Abstract
The study aimed to optimize ultrasonic (US: 40 kHz/200 W for 10, 20, 30, 40, and 50 min), and microwave (MW: 160 W for 45, 90, 125, 180, and 225 s) pretreatment conditions on protein extraction yield and degree of protein hydrolysis (DH) from almond de-oiled meal, an industrial by-product. First order model was used to describe the kinetics of almond protein hydrolysates obtained with Alcalase. The highest DH, 10.95% was recorded for the US-50 min and 8.87% for MW-45 s; while it was 5.76% for the untreated/control sample. At these optimized pretreatment conditions, a 1.16- and 1.18-fold increment in protein recovery was observed for the US and MW pretreatments, respectively in comparison to the conventional alkaline extraction. The molecular weight distribution recorded for pretreated samples disclosed a significant reduction in the band thickness in comparison with control. Both the pretreatments resulted in a significant increase (P < 0.05) in the antioxidant activity, and TCA solubility index when compared with the control. Results evinced that US and/or MW pretreatments before enzymatic hydrolysis can be a promising approach for the valorization of almond meal for its subsequent use as an ingredient for functional foods/nutraceuticals which otherwise fetches low value as an animal feed.
Keywords: Almond de-oiled residue, Alcalase hydrolysis, First order kinetics, Ultrasonic, Microwave pretreatment, ORAC, Economics
Introduction
Proteins are an important part of the diet, but an estimated 12.2% of the global population is at risk of protein deficiency which is further predicted to increase to 15.1% by 2050 (Medek et al. 2017). De-oiled cakes/meal generated after mechanical expression/cold press has emerged as a novel protein source for human consumption. Since a major part of such de-oiled meals comprises protein, one valorization option is to extract proteins for the bioactive peptide production which can fetch substantially more value as a functional ingredient in food formulations (Montesano et al. 2020).
Notably, almonds (Prunus dulcis; syn. Prunus amygdalus) are one of the most consumed and nutrient-dense tree nuts majorly composed of oil (~ 50%), proteins (~ 20%), and carbohydrates (~ 20%) (Barreca et al. 2020). After almond oil extraction, the by-product left is a high-quality plant protein (31- 48% protein) resource with all essential amino acids (Houmy et al. 2020). However, the presence of antinutritional factors like cyanogenic glycosides and allergic proteins makes direct consumption of these cakes impractical, especially in the case of wild almonds (Amygdalus amara), which is generally used for oil extraction. Extraction and hydrolysis of proteins could be a possible technique to reduce the allergenicity of proteins as it aids in the breakdown of peptide chains (Verhoeckx et al. 2015).
Enzymatic hydrolysis is a proven method for the production of bioactive peptides from different protein-rich substrates. Among several enzymes reported, Alcalase has been shown to produce higher bioactivity as well as a higher degree of hydrolysis (DH) for the hydrolysates/peptides obtained from plant proteins (Mirzapour et al. 2017), muscle proteins (Kumar et al. 2021), milk proteins (Cui et al. 2021) as well as from the almond proteins (Mirzapour et al. 2016). Recently, non-thermal novel techniques such as ultrasound/ultrasonic energy (US), microwave (MW), pulsed electric field, microfluidization, etc. have been reported to improve hydrolysates’ yield, production time, and bio-functional properties (Sari et al. 2022). Among documented reports, many studies conclude the viability of US and MW energy in enhancing the yield and functional properties of hydrolysates with the additional benefit of greater scalability to commercial applications (He et al. 2021). The cavitation phenomenon under ultrasonication could induce structural changes either by breaking the side chains or modifying the side groups of amino acids. Similarly, microwave energy aids in the unfolding of complex protein structure, leading to a greater accessibility of enzymes thereby enhancing the functional and biological properties. It has been observed that bioactive peptides released by the US and MW-assisted proteolysis exhibit greater nutritional value, bio functionalities, and more effective gastrointestinal (GI) absorption than native proteins (Zheng et al. 2021).
However, the effect of US and MW energy pretreatments on the DH, protein recovery, and bio-functional properties of enzymatic hydrolysates from almond meal proteins has not yet been studied. Therefore, present study aimed to optimize US and MW pretreatment conditions for almond de-oiled cake, and study their influence on the crucial bio-functional properties of the hydrolysate derived from its extracted proteins. The results in turn will pave the way for the development of functional food/nutraceuticals formulations with improved nutritional and antioxidative benefits of almond protein hydrolysate.
