Abstract
Background:
The spectrum of disease-modifying therapies (DMTs) for people with multiple sclerosis (PwMS) has expanded over years, but data on treatment strategies is largely lacking. DMT switches are common clinical practice.
Objective:
To compare switchers and non-switchers, characterize the first DMT switch and identify reasons and predictors for switching the first DMT.
Methods:
Data on 2722 PwMS from the German MS Registry were retrospectively analyzed regarding sociodemographic/clinical differences between 1361 switchers (PwMS discontinuing the first DMT) and non-switchers matched according to age, sex, and observation period. Frequencies of first and second DMTs were calculated and switch reasons identified. Predictors for DMT switches were revealed using univariable and multivariable regression models.
Results:
Switchers and non-switchers differed significantly regarding time to first DMT, education, calendar period of the first DMT start (2014–2017 versus 2018–2021), first DMT class used [mild-to-moderate efficacy (MME) versus high-efficacy (HE) DMT], time on first DMT, and disease activity at first DMT start or cessation/last follow-up. The majority of PwMS started with MME DMTs (77.1%), with the most common being glatiramer acetate, dimethyl/diroximel fumarate, and beta-interferon variants. Switchers changed treatment more often to HE DMTs (39.6%), most commonly sphingosine-1-phosphate receptor modulators, anti-CD20 monoclonal antibodies, and natalizumab. Fewer PwMS switched to MME DMTs (35.9%), with the most common being dimethyl/diroximel fumarate, teriflunomide, or beta-interferon. Among 1045 PwMS with sufficient data (76.8% of 1361 switchers), the most frequent reasons for discontinuing the first DMT were disease activity despite DMT (63.1%), adverse events (17.1%), and patient request (8.3%). Predictors for the first DMT switch were MME DMT as initial treatment [odds ratio (OR) = 2.83 (1.76–4.61), p < 0.001; reference: HE DMT], first DMT initiation between 2014 and 2017 [OR = 11.55 (6.93–19.94), p < 0.001; reference: 2018–2021], and shorter time on first DMT [OR = 0.22 (0.18–0.27), p < 0.001].
Conclusion:
The initial use of MME DMTs was among the strongest predictors of DMT discontinuation in a large German retrospective MS cohort, arguing for the need for prospective treatment strategy trials, not only but also on the initial broad use of HE DMTs in PwMS.
Keywords: discontinuation, disease-modifying therapy, multiple sclerosis, switch
Introduction
Multiple sclerosis (MS) is the most common neurological immune-mediated disease that mainly affects young adults.1–3 The average age of people diagnosed with MS is between 20 and 49 years. 4 Worldwide, approximately 2.9 million people suffer from MS, particularly in Northern America and Western Europe.3,5 In Germany, the number of people with MS (PwMS) is estimated at approximately 280,000. 3 The disease courses of PwMS are just as variable as the symptoms that might occur. Thus, MS relapses can appear at variable intervals and are accompanied by a temporary or permanent disease worsening. Commonly used MS classifications try to account for a variable presentation from disease onset and during the disease course [relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), or primary progressive MS].1,6 The symptoms can affect, for example, motor function, cognition, gastrointestinal tract, urogenital tract, etc.; specific symptoms are highly dependent on the location of inflammatory and degenerative lesions and functional disturbances in the central nervous system.7,8
Disease-modifying therapies (DMTs) that affect different targets in the immune-mediated pathogenesis play an important role in MS management, as these drugs are designed to reduce the occurrence of relapses and MS progression. The first DMT available for MS treatment, interferon beta-1b, was approved in the 1990s. 9 Since then, numerous new therapeutic approaches have been developed and corresponding preparations have been approved, such as the first monoclonal antibody (MAB) for intravenous application (natalizumab), the first oral therapeutic (fingolimod), and the first MAB for subcutaneous injection (ofatumumab).2,10 It is expected that a large number of other DMTs will be available in clinical practice in the near future. 11 On the one hand, in addition to individualized therapy initiation in PwMS, this spectrum of therapeutic options makes it possible to appropriately react to medical events, such as DMT intolerance, desire to have children, insufficient DMT efficacy, or accommodation of patient preferences. On the other hand, the wide range of DMTs carries an increased risk of side effects.12–14 As a result, therapy decisions require a more sophisticated decision-making approach than they did 10 years ago, and DMT switches in PwMS are regular interventions in the clinical practice and might be required for a wide spectrum of reasons.
Studies on the switching behavior of individual DMTs have been performed, as in the case of fingolimod or natalizumab.15,16 For example, in a previously published study, we concluded that PwMS treated with fingolimod showed a faster switch behavior than 10 years ago. Moreover, young and female patients, in particular, tended to stop fingolimod treatment within a shorter period of time. 15 Another study by Kalincik et al. compared PwMS who switched from injectable DMTs to natalizumab or fingolimod. Switching the DMT to natalizumab appeared to be more effective than switching to fingolimod considering the reduction of the relapse rate as well as short-term disability level. 16 The results of these studies provide valuable evidence for the management of switches of certain DMTs in clinical practice. However, comprehensive studies on the DMT switching behavior among PwMS in a real-world setting covering the entire and newer DMT spectrum, including CD20 antibodies, are scarce. Those studies are essential to better understand the full range of therapeutic patterns and eventually to draw conclusions for the treatment of PwMS and also for initial treatment decisions.
Therefore, the first aim of this Germany-wide study was to identify sociodemographic, clinical-neurological, and therapeutic differences between PwMS discontinuing their first DMT at least once (switchers) and those who never switched their first DMT (non-switchers). Secondly, the first DMT switch was characterized in consideration of all DMTs available in Germany, including the identification of reasons for switching. Predictors for discontinuing the first DMT were identified, and the treatment duration of the first DMT was analyzed depending on the number of switches, the calendar period of the first DMT onset, and the first DMT class.
Materials and methods
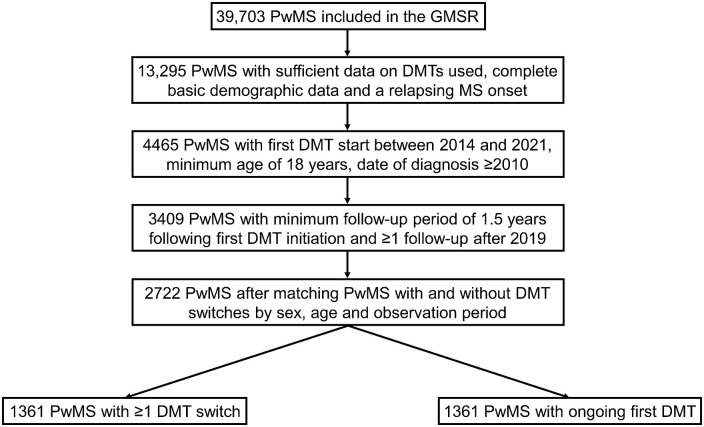
In 2001, the German MS Registry (GMSR) was initiated by the German MS Society and aimed to acquire sociodemographic, clinical, and therapeutic data on PwMS in Germany.17,18 Moreover, these comprehensive and comparable data intended to support research on MS. Following technical revisions in 2014, the GMSR also started collecting comprehensive data on DMTs (e.g. therapy duration, DMT class, reasons for treatment switch and/or discontinuation), presented in detail in an article by Ohle et al. 19 The cut-off date for this analysis was 31 December 2021, with data export occurring only on 1 December 2022 due to subsequent documentation in the GMSR. From the 39,703 PwMS included in the GMSR at the date of data export, 3409 had complete basic demographic data. Inclusion criteria comprised the following: sufficient data on DMTs used, relapsing MS onset, treatment initiation with the first DMT between 2014 and 2021, minimum age of 18 years, date of MS diagnosis in 2010 or later, minimum follow-up of 1.5 years after first DMT initiation, and at least one follow-up visit after 2019 (Figure 1). Finally, 2722 PwMS remained following the 1:1 matching of patients with and without DMT switches (N = 1361, respectively) by sex, age at first DMT start, and observation period. The selection of 1361 PwMS at a 1:1 ratio from a total population of 3409 ensures adequate comparability of both groups. This so-called population trimming approach leaves out a small portion of patients to ensure valid estimation of effects in the resulting ‘overlap population’. Leaving out such a small amount of patients, who have not enough matching controls based on covariates, is an approach described by Stürmer et al. 20 The sociodemographic and clinical profiles of the unmatched patients (N = 687) in comparison to the matched PwMS (N = 2722) have been included in Supplemental Table S1.
