Abstract
Infertility affects 15% of couples in reproductive age worldwide. In women in particular, infertility can be caused by various abnormalities, with polycystic ovary syndrome (PCOS) being the most common. Currently, there are many assisted reproductive techniques (ART) available to combat the burden of infertility. However, positive results are not guaranteed. The administration of inositol has been shown to increase positive reproductive outcomes in women undergoing ART. Here we present a series of clinical cases in which women with a history of infertility and previously failed ART, supplemented with a specific 3.6:1 MYO:DCI ratio, antioxidants, vitamins, and minerals for a period of 1 to 3 months before undergoing in vitro fertilization (IVF). In this series of case reports, we provide preliminary evidence that supplementation with a specific 3.6:1 MYO to DCI ratio, as well as antioxidants, vitamins, and minerals may contribute positively to female fertility in women undergoing IVF, with a history of primary or secondary infertility and previously failed ART.
Keywords: In vitro fertilization (IVF), female infertility, inositol, MYO:DCI ratio, polycystic ovary syndrome (PCOS)
Introduction
According to the World Health Organization, infertility is defined as the failure to achieve pregnancy after 12 months or longer of regular unprotected sexual intercourse. It is estimated to affect about 15% of reproductive-age couples worldwide. 1 Infertility in women may be caused by a wide range of abnormalities concerning the female reproductive and/or endocrine systems, with polycystic ovary syndrome (PCOS) being the main cause due to ovulatory disfunction.2,3
To help alleviate the burden of infertility, there are many assisted reproductive technologies (ART) available. In vitro fertilization (IVF) is one of the most used techniques which often involves intracytoplasmic sperm injection (ICSI). According to the Society for Reproductive Technology, an affiliate of the American Society for Reproductive Medicine, 55% of women aged less than 35-years-old were successful in achieving live births through IVF in 2019. 4 This percentage decreased as age increased, being 41% in those aged 35 to 37, 26.8% in those aged 38 to 40, 13.4% in those aged 41 to 42, and 4.1% in those older than 42. While it is well known that the most important factor associated with female infertility is age, uncertainty still surrounds the precise cause of this age associated decline in fertility. 5 However, the most important factors associated seem to be follicular reserve, oocyte quality, and increased reproductive tract defects. 6
For women with PCOS undergoing IVF/ICSI, the effects of inositol, a natural insulin sensitizer, have been studied by many authors and have been shown to improve reproductive outcomes. 7 Of its 9 stereoisomers, the 2 most relevant are myo-inositol (MYO) and D-chiro-inositol (DCI) as they have been shown to mediate the post receptor cascade of insulin. 3 Supplementation with MYO alone has been previously shown to improve hyperandrogenism as well as the overall metabolic profile of women with PCOS.8,9 However, a recent systemic review and meta-analysis demonstrated that supplementation with MYO alone is not sufficient to improve oocyte maturation, embryo quality, or pregnancy rate. 10 In a further study by the same group, supplementation with the combination of MYO and DCI, in a ratio of 3.6:1 respectively, were shown to improve pregnancy rates, as well as reduce the risk of ovarian hyperstimulation syndrome (OHSS). 11 Additionally, in another randomized controlled trial, supplementation with the same MYO:DCI ratio was seen to improve oocyte quality. 12 In a recently published randomized open-label study, using the same 3.6:1 MYO to DCI ratio it was seen to be effective in regularizing menstrual cycles and improving insulin resistance in 70 women with PCOS. 13 Another randomized study including 283 women with PCOS demonstrated the benefits of the 3.6:1 MYO to DCI ratio in improving the hormonal, glycemic and lipidic profile. 14
As inositols, the treatment with melatonin has also displayed beneficial effects on insulin levels and on hormonal regulation in women with PCOS. The supplementation of melatonin for 6 months has also been shown to restore the menstrual cycle in 40 women with PCOS, by significantly reducing the serum levels of androgens and anti-Mullerian hormone while raising the FSH levels. 15 Likewise, melatonin has proven to be efficient in improving ART outcomes including the embryo quality and clinical pregnancy rates. 16 The co-supplementation of melatonin and myo-inositol has been shown to act synergistically to improve oocyte and embryo quality, clinical pregnancy and implantation rates even after IVF failures.17 -20
Other natural antioxidants included in the food supplement under study, such as vitamins E, D, B12, B6, B2, B1, zinc, selenium, and natural polyphenols as Pomanox®—a powerful antioxidant patented from pomegranate extract—showed beneficial effects in improving female fertility, thanks to their ability to scavenge free radicals.21,22 Therefore, the combined supplementation with these antioxidants could be relevant in patients at advanced maternal age undergoing IVF since a well-known factor of physiological aging is the increased production of reactive oxygen species (ROS) in the ovary. 23
In this series of case reports, we provide preliminary evidence that treatment with a multi-ingredient food supplement with a specific 3.6:1 MYO to DCI ratio, as well as antioxidants, vitamins, and minerals may contribute positively to female fertility in women undergoing IVF and previously failed ART.
Case Reports
Here we present a series of 5 clinical cases involving women with a history of primary or secondary infertility and previously failed ART, who take a multi-component food supplement with a specific 3.6:1 MYO:DCI ratio, antioxidants, vitamins, and minerals (Ovosicare®) for a period of 1 to 3 months before undergoing IVF. Patients’ clinical data are summarized in Table 1. Informed consent was obtained from all participants included in this series of cases.
Table 1.
Clinical characteristics of the patients.
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Age (years) | 31 | 37 | 34 | 37 | 35 |
| BMI (kg/m2) | 26.0 | 25.3 | 22.0 | 26.0 | 31.4 |
| Obstetric history | G0 P0 | G0 P0 | G1 P1 | G1 A1 P0 | G0 P0 |
| PCOS diagnosis | Yes | No | Yes | Yes | Yes |
| Menstrual cycles | Irregular (every 50-60 d) | Regular (every 28 d) | Regular (every 30-31 d) | Irregular (every 45-60 d) | Irregular (every 30-35 d) |
| Relevant medical history | Obstruction of right fallopian tube | None | None | None | None |
| Toxic habits | None | None | None | None | None |
| Infertility time (years) | 2 | 3 | 1 | 2 | 3 |
| AMH (ng/mL) | 5.5 | 0.8 | 3.48 | 7.7 | 10.80 |
| FSH (IU/L) | 5 | 6.8 | 11.3 | 4 | 3.52 |
| LH (IU/L) | 9 | 4.5 | 7.9 | 5.3 | 7.70 |
| TSH (IU/L) | 1.9 | 1.2 | 1.94 | - | 2.30 |
| Estradiol (pg/mL) | - | - | 44 | 24 | 46.50 |
| Vitamin D | - | - | - | 10 (ng/mL) | 56.5 (nmol/L) |
| Semen analysis | Normal | Normal | Normal | - | Normal |
| Previous treatment | 2 Failed IVF cycles | 2 Failed IVF cycles | 4 Failed IUI using Ovusitol D® | 2 Failed ICSI cycles with metformin | 3 Failed IVF cycles |
| Type of ART | IVF | IVF | IVF | IVF/ICSI | IVF |
| Ovosicare® dosage | 2 Capsules per day | 2 Capsules per day | 2 Capsules per day | 2 Capsules per day | 1 Packet per day |
| Time taking Ovosicare® | 1 mo | 2 mo | 2 mo | 3 mo | 3 mo |
Abbreviations: A, abortion; AMH, anti-mullerian hormone; ART, assisted reproductive technique; BMI, body mass index; FSH, follicle-stimulating hormone; G, gravidity; ICSI, intracytoplasmic sperm injection; IUI, intrauterine insemination; IVF, in vitro fertilization; LH, luteinizing hormone; P, parity; PCOS, polycystic ovary syndrome; TSH, thyroid-stimulating hormone.
