Abstract
Productive replication of human immunodeficiency virus type 1 (HIV-1) in brain macrophages and microglia is a critical component of viral neuropathogenesis. However, how virus-macrophage interactions lead to neurological disease remains incompletely understood. Possibly, a differential ability of virus to replicate in brain tissue macrophages versus macrophages in other tissues underlies HIV-1 neurovirulence. To these ends, we established systems for the isolation and propagation of pure populations of human microglia and then analyzed the viral life cycles of divergent HIV-1 strains in these cells and in cultured monocytes by using identical viral inocula and indicator systems. The HIV-1 isolates included those isolated from blood, lung tissue, cerebrospinal fluids (CSF), and brain tissues of infected subjects: HIV-1ADA and HIV-189.6 (from peripheral blood mononuclear cells), HIV-1DJV and HIV-1JR-FL (from brain tissue), HIV-1SF162 (from CSF), and HIV-1BAL (from lung tissue). The synthesis of viral nucleic acids and viral mRNA, cytopathicity, and release of progeny virions were assessed. A significant heterogeneity among macrophage-tropic isolates for infection of monocytes and microglia was demonstrated. Importantly, a complete analysis of the viral life cycle revealed no preferential differences in the abilities of the HIV-1 strains tested to replicate in microglia and/or monocytes. Macrophage tropism likely dictates the abilities of HIV-1 to invade, replicate, and incite disease within its microglial target cells.
The human immunodeficiency virus (HIV) invades the central nervous system (CNS) early following viral infection but produces brain disease only years later. During the periods of progressive immunosuppression, from 15 to 25% of infected individuals develop cognitive and motor abnormalities referred to as the HIV type 1 (HIV-1)-associated dementia complex (ADC) (21, 44, 45). Interestingly, and despite the significant neurological impairments observed in ADC, cells of neural origin (neurons, oligodendrocytes, and astrocytes) are rarely infected in affected brain tissue (47). More than a decade of research led to a consensus that the clinical and neuropathologic abnormalities seen in ADC are likely a result of viral infection of mononuclear phagocytes (microglia and blood-derived brain macrophages) (27, 51, 56). Microglia, the resident macrophages of the brain, were first described nearly six decades ago by del Rio Hortega (7, 8), but as yet their functional significance in health and disease remains elusive. The role of mononuclear phagocytes in HIV neuropathogenesis is highlighted by HIV encephalitis (11, 55), in which multinucleated giant cells (MGC) and perivascular macrophages are hallmarks of brain pathology (2, 10, 46). Importantly, a recent study demonstrated that the number of immunocompetent brain macrophages is the best correlate for neurological disease in ADC (18).
It is well established that all HIV-1 neuroinvasive isolates are macrophage tropic (15). However, the degree of viral replication in brain tissue may not always predict disease progression. Thus, it was hypothesized that a class of “neurotropic” HIV-1 isolates that preferentially infect microglia and incite disease exists (43, 50). Infected microglial cells secrete neurotoxins that serve to amplify the pathological and clinical manifestations of ADC. Such a neuropathogenic mechanism is unique among neurovirological disorders. Indeed, for herpes simplex virus, cytomegalovirus, measles virus (subacute sclerosing panencephalitis), and other forms of viral encephalitis, productive viral replication occurs in neurons and is associated with direct neuronal cytopathicity (22). Neuronal loss is caused as a direct consequence of viral infection. Alternatively, for parainfectious encephalomyelitis (mumps, measles, and rubella viruses), the virus produces neurological disease through autoimmune mechanisms (23). For such diseases, virus cannot be demonstrated in the CNS yet an intense inflammatory reaction in the brain produces profound perivascular demyelination, presumably mediated by host immune responses elicited as a consequence of peripheral viral replication.
Whether or not HIV is truly neurotropic remains unresolved. A recent study of the viral determinants for simian immunodeficiency virus neurovirulence showed that although the envelope glycoprotein determined macrophage tropism, sequences from the transmembrane glycoprotein-encoding portion of the same gene along with nef conferred neurovirulence (35). Such genetic signatures have not as yet been demonstrated conclusively for HIV (12, 28, 31, 43, 46, 49). Nonetheless, if such subsets of HIV do exist and produce neurological impairments, perhaps by preferentially infecting microglia, such neurotropic viruses could have important implications in viral neuropathogenesis. In support of this concept, a recent report by Strizki and colleagues (50) demonstrated that specific HIV-1 strains show preferential abilities to replicate in microglia versus monocytes. However, the study examined only viral p24 production as a marker for HIV-1 replication. Quantitative assessments of the viral life cycle, beginning with the formation of proviral DNA and leading to the production of infectious progeny virions, had not been determined.
To investigate whether specific HIV-1 strains have a preferential ability to replicate in microglia, we quantitatively evaluated the viral life cycle in monocytes and microglia after virus exposure. We performed quantitative comparisons of the synthesis of viral nucleic acids in both cell types and determined productive replication by measuring reverse transcriptase (RT) activity in cell culture fluids. We show that a panel of viral isolates recovered from peripheral blood mononuclear cells (PBMCs), cerebrospinal fluid (CSF), and lung and brain tissues of infected patients exhibit differential levels of replication in monocytes that are identical to those observed in microglia.
MATERIALS AND METHODS
Reagents.
Viral isolates HIV-1JR-FL (29), HIV-1BAL (14), HIV-189.6 (6), and HIV-1SF162 (5) were obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases. HIV-1ADA was isolated from PBMC of a patient with AIDS as previously described (17). HIV-1DJV was obtained by monocyte cocultivation from brain tissue of a patient who died of dementia (20). All viral strains were propagated on monocytes. HIV-1LAI (41) and HIV-1YU-2 (34) proviral plasmid DNAs were generous gifts from Michael Emerman and Beatrice Hahn, respectively. The isolates HIV-1LAI and HIV-1YU-2 were rescued with a 293T packaging system (40). Briefly, episomal forms of the HIV-1 clones were used to transfect human kidney epithelial cells, the 293T cell line, in a transient-expression system. The resultant high-titer cell-free virions were used to infect monocytes or microglia. HIV-1JR-FL, HIV-1DJV, and HIVYU-2 were the neurotropic strains analyzed, as all were derived from brain tissue of infected subjects with HIV encephalitis. Table 1 provides a summary of the viral isolates used in this study. Antibodies to CD68 and HAM-56 (macrophages), glial fibrillary acidic protein (GFAP; astrocytes), and von Willebrand factor (endothelial cells) were purchased from Dako Corp., Carpinteria, Calif. The antibodies to neurofilament (NF), fluorescein isothiocyanate (FITC)-conjugated antibodies to mouse immunoglobulin G (IgG) F(ab′)2 fragments, and FITC- and rhodamine-conjugated antibodies to rabbit IgG F(ab′)2 fragments were procured from Boehringer Mannheim Corp., Indianapolis, Ind.
TABLE 1.
Panel of HIV-1 isolates used to compare monocyte and microglial susceptibilities to HIV-1 infection
| HIV-1 strain | Source | Tropism | Reference |
|---|---|---|---|
| HIV-1JR-FL | Brain tissue of patient with HIV-1 dementia | MOa | 29 |
| HIV-1DJV | Brain tissue of patient with HIV-1 dementia | MO | 20 |
| HIV-1BAL | Infant lung tissue | MO | 14 |
| HIV-1SF162 | CSFb of a patient with AIDS | MO | 5 |
| HIV-1ADA | PBMC of patient with AIDS | MO | 17 |
| HIV-189.6 | PBMC of patient with AIDS | MO | 6 |
| HIV-1YU-2 | Brain tissue of patient with AIDS | MO | 34 |
| HIV-1LAI | PBMC of patient with AIDS | T-cell | 41 |
MO, macrophage.
