Abstract
Herpes simplex virus (HSV) glycoprotein D (gD) is modified with mannose 6-phosphate (M6P) and binds to M6P receptors (MPRs). MPRs are involved in the well-characterized pathway by which lysosomal enzymes are directed to lysosomes via a network of endosomal membranes. Based on the impaired ability of HSV to form plaques under conditions in which glycoproteins could not interact with MPRs, we proposed that MPRs may function during HSV egress or cell-to-cell spread (C. R. Brunetti, R. L. Burke, B. Hoflack, T. Ludwig, K. S. Dingwell, and D. C. Johnson, J. Virol. 69:3517–3528, 1995). To further analyze M6P modification and intracellular trafficking of gD in the absence of other HSV proteins, adenovirus (Ad) vectors were used to express soluble and membrane-anchored forms of gD. Both membrane-bound and soluble gD were modified with M6P residues and were localized to endosomes that contained the 275-kDa MPR or the transferrin receptor. Similar results were observed in HSV-infected cells. Cell fractionation experiments showed that gD was not present in lysosomes. However, a mutant form of gD and another HSV glycoprotein, gI, that were not modified with M6P were also found in endosomes in HSV-infected cells. Moreover, a substantial fraction of the HSV nucleocapsid protein VP6 was found in endosomes, consistent with accumulation of virions in an endosomal compartment. Therefore, it appears that HSV glycoproteins and virions are directed to endosomes, by M6P-dependent as well as by M6P-independent mechanisms, either as part of the virus egress pathway or by endocytosis from the cell surface.
Herpes simplex virus (HSV) glycoprotein D (gD) is essential for virus entry into cells, as well as for cell fusion and cell-to-cell spread (7, 61). The functions of gD are best understood for virus entry, a process in which gD binds to “gD receptors,” cell surface molecules that are more restricted in number than the glycosaminoglycans to which the virus initially adsorbs (3, 18, 34, 36, 58). The hypothesis that gD is a receptor-binding protein is based on at least three types of evidence. (i) UV-inactivated wild-type HSV virions (containing gD) bind to a limited number of sites on the cell surface and block subsequent entry of infectious HSV particles into cells, whereas UV-inactivated virions lacking gD cannot block infection (1, 36). (ii) HSV can adsorb onto but not enter into cell lines constitutively expressing gD; the gD apparently binds to and sequesters cellular receptors (9, 38). (iii) Soluble forms of HSV type 1 (HSV-1) and HSV-2 gDs bind to a relatively restricted number of protease-sensitive sites on cells and block HSV-1 and HSV-2 entry (34).
Several potential gD binding proteins have been characterized and may represent different pathways for virus entry or sequential steps in the entry pathway. We reported that soluble gD and, to a lesser degree, membrane-anchored gD were modified with mannose 6-phosphate (M6P) residues and were able to interact with the 275- and 46-kDa M6P receptors (MPRs) (8). Blocking the ability of HSV to interact with MPRs, using antibodies, ligands, or a soluble form of the 275-kDa MPR, decreased HSV entry into adherent primate cells by 50 to 80% (7). In apparent contrast to these results, HSV could enter into mouse fibroblasts lacking both MPRs, and MPR ligands had no effect on virus entry or replication in these cells (7). Therefore, MPRs may represent cell surface receptors for HSV entry into some primate cells but not mouse cells. In other studies, anti-idiotype antibodies (produced with a gD-specific monoclonal antibody [MAb]) reacted with a 62-kDa cellular protein and inhibited HSV entry into cells (30). More recently, it was reported that gD binds to HVEM (70), a novel member of the tumor necrosis factor receptor family that had been identified as a receptor for HSV (44). Entry of HSV into HVEM-transfected CHO cells could be inhibited by anti-HVEM antibodies and soluble HVEM (44). However, soluble gD did not block entry into HVEM-transfected CHO cells and anti-HVEM antibodies and soluble HVEM did not block entry into monkey Vero cells (44, 70), and thus, there must be other gD receptors important for infection of primate cells.
The interactions between HSV gD and cellular receptors appear to be essential not only for entry of extracellular virus particles but also for the process of cell-to-cell spread. A mutant HSV-1 lacking the gD gene but grown on complementing cells can enter cells, but without gD it cannot spread between cells (17, 42). A second HSV-1 mutant which expresses a form of gD lacking N-linked oligosaccharides (and thus M6P residues) spreads less efficiently from cell to cell, especially in fibroblast and epithelial cell monolayers (7, 16a, 60). Cell-to-cell spread of wild-type HSV is also markedly reduced in fibroblasts defective for addition of M6P residues or when MPRs are blocked with bulky ligands (7). These studies suggest that MPRs are involved in cell-to-cell spread of HSV but do not clarify which stage of this process is inhibited: generalized movement of virus to the cell surface (virus egress), directed traffic of virions to specific cell surface domains (e.g., cell junctions), or subsequent movement across the cell junctions and entry into an adjacent cell.
Several models for herpesvirus egress have been proposed, most suggesting that virions acquire an envelope at the inner nuclear envelope. One group of models suggest that enveloped HSV particles subsequently move from the space between the nuclear membranes to the endoplasmic reticulum (ER) and Golgi apparatus and on to the cell surface without exchange of the virion envelope (11, 37). Other models suggest that enveloped particles lose their envelope by fusion with the outer nuclear envelope so that unenveloped capsids acquire a second envelope by budding into cytoplasmic vesicles, e.g., the Golgi apparatus (21, 39, 69). In many cases, support for these models comes from electron microscopic studies in which it is often not clear whether viruses are in the process of being enveloped or de-enveloped. However, there are some biochemical and genetic data supporting de-envelopment–reenvelopment models (6, 62, 67), yet other studies involving mutant HSV-1 suggested that cytosolic unenveloped capsids are part of a dead-end pathway (11, 32). Whatever the mechanism of alphaherpesvirus egress, the process appears to be highly inefficient, with the majority of enveloped virions accumulating in cytoplasmic vesicles of unknown derivation (14–16, 20, 39, 56, 69).
The observation that HSV gD interacts with MPRs suggested that the intracellular traffic of gD and gD-containing virions might be influenced by the well-established ability of MPRs to direct proteins to endosomes and lysosomes (26, 40, 45–46, 57). Directed transport of gD or gD-containing virions to the endosomal network might influence egress to defined domains of the cell surface (cell junctions) or promote reenvelopment of capsids into an endosomal compartment. As with HSV, the related alphaherpesvirus varicella-zoster virus (VZV) contains glycoproteins that are modified with M6P, and it has been suggested that movement of VZV particles to endosomes or lysosomes may be facilitated by MPRs (19, 24). Dileucine and tyrosine motifs in the cytoplasmic domains of VZV glycoproteins can also direct transport from the Golgi apparatus to the trans-Golgi network (TGN) and endosomes or cause endocytosis from the cell surface (2, 48, 72).
In order to further characterize the intracellular transport of HSV-1 gD and the relationship of this transport to mannose phosphorylation, we constructed adenovirus (Ad) vectors expressing soluble and membrane-bound forms of gD. In cells infected with these Ad vectors, and also in HSV-infected cells, soluble and membrane-bound forms of gD were modified with M6P and colocalized with the 275-kDa MPR and the transferrin receptor in endosomal compartments. There was no gD in lysosomes. However, a mutant form of gD and another HSV glycoprotein, gI (neither modified with M6P), were found in endosomes, consistent with the notion that there are M6P-independent mechanisms for endosomal localization. Since we also found HSV virions accumulating in endosomes, it appears that HSV glycoproteins and virus particles traffic through endosomes during virus egress.
MATERIALS AND METHODS
Cells and viruses.
