Abstract
High levels of low-density lipoprotein cholesterol (LDL-C) have been associated with an augmented mortality of ischemic stroke. The yearly deaths and mortality data of IS-hLDL-C were derived from the global burden of disease 2019 dataset. The joinpoint, age-period-cohort and decomposition analysis were utilized to evaluate the long-term patterns in the disease burden of IS-hLDL-C, and the effects of population growth and aging. Globally, in 2019, 0.61 million ischemic stroke-related deaths were attributable to high LDL-C, with the highest death burden in the high-middle socio-demographic index (SDI) region. From 1990 to 2019, the age-standardized death rate (ASDR) for IS-hLDL-C exhibited a downward trend, with an average annual percentage change of -1.69 [95% confidence interval: −1.90, −1.48)]. The fastest decreasing trends in ASDR were experienced in the high SDI region. In 119 (58.33%) countries, aging increased the disease burden of hLDL-IS, and population growth increased the disease burden of IS-hLDL-C in 163 (79.90%) countries. The trend in disease burden of IS-hLDL-C exhibited variation across countries and regions, particularly in territories with high to middle high SDI. Aging in upper to middle-income countries and population growth in low to middle-income countries further offset endeavors to reduce the burden of ischemic stroke deaths.
Keywords: High low-density lipoprotein cholesterol, ischemic stroke, joinpoint analysis, age-period-cohort analysis, decomposition analysis, epidemiology
Introduction
Stroke, a non-communicable disease, was reported as the second leading cause of death and the third leading cause of disability worldwide. 1 There were approximately 101 million prevalent cases of stroke, resulting in 6.55 million deaths and 143 million cases of disability in 2019. 1 According to pathology, stroke can be classified into three types: ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. 2 Ischemic stroke accounted for 62.4% of all stroke events in 2019. 1 According to the findings of the Global Burden of Disease (GBD) 2017, although the age-standardized death rate (ASDR) for ischemic stroke declined sharply from 1990 to 2017, the age-standardized incidence rate declined much less, suggesting that ischemic stroke prevention efforts are ineffective.1,3 Consequently, the United Nations (UN) has prioritized the reduction of the burden imposed by ischemic stroke as a primary objective for all non-communicable disease afflictions. 4
Ischemic stroke is highly preventable and 90% of the global stroke burden can be attributed to modifiable risk factors. 5 By regulating these modifiable risk factors, it would be possible to preclude three-quarters of the stroke burden worldwide. 6 Metabolic factors are an important component of modifiable risk factors, mainly including high low-density lipoprotein cholesterol (LDL-C), high systolic blood pressure and high body mass index (BMI). From 1990 to 2019, metabolic risk factors surfaced as primary contributors to elevated rates of age-standardized disability-adjusted life years (DALYs) in ischemic stroke. 1 Therefore, it is of utmost significance to devise prevention policies and healthcare management strategies that focus on modifiable risk factors, in line with the evolving patterns in the disease burden of ischemic stroke.
Hyperlipidemia is an established risk factor for a multitude of ailments.7 –9 At the global and national levels, the UN is promoting the reinforcement of early intervention and management measures for illnesses linked to hyperlipidemia, with a view to accomplishing the Sustainable Development Goals by 2030. 10 LDL-C is a metric of the cholesterol transported by serum LDL particles. Research has demonstrated that the number of deaths and the absolute count of DALYs associated with diseases attributed to elevated LDL-C persistently increase, imposing a substantial strain on global healthcare systems. 7 High LDL-C represents a critical risk factor for stroke and is positively correlated with the likelihood of onset of ischemic stroke.11,12 The burden of ischemic stroke varies between countries and regions.1,3,5 Ischemic stroke induced by high LDL-C is on the rise in low- and middle-income countries (LMICs), owing to population expansion, augmented urbanization, and evolving dietary habits. 13 Previous research has examined the epidemiological characteristics of the global disease burden linked to high LDL-C. 7 Nonetheless, the patterns of prevalence pertaining to ischemic stroke, attributable to high LDL-C, remain ambiguous. Moreover, the epidemiology of ischemic stroke is undergoing a transformation in various regions and nations, as a result of the expedited aging of the global populace.1,14 Hence, exploring the epidemiology of high LDL-C attributable ischemic stroke (IS-hLDL-C) would aid in the formulation of suitable prevention policies, aimed at enhancing access to pharmaceutical and other remedial measures in regions where the burden of stroke is high, as well as augmenting the primary prevention of ischemic stroke.
GBD2019 offered the most comprehensive estimate for the global burden disease of ischemic stroke, spanning from 1990 to 2019, and provided a framework for evaluating risk factors. 15 In this work, we employed joinpoint regression analysis to uncover long-term trends in the death burden of IS-hLDL-C, categorized by age, sex, country, and region. 16 Furthermore, we decomposed and analyzed the overall differences in the number of IS-hLDL-C deaths due to aging, population growth, and epidemiological changes. Lastly, we scrutinized the independent effects of age, period, and cohort for IS-hLDL-C.
Materials and methods
Overview and data resources
The GBD2019 employed a standardized and comparable methodology to estimate data on the burden of disease for 369 diseases or injuries, and 87 risk factors, across 204 countries and 21 regions worldwide.15,17 This comparable approach estimated disease-related epidemiological indicators and disease-caused disease burden indicators, including incidence, prevalence, mortality, and DALYs.
We collected and extracted yearly deaths and mortality data concerning all IS-hLDL-C, from the Global Health Data Exchange featured in the GBD2019 dataset, across a global scale, 21 GBD regions, 5 regions classified based on quintile of the socio-demographic index (SDI), and 204 countries and territories. All data contained in GBD2019 were publicly accessible on the IHME website, and can also be obtained via a web-based tool (https://vizhub.healthdata.org/gbd-results/).
Ischemic stroke definition
In the GBD2019 dataset, stroke was classified as ischemic stroke, cerebral hemorrhage, and subarachnoid hemorrhage. According to the clinical criteria stipulated by the World Health Organization (WHO), ischemic stroke is defined as a localized cerebral, spinal, or retinal infarction, resulting from constriction or obstruction of the vasculature supplying blood to the brain, leading to a bout of neurological impairment.1,5 The various stroke types in the GBD2019 project were classified based on the International Classification of Diseases 10th edition (ICD-10), with the ischemic stroke codes being G45–G46.8, I63–I63.9, I65–I66.9, I67.2–I67.848, and I69.3–I69.4. 15
Metabolic risk factor definitions
The GBD2019 dataset estimated the burden of injury and disease attributed to 87 risk factors. 17 High LDL-C was categorized as a metabolic risk factor, and was delineated as a blood LDL-C level surpassing the theoretical minimum risk exposure level (TMREL) of 1.3 mmol/L (50 mg/dL). 8
GBD2019 employed the risk assessment framework to ascertain the causal connection between disease and risk exposure, determining the TMREL to evaluate risk factor exposure levels and compute the population attributable burden. Risk was classified based on the method of measuring exposure, with high LDL-C being assessed on a continuous scale. The mortality associated with IS-hLDL-C, was evaluated using the population attributable fraction (PAF). The projected mortality rate attributed to ischemic stroke resulting from high LDL-C is equivalent to the PAF for the risk factors exposed in each age group, sex, geography, and year, multiplied by the total number of deaths resulting from ischemic stroke. 18 A comprehensive overview of these methods was provided in a previous study. 17 PAF calculation formula:
represents the PAF value for risk factor j at cause r, age group a, sex s, location l, and year t. represents the risk function linked to exposure levels κ and maximum exposure level λ for risk factor j at cause r, age group a, sex s, and location l. represents the exposure distribution function for age group a, sex s, location l, and year t. represents the theoretical minimum risk exposure level for risk factor j, age group a, and sex s. Attributable death (AD) formula:
N represents the overall number of individuals who die as a result of ischemic stroke.
