Abstract
Aims
Recent studies suggest that bioactive mediators called resolvins promote an active resolution of inflammation. Inflammatory signalling is involved in the development of the substrate for atrial fibrillation (AF). The aim of this study is to evaluate the effects of resolvin-D1 on atrial arrhythmogenic remodelling resulting from left ventricular (LV) dysfunction induced by myocardial infarction (MI) in rats.
Methods and results
MI was produced by left anterior descending coronary artery ligation. Intervention groups received daily intraperitoneal resolvin-D1, beginning before MI surgery (early-RvD1) or Day 7 post-MI (late-RvD1) and continued until Day 21 post-MI. AF vulnerability was evaluated by performing an electrophysiological study. Atrial conduction was analysed by using optical mapping. Fibrosis was quantified by Masson’s trichrome staining and gene expression by quantitative polymerase chain reaction and RNA sequencing. Investigators were blinded to group identity. Early-RvD1 significantly reduced MI size (17 ± 6%, vs. 39 ± 6% in vehicle-MI) and preserved LV ejection fraction; these were unaffected by late-RvD1. Transoesophageal pacing induced atrial tachyarrhythmia in 2/18 (11%) sham-operated rats, vs. 18/18 (100%) MI-only rats, in 5/18 (28%, P < 0.001 vs. MI) early-RvD1 MI rats, and in 7/12 (58%, P < 0.01) late-RvD1 MI rats. Atrial conduction velocity significantly decreased post-MI, an effect suppressed by RvD1 treatment. Both early-RvD1 and late-RvD1 limited MI-induced atrial fibrosis and prevented MI-induced increases in the atrial expression of inflammation-related and fibrosis-related biomarkers and pathways.
Conclusions
RvD1 suppressed MI-related atrial arrhythmogenic remodelling. Early-RvD1 had MI sparing and atrial remodelling suppressant effects, whereas late-RvD1 attenuated atrial remodelling and AF promotion without ventricular protection, revealing atrial-protective actions unrelated to ventricular function changes. These results point to inflammation resolution–promoting compounds as novel cardio-protective interventions with a particular interest in attenuating AF substrate development.
Keywords: Atrial fibrillation, Fibrosis, Resolvin, Myocardial infarction, Electrophysiology
Graphical Abstract
Graphical Abstract.
Effects of RvD1 on an atrial fibrillation (AF) substrate resulting from myocardial infarction (MI)–induced left ventricular (LV) dysfunction. MI is characterized by a non-contractile scar that produces LV dysfunction. Early treatment with RvD1 (pre-MI) reduces the scar area and prevents LV dysfunction, whereas later RvD1 therapy (starting 7 days post-MI) does not affect MI scar or LV dysfunction. MI and associated LV dysfunction cause increased atrial inflammatory signalling and recruitment of pro-inflammatory M1 macrophages. RvD1 therapy reduces atrial inflammatory signalling and M1 macrophage recruitment, while enhancing the presence of anti-inflammatory M2 macrophages and increasing pro-resolution signalling. MI-induced inflammatory signalling causes fibrosis and atrial conduction abnormalities that lead to an AF-maintaining substrate; these changes are prevented by RvD1 treatment.
Time for primary review: 65 days
See the editorial comment for this article ‘A new year's resolution to resolve atrial fibrillation: Resolvin D1 emerges as a powerful target against post-MI atrial remodelling’, by J.J. Velasco and F.G. Akar, https://doi.org/10.1093/cvr/cvae039.
1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia.1 Many conditions, including congenital heart disease, heart failure, obstructive pulmonary disease, and myocardial infarction (MI), are associated with increased risks of AF.1,2 Chronic inflammation contributes to AF risk factor–mediated effects3 and is associated with AF itself.4
Autacoids called specialized pro-resolving mediators (SPMs) attenuate chronic inflammation and ameliorate chronic disease processes.5,6 Resolvin-D1 [RvD1: 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (DHA)] is an endogenous SPM derived from a DHA derivative.5 Our group has previously identified gene expression changes consistent with active atrial inflammation in a rat model of MI-induced left-ventricular (LV) dysfunction and AF,7 but the potential impact of inflammation resolution promotion to prevent left atrial (LA) remodelling and AF incidence has never been tested in an LV disorder.
We hypothesized that RvD1 administration might prevent AF substrate development associated with LV dysfunction, a common clinical cause of AF. Here, we studied two paradigms: (i) RvD1 begun prior to MI (early-RvD1), as might occur in patients experiencing MI while taking chronic drug therapy and (ii) RvD1 begun 7 days post-MI (late-RvD1), to mimic drug administration following clinical presentation with recent-onset MI and/or LV dysfunction. An added value of this approach is the potential to study atrial effects free of infarct-sparing actions of RvD1, because the MI is largely mature and established by Day 7.8
2. Methods
2.1. MI and RvD1 therapy protocols
All procedures were approved by the Animal Ethics Committee of the Montreal Heart Institute, in accordance with the Canadian Council on Animal Care and the NIH Guide for the Care and Use of Laboratory Animals (protocol number: 2019-2457:2018-47-11). Adult male Wistar rats weighing 200–275 g (Charles River Laboratories, Montreal, QC, Canada) were randomized to six groups: Sham; Sham + early RvD1; Sham + late RvD1; MI; MI + early RvD1; and MI + late RvD1 (see Supplementary material online, Figure S1). Buprenorphine (0.03 mg/kg) and 2% isoflurane anaesthesia were used for left thoracotomy. MI was induced by left anterior descending coronary artery ligation. Buprenorphine (0.03 mg/kg) was administrated post-operatively to sham and MI animals. On Day 21 post-MI, echocardiography and transoesophageal electrophysiological studies (EPSs) were performed under 2% isoflurane anaesthesia. For euthanasia, while under continued anaesthesia, rats were euthanized by exsanguination. The excised heart was Langendorff-perfused for optical mapping (n = 6 rats/group). Tissues were flash-frozen and kept in liquid N2 or formalin-fixed and paraffin-embedded for subsequent analysis. Investigators performed experiments and analyses blinded to experimental groups. RvD1 was administered intraperitoneally at a dose of 2 µg/kg/day (based on previous work)9–12 from 24 h pre-MI (early-RvD1) or 7 days post-MI (late-RvD1) through Day 21.
2.2. Echocardiography
Under 2% isoflurane, echocardiograms were obtained by using a phased-array 10S probe (4.5–11.5 MHz) in a Vivid 7 Dimension system (GE Healthcare Ultrasound, Horten, Norway).13 Atrial size and volume were measured by using echocardiography 21 days post-surgery. M-mode echocardiography was used to measure LA dimensions at the end of cardiac systole (LADs) and diastole (LADd) in a parasternal long-axis view at the level of the aortic valve. LA fractional shortening (FS) was calculated as FS% = (LADs−LADd)/LADs × 100. LA area at end cardiac systole (LAAs) and diastole (LAAd) were measured with two-dimensional (2D) echocardiography in an apical four-chamber view. LA volume at end cardiac systole (LAVs) and diastole (LAVd) were obtained by a modified area length method with the Vivid-7 Dimension EchoPac system. LA fractional area change (FAC) was calculated as FAC% = (LAAs−LAAd)/LADs × 100, and LA ejection fraction (EF) was expressed as LAEF% = (LAVs−LAVd)/LAVs × 100.
The right atrial (RA) dimension at end cardiac systole (RADs) was measured in the apical four-chamber view. RA area at end cardiac systole (RAAs) and diastole (RAAd) were obtained in the same way as for LA by 2D echocardiography. RA FAC was calculated as FAC% = (RAAs−RAAd)/RADs × 100. Cardiac systole and diastole were defined by using a simultaneously recorded electrocardiogram (ECG).
