Abstract
Objective
This study examined whether bronchoscopy leads to clinicoradiological improvement in cystic fibrosis (CF) and the predictive factors. The study also investigated whether pulmonary atelectasis is a poor prognostic factor in CF.
Methods
This multicenter, case–control, observational, retrospective study included two groups of patients with CF: a case group (patients with persistent atelectasis who were followed-up at least for 2 years) and a control group (patients without atelectasis matched 1:1 by sex and age [±3 years]). We recorded demographic data, lung function test results, pulmonary complications, comorbidities, treatments (including bronchoscopies, surgery and transplantation), and deaths.
Results
Each group included 55 patients (case group: 20 men, mean age 25.4 ± 10.4 years; control group: 20 men, mean age 26.1 ± 11.4 years). Bronchoscopy did not lead to clinicoradiological improvement. Allergic bronchopulmonary aspergillosis (ABPA) was more frequent in the case group. Patients in the case group more frequently used inhaled steroids, their pre-atelectasis lung function was statistically worse, and they had more exacerbations during follow-up.
Conclusion
Moderate-to-severe pulmonary disease and ABPA can favor atelectasis. Pulmonary atelectasis can be a poor prognostic factor in CF because it increases exacerbations. Despite our results, we recommend enhancing treatment, including bronchoscopy, to prevent persistent atelectasis.
Keywords: Cystic fibrosis, atelectasis, prognosis, risk factor, exacerbation, bronchoscopy, allergic bronchopulmonary aspergillosis
Introduction
Cystic fibrosis (CF) is a rare hereditary disease with a chronic and progressive course. Respiratory impairment has an incidence of 95% in adulthood, although the severity of its presentation varies, and it constitutes the primary cause of morbidity and mortality in patients with CF. 1
The prognosis for CF has evolved favorably in recent years because of neonatal screening, the emergence of new therapeutic tools, early treatment of respiratory infections, the maintenance of adequate nutrition, and multidisciplinary care, achieving a median survival exceeding 40 years. 2
Among the respiratory complications of CF, atelectasis (lung collapse) is associated with the fewest medical publications, with no consensus on its etiology, prognosis, or treatment. Although a few studies examined the impact of pulmonary atelectasis on the prognosis of CF, the number of included patients was small.3–6 Given that CF occurs with other pulmonary complications associated with poor quality of life, morbidity, and prognosis deterioration,7–10 it is important to determine whether pulmonary atelectasis is a poor prognostic factor in CF. The published studies on pulmonary atelectasis in CF identified no clear factors that could predict the onset of atelectasis and its effect on CF.
Various risk factors for developing atelectasis have been postulated, such as allergic bronchopulmonary aspergillosis (ABPA), hyperglycemia, and hemoptysis, given that these factors promote an increase in the viscosity of bronchial secretions. 4 A history of poor lung function, chronic bronchial infection, and a lack of adherence to the mucociliary clearance technique have also been linked to the onset of atelectasis in CF. 5 A study by our group concluded that atelectasis could negatively affect survival in CF, being associated with worsening lung function, increased lung transplantation rates, and ultimately death. 6
The absence of radiological improvement can lead to decreased lung function and more exacerbations, consequently entailing a poor prognosis for patients with CF. 11
Little is known about the mechanisms by which pulmonary atelectasis affects CF. Our study’s objectives were therefore to analyze the usefulness of bronchoscopy as a therapeutic method for achieving clinicoradiological improvement of pulmonary atelectasis at the end of the study period, determine the clinical (e.g., pulmonary complications, comorbidities, respiratory function) and genetic factors that could predict the onset of atelectasis in CF, and determine whether pulmonary atelectasis is a poor prognostic factor in CF (as measured by exacerbations, lung surgery, lung transplantation, and death).
Patients and methods
This was a multicenter, case–control, observational, retrospective study of patients diagnosed with CF according to the criteria of the European Cystic Fibrosis Society, 12 The patients were treated in 1 of 14 hospitals in Spain from January 2015 to December 2019. The study included two patient groups. First, the case group consisted of children and adults with a history of persistent or long-term atelectasis that did not improve with standard treatment 13 who underwent follow-up for at least 2 years after the onset of this respiratory complication (all patients with atelectasis from each unit were included regardless of age). Persistent atelectasis was defined as a complete or partial collapse of the lungs that might affect gas exchange. 14 Meanwhile, the control group included patients with CF but no prior history of atelectasis who were recruited from the same hospital as the included patients. The patients (with or without atelectasis) were matched 1:1 by sex and age (±3 years). The reporting of this study conforms to STROBE guidelines. 15
The study excluded patients with CF who underwent transplantation before the onset of atelectasis. Demographic data were recorded, including genetics, CF complications (ABPA, hemoptysis, and pneumothorax), comorbidities (CF-related diabetes and exocrine pancreatic insufficiency), and the results of lung function tests at the onset of atelectasis. The number of CF exacerbations by the end of the study period and prognoses that involved lung surgery, lung transplantation, or death were also recorded. Lastly, we recorded the presence of chronic bronchial infection 2 and therapies for treating atelectasis, including bronchoscopy (1–6 months after the episode), and analyzed whether bronchoscopy was associated with clinicoradiological improvement. Clinical improvement was defined as amelioration or recovery from dyspnea, cough, rhonchi, and/or expectoration. Radiological improvement was considered if the images were reduced or disappeared, according to the radiologist’s opinion.13,16 All patients were treated in an outpatient clinic.