Statistical analysis
All analyses and data visualization for this study were conducted in the R program (version 4.2.3) and GraphPad Prism 9.0. Information about the R packages was collected in the supplementary materials.
Joinpoint regression analysis
A joinpoint regression model comprises a set of linear statistical models primarily used to quantify the trend of rate values over time, such as incidence, mortality, and prevalence. This modeling concept is also referred to as segmental linear regression, segmental regression, or multisegmental regression. The fundamental concept behind joinpoint regression is to estimate the pattern of change in disease rate values by dividing the long-term trend into segments, each of which is characterized by a continuous log-linear model, using the method of least squares. 16 The model relies on a log-linear model with Poisson distribution, where the rate values of incidence, mortality, and disease prevalence are treated as dependent variables and time (annual year) is regarded as the independent variable. The model computes the sum of squares of residuals between estimated and actual values through Monte Carlo permutations, enabling the identification of significant transitions in the overall trend of rate values, as well as their respective locations. This methodology facilitates the differentiation of long-term trends and local trends in rate values and depicts the local trend for each period by annual percentage change (APC) and overall trend by average annual percentage change (AAPC).
The equation of the Joinpoint regression model is:
represents the response variable at time point t (typically the natural logarithm of incidence rates or occurrence rates). is the intercept term of the model, representing the baseline level before the joinpoint. is the linear trend term of the model, indicating the change in the response variable per unit of time (typically years) before the joinpoint. (k = 1,2, …, n, refers to the count of joinpoint) is regression coefficients at each joinpoint, representing the linear trend in each time segment after the joinpoint. , , …, are the time points of the joinpoints, marking the points where the trend changes is the error term representing unaccounted random errors. Note: When (t–τ k ) > 0, (t–τ k ) is constant.
APC can be obtained by calculating the linear trend coefficient at each joinpoint.:
AAPC is a weighted average of APCs. AAPC estimation formula:
represents the year range of each APC.
Decomposition analysis
We employed the Das Gupta-3 decomposition method to decompose the overall differences in ischemic stroke deaths by population age structure, population growth, and epidemiological changes.19 –21
The deaths in each area are calculated by the following formula:
represents the deaths attributed to age structure, population, and mortality in a given year y. represents the proportion of the population in age category i out of 20 age categories in a given year y. represents the total population in a given year y. represents the mortality rate in age category i in a given year y.
In this study, to provide a more comprehensive understanding of the changes in the number of ischemic stroke deaths attributed to non-demographic factors, we decomposed the total changes into three components. These components include age structure, population growth, and epidemiological changes of IS-hLDL-C.
The number of deaths for each region and age group is calculated by the following formula:
represents the number of deaths of IS-hLDL-C within a specific age group and over a period of y years.
Age-period-cohort analysis
The classical age-period-cohort model, founded on the Poisson distribution, is a log-linear regression model that can effectively capture temporal trends in either incidence or mortality rates with respect to age, period, and cohort. As the identification of individual birth cohorts is reliant on an individual's age at death and the time period of death, this linear dependence gives rise to issues of identifiability. To overcome this challenge, we conducted an age-period-cohort analysis in this study using a web-based tool developed by the National Cancer Institute (https://analysistools.cancer.gov/apc/). To address the parameter identifiability problem of the model, the parameter estimation methods that are widely implemented or highly regarded include the estimable function method, the constrained generalized linear model method, the intrinsic estimation method, and the sequential method. 22 In this study, we employed the following four estimable functions: (1) Net drift, which is the annual average percentage change in the logarithm of mortality after considering period and cohort effects. (2) Local drift, which denotes the annual average percentage change in mortality over time for each age group. (3) Longitudinal age curves, which signify the longitudinal age-specific death rate in the reference cohort after adjusting for period bias. (4) The period/cohort risk ratio, which indicates the relative death risk of period/cohort after adjusting for age and nonlinear effects. This age-period-cohort analysis tool has been widely employed in the fields of epidemiology, sociology, and demography.23 –25
Ethical approval and consent to participate
Not applicable. This study used publicly available data from participant studies on human subjects approved by the Ethical Standards Committee. No separate ethical approval was required for this study.
