Abstract
The immediate and well‐documented benefits of carbohydrate restriction include improved glycemic control in individuals with diabetes mellitus. Starch, a significant source of carbohydrates, is categorized as rapidly digestible, slowly digestible, or resistant starch (RS). RS, which is a non‐viscous fermentable fiber, has shown promise in animal studies for antidiabetic effects by improving glucose metabolism. Although the exact mechanism by which RS affects glucose metabolism remains unclear, it is expected to positively impact glucose tolerance and insulin sensitivity. The fermentation of RS by colonic microbiota in the large bowel produces short‐chain fatty acids, which exert multiple metabolic effects on glucose regulation and homeostasis. Moreover, RS may influence glucose metabolism via bile acid modulation, independent of its fermentation. Diets rich in RS could aid in blood glucose homeostasis. However, it is uncertain whether they can alter the metabolic pathology associated with glucose regulation. In essence, RS has the potential to lower postprandial glucose levels similarly to a low‐glycemic index diet. Yet, its efficacy as a medical nutrition therapy for type 2 diabetes needs further investigation. To confirm the role of RS in glycemic control and to possibly recommend it as an additional dietary approach for people with type 2 diabetes mellitus, a well‐designed, large‐scale intervention is required.
Keywords: Diabetes mellitus, type 2; Diet; Resistant starch
Starch is a significant source of carbohydrates and resistant starch, which is a non‐viscous fermentable fiber, has shown to enhance glucose metabolism. The efficacy of resistant starch as a medical nutrition therapy for type 2 diabetes needs further investigation.
INTRODUCTION
One of the primary goals in treating diabetes mellitus is to prevent cardiovascular complications. Blood glucose control has been suggested as an effective means to prevent or slow down the progression of diabetic complications. Numerous randomized controlled trials have demonstrated that intensive glycemic control in patients with diabetes reduces the risk of microvascular complications, such as nephropathy and retinopathy. However, it may not improve patient survival or alter the course of macrovascular complications. In the UK Prospective Diabetes Study (UKPDS), participants with newly diagnosed type 2 diabetes followed for 10 years showed that intensive glycemic control (median HbA1c 7.0%) reduced the overall microvascular complication rate by 25% compared with conventional treatment (median A1C 7.9%) 1 . In other words, each 1% reduction in HbA1c was associated with a 25% reduction in the risk of microvascular complications. The ADVANCE trial, which randomized 11,140 patients with diabetes and had a median follow‐up of 5 years, found that the intensive treatment group (mean HbA1c 6.5%) had a 14% reduction in microvascular events compared with the standard treatment group (mean HbA1c 7.3%), with diabetic nephropathy, in particular, being significantly reduced 2 . In the ACCORD trial, 10,251 individuals with type 2 diabetes were assigned to one of two glycemic control strategies over a 3.5 year follow‐up. The intensive therapy group aimed for an HbA1c level of <6.0%, while the standard therapy group targeted an HbA1c range of 7–7.9%. The intensive therapy group showed a 21% lower incidence of microalbuminuria and a 32% risk reduction for macroalbuminuria incidence compared with the standard therapy group 3 . Optimal management of glycemic control may involve diet, exercise therapy, and anti‐diabetic medications. Non‐adherence to lifestyle regimens or medications in people with diabetes is associated with increased hospitalization and mortality 4 . Among the methods for achieving good glycemic control, exercise and diet are fundamental. However, lifestyle modification involves altering long‐term habits, and sometimes medication adherence is more effective for people with diabetes than lifestyle changes 5 . But anti‐diabetic agents cannot always control blood glucose adequately due to limited efficacy and contraindications. For example, metformin typically reduces HbA1c by 1–2%, sulfonylureas by 1–2%, thiazolidinediones by 0.5–1.4%, DPP‐4 inhibitors by 0.5–0.8%, and SGLT2 inhibitors by 0.5–1.0% 6 . Although insulin has no intrinsic limitation in controlling blood glucose, more than 50% of subjects in studies did not reach the glycemic goal with insulin therapy 7 . Therefore, diet control and exercise are required to achieve the target glycemic goal in type 2 diabetes. Lifestyle modification is challenging, and maintaining a dietary regimen can often be more difficult than sustaining an exercise routine 8 . The control of carbohydrate intake is a crucial aspect of regulating blood glucose levels in people with diabetes and the healthy population alike. One study reported that an increase in white rice consumption correlated with a higher risk of developing diabetes. Other studies have shown that replacing highly polished white rice with other cereals, healthier varieties of rice, or adding appropriate legumes can lower the glycemic index of a meal and, subsequently, blood glucose levels 9 .
