dissection.
Keywords: Aortic dissection, Endovascular aortic repair, Laser in situ fenestration
Fenestration of the septum between the true and false lumen can be necessary after aortic dissection when no natural preexisting fenestrations are present in the required locations. This can be used in the acute period for malperfusion and when a thoracic endovascular aortic repair (TEVAR) endograft or frozen elephant trunk (FET) is deployed into the false lumen, or in the chronic period when target vessels originate from the false lumen during fenestrated-branched endovascular aneurysm repair (FB-EVAR).1, 2, 3 Multiple endovascular techniques have previously been used to cross the dissection septum, including the stiff back end of a guidewire, a reentry catheter, an intrahepatic access needle, a radiofrequency guidewire, and a radiofrequency transseptal needle.1,2,4, 5, 6 As a new alternative technique, we have found that in situ laser fenestration is a feasible solution for fenestration of the dissection septum. We present two illustrative cases of in situ laser fenestration to allow for bridging of the true and false lumens—the first after inadvertent deployment of a FET into the false lumen and the second for access to a left renal artery originating from the false lumen during FB-EVAR. Both patients provided written informed consent for the report of their case details and imaging details.
Case report
Patient 1
A 56-year-old man initially presented to the cardiothoracic surgery service with a type A10 aortic dissection and underwent total arch replacement with FET deployment. On follow-up computed tomography angiography at 6 weeks postoperatively, it was noted that the FET had been deployed in the false lumen (Fig 1). Additionally, the patient was experiencing left upper extremity effort fatigue and chest pain concerning for a symptomatic dissection. Therefore, 2 months after the initial type A repair with FET, he underwent left carotid–subclavian transposition and endovascular repair, with a plan to bridge the true and false lumen with a TEVAR distal extension. Because no natural, preexisting fenestration was present near the distal aspect of the FET, in situ laser fenestration was used (Fig 2; Supplementary Video).
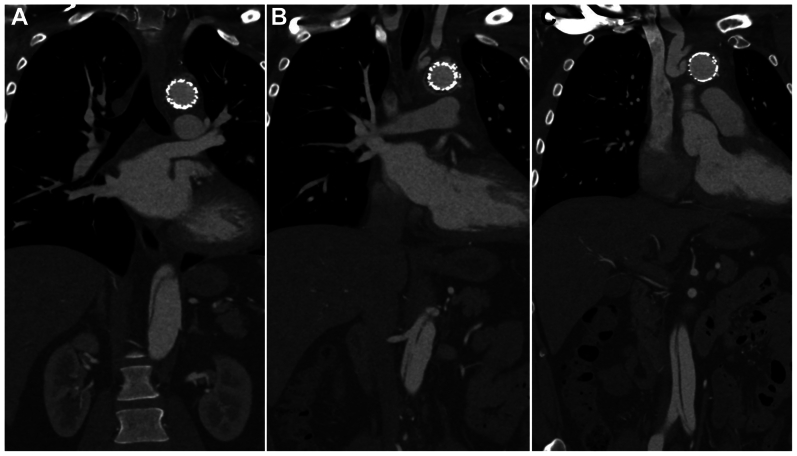
Fig 1.
Computed tomography angiogram demonstrating frozen elephant trunk (FET) placement in the false lumen and a compressed true lumen (white arrow).
Fig 2.
Computed tomography angiogram of patient 1 demonstrating aortic dissection in the septum, distal to the frozen elephant trunk (A), at the level of the renal arteries with a large septal fenestration (B), and in the infrarenal aorta (C).