Materials and methods
Materials and chemicals
Almond (Prunus amygdalus) de-oiled cake was collected from the almond oil manufacturing industry, India. The mechanically pressed cake had 7.28 ± 0.09% residual fat, 37.04 ± 0.19% protein, and 1.30 ± 0.06% moisture. Alcalase (AU ≥ 2.4), 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis 3-ethylbenzthiazoline-6-sulphonic acid (ABTS), 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH) and fluorescein were purchased from Sigma Aldrich (USA).
Ultrasonic and microwave pretreatments
The de-oiled almond cake was milled and passed through 500 μ sieve to get uniform-sized particles. Soluble almond protein extract (APE) was obtained from completely defatted flour using a modified procedure of Udenigwe et al. (2013). Briefly, flour was mixed with distilled water (1:10 ratio (w/v), and continuously stirred for 2 h at 50 °C, pH 8, using hot plate magnetic stirrer (Labquest, Borosil, Mumbai, India). For US pretreatment, 200 mL slurry was placed in an ultrasonic bath (Branson Ultrasonics Corporation, USA), operated at 110 W, 40 kHz, and varying time (10, 20, 30, 40, and 50 min) with a programmed temperature not exceeding 50 °C. For MW treatment, 200 mL suspensions were microwaved at 160 W power and 2,450 MHz frequency for 45, 90, 135, 180, and 225 s. The obtained slurry was centrifuged (Sigma Laborzentrifugen, Germany) to separate soluble proteins termed as US-APE and MW-APE indicating US and MW pretreatments, respectively. The sample not subjected to any pretreatment was considered as control (C-APE).
Enzymatic hydrolysis
US-APE, MW-APE, and C-APE were hydrolyzed with Alcalase at an enzyme substrate ratio of 1%, pH 8, and 50 °C for 3 h (Mirzapour et al. 2016). The digested proteins after terminating enzyme activity were recovered by centrifuging at 4000 g for 20 min at 4 °C. The almond protein hydrolysates (APH’s) thus obtained were termed US-APH, MW-APH, and C-APH, respectively.
Determination of DH and protein recovery
DH was calculated as the ratio of the number of peptide bonds cleaved to the total number of peptide bonds originally present in the almond protein as given by Adler-Nissen (1986).
| 1 |
where, V is consumption amount (mL) of 0.1 N NaOH solution; N is the molarity of alkali used (mol/L); α is the average degree of dissociation of α-NH2 of almond proteins at pH 8; M is the protein mass (g); htot is the total number of peptide bonds per unit mass, which is 7.58 mmol/g of almond protein (de Souza et al. 2020). The protein content was determined by Kjeldahl method as per AOAC (2012) with a conversion factor 5.18. The recovery of the protein after termination of the enzyme activity was calculated according to Eq. (2) given by Kumar et al. (2021).
| 2 |
Almond protein hydrolysis kinetics
The hydrolysis kinetics of US-APE, MW-APE, and C-APE were evaluated using the first-order model. The linearized form of the reaction kinetics was used to fit the data of DH at different pretreatment conditions following Eq. (3). A positive sign was used for the rate of reaction as the DH was expected to increase with time. The values of were plotted against the respective time of hydrolysis and the values of N0 and k were determined through the slope and intercept of a straight-line equation as: k = slope; N = eintercept. The coefficient of determination (R2) was used as an indicator of the model fit to the hydrolysis data. Data was logarithmically linearized.
| 3 |
where, N represents the DH at any time, t, N0 is the DH at t = 0, and k is the reaction rate constant.
Molecular weight distribution
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Zhao et al. (2021) with modifications. Samples (2 mg/mL) were run with buffer containing 5% β-mercaptoethanol under reducing conditions and heated at 90 °C/ 5 min. The separation was done with 4.5% stacking gel at 90 V for 15 min and 12.5% resolving gel at 120 V. All blue pre-stained protein standard (BioRad, India) containing ten blue-stained recombinant proteins (10–250 kDa), including three reference bands (25, 50, 75 kDa) were used as a molecular weight standard.
2,2,2-trichloroacetic acid (TCA) solubility index
The presence of low molecular weight peptides and free amino acids in the prepared hydrolysates was compared by calculating TCA soluble protein as described by Buˇcko et al. (2016). Analysis for protein was done following AOAC (2012). The TCA-soluble proteins were calculated by Eq. 4.
| 4 |
Antioxidant activity
DPPH radical scavenging activity assay
DPPH radical scavenging activity of the US-APE, MW-APE, and C-APE were determined as outlined by Kumar et al. (2022) with minor modifications. All hydrolysate samples were diluted to a concentration of 1 mg/mL protein concentration and the scavenging activity was expressed as μM Trolox equivalent antioxidant capacity (μM TEAC) per mg of protein. The absorbance was read at 517 nm in a multimode microplate reader (SpectraMax® M2e system, Molecular devises, USA).
