Abstract
Vitamin A, also known as retinol, is a fat-soluble vitamin that plays crucial role in various physiological functions In vivo. However, factors such as light, oxygen, and others may impact the stability of VA. To enhance its stability. This study microencapsulated VA, Gelatin, carboxymethyl cellulose, and salt were mixed in a ratio of 5:1:0.1 as the shell material. Additionally, 12% TG and 3.5% sucrose ester were added with core–shell ratio of 1:8. The experimental results indicated that VA microcapsules exhibited an encapsulation efficiency of 81.12%, after 9 weeks of storage this rate decreased to 75.38%, and the encapsulated VA oil did not exhibit extravasation. The addition of an appropriate amount of salt to the shell material enhanced the mechanical properties of the shell material, compared to the shell material without added salt, the leakage of VA in the salt-added sample decreased by 5.8% for 30 min and 14.5% for 60 min. In vitro release experiments showed that after 3 h of incubation in simulated gastric fluid, the microcapsules had an 18.52% release rate. In simulated intestinal fluid, this increased to 66.58%, indicating strong enteric solubility.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-024-05962-w.
Keywords: Vitamin A, Microencapsulation, Nutrient fortified salt, Characterization
Introduction
Vitamin A(VA) is an essential micronutrient that cannot be synthesized endogenously, requiring absorption from animal or plant sources. The absorption of VA is limited in the stomach of animals, with predominant uptake occurring within the mucous membrane of the small intestine. This process is facilitated by the mucosal interaction with bile acids and the breakdown products of fats. The coexistence of antioxidants and bile in the small intestine additionally amplifies the absorption of VA in the human body (Debelo et al. 2017). The recommended daily intake of VA is 400 μg for adult males, 500 μg for adult females, and 400 μg for children aged 1–3 years (Tan et al. 2015). VA intake in urban residents is seriously inadequate, with about 71% of the population at risk of inadequate intake. Especially in developing countries, VA deficiency poses a significant public health concern, potentially leading to night blindness, compromised immune system function, increased susceptibility to infectious diseases, and other health issues. Children and pregnant women are particularly vulnerable to the impact of VA deficiency. The addition of VA to dietary salt has been identified as an effective measure for the prevention and alleviation of VA deficiency, thereby enhancing the overall health status of the population.
The molecular structure of VA includes several conjugated double bonds and alkyl chains. Its high reactivity and sensitivity make it susceptible to the influence of oxidation, photolysis and other environmental factors. Exposure to ultraviolet radiation or oxidation can lead to processes such as oxidation, addition, cleavage, polymerization, and dehydration, resulting in structural modifications (Gonçalves et al. 2016). To ameliorate this phenomenon, microencapsulation technology is employed for the encapsulation of VA. It offers several advantages, such as substantially reducing the degradation of active components and prolonging the shelf life of food products. Widely applied in fats and oils, enzymes, this technology enhances sensory appeal, improving palatability and contributing to enhanced nutritional and economic values. Continual advancements in processes, equipment, and materials have matured microencapsulation technology, expanding its pervasive application in food industry.
The current methodologies employed for microencapsulation in the food industry encompass various techniques, broadly categorized into physical, chemical, and physicochemical methods. Physical methods consist of spray drying, extrusion, freeze-drying, multiple emulsion, suspension polymerization and molecular embedding. Chemical methods involve pore coagulation and interfacial polymerization. Physicochemical methods include complex coacervation and phase separation, among others (Li et al. 2018; Dan et al. 2015). The basic process of preparing microcapsules by complex coacervation began by preparing shell material, core material, and an emulsifier solution, which were homogeneously combined to create a stable mixture. Inducing a compound coalescence reaction enhanced the fusion of emulsified droplets, promoting microcapsule formation. Curing strengthened microcapsule walls for stability, followed by freeze-drying to remove moisture. The resulting dried microcapsules were finely ground for the desired particle size.
This study aimed to enhance VA stability by employing gelatin, carboxymethyl cellulose, and salt in complex coacervation as shell materials for microcapsule encapsulation. Preparation conditions were systematically determined. Investigating the rarely reported incorporation of salt to enhance mechanical properties of microcapsule shells, the findings provide valuable insights for improving shell quality and expanding market value in the seasoning industry.
Materials and methods
Materials
VA oil(food-grade, 99%) was procured from Guochen Biotechnology Company Limited(Henan, China), gelatin(Type A, C.P), carboxymethyl cellulose(CMC, C.P), sodium alginate(C.P), gum arabic(C.P), maltodextrin(C.P), octenylated starch, and ginkgo biloba polysaccharide were supplied by Yuxing Biotechnology Company Limited(Henan, China). Food-grade sucrose ester and transglutaminase were purchased from Kangda Food Engineering Company Limited (Shanghai, China). Tween 80 (C.P), Span 40 (99%), and glutaraldehyde (A.R) were obtained from Chemiol Chemical Reagent Company Limited(Tianjin, China).
All other chemicals used in this study were of chemical pure.
Experimental methods
Preparation method determination
As described in Table 1 (Chen et al. 2013; Lu et al. 2018; Borreguero et al. 2010; Özonur et al. 2006; Song et al. 2013), considering the lipid solubility of VA oil and its potential impact on the load, encapsulation efficiency(EE), particle size, and the activity of VA in the microcapsules, we employed the complex coacervation as the preparation technique for VA microcapsules.
Table 1.
The method, applicable range, characteristics, and current research status of microencapsulation
| Preparation method | Applicable range | Characteristics | Core material | Wall material | EE value (%) |
|---|---|---|---|---|---|
| Spray drying | Lipophilic/thermosensitiv substances | The drying process is brief; the particle size distribution is uneven, with some exhibiting concave fractures | Paraffin | Silicon dioxide |
82.2 (Chen et al. 2013) |
| Interfacial polymerization | Hydrophobic substances | Good compactness, high requirements for wall materials, poor mechanical performance | Butyl stearate | Polyurethane resin |
78.5 (Lu et al. 2018) |
| Suspension polymerization | Porous particles adsorbing liquid or encapsulating solid particles | Exhibits good heat resistance, prone to surface damage, and has low yield | Paraffin | Polybutyl acrylate |
75.6 (Borreguero et al. 2010) |
| Complex coacervation | Water-insoluble/bioactive substances | High yield, high efficiency, minimal impact on bioactivity | Paraffin | Gelatin / gum arabic |
80.0 (Özonur et al. 2006) |
| Pyridylmethylamine | Gelatin / sodium alginate |
61.21 (Song et al. 2013) |
Refined shell material selection
Improved and based on the methodologies established by Tang and Xuan (2022) and Zhang and Li (2018), this study employed a mixture of gelatin at specific concentrations with certain concentrations and volumes of CMC, sodium alginate, gum Arabic, maltodextrin, octene-esterified starch, and ginkgo biloba polysaccharide solutions as shell materials. The electrostatic interaction of these shell materials in aqueous solutions was assessed through conductivity titration, aiming to identify the most optimal shell material for selection (Rinaudo et al. 1997).
