Granulomatous disease prompts a search for antigenic triggers, given our understanding of granuloma formation as a defense mechanism against persistent antigen in tissue. Cutaneous granulomas in patients with primary immunodeficiency have been attributed to immune dysregulation because no infectious trigger has been identified in these patients.1 However, recent reports have emerged of skin biopsies in at least 9 children with primary immunodeficiency confirming the presence of vaccine-derived rubella virus (VDRV) related to the RA27/3 vaccine strain in granulomas and its absence in normal skin.2-4 Immune deficiency may allow for the persistence of vaccine-strain virus, which could accrue mutations.3 We report herein the cases of 2 children with cutaneous granulomas harboring VDRV, one with primary immunodeficiency, and the other with undefined immunodeficiency.
Report of Cases
Case 1.
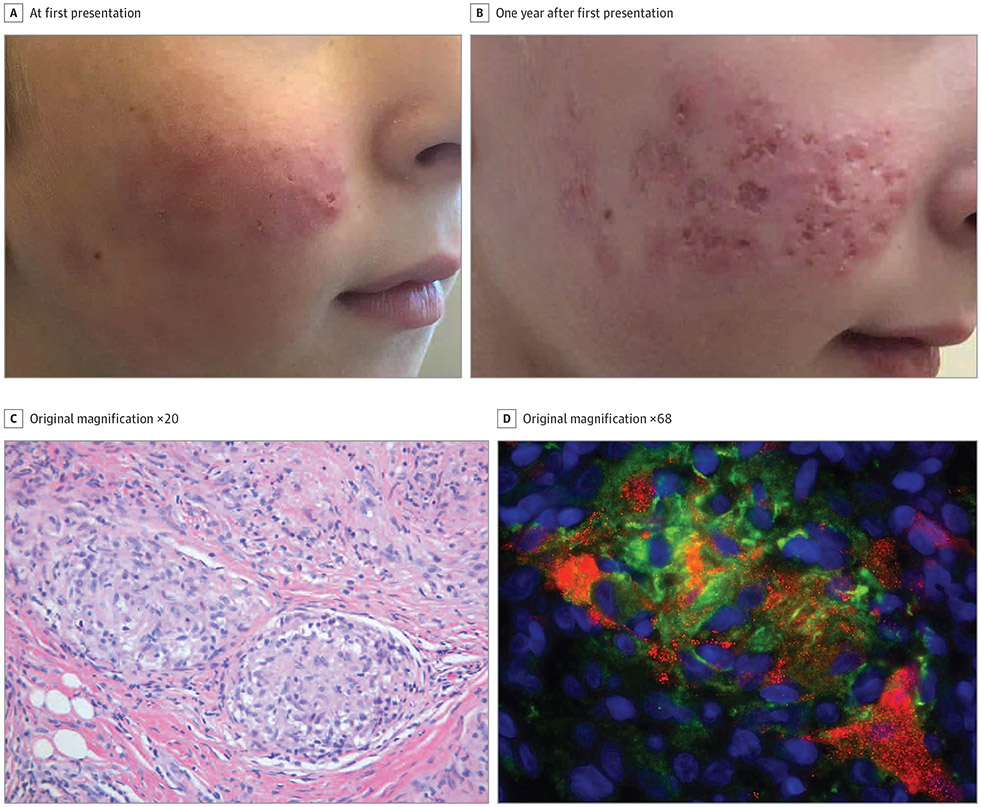
Patient 1, an 11-year-old boy with Nijmegen breakage syndrome, presented with a 1-year history of an erythematous plaque on the right cheek (Figure 1A). He had received the measles, mumps, and rubella (MMR) vaccine at ages 1 and 5 years. Skin biopsy demonstrated sarcoidal granulomas (Figure 1C). Tissue cultures were negative for bacteria, fungi, and mycobacteria. The biopsy results were positive for rubella virus by immunohistochemical analysis (Figure 1D) and reverse transcriptase-polymerase chain reaction (RT-PCR). Infectious virus was recovered from the biopsy by cell culture. Whole-genome sequencing of the isolated virus showed 2% nucleotide divergence from the sequence of the RA27/3 vaccine strain. A nasopharyngeal swab tested negative for rubella virus by RT-PCR. At last follow-up, the lesion remained untreated (Figure 1B).
