Abstract
Viability loss of probiotics often occur during processing, storage and gastrointestinal transit. In this study, the viability of freeze-dried Lactobacillus acidophilus LA-5® was assessed after controlled freeze drying and storage at 4 °C and 25 °C over six months using glycerol, skim milk and trehalose as protectants. The freeze-dried probiotic was filled into hard gelatin capsules and enteric coated with the co-polymer Eudragit L100-55 using a fluidised bed coater to determine if the freeze-dried probiotic will survive the enteric coating process and remain viable during gastric transit. Empty hard gelatin capsules were also enteric coated by dipping in the co-polymer solution. These were dried, filled with microcrystalline cellulose and tested for their resistance to simulated gastric condition. The results showed that controlled freezing of the probiotic bacteria did not cause significant loss in viability when the cells were cryopreserved in the protectants. Viable cell loss was greater during the drying stage. Relatively better cell survival was recorded when the freeze-dried samples that were cryopreserved with skim milk were stored over six months at 4 °C. Freeze-dried samples that were preserved with trehalose stored better at 25 °C. The results also demonstrated that capsules coated with Eudragit L100-55 did not disintegrate in simulated gastric fluid. However, the capsules disintegrated in a simulated intestinal fluid. The enteric coating process resulted in about 95% recovery of viable cells. The high viable cell recovery after the coating process is likely due to the coating solution and conditions impacting the capsule body and cap rather than the cells directly. The study highlights that enteric coated capsules can offer gastric protection whilst minimizing viability losses associated with the enteric coating process.
Keywords: Probiotic, Viability, Lactic acid bacteria, Enteric coated capsule, Targeted delivery, Tolerance, Freeze-dried, Formulation
Highlights
-
•
Probiotic viability loss greater during drying than freezing.
-
•
Probiotics are more stable at 4 °C storage than 25 °C storage.
-
•
Enteric coated capsules offer gastric protection to probiotics.
-
•
Enteric coated probiotics undergo minimal loss during processing.
1. Introduction
Probiotics are live microorganisms that are administered for their health benefits. Probiotics are usually members of the lactic acid bacteria. Probiotics exert their beneficial effects through various mechanisms, including lowering intestinal pH, decreasing colonization and invasion by pathogenic microorganisms and modifying the host immune response [[1], [2], [3]]. They are often incorporated into food and other matrices. To exert their health benefit, probiotics must overcome some technological and physiological challenges. For instance, they must withstand manufacturing processes and remain viable within a product. They are also expected to survive passage through the gastrointestinal tract and remain viable in sufficient numbers in the colon where they exert their action [4].
Freeze drying and spray drying are often used to incorporate probiotics in foods. Spray drying, although economical, can cause high mortality of probiotic bacteria as a result of simultaneous dehydration, temperature and oxygen stresses imposed to the bacteria during the process [[5], [6], [7]]. Freeze drying is considered the most common and favourite method for producing powdered probiotics [6,8]. However, the viability of freeze-dried probiotic bacteria is affected during processing and storage [9,10]. The freezing process, especially the cooling rate could damage the cells [11]. Very slow cooling rate can result in death of cells caused by changes in osmotic pressure whereas very fast freezing rate can cause cell death from mechanical damage of cell membranes caused by recrystallization of intracellular ice [12]. Addition of cryoprotectants during the freezing drying process has been shown to increase the survival of some probiotic bacteria [10,[13], [14], [15]]. However, most studies have evaluated the survival of the probiotic only after the freeze drying process, not the stages of the freezing drying process. Furthermore, freeze-dried and spray dried probiotics, which are marketed as powdered products have shown viability losses during gastric tolerance assays as the techniques do not adequately provide protection in gastric fluid [16,17].
Probiotics are also marketed in the form of tablets, granules and pellets. Due to reported significant viability losses during gastric transit, there have been attempts to enteric coat some probiotic granules, tablets and pellets [18,19]. However, significant losses have been noted to occur from tableting or pelletization process alone [18] and these losses can be increased during the coating process due to direct impact on the cells during the processes. Capsules have the advantage of not requiring a granulation and compression step, hence the heat exposure which could be associated with significant viability loss during granulation or tableting processes is avoided [20]. Capsules also allow for enteric coating for targeted delivery of the filled content without detrimental effects to the content [21,22]. The aim of this work was to develop an enteric coated probiotic capsule produced by controlled freeze drying of the bacteria cells and fluidised bed coating of the filled capsule with the co-polymer Eudragit L100-55. Viability of the probiotic bacteria during the stages of the freeze drying process and storage at 4 °C and 25 °C was evaluated. Additionally, dip coated capsules which can allow probiotics to be filled after the enteric coating process was investigated. There was a belief that enteric coated capsules would reduce the impact of processing conditions on the viability of the cells, which accordingly can withstand the gastric low pH conditions to ensure the probiotic survive the stomach and is released in the intestine.