Clinical case 1
A 31-year-old female patient attended the fertility clinic at the Hospital Universitario Materno Infantil de Canarias in Gran Canaria, Spain, to consult over primary infertility of 2 years of evolution. The patient had previously been diagnosed with PCOS; however, besides that, she had no other relevant past medical history and denied participating in any toxic habits. She presented with irregular menstrual cycles every 50 to 60 days and reported occasionally experiencing amenorrhea of up to 6 months.
During physical examination the patient presented normal. On transvaginal ultrasound a normal retroverted uterus and both ovaries (each with more than 15 antral follicles) were visualized. The patient, and her partner were both ordered sterility tests, in which she presented with normal blood tests, except for an inversion of the FSH/LH ratio and an AMH of 5.5 ng/mL, and he presented normal on both blood and semen analysis. On further evaluation by hysterosalpingography an obstruction of the right fallopian tube was observed.
Due to these results a decision was made with the patient to proceed with IVF. A first short protocol cycle of IVF was conducted stimulating with 150 IU/day of FSH. After 10 days of ovarian stimulation, a puncture was performed and 15 oocytes were obtained, 10 of them being mature oocytes in metaphase 2 (MII), 8 fertilized, 5 then evolved, and finally 2 blasts were transferred, without achieving gestation. A second cycle of IVF was then started, and the FSH dose was increased to 225 IU/day. After 8 days of ovarian stimulation, a puncture was performed obtaining 10 oocytes, 7 of them being mature, of which 4 were fertilized, 2 then further evolved and were then transferred. This cycle again did not achieve gestation.
Finally, prior to the third attempt, the patient was started on a daily inositol and antioxidants-based supplement (Ovosicare®) for 1 month. During this cycle, the patient was then stimulated with clomiphene citrate, in addition to the daily administration of 150 IU/day of FSH. After 10 days of stimulation, 17 oocytes were retrieved, 14 of them in metaphase II, of which 8 were fertilized, 7 then further evolved and 2 were transferred at the blastocyst stage. Additionally, 2 were frozen at the blastocyst stage and another 2 at the pre-embryo stage. Fourteen days after the transfer, the patient underwent a b-hCG blood test with a positive result. An appointment was made at 8 weeks of gestation and a single viable pregnancy was found.
The pregnancy was successful, however during the first trimester screening a high risk of preeclampsia was found and therefore the patient took aspirin 150 mg per day from weeks 13 to 37. Additionally, the patient continued taking the supplement during the first trimester of pregnancy, later switching to another complex. Fetal growth was adequate on ultrasound throughout the pregnancy. A spontaneous rupture of membranes occurred at 39 + 2 weeks, inducing labor. The vaginal delivery was uneventful.
Clinical case 2
A 37-year-old female patient attended the fertility clinic at ARPA MEDICA in Madrid, Spain, to consult over primary sterility of 3 years of evolution with 2 previously failed IVF cycles, both of which never achieved embryonic transfer. The patient presented with a BMI of 25.3 and was then referred to the nutritional service, where she lost weight and was able to achieve a BMI of 23.8. She has no other relevant past medical history and denied participating in toxic habits. She presented with regular menstrual cycles of 28 days.
On transvaginal ultrasound both ovaries were visualized, the right with 4 follicles (Figure 1) and the left with 3 follicles. The patient, and her male partner were both ordered sterility tests, in which she presented with the following results: AMH: 0.8 ng/mL, FSH: 6.8 IU/L, LH: 4.5 IU/L, TSH: 1.2 IU/L, as well as normal karyotyping. Her partner presented normal on blood tests, karyotyping, and semen analysis.
Figure 1.
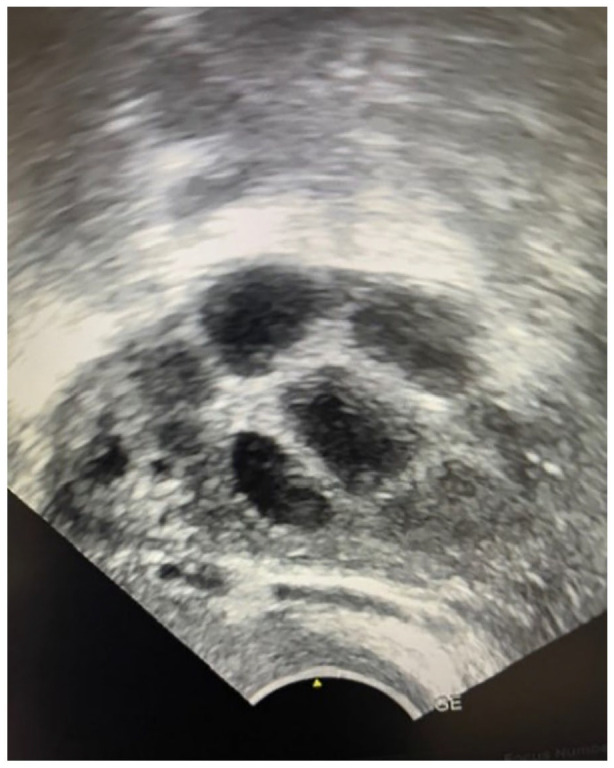
Four follicles visualized in the right ovary through ultrasound (Case 2).
Due to previous IVF results, a new IVF cycle was recommended, changing the medication regimen, and adding prior treatment with a daily inositol and antioxidants-based supplement (Ovosicare®) for 2 months. After this, a new short IVF cycle with agonist was started. After 8 days, the response to the new treatment was assessed via ultrasound in which follicular growth was observed. The right ovary contained follicles of 17, 18, and 19 mm in diameter, and the left ovary contained follicles of 16, and 19 mm. The endometrium was visualized in C1 12 mm. A puncture was performed, and 5 oocytes were obtained, of which 1 embryo further evolved and was then transferred at the blastocyst stage. This IVF cycle ultimately resulted in a live healthy newborn. The patient had a normal pregnancy and uneventful delivery at 39 weeks and gave birth to a girl of 3200 g.
Clinical case 3
A 34-year-old female patient attended the fertility clinic at CEFER LLEIDA in Catalonia, Spain to consult over secondary infertility of 1 year with 4 previous failed intrauterine inseminations (IUI) using Ovusitol D®. The patient had previously been diagnosed with PCOS and psoriasis, and her past medical history included a successful pregnancy.
