Abstract
Aims
Gut microbiota have been linked to blood lipid levels and cardiovascular diseases (CVDs). The composition and abundance of gut microbiota trophic networks differ between ethnicities. We aim to evaluate the relationship between gut microbiotal trophic networks and CVD phenotypes.
Methods and results
We included cross-sectional data from 3860 individuals without CVD history from 6 ethnicities living in the Amsterdam region participating in the prospective Healthy Life in Urban Setting (HELIUS) study. Genetic variants were genotyped, faecal gut microbiota were profiled, and blood and anthropometric parameters were measured. A machine learning approach was used to assess the relationship between CVD risk (Framingham score) and gut microbiota stratified by ethnicity. Potential causal relationships between gut microbiota composition and CVD were inferred by performing two-sample Mendelian randomization with hard CVD events from the Pan-UK Biobank and microbiome genome-wide association studies summary data from a subset of the HELIUS cohort (n = 4117). Microbial taxa identified to be associated with CVD by machine learning and Mendelian randomization were often ethnic-specific, but some concordance across ethnicities was found. The microbes Akkermansia muciniphila and Ruminococcaceae UCG-002 were protective against ischaemic heart disease in African-Surinamese and Moroccans, respectively. We identified a strong inverse association between blood lipids, CVD risk, and the combined abundance of the correlated microbes Christensenellaceae–Methanobrevibacter–Ruminococcaceae (CMR). The CMR cluster was also identified in two independent cohorts and the association with triglycerides was replicated.
Conclusion
Certain gut microbes can have a potentially causal relationship with CVD events, with possible ethnic-specific effects. We identified a trophic network centred around Christensenellaceae, Methanobrevibacter, and various Ruminococcaceae, frequently lacking in South-Asian Surinamese, to be protective against CVD risk and associated with low triglyceride levels.
Keywords: Trophic networks, Microbiome, Cardiovascular diseases, Atherosclerosis, Mendelian randomization, HELIUS study
Graphical Abstract
Graphical Abstract.
Time for primary review: 46 days
1. Introduction
Modifiable risk factors such as hypertension, dyslipidaemia, obesity, and hyperglycaemia have significant impact on cardiovascular disease (CVD) risk.1,2 Recent evidence has shown that these risk factors are influenced by the gut microbiome3 or gut-derived metabolites, including short-chain fatty acids (SCFA).4 However, the gut microbiome and its metabolites may also directly affect CVD risk. For example, dietary ingested choline or L-carnitine is processed by the gut microbiota into trymethylamine, further metabolized into trimethylamine N–oxide, whose concentrations vary according to diet and microbiota composition, and is associated with major adverse cardiovascular events.5,6
While diet is one of the most important determinants of the gut microbiota composition,7 after correction for diet, specific differences in the gut microbiota composition between ethnicities remain, as seen in a multi-ethnic cross-sectional study of the inhabitants of Amsterdam, The Netherlands.7 This study minimized geographical, environmental, and sample processing influence on gut microbiota results, and suggests that other factors related to ethnicity contribute to differences in the gut microbiota composition.
Changes in gut microbiota composition related to genetic variants in the host can also affect disease phenotypes, as already described for inflammatory bowel diseases.8,9 Mendelian randomization (MR) studies have been used to infer causality between gut microbiota and metabolic diseases such as Type 2 diabetes through genetic variants that promote the production capacity of gut microbiota for SCFA.10 In their pioneering study, Sanna et al.10 identified several bacteria able to produce the SCFA butyrate to have a causative influence on glucose metabolism. A causal role for gut microbiota in other CVD risk factors such as blood pressure and lipid levels has also been established by an MR approach.11 However, as a single bacterium is part of a complex ecosystem in the gut, recent literature suggests that rather than individual bacteria, groups of co-dependent bacteria may be the driving force behind beneficial pathways via cross-feeding and syntrophic interactions. These co-dependent networks are referred to as bacterial guilds or trophic networks and may influence host metabolism as they alter the availability of substrates for themselves and for their host.
Therefore, we hypothesized that ethnic-specific gut microbiota compositions affect CVD, and interrogated this hypothesis using a multi-layered approach. To identify microbes associated with CVD risk in our multi-ethnic population, we performed a machine learning analysis. Subsequently, we inferred causality between single taxa and hard CVD outcomes by performing two-sample Mendelian randomization in a multi-ethnic cohort with outcome data from the UK Biobank.10 Finally, we evaluated the microbes identified by machine learning and Mendelian randomization from a trophic network perspective to provide greater biological insights into the association between CVD and the gut microbiome composition.
2. Methods
2.1. Study population
The primary discovery cohort for this study was the Healthy Life in Urban Setting (HELIUS) study in Amsterdam, The Netherlands, as previously described.12,13 In short, in this prospective cohort study, health differences between the six most common local ethnicities, namely, Dutch, African-Surinamese, South-Asian Surinamese, Turkish, Moroccan, and Ghanaian backgrounds, are evaluated. Inhabitants of Amsterdam between the ages of 18 and 70 years were randomly sampled, stratified by ethnicity, through the municipality registry of Amsterdam. Ethnicity was defined based on the country of birth as well as the parents’ country of birth, although for the African- and South-Asian Surinamese populations self-reported ethnicity was used.13 After a positive response to a written invitation, the subjects received a confirmation letter of an appointment for a physical examination and a digital or paper version of the questionnaire (depending on the preference of the subject) to fill out at home. Of 24 789 HELIUS participants, 22 165 participants underwent a physical examination and completed the questionnaire, 10 283 participants were genotyped, and 6048 handed in a faeces sample. A total of 4117 subjects were selected and used in this study based on the availability of the clinical, microbiome, and genetic data. For machine learning analysis, 3860 subjects with faecal samples but without CVD history were selected. The HELIUS study was approved by the institutional review board of the Academic Medical Centre (AMC) Amsterdam, The Netherlands, and was conducted in accordance with the Declaration of Helsinki (6th, 7th revisions). All participants provided written informed consent. Researchers who wish to conduct analyses on this cohort can submit a proposal as outlined at http://www.heliusstudy.nl/en/researchers/collaboration, by email: heliuscoordinator@amsterdamumc.nl. The proposals will be examined by the HELIUS Executive Board. If the proposals comply with the ethical guidelines, general objectives, and informed consent forms of the HELIUS study, access will be granted.
2.2. Blood and faeces processing
Anthropometric data and blood samples were collected during the first study visit along with information on drug use. The participants were asked to collect faeces prior to or during the study visit, as previously described.13 Exclusion criteria for faeces sampling included having diarrhoea 1 week before the study visit or the use of antibiotics three months prior (or unknown antibiotics use). If samples were collected more than 6 h before the study visit, the participants were instructed to store the sample in a freezer. At the study visit, the samples were frozen at −20°C for up to 1 day and subsequently at −80° in the permanent freezer. DNA was extracted at the Wallenberg Laboratory (Sahlgrenska Academy at University of Gothenburg, Sweden) with a repeated bead-beating protocol.14 The microbiota composition of faecal samples was assessed by sequencing the V4 region of the 16S rRNA gene on an Illumina MiSeq (Illumina RTA v1.17.28; MCS v2.5, San Diego, CA, USA) chip using 515F and 806R primers designed for dual-indexing15 and the V2 Illumina kit (2 × 250 bp paired-end reads). Polymerase chain reaction (PCR) and pre-processing of raw sequencing data were performed, as previously described.2
2.3. Microbial data preparation
The faecal samples and 16S sequencing data processing has been previously described.2,16 In short, faecal samples were collected and stored at −80°C, before total genomic DNA was extracted and paired-end sequencing of the V4 region of the 16S rRNA gene was carried out with an Illumina MiSeq system (2 × 250 bp). The resulting sequencing reads were merged and quality filtered, before the contigs were dereplicated and the UNOISE3 algorithm was used to denoise unique sequences to generate Amplicon Sequence Variants (ASVs). The assign Taxonomy function from the DADA2 R package (v. 1.12.1) was used to assign taxonomy using the SILVA (v. 132) database. For the genome-wide association studies (GWAS), the gut microbiome sequencing data were summarized to phylum, family, genus, or species level, and individual ASVs. The taxa were then filtered to keep taxa with >20 counts in >5% of the subjects. The findCorrelation function from the R caret package v. 6.0-90 was used to remove highly correlated taxa, to reduce redundancy. The final microbial dataset used for GWAS consisted of 401 taxa (237 ASVs, 60 species, 21 families, 76 genera, 7 phyla). If <5% of subjects had zero abundance, a pseudocount of 1 was added and the data were log10 transformed. Alternatively, if >5% of subjects had zero abundance, the data were encoded into a presence/absence pattern and analysed as a binary trait. Detailed methods have previously been published.16,17 For the machine learning approach and trophic network analysis, the microbes were kept as ASVs but were filtered by abundance to keep the microbes with the highest average abundance. The sequencing data generated in this study have been deposited in the European Genome-Phenome Archive database under the accession code EGAD00001004106.