Health professionals exert considerable effort to successfully administer dietary regimens in real life 10 . One such effort involves controlling blood glucose levels through dietary fiber intake. Dietary fiber, which is a non‐digestible complex, bypasses digestion in the small intestine and proceeds to the large intestine, where it is fermented by colonic microflora 11 . Fiber is considered a total carbohydrate, but not an available carbohydrate, meaning it is not absorbed in the same way as simple sugars. In practical terms, ‘fiber’ usually refers to all fibrous components found in food, including non‐starch polysaccharides, resistant starch (RS), short‐chain oligosaccharides, among others 12 . Of these, RS is a non‐viscous, highly fermentable fiber that can be readily incorporated into food products.
There has been growing interest in the role of resistant starch in glucose metabolism and insulin sensitivity. Numerous studies suggest that RS can lower postprandial glucose levels when it replaces the available carbohydrate in a meal 12 , 13 . However, some studies have not observed these beneficial effects on glycemic control 14 , 15 . Therefore, the purpose of this article is to review the impact of RS on blood glucose management in type 2 diabetes.
STARCH
Starch is a major carbohydrate produced by plants, consisting of a pure glucose polymer. It is present in granular form in various foods and is primarily stored in plant seeds or roots. Foods rich in starch include cereals, legumes, potatoes, wheat, maize, rice, bananas, and mangoes. Starch is composed of linear amylose and highly branched amylopectin molecules. Amylose is a linear molecule made up of d‐glucopyranosyl units, which naturally twist within starch granules into a helical conformation, with six anhydroglucose units per turn 13 . In contrast, amylopectin is a branched molecule composed of anhydroglucose chains with many d‐glucose branch points and does not form a helical coil 16 .
In the gastrointestinal tract, starch granules are digested by salivary and pancreatic α‐amylase, as well as by brush border glucogenic enzymes such as maltase, glucoamylase, and sucrase‐isomaltase 17 , 18 . The catalysis of starch by α‐amylase represents the initial step in starch digestion after food consumption. The primary products of this process, β‐limit dextrins, and oligosaccharides, are subsequently cleaved into glucose by brush border enzymes. These glucose molecules are then absorbed via the sodium‐glucose cotransporter (SGLT1) at the luminal surface of enterocytes 18 . The susceptibility of natural food starch to amylolysis varies among botanical species and can be altered by home cooking and commercial food processing 18 , 19 . Additionally, the passage of food through the gastrointestinal tract involves other physiological processes that affect digestion and absorption, including gastric emptying, the presence of enzyme inhibitors, viscosity, and gut microbiota 20 . Despite these complexities, starches are generally classified into three types based on in vitro amylolysis and digestion rates: rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS). As indicated by their names, these starch types differ in the time they take to digest in the small intestine.
CLASSIFICATION OF STARCH
Rapidly digestible starch
RDS is defined as a type of starch that is rapidly converted to glucose within 20 min through enzymatic digestion 21 . Consuming foods high in RDS can quickly release glucose into the bloodstream, leading to a rapid increase in blood glucose and insulin levels. RDS has a significant correlation with the glycemic index, which is based on the in vivo postprandial glycemic response 22 . Foods subjected to moist‐heat cooking techniques, such as bread and potatoes, typically contain high proportions of RDS 23 .
