Atherosclerotic cardiovascular disease (ASCVD) is characterized by a build-up of plaque inside the arteries due to a chronic slowly evolving vascular wall inflammation and lipid accumulation that can lead to several major adverse cardiovascular events including myocardial infarction, stroke, or even death. Because the relative risk for major adverse cardiovascular events is decreased when low-density lipoprotein cholesterol (LDL-C) levels in blood are reduced,1 the European Society of Cardiology guidelines, along with other international directives, advocate the use of lipid-lowering statins therapy as a primary preventive measure for ASCVD in individuals exhibiting pathologically elevated levels of LDL-C in blood.2 However, the low adherence to statin therapies, marked by rates falling below 50% within the first year of initiation and further declining to 30% by the second year, is linked to an increased risk of mortality.2 The statin discontinuation, attributable to patient choice, lack of sufficient beneficial effect or tolerance, highlights the need for alternative therapies.
PCSK9, a protease secreted by the liver, binds to the hepatocyte-derived low-density lipoprotein receptor (LDLR), preventing its recycling and enhancing its degradation. The resulting low levels of LDLR drive the increase in circulating LDL-C in ASCVD patients. The discovery of a PCSK9 inactivating mutation driving shallow cholesterol levels sparked the race to develop PCSK9 inhibitors as a novel class of medications for reducing cholesterol levels. Targeting PSCK9 by monoclonal antibodies and RNA interference (RNAi)-based therapeutic inhibitors have been the first emerging approaches. Monoclonal antibodies Evolocumab and Alirocumab are administered subcutaneously twice monthly, lower LDL-cholesterol by 60%, showed long-term safety, and successfully reduce cardiovascular endpoints.3 Inclisiran is a long-acting RNAi therapeutic agent subcutaneously injected twice yearly that neutralizes the mRNA of PCSK9 and inhibits the protein synthesis of PCSK9. In the recently reported ORION-3 extension study, Inclisiran doses were well-tolerated, and the 4-year averaged mean reduction of LDL-C was 44.2%, with reductions in PCSK9 ranging from 62.2 to 77.8%.4 A third approach consists of permanently editing PCSK9 gene using CRISPR/Cas9, a highly potent and versatile engineering tool for rapid and precise editing of DNA sequences, to produce a markedly lower exposure to LDL-C.5 Contingent upon sustained efficacy and safety, this methodology could address the challenge of sustaining long-term adherence to lifestyle modifications and pharmacological regimens. Initial in vivo murine studies using an adenoviral vector encapsulating CRISPR/Cas9 and a guide RNA targeting PCSK9 gene showed a disruption of 90% of PCSK9 expression, leading to a 40% reduction in LDL-C.5 To improve accuracy, efficiency, and safety, base editing to introduce nonsense mutations through a single adenine–thymine (A–T) to guanine–cytosine (G–C) nucleotide base pair change into PCSK9 gene, and new delivery vectors such as lipid nanoparticles (LNPs) have been developed.5 These cumulative efforts led to the engineering of VERVE-101, a CRISPR base-editing therapy composed of a mRNA encoding for an adenine base editor protein and a guide RNA targeting the PCSK9 gene, co-encapsulated within LNPs absorbed primarily by hepatocytes through a receptor-mediated process. In non-human primates, a single intravenous infusion of VERVE-101 at a dose of 1.5 mg/kg durably lowered for 2.5 years PCSK9 by 69% and LDL-C by 50%, respectively. Despite the observed dose-dependent transient elevation in alanine transaminase (ALT), consistent with other therapeutics delivered through nonclinical studies, VERVE-101 was well-tolerated, opening the investigational door to evaluate its safety and efficacy in humans (Figure 1).6
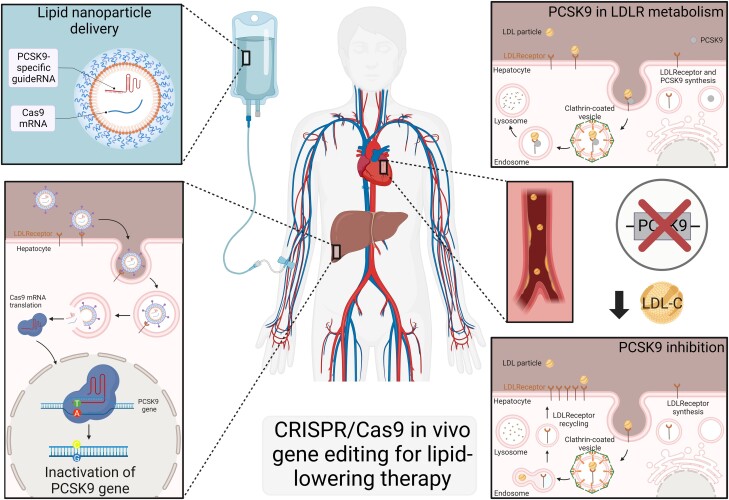
Figure 1.
