Abstract
With the widespread use of combination antiretroviral therapy (cART), the incidence of central nervous system (CNS) opportunistic infections and coinfections has significantly decreased. This review focuses on the clinical presentation, diagnostic laboratory and radiologic findings, as well as the treatment of neurosyphilis, progressive multifocal leukoencephalopathy, primary CNS lymphoma, and toxoplasmosis, which are CNS opportunistic infections and coinfections that are most relevant to clinicians in North America.
Keywords: human immunodeficiency virus, central nervous system, opportunistic infection, coinfection, lymphoma
In the era before combination antiretroviral therapy (cART), central nervous system (CNS) diseases due to opportunistic infections and coinfections were common in human immunodeficiency virus- (HIV-) infected patients. Many of these diseases, such as progressive multifocal leukoencephalopathy (PML), primary CNS lymphoma (PCNSL), and toxoplasmosis, are acquired immunodeficiency syndrome- (AIDS-) defining conditions and are associated with high mortality.1,2 With the widespread use of cART, the incidence of CNS opportunistic infections has decreased significantly and overall survival has improved.2,3 However, clinicians still need to be able to recognize these diseases because they continue to occur in those patients with significant HIV-associated immunosuppression, particularly in those not on cART. CNS opportunistic infections and coinfections such as neurosyphilis continue to perplex and challenge medical providers.
Neurosyphilis
Syphilis is caused by the bacterium Treponema pallidum subspecies pallidum, which can invade the central nervous system (CNS) to cause neurosyphilis. Although neurosyphilis has been traditionally considered a late or “tertiary” manifestation of syphilis, in reality, asymptomatic or symptomatic neurosyphilis can occur at any time after infection. Neurosyphilis is of particular concern in HIV-infected patients. Syphilis occurs commonly in this patient population and HIV-infected patients are at higher risk of failing therapy for early syphilis and developing early neurosyphilis (see discussion below on neurorelapse). Neurosyphilis may be more difficult to diagnose in the setting of concomitant HIV, and HIV-infected patients may be more likely to fail therapy for neurosyphilis. We address each of these issues in the following sections.
In the last decade, the number of cases of syphilis in the United States has increased, with a doubling in the rate of early syphilis cases.4 Syphilis has resurged in Europe and China as well.5–8 In 2011, 72% of primary and secondary syphilis cases reported to the U.S. Centers for Disease Control and Prevention (CDC) occurred in men who have sex with men (MSM), many of whom were also infected with HIV.4
The clinical manifestations of syphilis involving the CNS are extremely variable and include asymptomatic neurosyphilis, symptomatic meningitis, meningovascular syphilis (affecting brain or spinal cord), ocular syphilis, otologic syphilis, general paresis, and tabes dorsalis. The forms of neurosyphilis that are characterized by meningeal inflammation (asymptomatic, meningeal, and meningovascular) are most common in the initial few years after infection, while parenchymal forms of neurosyphilis (general paresis and tabes dorsalis) occur years or decades after infection; general paresis and tabes dorsalis are rarely seen in the modern antibiotic era. Ocular and otological manifestations may occur in both early and late stages, often in combination with meningitis. Thus, neurosyphilis should be considered in the differential diagnosis of any HIV-infected patient with acute aseptic meningitis, chronic meningitis, stroke involving the brain or spinal cord, transverse myelitis, chronic myelopathy, and dementia.
In addition to the clinical presentation, neurosyphilis is diagnosed by cerebrospinal fluid (CSF) abnormalities, including pleocytosis, elevated CSF protein, or reactive CSF-VDRL (Venereal Disease Research Laboratory; the gold standard diagnostic test). The diagnosis of asymptomatic neurosyphilis is based upon the presence of CSF abnormalities alone. CSF white blood cell (WBC) concentration in neurosyphilis is generally > 10/uL with a lymphocytic predominance. Because CSF-VDRL may be nonreactive in neurosyphilis (sensitivity ~ 30–70%9–11), a reactive CSF-VDRL establishes the diagnosis of neurosyphilis, but a nonreactive test does not exclude the diagnosis. Thus, in an HIV-infected person, the diagnosis of neurosyphilis may need to be based solely on identification of CSF pleocytosis. Because HIV itself can cause mild CSF pleocytosis,12 diagnosing neurosyphilis in HIV-infected patients can be particularly challenging. We have typically used a cutoff of > 20 WBC/uL as diagnostic of neurosyphilis in HIV-infected individuals.13 A lower cutoff has been used by others,14 and is most appropriate when the peripheral blood CD4+ T cell concentration is low, in patients who are taking cART, and in those with undetectable plasma HIV RNA, because these lower the risk of HIV-related CSF pleocytosis.12 Other tests that may aid in the diagnosis of asymptomatic neurosyphilis, but are not currently used clinically include (1) the proportion of CSF lymphocytes that are B cells,15 and (2) the CSF concentration of the chemokine, C-X-C motif ligand 13 (CXCL-13), which is a B-cell chemoattractant. Both are elevated in patients with neurosyphilis compared with patients with uncomplicated syphilis.16,17
Several studies have examined predictors of abnormal CSF in neurologically asymptomatic and symptomatic patients with syphilis. The odds of neurosyphilis are higher when serum rapid plasma reagin (RPR) titers are ≥ 1:32, and when peripheral blood CD4+ T cells are ≤ 350/uL.13,18,19 Also, HIV-infected patients with syphilis who are taking cART may be at lower risk of neurosyphilis compared with those not taking cART.14 A small, retrospective study suggested that high plasma HIV RNA concentration increased the risk of neurosyphilis among individuals with peripheral blood CD4+ T cells > 350/uL.20
The recommended first-line treatment for neurosyphilis by the Centers for Disease Control and Prevention (CDC) is high-dose intravenous (IV) penicillin G (Table 1). An alternative treatment is intramuscular (IM) procaine penicillin G with oral probenecid (Table 1–21). Case reports22–25 and a small study of HIV-infected patients with early neurosyphilis26 suggest that ceftriaxone may be an acceptable alternative to penicillin (Table 1). Treatment for ocular and otosyphilis is identical to neurosyphilis treatment, although steroids are sometimes recommended as an adjunctive therapy.27–31 The European32 and UK33 syphilis guidelines recommend doxycycline 200 mg orally twice daily for 28 days as an alternative neurosyphilis treatment regimen for those patients who are allergic to penicillin or who refuse parenteral therapy. Currently, the CDC guidelines do not recommend doxycycline as an alternative treatment for neurosyphilis, but recommend penicillin desensitization for penicillin allergic patients in whom ceftriaxone is not an option.21 Patients with resolution or stabilization of neurologic signs and symptoms and normalization of CSF abnormalities are thought to be successfully treated for neurosyphilis. Careful follow-up after neurosyphilis therapy with repeat CSF testing every 3 to 6 months is mandatory for all patients. The CDC guidelines state that CSF WBC count should decline at 6 months and all CSF abnormalities should resolve by 2 years after treatment.21 Patients who fail to meet these criteria should be retreated.