Radiological techniques
The study employed chest X-rays or chest computed tomography to diagnosis atelectasis.13,16
Lung function tests
Spirometry was performed according to the European Respiratory Society/American Respiratory Society guidelines.17–19 The predicted values (% pred.) and z-scores were calculated for forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC for each patient.
Regarding lung function indices, FVC pre-atelectasis, FEV1 pre-atelectasis, and FEV1/FVC pre-atelectasis (% pred. and z-scores) were recorded in the case group. Spirometry was performed before the first episode of atelectasis (the period should be shorter than 1 month). Meanwhile, FVC, FEV1, and FEV1/FVC (% pred. and z-scores) were recorded in the control group with the timing coinciding with the pre-atelectasis spirometry date in the case group.
Both spirometry sessions were completed during a stable period (with no complications for at least 28 days).
Ethics
The Research Ethics Committee of La Paz University Hospital approved this study in October 2015 (code PI-2130). The authors of this study signed the Researcher Commitment and Confidentiality Statement. The requirement for informed consent was waived because of the retrospective nature of the study.
Statistical analysis
Quantitative variables were expressed as the mean and standard deviation or median [interquartile range]. For categorical variables, we recorded frequencies and proportions. Categorical comparisons were performed using the chi-square test. To compare continuous data, we employed paired and unpaired Student’s t-tests for parametric analyses and the Mann–Whitney U test for non-parametric analyses. To determine data normality, the Kolmogorov–Smirnov test was employed. We set statistical significance at P < 0.05. The data were analyzed using SAS v. 9.3 (SAS Institute, Cary, NC, USA).
Results
Descriptive analysis
Both study groups included 55 patients. The case group consisted of 20 men and 35 women with a mean age of 25.4 ± 10.4 years and a mean age at atelectasis onset of 20.6 ± 11.3 years. The control group consisted of 20 men and 35 women with a mean age of 26.1 ± 11.4 years. The predominant genetic abnormalities in both groups were homozygous and heterozygous F508del (p.Phe508del) mutations (Table 1). All patients with atelectasis reported symptoms (e.g., dyspnea, cough, change of bronchial secretions).
Table 1.
Characteristics of patients with and without atelectasis.
| Atelectasis (n = 55) | Non-atelectasis (n = 55) | P | |
|---|---|---|---|
| Male | 20 (36.4%) | 20 (36.4%) | |
| Age at inclusion, years | 25.4 ± 10.4 | 26.1 ± 11.4 | 0.726 |
| Body mass index, kg/m2* | 19.8 ± 3.9 | 20.4 ± 3.7 | 0.582 |
| Genotype (%) | 0.196 | ||
| F508 homozygous | 12 (21.8) | 20 (36.4) | |
| F508del heterozygous | 33 (60.5) | 29 (52.7) | |
| Other¬ | 10 (18.2) | 6 (10.9) | |
| Previous or concomitant cystic fibrosis comorbidities and respiratory complications | |||
| Exocrine pancreatic insufficiency | 38 (69.1) | 43 (73.8) | 0.387 |
| Cystic fibrosis-related diabetes | 15 (27.3) | 13 (23.6) | 0.827 |
| Hemoptysis | 6 (10.9) | 4 (7.3) | 0.742 |
| Pneumothorax | 4 (7.3) | 0 | 0.116 |
| Allergic bronchopulmonary aspergillosis | 13 (23.6) | 1 (1.8) | 0.001 |
| Localization of atelectasis and imaging techniques | |||
| Chest X-rays for diagnosis and resolution | 5 (9.1%) | ||
| CT at diagnosis, n (%) | 43 (78.2%) | ||
| CT at resolution, n (%) | 2 (3.6%) | ||
| CT at diagnosis and resolution | 5 (9.1%) | ||
| Type of atelectasis | |||
| Lobar, n (%) | 32 (58.2%) | ||
| Segmental, n (%) | 13 (23.6%) | ||
| Subsegmental, n (%) | 3 (5.5%) | ||
| Laminar, n (%) | 1 (1.8%) | ||
| Bilobar, n (%) | 6 (10.9%) | ||
| Trilobar, n (%) | 3 (5.5%) | ||
CT, computed tomography.
Data are presented as the mean ± standard deviation or n (%).
Comparisons between groups were made using the unpaired Student’s t-test, Mann–Whitney U test, or chi-square test.
