ABSTRACT
Introduction
The aim of this study was to compare the effects of mitiglinide/voglibose with those of glimepiride on glycemic variability and vascular endothelial function in patients with type 2 diabetes.
Materials and Methods
It was a multicenter, open‐label, randomized, crossover study. Hospitalized patients received either mitiglinide/voglibose (three times daily administration of 10 mg mitiglinide and 0.2 mg voglibose) or glimepiride (once‐daily 2 mg) in random order, each for 5 days. The reactive hyperemia index (RHI) and the mean amplitude of glycemic excursions (MAGE) were measured as co‐primary endpoints using reactive hyperemia peripheral arterial tonometry and continuous glucose monitoring.
Results
The analysis included 30 patients (15 in each group). The RHI was 1.670 ± 0.369 during treatment with mitiglinide/voglibose and 1.716 ± 0.492 during treatment with glimepiride, with no significant difference between the two. MAGE was significantly lower in the mitiglinide/voglibose group (47.6 ± 18.5 mg/dL) than in the glimepiride group (100.6 ± 32.2 mg/dL). Although the mean blood glucose levels over the entire 24 h period were comparable between the two groups, the use of mitiglinide/voglibose was associated with a lower standard deviation of mean glucose, coefficient of variation, and mean postprandial glucose excursion compared with glimepiride. The time below range (<70 mg/dL) and the time above range (>180, >200, and 250 mg/dL) were lower in the mitiglinide/voglibose group, while the time in range (70–180 mg/dL) was higher.
Conclusions
In our short‐duration randomized crossover study, although not impacting vascular endothelial function, mitiglinide/voglibose demonstrated potential benefits in reducing glycemic variability, postprandial hyperglycemia, and hypoglycemia in patients with type 2 diabetes.
Keywords: continuous glucose monitoring, mitiglinide/voglibose fixed‐dose combination tablet, reactive hyperemia index
INTRODUCTION
In type 2 diabetes, prolonged hyperglycemia can lead to microvascular complications (e.g., retinopathy, nephropathy, and neuropathy) as well as macrovascular complications (e.g., stroke, ischemic heart disease, and peripheral occlusive arterial disease) due to systemic arteriosclerosis 1 . Vascular endothelial dysfunction is an early manifestation of arteriosclerosis, and large swings in blood glucose levels are considered more damaging to vascular endothelial cells than persistent hyperglycemia 2 . Glycemic variability induces oxidative stress, causes vascular endothelial dysfunction, and promotes arteriosclerosis 3 . The EndoPAT 2000 device, based on reactive hyperemia peripheral arterial tonometry (RH‐PAT), serves as a non‐invasive, highly objective, and reproducible method for the assessment of endothelial function. It offers the advantage of being more convenient than brachial flow‐mediated dilation (FMD) measurement. Furthermore, the utility of RH‐PAT has been reported in several studies, such as the Framingham Heart Study 4 . In addition, we have reported that glycemic variability affects the reactive hyperemia index (RHI) measured by RH‐PAT 5 . Hypoglycemia is also implicated in vascular endothelial dysfunction through increased production of reactive oxygen species, catecholamines 6 , and proinflammatory cytokines 7 , in addition to the activation of the sympathetic nervous system 8 . Furthermore, we have also reported that hypoglycemia reduces RHI in patients with type 2 diabetes 9 and such a hypoglycemia‐induced reduction in RHI is also associated with an increase in adrenaline level in patients with normal glucose tolerance 10 .
Continuous glucose monitoring (CGM) allows the visualization of glycemic variability. The international consensus guidelines on target values for CGM metrics were issued in 2019 11 . Oral hypoglycemic agents that are considered effective, especially in improving postprandial hyperglycemia, include α‐glucosidase inhibitors (α‐GIs) 12 and rapid‐acting insulin secretagogues 13 . The fixed‐dose mitiglinide/voglibose combination tablet (hereinafter referred to as mitiglinide/voglibose), which contains a rapid‐acting insulin secretagogue (mitiglinide calcium hydrate) and an α‐GI (voglibose), has evidenced promise in improving postprandial hyperglycemia and in reducing hypoglycemic events 14 . This tablet is highly effective in lessening glycemic variability. Since glycemic variability and hypoglycemia are associated with vascular endothelial dysfunction 5 , 6 , 7 , 8 , mitiglinide/voglibose may improve vascular endothelial function, though such a possibility has so far only been described in a small‐scale pilot study 15 . To our knowledge, there are no studies that have investigated the effects of mitiglinide/voglibose on both glycemic variability and vascular endothelial function. The aim of the present study was to compare the effects of mitiglinide/voglibose with those of glimepiride on glycemic variability and vascular endothelial function in patients with type 2 diabetes.
METHODS
Patients
Patients eligible for enrollment in this study were hospitalized patients with type 2 diabetes who met all the inclusion criteria, and did not meet any of the exclusion criteria. The inclusion and exclusion criteria are listed in Appendix S1. In brief, the inclusion criteria were patients whose HbA1c levels measured within 2 months before enrollment were ≥7.0 and <10.0%, patients on treatment with metformin alone or a combination of metformin and a DPP‐4 inhibitor.
This trial was registered in the Japan Registry of Clinical Trials (jRCTs071190047, Date of registration: 20/02/2020). The study was approved by the Ethics Committee of Nara Medical University (CRB5200002) and was carried out in accordance with the Declaration of Helsinki. Informed consent was obtained from all the participants.