Optimal pH determination
The pH of the reaction mixture was precisely modulated to 3.0, 3.5, 4.0, 4.5, and 5.0 by the incremental addition of distinct volumes of glacial acetic acid to the shell solution composed of an equimolar blend of gelatin (1.0%, w/v) and CMC (1.0%, w/v). Following thorough mixing, the conductivity of the resultant solution was measured through conductivity titration (Lai 2016).
Shell material proportions optimization
The mixtures were prepared using gelatin solution(1.0%, w/v) and CMC solution(1.0%, w/v) at varying mass ratios of 7:1, 6:1, 5:1, 4:1, and 3:1 under optimal pH conditions determined in Sect. “Optimal pH determination”. Subsequently, the mixtures were homogenized on a high-speed disperser(1750G, IKA Works, Guangdong, China) for 10 min to form emulsions. Following homogenization, the conductivity of the mixtures was determined via conductivity titration, and the transmittance was measured using a spectrophotometer (722S, SPSIC, Shanghai, China) at 565 nm.
Emulsion preparation
VA microcapsules were synthesized using a complex coacervation. Gelatin solution (1.0%, w/v) and CMC solution(1.0%, w/v) were dissolved in distilled water. An appropriate quantity of emulsifier and VA oil were introduced into the gelatin solution, followed by the gradual addition of glacial acetic acid solution(10%, v/v) to adjust the pH of the reaction system. The emulsion was homogenized using a high-speed disperser (1750 G) for 10 min (Li et al. 2023).
Emulsifier selection and dosage determination
The gelatin and CMC solution was prepared by combining them in a 5:1 ratio. The core and shell materials were mixed in a 1:3 ratio under a pH of 3.5. Monoglycerides, sucrose esters, Tween 80, and Span 40 were incorporated at 2% of the total core and shell material respectively. Subsequently, 10 mL of the resulting emulsion was dispensed into a 25 mL colorimetric tube, and the homogenized emulsion was subjected to a water bath (HH-6, Guohua, Changzhou, China) set at 50 °C for a duration of 1 h. Measurements of the heights of the cohesive and aqueous phases for various emulsifiers were recorded after 30 and 60 min of standing. Consequently, the emulsion stability index (ESI) (Liang et al. 2017) was documented within the feasible range of 0 (poor stability)–1(high stability).
Emulsion stability is calculated by the following equation.
| 1 |
V1: total water amount (ml). V2: free water amount (ml).
Sucrose esters were incorporated at concentrations of 0.5%, 1.0%, 1.5%, 2.0%, 2.5%, and 3.0% relative to the total core and shell materials. The resulting mixture was then dispensed into a 25 mL colorimetric tube. Emulsion stability was subsequently determined using the aforementioned equation.
Cross-linking agent selection and dosage optimization
The gelatin solution (1.0%, w/v) and CMC solution (1.0%, w/v) were combined in a ratio of 5:1. The core and shell materials were assembled in a mass ratio of 1:8 at pH 3.5. Sucrose ester was introduced at a concentration of 3.0%, and equimolar quantities of transglutaminase (TG) and glutaraldehyde were added under the same conditions, accounting for 1%, 3%, 5%, 7%, 8%, 12%, 16%, and 20% of the total core and shell materials respectively. The synthesized emulsion was homogenized at 50 °C for 1 h. Subsequently, the obtained wet microcapsules were freeze-dried in a lyophilizer(18–006, Xinzhi, Ningbo, China) at − 70 °C for 24 h. The resulting dried samples were then ground to obtain VA microcapsules. The optimal dose of the cross-linking agent was determined by evaluating the EE value.
EE value represented the proportion of core substance content to microcapsule content. Elevated EE values correlated with reduced exposure of core substance on the microcapsule surface. This characteristic represents the stability of microencapsulated products.
The formula for determining the EE value of VA microcapsules is as followed (Xia 2016).
| 2 |
W1: total oil amount (g). W2: surface oil amount (g).
Core–shell ratio selection
The gelatin solution (1.0%, w/v) and CMC solution (1.0%, w/v) were combined at a ratio of 5:1. The core and shell materials were introduced at mass ratios of 1:3 to 1:10, while sucrose esters were incorporated at 3.0% under pH 3.5. The resulting emulsion was homogenized and subjected to a water bath at 50 °C for 1 h. The ensuing wet microcapsules were freeze-dried in a lyophilizer at − 70 °C for 24 h, followed by grinding to produce VA microcapsules. The optimal core–shell ratio was determined by assessing EE value.
Microencapsulation of VA
Gelatin, carboxymethyl cellulose, and salt were prepared into aqueous solutions in specific mass ratios. VA oil and an emulsifier were added to the gelatin solution and stirred to dissolve. Acidic reagents commonly used in the food processing industry, such as acetic acid, citric acid, lactic acid, etc., were prepared into aqueous solutions of appropriate concentrations and subsequently added to the solution to adjust to the desired pH. Carboxymethyl cellulose and salt aqueous solutions were added followed by homogenization to obtain a homogeneous slurry. The slurry was incubated at 50 °C for 30 min, and the solution's pH was adjusted to 6 using food-grade reagents such as sodium hydroxide and potassium hydroxide. The cross-linking agent was added and stirred until dissolved. The wet microcapsules were then freeze-dried at − 70 °C for 24 h in a lyophilizer, resulting in the dried sample, i.e. VA microcapsules.
To determine the loss of vitamin A during the microencapsulation process, we followed the guidelines outlined in “GB 5009 National Standards for Food Safety” VA microcapsules were dissolved, saponified, extracted, and washed, and the determination was carried out using HPLC. The chromatographic conditions included a C30 column (250 mm length, 4.6 mm inner diameter, 3 μm particle size) maintained at 20 °C. The mobile phase consisted of A:water and B:methanol, with a gradient as specified in Supply File 5. UV detection was performed at 325 nm, the injection volume was 10 μL.
Orthogonal experiment
In the initial phase of experimentation, we verified that cross-linking agent dosage, emulsifier dosage, and core–shell Ratio were the primary factors influencing microencapsulation. Three representative levels were chosen from each factor, following a Latin square experimental design, where the bidirectional error design enhances the grouping and balancing of experimental groups, thus further reducing experimental errors, as illustrated in Supply File 1.
The orthogonal experiments were conducted in accordance with the specifications outlined in Supply File 2, ki denotes the mean EE value for each factor at its respective level, and R (range) represents the disparity between ki max and ki min. The ki value laid the groundwork for determining the optimal level of each factor, while the R value quantified the extent of influence exerted by each factor.
