Abstract
Background:
In REFLEX, subcutaneous interferon beta-1a (sc IFN β-1a) delayed the onset of multiple sclerosis (MS) in patients with a first clinical demyelinating event (FCDE).
Objectives:
This post hoc analysis aimed to determine whether baseline serum neurofilament light (sNfL) chain can predict conversion to MS and whether correlations exist between baseline sNfL and magnetic resonance imaging (MRI) metrics.
Methods:
sNfL was measured for 494 patients who received sc IFN β-1a 44 μg once weekly (qw; n = 168), three times weekly (tiw; n = 161), or placebo (n = 165) over 24 months. Median baseline sNfL (26.1 pg/mL) was used to define high/low sNfL subgroups. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox’s proportional hazard model to determine factors influencing the risk of conversion to MS. Kaplan–Meier estimates calculated median time-to-conversion to MS (McDonald 2005 criteria) or clinically definite MS (CDMS; Poser criteria). Correlations between sNfL and MRI findings were assessed using Spearman’s rank correlation coefficient (r).
Results:
Multivariable models indicated that high baseline sNfL was associated with the likelihood of converting to MS and inversely to time-to-conversion (HR = 1.3, 95% CI: 1.03–1.64; p = 0.024). Significant additional factors affecting conversion to McDonald MS were on-study treatment (sc IFN β-1a/placebo; qw: HR = 0.59, 95% CI: 0.46–0.76; tiw: HR = 0.45, 95% CI: 0.34–0.59), classification of FCDE (monofocal/multifocal; HR = 0.69, 95% CI: 0.55–0.85), and most baseline imaging findings (T2 and T1 gadolinium-enhancing [Gd+] lesions; HR = 1.02, 95% CI: 1.01–1.03 and HR = 1.07, 95% CI: 1.03–1.11); all p ⩽ 0.001. Conversion to CDMS showed similar results. At month 24, sNfL was strongly correlated with a mean number of combined unique active (r = 0.71), new T2 (r = 0.72), and new T1 Gd+ (r = 0.60) lesions; weak correlations were observed between sNfL and clinical outcomes for all treatment groups.
Conclusion:
Higher baseline sNfL was associated with an increased risk of MS conversion, a risk that was mitigated by treatment with sc IFN β-1a tiw.
Trial registration:
ClinicalTrials.gov identifier: NCT00404352. Date registered: 28 November 2006.
Keywords: interferon beta-1a, multiple sclerosis, serum neurofilament light chain
Introduction
Multiple sclerosis (MS) is a chronic, disabling disease that requires long-term treatment and regular monitoring while also being associated with negative effects on health-related quality of life due to reduced physical, cognitive, and psychosocial functioning.1–3
The current standard for measuring neuroaxonal damage associated with disease activity in patients with MS is magnetic resonance imaging (MRI)-assessed lesion burden and brain atrophy. 4 Alongside such imaging, various biomarkers have been explored to help identify patients at greater risk of aggressive disease phenotypes as well as aiding the selection of appropriate disease-modifying therapies (DMTs).5–7 Serum neurofilament light (sNfL) chain is meanwhile a very promising and useful biomarker of neuroaxonal damage in MS as well as drug response. Six years before the onset of clinical MS, sNfL levels are higher than in matched controls, suggesting the biomarker is hugely relevant for prognosis and that neuroaxonal damage occurs for years before MS onset. 8 The baseline level of sNfL appears to predict disease course in early MS 9 and future disability progression in those with established disease.10,11 The timely identification of patients with potentially more aggressive forms of MS or suboptimal treatment response could mean that the disease course may be favorably altered by providing early access to high-efficacy treatments.9,12
Subcutaneous interferon beta-1a (sc IFN β-1a) has proven efficacy in the treatment of patients with a first clinical demyelinating event (FCDE), in terms of significantly delaying the onset of MS (according to McDonald 2005 criteria) or clinically definite MS (CDMS; according to Poser criteria) in the REFLEX study. 13 Of note, a prior analysis of patients from this study found that those treated with sc IFN β-1a had reduced sNfL concentration as early as 6 months post-baseline. 14 Against this background, the current analysis was designed to further explore whether baseline sNfL concentration can predict conversion to MS in patients with an FCDE. Our primary research question was to determine whether baseline sNfL concentration can predict conversion to MS and whether any correlation exists between sNfL and MRI outcomes in such patients.
Methods
Study design and procedures
In this post hoc analysis, sNfL concentrations were measured in blood samples from patients who participated in the 2-year, randomized, double-blind REFLEX study (NCT00404352), in which eligible patients received sc IFN β-1a 44 μg (Rebif®, Merck Healthcare KGaA, Darmstadt, Germany) once weekly (qw), three times weekly (tiw), or placebo. Enrolment for REFLEX occurred at 67 study locations across 27 countries, beginning in November 2006, with study completion in July 2011.
As has been described previously, 13 patients were eligible for inclusion in the REFLEX study if they were aged 18–50 years, had an Expanded Disability Status Scale (EDSS) score of ⩽5.0, and had experienced a first event suggestive of MS ⩽60 days before study entry; and at least two clinically silent lesions of ⩾3 mm on T2-weighted brain MRI scan (at least one of which was ovoid, periventricular, or infratentorial). Exclusion criteria included a diagnosis of MS according to McDonald 2005 criteria, other diseases that could explain the signs and symptoms a patient was experiencing at the index event, and previous use of any other immunomodulatory or immunosuppressive therapy, or use of any corticosteroids within 30 days prior to initiating treatment in REFLEX. 13
Study endpoints and statistical analysis
The post hoc analyses described here were performed using data from the double-blind period of the REFLEX study up to CDMS conversion and included patients who had serum samples available from baseline and at least one other time point during the study. The sNfL concentration of samples taken at baseline, month 6, month 12, and month 24 was analyzed using a sensitive single molecule array assay (as described by Disanto et al. 15 ) and were run on a Simoa HD-1 instrument (Quanterix, Billerica, MA, USA) using a two-step Assay Neat 2.0 protocol.
Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated from univariable Cox’s proportional hazard models used to determine the factors influencing time to conversion to MS. A stepwise multivariable Cox’s proportional hazard model was used with factors or covariates selected from the univariable models (threshold p < 0.15) that were seen to have an influence on conversion to MS. These factors were on-study treatment, age at baseline, FCDE classification (monofocal/multifocal), and number of T1 gadolinium-enhancing (Gd+) and T2 lesions. Baseline sNfL concentration was also included as a factor, being categorically defined in relation to the median value of 26.1 pg/mL as either low (baseline sNfL ⩽ median) or high (baseline sNfL > median). For both models, variable selection was based on a two-sided Wald test.
The median time to conversion to MS (McDonald 2005 criteria) or CDMS in each treatment group by month 24 was calculated using Kaplan-Meier curve estimates.
Correlations between sNfL and MRI findings were assessed using Spearman’s rank correlation coefficient (r), according to two approaches: baseline sNfL with baseline lesion count and normalized brain volume; and sNfL at month 24 with (1) number of new MRI lesions between baseline and month 24 and (2) mean number of combined unique active (CUA), new T2, T1 Gd+, and T1 hypointense lesions per patient per scan during the 24-month double-blind period.
Correlation analyses were performed on observed data and the strength of correlation was defined as follows: very strong (r = 0.80–1.00), strong (r = 0.60–0.79), moderate (r = 0.40–0.59), weak (r = 0.20–0.39), or very weak (r = 0.00–0.19).
Sample size and statistical power considerations
In REFLEX, the primary endpoint was the time to conversion to MS (McDonald 2005 criteria) where a total of 450 patients were required to achieve 165 events for the comparison sc IFN β-1a 44 µg tiw versus placebo. This sample size was required to achieve 90% power with a two-sided 0.05 alpha error for detecting a HR of 0.6 for the primary comparison. The time to conversion was assumed to be exponentially distributed, and no adjustment for the multiplicity of the alpha error was applied.
Patients were randomly and equally allocated to each of the three treatment arms at enrolment to REFLEX and were included in this post hoc analysis based on the criteria mentioned above.
Results
Overall, 517 patients were randomly assigned to treatment in REFLEX, of whom 494 patients were included in this analysis (sc IFN β-1a qw, n = 168; sc IFN β-1a tiw, n = 161; placebo, n = 165). The mean ± standard deviation (SD) age for these 494 patients was 30.8 (±8.1) years, and 318 (64.4%) were female (Table 1).
Table 1.
Patient demographics and disease characteristics at baseline.
| Full sNfL analysis set (n = 494) | ||||
| Characteristic | Placebo (n = 165) | sc IFN β-1a qw (n = 168) | sc IFN β-1a tiw (n = 161) | Overall (n = 494) |
| Age, years | 30.7 (7.7) | 30.9 (8.2) | 30.8 (8.6) | 30.8 (8.1) |
| Female, n (%) | 108 (65.5) | 104 (61.9) | 106 (65.8) | 318 (64.4) |
| Time since FCDE, days | ||||
| Median (Q1–Q3) | 59.0 (56.0–60.0) | 59.0 (57.0–60.0) | 59.0 (56.0–60.0) | 59.0 (56.0–60.0) |
| EDSS score mean (median) | 1.53 (1.5) | 1.52 (1.5) | 1.57 (1.5) | 1.54 (1.5) |
| sNfL value, pg/mL | ||||
| Mean (SD) | 59.3 (90.8) | 54.6 (106.2) | 45.7 (62.4) | 53.3 (88.6) |
| Median (Q1–Q3) | 24.6 (15.6–61.8) | 26.7 (16.5–51.9) | 25.3 (15.4–48.4) | 26.1 (15.8–53.1) |
| Number of T1 Gd+ lesions | 1.2 (2.8) | 1.5 (3.5) | 1.3 (2.5) | 1.3 (3.0) |
| T1 Gd+ lesion volume, mm3 | 193.4 (594.2) | 199.8 (604.6) | 148.3 (409.1) | 108.9 (544.2) |
| Number of T1 hypointense lesions | 5.5 (7.7) | 6.0 (7.6) | 5.8 (6.9) | 5.8 (7.4) |
| T1 hypointense lesion volume, mm3 | 660.9 (1049.3) | 790.7 (1304.7) | 691.2 (1074.1) | 714.9 (1149.1) |