Results
Changes in mortality of ischemic stroke attributed to high low-density lipoprotein cholesterol
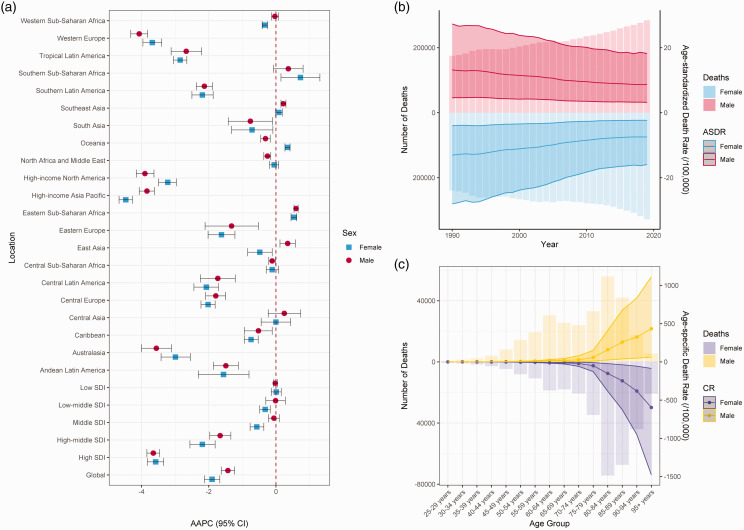
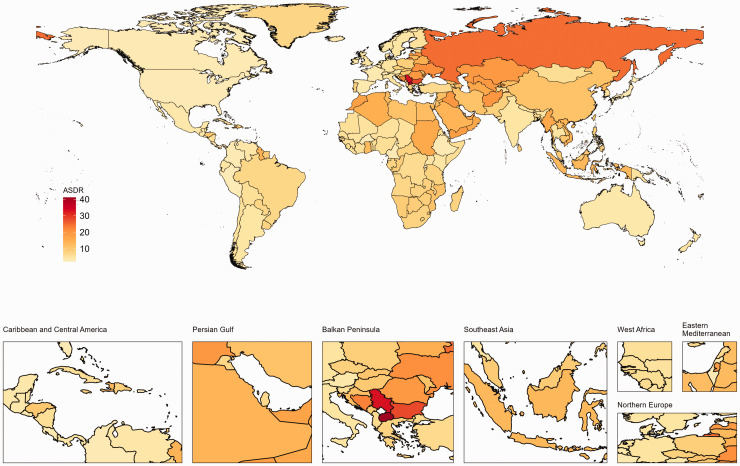
Table 1 and Figure 1(a) show the number of deaths in 1990 and 2019, and the AAPC for the ASDR of IS-hLDL-C in males and females from 1990 to 2019. In 2019, the number of IS-hLDL-C was 612,650 [95% uncertainty interval (UI), 229,107.4, 1,276,394.5] worldwide, which marked a 47.78% surge from 414,562 (95% UI, 160,213.3, 836,789.9) reported in 1990. Nonetheless, the ASDR for IS-hLDL-C per 100,000 individuals declined from 13.29 (95% UI, 4.33, 28.03) in 1990 to 8.08 (95% UI, 2.8, 16.97) in 2019. The ASDR of IS-hLDL-C exhibited a noteworthy downward trajectory over the past 30 years [AAPC (95% confidence interval (CI)) = −1.69 (−1.9, −1.48), p < 0.001] (Table 1). Regarding gender disparities, the ASDR of IS-hLDL-C decreased in both males and females over the last 30 years [males: AAPC (95% CI), −1.43 (−1.63, −1.23); females: AAPC (95% CI), −1.90 (−2.13 to −1.67)]. Notably, the ASDR (95% UI) was marginally greater in males [8.65 (3.19, 18.22)] than in females [7.47 (2.35, 15.96)] (Table S1 and Figure 1(b)). IS-hLDL-C increased in all five SDI regions, with the exception of the high SDI region. In 2019, the ASDR (95% UI) was highest in the high-middle SDI region [11.57 (3.87, 24.25)], whereas the lowest ASDR (95% UI) was recorded in the high SDI region [3.92 (1.12, 8.67)]. From 1990 to 2019, the most substantial reduction in ASDR (95% UI) of IS-hLDL-C was observed in the high SDI region [AAPC (95% CI) = −3.58 (−3.83, 3.33)], whereas the middle SDI region remained unchanged [AAPC (95% CI) = 0.00 (−0.13, 0.12)] (Table 1). From 1990 to 2019, the ASDR (95% UI) for IS-hLDL-C underwent multiple changes, with no significant trend was found in 1990–1994 [APC (95% CI) = −0.38 (−0.94, 0.19), p = 0.173], 1994–1998 being the first period of rapid decline [APC (95% CI) = −2.46 (−3.33, −1.58), p < 0.001], 1998–2003 was the second period of rapid decline [APC (95% CI) = −1.38 (−1.94, −0.81), p < 0.001], and 2003–2007 was the third period of rapid decline [APC (95% CI) = −3.19 (−4.07, −2.31), p < 0.001], and 2007–2014 was the fourth period of rapid decline [APC (95% CI) = −2.02 (−2.32, −1.72), p < 0.001], however, it remained stable in 2014–2019 [APC (95% CI) = −0.75 (−1.15, −0.35), p = 0.001] (Table S2). It is worth mentioning that in 2019, the age group with the highest proportion of IS-hLDL-C worldwide was individuals aged 80–84 years (Figure 1(c)). Among the 21 GBD regions, ASDR (95% UI) of IS-hLDL-C was highest in Eastern Europe [22.69 (8.32, 46.40)], and lowest in high-income North America [2.81 (0.71, 6.70)] in 2019. From 1990 to 2019, the ASDR of IS-hLDL-C either remained stable in Central Asia [AAPC (95% CI) = 0.13 (−0.29, 0.55)], Oceania [AAPC (95% CI) = 0.01 (−0.1, 0.11)], or increased slightly Eastern Sub-Saharan Africa [AAPC (95% CI) = 0.58 (0.51, 0.65)], Southeast Asia [AAPC (95% CI) = 0.16 (0.06, 0.27)], and Sub-Saharan Africa [AAPC (95% CI) = 0.63 (0.06, 1.21)]. In contrast, the remaining regions showed a declining trend, with Western Europe exhibiting the most rapid decline [AAPC (95% CI) = −3.8 (−4.06, −3.53)] (Table 1). At the national level, North Macedonia had the highest ASDR (95% UI) of IS-hLDL-C in 2019 [41.13 (9.84, 94.51)], while Burkina Faso had the lowest ASDR (95% UI) [2.11 (0.66, 4.98)]. Among 204 countries and territories, Singapore had the most rapid decrease in ASDR associated with IS-hLDL-C [AAPC (95% CI) = −5.1921 (−5.62, −4.76)], while Tajikistan had the fastest increase in ASDR [AAPC (95% CI) = 2.35 (1.52, 3.18)] (Table S3 and Figure 2).
Table 1.
The number of deaths and ASDR for ischemic stroke attributed to high low-density lipoprotein cholesterol in 1990 and 2019, and AAPC of ASDR from 1990 to 2019 in global, quintile SDI regions and 21 GBD regions.
| Location | 1990 |
2019 |
1990–2019 |
||
|---|---|---|---|---|---|
| Deaths number (95% UI) | ASDR (95% UI) | Deaths number | ASDR (95% UI) | AAPC (95% CI) | |
| Global | 414562.0 (160213.3–836789.9) | 13.29 (4.33–28.03) | 612650.0 (229107.4–1276394.5) | 8.08 (2.80–16.97) | −1.69 (−1.90, −1.48)* |
| Socio-demographic index Regions | |||||
| High SDI | 116747.9 (33302.4–251196.3) | 11.30 (3.31–24.38) | 90996.6 (22234.9–207697.6) | 3.92 (1.12–8.67) | −3.58 (−3.83, −3.33)* |
| High-middle SDI | 178994.0 (69886.7–359895.2) | 20.65 (6.96–42.98) | 227020.3 (78182.9–470954.7) | 11.57 (3.87–24.25) | −1.96 (−2.26, −1.66)* |
| Middle SDI | 76827.7 (36694.6–144353.3) | 10.22 (3.61–21.76) | 190151.8 (79938.8–382274.6) | 9.31 (3.32–20.08) | 0.00 (−0.13, 0.12) |
| Low-middle SDI | 32317.8 (14680.2–62589.5) | 7.84 (2.60–16.98) | 81175.7 (34742.8–162466.6) | 7.40 (2.63–15.72) | −0.19 (−0.41, 0.04) |
| Low SDI | 9497.4 (4193.1–18388.2) | 5.89 (1.87–13.04) | 23004.8 (10492.9–45473.4) | 5.92 (2.01–13.16) | −0.33 (−0.53, −0.14)* |
| GBD Regions | |||||
| Andean Latin America | 941.2 (372.8–1908.6) | 5.41 (1.82–11.67) | 1822.9 (601.5–4070.9) | 3.45 (1.07–7.83) | −1.53 (−1.97, −1.08)* |
| Australasia | 2094.3 (543.1–4667.1) | 9.96 (2.53–22.24) | 2299.0 (453.5–5506.8) | 3.84 (0.81–9.08) | −3.18 (−3.62, −2.73)* |
| Caribbean | 1936.6 (755.2–4005.6) | 8.44 (2.97–17.94) | 3595.3 (1280.2–7797.6) | 6.94 (2.47–15.05) | −0.65 (−0.78, −0.51)* |