Characterization of microcapsules
Determination of EE value
The determination of microcapsules surface oil followed the procedure outlined by Yang with improvements (Yang et al. 2015). Precisely 2 g of the sample was weighed, and 40 mL of petroleum ether was added, followed by vortex shaking for 3 min. The resulting solution underwent filtration through a Brinell funnel, this process was repeated thrice, with the collected filtrate being preserved. Subsequently, the solvent within the sample underwent evaporation using a rotary evaporator (OSB-2100, Quanjie, Shanghai, China), followed by drying in a dryer (PH-070A, Yiheng, Shanghai, China) at 105 °C for 2 h to eliminate residual solvent. The residual solvent was ultimately determined through a weight-based method.
For the determination of total oil in microcapsules, 2 g of the sample was precisely weighed and dissolved in 8 mL of water. Subsequently, 10 mL of 36% HCl by volume was added, and the sample was immersed in a water bath at 70 °C for 50 min. Following the completion of hydrolysis, the sample was allowed to cool to room temperature. Anhydrous ethanol 10 mL and ether 25 mL were added, and the mixture was shaken and agitated before being transferred to a separatory funnel. The supernatant was collected, and the solvent within the sample was evaporated using a rotary evaporator. The remaining substance was subjected to an oven at 105 °C for 2 h to eliminate any residual solvent, ultimately determined through a weight-based method.
Microcapsule morphology examination
A minute quantity of VA microcapsule suspension was applied onto a slide. The microcapsules morphology was examined through an optical microscope, and subsequent photographic documentation and recording of the samples were conducted.
Stability characterization
Storage stability assessment
Microcapsule samples, pre- and post-cross-linking, were stored under identical conditions for a duration of 30 days, with continuous observation and recording of the ensuing results.
Acid value
The acid value was determined through the dilution of 1 g of desiccated microcapsule samples with a 1:1 volume ratio mixed benzene-ethanol solution (50 mL). Subsequently, 0.01 g/mL phenolphthalein ethanol solution was added, followed by titration with a 0.10 mol/L potassium hydroxide solution until the indicator transitioned from red to colorless, indicating the endpoint of titration (Zhang et al. 2023a, b). Blanks, consisting of an equivalent quantity of VA and VA−VE(1%) samples, were employed for 9 consecutive days. The acid value for each sample was daily measured at the specified time.
TBA analysis of microcapsules
1 g of the sample was weighed, and 25 mL of trichloroacetic acid was added, followed by filtration. Subsequently, 10 mL of the filtrate was transferred into a 25 mL colorimetric tube. To this, 10 mL of a 0.02 mol/L thiobarbituric acid solution was added, thoroughly mixed, and subjected to incubation in a water bath at 90 °C for 40 min. After cooling, centrifugation was performed at 7100×g for 5 min (GL-21 M, Xiangyi, Changsha, China). The supernatant was then transferred into a nanochrome tube, and 5 mL of chloroform was added, followed by thorough shaking. Absorbance was determined at 532 nm, with the blank consisting of a sample-free solution (Lu 2021). Blank controls, comprising equal quantities of VA and VA−VE(1%), were employed for 11 consecutive days.
Verification of mechanical stability
20 g of VA microcapsules, with and without salt in the shell material, were weighed and placed in a 150 mL conical flask. Subsequently, 10 mL of anhydrous ethanol was added. The conical flask was positioned in an ultrasonic shaker (KQ-100E, Ultrasonic Instrument, Kunshan, China), and 5 mL of the solution was extracted after 30 and 60 min of ultrasonic shaking at 10 MHz. To this solution, 10 mL of potassium hydroxide and 30 mL of ethanol were added, followed by saponification. The VA content was determined using the colorimetric method outlined in GB/T 5009.82-2003. The relative content of VA in the other samples was calculated with the VA content in the microcapsule sample of the shell material without added salt after 60 min of treatment set as 100%.
In vitro release analysis
1 g of lyophilized microcapsules was combined with 100 mL of simulated gastric fluid (pH = 1.2) and intestinal fluid(pH = 6.8) in a 250 mL conical flask. The conical flask was then positioned in a thermostatic shaker (Eppendorf, Shanghai, China) and subjected to agitation at 100 r/min at 37 °C (Lu 2021). The VA content of 1 mL was assessed at intervals of 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 h, respectively, and release curves were constructed.
Data processing
Three parallel experiments were conducted, and distinctions between means were ascertained through analysis of variance (ANOVA) and Tukey’s test, maintaining a significance level of 95%(p < 0.05). The data were processed and graphed using Origin 9.1.
Results and analysis
Optimal microencapsulation process
Shell materials selection and EE
Materials employed in the fabrication of optimal microencapsulated products should exhibit high solubility, low viscosity, and afford protection to the core material against oxidation (Pérez-Alonso et al. 2003). Gelatin, serving as the primary shell material, possesses both polyanionic and polycationic characteristics contingent upon alterations in the aqueous solution’s pH. Leveraging the amphoteric property of gelatin (Milanović et al. 2014) and recognizing that acidic and basic ionizable amphoteric groups attain equal ionization at specific pH levels, complex coacervation approach was employed for microcapsule preparation. Shell materials with charges opposing gelatin were chosen (Shen et al. 2016). Given that CMC is anionic polyelectrolyte exhibiting thermal stability, preliminary experiments and Table 1 established that the shell performance of CMC and gelatin surpassed that of maltodextrin, octenyl succinate starch, gum arabic, ginkgo biloba polysaccharide, and sodium alginate.
In this study, the complex interaction with gelatin and CMC was augmented through the incorporation of salt as the shell material, aiming to achieve improved integrity and denseness. The embedding rate reached 93%. The investigation into the augmentation of stability in microcapsule shell materials through the incorporation of salt is currently underexplored in the existing literature. Our study demonstrated stable effectiveness with an EE from 81.05 to 89.06%.
The study aims to enhance the nutritional content of VA-fortified salt by incorporating a specific quantity of sodium chloride into the shell material. This not only increases the sodium chloride content but also improves encapsulation efficacy. During the complexation process, salt ions bind with oppositely charged sites, creating a shielding effect that mitigates electrostatic interactions, resisting electrostatic repulsion among protein molecules at low ion concentrations (Zhang et al. 2023a, b). This enhances the composite interaction between salt ions and gelatin, reinforcing the mechanical properties of the shell material and improving stability.
Optimal pH for microencapsulation
The optimal yield of microcapsules, produced through complex coacervation, is achieved when electrostatic equilibrium is attained between the positively charged gelatin and the negatively charged CMC at the pH value (Butstraen and Salaün 2014). Simultaneously, the electrical conductivity of the blended solution reaches its minimum. Experimental findings indicate a biphasic trend in the electrical conductivity of the shell solution concerning pH. Illustrated in Fig. 1a, the solution exhibited the lowest conductivity at pH 3.5, correlating with the highest efficiency in microcapsule yield obtained through the complex coacervation, this aligns with the findings presented by Lai (2016).