| Number of T2 lesions | 20.9 (19.9) | 24.0 (21.3) | 22.3 (19.0) | 22.4 (20.1) |
| T2 lesion volume, mm3 | 3300.6 (3980.4) | 3931.8 (4762.7) | 3093.0 (3434.4) | 3447.6 (4112.2) |
| Normalized brain volume, cm3 | 1545.9 (63.7) | 1536.0 (66.9) | 1536.2 (74.2) | 1539.4 (68.4) |
| Low baseline sNfL (⩽ median baseline value; n = 247) | ||||
| Characteristic | Placebo (n = 85) | sc IFN β-1a qw (n = 80) | sc IFN β-1a tiw (n = 82) | Overall (n = 247) |
| Age, years | 31.2 (7.5) | 31.2 (8.1) | 32.1 (7.7) | 31.5 (7.7) |
| Female, n (%) | 56 (65.9) | 55 (68.8) | 56 (68.3) | 167 (67.6) |
| Time since FCDE, days | ||||
| Median (Q1–Q3) | 59.0 (56.0–60.0) | 59.0 (57.0–60.0) | 59.0 (57.0–60.0) | 59.0 (57.0–60.0) |
| EDSS score, mean (median) | 1.52 (1.5) | 1.52 (1.5) | 1.49 (1.5) | 1.51 (1.5) |
| sNfL value, pg/mL | ||||
| Mean (SD) | 15.7 (5.7) | 15.8 (5.6) | 15.2 (5.3) | 15.6 (5.5) |
| Median (Q1–Q3) | 15.7 (12.2–20.0) | 16.1 (11.5–20.9) | 15.5 (11.7–18.5) | 15.7 (11.7–20.1) |
| Number of T1 Gd+ lesions | 0.3 (0.7) | 0.5 (1.0) | 0.4 (0.9) | 0.4 (0.9) |
| T1 Gd+ lesion volume, mm3 | 25.8 (72.0) | 53.9 (183.0) | 18.1 (66.4) | 32.4 (119.2) |
| Number of T1 hypointense lesions | 3.4 (4.9) | 5.1 (7.7) | 4.6 (5.7) | 4.3 (6.2) |
| T1 hypointense lesion volume, mm3 | 381.3 (794.0) | 566.4 (1217.6) | 596.1 (1186.6) | 512.6 (1079.0) |
| Number of T2 lesions | 15.1 (14.2) | 19.6 (18.3) | 16.9 (13.8) | 17.2 (15.6) |
| T2 lesion volume, mm3 | 1754.2 (2291.9) | 2261.6 (3150.7) | 2081.9 (2567.4) | 2027.3 (2682.7) |
| Normalized brain volume, cm3 | 1544.6 (64.6) | 1535.5 (63.5) | 1529.3 (72.3) | 1536.6 (67.0) |
| High baseline sNfL (>median baseline value; n = 247) | ||||
| Characteristic | Placebo (n = 80) | sc IFN β-1a qw (n = 88) | sc IFN β-1a tiw (n = 79) | Overall (n = 247) |
| Age, years | 30.2 (8.0) | 30.7 (8.2) | 29.4 (9.3) | 30.1 (8.5) |
| Female, n (%) | 52 (65.0) | 49 (55.7) | 50 (63.3) | 151 (61.1) |
| Time since FCDE, days | ||||
| Median (Q1–Q3) | 59.0 (56.0–60.0) | 59.0 (56.0–60.0) | 59.0 (55.0–60.0) | 59.0 (56.0–60.0) |
| EDSS score, mean (median) | 1.54 (1.5) | 1.52 (1.5) | 1.64 (1.5) | 1.57 (1.5) |
| sNfL value, pg/mL | ||||
| Mean (SD) | 105.7 (113.4) | 89.9 (137.8) | 77.3 (77.3) | 91.0 (113.4) |
| Median (Q1–Q3) | 63.1 (40.3–123.5) | 50.9 (35.7–92.8) | 48.9 (35.8–85.8) | 53.4 (36.2–98.7) |
| Number of T1 Gd+ lesions | 2.2 (3.7) | 2.4 (4.6) | 2.2 (3.2) | 2.3 (3.9) |
| T1 Gd+ lesion volume, mm3 | 371.4 (815.6) | 332.5 (796.2) | 283.4 (550.0) | 329.4 (731.4) |
| Number of T1 hypointense lesions | 7.7 (9.4) | 6.8 (7.4) | 7.1 (7.7) | 7.2 (8.2) |
| T1 hypointense lesion volume, mm3 | 958.0 (1200.8) | 994.6 (1353.9) | 790.0 (940.7) | 917.3 (1183.1) |
| Number of T2 lesions | 27.0 (23.1) | 28.0 (23.1) | 27.8 (22.0) | 27.6 (22.7) |
| T2 lesion volume, mm3 | 4943.7 (4690.2) | 5450.3 (5443.3) | 4142.5 (3893.6) | 4867.9 (4758.4) |
| Normalized brain volume, cm3 | 1547.2 (63.1) | 1536.5 (70.1) | 1543.5 (75.9) | 1542.2 (69.7) |
Mean (SD) unless stated otherwise.
EDSS, Expanded Disability Status Scale; FCDE, first clinical demyelinating event; Gd+, gadolinium enhancing; IFN, interferon; Q, quartile; qw, once weekly; sc, subcutaneous; sc IFN β-1a, subcutaneous interferon beta-1a; SD, standard deviation; sNfL, serum neurofilament light chain; tiw, three times weekly.
In the overall sNfL analysis set, the interquartile range for sNfL concentrations at baseline was 15.4–61.8 pg/mL, with a median of 26.1 pg/mL. Patients with low sNfL at baseline (i.e. ⩽26.1 pg/mL) had numerically fewer T1 Gd+, T1 hypointense, and T2 lesions compared with the high baseline sNfL cohort. Lesion volumes at baseline were also lower in the low sNfL subgroup compared with the high sNfL subgroup, but there were no observed differences in normalized brain volume.