ABTS radical scavenging capacity
The ABTS radical scavenging activity was determined according to Zhang et al. (2017) with modifications. ABTS radical stock solution (7 mM ABTS in 2.45 mM potassium persulfate), was incubated in the dark at 25 °C for 16 h and diluted to read an absorbance of 0.700 ± 0.02 at 734 nm using a multimode microplate reader. Trolox (0–160 μM) was used as standard and the results are expressed in terms of μM TEAC per mg of protein.
Oxygen radical absorbance capacity (ORAC) assay
ORAC assays of all hydrolysates were performed according to the method of Habinshuti et al. (2020) with minor modifications. The hydrolysate solutions were diluted to a protein concentration of 0.1 mg/mL for analysis. 50 μL of 78 nM fluorescein was mixed with 50 μL Trolox standard/ hydrolysate solutions (prepared in phosphate buffer (pH 7.4)) in a 96-well opaque plate. After incubation at 37 °C for 10 min, AAPH (221 mM, 25 μL) was added to each well, and the relative fluorescence intensity was recorded every minute for 90 min using a multimode microplate reader. The excitation and emission wavelengths chosen were 485 and 535 nm, respectively. Sample diluent was used to run a blank test, and 20 μM Trolox was used as a standard. The area under the curve (AUC) for each experiment was calculated by the data analysis software, SoftMax® Pro. The final ORAC values were expressed as μM TEAC per mg of protein with the help of Eq. 5.
| 5 |
where, C Trolox is the concentration of Trolox (20 μM), D is the sample dilution factor, and AUC-Sample, AUC-Blank, and AUC-Trolox is the area under the fluorescence intensity curve of the sample, blank, and Trolox, respectively.
Results and discussion
Kinetics of US and MW pretreatments on enzymolysis
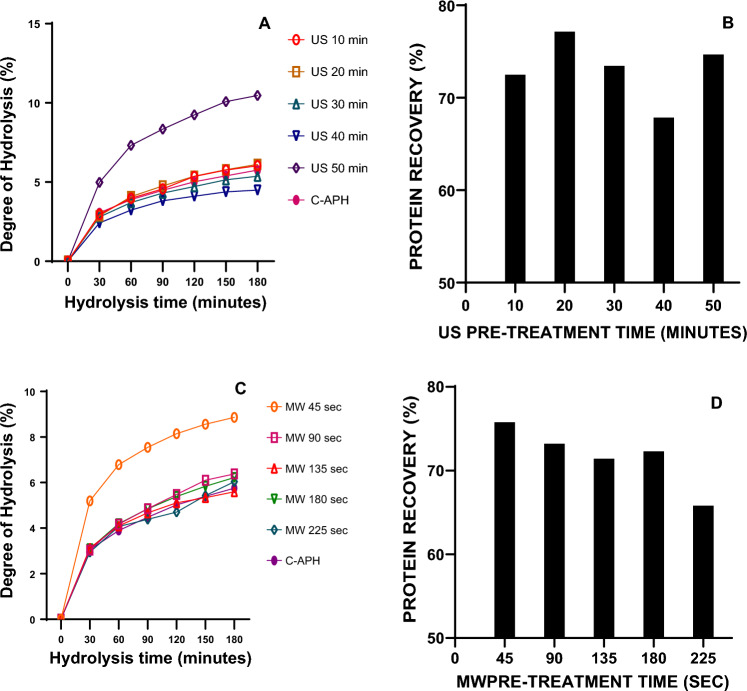
Experiments were defined to test the effect of ultrasonic inputs (frequency 40 kHz; power level 200 W and varying time intervals of 10, 20, 30, 40, and 50 min), and microwave energy inputs (power level 160 W, 2,450 MHz frequency for 45, 90, 125, 180, and 225 s) on output response of DH and protein yield. Both DH and protein recovery were found to vary greatly with the pretreatments given to almond de-oiled meal suspensions. The effect of US and MW energy on DH and protein recovery is shown in Fig. 1. Table 1 shows the DH under different treatments and control sample fitted to Eq. (3). Comparing the results of different conditions of pretreatments, US at 50 min resulted in a higher DH (10.95%) and the highest k value among various conditions analyzed for US pretreatments. Similarly, among MW pretreatments, 45 s resulted in high DH (8.87%) with a k value of 0.0046. Whereas, the control sample resulted in comparatively lower DH (5.76%) and protein recovery of 64.17%. The k values obtained from the first-order kinetics were in agreement with the experimental values (Fig. 1). It shows that first-order model was able to explain the kinetics of almond protein hydrolysis. The hydrolysis rates of US 50 min and MW 45 s potentiated DH with the highest k value in comparison with conventional hydrolysis. At these optimized conditions, 1.16- and 1.18-fold increment in protein recovery were also observed for US and MW pretreatments in comparison with conventional extraction of protein.