Fig. 1.

Impact of solution pH on the solution conductivity of the shell material (a) / Influence of the gelatin-to-CMC ratio on conductivity (b) / turbidity (c) of shell material solution
As the solution pH gradually increases from 2 to 3.5, a progressive decrease in conductivity is observed, indicating a reduction in free charges in the solution. Simultaneously, the electrostatic driving forces between large molecules gradually intensify, initiating the formation of complexes between gelatin and CMC. However, at pH levels exceeding 3.5, the enhanced electrostatic forces between large molecules lead to excessive coacervation, influencing the particle size of microcapsules. Additionally, the increased abundance of free charges weakens the electrostatic interaction between gelatin and CMC, preventing the formation of a stable shell.
Optimal gelatin-CMC ratio for microencapsulation
In Fig. 1b, c, it is evident that, within the gelatin-to-CMC ratios of 3:1–5:1, there was a notable increase in both conductivity and transmittance differences observed between the mixed shell materials before and after complex coacervation. Conversely, within the range of 5:1–7:1, a decreasing trend in both conductivity and transmittance differences was observed. Notably, at a specific gelatin-to-CMC ratio of 5:1, the conductivity and transmittance differences reached their maximum values, signifying this particular ratio as optimal for microcapsule preparation. These findings underscore the significance of the gelatin-to-CMC ratio in influencing the complex coacervation process and subsequent material properties.
Within a specific pH range, the complex coacervation reaction exhibited an initial increase followed by a decrease, potentially attributed to an excess of gelatin in the solution. This led to heightened solution conductivity and a weakened complex coalescence reaction. Concurrently, the differences in electrical conductivity and transmittance of the mixed shell materials decreased with escalating CMC content. Notably, at a 5:1 ratio, a balance was achieved where the charges of gelatin and CMC equilibrated, reducing free charges in the solution, resulting in decreased conductivity. This scenario, characterized by substantial conductivity and transmittance differences before and after complex coacervation, marked the completion of the complex coalescence reaction.
Microcapsule complex condensates were synthesized by incorporating NaCl at concentrations of 0.05 mol/L to 0.3 mol/L during the preparation process, resulting EE values reached to 94.46%, 95.33%, 94.81%, and 83.27% respectively. Analysis of coacervation yield data indicates that optimal ionic strength is achieved at a NaCl concentration of approximately 0.1 mol/L. However, when the concentration surpasses 0.3 mol/L, both the production rate and coacervation quality exhibit a noticeable reduction. This underscores the sensitivity of coacervation outcomes to NaCl concentration, emphasizing the need for precise control within the specified range for optimal microcapsule synthesis.
Optimal emulsifier for microencapsulation
The introduction of sucrose ester into the solution markedly reduces interfacial tension, thereby altering the system’s interfacial state. In Fig. 2a, the slope derived from the conductivity method serves as a metric for assessing emulsion stability. A lower parameter value indicates increased emulsion stability, the effects of Tween 80 and sucrose esters are similar and higher compared to the other two. As depicted in Fig. 2b, the emulsion stability parameter indicates that Tween 80 exhibits slightly better emulsion stability than sucrose ester, albeit the difference lacks significance. Consequently, sucrose ester emerges as a viable choice for an emulsifier.
Fig. 2.
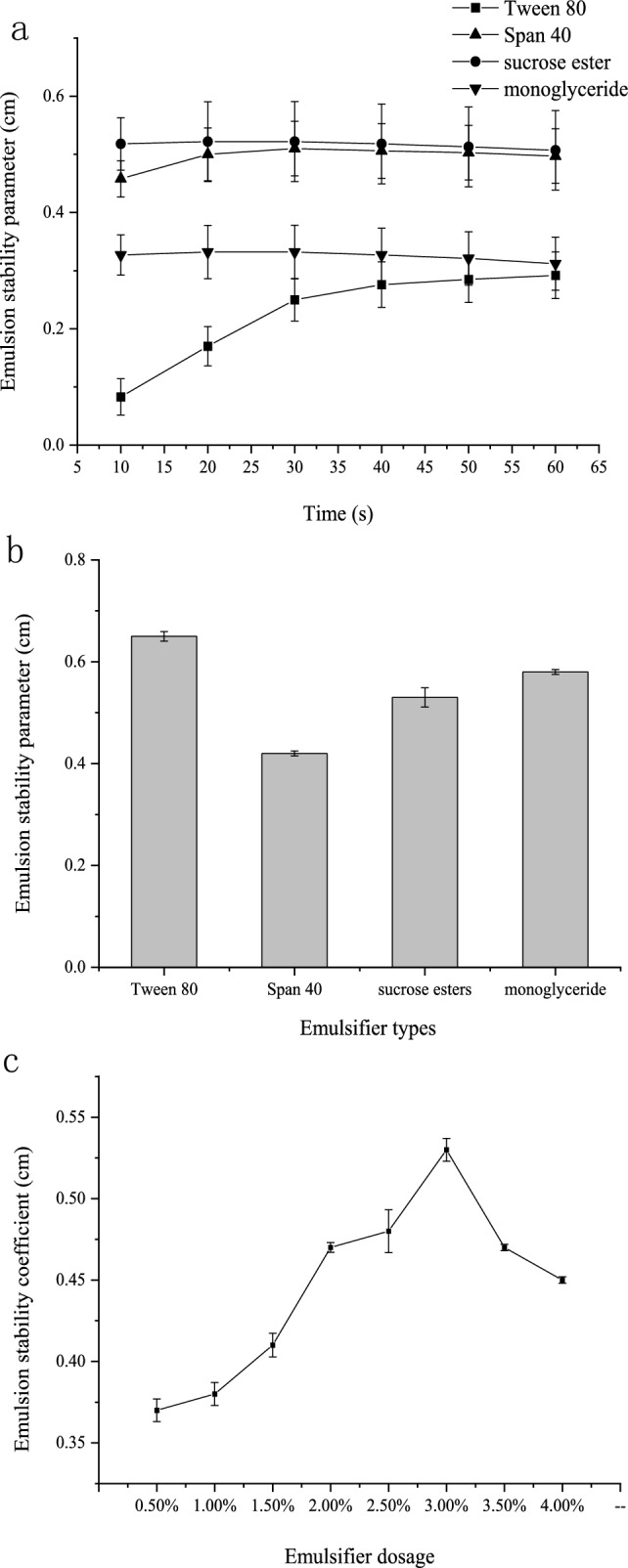
Effect of emulsifier types (a and b) / dosage (c) on emulsion stability
Compared to monoglycerides and Span 40, sucrose esters and Tween 80 have complex molecular structures with longer hydrophobic tails and multiple functional groups, enhancing surface activity at lower concentrations without compromising system stability. Sucrose esters, derived from natural sources like sucrose and fatty acids, possess superior naturalness and biocompatibility. In contrast, Tween 80, a synthetic non-ionic surfactant, may raise concerns or discomfort in specific applications.