Table 1.
Treatment for neurosyphilis
| Recommended therapy (10–14 d) | |
|---|---|
| Penicillin G | 24 million units IV every d (continuous infusion or divided into 6 doses) |
| Alternative therapies (10–14 d) | |
| Procaine penicillin G plus Probenecid | 2.4 million units IM 500 mg PO qid |
| or | |
| Ceftriaxone | 1–2 g IV every d |
Abbreviations: IM, intramuscularly; PO, by mouth; qid, 4 times a day
Neurorelapse is defined as development of neurosyphilis (including ocular or otologic syphilis) after appropriate treatment for early syphilis. Neurorelapse was recognized in the preantibiotic era and was more likely in patients who had received inadequate treatment for uncomplicated syphilis.34 Syphilologists at the time theorized that partial treatment downregulated the host immune response, which, in combination with treatment that did not eradicate bacteria from the CNS, eye, or inner ear, predisposed to symptomatic neurologic disease. Several case reports document neurorelapse occurring in HIV-infected patients.29,35–37 Neurorelapse may be more common in HIV-infected patients with syphilis because benzathine penicillin G, the usual treatment for uncomplicated syphilis, does not clear T. pallidum from the CNS, the eye or the inner ear38,39 and HIV-induced cell-mediated immunity impairs clearance of organisms that persist at these sites.35
HIV-infected individuals may be more likely than HIV-uninfected patients to fail neurosyphilis therapy based on clinical and serological criteria as well as on failure to normalize CSF abnormalities.14,40–45 In a prospective analysis of 13 HIV-uninfected and 46 HIV-infected patients with neurosyphilis, those who were HIV-infected were 2.5 times less likely to normalize CSF-VDRL after therapy.44 Among the HIV-infected subjects, those with peripheral blood CD4+ T cells ≤ 200/uL were 3.3 times less likely to normalize CSF-VDRL.44 These data emphasize the need for diligent follow-up after treatment for neurosyphilis in HIV-infected individuals.
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is caused by a polyoma virus, JC virus. The virus is very prevalent, with 70 to 90% of people acquiring it in childhood or adolescence.46,47 Afterwards, the virus remains latent in the kidney and perhaps the brain, reactivating during immunosuppression, such as in HIV-infection.48,49 Infection of oligodendrocytes results in demyelination and clinical disease.
The typical clinical presentation of PML is subacute onset of progressive focal neurological deficits (e.g., cognitive dysfunction, limb weakness, gait disturbance, incoordination and vision loss); one-third of patients have headache.50 Less common manifestations of CNS JC infection include neuronopathy51 and JC infection of cortical pyramidal neurons causing an acute encephalopathy.52 Typically, HIV-infected patients with PML have peripheral blood CD4+ T cells < 200/uL.50 In the cART era, individuals with PML and much higher or even near-normal peripheral blood CD4+ T cell concentrations have been reported.53,54
With respect to neuroimaging, brain computed tomography (CT) in patients with PML may be normal. More often, it shows multiple, often confluent, white matter hypodense lesions that are most commonly located in the parietooccipital regions with little, if any, mass effect50 (Fig. 1). Enhancement may be seen in 10% of CT scans and is usually faint and peripheral.50 Brain magnetic resonance imaging (MRI) is more sensitive than CT in revealing PML lesions.55 In contrast to white matter lesions seen in HIV-associated dementia, which are visible only on T2-weighted images, PML lesions are low intensity on T1-weighted and high intensity on T2-weighted images; restricted diffusion is observed56 and ~ 15% of patients show faint contrast enhancement,50 particularly in the setting of immune reconstitution (see section on immune reconstitution inflammatory syndrome).
Fig. 1.

Progressive multifocal leukoencephalopathy immune reconstitution inflammatory syndrome in an human immunodeficiency virus-infected man. (A) Fluid attenuation inversion recovery images show focal increased signal in the right posterior frontal lobe. The area of abnormality did not enhance with contrast (not shown). (B) T1-weighted contrast enhanced images after antiretroviral therapy for 11 weeks shows new contrast enhancement. Courtesy of Christina M. Marra, MD.
Other diseases that should be considered in a patient suspected of having PML include varicella zoster encephalitis, which may also cause demyelination57; substance abuse, particularly a form of heroin use called “chasing the dragon,” which can cause clinical and radiographic abnormalities similar to PML58; and a severe form of HIV-associated leukoencephalopathy with patchy or confluent white matter high signal intensity seen on brain MRI that occurs in patients failing cART who have high levels of brain and CSF HIV RNA.59 Leukoencephalopathy has also been described in individuals with CNS immune reconstitution60–62 or CNS escape, in which patients have detectable CSF HIV RNA in the setting of controlled peripheral viremia63; in these patients, CSF pleocytosis is observed, making PML less likely.
Recently published diagnostic criteria64 indicate that patients with characteristic clinical and neuroimaging findings, characteristic histological examination or identification of JC virus DNA by polymerase chain reaction (PCR) in CSF have a confirmed diagnosis of PML. However, a negative CSF PCR does not exclude the diagnosis. A negative JC virus PCR is more likely to occur in patients receiving cART as well as those with higher peripheral blood CD4+ T cell concentrations.65
There is no specific treatment for PML; however, treatment with cART has resulted in significantly improved survival.66–68 All HIV-infected patients with PML should be treated with cART aimed at complete suppression of plasma HIV viremia. Whether certain types of antiretroviral regimens are more effective than others is not known. Two retrospective analyses suggest that a regimen that includes a protease inhibitor may be more effective than one that does not.69,70 One multicenter, prospective, open-label trial of an individualized regimen of five antiretrovirals, including enfuvirtide for the first 6 months, in 28 HIV-infected patients with PML showed that the 1-year survival was 75%,71 significantly higher than expected.71,72 Poorer outcome is associated with mass effect on MRI73 and brainstem and cerebellar involvement,74,75 while better outcome is associated with higher peripheral blood CD4+ T cell count at diagnosis,55,67,71,76–78 PML as the AIDS-defining illness,77 lower concentration and decline in CSF JC virus DNA concentration during antiretroviral therapy,71,72,78–81 and the presence of JC virus-specific cytotoxic T cells in blood.82–84 HIV-infected patients with PML who survive generally have persistent neurologic deficits, but may regain independence.67,68,71,74
As a consequence of an immune reconstitution inflammatory syndrome (IRIS), PML may develop or worsen after beginning cART. In an observational study of 61 PML patients, 14 (23%) developed PML-IRIS.66 Another case review reported that PML-IRIS developed 1 week to 26 months (median 7 months) after beginning cART, with a shorter latency, greater number of MRI-defined brain lesions and poorer outcome in those with known PML who worsened after beginning cART compared with those who developed PML after beginning cART.85 Very low peripheral blood CD4+ T cells (< 50/uL) at the time of initiating cART significantly increases the risk of PML IRIS.86 Histopathologic studies of 11 patients with PML-IRIS who underwent brain biopsy showed that PML-IRIS brain lesions are enriched in cytotoxic CD8+ T cells that engage JC virus-infected oligodendrocytes.87 Despite differences in the outcome of patients with different manifestations of PML-IRIS described above, the prognosis of patients with PML IRIS may be similar to that of PML patients without IRIS.86
While PML-IRIS that develops after beginning cART can be difficult to distinguish from progressive disease, the onset of clinical worsening in IRIS may be more acute. PML-IRIS can be benign or fatal,85,88–90 and mayor may not respond to steroids, although these are commonly used.85,91 The National Institutes of Health Guidelines for Prevention and Treatment of Opportunistic Infections92 recommend that empiric steroids may be used with clinical benefit: They suggest a 3- to 5-day course of IV methylprednisolone 1 g per day, followed by an oral prednisone taper (60 mg/d, tapered over 1–6 wk), although they note that the dosage and duration of steroids have not been established. Follow-up imaging with contrast-enhanced MRI at 2 to 6 weeks may be helpful in documenting resolution of inflammation and edema and to obtain a new baseline, but the clinical status of the patient is likely the best indicator of treatment efficacy. A single case of rapid improvement in PML-IRIS after treatment with the CCR5 inhibitor, maraviroc, but not with methylprednisolone, has been reported.93 The authors speculated that blocking of CCR5+ leukocyte recruitment to the CNS might have been the underlying mechanism of improvement.