Human R970 cells (51) and monkey Vero cells (from the American Type Culture Collection) were propagated in α minimal essential medium (Life Technologies, Inc.) supplemented with 7% fetal bovine serum (FBS). MRC-5 human fibroblasts (from the American Type Culture Collection) and 293 cells (23) were grown in Dulbecco’s modified minimal essential medium (DMEM) (Life Technologies, Inc.) supplemented with 10% FBS. E1− Ad vectors were propagated and titered on 293 cell monolayers. Wild-type HSV-1 strain F was obtained from P. G. Spear (Northwestern University, Chicago, Ill.). HSV-1 (QAA), a mutant that lacks the three N-linked oligosaccharide sites on gD (59), was obtained from G. H. Cohen and R. J. Eisenberg (University of Pennsylvania, Philadelphia). HSV-1 cells were propagated and titered on Vero cells.
Antibodies.
MAb DL6, specific for HSV-1 gD (33), and rabbit antiserum NC-1 (anti-VP5) (13) were gifts of G. H. Cohen and R. J. Eisenberg. MAb LP2, specific for gD, was obtained from A. C. Minson (University of Cambridge, Cambridge, United Kingdom). Rabbit polyclonal serum specific for gD was produced by using soluble gD1t (a generous gift of Rae Lyn Burke, Chiron). Rabbit antiserum which recognizes human TAP (27) was obtained from H. Ploegh (Massachusetts Institute of Technology). MAb 3104, specific for HSV-1 gI (35), was a generous gift from A. Cross and N. Stow (Institute of Virology, Glasgow, United Kingdom). Mouse anti-transferrin receptor antibody was obtained from Sigma (Mississauga, Canada). Immunopurified rabbit antibodies specific for the 275-kDa MPR were generated with serum from rabbits injected with a soluble form of the 275-kDa MPR purified from FBS as described previously (7). The anti-MPR antibodies were immunopurified with soluble MPR coupled to Sepharose 4B by CNBr (Pharmacia, Baie d’Urfé, Canada). Antibodies were eluted with 100 mM glycine, pH 2.5, and precipitated by adding an equal volume of ammonium sulfate and centrifuging the material at 10,000 × g for 30 min. The antibodies were resuspended in phosphate-buffered saline (PBS) and dialyzed against PBS.
Construction of replication-defective recombinant Ad vectors expressing gD1 or gD1t.
Plasmids pCA3 and pCA4 contain the left end (16%) of the Ad type 5 (Ad5) genome with a deletion in the E1 region and differ in the orientation of the cloning polylinker (28). The full-length gD gene, encoding amino acids 1 to 394, or a truncated version of the gD1 gene (gD1t), lacking sequences encoding the transmembrane domain and the cytoplasmic tail and encompassing amino acids 1 to 312, were excised with restriction enzymes from plasmid pS5exp, which contains the HSV-1 (strain KOS) gD coding sequences, and the genes were subcloned into pCA4 and pCA3, respectively. The plasmids, denoted pCA3gD1t (with the truncated gD gene) and pCA4gD1 (with the full-length gD gene), contained the gD genes coupled to the human cytomegalovirus immediate-early promoter and an simian virus 40 poly(A) site flanked by Ad E1 sequences. Cotransfection of either pCA3gD1t or pCA4gD1 vectors with pBHG10 (5) into 293 cells produced recombinant AdgD1t(E1−) and AdgD1(E1−), respectively. Both viruses were plaque purified three times.
Labelling of proteins with [3H]mannose and analysis of M6P.
Approximately 1.5 × 107 R970 cells were infected with AdgD1t(E1−) or AdgD1(E1−) at 10 PFU/cell and incubated at 37°C for 48 h. The cells were labelled for 4 h at 37°C in medium containing 10% of the normal level of glucose, 1% FBS, and 30 μCi of d-[2-3H]mannose (Dupont, NEN)/ml. The medium was removed, and the cells were incubated in medium containing the normal amount of glucose for 3 h. The cells were lysed in Nonidet P-40 (NP-40)–sodium deoxycholate (DOC) buffer (1% NP-40, 0.5% DOC, 50 mM Tris-HCl [pH 7.5], 100 mM NaCl) containing 2 mg of bovine serum albumin (BSA)/ml, and 1 mM phenylmethylsulfonyl fluoride. gD was immunoprecipitated with a mixture of MAbs DL6 and LP2 and protein A-Sepharose and eluted with 2% sodium dodecyl sulfate SDS–100 mM Na citrate [pH 5.5] at 100°C for 10 min. The gD was characterized by gel electrophoresis and then incubated with 5,000 U of endoglycosidase H (endo H; New England Biolabs) for 6 h at 37°C. The samples were diluted to 2 ml with 2 mM Tris base and applied to a Centricon-30 membrane (Amicon, Beverly, Mass.) so that the liberated oligosaccharides passed through the membrane and the core glycoproteins (containing complex N-linked oligosaccharides) were retained. The oligosaccharides were hydrolyzed by boiling in 0.02 N HCl for 30 min, diluted to 10 ml with H2O, and applied to a 1-ml quaternary aminoethyl-Sephadex ion-exchange column (Pharmacia). Oligosaccharides without M6P residues do not bind to the ion-exchange column, whereas those bearing one or two M6P residues can be eluted with 2 mM Tris base containing 20 mM and 70 mM NaCl, respectively (3, 12).
Radiolabelling of cells with [35S]methionine and [35S]cysteine and immunoprecipitation of radiolabelled proteins.
R970 or MRC-5 cells were labelled with [35S]methionine and [35S]cysteine (Dupont, NEN) by washing the cells extensively with DMEM lacking methionine and cysteine and incubating the cells with medium lacking methionine and with 50 to 150 μCi of [35S]methionine and [35S]cysteine per ml for various periods. Chase periods involved incubating the cells in DMEM containing a 10-fold excess of methionine and cysteine. Extracts of radiolabelled cells were made with NP-40–DOC buffer containing 2 mg of BSA/ml and 1 mM phenylmethylsulfonyl fluoride. The extracts were clarified by centrifuging at 100,000 × g for 60 min at 4°C. Antibodies were mixed with the extracts, and the samples were incubated on ice for 60 min. Protein A-Sepharose (Pharmacia) was added, and the samples were incubated for a further 2 h at 4°C. The protein A-Sepharose was washed three times with NP-40–DOC buffer; resuspended in a solution containing 50 mM Tris (pH 6.8), 2% SDS, 10% glycerol, bromophenol blue, and 2% β-mercaptoethanol; boiled for 5 min; and then loaded onto 10% N,N′-diallyltartardiamide cross-linked polyacrylamide gels. The gels were dried, enhanced with Enlightning (Dupont, NEN), and exposed to Kodak XAR film.
Cell surface labelling.
Approximately 1.5 × 107 MRC-5 fibroblasts grown on a 150-mm2 dish were overlaid with a solution of 8 ml of PBS containing 1.2 mg of lactoperoxidase (Sigma), 1 mM CaCl2, 1 mM MgCl2, and 1 mCi of Na125I. Every 2 min for 12 min, 120 μl of 0.1% H2O2 was added to the cells; the cells were then incubated for a further 12 min at room temperature and subsequently washed three times with PBS containing 1 mM CaCl2, 1 mM MgCl2, 5 mg of BSA/ml, and 1% FBS.
Cell fractionation.
Approximately 1.5 × 107 MRC-5 cells grown in a 150-mm2 dish were radiolabelled with either [35S]methionine or Na125I, washed with PBS, and scraped into 10 ml of PBS. The cells were pelleted at 1,000 × g for 5 min, resuspended in 1.5 ml of homogenization buffer (0.25 M sucrose, 1 mM EDTA [pH 7.5]), and stored on ice for 5 min. The cells were homogenized with a Dounce homogenizer, and the nuclei and unbroken cells were pelleted at 4,000 × g for 20 min. Membranes were diluted in homogenization buffer and Percoll to produce 12-ml of solution with a final Percoll concentration of 18%. The gradient was centrifuged for 30 min at 20,000 rpm in a 50Ti rotor (Beckman Instruments Inc., Palo Alto, Calif.) at 4°C and fractionated into 12 1-ml fractions collected from the bottom of the tube, and the fractions were analyzed for radioactivity or enzyme markers.
Enzymatic assays of cell fractions.