Study design
The present study was conducted as a multicenter open‐label randomized crossover comparative study. The clinical trial was conducted between January 2020 and July 2022, at two clinical trial centers: the Hospital of Occupational and Environmental Health, Japan, and the Wakamatsu Hospital of the University of Occupational and Environmental Health, Japan. After enrollment, patients were randomly assigned to either the mitiglinide/voglibose prior group or the glimepiride prior group. In the mitiglinide/voglibose prior group, the patients underwent the run‐in period (continuing treatment with metformin used before enrollment [concomitant use of a DPP‐4 inhibitor was allowed]), received the fixed‐dose combination of mitiglinide 10 mg and voglibose 0.2 mg (three times daily before meals), and then switched after 5 days to glimepiride 2 mg (two 1 mg tablets administered after breakfast) for another 5 days. In the glimepiride prior group, the patients underwent the run‐in period, received glimepiride 2 mg for 5 days and then switched to the fixed‐dose combination of mitiglinide 10 mg and voglibose 0.2 mg for another 5 days. A washout period of 1 day was set before switching the drugs. The reason for using glimepiride at 2 mg as the control was based on the finding of a previous study that compared mitiglinide/voglibose with glimepiride at 1 mg 15 , which showed MBG levels of 8.01 mmol/L (144.2 mg/dL) and 8.24 mmol/L (P = 0.184), respectively. Because MBG tended to be lower for mitiglinide/voglibose, we decided to set the dose of glimepiride at 2 mg in this study. Randomization was centrally performed using blocked randomization through the envelope approach with an allocation ratio of 1:1. The contract research organization provided the allocation list to the allocation manager (who was a member of a department separate from the research implementation department). After the research physician enrolled the participants, the allocation manager assigned them according to the allocation list. CGM was performed three times during the study period. The vascular endothelial function was evaluated using a peripheral arterial tonometry (PAT) device (EndoPAT 2000, Itamar Medical, Caesarea, Israel) on the last day of each treatment period.
In this study, we used a fixed‐dose mitiglinide/voglibose combination tablet manufactured by Kissei Pharmaceutical Co., Ltd (Matsumoto, Japan), and glimepiride manufactured by Sanofi (Gentilly, France). The study protocol is illustrated in Figure 1.
Figure 1.
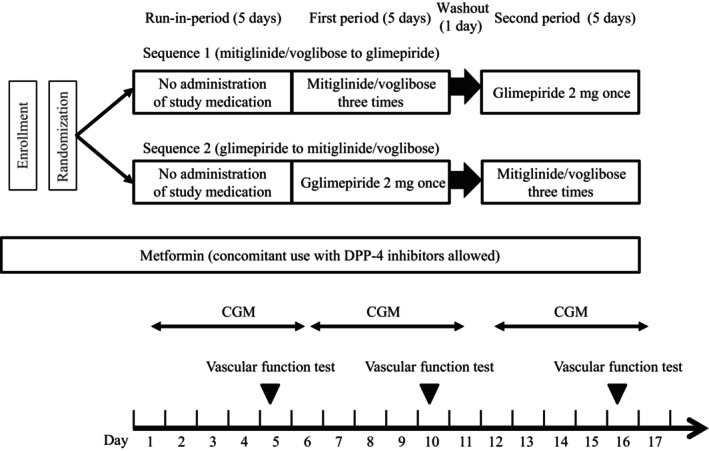
Study protocol. CGM, continuous glucose monitoring.
CGM system
The schedule for CGM is illustrated in Figure 2. The subjects were issued with a CGM system (iPro2 [Medtronic MiniMed, Northridge, CA, USA] was used until October 2021; Guardian Connect [Medtronic MiniMed] was used in and after November 2021) to monitor glycemic excursions throughout each study period. The CGM data obtained from the last 72 h of each period was used. Self‐monitoring of blood glucose was performed before meals and at bedtime using data from a self‐monitored blood glucose device (MEDISAFE MINI; Terumo, Inc., Tokyo, Japan) to calibrate interstitial fluid glucose concentrations determined using iPro2 or Guardian Connect. The extracted data recorded by CGM were: MBG, SD, CV, MAGE, estimated HbA1c, AOC <54 mg/dL, <70 mg/dL, AUC >140 mg/dL, >180 mg/dL, >200 mg/dL, >250 mg/dL, TBR <54 mg/dL, <70 mg/dL, TIR 70–180 mg/dL, TAR >140 mg/dL, 180 mg/dL, 200 mg/dL, 250 mg/dL, MPPGE, LBGI, high blood glucose index, and predicted percentage of BG <70 mg/dL. Some data were calculated separately for 24 h (0:00–24:00), daytime (07:00–24:00), and night‐time (00:00–07:00).
Figure 2.
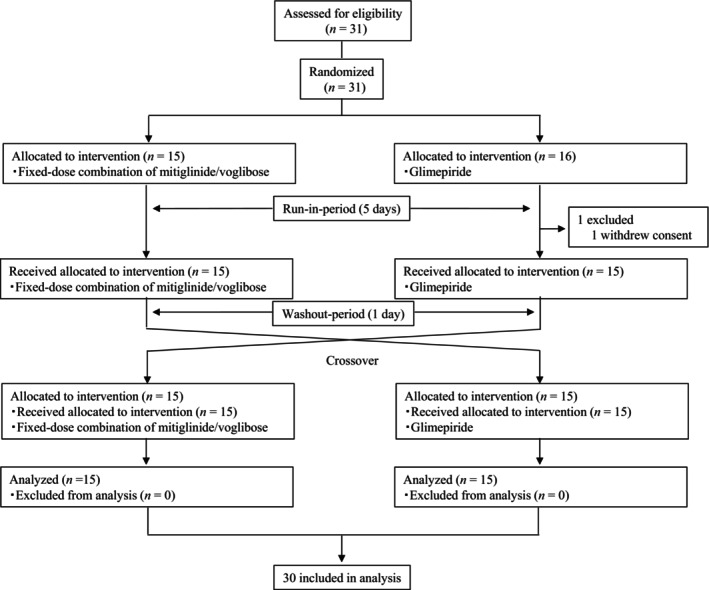
CONSORT flow chart showing patient recruitment. We enrolled 31 patients in this study, including 15 in the mitiglinide/voglibose prior group and 16 in the glimepiride prior group. One patient of the glimepiride prior group discontinued the study medication, leaving 30 patients for the final analysis (15 in each group).