On physical exploration/transvaginal ultrasound both ovaries were visualized, the right with 12 antral follicles and the left with 6, as well as an endometrioma of 16 mm. The patient, and her male partner were both ordered sterility tests, in which she presented with normal blood tests (FSH 11.3 IU/L, LH: 7.9 IU/L, Estradiol 44 pg/mL, AMH: 3.48 ng/ml, TSH: 1.94 IU/L, Glucose: 72 mg/dL), karyotyping, and hysterosalpingography, and he presented normal on blood tests, karyotyping, and semen analysis.
An IVF cycle was recommended with prior treatment using a daily inositol and antioxidants-based supplement (Ovosicare®) for 2 months. After starting ovarian stimulation, 16 mature oocytes are obtained, of which 7 were fertilized and further evolved. One was transferred at the blastocyst stage and the others were frozen. The patient later underwent a b-HCG blood test which resulted positive. Given this result, the patient stopped taking the supplement at this point. The pregnancy took a normal course, and the patient gave birth to a healthy girl.
Clinical case 4
A 37-year-old female patient attended the fertility clinic at Complejo Hospitalario San Millán – San Pedro in La Rioja, Spain to consult over primary infertility of 2 years of evolution. The patient presented clinically overweight, with a BMI of 26, and with irregular menstrual cycles every 45 to 60 days. She had also previously been diagnosed with PCOS, and had undergone a failed IUI, and a cycle of IVF which resulted in a spontaneous abortion at 6 weeks of gestation. Due to her overweight status, the patient consulted an endocrinologist and a pathological glycemia curve was detected with levels at 1 hour of 237 mg/dL and an elevated insulin of 33 µU/mL. Low vitamin D levels of 10 ng/mL were also observed.
To begin, she was prescribed treatment with metformin, in addition to diet and physical exercise to improve the patient’s metabolic state. Unfortunately, the patient could not tolerate the indicated dose of metformin and was taking a suboptimal dose. At this time, she was also advised to begin taking a daily inositol and antioxidants-based supplement (Ovosicare®).
In a second attempt at IVF/ICSI, a moderate ovarian hyperstimulation occurred and therefore stimulation was stopped. In a third and last attempt, the dosage of ovarian stimulation with FSH was lowered and the patient continued taking metformin and the inositol and antioxidants-based supplement (she had now been taking the supplement for more than 12 weeks). In this cycle, 9 mature oocytes were obtained and ultimately 1 blastocyst was transferred on day 5, which resulted in a successful pregnancy with a healthy newborn now at home.
Clinical case 5
A 35-year-old female patient attended the fertility clinic at the Hospital Universitario de Valme, in Sevilla, Spain to consult over primary infertility of 3 years of evolution. The patient presented clinically obese, with a BMI of 31.44, and with irregular menstrual cycles every 30 to 35 days. She had also previously been diagnosed with PCOS and had undergone 3 failed cycles of controlled ovarian stimulation with follitropin beta (dosage: 50 IU). Besides this, she had no other relevant past medical history and denied participating in any toxic habits.
On transvaginal ultrasound a regular anteverted uterus (46 × 28 × 38 mm) was visualized as well as both ovaries, the right with 11 follicles (13.36 cm3) and the left with 12 follicles (15.50 cm3). The patient, and her male partner were both ordered sterility tests, in which she presented with the following results: FSH: 3.52 IU/L, LH: 7.70 IU/L, LH/FSH Ratio: 2.18, Estradiol: 46.50 pg/mL, TSH: 2.30 UI/L, Prolactin: 15.1 ng/mL, Vitamin D: 56.5 nmol/l, AMH: 10.80 ng/mL, Testosterone: 0.43 ng/mL, Free testosterone: 0.97 pg/mL, Dehydroepiandrosterone-sulfate: 253 µg/dL, 17-Hydroxyprogesterone: 0.90 ng/mL, and Delta4-Androstenedione: 4.69 ng/mL. Her partner presented normal on semen analysis with a volume of 3.40 mL, reporting a sperm count of 58 million/mL, with 5% morphology, 48% progressive mobility, 55% total mobility and a REM value of 28.80 million/mL. Additionally, both appeared normal on karyotyping.
Due to the previously failed cycles of IVF, the patient was initiated on a treatment with metformin 850 mg (1 tablet/12 hours) + a daily inositol and antioxidants-based supplement (Ovosicare®) for 3 months. After this treatment, the patient then underwent a new short IVF cycle with agonist, triggering with Triptorelin 0.2 mg 36 hours prior to follicular puncture. The day of induction, hormone levels were the following: Estradiol 3799 pg/mL and Progesterone 1.51 ng/mL. A puncture was performed, obtaining 18 oocytes, 16 of them being mature oocytes in metaphase II, 12 then fertilized, and 4 further evolved to blastocyst stage (with the following quality grading according to ASEBIR: BA, BB, CC, and CC). 24 Due to risk of OHSS, all blastocysts were frozen. The patient was now given transdermal estradiol 200 µg every 48 hours. After 9 days she presented a trilaminar endometrium pattern of 8.8 mm and progesterone level of 0.05 ng/mL. A selective ultrasound-guided transfer of the BA embryo was now performed. Progesterone level on the day of transfer was 12 ng/mL. This transfer resulted in a successful pregnancy. The pregnancy took a normal course, and the patient gave birth through vaginal delivery to a healthy boy weighing 3750 g.
Discussion
Multiple factors are associated with the deterioration of fertility such as, age, obesity, endocrine and hormonal disorders, malnutrition, lifestyle choices and/or stress.25 -27 Currently, the proportion of women delaying childbearing has greatly increased, and so as the number of women over 35, with difficulties to getting pregnant.28,29 With aging, women experience a dramatic decrease in the chance of producing chromosomally-normal blastocysts. 30 This fact can be attributed to the gradual depletion of the ovarian reserve and also to the progressive decrease in oocyte/embryo quality.31 -33 As a result, diminished ovarian reserve and increased genetic damage of the oocyte pool are currently among the primary indications for ART. 34 In addition, the increasing prevalence of other endocrine disorders with age, causing ovulatory disfunctions such as thyroid disease, PCOS or hyperprolactinaemina, have also a marked prevalence in assisted reproduction programs. 34 This challenging scenario increased the interest in therapeutics that may improve ART outcomes. The potential benefits that arise from the periconceptional supplementation with natural compounds including inositols, antioxidants, vitamins and minerals may represent an opportunity for enhancing the chances of pregnancy in women undergoing ART.