Primary CNS Lymphoma
The primary CNS lymphomas (PCNSL) that occur in HIV-infected patients are of B cell origin (diffuse large cell or immunoblastic94); Epstein Barr virus (EBV) is detectable in virtually all of these tumors94 and it is thought that immunosuppression as well as EBV-induced B cell stimulation leads to tumor development.95 Historically, PCNSL occurred in 1 to 4% of HIV-infected patients, but with the widespread use of cART, the incidence of PCNSL has declined,96,97 and survival has improved.98,99
HIV-associated PCNSL occurs in patients with very low peripheral blood CD4+ T cell counts (< 50 cells/uL).100 Patients present with confusion, lethargy, memory loss, hemiparesis, speech and language disorders, seizures, and cranial nerve palsies.101,102 On brain imaging, PCNSL presents as ring or homogeneously enhancing lesion(s), often located periventricularly or in the frontal lobes, making it similar in appearance to toxoplasmosis and tuberculoma. PCNSL lesions may cross the midline in the corpus callosum and may be associated with patchy nodular ventricular enhancement.103 Thallium (Tl)-201 SPECT may be helpful in distinguishing between CNS infections and PCNSL in HIV-infected individuals. Focal areas of increased Tl-201 uptake are seen in patients with lymphoma, while no brain uptake is seen in patients with CNS infections.104,105 Delayed imaging and calculation of a retention index increase the specificity of the test.105
Although the diagnosis of PCNSL can only be proven by histopathology, detection of EBV DNA by PCR in the CSF is sensitive and specific when it is used in the appropriate clinical setting106 — an HIV-infected patient with a focal brain lesion with mass effect and enhancement. The positive predictive value of the test is highest in patients with a low likelihood of CNS toxoplasmosis, such as those who are Toxoplasma-seronegative or who have been taking trimethoprim-sulfamethoxazole for prophylaxis against toxoplasmosis.106 When the test is used more broadly in clinical settings, the positive predictive value of detection of EBV DNA in CSF for diagnosis of PCNSL is lower.107,108
HIV-associated PCNSL responds to steroids and to radiation therapy. In a retrospective analysis conducted before the availability of cART, median survival was 3 to 4 months with combination dexamethasone and radiation treatment, while untreated survival was only 3 to 4 weeks.109 Most deaths were due to opportunistic infection.101,102 More recent studies show that whole brain radiation therapy (at least 30 Gy)98,110,111 and cART98,111,112 improve survival, with the best survival seen in those who receive both modalities. Prolonged survival has also been reported in HIV-infected patients with PCNSL who received only cART.113–115 Some clinicians have suggested that whole brain radiation should be reserved for patients who do not respond to cART because of the toxic side effects of radiation, including leukoencephalopathy. Other investigators have advocated for addition of anti-viral agents such as ganciclovir or foscarnet, because of the association of PCNSL with EBV.116–121 Overall, the best treatment for HIV-associated primary CNS lymphoma is not known because no randomized trial has been thus far been conducted.
Toxoplasmosis
CNS toxoplasmosis in HIV-infected patients results from reactivation of latent infection by Toxoplasma gondii. It usually presents as one or more brain abscesses, and rarely, as meningoencephalitis. Toxoplasma gondii is an intracellular parasite that exists in three forms: (1) tachyzoites, replicating organisms that cause active disease; (2) bradyzooites, non-replicating organisms that are responsible for latent disease; and (3) oocysts, an infectious form that is shed in cat feces. Humans acquire infection by ingesting oocysts or by ingesting bradyzooites in undercooked meat. In 90% of immunocompetent individuals, the primary infection is asymptomatic; a minority of patients have a mononucleosis-like illness or regional lymphadenopathy.
CNS toxoplasmosis typically occurs in HIV-infected patients with CD4+ T cells < 200/uL. Patients who are not receiving trimethoprim-sulfamethoxazole prophylaxis, have detectable serum anti-Toxoplasma antibody (particularly if the titer is high), or are not receiving cART are at highest risk of developing CNS toxoplasmosis.122–124 Approximately 80% of patients respond to therapy, but patients commonly have residual deficits and they are at increased risk for subsequent dementia.125,126
Clinical manifestations of patients with CNS toxoplasmosis include headache, fever, hemiparesis, ataxia, change in level of consciousness and psychomotor retardation; ~ 30% of patients have seizures.127 Almost all of HIV-infected patients with Toxoplasma encephalitis will have detectable serum anti-Toxoplasma IgG, but IgM is rarely detectable because the disease represents reactivation of a chronic infection.128 Often, CSF examination is not helpful in establishing the diagnosis. On CT, there are round, isodense or hyperdense lesion(s) in the hemispheric gray–white junction, deep white matter, or basal ganglia. More than 90% of these lesions enhance with contrast in a ring, nodular, or homogenous pattern. MRI is more sensitive than CT and often identifies multiple lesions (Fig. 2).
Fig. 2.