CSF, cerebrospinal fluid.
Isolation of microglia.
Fetal brain tissue (gestational age, 14 to 20 weeks) was obtained from elective abortion procedures performed in full compliance with National Institutes of Health and University of Nebraska Medical Center ethical guidelines. The tissue was washed with cold Hanks balanced salt solution (MediaTech, Herndon, Va.) supplemented with Ca2+ and Mg2+ and then was digested with 0.25% trypsin (Sigma Chemical Co., St. Louis, Mo.) for 30 min at 37°C. Trypsin was neutralized with fetal bovine serum (FBS), and the tissue was further dissociated to obtain single-cell suspensions. The cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma) supplemented with a mixture containing 10% heat-inactivated FBS, 1,000 U of purified recombinant human macrophage colony stimulating factor (MCSF) per ml (a generous gift from Genetics Institute, Cambridge, Mass.), penicillin and streptomycin (50 μg/ml), and 100 μg of neomycin per ml. The mixed culture was maintained under 10% CO2 for 7 days, and the medium was fully replaced to remove any cell debris. The microglia cells released with further incubation were collected and purified by preferential adhesion. Microglia were cultured as adherent monolayers at a density of 5 × 104 cells/well in 96-well plates, and floating cells were removed after 4 h. The adherent microglia preparations (>98% pure) were identified by CD68 immunostaining. Replicate cultures were used for viral infections.
Isolation and cultivation of monocytes.
PBMCs were obtained from HIV-1-, HIV-2-, and hepatitis B virus-seronegative donors by leukopheresis and were purified by countercurrent centrifugation to generate pure populations of monocytes (17). Cell suspensions were found to be >98% pure monocytes by Wright-staining, nonspecific esterase, granular peroxidase, and CD68 immunostaining assays. Cells were cultured in DMEM supplemented with 10% heat-inactivated pooled human serum–10 μg of ciprofloxacin (Sigma) per ml–50 μg of gentamicin (Sigma) per ml–1,000 U of MCSF per ml. All reagents were prescreened and found to be negative for endotoxin (<10 pg/ml; Associates of Cape Cod, Woods Hole, Mass.) and mycoplasma contamination (Gen-Probe II; Gen-Probe, San Diego, Calif.).
HIV-1 infection of monocytes and microglia.
Monocytes and microglia were cultured on 96-well plates (Costar Corp., Cambridge, Mass.) at densities of 105 and 5 × 104 cells/well, respectively, for 7 days prior to infection with HIV-1. The cell-free viral inoculum used for each experiment was standardized for all experiments by RT activity (2 × 105 cpm/106 cells). Each experiment was performed in triplicate determinations. The culture medium was exchanged twice weekly, and samples were collected for RT activity measurement. RT activity was determined by incubating 10 μl of sample with a reaction mixture consisting of 0.05% Nonidet P-40 (Sigma), 0.25 μg of oligo(dT) per ml, 10 μg of poly(A) (Pharmacia Fine Chemicals, Piscataway, N.J.) per ml, 150 mmol of KCl per liter, 15 mmol of MgCl2 per liter, 5 mmol of dithiothreitol (Sigma) per liter, and [3H]dTTP (2 Ci/mmol; Amersham Corp., Arlington Heights, Ill.) in Tris-HCl buffer (pH 7.9) for 24 h at 37°C. Radiolabeled nucleotides were precipitated on paper filters in an automatic cell harvester (Skatron, Sterling, Va.) with cold 10% trichloroacetate and 95% ethanol. The incorporated radioactivity was measured by liquid scintillation spectroscopy (26). Adherent monolayers of monocytes and microglia were incubated with HIV-1 for 4 h at 37°C. The inoculum was withdrawn, and residual virus was washed off with fresh culture medium. Samples of culture supernatant were collected by medium exchange twice weekly for RT analysis. Viability of infected cells was measured by conversion of 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) to purple formazan.
PCR analysis of synthesis of viral nucleic acids.
Monocytes (3 × 106 cells/well) and microglia (5 × 105/well) were cultured on six-well plates (Costar Corp.) and infected with HIV-1 as described above. Prior to infection, the HIV-1 cell-free stocks were treated with DNase I for 30 min at 37°C (57). At 8, 24, 48, 72, and 96 h postinfection, samples were collected for RT analysis and the residual medium was washed off with fresh phosphate-buffered saline (PBS; Sigma). The cells were then scraped in 1 ml of PBS. The resultant cell pellet was used for the extraction of cellular DNA with the Iso-quick nucleic extraction kit (ORCA Research Inc., Bothell, Wash.). The DNA was resuspended at a concentration of 104 cell equivalents/μl. PCR was performed to identify early (primers to long terminal repeat [LTR] U3/R), intermediate (primers to pol I and J regions), and late (primers to LTR U3/gag) products of reverse transcription (57). PCR primers to gag and nef regions of the viral genome were used to detect the episomal forms containing one and two LTRs (one- and two-LTR circles, respectively) (3, 4, 57). Standard HIV-1 cDNAs were prepared by simultaneous amplification on serial twofold dilutions of DNA extracted from 8e5 cells that harbor defective HIV-1 proviruses (13). Extrachromosomal one-LTR standards were generated by PCR on HIV-1LAI-infected primary peripheral blood lymphocytes. Amplified products were hybridized to radiolabeled oligonucleotide probes and quantified on a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Immunocytochemistry.
Microglia were seeded on 8-mm-diameter chamber slides (Nunc Inc., Naperville, Ill.) and were cultured as described above. Cells were fixed in an ice-cold absolute acetone-methanol (1:1) mixture for 15 min at −20°C and then incubated with monoclonal antibodies (MAb) to CD68 or HAM-56 (at a dilution of 1:50) or to GFAP at a 1:100 dilution. Additional MAbs to HLA-DR, NF, CD14, and vimentin were from Boehringer Mannheim Corp. and were used at a 1:50 dilution. Antibodies to HIV-1 p24, galactocerebrocide (Gal-C; for oligodendrocytes), and von Willebrand factor were from Dako and were used at 1:5, 1:50, and 1:200 dilutions, respectively. Immunostaining was visualized by incubating the preparations with antibodies to mouse IgG F(ab′)2 fragments conjugated to FITC (at a 1:100 dilution) for p24, CD68, HLA-DR, CD14, and vimentin or antibodies to mouse IgM F(ab′)2 fragments conjugated to tetramethyl rhodamine isothiocyanate (TRITC; Boehringer) (at a 1:100 dilution) for HAM-56. Anti-rabbit IgG F(ab′)2 fragments conjugated to FITC (for GFAP) and rhodamine (for Gal-C and von Willebrand factor) were used at a 1:100 dilution. Antigen-reactive cells were viewed on a Nikon Microphot-FXA microscope (Nikon, Tokyo, Japan).
Electron microscopy.
Microglia were placed on 12-mm-diameter coverslips (105 cells/coverslip) and cultured as described above. After viral infection, cells were fixed at days 7 and 21 postinfection with 2.5% glutaraldehyde, washed with cacodylate buffer, postfixed in 1% osmium tetroxide, and then dehydrated and embedded in LX 112 (Ladd Research Industries, Burlington, Vt.). Ultrathin sections were stained with uranyl acetate and lead citrate and then examined on a Philips EM 410 electron microscope (Eindhoven, The Netherlands) as previously described (37).
RESULTS
Characterization of primary human fetal microglia.