Optimal emulsifier dosage
In Fig. 2c, the system exhibited peak emulsification stability, registering a maximum value of 0.53 at 3.0%. Consequently, 3% of the overall core and shell material was identified as the optimal emulsifier for VA microcapsules.
Increased emulsifier quantity directly improves emulsion stability, demonstrating a notable emulsification effect. Insufficient sucrose esters fail to adequately cover the interface, causing instability. Excessive sucrose esters lead to an overly thick film, increasing interfacial tension and instability. Beyond a threshold, excess emulsifier affects rheological properties, decreasing stability due to hindered shear dispersion. High concentrations of small-molecule emulsifiers displace proteins, reducing stability in later stages.
Optimal cross-linking agent for microencapsulation
Based on the data presented in Fig. 3b, no significant distinction was observed in the cross-linking efficacy between equal quantities of TG and glutaraldehyde. Nonetheless, TG is a natural enzyme, typically extracted from microorganisms or plants. In comparison with chemical crosslinking agents such as glutaraldehyde, this natural origin renders TG more favorably regarded in terms of safety. Moreover, the extent of TG crosslinking can be modulated by adjusting reaction conditions, affording flexibility in fine-tuning the degree of crosslinking during food processing. TG exhibits the capability to accomplish crosslinking under relatively mild conditions, contributing to the preservation of biological activity and sensory attributes of constituents within emulsions.
Fig. 3.

Influence of the core–shell ratio (a)/ cross-linking agent types (b)/ cross-linking agent dosage on the EE value of VA (c)
Therefore, in this study, we opted for TG as the crosslinking agent instead of glutaraldehyde.
Optimal cross-linking agent dosage
As shown in Fig. 3c, the dosage of the cross-linking agent was increased from 1 to 20%. During the range from 1 to 12%, EE value of the microcapsules exhibited a continuous rise, reaching its peak at 12% with an EE value of 72%. Consequently, the optimal cross-linking agent quantity was determined to be 12% of the total core and shell material.
Experimental findings underscored a positive correlation between the cross-linking effect and TG content in initial stage. However, cross-linking agent dosage increased, the cross-linking effect plateaued, and then slightly diminished. This phenomenon is attributed to elevated TG content thickening the microcapsule shell. Beyond a certain threshold, the reaction might reach saturation within a short period, resulting in excessive crosslinking and leading to the loosening and irregular structure of the gel network.
Optimal core–shell ratio
As depicted in Fig. 3a, a rising trend in microcapsule efficiency was observed within the core material to shell material ratios of 1:3–1:8, followed by a subsequent decline in the range of 1:8–1:10. A peak efficiency of 48.84% was achieved at a 1:8 ratio. Consequently, the optimal ratio for the preparation of microcapsules was determined to be 1:8.
Elevated core–shell ratios led to reduced EE values in uncured microcapsules, causing surface oil accumulation, hindering flow, and compromising storage stability. Initial-stage findings showed a positive correlation between EE value and core–shell ratio. Conversely, higher core–shell ratios resulted in mitigated EE values. Increasing shell ratio gradually enhanced microcapsule efficiency due to augmented shell thickness, hardness, and improved efficiency. However, excessive shell material viscosity and abundance hindered favorable microcapsule formation.
Orthogonal experiments
As per the information provided in Supply File 1, the hierarchical order of influence of various factors on microcapsule efficiency is as follows: emulsifier quantity > crosslinker quantity > core–shell ratio. The optimal configuration for this experiment is denoted as A2B3C2. The optimal procedural conditions for the preparation of VA microcapsules encompass a cross-linking agent dosage constituting 12% of the total core–shell material, an emulsifier dosage comprising 3.5% of the total core–shell material, a core-to-shell ratio of 1:8, resulting in a microencapsulation rate of 75.01%.
Microcapsule formation
Gelatin, carboxymethyl cellulose, and salt were weighed according to the mass ratio of 5:1:0.1 and then formulated into aqueous solutions respectively. VA oil and emulsifier sucrose ester were added to the aqueous gelatin solution, maintaining a mass ratio of 1:8 for the core material and shell material. The total weight of the shell material comprised the combined quantity of gelatin, carboxymethyl cellulose, and salt. Stirring and dissolution took place and adjusted to the pH to 3.5, acidic citric acid was added either directly to the original solution or configured into an appropriate concentration of aqueous solution and introduced drop by drop. Aqueous carboxymethyl cellulose solution and aqueous salt solution were added drop by drop, followed by homogenization at 14,200×g for 30 min to obtain the homogenized solution. The resulting homogenized solution was incubated at 50 °C for 30 min. The pH of the solution was adjusted to 6 using food-grade reagents such as sodium hydroxide and potassium hydroxide. Subsequently, 12% cross-linking agent TG was added and dissolved, and the solution was further incubated at 50 °C for 30 min. The solution was then freeze-dried, resulting in the production of VA microcapsules. As shown in Supply File 6, the loss rate of Vitamin A after microencapsulation was 9.85%.
In accordance with the “China Dietary and Nutrition Survey report”, the recommended daily intake for salt consumption per capita is 5 g/d, while the recommended daily intake for VA is 1.5 mg/day. According to the “Chinese Resident Dietary Guidelines Scientific Research Report”, the average daily intake of VA is only 66.67% of the recommended intake. We aim to supplement the deficiency in VA intake through daily salt consumption, reaching 90% of the recommended intake, to prevent potential cases of excessive VA intake in certain population groups. Therefore, we propose an addition of 70 mg/1000 g of this microencapsulated VA to edible salt.
Microcapsules characterization results
Stability experiments
Microencapsulated nutrient salts containing VA were subjected to storage under identical room temperature dry conditions for a duration of 9 weeks. The surface of the uncured microcapsules exhibited a light yellow hue, and the microcapsules displayed a sticky consistency, indicated that the extrusion of encapsulated VA oil and poor storage stability. Upon solidification, the microcapsule surface appeared white and dry, suggesting the absence of VA oil extrusion and demonstrating favorable storage stability. The incorporation of TG during entrapment and complex coacervation was found to enhance EE value. This cross-linking reaction not only facilitated the formation of high molecular weight protein polymers but also enhanced the binding strength and thermal stability of the cross-linked proteins. The selection of TG also ensuring their safety and harmlessness to humans.
The encapsulation rate measurement results, as presented in Fig. 4c, indicated that after 9 weeks of storage, the encapsulation rate of VA microcapsules was 75.38%, representing a decrease of only 5.74% from the initial value of 81.12%, demonstrated excellent storage stability.
Fig. 4.