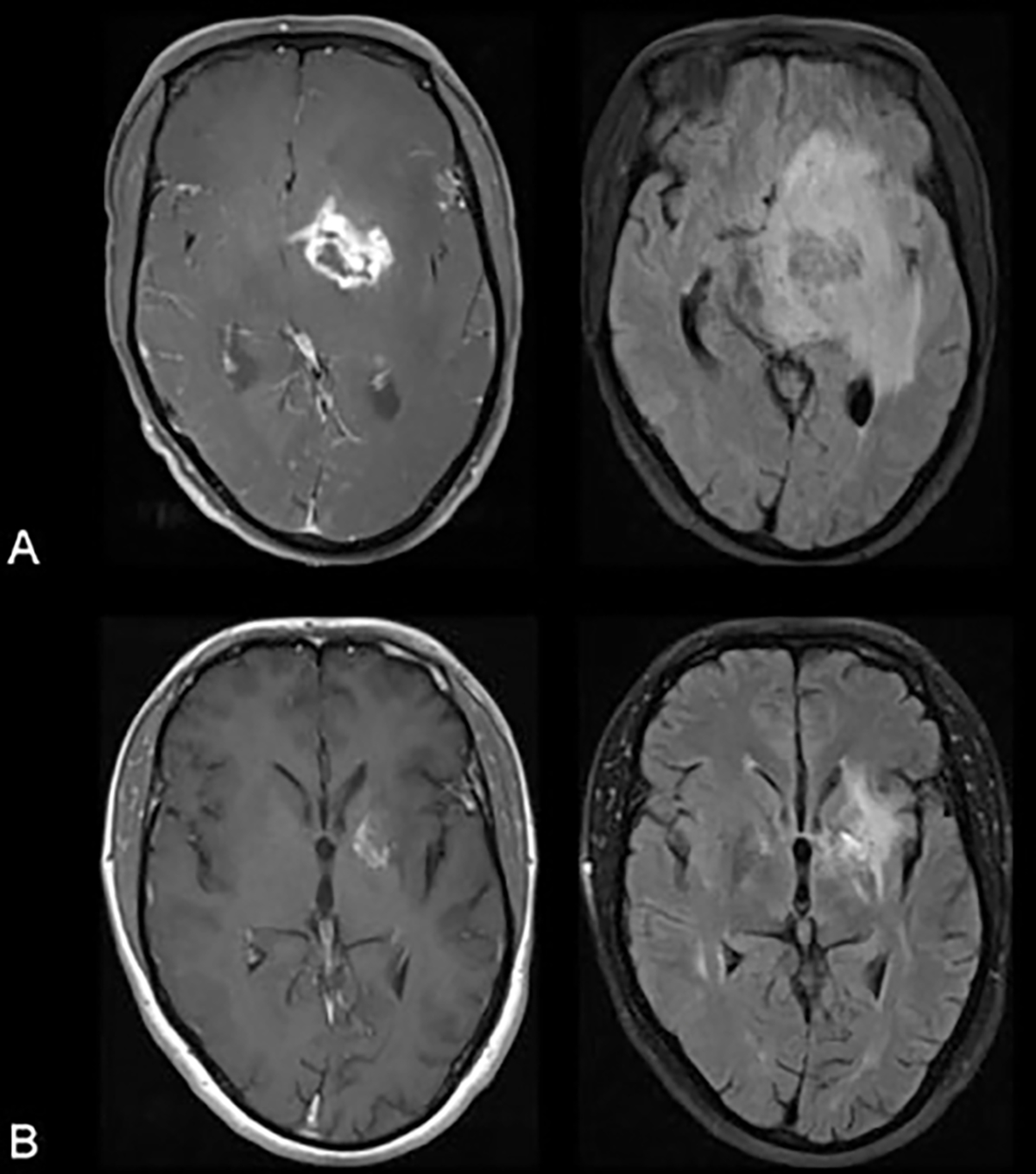
Biopsy proven cerebral toxoplasmosis in an human immunodeficiency virus-infected patient. (A) Contrast enhanced T1-weighted image (left) and fluid attenuation inversion recovery image (right) at presentation. (B) Corresponding images 5 months after beginning treatment for toxoplasmosis. Courtesy of Christina M. Marra, MD.
For patients at high risk for CNS toxoplasmosis (see above), a presumptive diagnosis is made by response to a treatment trial. Diagnosis is established if clinical improvement occurs within 1 to 2 weeks and radiographic improvement within 2 to 3 weeks. Clinical providers should consider brain biopsy if there is no response to a treatment trial or for patients at low risk for CNS toxoplasmosis because, for example, they are Toxoplasma seronegative or have been taking trimethoprim-sulfamethoxazole or cART.
Treatment for cerebral toxoplasmosis occurs in two stages: primary therapy (≥ 6 wk) and chronic suppressive therapy (secondary prophylaxis continued until immune recovery occurs).92 Primary therapy consists of a combination of pyrimethamine, sulfadiazine and folinic acid (Table 2); chronic suppressive therapy consists of lower doses of these medications (Table 3). Clindamycin may be used in place of sulfadiazine in patients with a sulfa allergy (Tables 2, 3). For patients not already on cART, potent antiretroviral therapy is an important component of maintenance therapy. Immune reconstitution inflammatory syndrome is uncommon in HIV-infected patients treated for Toxoplasma encephalitis.128 Secondary prophylaxis for Toxoplasma encephalitis can be discontinued in patients treated with cART who have a sustained increase in peripheral blood CD4+ T cells to > 200/uL for 3 to 6 months after completion of primary therapy. Some experts recommend repeat brain imaging before stopping secondary prophylaxis to confirm that Toxoplasma encephalitis is quiescent; in patients whose radiologic response to treatment is incomplete, a longer duration of primary therapy may be required.
Table 2.
Treatment for CNS toxoplasmosis in HIV
| Primary Therapy (≥ 6wk) | |
|---|---|
| Pyrimethamine plus | 200 mg PO load, then 75 PO every d |
| Sulfadiazine or Clindamycin plus | 1.5 g PO qid 600 mg PO or IV qid |
| Folinic acid (leucovorin) | 10–50 mg PO per day |
Abbreviations: CNS, central nervous system; HIV, human immunodeficiency virus; IV, intravenous; PO, by mouth; qid, 4 times a day.
Source: Table data from92.
Table 3.
Treatment for CNS toxoplasmosis in HIV
| Chronic suppressive therapy or secondary prophylaxis (duration determined by response to cART) | |
|---|---|
| Pyrimethamine plus | 25–50 mg PO every d |
| Sulfadiazine or Clindamycin plus | 1 g PO bid–qid 600 mg PO tid |
| Folinic acid (leucovorin) | 10 – 50 mg PO every d |
Abbreviations: bid, twice daily; cART, combination antiretroviral therapy; CNS, central nervous system; HIV, human immunodeficiency virus; IV, intravenous; PO, by mouth; qid, 4 times a day; tid, three times a day.
Source: Table data from92.
Acknowledgments
E.H. was supported by NIH grant T32 AI007140.