2. Materials and methods
2.1. Microorganism, freeze drying and storage
L. acidophilus LA-5® obtained from Chr. Hansen's Culture Collection (Hørsholm, Denmark) was grown anaerobically at 37 °C in de man rogosa sharpe (MRS) broth (Oxoid, Basingstoke, UK) supplemented with 0.05% w/v l-cysteine hydrochloride (Fisher Scientific, UK). At the stationary phase of growth, the culture was divided into equal aliquots and centrifuged (Heraues Stratosbiofuge, Thermo Scientific, UK) at 3500 g for 10 min at 4 °C. Cells at the stationary phase of growth remain metabolically active and are known to be more resistant than exponentially growing cells which may exhibit greater sensitivity [23]. The pellet obtained in each aliquot was washed twice with phosphate buffered saline (PBS). The pellets were resuspended and mixed in different protectants in ¼ strength ringer's solution (Sigma-Aldrich, UK): 15% v/v glycerol (Fisher Scientific, UK), 10% w/v trehalose (Sigma-Aldrich, UK) and 10% w/v reconstituted skim milk (Sigma-Aldrich, UK). Pellets resuspended in ¼ strength ringer's solution without protectant were used as control. 1 mL of each suspension was dispensed into vials, sealed and frozen over liquid nitrogen vapour as described by Beezer et al. [11] to a temperature of −50 °C. Briefly, a modified bath (Fig. 1) was filled with liquid nitrogen to an appropriate level. The sealed vials were clipped onto terry clips of racks and placed into the relevant fixture of the modified bath containing liquid nitrogen. The level of liquid nitrogen was measured and topped up to 7 cm below the level of the racks that the vials were attached to making sure the vials were not touching any liquid nitrogen. A thermometer was used to monitor the temperature of freezing as the vials were cooled gradually in the nitrogen vapour [11]. When the temperature reached −50 °C, the vials were released and transferred to a freeze dryer, Modulyo D-230 (Thermo Scientific, UK) whose sample shelf was equilibrated at −50 °C. The vials were kept at this temperature and under 0.5 mbar vacuum for 22 h for the primary drying stage after which a secondary drying stage began for a further 6 h at 10 °C. Viable cell count after freezing and freezing drying of the probiotic with the different protectants was determined and compared to viable cell count before freezing. This was carried out by either thawing the frozen cells at 40 °C for 3 min in a water bath or hydration of the freeze-dried samples in PBS. Followed by serial dilution, spread plating on MRS agar supplemented with 0.05%w/v l-cysteine hydrochloride and anaerobic incubation of the plates at 37 °C for 48 h. The freeze-dried samples obtained were separated into two groups. One group was stored at 4 °C and the other stored at 25 °C. The viability of the freeze-dried probiotic bacteria in the different protectants was determined after 3 days, 7 days, 10 days, 14 days, 28 days, 2 months, 3 months and 6 months of storage either at 4 °C or at 25 °C. This was measured by serial dilution, spread plating and colony counting on MRS agar supplemented with 0.05%w/v l-cysteine hydrochloride after anaerobic incubation at 37 °C for 48 h. Experiments were repeated in triplicates. Results are expressed as mean ± standard deviation.
Fig. 1.
Schematic representation of the modified bath used for freezing probiotic bacteria in liquid nitrogen. Cryovials were held in terry clips of racks, which were held above the surface of the liquid nitrogen.
2.2. Capsule filling
183 mg of the freeze-dried L. acidophilus LA-5® in skim milk was mixed with microcrystalline cellulose and hand filled into size 0 hard gelatin capsule (Capsugel, UK) of 0.68 mL capsule volume to produce capsules of mass 345 mg. Some capsules were also filled with microcrystalline cellulose using a capsule filling machine (Zanasi A25 Capsule filling machine, IMA Industria Macchine Automatiche, Italy) and were used as placebo to increase the capsule bulk for coating.