Of the 5 women included in this series, 4 had been diagnosed with PCOS (Cases 1, 3-5) and 1 presented diminished ovarian reserve (Case 2). PCOS is a common and complex endocrine disorder that negatively affects reproductive and metabolic factors, with 30% to 40% of cases being associated with decrease in insulin-mediated glucose uptake.35,36 Insulin resistance can be defined as a dysregulation of glucose and insulin metabolism, and affects metabolic homeostasis within liver, muscle and adipose tissue. 37 The compensatory hyperinsulinaemia developed in this context alters ovarian function, induces an enhanced androgen production and hampers ovarian follicular development. 37 Additionally, hyperinsulinemia causes excessive triglycerides and cholesterol synthesis, leading to fat storage mainly in liver and adipose tissue. Thus, insulin resistance plays a crucial role in the strong association of PCOS with adverse metabolic risk, including hyperglycemia, dyslipidemia, and obesity. The reproductive function in women with PCOS is not only dependent on metabolic status but also on body weight. It is well established that being overweight or obese increases the risk of infertility and implies a longer time for conception. 38 The Case 5 is a good example of the close association between PCOS, obesity and subfertility.
To date, several insulin sensitizing drugs, such as metformin, pioglitazone and inositols have been used to reduce insulin resistance, which in turn, may improve fertility. Metformin is an oral insulin sensitizer recommended as the first-line treatment in women with PCOS who have impaired glucose sensitivity. 39 Metformin has been shown to reduce OHSS and improve pregnancy outcomes.40,41 However, the persistent use of metformin is associated with adverse effects of the gastrointestinal system. 42 On the other hand, natural insulin sensitizers, such as inositols, appear to have fewer side effects while contributing positively to fertility. 43 Although, there is still limited evidence collected, inositols have been recently proposed as a valid alternative to metformin in international guidelines for the management of PCOS. 44
In a double-blind randomized controlled trial comparing the use of MYO to metformin as a pretreatment in women with PCOS undergoing IVF, Rajasekaran et al 45 found that MYO is equally as beneficial as metformin in reducing the risk of OHSS and has better ART outcomes. While MYO has been shown non-inferiority to improve the metabolic profile of women with insulin resistance compared to the gold standard—metformin,46,47 it is unclear whether supplementation with it alone can improve direct outcomes such as clinical pregnancy or live birth rates. In a systematic review and meta-analysis by Mendoza et al, 10 supplementation with MYO alone in women with PCOS was not associated with improved oocyte and embryo quality, or pregnancy rate. In a large randomized trial, including 1729 women planning to conceive, a combined MYO, probiotics and micronutrients supplement showed similar time-to-natural-conception and clinical pregnancy rates than standard supplement within a year. 48 These results may explain the recurrent failure of IUI after supplementation with MYO in monotherapy (Ovusitol D®) in clinical case number 3.
On the other hand, the combined supplementation with MYO and DCI has been seen to be more efficient to improve endocrine and metabolic profiles in women with PCOS than their respective monotherapies or even metformin in monotherapy. 43 In concrete, MYO combined with DCI has shown to be particularly efficacious in menstrual recovery. 43 Furthermore, the combination of these 2 isomers has proven to improve the oocyte quality and the pregnancy outcomes in women with PCOS undergoing ART.11,12,49,50 Despite a significant number of evidence suggests that MYO and DCI metabolic derivatives act synergically,51,52 there is still ongoing debate concerning their optimal ratio.
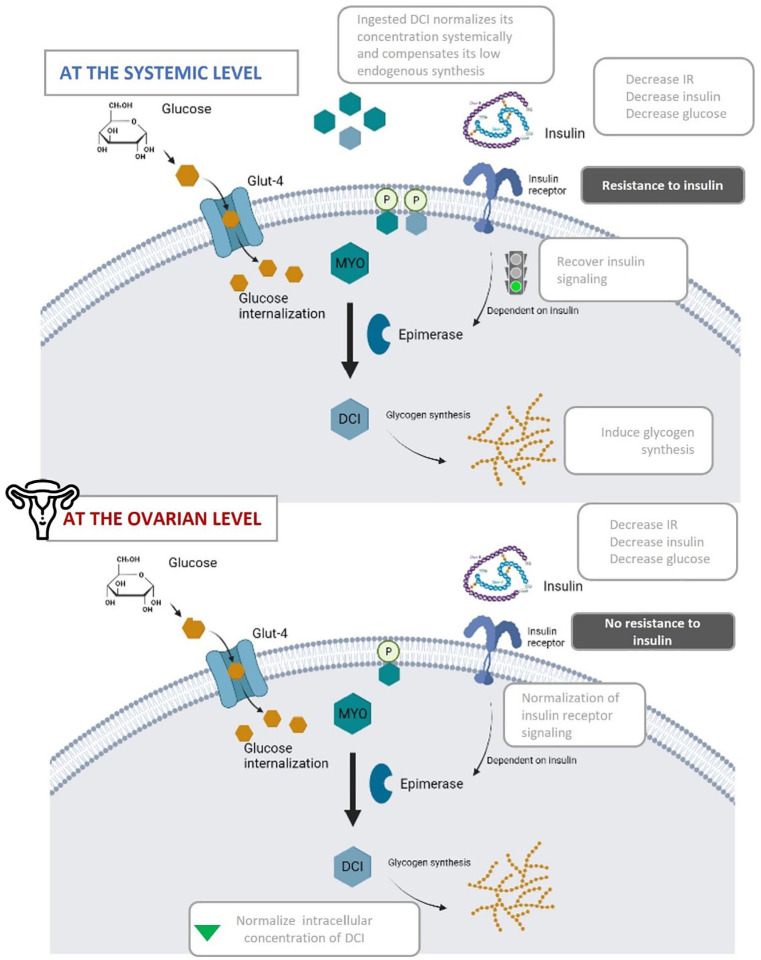
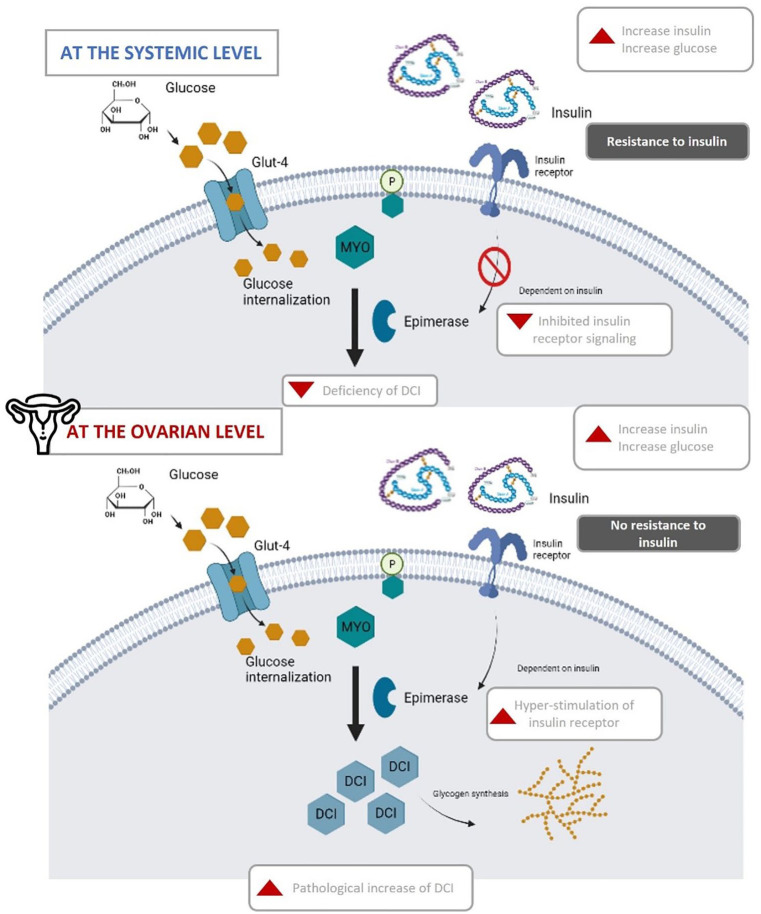
Endogenously, the isomerization of MYO to DCI happens through the activity of the epimerase enzyme. 53 This activity and its regulation are important as the activities of both isomers are very different. Both MYO and DCI stimulate glucose uptake via glucose transporter 4 (GLUT4) translocation to the plasma membrane, but only DCI can storage cytosolic glucose through glycogen synthesis. 54 Therefore, tissues with high metabolic activity, like brain, heart, or ovary, tend to have higher MYO concentrations, and conversely, tissues that tend to store glycogen, like fat, liver or muscle have higher DCI concentrations. 55 At the systemic level, women with insulin resistance often present low levels of DCI. 55 This can be explained by the fact that as insulin sensitivity is decreased, its signaling cascade is too.53,56 Insulin resistance impairs the activation of the epimerase enzyme, therefore impeding the conversion of MYO to DCI (Figure 3). With lower levels of DCI available, glycogen synthesis is diminished, increasing the overall blood glucose concentration, which in turn further aggravates the insulin resistance present. While this is true in most organs of the body, it has been demonstrated that the ovary is the only organ that does not show resistance to insulin, known as the ovarian paradox (Figure 2).57 -59 Since ovarian tissue remains sensitive to insulin, the compensatory hyperinsulinemia overstimulates ovarian epimerase activity, causing an excess of DCI in this organ at the expense of MYO. Consequently, the increment of DCI concentration induces androgen synthesis, while MYO depletion worsens FSH signaling and oocyte quality. 55