CRISPR/Cas9 in vivo gene editing for lipid-lowering therapy. Lipid nanoparticles encapsulated with a cargo comprising Cas9 mRNA encoding an adenine base editor and a PCK9-specific guide are delivered via a single intravenous administration. After infusion, apolipoprotein E opsonizes the nanoparticles, facilitating their uptake by hepatocytes via the low-density lipoprotein receptor through endocytosis and endosome formation. Following the breakdown of the lipid nanoparticle and disruption of the endosomal membrane, the active components are released into the cytoplasm. Cas9 mRNA is then translated in the cytoplasm and ultimately complexes with gRNAs to form a ribonucleoprotein. This complex enters the nucleus to induce gene editing, introducing a nonsense mutation by converting a single adenine–thymine (A–T) to guanine–cytosine (G–C) nucleotide base pair in the PCSK9 gene, thereby reducing PCSK9 gene levels. PCSK9 normally binds to the low-density lipoprotein receptor (LDLR), leading to the degradation of the receptor in the lysosomal compartment. Inhibition of the PCSK9 gene prevents further degradation of endocytosed LDLR, allowing it to be recycled back to the plasma membrane. This process results in increased clearance of LDL-C and a reduction in circulating LDL-C levels. Figure created using Biorender website.
In this context, the gene editing company Verve Therapeutics presented at the American Heart Association Scientific Sessions 2023 on the 12 November 2023 the eagerly awaited interim data from Heart-1, an open-label, phase 1b clinical trial designed to evaluate the safety and tolerability of VERVE-101 in patients living with heterozygous familial hypercholesterolaemia, established ASCVD and uncontrolled hypercholesterolaemia, on maximally tolerated oral lipid-lowering therapy (eight on statin therapy; two with prior use of PCSK9-targeted therapy) (https://www.vervetx.com/sites/default/files/2023-11/Verve_AHA_2023_LBS_for%20website.pdf).
This trial, the first-ever human in vivo data for base editing, included 10 participants (male: 80%, mean age: 54 years, mean LDL-C: 193 mg/dL) treated across a single intravenous infusion ascending dose study (0.1 mg/kg, n = 3; 0.3 mg/kg, n = 3; 0.45 mg/kg, n = 3, and 0.6 mg/kg, n = 1) in United-Kingdom and New Zealand centres. Study participants presented a high burden of disease at baseline, including a history of myocardial infarction and recurrent in-stent restenosis. While the two lower doses had minimal effect, in three patients receiving the higher dose, reductions in blood PCSK9 protein levels of 47, 59, and 84% were observed, while reductions in blood LDL-C of 39, 48, and 55% were reported after 180 days. This reduction in blood level LDL-C is similar to that observed after monoclonal antibodies and RNAi-based therapeutic PCSK9 inhibitors.3,4 Interestingly, the 55% LDL-C reduction, achieved with only 47% reduction in PCSK9 levels in the 0.6 mg/kg dose single participant cohort, extended up to 180 days. Nonetheless, this reduction LDL-C of 55% from a mean baseline of 193 mg/dL does not allow to reach an LDL-C target of <70 mg/dL for patients at high cardiovascular risk and suggests that a combination therapy may still be more efficient. Regarding the safety profile, a transient ALT elevation (4-to-6-fold increase) was observed at the two higher doses in the first 14 days but remained elevated close to the upper limit of normal at 28 days, while the bilirubin levels remained below the upper limit of normal. Nonetheless, three serious adverse cardiovascular events occurring in two participants were reported. One patient dosed in the 0.3 mg/kg cohort had a fatal cardiac arrest 5 weeks after receiving the infusion due to underlying ischaemic heart disease. One patient from the 0.45 mg/kg cohort, in the setting of unstable chest pain symptoms unreported to the investigators before dosing, experienced a myocardial infarction the day after treatment and non-sustained ventricular tachycardia more than four weeks after dosing. Although the investigator and independent data and safety monitoring board considered the fatal cardiac arrest and the tachycardia to be not related to treatment and consistent with a severe advanced ASCVD patient population, the aetiology of the myocardial infarction event was considered potentially related to treatment due to the proximity to dosing, raising a possible inflammation potentiated by the LNPs. In that view, Verve Therapeutics is evaluating a second PCSK9 gene editor, VERVE-102, with LNPs covalently linked with the N-acetylgalactosamine (GalNAc) sugar, which exhibits an affinity for the asialoglycoprotein receptor expressed on hepatocytes. This conjugation facilitates the cellular internalization process and enhance the hepatic selectivity to the therapeutic strategy.6