2.3. Coating of capsules
Enteric coating solution was prepared from an organic polymer dispersion of composition, Eudragit L100-55 5g, talc 2.5 g, triethyl citrate 0.5 g, isopropanol 69.85 g and water 2.15 g. The capsules were coated using a Strea-1 bottom spray fluidised bed coater (Aeromatic AG, Bubendorf, Switzerland). For each batch, the coater was loaded with 30 g of capsules and operated. The coating conditions used are: inlet temperature 40 °C, outlet temperature 32 °C, fan capacity 14, atomizing pressure 0.2 bar, flow rate 0.5797 g/min and coating level 5 mg/cm2 [24]. After gaining an estimated weight corresponding to a coating thickness of 5–20 mg/cm2, the capsules were removed from the coater and cured for 2 h in an oven set at 40 °C.
Some capsules were also dip coated manually. Capsules were separated into halves. The capsule head or body was held with tweezers and dipped into a coating solution prepared as previously described. The dip coated capsules were air-dried and further dips continued until the capsules had gained significant coating thickness. The capsules were placed on a mesh tray with open end down and cured overnight in an oven set at 40 °C.
2.4. Viability of L. acidophilus after enteric coating
Enumeration of viable cells after the fluidised bed coating process was determined by emptying the content of the coated capsule into 1 mL of PBS solution to hydrate. The mixture was serially diluted and spread plated onto MRS agar supplemented with 0.05%w/v l-cysteine hydrochloride. The plates were incubated anaerobically at 37 °C for 48 h. Colonies obtained after incubation were counted and recorded. Experiments were repeated in triplicates. Results are expressed as mean ± standard deviation.
2.5. Scanning electron microscopy (SEM)
Imaging of both the fluidised bed coated and dip coated capsules was done to characterize the surface uniformity of the coating and to determine if there was any cracking on the coated surface. The samples were sputter-coated with gold to a thickness of 10 nm. Imaging was performed using a scanning electron microscope (SEM, Quanta 200 FEG, FEI, Netherlands).
2.6. Disintegration of enteric-coated capsules
The disintegration test was conducted according to British Pharmacopoeia for capsules with a gastro-resistant shell using a disintegration apparatus (ErwekaZT-34, Copley, UK). Six capsules were exposed to 900 mL of 0.1 M HCl of pH 1.12 which was maintained at 37 °C for 2 h. After 2 h, the capsules were tested in phosphate buffer, pH 6.8 (KH2PO4 3.03 g/L; Na2HPO42H2O 3.95 g/L) for a further 1 h. The capsules were monitored and the time they disintegrated was noted. The dip-coated capsules were filled with 345 mg of microcrystalline cellulose and a disintegration test conducted as above.
2.7. Statistical analysis
Statistical analysis was performed in Origin Pro 8.6 (Microcal Software Inc.). T-test or Analysis of Variance (ANOVA) was conducted. P values less than 0.05 were regarded as significant difference between means.
3. Results
3.1. Freeze drying and storage of L. acidophilus
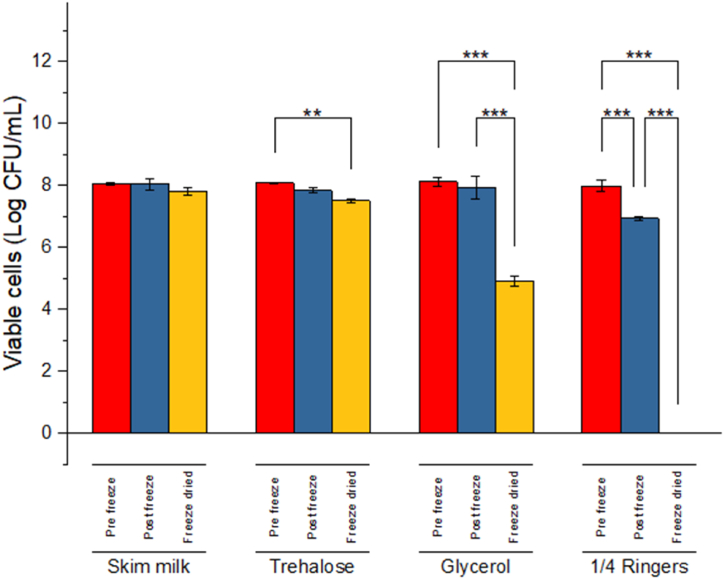
Fig. 2 shows the survival of L. acidophilus LA-5® at each stage of the freeze drying process: the pre-freezing, freezing and drying stages. The controlled freezing of the bacteria did not result in a significant decrease in viability when the cells were cryopreserved in skim milk, trehalose and glycerol. A statistically significant decrease in viability (p < 0.05), about 1 log CFU/mL was noted when the bacteria were frozen without cryoprotectant in ¼ strength ringer's solution. Significant differences in viable cells were noted between the control and the frozen cells with protectants. Viable cell loss was greater during the drying process than the freezing process for all the media used. Non-significant reduction in viable cells (p > 0.05) was observed when the cells were freeze-dried in skim milk. Significant loss in viable cells (p < 0.05), occurred when the cells were freeze-dried in trehalose and 15% v/v glycerol. The probiotic did not survive the freeze drying process in ¼ strength ringer's solution without protectant. Significant differences in viable cells (p < 0.05) were also noted amongst the protectants; between skim milk and glycerol as well as trehalose and glycerol after freeze drying.