Slowly digestible starch
SDS is a type of starch that takes more than 20 min to convert to glucose through enzymatic digestion 21 . Unlike RDS, which is also completely digested in the small intestine, SDS is digested slowly, resulting in a more sustained release of glucose. This gradual release is different from the concept of the glycemic index, which measures the glycemic response from 0 to 120 min and does not account for the carbohydrates contained within the food 20 , 24 . Physically inaccessible amorphous starches, most raw cereal starches, and retrograded forms found in cooked foods are classified under the RS2 category.
Resistant starch
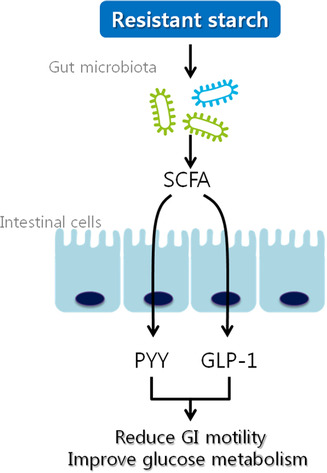
RS is defined as a type of starch that is resistant to hydrolysis by enzymatic digestion. RS remains unhydrolyzed even after 120 min of incubation with α‐amylase and pullulanase 21 , 24 . Unlike RDS and SDS, which are hydrolyzed in the small intestine to produce glucose, RS passes through the small intestine intact and is fermented similarly to dietary fiber in the large intestine by intestinal microorganisms. While RDS and SDS are digestible forms of starch, RS is non‐digestible, non‐viscous, and highly fermentable. Therefore, RS is classified as a type of dietary fiber due to its similar physiological effects on humans 25 . For instance, like other dietary fibers, RS can potentially slow the rate of gastric emptying. RS also mirrors the effects of dietary fiber in reducing the motility of the gastrointestinal tract through the short‐chain fatty acids (SCFAs) produced during its fermentation in the large intestine 25 , 26 . SCFAs have been studied for their physiological functions, including their ability to reduce gastrointestinal motility by inducing the secretion of incretin hormones such as glucagon‐like peptide 1 (GLP‐1) and peptide YY (PYY) 27 , 28 . Figure 1 illustrates the mechanisms by which RS exerts beneficial effects on glucose metabolism.
Figure 1.
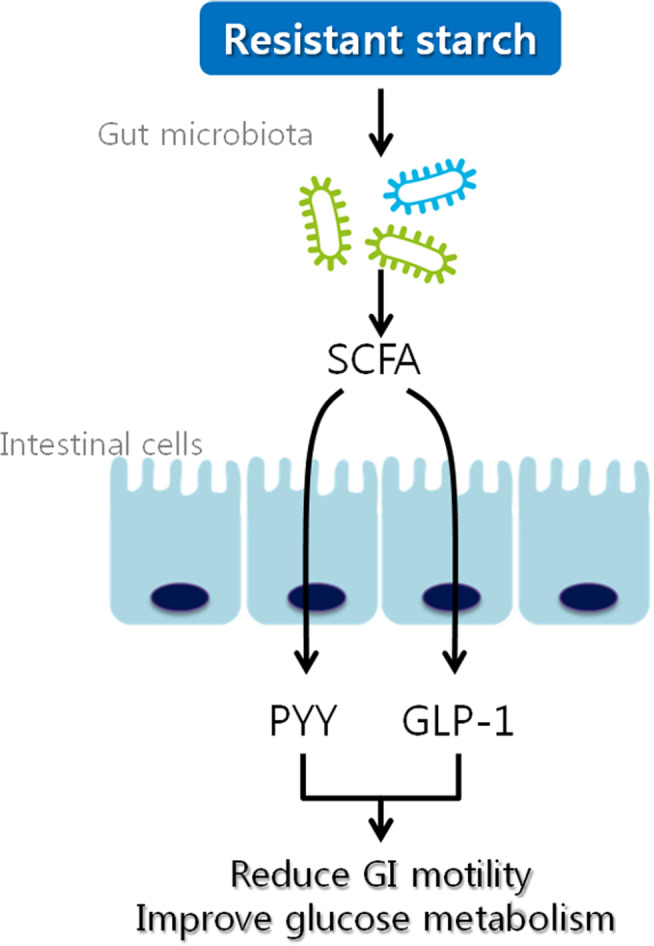
Mechanism of beneficial effects of RS on glucose metabolism. RS may stimulate the microbial production of SCFA and enhance the secretion of PYY and GLP‐1 by intestinal cells. PYY and GLP‐1 contribute to reduced gastrointestinal (GI) motility and stimulate insulin secretion, which can lead to improved glucose metabolism 29 , 30 . GLP‐1, glucagon‐like peptide 1; PYY, peptide YY; RS, resistant starch; SCFA, short‐chain fatty acids.