Noninvasive vascular function test
Vascular endothelial function was assessed with reactive hyperemia‐peripheral arterial tonometry (RH‐PAT) using the EndoPAT 2000 device (Itamar Medical, Caesarea, Israel) 16 . The RH‐PAT principles and procedures are detailed in Appendix S2.
Endpoints
The co‐primary endpoints were RHI and MAGE. The secondary endpoints were other CGM metrics, association between vascular endothelial function and glycemic variability, and safety (adverse events).
Diet and exercise
Based on the guidelines of the Japan Diabetes Association, energy intake during the study period was set as follows: the total energy intake: 25–30 kcal/kg of ideal body weight; the target composition of the energy intake was 50–60% from carbohydrates, 20% or lower from proteins, and the remaining percentage from fat. The time of starting breakfast, lunch, and dinner was recorded on days 3–5, days 8–10, and days 14–16 after the start of the current study. During the study period, the contents of exercise therapy were not changed, and efforts were made to maintain the intensity of exercise constant.
Statistical analysis
One previous study of 20 patients described a significant difference in glycemic variability between mitiglinide/voglibose and glimepiride 14 , but no previous studies have investigated vascular endothelial function. Therefore, the aim of our study was to conduct exploratory evaluation of the assessment parameters, and a sample size of 30 cases was determined as feasible within the planned registration period. All data were expressed as mean ± standard deviation. A linear mixed‐effects model was used to analyze the measured values, with treatment (mitiglinide/voglibose, glimepiride), period (first, second), and sequence (allocation group) as fixed effects and patients as random effects, to calculate the effect size between the treatments along with its 95% confidence interval and P‐values. Univariate linear regression analysis was performed to assess the association between RHI and CGM metrics. The number of occurrences and percentage of the safety analysis population for each category (entire duration, observation period, treatment type) were calculated. In addition, a direct probability test using Fisher's exact test was performed to compare the presence or absence of adverse events and side effects between the treatment groups. All P values were estimated in a two‐sided manner. A P value < 0.05 was considered statistically significant. All analyses were performed using the SAS software (version 9.4, SAS Institute, Cary, NC, USA).
RESULTS
Patient demographics
We enrolled 31 patients in this study, including 15 in the mitiglinide/voglibose prior group and 16 in the glimepiride prior group. One patient of the glimepiride prior group discontinued the study medication, leaving 30 patients for the final analysis (15 in each group) (Figure 2). Table 1 summarizes the background characteristics of the study participants. Of the 30 patients, 19 were males, with a mean age of the whole group of 58.2 ± 15.7 (±SD) years, body weight 68.4 ± 16.4 kg, and body mass index of 25.6 ± 5.5 kg/m2. The mean glycated hemoglobin (HbA1c) level was 8.58% ± 0.82%, and fasting plasma glucose (FPG) level was 149.4 ± 21.8 mg/dL. Sixteen patients (53.3%) used dipeptidyl‐peptidase‐4 (DPP‐4) inhibitors.
Table 1.
Baseline clinical characteristics of the study patients
| Patients (n = 30) | |
|---|---|
| Male/Female (n) | 19/11 |
| Age (years) | 58.2 ± 15.7 |
| Body weight (kg) | 68.4 ± 16.4 |
| Body mass index (kg/m2) | 25.6 ± 5.5 |
| Aspartate transaminase (IU/L) | 19.5 ± 8.6 |
| Alanine transaminase (IU/L) | 25.1 ± 14.7 |
| γ‐Glutamyl transferase (IU/L) | 36.2 ± 19.6 |
| Total cholesterol (mg/dL) | 175.7 ± 36.3 |
| LDL‐C (mg/dL) | 106.3 ± 34.0 |
| HDL‐C (mg/dL) | 50.1 ± 13.5 |
| Triglycerides (mg/dL) | 146.4 ± 66.7 |
| Blood urea nitrogen (mg/dL) | 13.6 ± 3.9 |
| Creatinine (mg/dL) | 0.69 ± 0.16 |
| eGFR (mL/min/1.73 m2) | 86.27 ± 21.68 |
| Albumin (g/dL) | 4.25 ± 0.25 |
| HbA1c (%) | 8.58 ± 0.82 |
| Fasting plasma glucose (mg/dL) | 149.4 ± 21.8 |
| Erythrocyte count (×104/μL) | 466.7 ± 51.4 |
| Hemoglobin (g/dL) | 13.97 ± 1.13 |
| Hematocrit (%) | 41.05 ± 3.27 |
| Leukocyte count (/μL) | 7,373.3 ± 2,301.0 |
| Platelet count (×104/μL) | 26.50 ± 7.01 |
| Use of metformin (%) | 30 (100.0) |
| Use of DPP‐4 inhibitors (%) | 16 (53.3) |
Data are mean ± standard deviation or n (%). DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Efficacy
Co‐primary endpoints
Table 2 lists various parameters before and during administration of mitiglinide/voglibose or glimepiride. The RHI was 1.670 ± 0.369 during treatment with mitiglinide/voglibose and 1.716 ± 0.492 during treatment with glimepiride, with no significant difference between the two. The mean amplitude of glycemic excursions (MAGE) at baseline was 99.6 ± 31.9 mg/dL, but decreased during treatment to 47.6 ± 18.5 mg/dL for mitiglinide/voglibose but remained at 100.6 ± 32.2 mg/dL for glimepiride, being significantly lower for mitiglinide/voglibose (P < 0.001).
Table 2.
