Abstract
Objective:
Exposure to infections during pregnancy may be a potential risk factor for later psychopathology, but large-scale epidemiological studies investigating associations between prenatal infection and long-term offspring behavioral problems in the general population are scarce. In our study, we aimed to investigate the following: (1) the association between prenatal infection and adolescent behavior, (2) putative underlying pathways (mediation), and (3) “second hits” interacting with prenatal infection to increase the risk of adolescent behavior problems (moderation).
Method:
Our study was embedded in a prospective Dutch pregnancy cohort (Generation R; n = 2,213 mother–child dyads). We constructed a comprehensive prenatal infection score comprising common infections for each trimester of pregnancy. At age 13 to 16 years, we assessed total, internalizing, and externalizing problems, and autistic traits using the Child Behavioral Checklist and the Social Responsiveness Scale, respectively. We investigated maternal lifestyle and nutrition, perinatal factors (placental health and delivery outcomes), and child health (lifestyle, traumatic events, infections) as mediators and moderators.
Results:
We observed associations of prenatal infection with adolescent total behavioral, internalizing, and externalizing problems. The association between prenatal infection and internalizing problems was moderated by higher levels of maternal psychopathology, alcohol and tobacco use, and a higher number of traumatic childhood events. We found no association between prenatal infection and autistic traits. Yet, children exposed to prenatal infections and maternal substance use, and/or traumatic childhood events, had a higher risk of autistic traits in adolescence.
Conclusion:
Prenatal infection may be a risk factor for later psychiatric problems as well as a disease primer making individuals susceptible to other hits later in life.
Study preregistration information:
Prenatal maternal infection and adverse neurodevelopment: a structural equation modelling approach to downstream environmental hits; https://osf.io/cp85a; cp85a.
Keywords: maternal immune activation, neurodevelopment, maternal health, pregnancy, child health
Exposure to infections during pregnancy is increasingly recognized as a potential risk factor for psychopathology and neurodevelopmental disorders in offspring, including increased risk for autism spectrum disorder (ASD), depression, and attention-deficit/hyperactivity disorder (ADHD).1 Infectious stimuli can cross the placenta directly or assert their effects via dysregulation of the maternal immune system.2 However, several recent reviews and meta-analyses dedicated to this topic, including the most recent and largest meta-analysis,3 noted the limited quantity and quality of studies on the association between prenatal infection and behavioral problems. In brief, this review noted that despite the growing body of literature on prenatal infection and psychopathology, most studies are small and do not adjust for important confounders. Thus, the reproducibility of the results is affected. Moreover, existing studies focus on clinical cases of infection, for example by studying hospitalized mothers with respiratory infections such as influenza or perinatal infections such as chorioamnionitis. Moreover, prior studies have tended to focus on clinical diagnoses of psychopathology (eg, ASD, ADHD). The generalizability to a broader range of infections (concerning both severity and type) and behavioral problems along the continuum is therefore limited.1,3–5 To date, large-scale epidemiological studies investigating the long-term association between prenatal infection and offspring behavioral problems in the general population are scarce.
In humans, it is challenging to study the potential pathway underlying the association between infections during pregnancy and later-life offspring behavioral problems because this pathway is highly complex, with several environmental factors affecting the association over the course of a lifetime.2 As such, prior studies have focused on the total effect of prenatal infection on behavioral problems. Different mediators, namely, causal factors on the pathway from prenatal infection to adolescent behavior problems, may play an important role. For example, because placental histopathology has been linked to both prenatal infection6 and ASD,7 placental abnormalities may be an important mediator underlying the association. Similarly, delivery outcomes such as preterm birth could be causalities on the pathway from prenatal infection to adverse neurodevelopment.8
In addition to a direct association between prenatal infection and behavioral problems, the “second-hit hypothesis” suggests that prenatal infections may act only as disease primer. As such, prenatal infections may not induce behavioral problems by themselves but increase an individual’s susceptibility to downstream stressors (“second hits”).2 Normally, when a “hit” occurs, the individual’s inflammatory response protects against such stressors. Yet, if the fetal immune system was exposed to prenatal infections and imprinted atypically, the individual’s lifelong susceptibility to disease may increase.2 Maternal psychopathology, deficient maternal nutrition, high body mass index (BMI), and maladaptive lifestyle factors (eg, smoking) during pregnancy may be “second hits” of interest (hereafter defined as moderating factors potentially interacting with prenatal infection to increase the risk of adolescent behavior problems).2,9,10
Here, we aimed to investigate the association between prenatal exposure to common infections and a range of adolescent behavioral problems at age 13–16 years in a large population-based cohort. We considered the effects of prenatal infections during the whole pregnancy, as well as in each trimester separately. We further aimed to examine putative pathways underlying the association between prenatal infection and adolescent behavior by studying potential perinatal mediators (eg, placenta growth and blood flow, birthweight, and gestational age at birth). Finally, we explored the possibility of “second hits,” namely, moderating factors interacting with prenatal infection to increase the risk of adolescent behavior problems. We examined maternal (eg, nutrition, lifestyle, pregnancy health) and childhood (eg, traumatic events, lifestyle, infections, and BMI) moderating factors.
METHOD
Preregistration
We preregistered our study prior to any analyses (https://osf.io/cp85a) (Supplement 1 and Tables S1-S3, available online).
Study Selection and Participants
This study was embedded in the Generation R Study, a large prospective population-based cohort investigating child development from pregnancy onwards.11 Pregnant individuals (N = 9,778) living in Rotterdam, the Netherlands, were recruited between April 2002 and January 2006. Prenatally, mothers were followed up during each trimester (~70% enrolled in the first trimester). Postnatally, mothers and their children were assessed in multiple follow-up waves at mean ages of 6, 10, 14, and currently at age 17 years. The Medical Ethics Committee of the Erasmus Medical Centre approved all study procedures. Parents and their children provided written informed consent.
To be included in our study, mothers had to be enrolled in their first trimester, and information on prenatal infection during each trimester as well as information on the parent-reported behavior outcomes at the 13 to 16 years assessment wave had to be available. For siblings and twins, 1 sibling/twin was excluded at random. After applying these criteria, 2,213 mother–child pairs were included in our study (Figure 1).
FIGURE 1. Flow Chart of Study Population.