The resistance of starch to digestion is influenced by the ratio of amylose to amylopectin. Amylose is digested more slowly, while amylopectin is rapidly digested, particularly after retrogradation. Generally, the RS content in granular starch has a positive correlation with its amylose content. It has been reported that a strong correlation exists between the amylose and RS content in maize starch 31 .
CLASSIFICATION AND STRUCTURE OF RS
Based on structure and properties, resistant starch is classified into five subtypes: RS1, RS2, RS3, RS4, and RS5, as detailed in Table 1. The initial classification of RS encompassed only three types – RS1, RS2, and RS3. The categories RS4 and RS5 were added in later years 31 , 32 .
Table 1.
Classification of types of resistant starch (RS)
| Type of RS | Description | Example |
|---|---|---|
| RS1 | Physically inaccessible starch | Whole or partly milled grains and seeds |
| RS2 | Raw granular starch | Uncooked potato starch, green banana starch, gingko starch, high‐amylose maize starch |
| RS3 | Retrograded starch | Cooked and cooled potatoes and cornflakes |
| RS4 | Chemically modified starch | Cross‐linked starch and octenyl succinate starch |
| RS5 | Amylose–lipid complexes | Stearic acid‐complexed high‐amylose starch |
RS1
RS1 refers to starch that is physically inaccessible to digestion. This includes whole or partially milled grains and seeds, as well as certain highly processed starchy foods. RS1 can be completely digested in the small intestine if it is properly milled. As it is heat‐stable, RS1 does not break down during normal cooking processes 32 .
RS2
RS2 is a form of raw granular starch. In such granules, the starch molecules are tightly packed in a radial pattern and are relatively dehydrated 31 , 32 . This compact structure shields RS2 from various digestive enzymes, including amylases. In our diets, we consume raw starch in foods such as green bananas, raw potatoes, and high‐amylose maize starch. The mechanisms behind the resistance properties of raw starch are not fully understood yet. Factors such as the size and shape of the starch granules, the surface texture of the granules, amylose content, starch crystallinity, and pore size all vary and can influence resistance. Therefore, the resistance of RS2 to digestion is multifaceted and depends on a combination of these factors.
RS3
RS3, also known as retrograded starch, is the most resistant starch fraction. It primarily consists of retrograded amylose that forms when gelatinized starch cools to room temperature. Consequently, most foods prepared with moist heat contain some physically modified starches, including RS3. As RS3 remains at room temperature over time, the amylose double helices aggregate to form a highly thermostable crystalline structure. This structure cannot be rehydrated at temperatures below 150°C and does not dissociate during cooking. RS3 is a significant starch fraction due to its thermal stability, and it is commonly used as an ingredient in a variety of foods 33 . Compared with granular starch, RS3 has a higher water‐holding capacity. Cooked and cooled potatoes and cornflakes are typical examples of foods containing RS3 23 .
RS4
RS4 refers to chemically modified starch. This category includes starches that have been etherized, esterified, or cross‐linked with chemicals to reduce their digestibility 31 , 32 . Chemical modifications alter the structure and composition of starch granules, making them resistant to amylolytic enzymes. These modifications disrupt the normal arrangement of the starch chains through substitution, rendering the starch inaccessible to these enzymes. The primary types of modifications that contribute to this resistance are conversion, substitution, and cross‐linking of the starch molecules, all of which hinder enzymatic hydrolysis.