| Central Asia | 6008.6 (2740.5–11472.5) | 14.29 (5.72–28.68) | 8364.8 (4162.9–15191.3) | 14.48 (5.57–30.04) | 0.13 (−0.29, 0.55) |
| Central Europe | 31915.6 (12191.1–64186.0) | 24.81 (8.54–51.27) | 31856.9 (9047.6–69597.5) | 14.14 (4.23–30.46) | −1.92 (−2.15, −1.69)* |
| Central Latin America | 4462.6 (1811.2–8930.9) | 6.64 (2.24–14.20) | 8407.9 (2708.4–18340.3) | 3.81 (1.17–8.42) | −1.90 (−2.28, −1.53)* |
| Central Sub-Saharan Africa | 968.5 (444.5–1912.5) | 6.83 (2.08–15.49) | 2351.4 (988.9–4782.7) | 6.70 (1.96–15.63) | −0.08 (−0.16, 0.01) |
| East Asia | 70757.8 (35147.8–136058.8) | 11.05 (4.09–23.20) | 188178.1 (73577.7–392766.9) | 10.86 (3.64–23.51) | −0.08 (−0.41, 0.25) |
| Eastern Europe | 85649.2 (34745.7–169585.9) | 34.99 (12.65–70.81) | 79642.2 (28845.4–163293.5) | 22.69 (8.32–46.4) | −1.41 (−2.12, −0.70)* |
| Eastern Sub-Saharan Africa | 2401.0 (998.5–4945.7) | 4.69 (1.45–10.76) | 6404.8 (2657.2–13387.2) | 5.57 (1.72–13.06) | 0.58 (0.51, 0.65)* |
| High-income Asia Pacific | 19065.9 (5756.3–41641.8) | 11.74 (3.19–26.43) | 22260.0 (4566.1–53204.6) | 3.46 (0.91–7.91) | −4.13 (−4.34, −3.93)* |
| High-income North America | 29043.3 (7893.0–61692.8) | 7.84 (2.25–16.51) | 20640.4 (4661.4–50466.3) | 2.81 (0.71–6.70) | −3.48 (−3.73 to −3.24)* |
| North Africa and Middle East | 16779.5 (8186.4–31123.0) | 12.79 (4.71–27.17) | 44064.2 (21234.7–81159.6) | 12.19 (4.71–25.11) | −0.17 (−0.32, −0.01)* |
| Oceania | 127.3 (62.3–236.5) | 6.13 (2.15–13.64) | 318.0 (163.2–570.2) | 6.18 (2.22–13.18) | 0.01 (−0.10, 0.11) |
| South Asia | 22524.1 (9247.7–43887.4) | 6.31 (1.89–14.23) | 56070.0 (23080.0–114094.2) | 5.14 (1.71–11.24) | −0.70 (−1.27, −0.14)* |
| Southeast Asia | 18977.1 (8112.3–36868.8) | 10.18 (3.36–22.49) | 51253.6 (21387.1–103865.9) | 10.64 (3.63–23.47) | 0.16 (0.06, 0.27)* |
| Southern Latin America | 3753.5 (1291.8–7930.8) | 9.40 (2.90–20.48) | 4288.9 (1182.9–9502.6) | 4.96 (1.4–10.91) | −2.20 (−2.45, −1.94)* |
| Southern Sub-Saharan Africa | 1480.8 (600.0–3126.9) | 6.79 (2.13–15.44) | 3443.2 (1282.8–7340.0) | 8.14 (2.43–18.94) | 0.63 (0.06, 1.21)* |
| Tropical Latin America | 11494.6 (5328.3–22038.6) | 16.29 (6.04–33.55) | 15984.5 (5637.3–33618.6) | 7.12 (2.37–15.22) | −2.79 (−2.96, −2.61)* |
| Western Europe | 80044.8 (20726.4–178376.4) | 13.56 (3.65–30.07) | 53233.1 (11026.1–123640.4) | 4.42 (1.05–10.03) | −3.80 (−4.06, −3.53)* |
| Western Sub-Saharan Africa | 4135.9 (1829.0–8034.1) | 6.38 (2.16–13.79) | 8170.7 (3647.1–15985.0) | 5.92 (1.99–13.04) | −0.26 (−0.36, −0.15)* |
ASDR: age-standardized death rate (per 100,000); AAPC: average annual percentage change; UI: uncertainty interval; CI: confidence interval; GBD: global burden of disease; SDI: socio-demographic index. “*” indicates p value <0.05.
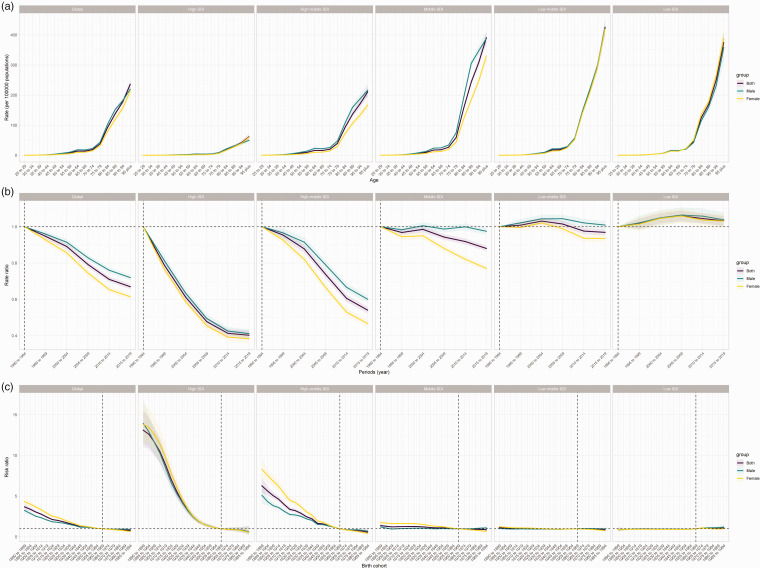
Figure 1.
Death burden of ischemic stroke attributed to high low-density lipoprotein cholesterol. (a) AAPC (95% CI) of ASDR for ischemic stroke attributed to high low-density lipoprotein cholesterol in global, SDI regions and GBD regions from 1990 to 2019. (b) The deaths and ASDR of ischemic stroke attributed to high low-density lipoprotein cholesterol globally, 1990–2019. (c) Age-specific death rate of ischemic stroke attributed to high low-density lipoprotein cholesterol in 2019, globally. AAPC: average annual percentage change; CI: confidence interval; ASDR: age-standardized death rate; CR: crude rate.
Figure 2.
The age-standardized death rate (ASDR) for ischemic stroke attributed to high LDL cholesterol in 204 countries in 2019.
Decomposition analysis of the change in the deaths of ischemic stroke attributed to high low-density lipoprotein cholesterol
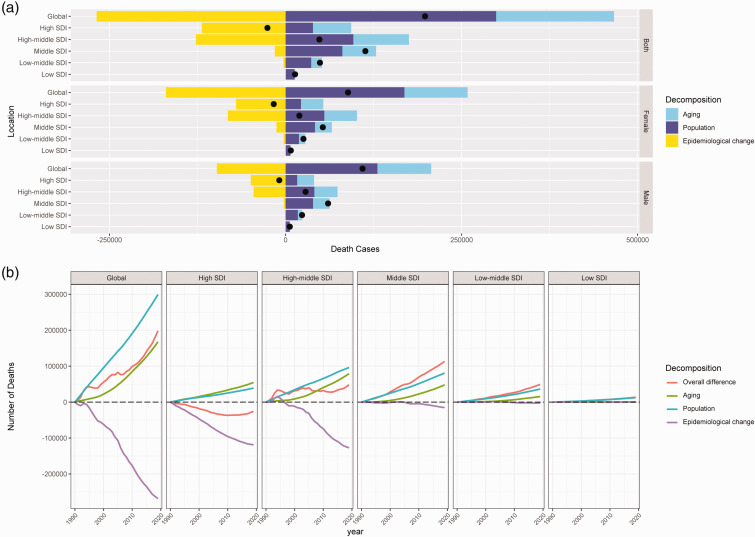
Table 2 and Figure 3 illustrate the proportion of aging, population growth, and mortality changes associated with the overall difference in IS-hLDL-C. The decomposition analysis revealed a global increase in deaths from IS-hLDL-C of 198088.04 between 1990 and 2019, with aging and population growth accounting for 84.51% and 150.96% of the overall difference, respectively (Table 2 and Figure 3(a)). In the five SDI regions, deaths of IS-hLDL-C rose in the high-middle, middle, low-middle, and low SDI regions, while demonstrating a decline in the high SDI region (Figure 3(b)). Among the 21 GBD regions, deaths resulting from IS-hLDL-C surged in the majority of regions (80.95%), while experiencing a decline in Central Europe, Eastern Europe, high-income North America, and Western Europe (Table 2). At the national level, the overall difference has decreased in 37 countries (18.14%) and increased in 167 countries (81.86%) over the past 30 years. The deaths of IS-hLDL-C increased in 119 countries (58.33%) due to aging and in 163 countries (79.90%) due to population growth. Excluding aging and population growth, IS-hLDL-C increased in 52 countries (25.49%). Notably, China, India, and Indonesia experienced the most significant increases in deaths of IS-hLDL-C, with aging (51.19%) and population growth (54.97%) being identified as the main drivers of the increased disease burden of IS-hLDL-C in China. Germany, the USA, the UK, and Ukraine had the most substantial decreases in deaths from IS-hLDL-C, with mortality (217.75%) being identified as the primary factor behind the reduction in the disease burden of iIS-hLDL-C in Germany (Table S4).
Table 2.