2.4. Genotype data
The Illumina GSA 24v1-0 array was used to genotype participants at the Erasmus MC Human Genomic Facility (Rotterdam, The Netherlands). GenomeStudio software was used for genotyping of the array according to in-house protocols from the Human Genomic Facility. The Sanger Imputation Service was used to impute autosomal chromosomes according to instructions (https://imputation.sanger.ac.uk/). For phasing, EAGLE2 was used and the Positional Burrows-Wheeler Transform method with the HAPLOTYPE Reference Consortium (release 1.1) was used, including the 1000 Genomes. After imputation, single nucleotide polymorphisms (SNPs) with an INFO score ≤0.3 were removed. The SNPs were annotated with rs numbers if possible; if this was not known, the chromosomal location was used for annotation based on GRCh37 coordinates. Detailed methods including details on further quality control and genotype-to-microbiome associations have previously been published.16,18 Relevant summary statistics can be found in Supplementary material online, Table S1.
2.5. Framingham score and CVD outcomes
The Framingham score was used to assess CVD risk in our cohort. This score predicts the 10-year risk to develop CVDs in subjects who are free of CVD and cancer at baseline. This score fitted best with our relatively young population. Other scores such as the European SCORE19 and Pooled Cohort Studies Equations20 were similar in our population with correlation test R2 ranges between 0.79 and 0.98 (see Supplementary material online, Figure S1).
This score uses the following parameters: sex, age, systolic blood pressure, use of antihypertensive medication, smoking (defined by self-report and categorized as currently smoking vs. non-smoking), diabetes mellitus (defined as a fasting glucose ≥7 mmol/L or use of glucose-lowering medication), total cholesterol, and HDL cholesterol levels.21,22
For the two-sample Mendelian randomizations, summary statistics of hard cardiovascular outcomes were obtained from the multi-ethnic Pan-UK Biobank (Pan-UKBB) cohort (https://pan.ukbb.broadinstitute.org, 2020). Three phenotypes were relevant to our study: ischaemic heart disease (phecode: 411), myocardial infarction (phecode: 411.2), and coronary atherosclerosis (phecode: 411.4). These phenotypes are composites of different International Classification of Diseases and related health problems (ICD) codes into a single disease endpoint (see Supplementary material online, Table S6). This study used participants from European, Central/South Asian, African, and Middle Eastern ancestry from the Pan-UKBB.
2.6. Machine learning
Continuous outcomes such as the Framingham score were estimated with a machine learning approach using a regression predictor, for N = 3860 participants. For this, the gradient boosted trees method XGBoost (version 0.90) was used. The participants with a reported CVD event in their medical history were excluded. Nested cross-validation was performed to avoid overfitting and ensure robustness of results, which was employed over 100 iterations on a completely reshuffled dataset to improve robustness, and the average predictive performance was reported as root mean squared error (RMSE). We have used the stability selection approach as previously described,23 to ensure reliability of the findings. We also use a specialized regularization strategy that makes our methodology applicable to the high-dimensional regime.
Machine learning was performed in Python (version 3.7.4) using the following packages: pandas (version 0.25.1), numpy (version 1.16.4), and scikit-learn (version 0.21.2); for hyperparameters and more detailed methods, see Supplementary material online, Methods.
2.7. Mendelian randomization
In order to infer potential causal relationships between bacteria as exposure and CVD outcomes, we used the inverse variance weighted (IVW) method;24 however, if only one SNP was available, the Wald ratio was used, as a two-sample Mendelian randomization analysis of the summary association statistics. Mendelian randomization relies on three core assumptions: (i) the genetic instrument variables are related to the risk factor; (ii) the confounders are not related to the exposure, outcome, and genetic instruments; and (iii) there is no relationship between the genetic instrument and outcome other than through the risk factor. A threshold of P < 1 × 10−8 was initially used to select SNPs associated with the exposure and a second threshold of P < 1 × 10−5 was used to select variants associated to the exposure to avoid reliance on individual SNPs during Mendelian randomization, as previously described.10 Only SNPs with an F statistic >10 in regard to the association with the exposure were used. These thresholds strengthen the first assumption of Mendelian randomization analysis. The GWAS on the gut microbiome was performed with appropriate confounder correction [age, sex body mass index (BMI), top 10 genetic principal components, metformin use, proton-pump inhibitor use, educational level, and 16S rRNA sequencing run] to reduce the risk of confounded MR results.16 Finally, the third assumption cannot be formally tested although it is important to consider in the interpretation of the results. Clumping was performed using PLINK v. 2.0 with default parameters. This linkage disequilibrium-reduced SNP set was used for the MR analysis. Furthermore, MR Egger, leave-one-out, and weighted median were used as sensitivity analyses approaches for significant results. Two-sample MR was performed by using the ‘TwoSampleMR’ R library (https://github.com/MRCIEU/TwoSampleMR) v. 0.5.5. The Pan-UKBB subgroups were matched to the HELIUS groups as follows: the ‘Meta’ group in the Pan-UKBB with the total group in HELIUS, the ‘EUR’ group with the Dutch and Turkish separately in HELIUS, the ‘AFR’ group with the African-Surinamese and Ghanaian groups in HELIUS together as well as separately, ‘CSA’ with the South-Asian Surinamese in HELIUS, and the ‘MID’ with the Moroccan group in HELIUS. This is in line with previous results that showed that African populations from the HELIUS cohort are genetically similar to Africans in the 1000 genome cohort; the same holds true for the Dutch and South-Asian with regard to their respective geographical origins.18 Similarly, UKBB ethnicities were also matched to the 1000 genome cohorts, which also confirmed the geographical origin.25 Reported P-values are nominal and should be interpreted as explorative results. For genetical instruments, see Supplementary material online, Tables S3–S5. Beta coefficients correspond to the log of the odds ratio (OR) per standard deviation increase.
To confirm that causal relationships were not bidirectional, we performed bidirectional MR using CVD outcomes as exposure and microbiome data as outcome.
2.8. Co-occurring networks
To identify correlated microbial networks with potential causative effect on CVD, we selected the 30 bacteria with the strongest Spearman’s correlation to bacteria identified from the MR analyses (Figure 1). These groups of bacteria were summed and used as one composite signal (SumTn). Subsequently, a correlation analysis was performed for the most important cardiovascular and metabolic parameters and the SumTn. An alternative approach for defining composite signals was to conduct hierarchical clustering (Euclidean distance, complete linkage) of Spearman ρ coefficients of the 250 most abundant ASVs. Specifically, several bacteria causally linked to CVD were found to be part of the Christensenellaceae–Methanobrevibacter–Ruminococcaceae (CMR) cluster. This trophic network was subsequently also summed up together and used as one composite signal CMR and correlated with important cardiovascular and metabolic parameters. The CMR cluster was validated in the METSIM cohort26 (a cohort of 531 Finish men) and in a Korean cohort27 (623 healthy and hypertensive Korean men and women). We used the same methods for clustering in all three cohorts.
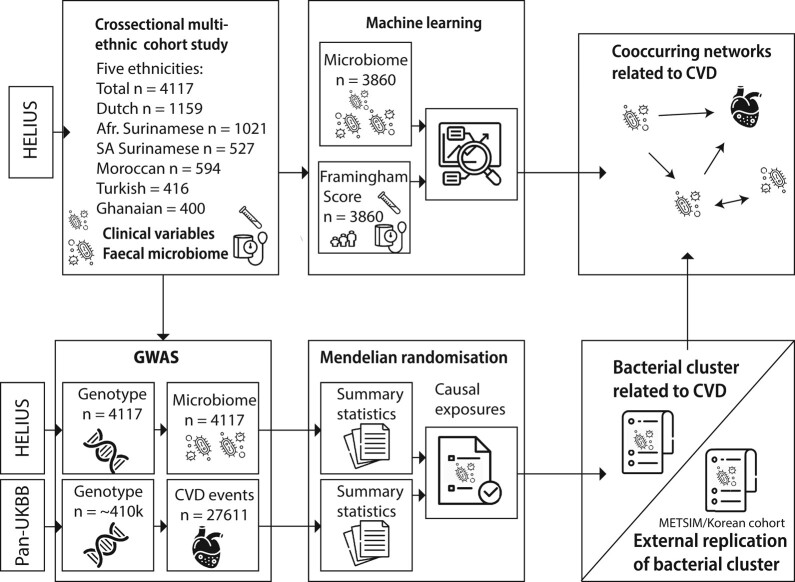
Figure 1.