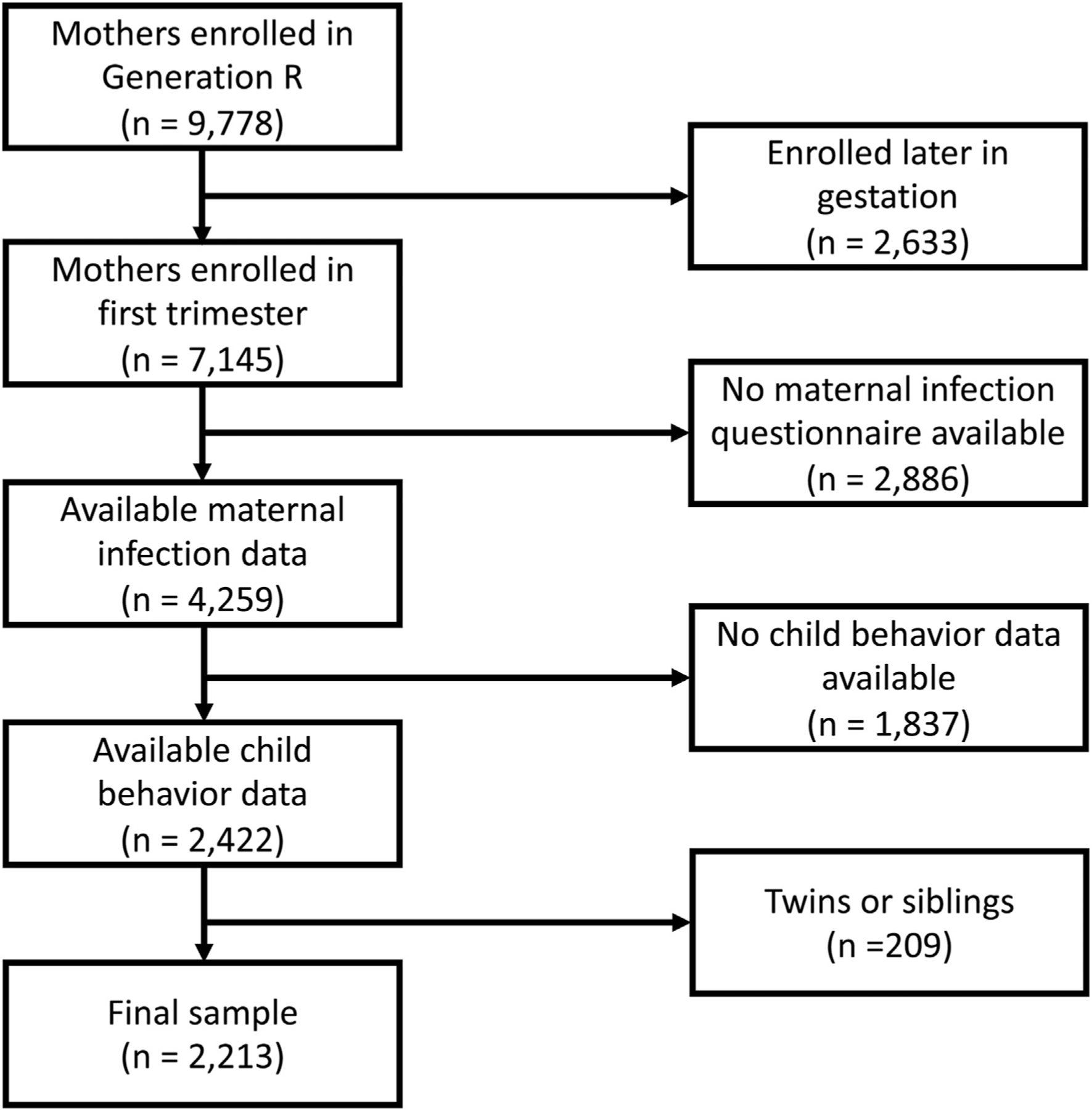
Note: This figure shows the flowchart of the study population (application of the inclusion and exclusion criteria).
Exposure: Prenatal Infection Assessment
To define prenatal infections, we used complete cases of questionnaire data collected at 3 time points during pregnancy, specifically at the end of trimesters 1, 2, and 3. Women were asked to report on the following infection items: upper respiratory infections (pharyngitis, rhinitis, sinusitis, ear infection), lower respiratory infections (pneumonia, bronchitis), gastrointestinal infections (diarrhea, enteritis), cystitis/pyelitis, dermatitis (boils, erysipelas), eye infections, herpes zoster, flu, sexually transmitted diseases (STD), and a period of fever (>38°C/100.4°F) within the past 3 months (first and third trimester) or 2 months (second trimester) (Supplement 2, Figure S1, available online).
We constructed 4 prenatal infection sum scores: 1 score for each trimester (trimester-based), and 1 score for the whole pregnancy. For the trimester-based scores, each confirmation of a condition within that trimester (“yes”) was scored as 1 point. A “no” response was rated with 0 points. A maximum sum score of 10 points could be derived per trimester (maximum 30 points for the whole pregnancy). Fever was additionally used as a separate severity marker. We constructed a binary fever variable (yes/no) for each trimester and a sum score for the whole pregnancy (maximum 3 points per pregnancy).
The Generation R cohort has data on prenatal C-reactive protein (CRP) levels available at a single time-point (mean 12 weeks of gestation), although not at timing of infection. Given the short half-life (~12 hours) of CRP12 and the phenomenon of healthy volunteer bias (ie, infected participants are less likely to attend a research visit), we were unable to use CRP levels in our prenatal infection score (Supplement 3, Figure S3, displays the correlation matrix between infections and CRP, available online).
Outcome: Adolescent Behavior Problems
Child Behavioral Checklist.
To examine internalizing, externalizing and total behavioral problems, we used parental ratings on the Child Behavioral Checklist (CBCL) at age 13 to 16 years.13 Parents reported on the adolescents’ behavior over the past 6 months. Eight empirically based scales were derived from 112 items: anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule-breaking behavior, and aggressive behavior. Those scales were then combined into externalizing problems (rule-breaking behavior, aggressive behavior), and internalizing problems (anxious/depressed, withdrawn/depressed, somatic complaints). The total behavioral problems score comprises externalizing and internalizing problems, and additionally includes social problems, thought problems, and attention problems. Higher scores indicate higher levels of behavioral problems (Supplement 2, Figure S2, available online).