Decomposition of ischemic stroke deaths attributed to high low-density lipoprotein cholesterol from 1990 to 2019 according to population growth, aging, and epidemiological changes at the global level, 21 GBD region level, and quintile SDI region level.
| Location | Overall difference | Aging (Percent) | Population (Percent) | Epidemiological changes (Percent) |
|---|---|---|---|---|
| Global | 198088.04 | 167395.82 (84.51%) | 299031.12 (150.96%) | −268338.90 (−135.46%) |
| Socio-demographic Index Regions | ||||
| High SDI | −25751.33 | 54411.95 (−211.30%) | 38818.76 (−150.74%) | −118982.04 (462.04%) |
| High-middle SDI | 48026.27 | 79017.95 (164.53%) | 96178.87 (200.26%) | −127170.55 (−264.79%) |
| Middle SDI | 113324.09 | 47836.90 (42.21%) | 80670.81 (71.19%) | −15183.61 (−13.40%) |
| Low-middle SDI | 48857.91 | 15353.16 (31.42%) | 36356.94 (74.41%) | −2852.19 (−5.84%) |
| Low SDI | 13507.43 | 800.49 (5.93%) | 12515.95 (92.66%) | 190.99 (1.41%) |
| GBD Regions | ||||
| Andean Latin America | 881.75 | 465.78 (52.82 %) | 1100.88 (124.85%) | −684.90 (−77.68%) |
| Australasia | 204.71 | 1358.11 (663.43%) | 1157.68 (565.52%) | −2311.08 (−1128.94%) |
| Caribbean | 1658.73 | 833.42 (50.24 %) | 1371.93 (82.71 %) | −546.62 (−32.95%) |
| Central Asia | 2356.23 | −1114.74 (−47.31%) | 3566.78 (151.38%) | −95.81 (−4.07%) |
| Central Europe | −58.65 | 15059.28 (−25674.40%) | 3734.01 (−6366.06%) | −18851.94 (32140.46%) |
| Central Latin America | 3945.35 | 2959.86 (75.02 %) | 4954.05 (125.57%) | −3968.56 (−100.59 %) |
| Central Sub-Saharan Africa | 1382.88 | 63.73 (4.61%) | 1449.16 (104.79%) | −130.01 (−9.40%) |
| East Asia | 117420.24 | 61234.56 (52.15 %) | 65185.07 (55.51%) | −8999.39 (−7.66%) |
| Eastern Europe | −6006.98 | 27290.38 (−454.31%) | 4559.34 (−75.90%) | −37856.70 (630.21 %) |
| Eastern Sub-Saharan Africa | 4003.78 | −58.80 (−1.47 %) | 3488.24 (87.12%) | 574.33 (14.34%) |
| High-income Asia Pacific | 3194.09 | 26685.46 (835.46 %) | 7204.59 (225.56 %) | −30695.96 (−961.02%) |
| High-income North America | −8402.91 | 8714.28 (−103.71%) | 9362.97 (−111.43%) | −26480.16 (315.13%) |
| North Africa and Middle East | 27284.79 | 3067.68 (11.24%) | 25374.74 (93.00 %) | −1157.64 (−4.24%) |
| Oceania | 190.67 | 14.19 (7.44 %) | 175.53 (92.06 %) | 0.95 (0.50 %) |
| South Asia | 33545.98 | 13771.55 (41.05 %) | 27278.34 (81.32 %) | −7503.91 (−22.37 %) |
| Southeast Asia | 32276.58 | 9380.77 (29.06 %) | 21508.29 (66.64 %) | 1387.53 (4.30 %) |
| Southern Latin America | 535.43 | 1348.46 (251.85 %) | 1954.99 (365.13 %) | −2768.02 (−516.97%) |
| Southern Sub-Saharan Africa | 1962.42 | 90.18 (4.60 %) | 1530.82 (78.01%) | 341.42 (17.40 %) |
| Tropical Latin America | 4489.88 | 7311.83 (162.85 %) | 10471.15 (233.22%) | −13293.10 (−296.07%) |
| Western Europe | −26811.72 | 35214.69 (−131.34 %) | 16408.33 (−61.20%) | −78434.74 (292.54%) |
| Western Sub-Saharan Africa | 4034.80 | −599.72 (−14.86 %) | 5329.16 (132.08%) | −694.64 (−17.22%) |
Note: “−” of percentage indicates the direction of deaths change related to each factor is opposite to the overall difference. GBD: global burden of disease. SDI: socio-demographic index.
Figure 3.
The decomposition analysis of ischemic stroke attributed to high low-density lipoprotein cholesterol in global and quintile SDI regions. (a, difference between 1990 and 2019; b, differences from 1990 to 2019). SDI: socio-demographic index.
Age, period and cohort effects on ischemic stroke attributed to high low-density lipoprotein cholesterol
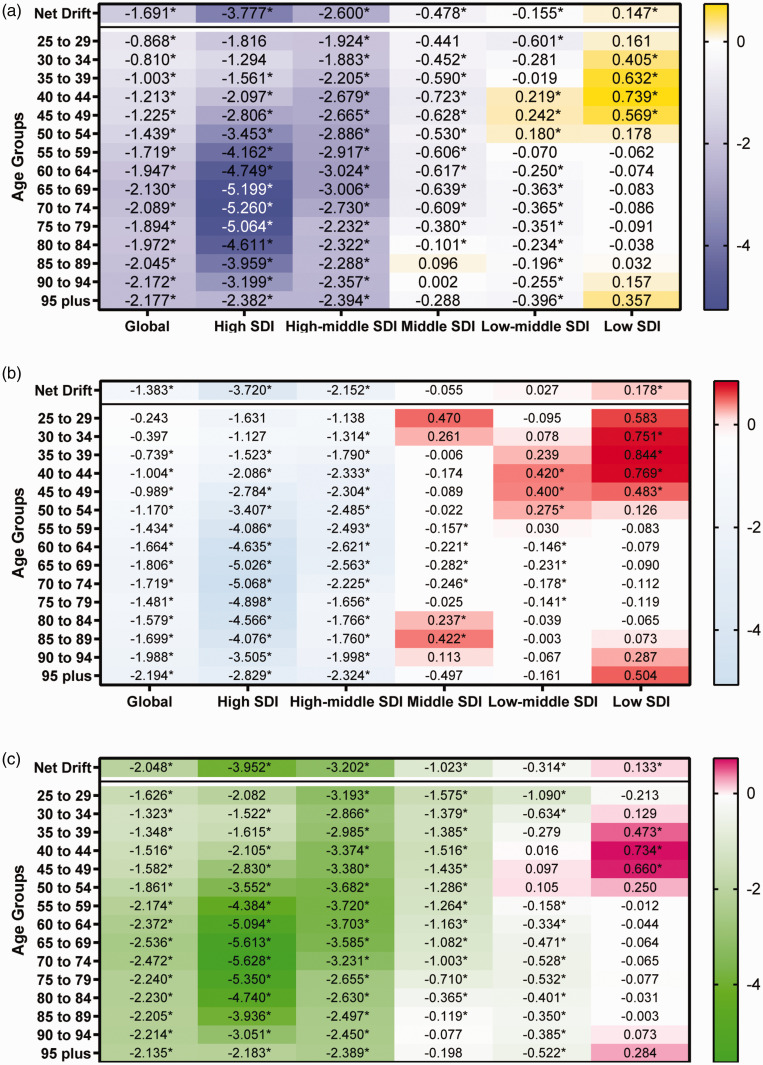
Table S5 and Figure 4 estimate the annual percentage change in IS-hLDL-C across age groups (local drift) and all age group (net drift) globally. Globally, there was a decreasing trend in mortality of IS-hLDL-C across all age group [net drift (95% CI) = −1.69 (−1.77, −1.61), p < 0.001]. The rate of decline was higher in females [net drift (95% CI) =−2.05 (−2.16, −1.94), p < 0.001] than in males [net drift (95% CI) =−1.38 (−1.46, −1.31), p < 0.001], suggesting a more pronounced improvement in high mortality attributable to IS-hLDL-C in females over the past 30 years. The local drifts showed the most significant reduction in mortality among males in the 95+ age group [local drift (95% CI) = −2.18 (−2.43, −1.93), p < 0.001]. Among females, we observed that the rate of decline in mortality tended to increase gradually with age, reaching its peak in the 65-69 age group [local drift (95% CI) = −2.54 (−2.67, −2.40), p < 0.001], and then began to slow down in older age groups of 69 years and above. Among the five SDI regions, there was an increasing trend in the mortality rate resulting from IS-hLDL-C across all age groups only in the low SDI region [net drift (95% CI) =0.15 (0.05, 0.24), p < 0.001]. In contrast, the remaining four SDI regions showed a significant decreasing trend, with the high SDI region [net drift (95% CI) = −3.78 (−4.01, −3.55), p < 0.001] and high-middle SDI region [net drift (95% CI) = −2.60 (−2.73, −2.47), p < 0.001] experiencing the most significant reduction in mortality. It is noteworthy that, among males, the mortality rate resulting from IS-hLDL-C remained stable in all age groups in the middle SDI region [net drift (95% CI) = −0.05 (−0.12, 0.01), p > 0.05] and the middle-low SDI region [net drift = −0.03 (−0.05, 0.10), p > 0.05]. Based on local drifts, we found a stable mortality trend in the 25–29 and 30–34 age groups in the high SDI region, while all other age groups showed a decreasing trend in mortality, with the most significant decline in the 70-74 age group [−5.26 (−5.49, −5.03), p < 0.001]. In contrast, in the low SDL region, we observed an increasing trend in mortality among the 30-34, 35-39, 40-44, and 45-49 age groups, while the mortality rates for other age groups remained stable or decreased.