Over the 24-month study period, concentrations of sNfL were reduced in all three groups; these reductions occurred as early as 6 months post-baseline and were more visibly reduced in patients with high baseline sNfL and for those treated with sc IFN β-1a (Supplemental Figure S1).
sNfL at baseline and conversion to MS
The multivariable stepwise analysis determined that high sNfL at baseline was correlated with the likelihood of conversion to MS (McDonald 2005 criteria); significant factors in this multivariable model were baseline sNfL subgroup (high/low: HR = 1.3, 95% CI: 1.03–1.64; p = 0.024), on-study treatment versus placebo (sc IFN β-1a qw: HR = 0.59, 95% CI: 0.46–0.76; sc IFN β-1a tiw: HR = 0.45, 95% CI: 0.34–0.59; both p < 0.001), classification of FCDE (monofocal/multifocal: HR = 0.69, 95% CI: 0.55–0.85; p < 0.001), baseline number of T1 Gd+ (HR = 1.07, 95% CI: 1.03–1.11; p = 0.001) and T2 lesions (HR = 1.02, 95% CI: 1.01–1.03; p < 0.001), and age at baseline (<30/⩾30 years: HR = 1.47, 95% CI: 1.19–1.82; p < 0.001) (Table 2).
Table 2.
Multivariable analysis a of time to conversion to MS or clinically definite MS.
| Factor | Parameter estimate | SE | HR (95% CI) | p Value b |
|---|---|---|---|---|
| Time to conversion to MS (McDonald 2005 criteria) | ||||
| Treatment | ||||
| (sc IFN β-1a qw/placebo) | −0.53 | 0.13 | 0.59 (0.46–0.76) | <0.001 |
| (sc IFN β-1a tiw/placebo) | −0.80 | 0.14 | 0.45 (0.34–0.59) | <0.001 |
| Age at baseline (<30/⩾30 years) | 0.39 | 0.11 | 1.47 (1.19–1.82) | <0.001 |
| FCDE classification (monofocal/multifocal) | −0.38 | 0.11 | 0.69 (0.55–0.85) | <0.001 |
| Number of T1 Gd+ lesions at baseline | 0.07 | 0.02 | 1.07 (1.03–1.11) | 0.001 |
| Number of T2 lesions at baseline | 0.02 | 0.00 | 1.02 (1.01–1.03) | <0.001 |
| Baseline median sNfL subgroup (high/low) c | 0.26 | 0.12 | 1.3 (1.03–1.64) | 0.024 |
| Time to conversion to clinically definite MS (Poser criteria) | ||||
| Treatment | ||||
| (sc IFN β-1a qw/placebo) | −0.80 | 0.21 | 0.45 (0.30–0.68) | <0.001 |
| (sc IFN β-1a tiw/placebo) | −0.77 | 0.22 | 0.46 (0.30–0.71) | <0.001 |
| Number of T1 Gd+ lesions at baseline | 0.09 | 0.03 | 1.10 (1.04–1.16) | <0.001 |
A stepwise multivariable Cox’s proportional hazard model was performed using factors selected from the univariable model (threshold p < 0.15). Only statistically significant factors in the multivariable model are shown.
Two-sided Wald test.
Low baseline sNfL: baseline sNfL ⩽ median; high baseline sNfL: baseline sNfL > median.
CI, confidence interval; FCDE, first clinical demyelinating event; Gd+, gadolinium enhancing; HR, hazard ratio; IFN, interferon; MS, multiple sclerosis; qw, once weekly; sc, subcutaneous; sc IFN β-1a, subcutaneous interferon beta-1a; SE, standard error; sNfL, serum neurofilament light chain; tiw, three times weekly.
Regarding multivariable models of conversion to CDMS (Poser criteria), the only remaining significant factors were on-study treatment versus placebo (sc IFN β-1a qw: HR = 0.45, 95% CI: 0.30–0.68; sc IFN β-1a tiw: HR = 0.46, 95% CI: 0.30–0.71; both p < 0.001), and number of T1 Gd+ (HR = 1.10, 95% CI: 1.04–1.16; p < 0.001) and T2 lesions (HR = 1.01, 95% CI: 1.00–1.02; p = 0.053) at baseline. Results for the other factors, for example, age at baseline and baseline sNfL subgroup, were significant in the univariable models but were not significant in the stepwise multivariable Cox’s proportional hazard model and, as such, were not subsequently included in the multivariable model.
Low versus high sNfL at baseline and time to conversion to MS
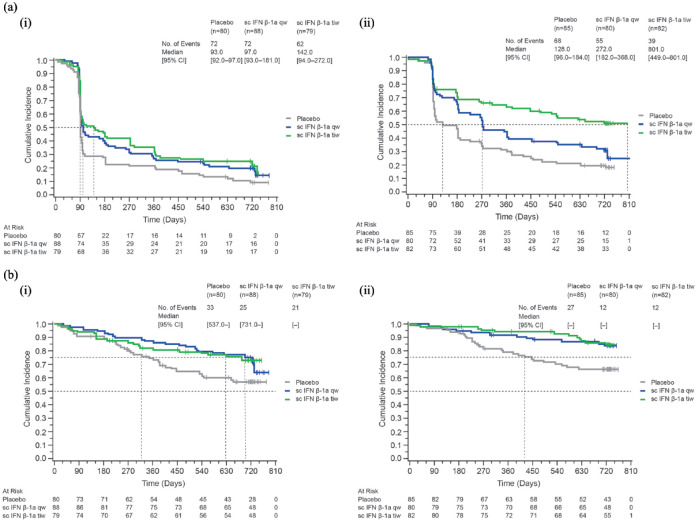
The median time to conversion to MS (McDonald 2005 criteria) was longer in patients in the low sNfL subgroup (128, 272, and 801 days for placebo, sc IFN β-1a qw, and tiw, respectively) than in the high sNfL subgroup (93, 97, and 142 days for placebo, sc IFN β-1a qw, and tiw, respectively). Corresponding median time to conversion to CDMS could not be reported since the proportion of patients who converted was below 50%; however, at the 25% level, the time to conversion to CDMS was longer in the low sNfL subgroup (445 days for placebo, and not estimable for sc IFN β-1a since this level was not reached for either therapeutic dose) than in the high sNfL subgroup (345, 699, and 651 days for placebo, sc IFN β-1a qw, and tiw, respectively) [Figure 1(a) and (b) and Supplemental Table S1).