References
- 1.Mocroft AJ, Lundgren JD, d’Armino Monforte A, et al. ; The AIDS in Europe Study Group. Survival of AIDS patients according to type of AIDS-defining event. Int J Epidemiol 1997;26(2):400–407 [DOI] [PubMed] [Google Scholar]
- 2.Garvey L, Winston A, Walsh J, et al. ; UK Collaborative HIV Cohort (CHIC) Study Steering Committee. HIV-associated central nervous system diseases in the recent combination antiretroviral therapy era. Eur J Neurol 2011;18(3):527–534 [DOI] [PubMed] [Google Scholar]
- 3.Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology 2010;75(13):1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2011. Atlanta, GA: Department of Health and Human Services; 2012 [Google Scholar]
- 5.Van de Laar M, Spiteri G. Increasing trends of gonorrhoea and syphilis and the threat of drug-resistant gonorrhoea in Europe. Euro Surveill 2012;17(29):20225. [PubMed] [Google Scholar]
- 6.Tucker JD, Yin YP, Wang B, Chen XS, Cohen MS. An expanding syphilis epidemic in China: epidemiology, behavioural risk and control strategies with a focus on low-tier female sex workers and men who have sex with men. Sex Transm Infect 2011;87(Suppl 2):ii16–ii18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchacz K, Klausner JD, Kerndt PR, et al. HIV incidence among men diagnosed with early syphilis in Atlanta, San Francisco, and Los Angeles, 2004 to 2005. J Acquir Immune Defic Syndr 2008;47(2):234–240 [PubMed] [Google Scholar]
- 8.Gao L, Zhang L, Jin Q. Meta-analysis: prevalence of HIV infection and syphilis among MSM in China. Sex Transm Infect 2009;85(5):354–358 [DOI] [PubMed] [Google Scholar]
- 9.Davis LE, Schmitt JW. Clinical significance of cerebrospinal fluid tests for neurosyphilis. Ann Neurol 1989;25(1):50–55 [DOI] [PubMed] [Google Scholar]
- 10.Rolfs RT, Joesoef MR, Hendershot EF, et al. ; The Syphilis and HIV Study Group. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. N Engl J Med 1997;337(5):307–314 [DOI] [PubMed] [Google Scholar]
- 11.Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis 2012;39(6):453–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra CM, Maxwell CL, Collier AC, Robertson KR, Imrie A. Interpreting cerebrospinal fluid pleocytosis in HIV in the era of potent antiretroviral therapy. BMC Infect Dis 2007;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marra CM, Maxwell CL, Smith SL, et al. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis 2004;189(3):369–376 [DOI] [PubMed] [Google Scholar]
- 14.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS 2008;22(10):1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marra CM, Tantalo LC, Maxwell CL, Dougherty K, Wood B. Alternative cerebrospinal fluid tests to diagnose neurosyphilis in HIV-infected individuals. Neurology 2004;63(1):85–88 [DOI] [PubMed] [Google Scholar]
- 16.Marra CM, Tantalo LC, Sahi SK, Maxwell CL, Lukehart SA. CXCL13 as a cerebrospinal fluid marker for neurosyphilis in HIV-infected patients with syphilis. Sex Transm Dis 2010;37(5):283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rupprecht TA, Kirschning CJ, Popp B, et al. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect Immun 2007;75(9):4351–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libois A, De Wit S, Poll B, et al. HIVand syphilis: when to perform a lumbar puncture. Sex Transm Dis 2007;34(3):141–144 [DOI] [PubMed] [Google Scholar]
- 19.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Lumbar puncture in HIV-infected patients with syphilis and no neurologic symptoms. Clin Infect Dis 2009;48(6):816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YJ, Chi CY, Chou CH, et al. Syphilis and neurosyphilis in human immunodeficiency virus-infected patients: a retrospective study at a teaching hospital in Taiwan. J Microbiol Immunol Infect 2012;45(5):337–342 [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control. Sexually transmitted diseases treatment guidelines, 2010. MMWR Morb Mortal Wkly Rep 2010;59(RR–12):1–110 [PubMed] [Google Scholar]
- 22.Hook EWIII III, Baker-Zander SA, Moskovitz BL, Lukehart SA, Handsfield HH. Ceftriaxone therapy for asymptomatic neurosyphilis. Case report and Western blot analysis of serum and cerebrospinal fluid IgG response to therapy. Sex Transm Dis 1986; 13(3, Suppl):185–188 [PubMed] [Google Scholar]
- 23.Gentile JH, Viviani C, Sparo MD, Arduino RC. Syphilitic meningomyelitis treated with ceftriaxone: case report. Clin Infect Dis 1998;26(2):528. [DOI] [PubMed] [Google Scholar]
- 24.Milger K, Fleig V, Kohlenberg A, Discher T, Lohmeyer J. Neurosyphilis manifesting with unilateral visual loss and hyponatremia: a case report. BMC Infect Dis 2011;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shann S, Wilson J. Treatment of neurosyphilis with ceftriaxone. Sex Transm Infect 2003;79(5):415–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marra CM, Boutin P, McArthur JC, et al. A pilot study evaluating ceftriaxone and penicillin G as treatment agents for neurosyphilis in human immunodeficiency virus-infected individuals. Clin Infect Dis 2000;30(3):540–544 [DOI] [PubMed] [Google Scholar]
- 27.Dobbin JM, Perkins JH. Otosyphilis and hearing loss: response to penicillin and steroid therapy. Laryngoscope 1983;93(12):1540–1543 [DOI] [PubMed] [Google Scholar]
- 28.Linstrom CJ, Gleich LL. Otosyphilis: diagnostic and therapeutic update. J Otolaryngol 1993;22(6):401–408 [PubMed] [Google Scholar]
- 29.McLeish WM, Pulido JS, Holland S, Culbertson WW, Winward K. The ocular manifestations of syphilis in the human immunodeficiency virus type 1-infected host. Ophthalmology 1990;97(2):196–203 [DOI] [PubMed] [Google Scholar]
- 30.Kiss S, Damico FM, Young LH. Ocular manifestations and treatment of syphilis. Semin Ophthalmol 2005;20(3):161–167 [DOI] [PubMed] [Google Scholar]
- 31.Tucker JD, Li JZ, Robbins GK, et al. Ocular syphilis among HIV-infected patients: a systematic analysis of the literature. Sex Transm Infect 2011;87(1):4–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, Young H; IUST. IUSTI: 2008 European Guidelines on the Management of Syphilis. Int J STD AIDS 2009;20(5):300–309 [DOI] [PubMed] [Google Scholar]
- 33.Kingston M, French P, Goh B, et al. ; Syphilis Guidelines Revision Group 2008, Clinical Effectiveness Group. UK National Guidelines on the Management of Syphilis 2008. Int J STD AIDS 2008;19(11):729–740 [DOI] [PubMed] [Google Scholar]
- 34.Moore JE, Gieske M. Syphilitic iritis. A study of 249 patients. Am J Ophthalmol 1931;14:110–126 [Google Scholar]