Lysosomal β-hexosaminidase activity was determined as described previously (24). Briefly, samples were diluted with 50 mM Na citrate (pH 4.5)–0.1% Triton X-100 and incubated at 37°C for 60 min with 1.67 mM p-nitrophenyl N-acetyl β-glucosaminide (Sigma). The reaction was terminated by the addition of 200 μl of 1 M Na carbonate (pH 10), and the absorbance was read at 400 nm. Golgi galactosyltransferase activity was determined as described previously (24) after the fractions were diluted with 50 mM Tris-HCl (pH 7.6), 20 mM MnCl2, 0.4% Triton X-100, 0.0031 nM [14C]UDP-galactose (DuPont, NEN), and 17.5 μg of ovalbumin/ml and incubated at 37°C for 60 min. Cold 10% (wt/vol) trichloroacetic acid was added, and the samples were incubated for 30 min on ice and then passed through GF/C glass fiber filters. The filters were washed three times with 5% (wt/vol) trichloroacetic acid and dried, and radioactivity was counted.
Coupling of FITC to rabbit IgG.
Immunoglobulin G (IgG) was purified from rabbit antiserum directed against VP5 (anti-NC1 serum) by using protein A-Sepharose, and the IgG was eluted with 100 mM glycine (pH 3.0). The IgG was dialyzed against 100 mM carbonate-bicarbonate buffer (pH 9.0), 0.025 mg of a 1 mg/ml solution of fluorescein isothiocyanate (FITC; Sigma) in dimethyl sulfoxide was added to 300 μl of purified IgG (2 mg/ml), and the mixture was incubated at 22°C for 2 h. The IgG was separated from the uncoupled FITC by using a Sephadex G50 gel filtration column.
Confocal immunofluorescence microscopy.
R970 cells grown on glass coverslips were infected with AdgD1(E1−) or AdgD1t(E1−) at 100 PFU/cell for 36 to 48 h. Alternatively, the cells were infected with either HSV-1 (F) or HSV-1 (QAA) at 10 PFU/cell for 8 to 16 h. The cells on coverslips were fixed with 4% paraformaldehyde for 10 min, washed twice with PBS, permeabilized with 0.2% Triton X-100 in PBS for 5 min, and then washed twice with PBS. The cells were incubated for 1 h at 22°C in 1% (wt/vol) BSA–2% goat serum in PBS (blocking buffer) and then incubated with primary antibodies diluted in blocking buffer for 1 h at room temperature. The samples were washed three times with blocking buffer and then incubated with secondary goat anti-rabbit Texas red and goat anti-mouse FITC antibodies (Jackson ImmunoResearch Laboratories, West Grove, Pa.) diluted in blocking buffer. In some experiments, the cells were stained with anti-275-kDa MPR antibodies and Texas red-conjugated anti-rabbit IgG, washed with blocking buffer containing 10% normal rabbit serum, and then incubated with FITC-conjugated anti-NC1 IgG. The coverslips were mounted on microscope slides with Vectashield (Vector Labs, Burlingame, Calif.) and viewed with a Zeiss confocal microscope.
RESULTS
Construction of Ad vectors expressing full-length or soluble gD.
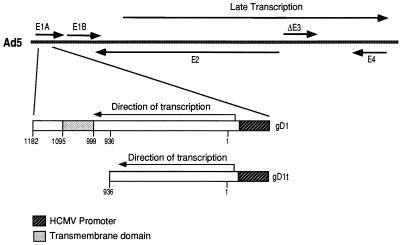
Previously, we compared M6P modification of soluble and membrane-bound forms of gD (8), but these studies were complicated because the soluble gD was produced in transfected CHO cells and the membrane-bound gD was produced in HSV-infected human R970 cells. In order to compare M6P modification and intracellular transport of these proteins in the same cell type, replication-defective (E1−) Ad vectors expressing either membrane-bound or soluble forms of gD were constructed. This also allowed us to examine M6P modification and intracellular transport of gD in the absence of HSV infection, which causes inhibition of host protein synthesis (54, 63, 64), alterations in the cytoskeleton, cell rounding, and disruption of the Golgi apparatus and other cellular membranes (10). A soluble form of HSV-1 gD, denoted gDt, composed of amino acids 1 to 312 and including the extracellular domain but lacking the transmembrane and cytoplasmic domains, was constructed by using restriction enzymes (HindIII and NarI) to remove a truncated form of the gD gene from a plasmid containing the entire gD coding sequence. This truncated gD gene was inserted into pCA3, which contains the human cytomegalovirus promoter, the simian virus 40 poly(A) site, and flanking Ad E1 sequences (28). Sequences encoding the full-length, membrane-bound form of gD were also inserted into a similar Ad shuttle plasmid, pCA4 (28). The gD-containing pCA3 or pCA4 shuttle plasmids were cotransfected into 293 cells along with pBHG10, which supplies the right end of the Ad5 genome (5). Recombinant Ad vectors, AdgD1(E1−) and AdgD1t(E1−), expressing membrane-anchored and soluble gD, respectively, were isolated (Fig. 1). These replication-defective Ad vectors, like other such viruses, produce very low levels of Ad proteins and little or no cytopathic effect (28).
FIG. 1.
Features of recombinant Ad vectors expressing gD1 or gD1t. Sequences encoding a full-length, membrane-anchored gD (gD1; residues 1 to 394; nucleotides 1 to 1182) or a truncated version of gD missing the transmembrane and cytosolic domains (gD1t; residues 1 to 312; nucleotides 1 to 936) were inserted into the E1 region of the Ad5 genome. The gD genes were coupled to the human cytomegalovirus (HCMV) immediate-early promoter in the right-to-left orientation, opposite to the direction of E1 transcription. The Ad vectors, AdgD1(E1−) and AdgD1t(E1−), are nonreplicating Ad propagated on 293 cells that supply E1 proteins.
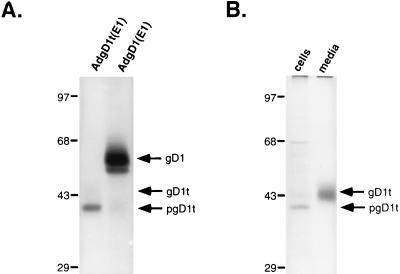
To test for expression of the two different forms of gD, human R970 cells were infected with either AdgD1(E1−) or AdgD1t(E1−) and labelled with [35S]cysteine and [35S]methionine for 2 h and gD proteins were immunoprecipitated. Two protein bands were precipitated by using anti-gD antibodies from cells infected with AdgD(E1−), apparently corresponding to the mature and immature forms of the membrane-bound form of gD (Fig. 2A). In contrast, a single band of gDt was observed in extracts of AdgD1t(E1−)-infected cells. This band appeared to be the immature form of the truncated protein because a slower-migrating form, presumably the mature form, was observed in the medium of AdgD1t(E1−)-infected cells (Fig. 2). Comparisons of the amount of gD1t which was secreted and that which remained cell associated in AdgD1t(E1−)-infected cells suggested that the majority of the gD1t (80% of the labelled protein) was secreted from the cell after a 2-h chase period (Fig. 2B). However, Western blot analysis indicated that a substantial fraction (∼30%) of gD1t remained cell associated (not shown). The steady-state levels of gD1 and gD1t in Ad-infected cells observed in Western blots were similar to the levels of gD1 found in cells infected with HSV-1 8 to 10 h after infection (not shown).
FIG. 2.
Expression of gD by recombinant Ad vectors. (A) Human R970 cells were infected with AdgD1(E1−) or AdgD1t(E1−) at 10 PFU/cell, and after 44 h the cells were labelled for 2 h with [35S]cysteine and [35S]methionine. Detergent extracts of the cells were made, and gD was immunoprecipitated from cellular extracts with a mixture of anti-gD MAbs DL6 and LP2. (B) Human R970 cells were infected as for panel A, and then the cells were labelled for 30 min with [35S]cysteine and [35S]methionine. The medium was removed, medium containing excess unlabelled cysteine and methionine was added, and the cells were incubated for an additional 2 h. The gD present in the cell culture supernatant or in detergent extracts of the cells was immunoprecipitated with a mixture of anti-gD MAbs DL6 and LP2, and the proteins were separated on SDS-polyacrylamide gels.