*Body mass index was calculated as weight in kilograms divided by height in meters squared.
¬Including G540X/2869INSG, Q890X/Q890X, 711 + 1 G>TI507del, 2789 + 5 G>A/H1085R, 712-1 G>T/R347H, p.leu365Pro/p.Arg1066Cys, 110delC/J148T.
G542X/711 + 1G>T, G542X/V232D, R1162X/3272-26A>G, R1162X/3272-26A>G, I507delR553X, Q890X/Q980X, N1303K/712 + 1G>T, G673X/1341G, G542X/V323D and G542X/1078delT.
Utility of bronchoscopy as a therapeutic method
To treat atelectasis, bronchoscopy (with or without mucolytic substances [saline and/or recombinant human DNase injection]) was performed in 22 (40.7%) patients. Eleven patients noted clinical amelioration of atelectasis, and eight (36.4%) experienced radiological recovery according to the radiologist’s opinion (Table 2).
Table 2.
Impact of atelectasis treatment on symptoms and radiological findings.
| Impact on symptoms |
P | Impact on radiological findings |
P | |||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Bronchoscopy with/without any substance injected | 11 (50%) | 11 (50%) | 0.821 | 8 (36.4%) | 14 (63.6%) | 0.909 |
| Bronchoscopy and rhDNase injection | 4 (44.4%) | 5 (55.6%) | 0.99 | 4 (44.4%) | 5 (55.6%) | 0.99 |
| Bronchoscopy and saline injection | 6 (46.2%) | 7 (53.8%) | 0.869 | 5 (38.5%) | 8 (61.5%) | 0.848 |
rhDNase, recombinant human DNase.
Data are presented as n (%).
Comparisons between the groups were made using the chi-square test.
Although not a study objective, we analyzed the recommended treatments in both groups, verifying at the end of the study that patients with atelectasis (case group) received significantly more inhaled corticosteroids than control subjects (42% vs. 28%, P = 0.01; Table 3).
Table 3.
Treatment of atelectasis in patients with and without atelectasis
| Atelectasis (n = 55) | Non-atelectasis (n = 55) | P | |
|---|---|---|---|
| Physiotherapy (%) | 53 (96.4%) | 49 (89.1%) | 0.270 |
| Inhaled therapy | |||
| SABA (%) | 32 (60.4%) | 35 (63.6%) | 0.843 |
| LABA (%) | 32 (60.4%) | 25 (45.5%) | 0.129 |
| LAMA (%) | 10 (18.9%) | 9 (16.4%) | 0.804 |
| IC (%) | 42 (76.4%) | 28 (50.9%) | 0.010 |
| rhDNase, (%) | 30 (54.5%) | 21 (38.2%) | 0.126 |
| Hypertonic saline (%) | 40 (72.7%) | 31 (56.4%) | 0.110 |
| Inhaled antibiotics (%) | 38 (69.1%) | 35 (63.6%) | 0.687 |
| Colistin, (%) | 26 (47.3%) | 21 (38.2%) | 0.441 |
| Tobramycin, (%) | 9 (16.4%) | 11 (0.2%) | 0.805 |
| Aztreonam, (%) | 11 (0.2%) | 6 (10.9%) | 0.291 |
SABA, short-acting β2-agonist; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; IC, inhaled corticosteroids; rhDNase, recombinant human DNase.
Data are presented as n (%).
Comparisons between the groups were made using the chi-square test.
Clinical factors that could predict the future onset of pulmonary atelectasis
Lung function tests
FVC, FEV1, and FEV1/FVC pre-atelectasis (% pred. and z-score) were significantly lower in the case group than in the control group (all P < 0.05, Table 4).
Table 4.
Lung function test results in patients with and without atelectasis.
| Atelectasis (n = 55) | Non-atelectasis (n = 55) | P | |
|---|---|---|---|
| FVC pre-atelectasis (% pred.) | 73.7 ± 22.9 | 93.5 ± 27.9 | <0.001 |
| FVC pre-atelectasis (z-score) | −2.6 ± 1.8 | −0.9 ± 1.7 | <0.001 |
| FEV1 pre-atelectasis (% pred.) | 58.7 ± 26.5 | 84 ± 29.6 | <0.001 |
| FEV1 pre-atelectasis (z-score) | −3.5 ± 1.9 | −1.7 ± 1.9 | <0.001 |
| FEV1/FVC pre-atelectasis (% pred.) | 68.2 ± 15.8 | 78.4 ± 13.6 | 0.001 |
| FEV1/FVC pre-atelectasis (z-score) | −2.3 ± 1.6 | −1.3 ± 1.5 | <0.001 |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Data are presented as the mean ± standard deviation.
Comparisons between groups were made using the unpaired Student’s t-test or Mann–Whitney U test.
Pre-atelectasis pulmonary function tests were performed before the first episode of atelectasis (the period should be shorter than 1 month).