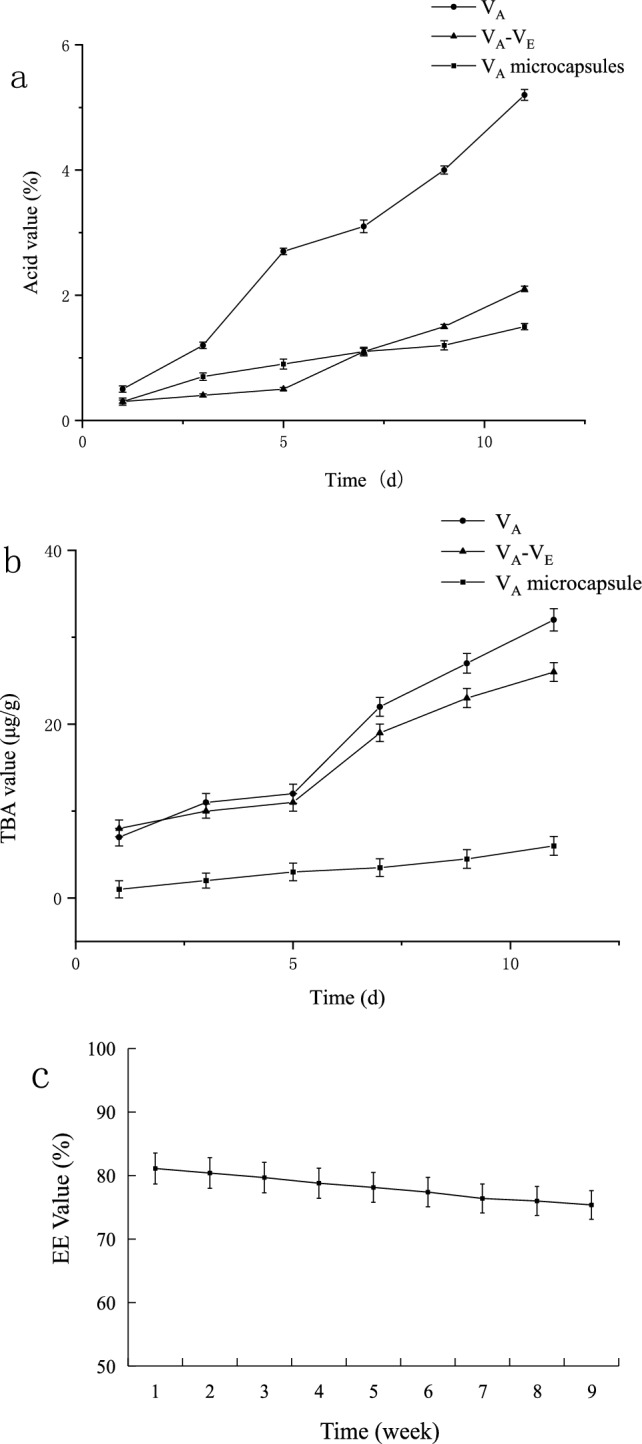
Impact of storage time on acid value (a) / TBA value (b) / EE value (c)
Morphological observation
In accordance with Supply File 3, The results demonstrate that almost all microcapsules exhibit a regular spherical morphology, with no leakage of core material and no rupture of the shell material. The surfaces of these particles displayed an absence of seams or cracks, thereby facilitating enhanced protection for the encapsulated core material and minimizing its susceptibility to oxidation. The irregularities observed in certain microcapsule particles may be attributed to alterations in microcapsule morphology induced by the evaporation of water during vacuum extrusion and freeze-drying (Assegehegn et al. 2019).
Acid value determination
Blanks consisting of equal proportions of VA and VA−VE(1% i.e. VA containing 1% VE) were prepared. The acid values of each sample were measured over a span of 9 consecutive days at the specified time daily, with the results depicted in Fig. 4a. As time elapsed, the acid values of both VA and VA−VE significantly surpassed those of VA microcapsules, signifying the effective role played by microcapsules shell in impeding the generation of free fatty acids in oils and fats. Moreover, the inhibitory capacities of VA and VA−VE were essentially comparable.
The crosslinking interaction between gelatin and CMC can yield a microcapsule shell that could creat a physical barrier to prevent direct contact of the VA oil core with air, moisture, and other external factors. Simultaneously, the abundant hydroxyl functional groups in the molecules of gelatin and CMC contribute to the hydroxy functionality, aiding in maintaining the structural integrity of the microcapsule shell. This functionality reduces water permeation, thereby decelerating the acidification process of the VA oil core, this corresponds with the research outcomes reported by Lu (2021).
TBA value determination
Samples comprising identical quantities of VA and VA−VE(1%) were employed as blanks and subjected to measurement over an uninterrupted span of 11 days, as illustrated in Fig. 4b. The depicted results reveal a noteworthy increase in the TBA values of both VA−VE and VA over time. While the TBA values of the VA microcapsules also experienced an increase, the magnitude of this elevation was not particularly significant. These observations indicate that VA with a VE content of 1% exhibits a lesser efficacy in inhibiting the production of fatty aldehydes compared to VA microcapsules. The encapsulation process, specifically the optimal shell material, was identified as efficacious in safeguarding the core material and retarding the lipid oxidation process.
The TBA value is commonly employed to assess the lipid peroxidation content in food and other samples. The cross-linked structure of gelatin and CMC can give rise to a stable microcapsules shell, thereby effectively mitigating the permeation of oxygen and other oxidizing agents. Consequently, this diminishes the likelihood of lipid peroxidation (Fan et al. 2014).
Mechanical stability experiment
As shown in Supply File 4a, the relative quantity of VA in the remaining samples was determined, the VA content in the microencapsulated sample of the shell material without salt addition was considered as 100% after a 60-min treatment. In comparison with the shell material lacking salt addition, a reduction of 5.8% at 30 min and 14.5% at 60 min was observed in the leakage of VA from the shell material with salt addition. Consequently, microcapsules experienced fewer ruptures, resulting in diminished content leakage.
As depicted in Fig. 5a, b, the outcomes revealed a significant increase in the relative leakage of VA following ultrasonic treatment compared to the control group. Conversely, there was a notable decrease in leakage after the addition of salt compared to the control group. Importantly, no significant alteration was observed in ultrasonication with salt addition when compared to the condition without ultrasonication. It can be inferred that ultrasonication did not exert a significant influence on microcapsules with salt addition. To substantiate these findings, the surface microstructure of the samples was examined using optical microscopy and scanning electron microscopy. Supply File 4b) indicated that the addition of salt enhanced the surface densities of the microcapsules, demonstrating a more favorable effect.
Fig. 5.