Figure 1.
Selection of people with MS. At the date of patient selection (1 December 2022), the GMSR contained data on 39,703 PwMS. In total, 3409 patients met the following inclusion criteria: complete basic demographic data, sufficient data on DMTs used, relapsing MS onset, first DMT start between 2014 and 2021, minimum age of 18 years, date of MS diagnosis from 2010, minimum follow-up of 1.5 years following the first DMT start and at least one follow-up visit after 2019. Matching patients with and without DMT switches by age at first DMT start, sex, and observation period revealed a study cohort of 2722 PwMS. Of those patients, 1361 stopped DMT at least once and 1361 persisted using one DMT without a break or cessation until the end of observation period.
DMT, disease-modifying therapy; GMSR, German Multiple Sclerosis Registry; MS, multiple sclerosis; PwMS, people with multiple sclerosis.
Statistical analysis
PwMS were divided into two groups, switchers and non-switchers, and then further divided into subgroups (based on the number of switches), the calendar period of DMT start and the efficacy class of the first DMT. They were also compared according to their sociodemographic, clinical, and therapeutic characteristics. The following patient subgroups were analyzed: PwMS with one versus two versus three or more DMT switches, patients whose first DMT started in 2014–2017 versus 2018–2021 and PwMS who started their first treatment with high-efficacy (HE) DMTs versus mild-to-moderate efficacy (MME) DMTs. Categorization of date ranges was based on the technical revision of the GMSR in 2014 and the approval of ocrelizumab as the first humanized anti-CD20 MAB for MS therapy in Germany in 2018. The classification of DMT efficacy is based on the guidelines for the therapy of MS by the German Society of Neurology. 21 HE DMTs include alemtuzumab, cladribine, cyclophosphamide, fingolimod, natalizumab, ocrelizumab, ofatumumab, ozanimod, ponesimod, rituximab, and siponimod. MME DMTs include dimethyl fumarate (DMF), diroximel fumarate (DRF), glatiramer acetate, interferon beta, and teriflunomide. Alemtuzumab and cladribine are typically administered in two treatment cycles, with additional cycles being rare. This is followed by a period of DMT-free wait-and-watch follow-up period. For this analysis, we considered any alternative therapy initiated after at least one treatment cycle of alemtuzumab or cladribine as a switch, which likely indicates breakthrough disease during the wait-and-watch follow-up period. Patients who discontinued DMT after completing at least one cycle of alemtuzumab or cladribine without starting another DMT until the end of the observation period were not considered switchers. For the comparisons of the patient subgroups, means and standard deviations, proportions of patients, and annualized relapse rates (ARRs; number of clinically diagnosed relapses per observation period in years) with 95% confidence intervals (CIs) were calculated. Chi-square tests, Fisher’s exact tests, Kruskal-Wallis tests, and Student’s t-tests were used when appropriate. The significance level of p values was set to α = 0.05. To visualize the first DMT switch (frequencies of DMTs used), alluvial graphs were created. For patients who never switched the first DMT, delay was censored on the date of the last neurological consultation. The reported reasons for discontinuing the first DMT were divided into the following categories: disease activity despite DMT, adverse events, patient request, physician’s decision, pregnancy, positive human polyomavirus 2 (JCV) antibody test in serum, DMT break, wish to have children, lack of adherence, market removal of DMT, and other. If there was no reported reason for discontinuing the first DMT, a surrogate parameter was utilized to evaluate disease activity despite DMT as a switch reason by meeting at least one of the following criteria: any relapse, increases in Expanded Disability Status Scale (EDSS) scores, magnetic resonance imaging (MRI) activity, increase in symptoms or SPMS within half a year before the end of therapy. Transitioning to SPMS, representing a gradual change for patients who previously suffered from RRMS (irreversible disability progression, reduced recovery from relapses), may indicate inadequate DMT efficacy in halting disease worsening or progression, as described by D’Amico et al. 22 Univariable and multivariable regression models were used to identify the variables associated with the first DMT switch. Survival curves included time on first DMT across multiple switches in relation to the order of DMTs used, first DMT efficacy class and period of the first DMT initiation. Data transformation and analyses were conducted using R v4.0 (R Foundation for Statistical Computing, Vienna, Austria; packages used: glm, comparegroups, survival, survmin, alluvial, finalfit23–26). Figures were created via R v4.0 and Microsoft Excel v2202 (Microsoft Corporation, Redmond, WA, USA).
Results
Study population
The 2722 matched (included) and 687 unmatched patients (excluded) differed in sex distribution, time from diagnosis to first DMT start, observation period since first DMT start, calendar period of first DMT start, time on first DMT, and ARR at first DMT start (Supplemental Table S1). In the study population of 2722 PwMS, the mean age at MS onset was 33.4 ± 10.3 years (Table 1). In regard to the matching criteria, 70.0% were female, the mean age at initiation of the first DMT was 36.7 ± 10.6 years, and the mean observation period since the first DMT was 4.4 ± 1.8 years. A total of 1361 patients (50.0%) discontinued their first DMT at least once (hereinafter referred to as switchers) and 1361 PwMS (50.0%) remained on their first DMT (hereinafter referred to as non-switchers). The mean time from the start to the cessation of the first DMT was 3.0 ± 2.1 years. More than half of the 1361 switchers (55.5%) changed the initial DMT within 1.5 years after the DMT start (for comparison: 27.8% of all patients), with a mean age at the first DMT switch of 37.9 ± 10.8 years. Exactly one switch was performed by 917 PwMS (67.4%), while 326 PwMS switched twice (24.0%) and 118 switched at least three times (8.7%). The comparison of these three switcher subgroups is shown in Supplemental Table S2. Switchers and non-switchers differed significantly in the time from diagnosis to first DMT initiation, education, calendar period of first DMT initiation, first DMT class used, time on first DMT, and disease activity at first DMT start and cessation/last follow-up (relapse and MRI), see Table 1.
Table 1.
Baseline comparison between DMT-switching and non-switching patients with MS matched by sex, age, and observation period.