Figure 3.
After Ovosicare® supplementation, the endogenous concentration of both metabolic derivatives of MYO and DCI—inositol phosphoglycans—are restored, recovering glycogen synthesis and therefore, reducing systemic hyperglycemia and hyperinsulinemia. After recovering insulin signaling, epimerase enzyme catalyzes the conversion of MYO to DCI. Once the homeostatic metabolic regulation is restored at the systemic level, the insulin receptor is no longer overstimulated and normalizes the concentration of DCI at the ovarian level.
Figure 2.
MYO to DCI epimerase conversion in the presence of insulin resistance at both the systemic and ovarian levels. In normal conditions, after insulin binds to the insulin receptor, inositol derivatives—inositol phosphoglycans—are hydrolyzed from cytoplasmic membrane, releasing MYO which induces glucose internalization via Glut-4 receptor. Meanwhile, the epimerase enzyme catalyzes the isomerization of MYO to DCI, which induces glycogen synthesis. Intracellularly, insulin resistance down-regulates insulin signaling in classic insulin target tissues leading to a systemic deficiency of DCI. As ovaries maintain the normal insulin sensitivity (deemed “ovarian paradox”), the overstimulation of the insulin receptor increases the isomerization of MYO to DCI, resulting in a pathological increase of DCI.
Thus, to overcome abnormal ratios of MYO to DCI, an adequate administration of both inositols may act synergistically to bypass a defective activity of epimerase, and thus, restore insulin sensitivity and glucose disposal. Currently, the most used ratio of MYO to DCI is 40:1, since it is commonly defined as the physiological ratio, albeit there exist different tissue-specific ratios. 60 Nordio et al 61 found that the 40:1 MYO to DCI ratio comparing with other ratios was the best to restoring ovulation and normalizing metabolic and hormonal parameters in women with PCOS. However, in a recent work, Mendoza et al 12 hypothesized that supplementation with higher doses of DCI may be beneficial for fertility, since it may compensate the low endogenous DCI synthesis found at the systemic level, and hence, restore ovarian homeostasis. The supplementation of DCI with dosages higher than 1200 mg/day has been described as detrimental in the literature. 62 In Mendoza’s work, the daily dosage of DCI (300 mg) is considered to be safe even in longer-term regimens (>12 months), according to the safety recommendations of Gambioli et al. 63 In a double-blind, multicenter, randomized clinical trial involving 60 patients, they compared the effect of supplementation with MYO and DCI in 2 different ratios (3.6:1 vs 40:1) in women with PCOS undergoing ICSI. In this study, the pregnancy and live birth rates were significantly higher in the group receiving the 3.6:1 ratio than in the group receiving the 40:1 ratio (65.5% vs 25.9% and 55.2% vs 14.8%, respectively). 12 Moreover, the use of MYO with higher doses of DCI (in a ratio of 3.6:1) has been shown to improve oocyte quality by reducing testosterone and increasing insulin sensitivity in women with PCOS undergoing ICSI. 11 The same 3.6:1 MYO to DCI ratio it was seen to be effective in regularizing menstrual cycles and improving insulin resistance in women with PCOS. 13 Similarly, another randomized study including 283 women with PCOS demonstrated the benefits of the 3.6:1 MYO to DCI ratio in improving the hormonal, glycemic, and lipidic profile. 14 Although a large body of evidence have been published using the metabolic and hormonal benefits of the 40:1 ratio,49,61,64,65 until now, the 3.6:1 ratio is the unique ratio with proven benefits in direct parameters such as pregnancy and live birth rates.
The combined supplementation of MYO and DCI will ensure an adequate endogenous tissue content of MYO and DCI metabolic derivatives (Figure 3). The internalized DCI stimulates glycogen synthetase which enhances glycogen storage and diminishes systemic hyperglycemia and hyperinsulinemia. Consequently, insulin sensitivity will be improved, by recovering epimerase signaling, leading to the conversion of MYO to DCI, and therefore, normalizing the concentration of DCI systemically. Once the homeostatic metabolic regulation is restored at the systemic level, it is expected that the insulin receptor in ovarian tissue will no longer be hyper-stimulated, normalizing its activity and counter intuitively leading to decreased levels of DCI within the ovary.