Effects of treatment on RHI and CGM metrics
| No administration of study medication | Mitiglinide/voglibose | Glimepiride | Effect size between the treatments | P‐value | |
|---|---|---|---|---|---|
| RHI | 1.848 ± 0.546 | 1.670 ± 0.369 | 1.716 ± 0.492 | −0.046 (−0.222, 0.131) | 0.601 |
| MAGE (mg/dL) | 99.6 ± 31.9 | 47.6 ± 18.5 | 100.6 ± 32.2 | −52.6 (−62.0, −43.2) | <0.001 |
| MBG, 0:00–24:00 (mg/dL) | 155.5 ± 24.8 | 130.2 ± 21.7 | 129.1 ± 24.4 | 1.3 (−1.9, 4.5) | 0.424 |
| MBG, 0:00–7:00 (mg/dL) | 128.0 ± 21.3 | 124.8 ± 24.0 | 106.5 ± 24.4 | 18.2 (14.1, 22.3) | <0.001 |
| MBG, 7:00–24:00 (mg/dL) | 167.1 ± 27.6 | 132.5 ± 21.3 | 138.4 ± 26.1 | −5.7 (−9.7, −1.8) | 0.006 |
| SD, 0:00–24:00 (mg/dL) | 36.8 ± 11.5 | 18.3 ± 6.9 | 35.9 ± 10.8 | −17.3 (−20.3, −14.4) | <0.001 |
| SD, 0:00–7:00 (mg/dL) | 11.7 ± 6.5 | 11.2 ± 6.8 | 11.5 ± 6.4 | −0.1 (−2.0, 1.9) | 0.947 |
| SD, 7:00–24:00 (mg/dL) | 37.3 ± 11.0 | 19.3 ± 7.1 | 37.5 ± 10.9 | −17.9 (−20.8, −15) | <0.001 |
| CV, 0:00–24:00 (%) | 23.5 ± 5.7 | 13.8 ± 4.0 | 27.6 ± 6.6 | −13.6 (−15.6, −11.6) | <0.001 |
| CV, 0:00–7:00 (%) | 9.1 ± 4.0 | 8.6 ± 4.2 | 10.7 ± 5.0 | −1.9 (−3.4, −0.3) | 0.020 |
| CV, 7:00–24:00 (%) | 22.1 ± 4.5 | 14.3 ± 3.8 | 26.8 ± 5.5 | −12.3 (−14.1, −10.6) | <0.001 |
| Estimated HbA1c (%) | 7.03 ± 0.58 | 6.43 ± 0.51 | 6.41 ± 0.57 | 0.03 (−0.05, 0.11) | 0.424 |
| AOC < 54, 0:00–24:00 (mg/dL) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.22 ± 0.83 | −0.22 (−0.53, 0.09) | 0.164 |
| AOC < 54, 0:00–7:00 (mg/dL) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.05 ± 0.25 | −0.05 (−0.14, 0.05) | 0.330 |
| AOC < 54, 7:00–24:00 (mg/dL) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.17 ± 0.72 | −0.17 (−0.45, 0.10) | 0.204 |
| AOC < 70, 0:00–24:00 (mg/dL) | 0.00 ± 0.00 | 0.08 ± 0.31 | 3.81 ± 9.07 | −3.71 (−7.14, −0.27) | 0.035 |
| AOC < 70, 0:00–7:00 (mg/dL) | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.77 ± 5.61 | −1.77 (−3.90, 0.35) | 0.100 |
| AOC < 70, 7:00–24:00 (mg/dL) | 0.00 ± 0.00 | 0.07 ± 0.30 | 2.03 ± 4.62 | −1.87 (−3.57, −0.17) | 0.034 |
| AUC > 140, 0:00–24:00 (mg/dL) | 602.85 ± 443.59 | 184.69 ± 263.7 | 335.23 ± 300.95 | −148.39 (−199.02, −97.75) | <0.001 |
| AUC > 140, 0:00–7:00 (mg/dL) | 36.89 ± 62.28 | 39.65 ± 75.21 | 16.94 ± 48.34 | 22.26 (4.19, 40.33) | 0.018 |
| AUC > 140, 7:00–24:00 (mg/dL) | 566.91 ± 392.55 | 145.22 ± 194.67 | 318.19 ± 267.43 | −170.47 (−219.25, −121.70) | <0.001 |
| AUC > 180, 0:00–24:00 (mg/dL) | 239.51 ± 255.35 | 32.18 ± 73.79 | 116.57 ± 148.37 | −83.6 (−119.06, −48.15) | <0.001 |
| AUC > 180, 0:00–7:00 (mg/dL) | 3.44 ± 13.54 | 5.57 ± 15.89 | 2.89 ± 15.40 | 2.59 (−2.94, 8.11) | 0.346 |
| AUC > 180, 7:00–24:00 (mg/dL) | 236.69 ± 247.41 | 26.66 ± 59.56 | 113.58 ± 141.73 | −86.14 (−122.74, −49.54) | <0.001 |
| AUC > 200, 0:00–24:00 (mg/dL) | 144.99 ± 183.65 | 11.43 ± 28.97 | 62.82 ± 92.58 | −51.28 (−78.11, −24.44) | <0.001 |
| AUC > 200, 0:00–7:00 (mg/dL) | 1.57 ± 7.17 | 1.61 ± 5.20 | 1.22 ± 6.69 | 0.35 (−1.31, 2.01) | 0.668 |
| AUC > 200, 7:00–24:00 (mg/dL) | 143.87 ± 179.63 | 9.83 ± 24.84 | 61.53 ± 89.88 | −51.57 (−78.64, −24.5) | <0.001 |
| AUC > 250, 0:00–24:00 (mg/dL) | 33.48 ± 60.8 | 0.17 ± 0.52 | 9.27 ± 22.82 | −9.1 (−17.52, −0.68) | 0.035 |
| AUC > 250, 0:00–7:00 (mg/dL) | 0.11 ± 0.60 | 0.01 ± 0.04 | 0.02 ± 0.09 | −0.01 (−0.03, 0.01) | 0.341 |
| AUC > 250, 7:00–24:00 (mg/dL) | 33.5 ± 60.5 | 0.16 ± 0.48 | 9.24 ± 22.76 | −9.08 (−17.49, −0.68) | 0.035 |
| TBR < 54 (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.19 ± 0.64 | −0.19 (−0.44, 0.05) | 0.119 |
| TBR < 70 (%) | 0.00 ± 0.00 | 0.06 ± 0.23 | 2.64 ± 6.53 | −2.58 (−5.04, −0.11) | 0.041 |
| TIR 70–180 (%) | 76.05 ± 17.24 | 93.6 ± 12.30 | 83.31 ± 13.03 | 10.13 (6.86, 13.41) | <0.001 |
| TAR > 140 (%) | 55.81 ± 24.45 | 30.42 ± 30.32 | 34.14 ± 21.91 | −3.64 (−9.53, 2.26) | 0.217 |
| TAR > 180 (%) | 23.95 ± 17.24 | 6.34 ± 12.29 | 14.05 ± 13.28 | −7.56 (−10.29, −4.82) | <0.001 |
| TAR > 200 (%) | 15.98 ± 14.39 | 2.73 ± 6.53 | 8.69 ± 10.21 | −5.87 (−8.27, −3.47) | <0.001 |
| TAR > 250 (%) | 4.64 ± 6.97 | 0.11 ± 0.38 | 1.59 ± 3.14 | −1.49 (−2.59, −0.39) | 0.010 |
| MPPGE (mg/dL) | 82.3 ± 27.7 | 27.9 ± 14.5 | 79.0 ± 25.9 | −50.8 (−58.4, −43.3) | <0.001 |
| LBGI | 0.35 ± 0.39 | 0.50 ± 0.44 | 1.77 ± 1.55 | −1.25 (−1.76, −0.74) | <0.001 |
| HBGI | 5.95 ± 3.47 | 2.17 ± 2.13 | 4.60 ± 2.36 | −2.39 (−2.97, −1.82) | <0.001 |
| Predicted % of BG < 70 (mg/dL) | 0.43 ± 0.62 | 0.14 ± 0.43 | 4.67 ± 6.44 | −4.54 (−6.87, −2.20) | <0.001 |