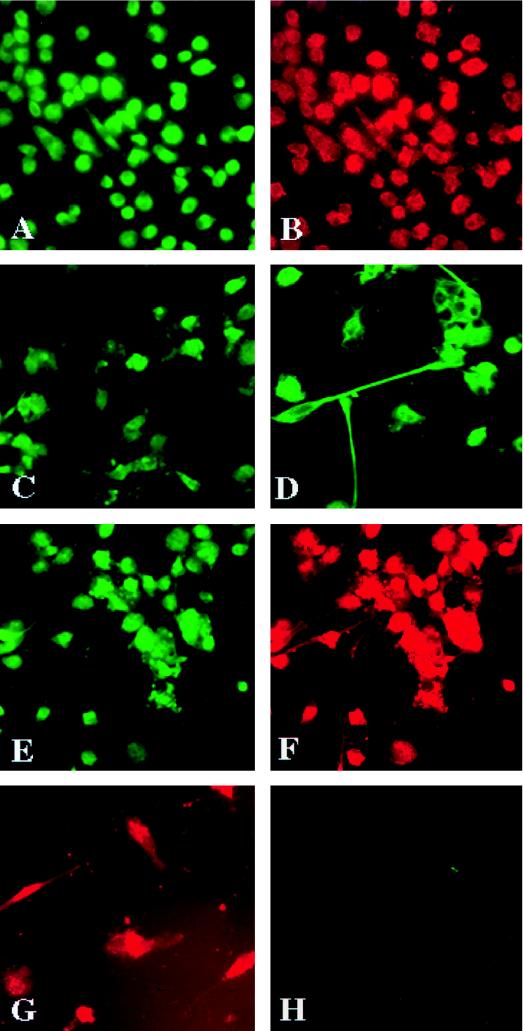
Detailed morphological and immunocytochemical characterizations of purified microglia cells were performed. The microglia were collected as a nonadherent layer from the mixed brain cell cultures and seeded on chamber slides as adherent cells. The microglia differentiated with time from an ameboid form and then developed processes that extended to several times the size of their initial small oval cell bodies (Fig. 1A and D, respectively). Cultured microglia were stained with antibodies to cell-specific markers for macrophages (CD68 and HAM-56) (Fig. 1A and B), for astrocytes (GFAP) (Fig. 1H), for endothelial cells (von Willebrand factor), for oligodendrocytes (Gal-C), and for neurons (NF) (data not shown) to establish purity. The cells were >98% pure on the basis of these characteristics. Double immunostaining with HAM-56 and GFAP showed that only HAM-56-positive cells were present in the culture and that none of the cells reacted with GFAP (Fig. 1G and H). Double immunostaining with HLA-DR, a marker for cell activation, and HAM-56 showed that the percentage of HLA-DR- and HAM-56-double-positive cells was low (<25%) and that the cells expressed HLA-DR weakly (Fig. 1E and F). This result was distinct from that previously reported by Lee et al. (33), demonstrating that cultured fetal microglia were highly positive for HLA-DR. Staining for vimentin, a marker for cytoskeletal proteins, clearly showed the cell bodies and unique processes of microglial cells (Fig. 1D). Interestingly, about 50% of the microglial cells were immunoreactive for CD14, the receptor for bacterial lipopolysaccharide (36), and a marker believed to distinguish adult monocytes from microglia (52, 53).
FIG. 1.
Immunocytochemical characterization of microglia cells. Microglia cells were collected from mixed cultures of glia and purified by preferential adhesion. The cells were cultured on Chamber-Tech slides as adherent monolayers for 7 days. They were then fixed with cold acetone-methanol (1:1) for 15 min and treated with anti-CD68 (A) or anti-HAM-56 (B) to confirm their identity. The cells were immunoreactive for CD14 (C). Incubation with vimentin (D) demonstrated processes extending from the cell bodies. Double immunostaining for HLA-DR and HAM-56 revealed the presence of major histocompatibility complex class II on some cells. The preparations were devoid of any astrocytes as demonstrated by double labeling with HAM-56 (G) and GFAP (H), an astrocyte marker. Positive staining was visualized by anti-mouse IgG labeled with FITC for CD68, vimentin, CD14, and HLA-DR. Anti-mouse IgM labeled with TRITC was used for HAM-56. GFAP staining was visualized with anti-rabbit rhodamine. All observations were made on a Nikon Microphot FXA microscope with appropriate filters. Original magnifications, ×200 (A, B, C, D, E, and F) and ×400 (G and H).
Comparative analysis of HIV-1ADA infection of monocytes and microglia.
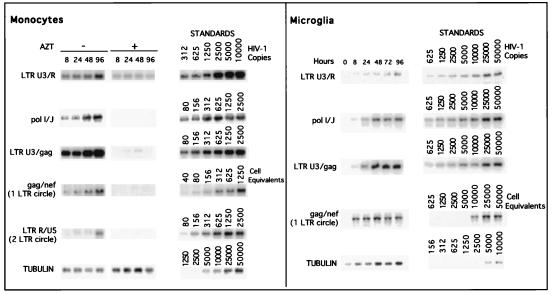
With the establishment of highly purified human microglia, we compared levels of viral infection by using a prototypic macrophage-tropic viral strain, HIV-1ADA. The accumulations of viral nucleic acids by PCR in monocytes and microglia infected with equivalent viral inocula were determined. The results are shown in Fig. 2. The use of zidovudine (AZT) served as an internal control. In cells treated with AZT, intermediate and late products of reverse transcription were not detected. The viral DNA products identified in the HIV-1ADA-inoculated cultures represent only de novo HIV-1 DNA synthesis. In both monocytes and microglia both intermediate and late products were detected at 8 h postinfection (Fig. 2). The episomal forms of the viral nucleic acids (one-LTR circles) were also observed in monocytes and microglia at 8 h postinfection. As the episomal forms of the viral DNA indicate the completion of viral nucleic acid synthesis and the successful nuclear import across the nuclear membrane, our data show that under the culture conditions used, both monocytes and microglia support a complete cycle of synthesis of viral DNA, as early as 8 h after viral exposure. The experiment was repeated for three individual donors for monocytes and microglia, and similar results were obtained. The ratios of early, intermediate, and late products of viral cDNA synthesis to a mitochondrial gene (the internal control for extraction of extrachromosomal DNA) were also determined, and similar kinetics were observed for all products of HIV-1 proviral DNA synthesis (data not shown). The peak accumulations of late products were at 48 h postinfection for monocytes (Fig. 2) and microglia. Late-product accumulation reached a peak at 48 h and did not increase significantly thereafter.
FIG. 2.
Identification of HIV-1 DNA in infected monocytes and microglia. Cells were infected, as described in Materials and Methods, with HIV-1ADA. At specified times postinfection, supernatant samples were collected for RT determination and the cells were fractured for viral DNA analysis. Primers that specifically amplified early, intermediate, and late products of RT were used. The PCR-amplified products were quantified on a PhosphorImager after hybridization. A representative experiment is shown.