Ventricular size and volume were also measured 21 days post-surgery. Parasternal long-axis views were recorded to obtain the M-mode echocardiographic measurements of LV and right ventricular (RV) anterior and posterior wall thicknesses (LVAW, LVPW, RVAW, and RVPW) and dimensions (LVD and RVD) at diastole and systole. The M-mode was also used to measure LV and RV ejection time, EF, and FS. MI mostly perturbed the LV function, while RV was not significantly affected; hence, we predominantly show data testifying of the LV structural and functional remodelling.
2.3. Transoesophageal stimulation
EPS was performed with a 4F quadripolar catheter (2 mm inter-polar distance; St. Jude Medical #401993). Twelve 50 Hz 3 s pacing bursts were applied (4× threshold current, 2 ms pulse width), separated by 2 s rest periods.14 AF was defined as a rapid irregular atrial rhythm (>800 b.p.m.), and atrial flutter (AFl) was defined as a regular atrial tachyarrhythmia at a cycle length (CL) between 600 and 800 b.p.m. AF duration was calculated as the mean duration of all induced AF episodes. Acquisition and analysis of ECG and catheter signals was performed with Iox2 software (version 2.8.0.13, EMKA technologies, Paris, France).
2.4. Optical mapping
The heart was excised and Langendorff-perfused via the aorta with Krebs solution (10 mL/min; 37°C). Blebbistatin (15 µM) was then administered and Di-4-ANEPPS (10 µM. 0.1 mL) was injected. Fluorescence signals were acquired at 1–2 kHz with a charge-coupled camera (CardioCCD, RedShirtImaging, Decatur, GA, USA) focused on a 10 × 10 mm region in the RA or LA free wall. To determine an effective refractory period (ERP), atria were paced at a basic CL (BCL) of 150 ms, with an S2 extrastimulus after every seven S1s, decrementing by 1 ms from 80 ms until failure to capture. To evaluate AF inducibility in situ, 25 Hz stimulation was performed at BCLs 300, 250, 150, 100, 80, and 60 ms. The inducible AF episodes were defined as lasting >3 s. Conduction velocity (CV) and action potential duration to 80% repolarization (APD80) were obtained with a MATLAB® algorithm as previously described.15
2.5. Histological analyses
Formalin-fixed samples were stained with Masson’s trichrome to quantify fibrous tissue content, and immunohistochemistical analyses were performed to identify CD68 (diluted 1:1000; Thermo Fisher PA5-81594) CD206 (1:750; ABCAM ab64693), CD31 (1:1000; Novus Biologicals NB100-2284), GPR32 (1:500; GeneTex GTX71225), and ALX/FPR2 (1:500; Novus Biologicals NLS1878SS). In this study involving non-tumoural cells, CD68 and CD206 were used and validated as specific markers of M1 and M2, respectively, in atrial and ventricular tissues from sham and MI rats.16–21 Images were analysed with Image Pro Premier 9.3 Software (Media Cybernetics, Rockville, MD, USA) for the detection of fibrous tissue content and automated quantification of inflammation-related cells. An observer blinded to group identity quantified and reported targeted inflammation-related cells as the number of cells/mm2. To quantify MI size, transverse and longitudinal sections were quantified as the percentage of LV tissue.
2.6. Polymerase chain reaction
LA and RA tissue samples were freshly isolated, snap-frozen in liquid N2, and homogenized in an RA1 lysis buffer. RNAs were extracted with the NucleoSpin RNA II® Kit (Macherey-Nagel GmbH & Co., Düren, Germany). mRNA concentration was assessed on Nanodrop-2000 (Thermo Fisher Scientific, Waltham, MA, USA), and 250 ng was retrotranscribed with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). A StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) was used for reverse transcriptase–polymerase chain reaction (RT–PCR). TaqMan probes were used for apoptosis-associated speck-like proteins [Asc, L-type calcium-channel (Cacn1ac), caspase-1 (Casp1), caspase-8 (Casp8), chemokine ligand-2 (Ccl2), collagen-1 alpha-1 (Col1a1), C-X-C motif chemokine ligands 1 and 2 (Cxcl1, Cxcl2), interleukin-1 beta (Il1b), interleukin-6 (Il6), potassium channel subunits (Kcna5, Kcnj2, and Kcnq1), NOD-like receptor family, pyrin domain containing-3 (Nlrp3), myosin heavy chain-beta (Myh7), and thrombospondin-1 (Thbs1)]. SYBR green primers were used for connexins 40 and 43 (Cx40 and Cx43), phospholamban (Pln), ryanodine receptor-2 (Ryr2), sodium channel alpha-subunit 5 (Scn5a), sarcoplasmic reticulum calcium ATPase-2a (Serca2a), transforming growth factor-beta 1 (Tgfb1), α-smooth muscle actin (Acta2), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh). TaqMan probes and SYBR green primer sequences (forward and reverse) are provided in Supplementary material online, Tables S1 and S2. Gene expression levels were calculated with the 2−ΔCt method, and Gapdh was the internal standard.
2.7. Digital PCR analysis on human samples
Total RNA was extracted from RA appendages (RAAs) obtained from sinus rhythm (Ctl) and chronic AF (cAF) patients using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Subsequently, 500 ng RNA was transcribed into cDNA using a reverse transcription kit (Applied Biosystems; Thermo Fisher Scientific) according to the manufacturer’s instructions. Digital PCR (dPCR) was carried out with TaqMan probes from Thermo Fisher Scientific for GPR32; Hs00265986_s1 and FPR2; Hs00265954_m1. The reactions were run on a QIAcuity Digital PCR System (Qiagen, Hilden, Germany) using a 96-well Nanoplate with 8500 partitions and the QIAcuity master mix according to the manufacturer’s instructions. The cycling programmes used for the reactions were as follows: 2 min at 50°C, followed by 10 min at 95°C, a total of 35 cycles (15 s at 95°C and 1 min at 60°C), and 30 s at 60°C. The relative mRNA levels to controls were calculated from the copies/µL values and normalized to the average of HMBS, B2M, GATA4, and GAPDH.
2.8. RNA-sequencing
The Macherey-Nagel NucleoSpin RNA II® isolation kit (Macherey-Nagel GmbH & Co.) was used to isolate mRNAs from flash-frozen atrial tissue samples. RNA concentrations were measured with an ND-2000 spectrophotometer. RNA quality was assessed on an Agilent Bioanalyzer. Messenger RNAs were further processed, and a library [NEB rRNA-depleted (HMR) stranded] was prepared and checked with the Illumina Library QC. RNA sequencing (RNA-seq) was performed on a NovaSeq 6000 PE100 system. RNA-seq analysis was performed through GenAP on Compute Canada servers. The R1 and R2 fastq files were compiled through a workflow including Trimmomatic, RNA Star, featureCounts, and DESeq2 with FastQC quality control between steps. The Gene Ontology analysis of the differentially expressed genes (fold change >1.1, P-value <0.05) was performed with KEGG through Kobas 3.0.22
2.9. Human atrial tissue
Experimental protocols were approved by the ethical review board of University Hospital Essen, Germany (no. 12-5268-BO). All studies were performed according to the principles outlined in the Declaration of Helsinki. All patients included in this study gave written informed consent to participate in this investigation. Patients (>18 years) undergoing open-heart surgery without a previous history of AF and in normal sinus rhythm at the time of surgery were compared with those in AF with a >6 month AF history (see Supplementary material online, Table S3).
2.10. Immunoblot—rat atrial tissue
Proteins were separated by electrophoresis on 4–15% sodium dodecyl sulfate polyacrylamide gels and transferred onto polyvinylidene difluoride membranes. Membranes were blocked using Tris-buffered saline (TBS) containing 0.2% (volume/volume) Tween-20 and 5% (weight/volume) non-fat milk. Primary antibodies diluted in TBS containing 0.2% Tween-20 were used to incubate the membranes overnight at 4°C. Horseradish peroxidase-conjugated was the secondary antibody. Bands were detected with enhanced chemiluminescence (ECL) on Mandel Bioflex MSI Film (Mandel Scientific, Guelph, ON, Canada). All expression data were relative to total protein on membrane stained with No-Stain™ Protein Labeling Reagent (Thermo Fisher Scientific, Carlsbad, CA, USA). The images of membranes were captured UV light transillumination. Quantity One or Image lab software (Bio-Rad, Hercules, CA, USA) was used to quantify protein levels.