Pulmonary complications/comorbidities/genetics
In terms of previous respiratory complications, ABPA was more frequent in the case group than in the control group (23.6% vs. 1.8%, P = 0.001). There were no intergroup differences in terms of comorbidities. Similarly, there were no genetic differences between the groups (Table 1).
Pulmonary atelectasis as a poor prognostic factor in CF
Exacerbations and chronic bronchial infection
The average number of exacerbations was 3 [1–5] in the case group in the year before the end of the study, versus 2 [1–3] in the control group (P = 0.008). Likewise, 47 (87.7%) patients were diagnosed with chronic bronchial infection at the end of the study. The chronic bronchial infections are detailed in Table 5.
Table 5.
Chronic bronchial infection and cystic fibrosis exacerbations in patients with and without atelectasis.
| Atelectasis (n = 55) | Non-atelectasis (n = 55) | P | |
|---|---|---|---|
| Chronic bronchial infection | 47 (85.5%) | 47 (85.5%) | 0.99 |
| Pseudomonas aeruginosa | 30 (54.5%) | 32 (58.2) | 0.252 |
| MSSA | 23 (41.8%) | 29 (52.7%) | 0.180 |
| MRSA | 4 (7.3%) | 8 (14.5%) | 0.776 |
| Nontuberculous mycobacteria | 3 (5.5%) | 3 (5.5%) | 0.99 |
| Other § | 19 (34.5%) | 20 (36.4%) | 0.841 |
| Number of exacerbations | 3 [1–5] | 2 [1–3] | 0.008 |
MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
Data are presented as the median [interquartile range] or n (%).
Comparisons between groups were made using the Mann–Whitney U test or chi-square test.
Including Aspergillus spp. (nine patients), Candida spp. (five patients), Achromobacter xylosoxidans (three patients), and Stenotrophomonas maltophilia (two patients) in the atelectasis group and Aspergillus spp. (nine patients) and Haemophilus influenza (five patients) in the non-atelectasis group.
Lung surgery, lung transplantation, and deaths
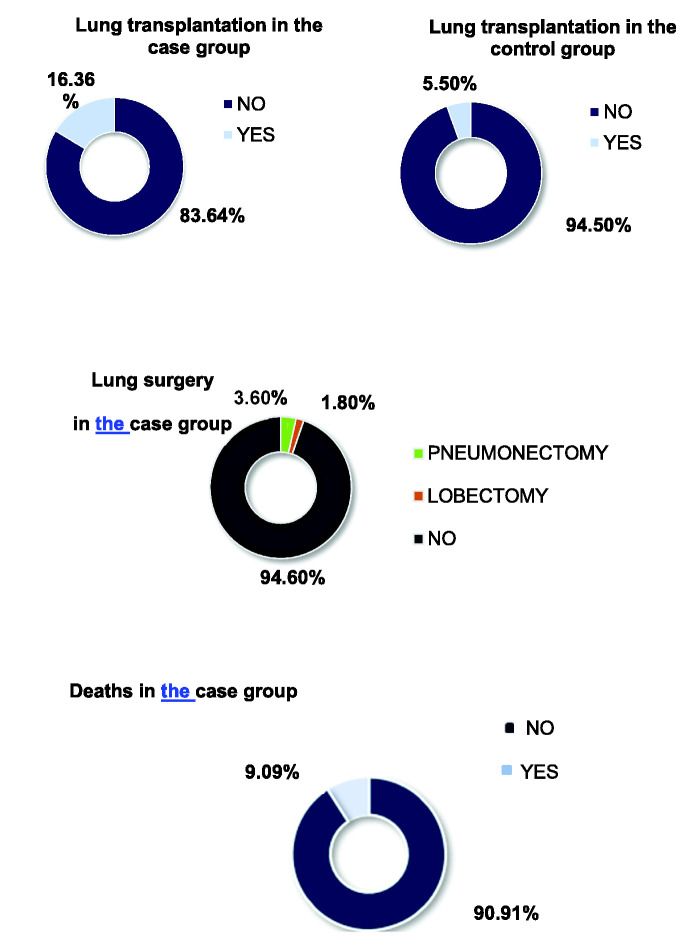
Concerning the prognosis, 21 (38.2%) patients in the case group underwent pulmonary surgery, required lung transplantation, or died. Pulmonary surgery was performed in three patients in the case group (one pneumonectomy and two lobectomies). In this group, four patients were on the lung transplant waiting list, and nine underwent transplantation. In the control group, only three patients underwent transplantation, and none was on the waiting list. Regarding deaths, five (9.1%) patients in the case group died, whereas no deaths were reported in the control group (P = 0.055). Deaths were secondary to complications after lung transplantation (two patients), severe exacerbation (one patient), renal failure (one patient), and pneumothorax (one patient; Figure 1). Although there were no statistically significant differences, the numbers of surgeries, transplantations, and deaths were higher in the case group.
Figure 1.