| Variables | Total (N = 2722) | Switchers (N = 1361) | Non-switchers (N = 1361) | p |
|---|---|---|---|---|
| Age at MS symptom onset [years], mean (SD)* | 33.4 (10.3) | 33.2 (10.2) | 33.7 (10.4) | 0.233t |
| Disease duration from onset to first DMT [years], mean (SD)* | 2.8 (4.7) | 2.8 (5.0) | 2.8 (4.3) | 0.861t |
| Disease duration from diagnosis to first DMT [years], mean (SD) | 0.95 (1.7) | 0.7 (1.4) | 1.2 (1.9) | <0.001 t |
| Partnership status, N (%)* | 0.113Chi | |||
| Single | 622 (31.4) | 331 (33.1) | 291 (29.7) | |
| Any partnership | 1360 (68.6) | 670 (66.9) | 690 (70.3) | |
| Employment status, N (%)* | 0.639Fi | |||
| In training | 148 (7.5) | 73 (7.4) | 75 (7.7) | |
| Employed – full time | 1048 (53.4) | 522 (52.8) | 526 (54.1) | |
| Employed –part time | 377 (19.2) | 192 (19.4) | 185 (19.0) | |
| Retired – disability | 161 (8.2) | 88 (8.9) | 73 (7.5) | |
| Retired – old age | 38 (1.9) | 15 (1.5) | 23 (2.4) | |
| Other | 189 (9.6) | 98 (9.9) | 91 (9.4) | |
| Educational level, N (%)* | 0.039 Fi | |||
| NSCE | 22 (1.2) | 12 (1.3) | 10 (1.1) | |
| CSE/GCSE | 1093 (57.7) | 568 (59.9) | 525 (55.6) | |
| Advanced technical college entrance qualification | 187 (9.9) | 101 (10.6) | 86 (9.1) | |
| A level | 592 (31.3) | 268 (28.2) | 324 (34.3) | |
| Calendar period of first DMT start, N (%) | 0.011 Chi | |||
| 2014–2017 | 1925 (70.7) | 993 (73.0) | 932 (68.5) | |
| 2018–2021 | 797 (29.3) | 368 (27.0) | 429 (31.5) | |
| First DMT class, N (%) | <0.001 Chi | |||
| Mild-to-moderate efficacy | 2100 (77.1) | 1183 (86.9) | 917 (67.4) | |
| High efficacy | 622 (22.9) | 178 (13.1) | 444 (32.6) | |
| Time on first DMT [years], mean (SD) | 3.0 (2.1) | 1.7 (1.4) | 4.4 (1.7) | <0.001 t |
| EDSS at first DMT start, N (%)* | 0.807Fi | |||
| Mild (0.0–2.5) | 849 (82.1) | 411 (81.7) | 438 (82.5) | |
| Moderate/severe (⩾3.0) | 185 (17.9) | 92 (18.3) | 93 (17.5) | |
| EDSS worsening within 6 months before first DMT cessation/last follow-up, N (%)* | 7 (0.4) | 5 (0.8) | 2 (0.2) | 0.110Chi |
| ARR at first DMT start (95% CI) | 0.23 (0.21–0.25) | 0.26 (0.23–0.29) | 0.20 (0.18–0.23) | 0.005 Kru |
| Relapses within 6 months after first DMT start, N (%) | 211 (7.8) | 147 (10.8) | 64 (4.7) | <0.001 Chi |
| ARR at first DMT cessation/last follow-up (95% CI) | 0.22 (0.19–0.24) | 0.36 (0.32–0.39) | 0.07 (0.06–0.09) | <0.001 Kru |
| MRI results at first DMT start, N (%)* | 0.724Chi | |||
| Stable | 60 (29.9) | 30 (28.3) | 30 (31.6) | |
| Unstable | 141 (70.1) | 76 (71.7) | 65 (68.4) | |
| MRI results within 6 months after first DMT start, N (%)* | 0.990Chi | |||
| Stable | 319 (62.5) | 234 (62.4) | 85 (63.0) | |
| Unstable | 191 (37.5) | 141 (37.6) | 50 (37.0) | |
| MRI results at first DMT cessation/last follow-up, N (%)* | <0.001 Chi | |||
| Stable | 708 (75.9) | 237 (59.0) | 471 (88.7) | |
| Unstable | 225 (24.1) | 165 (41.0) | 60 (11.3) | |
| Matching variables | ||||
| Sex, N (%) | p > 0.999Chi | |||
| Female | 1906 (70.0) | 953 (70.0) | 953 (70.0) | |
| Male | 816 (30.0) | 408 (30.0) | 408 (30.0) | |
| Age at first DMT start [years], mean (SD) | 36.7 (10.6) | 36.2 (10.6) | 36.6 (10.6) | 0.332t |
| Observation period [years], mean (SD) | 4.4 (1.8) | 4.5 (1.8) | 4.4 (1.7) | 0.186t |
Denominators may differ due to missing values.
Bold italized indicates statistical significance.
ARR, annualized relapse rate; Chi, chi-square test; CI, confidence interval; CSE/GCSE, certificate of secondary education/ general CSE; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; Fi, Fisher’s exact test; Kru, Kruskal-Wallis test; MRI, magnetic resonance imaging; MS, multiple sclerosis; N, number of patients; NSCE, no school-leaving certificate; SD, standard deviation; t, Student’s t test.
Characterization of the first DMT switch
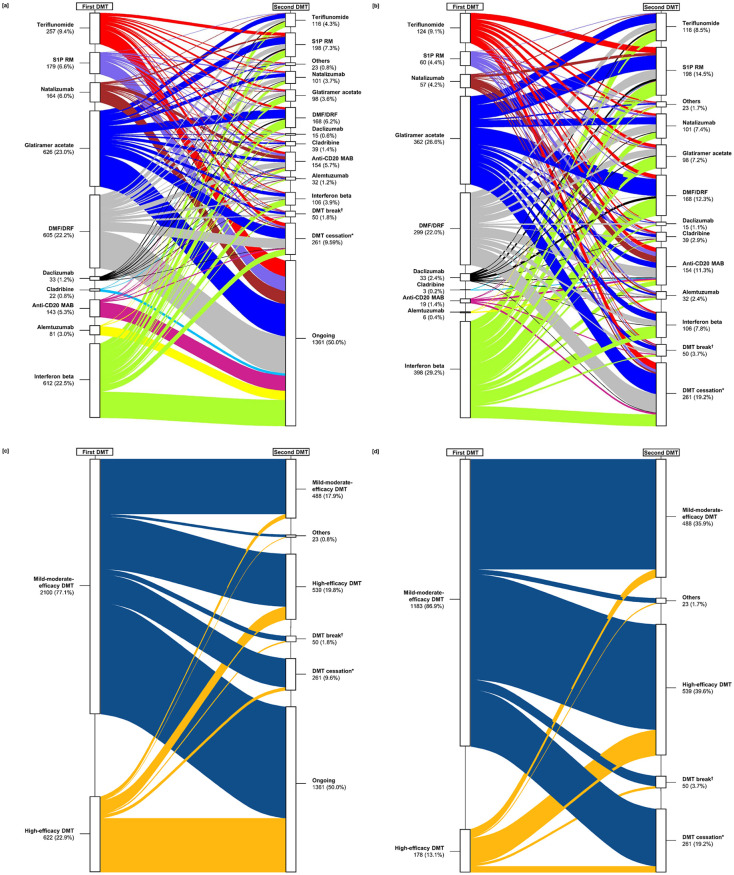
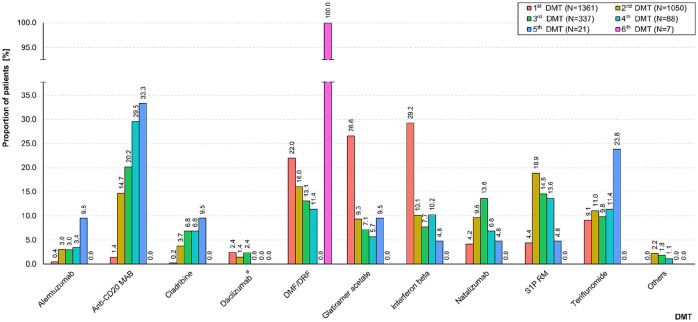
In the total study population (N = 2722), most patients started with the MME DMT glatiramer acetate (23.0%), interferon beta (22.5%), or DMF/DRF (22.2%). PwMS switched slightly more often from the first DMT to HE DMTs than to MME DMTs (19.8% and 17.9%, respectively). As shown in Figure 2, the most common subsequent (second) DMTs were sphingosine-1-phosphate receptor modulators [S1P RM; 7.3% in total; fingolimod (6.9%), ozanimod (0.1%), ponesimod (0.0%), siponimod (0.2%)], DMF/DRF (6.2%), and anti-CD20 MAB [5.7% in total; ocrelizumab (5.5%), ofatumumab (0.0%), rituximab (0.2%, off-label use)]. A total of 261 PwMS (9.6%) stopped the first DMT without reinitiating another one until the end of the observation period (median: 0.8 years, see Supplemental Table S3) and 50 patients (1.8%) paused the first DMT for a median time of 3.1 months. As shown in Table 2, the most common reason for discontinuing the first DMT among 1045 PwMS with sufficient data (76.8% of 1361 switchers) was disease activity despite DMT (63.1%), followed by adverse events (17.1%) and patient request (8.3%). When analyzing the frequencies of the single DMTs used across five subsequent switches, a decrease in the use of glatiramer acetate and DMF/DRF resulted. In addition, there was an increase in treatments with alemtuzumab, anti-CD20 MAB, cladribine, and teriflunomide across the switches (Figure 3).