Study design. To study the relation between microbes and CVD risk, we predicted the Framingham score with faecal microbiome data using a machine learning approach. To study causal relations between gut microbes and CVD events, we performed a two-step MR approach. For this, summary statistics from a GWAS in the multi-ethnic HELIUS cohort with microbes and in the multi-ethnic Pan-UKBB cohort were used. Bacteria correlated to bacteria deemed to be causally related with CVD events based on Mendelian randomization were compared with results from the machine learning approach. As a third method, an unbiased hierarchical clustering approach was used to identify co-occurring bacterial trophic networks. GWAS, genome-wide association study. Images from Flaticon.com.
2.9. Statistical analysis
A GWAS of genotype and microbiome data was performed using PLINK v. 2.0, considering the following parameters: -glm and -covar-variance-standardize. The association was adjusted for covariates (top 10 genetic principal components calculated by pca in PLINK v. 2.0), age, sex, BMI, proton-pump inhibitor use, and metformin use, since all these parameters are known to modulate the gut microbiota composition.28 The GWAS was conducted in both the total cohort and separately per ethnic subset. Further details on the microbiome GWAS can be found in the previously published study.16 Spearman ρ correlations were used to evaluate the associations among microbes and metabolic parameters. Mann–Whitney U testing was used for non-parametric comparison of groups with normal or elevated triglyceride levels. Linear regression was used to assess the dose-dependent effect of CMR abundance and elevated triglyceride prevalence and to model interaction, which was compared with a non-interaction model using an analysis of variance (ANOVA). The false discovery rate (FDR) method was used to correct for multiple correlation comparisons and q < 0.05 was considered statistically significant. FDR correction was applied within each ethnicity. All other statistical analyses were performed in R (v 3.6.1) and ggplot2 was used for data visualization. One author had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
3. Results
3.1. Baseline characteristics
Table 1 displays clinical characteristics of patients stratified by ethnic groups. The median age was 51 years, with the Dutch and South-Asian Surinamese being the oldest (median age 54 years), while the Moroccan and Turkish groups were the youngest (median age 46 years). The Turkish group had the highest BMI, while the Dutch had the lowest. African-Surinamese and Moroccans had the highest and lowest Framingham scores, respectively. Most antihypertensive usage was found in the South-Asian Surinamese group with 20.2% and the least in the Moroccan group with 1.5% (Table 1 and Supplementary material online, Table S2).
Table 1.
Baseline characteristics in the HELIUS cohort, stratified per ethnicity
| Overall | Dutch | South.Asian.Surinamese | African.Surinamese | Ghanaian | Turkish | Moroccan | |
|---|---|---|---|---|---|---|---|
| N | 4117 | 1159 | 527 | 1021 | 400 | 416 | 594 |
| Age [median (IQR)] | 51.00 [42.00, 58.00] | 54.00 [43.00, 62.00] | 54.00 [46.00, 60.00] | 53.00 [47.00, 59.00] | 49.00 [43.00, 55.00] | 46.00 [36.00, 51.00] | 46.00 [37.00, 54.00] |
| Sex: female (%) | 2175 (52.8) | 571 (49.3) | 273 (51.8) | 617 (60.4) | 231 (57.8) | 208 (50.0) | 275 (46.3) |
| Weight [median (IQR)] | 76.70 [67.45, 86.76] | 77.10 [67.65, 87.05] | 70.20 [62.20, 79.70] | 77.80 [68.64, 88.80] | 76.03 [68.38, 85.66] | 77.95 [68.61, 88.75] | 77.68 [69.50, 88.46] |
| BMI, kg/m2 [median (IQR)] | 26.66 [23.84, 30.15] | 24.83 [22.33, 27.80] | 26.18 [23.66, 29.07] | 27.45 [24.46, 31.54] | 27.92 [25.14, 30.96] | 28.67 [25.60, 31.74] | 27.56 [25.10, 31.00] |
| Waist circumference, cm [mean (SD)] | 94.05 (13.03) | 92.09 (13.22) | 93.76 (12.47) | 94.46 (13.74) | 94.07 (11.26) | 96.17 (12.99) | 95.92 (12.54) |
| Systolic blood pressure, mmHg [median (IQR)] | 127.50 [116.50, 140.00] | 125.00 [115.00, 137.00] | 130.50 [118.50, 143.00] | 131.50 [120.00, 143.50] | 135.75 [125.00, 148.00] | 122.50 [112.50, 132.00] | 122.50 [113.00, 134.88] |
| Fasting glucose, mmol/L [median (IQR)] | 5.30 [5.00, 5.80] | 5.30 [5.00, 5.70] | 5.60 [5.10, 6.40] | 5.30 [4.90, 5.80] | 5.20 [4.80, 5.70] | 5.30 [5.00, 5.70] | 5.40 [5.00, 5.90] |
| HbA1c, mmol/mol [median (IQR)] | 38.00 [35.00, 42.00] | 37.00 [34.00, 39.00] | 41.00 [37.00, 47.00] | 39.00 [36.00, 43.00] | 39.00 [34.00, 43.00] | 37.00 [34.00, 41.00] | 37.00 [34.00, 41.00] |
| Total cholesterol, mmol/L [median (IQR)] | 4.96 [4.31, 5.67] | 5.17 [4.54, 5.89] | 4.91 [4.21, 5.70] | 4.89 [4.25, 5.61] | 4.96 [4.30, 5.63] | 4.90 [4.26, 5.55] | 4.74 [4.12, 5.37] |
| HDL-c, mmol/L [median (IQR)] | 1.39 [1.13, 1.71] | 1.49 [1.21, 1.81] | 1.26 [1.04, 1.49] | 1.45 [1.20, 1.78] | 1.59 [1.31, 1.89] | 1.21 [1.02, 1.49] | 1.26 [1.03, 1.52] |
| Triglycerides, mmol/L [median (IQR)] | 0.87 [0.59, 1.27] | 0.92 [0.63, 1.32] | 1.07 [0.73, 1.51] | 0.76 [0.54, 1.05] | 0.63 [0.46, 0.89] | 1.02 [0.69, 1.57] | 0.92 [0.63, 1.33] |
| LDL-c, mmol/L [median (IQR)] | 3.05 [2.45, 3.67] | 3.16 [2.55, 3.80] | 3.02 [2.39, 3.75] | 3.00 [2.36, 3.61] | 2.99 [2.42, 3.67] | 3.06 [2.46, 3.59] | 2.98 [2.42, 3.52] |
| Creatinine, µmol/L [median (IQR)] | 73.00 [63.00, 84.00] | 74.00 [66.00, 84.00] | 72.00 [62.00, 86.00] | 76.00 [66.00, 88.00] | 77.00 [66.75, 91.00] | 66.00 [57.00, 78.00] | 67.00 [56.00, 77.00] |
| Framingham score [mean (SD)] | 9.77 (5.92) | 9.67 (5.95) | 11.99 (6.13) | 10.70 (5.76) | 9.06 (4.79) | 8.10 (5.24) | 7.72 (6.04) |
| Antihypertensive use: no (%) | 3772 (91.6) | 1111 (95.9) | 480 (91.1) | 873 (85.5) | 319 (79.8) | 404 (97.1) | 585 (98.5) |
| Statin use: no (%) | 3615 (87.8) | 1042 (89.9) | 384 (72.9) | 913 (89.4) | 364 (91.0) | 369 (88.7) | 543 (91.4) |
3.2. Association between gut microbiota and CVD risk
As overt CVD outcome data were not available in the HELIUS study, we used the Framingham score as a surrogate marker for CVD to study the relationship between CVD and the gut microbiome. We performed a gradient boosted machine learning analysis for regression predictive modelling on all subjects together (total group) and on ethnic subgroups separately to predict the Framingham score based on the gut microbiota composition. The total group (RMSE: 0.386 ± 0.006, P = 1.0 × 10−4n = 3860), Dutch (RMSE: 0.501 ± 0.018, P = 1.0 × 10−4, n = 1196), and African-Surinamese (RMSE: 0.514 ± 0.018, P = 9.2 × 10−3n = 998, Figure 2) had the most relevant microbiome profiles for Framingham score estimation. The microbiome composition of the Moroccan (RMSE: 0.458 ± 0.034, P = 0.09, n = 489), Figure 3, and South-Asian Surinamese (RMSE: 0.582 ± 0.059, P < 0.05 n = 460) subgroup had similar relevance value for CVD risk. For the Ghanaian (RMSE: 0.550 ± 0.045, P = 0.21, n = 383) and Turkish (RMSE: 0.522 ± 0.047, P = 0.23 n = 334) groups, the microbiome was not found to be relevant for the Framingham score. The 30 most contributing features differed between ethnicities (see Supplementary material online, Figures S2–S5, for relative feature importance).