The women included in our series, had an average age of 34.8 years, and represent a physiologically aging population. As women age, there is an increased production of ROS in the ovary which steadily leads to a decline in fertility.66,67 Beyond the age of 32, ovarian aging occurs gradually because of the mitochondrial buildup from oxidative stress-related damage.68,69 This influences oocyte quality, increasing the number of chromosomic alterations. For circumvent the reduction of antioxidant defenses with age, the supplementation with antioxidants may benefit subfertile women. The food supplement under study included a complete mix of powerful antioxidants including melatonin, Pomanox®, vitamins B1, B2, B3, B6, B9, B12, D, E, zinc, iodine and selenium that may contribute to reduce ROS and improve oocyte/embryo quality and IVF outcomes. The inositols have also demonstrated to possess antioxidant properties.70,71
Melatonin, is a hormone synthesized and secreted by the pineal gland with potent antioxidant properties, and seems to have beneficial effects in women experiencing infertility72,73 and in women with PCOS. 74 Follicular melatonin protects granulosa cells, oocytes, and ovaries from oxidative stress, delaying ovarian aging. In a randomized pilot study carried out in 40 women undergoing IVF, the supplementation with melatonin was seen to improve intrafollicular oxidative balance and oocyte quality, and slightly increasing the rate of pregnancies/live births. 75 In patients with poor fertilization rate and poor embryo development rate in previous ART cycles, the melatonin treatment has shown to reduce oxidative stress and protect granulosa cells, while improving cellular functions by altering their transcriptomes. 76 Importantly, a meta-analysis including 10 randomized clinical trials concluded that melatonin treatment in ART significantly increases the number of oocytes collected, matured oocytes, good quality embryo, and can impact positively on the clinical pregnancy rate but not on live birth rate. 16 In addition, melatonin may act synergistically with inositols. New evidence highlighted that the co-supplementation of MYO and melatonin may effectively improve the quality of oocytes and embryos, increase the implantation rates and improve the pregnancy rates, even after previous IVF failures.17 -20 Thus, specifically for the women represented in this case series—with a previous failure ART—the synergy produced from the combination of melatonin and inositols might have contributed to improve the success of ART.
Some studies with other antioxidant mixtures showed to improve female fertility. In a randomized controlled trial conducted on 56 women undergoing IVF and 13 age-matched controls, supplementation with antioxidants, such as vitamins A, C, and E, was seen to significantly increase the levels of vitamins C, and E and GSH in serum and vitamin C and glutathione peroxidase (GSH-Px) in follicular fluid. 77 Additionally, supplemented women were seen to have reduced levels of lipid peroxidation in serum and follicular fluid. This seems to indicate that oral supplementation with antioxidants strengthen the antioxidant defense system and reduce oxidative stress. 78 Moreover, treatment of infertile women aged >39 years prior to IVF cycles with a complete supplementation with antioxidant effects, including vitamins B12, B6, C and E, cooper, manganese, zinc, and selenium, has shown to protect the follicular microenvironment from oxidative stress, thus enhancing the number of good quality oocytes recovered by ovum pick up. 79 In the case of infertile women with occult premature ovarian insufficiency (OPOI), the supplementation with selenium and vitamin E successfully increased their levels of AMH, antral follicle count and mean ovarian volume. 80 Likewise, an acute rise in vitamin D status in women has demonstrated to produce an increment in serum AMH. Furthermore, pomegranate supplementation in the form of extract or concentrated juice has demonstrated efficacy on improving metabolic, oxidative, inflammatory and blood pressure outcomes in women with PCOS and healthy subjects.21,81 -83
In our series, all the patients were able to achieve positive results in ART after taking the food supplement for 1 to 3 months prior to ovarian puncture. Here it is important to highlight that each of the patients had previously undergone some type of ART and failed to achieve pregnancy. More specifically 3 of the patients had 2 previously failed IVF, 1 patient had 3 previously failed IVF, and 1 patient had 4 previously failed intrauterine inseminations. The clinical outcomes from this series suggest that prior supplementation with MYO and DCI in a specific 3.6:1 ratio combined with antioxidants, vitamins, and minerals could be beneficial for subfertile women undergoing ART. Although the role of chance cannot be discarded, all the patients in our series were able to achieve pregnancy only after 1 to 3 months of treatment with Ovosicare®. Although lack of generalizability (due to the low number of patients and the lack of controls) is a serious limitation of this report, we hope that the observations described by this case series may inspire further research into the use of this supplement as a pretreatment for patients undergoing ART. A randomized pilot study performed in a larger representative population and including a control group is required to establish the cause-effect relationship between supplementation and the success of ART.
Conclusion
To summarize, this series of cases represent a frequent female profile eligible for ART, with several poor prognostic factors of ART success; women with an average age of 35 years and gestational desire, undergoing recurrent failed ART, with diminished ovarian reserve, endocrine alterations and/or impaired oocyte quality. In all cases, pregnancy was achieved in a short period of time (1-3 months), after receiving supplementation with MYO to DCI 3.6:1 ratio and antioxidants based food supplement and undergoing ART. These results seem to suggest a clear benefit to female fertility from using this combination of ingredients. However, more studies are needed to reassess the effectiveness of the supplementation with MYO:DCI on clinical pregnancy and live birth rates in ART, these preliminary results confirm the beneficial effects of this preconception supplementation.
Footnotes
Author Contributions: All authors contributed equally to the work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. World Health Organization. Infertility. Assessed on March 2024. https://www.who.int/news-room/fact-sheets/detail/infertility [Google Scholar]
- 2. Collée J, Mawet M, Tebache L, Nisolle M, Brichant G. Polycystic ovarian syndrome and infertility: overview and insights of the putative treatments. Gynecol Endocrinol. 2021;37:869-874. [DOI] [PubMed] [Google Scholar]
- 3. Merviel P, James P, Bouée S, et al. Impact of myo-inositol treatment in women with polycystic ovary syndrome in assisted reproductive technologies. Reprod Health. 2021;18:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Society for Assisted Reproductive Technology. Final national summary report for 2019. SART national summary report. Assessed on March 2024. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2019 [Google Scholar]
- 5. Tan TY, Lau SK, Loh SF, Tan HH. Female ageing and reproductive outcome in assisted reproduction cycles. Singapore Med J. 2014;55:305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiao J, Wang Z-B, Feng H-L, et al. The root of reduced fertility in aged women and possible therapentic options: current status and future perspects. Mol Aspects Med. 2014;38:54-85. [DOI] [PubMed] [Google Scholar]