Figure 1. Clinical and Histopathologic Images of Patient 1.
A, Right cheek with erythematous papules coalescing into plaques. B, Right cheek with the granulomatous plaque 1 year after first presentation. C, Lesional biopsy specimen showing sarcoidal granulomas (hematoxylin-eosin).
D, Immunofluorescent immunohistochemical staining of rubella virus capsid (red) in CD206-positive (green) M2 macrophages; nuclei counterstained with DAPI (blue).
Case 2.
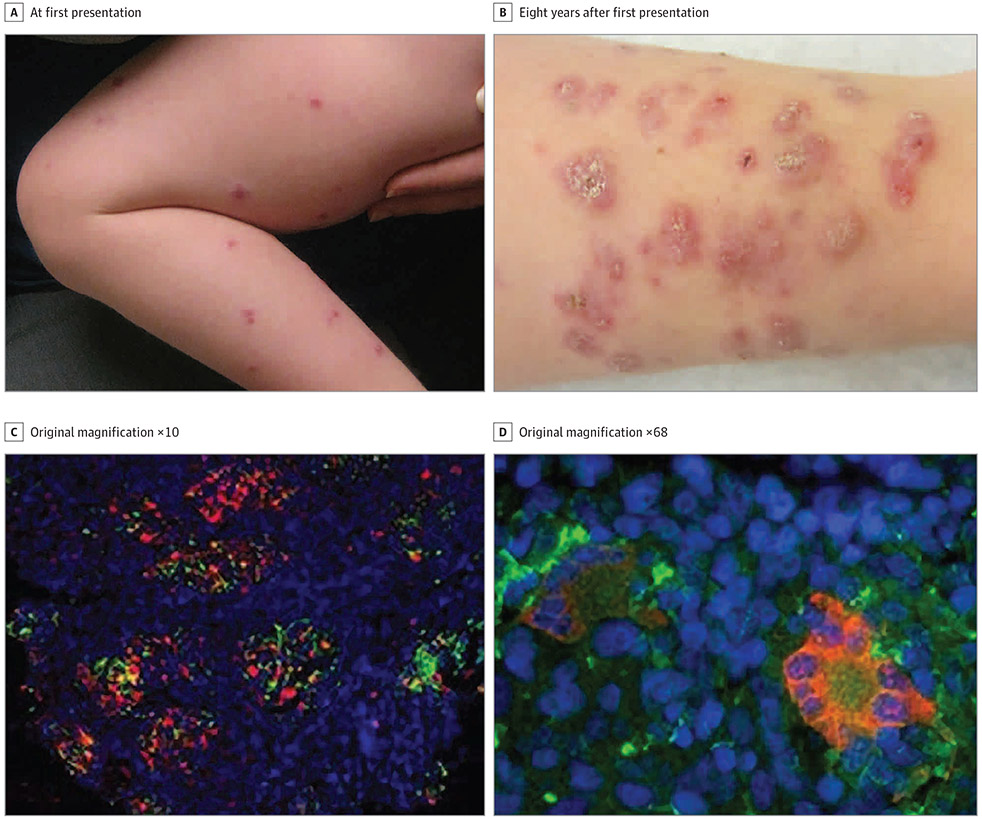
Patient 2 presented at age 2.5 years with 1-year history of skin eruption. She was otherwise healthy. The parents noted that a few months after she received her MMR vaccine at age 12 months, red papules erupted on her legs (Figure 2A). Histopathologic analysis showed sarcoidal granulomas. Test results for systemic sarcoidosis were negative, and she was lost to follow-up until age 10 years (Figure 2B). At that time, biopsies again showed sarcoidal granulomas, but test results for systemic sarcoidosis were again negative. Genetic evaluation found a NOD2 mutation, c2104C>T (pArg702Trp), associated with increased risk of Crohn disease, but to our knowledge not previously described in association with Blau syndrome or early-onset sarcoidosis.5 She had no clinical features of Blau syndrome, early-onset sarcoidosis, or Crohn disease. The biopsy specimen tested strongly positive for rubella by immunohistochemical analysis (Figure 2C and D). A nasopharyngeal swab tested negative for rubella virus. Antibody titers demonstrated immunity to MMR, but low immunity to Streptococcus pneumoniae, Haemophilus influenzae, and tetanus toxoid despite the patient receiving these vaccines on schedule. Isolated cutaneous granulomas may be a previously unknown feature of her particular NOD mutation variant, or perhaps further immunology evaluation may yield another explanation.