Fig. 2.
Viability of L. acidophilus at different stages of the freeze drying process in different protectants: 10% w/v skimmed milk, 10% w/v trehalose, 15% v/v glycerol and a control, ¼ strength ringer's solution. Data are expressed as mean ± SD (n = 3). Asterisks *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
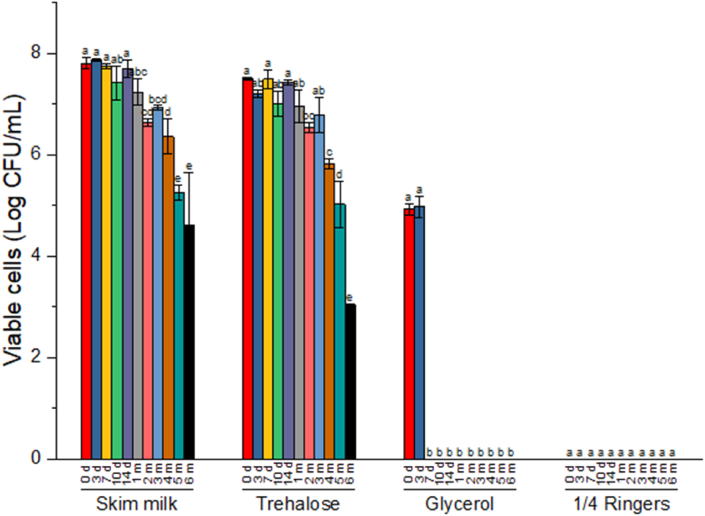
Fig. 3, Fig. 4 show the viability of the cells freeze-dried in the different protectants during six months storage at 4 °C and 25 °C respectively. Differences were observed among the protectants and the two storage conditions. For ¼ strength ringer's solution and 15% v/v glycerol, total loss of viability was observed either immediately or by 7 days after freeze-drying respectively. Viability was generally maintained for 2 months with no significant (p > 0.05) decrease observed in the cells' viability compared to day 0 for cells freeze-dried in skim milk or trehalose when stored at 4 °C. Viable cell recovery was higher in skim milk than in trehalose at 4 °C storage. At storage temperature of 25 °C, cells freeze-dried in skim milk or trehalose, maintained viability for 14 days without significant reduction in viability (p > 0.05) when compared to day 0 of storage. However, trehalose performed better in protecting the cells at this storage condition.
Fig. 3.
Viability of freeze-dried L. acidophilus LA-5® with different protectants during storage at 4 °C for 6 months. Data are expressed as mean ± SD (n = 3). d = days, m = month.
Fig. 4.
Viability of freeze-dried L. acidophilus LA-5® with different protectants during storage at 25 °C for 6 months. Data are expressed as mean ± SD (n = 3). d = days, m = month.
3.2. Enteric coated L. acidophilus
The viability of the bacteria before and after fluidised bed enteric coating is shown in Fig. 5. A reduction of less than 0.5 log CFU/mL of viable cells which represented about 95% recovery of viable cells was observed after the enteric coating process.
Fig. 5.
Viable cells before and after enteric coating using the fluidised bed coater.
The SEM images of the capsules coated by fluidised bed coating and the dip-coated capsules (with coating thickness of 99–100 μm and 33–42 μm respectively) are shown in Fig. 6 (A and B respectively). The micrographs show a uniform coating around both the curved and flat surface of the capsules. No pores or cracks were observed.
Fig. 6.
Scanning electron micrographs of the surface of the coated capsules. [A] fluidised bed coated and [B] dip coated.
Disintegration test performed on the uncoated capsules, showed they disintegrated within 4 min in 0.1 M HCl (pH 1.12 ± 0.01 at 37 °C). After coating the capsules with Eudragit L100-55, the capsules remained intact in the acidic medium for 2 h for both fluidised bed-coated and dip-coated capsules. The capsules disintegrated in phosphate buffer (pH 6.8 at 37 °C) within 25 min and 14 min respectively showing the faster disintegration of the dip-coated capsules. About 99% viable cell recovery was obtained after the disintegration test.