Figure 1.
Kaplan–Meier cumulative incidence curves for time to conversion to MS (McDonald 2005 criteria) or clinically definite MS (Poser criteria) by treatment group, for each baseline sNfL subgroup. (a) Time to conversion to MS (McDonald 2005 criteria). (i) Low baseline sNfL (⩽ median baseline value). (ii) High baseline sNfL (> median baseline value). (b) Time to conversion to clinically definite MS (Poser criteria). (i) Low baseline sNfL (⩽ median baseline value). (ii) High baseline sNfL (> median baseline value).
CI, confidence interval; IFN, interferon; MS, multiple sclerosis; qw, once weekly; sc, subcutaneous; sc IFN β-1a, subcutaneous interferon beta-1a; sNfL, serum neurofilament light chain; tiw, three times weekly.
Correlation between sNfL at baseline and MRI findings/clinical outcomes
Moderate, positive correlations were observed between baseline sNfL and baseline MRI findings, including the volume of T2 lesions and the volume and number of T1 Gd+ lesions (r = 0.55, 0.49, and 0.46, respectively; all p < 0.0001) [Table 3 and Supplemental Figure S2(a)]. There were weak correlations between baseline sNfL and other baseline MRI findings, including the volume and number of T1 hypointense lesions and number of T2 lesions (r = 0.36, 0.35, and 0.30, respectively; all p < 0.0001). Very weak correlations were seen between baseline sNfL and normalized brain volume (r = 0.01, p = 0.8180) (Table 3).
Table 3.
Correlation between sNfL concentration and MRI findings at baseline.
| Characteristic | Overall Spearman’s rank correlation coefficient (r) (n = 494) | p Value |
|---|---|---|
| Volume of T2 lesions, mm3 | 0.545 | <0.0001 |
| Volume of T1 Gd+ lesions, mm3 | 0.490 | <0.0001 |
| Number of T1 Gd+ lesions | 0.464 | <0.0001 |
| Volume of T1 hypointense lesions, mm3 | 0.358 | <0.0001 |
| Number of T2 lesions | 0.345 | <0.0001 |
| Number of T1 hypointense lesions | 0.298 | <0.0001 |
| Normalized brain volume*, cm3 | 0.010 | 0.8180 |
Bold, italicized values indicate moderate correlations.
Based on n = 488.
Gd+, gadolinium enhancing; MRI, magnetic resonance imaging; sNfL, serum neurofilament light chain.
During the 24-month double-blind treatment period, the mean number of MRI lesions was significantly lower in the sc IFN β-1a tiw group compared with placebo (Supplemental Table S2). In the placebo group, for the same timeframe, strong correlations were observed between sNfL at month 24 and the mean number of CUA, new T2 lesions, and new T1 Gd+ lesions [Table 4 and Supplemental Figure S2(b)]. The lower Spearman’s rank correlation coefficients observed in the sc IFN β-1a tiw treatment group indicate a lower number and volume of T1 and T2 lesions compared with sc IFN β-1a qw and placebo. Very weak correlations were observed between sNfL at month 24 and clinical outcomes (EDSS, brain volume, and qualifying relapses) in the placebo and sc IFN β-1a-treated groups (Table 4).
Table 4.
Correlation between month 24 sNfL and MRI and clinical findings at month 24, according to treatment group.
| Characteristic | Spearman’s rank correlation coefficient (r) | ||
|---|---|---|---|
| Placebo | sc IFN β-1a qw | sc IFN β-1a tiw | |
| Lesion count per patient per scan a during the 24-month double-blind period | n = 91 | n = 113 | n = 115 |
| Mean number of CUA lesions | 0.708*** | 0.485*** | 0.133 |
| Mean number of new T2 lesions | 0.715*** | 0.458*** | 0.105 |
| Mean number of new T1 Gd+ lesions | 0.598*** | 0.400*** | 0.308** |
| Mean number of new T1 hypointense lesions | 0.494*** | 0.477*** | 0.182 |
| Lesion count at month 24 | n = 133 | n = 139 | n = 138 |
| Number of new T2 lesions | 0.564*** | 0.372*** | 0.274** |
| Volume of total T2 lesions, mm3 | 0.453*** | 0.286** | 0.215* |
| Number of new T1 Gd+ lesions | 0.344*** | 0.360*** | 0.231* |
| Volume of total T1 Gd+ lesions, mm3 | 0.379*** | 0.355*** | 0.232* |
| Number of new T1 hypointense lesions | 0.319** | 0.259* | 0.292** |
| Volume of total T1 hypointense lesions, mm3 | 0.430*** | 0.267* | 0.194* |
| EDSS (change from baseline) at month 24 |
n = 138 0.014 |
n = 140 −0.148 |
n = 145 −0.011 |
| Brain volume (% change from baseline) at month 24 |
n = 127 −0.135 |
n = 136 −0.081 |
n = 135 −0.218 |
| Qualifying relapses during a 24-month, double-blind period |
n = 141 0.012 |
n = 141 −0.031 |
n = 148 0.080 |
Bold, italicized values indicate moderate correlations; bolded blue values indicate strong correlations.