The gene editing therapy field, still in its infancy, elicits substantial excitement owing to its potential to engender novel therapeutic modalities for many pathological conditions. A recent illustration of a successful delivery of the CRISPR/Cas9-based in vivo gene editing in humans is evidenced in treating Transthyretin amyloidosis, a progressive disease characterized by the abnormal build-up of misfolded transthyretin protein. Using a guide RNA targeting the transthyretin sequence encapsulated in liver-targeted LNPs, an 87% decrease in serum transthyretin protein concentrations in six patients was described 28 days after infusion, while only mild adverse effects were reported.7 Nevertheless, following the announcement of the VERVE-101 interim data, the gene editing company Verve Therapeutics stock initially fell by 40% in November 2023 followed by gradual increase of its market value, showing that safety is the first concern to be addressed. It is essential to determine if patients experience any adverse effects resulting from unintended modifications and to monitor immune reactions in patients. Further investigations into the long-term safety and efficacy are needed and will be facilitated by the recent FDA approval of base editing treatment. In addition, emerging evidence have identified that substantial residual risk may be attributed to other lipoprotein particles such as high-density lipoprotein cholesterol, triglycerides- to- HDLc, or remnant cholesterol, a triglyceride-rich lipoproteins. Remnant cholesterol’s property to infiltrate into the arterial intima and its accumulation in macrophages are associated with the presence of flow-limiting atherosclerotic cardiovascular disease in high-risk patients.8 Since monoclonal antibodies against PCSK9 demonstrate a beneficial effect on lipid profile beyond LDL-C levels reduction,9 it is needed to establish whether PCSK9 gene editing improved this lipid residual risk. Finally, the interplay between LDL-C and target cells needs to be assessed. As such, the interaction between lipoprotein and platelets enhances their reactivity and adhesion, suggesting a crucial role in thrombosis formation.10 It is, therefore, essential to monitor tightly PCSK9 inhibition biology to establish clinically meaningful outcome measures. Answering these questions will be crucial to determine the direction of the future of lipid-lowering therapy and whether we can treat the root cause of the number one cause of death in the western world with a single infusion.
Contributor Information
Simon Tual-Chalot, Biosciences Institute, Vascular Biology and Medicine Theme, Faculty of Medical Sciences, Newcastle University, Centre for Life, Newcastle Upon Tyne NE1 3BZ, UK.
Konstantinos Stellos, Biosciences Institute, Vascular Biology and Medicine Theme, Faculty of Medical Sciences, Newcastle University, Centre for Life, Newcastle Upon Tyne NE1 3BZ, UK; Department of Cardiovascular Research, European Center for Angioscience, Medical Faculty Mannheim, Heidelberg University, Ludolf-Krehl-Straße 13-17, D-68167 Mannheim, Germany; Preventive Cardiology Clinic, Department of Cardiology, University Hospital Mannheim, Medical Faculty Mannheim, Heidelberg University, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site Heidelberg/Mannheim, Mannheim, Germany; Mannheim Institute for Innate Immunoscience (MI3), Medical Faculty Mannheim, Heidelberg University, Ludolf-Krehl-Straße 13-17, D-68167 Mannheim, Germany.
Funding
S. Tual-Chalot is supported by a British Heart Foundation grant (PG/23/11093) and the Royal Society (RG\R1\241197). K. Stellos is supported by grants from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (MODVASC, grant agreement No 759248), the German Research Foundation DFG (CRC1366 C07, project number 394046768), the Health+Life Science Alliance Heidelberg Mannheim GmbH, and the Biotechnology and Biological Sciences Research Council (BBSRC) of the UK Research and Innovation (UKRI).
Data availability
No new data were generated or analysed in support of this research.