- 35.Musher DM. Syphilis, neurosyphilis, penicillin, and AIDS. J Infect Dis 1991;163(6):1201–1206 [DOI] [PubMed] [Google Scholar]
- 36.Inungu J, Morse A, Gordon C. Neurosyphilis during the AIDS epidemic, New Orleans, 1990–1997. [letter]J Infect Dis 1998; 178(4):1229. [DOI] [PubMed] [Google Scholar]
- 37.Shalaby IA, Dunn JP, Semba RD, Jabs DA. Syphilitic uveitis in human immunodeficiency virus-infected patients. Arch Ophthalmol 1997;115(4):469–473 [DOI] [PubMed] [Google Scholar]
- 38.Lukehart SA, Hook EWIII III, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med 1988;109(11):855–862 [DOI] [PubMed] [Google Scholar]
- 39.Tramont EC. Persistence of Treponema pallidum following penicillin G therapy. Report of two cases. JAMA 1976;236(19):2206–2207 [PubMed] [Google Scholar]
- 40.Gordon SM, Eaton ME, George R, et al. The response of symptomatic neurosyphilis to high-dose intravenous penicillin G in patients with human immunodeficiency virus infection. N Engl J Med 1994;331(22):1469–1473 [DOI] [PubMed] [Google Scholar]
- 41.Malone JL, Wallace MR, Hendrick BB, et al. Syphilis and neurosyphilis in a human immunodeficiency virus type-1 seropositive population: evidence for frequent serologic relapse after therapy. Am J Med 1995;99(1):55–63 [DOI] [PubMed] [Google Scholar]
- 42.Dibbern DA Jr, Ray SC. Recrudescence of treated neurosyphilis in a patient with human immunodeficiency virus. Mayo Clin Proc 1999;74(1):53–56 [DOI] [PubMed] [Google Scholar]
- 43.Marra CM, Longstreth WT Jr, Maxwell CL, Lukehart SA. Resolution of serum and cerebrospinal fluid abnormalities after treatment of neurosyphilis. Influence of concomitant human immunodeficiency virus infection. Sex Transm Dis 1996;23(3):184–189 [DOI] [PubMed] [Google Scholar]
- 44.Marra CM, Maxwell CL, Tantalo L, et al. Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter? Clin Infect Dis 2004;38(7):1001–1006 [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention (CDC). Symptomatic early neurosyphilis among HIV-positive men who have sex with men—four cities, United States, January 2002-June 2004. MMWR Morb Mortal Wkly Rep 2007;56(25):625–628 [PMC free article] [PubMed] [Google Scholar]
- 46.Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis 1973;127(4):467–470 [DOI] [PubMed] [Google Scholar]
- 47.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 2009;199(6):837–846 [DOI] [PubMed] [Google Scholar]
- 48.Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 1992;5(1):49–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan CS, Ellis LC, Wüthrich C, et al. JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J Virol 2010;84(18):9200–9209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol 1998;4(1):59–68 [DOI] [PubMed] [Google Scholar]
- 51.Koralnik IJ, Wüthrich C, Dang X, et al. JC virus granule cell neuronopathy: A novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol 2005;57(4):576–580 [DOI] [PubMed] [Google Scholar]
- 52.Wüthrich C, Dang X, Westmoreland S, et al. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol 2009;65(6):742–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mascarello M, Lanzafame M, Lattuada E, Concia E, Ferrari S. Progressive multifocal leukoencephalopathy in an HIV patient receiving successful long-term HAART. J Neurovirol 2011;17(2):196–199 [DOI] [PubMed] [Google Scholar]
- 54.Manfredi R, Piergentili B, Marinacci G, Calza L. Atypical progressive multifocal leukoencephalopathy in HIV with a high CD4 count: the use of magnetic resonance imaging plus spectrometry studies. Int J STD AIDS 2012;23(3):e35–e38 [DOI] [PubMed] [Google Scholar]
- 55.Berenguer J, Miralles P, Arrizabalaga J, et al. ; GESIDA 11/99 Study Group. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis 2003;36(8):1047–1052 [DOI] [PubMed] [Google Scholar]
- 56.Küker W, Mader I, Nägele T, et al. Progressive multifocal leukoencephalopathy: value of diffusion-weighted and contrast-enhanced magnetic resonance imaging for diagnosis and treatment control. Eur J Neurol 2006;13(8):819–826 [DOI] [PubMed] [Google Scholar]
- 57.Amlie-Lefond C, Kleinschmidt-DeMasters BK, Mahalingam R, Davis LE, Gilden DH. The vasculopathy of varicella-zoster virus encephalitis. Ann Neurol 1995;37(6):784–790 [DOI] [PubMed] [Google Scholar]
- 58.Kriegstein AR, Shungu DC, Millar WS, et al. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (“chasing the dragon”). Neurology 1999;53(8):1765–1773 [DOI] [PubMed] [Google Scholar]
- 59.Langford TD, Letendre SL, Marcotte TD, et al. ; HNRC Group. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS 2002;16(7):1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rackstraw S, Meadway J, Bingham J, Al-Sarraj S, Everall I. An emerging severe leukoencephalopathy: is it due to HIV disease or highly active antiretroviral therapy? Int J STD AIDS 2006;17(3):205–207 [DOI] [PubMed] [Google Scholar]
- 61.Costello DJ, Gonzalez RG, Frosch MP. Case records of the Massachusetts General Hospital. Case 18–2011. A 35-year-old HIV-positive woman with headache and altered mental status. N Engl J Med 2011;364(24):2343–2352 [DOI] [PubMed] [Google Scholar]
- 62.Zaffiri L, Verma R, Struzzieri K, Monterroso J, Batts DH, Loehrke ME. Immune reconstitution inflammatory syndrome involving the central nervous system in a patient with HIV infection: a case report and review of literature. New Microbiol 2013;36(1):89–92 [PubMed] [Google Scholar]
- 63.Venkataramana A, Pardo CA, McArthur JC, et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 2006;67(3):383–388 [DOI] [PubMed] [Google Scholar]
- 64.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80(15):1430–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marzocchetti A, Di Giambenedetto S, Cingolani A, Ammassari A, Cauda R, De Luca A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol 2005;43(8):4175–4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falcó V, Olmo M, del Saz SV, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr 2008;49(1):26–31 [DOI] [PubMed] [Google Scholar]
- 67.Engsig FN, Hansen AB, Omland LH, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis 2009;199(1):77–83 [DOI] [PubMed] [Google Scholar]
- 68.Khanna N, Elzi L, Mueller NJ, et al. ; Swiss HIV Cohort Study. Incidence and outcome of progressive multifocal leukoencephalopathy over 20 years of the Swiss HIV Cohort Study. Clin Infect Dis 2009;48(10):1459–1466 [DOI] [PubMed] [Google Scholar]
- 69.Dworkin MS, Wan PC, Hanson DL, Jones JL. Progressive multifocal leukoencephalopathy: improved survival of human immunodeficiency virus-infected patients in the protease inhibitor era. J Infect Dis 1999;180(3):621–625 [DOI] [PubMed] [Google Scholar]