Rates of patients undergoing lung surgery and lung transplantation and the rate of death.
Discussion
The reported prevalence of atelectasis is 3.91% to 11%.3,20–22 There was no predominance in terms of sex for this complication in our study, but the results in the literature vary widely.4,20–22 The mean age exceeded 18 years in both groups. This predominance could be explained by the fact that the airways are more distorted because of chronic inflammation in adulthood, which could cause new-onset atelectasis or exacerbate existing atelectasis.23,24 A number of authors concluded that pulmonary atelectasis in CF is usually irreversible, causing a worsening of clinical, radiological, and functional findings.3,23,24
An important aspect in our study was the impact of treatment, including therapeutic bronchoscopy, on atelectasis. Bronchoscopy, with the concomitant use of mucolytic substances, is an essential tool for treating persistent atelectasis despite the optimization of medical treatment, with a reported success rate of approximately 80%, achieving partial or total re-expansion of the lungs.13,16,25 In our series, 50% of the patients experienced clinical improvement, and 36.4% experienced radiological improvement. Both rates were lower than those published in the literature (approximately 87%).26–29 There were no differences in our study in the rates of clinical or radiological improvement between patents who underwent bronchoscopy with or without mucolytic substances. The differences between our results and previously published findings could be because certain units did not recommend bronchoscopy as a therapeutic technique. Another explanation could be that this technique was recommended later. Nevertheless, our opinion, as well as that of other authors,13,16,29,30 is that bronchoscopy should be recommended earlier in an attempt to resolve lung collapse to prevent long-term atelectasis.
Various studies emphasized therapies that promote the elimination of secretions.4,13,20,28,31 In our case, there were no differences in the recommended treatments needed to achieve clinical or radiological improvement by the end of study. It is therefore unclear whether patients who do not use the clearance technique could have significantly worsened and not recovered from this complication. 29 Nevertheless, there were clear differences in the use of inhaled glucocorticoids in our study. However, their use in bronchiectasis is controversial. According to the latest Spanish registry, only 39.9% of patients receive inhaled glucocorticoids. 32 The comparison of the two groups in the last year of follow-up revealed considerable (76.4%) use of these drugs in the case group, which could be explained by a desire to reduce bronchial inflammation.
Among the factors that could predict the formation of atelectasis, we analyzed spirometry data, various pulmonary complications, comorbidities, and genetics. Forced spirometry remains the most useful test available for assessing disease progression. An annual loss of % pred. FEV1 exceeding 10% is the best single predictor of survival (FEV1 < 30%, 2-year mortality rate of 50%), and it helps select candidates for lung transplantation. 28 Our global study observed that the case group started with poorer FEV1, FVC, and FEV1/FVC (% pred. and z-score). Therefore, patients with CF could develop atelectasis if they have at least moderate pulmonary obstruction. It has been reported that moderate-to-severe respiratory functional impairment is associated with greater structural deterioration of the lung parenchyma, which can subsequently lead to atelectasis.
Among the non-infectious pulmonary complications, we observed an increase in the percentage of patients with hemoptysis compared with the registry data (2.7%), 32 which could be attributable to atelectasis causing sustained bronchial inflammation, which favors hemoptysis. However, the rate of this complication did not significantly differ between our study groups. Furthermore, 7.3% of patients in the case group had pneumothorax, which exceeded the rate of the national registry, 32 whereas there were no cases of pneumothorax in the control group. Atelectasis secondary to pneumothorax has been reported in patients without CF, although no clear relationship between the two diseases has been established. 14 The results of our study therefore do not indicate that pneumothorax causes future atelectasis. The most frequent complication in the case group was ABPA (23.6%), which occurred at a significantly rate higher than that reported in Spain (2.61%) and significantly higher than that of the control group. 32 According to other publications, ABPA can be associated with the subsequent onset of atelectasis secondary to increased sputum viscosity and destruction of the lung parenchyma.33–35 These findings could be explained by the fact that our study recruited all patients since the start of Spanish CF units, as many currently available treatments were not available at the start of this period and the likelihood of pulmonary complications was higher.
When considering certain genetic mutations related to more severe disease, there were no differences between the two study groups.36,37 In our genetic study, the predominant mutation in the patients who presented with atelectasis was the heterozygous F508del (p.Phe508del) mutation (54.5%), followed by the homozygous F508del mutation (21.8%), which were almost identical to the findings in the latest European registry. 32 No relationship has been observed between genotype and pulmonary complications, possibly because the genotype–phenotype correlation is weaker for lung disease than for other comorbidities, although there are no specific studies for lung atelectasis, excluding our study. 34
Likewise, we examined certain common comorbidities in this disease, such as pancreatic insufficiency and CF-related diabetes. Pancreatic insufficiency typically occurs in the “classic, typical, or severe” phenotype. 38 Although this condition was present in 69.1% of our patients, there was no correlation with atelectasis.4,21–23 A published article indicated that the formation of pulmonary atelectasis was related to acute pancreatitis, although not in patients with CF. 39 CF-related diabetes was detected in our series at a rate similar to that published by the European registry (approximately 23.3%). 32 Although hyperglycemia has been postulated as a possible risk factor for atelectasis because of increased viscosity of the bronchial mucus, 28 we did not find an association between hyperglycemia and atelectasis in our patients.