Fig. 1.
Effect of US and MW pretreatments on the DH and protein recovery of APHs. A and B indicate DH and protein recovery for various US pretreatment conditions, respectively C and D indicate DH and protein recovery for various MW pretreatments conditions, respectively
Table 1.
Kinetics parameters of Eq. (3) adjusted to the DH following US and MW pretreatments on almond protein extracts
| Pre-treatments | N0 | k | R2 |
|---|---|---|---|
| Ultrasonication at 40 kHz | |||
| 10 min | 2.87E + 00 | 0.0046 | 0.9201 |
| 20 min | 2.86E + 00 | 0.0047 | 0.8903 |
| 30 min | 2.75E + 00 | 0.0041 | 0.9067 |
| 40 min | 2.43E + 00 | 0.0039 | 0.8612 |
| 50 min | 4.99E + 00 | 0.0048 | 0.8875 |
| MW at 160 W | |||
| 45 s | 5.25E + 00 | 0.0046 | 0.865 |
| 90 s | 2.96E + 00 | 0.0047 | 0.9185 |
| 135 s | 3.13E + 00 | 0.0036 | 0.8786 |
| 180 s | 3.04E + 00 | 0.0044 | 0.9119 |
| 225 s | 2.85E + 00 | 0.0043 | 0.9307 |
| Control (without pre-treatment) | |||
| 2.942 | 0.0041 | 0.938 | |
N0 is the initial DH, k is the rate constant and R2 is the model fit performance
Ultrasonic power is well known to increase the yield and DH during enzymatic hydrolysis which was explained by the effect of turbulence and shear force which induces disintegration of the complex molecular structure of proteins, making them more soluble and providing easy access for proteolytic enzymes to act (Naik et al. 2022). Similarly, the cell wall rupture induced by microwave heating results in an increased breakdown of protein buried inside the compact structure thereby increasing the soluble protein concentration, which could produce more active sites for the enzymes (Varghese and Pare, 2019). It was also evident from the present study that optimization of pretreatment conditions is crucial for higher yield and functional characteristics of the hydrolysates produced. Similar results were obtained with lotus seed protein hydrolysates, where an increment in DH was observed with MW pretreatment before enzymolysis (Gohi et al. 2019). An improvement in the protein extraction yield together with enhanced DH may contributes to the enhanced nutritional profile of protein hydrolysates prepared with US and MW pretreatments.
Molecular weight distribution
The electrophoretic profile of almond protein hydrolysates is presented in Fig. 2A. As expected, the electrophoresis pattern showed several low molecular weight bands ranging from 10 to 40 kDa. The band profiles of all three samples were similar, however variations are visible in the relative band intensity/thickness. Notably, the band thickness got weaker after pretreatment with MW and US in comparison with C-APH. The plausible reason is the abundance of protein in the untreated hydrolysate leading to an increased band thickness. The pretreatment effectively could unfold the compact native structure of almond proteins further enhancing the proteolytic action of Alcalase, thereby releasing more small molecular weight peptides/free amino acids from the terminal end of the protein. The SDS page bands further explain the improved DH as obtained with the hydrolysates under MW and US pretreatments. Our results are consistent with the reports by Adjei-Fremah et al. (2019), who revealed that non-thermal processing could reduce the intensity of protein bands.
Fig. 2.