- 70.Fanjul F, Riveiro-Barciela M, Gonzalez J, et al. Evaluation of progressive multifocal leukoencephalopathy treatments in a Spanish cohort of HIV-infected patients: do protease inhibitors improve survival regardless of central nervous system penetration-effectiveness (CPE) score? HIV Med 2012 [DOI] [PubMed] [Google Scholar]
- 71.Gasnault J, Costagliola D, Hendel-Chavez H, et al. ; ANRS 125 Trial Team. Improved survival of HIV-1-infected patients with progressive multifocal leukoencephalopathy receiving early 5-drug combination antiretroviral therapy. PLoS ONE 2011;6(6):e20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Luca A, Giancola ML, Ammassari A, et al. The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS-associated progressive multifocal leukoencephalopathy. J Infect Dis 2000;182(4):1077–1083 [DOI] [PubMed] [Google Scholar]
- 73.Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol 1999;20(10):1896–1906 [PMC free article] [PubMed] [Google Scholar]
- 74.Lima MA, Bernal-Cano F, Clifford DB, Gandhi RT, Koralnik IJ. Clinical outcome of long-term survivors of progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry 2010;81(11):1288–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piza F, Fink MC, Nogueira GS, Pannuti CS, Oliveira AC, Vidal JE. JC virus-associated central nervous system diseases in HIV-infected patients in Brazil: clinical presentations, associated factors with mortality and outcome. Braz J Infect Dis 2012;16(2):153–156 [PubMed] [Google Scholar]
- 76.Marra CM, Rajicic N, Barker DE, et al. ; Adult AIDS Clinical Trials Group 363 Team. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS 2002;16(13):1791–1797 [DOI] [PubMed] [Google Scholar]
- 77.Berger JR, Levy RM, Flomenhoft D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann Neurol 1998;44(3):341–349 [DOI] [PubMed] [Google Scholar]
- 78.Bossolasco S, Calori G, Moretti F, et al. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin Infect Dis 2005;40(5):738–744 [DOI] [PubMed] [Google Scholar]
- 79.Yiannoutsos CT, Major EO, Curfman B, et al. Relation of JC virus DNA in the cerebrospinal fluid to survival in acquired immunodeficiency syndrome patients with biopsy-proven progressive multifocal leukoencephalopathy. Ann Neurol 1999;45(6):816–821 [DOI] [PubMed] [Google Scholar]
- 80.García De Viedma D, Díaz Infantes M, Miralles P, et al. JC virus load in progressive multifocal leukoencephalopathy: analysis of the correlation between the viral burden in cerebrospinal fluid, patient survival, and the volume of neurological lesions. Clin Infect Dis 2002;34(12):1568–1575 [DOI] [PubMed] [Google Scholar]
- 81.Clifford DB, Nath A, Cinque P, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol 2013;19(4):351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain 2004;127(Pt 9):1970–1978 [DOI] [PubMed] [Google Scholar]
- 83.Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 2009;73(19):1551–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khanna N, Wolbers M, Mueller NJ, et al. ; Swiss HIV Cohort Study. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J Virol 2009;83(9):4404–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 2009;72(17):1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrison DM, Newsome SD, Skolasky RL, McArthur JC, Nath A. Immune reconstitution is not a prognostic factor in progressive multifocal leukoencephalopathy. J Neuroimmunol 2011;238(1–2):81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin-Blondel G, Bauer J, Cuvinciuc V, et al. In situ evidence of JC virus control by CD8+ T cells in PML-IRIS during HIV infection. Neurology 2013;81(11):964–970 [DOI] [PubMed] [Google Scholar]
- 88.Safdar A, Rubocki RJ, Horvath JA, Narayan KK, Waldron RL. Fatal immune restoration disease in human immunodeficiency virus type 1-infected patients with progressive multifocal leukoencephalopathy: impact of antiretroviral therapy-associated immune reconstitution. Clin Infect Dis 2002;35(10):1250–1257 [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann C, Horst HA, Albrecht H, Schlote W. Progressive multifocal leucoencephalopathy with unusual inflammatory response during antiretroviral treatment. J Neurol Neurosurg Psychiatry 2003;74(8):1142–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.D’Amico R, Sarkar S, Yusuff J, Azar E, Perlman DC. Immune reconstitution after potent antiretroviral therapy in AIDS patients with progressive multifocal leukoencephalopathy. Scand J Infect Dis 2007;39(4):347–350 [DOI] [PubMed] [Google Scholar]
- 91.Martinez JV, Mazziotti JV, Efron ED, et al. Immune reconstitution inflammatory syndrome associated with PML in AIDS: a treatable disorder. Neurology 2006;67(9):1692–1694 [DOI] [PubMed] [Google Scholar]
- 92.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed November 22, 2013
- 93.Martin-Blondel G, Cuzin L, Delobel P, et al. Is maraviroc beneficial in paradoxical progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome management? AIDS 2009;23(18):2545–2546 [DOI] [PubMed] [Google Scholar]
- 94.So YT, Beckstead JH, Davis RL. Primary central nervous system lymphoma in acquired immune deficiency syndrome: a clinical and pathological study. Ann Neurol 1986;20(5):566–572 [DOI] [PubMed] [Google Scholar]
- 95.Ambinder RF. Epstein-Barr virus associated lymphoproliferations in the AIDS setting. Eur J Cancer 2001;37(10):1209–1216 [DOI] [PubMed] [Google Scholar]
- 96.Kirk O, Pedersen C, Cozzi-Lepri A, et al. ; EuroSIDA Study Group. Non-Hodgkin lymphoma in HIV-infected patients in the era of highly active antiretroviral therapy. Blood 2001;98(12):3406–3412 [DOI] [PubMed] [Google Scholar]
- 97.Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis 2006;19(1):14–19 [DOI] [PubMed] [Google Scholar]
- 98.Newell ME, Hoy JF, Cooper SG, et al. Human immunodeficiency virus-related primary central nervous system lymphoma: factors influencing survival in 111 patients. Cancer 2004;100(12):2627–2636 [DOI] [PubMed] [Google Scholar]
- 99.Kreisl TN, Panageas KS, Elkin EB, Deangelis LM, Abrey LE. Treatment patterns and prognosis in patients with human immunodeficiency virus and primary central system lymphoma. Leuk Lymphoma 2008;49(9):1710–1716 [DOI] [PubMed] [Google Scholar]
- 100.Pluda JM, Venzon DJ, Tosato G, et al. Parameters affecting the development of non-Hodgkin’s lymphoma in patients with severe human immunodeficiency virus infection receiving antiretroviral therapy. J Clin Oncol 1993;11(6):1099–1107 [DOI] [PubMed] [Google Scholar]