When factors related to a poor prognosis were studied, patients with atelectasis had more exacerbations over time than control patients. This difference could be attributable to several factors, such as the sustained accumulation of retained secretions in the areas of atelectasis and the chronic inflammatory environment, both of which favor the persistence of complications. 31 Exacerbations are associated with higher healthcare costs, an accelerated decline in lung function, an increased risk of mortality, and a reduction in quality of life.40,41 Chronic bronchial infection was present in more than 80% of patients with or without atelectasis, in line with the rate of infections. Pseudomonas aeruginosa was the most frequently found microorganism in both groups, and it is associated with a poor prognosis. 42 Nevertheless, there were no statistically significant differences between the groups, likely because of the small number of infected patients included in our study.
Lung resection is indicated for patients who present with localized and persistent bronchiectasis or atelectasis (with minimal damage to other areas of the lung parenchyma), persistent symptoms requiring hospitalization despite medical treatment optimization, hemoptysis refractory to embolization, and bronchopulmonary fistula. 43 Prior studies reported different results regarding improvements after lung surgery for atelectasis, ranging from improvements in 100% of the operated patients to a lack of functional or clinical improvement.43,44 More lung transplantations were performed in the case group than in the control group, albeit without significance. In one study on atelectasis in CF, no patient underwent transplantation, either because this variable was not analyzed or because of the scarcity of cases. Only one patient in the study by Flight et al. underwent transplantation; however, the patient died during surgery. 4 Similarly, five patients in the case group in this study died (all 18 years or older), versus none in the control group, with the difference approaching significance (P = 0.055). However, other causes could be involved. Considering that the death rate in the CF national registry is 0.56% and the lung transplant rate in the registry is 8%, 32 atelectasis could be considered an indicator of poor prognosis for patients diagnosed with CF. Specific treatment should be increased, including the recommendation of therapeutic bronchoscopy, to prevent this complication from becoming persistent.
The main limitation of this study was its retrospective and observational nature with the consequent possible variability of practitioners and patients. In addition, the sample size might have been insufficient to identify significant differences in some of the analyses. However, the study’s retrospective nature is also a strength because it allowed us to enroll a larger number of patients with multiple variables within a short period. Furthermore, this was a multicenter study in which 14 national monographic CF units participated, and we found no previous study comparing patients with CF according to the presence or absence of atelectasis. We did not study the patients’ oxygenation status or symptoms when atelectasis was diagnosed, and this could be examined in an upcoming extension of this study. We could more readily establish associations rather than correlations between certain variables and poor prognosis in patients with CF. Although there were no significant differences according to the use of bronchoscopy, future studies could help resolve this issue, as other authors have proposed.13,16,29,30
We conclude that patients with CF who have moderate-to-severe pulmonary obstruction or who have been previously diagnosed with ABPA could develop pulmonary atelectasis. Once pulmonary atelectasis has occurred, patients’ condition worsens, they experience more exacerbations, they are placed on transplantation waiting lists, they undergo transplantation or other operations, and they are at increased risk of death. More studies are needed to determine the role of bronchoscopy as a therapeutic method for treating atelectasis and the low adherence to therapies such as physiotherapy. Considering these findings, we can conclude that atelectasis as a pulmonary complication represents a poor prognostic factor in CF.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605241233520 for Should atelectasis be considered a pulmonary complication and indicator of poor prognosis in cystic fibrosis? by María Martínez Redondo, Carlos Carpio Segura, Ester Zamarrón De Lucas, Rodolfo Álvarez-Sala Walther and Concepción Prados Sánchez in Journal of International Medical Research
Author contributions: María Martínez Redondo and Concepción Prados Sánchez created the study, wrote the manuscript, and collected the data.
Ester Zamarrón de Lucas, Rodolfo Álvarez-Sala Walther, and Concepción Prados Sánchez reviewed the manuscript.
Carlos Carpio Segura performed the statistical analysis.
The Working Group of the Spanish Society for Cystic Fibrosis on Atelectasias included the following members: María Isabel Barrio Gómez de Agüero, Marta Ruiz de Valbuena, Cristina de Manuel Gómez, Silvia Castillo Corullón, Antonio Salcedo Posadas, Carlos Martín de Vicente, Rosa María Girón Moreno, María Teresa Martínez Martínez, Layla Diab Cáceres, María del Carmen Luna Paredes, Luiz Maíz Carro, Esther Quintana Gallego, Laura Carrasco Hernández, Amparo Solé Jover, Jordi Costa Colomer, Marta García Clemente, Marina Blanco Aparicio, David Iturbe Fernández, and Rosa Cordovilla Pérez. The members of this group contributed to the collection of the variables and review of the final manuscript.