M6P content of soluble and membrane-bound forms of gD.
In previous experiments, we found that approximately 58% of the oligosaccharides associated with a soluble form of gD, produced in recombinant CHO cells and secreted into the cell culture supernatant, were modified with M6P residues (8). Since there are three oligosaccharides on each gD molecule, it was reasonable to believe that virtually every soluble gD molecule was modified with one or two M6P residues. By contrast, only 1.0% of the full-length gD produced in HSV-infected R970 cells was modified with M6P residues (8). Similar levels of phosphorylation of VZV glycoproteins (0.6 to 3% of M6P) have been reported (19). To compare the amount of M6P modification of soluble gD with that of full-length gD in the same cell type, we infected human R970 cells with AdgD1(E1−) or AdgD1t(E1−). The cells were labelled with [3H]mannose for 4 h beginning 40 h after infection, and then the label was chased for 3 h. gD was immunoprecipitated from detergent extracts of the cells and from the medium, and then the gD proteins were treated with endoglycosidase H to release high-mannose oligosaccharides. The liberated high-mannose oligosaccharides were separated from complex oligosaccharides (which remain on the protein) with Centricon-30 membranes. The high-mannose oligosaccharides were subjected to mild acid hydrolysis to remove GlcNAc residues, and uncharged oligosaccharides (without M6P) were separated from charged oligosaccharides (containing M6P) with quaternary aminoethyl-Sephadex (3, 12). Complex oligosaccharides were quantified by counting the labelled material that was retained by the Centricon-30 membrane.
Approximately 8.4% of the membrane-bound gD1 was modified with M6P residues, again based on the assumption that there are three N-linked oligosaccharides per gD molecule (Table 1). The fraction of soluble gD1t that was found in cells was more extensively modified with M6P residues (30.3%), whereas the fraction of soluble gD1t that was secreted from cells was modified less extensively (2.4%). These results demonstrate that both soluble and membrane-bound forms of gD can be modified with M6P and this can vary from 1.0% in HSV-infected cells to 30 or 100% for gD that is secreted. The lower content of M6P in gD from HSV-infected cells may be related to the effects of HSV on the phosphorylation machinery. The gD analyzed in previous experiments (8) was extracted relatively late in the infection for technical reasons, and there may be more M6P associated with gD produced early in the infection. As in our previous experiments, the soluble form of gD was a better substrate for the phosphotransferase (which modifies mannose residues) than was membrane-bound gD.
TABLE 1.
M6P content of gD molecules expressed by AdgD1 and AdgD1t
| Virus | Protein analyzed | Cell line | % Complexa | % HMa | % 1 M6Pb | % 2 M6Pb | % gDc |
|---|---|---|---|---|---|---|---|
| AdgD1 | gD1 | R970 | 95.4 | 4.6 | 1.9 | 0.9 | 8.4 |
| AdgD1t | gD1t | R970 | 50.3 | 49.7 | 5.5 | 4.6 | 30.3 |
| AdgD1t | gD1t (medium) | R970 | 98.5 | 1.5 | 0.8 | NDd | 2.4 |
The percentage of complex or high-mannose (HM) oligosaccharides was determined as described in Materials and Methods based on the assumption that HM oligosaccharides contain an average of 5.1 mannose residues/oligosaccharide and complex oligosaccharides contain three mannose residues/oligosaccharide.
1 M6P and 2 M6P, percentage of oligosaccharides bearing one or two M6P residues, respectively.
Value obtained by multiplying the total percentage of M6P-modified oligosaccharides by 3, the number of N-linked oligosaccharides per gD molecule.
ND, none detected.
The subcellular localization of gD coincides with that of the 275-kDa MPR.
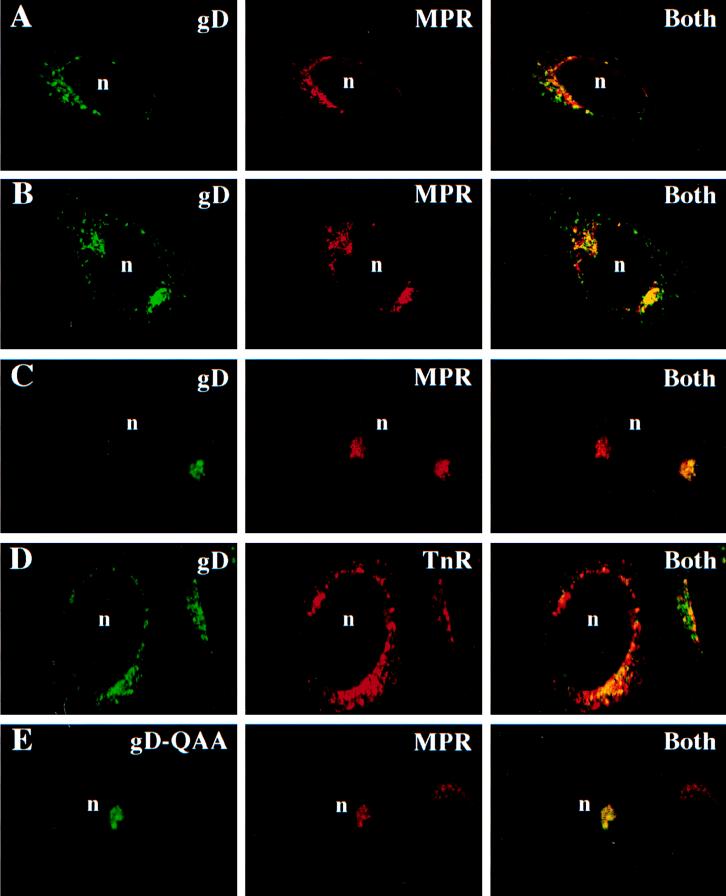
Since both full-length and soluble forms of gD were modified with M6P residues, it was reasonable to believe that a fraction of the gD in cells would bind to MPRs and be directed to an endosomal or lysosomal compartment. Immunofluorescence confocal microscopy was performed to determine whether intracellular gD colocalizes with the 275-kDa MPR. R970 cells were infected with either AdgD1(E1−) or AdgD1t(E1−), fixed and permeabilized, and then incubated with rabbit antibodies specific for the 275-kDa MPR and simultaneously with a mouse MAb directed to gD. The primary antibodies were detected with fluorescent secondary antibodies, goat anti-mouse FITC (green signal indicated gD) and goat anti-rabbit Texas red (red signal indicated the 275-kDa MPR). In AdgD1(E1−)-infected cells, the membrane-anchored form of gD was found on the cell surface and in cytosolic vesicles, primarily close to the nucleus (Fig. 3A). A substantial fraction of the cytoplasmic vesicles containing gD also contained the 275-kDa MPR, as can be seen by the yellow fluorescence derived from superimposed green and red fluorescence (Fig. 3A, right). Similarly, soluble gD1t was largely localized to cytoplasmic, perinuclear vesicles and a relatively large fraction of the glycoprotein was found in endosomes containing the 275-kDa MPR (Fig. 3B).
FIG. 3.
HSV gD accumulates in endosomes. R970 cells grown on glass coverslips were infected with AdgD1(E1−) (A) or AdgD1t(E1−) (B) at 100 PFU/cell for 44 h or with wild-type HSV-1 (C and D) or HSV (QAA) (E) at 1 PFU/cell for 8 h. The cells were fixed with paraformaldehyde and permeabilized with 0.2% Triton X-100. The following primary antibodies were applied to the cells: affinity-purified rabbit anti-275-kDa MPR antibodies and, simultaneously, anti-gD MAb DL6 (A, B, C, and E) and rabbit anti-gD polyclonal antibodies and a mouse anti-transferrin receptor (TnR) MAb (D). The cells were washed, and secondary antibodies, goat anti-rabbit Texas red (red signal) and goat anti-mouse FITC (green signal), were applied to the cells. In panel D the red signal produced by rabbit anti-gD antibodies was switched to a green signal and the green signal from the mouse anti-transferrin receptor MAb was switched to green for consistency. The cells were washed, and the coverslips were mounted on glass slides and viewed with a Zeiss confocal microscope. “Both” indicates images in which both signals (green for gD and red for MPR or TnR) were superimposed. n, cell nucleus.