Authors
 Biography: Dr Simon Tual-Chalot (@SimonTualChalot) received his PhD in Cardiovascular Physiology in 2011 from the University of Angers, France, and revealed the importance of extracellular vesicles in driving endothelial dysfunction in hypoxia-related diseases. He joined Newcastle University in 2012, supported by a Marie Curie Intra-European Fellowship to work on the cardioprotective role of endoglin. In 2015 he became a joint investigator on a British Heart Foundation grant, and gained a ‘Young Investigator Award’ from Cure HHT, to continue investigations on how endothelial endoglin regulates cardiovascular integrity. Since 2018, Dr Tual-Chalot has been a member of the Stellos lab, working on the role of RNA-based mechanisms in endothelial cells. In 2024, Dr Tual-Chalot started his own group, supported by the British Heart Foundation and the Royal Society, studying the epitranscriptomic control of endothelial function during vascular ageing. He is an active member of society relevant to Ageing, Cardiovascular Research and ECR development. In 2021, he was appointed as the nucleus member of the working group ‘Small Vessels’ of the European Society of Hypertension, a research network that promotes cooperative research projects and generates position papers and educational material.
Biography: Dr Simon Tual-Chalot (@SimonTualChalot) received his PhD in Cardiovascular Physiology in 2011 from the University of Angers, France, and revealed the importance of extracellular vesicles in driving endothelial dysfunction in hypoxia-related diseases. He joined Newcastle University in 2012, supported by a Marie Curie Intra-European Fellowship to work on the cardioprotective role of endoglin. In 2015 he became a joint investigator on a British Heart Foundation grant, and gained a ‘Young Investigator Award’ from Cure HHT, to continue investigations on how endothelial endoglin regulates cardiovascular integrity. Since 2018, Dr Tual-Chalot has been a member of the Stellos lab, working on the role of RNA-based mechanisms in endothelial cells. In 2024, Dr Tual-Chalot started his own group, supported by the British Heart Foundation and the Royal Society, studying the epitranscriptomic control of endothelial function during vascular ageing. He is an active member of society relevant to Ageing, Cardiovascular Research and ECR development. In 2021, he was appointed as the nucleus member of the working group ‘Small Vessels’ of the European Society of Hypertension, a research network that promotes cooperative research projects and generates position papers and educational material.
 Biography: Konstantinos Stellos (@K_Stellos) is a physician-scientist who currently works as Professor of Medicine and Chair of the Department of Cardiovascular Research at the Heidelberg University in Germany. He also runs a preventive cardiology clinic at the University Hospital Mannheim of the Heidelberg University and works part-time as the Chair of Cardiovascular Medicine & Epitranscriptomics at the Newcastle University, Newcastle Upon Tyne, UK. His research focuses on the crosstalk among RNA and vascular biology, immunology, and cardiovascular medicine (www.StellosLab.com). His research work is published in discovery journals including Nature Medicine, Annals of Internal Medicine, and Immunity as well as in all major cardiovascular journals including Circulation, European Heart Journal, Journal of the American College of Cardiology and JAMA Cardiology. He is a recipient of a 1.5 M € European Research Council (ERC) Grant and his research work has been funded by several grants by the German Research Foundation (DFG). His research has been awarded with several international awards including the European Society of Cardiology Outstanding Achievement Award 2020 and the European Heart Journal Top Reviewer Award 2022. His trainees have received numerous awards including the Top Project Award 2019 by the Royal Society of Biology of the UK, the British Atherosclerosis Society Early Career Investigator Award 2019, the American Heart Association Early Career Investigator Awards 2019 and 2022, the German Cardiac Society Young Investigator Award 2023 and 2024, and several of them have started their own research labs (Gatsiou Lab, Tual-Chalot Lab, Vlachogiannis Lab and others). He serves the scientific community by several positions in international organizations and peer-reviewed journals. He is an Associate Editor of the the European Society of Cardiology basic science journal Cardiovascular Research, Assistant Editor of the journal Circulation: Genomic and Precision Medicine, Editorial Board member of the European Heart Journal, Secretary of the European Society of Cardiology Working Group on Atherosclerosis & Vascular Biology, and Chair of the American Heart Association (AHA) Council on Genomic & Precision Medicine Professional/Public Education & Publications’ Committee.