RS5
RS5 is a type of resistant starch that arises from the formation of amylose–lipid complexes. These complexes can form during the processing of food and can also be produced under controlled laboratory conditions 23 , 31 . Amylose‐lipid complexes typically originate from starches that have a high amylose content. The structure and development of RS5 differ based on the botanical source of the starch. RS5 consists of water‐insoluble linear poly‐α‐1,4‐glucan polysaccharides, which are resistant to degradation by α‐amylase 34 . These polysaccharides encourage the production of short‐chain fatty acids (SCFAs), particularly butyrate, which is considered the most beneficial SCFA.
CLINICAL STUDIES OF RS IN TYPE 2 DIABETES MELLITUS
RS is considered a type of fiber resistant to the action of digestive enzymes and is thought to play an important role in the body's glucose and insulin responses to food. However, there have been few human studies on the effects of RS on glycemic control and hormonal responses, such as insulin and GLP‐1. Moreover, studies on the effects of RS on glucose responses in patients with diabetes are extremely rare, and the results are inconsistent, as shown in Table 2. Since this review focuses on individuals with type 2 diabetes, three articles have been selected for further discussion as follows.
Table 2.
Summary of human studies using resistant starch
| First author (year of publication) | Subjects (number) | Intervention/duration | RS | Insulin | Prandial glucose | Others |
|---|---|---|---|---|---|---|
| Roberson et al. (2005) 35 | Healthy (10) | Randomized, crossover trial/4 weeks | 30 g RS2 | ↓ | NS | No effect on fasting glucose levels |
| Bodinham et al. (2010) 15 | Healthy (20) | Randomized, crossover trial/two meals | 48 g HAM‐RS2 | ↓ | NS | No significant change of insulin sensitivity |
| Bodinham et al. (2014) 41 | T2DM (17) | Randomized, crossover trial/12 weeks | 40 g HAM‐RS2 | NS | ↓ |
No significant change of HbA1c Reduced GLP‐1 on RS regimen |
| Hallström et al. (2011) 37 | Healthy (14) | Randomized, crossover trial/12 weeks | 7.7 g RS | ↑ | ↓ | |
| Li et al. (2010) 38 | Healthy (16) | Crossover trial/one meal | 8 g RS | ↓ | ↓ | |
| Al‐Tamimi et al. (2010) 39 | Healthy (13) | Randomized, crossover trial/one meal | 20 g RS4XL | ↓ | ↓ | |
| Lin et al. (2015) 40 | Healthy (40) | Randomized, 2‐regimen, crossover/one meal |
PPB‐R‐203 RS3 Matched daily energy needs |
↓ | ↓ | Reduced AUC on RS regimen |
| T2DM (44) | NA | ↓ | ||||
| Kwak et al. (2012) 36 | IFG, IGT, T2DM (90) | Randomized, placebo controlled | 6.51 g RS | NS | ↓ | Improved surrogate markers of endothelial function on RS regimen |
AUC, area under the curve; HAM, high‐amylose maize; NA, not applicable; NS, not significant; RS, resistant starch.
Study by Lin et al.
Lin et al. 40 conducted a randomized, two‐regimen, crossover, comparative study with 44 subjects with type 2 diabetes who were given either the new resistant starch formula, PPB‐R‐203, or a control diet. PPB‐R‐203 is a novel RS3 product. Additionally, 40 healthy subjects were administered PPB‐R‐203 or a control diet under identical conditions. A single portion of PPB‐R‐203 contains 20 g of carbohydrates, consisting of 10% degraded starch, 20% amylase, and 70% amylopectin. Since both test and control diets had the same dietary composition, they differed only in starch content. The glucose levels of the diabetic patients were continuously monitored for 3 days using a glucose monitoring system. In patients with type 2 diabetes, the mean blood glucose level and the area under the curve (AUC) for total blood glucose and hyperglycemia (blood glucose >180 mg/dL) were significantly reduced on the PPB‐R‐203 diet compared with the control diet. A similar effect was observed in healthy participants. They also measured insulin levels in healthy participants, finding that those on the PPB‐R‐203 diet had lower insulin levels than those on the test diet. Unfortunately, insulin levels were not measured in patients with type 2 diabetes.