Data are mean ± standard deviation or effect size between the treatments with 95% confidence interval within parentheses. P‐values were calculated using a linear mixed‐effects model for comparing the two treatment groups and adjusting for type of treatment (mitiglinide/voglibose, glimepiride), period (first, second), and sequence (allocation group) as fixed effects and patients as random effects. AOC, area over the curve; AUC, area under the curve; BG, blood glucose; CV, coefficient of variation; HBGI, high blood glucose index; LBGI, low blood glucose index; MAGE, mean amplitude of glycemic excursions; MBG, mean blood glucose; MPPGE, mean postprandial glucose excursion; RHI, reactive hyperemia index; SD, standard deviation of mean glucose; TAR, time above range; TBR, time below range; TIR, time in range.
Secondary endpoints
Univariate linear regression analyses showed no significant parameters associated with the RHI (Table 3). With regard to the effects of the type of treatment on CGM parameters, glimepiride was associated with lower mean blood glucose (MBG) levels at nighttime, while the MBG levels were lower during the daytime in the mitiglinide/voglibose group. However, over the entire 24 h period, MBG levels were comparable between the two groups. The estimated HbA1c levels, associated with all‐time MBG levels, were not different between the two treatments. The standard deviation of mean glucose (SD) and coefficient of variation of mean glucose (CV) were lower, similar to MAGE, in the mitiglinide/voglibose group. Mitiglinide/voglibose significantly suppressed mean postprandial glucose excursion (MPPGE). No measurement before or during mitiglinide/voglibose administration was associated with an area over the curve (AOC) of <54 mg/dL or the time below range (TBR) of <54 mg/dL, whereas glimepiride was associated with measurements meeting these values. The percentages of AOC <70 mg/dL and TBR <70 mg/dL were significantly higher for glimepiride. Glimepiride was also associated with a higher low blood glucose index (LBGI) and predicted percentage of blood glucose (BG) <70, which is associated with a risk of hypoglycemia. Furthermore, the use of mitiglinide/voglibose was associated with a significantly higher time in range (TIR; 70–180 mg/dL) (93.6% ± 12.3%) compared with glimepiride (83.31% ± 13.03%). For indices of hyperglycemia, glimepiride use was associated with significantly higher percentages of area under the curve (AUC) >180, >200, and 250 mg/dL, as well as percentages of time above range (TAR) >180, >200, and 250 mg/dL, compared with mitiglinide/voglibose.
Table 3.
Association between RHI and CGM metrics in univariate linear regression analysis
| β (95% CI) | P‐value | |
|---|---|---|
| MAGE | 0.00219 (−0.00084, 0.00522) | 0.153 |
| MBG, 0:00–24:00 | 0.00320 (−0.00176, 0.00816) | 0.202 |
| SD, 0:00–24:00 | 0.00522 (−0.00378, 0.01422) | 0.251 |
| CV, 0:00–24:00 | 0.00474 (−0.00831, 0.01780) | 0.470 |
| Estimated HbA1c | 0.13640 (−0.07511, 0.34791) | 0.202 |
| AOC < 54, 0:00–24:00 | 0.06025 (−0.13274, 0.25324) | 0.534 |
| AOC < 70, 0:00–24:00 | −0.00700 (−0.02415, 0.01014) | 0.417 |
| AUC > 140, 0:00–24:00 | 0.00021 (−0.00018, 0.00060) | 0.292 |
| AUC > 180, 0:00–24:00 | 0.00045 (−0.00047, 0.00137) | 0.333 |
| AUC > 200, 0:00–24:00 | 0.00070 (−0.00087, 0.00226) | 0.377 |
| AUC > 250, 7:00–24:00 | 0.00277 (−0.00406, 0.00960) | 0.420 |
| TBR < 54 | 0.00950 (−0.23776, 0.25676) | 0.939 |
| TBR < 70 | −0.01375 (−0.03749, 0.00999) | 0.251 |
| TIR 70–180 | −0.00293 (−0.01138, 0.00551) | 0.490 |
| TAR > 140 | 0.00198 (−0.00239, 0.00634) | 0.368 |
| TAR > 180 | 0.00487 (−0.00372, 0.01346) | 0.261 |
| TAR > 200 | 0.00629 (−0.00636, 0.01893) | 0.324 |
| TAR > 250 | 0.01586 (−0.03282, 0.06453) | 0.517 |
| MPPGE | 0.00291 (−0.00048, 0.00630) | 0.091 |
| LBGI | −0.06620 (−0.15256, 0.02017) | 0.130 |
| HBGI | 0.02722 (−0.01748, 0.07192) | 0.228 |
| Predicted % of BG < 70 | −0.01228 (−0.03463, 0.01007) | 0.278 |
| Glimepiride (vs mitiglinide/voglibose) | −0.04567 (−0.27042, 0.17909) | 0.686 |
Safety
No adverse events were observed before the administration of the investigational drugs. During treatment, four patients of the glimepiride group reported hypoglycemia (mild in three, moderate in one), which resolved in all four with appropriate treatment. In the mitiglinide/voglibose group, one patient reported mild abdominal distention, which disappeared after the drug administration period. There were no statistically significant differences between the two groups in terms of side effects (P = 0.353).