2.11. Immunoblot—patient atrial tissue
RAA tissue was harvested from patients (control and cAF), following heart procurement, flash-frozen in liquid nitrogen, and stored at −80°C until further use. Frozen RAA tissue was ground to a fine powder using a ceramic mortar and pestle in liquid nitrogen. Ground tissue was homogenized in a manual glass Dounce homogenizer in an ice-cold tissue homogenization buffer containing Tris-HCl 10 mM, EDTA 1 mM, aprotinin 10 μg/L, leupeptin 2 μg/L, and pepstatin 100 μg/L. Sucrose 250 mM was added to the buffer aliquot on the day of homogenization. Protein concentration in each sample was determined using a Bradford assay. A sample buffer containing 7% beta-mercaptoethanol was used as a reducing agent, and the protein was denatured by heating at 50°C for 30 min. Then, 26 µg of protein was loaded onto Mini-PROTEAN TGX precast gradient gels (4–15%, Bio-Rad, Product #4561084). Protein was resolved onto the gel at 90 V for 2 h and transferred onto a 0.2 μm polyvinylidene difluoride (PVDF) membrane at 100 V on ice. Total membrane protein was determined by using a No-Stain Protein Labeling Reagent (Invitrogen, Product #A4449). Membranes were washed with TBS buffer containing 0.1% Tween-20 and blocked using 5% skim milk in TBS-T overnight at 4°C. Primary rabbit polyclonal antibodies for ALX/FPR2 (Invitrogen, Product #720293) and GPR32 (Thermo Fisher Scientific, Product #PA3-021) were used at 1:1000 dilution and incubated on the membrane for 90 min at room temperature. Following washing 3× for 5 min each in TBS-T, membranes were incubated with goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody at 1:3500 dilution for 60 min at room temperature. Membranes were exposed to the Pierce ECL Prime Western Blotting Substrate (Thermo Fisher Scientific, Product #32109), and a chemiluminescent signal was detected using the Bio-Rad ChemiDoc Imaging System. Protein bands of interest and total membrane protein levels were quantified by densitometry using ImageJ software (National Institutes of Health, NIH, USA). ALX/FPR2 and GPR32 protein expressions from each sample were normalized to the total membrane protein.
2.12. Drugs and reagents
Blebbistatin and di-4-ANEPPS were obtained from Cedarlane (Burlington, ON, Canada). CD31 was obtained from Novus Biologicals (Toronto, ON, Canada); CD206 antibody was obtained from Abcam (Toronto, ON, Canada); and resolvin D1 was obtained from Cayman Chemical (Ann Arbor, MI, USA). ACTA2, ASC, CACN1AC, CASP1, CASP8, CCL2, COL1a1, CXCL1, CXCL2, IL1B, IL6, KCNA5, KCNJ2, KCNQ1, NLRP3, and GAPDH qPCR probes, as well as CD68 antibody, were obtained from Thermo Fisher Scientific.
Primary antibodies for immunoblot experiments included: ALX/FPR2 (Bioss Antibodies bs-3654R), ASC (Novus Biologicals NBP1-78977), COL3A1 (Novus Biologicals NB600-594), GPR32 (Genetax GTX71225), IL6 (Thermo Fisher Scientific ARC0962), IL1β (Thermo Fisher Scientific PA5-46956), NLRP3 (Novus Biologicals NBP1-77080SS), CASPASE-1 (Santa Cruz sc-56036), and TGF-β3 (Thermo Fisher Scientific PA5-51070).
2.13. Statistical analysis
One-way or two-way analysis of variance (ANOVA) or non-paired Student’s t-tests (for comparisons of only two groups of data) were used for statistical comparisons, with post hoc Tukey or Bonferroni-corrected Student’s t-tests when ANOVA revealed significant group effects. Shapiro–Wilk tests were performed to assess distribution normality. Non-normally distributed continuous data or data for which normality could not be assessed were compared using Mann–Whitney U tests. Fisher exact test was applied for categorical variables like AF inducibility, and human categorical data were reported in Supplementary material online, Table S3. Results are expressed as mean ± SEM. Statistical significance was defined as two-tailed P-values <0.05.
3. Results
3.1. Effect of RvD1 on MI-induced AF vulnerability and cardiac structural/functional remodelling
MI rats had increased R-R, P-R, P-wave, QRS, and QT measurements (see Supplementary material online, Figure S2). Early-RvD1, but not late-RvD1, attenuated MI-induced changes in P wave and QT durations. Transoesophageal stimulation (see Supplementary material online, Figure S3) induced AF in 2/18 (11%) Sham rats and 18/18 (100%) MI-only rats (Figure 1A). AF could not be induced in any of the 18 Sham + early-RvD1–treated and 12 Sham + late-RvD1–treated animals. Tachystimulation did not influence R-R intervals measured at rest, during the first second of normal rhythm recorded after tachypacing vs. the last second of the EPS session recording. (see Supplementary material online, Figure S4). Both early-RvD1 and late-RvD1 treatments significantly decreased MI-induced AF inducibility (Figure 1A). AF duration was highly variable in MI animals, and while mean duration was quantitatively shorter in RvD1-treated MI rats, statistical comparisons were limited because there were only two inducible animals in the Sham group (see Supplementary material online, Figure S5A).
Figure 1.
AF vulnerability, cardiac function, and atrial fibrosis. (A) Inducibility of AF and AFl during transoesophageal EPS in vivo. (B) Scar area on transverse sections 5 mm from the apex. (C) LVEF and (D) WMSI. (E–G). Fibrosis and atrial diameters (by echocardiography) for right (E and G) and left (F and H) atria. (Statistical analysis: one-way ANOVA followed by Bonferroni correction. Each point results from an individual animal.) The horizontal lines are mean ± SEM. n = 6 rats/group.
Compared with MI-only rats, infarct area was significantly reduced in early-RvD1, but not in late-RvD1, rats (Figure 1B; Supplementary material online, Figure S5B–E).
MI significantly decreased LVEF and increased wall motion score index (WMSI); early-RvD1, but not late-RvD1, significantly attenuated MI-induced reductions in these variables (Figure 1C and D). Compared with the Sham group, MI-only rats showed significant changes LV dimensions and FS, as well as LA dimensions (see Supplementary material online, Figures S6 and S7). Early-RvD1, but not late-RvD1, significantly attenuated most of the LV changes (see Supplementary material online, Figures S6 and S7).
Atrial and ventricular pressures in vivo are shown in Supplementary material online, Figures S8 and S9. MI significantly decreased LV and RV systolic pressure, while increasing LA pressure. Early-RvD1, but not late-RvD1 significantly attenuated MI-induced haemodynamic changes in LV and LA (see Supplementary material online, Figure S9A and C).
To evaluate post-MI neovascularization, we analysed the immunohistochemical expression of the endothelial cell marker CD31 in the infarct zone in MI rats with and without RvD1 therapy (see Supplementary material online, Figure S10). Early-RvD1 rats, and to a lesser extent late-RvD1 rats, showed increased endothelial cell marker expression, suggesting increased neovascularization and/or preserved vasculature (see Supplementary material online, Figure S10A and C).
3.2. Effect of RvD1 on MI-induced atrial fibrosis
RA and LA fibrous tissue contents were significantly increased in MI vs. Sham rats (Figure 1E and F; Supplementary material online, Figure S11A and B). Early-RvD1 and late-RvD1 treatments attenuated MI-induced LA fibrosis (Figure 1E and F). MI significantly increased LA, but not RA, dimensions; RvD1 did not prevent LA dilation (Figure 1G and H).