Study by Bodinham et al.
Bodinham et al. 41 conducted a study with 17 individuals with type 2 diabetes who received 40 g of RS2 or a control diet for 12 weeks, followed by a 12 week washout period. This single‐blind, randomized, crossover study aimed to compare the effects of increased RS intake on glycemic control, insulin sensitivity, postprandial metabolites, and body fat changes in patients with type 2 diabetes. The RS2 test diet (HAM‐RS2) consisted of 67 g of Hi‐maize 260 (60% RS and 40% rapidly digestible starch), while the control diet was made up of 27 g of Amoica (100% rapid digestible starch). Both were provided in ready‐to‐use sachets. There was no difference in total calorie and carbohydrate intake between the groups during the study. At the study's conclusion, no significant difference was observed in fasting plasma glucose, HbA1c, insulin sensitivity, or beta‐cell function, as assessed by the Homeostasis Model Assessment (HOMA), between the RS2 diet group and the control group.
Hepatic glucose production and insulin sensitivity were measured using a euglycemic hyperinsulinemic clamp, showing no differences between the groups. Additionally, insulin concentrations throughout the clamp study were comparable. A meal tolerance test required participants to consume one of the supplements, resulting in a significantly lower glucose area under the curve for 0–120 min (AUC0–120 min) in the RS2 group compared with the control during the test. However, this did not lead to a reduction in plasma insulin concentration. The RS2 diet did show a tendency to increase glucose uptake into muscle tissue and significantly raised glucagon‐like peptide 1 concentrations compared with the control diet (P = 0.009). The authors suggested that the reduction in glucose AUC0–120 min observed during the meal tolerance test was due to an incretin effect, and GLP‐1 also appeared to enhance muscle glucose uptake. In conclusion, the RS2 diet improved meal glucose handling without improving insulin sensitivity. However, there was no improvement in HbA1c after 12 weeks in well‐controlled individuals with type 2 diabetes mellitus (mean HbA1c 6.4%).
Study by Kwak et al.
Kwak et al. 36 sought to determine whether a 4 week diet of rice containing resistant starch would reduce blood glucose and oxidative stress and improve endothelial function. They recruited 90 subjects with prediabetes or newly diagnosed type 2 diabetes. In the test group, 41 subjects consumed rice with 6.51 g of corn starch‐derived RS, while 44 subjects in the placebo group received rice without RS. Following the 4 week dietary intervention, the test group exhibited significant decreases in HOMA‐IR, postprandial glucose at 60 and 120 min, and glucose areas under the response curve during a standard meal test compared with the placebo group. Different markers of endothelial function were assessed in this study than in the previous two. The test group saw significant improvements in malondialdehyde levels, the reactive hyperemia peripheral arterial tonometry (RH‐PAT) index, and serum total nitric oxide concentrations. This suggests that a 4 week dietary intervention with RS‐containing rice may improve endothelial function by reducing oxidative stress.
CONCLUSION
Resistant starch has recently been recognized as an important source of fiber. With the decline in natural fiber consumption, RS‐enriched foods present a new and exciting potential as a source of fiber. It is technically feasible to produce RS‐enriched foods by modifying processing conditions, such as heating and cooling, making RS an ideal ingredient for creating palatable carbohydrate‐rich foods, compared with those made with natural fibers. Additionally, RS is anticipated to have beneficial effects on glucose metabolism, similar to fiber.