Patient 2
The second case involved a 67-year-old man with a history of morbid obesity, hypertension, and atrial fibrillation, who underwent zone 2 TEVAR with left carotid–subclavian bypass for type B3-10 aortic dissection 4 years prior. He presented with an enlarging extent III thoracoabdominal aortic aneurysm with interval growth of the abdominal portion of his aneurysm to 6.0 cm. Given his comorbidities and his wish to avoid open repair, FB-EVAR was planned. The dissected thoracoabdominal aorta had highly complex anatomy, including dissection extending into the celiac and superior mesenteric arteries, two left renal arteries with one originating from the false lumen, a false lumen that perfused the right renal artery, and bilateral common iliac artery aneurysms. No natural fenestration was present at the level of the false lumen perfusing the inferior left renal artery (Fig 3); therefore, fenestration of the septum at that level was required to facilitate placement of a bridging stent from the physician-modified endograft. For the right renal artery originating from the false lumen, a preexisting septal fenestration was present near the origin, which was enlarged to 12 mm with balloon angioplasty and used for placement of a bridging stent to the right renal artery.
Fig 3.
Computed tomography angiogram of patient 2 demonstrating the large false lumen (white arrow), dissected superior mesenteric artery (dashed arrow), and left renal artery originating from the false lumen (black arrow).
Surgical technique
In both cases, wire access was obtained in the true and false lumens, and positioning was confirmed with intravascular ultrasound and angiography. The fluoroscopy obliquity was adjusted to be orthogonal to the long axis of the aortic septum at the level of the planned fenestration based on the preoperative computed tomography angiography findings. True and false lumen angiograms were performed to confirm the obliquity was correct before laser fenestration. An articulating sheath was then placed at the level of the desired fenestration. A 2.3-mm Turbo Elite laser ablation catheter (Spectranetics) was adjusted to be flush with the aortic septum. Laser fenestration was performed, and the laser probe was visualized breaking through the septum (Fig 4). A stiff wire was advanced through the new fenestration and enlarged with a 10 × 4-mm balloon. In the first case, a TEVAR extension 32 × 24 × 158-mm endograft was bridged from the false lumen elephant trunk into the thoracic true lumen (Fig 5, A). In the second case, the target vessel was cannulated through the newly created fenestration and then bridged to the physician-modified endograft with a balloon expandable stent (Fig 5, B). Completion angiography confirmed adequate apposition and brisk true lumen filling with no endoleak. At follow-up for the first case at 6 months, the patient continued to have symptomatic relief, and imaging studies demonstrated stable dissection with no aneurysmal changes or endoleak. For the second patient, at 1 year of follow-up, imaging studies showed that all stent grafts were patent, with aneurysm sac regression to 5.4 cm.
Fig 4.
Intraoperative fluoroscopy images of patient 1 demonstrating the laser probe against the septum before fenestration (A) and the laser probe advanced through the septum after fenestration with guidewire placed into the frozen elephant trunk (FET; B). In patient 2, images show the laser probe against the septum before fenestration (C) and the laser probe advanced through the septum after fenestration (D).
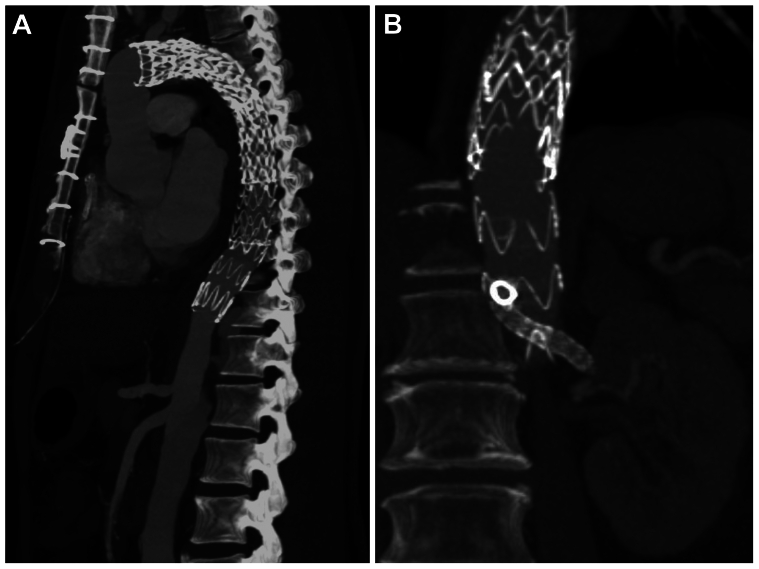
Fig 5.