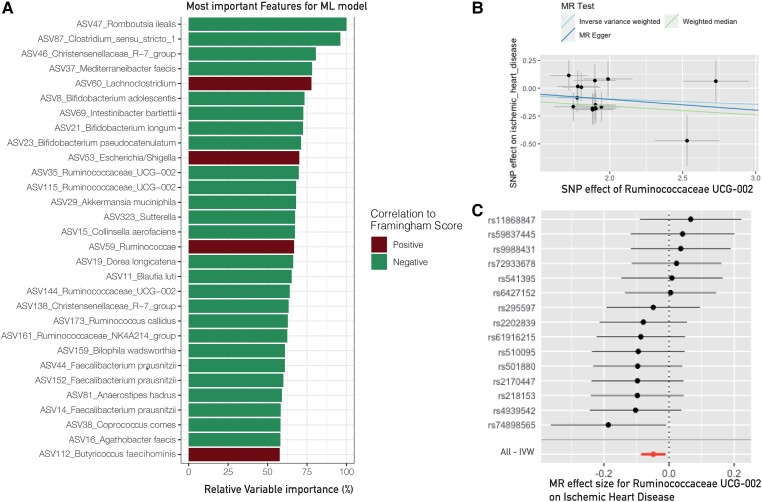
Figure 2.
Results for the African-Surinamese subgroup. (A) The 30 most important features to predict Framingham score based on the faecal gut microbiota composition. *The bacteria were present in the SumTn of A. muciniphila, indicating that this bacterium is part of the trophic network that has a protective effect against ischaemic heart disease (n = 998). (B) Scatter plot of instruments with sensitivity analyses. (C) Forestplot with the effect of all SNPs used for the Mendelian randomization for A. muciniphila. IVW, inverse variance weighted.
Figure 3.
Results for the Moroccan subgroup. (A) The 30 most important features to predict Framingham score based on the faecal gut microbiota composition (n = 489). *The bacterium was present in the SumTn of Ruminoccocaceae UCG-002, which was protective against ischaemic heart disease, indicating that this bacterium may be part of the trophic network that has a protective effect against ischaemic heart disease. (B) Scatter plot of instruments with sensitivity analyses. (C) Forestplot with the effect of all SNPs used for the Mendelian randomization for Ruminoccocaceae UCG-002. IVW, inverse variance weighted.
3.3. Relations between the gut microbiome and CVD phenotypes
We performed a two-sample MR using the HELIUS cohort for exposure (microbial taxa abundance) and three CVD outcomes (ICD codes, see Supplementary material online, Table S6) from the Pan-UKBB. Subsequently, results from this analysis were compared with machine learning results and microbiome clustering to better understand the role of bacterial clusters or trophic networks in relation to CVD. In total, 303 MR analyses were performed (see Supplementary material online, Tables S3–S5) of which 21 were nominally statistically significant.
3.3.1. Ischaemic heart disease
In the total group, Dorea formicigenerans was potentially protective against ischaemic heart disease (ASV 72, IVW: OR = 0.92, P = 3.8 × 10−2). In the Dutch group, Acidaminococcus (ASV 167, OR = 0.99, P = 2.7 × 10−2) was shown to be protective against this phenotype. The OR represent increase or decrease of risk per standard deviation change.
In the Moroccan subgroup, three bacteria showed to be associated with a protective effect against ischaemic heart disease. Namely, the two genera Ruminococcaceae UCG-002 (ASV 176, OR = 0.89, P = 1.2 × 10−2, Figure 3) and Ruminococcaceae UCG-005 (ASV 105, OR = 0.89, P = 4.9 × 10−3) as well as Roseburia (ASV 119, OR = 0.84, P = 1.4 × 10−2). Ruminococcaceae UCG-002 and Ruminococcaceae UCG-005 also showed a significant result for the weighted median but not for the MR Egger as a sensitivity test (see Supplementary material online, Table S3 for remaining results and sensitivity analyses). Lachnospira pectinoschiza was also shown to have a potential protective effect (ASV 54, OR = 0.87, P = 2.4 × 10−2).
In the South-Asian Surinamese subgroup, Alloprevotella (ASV 66, OR = 0.96, P = 3.9 × 10−3) was significantly associated with a protective effect against ischaemic heart disease, whereas Solobacterium (ASV 191, OR = 1.03, P = 5.3 × 10−3) contributed to ischaemic heart disease.
In the African-Surinamese, Akkermansia muciniphila showed to be associated with a protective effect against ischaemic heart disease (ASV 29, OR = 0.94, P = 4.2 × 10−2), whereas Desulfovibrionaceae (ASV 617, Wald Ratio: OR = 1.89, P = 1.8 × 10−2) contributed to the disease. In the Ghanaian group, Alloprevotella (ASV 66, OR 1.04, P = 2.5 × 10−2) and Lachnospiraceae (ASV 30, OR = 1,39, P = 4.2 × 10−2) contributed to this phenotype. In the Turkish groups, no significant role of the microbiome was observed.
3.3.2. Myocardial infarction
Narrowing our analysis to a more focused clinical phenotype (i.e. myocardial infarction), we found in the African-Surinamese Ruminiclostrididium 6 (ASV 84, IVW: OR = 0.90, P = 3.8 × 10−2) and Bacteroidetes (OR = 0.32, P = 4.8 × 10−2) were potentially protective against myocardial infarction. In the Ghanaian group, Blautia faecis (ASV 40, OR = 0.54, P = 2.1 × 10−2) was potentially protective whereas a relationship between Dorea longicatena (ASV 19, OR = 1.45, P = 4.4 × 10−2) and myocardial infarction was found.
However, the relationship with this phenotype disappeared after correction for multiple testing. In the South-Asian Surinamese group, Solobacterium appeared to be contributing to myocardial infarction (ASV 191, OR = 1.04, P = 7.2 × 10−3).
3.3.3. Coronary atherosclerosis
The third phenotype, which had the smallest selection of ICD codes and thus the most focused phenotype, was coronary atherosclerosis (see for overlap between phenotypes in ICD codes Supplementary material online, Table S6).
In the Moroccan group, Ruminococcaceae UCG-005 (ASV 105, IVW: OR = 0.84, P = 1.6 × 10−4), L. pectinoschiza (ASV 54, OR = 0.86, P = 2.7 × 10−2), and Collinsella aerofaciens (ASV 15, OR = 0.33, P = 2.6 × 10−2) were all associated with a protective effect against this phenotype. In the South-Asian Surinamese group, Alloprevotella (ASV 66, OR = 0.95, P = 2.5 × 10−3) was potentially protective against coronary atherosclerosis (see Supplementary material online, Table S5, for all MR results and sensitivity analyses).
In the Dutch, Turkish, African-Surinamese, and Ghanaian groups, no significant associations were found (see Supplementary material online, Table S5).
3.3.4. Bidirectional MR
We performed bidirectional MR to assess if there is a potential bidirectional relationship between microbes and CVD outcomes. This was performed for the total and Dutch groups using gut microbiota as outcome and CVD event as exposure (see Supplementary material online, Table S7). For the African-Surinamese, South-Asian Surinamese, Moroccan, and Turkish groups, no SNPs passed the F statistic threshold for the three phenotypes. Significant taxa identified in the initial MR were not significant in the bidirectional analysis MR in the total group. In the Dutch group, ischaemic heart disease was significantly contributing to the relative abundance of Acidaminococcus (ASV 167), suggesting that this microbe does not have a causal effect on ischaemic heart disease, but that ischaemic heart disease or a mechanism related to ischaemic heart disease may influence the abundance of this bacteria.
3.4. Trophic networks related to cardiovascular health
To determine the involvement of taxa identified through machine learning and MR in a trophic network, we employed two approaches. First, we identified the 30 most strongly positively correlated ASVs for MR bacteria and merged them into a composite signal termed SumTn. Secondly, we utilized hierarchical clustering of Spearman’s ρ coefficients for the 250 most abundant ASVs as an alternative method for defining composite signals. Within this framework, we observed that certain MR bacteria associated with CVD (ASVs 105 and 176) were interconnected within the CMR trophic network cluster (Figure 4 and Supplementary material online, Figures S8–S14). This cluster was then consolidated into a composite signal called CMR, which we correlated with cardiovascular and metabolic parameters. By comparing both approaches for generating composite signals of co-abundant bacteria, we assessed whether these composite methods enhanced the association with the metabolic parameters of interest when compared with each individual MR taxon.
Figure 4.