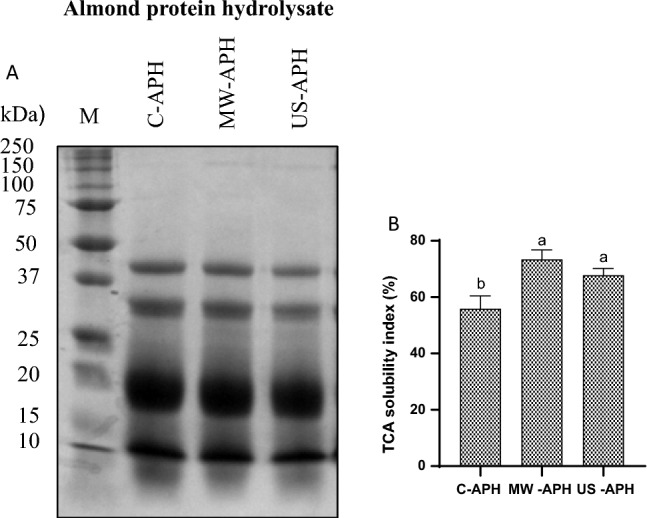
Effect of US and MW pretreatments on SDS-PAGE profiles A and TCA solubility index B of almond protein hydrolysates. M: reference marker 10–250 kDa (lane 1) Data is represented as Mean ± SD (n = 3). Bars bearing at least one common small superscript letter (a, b…etc.) do not differ significantly between treatments (control, MW, and US) at P < 0.05 in Duncan's multiple comparison post hoc test
TCA solubility index
TCA soluble protein concentration depicts the amount of small molecular weight peptides and free amino acids released during proteolysis which further indicates the degree of proteolysis (Rutherfurd 2010). A higher solubility index of hydrolysates in TCA also indicates the presence of low molecular weight peptides, thus improving the gastrointestinal availability of peptides. The TCA solubility index of all APH’s is presented in Fig. 2B. Significantly higher percentage (P < 0.05) of TCA soluble proteins was observed in the almond protein hydrolysates obtained after pretreatment with microwave (73.46 ± 3.33%) than the control (C-APH: 55.88 ± 4.59%). Even ultrasonic prior-treatment resulted in higher TCA soluble proteins (67.87 ± 2.34%) in the hydrolysate than the control; however, there was no significant difference between the MW-APH & US-APH. This data trend was consistent with the DH obtained for the respective hydrolysates. The underlying mechanism could be ascribed to the pretreatments given which in turn provided structural changes in the protein further increasing DH and TCA soluble protein concentration. A similar trend for the DH and TCA solubility index was observed for the Alcalase hydrolysates from the Mung bean (Vigna radiate) by Xie et al. (2019), wherein a consistent increase in the TCA soluble proteins with an increment in the DH is reported.
Antioxidant activity
Alcalase is a versatile endo-peptidase with broad cleavage sites for the production of antioxidant peptides from various protein substrates (Tacias-Pascacio et al. 2020). From Table 2, it is clear that US-APH showed the highest TEAC values for the DPPH (75.70 ± 1.53), ABTS (122.47 ± 4.36), and ORAC (150.61 ± 6.89) assays. Similarly, MW-APH showed significantly higher (P < 0.05) TEAC values as compared to the control hydrolysate (C-APH). The presence of high proportion TCA soluble peptides and greater DH might have resulted in the significantly higher (P < 0.05) antioxidant activity in the US-APH and MW-APH as compared to the control (C-APH). Although the results of all three antioxidant estimation methods followed a similar trend, the variation in the TEAC value could be probably attributed to the differences in the mechanism of antioxidant activity by different estimation methods. In general, the ORAC values are higher than the ABTS and DPPH for protein hydrolysates, which is explained by the differences in the electron transfer capacity (measured by ORAC) and hydrogen atom transfer capacity (measured by DPPH and ABTS) of the samples. US-APH had 1.7 times ORAC, 1.33 times ABTS, and 1.41 times DPPH values than C-APH. The antioxidant activity reported in this study considerably exceeds the scavenging capacity of FoodPro Alkaline Protease-assisted skim extracts of almond proteins (ORAC and ABTS values of 392.2 and 650.5 µM TEAC/g, respectively) reported by de Souza et al. (2020). The most plausible reason for the higher antioxidant activity in the US & MW samples is the significant impact of the US and MW pretreatments which contributes to the unfolding of proteins, thereby enhancing the DH and functional attributes as well as release of antioxidant peptides. Our findings could not be compared with the almond protein hydrolysates as US and MW treatments have not been evaluated earlier. Similar observations were made by Habinshuti et al. (2020), where US and MW treatments on sweet potato protein released diverse peptides with enhanced antioxidant activity in comparison with the control hydrolysates. Parallel to the findings of the present study, Alcalase hydrolyzed Iranian wild almonds was shown to have significantly high antioxidant activity by ABTS assay (IC50 value of 50.2 ± 2.2 µg/mL) than other proteases (Mirzapour et al. 2016). It may also be hypothesized that apart from antioxidant-rich peptides, the presence of water-soluble polyphenols in the alkaline extract might have contributed to the higher antioxidant activity of the hydrolysates to some extent in the present study. The composition and position of amino acids in peptide sequences contribute to a higher antioxidant activity as compared to free amino acids (Sarmadi et al. 2010). This further reinforces the wisdom of using minimally hydrolyzed (5–10% DH) proteins rather than extensively hydrolyzed ones in nutraceuticals and functional foods.