- 7. Genazzani AD. Inositol as putative integrative treatment for PCOS. Reprod Biomed Online. 2016;33:770-780. [DOI] [PubMed] [Google Scholar]
- 8. Zheng X, Lin D, Zhang Y, et al. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine. 2017;96:e8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unfer V, Nestler JE, Kamenov ZA, Prapas N, Facchinetti F. Effects of inositol(s) in women with PCOS: A systematic review of randomized controlled trials. Int J Endocrinol. 2016;2016:1849162-1849212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendoza N, Pérez L, Simoncini T, Genazzani A. Inositol supplementation in women with polycystic ovary syndrome undergoing intracytoplasmic sperm injection: a systematic review and meta-analysis of randomized controlled trials. Reprod Biomed Online. 2017;35:529-535. [DOI] [PubMed] [Google Scholar]
- 11. Mendoza N, Diaz-Ropero MP, Aragon M, et al. Comparison of the effect of two combinations of myo-inositol and D-chiro-inositol in women with polycystic ovary syndrome undergoing ICSI: a randomized controlled trial. Gynecol Endocrinol. 2019;35:695-700. [DOI] [PubMed] [Google Scholar]
- 12. Mendoza N, Galan MI, Molina C, et al. High dose of d-chiro-inositol improves oocyte quality in women with polycystic ovary syndrome undergoing ICSI: a randomized controlled trial. Gynecol Endocrinol. 2020;36:398-401. [DOI] [PubMed] [Google Scholar]
- 13. Kachhawa G, Senthil Kumar KV, Kulshrestha V, et al. Efficacy of myo-inositol and d-chiro-inositol combination on menstrual cycle regulation and improving insulin resistance in young women with polycystic ovary syndrome: a randomized open-label study. Int J Gynecol Obstet. 2022;158:278-284. [DOI] [PubMed] [Google Scholar]
- 14. Vyas L, Raiturker AP, Sud S, et al. Management of polycystic ovary syndrome among Indian women using myo-inositol and D-chiro-inositol. Bioinformation. 2022;18:103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shabani A, Foroozanfard F, Kavossian E, et al. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2019;250:51-56. [DOI] [PubMed] [Google Scholar]
- 16. Hu K-L, Ye X, Wang S, Zhang D. Melatonin application in assisted reproductive technology: a systematic review and meta-analysis of randomized trials. Front Endocrinol. 2020;11:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlomagno G, Nordio M, Chiu TT, Unfer V. Contribution of myo-inositol and melatonin to human reproduction. Eur J Obstet Gynecol Reprod Biol. 2011;159:267-272. [DOI] [PubMed] [Google Scholar]
- 18. Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improvingin vitrofertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2016;32:69-73. [DOI] [PubMed] [Google Scholar]
- 19. Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. 2011;27:857-861. [DOI] [PubMed] [Google Scholar]
- 20. Rizzo P, Raffone E, Benedetto V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial. Eur Rev Med Pharmacol Sci. 2010;14:555-561. [PubMed] [Google Scholar]
- 21. Esmaeilinezhad Z, Barati-Boldaji R, Brett NR, et al. The effect of synbiotics pomegranate juice on cardiovascular risk factors in PCOS patients: a randomized, triple-blinded, controlled trial. J Endocrinol Invest. 2020;43:539-548. [DOI] [PubMed] [Google Scholar]
- 22. Vašková J, Klepcová Z, Špaková I, et al. The importance of natural antioxidants in female reproduction. Antioxidants. 2023;12:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Homer HA. The role of oocyte quality in explaining “unexplained” infertility. Semin Reprod Med. 2020;38:021-028. [DOI] [PubMed] [Google Scholar]
- 24. Cuevas Saiz I, Carme Pons Gatell M, Vargas MC, et al. The embryology interest group: updating ASEBIR’s morphological scoring system for early embryos, morulae and blastocysts. -med Reprod Y Èmbriol Clin. 2018;5:42-54. [Google Scholar]
- 25. Skoracka K, Ratajczak AE, Rychter AM, Dobrowolska A, Krela-Kaźmierczak I. Female fertility and the nutritional approach: the most essential aspects. Adv Nutr. 2021;12:2372-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unuane D, Tournaye H, Velkeniers B, Poppe K. Endocrine disorders & female infertility. Best Pract Res Clin Endocrinol Metab. 2011;25:861-873. [DOI] [PubMed] [Google Scholar]
- 28. Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17:848-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt L, Sobotka T, Bentzen JG, Nyboe Andersen A. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update. 2012;18:29-43. [DOI] [PubMed] [Google Scholar]
- 30. Franasiak JM, Forman EJ, Hong KH, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656-663.e1. [DOI] [PubMed] [Google Scholar]
- 31. Cimadomo D, Fabozzi G, Vaiarelli A, et al. Impact of maternal age on oocyte and embryo competence. Front Endocrinol. 2018;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miao Y-L, Kikuchi K, Sun Q-Y, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573-585. [DOI] [PubMed] [Google Scholar]
- 33. Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril. 2015;103:317-322. [DOI] [PubMed] [Google Scholar]
- 34. Herman T, Csehely S, Orosz M, et al. Impact of endocrine disorders on IVF outcomes: results from a large, single-centre, prospective study. Reprod Sci. 2023;30:1878-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salley KES, Wickham EP, Cheang KI, et al. POSITION STATEMENT: glucose intolerance in polycystic ovary syndrome—a position statement of the androgen excess society. J Clin Endocrinol Metab. 2007;92:4546-4556. [DOI] [PubMed] [Google Scholar]
- 36. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao H, Zhang J, Cheng X, Nie X, He B. Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J Ovarian Res. 2023;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wootton RE, Lawn RB, Magnus MC, et al. Associations between health behaviours, fertility and reproductive outcomes: triangulation of evidence in the Norwegian mother, father and child cohort study (MoBa). BMC Med. 2023;21:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang FF, Pan JX, Wu Y, et al. American, European, and Chinese practice guidelines or consensuses of polycystic ovary syndrome: a comparative analysis. J Zhejiang Univ Sci B. 2018;19:354-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang T, Glanville J, Orsi N, Barth JH, Balen AH. The use of metformin for women with PCOS undergoing IVF treatment. Hum Reprod. 2006;21:1416-1425. [DOI] [PubMed] [Google Scholar]
- 41. Tso LO, Costello MF, Albuquerque LET, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;12(12):CD006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Renato P. Metformin in women with PCOS, Pros. Endocrine. 2015;48:422-426. [DOI] [PubMed] [Google Scholar]
- 43. Zhao H, Xing C, Zhang J, He B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health. 2021;18:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teede HJ, Tay CT, Laven JJE, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endoc. 2023;189:G43-G64. [DOI] [PubMed] [Google Scholar]
- 45. Rajasekaran K, Malhotra N, Mahey R, Khadgawat R, Kalaivani M. Myoinositol versus metformin pretreatment in gnrh-antagonist cycle for women with PCOS undergoing IVF: a double-blinded randomized controlled study. Gynecol Endocrinol. 2022;38:140-147. [DOI] [PubMed] [Google Scholar]
- 46. Greff D, Juhász AE, Váncsa S, et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. 2023;21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fatima K, Jamil Z, Faheem S, et al. Effects of myo-inositol vs. metformin on hormonal and metabolic parameters in women with PCOS: a meta-analysis. Ir J Med Sci. 2023;192:2801-2808. [DOI] [PubMed] [Google Scholar]