Figure 2. Clinical and Histopathologic Images of Patient 2.
A. Scaly erythematous to violaceous papulonodules over the extremities at initial presentation. B, More confluent, scaly, and scarring erythematous to violaceous plaques over bilateral lower extremities 8 years later. C, Immunofluorescent immunohistochemical staining of rubella virus capsid (red) in CD206-positive (green) M2 macrophages; nuclei counterstained with DAPI (blue). D, M2 syncytia (Langhans cells) containing rubella virus capsid.
Discussion
These 2 cases add to the growing number of reports regarding VDRV in association with granulomas in patients with immune system defects. The finding of VDRV in granulomas raises the question of whether rubella vaccine is an antigenic trigger or a bystander. The cases described in the literature report the localization of VDRV in affected skin, its absence in normal skin, and its ability to persist in anti-inflammatory M2 macrophages, as well as keratinocytes, while accumulating mutations. All reports suggest a causal relationship between rubella virus persistence in the skin and granuloma development.1-4 It is also possible that rubella virus is a bystander in the granulomatous process, perhaps representing a locus minoris resistentiae phenomenon.6 Continued investigation of the relationship between granulomatous disease and VDRV is needed. Another important question is whether VDRV is transmissible, since live virus has been cultured from granulomas. We counseled both patients’ parents to take precautions around those who are immunocompromised or pregnant, but more studies are needed to elucidate the natural history and infectious potential of VDRV.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
Contributor Information
Julie Dhossche, Department of Dermatology, Oregon Health and Science University, Portland, Oregon.
Luke Johnson, Department of Dermatology, University of Utah, Salt Lake City, Utah.
Kevin White, Department of Dermatology, Oregon Health and Science University, Portland, Oregon.
Tracy Funk, Department of Dermatology, Oregon Health and Science University, Portland, Oregon.
Sabra Leitenberger, Department of Dermatology, Oregon Health and Science University, Portland, Oregon.
Ludmila Perelygina, Centers for Disease Control and Prevention, Division of Viral Diseases, Atlanta, Georgia.
Alfons Krol, Department of Dermatology, Oregon Health and Science University, Portland, Oregon.
References
- 1.Mitra A, Pollock B, Gooi J, Darling JC, Boon A, Newton-Bishop JA. Cutaneous granulomas associated with primary immunodeficiency disorders. Br J Dermatol. 2005;153(1):194–199. doi: 10.1111/j.1365-2133.2005.06619.x [DOI] [PubMed] [Google Scholar]
- 2.Bodemer C, Sauvage V, Mahlaoui N, et al. Live rubella virus vaccine long-term persistence as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency. Clin Microbiol Infect. 2014;20(10):O656–O663. doi: 10.1111/1469-0691.12573 [DOI] [PubMed] [Google Scholar]
- 3.Perelygina L, Plotkin S, Russo P, et al. Rubella persistence in epidermal keratinocytes and granuloma M2 macrophages in patients with primary immunodeficiencies. J Allergy Clin Immunol. 2016;138(5):1436–1439.e11. doi: 10.1016/j.jaci.2016.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neven B, Pérot P, Bruneau J, et al. Cutaneous and visceral chronic granulomatous disease triggered by a rubella virus vaccine strain in children with primary immunodeficiencies. Clin Infect Dis. 2017;64(1):83–86. doi: 10.1093/cid/ciw675 [DOI] [PubMed] [Google Scholar]
- 5.Caso F, Galozzi P, Costa L, Sfriso P, Cantarini L, Punzi L. Autoinflammatory granulomatous diseases: from Blau syndrome and early-onset sarcoidosis to NOD2-mediated disease and Crohn’s disease. RMD Open. 2015;1(1):e000097. doi: 10.1136/rmdopen-2015-000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo Schiavo A, Ruocco E, Russo T, Brancaccio G. Locus minoris resistentiae: An old but still valid way of thinking in medicine. Clin Dermatol. 2014;32(5):553–556. doi: 10.1016/j.clindermatol.2014.04.001 [DOI] [PubMed] [Google Scholar]