3.3. Optical mapping in situ
Programmed in situ atrial burst pacing (see Supplementary material online, Figure S12A) showed that MI-only rats had high AF inducibility (6/6 rats) vs. Sham rats (1/6 rats; Figure 2A). MI-only rats showed significantly reduced CV vs. Sham rats (Figure 2B and C; Supplementary material online, Figure S12B). LA ERP (Figure 2D) and APD (see Supplementary material online, Figure S12C) were significantly abbreviated in MI-only rats. Two MI-only rats developed stable rotors around the lines of conduction disturbance in LA during AF (see Supplementary material online, Figures S12A and S13). In the RA, CV (see Supplementary material online, Figure S14A), APD (see Supplementary material online, Figure S14B), and ERP (see Supplementary material online, Figure S14C) were also significantly decreased in MI-only rats.
Figure 2.
Atrial optical mapping and conduction-related genes. (A) AF induction by in situ EPS of the LA. (B) Representative LA activation maps at BCL 60 ms. (C) LA CV at BCL 60 ms. (D) LA ERP in MI, Sham, and RvD1-treated rats. (Statistical analysis: (A) Fisher’s exact test. (C) and (D) One-way ANOVA followed by Bonferroni correction.) Each point results from an individual animal. The horizontal lines are mean ± SEM. n = 6 rats/group.
Enhanced AF vulnerability was also seen during RA EPS (see Supplementary material online, Figure S14D) for MI-rats (5/6 rats) vs. Sham rats (2/6; Supplementary material online, Figure S14E). AF duration was increased by MI, but statistical comparisons were limited by the small numbers of inducible rats in the Sham and MI RvD1-treated groups (see Supplementary material online, Figure S14F).
Early-RvD1 and late-RvD1 attenuated MI-induced electrophysiological remodelling in terms of CV, ERP, APD, AF inducibility, and AF duration (Figure 2A–D; see Supplementary material online, Figures S12 and S14).
Note that during in situ EPSs, no significant changes were observed in terms of baseline atrial rate, when comparing the atrial ECGs among experimental groups (see Supplementary material online, Figure S15).
3.4. RvD1 effects on the expression of conduction and Ca2+-handling–related genes
The atrial expressions of selected genes involved in arrhythmogenic electrical remodelling including connexins (Cx40 and Cx43), calcium handling (Ryr2, Scn5a, Serca, and Plb), and ion channels (Kcna5, Kcnq1, Kcnj2, and Cacn1ac) were evaluated by quantitative PCR (qPCR) (see Supplementary material online, Figures S11C–F and S16). Compared with Sham rats, MI-only rats showed significantly increased expression of Cx40 (see Supplementary material online, Figure S11C) and Kcna5 (see Supplementary material online, Figure S16A) in LA, with decreased LA expression of Cacn1ac (see Supplementary material online, Figure S16D), and Ryr2, Scn5a, (see Supplementary material online, Figure S11E and F) Serca2a, and Plb (see Supplementary material online, Figure S16F and H) in RA and LA, respectively. MI did not change the expression of Kcnq1 and Kcnj2 (see Supplementary material online, Figure S16B and C). Early-RvD1 and late-RvD1 treatment prevented MI-induced changes and moved the expression of these genes towards sham values (see Supplementary material online, Figures S11C–F and S16).
3.5. Effect of RvD1 on inflammation-related genes/cells
The immunohistochemistry of anti-CD68 and anti-CD206 was performed to quantify M1 and M2 macrophages, in LA (Figure 3A and B; see Supplementary material online, Figure S17A), RA (Figure 2C; see Supplementary material online, Figure S17B), and LV tissue (Figure 3D; see Supplementary material online, Figure S18). MI-only rats showed significantly increased M1 macrophage content in LA, RA, and LV vs. Sham rats. Early-RvD1 attenuated MI-induced M1 macrophage recruitment while increasing M2 macrophage content in LA, RA, and LV (Figure 3; see Supplementary material online, Figures S17 and S18). Late-RvD1 significantly increased M2 macrophage expression vs. MI-only rats in LA and LV and attenuated M1 macrophage increases in LV. The inducible nitric oxide synthase (iNOS) has been described as a functional marker of the M1 phenotype.23 Pro-inflammatory macrophages, expressing iNOS, were significantly increased in LA and RA from MI rats compared with Sham rats (see Supplementary material online, Figure S19A, B, and E). The number of iNOS-positive cells significantly diseased in the LA and RA of RvD1-treated rats. The number of neutrophil marker myeloperoxidase staining cells was small and did not significantly vary between groups, in RA and LA tissues (see Supplementary material online, Figure S19C, D, and F).
Figure 3.
Macrophage expression. Immunohistochemical staining for pro-inflammatory (M1) macrophage marker CD68 and anti-inflammatory (M2) macrophage marker CD206 (A). The arrows indicate immunochemically identified macrophages. (B–D) The quantification of immunohistochemical staining for LA (B), RA (C), and LV (D). The representative histological images are shown in Supplementary material online, Figures S17 and S18. (Statistical analysis: one-way ANOVA followed by Bonferroni correction.) Each point represents results from an individual animal. The horizontal lines are mean ± SEM. n = 6 rats/group.
The expression of selected inflammation-related genes was evaluated by qPCR (Figure 4). Although no significant changes were observed in the RA, MI-rats showed statistically significant up-regulation of Il6 (Figure 4A), Il1b (Figure 4B), Cxcl1 (Figure 4C), and Cxcl2 (Figure 4D) in the LA. Early-RvD1 reduced the MI-induced up-regulation of all pro-inflammatory markers. Late-RvD1 showed qualitatively similar changes to early-RvD1, with significant decreases only for Cxcl2.
Figure 4.
Inflammasome-related and fibrosis-related genes. Gene expression level evaluated by RT–qPCR analysis for inflammation-related genes Il6 (A), Il1b (B), Cxcl1 (C), and Cxcl2 (D) and for NLRP3 inflammasome components Nlrp3 (E), Asc (F), Casp1 (G), and Casp8 (H) in RA and LA from Sham, MI, and RvD1-treated rats. (Each point represents the level of expression from an individual animal. The results are expressed as mean ± SEM. Statistically significant differences were defined as two-tailed P-values <0.05; n = 6 rats/group.)
The NLRP3 inflammasome-related genes, Nlrp3, Asc, Casp1, and Casp8, were increased in MI-only rats vs. Sham rats in RA and LA, with significant changes for all but Asc in the RA (Figure 4E–H). Early-RvD1 and late-RvD1 attenuated the MI-induced up-regulation of NLRP3 inflammasome components.
Inflammatory cytokine Ccl2 and fibrosis-related gene expression were also studied by qPCR. Acta2, Tgfb1, and Col1a1 were significantly up-regulated in MI-only rats compared with Sham rats in the LA (see Supplementary material online, Figure S20A–D). In the RA, MI-only rats also increased the expression of these genes, with statistical significance for Ccl2, Acta2, and Col1a1. Early-RvD1 and late-RvD1 attenuated the MI-induced up-regulation of these genes, with statistically significant decreases for early-RvD1 in Ccl2, Col1a1, and Acta2 in RA and Acta2 in LA (see Supplementary material online, Figure S20A, B, and D).
3.6. Effect of RvD1 on the protein expression of inflammation resolution, fibrosis resolution, and pro-resolution biomarkers
Original immunoblots evaluating the LA expression of proteins involved in NLRP3 inflammasome (NLRP3, ASC, and PRO-CASP1), inflammation (IL6), and fibrosis (TGFβ3 and COL3A1) are illustrated in Figure 5A. Mean data are shown in Figure 5B–F. MI-only rats significantly increased the protein expression of ASC (Figure 5A and C) and PROCASP1 (Figure 5A and D), indicating the priming of NLRP3 inflammasome components and TGFβ3-monomer (Figure 5A and F) compared with Sham rats. Early-RvD1 and late-RvD1 significantly attenuated the MI-induced protein expression augmentations (Figure 5) without affecting CASP1-p12, TGFβ3-dimer, or COL3A1 (see Supplementary material online, Figure S21).