These studies were extended to HSV-infected cells. Again, a large fraction of gD, often a majority of the protein, was found in cytoplasmic vacuoles that colocalized with the 275-kDa MPR (Fig. 3C). This pattern of gD colocalization with the 275-kDa MPR was maintained for 3 to 5 h in HSV- or AdgD(E1−)-infected cells treated with cycloheximide to block protein synthesis (data not shown). Thus, the colocalization of gD to the MPR-positive compartment was a relatively stable event, and gD was not passing through this compartment in a transient fashion. At later times after infection with HSV-1, especially when higher multiplicities of infection were used, there was less colocalization of gD with the 275-kDa MPR (not shown), perhaps related to observations that HSV infection leads to destruction of the Golgi apparatus (10).
While the bulk of the 275-kDa MPR is localized to late endosomes, the protein is also found in the TGN (25), and thus, it was possible that the colocalization of gD with the 275-kDa MPR was simply due to gD that was trafficking through the TGN. To further examine the intracellular localization of gD, HSV-infected cells were simultaneously stained with anti-transferrin receptor antibodies and anti-gD antibodies. The transferrin receptor is found on the cell surface and inside cells, almost exclusively in early endosomal compartments rather than in late endosomes or in the TGN (29, 49, 55, 71). Again, a substantial fraction of HSV gD colocalized with the transferrin receptor (Fig. 3D), confirming that gD is in both early and late endosomes in HSV-infected cells.
Although we found that gD was present in late and early endosomes, it was not clear whether this was related to M6P modification and the involvement of MPRs in targeting to endosomes. The HSV-1 mutant, QAA, expresses a mutant form of gD lacking the sites for addition of N-linked oligosaccharides (59), and without N-linked oligosaccharides, there is no possibility for the addition of M6P residues on gD. QAA gD does not bind to the purified 275-kDa MPR (6a). To test whether this mutant form of gD was localized to endosomes, cells were infected with HSV-1 (QAA) and the distributions of gD and the 275-kDa MPR were determined by immunofluorescence microscopy. QAA gD was found to have a distribution similar to that of wild-type gD (i.e., largely in cytoplasmic vesicles surrounding the nucleus and on the cell surface), and a large fraction of QAA gD, a majority of the protein in some cells, was colocalized with the 275-kDa MPR (Fig. 3E). Similar results were observed when the distribution of QAA gD was compared to that of the transferrin receptor (not shown). Therefore, gD, produced either in HSV-infected cells or in cells infected with Ad vectors, localized to endosomes and accumulation in endosomes did not require M6P modification, at least in the context of HSV-infected cells.
HSV gI and a nucleocapsid protein colocalize with the 275-kDa MPR.
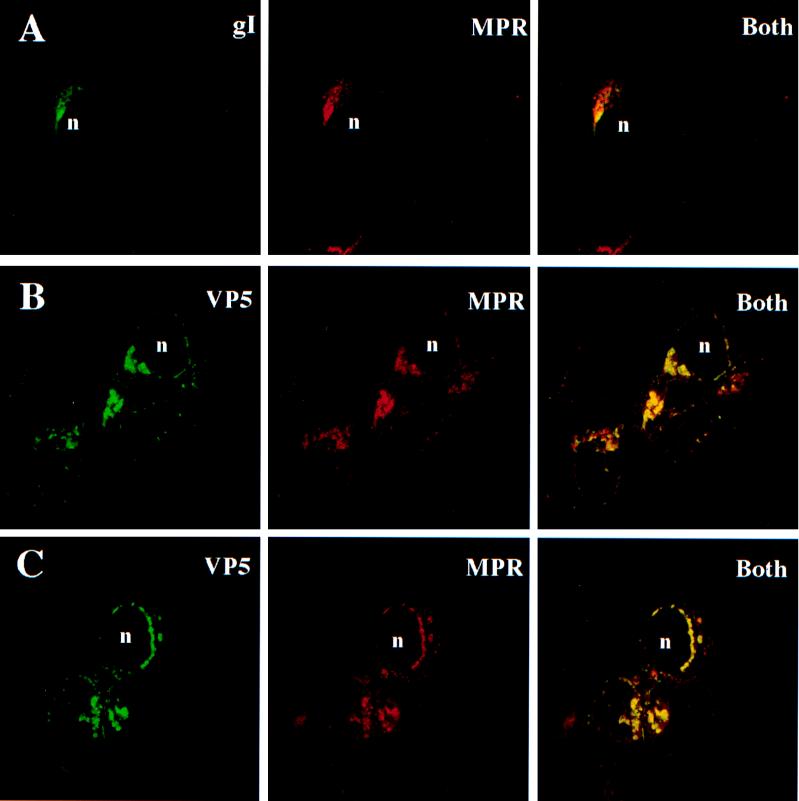
The studies were extended to another HSV glycoprotein, gI. Previously, we could not detect M6P associated with gI that was extracted from HSV-infected cells (8, 8a). HSV-infected cells were stained with an anti-gI MAb and, as with gD, a substantial fraction of gI colocalized with the 275-kDa MPR (Fig. 4A). There was a ring of gI associated with vesicles surrounding the nucleus, and many of these vesicles stained with antibodies specific for the 275-kDa MPR (Fig. 4A).
FIG. 4.
HSV gI and VP5 capsid protein colocalize with 275-kDa MPR. R970 cells were infected with HSV-1, and 12 to 16 h later, the cells were fixed and subsequently permeabilized with 0.2% Triton X-100. (A) Cells incubated simultaneously with affinity-purified rabbit anti-275-kDa MPR and anti-gI MAb 3104. (B and C) Cells incubated with rabbit anti-275-kDa MPR antibodies for 60 min, washed, and incubated with Texas red-conjugated goat anti-rabbit IgG. The cells were washed and subsequently incubated with rabbit anti-VP5 antibodies that had been directly conjugated with FITC. The cells were washed again and viewed with a confocal microscope. n, cell nucleus.
Since fractions of gD and other HSV glycoproteins are components of the virion envelope and the majority of virions often accumulate in cytoplasmic vesicles, we sought to determine whether virions were also present in endosomes. The major HSV nucleocapsid protein, VP5, is found in the cytoplasm and in the nucleus and is a component of enveloped virions that are in the process of virus egress (47, 65, 66). It is apparent from our studies, as well as those of others, that a relatively large fraction of the VP5 in the cytoplasm is distributed in an intensely staining, punctate pattern near the nuclear envelope as well as throughout the cytoplasm (Fig. 4B and C). Electron microscopy of HSV-infected R970 cells has shown numerous enveloped nucleocapsids within cytoplasmic vesicles, especially close to the nucleus, and only very few unenveloped capsids (32, 42). Unenveloped capsids in the cytoplasm tend to be more evenly distributed and are unlikely to produce the intense, punctate staining that might be obtained with cytosolic vesicles containing numerous enveloped particles. Consistent with this interpretation, the pattern of VP5 staining in the nucleus was diffuse, with no evidence of intense, punctate staining (Fig. 4B and C), yet it is well known that there are free VP5 and numerous nucleocapsids in the nucleus. Therefore, it is highly likely that the intense, punctate VP5 staining in the cytoplasm is associated with enveloped nucleocapsids that accumulate in membrane vesicles rather than nonenveloped nucleocapsids in the cytosol or free VP5.
Using confocal immunofluorescence microscopy, we examined the subcellular distribution of VP5 and the 275-kDa MPR. The majority of cytosolic vesicles that stained with anti-VP5 antibodies also stained with anti-275-kDa MPR antibodies (Fig. 4B and C). Anti-VP5 antibodies did not stain uninfected cells (not shown). Since the MPR is exclusively membrane associated, the colocalization of VP5 with the 275-kDa MPR is further evidence that the punctate, cytosolic VP5 is associated with enveloped, rather than unenveloped, capsids. Therefore, these results indicate that many of the enveloped virions that accumulate in the cytoplasm of HSV-infected cells are present in endosomes or endosomal compartments, either as part of the egress process or as a dead-end pathway.