Heatmap of 16S faecal microbiome data of combined ethnicities. Six clusters were identified with this heatmap. The Prevotella enterotype-associated cluster, bacteria related to metformin, the CMR cluster core and a larger bacterial cluster, the Bacteroides cluster and a small intestinal bacterial cluster. Bacteria related to the heatmap based on n = 4117 individuals of different ethnicities. Hierarchical cluster based on Euclidean distances from a Spearman correlation.
The Moroccan group displayed a protective effect of Ruminococcaceae UCG-002 and Ruminococcaceae UCG-005 (ASV 176 and ASV 105, respectively) against ischaemic heart disease and myocardial infarction, both of which were also present in the CMR cluster. When examining the correlation coefficient (Spearman’s ρ), it was observed that the SumTn of Ruminococcaceae UCG-002 (ASV 176) exhibited amplification in the Moroccan subgroup across 13 out of 14 correlations, with four of them demonstrating significance after correcting for multiple comparisons (refer to Table 2). Among the SumTn correlation results, eight showed higher coefficients within the CMR cluster compared with the SumTn group. Notably, the inverse correlation with the Framingham score became statistically significant by using the SumTn bacteria (Ruminococcaceae UCG-002: ρ = −0.089, P = 0.042, q = 0.158; SumTn: ρ = −0.107, P = 0.015, q = 0.036; CMR cluster: ρ = −0.116, P = 0.008, q = 0.026). A similar effect was observed for triglyceride levels, smoking, HbA1c, and Lp(a) as indicated in Table 2.
Table 2.
Correlation to cardiovascular and metabolic markers is amplified with the sum of the trophic network (SumTn) or sum of the CMR cluster
| Ruminococcaceae UCG-002/ASV 176 | SumTn | CMR cluster | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | ρ | P-value | q value | ρ | P-value | q value | ρ | P-value | q value | |
| Sex | 594 | −0.005 | 0.910 | 0.910 | 0.038 | 0.352 | 0.376 | 0.037 | 0.374 | 0.427 |
| Age | 594 | 0.025 | 0.539 | 0.578 | 0.035 | 0.393 | 0.393 | 0.027 | 0.507 | 0.541 |
| Framingham score | 518 | −0.089 | 0.042 | 0.158 | −0.107 | 0.015 | 0.036 | −0.116 | 0.008 | 0.026 |
| Triglycerides | 594 | −0.116 | 0.004 | 0.034 | −0.147 | 3.19E-04 | 0.002 | −0.154 | 1.71E-04 | 0.001 |
| LDL-c | 594 | −0.055 | 0.184 | 0.276 | −0.061 | 0.139 | 0.171 | −0.080 | 0.051 | 0.079 |
| Systolic blood pressure | 594 | −0.064 | 0.120 | 0.276 | −0.071 | 0.083 | 0.120 | −0.080 | 0.052 | 0.079 |
| Smoking | 580 | 0.102 | 0.014 | 0.070 | 0.122 | 0.003 | 0.014 | 0.125 | 0.003 | 0.010 |
| BMI | 594 | −0.049 | 0.232 | 0.317 | −0.076 | 0.065 | 0.115 | −0.079 | 0.055 | 0.079 |
| Waist circumference | 594 | −0.060 | 0.141 | 0.276 | −0.092 | 0.025 | 0.050 | −0.093 | 0.023 | 0.047 |
| Fasting glucose | 594 | −0.025 | 0.539 | 0.578 | −0.073 | 0.077 | 0.120 | −0.064 | 0.119 | 0.147 |
| Diabetes | 594 | −0.047 | 0.256 | 0.320 | −0.042 | 0.304 | 0.348 | −0.022 | 0.592 | 0.592 |
| HbA1c | 594 | −0.076 | 0.063 | 0.188 | −0.099 | 0.016 | 0.036 | −0.094 | 0.022 | 0.047 |
| CRP | 93 | −0.144 | 0.167 | 0.276 | −0.155 | 0.137 | 0.171 | −0.170 | 0.103 | 0.138 |
| Lpa | 93 | −0.139 | 0.183 | 0.276 | −0.255 | 0.014 | 0.036 | −0.245 | 0.018 | 0.047 |
| Ruminococcaceae UCG-002/ASV 29 | 1.000 | 0.000 | 0.000 | 0.690 | 4.05E-85 | 3.24E-84 | 0.6736108 | 8.99E-80 | 7.19E-79 | |
| SumTN/Sum CMR | 1.000 | 0.000 | 0.000 | 1 | 0 | 0 | ||||
Correlations shown are only performed in the Moroccan subgroup.
Within the African-Surinamese group, A. muciniphila (ASV 29) exhibited a protective effect against ischaemic heart disease, as illustrated in Figure 2. Furthermore, when comparing the correlation coefficient of A. muciniphila with the SumTn group, it was found to be higher in 9 out of 14 parameters concerning metabolic and cardiovascular health, with 7 of them demonstrating statistical significance (Table 3). These findings highlight a strong association between these combined bacteria and metabolic parameters. It is important to note that there is significant overlap between the ASVs associated with the SumTn from ASV 29, ASV 176, and the CMR cluster, as indicated in Supplementary material online, Table S9.
Table 3.
Correlation to cardiovascular and metabolic markers is amplified with the sum of the trophic network (SumTn) or sum of the CMR cluster
| A. muciniphila | SumTn | CMR cluster | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | ρ | P-value | q value | ρ | P-value | q value | ρ | P-value | q value | |
| Sex | 1021 | 0.148 | 2.00−06 | 1.50E−05 | 0.208 | 2.01E−11 | 1.07E−10 | 0.119 | 1.37E−04 | 4.10E−04 |
| Age | 1021 | −0.003 | 0.919 | 0.962 | 0.001 | 0.986 | 0.986 | −0.028 | 0.370 | 0.512 |
| Framingham score | 968 | −0.073 | 0.024 | 0.072 | −0.122 | 1.49E−04 | 4.12E−04 | −0.104 | 0.001 | 0.003 |
| Triglycerides | 1021 | −0.076 | 0.015 | 0.072 | −0.174 | 2.35E−08 | 9.39E−08 | −0.17565 | 1.61E−08 | 5.79E−08 |
| LDL-c | 1021 | 0.028 | 0.363 | 0.605 | 0.029 | 0.355 | 0.474 | 0.024 | 0.440 | 0.563 |
| Systolic blood pressure | 1020 | −0.059 | 0.059 | 0.111 | −0.087 | 0.005 | 0.011 | −0.073 | 0.019 | 0.034 |
| Smoking | 1021 | 0.060 | 0.056 | 0.111 | 0.114 | 2.65E−04 | 0.001 | 0.077 | 0.013 | 0.027 |
| BMI | 1019 | 0.006 | 0.840 | 0.962 | −0.023 | 0.463 | 0.569 | −0.012 | 0.707 | 0.796 |
| Waist circumference | 1020 | −0.021 | 0.510 | 0.695 | −0.079 | 0.012 | 0.021 | −0.059 | 0.059 | 0.097 |
| Fasting Glucose | 1021 | −0.063 | 0.044 | 0.111 | −0.118 | 1.54E−04 | 4.12E−04 | −0.085 | 0.007 | 0.015 |
| Diabetes | 1021 | −0.001 | 0.962 | 0.962 | −0.058 | 0.064 | 0.103 | −0.046 | 0.141 | 0.212 |
| HbA1c | 1021 | −0.072 | 0.022 | 0.072 | −0.043 | 0.166 | 0.241 | −0.023 | 0.469 | 0.563 |
| CRP | 198 | −0.048 | 0.501 | 0.695 | −0.041 | 0.566 | 0.647 | 0.008 | 0.910 | 0.963 |
| Lpa | 198 | −0.034 | 0.638 | 0.798 | −0.002 | 0.978 | 0.986 | −0.003 | 0.963 | 0.963 |
| A. muciniphila/ | 1.000 | 0.000 | 0.000 | 0.495 | 3.22E−64 | 2.58E−63 | 0.378 | 4.05E−36 | 1.82E−35 | |
| ASV 29 | ||||||||||
| SumTn/Sum CMR | 1 | 0 | 0 | 1 | 0 | 0 | ||||
Correlations shown are only performed for the African-Surinamese subgroup.