The authors declare that there are no conflicts of interest.
Funding: This study received no funding.
Data availability statement
Data will be made available if the editor considers it necessary to understand the manuscript.
ORCID iD
Concepción Prados Sánchez https://orcid.org/0000-0002-5545-6529
References
- 1.Lerín M, Prados C, Martínez MT, et al. Cystic fibrosis in adult age. Rev Clin Esp (Barc) 2014; 214: 289–295. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson KM, Collaco JM. Cystic Fibrosis. Pediatr Rev 2021; 42: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huisman C, De Graaff CS, Boersma WG. Unilateral air bronchogram in a patient with cystic fibrosis. Chest 2002; 121: 1343–1344. [DOI] [PubMed] [Google Scholar]
- 4.Flight WG, Hildage J, Webb AK. Progressive unilateral lung collapse in cystic fibrosis–a therapeutic challenge. J R Soc Med 2012; 105: S44–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez Redondo M, Prados Sánchez C, Salcedo Posadas A, et al. Características de las atelectasias como complicación pulmonar en la fibrosis quística. Rev Patol Respir 2017; 20: 79–87. [Google Scholar]
- 6.Prados-Sánchez C, Martínez-Redondo M, Máiz-Carro L, et al. Atelectasis with torpid evolution in patients with cystic fibrosis. Ann Pulmonol 2018; 2: 27–32. [Google Scholar]
- 7.Mingora CM, Flume PA. Pulmonary complications in cystic fibrosis: Past, present, and future: Adult cystic fibrosis series. Chest 2021; 160: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lattanzi C, Messina G, Fainardi V, et al. Allergic bronchopulmonary aspergillosis in children with cystic fibrosis: An update on the newest diagnostic tools and therapeutic approaches. Pathogens 2020; 9: 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Máiz L, Baranda F, Coll R, SEPAR (Spanish Society of Pneumology and Thoracic Surgery) et al. Normativa del diagnóstico y el tratamiento de la afección respiratoria en la fibrosis quística. Arch Bronconeumol 2001; 37: 316–324. [DOI] [PubMed] [Google Scholar]
- 10.Heltshe SL, Goss CH, Thompson V, et al. Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. Thorax 2016; 71: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Judge EP, Dodd JD, Masterson JB, et al. Pulmonary abnormalities on high-resolution CT demonstrate more rapid decline than FEV 1 in adults with cystic fibrosis. Chest 2006; 130: 1424–1432. [DOI] [PubMed] [Google Scholar]
- 12.Boeck KD, Wilschanski M, Castellani C, Diagnostic Working Group et al. Cystic fibrosis: Terminology and diagnostic algorithms. Thorax 2006; 61: 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado Pecellín I, Moreno Ortega M, Carrasco Hernández L, et al. Persistent atelectasis in a patient with cystic fibrosis: Are antibiotics always needed? Arch Bronconeumol 2019; 55: 54–55. [DOI] [PubMed] [Google Scholar]
- 14.Torres Borrego J, López-Silvarrey Varela A, Rueda ES, et al. Atelectasias. Sindrome de lóbulo medio. Protoc Diagn Ter Pediatr 2017; 1: 103–113. [Google Scholar]
- 15.Von Elm E, Altman DG, Egger M, STROBE Initiative et al. The Strengthening the Reporting of Observational Study in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 16.Peroni DG, Boner AL. Atelectasis: mechanisms, diagnosis and management. Pediatr Respir Rev 2000; 1: 274–278. [DOI] [PubMed] [Google Scholar]
- 17.Brusasco V, Crapo R, Viegi G; American Thoracic Society, European Respiratory Society. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J 2005; 26: 1–2. [DOI] [PubMed] [Google Scholar]
- 18.Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V; ATS/ERS Task Force et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin AM, McGrath E, Barry R, et al. Treatment of lobar atelectasis with bronchoscopically administered recombinant human deoxyribonuclease in cystic fibrosis? Clin Respir J 2008; 2: 123–126. [DOI] [PubMed] [Google Scholar]
- 21.Stern RC, Boat TF, Orenstein DM, et al. Treatment and prognosis of lobar and segmental atelectasis in cystic fibrosis. Am Rev Respir Dis 1978; 118: 821–826. [DOI] [PubMed] [Google Scholar]
- 22.Di Sant’Agnese PA. Bronchial obstruction with lobar atelectasis and emphysema in cystic fibrosis of the pancreas. Pediatrics 1953; 12: 178–190. [PubMed] [Google Scholar]
- 23.Lowe DA, Vasquez R, Maniaci V. Foreign body aspiration in children. Clin Pediatr Emerg Med 2015; 16: 140–148. [Google Scholar]