Computed tomography angiograms demonstrating thoracic endovascular aortic repair (TEVAR) extension through the laser-fenestrated septum into the true lumen in patient 1 (A), and the bridging stent from the physician-modified endograft to the left renal artery through the laser-fenestrated septum in patient 2 (B).
Discussion
The need for fenestration of the dissection septum between true and false lumens has been previously described for a variety of conditions associated with aortic dissection. Patients who present with acute aortic dissection and malperfusion have an increased risk of mortality, and relief of the malperfusion can be accomplished with fenestration of the septum and expansion of the true lumen as the primary treatment.7 TEVAR and FET deployment in the false lumen can lead to severe compression of the true lumen with resultant visceral, renal, and peripheral malperfusion, requiring distal extension into the true lumen.1,8 For chronic postdissection aneurysms undergoing FB-EVAR, septal fenestration can be necessary to bridge target vessels originating from the false lumen.3
Multiple techniques have been described to perform septal fenestration. Before the development of endovascular techniques, open fenestration was used to create a common channel between the true and false lumens and perfuse branches fed by the false lumen.9 However, endovascular techniques have become much more common, including using the stiff back end of a guidewire, a reentry catheter, an intrahepatic access needle, a radiofrequency NRG transseptal needle (Baylis Medical), and a radiofrequency wire (PowerWire; Baylis Medical).1,2,4, 5, 6 The radiofrequency wire is very controlled and creates a precise fenestration, which limits bleeding if perforation occurs; however, the fenestration can be challenging to cross with a catheter or balloon. The benefits of laser fenestration include that it can be used inside an articulating sheath, creates a larger fenestration, and allows for a smoother wire exchange.
In situ laser fenestration was initially described for in situ fenestration of zone 2 TEVAR to preserve the left subclavian artery.10 Its use was then expanded for in situ laser fenestrated endograft placement during FB-EVAR and later for in situ fenestration for repair of ruptured thoracoabdominal aortic aneurysms.11, 12, 13, 14 The technique for septal fenestration first involves obtaining wire access into the true and false lumens and then confirming the position using intravascular ultrasound and angiography. After an articulating sheath is introduced, the fluoroscopic obliquity is adjusted to be perfectly orthogonal to the aortic septum. This is a key step for visualization when positioning the sheath and laser ablation catheter flush to the aortic septum. The use of an articulating sheath such as a TourGuide steerable sheath (Medtronic) can provide additional support and allow for optimal positioning. The laser probe is then used to create a fenestration in the desired location. Finally, the fenestration is balloon dilated to allow for easy tracking of the stent. This has become our preferred technique for aortic septal fenestration. We have used this technique several times in situations similar to the two cases described.
Conclusions
In situ laser fenestration of the aortic dissection septum is a feasible option to enable passage between true and false lumens during endovascular repair of an aortic dissection. The key to performing this technique is optimal adjustment of the fluoroscopic obliquity when positioning the sheath and laser probe flush to the aortic septum, which allows for accurate positioning and controlled fenestration.
Disclosures
S.M.H. is a consultant for W.L. Gore & Associates, Cook Medical Inc, Terumo, and Vestek and on the scientific advisory board for W.L. Gore & Associates and Vestek. G.A.M. is a consultant for W.L. Gore & Associates and Silk Road. A.D.D. and E.M. have no conflicts of interests.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Appendix (online only)
Video of patient 1 depicting the preoperative computed tomography angiography scan, intraoperative fluoroscopy images, and postoperative computed tomography angiography scan.