Social Responsiveness Scale.
We measured autistic traits in adolescents at age 13 to 16 years, using the abbreviated parent-reported Social Responsiveness Scale (SRS; 18-items).14 The abbreviated SRS is a validated questionnaire assessing interpersonal behavior, communication, and repetitive/stereotypic behavior in adolescents. Higher scores indicate higher levels of autistic traits (Supplement 2, Figure S2, available online).
Mediators
Levels of placental growth factor (PIGF) were measured in the first 18 weeks of gestation in maternal venous blood samples. The arteria umbilicalis pulsatility index and arteria uterine resistance index were obtained by ultrasound examinations in the second trimester. The 5-minute Apgar score, umbilical cord blood pH, placental weight at birth, gestational age at birth, and birthweight were obtained from hospital registries (standardized delivery registrations of midwives and obstetricians). Supplements 4 and 5, available online, provide more information on these mediators.
Moderators
Pre-pregnancy BMI was calculated based on height and weight before pregnancy from the enrollment questionnaire. The enrollment questionnaire also included questions regarding psychoactive substance use, tobacco use, and alcohol consumption during pregnancy. Maternal psychopathology was assessed at enrollment using the Global Severity Index from the Brief Symptom Inventory.15 The diet quality score was calculated based on the food frequency questionnaire administered during the first 18 weeks of gestation.16 Iron and vitamin D levels were measured in the first 18 weeks of gestation in maternal venous blood samples. Information about breastfeeding was obtained from questionnaires 12 months after birth. Childhood BMI was measured at the 9 to 12 years research visit. A sum score for childhood infection was constructed based on questionnaire data asking about antibiotic use at 2 and 6 months and at 1, 2, 3, 4, 5, and 9 years of age (for details on score construction, see Table S4). Childhood trauma was assessed using cumulative scores of postnatal life events and postnatal direct victimization (https://github.com/SereDef/cumulative-ELS-score).17 Adolescent alcohol and tobacco use were assessed in self-reported questionnaires at 13 to 16 years. Supplement 6, available online, provides more information.
Covariates
Child age was established from the date of birth and the date of questionnaire completion. Hospital registries provided information on child sex. Maternal age and national background (“Dutch” or “non-Dutch”) were established via the enrollment questionnaire. Household income at enrollment (<€2,200 per month or >€2,200 per month), parental education (primary [no education or primary school], intermediate [secondary school or lower vocational training] and high [higher vocational training or university]), and maternal IQ (Raven’s Advanced Progressive Matrices Test set I18) were assessed when the child was 5 to 8 years of age. Child IQ was assessed using the vocabulary, matrix reasoning, digit span, and coding subscales of the Wechsler Intelligence Scale for Children–Fifth Edition at age 13 to 16 years.19 Figure 2 shows an overview of the measurement time of each exposure, outcome, mediator, moderator, and covariate variable.
FIGURE 2. Overview of Included Variables.

Note: This plot, in 2 panels, shows when each variable (exposure, outcomes, mediators, moderators, and covariates) was measured. BMI = body mass index; IQ = intelligence quotient; pH = potential of hydrogen.
Statistical Analyses
We performed a non-response analysis to explore subsample selection biases. We compared the demographical variables of study participants to those of the whole Generation R sample. We used χ2 tests for categorical variables and unpaired t tests for continuous variables. We square root transformed the CBCL and SRS scores to satisfy the normality assumption. To enable direct comparison between variables, we standardized all variables to z scores.
Direct Association.
We used linear regressions to investigate the associations between prenatal infection and behavioral problems. As exposure, we first used the total infection sum score for the whole pregnancy. We ran separate analyses for the 4 outcome variables: CBCL total behavioral, internalizing and externalizing problems, and SRS total score. To investigate potential sensitive periods, we subsequently examined associations between the 3 trimester-based infection sum scores and the 4 behavioral outcomes in individual models.
Mediation.
We applied separate mediation analyses for each behavioral outcome (CBCL total behavioral, internalizing, and externalizing problems; and SRS total score). For each analysis, we included the following mediators into the same model: arteria umbilicalis pulsatility index, arteria uterine resistance index, placental growth factor, placental weight, birthweight, and gestational age at birth. We used bootstrapping (n = 1,000) to obtain 95% confidance intervals and p values for each indirect path in the mediation model.
Moderation.
We conducted separate moderation analyses for various maternal and child moderators. We added interaction terms between prenatal infection and the following maternal variables: psychopathology, dietary food score, iron levels, vitamin D levels, pre-pregnancy BMI, substance use, alcohol use, tobacco use; and interaction terms between prenatal infection and the following child variables: BMI, tobacco use, alcohol use, breastfeeding, infections, postnatal life events score, and postnatal direct victimization score.
Sensitivity Analyses.
To investigate the effect of infection severity, we repeated the linear regressions for the direct associations using “fever” instead of “prenatal infection” as exposure. To investigate the role of child sex, we included an interaction term between prenatal infection and child sex. We repeated the analyses for all infection types separately to investigate whether there was an infection-specific effect.
Additional Information.
All models were adjusted for the following covariates: maternal age,20 maternal education,21 paternal education,22 household income,23 maternal national background,24 maternal IQ,25 child IQ,26 child sex,27 and child age.28 The rationale behind these covariates was that these may be associated with prenatal infection and/or behavioral problems in adolescence but are not on the causal pathway underlying the association. Figure S4 and Supplement 4, available online, show an overview of all aims, variables, and analyses. The p values presented throughout the article are false discovery rate–Benjamini Hochberg corrected, with a pcorrected value ≤.05 considered significant. All analyses were conducted in R (version 3.6.1). Missing moderator (maximum 30%) and covariate (maximum 15%) data were imputed in R with multiple imputation using chained equations (30 iterations and 8 imputations; “mice” package). For missing mediator data, we applied the default method in the “lavaan” package (full information maximum likelihood), where these models were conducted.
RESULTS
Participant Demographics
A total of 2,213 mother–child pairs were included in our study. Table 1 contains descriptive information. Supplement 7 and Table S5, available online, contain the frequencies of the moderators and mediators, respectively. Supplement 8, available online, describes the non-response analysis.