Figure 2.
Characterization of the first DMT switch considering (a, c) all MS patients (N = 2722) and specifically (b, d) DMT-switching patients (N = 1361). The boxes on the left side represent the proportion of patients stratified by the first DMT used. Complementary to this, the immediately subsequent DMTs are shown on the right side (second DMTs). Boxes (a) and (b) show specific DMTs or DMT groups and boxes (c) and (d) visualize DMT efficacy categories. The color line sizes correspond to the proportions of patients using the respective DMTs. PwMS using mild-to-moderate efficacy DMTs more often switched to high-efficacy DMTs or stopped the therapy than patients initially treated with high-efficacy DMTs.
†Median time of DMT break (25% quantile, 75% quantile): 3.1 (0.9, 10.7) months.
*Median time from DMT cessation until the end of observation period (25% quantile, 75% quantile): 0.8 (0.3, 1.8) years.
Anti-CD20 MAB, anti-CD 20 monoclonal antibodies: ocrelizumab/ofatumumab/rituximab; DMF, dimethyl fumarate; DMT, disease-modifying therapy; DRF, diroximel fumarate; MS, multiple sclerosis; N, number of patients; PwMS, people with multiple sclerosis; S1P RM, sphingosine-1-phosphate receptor modulators: fingolimod/ozanimod/ponesimod/siponimod.
Table 2.
Reasons for first DMT discontinuation among MS patients.
| Reasons for first DMT discontinuation, N (%) | 1045 (100.0) |
| Disease activity despite DMT (reported + surrogate): | 659 (63.1) |
| Disease activity despite DMT (reported) | 249 (23.8) |
| Disease activity despite DMT (surrogate)* | 410 (39.2) |
| Adverse events | 179 (17.1) |
| Patient request | 87 (8.3) |
| Physician’s decision | 22 (2.1) |
| Pregnancy | 22 (2.1) |
| JCV status positive | 17 (1.6) |
| DMT break | 10 (1.0) |
| Wish to have children | 10 (1.0) |
| Lack of adherence | 8 (0.8) |
| Market removal of DMT | 8 (0.8) |
| Other | 23 (2.2) |
Lack of therapy efficacy indicated by fulfilling ⩾1 of the following criteria: any relapse, increases in EDSS, MRI activity, increase in symptoms, or SPMS within half a year before the end of therapy (surrogate was only used in patients who discontinued the first DMT without providing a reason).
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; JCV, human polyomavirus 2; MRI, magnetic resonance imaging; MS, multiple sclerosis; N, number of patients; SPMS, secondary progressive MS.
Figure 3.
Frequencies of DMTs used across subsequent switches. Colored bars show the frequencies of DMTs used among treatment-switching PwMS (N = 1361), stratified by the sequence of DMT switches (from the first to the sixth DMT, respectively). Across the subsequent DMT switches, a decrease in the use of glatiramer acetate and DMF/DRF was observed, while the treatment with alemtuzumab, anti-CD20 MAB, cladribine, and teriflunomide increased.
*Market removal in March 2018.
Anti-CD20 MAB, anti-CD 20 monoclonal antibodies: ocrelizumab/ofatumumab/rituximab; DMF, dimethyl fumarate; DMT, disease-modifying therapy; DRF, diroximel fumarate; N, number of patients; PwMS, people with multiple sclerosis; S1P RM, sphingosine-1-phosphate receptor modulators: fingolimod/ozanimod/ponesimod/siponimod.
Predictors for the discontinuation of the first DMT among MS patients
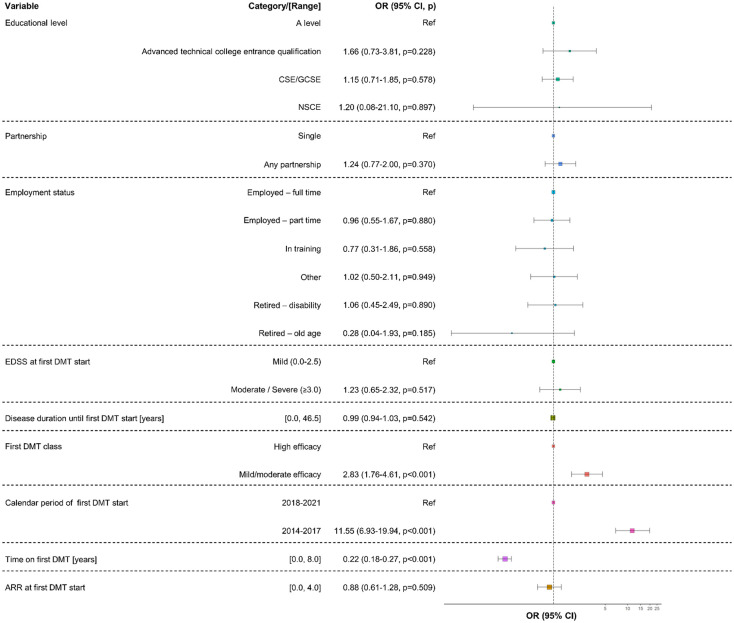
According to the univariable regression model, stopping the first DMT was significantly associated with five variables: lower educational level [certificate of secondary education: odds ratio (OR) = 1.31 (95% CI: 1.07–1.60), p = 0.009; advanced technical college entrance qualification: OR = 1.41 (1.02–1.98), p = 0.038; reference: A level], higher ARR at first DMT start [OR = 1.24 (1.07–1.44), p = 0.005], MME DMT as initial treatment [OR = 3.22 (2.65–3.91), p < 0.001; reference: HE DMT], first DMT start between 2014 and 2017 [OR = 1.23 (1.05–1.47), p < 0.010; reference: 2018–2021], and shorter time on the first DMT [OR = 0.38 (0.36–0.41), p < 0.001], as shown in Supplemental Table S4. Of these predictors, three remained significant in the multivariable regression model: MME DMT as initial treatment [OR = 2.83 (1.76–4.61), p < 0.001; reference: HE DMT], first DMT initiation between 2014 and 2017 [OR = 11.55 (6.93–19.94), p < 0.001; reference: 2018–2021], and shorter time on the first DMT [OR = 0.22 (0.18–0.27), p < 0.001], and shown in Figure 4.
Figure 4.
Predictors of the first DMT switch among MS patients. A multivariable logistic regression model was used to identify variables associated with the first DMT switch among 2722 PwMS. The forest plot contains colored boxes indicating the ORs of the variables analyzed for discontinuing the first DMT. Box sizes represent the number of patients included. Whiskers symbolize the 95% CIs of ORs. Initiating the first DMT between 2014 and 2017, shorter time on the first DMT and mild-to-moderate efficacy drugs as first DMT have been identified to favor switching or stopping the first DMT.
ARR, annualized relapse rate; CI, confidence interval; CSE/GCSE, certificate of secondary education/general CSE; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; NSCE, no school-leaving certificate; OR, odds ratio; p, p-value; Ref, reference.