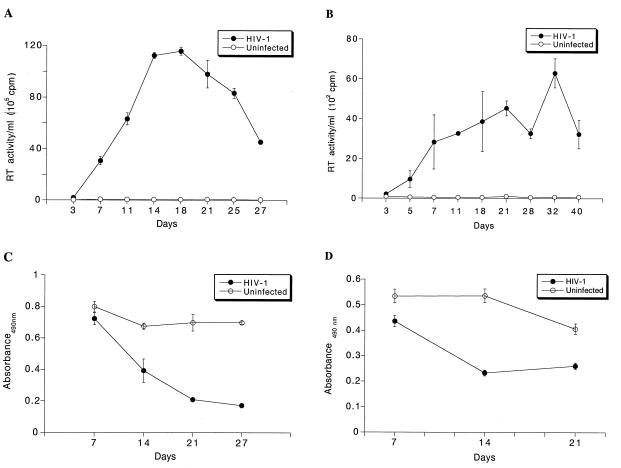
We next examined the replication profiles of HIV-1ADA in monocytes and microglia over a period of 3 to 4 weeks (Fig. 3A and B, respectively). As virus-induced cytopathicity could affect the levels of virus produced, we also examined the viability of cells at specific times during the course of infection (Fig. 3C and D). Both monocytes and microglia supported productive replication of HIV-1ADA weeks after virus exposure. At days 14 and 21 postinfection, there was significant cell death observed for both cell types, and the RT production subsequently decreased. This experiment was repeated with three separate donors of microglia and was performed with three or more replicates. Thus, the synthesis of HIV-1 proviral DNA in monocytes and microglia infected with HIV-1ADA occurs at equivalent rates and both cell types support productive viral replication.
FIG. 3.
Replication profiles of HIV-1ADA-infected monocytes and microglia. Adherent layers of monocytes (105 cells/well) and primary human fetal microglia (5 × 104 cells/well) were cultured with MCSF for 7 days and then infected with HIV-1ADA. Virus was removed after a 4-h incubation. Culture fluids were collected every 2 days for RT analysis for both monocytes (A) and microglia (B). All experiments were performed with three separate donor cells and analyzed in triplicate determinations. Cell viability was measured in triplicate by determining MTT activity at weekly intervals for the infected monocytes (C) and microglia (D). Error bars represent standard deviations.
Morphological, ultrastructural, and immunocytochemical studies of HIV-1ADA-infected monocytes and microglia.
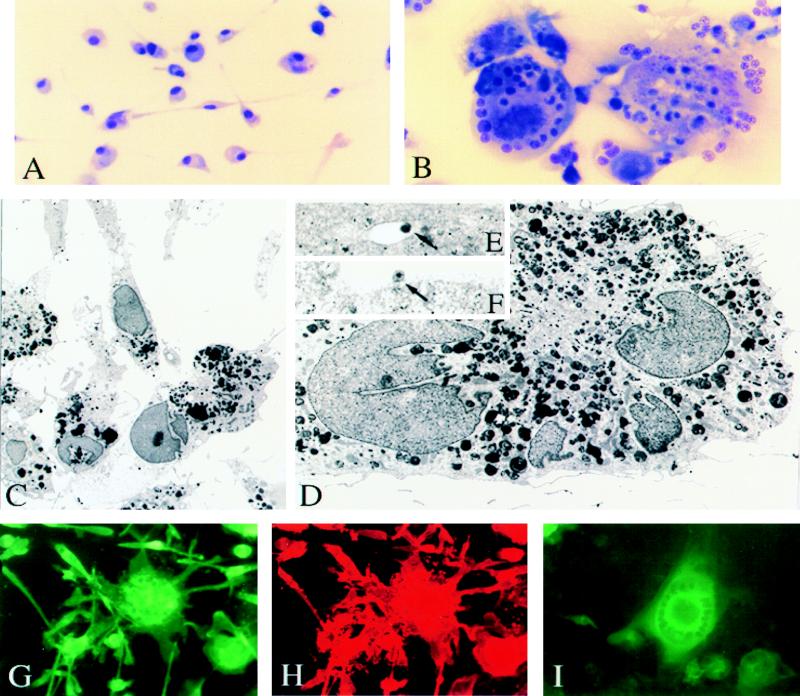
Morphological and immunocytochemical characterizations of HIV-1ADA-infected monocytes and microglia were performed. HIV-1 infection of microglia caused the fusion of individual cells and the formation of multinucleated syncytia, similar to infection of monocytes (Fig. 4) (5). The cytopathic effects were virtually indistinguishable in monocytes and microglia despite the fact that uninfected microglia bear longer processes and are bipolar compared to ameboid monocytes with relatively short processes.
FIG. 4.
Morphological, ultrastructural, and immunocytochemical characterization of HIV-1ADA-infected microglia. Adherent monolayers of microglia were infected with HIV-1ADA or were left as uninfected controls. At 14 days after viral inoculation, cells were fixed with a mixture of acetone and methanol (1:1) for 15 min and then stained by a modified Wright’s stain method. Uninfected (A) and HIV-1-infected (B) microglia are shown. Original magnification (A and B), ×200. Ultrastructural features of virus-infected and uninfected human microglia are shown in panels C to F. Cytoplasm of uninfected microglia cells (C) contains cisternae of the endoplasmic reticulum, prominent Golgi apparatus, mitochondria, vacuoles, and phagosomes. Long cytoplasmic processes contact one another forming gap junctions. HIV-1-infected multinucleated microglia cells (D) show the same ultrastructural characteristics as uninfected ones, including multiple phagosomes with dense products of myelin degradation. In addition, typical mature (F; arrow) and immature (E; arrow) lentiviral particles are detected within cytoplasmic vacuoles of HIV-1-infected microglia cells. Panels E and F present higher magnifications of the cell shown on panel D. Original magnifications, ×2,000 (C), ×45,000 (D), ×46,000 (E), and ×40,000 (F). The results of immunocytochemical studies of HIV-1ADA-infected microglia are shown in panels G to I. HIV-1ADA-infected microglia were stained for monocyte lineage markers CD68 (G) and HAM-56 (H). MGC immunoreactive for both CD68 and HAM-56 are illustrated. HIV-1 p24 immunoreactivity is shown for the MGC and also for single cells on day 7 following viral infection (I). Original magnification (G to I), ×200.
By transmission electron microscopy (TEM), microglia cells showed typical ultrastructural features of fully differentiated macrophages, with well-developed cisternae of the endoplasmic reticulum, prominent Golgi apparatus, numerous elongated mitochondria, lysosomes, lipid droplets, vacuoles, and intermediate cytoplasmic filaments (Fig. 4C). Most of the microglial phagosomes contained dense products of myelin degradation. The cells had long cytoplasmic processes and tightly contacted one another, forming gap junctions. The cytoplasmic membranes of the microglia were uneven with conspicuous filopodia. Typical cytopathic effects (in MGC) following viral infection were seen by TEM (Fig. 4D). Such cells with several nuclei showed the same ultrastructural characteristics as uninfected cells, including numerous phagosomes with myelin remnants. In addition, typical mature and immature lentiviral particles in association with cytoplasmic vacuoles or cell membranes (Fig. 4E and F) were detected.
Immunocytochemical evaluation of monolayer cultures of microglia infected with HIV-1ADA showed that >50% of cells were consistently HIV-1 p24 positive 7 days after infection (Fig. 4I). MGC double immunoreactive for CD68 and HAM-56 were seen (Fig. 4G and H). Cells obtained from multiple donors (eight in all) of varying gestational ages (15 to 20 weeks) were cultured and infected on a routine basis and yielded similar results in terms of purity, morphology, and susceptibility to infection with HIV-1ADA.
Analysis of viral DNA synthesis in monocytes and microglia infected with a panel of HIV-1 isolates.
An analysis of HIV-1ADA-infected monocytes and microglia showed that there were no easily detectable differences in the two cell types with regard to the cell’s ability to support productive viral replication. To expand upon these observations, we investigated the synthesis of viral DNA in monocytes (Fig. 5) and microglia (Fig. 6) infected with a panel of macrophage-tropic and lymphotropic viruses that included HIV-1BAL, HIV-1DJV, HIV-1SF162, HIV-1JR-FL, HIV-189.6, and HIV-1LAI.