TABLE 1.
Descriptive Characteristics of Participants
| Mothers | |
| General characteristics | |
| Age at enrollment. mean ± SD | 31.3 ± 4.3 |
| Pre-pregnancy BMI, mean ± SD | 23.2 ± 4.0 |
| Maternal IQ, mean ± SD | 100.2 ± 13.3 |
| National background | |
| Dutch, n (%) | 1,549 (70.0) |
| Non-Dutch, n (%) | 656 (29.6) |
| Maternal education | |
| High, n (%) | 1,400 (63.3) |
| Intermediate, n (%) | 631 (28.5) |
| Low, n (%) | 15 (0.7) |
| Household income | |
| < €2000 (%) | 197 (8.9) |
| > €2000 (%) | 1,747 (78.9) |
| Exposure | |
| Total pregnancy prenatal infection sum score, mean ± SD | 2.9 ± 2.1 |
| Children | |
| General characteristics | |
| Sex, female, n (%) | 1,126 (50.9) |
| Birth weight, g, mean ± SD | 3460.3 ± 541 |
| Gestational age at birth, wk, mean ± SD | 40 ± 1.6 |
| Average child age at assessment, y, mean ± SD | 13.5 ± 0.3 |
| Child IQ, mean ± SD | 103.8 ± 13.3 |
| Outcome | |
| CBCL, total behavioral problem score, mean ± SD | 18.2 ± 16.3 |
| CBCL, externalizing problem score, mean ± SD | 4.2 ± 5.2 |
| CBCL, internalizing problem score, mean ± SD | 5.5 ± 5.7 |
Note: BMI = body mass index; CBCL = Child Behavioral Checklist.
Direction Association
After adjusting for multiple testing, we found direct associations between the total infection sum score for the whole pregnancy and CBCL total behavioral problems (b = 0.106, 95% CI = 0.065–0.148, pcorrected < .001), CBCL internalizing problems (b = 0.111, 95% CI = 0.069–0.152, pcorrected < .001), and CBCL externalizing problems (b = 0.057, 95% CI = 0.015–0.099, pcorrected < .050) (Table 2, and Supplement 9 and Figure S5, available online). We found no direct association between the total infection sum score for the whole pregnancy and SRS total score (b = 0.021, 95% CI = –0.020 to 0.062, pcorrected = .623). We observed similar associations for the trimester-based infection sum scores (Table 2).
TABLE 2.
Standardized Coefficients for Direct Associations of Prenatal Infection With Adolescent Behavior
| Outcome | Timing of exposure | β | 95% CI | Raw p | Adjusted p |
|---|---|---|---|---|---|
| CBCL total behavioral problems | Total pregnancy | 0.106 | 0.065 to 0.148 | <.001* | <.001** |
| First trimester | 0.068 | 0.027 to 0.110 | .001* | .009** | |
| Second trimester | 0.078 | 0.037 to 0.119 | <.001* | .002** | |
| Third trimester | 0.083 | 0.042 to 0.125 | <.001* | .002** | |
| CBCL internalizing behavioral problems | Total pregnancy | 0.111 | 0.069 to 0.152 | <.001* | <.001** |
| First trimester | 0.077 | 0.035 to 0.118 | <.001* | .003** | |
| Second trimester | 0.069 | 0.027 to 0.110 | .001 | .009** | |
| Third trimester | 0.093 | 0.051 to 0.134 | <.001* | <.001** | |
| CBCL externalizing behavioral problems | Total pregnancy | 0.057 | 0.015 to 0.099 | .007* | .038** |
| First trimester | 0.030 | −0.011 to 0.071 | .153 | .378 | |
| Second trimester | 0.048 | 0.007 to 0.090 | .023* | .094 | |
| Third trimester | 0.045 | 0.004 to 0.087 | .031* | .123 | |
| Total SRS | Total pregnancy | 0.021 | −0.020 to 0.062 | .320 | .623 |
| First trimester | 0.013 | −0.028 to 0.054 | .535 | .843 | |
| Second trimester | 0.005 | −0.035 to 0.046 | .796 | .968 | |
| Third trimester | 0.027 | −0.088 to 0.126 | .733 | .460 |
Note: All models were corrected for maternal age, maternal education, paternal education, household income, maternal national background, maternal IQ, child sex, child IQ, and child age at measurement. Adjusted p values are Benjamini–Hochberg corrected. CBCL = Child Behavioral Checklist; SRS = Social Responsiveness Scale.
p < 0.05;
p < 0.05 after multiple testing correction.
Mediation
None of the placental health variables (arteria umbilicalis pulsatility index, arteria uterine resistance index, placental growth factor, and placental weight) or delivery outcomes (birthweight, gestational age at birth, 5-minute Apgar score, and umbilical cord blood pH) mediated the associations between prenatal infection and adolescent behavior (Supplement 10 and Table S6, available online).
Moderation
The association between prenatal infection during pregnancy and CBCL total behavioral problems was moderated by higher levels of maternal psychopathology (b = 0.067, 95% CI = 0.033–0.101, pcorrected < .010), maternal alcohol use (b = 0.153, 95% CI = 0.086–0.218, pcorrected < .001), maternal tobacco use (b = 0.145, 95% CI = 0.034–0.256, pcorrected < .050), child postnatal life events (b = 0.058, 95% CI = 0.019–0.096, pcorrected < .050), and child postnatal direct victimization (b = 0.075, 95% CI = 0.035–0.114, pcorrected < .010) (Table 3).
TABLE 3.