Table 2.
Antioxidant activities of almond protein hydrolysates as µM TE/mg protein
| DPPH | ABTS | ORAC | |
|---|---|---|---|
| C-APH | 53.56 ± 0.71c | 84.72 ± 3.47c | 88.54 ± 6.52c |
| MW-APH | 68.76 ± 1.90b | 103.14 ± 2.00b | 118.68 ± 2.36b |
| US-APH | 75.70 ± 1.53a | 122.47 ± 4.36a | 150.61 ± 6.89a |
Antioxidant capacity of C-APH, MW-APH, and US-APH as evaluated by DPPH, ABTS, and ORAC methods. Data is represented as mean ± SD (n = 3). Values bearing different lowercase letters indicate a significant difference (P < 0.05) between the different hydrolysates for the same antioxidant activity assay
The estimated cost analysis for the production of almond protein hydrolysates was carried out as described by Singh et al. (2022). It should be noted that the industry scale evaluations and economic feasibility of US and MW assisted production of almond protein hydrolysate could only be done based on the assumptions and approximations. The de-oiled almond meal was purchased at $ 0.5/kg. The value of other materials used (chemicals and Alcalase) in the study was calculated considering the prices available on the bulk purchase. Recommendation from the Ministry of Labour & Employment, Government of India, (2023) was used for calculating the labour cost. The utility costs (electricity and water) were based on the utility hours and current industrial prices. While, there are some costs (contingency cost, average yield lost cost, waste treatment, net profit ratio, equipment cost, and annual depreciation) which have not been considered owing to the fact that the liquid hydrolysate is an intermediate product. However, future work of the present study comprises processing of these hydrolysates to a powder form product, their physicochemical, functional characterization, and complete cost economics analysis. It was found (Table 3) that the per kg production of C-APH, MW-APH, and US-APH would cost around $ 6.80, 6.81, and 6.82, respectively. He et al. (2015) reported $ 4.05/kg cost for the production of fish protein hydrolysate using a microwave-intensified enzymatic process from fish processing by-products. The authors used Superpro Designer (version 8.0), a simulation software considering 100,000 kg/day availability of fish processing by-products. Such a high volume of production might have contributed to a decrease in the fixed cost per unit, overall reducing the production cost.
Table 3.
Cost economic analysis (in USD) of almond protein hydrolysates following US and MW pretreatments
| Expenses/kg | C-APH | MW-APH | US-APH |
|---|---|---|---|
| Labour cost | 1.82 | 1.82 | 1.82 |
| Raw material cost | |||
| 1. Almond de-oiled cake | 0.5 | 0.5 | 0.5 |
| 2. Chemicals | 4.36 | 4.36 | 4.36 |
| 3. Protease enzyme | 0.12 | 0.13 | 0.12 |
| Utility cost | |||
| Electricity & water | 0.003 | 0.0037 | 0.02 |
| Total cost of production | 6.80 | 6.81 | 6.82 |
Conclusion
The current data support the use of ultrasonication and microwave pretreatments for enhancing the DH of protein extracted from de-oiled almond meal. The optimal conditions for the highest DH were found with US pretreatment of 50 min at 40 kHz and MW pretreatment of 160 W at 45 s. Protein hydrolysates from almond de-oiled cake following MW and US pretreatments can be used as a functional ingredient in functional foods owing to their excellent degree of hydrolysis yielding low molecular weight peptides in comparison to the conventional hydrolysis. Future work comprises processing of these hydrolysates to a shelf stable powder form, physicochemical and functional characterization, and its incorporation in the suitable plant-based protein supplements.
Acknowledgements
Authors express sincere gratitude to the Director, National Institute of Food Technology Entrepreneurship and Management, Kundli (NIFTEM-K) for providing necessary facilities and support to carry out the research work. The first author is grateful to the institute, National Institute of Food Technology Entrepreneurship and Management, Kundli (NIFTEM-K) for providing PhD Fellowship.
Author contributions
TPS: Methodology, Investigation, Formal analysis, Data curation, Validation, Writing—Original draft. RS: Methodology, Data curation, Writing—review & editing. PT: Investigation, Formal analysis, Data curation. GT: Investigation, Formal analysis, Data curation. JP: Investigation, Formal analysis, Data curation. NNK: Investigation, Formal analysis, Data curation. KV: Data curation, Formal analysis. PCB: Conceptualization, Methodology, Validation, Data curation, Supervision, Resources, Writing—review & editing. SP: Data curation, Writing—review & editing.