Soluble gD1t and membrane-anchored gD1 are not transported to lysosomes.
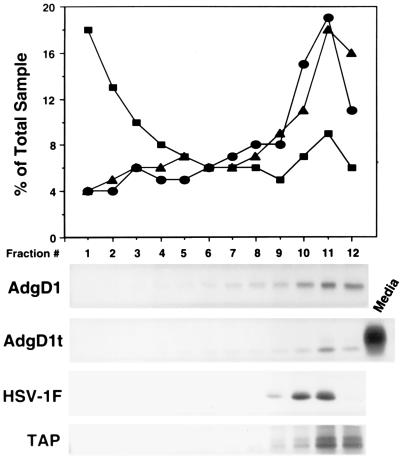
Since gD colocalizes with the 275-kDa MPR in endosomes, it was also of interest to determine whether gD was delivered to lysosomes. Though MPRs deliver lysosomal enzymes to lysosomes, the MPRs uncouple from the M6P-modified glycoproteins in late endosomes. Lysosomes are relatively dense subcellular organelles which can be effectively separated from other organelles, e.g., ER, Golgi apparatus, plasma membrane, and endosomes, by using Percoll density gradient centrifugation (24, 53). Cells infected with AdgD1(E1−), AdgD1t(E1−), or HSV-1 were labelled with [35S]cysteine and [35S]methionine for 30 min, and then the label was chased for 2 h. The cells were disrupted with a Dounce homogenizer, the cellular membranes were applied to 18% Percoll gradients, and the gradients were centrifuged. The gradient fractions were analyzed for organelle-specific markers or for the presence of radiolabelled gD that was immunoprecipitated by using gD-specific antibodies. Figure 5 shows that the lysosomal marker, β-hexosaminidase, was predominantly at the bottom of the gradient (fractions 1 to 3) while the Golgi marker, galactosyltransferase, was found at the top of the gradient (fractions 10 to 12). Plasma membranes, labelled by lactoperoxidase-catalyzed iodination with 125I, were also in the less dense fractions at the top of the gradient (Fig. 5). A specific marker for the ER, TAP (transporter associated with antigen presentation), was immunoprecipitated from cell extracts and was similarly found at the top of the gradients. Membrane-anchored gD expressed with AdgD1(E1−) or after HSV infection was found at the top of the gradient. Similarly, the soluble form of gD was present in less dense fractions of cells. There was no evidence that HSV-1 infection of these cells caused redistribution of the lysosomal marker at early and intermediate times after infection (1 to 9 h postinfection). Therefore, soluble and membrane-anchored forms of gD do not accumulate to any extent in lysosomes.
FIG. 5.
Subcellular fractionation of AdgD1t(E1−)-, AdgD1(E1−)-, and HSV-1 (F)-infected cells. Human MRC-5 fibroblasts were infected with AdgD1(E1−) or AdgD1t(E1−) at 20 PFU/cell for approximately 40 h or with HSV-1 (F) for 8 h. Cells were labelled for 30 min with [35S]cysteine and [35S]methionine followed by incubation for 2 h in unlabelled medium or with Na 125I and lactoperoxidase or were left unlabelled (for enzyme assays). The cells were disrupted with a Dounce homogenizer, nuclei were removed by centrifugation, and cellular membranes were applied to an 18% Percoll gradient, which was centrifuged for 30 min. The gradients were fractionated from the bottom, and fractions from unlabelled cells were assayed for galactosyltransferase activity (circles) (Golgi marker) and β-hexosaminidase activity (squares) (lysosomal marker). Fractions from cells labelled with 125I (triangles) (plasma membrane marker) were analyzed by using a liquid scintillation counter. Fractions from cysteine- or methionine-labelled cells were immunoprecipitated with gD-specific MAbs LP2 and DL6 or with rabbit polyclonal serum specific for TAP (ER marker).
DISCUSSION
Soluble lysosomal enzymes are modified with M6P in the Golgi apparatus and are bound by MPRs and directed to the endocytic compartment via clathrin-coated vesicles (CCVs) (reviewed in references 31, 40, 45, 50, and 57). In a low-pH, late-endocytic compartment the MPRs dissociate from M6P-containing lysosomal enzymes, which are delivered to lysosomes while the MPRs are recycled to the TGN. MPRs can also traffic to the plasma membrane, where M6P-containing lysosomal enzymes can be captured in coated pits, endocytosed, and delivered to lysosomes. In addition, there are M6P-independent lysosomal targeting mechanisms. For example, Lamp1 and LAP, membrane-bound lysosomal proteins, are delivered specifically to lysosomes without modification by M6P. Signals in the cytoplasmic domains of these membrane proteins, including tyrosine-based and dileucine, motifs direct incorporation into CCVs, either at the cell surface or in the TGN, so that the proteins are transported to endosomes and, ultimately, to lysosomes (31, 43).
Both HSV gD and several VZV glycoproteins are modified with M6P (8, 19), suggesting that alphaherpesviruses utilize the endosomal-lysosomal targeting pathway for their replication. In a previous study, most or all of the soluble gD2 produced in transfected CHO cells was modified with M6P while only approximately 1% of the membrane-anchored gD1 produced in HSV-infected R970 cells was phosphorylated (8). In order to compare soluble and membrane-bound forms of gD in the same cells and to determine whether virus infection altered M6P modification, we expressed these proteins with nonreplicating Ad vectors and found that 8% of the membrane gD was modified with M6P while 31% of the secreted, soluble gD was phosphorylated. In the previous study, HSV-1-infected cells were labelled with [3H]mannose until relatively late in the infection, in order to produce sufficient quantities of labelled material for M6P analysis (8). gD produced earlier during the infection may be more extensively modified by M6P. HSV disruption of the Golgi apparatus or other organelles late in the infection (10) may contribute to reduced levels of phosphorylation. Again, in this study, the membrane-bound form of gD was modified less extensively than was the soluble secreted gD, probably related to a preference of the phosphotransferase for soluble proteins. For example, when cathepsin D, a soluble lysosomal enzyme, was fused to a membrane anchor, M6P modification was reduced 10-fold (40a).
Given what is known about the specificity of M6P addition to lysosomal enzymes, it appears highly unlikely that phosphorylation of mannose residues on HSV gD and those of VZV glycoproteins is accidental or serendipitous. The domains of soluble lysosomal enzymes that are recognized by the phosphotransferase are highly specific and are restricted to lysosomal enzymes (4). In an in vitro assay involving partially purified mannose phosphotransferase, soluble gD2 was phosphorylated as a high-affinity substrate similar to cathepsin D, an authentic lysosomal enzyme. Moreover, when M6P modification was inhibited (in pseudo-Hurler fibroblasts) or when MPR was blocked with a synthetic ligand, HSV cell-to-cell spread was inhibited (7), supporting the hypothesis that interactions between HSV glycoproteins and MPRs play a role in some aspect of intracellular transport or movement of virus across cell junctions. This observation led us to investigate whether viral glycoproteins and virions traffic through endosomal compartments.
Substantial fractions of both soluble and membrane-bound forms of gD, expressed with Ad vectors or in HSV-infected cells, were found in late endosomes containing the 275-kDa MPR and in early endosomes containing the transferrin receptor. On their own, these observations might suggest that MPRs direct gD to endosomal compartments. However, QAA gD and gI, which do not contain M6P, were also found in endosomes. This is perhaps not surprising given that there is a high degree of redundancy in these interacellular trafficking signals. Signals such as di-Leu or Tyr motifs may cause these glycoproteins to traffic to endosomes. HSV-1 and HSV-2 gDs do not contain cytoplasmic di-Leu or Tyr motifs similar to those found in lysosomal enzymes (31, 41, 68), although there are Tyr residues at the C termini of gD1 and gD2 that could potentially participate in targeting to endosomes. VZV glycoproteins, gE and gI, contain Tyr and di-Leu motifs that have been shown to direct the glycoproteins to endosomes (2, 48, 72), and it is likely, by extension, that HSV gE and gI also contain such motifs. Moreover, gD may have signals in the extracellular (lumenal) domain that mediate sorting to endosomes. Cathepsin D, without a cytoplasmic domain, can reach lysosomes, albeit less (45%) efficiently, in the absence of M6P (22). There are determinants for M6P-independent lysosomal sorting of cathepsin D that overlap the domain recognized by the phosphotransferase (22), and these may also be present in gD. Consistent with M6P-independent sorting signals in gD, only 8% of membrane-bound gD was modified with M6P in AdgD1-infected cells, yet a larger fraction (as much as 50%) of the protein colocalized with MPRs in endosomes.