MRI metrics collected at several time points during a 24-month period were used for the correlation analysis; data reported for patients with sNfL and MRI/clinical data were available for analysis.
p < 0.05, **p < 0.001, ***p < 0.0001.
CUA, combined unique active; EDSS, Expanded Disability Status Scale; Gd+, gadolinium enhancing; IFN, interferon; MRI, magnetic resonance imaging; qw, once weekly; sc, subcutaneous; sc IFN β-1a, subcutaneous interferon beta-1a; sNfL, serum neurofilament light chain; tiw, three times weekly.
Discussion
Recently, sNfL has received growing interest around how this biomarker may predict the future disease course in early MS, as well as how sNfL concentrations are affected in response to DMTs. In this analysis of REFLEX, sNfL concentrations of patients experiencing a first event suggestive of early MS were correlated with current and future MRI outcomes along with conversion to MS, as defined by McDonald 2005 and Poser criteria. Using a multivariable analysis, results from the study show that treatment with sc IFN β-1a delayed the time to conversion and reduced the proportion of patients who converted to MS over the 24-month study period.
Conversion to MS (McDonald 2005 criteria) or CDMS (Poser criteria)
Several studies have reported higher sNfL concentrations in patients with clinically isolated syndrome or a FCDE. However, there have been few published examples of how sNfL may be used prognostically to determine the risk of conversion to MS.12,16,17 It should be noted that these studies explored the risk of conversion to MS according to McDonald 201012,17 or 2017 criteria, 16 whereas our analysis of REFLEX investigated conversion according to the earlier 2005 criteria that were in place at the time of study initiation. We found that higher baseline sNfL concentration was associated with an increased risk of conversion to MS, thus indicating that sNfL may be a prognostic biomarker of disease in patients with a FCDE. One such study found a 1-point increase in the HR for the risk of conversion to CDMS for every 100 pg/mL increase in sNfL using a univariable model and found the adjusted HR for CDMS conversion remained significant in a multivariable model. 17
Factors such as receiving treatment with sc IFN β-1a, the classification of FCDE (mono- or multi-focal), and low numbers of MRI lesions at baseline were also found to be independently associated with a reduced risk of conversion to MS. Indeed, the respective multivariable models both showed significance for on-study treatment (versus placebo) and for the number of T1 Gd+ and T2 lesions for the conversion to both MS (McDonald 2005 criteria) and CDMS (Poser criteria), respectively.
Correlations between sNfL and MRI findings/clinical outcomes
The presence of MRI lesions is commonly associated with a more active disease course, and as such it would be expected that higher sNfL concentrations would be present. Indeed, there was a consistent association between sNfL concentration and both MRI and clinical outcomes during the study. At baseline, for example, higher sNfL concentrations were observed in patients who had higher numbers of T1 Gd+, T1 hypointense, and T2 MRI lesions, possibly reflecting recent/ongoing neuroaxonal damage 18 and/or greater disease activity. 19 Moderate, albeit significant, correlations were observed between baseline sNfL concentration and baseline T2 lesion volumes, T1 Gd+ lesion volumes, and T1 Gd+ lesion numbers, findings that are consistent with previous observations 15 and studies of plasma NfL. 18 These findings were also similar to those of patients treated with intramuscular IFN β-1a, which showed moderate correlations in the number of T1 Gd+ lesions and T2 lesion volumes. 5
During the 24-month, double-blind study period and at the month 24 timepoint, strong correlations were observed between sNfL and CUA, T1 Gd+, and T2 lesions in the placebo group. It should be noted that patients in the placebo group had a similar number of lesions as compared to the treated subgroups at baseline.
Studies have found increased sNfL close to the onset of clinical relapse,15,16,20 with the appearance of T1 Gd+ and new and/or enlarging T2 lesions,15,16,19,20 and EDSS progression.12,20,21 sNfL concentration at the time of diagnosis was also found to predict disease activity over a succeeding 2-year period. 22 Although some studies have found correlations between sNfL and EDSS worsening, these observations were not made in this post hoc analysis of REFLEX, which may be due to the study population. In early MS, there are very few changes to disability and any changes that are present can be difficult to detect. Other studies have also found no correlations between sNfL and EDSS,16,17,19 with some studies indicating a correlation using univariable and multivariable models.11,15
Taken together, the results from this analysis suggest that patients with a FCDE and high baseline sNfL have a higher probability of disease worsening. Indeed, since the REFLEX study was conducted, treatment practices have changed, and early treatment may be used in patients with high levels of sNfL to maintain neurological function.23–25
Study limitations
This analysis is limited by the use of the McDonald 2005 criteria, which were the current means for determining conversion to MS at the time the REFLEX study commenced (November 2006). It should also be noted that the sNfL data in this analysis were collected over a short 2-year timeframe, during which patients may not have converted to MS based on their disease phenotype. However, longer-term studies have found that at 10 years, there was no statistically significant difference in the risk of CDMS between patients with low versus high sNfL concentrations. 5 It should also be considered that the present analysis of REFLEX focuses on a patient population that had experienced a FCDE. For this population, the time-to-conversion analysis was deemed to be important and therefore the multivariable analyses explored the primary and secondary endpoints of time-to-conversion to McDonald MS (2005 criteria) and CDMS (Poser criteria). For this analysis, the multivariable models were not expanded to other variables, thus raising an interesting question about the interactions with lesion volume and brain atrophy, which needs further exploration. Lastly, not using percentiles/Z-scores – adjusting for age and BMI based on healthy controls – was a shortcoming of the work. However, large reference databases are not available for the homebrew assay used to measure sNfL in this study; consequently, we were not able to use percentiles/Z-scores. 11 However, all models were adjusted for age.