Figure 5.
Inflammation-related and fibrosis-related protein expression. A western blot analysis of the LA protein expression of NLRP3-related compounds: NLRP3 (A and B), ASC (A and C), PRO-CASP1 (A and D); proinflammatory interleukin IL6 (A and E); and fibrosis-related protein TGFβ3 (A and F). The images correspond to protein bands from gels on which they were run compared with total proteins on the blot (A). Uncropped membrane images are available in Supplementary material online, Figure S22. (Each point represents the level of expression from an individual animal. The results are expressed as mean ± SEM. Statistically significant differences were defined as two-tailed P-values <0.05; n = 6 rats/group.)
3.7. Effect of RvD1 on protein expression of RvD1 receptors
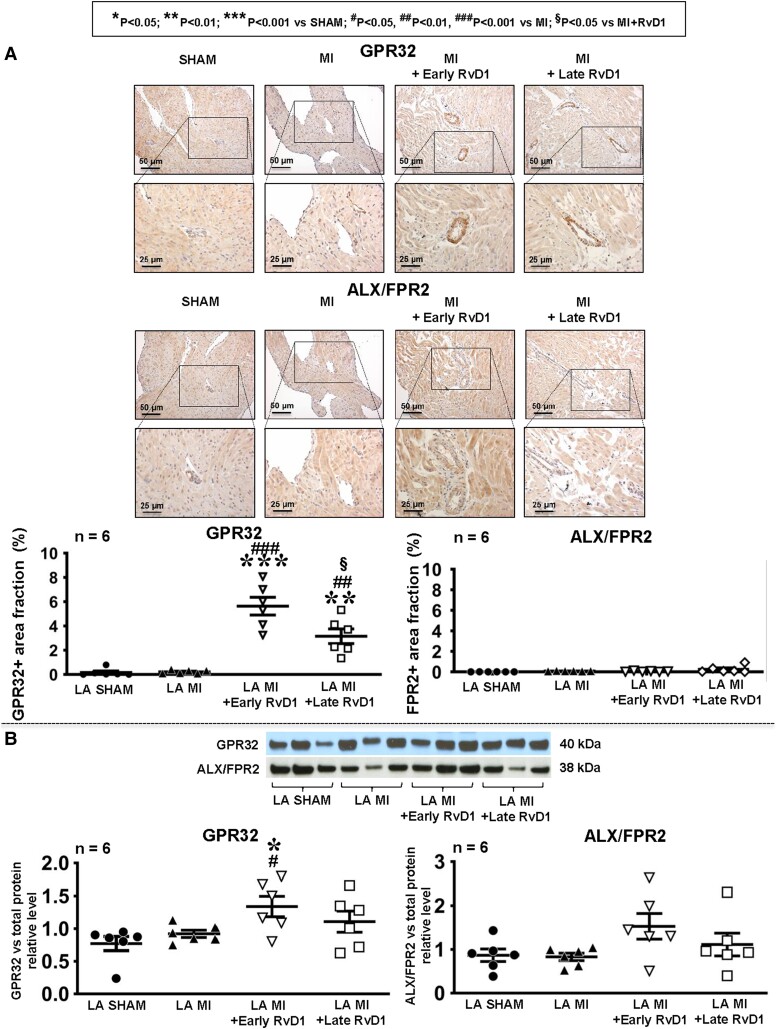
Immunohistochemistry was performed to detect the presence and expression level of RvD1 receptors GPR32 and ALX/FPR2 in LA (Figure 6A). No specific staining was seen for ALX/FRP2 under any condition. Under sham conditions and vehicle MI, no specific staining for GPR32 was noted. On the other hand, RvD1-treated MI rats showed specific staining largely located in the region of arterioles. Clear bands at the expected molecular masses were seen for ALX/FRP2 and GPR32 (see Supplementary material online, Figure S22). The GPR32 expression was not altered by MI and (unlike the immunochemistry results) MI + late RvD1. ALX/FRP2 did not show any significant differences across conditions (Figure 6B).
Figure 6.
The LA expression of RvD1 receptors. (A) Immunohistochemical staining of RvD1 receptors GPR32 and ALX/FPR2 in LA from Sham, MI, and RvD1-treated rats, 21st day post-MI. The graphs show the area fraction (cross-sectional area occupied by immunostaining relative to the total cross-sectional area as a percentage, by colorimetric analysis) of the atrial GPR32-positive and ALX/FPR2-positive zones in LA. (B) A western blot analysis of the LA protein expression of RvD1 receptors GPR32 (left panel) and ALX/FPR2 (right panel). The images correspond to protein bands from gels on which they were run. Uncropped membrane images are available in Supplementary material online, Figure S22. Total protein blot images are available in Supplementary material online, Figure S23. (Statistical analysis: one-way ANOVA followed by Bonferroni correction.) Each point represents results from an individual animal. The horizontal lines are mean ± SEM. n = 6 rats/group.
To assess the potential translational relevance of RvD1 receptors, we evaluated the GPR32 and ALX/FPR2 expressions by immunoblot in patients with AF > 6 months (cAF) compared with those in sinus rhythm with no AF history (CTRL) (Figure 7A–C) and dPCR (Figure 7D and E). The expression levels of GPR32 and ALX/FPR2 proteins were significantly decreased in cAF patients compared with CTRL. Full-lane original blots for resolvin receptors are shown in Supplementary material online, Figures S22–S24.
Figure 7.
The expression of RvD1 receptors in AF patients. A western blot analysis of the atrial protein expression of GPR32 (A and B) and ALX/FPR2 (A and C). The images correspond to protein bands from gels on which they were run compared with GAPDH. Uncropped-membrane images are available in Supplementary material online, Figure S24. The gene expressions of GPR32 (D) and ALX/FPR2 (E) were also analysed with dPCR. (Statistical analysis: Student’s t-test.) Each point represents results from an individual patient. The horizontal lines are mean ± SEM. n = 11 patients/group for immunoblot, n = 6 patients/group for dPCR.
3.8. LA RNA-seq
RNA-seq was performed on LA tissue isolated from Sham, MI, MI + early-RvD1, and MI + late-RvD1 rats (see Supplementary material online, Figure S25). Among the top 10 significantly up-regulated genes by MI compared with Sham, four were related to pro-inflammatory activity (Plekho2, Chrdl2, Tmem135, and Kdm6b). In contrast, three genes associated with inflammation resolution or inflammation termination process (Lair1, Syne1, and Mlip) were among the top 10 significantly down-regulated genes by MI compared with Sham (see Supplementary material online, Figure S25A). Compared with MI-only rats, MI with early-RvD1 treatment showed significantly up-regulated genes involved in inflammation resolution and the cessation of inflammation (Cyp11a1, Lair1, and Anxa4), with significant down-regulation of Adamtsl2 and Setd4, involved in inflammation (see Supplementary material online, Figure S25B). Late-RvD1 treatment compared with MI-only rats led to significantly increased expression of Lair1 and significant down-regulation of inflammation-associated genes Chrdl2, Plekho2, Mmp1b, and Mmp1 (see Supplementary material online, Figure S25C).