The impact of RS on glycemic control in patients with type 2 diabetes remains unclear. Animal studies have shown promising results, suggesting that an RS‐enriched diet could be incorporated into diabetes management regimens. However, the outcomes of using an RS diet in healthy individuals and diabetic patients do not always align with animal data. Moreover, the types of RS used, the amounts of RS, and the duration of the studies, which are often very short, vary greatly. These factors complicate the interpretation of the glucose and insulin response to an RS diet. Another variable to consider is the gut microbiota. RS intake has been shown to significantly affect microbiota changes. The interplay between gut microbiota and RS metabolism is crucial in determining the metabolic benefits of RS on glucose and insulin response. Given the differences between animals and humans in gut anatomy and microbiota composition, the direct translation of animal study results to human contexts is challenging. Therefore, further well‐designed, high‐quality human research is necessary to establish the effects of RS on glucose metabolism in people with type 2 diabetes.
DISCLOSURE
Doo‐Man Kim is an Editorial Board member of Journal of Diabetes Investigation and a corresponding author of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication.
REFERENCES
- 1. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK prospective diabetes study (UKPDS) group. Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 2. ADVANCE Collaborative Group , Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 3. Action to Control Cardiovascular Risk in Diabetes Study , Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006; 166: 1836–1841. [DOI] [PubMed] [Google Scholar]
- 5. Broadbent E, Donkin L, Stroh JC. Illness and treatment perceptions are associated with adherence to medications, diet, and exercise in diabetic patients. Diabetes Care 2011; 34: 338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med 2013; 159: 262–274. [DOI] [PubMed] [Google Scholar]
- 7. Giugliano D, Maiorino MI, Bellastella G, et al. Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta‐analysis of randomized controlled trials. Diabetes Care 2011; 34: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skender ML, Goodrick GK, Del Junco DJ, et al. Comparison of 2‐year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. J Am Diet Assoc 1996; 96: 342–346. [DOI] [PubMed] [Google Scholar]
- 9. Bhavadharini B, Mohan V, Dehghan M, et al. White rice intake and incident diabetes: a study of 132,373 participants in 21 countries. Diabetes Care 2020; 43: 2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooper HC, Booth K, Gill G. Patients’ perspectives on diabetes health care education. Health Educ Res 2003; 18: 191–206. [DOI] [PubMed] [Google Scholar]
- 11. Cummings JH, Mann JI, Nishida C, et al. Dietary fibre: an agreed definition. Lancet 2009; 373: 365–366. [DOI] [PubMed] [Google Scholar]
- 12. Robertson MD. Dietary‐resistant starch and glucose metabolism. Curr Opin Clin Nutr Metab Care 2012; 15: 362–367. [DOI] [PubMed] [Google Scholar]
- 13. Anderson GH, Cho CE, Akhavan T, et al. Relation between estimates of cornstarch digestibility by the Englyst in vitro method and glycemic response, subjective appetite, and short‐term food intake in young men. Am J Clin Nutr 2010; 91: 932–939. [DOI] [PubMed] [Google Scholar]
- 14. Nichenametla SN, Weidauer LA, Wey HE, et al. Resistant starch type 4‐enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross‐over intervention. Mol Nutr Food Res 2014; 58: 1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bodinham CL, Frost GS, Robertson MD. Acute ingestion of resistant starch reduces food intake in healthy adults. Br J Nutr 2010; 103: 917–922. [DOI] [PubMed] [Google Scholar]
- 16. Hoover R. Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr Polym 2001; 45: 253–267. [Google Scholar]
- 17. Pencek RR, Koyama Y, Lacy DB, et al. Transporter‐mediated absorption is the primary route of entry and is required for passive absorption of intestinal glucose into the blood of conscious dogs. J Nutr 2002; 132: 1929–1934. [DOI] [PubMed] [Google Scholar]