3.5. CMR cluster replication cohort
The presence of the CMR cluster was also validated using the same clustering approach in the independent METSIM cohort and in a Korean cohort (see Supplementary material online, Figures S15 and S16 and Table S12) showing that the CMR trophic network cluster can reproducibly be identified in other ethnicities (Finnish and Korean). In fact, the exact same representative sequences are found in both cohorts for most ASVs of their respective CMR clusters, with ASVs 105 and 176 (uncultured Ruminococcaceae) from the HELIUS cohort being identical with ASVs 120 and 245 of the METSIM cohort. The main difference with regard to the CMR clusters is the inclusion of various Tenericutes ASVs in the METSIM cohort. However, by extending the top 250 most abundant ASVs by including less abundant ASVs, we also found Tenericutes ASVs to belong to the CMR cluster in the HELIUS cohort, and that Tenericutes ASVs 273, 235, and 525 from HELIUS match the sequence of the METSIM Tenericutes ASVs 91, 188, and 243. From the literature it is known that Tenericutes are indeed strongly associated with what is here called the CMR trophic network cluster.29 In addition to this, the inverse association of the CMR cluster was also replicated in the Korean study, as was the negative correlation with triglycerides (ρ −0.23, P = 5.10 × 10−6, Supplementary material online, Table S16). See Supplementary material online, Tables S13 and S14, which summarize the overlapping findings from the different applied methods.
3.6. Triglyceride levels and the CMR cluster
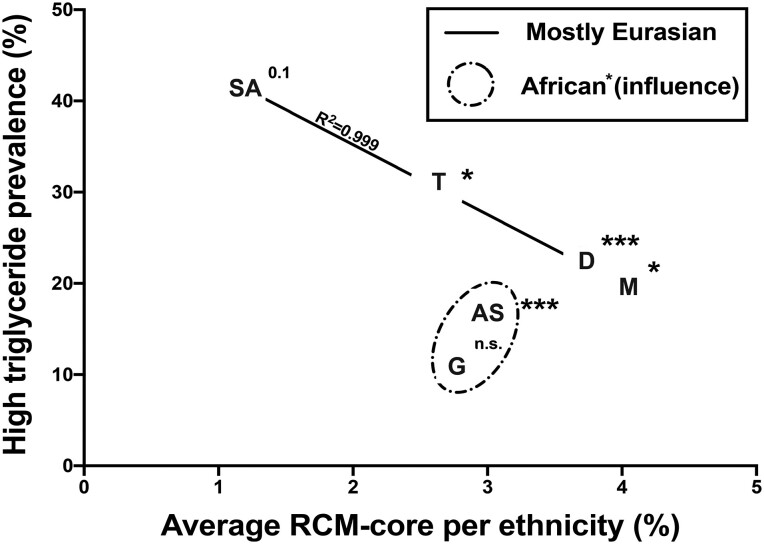
The identified CMR cluster was especially strongly inversely correlated with triglyceride levels. To better understand if this can be interpreted as a dose-dependent effect, we calculated the average combined CMR abundance per ethnicity and plotted this against the prevalence of elevated triglyceride levels (triglyceride levels ≥1.7 mmol/L or lipid-lowering drugs) per ethnicity (Figure 5) by Mann–Whitney U testing. In addition, we compared individual CMR abundances per ethnicity between individuals with high triglyceride or low triglyceride levels. In Eurasian (South-Asian Surinamese, Turkish, Dutch, and Moroccan) ethnicities, a dose-dependent effect seems to be observed between the prevalence of CMR per ethnicity and the prevalence of high triglyceride levels (Figure 5).
Figure 5.
High plasma triglyceride level prevalence and average CMR abundance between ethnicities. South-Asian Surinamese had the lowest abundance of CMR but the highest prevalence of elevated triglyceride levels, followed by the Turkish, Dutch, and Moroccan subgroups. Eurasians and Sub-Saharan groups have different CMR-dependent triglyceride associations. Asterisks and P-value indicate results from Mann–Whitney U testing comparing CMR abundance between individuals with normal fasting triglyceride levels or increased triglyceride levels (≥1.7 mmol/L or use of lipid-lowering drugs). Higher CMR abundance is seen in individuals with lower prevalence of elevated triglyceride levels. R2 indicates the result of a linear regression between average CMR abundance from Eurasian subgroups (e.g. SA, T, D, and M). SA, South-Asian Surinamese; T, Turkish; D, Dutch; M, Moroccan; AS, African-Surinamese; G, Ghanaian.
In the METSIM and Korean validation cohort, Christensenellaceae and Methanobrevibacter were similarly reported26 or shown (see Supplementary material online, Table S16) to be associated with lower triglyceride levels (ρ = −0.23, P = 5.10 × 10−5). Moreover, many of the exact same Ruminococcaceae as reported in this study were associated with Christensenellaceae and Methanobrevibacter in the validation cohorts, and in the METSIM cohort they were also reported to be depleted in the pre-diabetic subjects.
3.7. Ethnicity interaction
In order to not only identify ethnicity-specific effects as reported previously for MR and machine learning analyses, we also conducted a linear regression to identify ethnicity interactions in the relationship between microbes and triglycerides. We chose the nominally significant microbial taxa from the MR results and compared a basic linear model (microbial abundance and fasting serum triglyceride concentration) with a linear model including ethnicity, and compared the two models with an ANOVA. Here, we found several bacteria that also had an ethnicity interaction, including L. pectinoschiza, D. Formicigenerans, and Acidaminococcus, in the ischaemic heart disease outcome group. In the myocardial infarction group, B. faecis, D. Longicatena, and the genus Sutterella showed significant interaction. For coronary atherosclerosis, L. pectinoschiza and C. aerofaciens had an interaction with ethnicity (see Supplementary material online, Tables S8–S10).
4. Discussion
To date, this is the first study assessing the role of single and co-abundant bacteria in relation to CVD in a multi-ethnic cohort. In our study, we show that several of the bacteria associated with the Framingham score and microbes that are potentially causally related to CVD in different ethnic groups are correlated and form the CMR cluster. The use of the CMR cluster, containing these causative bacteria, as a combined composite signal amplified the negative association between the Framingham score and elevated triglyceride incidence, which was replicated in two other cohorts. The CMR cluster encompassing the bacteria found to be causatively associated with CVD was not only found to be associated with CVD risk in the African-Surinamese and Moroccan individuals but was also found to be of relevance in all Eurasian ethnicities. Furthermore, a dose-dependent effect was observed between the CMR abundance per ethnicity and the prevalence of elevated triglyceride levels in Eurasian ethnicities (Figure 5), further providing proof of the potential protective importance of this cluster.
The identified SumTn in the African-Surinamese and Moroccan groups, which is similar to the CMR cluster, has already been shown to be strongly associated with parameters of health. Christensenellaceae has previously been shown to be inversely associated with BMI and age,29,30 and supplementation of Christensenella minuta into human donor faeces introduced into germ-free mice reduced weight gain.29 A lower BMI is associated with improved metabolic parameters and a lower CVD risk.31Christensenellaceae are known to co-occur with Methanobrevibacter,29,30,32 which is based on the cross-feeding relationship between these two groups as C. minuta produces H2, which is a substrate for Methanobrevibacter smithii.30 Interestingly, various Ruminococcaceae, a family abundantly represented in the CMR cluster, are also known to ferment complex dietary carbohydrates to hydrogen and may thus in this fashion contribute to this trophic network.33 Furthermore, previous studies also observed a direct protective effect against CVD of bacteria identified herein. In the African-Surinamese group, A. muciniphila had a negative association with the Framingham score and protected causally against the ischaemic heart disease phenotype. A similar effect has been observed in apolipoprotein E-deficient mice, where A. muciniphila reversed atherosclerosis induced by a Western diet through induction of intestinal tight junction expression and subsequent attenuation of endotoxemia-induced inflammation.34
Interestingly, in our study, the A. muciniphila SumTn was inversely associated with triglyceride levels, and in the CMR cluster this association was even stronger. This is of interest, as increased triglyceride levels are a risk factor to develop CVD35 and might point towards a potential additional protective mechanism. Indeed, 4 weeks oral gavage of A. muciniphila lowered body weight, total blood cholesterol, and triglyceride levels in a hyperlipidaemic ApoE3 E3L CETP mice model.36 The results imply a mechanistic role for this species in lipid metabolism.
Using MR, we assume in our study that the genetic constitution of participants can influence gut microbiota, which in turn can have a casual effect on CVD. This concept has previously been introduced by studying the microbiome of monozygotic and dizygotic twins, where it was shown that monozygotic twins have a more similar gut microbiota compared with one another than dizygotic twins. Of additional interest is that the highest heritability based on phylogeny was seen for the families Ruminococcaceae and Lachnospiraceae, whereas Christensenellaceae was the most highly estimated heritable taxon.29 This strongly suggests that the human genetic constitution can influence the gut microbiota composition. As the genotype differs between ethnicities, genetic differences may explain observed ethnic differences in gut microbiota composition.7 In addition to this, the genotype influence on gut microbiota composition may in part explain why different bacteria were found to be of importance in different ethnicities in our study. However, age also affects gut microbiota37 and there were differences in the average age of the different ethnicities included in this study, yet this does not account for the associations found with the CMR cluster. This cluster is, for example, slightly positively correlated with age. For instance, the Asian Surinamese have the highest age on average, yet have the lowest abundance of the CMR cluster. Furthermore, despite the various age differences, the CMR cluster appears to be associated with cardiovascular health in all ethnicities except in Ghanaians. Interestingly, some of the bacteria found in our study were already identified as being related to CVDs in previous MR studies. The species Alistipes shahi and unclassified Alistipes have been shown to reduce triglyceride levels in two independent cohorts.38 This is in line with findings in our study, where A. shahi was part of the A. muciniphila SumTn, which enhanced the inverse association with triglyceride levels compared with A. muciniphila only. Furthermore, we found that Lachnospiraceae were contributing to ischaemic heart disease in African-Surinamese subjects. Lachnospiraceae have previously been shown to increase the risk of heart failure, which can be the result of ischaemic heart disease.39 This suggests that previously identified bacteria can be part of a relevant bacterial cluster that could affect CVD events.