Figure 4.
The net and local drifts for age-specific death rate of ischemic stroke attributed to high low-density lipoprotein cholesterol in global and quintile SDI regions, from 1990 to 2019. (a, Both; b, Male; c, Female). SDI: socio-demographic index; “*” refers to p < 0.05.
The age-period-cohort model estimated the effects of age, period, and cohort on IS-hLDL-C, respectively. Table S6 and Figure 5(a) depict the age effect of IS-hLDL-C. The longitudinal age curves revealed that the global mortality of IS-hLDL-C increased with age, maintaining a consistent trend across the five SDI regions. In the lower SDI region, the age effect exhibited an exponential increase for individuals aged 75 years or older, particularly in the middle, low-middle, and low SDI regions. In contrast, the age effect increased more smoothly in the high SDI region.
Figure 5.
The age-period-cohort analysis of ischemic stroke attributable to high low-density lipoprotein cholesterol in global and quintile SDI regions. (a, Longitudinal age curve; b, Period risk ratios; c, Cohort risk ratios).
Table S7 and Figure 5B present the period effect of IS-hLDL-C. Globally, there was a decreasing trend in mortality from IS-hLDL-C between 1990 and 2019. This downward trend was primarily concentrated in high SDI and high-middle SDI regions. However, the mortality rate decline in the high SDI region slowed from 2009 onward. It is noteworthy that the mortality rate resulting from ischemic stroke increased in the low SDI region and remained stable in the low-middle SDI region.
Table S8 and Figure 5 C illustrate the cohort effect of IS-hLDL-C. Our analysis suggested that globally, mortality of IS-hLDL-C has the potential to be reduced in the newborn cohorts. The newborn cohorts had a greater reduction in relative risk of IS-hLDL-C in high- and high-middle SDI regions. Our findings indicated that the relative risk of experiencing IS-hLDL-C in the newborn cohorts remained unchanged in the low SDI region.
Discussion
Dyslipidemia, particularly heightened levels of LDL-C in plasma, is widely regarded as a significant predisposing factor for ischemic heart disease (IHD) and ischemic stroke.26,27 The morbidity resulting from dyslipidemia escalated by over 40% in the past 30 years, presenting a significant challenge to healthcare systems worldwide. As per the projections of the WHO, dyslipidemia was responsible for over one-third of global mortalities attributed to IHD or ischemic stroke in 2008. 28 From 1990 to 2019, the number of deaths from IS-hLDL-C increased globally. However, the ASDR for IS-hLDL-C decreased in both males and females globally. Decomposition analysis demonstrated that the increased disease burden of IS-hLDL-C could primarily be attributed to aging and population growth. Excluding the effects of aging and population growth, the mortality of IS-hLDL-C declined, which elucidated the inverse alteration in absolute numbers and age-standardized rates. From a global perspective, the utilization of oral lipid-lowering medications and other general lipid-lowering measures could aid in mitigating the disease burden associated with IS-hLDL-C. 29
Stroke was influenced by multiple risk factors, such as behavioral, metabolic, and environmental factors,30,31 wherein metabolic risk factors accounted for 72% of the DALYs attributable to ischemic stroke. 32 Historically, metabolic risk factors, particularly heightened levels of LDL-C in plasma, were recognized as a major obstacle to reducing the burden of disease in high-income countries. Nonetheless, the disease burden associated with high LDL-C surged in LMICs due to the development in socioeconomic levels and alterations in the diet and lifestyle of the populace. Indeed, heightened levels of LDL cholesterol in plasma were a substantial contributor to IHD and ischemic stroke in both developed and developing nations. 33 Using the quintile of SDI as a basis for classification, countries and regions are categorized as high SDI, middle-high SDI, medium SDI, middle-low SDI, and low SDI regions. In this study, we paid specific attention to the relationship between SDI levels and the disease burden of IS-hLDL-C. In the high SDI region, the mortality of IS-hLDL-C was considerably lower in 2019 than in 1990. Simultaneously, in the high SDI region, the ASDR for ischemic stroke due to high LDL cholesterol has significantly declined over the last 30 years. Higher levels of medical care in high SDI regions have allowed for earlier intervention in the lipid levels of the population, and this primary prevention strategy for ischemic stroke may directly contribute to a reduction in mortality. 34 Several studies conducted in high-income nations in Europe and North America have indicated that the prophylactic utilization of statins and implementation of lifestyle changes may have resulted in a reduction in population plasma cholesterol levels.35,36 Conversely, in the low-SDI region, the number of deaths attributable to IS-hLDL-C has significantly increased, despite a slight reduction in ASDR. Individuals with lower socioeconomic status in low SDI regions had limited access to statins (and other medications), let alone the awareness and availability of prophylactic medications, resulting in only a small proportion of the population having access to pharmacological interventions. 37 This may have partly accounted for an escalation in mortality from IHD and ischemic stroke.