FIG. 5.
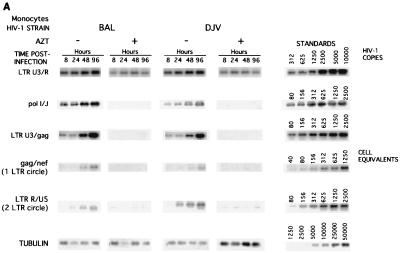
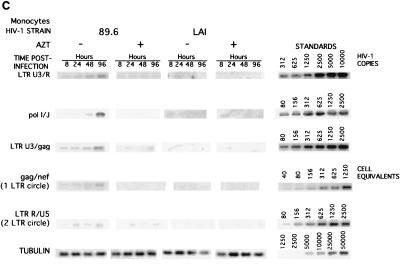
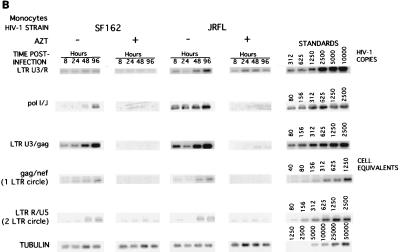
Comparative analysis of the synthesis of viral nucleic acids in monocytes infected with a panel of isolates. Monocytes were infected as described in Materials and Methods with the panel of HIV-1 isolates: HIV-1BAL (BAL), HIV-1DJV (DJV), HIV-1JR-FL (JRFL), HIV-1SF162 (SF162), HIV-189.6 (89.6), and HIV-1LAI (LAI). At 8, 24, 48, and 96 h postinfection, supernatant samples were collected for analysis of RT and the cells were collected for isolation of DNA in PBS. The respective primers were used for amplifying early (LTR U3/R), intermediate (pol I and J), and late (LTR U3/gag) products of reverse transcription. The episomal forms of the viral DNA were amplified with primers to the gag and nef regions. The PCR-amplified products were quantified on a PhosphorImager after hybridization. A representative experiment is shown. HIV-1BAL- and HIV-1DJV- (A), HIV-1SF162- and HIV-1JR-FL- (B), and HIV-189.6- and HIV-1LAI-infected monocytes (C) were analyzed.
FIG. 6.
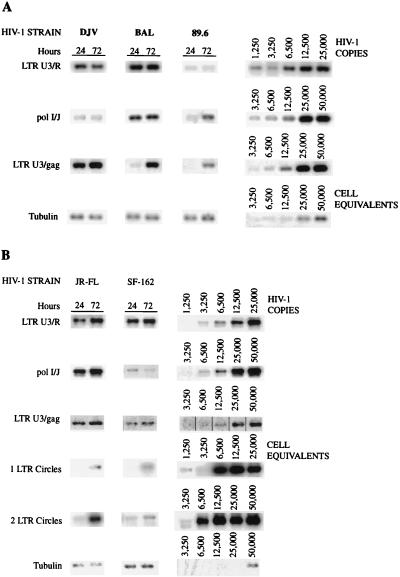
Comparative analysis of the synthesis of viral DNA in microglia infected with a panel of HIV-1 isolates. Adherent layers of microglia were infected with the panel of HIV-1 isolates, and at 24 and 72 h postinfection, supernatant fluids were collected for analysis of RT activity and the cells were collected for extraction of cellular DNA for PCR analysis. Early, intermediate, and late products of RT were amplified as described in Materials and Methods. Episomal forms of HIV-1 DNAs were detected with primers to gag and nef and LTR R/U5 regions. The amplified products were analyzed by electrophoresis on agarose gels and then hybridized to radiolabeled oligonucleotides to detect specific regions of HIV-1 proviral DNA. The accumulated viral DNA products were then quantified on a PhosphorImager. HIV-1DJV (DJV)-, HIV-1BAL (BAL)-, and HIV-189.6 (89.6)-infected microglia (A) and HIV-1JR-FL (JR-FL)- and HIV-1SF162 (SF-162)-infected microglia and infected microglia (B) are shown.
The synthesis of early, intermediate, and late products of reverse transcription and the presence of episomal forms of the viral nucleic acids were determined to assess successful nuclear import of the preintegration complex of HIV-1 proviral DNA. As shown with HIV-1ADA, in cells treated with AZT, intermediate and late products were not detected. This was consistent for all isolates. Thus, the products identified in the monocyte cultures inoculated with HIV-1 represent de novo synthesis. In monocytes, both intermediate and late products were detected as early as 8 h postinfection for HIV-1BAL, HIV-1DJV, HIV-1SF162, HIV-1JR-FL, and HIV-189.6 (Fig. 5A, B, and C). HIV-1 late products were not observed in HIV-1LAI-infected monocytes (Fig. 5C). The episomal forms of the viral nucleic acids containing one LTR were detected in monocytes at 24 h postinfection for all macrophage-tropic strains (Fig. 5). HIV-1YU-2 showed results similar to those illustrated for HIV-1BAL and HIV-1DJV (data not shown). Analysis of viral mRNA was performed in infected monocytes by techniques described previously for in situ hybridization (16, 39), and similar trends were observed (data not shown).
Next, the accumulations of viral nucleic acids in microglia infected with HIV-1BAL, HIV-1DJV, HIV-189.6 (Fig. 6A), HIV-1JR-FL, and HIV-1SF162 (Fig. 6B) were determined. There was considerable accumulation of the late products of HIV-1 proviral DNA (identified by using LTR U3/gag regions) in microglia for all isolates. The trend was similar to that observed in virus-infected monocytes. The accumulation of episomal forms of the viral nucleic acids containing one LTR was detected at 72 h in microglia for both HIV-1JR-FL and HIV-1SF162. As the episomal forms of viral DNA indicate completion of viral nucleic acid synthesis and successful nuclear import, the data show that with comparable levels of virus inocula both microglia and macrophages support a complete cycle of viral DNA synthesis. The extrachromosomal forms of viral cDNA containing two LTRs, which are low-abundance circle forms of the viral DNA (two-LTR circle), were also detected in microglia for both HIV-1JR-FL and HIV-1SF162 as early as 24 h postinfection (Fig. 6). In monocytes the two-LTR circle forms were detected at 48 h postinfection (Fig. 5). For quantitative comparisons the ratios of the late products of reverse transcription to that of a mitochondrial gene were also calculated, and these ratios also reflected significant differences between viral strains (a range of 0.01 [for HIV-1SF162] to 0.04 [for HIV-1ADA]). In addition, the kinetics of HIV-1 RT products was evident for all isolates used, as reflected by an increase in the early, intermediate, and late products with prolonged postinfection time points. These were observed for all viral isolates (data not shown). Moreover, the differences in the levels of productive infection between the isolates were also evident. HIV-1DJV, HIV-1BAL, HIV-1JR-FL, and HIV-1SF162 rapidly complete their viral life cycles in both monocytes and microglia. The rates of synthesis of proviral DNA in microglia infected with HIV-1BAL, HIV-1DJV, HIV-1JR-FL, HIV-1SF162, and HIV-189.6 are comparable to those observed in monocytes.
Analysis of progeny virion production in monocytes and microglia infected with a panel of HIV-1 isolates.