Funding
Not applicable.
Data availability
The data set used and/or analysed during the study will be made available by the corresponding author upon reasonable request.
Declarations
Conflicts of interest
Authors have no conflicts of interest to disclose.
Ethical approval
Not applicable.
Consent to participate
All authors have read and approved the manuscript and are aware of its submission to JFST.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adjei-Fremah S, Worku M, De Erive MO, He F, Wang T, Chen G. Effect of microfluidization on microstructure, protein profile and physicochemical properties of whole cowpea flours. Innov Food Sci Emerg Technol. 2019;57:102207. doi: 10.1016/j.ifset.2019.102207. [DOI] [Google Scholar]
- Adler-Nissen J. Enzymic hydrolysis of food proteins. UK: Elsevier Applied Science Publishers; 1986. [Google Scholar]
- Barreca D, Nabavi SM, Sureda A, Rasekhian M, Raciti R, Silva AS, Annunziata G, Arnone A, Tenore GC, Süntar İ, Mandalari G. Almonds (Prunus Dulcis Mill. D. A. Webb): a source of nutrients and health-promoting compounds. Nutrients. 2020;12:672. doi: 10.3390/nu12030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bučko S, Katona J, Popović L, Petrović L, Milinković J. Influence of enzymatic hydrolysis on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. Food Hydrocoll. 2016;60:271–278. doi: 10.1016/j.foodhyd.2016.04.005. [DOI] [Google Scholar]
- Cui Q, Sun Y, Zhou Z, Cheng J, Guo M. Effects of enzymatic hydrolysis on physicochemical properties and solubility and bitterness of milk protein hydrolysates. Foods. 2021;10:2462. doi: 10.3390/foods10102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza TSP, Dias FFG, Oliveira JPS, de Moura Bell JMLN, Koblitz MGB. Biological properties of almond proteins produced by aqueous and enzyme-assisted aqueous extraction processes from almond cake. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-020-67682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohi BFCA, Du J, Zeng HY, Cao XJ, Zou KM. Microwave pretreatment and enzymolysis optimization of the Lotus seed protein. Bioeng. 2019;6:28. doi: 10.3390/bioengineering6020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habinshuti I, Mu TH, Zhang M. Ultrasound microwave-assisted enzymatic production and characterization of antioxidant peptides from sweet potato protein. Ultrason Sonochem. 2020;69:105262. doi: 10.1016/j.ultsonch.2020.105262. [DOI] [PubMed] [Google Scholar]
- He S, Franco CM, Zhang W. Economic feasibility analysis of the industrial production of fish protein hydrolysates using conceptual process simulation software. J Bioprocess Biotechniques. 2015;5:1. [Google Scholar]
- He L, Gao Y, Wang X, Han L, Yu Q, Shi H, Song R. Ultrasonication promotes extraction of antioxidant peptides from oxhide gelatin by modifying collagen molecule structure. Ultrason Sonochem. 2021;78:105738. doi: 10.1016/j.ultsonch.2021.105738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Jyoti A, Tarafdar A, Kumar A, Badgujar PC. Comparative functional and spectroscopic analysis of spent hen meat hydrolysate by individual and combined treatment of microbial proteases. Prep Biochem Biotechnol. 2021;51:618–627. doi: 10.1080/10826068.2020.1848865. [DOI] [PubMed] [Google Scholar]
- Kumar D, Tarafdar A, Dass SL, Pareek S, Badgujar PC. Antioxidant potential and amino acid profile of ultrafiltration derived peptide fractions of spent hen meat protein hydrolysate. J Food Sci Technol. 2022;60(3):1–7. doi: 10.1007/s13197-022-05437-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medek DE, Schwartz J, Myers SS. Estimated effects of future atmospheric CO2 concentrations on protein intake and the risk of protein deficiency by country and region. Environ Health Perspect. 2017;125:087002. doi: 10.1289/EHP41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzapour M, Rezaei K, Sentandreu MA, Moosavi-Movahedi AA. In vitro antioxidant activities of hydrolysates obtained from Iranian wild almond (Amygdalus scoparia) protein by several enzymes. Int J Food Sci Technol. 2016;51:609–616. doi: 10.1111/ijfs.12996. [DOI] [Google Scholar]