From 1990 to 2019, the disease burden of ischemic stroke attributed to high LDL-C was high in upper to middle-income countries (UMICs), specifically in Eastern Europe and Central Europe. On the contrary, the disease burden was lower in high-income regions such as North America, high-income Asia-Pacific, and Australasia. At the national level, the ASDR for IS-hLDL-C showed the greatest decrease in Singapore and the utmost increase in Tajikistan between 1990 and 2019. For economically developed regions and countries such as Singapore and high-income areas in North America, a well-established primary care infrastructure, high health insurance coverage, a high level of medical services, inexpensive drugs, early disease screening, and public education could effectively reduce the disease burden of IS-hLDL-C, particularly through the utilization of lipid-lowering drugs like statins.38,39 From 2002 to 2013, the utilization of statins significantly increased in Denmark and the United States, especially in the latter, where it escalated by 79%.40,41 The utilization of generic statins also significantly reduced the total cost, which greatly contributed to the widespread use of lipid-lowering drugs among the population.40,41 Additionally, the update of the guidelines clarified the populations that needed lipid-lowering therapy, which further expanded the scope of the beneficiary population. 42
The period effect indicates a significant reduction in mortality from IS-hLDL-C in high SDI and high-middle SDI regions and an increase in mortality in the low SDI region. This trend suggested that efforts to decrease LDL-C levels are active and effective. From 1990 to 2019, the ASDR dropped the most in the high SDI region, and a decomposition analysis suggested that aging and population growth were the primary drivers of this change. According to UN reports, the total population aged 65 and above has increased by approximately 3% over the last three decades, and these changes were in line with the substantial increase in statin use at the start of the 21st century.34,43 Nevertheless, after 2009, the reduction in the disease burden of IS-hLDL-C in the high SDI region decelerated considerably. We suggested that in the past, statins and other measures alone may not have been sufficient in reducing blood cholesterol levels and that additional lipid-lowering agents such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and ezetimibe were necessary to achieve further lipid reduction.44 –46
Regions and countries with lower economic levels, such as Central Asia, South Africa, and sub-Saharan Africa, went through a phase of economic development during which changes in diet, living environment, and lifestyle heightened the population's risk of exposure to metabolic factors relevant to ischemic stroke. This led to a notable increase in the disease burden of ischemic stroke attributed to metabolic factors. Furthermore, in low-SDI regions, the disease burden of IS-hLDL-C was aggravated by the misallocation of healthcare resources, underinvestment, and inadequate population health awareness. 47 Lastly, the epidemiology of disease in low-SDI regions was still in its early stages of transformation, primarily from communicable to non-communicable chronic diseases. 48 This resulted in a dual disease burden in low-SDI regions. Zhao et al. based on data from the Sino-MONICA-Beijing project discovered a considerable increase in the incidence of ischemic stroke in LMICs during the transition period of stroke epidemiology. 48 Despite the increased awareness of the risks of hyperlipidemia, risk factors for ischemic stroke and stroke prevention, and advancements in medical technology, mortality and DALY rates from IS-hLDL-C have escalated in these low-income regions and countries. 49 Consequently, LMICs should allocate more healthcare resources to the prevention and treatment of IS-hLDL-C. In particular, LMICs ought to augment their investment in and implementation of these low-cost generic statins to enhance their accessibility and affordability to the population. Moreover, it is crucial to further heighten awareness of dyslipidemia among primary care physicians and the public.
As for countries with developing economies, such as China, India, and Southeast Asia, the ASDR for IS-hLDL-C remained unaltered between 1990 and 2019. As urbanization, industrialization, and globalization trends intensify in these developing countries, aging and population growth play a role in influencing the life expectancy of the population, thus augmenting the risk of exposure to lipid-related factors. 50 Moreover, the escalated consumption of high-sugar and high-fat foods, as well as the changes in lifestyle, substantially contributed to the abnormal blood lipid levels in the inhabitants of developing countries. 51 Thus, it is imperative to make endeavors to integrate and coordinate primary healthcare resources, formulate appropriate health policies, enhance primary healthcare providers' awareness of stroke prevention and the implementation of basic lipid-lowering therapy, and improve healthcare testing systems in the middle-SDI region to boost the prevention and management of IS-hLDL-C.
In this study, we conducted a stratified analysis by sex and age, and between 1990 and 2019, the ASDR from IS-hLDL-C decreased worldwide in both males and females, with slightly higher rates observed in males than in females. Nonetheless, the disease burden of IS-hLDL-C heightened with age in both males and females. Our findings revealed that the mortality of IS-hLDL-C in the elderly was higher in regions with higher economic levels, which might be associated with the aging population and low birth rate in developed countries. By employing the age effect of the age-period-cohort model, we observed that the age effect rises exponentially in regions with lower economic development, particularly among individuals aged 75 years or older.
Several advantages are worth noting. Firstly, the strength of this study is the systematic use of GBD2019 data and methods to estimate the global disease burden of IS-hLDL-C in 204 countries and territories, which is based on the most up-to-date source of data using multinational site data. This provides access to its data sources, study methods and results, making it one of the most comprehensive sources of high LDL-C disease burden available. Secondly, in the scenario of mounting cardiovascular ailments triggered by dyslipidemia, this study's primary clinical implications suggest that physicians should offer counsel to geriatric patients about focusing on the prevention of ischemic stroke through regular lipid-level screening and better utilization of lipid-lowering drugs. It also highlights the need for LMICs to allocate medical resources efficiently, design appropriate healthcare policies, and reinforce grassroots education toward reducing the high LDL-C-induced disease burden of ischemic stroke. Thirdly, the study comprehensively elaborates on the effects and long-term trends of age, period, cohort, and demographics on the disease burden of IS-hLDL-C using joinpoint analysis, age-period-cohort models, and decomposition analysis. This serves as a reference value for the government in formulating corresponding public health policies.
Nonetheless, the present study has several limitations. Firstly, it should be noted that the GBD2019 is inherently defective, and although attempts are made to adjust and modify it to improve the estimation of risk exposure, as well as the collection and quality assessment of data pertaining to the disease burden of IS-hLDL-C, the quality and availability of the data cannot be guaranteed due to insufficiently developed population-based ischemic stroke registration systems and mortality information systems in some less developed countries during the survey period. Secondly, the data analysis might be incomplete since data on IS-hLDL-C in individuals younger than 25 years is excluded. Thirdly, GBD is not based on rigorously designed randomized controlled trials or prospective studies, and further validation of the biological effects and dose-response relationships between risk and outcomes may be required in the future. Finally, similar to other age-period-cohort analyses, our study is inevitably subject to ecological fallacies, as interpretations of population-level findings may not necessarily be applicable to individuals. Therefore, this work needs validation in the future through individual studies.
Conclusion
In conclusion, although the ASDR of IS-hLDL-C decreased globally from 1990 to 2019, in this work we found that the burden of disease for IS-hLDL-C remains high in UMICs, while it is steadily increasing in LMICs. UMICs need to be wary of aging while LMICs need to focus on population growth, and different lipid-lowering measures and rational allocation of healthcare resources will be effective in reducing the disease burden of ischemic stroke.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231211448 for Global, regional, and national burden of ischemic stroke attributed to high low-density lipoprotein cholesterol, 1990–2019:A decomposition analysis and age-period-cohort analysis by Jian Zhang, Shijie Zhu, Chunlong Liu, Yaofeng Hu, Aoran Yang, Yonghui Zhang and Yang Hong in Journal of Cerebral Blood Flow & Metabolism
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Natural Science Foundation of Liaoning Province (No.2022-YGJC-34 and No.20180530024).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: All authors revised and approved the final manuscript. Shijie Zhu, Yang Hong: Conceptualization, Data curation, Resources. Jian Zhang, Shijie Zhu and Chunlong Liu: Writing – original draft, Writing – review & editing, Formal analysis, Methodology, Validation. Yaofeng Hu and Aoran Yang: Validation, Data curation, Resources. Yonghui Zhang: Writing – review & editing, Resources. Writing – review & editing, Validation. Yang Hong: Funding acquisition.
ORCID iD: Shijie Zhu https://orcid.org/0000-0003-1931-7430
Availability of data and materials
All data involved in the current study are publicly available data.
References
- 1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hisham NF, Bayraktutan U. Epidemiology, pathophysiology, and treatment of hypertension in ischaemic stroke patients. J Stroke Cerebrovasc Dis 2013; 22: e4–14. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology 2020; 54: 171–179. [DOI] [PubMed] [Google Scholar]
- 4.Nations U. UN Secretary General’s Report on Progress on the prevention and control of noncommunicable diseases - December 2017. Published online 2018. https://digitallibrary.un.org/record/1474584 (accessed 10 September 2023).