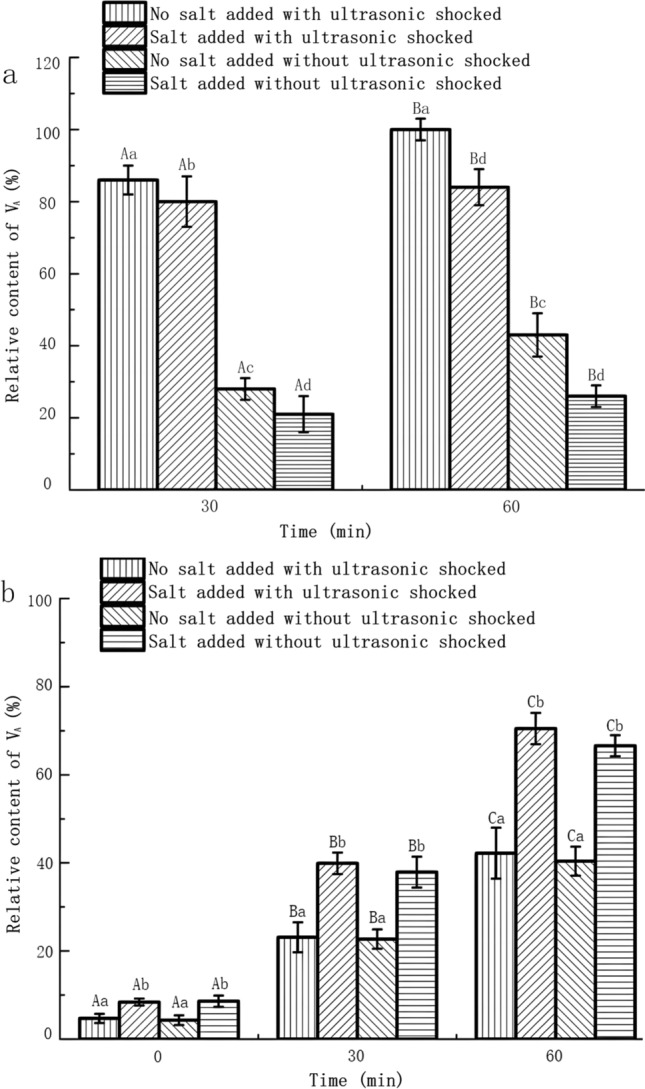
The influence of ultrasonic shocked on the relative content of VA (a) / conductivity (b) in microcapsules with or without edible salt added in the shell material
In vitro release of microcapsules
Supply File 4c illustrates the dynamic release of VA from microcapsules in gastric and intestinal fluid media. Following a 3-h incubation of microcapsules in simulated gastric fluid at pH 1.2, a modest quantity of core material (18.52%) was released, and the release rate was gradual, suggesting that the shell material did not undergo swelling or rupture under these conditions. Subsequently, 66.58% of the microcapsules remained intact in intestinal fluid at pH 6.8 after a 3-h release period, underscoring the favorable intestinal solubility of the microcapsules.
The release of the core substance in the intestinal fluid is higher than that in the gastric fluid, probably due to the hydrolytic effect on denatured proteins(gelatin) in the microcapsule shell material (Ketnawa et al. 2017), resulting in an increased release of the core substance in the intestinal fluid.
Conclusion and discussion
VA is an unsaturated monohydroxy compound with a lipid ring which comprises a series of conjugated double bonds, imparting an unsaturated structure. This distinctive structure grants VA its crucial functions in physiological processes such as the visual system, cell differentiation, and immune function. Deficiency in VA may lead to a range of clinical symptoms, including night blindness and ultimately blindness. Additionally, compromised immune function is a consequence of VA deficiency, potentially resulting in increased susceptibility to infections and heightened severity of infectious diseases. In children, prolonged VA deficiency may impact growth and development, as well as compromise the health of the immune system, posing a threat to life. The molecular structure of VA making it highly sensitive to environmental conditions and prone to instability. Specifically, the conjugated double bond structure within the molecule renders VA susceptible to oxidative effects, leading to a gradual loss of biological activity. Furthermore, the lipid ring structure makes it photosensitive to ultraviolet radiation, accelerating photooxidation reactions. These factors collectively contribute to the ease with which VA becomes inactive during food processing, storage, and cooking.
In this study, VA-fortified salt is obtained by mixing salt and VA microcapsules which was produced using the complex coacervation with the ratio of 70 mg:1000 g, this process effectively slows down the acidification and oxidation of VA. We characterized the morphology, acid value, TBA value, EE value, and mechanical stability of the microcapsules. Through orthogonal experiments, we determined that 12% of TG as a crosslinking agent and 3.5% sucrose ester as an emulsifier, with a core-wall ratio of 1:8, is the optimal process for microencapsulation of VA. Additionally, adding salt in a 5:1:0.1 ratio to the wall material provides better stability, resulting in an 81.12% encapsulation rate, with no surface cracks which exhibited the best storage and mechanical stability, In vitro release studies indicate that after 3 h incubation in simulated gastric fluid, microcapsules had an 18.52% release rate. In simulated intestinal fluid, this increased to 66.58%, demonstrating strong intestinal solubility and improved utilization of VA.
This study provides a unique low-sodium salt rich in VA microcapsules along with the method involving the addition of salt in the microcapsule wall material to enhance stability was employed which was scarcely any reports related or similar in nature currently. The use of salt as a carrier enhances the intake of VA in the population, offering universality, effective supplementation benefits, and practicality. It is of significant importance for alleviating the widespread issue of VA deficiency globally, especially in developing countries. The strategy of fortifying salt with nutrients is one of the effective means to address VA deficiency in the field of public health. This approach possesses a certain degree of market value and practical applicability.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- VA
Vitamin A
- VE
Vitamin E
- C.P
Chemical pure
- CMC
Carboxymethyl cellulose
- EE
Encapsulation efficiency
- TBA
Thiobarbituric acid
- A.R
Analytical reagent
- TG
Transglutaminase
Author contributions
Concept and design: NX; Implementation of the research process: NX, TZ; Data collection and analysis: YD; Drafting of article: YL; Critical revision of the article for important intellectual content: SY; All the authors approved the final article.
Funding
This work was supported by National Key R&D Program of China (2021YFD2100100) the National Natural Science Foundation of China (32171836).
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
There are no conflicts or competing interests reported to declare.
Ethical approval
No humans and animals were involved in this study so that ethical approval does not apply to this article.