4. Discussion
4.1. Freeze drying and storage of L. acidophilus
Freezing of the bacteria over liquid nitrogen vapour showed very good recoveries which concurs with previous reports [11,25]. Controlled cooling rate was achieved which reduced the amount of damage to the cells. The results demonstrated the significance of the protectants in the freeze drying of the bacteria as there was no survival of the freeze-dried sample in ¼ strength ringer's solution without protectant. 15% v/v glycerol was demonstrated to be a good cryoprotectant for freezing of the bacteria but not a good protectant for freeze drying. Glycerol, a known cryoprotectant acts by protecting the cells from both intracellular and extracellular damage. It reduces the amount of frozen water, by decreasing the freezing-point of water and thereby, lessening the concentration of salt dissolved in solution which effectively inhibit osmotic shock [26,27]. For instance, addition of glycerol to water has been shown to decrease the freezing point of water to a minimum of −46 °C, preventing the formation of large ice crystals within the cells and subsequently reducing cell damage [28]. The results showed better performance of trehalose in protecting the cells when the freeze-dried samples were stored at 25 °C. Trehalose has a high glass transition temperature, Tg, of 110 °C [29] in the anhydrous form and this may enable an amorphous trehalose obtained as a result of freeze drying to remain stable at 25 °C. Trehalose is also capable of forming some crystalline hydrates which can result in keeping the remaining amorphous sugar dry, thereby maintaining stability of the freeze-dried matrix [30]. Skim milk, is also known to be stable above its glass transition temperature of 92 °C [31]. The presence of moisture, however, has been found to significantly alter its glass transition. For instance, a moisture content of 4.52 g per 100 g of dry powder has been shown to lower the Tg of skim milk to 46.7 °C [32]. Between the two, trehalose may offer better protective effect for storage in humid environments. The two protectants were nonetheless better when stored at 4 °C than at 25 °C even though there were still significant losses in viability over the duration of storage at 4 °C. The results demonstrate that storage of freeze-dried probiotic bacteria at 4 °C could ensure high viability of the cells for long periods than at 25 °C but this may imply increase cost of transportation and storage. Other studies [10,33,34] have also shown the advantage of storing freeze-dried bacteria at 4 °C than at higher temperatures. The loss in viability of the cells during storage can be attributed to factors such as the water activity in the freeze-dried bacteria and also the absence of antioxidants. It has previously been shown the combined effect of water activity, oxygen level and antioxidant on storage stability [15].
4.2. Enteric coated L.acidophilus
Probiotics are frequently sold as dried form; freeze-dried powders packed into capsules or sachet, as pellets, granules and tablets. This offers a convenient means of storage and transport for both the manufacturer and consumer and reduces the overall cost to both. For probiotics to achieve their beneficial function in a host, it is important that they arrive in sufficient quantity at the target site of the host [4]. Accordingly, it is vital that appropriate protection is provided to probiotics to achieve their functional effect. Gastro-resistant forms of probiotics can be achieved by coating tablets, granules, pellets, or capsules with enteric polymers. Capsules offer the advantage of less technological stress during processing as well as the possibility of enteric coating the capsule shells before filling. The enteric capsule shell avoids the need for process development and exposure of the probiotic to heat during the coating process. A simple and cost-efficient means of offering gastric protection to probiotics packaged into capsules by coating with an enteric co-polymer was explored in this study. Using the co-polymer Eudragit L100-55, it was demonstrated that the coated capsules did not disintegrate in the acidic medium, which could be detrimental to the viability of enclosed probiotic. However, when the capsules were exposed to the weakly alkaline medium simulating the intestinal fluid, disintegration of the coatings occurred. Both the dip-coated and fluidised bed-coated capsules had uniform coatings and were efficient in providing gastro-resistance to the probiotic. However, a faster disintegration of the dip-coated capsules in the buffer was observed due to the smaller coating thickness.