DISCUSSION
This randomized crossover study compared the effects of mitiglinide/voglibose and glimepiride on vascular endothelial function and glycemic variability in patients with type 2 diabetes. The co‐primary endpoints were RHI and MAGE. The MAGE at baseline was 99.6 ± 31.9 mg/dL, and significantly decreased during mitiglinide/voglibose treatment (47.6 ± 18.5 mg/dL) compared with no change during glimepiride treatment (100.6 ± 32.2 mg/dL) (P < 0.001). Furthermore, MAGE was not affected by the phase of treatment (first, second) and sequence (allocation group). Other indices of glycemic variability, such as SD and CV, were also significantly lower for mitiglinide/voglibose. Fujimoto et al. 14 compared the effects of mitiglinide/voglibose and glimepiride (1.0 mg/day) using a crossover design, similar to our study, and reported that MAGE for mitiglinide/voglibose was 62.46 mg/dL, significantly lower than for glimepiride (95.04 mg/dL). However, the authors reported as limitations of their study the lack of a washout period, evaluation of period effect or carryover effect, the short duration of drug administration, and the short application of CGM. Our present study design addressed these limitations, incorporating CGM measurements before drug administration. Our study clearly showed that mitiglinide/voglibose reduced MAGE by 50% from the baseline.
A previous study indicated that the MAGE cut‐off value of 3.4 mmol/L (61.3 mg/dL) was associated with coronary artery disease (CAD) in patients with type 2 diabetes experiencing chest pain 3 . In our study, MAGE during mitiglinide/voglibose treatment was well below the above cut‐off value (61.3 mg/dL). Since a recently reported meta‐analysis concluded that MAGE in hospitalized patients with acute coronary syndrome or stable CAD (n = 2,666) could accurately predict the subsequent onset of major adverse cardiovascular events 17 , the selection of drugs that suppress glycemic variability seems important in order to prevent cardiovascular diseases in patients with type 2 diabetes.
Our study showed no difference in RHI between the mitiglinide/voglibose and glimepiride groups. Regarding the effects of mitiglinide/voglibose on vascular endothelial function, only one report demonstrated that fasting and postprandial FMD improved at 3 months after switching from glimepiride to mitiglinide/voglibose 15 . However, the above study was a small‐scale single‐arm pilot study involving six patients only. To our knowledge, no other studies have compared the effects of mitiglinide/voglibose and glimepiride on vascular endothelial function by using a crossover design. Previous studies showed that glycemic variability detected by CGM is associated with vascular endothelial dysfunction 18 , and our group has also reported the association of RHI with MAGE in patients with type 2 diabetes 5 . Furthermore, hypoglycemia may impair vascular endothelial function in patients with type 2 diabetes through increased production of reactive oxygen species, catecholamines 6 , and proinflammatory cytokines 7 , as well as activation of the sympathetic nervous system 8 . We also reported previously that hypoglycemia reduces RHI 9 , 10 and is a factor that adversely affects the normalization of vascular endothelial function after educational hospitalization in patients with type 2 diabetes 19 .
We conducted the present study based on the hypothesis that administration of mitiglinide/voglibose would suppress glycemic variability, thereby improve RHI, or that mitiglinide/voglibose would more effectively prevent the decrease in RHI than glimepiride due to the lower incidence of hypoglycemia during mitiglinide/voglibose treatment. However, the results showed no difference in RHI between mitiglinide/voglibose and glimepiride. This finding is probably due to the short treatment duration (5 days). While the administration of mitiglinide/voglibose suppressed glycemic variability, improvement in vascular endothelial function may require a longer treatment/time. In this regard, Tuttolomondo et al. 20 conducted a randomized trial that evaluated the effect of dulaglutide on vascular endothelial function. Their findings included significantly lower HbA1c levels at 3 months after the addition of dulaglutide compared with after conventional therapy, whereas RHI remained unchanged at 3 months though it significantly improved at 9 months.
Another important finding of our study was that although hypoglycemia occurred more frequently during glimepiride treatment, only mild hypoglycemia (<70 mg/dL) was recorded in the present study. For example, no statistical differences were observed in the incidence of hypoglycemia of <54 mg/dL. In addition, during treatment with glimepiride, TBR <70 mg was 2.64% and TBR <54 mg/dL was 0.19%. These values sufficiently met the CGM management targets proposed by the international consensus, which are <4% for TBR <70 mg/dL and <1% for TBR <54 mg/dL 11 . Based on the results of our study, hypoglycemia caused by glimepiride was mild, which may have not been enough to reduce RHI.