Standardized Coefficients for Moderating Effects of Moderators
| Outcome | Moderator | β | 95% CI | Raw p | Adjusted p |
|---|---|---|---|---|---|
| CBCL total behavioral problems | Maternal psychopathology | 0.067 | 0.033 to 0.101 | <.001* | .002** |
| Maternal dietary food score | 0.015 | −0.029 to 0.058 | .511 | .825 | |
| Maternal iron levels | −0.014 | −0.056 to 0.028 | .516 | .825 | |
| Maternal vitamin D levels | −0.038 | −0.079 to 0.004 | .075 | .232 | |
| Pre-pregnancy BMI | −0.017 | −0.059 to 0.023 | .398 | .726 | |
| Maternal substance use (yes) | 0.183 | 0.032 to 0.332 | .017* | .075 | |
| Maternal alcohol use (pregnancy) | 0.153 | 0.086 to 0.218 | <.001* | <.001** | |
| Maternal tobacco use (pregnancy) | 0.145 | 0.034 to 0.256 | .01* | .050** | |
| Child BMI | 0.007 | −0.035 to 0.050 | .722 | .961 | |
| Child tobacco use (yes) | 0.279 | −0.852 to 1.411 | .622 | .906 | |
| Child alcohol use (yes) | 0.172 | −0.023 to 0.368 | .083 | .244 | |
| Breastfeeding (no) | 0.133 | −0.032 to 0.299 | .111 | .306 | |
| Childhood infections | 0.002 | −0.074 to 0.078 | .951 | .984 | |
| Postnatal life events score | 0.058 | 0.019 to 0.096 | .003* | .020** | |
| Postnatal direct victimization score | 0.075 | 0.035 to 0.114 | <.001* | .002** | |
| CBCL internalizing behavioral problems | Maternal psychopathology | 0.062 | 0.028 to 0.095 | <.001* | .003** |
| Maternal dietary food score | 0.026 | −0.020 to 0.073 | .258 | .541 | |
| Maternal iron levels | 0.004 | −0.036 to 0.046 | .822 | .973 | |
| Maternal vitamin D levels | −0.007 | −0.049 to 0.037 | .762 | .964 | |
| Pre-pregnancy BMI | −0.028 | −0.068 to 0.011 | .162 | .389 | |
| Maternal substance use (yes) | 0.215 | 0.064 to 0.366 | .005* | .031** | |
| Maternal alcohol use (pregnancy) | 0.127 | 0.062 to 0.193 | <.001* | .002** | |
| Maternal tobacco use (pregnancy) | 0.183 | 0.071 to 0.293 | .001* | .009** | |
| Child BMI | −0.007 | −0.050 to 0.036 | .758 | .964 | |
| Child tobacco use (yes) | 0.346 | −0.711 to 1.404 | .516 | .825 | |
| Child alcohol use (yes) | 0.270 | 0.068 to 0.472 | .009* | .048* | |
| Breastfeeding (no) | 0.126 | −0.037 to 0.290 | .127 | .339 | |
| Childhood infections | −0.007 | −0.073 to 0.058 | .816 | .973 | |
| Postnatal life events score | 0.057 | 0.019 to 0.096 | .003* | .021* | |
| Postnatal direct victimization score | 0.074 | 0.035 to 0.114 | <.001* | .002* | |
| CBCL externalizing behavioral problems | Maternal psychopathology | 0.024 | −0.001 to 0.057 | .166 | .396 |
| Maternal dietary food score | 0.033 | −0.012 to 0.079 | .154 | .383 | |
| Maternal iron levels | 0.005 | −0.036 to 0.047 | .793 | .965 | |
| Maternal vitamin D levels | 0.017 | −0.026 to 0.061 | .429 | .761 | |
| Pre-pregnancy BMI | −0.031 | −0.072 to 0.009 | .133 | .353 | |
| Maternal substance use (yes) | 0.142 | −0.008 to 0.292 | .064 | .227 | |
| Maternal alcohol use (pregnancy) | 0.053 | −0.012 to 0.119 | .110 | .312 | |
| Maternal tobacco use (pregnancy) | 0.122 | 0.011 to 0.233 | .031* | .126 | |
| Child BMI | −0.019 | −0.062 to 0.024 | .391 | .738 | |
| Child tobacco use (yes) | 0.344 | −0.851 to 1.539 | .563 | .871 | |
| Child alcohol use (yes) | 0.231 | 0.420 to 0.040 | .018* | .081 | |
| Breastfeeding (no) | 0.056 | 0.012 to 0.101 | .420 | .756 | |
| Childhood infections | −0.022 | −0.070 to 0.026 | .362 | .706 | |
| Postnatal life events score | 0.032 | −0.006 to 0.071 | .098 | .294 | |
| Postnatal direct victimization score | 0.023 | −0.016 to 0.062 | .253 | .548 | |
| Total SRS | Maternal psychopathology | 0.031 | −0.002 to 0.046 | .066 | .193 |
| Maternal dietary food score | 0.035 | −0.013 to 0.083 | .147 | .486 | |
| Maternal iron levels | 0.014 | −0.028 to 0.056 | .515 | .720 | |
| Maternal vitamin D levels | −0.007 | −0.048 to 0.034 | .735 | .777 | |
| Pre-pregnancy BMI | −0.022 | −0.063 to 0.020 | .306 | .609 | |
| Maternal substance use (yes) | 0.221 | 0.073 to 0.369 | .003* | .021* | |
| Maternal alcohol use during pregnancy | 0.040 | −0.025 to 0.106 | .227 | .487 | |
| Maternal tobacco use during pregnancy | 0.099 | −0.011 to 0.210 | .077 | .235 | |
| Child BMI | 0.008 | −0.036 to 0.052 | .072 | .232 | |
| Child tobacco use (yes) | 0.307 | −0.634 to 1.249 | .052 | .188 | |
| Child alcohol use (yes) | 0.195 | 0.014 to 0.377 | .035* | .135 | |
| Breastfeeding (no) | 0.091 | −0.054 to 0.237 | .219 | .486 | |
| Childhood infections | −0.017 | −0.064 to 0.030 | .474 | .798 | |
| Postnatal life events score | 0.052 | 0.013 to 0.090 | .008* | .042* | |
| Postnatal direct victimization score | 0.054 | 0.015 to 0.094 | .006* | .035* |
Note: All models were corrected for maternal age, maternal education, paternal education, household income, maternal national background, maternal IQ, child sex, child IQ, and child age at measurement. Adjusted p values are Benjamini–Hochberg corrected. BMI = body mass index; CBCL = Child Behavioral Checklist; SRS = Social Responsiveness Scale.
p < 0.05;
p < 0.05 after multiple testing correction.