The VP5 nucleocapsid protein was also found in endosomes, even a majority of that fraction of the protein found in the cytoplasm. Therefore, apparently, virions accumulate in endosomes. This observation might partially explain why glycoproteins not modified with M6P, e.g., gI and QAA gD, are present in endosomes, whether or not there are other trafficking signals. At present, our studies do not allow us to discern whether virions accumulate in endosomes by the action of MPRs. Although only a relatively small fraction of gD is phosphorylated, there are thousands of copies of gD per virion, and this may be sufficient to allow MPRs to target virions to endosomes from the Golgi apparatus or even from the cell surface. Thus, without M6P and MPRs, i.e., in the case of pseudo-Hurler cells (7), intracellular transport to the cell surface via endosomes might be inhibited.
Previously, it was suggested that VZV accumulates in low-pH vesicles, which may be prelysosomes or lysosomes, and that this may account for low levels of infectious VZV in some cultured cells (19, 21). In our studies we found no evidence for HSV glycoproteins in dense lysosomes and there was no evidence for disruption of lysosomes after HSV infection. Since HSV gD contains M6P, one might expect that the glycoproteins would be delivered to lysosomes; however, MPRs (integral membrane proteins) escape lysosomes, and presumably gD also has mechanisms to stay out of lysosomes. There was also evidence that gD that is part of the virion envelope was not found in lysosomes, and thus, virions apparently also escape delivery to lysosomes. Consistent with these observations, HSV grows to relatively high titers in these cultured cells.
The existing evidence suggests that there is active traffic of alphaherpesvirus glycoproteins and virions to endosomes. This transport appears to be important for some aspect of virus egress or cell-to-cell spread of HSV, but at present, it is not clear how endosomes figure in these processes. One possibility is that virions acquire a second envelope in the cytoplasm, derived from endosomal membranes. Previous electron microscopic studies of alphaherpesvirus maturation have suggested reenvelopment at Golgi apparatus-derived membranes (21, 39, 69), largely based on their morphology. Endosomal compartments contain tubules, budding intermediates, and vesicles resembling the Golgi apparatus. Therefore, if reenvelopment models of HSV egress are correct, it is possible that HSV acquires an envelope by budding into endosomes, and viral glycoproteins might be directed there to facilitate this process. A second possibility is that enveloped virions are directed into endosomes on their path from the Golgi apparatus to the cell surface. HSV particles are large structures which may not be handled well by the exocytic pathway, and thus, entry into endosomes may facilitate transport to the plasma membrane or even to specialized cell surface domains, such as cell junctions. In polarized epithelial cells and keratinocytes, important cells in the life cycle of HSV, traffic of proteins to the basolateral surface is directed by many of the same signals involved in transport to endosomes or CCVs (reviewed in reference 52). Thus, our observations that cell-to-cell spread of HSV was reduced in pseudo-Hurler cells or by bulky MPR ligands (7) may be related to accumulation of saccharides, proteins, or other debris in endosomes, inhibiting movement of virus particles to the cell surface or cell junctions. Thirdly, the accumulation of HSV glycoproteins and virions in endosomes may be due to endocytosis from the cell surface. HSV particles frequently accumulate on the cell surface, and these could be readily endocytosed, with or without MPRs that are exclusively in cell surface coated pits (31, 40). Recent studies by Olson and Grose have demonstrated that the VZV gE is rapidly endocytosed from the cell surface (48), and this may also be the case for gD. Efforts are under way to determine the origin of the endosomes containing HSV glycoproteins and virions.
ACKNOWLEDGMENTS
We thank John Rudy for excellent technical assistance. We are grateful to Roselyn Eisenberg and Gary Cohen for antisera and the QAA mutant. Jay Brown, Tony Minson, and Hidde Ploegh also kindly provided antisera.
This work was supported by grants from the National Cancer Institute of Canada (NCIC) and the Medical Research Council of Canada (MRC). C.R.B. and K.S.D. held research studentships from the NCIC and MRC, respectively. D.C.J. was a senior research scholar and F.L.G. was a Terry Fox research scholar of the NCIC during this work.
REFERENCES
- 1.Addison C, Rixon F J, Palfreyman J W, O’Hara M, Preston V G. Characterization of a herpes simplex virus mutant which has a temperature sensitive defect in penetration into cells and assembly of capsids. Virology. 1984;138:246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- 2.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 3.Banfield B W, Leduc Y, Esford L, Visalli R J, Brandt C R, Tufaro F. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology. 1995;208:531–539. doi: 10.1006/viro.1995.1184. [DOI] [PubMed] [Google Scholar]
- 4.Baranski T J, Koelsch G, Hartsuck J A, Kornfeld S. Mapping and molecular modeling of a recognition domain for lysosomal enzyme targeting. J Biol Chem. 1991;266:23365–23372. [PubMed] [Google Scholar]
- 5.Bett A J, Haddara W, Prevec L, Graham F L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Brunetti, C. R. Unpublished data.
- 7.Brunetti C R, Burke R L, Hoflack B, Ludwig T, Dingwell K S, Johnson D C. Role of mannose-6-phosphate receptors in herpes simplex virus entry into cells and cell-to-cell transmission. J Virol. 1995;69:3517–3528. doi: 10.1128/jvi.69.6.3517-3528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunetti C R, Burke R L, Kornfeld S, Gregory W, Masiarz F R, Dingwell K S, Johnson D C. Herpes simplex virus glycoprotein D acquires mannose 6-phosphate residues and binds to mannose 6-phosphate receptors. J Biol Chem. 1994;269:17067–17074. [PubMed] [Google Scholar]
- 8a.Brunetti, C. R., S. Kornfeld, and D. C. Johnson. Unpublished data.
- 9.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 into BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campadelli-Fiume G, Brandimarti R, Di Lazzaro C, Ward P L, Roizman B. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through Golgi following infection with herpes simplex virus 1. Proc Natl Acad Sci USA. 1993;90:2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campadelli-Fiume G, Farabegoli F, Di Gaeta S, Roizman B. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J Virol. 1991;65:1589–1595. doi: 10.1128/jvi.65.3.1589-1595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantor A B, Kornfeld S. Phosphorylation of Asn-linked oligosaccharides located at novel sites on the lysosomal enzyme cathepsin D. J Biol Chem. 1992;267:23357–23363. [PubMed] [Google Scholar]
- 13.Cohen G H, Ponce de Leon M, Diggelmann H, Lawrence W C, Vernon S K, Eisenberg R J. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980;34:521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook M L, Stevens J G. Replication of varicella-zoster virus in cell cultures. An ultrastructural study. J Ultrastruct Res. 1970;32:334–350. doi: 10.1016/s0022-5320(70)80014-4. [DOI] [PubMed] [Google Scholar]
- 15.Darlington R W, Moss L H. The envelope of herpesvirus. Prog Med Virol. 1969;11:16–45. [PubMed] [Google Scholar]
- 16.Dick J W, Rosenthal K S. A block in glycoprotein processing correlates with small plaque morphology and virion targeting to cell-cell junctions for an oral and an anal strain of herpes simplex virus type-1. Arch Virol. 1995;140:2163–2181. doi: 10.1007/BF01323238. [DOI] [PubMed] [Google Scholar]
- 16a.Dingwell, K. S. Unpublished data.