In conclusion, we found that higher baseline sNfL concentration was associated with an increased risk of conversion to MS (McDonald 2005 criteria) or CDMS (Poser criteria) in patients with a FCDE. Age, multifocal disease, and number of T1 or T2 lesions at baseline were also confirmed as significant determinants for risk of conversion. Conversion was delayed in all patients treated with sc IFN β-1a tiw regardless of baseline sNfL concentrations; yet, this delay in conversion was particularly noticeable for patients who had baseline sNfL values below the median of 26.1 pg/mL. Concerning MRI parameters, a higher sNfL concentration at baseline was moderately correlated with lesion load and T1 Gd+ lesion count in such patients, and this association was also present for placebo recipients at month 24. However, these correlations were weak in the sc IFN β-1a tiw group, consistent with a significant reduction in lesion counts compared with placebo. Together, these exploratory findings highlight the complex interplay between sNfL concentration, MRI outcomes, and risk of conversion to MS in patients with a FCDE, and the benefits of treatment with sc IFN β-1a in such patients.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864241239101 for Serum neurofilament light chain correlations in patients with a first clinical demyelinating event in the REFLEX study: a post hoc analysis by Jens Kuhle, David Leppert, Giancarlo Comi, Nicola de Stefano, Ludwig Kappos, Mark S. Freedman, Andrea Seitzinger and Sanjeev Roy in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors would like to thank patients and their families, investigators, co-investigators, and the study teams at each of the participating centers and Merck Healthcare KGaA, Darmstadt, Germany. Medical writing assistance was provided by Claire Mwape of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Merck Healthcare KGaA, Darmstadt, Germany.
Footnotes
ORCID iD: Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jens Kuhle, Department of Neurology, University Hospital Basel, Petersgraben 4, Basel CH-4031, Switzerland; Multiple Sclerosis Centre and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB), Departments of Biomedicine and Clinical Research, University Hospital and University of Basel, Spitalstrasse 2, Basel CH-4031, Switzerland.
David Leppert, Multiple Sclerosis Centre and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB), Departments of Biomedicine and Clinical Research, University Hospital and University of Basel, Basel, Switzerland.
Giancarlo Comi, Casa di Cura Privata del Policlinico, Università Vita-Salute San Raffaele, Milan, Italy.
Nicola de Stefano, Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy.
Ludwig Kappos, Multiple Sclerosis Centre and Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB), Departments of Biomedicine and Clinical Research, University Hospital and University of Basel, Basel, Switzerland.
Mark S. Freedman, Department of Medicine and the Ottawa Hospital Research Institute, University of Ottawa, Ottawa, ON, Canada
Andrea Seitzinger, Biostatistics, Merck Healthcare KGaA, Darmstadt, Germany.
Sanjeev Roy, Global Clinical Development – Immunology, Ares Trading S.A. (an affiliate of Merck KGaA), Eysins, Switzerland.
Declarations
Ethics approval and consent to participate: The REFLEX study was undertaken in compliance with the Declaration of Helsinki and standards of Good Clinical Practice according to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Before initiation of the study at each center, the relevant institutional review board or independent ethics committee reviewed and approved the study protocol, patient information leaflet, informed consent forms, and investigator brochure. All participants provided written informed consent at the screening visit of REFLEX, and additional approvals were sought from the Swiss Ethics Committee for the current post hoc analysis of sNfL (Swiss EC, approval number: 2018-01394).
Consent for publication: Not applicable.
Author contributions: Jens Kuhle: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing – review & editing.
David Leppert: Funding acquisition; Methodology; Writing – review & editing.
Giancarlo Comi: Conceptualization; Investigation; Writing – review & editing.
Nicola de Stefano: Conceptualization; Investigation; Writing – review & editing.
Ludwig Kappos: Conceptualization; Supervision; Writing – review & editing.
Mark S. Freedman: Conceptualization; Formal analysis; Methodology; Visualization.
Andrea Seitzinger: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing – review & editing.
Sanjeev Roy: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Merck (CrossRef Funder ID: 10.13039/100009945).