Processing conditions such as temperature, oxygen stress, osmotic stress, encapsulating medium, pH of encapsulating material and drying conditions may affect viability of probiotics [6,35]. Furthermore, conditions of the human body may impact their viability when ingested [16]. Several processing techniques, particularly encapsulation have been developed to improve survival of probiotics and enhance their colonization [19,[36], [37], [38], [39]]. However, these methods tend to apply the encapsulating or coating material directly to the probiotic bacteria. This results in viability losses during the process because the stress factors impact the cells directly. Besides, coating materials and media including organic solvents which are employed in the encapsulation or coating process may be destructive to the cells and likely to alter the functional properties of the encapsulated probiotics. This study demonstrates that by coating capsule shells with an enteric polymer, the destructive effect of the encapsulation process is partly avoided. Eudragit is a copolymer available commercially. It is made up of methyl acrylate with different acidic and alkaline end groups which dissolves by salt formation. The release of an entrapped ingredient in the co-polymer is usually pH dependent based on the properties of the co-polymer. For example, Eudragit L 100-55 would dissolve at pH of approximately 5.5 or greater. Thus, it dissolves once it reaches the duodenum [40]. Improvement in intestinal absorption of drugs coated with Eudragit co-polymers have been reported [22,[41], [42], [43]]. For instance, it has been shown that relative to subcutaneous injection, lipid filled Eudragit L100 enteric capsules can deliver insulin with 99% and 150% bioavailabilities for fast and slow acting forms of the peptide hormone respectively [43]. In this study, it was demonstrated that enteric coated capsules of probiotics did not release in simulated acidic conditions of the stomach. Release occurred in the simulated intestinal fluid, where viability of the enteric coated probiotic remained high. This indicates that when administered, the probiotic capsules coated with the co-polymer will release their contents in the intestinal tract, the location in which they can survive in adequate quantities and have a beneficial effect.
Future research will investigate the impact of the processing conditions on the functionality of the probiotic such as antimicrobial and organic acid production, adhesion, biofilm formation and autoaggregation. This will determine if these functionalities are better retained when the probiotics are not in direct contact with the coating or encapsulating material.
5. Conclusion
In this study, L. acidophilus LA-5® was produced by controlled freeze drying, filled into hard gelatin capsule and enteric coated. The results indicated that, viability loss was greater during drying than freezing. The results also demonstrated that storing probiotics at 4 °C was superior in maintaining viability than storing at 25 °C. Enteric coating freeze-dried probiotic capsules with Eudragit L100-55 offered the probiotic cells gastric protection with approximately 5% loss in viability during the process. Pre-coating of capsules before loading with probiotic cells could also offer gastric protection as content of the capsule were not released in the acidic environment. This suggests that when the probiotic capsule is swallowed, the contents will be released in the intestinal tract where it could thrive and produce health benefits. It can be concluded that probiotics filled into enteric coated hard gelatin capsules provide a promising delivery system for their administration.
Data availability statement
The author confirms that the data supporting the findings of this research are available within the article. More detailed content can be requested from the corresponding author.
CRediT authorship contribution statement
Mansa Fredua-Agyeman: Writing – review & editing, Writing – original draft, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author would like to thank Prof. Simon Gaisford and Dr. Hamid Merchant for inspiring this work.
References
- 1.Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16(10):605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 2.Collins M.D., Gibson G.R. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999;69(5) doi: 10.1093/ajcn/69.5.1052s. 1052S-7S. [DOI] [PubMed] [Google Scholar]
- 3.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 4.Wendel U. Assessing viability and stress tolerance of probiotics-A review. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.818468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anal K.A., Singh H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007;18(5):240–251. [Google Scholar]