A limitation of the study was the use of the Framingham score as a surrogate marker for CVD based on clinical parameters in the HELIUS cohort, as overt CVD cases were not available in this relatively young cohort. In addition, the Framingham score is not validated in all ethnic groups we tested, which might influence found differences. The combination of outcomes from the Pan-UKBB however made it possible to infer potential causality of several bacteria on hard CVD outcomes. The multi-ethnic design of the HELIUS study improves the representation of findings. Furthermore, the exact same methods were used to process all samples, which resulted in reliable data. Matched ethnicities between the UKBBand the HELIUS cohort were relatively similar, although not exactly equal, and may introduce some bias. Another limitation of this study includes possible horizontal pleiotropy, which is indicated by some sensitivity analyses. Horizontal pleiotropy can be a problem in MR studies, especially if the studied population is relatively small compared with other GWAS, if study results rely only on MR, and if there is no biological mechanisms of observed results.40 This must be considered, and could be due to low statistical power. In addition to this, testing for interactions between ethnicities provided varying results, which also may be due to the sample size, as a higher sample size is necessary for interaction estimation. However, our analyses are based on the biological hypothesis that the host genetic makeup influences gut microbiota composition and that gut microbiota derived metabolites or interactions with lipid absorption can affect host metabolism. In addition to this, we did not rely on the MR only, but support our hypothesis by a machine learning approach and conventional statistical analyses. Most machine learning models in our study showed low RMSE values and low P-values, indicating an overall good fit of the models. RMSE was the highest in the Ghanaian and Turkish groups and the lowest in the total and Moroccan groups, suggesting that the fit in these models was the best. However, RMSE may also be affected by sample size, which limits generalization of interaction effects by ethnicity. In addition to this, we were able to successfully replicate the CMR cluster in two independent external cohorts and replicate the inverse association of the CMR cluster with triglycerides, supporting our hypothesis that this cluster may have cardio-protective effects. This may be the reason why Christensenellaceae and Methanobrevibacter are so commonly found to be more abundant in centenarians.41–44 In our study, we used summary statistics of two GWAS that were both corrected for common covariates such as age, sex, and BMI.16,45 However, recent research has indicated that correction for covariates in GWAS for MR studies can introduce bias.46 The results of this study must therefore be considered in light of potential bias due to the use of covariate-adjusted summary statistics. Future studies should avoid correcting for covariates or publish uncorrected summary statistics along with corrected summary statistics to limit the risk of bias.
In this study we demonstrated that ethnic-specific gut microbiota profiles can estimate CVD risk, and certain gut microbes may have a causal relation to CVD events. However, by switching from a single microbe to a trophic network approach, stronger associations with CVD risk in all but the Ghanaian ethnicity were observed. The CMR trophic network appears to represent a relevant estimator of CVD risk in nearly all ethnicities, though the link with human genetics remains equally relevant as microbes in the CMR cluster are strongly associated with human genetics, as determined using twin and GWA studies.29,32 The dose-dependent relationship of elevated triglyceride prevalence and the CMR cluster in the Eurasian ethnicities highlight the importance of gut microbiome differences between the studied ethnicities. Species encompassed by a trophic network together represent a much larger fraction of the entire gut microbiome than single species, and can hence theoretically more significantly influence host metabolism. Future studies should focus on the metabolic functions in vivo and in vitro of the CMR cluster in relation to host metabolism in order to better understand the interaction between host genetics, gut microbiota, and host CVD risk.
Supplementary Material
Acknowledgements
We are most grateful to the participants of the HELIUS study and the management team, research nurses, interviewers, research assistants, and other staff who have taken part in gathering the data for this study. We would like to acknowledge V. Tremaroli, R. Jakubowicz, and M. Krämer for DNA extraction, PCR amplification, and sequencing. We thank the AMC Biobank for sample storage support. We thank L. F. Reeskamp for statistical support and A. Prodan for the introduction to machine learning methods. The graphical abstract and Figure 1 have been created by images from Flaticon.com.
Contributor Information
Moritz V Warmbrunn, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Gastroenterology Endocrinology Metabolism (AGEM) Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Ulrika Boulund, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Gastroenterology Endocrinology Metabolism (AGEM) Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Judith Aron-Wisnewsky, Nutrition and Obesities: Systemic Approaches Research Unit (Nutriomics), Sorbonne Université, Institut National de la Santé et de la Recherche Médicale, Paris, France; Nutrition Department, Assistantea Publique Hôpitaux de Paris, Pitié-Salpêtrière Hospital, Centres de Recherche en Nutrition Humaine, Paris, Ile de France, France.
Marcus C de Goffau, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; HorAIzon BV, 2625 GZ Delft, The Netherlands; Tytgat Institute for Liver and Intestinal Research, Amsterdam University Medical Centers, Meibergdreef 69, 1105 BK Amsterdam, The Netherlands.
Rosamel E Abeka, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Mark Davids, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Lucas R F Bresser, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; HorAIzon BV, 2625 GZ Delft, The Netherlands.
Evgeni Levin, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; HorAIzon BV, 2625 GZ Delft, The Netherlands.
Karine Clement, Nutrition and Obesities: Systemic Approaches Research Unit (Nutriomics), Sorbonne Université, Institut National de la Santé et de la Recherche Médicale, Paris, France; Nutrition Department, Assistantea Publique Hôpitaux de Paris, Pitié-Salpêtrière Hospital, Centres de Recherche en Nutrition Humaine, Paris, Ile de France, France.
Henrike Galenkamp, Department of Public Health, Amsterdam UMC, University of Amsterdam, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Bart Ferwerda, Department of Clinical Epidemiology and Biostatistics, Amsterdam University Medical Centers, Amsterdam, The Netherlands.
Bert-Jan J H van den Born, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Alexander Kurilshikov, Department of Pediatrics, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Jingyuan Fu, Department of Genetics, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Aeilko H Zwinderman, Department of Public Health, Amsterdam UMC, University of Amsterdam, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Maarten R Soeters, Department of Endocrinology and Metabolism, Internal Medicine, Amsterdam University Medical Centers, Amsterdam, The Netherlands.
Daniel H van Raalte, Department of Internal Medicine, Amsterdam University Medical Center (UMC), Vrije Universiteit (VU) University Medical Center, Amsterdam, The Netherlands.
Hilde Herrema, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Albert K Groen, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Max Nieuwdorp, Department of Internal and Vascular Medicine, Amsterdam University Medical Centers, Location AMC, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Author contributions
Study conceptualization: M.V.W., U.B., J.A.-W., D.H.v.R., A.K.G., M.N. Statistical analysis, data interpretation: M.V.W., U.B., M.C.de.G., R.E.A., E.L., L.R.F.B. Data curation and handling: H.H., K.C., H.G., B.F., B.J.H.v.d.B., A.K., J.F., A.H.Z.. Manuscript drafting: M.V.W., U.B., D.H.v.R., H.H., A.K.G., M.N., M.R.S., J.F. Funding: A.K.G., M.N.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Netherlands Cardiovascular Research Committee (CVON) IN-CONTROL-II grant (2018-27). M.N. is supported by a ZonMw Vici grant 2020 (09150182010020) and a Le Ducq foundation grant 17CVD01. H.H. is supported by a Senior Fellowship of the Dutch Diabetes Research Foundation (2019.82.004). J.F. is supported by the Dutch Heart Foundation IN-CONTROL (CVON2018-27), the ERC Consolidator grant (grant agreement no. 101001678), NWO-VICI grant VI.C.202.022, the AMMODO Science Award 2023 for Biomedical Sciences from Stichting Ammodo, and the Netherlands Organ-on-Chip Initiative, an NWO Gravitation project (024.003.001) funded by the Ministry of Education, Culture, and Science of the government of The Netherlands. The HELIUS study is conducted by the Amsterdam University Medical Center, location AMC, and the Public Health Service of Amsterdam. Both organizations provided core support for HELIUS. The HELIUS study is also funded by the Dutch Heart Foundation (K. Stronks, 2010T084), the Netherlands Organization for Health Research and Development (ZonMw: K. Stronks, 200500003), the European Union (FP-7, K. Stronks, 278901), and the European Fund for the Integration of non-EU immigrants (EIF, K. Stronks, 2013EIF013). U.B. is supported by European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 813781.