Consent for participation and publication
All authors approved the manuscript, this submission and the publication of individual’s data or image in this study. This manuscript describes original work and is not under consideration by any other journal. The work described has not been published before and it is not under consideration for publication elsewhere. Its submission to JFST publication has been approved by all authors as well as the responsible authorities at the institute where the work has been carried out.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Assegehegn G, Brito-de E, la Fuente JM, Franco CG. The importance of understanding the freezing step and its impact on freeze-drying process performance. J Pharm Sci. 2019;108(4):1378–1395. doi: 10.1016/j.xphs.2018.11.039. [DOI] [PubMed] [Google Scholar]
- Borreguero AM, Carmona M, Sanchez ML, et al. Improvement of the thermal behaviour of gypsum blocks by the incorporation of microcapsules containing PCMS obtained by suspension polymerization with an optimal core/coating mass ratio. Appl Therm Eng. 2010;30(10):1164–1169. doi: 10.1016/j.applthermaleng.2010.01.032. [DOI] [Google Scholar]
- Butstraen C, Salaün F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohyd Polym. 2014;99:608–616. doi: 10.1016/j.carbpol.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Chen Z, Cao L, Fang G, et al. Synthesis and characterization of microencapsulated paraffin microcapsules as shape-stabilized thermal energy storage materials. Nanoscale Microscale Thermophys Eng. 2013;17(2):112–123. doi: 10.1080/15567265.2012.761305. [DOI] [Google Scholar]
- Dan L, Bao XL, Yang F. Preparation of uniform starch microcapsules by premix membrane emulsion for controlled release of avermectin. Carbohyd Polym. 2015;136:341–349. doi: 10.1016/j.carbpol.2015.09.050. [DOI] [PubMed] [Google Scholar]
- Debelo H, Ntnyovo JA, Ferruzzi MG (2017) VA. Oxford Univ Press, Oxford
- Fan WJ, Zhang YK, Zhang YK, Chen YC, Sun JX, Yi YW. TBARS predictive models of porksausages stored at differenttemperatures. Meat Sci. 2014;96(1):1–4. doi: 10.1016/j.meatsci.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Gonçalves A, Estevinho BN, Rocha F. Microencapsulation of VA: a review. Trends Food Sci Technol. 2016;51:76–87. doi: 10.1016/j.tifs.2016.03.001. [DOI] [Google Scholar]
- Ketnawa S, Benjakul S, Martínez-Alvarez O, Rawdkuen S. Fish skin gelatin hydrolysates produced by visceral peptidase and bovine trypsin: bioactivity and stability. Food Chem. 2017;215:383–390. doi: 10.1016/j.foodchem.2016.07.145. [DOI] [PubMed] [Google Scholar]
- Lai MF. Complex coacervation of gelatin and sodium Carboxymethyl cellulose and preparation of microcapsules. Wuxi: Jiangnan University; 2016. [Google Scholar]
- Li Y, Wu C, Wu T. Preparation and characterization of citrus essential oils loaded in chitosan microcapsules by using different emulsifiers. J Food Eng. 2018;217:108–114. doi: 10.1016/j.jfoodeng.2017.08.026. [DOI] [Google Scholar]
- Li Y, Wang FF, Zang M, et al. Preparation and characterization of vitamin A microcapsules by complex coacervation. J Dalian Dalian Polytechn Univ. 2023;42(04):235–241. doi: 10.19670/j.cnki.dlgydxxb.2023.0401. [DOI] [Google Scholar]
- Liang Y, Gillies G, Matia ML. Structure and stability of sodium caseinate stabilized oil in water emulsions as influenced by heat treatment. Food Hydrocolloids. 2017;66:307–317. doi: 10.1016/j.foodhyd.2016.11.041. [DOI] [Google Scholar]
- Lu YH. Study on microencapsulation, physicochemical properties and stability of tree peony seed oil. Jinan: Qilu University of Technology; 2021. [Google Scholar]
- Lu S, Shen T, Xing J, et al. Preparation and characterization of cross-linked polyurethane shell microencapsulated phase change materials by interfacial polymerization. Mater Lett. 2018;211:36–39. doi: 10.1016/j.matlet.2017.09.074. [DOI] [Google Scholar]
- Milanović J, Petrović L, Sovilj V, Katona J. Complex coacervation in gelatin/sodium caseinate mixtures. Food Hydrocolloids. 2014;37:196–202. doi: 10.1016/j.foodhyd.2013.10.016. [DOI] [Google Scholar]
- Özonur Y, Mazman M, Paksoy HÖ, et al. Microencapsulation of coco fatty acid mixture for thermal energy storage with phase change material. Int J Energy Res. 2006;30(10):741–749. doi: 10.1002/er.1177. [DOI] [Google Scholar]
- Pérez-Alonso C, Báez-González JG, Beristain CI, Vernon-Carter EJ, Vizcarra-Mendoza MG. Estimation of the activation energy of carbohydrate polymers blends as selection criteria for their use as wall material for spray-dried microcapsules. Carbohyd Polym. 2003;53(2):197–203. doi: 10.1016/S0144-8617(03)00052-3. [DOI] [Google Scholar]
- Rinaudo M, Roure I, Milas M, Malovikova A. Electrostatic interactions in aqueous solutions of ionic polysaccharides. Int J Polym Anal Charact. 1997;4(1):57–69. doi: 10.1080/10236669708033937. [DOI] [Google Scholar]
- Shen LL, Chen JP, Bai YJ, Ma ZC, Huang J, Feng W. Physical properties and stabilization of microcapsules containing thyme oil by complex coacervation. J Food Sci. 2016;81(9):58–62. doi: 10.1111/1750-3841.13397. [DOI] [PubMed] [Google Scholar]
- Song SJ, Li Z, Liu WH, et al. Study on the preparation and properties of gelatin sodium alginate gum sustained releasing microspheres for acetamiprid. J Agric Univ Hebei. 2013;36(02):90–93. doi: 10.13320/j.cnki.jauh.2013.02.018. [DOI] [Google Scholar]
- Tan LB, Green MH, Ross AC. Vitamin A kinetics in neonatal rats vs. adult rats: comparisons from model-based compartmental analysis. J Nutr. 2015;145(3):403–410. doi: 10.3945/jn.114.204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XY, Xuan JY. Preparation and physicochemical properties of tuna oilmicrocapsules using complex coacervation. Chin Oil Fats. 2022;47(11):103–109. [Google Scholar]
- Xia HT. Preparation and characterization of olive oil microcapsule by complex coacervation. Harbin: Northeast Agricultural University; 2016. [Google Scholar]
- Yang X, Gao N, Hu LD. Development and evaluation of novel microcapsules containing poppy-seed oil using complex coacervation(Article) J Food Eng. 2015;161:87–93. doi: 10.1016/j.jfoodeng.2015.03.027. [DOI] [Google Scholar]
- Zhang B, Li JD. Study on stability of VA microcapsules prepared with 4 different embedded materials. Food Drug Law J. 2018;20(06):456–460. [Google Scholar]
- Zhang CH, Huang LX, Xie PJ, et al. Storage stability of olive oil microencapsulated by ultrasonic-spray drying. Biomass Chem Eng. 2023;57(03):15–22. [Google Scholar]
- Zhang J, Du H, Ma N, et al. Effect of ionic strength and mixing ratio on complex coacervation of soy protein isolate/Flammulina velutipes polysaccharide. Food Sci Human Wellness. 2023;12(01):183–191. doi: 10.1016/j.fshw.2022.07.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