Time on DMT in relation to number of switches, calendar period of first DMT initiation and first DMT class
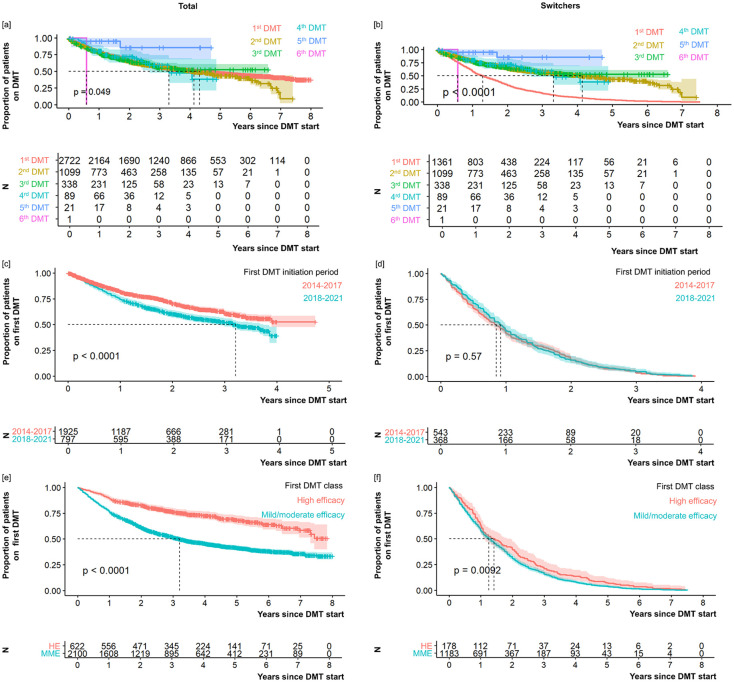
As shown in Table 1, the mean time on the first DMT was 3.0 ± 2.1 years among the 2722 PwMS analyzed. When considering the switchers only (N = 1361), a much lower mean time of 1.7 ± 1.4 years resulted. Plotting the treatment duration as a survival curve across multiple switches indicated a (slightly) significant association with the number of switches for all PwMS analyzed (p = 0.049) and particularly for switchers only (p < 0.0001), as shown in Figure 5. For the switchers group, the treatment duration was shorter for the initial DMT than for the subsequent ones. When dividing the patients by the calendar period of their first DMT initiation (Supplemental Table S5) and by the efficacy class of their first DMT (Supplemental Table S6), patients with DMT initiation between 2014 and 2017 (all patients: log-rank p < 0.0001) and those who were initially treated with a HE DMT (all patients: log-rank p < 0.0001; switchers: log-rank p = 0.009) remained on their first DMT longer.
Figure 5.
Treatment duration with regard to the number of DMT switches (a, b), the period of the first DMT (c, d), and the efficacy category of the first DMT (e, f). Graphs show proportions (upper graph parts, respectively) and numbers (lower graph parts, respectively) of patients (left: total study population of 2722 PwMS; right: 1361 switchers) being on DMT (y-axis) across time following DMT initiation (x-axis), visualized as survival curves. The treatment duration mainly varies, particularly for the initial switch (total study population: p = 0.049; switchers only: p < 0.0001), while the retention time for subsequent DMTs is comparable. Furthermore, survival curves revealed a discontinuation of DMT after shorter treatment duration in patients who initiated their first DMT between 2018 and 2021 (total study population: p < 0.0001) and in those who received a mild-to-moderate efficacy DMT as first treatment (total study population: p < 0.0001; switchers only: p = 0.0092). The date of the last neurological consultation was chosen as censoring.
DMT, disease-modifying therapy; HE, high efficacy; MME, mild-to-moderate efficacy; MS, multiple sclerosis; N, number of patients; p, log-rank p-value; PwMS, people with MS.
Discussion
The DMT options and therapy approaches for MS have expanded over the years.27–30 In clinical practice, two primary treatment strategies often debated are treatment escalation and early HE treatment.31–33 The escalation approach suggests that MME DMTs are initially prescribed, with the option to escalate to HE DMTs if disease activity or progression occurs. The advantage of treatment escalation is that it avoids immediate exposure to the potential risks of HE DMTs, making it a more conservative strategy. On the other hand, early HE treatment involves the initiation of HE DMTs right from the start, even in patients with relatively mild or early-stage MS. This therapy approach aims to prevent or minimize relapses and delay the accumulation of disability, potentially leading to better long-term outcomes.31,32 The choice between these strategies often depends on the patient’s disease characteristics, such as the level of disability, disease aggressiveness, and individual risk factors. Real-world data are essential to assess the current situation of the DMT landscape in PwMS and develop as well as improve MS treatment approaches. 34 The aim of our investigation was to elucidate the time before and after the first DMT switch and to identify reasons for discontinuation of the first DMT, using real-world data of more than 2700 patients from the GMSR. For this purpose, the practice of DMT switching in Germany was investigated, in addition to findings from other large MS registries,33,35–37 and results provide new evidence for the necessary adjustment of current clinical therapy approaches.
In our analysis, the patients examined stopped their first DMT after an average of 3 years, with over a quarter switching after 1.5 years. Similar results were found in a study by Saccà et al., 38 who examined a cohort of 2954 newly diagnosed PwMS from 24 Italian MS centers. More than 30% of the study population changed first DMT after 2 years. After 3 years, this proportion had risen to 48%. 38 Furthermore, in a US claims-based study by Fox et al., 39 which included over 14,000 MS patients, 51.3% of MS patients changed DMTs at least once, and 26.5% at least twice during an observation period of 2–10.5 years. These representative studies undoubtedly indicate that multiple therapy changes after initial DMT are common clinical practice globally.
The majority of patients in this GMSR-based study started with injectables (interferon beta variants or glatiramer acetate) or with the orally administered DMF or DRF. When discontinuing the first DMT, HE DMTs like S1P RM, anti-CD20 MAB, and natalizumab, as well as MME DMTs like DMF/DRF, teriflunomide, and interferon beta, were the most frequently used follow-up DMTs. Similar observations regarding follow-up therapies were also obtained in the multicenter, cross-sectional non-interventional study by Patti et al. 40 (i.e. SWITCH study) in which 303 patients from Italian MS centers switched their DMT, while 30 took a temporary break from therapy and three patients stopped their therapy permanently. 40 Among those switchers, the most common follow-up therapies after discontinuation of interferon beta or glatiramer acetate were DMF, fingolimod, and teriflunomide. 40 In the claims-based study by Fox et al., glatiramer acetate and interferon beta-1a also represented the most common initial DMTs (used in 76.8% of patients) and the first follow-up therapies after a switch were predominantly glatiramer acetate (32.0%), DMF (15.9%), and interferon beta-1a (13.8%). 39 The absence of anti-CD20 MAB in the studies by Patti et al. and Fox et al. is explained by the period of data collection; Patti et al. collected data between June 2016 and June 2017, and Fox et al. collected data from January 2006 to March 2018).39,40 Moreover, a retrospective analysis by Duquette et al. 41 indicated that, in a cohort of more than 12,000 privately insured PwMS from Canada, compliance rates (measured by medication possession ratio) were lower when using injectables (interferon beta and glatiramer acetate; 6 months: 53%, 24 months: 35%) as compared when using fingolimod (6 months: 75%, 24 months: 70%) or teriflunomide (6 months: 76%, 24 months: 68%). 41 To conclude, injectable DMTs still represent popular initial therapies but are usually replaced by DMTs of higher efficacy or oral DMTs at first switch, which is also related to the availability of the increasing number of DMTs (market entries) over time.