We also compared mitiglinide/voglibose and glimepiride by analyzing the CGM parameters measured during 0:00–24:00, 07:00–24:00, and 00:00–7:00 h, analyzed the width of postprandial glycemic variability, and examined new indices, such as TIR, TAR, and TBR. To our knowledge, our study is the first to analyze the combination of these parameters and indices. Since the estimated HbA1c levels were similar between the two treatment groups, no difference in all‐day MBG was observed. However, the daytime MBG was significantly lower for mitiglinide/voglibose, whereas the nighttime MBG was lower for glimepiride. The effects of the two drugs differed. A possible factor for the lower daytime MBG in the mitiglinide/voglibose group is that administration of mitiglinide/voglibose significantly reduced postprandial glucose levels. In this study of patients admitted to the hospital, the time of starting meals was recorded, and thus we were able to calculate MPPGE more accurately. The MPPGE was 82.3 ± 27.7 mg/dL at baseline; it remained almost unchanged at 79.0 ± 25.9 mg/dL during treatment with glimepiride, but it was extremely low at 27.9 ± 14.5 mg/dL during treatment with mitiglinide/voglibose. This finding confirmed that mitiglinide/voglibose had an immediate and potent inhibitory effect on the increase in postprandial glucose level. Katsuno et al. 21 reported that in Japanese patients with type 2 diabetes, the addition of voglibose to mitiglinide inhibited postprandial glucose spikes and further increased glucagon‐like peptide‐1 (GLP‐1) levels; however, these effects were not observed after administering a double dose of mitiglinide. The α‐GI, which changes the site of glucose absorption from the upper intestine to the lower intestine, has been reported to eventually enhance the secretion of GLP‐1, which is secreted in the lower intestine 22 . In Japanese individuals, impaired early‐phase insulin secretion is markedly involved in the pathology of type 2 diabetes, and the secretory ability of pancreatic β cells is weaker in Japanese individuals than in Europeans and Americans 23 , 24 . Thus, the concomitant use of mitiglinide and α‐GI, which have different mechanisms of action, appears to be useful in Japanese patients with type 2 diabetes. In addition, because the use of mitiglinide/voglibose can reduce the number of medications, it may be a treatment that can contribute to improved medication adherence in patients with type 2 diabetes.
The CGM management targets proposed by the international consensus for type 2 diabetes are more than 70% for TIR, less than 25% for TAR >180 mg/dL, less than 5% for TAR >250 mg/dL, less than 4% for TBR <70 mg/dL, and less than 1% for TBR <54 mg/dL 11 . In the present study, the management targets for TIR, TAR, and TBR were achieved by treatment with both types of medications; mitiglinide/voglibose and glimepiride. However, during the treatment with mitiglinide/voglibose, TIR was significantly higher and TAR and TBR were significantly lower. Especially, TBR <70 mg/dL was 0.06%, and TBR <54 mg/dL was not detected during treatment with mitiglinide/voglibose. Therefore, this drug significantly improved postprandial hyperglycemia and was associated with an extremely low risk of hypoglycemia. Recent clinical trials have shown that decreased TIR is associated with the onset and progression of microvascular complications 25 , intima‐media thickness 26 , and increased risks of all‐cause and cardiovascular mortality 27 in patients with diabetes. We have also previously analyzed CGM data of 999 Japanese patients with type 2 diabetes and reported that glycemic variability and hypoglycemia were associated with the onset and progression of microvascular complications 28 and macroangiopathy 29 , 30 . These studies also highlighted the potential usefulness of mitiglinide/voglibose in type 2 diabetes.
This study has several limitations. First, it was a short‐term study involving hospitalized patients, and accordingly we could not evaluate the long‐term benefits of the tested drugs. It is possible that the effects of the medications on RHI would be different during long‐term use. Second, because the service of iPro2 was terminated during the study period, more than one type of CGM device had to be used in the study. However, only a single type of CGM device was used per patient during the study, and the study was conducted using a crossover design. Thus, we consider no problems with the comparison of the CGM parameters between mitiglinide/voglibose and glimepiride. Third, we did not measure the levels of C‐peptide, GLP‐1, and glucose‐dependent insulinotropic polypeptide, which are hormones that may affect BG dynamics.
In conclusion, in our short‐duration randomized crossover study, we observed no difference in the effects of mitiglinide/voglibose and glimepiride on vascular endothelial function in patients with type 2 diabetes. However, the mitiglinide/voglibose tablet significantly reduced glycemic variability in terms of MAGE and other parameters. Our study also demonstrated for the first time that the use of mitiglinide/voglibose was associated with a significantly higher TIR, a significantly lower incidence of hyperglycemia (defined as BG level of ≥180 mg/dL), significantly milder postprandial glycemic variability, and extremely low risk of hypoglycemia. Based on these findings, mitiglinide/voglibose appears to be useful therapeutically through improvement of postprandial hyperglycemia and the control of glycemic variability in patients with type 2 diabetes.
DISCLOSURE
Y.O. has received lecture fees from Kissei Pharmaceutical Co., Ltd and Sanofi. The remaining authors have no competing interest.
Approval of the research protocol: The study was approved by the Ethics Committee of Nara Medical University (CRB5200002).
Informed consent: Informed consent was obtained from all the participants.
Registry and the registration no. of the study/trial: This trial was registered in the Japan Registry of Clinical Trials (jRCTs071190047, Date of registration: 20/02/2020).
Animal studies: N/A.
Supporting information
Appendix S1. Inclusion and exclusion criteria.
Appendix S2. Noninvasive vascular function test.
ACKNOWLEDGMENTS
The authors thank the EPS Corporation for data analysis. The authors also acknowledge Ms N. Sakaguchi for the excellent technical assistance. This study was supported by funds from Kissei Pharmaceutical Co., Ltd. The Pharmaceutical firm did not interfere with the study design, conduct of the study, analysis of data, report of the study findings or discussion of the potential usefulness of their product.