A Venn diagram showing all dysregulated genes with fold change >2, in the LA from MI, MI + early-RvD1, and MI + late-RvD1 compared with Sham, is provided in Supplementary material online, Figure S26. The diagram shows that 92 genes were down-regulated and 82 genes were up-regulated in MI-only rats compared with Sham rats. RvD1 attenuated the MI-induced gene expression differences compared with Sham rats. In MI + early-RvD1, only 11 genes (2 down-regulated and 9 up-regulated) were significantly dysregulated (vs. 174 for MI-only) compared with Sham. In MI + late-RvD1, 42 genes were down-regulated and 17 up-regulated compared with Sham. The genes involved in suppressing inflammation, including Apln, Arid5b, Gtf3a, Lair1, Mlip, Ncoa2, Prdx4, Selenok, Timd4, Tmem59, and Tmem74, were down-regulated in MI-only vs. only four (Bln2, Plekha8, Sh2d1b2, and Txndc17) in MI + late-RvD1 and none in MI + early RvD1 (see Supplementary material online, Figure S26A). A large number of genes up-regulated in MI-only rats are involved in inflammation/fibrosis promotion (red font in Supplementary material online, Figure S26B) and cellular remodelling (black font). RvD1 suppressed the up-regulation of these genes (see Supplementary material online, Figure S26B).
Overall, compared with Sham, MI-only was associated with dysregulation of 83 pathways, including 26 involved in inflammation signalling (see Supplementary material online, Figure S27). MI-only rats treated with early-RvD1 showed five dysregulated pathways compared with Sham rats, among which three pathways associated with pro-resolution or inflammation termination processes were affected by early-RvD1. Compared with Sham rats, MI rats treated with late-RvD1 showed 25 dysregulated pathways, mainly reflecting the persistence of contractile and cellular abnormalities but no proinflammatory profibrotic pathways, and the prominent pro-resolution linoleic acid pathway was activated (see Supplementary material online, Figure S27).
4. Discussion
4.1. Main findings
Here, we found that RvD1 therapy attenuated the AF substrate caused by MI-induced LV dysfunction. Early-RvD1 showed global cardio-protective properties, reducing MI size attenuation, preserving LV function preservation, and preventing atrial cardiomyopathy. Despite significant atrial remodelling attenuation and AF prevention, late-RvD1, administered 7 days post-MI when the infarct is relatively mature, did not reduce MI size or prevent LV dysfunction. Both RvD1 regimens prevented pro-inflammatory macrophage polarization while attenuating the overexpression of pro-inflammatory genes/pathways and enhancing pro-resolution signalling (Graphical Abstract).
4.2. Inflammation resolution–promoting interventions and AF
There is accumulating evidence for the role of unresolved inflammation and the beneficial effects of the promotion of resolution in heart disease.24 The strongest evidence is in MI, a pathology involving extensive tissue necrosis and active inflammation culminating in wound healing.25,26 Our observation of important cardioprotection and infarct size limitation with early-RvD1 administration is consistent with these observations.26 There are also data pointing to the role of non-resolved inflammation in heart failure with preserved EF.27
In AF, the only prior study to address the effects of resolution promotion in AF was our investigation of right heart disease, in which we found beneficial effects on the AF substrate occurring in the RA.28 The present work was designed to establish the effects of the SPM on left heart disease (LHD), the most common cause of AF, with the use of the lead compound RvD1. Our observations indicate substantial protection from SPMs in LHD, which includes not only substantial cardio-protective actions but also an important prevention of AF substrate development even with unchanged ventricular function with late-RvD1.
4.3. Myocardial inflammation, AF, and the role of resolution promotion
Inflammation has been recognized to be a pathophysiological component of AF for at least 20 years.29 Work in this area has recently intensified,30–32 particularly with the emerging evidence about the role of cardiomyocyte-mediated NLRP3 inflammasome activation in clinical AF, including paroxysmal, persistent, and post-operative forms33,34 as well as in atria from patients who have Type-2 diabetes mellitus or obesity and an increased risk of AF.35,36 In the present study, we saw evidence of enhanced LA NLRP3 inflammasome priming in MI based on increased gene and protein expressions of ASC and procaspase-1, which were suppressed by RvD1 (Figure 5). Furthermore, RNA-sequ data suggested increased pro-inflammatory gene and pathway expressions and reduced pro-resolution gene expression in MI, patterns that were reversed with RvD1 treatment (see Supplementary material online, Figures S25–S27). These results are consistent with previous work, which showed that genes like Lair1, Syne1, and Mlip,37–40 which promote inflammation resolution, are up-regulated by SPMs. RvD1 therapy also changed macrophage polarization to an anti-inflammatory phenotype, by reducing the presence of pro-inflammatory M1 macrophages and enhancing the presence of anti-inflammatory M2 macrophages (Figure 3). Our results provide further evidence for the role of inflammation in AF and open the door to potential novel therapeutic interventions that target AF by acting against atrial inflammatory signalling.
4.4. The translational and clinical context
AF is extremely common, and its prevalence is increasing, expected to rise by 67% by 2050.41 Recent work suggests that early and aggressive AF management can reduce long-term risks and complications, highlighting the importance of effective rhythm control management.42 The repertoire of available therapeutic approaches is limited, with both anti-arrhythmic drug therapy and ablation approaches having significant limitations.43 Novel options are needed in order to safely and effectively target mechanisms that have not previously been addressed. The finding that SPMs are an effective intervention in preventing the development of the AF substrate in the context of LV dysfunction identifies a promising new avenue for further exploration. Although our study showed no evident LV-sparing effects of late-RvD1, there was a clear and statistically significant effect of early-RvD1 treatment (Figure 1B–D). However, both late-RvD1 and early-RvD1 significantly decreased atrial arrhythmogenic remodelling, compared with the MI-only condition (Figure 1A and F). These data highlight the importance of the time-dependent effect of SPM supplementation following injury,44 while emphasizing the lack of reversibility of LV changes once the MI is mature.45,46 They also indicate that LA changes secondary to MI-induced LV dysfunction may be attenuated despite apparently irreversible ventricular damage and persistent LV dysfunction.
More work is needed to address the mechanisms underlying the effectiveness of RvD1 in our models. If the resolution of atrial inflammation is incomplete post-MI, even when LV function is preserved, it would be important to clarify the reasons why resolution fails and to pinpoint the specific pathways involved. More detailed studies are needed of the specific biochemical pathways involved in inflammation and inflammation resolution,31 to pinpoint any specific deficiencies that are present. While the development of resolvins as potential therapeutic agents is rooted in the concept of inflammation resolution, RvD1 clearly has substantial anti-inflammatory properties, and the involvement of pathways not specifically linked to inflammation resolution per se should be considered and investigated, particularly if specific defects in resolution pathways prove to be elusive.
Further study is also needed to assess the receptor systems and downstream signalling that mediate RvD1 effects. Interestingly, we were able to identify the putative RvD1 receptors GPR32 and ALX/FPR32 in humans and found that they are down-regulated at the protein level in AF patients (Figure 7). This observation raises the possibility that RvD1 receptor down-regulation in AF might contribute to the enhanced inflammatory signalling that is involved in clinical AF.33,34 We found that RvD1-treated rats showed the up-regulation of GPR32 (Figure 6), which could possibly contribute to its beneficial actions.
4.5. Limitations
This study did not determine the time course of the evolution of the atrial and ventricular inflammatory and fibrotic profiles following MI and early-RvD1 or late-RvD1 intervention. Such studies were out of the scope of the present investigation but might be an interesting approach to further understand the cardio-protective action of RvD1. Future investigations should also focus on the mechanistic origin of the atrial inflammatory profile. MI-induced LV dysfunction leads to LA pressure and volume loading, and the resulting sustained increase in wall stress might induce atrial inflammation. Although it is known that MI leads to LA volume loading and that this abnormal chronic stress can lead to atrial inflammation, the mechanistic basis and the specific cell types involved in transducing this effect are unknown. In addition, paracrine and bloodborne factors engaged by MI and its haemodynamic consequences might have contributed to the observed atrial inflammatory status. We have not examined the detailed biochemical and cellular signatures of active inflammation resolution per se, which would be needed to differentiate a simple reduction in inflammatory signalling from the one caused by an active resolution process.
Our work was limited to a rat model, and observations in further species, particularly large animals, would be of interest. Further studies of RvD1 in other pre-clinical models of AF would similarly be relevant.