- 6.Kieps J., Dembczynski R. Current trends in the production of probiotic formulations. Foods. 2022;11(15) doi: 10.3390/foods11152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S., Vignolles M.L., Chen X.D., Le Loir Y., Jan G., Schuck P., et al. Spray drying of probiotics and other food-grade bacteria: a review. Trends Food Sci. Technol. 2017;63:1–17. doi: 10.1016/j.tifs.2017.02.007. [DOI] [Google Scholar]
- 8.Morgan C.A., Herman N., White P.A., Vesey G. Preservation of micro-organisms by drying; a review. J. Microbiol. Methods. 2006;66(2):183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Ermis E. A review of drying methods for improving the quality of probiotic powders and characterization. Dry. Technol. 2022;40(11):2199–2216. doi: 10.1080/07373937.2021.1950169. [DOI] [Google Scholar]
- 10.Jalali M., Abedi D., Varshosaz J., Najjarzadeh M., Mirlohi M., Tavakoli N. Stability evaluation of freeze-dried Lactobacillus paracasei subsp. tolerance and Lactobacillus delbrueckii subsp. bulgaricus in oral capsules. Res Pharm Sci. 2012;7(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- 11.Beezer A.E., Newell R.D., Tyrrell H.J. Application of flow microcalorimetry to analytical problems: the preparation, storage and assay of frozen inocula of Saccharomyces cerevisiae. J. Appl. Bacteriol. 1976;41(2):197–207. doi: 10.1111/j.1365-2672.1976.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 12.Meryman H.T. Cryoprotective agents. Cryobiology. 1971;8(2):173–183. doi: 10.1016/0011-2240(71)90024-1. [DOI] [PubMed] [Google Scholar]
- 13.Bellali S., Bou Khalil J., Fontanini A., Raoult D., Lagier J.C. A new protectant medium preserving bacterial viability after freeze drying. Microbiol. Res. 2020;236 doi: 10.1016/j.micres.2020.126454. [DOI] [PubMed] [Google Scholar]
- 14.Savedboworn W., Teawsomboonkit K., Surichay S., Riansa-Ngawong W., Rittisak S., Charoen R., et al. Impact of protectants on the storage stability of freeze-dried probiotic Lactobacillus plantarum. Food Sci. Biotechnol. 2019;28(3):795–805. doi: 10.1007/s10068-018-0523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtmann L., Carlsen C.U., Risbo J., Skibsted L.H. Storage stability of freeze-dried Lactobacillus acidophilus (La-5) in relation to water activity and presence of oxygen and ascorbate. Cryobiology. 2009;58(2):175–180. doi: 10.1016/j.cryobiol.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Fredua-Agyeman M., Gaisford S. Comparative survival of commercial probiotic formulations: tests in biorelevant gastric fluids and real-time measurements using microcalorimetry. Benef. Microbes. 2015;6(1):141–151. doi: 10.3920/BM2014.0051. [DOI] [PubMed] [Google Scholar]
- 17.da Silva M.N., Tagliapietra B.L., Flores V.D., Richards N.S.P.D. In vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 2021;4:320–325. doi: 10.1016/j.crfs.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brachkova M.I., Duarte A., Pinto J.F. Evaluation of the viability of Lactobacillus spp. after the production of different solid dosage forms. J. Pharmaceut. Sci. 2009;98(9):3329–3339. doi: 10.1002/jps.21609. [DOI] [PubMed] [Google Scholar]
- 19.Pyar H., Peh K.K. Enteric coating of granules containing the probiotic Lactobacillus acidophilus. Acta Pharm. 2014;64(2):247–256. doi: 10.2478/acph-2014-0011. [DOI] [PubMed] [Google Scholar]
- 20.Bansal T., Garg S. Probiotics: from functional foods to pharmaceutical products. Curr. Pharmaceut. Biotechnol. 2008;9(4):267–287. doi: 10.2174/138920108785161587. [DOI] [PubMed] [Google Scholar]
- 21.Dvorackova K., Rabiskova M., Gajdziok J., Vetchy D., Muselik J., Bernatoniene J., et al. Coated capsules for drug targeting to proximal and distal part of human intestine. Acta Pol. Pharm. 2010;67(2):191–199. [PubMed] [Google Scholar]
- 22.Arpac B., Devrim Gokberk B., Kucukturkmen B., Ozakca Gunduz I., Palabiyik I.M., Bozkir A. Design and in vitro/in vivo evaluation of polyelectrolyte complex nanoparticles filled in enteric-coated capsules for oral delivery of insulin. J. Pharmaceut. Sci. 2023;112(3):718–730. doi: 10.1016/j.xphs.2022.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal A., Rangarajan N., Weisshaar J.C. Resistance of early stationary phase E. coli to membrane permeabilization by the antimicrobial peptide Cecropin A. Biochim. Biophys. Acta Biomembr. 2019;1861(10) doi: 10.1016/j.bbamem.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Titapiwatanakun V., Wanjing L., Gaisford S., Basit A.W. Effect of CO2 laser irradiation on Eudragit® L100-55, L100, and S100 coatings to modify drug release. Thai Journal of Pharmaceutical Sciences. 2020;45(5):318–325. [Google Scholar]