- 101.Lowenthal DA, Straus DJ, Campbell SW, Gold JW, Clarkson BD, Koziner B. AIDS-related lymphoid neoplasia. The Memorial Hospital experience. Cancer 1988;61(11):2325–2337 [DOI] [PubMed] [Google Scholar]
- 102.Remick SC, Diamond C, Migliozzi JA, et al. Primary central nervous system lymphoma in patients with and without the acquired immune deficiency syndrome. A retrospective analysis and review of the literature. Medicine (Baltimore) 1990;69(6):345–360 [DOI] [PubMed] [Google Scholar]
- 103.Goldstein JD, Zeifer B, Chao C, et al. CT appearance of primary CNS lymphoma in patients with acquired immunodeficiency syndrome. J Comput Assist Tomogr 1991;15(1):39–44 [DOI] [PubMed] [Google Scholar]
- 104.Ruiz A, Ganz WI, Post MJ, et al. Use of thallium-201 brain SPECT to differentiate cerebral lymphoma from toxoplasma encephalitis in AIDS patients. AJNR Am J Neuroradiol 1994;15(10):1885–1894 [PMC free article] [PubMed] [Google Scholar]
- 105.Lorberboym M, Wallach F, Estok L, et al. Thallium-201 retention in focal intracranial lesions for differential diagnosis of primary lymphoma and nonmalignant lesions in AIDS patients. J Nucl Med 1998;39(8):1366–1369 [PubMed] [Google Scholar]
- 106.Antinori A, Ammassari A, De Luca A, et al. Diagnosis of AIDS-related focal brain lesions: a decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology 1997;48(3):687–694 [DOI] [PubMed] [Google Scholar]
- 107.Ivers LC, Kim AY, Sax PE. Predictive value of polymerase chain reaction of cerebrospinal fluid for detection of Epstein-Barr virus to establish the diagnosis of HIV-related primary central nervous system lymphoma. Clin Infect Dis 2004;38(11):1629–1632 [DOI] [PubMed] [Google Scholar]
- 108.Corcoran C, Rebe K, van der Plas H, Myer L, Hardie DR. The predictive value of cerebrospinal fluid Epstein-Barr viral load as a marker of primary central nervous system lymphoma in HIV-infected persons. J Clin Virol 2008;42(4):433–436 [DOI] [PubMed] [Google Scholar]
- 109.Baumgartner JE, Rachlin JR, Beckstead JH, et al. Primary central nervous system lymphomas: natural history and response to radiation therapy in 55 patients with acquired immunodeficiency syndrome. J Neurosurg 1990;73(2):206–211 [DOI] [PubMed] [Google Scholar]
- 110.Nagai H, Odawara T, Ajisawa A, et al. Whole brain radiation alone produces favourable outcomes for AIDS-related primary central nervous system lymphoma in the HAART era. Eur J Haematol 2010;84(6):499–505 [DOI] [PubMed] [Google Scholar]
- 111.Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS 2003;17(12):1787–1793 [DOI] [PubMed] [Google Scholar]
- 112.Hoffmann C, Tabrizian S, Wolf E, et al. Survival of AIDS patients with primary central nervous system lymphoma is dramatically improved by HAART-induced immune recovery. AIDS 2001;15(16):2119–2127 [DOI] [PubMed] [Google Scholar]
- 113.McGowan JP, Shah S. Long-term remission of AIDS-related primary central nervous system lymphoma associated with highly active antiretroviral therapy. AIDS 1998;12(8):952–954 [PubMed] [Google Scholar]
- 114.Aboulafia DM, Puswella AL. Highly active antiretroviral therapy as the sole treatment for AIDS-related primary central nervous system lymphoma: a case report with implications for treatment. AIDS Patient Care STDS 2007;21(12):900–907 [DOI] [PubMed] [Google Scholar]
- 115.Travi G, Ferreri AJ, Cinque P, et al. Long-term remission of HIV-associated primary CNS lymphoma achieved with highly active antiretroviral therapy alone. J Clin Oncol 2012;30(10):e119–e121 [DOI] [PubMed] [Google Scholar]
- 116.Raez L, Cabral L, Cai JP, et al. Treatment of AIDS-related primary central nervous system lymphoma with zidovudine, ganciclovir, and interleukin 2. AIDS Res Hum Retroviruses 1999;15(8):713–719 [DOI] [PubMed] [Google Scholar]
- 117.Slobod KS, Taylor GH, Sandlund JT, Furth P, Helton KJ, Sixbey JW. Epstein-Barr virus-targeted therapy for AIDS-related primary lymphoma of the central nervous system. Lancet 2000; 356(9240):1493–1494 [DOI] [PubMed] [Google Scholar]
- 118.Aboulafia DM. Interleukin-2, ganciclovir, and high-dose zidovudine for the treatment of AIDS-associated primary central nervous system lymphoma. Clin Infect Dis 2002;34(12):1660–1662 [DOI] [PubMed] [Google Scholar]
- 119.Bossolasco S, Falk KI, Ponzoni M, et al. Ganciclovir is associated with low or undetectable Epstein-Barr virus DNA load in cerebrospinal fluid of patients with HIV-related primary central nervous system lymphoma. Clin Infect Dis 2006;42(4):e21–e25 [DOI] [PubMed] [Google Scholar]
- 120.Aboulafia DM, Ratner L, Miles SA, Harrington WJ Jr; AIDS Associated Malignancies Clinical Trials Consortium. Antiviral and immunomodulatory treatment for AIDS-related primary central nervous system lymphoma: AIDS Malignancies Consortium pilot study 019. Clin Lymphoma Myeloma 2006;6(5):399–402 [DOI] [PubMed] [Google Scholar]
- 121.Marretta L, Stocker H, Drauz D, et al. Treatment of HIV-related primary central nervous system lymphoma with AZT high dose, HAART, interleukin-2 and foscarnet in three patients. Eur J Med Res 2011;16(5):197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Antinori A, Larussa D, Cingolani A, et al. ; Italian Registry Investigative NeuroAIDS. Prevalence, associated factors, and prognostic determinants of AIDS-related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis 2004;39(11):1681–1691 [DOI] [PubMed] [Google Scholar]
- 123.Marra CM, Krone MR, Koutsky LA, Holmes KK. Diagnostic accuracy of HIV-associated central nervous system toxoplasmosis. Int J STD AIDS 1998;9(12):761–764 [DOI] [PubMed] [Google Scholar]
- 124.Kiderlen TR, Liesenfeld O, Schürmann D, Schneider T. Toxoplasmic encephalitis in AIDS-patients before and after the introduction of highly active antiretroviral therapy (HAART). Eur J Clin Microbiol Infect Dis 2011;30(12):1521–1525 [DOI] [PubMed] [Google Scholar]
- 125.Arendt G, von Giesen HJ, Hefter H, Neuen-Jacob E, Roick H, Jablonowski H. Long-term course and outcome in AIDS patients with cerebral toxoplasmosis. Acta Neurol Scand 1999;100(3):178–184 [DOI] [PubMed] [Google Scholar]
- 126.Luft BJ, Hafner R, Korzun AH, et al. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N Engl J Med 1993;329(14):995–1000 [DOI] [PubMed] [Google Scholar]
- 127.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 1992;327(23):1643–1648 [DOI] [PubMed] [Google Scholar]
- 128.Martin-Blondel G, Alvarez M, Delobel P, et al. Toxoplasmic encephalitis IRIS in HIV-infected patients: a case series and review of the literature. J Neurol Neurosurg Psychiatry 2011;82(6):691–693 [DOI] [PubMed] [Google Scholar]