We believe that the atrial conduction changes that we observed in this study were due primarily to atrial fibrosis, well established to cause conduction abnormalities.47 However, we cannot exclude a contribution of changes in other factors affecting conduction, such as alterations in Na+-current or connexins. We noted the LA APD abbreviation after MI, which was abrogated by RvD1 treatment (see Supplementary material online, Figure S12). A full exploration of the basis for these changes would require detailed ion current and transporter patch clamp and protein expression studies that go beyond the scope of the present manuscript.
We found that previously identified RvD1 receptors are expressed in the human atrium and are down-regulated in cAF patients (Figure 7). In the rat, receptor expression levels were low in sham animals and not altered by MI, while GRP32 was up-regulated by RvD1 treatment in the presence of MI (Figure 6). Much work remains to be done to clarify the molecular mechanisms responsible for RvD1 actions in the atrium; this will require a detailed exploration that goes beyond the scope of the present study.
5. Conclusions
Our results show that RvD1, an endogenous SPM, reduces atrial electrical remodelling resulting from LV dysfunction. RvD1 attenuates cardiac inflammatory signalling and promotes macrophage polarization away from an inflammatory phenotype, while reducing fibrosis and attenuating AF vulnerability. Resolvin-based intervention promoting the resolution of inflammation is a potential therapeutic strategy to prevent the development or progression of the atrial arrhythmogenic substrate associated with LV dysfunction.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
R.H. designed the work with S.N., performed all animal experiments, analysed the results, prepared figures, and wrote the paper. F.X. performed the experiments and analysed the data related to optical mapping. P.N. performed the experiments and analysed the data related to qPCR and RNA sequencing. J.X. and D.K.S. performed the experiments and analysed the data related to western blots in rat atria. E.L.Q. performed the experiments and analysed the data related to immunohistochemistry. A.S. and I.H.A.-T. performed the experiments and analysed the data related to western blots in human atria. M.K. performed human surgeries and provided human atrial tissue. C-A.L. performed sham and MI surgeries in rats and provided post-operative injections and animals’ healthcare. D.G.F.A. performed the blind haemodynamic pressure measurements and analyses. M.G.S. co-supervised the experiments and blind analyses of histological experiments with J.-F.T. T.H. guided the experiments and data analysis related to immunohistochemistry. J.-F.T. co-supervised the experiments and blind analyses of histological experiments with M.G.S. J.-C.T. supervised the experiments and blind analyses of echocardiography. D.D. supervised the experiments and blind analyses of dPCR and western blots in the human atria. S.N. conceptualized this study, provided funding for the experiments, designed the work in partnership with R.H., supervised the experiments and research group, wrote the manuscript, validated figures, and approved the final version of the article.
Supplementary Material
Acknowledgements
The authors are grateful to Nathalie L’Heureux, Chantal St-Cyr, Marie-Hélène Clavet, Billie Chouinard, Colombe Roy, Joanne Vincent, Simone Olesch, Ramona Löcker, Dr. YanFen Shi, Marie-Ève Higgins, and the animal care facility of the Montreal Heart Institute for technical support; Francis LeBlanc for advice regarding the use of RNA-seq analysis software; and Lucie Lefebvre and Carlos Lobos-Yevenes for secretarial assistance.
Contributor Information
Roddy Hiram, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8.
Feng Xiong, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8; Department of Pharmacology and Therapeutics, McGill University, 3655 Prom. Sir William Osler, Montreal, Canada H3G 1Y6.
Patrice Naud, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8.
Jiening Xiao, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8.
Deanna K Sosnowski, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8; Department of Pharmacology and Therapeutics, McGill University, 3655 Prom. Sir William Osler, Montreal, Canada H3G 1Y6.
Ewen Le Quilliec, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8.
Arnela Saljic, Institute of Pharmacology, West German Heart and Vascular Center, Faculty of Medicine, University Duisburg-Essen, Hufelandstr 55, Essen, Germany D-45122; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Norregade 10 P.O. Box 2177, Copenhagen, Denmark.
Issam H Abu-Taha, Institute of Pharmacology, West German Heart and Vascular Center, Faculty of Medicine, University Duisburg-Essen, Hufelandstr 55, Essen, Germany D-45122.
Markus Kamler, Department of Thoracic and Cardiovascular Surgery, West German Heart and Vascular Center Essen, University Hospital Essen, Hufelanstr 55, Essen, Germany 45122.
Charles-Alexandre LeBlanc, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8.
Doa’a G F Al-U’Datt, Department of Physiology and Biochemistry, Faculty of Medicine, Jordan University of Science and Technology, P.O. Box 3030 Irbid, Jordan 22110.
Martin G Sirois, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8.
Terence E Hebert, Department of Pharmacology and Therapeutics, McGill University, 3655 Prom. Sir William Osler, Montreal, Canada H3G 1Y6.
Jean-François Tanguay, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8.
Jean-Claude Tardif, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8.
Dobromir Dobrev, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8; Department of Pharmacology and Therapeutics, McGill University, 3655 Prom. Sir William Osler, Montreal, Canada H3G 1Y6; Institute of Pharmacology, West German Heart and Vascular Center, Faculty of Medicine, University Duisburg-Essen, Hufelandstr 55, Essen, Germany D-45122; Department of Physiology and Biochemistry, Faculty of Medicine, Jordan University of Science and Technology, P.O. Box 3030 Irbid, Jordan 22110.
Stanley Nattel, Department of Medicine, Montreal Heart Institute (MHI), Université de Montréal, 5000 Belanger Street, Montreal, Quebec, CanadaH1T 1C8; Department of Pharmacology and Therapeutics, McGill University, 3655 Prom. Sir William Osler, Montreal, Canada H3G 1Y6; Institute of Pharmacology, West German Heart and Vascular Center, Faculty of Medicine, University Duisburg-Essen, Hufelandstr 55, Essen, Germany D-45122; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Norregade 10 P.O. Box 2177, Copenhagen, Denmark; IHU Liryc and Fondation Bordeaux Université, 166 cours de l'Argonne, Bordeaux, France 33000.
Funding
This study was supported by the Canadian Foundation for Innovation (42228), the Canadian Institutes of Health Research (16-00012708), and the Heart and Stroke Foundation of Canada (G-22-0031958).
Data availability
All raw data supporting the findings of this study are available from the corresponding authors upon reasonable request.
Translational perspective.
Our findings support the importance of inflammation in the development of chronic cardiac conditions like atrial fibrillation (AF). Preventive administration of RvD1 may attenuate atrial remodelling associated with left ventricular (LV) dysfunction. In this study, we confirmed the presence of RvD1 receptors in rat, canine, and human atrial tissue. We demonstrated that RvD1 suppresses adverse cardiac remodelling post-myocardial infarction. RvD1 influences key pathways, genes, and protein expression to promote the resolution of inflammation, reduce arrhythmogenic atrial electrical remodelling, and prevent atrial fibrosis caused by LV dysfunction. Bioactive specialized pro-resolving mediators such as RvD1 constitute a potential new therapeutic approach to the attenuation of chronic inflammation and prevention of AF.