- 24.Máiz L, Nieto R, Cantón R, et al. Fungi in bronchiectasis: A concise review. Int J Mol Sci 2018; 19: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tosco A, Poli P, Casale A, et al. The role of bronchoscopy in the management of children with cystic fibrosis. J Bronchology Interv Pulmonol 2023; 30: 258–267. [DOI] [PubMed] [Google Scholar]
- 26.Salamone I, Mondello B, Lucanto MC, et al. Bronchial tree-shaped mucous plug-in cystic fibrosis: imaging-guided management. Respirol Case Rep 2017; 5: e00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talamoni HL, Pisapia ND, Buendía JA. Broncoscopía flexible en niños con atelectasias persistentes. Arch Argent Pediatr 2015; 113: e106–108. [DOI] [PubMed] [Google Scholar]
- 28.García Novo MD, Salcedo Posadas A, Girón Moreno RM, et al. Tratado de fibrosis quística. Madrid: Justim S.L.; 2012. [Google Scholar]
- 29.Daccò V, Sciarrabba CS, Corti F, et al. A successful treatment of a lobar atelectasis in a patient with cystic fibrosis. Pediatr Pulmonol 2022; 57: 2868–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y, Wang Y, Gong K. Risk predictor model for long-term atelectasis in children with pneumonia. BMN Pulm Med 2023; 23: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagakumar P, Hilliard T. Recurrent lobar atelectasis in a child with cystic fibrosis. J R Soc Med 2012; 105: S50–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ECFSPR. Annual Report 2020. Orenti A, Zolin A, Jung A, et al. 2022.
- 33.Benkalfate N, Dirou S, Germaud P, et al. Total unilateral pulmonary collapse secondary to allergic bronchopulmonary aspergillosis: A case series of an unusual cause of complete atelectasis. BMC Pulm Med 2021; 21: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Redondo M, Prados Sánchez C, García-Rio F, Spanish Working Group Atelectasis in CF et al. The relation of CFTR-Genotype and associated comorbidities to development of pulmonary atelectasis in cystic fibrosis patients. Arch Bronconeumol 2022; 58: 666–668. [DOI] [PubMed] [Google Scholar]
- 35.Kumar R, Poongadan MN, Singh M. Allergic bronchopulmonary aspergillosis presenting as lobar or total lung collapse. Pneumonol Alergol Pol 2015; 83: 144–150. [DOI] [PubMed] [Google Scholar]
- 36.Egan ME. Genetics of cystic fibrosis: Clinical implications. Clin Chest Med 2016; 37: 9–16. [DOI] [PubMed] [Google Scholar]
- 37.Rueda-Nieto S, Mondejar-Lopez P, Mira-Escolano MP, et al. Analysis of the genotypic profile and its relationship with the clinical manifestations in people with cystic fibrosis: study from a rare disease registry. Orphanet J Rare Dis 2022; 17: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabel ME, Galante GJ, Freedman SD. Gastrointestinal and hepatobiliary disease in cystic fibrosis. Semin Respir Crit Care Med 2019; 40: 825–841. [DOI] [PubMed] [Google Scholar]
- 39.Dugernier T, Deby-Dupont G, Roeseler J, et al. Respiratory complications in severe acute pancreatitis. Acta Gastro-Enterol Belg 1991; 54: 225–232. [PubMed] [Google Scholar]
- 40.Szentpetery S, Flume PA. Optimizing outcomes of pulmonary exacerbations in cystic fibrosis. Curr Opin Pulm Med 2018; 24: 606–611. [DOI] [PubMed] [Google Scholar]
- 41.Solem CT, Vera-Llonch M, Liu S, et al. Impact of pulmonary exacerbations and lung function on generic health-related quality of life in patients with cystic fibrosis. Health Qual Life Outcomes 2016; 14: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aurora P, Duncan JA, Lum S, London Cystic Fibrosis Collaboration (LCFC) et al. Early Pseudomoas aeruginosa predicts poorer pulmonary function in preschool children with cystic fibrosis. J Cyst Fibros 2022; 21: 988–995. [DOI] [PubMed] [Google Scholar]
- 43.Villac Adde F, Vidal Campos S, De Oliveira Braga Teixeira RH, et al. Indications for lung resection surgery and lung transplant in South American children with cystic fibrosis. Paediatr Respir Rev 2018; 25: 37–42. [DOI] [PubMed] [Google Scholar]
- 44.Camargos P, Bourgeois ML, Revillon Y, et al. Lung resection in cystic fibrosis: a survival analysis. Pediatr Pulmonol 2008; 43: 72–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605241233520 for Should atelectasis be considered a pulmonary complication and indicator of poor prognosis in cystic fibrosis? by María Martínez Redondo, Carlos Carpio Segura, Ester Zamarrón De Lucas, Rodolfo Álvarez-Sala Walther and Concepción Prados Sánchez in Journal of International Medical Research
Data Availability Statement
Data will be made available if the editor considers it necessary to understand the manuscript.