- 5.Zhang R, Liu H, Pu L, et al. Global burden of ischemic stroke in young adults in 204 countries and territories. Neurology 2023; 100: e422–e34. [DOI] [PubMed] [Google Scholar]
- 6.Katan M, Luft A. Global burden of stroke. Semin Neurol 2018; 38: 208–211. [DOI] [PubMed] [Google Scholar]
- 7.Du H, Shi Q, Song P, et al. Global burden attributable to high low-density lipoprotein-cholesterol from 1990 to 2019. Front Cardiovasc Med 2022; 9: 903126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karr S. Epidemiology and management of hyperlipidemia. Am J Manag Care 2017; 23: S139–s48. [PubMed] [Google Scholar]
- 9.Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 2013; 40: 195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy KS. Global burden of disease study 2015 provides GPS for global health 2030. Lancet 2016; 388: 1448–1449. [DOI] [PubMed] [Google Scholar]
- 11.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007; 370: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 12.Ansell BJ. Cholesterol, stroke risk, and stroke prevention. Curr Atheroscler Rep 2000; 2: 92–96. [DOI] [PubMed] [Google Scholar]
- 13.He J, Gu D, Reynolds K, et al. Serum total and lipoprotein cholesterol levels and awareness, treatment, and control of hypercholesterolemia in China. Circulation 2004; 110: 405–411. [DOI] [PubMed] [Google Scholar]
- 14.Béjot Y, Daubail B, Giroud M. Epidemiology of stroke and transient ischemic attacks: current knowledge and perspectives. Rev Neurol (Paris) 2016; 172: 59–68. [DOI] [PubMed] [Google Scholar]
- 15. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020; 396: 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Statist Med 2000; 19: 335–351. [DOI] [PubMed] [Google Scholar]
- 17. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020; 396: 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol 2019; 74: 2529–2532. [DOI] [PubMed] [Google Scholar]
- 19.Gupta DP. Standardization and decomposition of rates from cross-classified data. Genus 1994; 50: 171–196. [PubMed] [Google Scholar]
- 20.Gupta PD. Standardization and decomposition of rates: a users's manual. Washington d, 1993. [Google Scholar]
- 21.Liu C, Zhu S, Zhang J, et al. Global, regional, and national burden of liver cancer due to non-alcoholic steatohepatitis, 1990–2019: a decomposition and age-period-cohort analysis. J Gastroenterol 2023. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev 2014; 23: 2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao J, Eshak ES, Liu K, et al. Age-period-cohort analysis of stroke mortality attributable to high sodium intake in China and Japan. Stroke 2019; 50: 1648–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Yu C, Bi Y, et al. Trends and age-period-cohort effect on incidence and mortality of prostate cancer from 1990 to 2017 in China. Public Health 2019; 172: 70–80. [DOI] [PubMed] [Google Scholar]
- 25.Zhu S, Zhang F, Zhao G, et al. Trends in the global burden of oral cancer joint with attributable risk factors: results from the global burden of disease study 2019. Oral Oncol 2022; 134: 106189. [DOI] [PubMed] [Google Scholar]
- 26.Dai H, Much AA, Maor E, et al. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: results from the global burden of disease study 2017. Eur Heart J Qual Care Clin Outcomes 2022; 8: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan S, Tang B, Zheng J, et al. Circulating lipoprotein lipids, apolipoproteins and ischemic stroke. Ann Neurol 2020; 88: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Global Health Observatory, World Health Organization. Noncommunicable diseases: risk factors. https://www.who.int/data/gho/data/themes/ topics/topic-details/GHO/ncd-risk-factors (2021, accessed 10 September 2023). .
- 29.Pencina MJ, Pencina KM, Lloyd-Jones D, et al. The expected 30-year benefits of early versus delayed primary prevention of cardiovascular disease by lipid lowering. Circulation 2020; 142: 827–837. [DOI] [PubMed] [Google Scholar]
- 30.Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res 2017; 120: 472–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Hajifathalian K, Ezzati M, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet 2014; 383: 970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol 2019; 18: 417–418. [DOI] [PubMed] [Google Scholar]
- 33.Pirillo A, Casula M, Olmastroni E, et al. Global epidemiology of dyslipidaemias. Nat Rev Cardiol 2021; 18: 689–700. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE study): a prospective epidemiological survey. Lancet 2011; 378: 1231–1243. [DOI] [PubMed] [Google Scholar]
- 35.Hulmán A, Tabák AG, Nyári TA, et al. Effect of secular trends on age-related trajectories of cardiovascular risk factors: the whitehall II longitudinal study 1985–2009. Int J Epidemiol 2014; 43: 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posner BM, Franz MM, Quatromoni PA, et al. Secular trends in diet and risk factors for cardiovascular disease: the Framingham study. J Am Diet Assoc 1995; 95: 171–179. [DOI] [PubMed] [Google Scholar]
- 37.Chow CK, Nguyen TN, Marschner S, et al. Availability and affordability of medicines and cardiovascular outcomes in 21 high-income, middle-income and low-income countries. BMJ Glob Health 2020; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olejaz M, Juul Nielsen A, Rudkjøbing A, et al. Denmark health system review. Health syst transit. 2012; 14: i–xxii, 1–192. [PubMed] [Google Scholar]
- 39.Clarfield AM, Manor O, Nun GB, et al. Health and health care in Israel: an introduction. Lancet 2017; 389: 2503–2513. [DOI] [PubMed] [Google Scholar]
- 40.Salami JA, Warraich H, Valero-Elizondo J, et al. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the medical expenditure panel survey. JAMA Cardiol 2017; 2: 56–65. [DOI] [PubMed] [Google Scholar]
- 41.Mortensen MB, Falk E, Schmidt M. Twenty-year nationwide trends in statin utilization and expenditure in Denmark. Circ Cardiovasc Qual Outcomes 2017; 10:7. [DOI] [PubMed] [Google Scholar]
- 42.Pencina MJ, Navar-Boggan AM, D'Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370: 1422–1431. [DOI] [PubMed] [Google Scholar]
- 43.Walley T, Folino-Gallo P, Stephens P, et al. Trends in prescribing and utilization of statins and other lipid lowering drugs across Europe 1997–2003. Br J Clin Pharmacol 2005; 60: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ 2022; 377: e069066. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Wen D, Chen Y, et al. PCSK9 inhibitors for secondary prevention in patients with cardiovascular diseases: a Bayesian network meta-analysis. Cardiovasc Diabetol 2022; 21: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steffens D, Bramlage P, Scheeff C, et al. PCSK9 inhibitors and cardiovascular outcomes. Expert Opin Biol Ther 2020; 20: 35–47. [DOI] [PubMed] [Google Scholar]
- 47.Lee H, Nam YS, Lee KM. Development-assistance strategies for stroke in low- and middle-income countries. J Korean Med Sci 2015; 30 Suppl 2: S139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao D, Liu J, Wang W, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing project. Stroke 2008; 39: 1668–1674. [DOI] [PubMed] [Google Scholar]
- 49.Giroud M, Jacquin A, Béjot Y. The worldwide landscape of stroke in the 21st century. Lancet 2014; 383: 195–197. [DOI] [PubMed] [Google Scholar]
- 50.Thomas H, Diamond J, Vieco A, et al. Global atlas of cardiovascular disease 2000–2016: the path to prevention and control. Glob Heart 2018; 13: 143–163. [DOI] [PubMed] [Google Scholar]
- 51.Kim SH, Song YH, Park S, et al. Impact of lifestyle factors on trends in lipid profiles among Korean adolescents: the Korea National Health and Nutrition Examination surveys study, 1998 and 2010. Korean J Pediatr 2016; 59: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231211448 for Global, regional, and national burden of ischemic stroke attributed to high low-density lipoprotein cholesterol, 1990–2019:A decomposition analysis and age-period-cohort analysis by Jian Zhang, Shijie Zhu, Chunlong Liu, Yaofeng Hu, Aoran Yang, Yonghui Zhang and Yang Hong in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
All data involved in the current study are publicly available data.