- 25.Fredua-Agyeman M., Stapleton P., Basit A.W., Beezer A.E., Gaisford S. In vitro inhibition of Clostridium difficile by commercial probiotics: a microcalorimetric study. Int J Pharmaceut. 2017;517(1–2):96–103. doi: 10.1016/j.ijpharm.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Lovelock J.E. Het mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim. Biophys. Acta. 1953;11:28–36. doi: 10.1016/0006-3002(53)90005-5. [DOI] [PubMed] [Google Scholar]
- 27.Lovelock J.E., Bishop M.W.H. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183:1394–1395. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- 28.Hubalek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46(3):205–229. doi: 10.1016/s0011-2240(03)00046-4. doi: S0011224003000464 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Hernandez Garcia A. Anhydrobiosis in bacteria: from physiology to applications. J. Biosci. 2011;36(5):939–950. doi: 10.1007/s12038-011-9107-0. [DOI] [PubMed] [Google Scholar]
- 30.Crowe J.H., Carpenter J.F., Crowe L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998;60:73–103. doi: 10.1146/annurev.physiol.60.1.73. [DOI] [PubMed] [Google Scholar]
- 31.Roos Y.H. Importance of glass transition and water activity to spray drying and stability of dairy powders. Lait. 2002;82(4):475–484. doi: 10.1051/lait:2002025. [DOI] [Google Scholar]
- 32.Ozmen L., Langrish T.A.G. Comparison of glass transition temperature and sticky point temperature for skim milk powder. Dry. Technol. 2002;20(6):1177–1192. doi: 10.1081/drt-120004046. [DOI] [Google Scholar]
- 33.Carvalho A.S., Silva J., Ho P., Teixeira P., Malcata F.X., Gibbs P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004;14(10):835–847. doi: 10.1016/j.idairyj.2004.02.001. [DOI] [Google Scholar]
- 34.Savini M., Cecchini C., Verdenelli M.C., Silvi S., Orpianesi C., Cresci A. Pilot-scale production and viability analysis of freeze-dried probiotic bacteria using different protective agents. Nutrients. 2010;2(3):330–339. doi: 10.3390/nu2030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terpou A., Papadaki A., Lappa I.K., Kachrimanidou V., Bosnea L.A., Kopsahelis N. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019;11(7) doi: 10.3390/nu11071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patarroyo J.L., Florez-Rojas J.S., Pradilla D., Valderrama-Rincon J.D., Cruz J.C., Reyes L.H. Formulation and characterization of gelatin-based hydrogels for the encapsulation of kluyveromyces lactis-applications in packed-bed reactors and probiotics delivery in humans. Polymers. 2020;12(6) doi: 10.3390/polym12061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su J., Wang X., Li W., Chen L., Zeng X., Huang Q., et al. Enhancing the viability of Lactobacillus plantarum as probiotics through encapsulation with high internal phase emulsions stabilized with whey protein isolate microgels. J. Agric. Food Chem. 2018;66(46):12335–12343. doi: 10.1021/acs.jafc.8b03807. [DOI] [PubMed] [Google Scholar]
- 38.Anselmo A.C., McHugh K.J., Webster J., Langer R., Jaklenec A. Layer-by-Layer encapsulation of probiotics for delivery to the microbiome. Adv. Mater. 2016;28(43):9486–9490. doi: 10.1002/adma.201603270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poelvoorde N., Huyghebaert N., Vervaet C., Remon J.P. Optimisation of an enteric coated, layered multi-particulate formulation for ileal delivery of viable recombinant Lactococcus lactis. Eur. J. Pharm. Biopharm. 2008;69(3):969–976. doi: 10.1016/j.ejpb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Nikam A., Sahoo P.R., Musale S., Pagar R.R., Paiva-Santos A.C., Giram P.S. A systematic overview of Eudragit((R)) based copolymer for smart healthcare. Pharmaceutics. 2023;15(2) doi: 10.3390/pharmaceutics15020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terao T., Matsuda K., Shouji H. Improvement in site-specific intestinal absorption of furosemide by Eudragit L100-55. J. Pharm. Pharmacol. 2001;53(4):433–440. doi: 10.1211/0022357011775721. [DOI] [PubMed] [Google Scholar]
- 42.Tayel S.A., El-Nabarawi M.A., Tadros M.I., Abd-Elsalam W.H. Duodenum-triggered delivery of pravastatin sodium: II. Design, appraisal and pharmacokinetic assessments of enteric surface-decorated nanocubosomal dispersions. Drug Deliv. 2016;23(9):3266–3278. doi: 10.3109/10717544.2016.1172367. [DOI] [PubMed] [Google Scholar]
- 43.Strachan J.B., Dyett B., Chan S., McDonald B., Vlahos R., Valery C., et al. A promising new oral delivery mode for insulin using lipid-filled enteric-coated capsules. Biomater. Adv. 2023;148 doi: 10.1016/j.bioadv.2023.213368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The author confirms that the data supporting the findings of this research are available within the article. More detailed content can be requested from the corresponding author.