- 48. Chan S-Y, Barton SJ, Loy SL, et al. Time-to-conception and clinical pregnancy rate with a myo-inositol, probiotics, and micronutrient supplement: secondary outcomes of the NiPPeR randomized trial. Fertil Steril. 2023;119:1031-1042. [DOI] [PubMed] [Google Scholar]
- 49. Colazingari S, Treglia M, Najjar R, Bevilacqua A. The combined therapy myo-inositol plus d-chiro-inositol, rather than d-chiro-inositol, is able to improve IVF outcomes: results from a randomized controlled trial. Arch Gynecol Obstet. 2013;288:1405-1411. [DOI] [PubMed] [Google Scholar]
- 50. Facchinetti F, Bizzarri M, Benvenga S, et al. Results from the international consensus conference on myo-inositol and d-chiro-inositol in obstetrics and gynecology: the link between metabolic syndrome and PCOS. Eur J Obstet Gynecol Reprod Biol. 2015;195:72-76. [DOI] [PubMed] [Google Scholar]
- 51. Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651-657. [DOI] [PubMed] [Google Scholar]
- 52. Paul C, Laganà AS, Maniglio P, Triolo O, Brady DM. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Gynecol Endocrinol. 2016;32:431-438. [DOI] [PubMed] [Google Scholar]
- 53. Sun TH, Heimark DB, Nguygen T, Nadler JL, Larner J. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem Biophys Res Commun. 2002;293:1092-1098. [DOI] [PubMed] [Google Scholar]
- 54. Nestler JE, Unfer V. Reflections on inositol(s) for PCOS therapy: steps toward success. Gynecol Endocrinol. 2015;31:501-505. [DOI] [PubMed] [Google Scholar]
- 55. Dinicola S, Unfer V, Facchinetti F, et al. Inositols: from established knowledge to novel approaches. Int J Mol Sci. 2021;22(19):10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Unfer V, Dinicola S, Laganà AS, Bizzarri M. Altered ovarian inositol ratios may account for pathological steroidogenesis in PCOS. Int J Mol Sci. 2020;21:7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carlomagno G, Unfer V, Roseff S. The D-chiro-inositol paradox in the ovary. Fertil Steril. 2011;95:2515-2516. [DOI] [PubMed] [Google Scholar]
- 58. Facchinetti F, Unfer V, Dewailly D, et al. Inositols in polycystic ovary syndrome: an overview on the advances. Trends Endocrinol Metab. 2020;31:435-447. [DOI] [PubMed] [Google Scholar]
- 59. Heimark D, McAllister J, Larner J. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr J. 2014;61:111-117. [DOI] [PubMed] [Google Scholar]
- 60. Larner J. D-chiro-inositol–its functional role in insulin action and its deficit in insulin resistance. Int J Exp Diabetes Res. 2002;3:47-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nordio M, Basciani S, Camajani E. The 40:1 myo-inositol/D-chiro-inositol plasma ratio is able to restore ovulation in PCOS patients: comparison with other ratios. Eur Rev Med Pharmacol Sci. 2019;23:5512-5521. [DOI] [PubMed] [Google Scholar]
- 62. Nordio M, Bezerra Espinola MS, Bilotta G, Capoccia E, Montanino Oliva M. Long-lasting therapies with high doses of D-chiro-inositol: the downside. J Clin Med. 2023;12:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gambioli R, Forte G, Aragona C, et al. The use of D-chiro-inositol in clinical practice. Eur Rev Med Pharmacol Sci. 2021;25:438-446. [DOI] [PubMed] [Google Scholar]
- 64. Nordio M, Proietti E. The combined therapy with myo-inositol and D-chiro-inositol reduces the risk of metabolic disease in PCOS overweight patients compared to myo-inositol supplementation alone. Eur Rev Med Pharmacol Sci. 2012;16:575-581. [PubMed] [Google Scholar]
- 65. Benelli E, Del Ghianda S, Di Cosmo C, Tonacchera M. A combined therapy with myo-inositol and D-Chiro-inositol improves endocrine parameters and insulin resistance in PCOS young overweight women. Int J Endocrinol. 2016;2016:3204083-3204085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang L, Chen Y, Liu Y, et al. The role of oxidative stress and natural antioxidants in ovarian aging. Front Pharmacol. 2020;11:617843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sasaki H, Hamatani T, Kamijo S, et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front Endocrinol. 2019;10:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chiang JL, Shukla P, Pagidas K, et al. Mitochondria in ovarian aging and reproductive longevity. Ageing Res Rev. 2020;63:101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alviggi C, Humaidan P, Howles CM, Tredway D, Hillier SG. Biological versus chronological ovarian age: implications for assisted reproductive technology. Reprod Biol Endocrinol. 2009;7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shi L, Yu X-T, Li H, Wu G-S, Luo H-R. D-chiro-inositol increases antioxidant capacity and longevity of Caenorhabditis elegans via activating Nrf-2/SKN-1 and FOXO/DAF-16. Exp Gerontol. 2023;175:112145. [DOI] [PubMed] [Google Scholar]
- 71. Baldassarre MPA, Di Tomo P, Centorame G, et al. Myoinositol reduces inflammation and oxidative stress in human endothelial cells exposed in vivo to chronic hyperglycemia. Nutrients. 2021;13:2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gomes PRL, Motta-Teixeira LC, Gallo CC, et al. Maternal pineal melatonin in gestation and lactation physiology, and in fetal development and programming. Gen Comp Endocrinol. 2021;300:113633. [DOI] [PubMed] [Google Scholar]
- 73. Fernando S, Rombauts L. Melatonin: shedding light on infertility?–a review of the recent literature. J Ovarian Res. 2014;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mojaverrostami S, Asghari N, Khamisabadi M, Heidari Khoei H. The role of melatonin in polycystic ovary syndrome: a review. Int J Reprod BioMed. 2019;17(12):865-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Espino J, Macedo M, Lozano G, et al. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants. 2019;8:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tamura I, Tamura H, Kawamoto-Jozaki M, et al. Effects of melatonin on the transcriptome of human granulosa cells, fertilization and blastocyst formation. Int J Mol Sci. 2022;23:6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ozkaya MO, Nazıroğlu M. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil Steril. 2010;94:2465-2466. [DOI] [PubMed] [Google Scholar]
- 78. Schaefer E, Nock D. The impact of preconceptional multiple-micronutrient supplementation on female fertility. Clin Med Insights Womens Health. 2019;12:1179562X19843868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Luddi A, Capaldo A, Focarelli R, et al. Antioxidants reduce oxidative stress in follicular fluid of aged women undergoing IVF. Reprod Biol Endocrinol. 2016;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Safiyeh FD, Mojgan M, Parviz S, Sakineh MA, Behnaz SO. The effect of selenium and vitamin E supplementation on anti-Mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: a randomized controlled clinical trial. Complement Ther Med. 2021;56:102533. [DOI] [PubMed] [Google Scholar]
- 81. Abedini M, Ghasemi-Tehrani H, Tarrahi MJ, Amani R. The effect of concentrated pomegranate juice consumption on risk factors of cardiovascular diseases in women with polycystic ovary syndrome: a randomized controlled trial. Phytother Res. 2021;35:442-451. [DOI] [PubMed] [Google Scholar]
- 82. Lorzadeh E, Heidary Z, Mohammadi M, et al. Does pomegranate consumption improve oxidative stress? A systematic review and meta-analysis of randomized controlled clinical trials. Clin Nutr ESPEN. 2022;47:117-127. [DOI] [PubMed] [Google Scholar]
- 83. Al-Dujaili EAS, Casey C, Stockton A. Antioxidant properties and beneficial cardiovascular effects of a natural extract of pomegranate in healthy volunteers: a randomized preliminary single-blind controlled study. Antioxidants. 2022;11:2124. [DOI] [PMC free article] [PubMed] [Google Scholar]