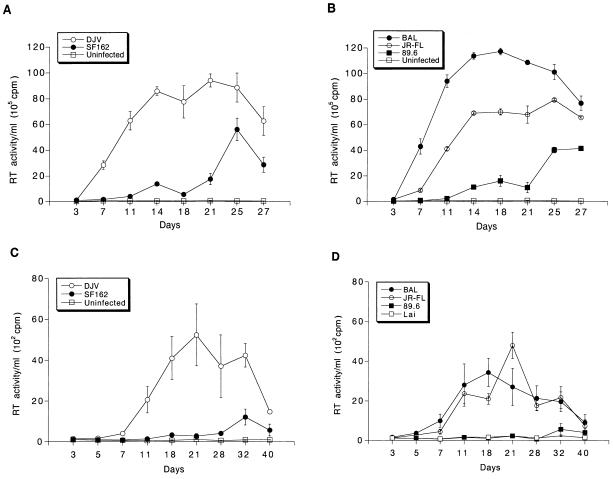
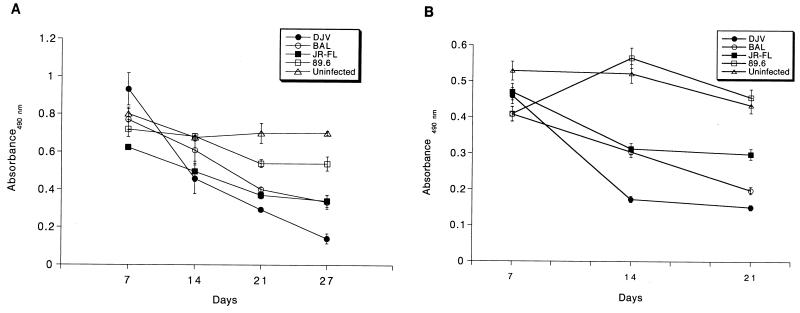
The production of progeny virions was measured by determining RT activity in culture fluids following HIV-1 infection in monocytes and microglia. Figure 7 shows the kinetics of productive replication of the panel of HIV-1 isolates. All macrophage-tropic strains productively infected both microglia and monocytes, albeit at different levels. HIV-1LAI did not give rise to any productive infection. Lee and colleagues (32) showed that HIV-1IIIB, when used at a high multiplicity of infection, led to infection of microglia. However, we did not find this for HIV-1LAI, consistent with previous reports concerning T-tropic isolates (54). More importantly, the pattern of the level of viral replication was identical for monocytes and microglia. HIV-1ADA, HIV-1DJV, and HIV-1BAL elicited very high levels of productive infection in monocytes (Fig. 7A and B) and microglia (Fig. 7C and D). HIV-1SF162 and HIV-189.6 gave incrementally reduced RT activity levels. HIV-1YU-2, a molecular clone directly obtained from infected brain tissue, also elicited very high levels of progeny virus production in both microglia and monocytes (data not shown). HIV-1YU-2 was rescued in a high-efficiency packaging cell system (34, 40) and was used directly for infection. The levels of virus produced were higher for monocytes than for microglia, including HIV-1YU-2. HIV-1SF162 and HIV-189.6 both reached peak RT activity later in microglia (day 40 and day 32, respectively). A similar result was seen in monocytes, as peak RT values were obtained on days 25 and 27, respectively. However, in general, peak RT values were delayed in microglia compared to monocytes. One-way analysis of group variance for both microglia and monocytes showed that the differences within the strain groups were significant (P < 0.0001). There was clear consistency for viral strains in both microglia and monocytes. The viabilities of the infected cells at specific time intervals after viral exposure were also similar for monocytes and microglia (Fig. 8). HIV-1DJV was highly cytopathic and led to significant cell death after 14 days of infection in both monocytes (Fig. 8A) and microglia (Fig. 8B). HIV-189.6 was the least cytopathic in both cell types. Interestingly, there was also consistency in the cytopathic effects observed in monocytes and microglia. These data demonstrate a heterogeneity among macrophage-tropic HIV-1 strains for infection of monocytes and microglia. HIV-1ADA, HIV-1DJV, and HIV-1BAL were highly productive in both cell types while HIV-189.6 was significantly less so. HIV-1LAI did not lead to productive viral replication in either monocytes or microglia. For each of the strains tested both cell types were equally susceptible to viral replication.
FIG. 7.
Replication profiles of the panel of HIV-1 isolates in blood monocytes and microglia. Adherent monolayers of macrophages (105 cells/well) or microglia (5 × 104 cells/well in a 96-well plate) were infected with comparable viral inocula. The infection proceeded for 4 h, after which the virus was removed. Culture supernatant samples were withdrawn at specific time intervals over a period of 3 to 4 weeks. RT activity was assayed for both virus-inoculated and control uninfected cells (A and C). Monocytes (A and B) and microglia cells (C and D) were infected with a panel of HIV-1 strains including HIVADA (ADA), HIV-1DJV (DJV), and HIV-1SF162 (SF162) (A and C), HIV-1BAL (BAL), HIV-1JR-FL (JR-FL), and HIV-189.6 (89.6) (B and D), and HIV-1LAI (LAI) (D). Each isolate was examined in triplicate. Representative results obtained with a single donor each are shown for monocytes and microglia. Error bars represent standard deviations.
FIG. 8.
Viability profiles of HIV-1-infected monocytes and microglia. Monocytes (A) and microglia (B) infected with the panel of HIV-1 isolates were maintained for 4 weeks. At days 7, 14, and 21 postinfection, the supernatant was withdrawn and the cells were incubated with 2 mg of MTT supplemented with 10% FBS for 45 min at 37°C. At the end of the incubation period, the MTT reagent was washed off and the formazan crystals were dissolved in 100 μl of dimethyl sulfoxide. The absorbance was recorded at 490 nm. Each assay was performed in triplicate. Error bars represent standard deviations.
DISCUSSION
In this study we investigated whether the susceptibility to HIV-1 infection differed between primary human microglia and monocytes. Such differences, if found, could reflect a neurotropic potential for HIV-1. We first established highly purified and well-characterized microglia systems and studied HIV-1 replication at three different stages of the viral life cycle. These included the synthesis of viral DNA and viral mRNA (data not shown) and progeny virion production as indicated by RT activity in culture supernatants. From all the data taken together, we found that there was no significant difference in the susceptibilities of the two cell types to HIV-1 infection. Thus the ability of a given isolate to infect microglia or monocytes may not be generally utilized as a signature to distinguish between neurovirulent and macrophage-tropic viral strains. Prior studies suggested that qualitative, not quantitative, properties of HIV-1 mainly determine clinical differences between demented and nondemented patients (24). A report by Strizki et al. (50) suggested that there is preferential HIV-1 infection of microglia for select viral strains. However, if such a finding were to be made for a small percentage of viral variants, it is unlikely to be a generalized observation to explain neurovirulent HIV-1 phenotypes. The ability of HIV-1 to produce disease within the CNS likely reflects multiple properties of virus-host interactions that include but are not limited to host genetic properties, the degree of immunosuppression, immunologic factors (inside and outside the brain), the integrity of the blood-brain barrier, the presence of opportunistic infections, and the virulence of the viral strain(s) that affects the CNS, including the host cell range of the virus. The Strizki findings may have added relevance to HIV neuropathogenesis in the developed CNS, because the microglia obtained in their work were from mature brain specimens.
Given their functional and morphological plasticity (25), microglia may play a unique role in HIV neuropathogenesis. The question as to whether microglia differ functionally from monocytes is unresolved. This question arises from the common origin of the two cell types. Despite their residence in the brain, microglia cells originate outside the CNS and migrate into the CNS in late embryonic life. It has also been previously demonstrated that microglia tropism maps at a region of HIV-1 env that similarly controls macrophage tropism (48). Initial reports showed that fetal microglia obtained from first-trimester tissue specimens were refractory to infection with HIV-1 (42); however, it was later shown that cultured microglia from second-trimester tissue and also adult microglia can be infected with macrophage-tropic but not lymphocyte cell-tropic isolates (54). Subsequently, two groups have shown that at high infectivity doses, microglia cells can be susceptible to infection with lymphocyte cell-tropic isolates but are subject to donor variation (32, 50). We did not find any infection of microglia with lymphocyte-tropic isolate HIV-1LAI at a viral inoculate level equivalent to those for other isolates. Importantly, donor-to-donor variations were also not demonstrated. The data support the notion that macrophage and microglial tropisms for HIV-1 are similar.