Competing interests: JK has received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Alnylam, Bayer, Biogen, Celgene (BMS), Immunic, Merck, Neurogenesis, Novartis, Octave Bioscience, Quanterix, Roche, Sanofi, and Stata DX. DL is the CMO of GeNeuro. GC has received consulting fees from Bayer, Biogen, Merck, Novartis, Receptos, Roche, Sanofis, and Teva; lecture fees from Bayer, Biogen, Merck, Novartis, Sanofi, Serono Symposia International Foundation, and Teva; and trial grant support from Bayer, Biogen, Merck, Novartis, Receptos, Roche, Sanofi, and Teva. NdS is a consultant for Biogen, Merck, Novartis, Roche, Sanofi, and Teva; has grants or grants pending from FISM and Novartis, is on the speakers’ bureaus of Biogen, Merck, Novartis, Roche, Sanofi, and Teva; and has received travel funds from Merck, Novartis, Roche, Sanofi, and Teva. LK has received no personal compensation. His institutions (University Hospital Basel/Foundation Clinical Neuroimmunology and Neuroscience Basel) have received and used exclusively for research support: payments for the steering committee and advisory board participation, consultancy services, and participation in educational activities from Actelion, Bayer, Celgene (BMS), df-mp Molnia & Pohlmann, Eli Lilly and Company, EMD Serono Research and Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Genentech, GlaxoSmithKline, Janssen, Japan Tobacco, Merck, MH Consulting, Minoryx, Novartis, Roche, Senda Biosciences Inc., Sanofi, Santhera, Shionogi BV, TG Therapeutics, and Wellmera, and license fees for Neurostatus-UHB products; grants from Novartis, Innosuisse, and Roche. MSF has received honoraria or consultation fees from Alexion (AstraZeneca), Atara Biotherapeutics, Bayer, Biogen, EMD Inc., Mississauga, Ontario, Canada, an affiliate of Merck KGaA, Novartis, Roche, Sandoz, Sanofi and Teva Canada Innovation; was a member of a company advisory board, board of directors, or another similar group for Alexion (AstraZeneca), Atara Biotherapeutics, Bayer, Actelion (Janssen/J&J), EMD Inc., Mississauga, Ontario, Canada, an affiliate of Merck KGaA, Novartis, Quanterix, Roche, and Sanofi; has been a participant in a company-sponsored speaker’s bureau for EMD Inc., Mississauga, Ontario, Canada, an affiliate of Merck KGaA, EMD Serono Research and Development Institute Inc., Billerica, MA, USA and Sanofi; and has received research or educational grants from EMD Inc., Mississauga, Ontario, Canada, an affiliate of Merck KGaA, and Sanofi. AS is an employee of Merck Healthcare KGaA, Darmstadt, Germany. SR is an employee of Ares Trading S.A., Eysins, Switzerland, an affiliate of Merck KGaA.
Availability of data and materials: Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data-sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
References
- 1. Oreja-Guevara C. Overview of magnetic resonance imaging for management of relapsing-remitting multiple sclerosis in everyday practice. Eur J Neurol 2015; 22(Suppl. 2): 22–27. [DOI] [PubMed] [Google Scholar]
- 2. Orme M, Kerrigan J, Tyas D, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health 2007; 10: 54–60. [DOI] [PubMed] [Google Scholar]
- 3. Jongen PJ. Health-related quality of life in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs 2017; 31: 585–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis – establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015; 11: 597–606. [DOI] [PubMed] [Google Scholar]
- 5. Plavina T, Singh CM, Sangurdekar D, et al. Association of serum neurofilament light levels with long-term brain atrophy in patients with a first multiple sclerosis episode. JAMA Netw Open 2020; 3: e2016278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferreira-Atuesta C, Reyes S, Giovanonni G, et al. The evolution of neurofilament light chain in multiple sclerosis. Front Neurosci 2021; 15: 642384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Comabella M, Montalban X. Body fluid biomarkers in multiple sclerosis. Lancet Neurol 2014; 13: 113–126. [DOI] [PubMed] [Google Scholar]
- 8. Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol 2020; 77: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varhaug KN, Torkildsen Ø, Myhr K-M, et al. Neurofilament light chain as a biomarker in multiple sclerosis. Front Neurol 2019; 10: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141: 2382–2391. [DOI] [PubMed] [Google Scholar]
- 11. Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol 2022; 21: 246–257. [DOI] [PubMed] [Google Scholar]
- 12. Thebault S, Abdoli M, Fereshtehnejad S-M, et al. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep 2020; 10: 10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Comi G, de Stefano N, Freedman MS, et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol 2012; 11: 33–41. [DOI] [PubMed] [Google Scholar]
- 14. Kuhle J, Leppert D, Comi G, et al. Effect of interferon β-1a treatment on serum neurofilament light chain levels in patients with a first clinical demyelinating event in the REFLEX trial (P1417). Mult Scler 2019; 25: 785–786. [Google Scholar]
- 15. Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalla Costa G, Martinelli V, Sangalli F, et al. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology 2019; 92: e733–e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arrambide G, Espejo C, Eixarch H, et al. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 2016; 87: 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92: e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varhaug KN, Barro C, Bjørnevik K, et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2018; 5: e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89: 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol 2019; 76: 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bittner S, Steffen F, Uphaus T, et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: a longitudinal multicentre cohort study. EBioMedicine 2020; 56: 102807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cerqueira JJ, Compston DAS, Geraldes R, et al. Time matters in multiple sclerosis: can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis? J Neurol Neurosurg Psychiatry 2018; 89: 844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol 2018; 31: 233–243. [DOI] [PubMed] [Google Scholar]
- 25. Yamout B, Sahraian M, Bohlega S, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult Scler Relat Disord 2020; 37: 101459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864241239101 for Serum neurofilament light chain correlations in patients with a first clinical demyelinating event in the REFLEX study: a post hoc analysis by Jens Kuhle, David Leppert, Giancarlo Comi, Nicola de Stefano, Ludwig Kappos, Mark S. Freedman, Andrea Seitzinger and Sanjeev Roy in Therapeutic Advances in Neurological Disorders