In clinical practice, each switch carries individual risks, such as lack of tolerability of the subsequent drug, return of disease activity during the washout period, or subsequent therapy adherence.42–48 Every therapy switch is based on both an expectation and a reason. 49 In the non-interventional, cross-sectional study by Mäurer et al. 50 involving 595 PwMS from 50 sites in Germany, post-switch expectations of patients and physicians differed. The most common expectations of physicians regarding new DMTs were the prevention of relapses (71.1%) and new MRI activity (61.3%), while patients most often reported good tolerability (53.9%) and effects on progression of disability (50.8%). Our analyses, based on data obtained by health care professionals, revealed that the most frequent reasons for switching the first DMT were disease activity despite therapy (62.3%), adverse events (17.5%), and patient request (8.2%). In addition to escalating to HE DMTs, switches within the same efficacy class and even the same substance class were also observed. These ‘horizontal’ switches can also be attributed to tolerability and adherence problems. An analysis of 110,326 PwMS from Big MS Data Network (including data from five clinical MS registries) revealed similar results: lack of efficacy (23.2%), side effects (16.1%), and lack of tolerability (13.8%) were reported as the most frequent reasons for switching the DMT. 51 In addition to the most common switch reasons (lack of efficacy in 58.4%, poor safety/tolerability in 33.0%), the SWITCH study by Patti et al. 40 also evaluated reasons for temporary treatment interruptions, with pregnancy being the most commonly reported reason (over 40% of patients).
With the increasingly popular approach of early MS treatment with HE DMTs,30,33,35–37 the predictors identified seem plausible. Data suggest a positive long-term effect when MS therapy is initiated with a highly potent DMT. New HE DMTs, such as ocrelizumab, ofatumumab, and cladribine, have been approved in recent years, therefore expanding the range of established highly potent DMTs, such as natalizumab, and increasingly leading clinicians away from the escalation therapy approach. 52 Compared to 10 years ago, a wider range of therapies and, thus, switch options are available. The study by Saccà et al. 38 analyzing data from 2954 Italian PwMS also indicated that there is a risk reduction of 50% or 87% when switching the DMT due to insufficient efficacy when MS therapy was initiated with fingolimod (p = 0.009) or natalizumab (p < 0.001), respectively, instead of interferon beta. 38 The risk for switching the DMT due to insufficient efficacy was also reduced when starting with DMF (by 40%; p = 0.037) or teriflunomide (by 79%; p = 0.031) compared to interferon beta. Focusing on switches due to a lack of tolerability, the risk for DMT switches was reduced when starting with fingolimod [hazard ratio (HR) = 0.35, p = 0.002], glatiramer acetate (HR = 0.61, p = 0.001) and DMF (HR = 0.57, p = 0.022) compared to interferon beta. However, it was higher when starting with natalizumab (HR = 1.43, p = 0.022); the reason for switching from natalizumab to alternative therapy in 49 out of 57 patients (86%) was a positive JCV antibody test. 38 Returning to the predictors of DMT switches, some interesting results can be found in the literature. In the study by Saccà et al., 38 several variables were noted to be independently associated with a higher rate of switches due to inefficiency: delayed MS diagnosis (HR = 1.23, p = 0.021), younger age (HR = 0.96, p < 0.001), spinal cord lesions (HR = 1.46, p = 0.001), and a higher EDSS score at baseline (HR = 1.17, p = 0.001). 38 The EDSS score was also associated with a DMT switch in a multivariable logistic regression model by Patti et al. (SWITCH study). However, no reasonable estimate for this variable was found that could predict this result. 40 In a study by Teter et al. 53 of 606 PwMS treated with interferon beta or glatiramer acetate, of whom 214 patients switched their DMT, predictors of a DMT switch included the occurrence of at least two relapses (OR = 2.8, p = 0.040), MRI worsening (OR = 6.3, p < 0.001), EDSS worsening (OR = 2.2, p = 0.009), and a combination of EDSS and MRI worsening (OR = 2.5, p = 0.031), compared with only one recorded relapse. 53 These differences in identified predictors of switches may be due to many factors, such as the type of statistical model used, the variables included in the model, and the population studied (size, composition).
Our analysis has several limitations. First, switch reasons are only available for 1045 of 1361 switchers (76.8%). Switch reasons are part of the pharmacovigilance module of the GMSR and have only been recorded since 2019. Since then, the amount of data collected has been growing steadily. Second, EDSS scores at first switch and MRI data, which may represent important switch criteria, were available only for a limited number of patients studied. In addition, the comprehensive range of DMTs was grouped into MME DMTs and HE DMTs following the guidelines by the German Neurological Society, 21 a classification not generally accepted and not entirely based on evidence but expert opinion. Additional analyses for single DMTs might provide added insights but were not considered. Third, only a German cohort was studied, limiting generalizability.
Our study has several strengths. First, the infrastructure of the GMSR allowed for a large study population and a comprehensive matching procedure to compare switchers and non-switchers. The GMSR gathers data from more than 80,000 PwMS, which accounts for around 29% of the estimated 280,000 PwMS in Germany. 3 Furthermore, the GMSR represents the German health care system covering patients with statutory health insurance (which accounts for approximately 87% of the German population), private health insurance, and other reimbursements. 19 In addition, in this study, a wide range of available DMTs was analyzed over an 8-year period to reflect real-life scenarios on prescribed DMTs and treatment strategies.
Conclusion
In conclusion, our Germany-wide real-world study found that the majority of patients examined started with an MME DMT as initial therapy, irrespective of the calendar period analyzed. This illustrates that a majority of initial treatment decisions follow an escalation approach, switching to an HE DMT when considered needed. This fits with a second finding of our study, which indicated that MS disease activity despite DMT use represented the most common reason for DMT switches.
The current German MS treatment guidelines advise a treat-to-target strategy, 21 aiming to use the optimal DMT for the individual PwMS based on the anticipated inflammatory activity of the disease. However, this approach is under debate, as:
(1) The physician’s ability to predict the individual MS disease course at onset is limited.
(2) The safety profile of some of the newer HE DMTs is favorable, and the tolerability superior to some of the MME DMTs.
(3) Short-term direct head-to-head studies demonstrate superiority of some of the HE DMTs compared to some of the MME DMTs. 54
(4) Real-world data suggest superiority of the early use of HE DMTs on disability progression. 36
Consequently, our real-world registry study – indicating a high frequency of DMT switches due to insufficient disease control – supports a view that prospective, longer-term treatment strategy studies are needed. This is of importance, considering that MS is a lifelong disease that rarely has an entirely benign course if PwMS are carefully followed. In addition to comparing the early use of HE DMTs with an escalating or treat-to-target strategy, such studies would need to also consider anticipated switch or treatment interruption strategies, as one of the main limitations to the broad use of HE DMTs might be the lack of a deescalating or discontinuation plan for most of the approved compounds. It is reassuring to see that these topics, including DMT discontinuation in predefined populations, 55 are gaining increasing attention among PwMS and MS researchers.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-3-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-4-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-5-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-6-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-7-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Acknowledgments
We would like to thank all the patients who gave their informed consent. Furthermore, this study would not have been possible without the efforts of the centers participating in the registries. The centers are listed in the Supplemental Material.
Footnotes
ORCID iDs: Niklas Frahm  https://orcid.org/0000-0002-4655-774X
https://orcid.org/0000-0002-4655-774X
Ksenija Schirduan  https://orcid.org/0009-0004-0666-7444
https://orcid.org/0009-0004-0666-7444
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Niklas Frahm, German MS Registry, MS Forschungs- und Projektentwicklungs-gGmbH (MS Research and Project Development gGmbH [MSFP]), Krausenstr. 50, Hannover, Niedersachsen 30171, Germany.
David Ellenberger, German MS Registry, MS Forschungs- und Projektentwicklungs-gGmbH (MS Research and Project Development gGmbH [MSFP]), Hannover, Germany.
Alexander Stahmann, German MS Registry, MS Forschungs- und Projektentwicklungs-gGmbH (MS Research and Project Development gGmbH [MSFP]), Hannover, Germany.