Clinical Trial Registry
Japan Clinical Trials Registry
jRCTs071190047
REFERENCES
- 1. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: The role of protein kinase C and NAD(P)H‐oxidase activation. Diabetes 2003; 52: 2795–2804. [DOI] [PubMed] [Google Scholar]
- 3. Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol 2011; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamburg NM, Keyes MJ, Larson MG, et al. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008; 117: 2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torimoto K, Okada Y, Mori H, et al. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol 2013; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin WL, Azuma K, Mita T, et al. Repetitive hypoglycaemia increases serum adrenaline and induces monocyte adhesion to the endothelium in rat thoracic aorta. Diabetologia 2011; 54: 1921–1929. [DOI] [PubMed] [Google Scholar]
- 7. Razavi Nematollahi L, Kitabchi AE, Stentz FB, et al. Proinflammatory cytokines in response to insulin‐induced hypoglycemic stress in healthy subjects. Metabolism 2009; 58: 443–448. [DOI] [PubMed] [Google Scholar]
- 8. Hijmering ML, Stroes ESG, Olijhoek J, et al. Sympathetic activation markedly reduces endothelium‐dependent, flow‐mediated vasodilation. J Am Coll Cardiol 2002; 39: 683–688. [DOI] [PubMed] [Google Scholar]
- 9. Torimoto K, Okada Y, Tanaka Y. Hypoglycemia abrogates the vascular endothelial protective effect of exenatide in type 2 diabetes mellitus. Diabetes Ther 2019; 10: 1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka K, Okada Y, Torimoto K, et al. Hypoglycemia induces vascular endothelial dysfunction in subjects with normal glucose tolerance. Sci Rep 2022; 12: 2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019; 42: 1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurozumi A, Okada Y, Mori H, et al. Efficacy of α‐glucosidase inhibitors combined with dipeptidyl‐peptidase‐4 inhibitor (alogliptin) for glucose fluctuation in patients with type 2 diabetes mellitus by continuous glucose monitoring. J Diabetes Investig 2013; 4: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osonoi T, Saito M, Tamasawa A, et al. Effects of sitagliptin or mitiglinide as an add‐on to acarbose on daily blood glucose fluctuations measured by 72 h subcutaneous continuous glucose monitoring in Japanese patients with type 2 diabetes: A prospective randomized study. Expert Opin Pharmacother 2014; 15: 1325–1335. [DOI] [PubMed] [Google Scholar]
- 14. Fujimoto K, Shibayama Y, Yamaguchi E, et al. Glucose excursions and hypoglycemia in patients with type 2 diabetes treated with mitiglinide/voglibose versus glimepiride: A randomized cross‐over trial. J Diabetes 2018; 10: 675–682. [DOI] [PubMed] [Google Scholar]
- 15. Murakami M, Bouchi R, Ohara N, et al. Beneficial effect of combination therapy with mitiglinide and voglibose on fasting and postprandial endothelial dysfunction in patients with type 2 diabetes: A pilot study. Integr Obesity Diabetes 2017; 3: 1–4. [Google Scholar]
- 16. Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2007; 44: 2137–2141. [DOI] [PubMed] [Google Scholar]
- 17. Pu Z, Lai L, Yang X, et al. Acute glycemic variability on admission predicts the prognosis in hospitalized patients with coronary artery disease: A meta‐analysis. Endocrine 2020; 67: 526–534. [DOI] [PubMed] [Google Scholar]
- 18. Buscemi S, Re A, Batsis JA, et al. Glycaemic variability using continuous glucose monitoring and endothelial function in the metabolic syndrome and in type 2 diabetes. Diabet Med 2010; 27: 872–878. [DOI] [PubMed] [Google Scholar]
- 19. Goshima Y, Okada Y, Torimoto K, et al. Changes in endothelial function during educational hospitalization and the contributor to improvement of endothelial function in type 2 diabetes mellitus. Sci Rep 2020; 10: 15384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuttolomondo A, Cirrincione A, Casuccio A, et al. Efficacy of dulaglutide on vascular health indexes in subjects with type 2 diabetes: A randomized trial. Cardiovasc Diabetol 2021; 20: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katsuno T, Watanabe N, Nagai E, et al. Comparison of efficacy of concomitant administration of mitiglinide with voglibose and double dose of mitiglinide in patients with type 2 diabetes mellitus. J Diabetes Investig 2011; 2: 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moritoh Y, Takeuchi K, Hazama M. Chronic administration of voglibose, an alpha‐glucosidase inhibitor, increases active glucagon‐like peptide‐1 levels by increasing its secretion and decreasing dipeptidyl peptidase‐4 activity in ob/ob mice. J Pharmacol Exp Ther 2009; 329: 669–676. [DOI] [PubMed] [Google Scholar]
- 23. Mitsui R, Fukushima M, Nishi Y, et al. Factors responsible for deteriorating glucose tolerance in newly diagnosed type 2 diabetes in Japanese men. Metabolism 2006; 55: 53–58. [DOI] [PubMed] [Google Scholar]
- 24. Kadowaki T, Miyake Y, Hagura R, et al. Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia 1984; 26: 44–49. [DOI] [PubMed] [Google Scholar]
- 25. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019; 42: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima‐media thickness in type 2 diabetes. Diabetes Technol Ther 2020; 22: 72–78. [DOI] [PubMed] [Google Scholar]
- 27. Lu J, Wang C, Shen Y, et al. Time in range in relation to all‐cause and cardiovascular mortality in patients with type 2 diabetes: A prospective cohort study. Diabetes Care 2021; 44: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wakasugi S, Mita T, Katakami N, et al. Associations between continuous glucose monitoring‐derived metrics and diabetic retinopathy and albuminuria in patients with type 2 diabetes. BMJ Open Diabetes Res Care 2021; 9: e001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wakasugi S, Mita T, Katakami N, et al. Associations between continuous glucose monitoring‐derived metrics and arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2021; 20: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taya N, Katakami N, Mita T, et al. Associations of glucose variability with intima‐media thickness and ultrasonic tissue characteristics of the carotid arteries: A cross‐sectional analysis in patients with type 2 diabetes. Cardiovasc Diabetol 2021; 20: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Inclusion and exclusion criteria.
Appendix S2. Noninvasive vascular function test.