Biography: Konstantinos Stellos (@K_Stellos) is a physician-scientist who currently works as Professor of Medicine and Chair of the Department of Cardiovascular Research at the Heidelberg University in Germany. He also runs a preventive cardiology clinic at the University Hospital Mannheim of the Heidelberg University and works part-time as the Chair of Cardiovascular Medicine & Epitranscriptomics at the Newcastle University, Newcastle Upon Tyne, UK. His research focuses on the crosstalk among RNA and vascular biology, immunology, and cardiovascular medicine (www.StellosLab.com). His research work is published in discovery journals including Nature Medicine, Annals of Internal Medicine, and Immunity as well as in all major cardiovascular journals including Circulation, European Heart Journal, Journal of the American College of Cardiology and JAMA Cardiology. He is a recipient of a 1.5 M € European Research Council (ERC) Grant and his research work has been funded by several grants by the German Research Foundation (DFG). His research has been awarded with several international awards including the European Society of Cardiology Outstanding Achievement Award 2020 and the European Heart Journal Top Reviewer Award 2022. His trainees have received numerous awards including the Top Project Award 2019 by the Royal Society of Biology of the UK, the British Atherosclerosis Society Early Career Investigator Award 2019, the American Heart Association Early Career Investigator Awards 2019 and 2022, the German Cardiac Society Young Investigator Award 2023 and 2024, and several of them have started their own research labs (Gatsiou Lab, Tual-Chalot Lab, Vlachogiannis Lab and others). He serves the scientific community by several positions in international organizations and peer-reviewed journals. He is an Associate Editor of the the European Society of Cardiology basic science journal Cardiovascular Research, Assistant Editor of the journal Circulation: Genomic and Precision Medicine, Editorial Board member of the European Heart Journal, Secretary of the European Society of Cardiology Working Group on Atherosclerosis & Vascular Biology, and Chair of the American Heart Association (AHA) Council on Genomic & Precision Medicine Professional/Public Education & Publications’ Committee.
References
- 1. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 2. Mortensen MB, Nordestgaard BG. Comparison of five major guidelines for statin use in primary prevention in a contemporary general population. Ann Intern Med 2018;168:85–92. [DOI] [PubMed] [Google Scholar]
- 3. Banach M, Penson PE. What have we learned about lipids and cardiovascular risk from PCSK9 inhibitor outcome trials: ODYSSEY and FOURIER? Cardiovasc Res 2019;115:e26–e31. [DOI] [PubMed] [Google Scholar]
- 4. Ray KK, Troquay RPT, Visseren FLJ, Leiter LA, Scott Wright R, Vikarunnessa S, Talloczy Z, Zang X, Maheux P, Lesogor A, Landmesser U. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol 2023;11:109–119. [DOI] [PubMed] [Google Scholar]
- 5. Musunuru K. Moving toward genome-editing therapies for cardiovascular diseases. J Clin Invest 2022;132:e148555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oostveen RF, Khera AV, Kathiresan S, Stroes ESG, Fitzgerald K, Harms MJ, Oakes BL, Kastelein JJP. New approaches for targeting PCSK9: small-interfering ribonucleic acid and genome editing. Arterioscler Thromb Vasc Biol 2023;43:1081–1092. [DOI] [PubMed] [Google Scholar]
- 7. Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, Seitzer J, O'Connell D, Walsh KR, Wood K, Phillips J, Xu Y, Amaral A, Boyd AP, Cehelsky JE, McKee MD, Schiermeier A, Harari O, Murphy A, Kyratsous CA, Zambrowicz B, Soltys R, Gutstein DE, Leonard J, Sepp-Lorenzino L, Lebwohl D. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med 2021;385:493–502. [DOI] [PubMed] [Google Scholar]
- 8. Delialis D, Georgiopoulos G, Aivalioti E, Mavraganis G, Dimopoulou AM, Sianis A, Aggelidakis L, Patras R, Petropoulos I, Ioannou S, Syrigou R, Chatzidou S, Kanakakis I, Stellos K, Stamatelopoulos K. Remnant cholesterol and atherosclerotic disease in high cardiovascular risk patients. Beyond LDL cholesterol and hypolipidemic treatment. Hellenic J Cardiol 2022;66:26–31. [DOI] [PubMed] [Google Scholar]
- 9. Cordero A, Fernández Olmo MR, Cortez Quiroga GA, Romero-Menor C, Fácila L, Seijas-Amigo J, Rondán Murillo J, Sandin M, Rodríguez-Mañero M, Bello Mora MC, Valle A, Fornovi A, Freixa Pamias R, Bañeras J, Blanch García P, Clemente Lorenzo MM, Sánchez-Álvarez S, López-Rodríguez L, González-Juanatey JR. Effect of PCSK9 inhibitors on remnant cholesterol and lipid residual risk: the LIPID-REAL registry. Eur J Clin Invest 2022;52:e13863. [DOI] [PubMed] [Google Scholar]
- 10. Stellos K, Sauter R, Fahrleitner M, Grimm J, Stakos D, Emschermann F, Panagiota V, Gnerlich S, Perk A, Schönberger T, Bigalke B, Langer HF, Gawaz M. Binding of oxidized low-density lipoprotein on circulating platelets is increased in patients with acute coronary syndromes and induces platelet adhesion to vascular wall in vivo–brief report. Arterioscler Thromb Vasc Biol 2012;32:2017–2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.