Firas Fneish, German MS Registry, MS Forschungs- und Projektentwicklungs-gGmbH (MS Research and Project Development gGmbH [MSFP]), Hannover, Germany.
Daniel Lüftenegger, Biogen GmbH, München, Germany.
Hans C. Salmen, Biogen GmbH, München, Germany
Ksenija Schirduan, Biogen GmbH, München, Germany.
Tom P. A. Schaak, Biogen GmbH, München, Germany
Peter Flachenecker, Neurological Rehabilitation Center Quellenhof, Bad Wildbad, Germany.
Christoph Kleinschnitz, Department of Neurology and Center of Translational and Behavioral Neurosciences (C-TNBS), University Hospital Essen, Essen, Germany.
Friedemann Paul, Experimental and Clinical Research Center, Max Delbrueck Center for Molecular Medicine and Charité – Universitätsmedizin Berlin, Berlin, Germany.
Dagmar Krefting, Department of Medical Informatics, University Medical Center Göttingen, Göttingen, Germany.
Uwe K. Zettl, Department of Neurology, Neuroimmunological Section, University Medical Center of Rostock, Rostock, Germany
Melanie Peters, German MS Registry, Gesellschaft für Versorgungsforschung mbH (Society for Health Care Research [GfV]), Hannover, Germany.
Clemens Warnke, Department of Neurology, Medical Faculty, University Hospital of Cologne, Cologne, Germany.
Declarations
Ethics approval and consent to participate: The registration of the GMSR took place at the German Registry for Clinical Trials [Deutsches Register Klinischer Studien (DRKS); No. DRKS00011257]. The initial ethics vote was approved by University of Würzburg’s institutional review board (Permit No. 142/12). All participants provided written consent for the utilization of their anonymized data for research purposes.
Consent for publication: Not applicable.
Author contributions: Niklas Frahm: Conceptualization; Methodology; Writing – original draft.
David Ellenberger: Conceptualization; Methodology; Writing – review & editing.
Alexander Stahmann: Conceptualization; Methodology; Writing – review & editing.
Firas Fneish: Writing – review & editing.
Daniel Lüftenegger: Conceptualization; Writing – review & editing.
Hans C. Salmen: Writing – review & editing.
Ksenija Schirduan: Writing – review & editing.
Tom P. A. Schaak: Conceptualization; Writing – review & editing.
Peter Flachenecker: Writing – review & editing.
Christoph Kleinschnitz: Writing – review & editing.
Friedemann Paul: Writing – review & editing.
Dagmar Krefting: Writing – review & editing.
Uwe K. Zettl: Writing – review & editing.
Melanie Peters: Conceptualization; Methodology; Supervision; Writing – review & editing.
Clemens Warnke: Conceptualization; Methodology; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The German MS Registry of the German MS Society was initiated and funded by the German MS Foundation and the German MS Society in 2001. It is operated by a not-for-profit company, the MSFP. MSFP receives funding from a broad range of public and private sponsors, including the German MS Society, the German MS Foundation, the Innovation Fund of the German Federal Joint Committee, and the German Retirement Insurance. In 2023, Biogen, Bristol-Myers Squibb, Merck, Novartis, and Roche are participating in the multi-stakeholder funding approach to support the registry’s operation and to allow the collection and reporting of (pharmacovigilance) data required as part of the EMA-minimal data set. Industry funding does not result in restrictions to publishing data, nor do the funders have access to the raw data or have any influence over the scientific conduct of the registry. The evaluations contained in this paper were supported and funded by Biogen.
Competing interests: NF is an employee of the MSFP. Moreover, he is an employee of Rostock’s University Medical Center and received travel funds for research meetings from Novartis. MP, DE, and FF had no personal financial interests to disclose other than being employees of the German MS Registry. AS has no personal financial interests to disclose other than being the leader of the German MS Registry, which receives funding from a range of public and corporate sponsors, recently including G-BA, the German MS Trust, German MS Society, Biogen, Bristol Myers Squibb, Merck, Novartis, and Roche. DL, HS, and KS are employees of Biogen and hold stock or stock options in Biogen. PF has received speaker’s fees and honoraria for advisory boards from Almirall, Bayer, Biogen Idec, Celgene, Genzyme, Novartis, Merck Serono, Roche, and Teva. He has participated in pharmaceutical company-sponsored trials by Roche. None resulted in a conflict of interest. TS was employee of Biogen and held stock or stock options in Biogen. CK has received speaker’s fees, honoraria for attending advisory boards, and financial support for conducting research projects from Merck Serono GmbH, Germany and Merck KgaA, Germany. None resulted in a conflict of interest. FP has received speaking fees, travel support, honoraria from advisory boards, and/or financial support for research activities from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, Shire, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, Guthy-Jackson Charitable Foundation, and National Multiple Sclerosis of the USA. He serves as academic editor for PLoS One and associate editor for Neurology, Neuroimmunology, and Neuroinflammation. DK declares no competing interests relevant to the content of the submitted manuscript. UZ has received speaking fees, travel support, and/or financial support for research activities from Alexion, Almirall, Bayer, Biogen, Bristol-Myers-Squibb, Janssen, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, Teva as well as EU, BMBF, BMWi, and DFG. CW has received institutional support from Novartis, Biogen, Alexion, Janssen, and Roche.
Availability of data and materials: Anonymized data will be made available on request for any qualified investigator under the terms of the registry’s usage and access guidelines and subject to the informed consent of the patients.
References
- 1. Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers 2018; 4: 43. [DOI] [PubMed] [Google Scholar]
- 2. Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med 2020; 133: 1380–1390.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MS International Federation (MSIF). Number of people with MS | Atlas of MS, https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms (accessed 15 January 2024).
- 4. Gilmour H, Ramage-Morin PL, Wong SL. Multiple sclerosis: prevalence and impact. Health Rep 2018; 29: 3–8. [PubMed] [Google Scholar]
- 5. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler 2020; 26: 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rommer PS, Eichstädt K, Ellenberger D, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Mult Scler 2019; 25: 1641–1652. [DOI] [PubMed] [Google Scholar]
- 8. Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet 2018; 391: 1622–1636. [DOI] [PubMed] [Google Scholar]
- 9. Zettl UK, Hecker M, Aktas O, et al. Interferon β-1a and β-1b for patients with multiple sclerosis: updates to current knowledge. Expert Rev Clin Immunol 2018; 14: 137–153. [DOI] [PubMed] [Google Scholar]
- 10. Rommer P, Zettl UK. Treatment options in multiple sclerosis and neuromyelitis optica spectrum disorders. Curr Pharm Des 2022; 28: 428–436. [DOI] [PubMed] [Google Scholar]
- 11. Cree BAC, Hartung H-P, Barnett M. New drugs for multiple sclerosis: new treatment algorithms. Curr Opin Neurol 2022; 35: 262–270. [DOI] [PubMed] [Google Scholar]
- 12. Gross RH, Corboy JR. Monitoring, switching, and stopping multiple sclerosis disease-modifying therapies. Continuum (Minneap Minn) 2019; 25: 715–735. [DOI] [PubMed] [Google Scholar]
- 13. Moiola L, Rommer PS, Zettl UK. Prevention and management of adverse effects of disease modifying treatments in multiple sclerosis. Curr Opin Neurol 2020; 33: 286–294. [DOI] [PubMed] [Google Scholar]
- 14. Rommer PS, Zettl UK. Managing the side effects of multiple sclerosis therapy: pharmacotherapy options for patients. Expert Opin Pharmacother 2018; 19: 483–498. [DOI] [PubMed] [Google Scholar]
- 15. Frahm N, Fneish F, Ellenberger D, et al. Therapy switches in fingolimod-treated patients with multiple sclerosis: long-term experience from the German MS Registry. Neurol Ther 2022; 11: 319–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalincik T, Horakova D, Spelman T, et al. Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol 2015; 77: 425–435. [DOI] [PubMed] [Google Scholar]
- 17. Flachenecker P, Stuke K, Elias W, et al. Multiple-sklerose-register in Deutschland. Dtsch Ärztebl 2008; 105: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stuke K, Flachenecker P, Zettl UK, et al. Symptomatology of MS: results from the German MS Registry. J Neurol 2009; 256: 1932–1935. [DOI] [PubMed] [Google Scholar]
- 19. Ohle L-M, Ellenberger D, Flachenecker P, et al. Chances and challenges of a long-term data repository in multiple sclerosis: 20th birthday of the German MS Registry. Sci Rep 2021; 11: 13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stürmer T, Webster-Clark M, Lund JL, et al. Propensity score weighting and trimming strategies for reducing variance and bias of treatment effect estimates: a simulation study. Am J Epidemiol 2021; 190: 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hemmer B, et al. Diagnose und Therapie der Multiplen Sklerose, Neuromyelitis-optica-Spektrum-Erkrankungen und MOG-IgG-assoziierten Erkrankungen, S2k-Leitlinie, https://register.awmf.org/assets/guidelines/030-050l_S2k_Diagnose-Therapie-Multiple-Sklerose-Neuromyelitis-Optica-Spektrum-MOG-IgG-assoziierte-Erkrankungen_2023-05.pdf (2023, accessed 15 January 2024).