The association between prenatal infection during pregnancy and CBCL internalizing problems was moderated by higher levels of maternal psychopathology (b = 0.062, 95% CI = 0.028–0.095, pcorrected < .010), maternal substance use (b = 0.215, 95% CI = 0.064–0.366, pcorrected < .050), maternal alcohol use (b = 0.127, 95% CI = 0.062–0.193, pcorrected < .010), maternal tobacco use (b = 0.183, 95% CI = 0.071–0.293, pcorrected < .010), child alcohol use (b = 0.270, 95% CI = 0.068–0.472, pcorrected < .50), child postnatal life events (b = 0.057, 95% CI = 0.019–0.096, pcorrected < .050), and child postnatal direct victimization (b = 0.074, 95% CI = 0.035–0.114, pcorrected < .010). The association between prenatal infection during pregnancy and CBCL externalizing problems was not moderated by any of the investigated factors.
The association between prenatal infection during pregnancy and SRS total score was moderated by higher levels of maternal substance use (b = 0.221, 95% CI = 0.073–0.369, pcorrected < .050), child postnatal life events (b = 0.013, 95% CI = 0.013–0.090, pcorrected < .050), and child postnatal direct victimization (b = 0.054, 95% CI = 0.015–0.094, pcorrected < .050).
Sensitivity Analyses
Our sensitivity analyses showed no effect of fever (Supplement 11 and Table S7, available online) and no moderating effect of child sex (Table S8, available online). The individual results per infection type for each behavior outcome can be found in Table S9 (available online).
DISCUSSION
In this large population-based cohort, we examined the relationship between prenatal infection and behavioral problems at age 13 to 16 years. We further examined the putative mechanism underlying this association (mediation) and the presence of “second hits” (moderation) that interact with prenatal infections to increase the risk of behavioral problems. We highlight 3 key findings. First, we observed associations between prenatal infection and total behavioral, internalizing, and externalizing problems. We found no association between prenatal infection and autistic traits. We observed similar associations for trimester-based scores. Second, none of the investigated perinatal variables were on the pathway from prenatal infection to adolescent behavior. Third, we observed that maternal psychopathology, maternal alcohol use, maternal tobacco use, child postnatal life events, and child postnatal direct victimization interacted with prenatal infections to strengthen the associations with adolescent total behavioral and internalizing problems. Maternal substance use and child alcohol use further moderated the association between prenatal infection and internalizing problems. Maternal substance use, child postnatal life events and child postnatal direct victimization interacted with prenatal infection to increase the risk of adolescent autistic traits.
Our finding of direct associations between prenatal infection and adolescent total behavioral, internalizing, and externalizing problems are in line with previous research.29 The relationship between prenatal infection and child internalizing behavior, particularly depressive and anxious symptomology, has been well documented across the life-span.29 The association between prenatal infection and externalizing symptoms is less well established. Although several studies have indicated that prenatal infection is associated with reduced impulse control in toddlers30 and a higher risk of ADHD in the child,31 other studies have not observed an association on externalizing problems, suggesting that the association may be better explained by unmeasured familial confounding32. However, heterogeneity across studies is high.33 Our study adds to the existing evidence, suggesting that the previously reported associations between severe infections and clinical diagnoses of psychiatric conditions also extend to common infections and continuously measured internalizing and externalizing behavior. These effects persisted after correction for multiple confounders and multiple testing—which were novel features of our study—increasing the robustness of the results.
Infections during pregnancy may affect the presence of internalizing and externalizing problems in children through various pathways.1,2 Vertical transmission, whereby the pathogen directly crosses the placenta and affects fetal development, is 1 potential pathway. Another possible pathway is the maternal immune system’s inflammatory response to the infection, rather than the pathogen itself. This response could cause immune cells to cross the placenta, to activate fetal immune cells, and consequently to affect fetal neurodevelopment.
Moreover, although various environmental risk factors have been linked to prenatal infections or adolescent behavior, our study is among the first to examine the joint role of multiple mediators in the relationship between prenatal infection and adolescent behavior. Because we found no mediating effects of placental health and birth outcomes, our findings suggest that perinatal outcomes do not to lie on the pathway from prenatal infection to adolescent behavioral problems.
In line with the “second-hits hypothesis,” we investigated various moderating factors. We observed that maternal lifestyle and traumatic childhood events strengthened the association between prenatal infection and adolescent behavioral problems. Maternal psychopathology may assert its effect via suboptimal parenting practices and/or genetic vulnerability.34 Similarly, a maladaptive lifestyle may have an exacerbating effect on the association between prenatal infection and adolescent behavior. Lifestyle factors such as alcohol and tobacco use might interact with the immune system.2 Postnatal traumatic events may lead to a chronic stress response in the child, which could interact with the altered immune response caused by prenatal exposure to infection.35 The relatively high heritability of externalizing symptoms compared to internalizing problems (eg, h2 = 74% for conduct disorder,36 h2 = 37% for depression37) may be the reason why none of the environmental factors investigated here interacted with prenatal infection to increase the risk for externalizing problems.
Although earlier work investigating prenatal infection and fever reported a positive association with ASD,38 we found no association between prenatal infection and autistic traits. To complement previous research on clinical ASD, we studied autistic traits in the general population, and adjusted for several important confounders and multiple testing to reduce the risk of false-positive results. In line with our findings, a meta-analysis showed no effect of prenatal infections on autism risk.39 Moreover, a recent large-scale Swedish register-based study concluded that the association between prenatal infection and autism may not reflect a causal relationship, but may be better explained by shared familial factors (e.g., genetic variation or shared environment).40 Although we did not observe a direct association between prenatal infection and autistic traits, prenatal maternal substance use and childhood traumatic experiences interacted with prenatal infection to increase the risk of autistic traits. Thus, prenatal infection may be a disease primer for autistic traits, making individuals susceptible to other hits later in life.