References
- 1. Thihalolipavan S, Morin DP. Atrial fibrillation and congestive heart failure. Heart Fail Clin 2014;10:305–318. [DOI] [PubMed] [Google Scholar]
- 2. Hiram R, Naud P, Xiong F, Al-U’Datt D, Algalarrondo V, Sirois MG, Tanguay JF, Tardif JC, Nattel S. Right atrial mechanisms of atrial fibrillation in a rat model of right heart disease. J Am Coll Cardiol 2019;74:1332–1347. [DOI] [PubMed] [Google Scholar]
- 3. Harada M, Nattel S. Implications of inflammation and fibrosis in atrial fibrillation pathophysiology. Card Electrophysiol Clin 2021;13:25–35. [DOI] [PubMed] [Google Scholar]
- 4. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res 2020;127:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun 2016;7:12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cardin S, Guasch E, Luo X, Naud P, Le Quang K, Shi YF, Tardif JC, Comtois P, Nattel S. Role for microRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol 2012;5:1027–1035. [DOI] [PubMed] [Google Scholar]
- 8. Hochman JS, Bulkley BH. Expansion of acute myocardial infarction: an experimental study. Circulation 1982;65:1446–1450. [DOI] [PubMed] [Google Scholar]
- 9. Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol 2014;193:4235–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu ZZ, Ji RR. Resolvins are potent analgesics for arthritic pain. Br J Pharmacol 2011;164:274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J 2011;25:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halade GV, Kain V, Serhan CN. Immune responsive resolvin D1 programs myocardial infarction-induced cardiorenal syndrome in heart failure. FASEB J 2018;32:3717–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guichard JB, Xiong F, Qi XY, L’Heureux N, Hiram R, Xiao J, Naud P, Tardif JC, Da Costa A, Nattel S. Role of atrial arrhythmia and ventricular response in atrial fibrillation induced atrial remodelling. Cardiovasc Res 2021;117:462–471. [DOI] [PubMed] [Google Scholar]
- 14. Reil JC, Hohl M, Selejan S, Lipp P, Drautz F, Kazakow A, Münz BM, Steendijk P, Reil GH, Allessie MA, Böhm M, Neuberger HR. Aldosterone promotes atrial fibrillation. Eur Heart J 2012;33:2098–2108. [DOI] [PubMed] [Google Scholar]
- 15. Xiong F, Qi X, Nattel S, Comtois P. Wavelet analysis of cardiac optical mapping data. Comput Biol Med 2015;65:243–255. [DOI] [PubMed] [Google Scholar]
- 16. Nakagawa M, Karim MR, Izawa T, Kuwamura M, Yamate J. Immunophenotypical characterization of M1/M2 macrophages and lymphocytes in cisplatin-induced rat progressive renal fibrosis. Cells 2021;10:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015;2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biorad. Macrophage polarization: mini review. https://www.bio-rad-antibodies.com/macrophage-polarization-minireview.html.
- 19. Minami K, Hiwatashi K, Ueno S, Sakoda M, Iino S, Okumura H, Hashiguchi M, Kawasaki Y, Kurahara H, Mataki Y, Maemura K, Shinchi H, Natsugoe S. Prognostic significance of CD68, CD163 and Folate receptor-β positive macrophages in hepatocellular carcinoma. Exp Ther Med 2018;15:4465–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamoshida G, Kikuchi-Ueda T, Nishida S, Tansho-Nagakawa S, Ubagai T, Ono Y. Pathogenic bacterium Acinetobacter baumannii inhibits the formation of neutrophil extracellular traps by suppressing neutrophil adhesion. Front Immunol 2018;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY, Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 2011;39:W316–W322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palmieri EM, Gonzalez-Cotto M, Baseler WA, Davies LC, Ghesquière B, Maio N, Rice CM, Rouault TA, Cassel T, Higashi RM, Lane AN, Fan TWM, Wink DA, McVicar DW. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun 2020;11:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hiram R. Resolution-promoting autacoids demonstrate promising cardioprotective effect against heart disease. Mol Biol Rep 2022;49:5179–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chalise U, Becirovie-Agic M, Lindsey ML. Neutrophil crosstalk during cardiac wound healing after myocardial infarction. Curr Opin Physiol 2021;24:100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 2015;84:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tourki B, Halade GV. Heart failure syndrome with preserved ejection fraction is a metabolic cluster of non-resolving inflammation in obesity. Front Cardiovasc Med 2021;8:695952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hiram R, Xiong F, Naud P, Xiao J, Sirois M, Tanguay JF, Tardif JC, Nattel S. The inflammation-resolution promoting molecule resolvin-D1 prevents atrial proarrhythmic remodelling in experimental right heart disease. Cardiovasc Res 2021;117:1776–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 30. Ajoolabady A, Nattel S, Lip GYH, Ren J. Inflammasome signaling in atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol 2022;79:2349–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dobrev D, Heijman J, Hiram R, Li N, Nattel S. Inflammatory signalling in atrial cardiomyocytes: a novel unifying principle in atrial fibrillation pathophysiology. Nat Rev Cardiol 2023;20:145–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott L Jr, Li N, Dobrev D. Role of inflammatory signaling in atrial fibrillation. Int J Cardiol 2019;287:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha I, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Müller FU, El-Armouche A, Tony Eissa N, Beeton C, Nattel S, Wehrens XHT, Dobrev D, Li N. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 2018;138:2227–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heijman J, Muna AP, Veleva T, Molina CE, Sutanto H, Tekook M, Wang Q, Abu-Taha IH, Gorka M, Künzel S, El-Armouche A, Reichenspurner H, Kamler M, Nikolaev V, Ravens U, Li N, Nattel S, Wehrens XHT, Dobrev D. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ Res 2020;127:1036–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fender AC, Kleeschulte S, Stolte S, Leineweber K, Kamler M, Bode J, Li N, Dobrev D. Thrombin receptor PAR4 drives canonical NLRP3 inflammasome signaling in the heart. Basic Res Cardiol 2020;115:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott L Jr, Fender AC, Saljic A, Li L, Chen X, Wang X, Linz D, Lang J, Hohl M, Twomey D, Pham TT, Diaz-Lankenau R, Chelu MG, Kamler M, Entman ML, Taffet GE, Sanders P, Dobrev D, Li N. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc Res 2021;117:1746–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cattin ME, Wang J, Weldrick JJ, Roeske CL, Mak E, Thorn SL, DaSilva JN, Wang Y, Lusis AJ, Burgon PG. Deletion of MLIP (muscle-enriched A-type lamin-interacting protein) leads to cardiac hyperactivation of Akt/mammalian target of rapamycin (mTOR) and impaired cardiac adaptation. J Biol Chem 2015;290:26699–26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dong J, Liu J, Wen Y, Tobin SE, Zhang C, Zheng H, Huang Z, Feng Y, Zhang D, Liu S, Zhang Z, Li J. Down-regulation of Lnc-CYP7A1-1 rejuvenates aged human mesenchymal stem cells to improve their efficacy for heart repair through SYNE1. Front Cell Dev Biol 2020;8:600304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumawat K, Geerdink RJ, Hennus MP, Abdul Roda M, van Ark I, Leusink-Muis T, Folkerts G, van Oort-Jansen A, Mazharian A, Watson SP, Coenjaerts FE, Bont L, Meyaard L. LAIR-1 limits neutrophilic airway inflammation. Front Immunol 2019;10:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997;7:283–290. [DOI] [PubMed] [Google Scholar]
- 41. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke 2021;16:217–221. [DOI] [PubMed] [Google Scholar]
- 42. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kick KH, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G; EAST-AFNET 4 Trial Investigators . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 43. Nattel S, Sager PT, Hüser J, Heijman J, Dobrev D. Why translation from basic discoveries to clinical applications is so difficult for atrial fibrillation and possible approaches to improving it. Cardiovasc Res 2021;117:1616–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lamon-Fava S, So J, Mischoulon D, Ziegler TR, Dunlop BW, Kinkead B, Schettler PJ, Nierenberg AA, Felger JC, Maddipati KR, Fava M, Rapaport MH. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins Leukot Essent Fatty Acids 2021;164:102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abudukelimu A, Barberis M, Redegeld FA, Sahin N, Westerhoff HV. Predictable irreversible switching between acute and chronic inflammation. Front Immunol 2018;9:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Recchiuti A, Mattoscio D, Isopi E. Roles, actions, and therapeutic potential of specialized pro-resolving lipid mediators for the treatment of inflammation in cystic fibrosis. Front Pharmacol 2019;10:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nattel S. How does fibrosis promote atrial fibrillation persistence: in silico findings, clinical observations and experimental data. Cardiovasc Res 2016;110:295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data supporting the findings of this study are available from the corresponding authors upon reasonable request.