- 17.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller A O, Lee W C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabel C, Dubey L, Steinberg S, Sherman D, Gershon M, Gershon A. Varicella-zoster virus glycoproteins are phosphorylated during posttranslational maturation. J Virol. 1989;63:4264–4276. doi: 10.1128/jvi.63.10.4264-4276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershon A, Cosio L, Brunnell P A. Observations on the growth of varicella-zoster virus in human diploid cells. J Gen Virol. 1973;18:21–31. doi: 10.1099/0022-1317-18-1-21. [DOI] [PubMed] [Google Scholar]
- 21.Gershon A A, Sherman D L, Shu Z, Gabel C A, Ambron R T, Gershon M C. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glickman J N, Kornfeld S. Mannose-6-phosphate-independent targeting of lysosomal enzymes in I-cell disease B lymphoblasts. J Cell Biol. 1993;123:99–108. doi: 10.1083/jcb.123.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham F L, Smiley J, Russel W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 24.Green S A, Zimmer K-P, Griffiths G, Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987;10:1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 26.Gruenberg J, Maxfield F R. Membrane transport in the endocytic pathway. Curr Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 27.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D C. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 28.Hitt M, Bett A J, Addison C L, Prevec L, Graham F L. Techniques for human adenovirus vector construction and characterization. In: Adolph K W, editor. Methods in molecular genetics. Vol. 78. Orlando, Fla: Academic Press; 1995. pp. 13–30. [Google Scholar]
- 29.Hopkins C R, Trowbridge I S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang T, Campadelli-Fiume G. Anti-idiotypic antibodies mimicking glycoprotein D of herpes simplex virus identify a cellular protein required for virus spread from cell to cell and virus-induced polykaryocytosis. Proc Natl Acad Sci USA. 1996;93:1836–1840. doi: 10.1073/pnas.93.5.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunziker W, Geuze H J. Intracellular trafficking of lysosomal membrane proteins. Bioessays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- 32.Hutchinson L, Johnson D C. Herpes simplex virus glycoprotein K promotes egress of virus particles. J Virol. 1995;69:5401–5413. doi: 10.1128/jvi.69.9.5401-5413.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isola V J, Eisenberg R J, Siebert G R, Heilman C J, Wilcox W C, Cohen G H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989;63:2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson D C, Burke R L, Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990;64:2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson D C, Frame M C, Ligas M W, Cross A M, Stow N D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson D C, Ligas M W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson D C, Spear P G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982;43:1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson R M, Spear P G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones F, Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988;62:2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kornfeld S. Structure and function of the mannose-6-phosphate/insulin-like growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 40a.Kornfeld, S. Personal communication.
- 41.Laskey L A, Dowbenko D J. DNA sequence analysis of the type-common glycoprotein D genes of herpes simplex virus types 1 and 2. DNA. 1984;3:23–29. doi: 10.1089/dna.1.1984.3.23. [DOI] [PubMed] [Google Scholar]
- 42.Ligas M W, Johnson D C. A herpes simplex virus mutant in which the glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marks S M, Ohno H, Kirchhausen T, Bonifacino J S. Protein sorting by tyrosine-based signals, adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery R I, Warner M S, Lum B, Spear P G. Herpes simplex virus 1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 45.Munier-Lehman H, Mauxion F, Hoflack B. Function of the two mannose-6-phosphate receptors in lysosomal enzyme transport. Biochem Soc Trans. 1996;24:133–136. doi: 10.1042/bst0240133. [DOI] [PubMed] [Google Scholar]
- 46.Neufeld E F. Lysosomal storage diseases. Annu Rev Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- 47.Newcomb W W, Trus B L, Booy F P, Stevens A C, Wall J S, Brown J C. Structure of the herpes simplex virus capsid: molecular composition of the pentons and triplexes. J Mol Biol. 1993;232:499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 48.Olson J K, Grose C. Endocytosis and recycling of varicella zoster virus Fc receptor glycoprotein E: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peranen J, Laakkonen P, Hyvonen M, Kaariainen L. The alphavirus replicase protein nsP1 is membrane-associated and has affinity to endocytic organelles. Virology. 1995;208:610–620. doi: 10.1006/viro.1995.1192. [DOI] [PubMed] [Google Scholar]
- 50.Peters C, Von Figura K. Biogenesis of lysosomal membranes. FEBS Lett. 1994;346:108–114. doi: 10.1016/0014-5793(94)00499-4. [DOI] [PubMed] [Google Scholar]
- 51.Rhim J S, Cho H Y, Huebner R J. Non-producer cells induced by murine sarcoma cells. Int J Cancer. 1975;15:23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- 52.Robinson W, et al. Coats and vesicle budding. Trends Cell Biol. 1997;7:99–102. doi: 10.1016/S0962-8924(96)10048-9. [DOI] [PubMed] [Google Scholar]
- 53.Rohrer J, Schweizer A, Johnson K F, Kornfeld S. A determinant in the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor prevents trafficking to lysosomes. J Cell Biol. 1995;130:1297–1306. doi: 10.1083/jcb.130.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roizman B, Borman G S, Kamali-Rousta M. Macromolecular synthesis in cells infected with herpes simplex virus. Nature. 1965;206:1374–1375. doi: 10.1038/2061374a0. [DOI] [PubMed] [Google Scholar]
- 55.Schmid S L, Fuchs R, Male P, Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988;52:73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz J, Roizman B. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969;38:42–49. doi: 10.1016/0042-6822(69)90126-3. [DOI] [PubMed] [Google Scholar]
- 57.Schweitzer A, Kornfeld S, Rohrer J. Cysteine-34 of the cytoplasmic tail of the cation-dependent mannose-6-phosphate receptor is reversibly palmitoylated and required for normal trafficking and lysosomal enzyme sorting. J Cell Biol. 1996;132:577–584. doi: 10.1083/jcb.132.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shieh M T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sodora D L, Cohen G H, Eisenberg R J. Influence of asparagine-linked oligosaccharides on antigenicity, processing, and cell surface expression of herpes simplex virus type 1 glycoprotein D. J Virol. 1989;63:5184–5193. doi: 10.1128/jvi.63.12.5184-5193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sodora D L, Eisenberg R J, Cohen G H. Characterization of a recombinant herpes simplex virus which expresses a glycoprotein D lacking asparagine-linked oligosaccharides. J Virol. 1991;65:4432–4441. doi: 10.1128/jvi.65.8.4432-4441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spear P G. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press; 1993. pp. 201–232. [Google Scholar]
- 62.Steinhart W L, Nicolet C M, Howland J L. Incorporation of [32P]-phosphate into membrane phospholipids during infection of cultured human fibroblasts by herpes simplex virus type 1. Intervirology. 1981;16:80–85. doi: 10.1159/000149251. [DOI] [PubMed] [Google Scholar]
- 63.Summers W P, Wagner M, Summers W C. Possible peptide chain termination mutants in thymidine kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci USA. 1975;72:4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sydiskis R J, Roizman B. The disaggregation of host polyribosomes in productive and abortive infection with herpes simplex virus. Virology. 1966;32:678–686. doi: 10.1016/0042-6822(67)90043-8. [DOI] [PubMed] [Google Scholar]
- 65.Thomsen D R, Newcomb W W, Brown J C, Homa F L. Assembly of the herpes simplex virus capsid: requirement for the carboxyl-terminal twenty-five amino acids of the proteins encoded by the UL26 and UL26.5 genes. J Virol. 1995;69:3690–3703. doi: 10.1128/jvi.69.6.3690-3703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trus B L, Newcomb W W, Booy F P, Brown J C, Stevens A C. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simplex virus capsid. Proc Natl Acad Sci USA. 1992;89:11508–11512. doi: 10.1073/pnas.89.23.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Genderen I L, Bradimarti R, Torrisi M R, Campadelli G, van Meer G. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology. 1994;200:831–832. doi: 10.1006/viro.1994.1252. [DOI] [PubMed] [Google Scholar]
- 68.Watson R, Weis J, Salstrom J, Enquist L. Herpes simplex virus type 1 glycoprotein D gene: nucleotide sequence and expression in E. coli. Science. 1982;218:381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- 69.Whealy M E, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamashiro D J, Tycko B, Fluss S R, Maxfield F R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]