- 18. Tester RF, Karkalas J, Qi X. Starch structure and digestibility enzyme‐substrate relationship. World's Poultry Sci J 2004; 60: 186–195. [Google Scholar]
- 19. Butterworth P, Warren F, Ellis P. Human α‐amylase and starch digestion: an interesting marriage. Starch 2011; 63: 395–405. [Google Scholar]
- 20. Zhang G, Hamaker BR. Slowly digestible starch: concept, mechanism, and proposed extended glycemic index. Crit Rev Food Sci Nutr 2009; 49: 852–867. [DOI] [PubMed] [Google Scholar]
- 21. Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 1992; 46(Suppl 2): S33–S50. [PubMed] [Google Scholar]
- 22. Englyst HN, Veenstra J, Hudson GJ. Measurement of rapidly available glucose (RAG) in plant foods: a potential in vitro predictor of the glycaemic response. Br J Nutr 1996; 75: 327–337. [DOI] [PubMed] [Google Scholar]
- 23. Raigond P, Ezekiel R, Raigond B. Resistant starch in food: a review. J Sci Food Agric 2015; 95: 1968–1978. [DOI] [PubMed] [Google Scholar]
- 24. McCleary BV, Monaghan DA. Measurement of resistant starch. J AOAC Int 2002; 85: 665–675. [PubMed] [Google Scholar]
- 25. Champ MM. Physiological aspects of resistant starch and in vivo measurements. J AOAC Int 2004; 87: 749–755. [PubMed] [Google Scholar]
- 26. Cuche G, Cuber JC, Malbert CH. Ileal short‐chain fatty acids inhibit gastric motility by a humoral pathway. Am J Physiol Gastrointest Liver Physiol 2000; 279: G925–G930. [DOI] [PubMed] [Google Scholar]
- 27. Massimino SP, McBurney MI, Field CJ, et al. Fermentable dietary fiber increases GLP‐1 secretion and improves glucose homeostasis despite increased intestinal glucose transport capacity in healthy dogs. J Nutr 1998; 128: 1786–1793. [DOI] [PubMed] [Google Scholar]
- 28. Behall KM, Hallfrisch J. Plasma glucose and insulin reduction after consumption of breads varying in amylose content. Eur J Clin Nutr 2002; 56: 913–920. [DOI] [PubMed] [Google Scholar]
- 29. Bindels LB, Dewulf EM, Delzenne NM. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci 2013; 34: 226–232. [DOI] [PubMed] [Google Scholar]
- 30. Bindels LB, Walter J, Ramer‐Tait AE. Resistant starches for the management of metabolic diseases. Curr Opin Clin Nutr Metab Care 2015; 18: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Birt DF, Boylston T, Hendrich S, et al. Resistant starch: promise for improving human health. Adv Nutr 2013; 4: 587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch – a review. Compr Rev Food Sci Food Saf 2006; 5: 1–17. [DOI] [PubMed] [Google Scholar]
- 33. Haralampu SG. Resistant starch – a review of the physical properties and biological impact of RS3. Carbohydr Polym 2000; 41: 285–292. [Google Scholar]
- 34. Frohberg C, Quanz M. Use of linear poly‐alpha‐1,4‐glucans as resistant starch. 2008. US 2008/0249297 A1.
- 35. Robertson MD, Bickerton AS, Dennis AL, et al. Insulin‐sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 2005; 82: 559–567. [DOI] [PubMed] [Google Scholar]
- 36. Kwak JH, Paik JK, Kim HI, et al. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis 2012; 224: 457–464. [DOI] [PubMed] [Google Scholar]
- 37. Hallstrom E, Sestili F, Lafiandra D, et al. A novel wheat variety with elevated content of amylose increases resistant starch formation and may beneficially influence glycaemia in healthy subjects. Food Nutr Res 2011; 55: 7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li M, Piao JH, Tian Y, et al. Postprandial glycaemic and insulinaemic responses to GM‐resistant starch‐enriched rice and the production of fermentation‐related H2 in healthy Chinese adults. Br J Nutr 2010; 103: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 39. Al‐Tamimi EK, Seib PA, Snyder BS, et al. Consumption of cross‐linked resistant starch (RS4(XL)) on glucose and insulin responses in humans. J Nutr Metab 2010; 2010: 651063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin CH, Chang DM, Wu DJ, et al. Assessment of blood glucose regulation and safety of resistant starch formula‐based diet in healthy normal and subjects with type 2 diabetes. Medicine (Baltimore) 2015; 94: e1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bodinham CL, Smith L, Thomas EL, et al. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr Connect 2014; 3: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