References
- 1.Han S.M., Gasper W.J., Chuter T.A. Endovascular rescue after inadvertent false lumen stent graft implantation. J Vasc Surg. 2016;63:518–522. doi: 10.1016/j.jvs.2014.11.072. [DOI] [PubMed] [Google Scholar]
- 2.Plotkin A., Hanks S.E., Han S.M., Fleischman F., Weaver F.A., Magee G.A. Endovascular septal fenestration using a radiofrequency wire to salvage inadvertent false lumen deployment of a frozen elephant trunk stent graft. J Vasc Surg Cases Innov Tech. 2019;5:553–556. doi: 10.1016/j.jvscit.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenorio E.R., Lima G.B., Marcondes G.B., Oderich G.S. Sizing and planning fenestrated and branched stent-grafts in patients with chronic post-dissection thoracoabdominal aortic aneurysms. J Cardiovasc Surg. 2020;61:416–426. doi: 10.23736/S0021-9509.20.11365-X. [DOI] [PubMed] [Google Scholar]
- 4.Wuest W., Goltz J., Ritter C., Yildirim C., Hahn D., Kickuth R. Fenestration of aortic dissection using a fluoroscopy-based needle re-entry catheter system. Cardiovasc Intervent Radiol. 2011;34:S44–S47. doi: 10.1007/s00270-009-9783-4. [DOI] [PubMed] [Google Scholar]
- 5.Tashiro J., Baqai A., Goldstein L.J., Salsamendi J.T., Taubman M., Rey J. "Cheese wire" fenestration of a chronic aortic dissection flap for endovascular repair of a contained aneurysm rupture. J Vasc Surg. 2014;60:497–499. doi: 10.1016/j.jvs.2013.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Wong R.H.L., Yu P.S.Y., Kwok M.W.T., et al. Endovascular fenestration for distal aortic sealing after frozen elephant trunk with thoraflex. Ann Thorac Surg. 2017;103:e479–e482. doi: 10.1016/j.athoracsur.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Norton E.L., Williams D.M., Kim K.M., et al. Management of acute type B aortic dissection with malperfusion via endovascular fenestration/stenting. J Thorac Cardiovasc Surg. 2020;160:1151–1161.e1. doi: 10.1016/j.jtcvs.2019.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takagi D., Wada T., Igarashi W., et al. Endovascular rescue for malpositioned frozen elephant trunk into the false lumen. J Card Surg. 2021;36:3948–3951. doi: 10.1111/jocs.15846. [DOI] [PubMed] [Google Scholar]
- 9.Panneton J.M., Teh S.H., Cherry K.J., et al. Aortic fenestration for acute or chronic aortic dissection: an uncommon but effective procedure. J Vasc Surg. 2000;32:711–721. doi: 10.1067/mva.2000.110054. [DOI] [PubMed] [Google Scholar]
- 10.Murphy E.H., Dimaio J.M., Dean W., Jessen M.E., Arko F.R. Endovascular repair of acute traumatic thoracic aortic transection with laser-assisted in-situ fenestration of a stent-graft covering the left subclavian artery. J Endovasc Ther. 2009;16:457–463. doi: 10.1583/09-2746.1. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L.L., Weaver F.A., Rowe V.L., Ziegler K.R., Magee G.A., Han S.M. Antegrade in situ fenestrated endovascular repair of a ruptured thoracoabdominal aortic aneurysm. J Vasc Surg Cases Innov Tech. 2020;6:416–421. doi: 10.1016/j.jvscit.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manzur M., Magee G.A., Ziegler K.R., Weaver F.A., Rowe V.L., Han S.M. Caudally directed in situ fenestrated endografting for emergent thoracoabdominal aortic aneurysm repair. J Vasc Surg Cases Innov Tech. 2021;7:553–557. doi: 10.1016/j.jvscit.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Houérou T., Fabre D., Alonso C.G., et al. In situ antegrade laser fenestrations during endovascular aortic repair. Eur J Vasc Endovasc Surg. 2018;56:356–362. doi: 10.1016/j.ejvs.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 14.DiBartolomeo A.D., Han S.M. Techniques of antegrade in situ laser fenestration for endovascular aortic repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg Cases Innov Tech. 2022;8:787–793. doi: 10.1016/j.jvscit.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of patient 1 depicting the preoperative computed tomography angiography scan, intraoperative fluoroscopy images, and postoperative computed tomography angiography scan.