The strengths of our study include the large population-based cohort, the broad range of infections, the long follow-up, and the inclusion of moderators, mediators, and relevant covariates. Moreover, we applied multiple testing corrections to minimize the probability of false-positive results. The study also has limitations. One limitation was that prenatal infections were recorded retrospectively, which may have introduced some recall bias. However, we attempted to minimize this bias by asking participants to recall infections after each trimester, which resulted in a recall period of approximately 2 to 3 months. In addition, self-report questionnaires rather than blood serum biomarkers were used. Self-report may be subject to recall bias. Yet, self-report questionnaires may also be advantageous, because they are not affected by healthy volunteer bias as they could be filled in during any point in the trimester at home, whereas infected participants are less likely to attend research visits where the blood withdrawal measurement occurs. Nonetheless, future research would benefit from serological evidence for bacterial and viral infections in each trimester of pregnancy to ascertain the medical diagnosis. Another limitation was that we were unable to investigate systemic immune responses because we did not have blood samples taken at the time of infection. This could have limited our understanding of the mechanisms underlying the observed associations. Furthermore, when using fever as a marker of infection severity, we found no associations with adolescent behavior, which may have been due to inadequate statistical power. Our study was designed primarily to detect potential long-term effects of exposure to common infections on child behavioral problems, given that most prior literature has focused on the more severe infections.
In conclusion, the results of this large population-based cohort show that prenatal infection may increase the risk for some adolescent behavioral problems. Maternal psychopathology, a maladaptive lifestyle during pregnancy, child alcohol use, and traumatic childhood events may interact with prenatal infections to further increase the risk for behavior problems. Although it may be difficult for pregnant individuals to prevent exposure to common infections, public health measures may be used to address the need to increase resilience and resistance to infections and to prevent the spread of diseases. Public health measures aimed at the pregnant individual may comprise promoting healthy lifestyle choices such as getting enough rest and exercise, maintaining a balanced diet, and staying up to date with vaccinations during pregnancy. In addition, community-level public health efforts implemented to reduce the transmission of infections may include improving ventilation and providing education and resources on hand hygiene, mask wearing and social distancing in public settings.
Supplementary Material
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center in close collaborations with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. The general design of the Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Netherlands, the Organization for Health Research and Development (ZonMw) and the Ministry of Health, Welfare and Sport. This project was funded by NIH grant: 1R01MH124776–01A1.
The authors gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
Diversity & Inclusion Statement:
We worked to ensure race, ethnic, and/or other types of diversity in the recruitment of human participants. We worked to ensure that the study questionnaires were prepared in an inclusive way. We worked to ensure sex and gender balance in the recruitment of human participants.
Footnotes
This work has been prospectively registered: https://osf.io/cp85a.
Disclosure: Prof. White, Dr. Cecil, Profs. Reiss and Jaddoe, Dr. Gigase, Prof. Hillegers, Dr. de Witte, Prof. Bergink, Dr. Rommel, and Mss. Suleri and Blok have reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Mss Anna Suleri, Erasmus University Medical Center, Rotterdam, the Netherlands.
Profs Tonya White, Erasmus University Medical Center, Rotterdam, the Netherlands.
Mss Elisabet Blok, Erasmus University Medical Center, Rotterdam, the Netherlands.
Drs Charlotte A.M. Cecil, Erasmus University Medical Center, Rotterdam, the Netherlands.
Profs Irwin Reiss, Erasmus University Medical Center, Rotterdam, the Netherlands.
Profs Vincent W.V. Jaddoe, Erasmus University Medical Center, Rotterdam, the Netherlands.
Drs F.A.J. Gigase, Erasmus University Medical Center, Rotterdam, the Netherlands.
Profs Manon H.J. Hillegers, Erasmus University Medical Center, Rotterdam, the Netherlands.
Drs Lot de Witte, Icahn School of Medicine at Mount Sinai, New York.
Profs Veerle Bergink, Erasmus University Medical Center, Rotterdam, the Netherlands, Icahn School of Medicine at Mount Sinai, New York.
Drs Anna-Sophie Rommel, Icahn School of Medicine at Mount Sinai, New York.
REFERENCES
- 1.Al-Haddad BJS, Jacobsson B, Chabra S, et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry. 2019;76(6):594–602. 10.1001/jamapsychiatry.2019.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han VX, Patel S, Jones HF, Dale RC. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol. 2021;17(9): 564–579. 10.1038/s41582-021-00530-8 [DOI] [PubMed] [Google Scholar]
- 3.Fung SG, Fakhraei R, Condran G, et al. Neuropsychiatric outcomes in offspring after fetal exposure to maternal influenza infection during pregnancy: a systematic review. Reprod Toxicol. 2022;113:155–169. 10.1016/j.reprotox.2022.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Blomström Å, Karlsson H, Gardner R, Jörgensen L, Magnusson C, Dalman C. Associations between maternal infection during pregnancy, childhood infections and the risk of subsequent psychotic disorder—a Swedish cohort study of nearly 2 million individuals. Schizophr Bull. 2016;42(1):125–133. 10.1093/schbul/sbv112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoun S, Ellul P, Peyre H, et al. Fever during pregnancy as a risk factor for neurodevelopmental disorders: results from a systematic review and meta-analysis. Mol Autism. 2021;12(1):60. 10.1186/s13229-021-00464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weckman AM, Ngai M, Wright J, McDonald CR, Kain KC. The impact of infection in pregnancy on placental vascular development and adverse birth outcomes. Front Microbiol. 2019;10:1924. 10.3389/fmicb.2019.01924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straughen JK, Misra DP, Divine G, et al. The association between placental histopathology and autism spectrum disorder. Placenta. 2017;57:183–188. 10.1016/j.placenta.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 8.Lahti M, Eriksson JG, Heinonen K, et al. Late preterm birth, post-term birth, and abnormal fetal growth as risk factors for severe mental disorders from early to late adulthood. Psychol Med. 2015;45(5):985–999. 10.1017/S0033291714001998 [DOI] [PubMed] [Google Scholar]