References
- 1. Dahlof B. Cardiovascular disease risk factors: epidemiology and risk assessment. Am J Cardiol 2010;105:3A–9A. [DOI] [PubMed] [Google Scholar]
- 2. Verhaar BJH, Collard D, Prodan A, Levels JHM, Zwinderman AH, Backhed F, Vogt L, Peters MJL, Muller M, Nieuwdorp M, van den Born BH. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur Heart J 2020;41:4259–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matey-Hernandez ML, Williams FMK, Potter T, Valdes AM, Spector TD, Menni C. Genetic and microbiome influence on lipid metabolism and dyslipidemia. Physiol Genomics 2018;50:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerard C, Vidal H. Impact of gut microbiota on host glycemic control. Front Endocrinol (Lausanne) 2019;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, Tremaroli V, Bakker GJ, Attaye I, Pinto-Sietsma SJ, van Raalte DH, Snijder MB, Nicolaou M, Peters R, Zwinderman AH, Backhed F, Nieuwdorp M. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med 2018;24:1526–1531. [DOI] [PubMed] [Google Scholar]
- 8. Khachatryan ZA, Ktsoyan ZA, Manukyan GP, Kelly D, Ghazaryan KA, Aminov RI. Predominant role of host genetics in controlling the composition of gut microbiota. PLoS One 2008;3:e3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, Zhu W, Sartor RB, Boedeker EC, Harpaz N, Pace NR, Li E. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis 2011;17:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Vosa U, Mujagic Z, Masclee AAM, Jonkers D, Oosting M, Joosten LAB, Netea MG, Franke L, Zhernakova A, Fu J, Wijmenga C, McCarthy MI. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet 2019;51:600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groot HE, van de Vegte YJ, Verweij N, Lipsic E, Karper JC, van der Harst P. Human genetic determinants of the gut microbiome and their associations with health and disease: a phenome-wide association study. Sci Rep 2020;10:14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stronks K, Snijder MB, Peters RJ, Prins M, Schene AH, Zwinderman AH. Unravelling the impact of ethnicity on health in Europe: the HELIUS study. BMC Public Health 2013;13:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snijder MB, Galenkamp H, Prins M, Derks EM, Peters RJG, Zwinderman AH, Stronks K. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, The Netherlands. BMJ Open 2017;7:e017873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mobini R, Tremaroli V, Stahlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, Berteus Forslund H, Perkins R, Backhed F, Jansson PA. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 2017;19:579–589. [DOI] [PubMed] [Google Scholar]
- 15. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013;79:5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boulund U, Bastos DM, Ferwerda B, van den Born BJ, Pinto-Sietsma SJ, Galenkamp H, Levin E, Groen AK, Zwinderman AH, Nieuwdorp M. Gut microbiome associations with host genotype vary across ethnicities and potentially influence cardiometabolic traits. Cell Host Microbe 2022;30:1464–1480.e6. [DOI] [PubMed] [Google Scholar]
- 17. Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients 2020;12:2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferwerda B, Abdellaoui A, Nieuwdorp M, Zwinderman K. A genetic map of the modern urban society of Amsterdam. Front Genet 2021;12:727269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njolstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM, SCORE project group . Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 20. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pencina MJ, D’Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham heart study. Circulation 2009;119:3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 23. Meinshausen N, Buhlmann P. Stability selection. J R Stat Soc B 2010;72:417–473. [Google Scholar]
- 24. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. UKBB . Quality Control. Pan UK Biobank. 2023. https://pan.ukbb.broadinstitute.org/docs/qc/index.html#ancestry-definitions
- 26. Org E, Blum Y, Kasela S, Mehrabian M, Kuusisto J, Kangas AJ, Soininen P, Wang Z, Ala-Korpela M, Hazen SL, Laakso M, Lusis AJ. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol 2017;18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song JS, Kim JOR, Yoon SM, Kwon M-J, Ki C-S. The association between gut microbiome and hypertension varies according to enterotypes: a Korean study. Front Microbiom 2023;2:1072059. [Google Scholar]
- 28. Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut 2020;69:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell 2014;159:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruaud A, Esquivel-Elizondo S, de la Cuesta-Zuluaga J, Waters JL, Angenent LT, Youngblut ND, Ley RE. Syntrophy via interspecies H2 transfer between Christensenella and Methanobrevibacter underlies their global cooccurrence in the human gut. mBio 2020;11:e03235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, Lloyd-Jones DM. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol 2018;3:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hansen EE, Lozupone CA, Rey FE, Wu M, Guruge JL, Narra A, Goodfellow J, Zaneveld JR, McDonald DT, Goodrich JA, Heath AC, Knight R, Gordon JI. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc Natl Acad Sci U S A 2011;108:4599–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klimenko NS, Tyakht AV, Popenko AS, Vasiliev AS, Altukhov IA, Ischenko DS, Shashkova TI, Efimova DA, Nikogosov DA, Osipenko DA, Musienko SV, Selezneva KS, Baranova A, Kurilshikov AM, Toshchakov SM, Korzhenkov AA, Samarov NI, Shevchenko MA, Tepliuk AV, Alexeev DG. Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients 2018;10:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation 2016;133:2434–2446. [DOI] [PubMed] [Google Scholar]
- 35. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 36. Katiraei S, de Vries MR, Costain AH, Thiem K, Hoving LR, van Diepen JA, Smits HH, Bouter KE, Rensen PCN, Quax PHA, Nieuwdorp M, Netea MG, de Vos WM, Cani PD, Belzer C, van Dijk KW, Berbee JFP, van Harmelen V. Akkermansia muciniphila exerts lipid-lowering and immunomodulatory effects without affecting neointima formation in hyperlipidemic APOE*3-Leiden.CETP mice. Mol Nutr Food Res 2020;64:e1900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bosco N, Noti M. The aging gut microbiome and its impact on host immunity. Genes Immun 2021;22:289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu X, Tong X, Zou Y, Lin X, Zhao H, Tian L, Jie Z, Wang Q, Zhang Z, Lu H, Xiao L, Qiu X, Zi J, Wang R, Xu X, Yang H, Wang J, Zong Y, Liu W, Hou Y, Zhu S, Jia H, Zhang T. Mendelian randomization analyses support causal relationships between blood metabolites and the gut microbiome. Nat Genet 2022;54:52–61. [DOI] [PubMed] [Google Scholar]
- 39. Dai H, Hou T, Wang Q, Hou Y, Wang T, Zheng J, Lin H, Zhao Z, Li M, Wang S, Zhang D, Dai M, Zheng R, Lu J, Xu Y, Chen Y, Ning G, Wang W, Bi Y, Xu M. Causal relationships between the gut microbiome, blood lipids, and heart failure: a Mendelian randomization analysis. Eur J Prev Cardiol 2023;30:1274–1282. [DOI] [PubMed] [Google Scholar]
- 40. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology 2017;28:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li C, Luan Z, Zhao Y, Chen J, Yang Y, Wang C, Jing Y, Qi S, Li Z, Guo H, Xu W, Zhao B, Wu C, Wang S, Yang Y, Sun G. Deep insights into the gut microbial community of extreme longevity in south Chinese centenarians by ultra-deep metagenomics and large-scale culturomics. NPJ Biofilms Microbiomes 2022;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu L, Zeng T, Zinellu A, Rubino S, Kelvin DJ, Carru C. A cross-sectional study of compositional and functional profiles of gut microbiota in sardinian centenarians. mSystems 2019;4:e00325-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M, Brigidi P, Candela M. Gut microbiota and extreme longevity. Curr Biol 2016;26:1480–1485. [DOI] [PubMed] [Google Scholar]
- 44. Sepp E, Smidt I, Roop T, Stsepetova J, Koljalg S, Mikelsaar M, Soidla I, Ainsaar M, Kolk H, Vallas M, Jaagura M, Mandar R. Comparative analysis of gut microbiota in centenarians and young people: impact of eating habits and childhood living environment. Front Cell Infect Microbiol 2022;12:851404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Biobank U. Covariates. 2023. https://pan.ukbb.broadinstitute.org/docs/qc/index.html#covariates
- 46. Hartwig FP, Tilling K, Davey Smith G, Lawlor DA, Borges MC. Bias in two-sample Mendelian randomization when using heritable covariable-adjusted summary associations. Int J Epidemiol 2021;50:1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.