HIV-1 is selectively localized within perivascular and infiltrated parenchymal blood-derived macrophages and microglia (9, 30, 51), suggesting a hematogenous entry of the virus to the brain. Our data also suggest that it is the macrophage, rather than the viral isolate, that regulates penetration by HIV-1 into the CNS. The sequestration of virus in mononuclear phagocytes is a reservoir for HIV-1. The evolution of quasispecies of virus could occur separately within the CNS. The fact that there exists genetic variation in different HIV-1 isolates obtained from different patients and from the same individual is consistent with this hypothesis (19). However, when HIV-1 isolates originally obtained from brain tissue or blood were compared, no significant difference in the comparative infectivity in microglia and monocytes was found.
An important issue for this work is that we utilized primary fetal microglia as target cells for HIV-1 infection. It is possible that fetal cells behave differently from cultured adult microglia. Indeed, the developing CNS is more susceptible to HIV-1 infection than the adult CNS (21). Future studies in which adult and fetal microglia are compared (side by side) for viral infection may prove helpful in elucidating the unique abilities that fetal brain tissue has for HIV-1 infection and for eliciting neurological disease.
The presence of molecular variants of the virus is a reflection of the fact that retroviral RT does not have any proofreading function. For HIV-1 there is a potential change of nucleotides at a rate of one for every 10,000 bp. That would bring about one base substitution per genome in every replication cycle (38). Studies comparing sequences of HIV-1 isolates from the brain and blood show that the envelope region of HIV-1 sequesters such mutations, indicating selection pressures. There are some conserved elements identified in the V3 loop of isolates derived from brains of demented patients compared to the consensus sequence shown by Korber et al., LaRosa et al., Power et al., and Shimizu et al. (28, 31, 43, 49). This suggests a potential overlapping pathway for selection of neurovirulent isolates in different individuals. The importance of the V3 loop sequences in determining tropism is controversial (46), as recently it was demonstrated that the residues originally thought to be critical for neurovirulence were also found in the majority of nondemented patient samples. Also, all of the brain-derived isolates lacked the proline residue at position 305, described initially by di Stefano et al. and Power et al. (12, 43).
Thus, the issue of HIV-1 neurovirulence has been controversial. Needless to say, there is evidence to suggest that it is the qualitative property of the virus that determines disease progression in patients. However, there have not been any consistent data regarding a particular molecular property of the virus to assign a signature for neurovirulence. We suggest that it is the onset of the immune functions elicited by infection of microglia by certain isolates, as distinct from those elicited by infection of monocytes, that may be the basis for clinical differences of disease progression. It is generally accepted that the myriad of pathological manifestations of HIV-1 infection result from the interplay of molecules mediating intercellular interactions between infected microglia and macrophages, astrocytes, oligodendrocytes, and neurons, more specifically through the elaboration of various cytokines and potential neurotoxins (1, 15, 45). Clearly, the network of microglia in the brain and their close proximity to the other neural elements make them central candidates for the differential immune-mediated manifestations of HIV-1-induced neurological disease.
ACKNOWLEDGMENTS
We thank Chun Chao and Shuxian Hu of the Hennepin County Medical Center in Minneapolis, Minn., for advice and guidance in establishing the microglia isolation and culture at the University of Nebraska Medical Center and Karen Spiegel for excellent editorial and graphic support.
This work was supported in part by NIH grants P01 NS31492-05, R01 NS34239-04, 1 R01 NS36126-01, 1 P01 MH57556-01, and 1-R29-NS34572-03, the Charles A. Dana Foundation, and the University of Nebraska Biotechnology start-up funds. Adeline Nukuna is a Nicholas B. Badami Fellow.
REFERENCES
- 1.Benveniste E N. Cytokine circuits in the brain: implications for AIDS dementia complex. In: Price R W, Perry S W, editors. HIV, AIDS and the brain. New York, N.Y: Raven Press; 1994. pp. 71–88. [PubMed] [Google Scholar]
- 2.Budka H. Multinucleated giant cells in the brain: a hallmark of the acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1986;69:253–256. doi: 10.1007/BF00688301. [DOI] [PubMed] [Google Scholar]
- 3.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukrinsky M I, Sharova N, Dempsey M I, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng-Mayer C, Levy J A. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Ann Neurol. 1988;23:S58–S61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 6.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7515–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.del Rio Hortega P. El tercer elemento de los centros nerviosos. I. La microglia en estado normal. II. Intervencion de la microglia en los procesos patologicos. III. Naturaleza probable de la microglia. Biol Soc Esp Biol. 1919;9:69–120. [Google Scholar]
- 8.del Rio Hortega P. Microglia. In: Penfield W, editor. Cytology and cellular pathology of the nervous system. Vol. 2. New York, N.Y: Hoeber; 1932. pp. 482–534. [Google Scholar]
- 9.Dickson, D. W., S. C. Lee, W. Hatch, L. A. Mattiace, C. F. Brosnan, and W. D. Lyman. Macrophages and microglia in HIV-related CNS neuropathology, p. 99–131. In R. W. Price and S. W. Perry (ed.), HIV, AIDS and the brain. Raven Press, New York, N.Y. [PubMed]
- 10.Dickson D W. Multinucleated giant cells in acquired immune deficiency syndrome: origin from endogenous microglia? Acta Pathol Lab Med. 1986;110:967–968. [PubMed] [Google Scholar]
- 11.Dickson D W, Mattiace L A, Katsuhiro K, Hutchins K, Lyman W D, Brosnan C F. Biology of disease. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991;64:135–156. [PubMed] [Google Scholar]
- 12.di Stefano M D, Wilt S, Gray F, Dubois-Dalcq M, Chiodi R. HIV type 1 V3 sequences and the development of dementia during AIDS. AIDS Res Hum Retroviruses. 1996;12:471–482. doi: 10.1089/aid.1996.12.471. [DOI] [PubMed] [Google Scholar]
- 13.Folks T M, Powell D, Lightfoot M, Koenig S, Fauci A S, Benn S, Rabson A, Daugherty D, Gendelman H E, Hoggan M D, Venkatesan S, Martin M A. Biological and biochemical characterization of a clone Leu-3 cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 15.Gendelman H E, Ghorpade A, Persidsky Y. The neuropathogenesis of HIV-1 dementia. In: Peterson P, Remington J, editors. In the defense of the brain. Cambridge, Mass: Blackwell Scientific Publications; 1997. pp. 290–304. [Google Scholar]
- 16.Gendelman H E, Koenig S, Aksamit A, Venkatesan S. In situ hybridization for detection of viral nucleic acid in cell cultures and tissues. In: Uhl G E, editor. In situ hybridization in brain. New York, N.Y: Plenum Press; 1986. pp. 203–221. [Google Scholar]