- 9.Prins JR, Eskandar S, Eggen BJL, Scherjon SA. Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment? J Reprod Immunol. 2018;126:18–22. 10.1016/j.jri.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Money KM, Barke TL, Serezani A, et al. Gestational diabetes exacerbates maternal immune activation effects in the developing brain. Mol Psychiatry. 2018;23(9):1920–1928. 10.1038/mp.2017.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243–1264. 10.1007/s10654-016-0224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ablij HC, Meinders AE. C-reactive protein: history and revival. Eur J Intern Med. 2002; 13(7):412–422. 10.1016/S0953-6205(02)00132-2 [DOI] [PubMed] [Google Scholar]
- 13.Achenbach T, Rescorla L. ASEBA School-Age Forms & Profiles. Aseba; 2001. [Google Scholar]
- 14.Constantino J Social Responsiveness Scale (SRS), Manual. Western Psychological Services; 2002. [Google Scholar]
- 15.de Beurs E Brief Symptom Inventory. Handleiding; 2004. [Google Scholar]
- 16.Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, et al. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998;52(8):588–596. 10.1038/sj.ejcn.1600611 [DOI] [PubMed] [Google Scholar]
- 17.Schuurmans IK, Luik AI, de Maat DA, Hillegers MHJ, Ikram MA, Cecil CAM. The association of early life stress with IQ-achievement discrepancy in children: a population-based study. Child Dev. Published online July 13, 2022. 10.1111/cdev.13825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raven J Advanced Progressive Matrices: Sets I and II. HK Lewis; 1962. [Google Scholar]
- 19.Blok E, Schuurmans I, Tijburg A, et al. Cognitive performance in children and adolescents with psychopathology traits: a cross-sectional multicohort study in the general population. Dev Psychopathol. 2022;1–15. 10.1017/S0954579422000165 [DOI] [PubMed] [Google Scholar]
- 20.Duncan GJ, Lee KTH, Rosales-Rueda M, Kalil A. Maternal age and child development. Demography. 2018;55(6):2229–2255. 10.1007/s13524-018-0730-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K, Morrison J, Pikhart H. Children’s behavioural problems and its associations with socioeconomic position and early parenting environment: findings from the UK Millennium Cohort Study. Epidemiol Psychiatr Sci. 2020;29:e155. 10.1017/S2045796020000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torvik FA, Eilertsen EM, McAdams TA, et al. Mechanisms linking parental educational attainment with child ADHD, depression, and academic problems: a study of extended families in the Norwegian Mother, Father and Child Cohort Study. J Child Psychol Psychiatry. 2020;61(9):1009–1018. 10.1111/jcpp.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulain T, Vogel M, Kiess W. Review on the role of socioeconomic status in child health and development. Curr Opin Pediatr. 2020;32(2):308–314. 10.1097/MOP.0000000000000876 [DOI] [PubMed] [Google Scholar]
- 24.Flink IJE, Jansen PW, Beirens TMJ, et al. Differences in problem behaviour among ethnic minority and majority preschoolers in the Netherlands and the role of family functioning and parenting factors as mediators: the Generation R Study. BMC Public Health. 2012;12:1092. 10.1186/1471-2458-12-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitley E, Gale CR, Deary IJ, Kivimaki M, Batty GD. Association of maternal and paternal IQ with offspring conduct, emotional, and attention problem scores. Transgenerational evidence from the 1958 British Birth Cohort Study. Arch Gen Psychiatry. 2011;68(10):1032–1038. 10.1001/archgenpsychiatry.2011.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalsgaard S, Thorsteinsson E, Trabjerg BB, et al. Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiatry. 2020;77(2):155–164. 10.1001/jamapsychiatry.2019.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen A, Schwarz D, Radcliffe J, Rogan WJ. Maternal IQ, child IQ, behavior, and achievement in urban 5–7 year olds. Pediatr Res. 2006;59(3):471–477. 10.1203/01.pdr.0000199910.16681.f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solmi M, Radua J, Olivola M, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27(1):281–295. 10.1038/s41380-021-01161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel S, Cooper MN, Jones H, Whitehouse AJO, Dale RC, Guastella AJ. Maternal immune-related conditions during pregnancy may be a risk factor for neuropsychiatric problems in offspring throughout childhood and adolescence. Psychol Med. Published online June. 2020;1:1–11. 10.1017/S0033291720001580 [DOI] [PubMed] [Google Scholar]
- 30.Graham A, Rasmussen J, Rudolph M, Heim C, Gilmore J, et al. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry. 2018;83(2):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Instanes JT, Halmøy A, Engeland A, Haavik J, Furu K, Klungsøyr K. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiatry. 2017;81(5):452–459. 10.1016/j.biopsych.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg Y, D’Onofrio BM, Rickert ME, et al. Maternal infection requiring hospitalization during pregnancy and attention-deficit hyperactivity disorder in offspring: a quasi-experimental family-based study. J Child Psychol Psychiatry. 2019;60(2):160–168. 10.1111/jcpp.12959 [DOI] [PubMed] [Google Scholar]
- 33.Zhu CY, Jiang HY, Sun JJ. Maternal infection during pregnancy and the risk of attention-deficit/hyperactivity disorder in the offspring: a systematic review and meta-analysis. Asian J Psychiatry. 2021;68:102972. 10.1016/j.ajp.2021.102972 [DOI] [PubMed] [Google Scholar]
- 34.Dean K, Stevens H, Mortensen PB, Murray RM, Walsh E, Pedersen CB. Full spectrum of psychiatric outcomes among offspring with parental history of mental disorder. Arch Gen Psychiatry. 2010;67(8):822–829. 10.1001/archgenpsychiatry.2010.86 [DOI] [PubMed] [Google Scholar]
- 35.Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry. 2014;23(10):943–956. 10.1007/s00787-014-0566-3 [DOI] [PubMed] [Google Scholar]
- 36.de Zeeuw EL, van Beijsterveldt CEM, Lubke GH, Glasner TJ, Boomsma DI. Childhood ODD and ADHD behavior: the effect of classroom sharing, gender, teacher gender and their interactions. Behav Genet. 2015;45(4):394–408. 10.1007/s10519-015-9712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong ASF, López-López JA, Hammerton G, et al. Genetic and environmental risk factors associated with trajectories of depression symptoms from adolescence to young adulthood. JAMA Netw Open. 2019;2(6):e196587. 10.1001/jama-networkopen.2019.6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tioleco N, Silberman AE, Stratigos K, et al. Prenatal maternal infection and risk for autism in offspring: a meta-analysis. Autism Res. 2021;14(6):1296–1316. 10.1002/aur.2499 [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore). 2017;96(18):e6696. 10.1097/MD.0000000000006696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brynge M, Sjöqvist H, Gardner RM, Lee BK, Dalman C, Karlsson H. Maternal infection during pregnancy and likelihood of autism and intellectual disability in children in Sweden: a negative control and sibling comparison cohort study. Lancet Psychiatry. 2022;9(10):782–791. 10.1016/S2215-0366(22)00264-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