- Mirzapour M, Rezaei K, Sentandreu MA. Identification of potent ACE inhibitory peptides from wild almond proteins. J Food Sci. 2017;82:2421–2431. doi: 10.1111/1750-3841.13840. [DOI] [PubMed] [Google Scholar]
- Montesano D, Gallo M, Blasi F, Cossignani L. Biopeptides from vegetable proteins: new scientific evidences. Curr Opin Food Sci. 2020;31:31–37. doi: 10.1016/j.cofs.2019.10.008. [DOI] [Google Scholar]
- Naik M, Natarajan V, Modupalli N, Thangaraj S, Rawson A. Pulsed ultrasound assisted extraction of protein from defatted Bitter melon seeds (Momardica charantia L.) meal: Kinetics and quality measurements. LWT. 2022;155:112997. doi: 10.1016/j.lwt.2021.112997. [DOI] [Google Scholar]
- Rutherfurd SM. Methodology for determining degree of hydrolysis of proteins in hydrolysates: a review. J AOAC Int. 2010;93:1515–1522. doi: 10.1093/jaoac/93.5.1515. [DOI] [PubMed] [Google Scholar]
- Sari TP, Sirohi R, Krishania M, Bhoj S, Samtiya M, Duggal M, Kumar D, Badgujar PC. Critical overview of biorefinery approaches for valorization of protein rich tree nut oil industry by-product. Bioresour Technol. 2022;362:127775. doi: 10.1016/j.biortech.2022.127775. [DOI] [PubMed] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Singh U, Kaur D, Mishra V, Krishania M. Combinatorial approach to prepare antioxidative protein hydrolysate from corn gluten meal with dairy whey: preparation, kinetics, nutritional study and cost analysis. LWT. 2022;153:112437. doi: 10.1016/j.lwt.2021.112437. [DOI] [Google Scholar]
- Tacias-Pascacio VG, Morellon-Sterling R, Siar EH, Tavano O, Berenguer-Murcia A, Fernandez-Lafuente R. Use of Alcalase in the production of bioactive peptides: a review. Int J Biol Macromol. 2020;165:2143–2196. doi: 10.1016/j.ijbiomac.2020.10.060. [DOI] [PubMed] [Google Scholar]
- Udenigwe CC, Je JY, Cho YS, Yada RY. Almond protein hydrolysate fraction modulates the expression of proinflammatory cytokines and enzymes in activated macrophages. Food Funct. 2013;4(5):777–783. doi: 10.1039/c3fo30327f. [DOI] [PubMed] [Google Scholar]
- Varghese T, Pare A. Effect of microwave assisted extraction on yield and protein characteristics of soymilk. J Food Eng. 2019;262:92–99. doi: 10.1016/j.jfoodeng.2019.05.020. [DOI] [Google Scholar]
- Verhoeckx KC, Vissers YM, Baumert JL, Faludi R, Feys M, Flanagan S, Herouet-Guicheney C, Holzhauser T, Shimojo R, van der Bolt N, Wichers H. Food processing and allergenicity. Food Chem Toxicol. 2015;80:223–240. doi: 10.1016/j.fct.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Xie J, Du M, Shen M, Wu T, Lin L. Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from Mung bean (Vigna radiate) Food Chem. 2019;270:243–250. doi: 10.1016/j.foodchem.2018.07.103. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang Q, Xu Y, Xia W. Effects of washing and membrane removal pretreatments on the antioxidant properties of grass carp (Ctenopharyngodon idella) protein hydrolysates produced by in vitro digestion. Int J Food Sci. 2017;52:1260–1268. doi: 10.1111/ijfs.13393. [DOI] [Google Scholar]
- Zhao Q, Yan W, Liu Y, Li J. Modulation of the structural and functional properties of perilla protein isolate from oilseed residues by dynamic high-pressure microfluidization. Food Chem. 2021;365:130497. doi: 10.1016/j.foodchem.2021.130497. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Zhang M, Fan H, Liu Y. Effect of microwave combined with ultrasonic pretreatment on flavor and antioxidant activity of hydrolysates based on enzymatic hydrolysis of bovine bone. Food Biosci. 2021;44:101399. doi: 10.1016/j.fbio.2021.101399. [DOI] [Google Scholar]
- Houmy N, Melhaoui R, Belhaj K, Richel A, Sindic M, Hano C, Kodad S, Mihamou A, Addi M, Abid M, Elamrani A (2020) Chemical characterization of almond meal as a co-product of the mechanical extraction of almond oil. In E3S Web of Conferences. 183, 04004. EDP Sciences. 10.1051/e3sconf/202018304004
- Official Methods of Analysis of AOAC International 950.38, (2012). 19th Ed., AOAC International, Gaithersburg, MD, USA
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set used and/or analysed during the study will be made available by the corresponding author upon reasonable request.