- 17.Gendelman H E, Orenstein J M, Martin M A, Ferruca C, Mitra R, Phillips T, Wahl L A, Lane H C, Fauci A J, Burke D S, Skillman D R, Meltzer M S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1498–1506. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glass J D, Fedor H, Wesselingh S L, McArthur J C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 19.Goodenow M, Huet T, Saurin W, Kwok S, Sninsky J, Wain-Hobson S. HIV-1 isolates are rapidly evolving quasispecies: evidence for viral mixtures and preferred nucleotide substitutions. J Acquired Immune Defic Syndr. 1989;2:344–352. [PubMed] [Google Scholar]
- 20.Heinzinger N, Baca-Regan L, Stevenson M, Gendelman H E. Efficient synthesis of viral nucleic acids following monocyte infection by HIV-1. Virology. 1995;206:731–735. doi: 10.1016/s0042-6822(95)80097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen R S, Cornblath D R, Epstein L G, Foa R P, McArthur J C, Price R W, Asbury A K K, Beckett A, Benson D F, Bridge T P, Leventhal C M, Satz P, Saykin A J, Sidtis J J, Tross S. Nomenclature and research case definitions for neurological manifestations of human immunodeficiency virus type 1 (HIV-1) infection. Neurology. 1991;41:778–785. [Google Scholar]
- 22.Johnson R T. Viral infections of the nervous system. New York, N.Y: Raven Press; 1982. pp. 129–159. [Google Scholar]
- 23.Johnson R T. Viral infections of the nervous system. New York, N.Y: Raven Press; 1982. pp. 87–129. [Google Scholar]
- 24.Johnson R T, Glass J D, McArthur J C, Chesebro B W. Quantitation of human immunodeficiency virus in brains of demented and nondemented patients with acquired immune deficiency syndrome. Ann Neurol. 1996;39:392–395. doi: 10.1002/ana.410390319. [DOI] [PubMed] [Google Scholar]
- 25.Jordan F L, Thomas W E. Brain macrophages: questions of origin and interrelationships. Brain Res Rev. 1988;13:165–178. doi: 10.1016/0165-0173(88)90019-7. [DOI] [PubMed] [Google Scholar]
- 26.Kalter D C, Nakamura M, Turpin J A, Baca L M, Dieffenbach C, Ralph P, Gendelman H E, Meltzer M S. Enhanced HIV replication in MCS-treated monocytes. J Immunol. 1991;146:298–307. [PubMed] [Google Scholar]
- 27.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 28.Korber B T M, Kunstman K J, Patterson B K, Furtado M, McEvilly M M, Levy R, Wolinsky S M. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 30.Kure K, Weidenheim K M, Lyman W D, Dickson D W. Morphology and distribution of HIV-1 positive microglia in subacute AIDS encephalitis: pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 1990;80:393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- 31.LaRosa G J, Davide J P, Weinhold D, Waterbury J A, Profy A T, Lewis J A, Langlois A J, Dreesman G R, Boswell R N, Shadduck P, Holley L H, Karplus M, Bolognesi D P, Matthews T J, Emini E A, Putney S D. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990;249:932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- 32.Lee S C, Hatch W C, Liu W, Kress Y, Lyman W D, Dickson D W. Productive infection of human fetal microglia by HIV-1. Am J Pathol. 1993;143:1032–1039. [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S C, Liu W, Brosnan C F, Dickson D W. Characterization of primary human fetal dissociated central nervous system cultures with an emphasis on microglia. Lab Invest. 1992;67:465–476. [PubMed] [Google Scholar]
- 34.Li Y, Kappes J C, Conway J A, Price R W, Shaw G M, Hahn B H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manskowski J L, Flaherty M T, Spelman J P, Hauer D A, Didier P J, Amedee A M, Murphey-Corb M, Kirstein L M, Muñoz A, Clements J E, Zink M C. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchant A, Duchow J, Delville J-P, Goldman M. Lipopolysaccharide induces up-regulation of CD14 molecule on monocytes in human whole blood. Eur J Immunol. 1992;22:1663–1665. doi: 10.1002/eji.1830220650. [DOI] [PubMed] [Google Scholar]
- 37.Nottet H S L M, Persidsky Y, Sasseville V G, Nukuna A N, Bock P, Zhai Q H, Sharer L R, McComb R D, Swindells S, Soderland C, Gendelman H E. Mechanism for the transendothelial migration of HIV-1 infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- 38.O’Brien W A. Genetic and biologic basis of HIV-1 neurotropism. In: Price R W, Perry S W, editors. HIV, AIDS and the brain. New York, N.Y: Raven Press; 1994. pp. 47–70. [PubMed] [Google Scholar]
- 39.Pardue M L. In situ hybridization. In: Hames B D, Higgins S J, editors. Nucleic acid hybridization, a practical approach. Oxford, United Kingdom: IRL Press; 1985. pp. 179–202. [Google Scholar]
- 40.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 42.Peudenier S, Hery C, Montagnier L, Tardieu M. Human microglial cells: characterization in cerebral tissue and in primary culture, and study of their susceptibility to HIV-1 infection. Ann Neurol. 1991;29:152–161. doi: 10.1002/ana.410290207. [DOI] [PubMed] [Google Scholar]
- 43.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price R W, Brew B, Sindtis J, Rosenblum M, Scheck A, Cleary P. The brain and AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- 45.Price R W. Understanding the AIDS dementia complex (ADC). The challenge of HIV and its effects on the central nervous system. In: Price R W, Perry S W, editors. HIV, AIDS and the brain. New York, N.Y: Raven Press; 1994. pp. 1–45. [PubMed] [Google Scholar]
- 46.Reddy R T, Achim C L, Sirko D A, Tehranchi S, Kraus F G, Staal F W, Wiley C A. Sequence analysis of the V3 loop in brain and spleen of patients with HIV encephalitis. AIDS Res Hum Retroviruses. 1996;12:477–482. doi: 10.1089/aid.1996.12.477. [DOI] [PubMed] [Google Scholar]
- 47.Sharer L R, Cho E, Epstein L G. Multinucleated giant cells and HTLV-III in AIDS encephalopathy. Hum Pathol. 1985;16:760. doi: 10.1016/s0046-8177(85)80245-8. [DOI] [PubMed] [Google Scholar]
- 48.Sharpless N E, O’Brien W A, Verdin E, Kufta C V, Chen I S Y, Dubois-Dalcq M. Human immunodeficiency virus type 1 tropism for brain microglial cells is determined by a region of the env glycoprotein that also controls macrophage tropism. J Virol. 1992;66:2588–2593. doi: 10.1128/jvi.66.4.2588-2593.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu N S, Shimizu N G, Takeuchi Y, Hoshino H. Isolation and characterization of human immunodeficiency virus type 1 variants infectious to brain-derived cells: detection of common point mutations in the V3 region of the env gene of the variants. J Virol. 1994;68:6130–6135. doi: 10.1128/jvi.68.9.6130-6135.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strizki J M, Albright A V, Sheng H, O’Connor M, Perrin L, González-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K, Wesselingh S L, Griffith D E, McArthur J C, Johnson R T, Glass J D. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 52.Ulvestad E, Williams K, Mork S, Antel J, Nyland H. Phenotypic differences between human monocytes/macrophages and microglial cells studied in situ and in vitro. J Neuropathol Exp Neurol. 1994;53:492–501. doi: 10.1097/00005072-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Ulvestad E, Williams K, Bjerkvig R, Tiekotter K, Antel J, Matre R. Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukocyte Biol. 1994;56:732–740. doi: 10.1002/jlb.56.6.732. [DOI] [PubMed] [Google Scholar]
- 54.Watkins B A, Dorn H H, Kelly W B, Armstrong R C, Potts B J, Michaels F, Kufta C V, Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249:549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- 55.Wiley C A, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immune deficiency syndrome. Ann Neurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- 56.Wiley C A, Schrier R D, Nelson J A, Lampert P W, Oldstone M B A. Cellular localization of human immunodeficiency virus infection within brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]