- 22. D’Amico E, Ziemssen T, Cottone S. To stop or not to stop disease modifying therapies in secondary progressive multiple sclerosis, that is the question. Expert Rev Neurother 2017; 17: 847–849. [DOI] [PubMed] [Google Scholar]
- 23. Bojanowski M, Edwards R. R package for creating alluvial diagrams, https://github.com/mbojan/alluvial (2016, accessed 15 January 2024).
- 24. Harrison E, Drake T, Ots R. Quickly create elegant regression results tables and plots when modelling, https://cran.r-project.org/web/packages/finalfit (2023, accessed 15 January 2024).
- 25. Subirana I, Sanz H, Vila J. Building bivariate tables: the compareGroups package for R. J Stat Softw 2014; 57: 1–16.25400517 [Google Scholar]
- 26. Therneau TM. A package for survival analysis in R, https://github.com/therneau/survival (accessed 15 January 2024).
- 27. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol 2018; 25: 215–237. [DOI] [PubMed] [Google Scholar]
- 28. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 777–788. [DOI] [PubMed] [Google Scholar]
- 29. Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 789–800. [DOI] [PubMed] [Google Scholar]
- 30. Wiendl H, Gold R, Berger T, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord 2021; 14: 17562864211039648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Casanova B, Quintanilla-Bordás C and Gascón F. Escalation vs. early intense therapy in multiple sclerosis. J Pers Med 2022; 12: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inojosa H, Proschmann U, Akgün K, et al. The need for a strategic therapeutic approach: multiple sclerosis in check. Ther Adv Chronic Dis 2022; 13: 20406223211063032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol 2021; 78: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jalusic KO, Ellenberger D, Rommer P, et al. Effect of applying inclusion and exclusion criteria of phase III clinical trials to multiple sclerosis patients in routine clinical care. Mult Scler 2021; 27: 1852–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 2019; 321: 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 2020; 19: 307–316. [DOI] [PubMed] [Google Scholar]
- 37. Prosperini L, Mancinelli CR, Solaro CM, et al. Induction versus escalation in multiple sclerosis: a 10-year real world study. Neurotherapeutics 2020; 17: 994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saccà F, Lanzillo R, Signori A, et al. Determinants of therapy switch in multiple sclerosis treatment-naïve patients: a real-life study. Mult Scler 2019; 25: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 39. Fox RJ, Mehta R, Pham T, et al. Real-world disease-modifying therapy pathways from administrative claims data in patients with multiple sclerosis. BMC Neurol 2022; 22: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patti F, Chisari CG, D’Amico E, et al. Clinical and patient determinants of changing therapy in relapsing-remitting multiple sclerosis (SWITCH study). Mult Scler Relat Disord 2020; 42: 102124. [DOI] [PubMed] [Google Scholar]
- 41. Duquette P, Yeung M, Mouallif S, et al. A retrospective claims analysis: compliance and discontinuation rates among Canadian patients with multiple sclerosis treated with disease-modifying therapies. PLoS One 2019; 14: e0210417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calabrese M, Pitteri M, Farina G, et al. Dimethyl fumarate: a possible exit strategy from natalizumab treatment in patients with multiple sclerosis at risk for severe adverse events. J Neurol Neurosurg Psychiatry 2017; 88: 1073–1078. [DOI] [PubMed] [Google Scholar]
- 43. González-Suarez I, Rodríguez de, Antonio L, Orviz A, et al. Catastrophic outcome of patients with a rebound after natalizumab treatment discontinuation. Brain Behav 2017; 7: e00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacot de, Alcântara I, Voruz P, Allali G, et al. Personality as a predictor of disability in multiple sclerosis. Arch Clin Neuropsychol 2023; 38: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klauer T, Zettl UK. Compliance, adherence, and the treatment of multiple sclerosis. J Neurol 2008; 255(Suppl. 6): 87–92. [DOI] [PubMed] [Google Scholar]
- 46. Kołtuniuk A, Pytel A, Krówczyńska D, et al. The quality of life and medication adherence in patients with multiple sclerosis-cross-sectional study. Int J Environ Res Public Health 2022; 19: 14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kołtuniuk A, Chojdak-Łukasiewicz J. Adherence to therapy in patients with multiple sclerosis-review. Int J Environ Res Public Health 2022; 19: 2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nygaard GO, Torgauten H, Skattebøl L, et al. Risk of fingolimod rebound after switching to cladribine or rituximab in multiple sclerosis. Mult Scler Relat Disord 2022; 62: 103812. [DOI] [PubMed] [Google Scholar]
- 49. Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases 2015; 3: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mäurer M, Tiel-Wilck K, Oehm E, et al. Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis. Ther Adv Neurol Disord 2019; 12: 1756286419892077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hillert J, Magyari M, Soelberg Sørensen P, et al. Treatment switching and discontinuation over 20 years in the big multiple sclerosis data network. Front Neurol 2021; 12: 647811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simpson A, Mowry EM, Newsome SD. Early aggressive treatment approaches for multiple sclerosis. Curr Treat Options Neurol 2021; 23: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Teter B, Agashivala N, Kavak K, et al. Characteristics influencing therapy switch behavior after suboptimal response to first-line treatment in patients with multiple sclerosis. Mult Scler 2014; 20: 830–836. [DOI] [PubMed] [Google Scholar]
- 54. Stankiewicz JM, Weiner HL. An argument for broad use of high efficacy treatments in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2020; 7: e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Corboy JR, Fox RJ, Kister I, et al. Risk of new disease activity in patients with multiple sclerosis who continue or discontinue disease-modifying therapies (DISCOMS): a multicentre, randomised, single-blind, phase 4, non-inferiority trial. Lancet Neurol 2023; 22: 568–577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-3-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-4-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-5-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-6-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-7-tan-10.1177_17562864241239740 for Treatment switches of disease-modifying therapies in people with multiple sclerosis: long-term experience from the German MS Registry by Niklas Frahm, David Ellenberger, Alexander Stahmann, Firas Fneish, Daniel Lüftenegger, Hans C. Salmen, Ksenija Schirduan, Tom P. A. Schaak, Peter Flachenecker, Christoph Kleinschnitz, Friedemann Paul, Dagmar Krefting, Uwe K. Zettl, Melanie Peters and Clemens Warnke in Therapeutic Advances in Neurological Disorders