Abstract
Nowadays, finding natural and inexpensive resources that can be easily used to make food films has been considered. Despite the widespread use of synthetic resins, natural resins are rarely used. Opopanax resin (OR) was used in this study as a new biosource to prepare the hydrophobic edible film. Ethylcellulose (EC) was blended well with the resin, allowing the formation of a composite film. Film preparation was possible using different amounts of OR and EC. It was interesting that OR had a plasticizing effect on EC film. While using up to 33% w/w glycerol could not produce an elastic EC film, using only 8.5% w/w OR produced a stiff and flexible EC film with lower water sensitivity. Fourier transform infrared (FTIR) spectroscopy analysis showed that the strength of C–O–C and CH bonds in OR + EC film was higher than in EC film. Despite the higher water sensitivity of OR-based composite films than EC-based composite films, they had lower water vapor permeability (WVP) and higher contact angle due to their smoother and more homogeneous film structures with lower porosity, confirmed by scanning electron microscopy (SEM) images. The mechanical properties showed that the film with the highest resin content had the lowest tensile strength (~ 0.4 MPa) and the higher elongation at break (~ 67%) and, therefore, the highest flexibility. The use of natural resins as a biosource is a promising approach in food packaging to prepare hydrophobic films with desirable mechanical properties.
Keywords: Natural film, Plasticizer, Resin, Ethylcellulose
Introduction
Edible films and coatings are thin layers that may be eaten with or separated from the food. Edible films are mainly produced by spreading the film-making solution on a surface and drying it. These films are a thin layer obtained as a solid sheet that can be used to package a food product. Nowadays, finding natural and inexpensive resources that can be easily used to make food films has been considered (Pak et al. 2020; Senarathna et al. 2022).
Resin is a natural or synthetic compound that is very viscous and hardens over time. The resin is soluble in alcohol but does not dissolve in water, unlike gum. Resins are polymers or relatively high molecular weight copolymers that have active groups within their chain. These polymers are mostly liquid with high viscosity at ambient temperature and have a honey-like, clear, light yellow to brownish appearance (Purwanti et al. 2019). The natural resin can be mixed with biopolymers, plasticizers, emulsifiers, and reinforcing agents to develop edible films and coatings (Yong and Liu 2021). Cho et al. (2023) used polyacrylonitrile (PAN) and shellac to produce composite films. The produced composite film had outstanding mechanical performances (73.8% higher tensile strength, 60% higher storage modulus) compared with control PAN film. Isfran et al. (2023) produced propolis extract/chitosan coating to control the melanosis of refrigerated shrimps during storage. Olewnik-Kruszkowska et al. (2022) used polylactide (PLA) and propolis extract to produce composite film using poly(ethylene glycol) as a plasticizer for extending the shelf life of stored food products. Buccoadhesive films of Myrrh extract/ polyvinyl pyrrolidone/Sodium carboxy methylcellulose was developed by Auda et al. (2017). They also exhibited antibacterial activities against different types of bacterial strains as well as antifungal activity.
Opopanax is a plant of the Apiaceae (Umbelliferae) family with the scientific name of Prangos ferulacea (L.) Lindl. Opopanax is a perennial and aromatic plant native to the mountains of southern Iran. Scratching the stem and roots of the opopanax plant leads to exudate resin-gum on its stem or root, which will gradually harden due to exposure to air. Opopanax resin-gum, which has been used in traditional medicine to treat many disorders, contains 63.5% resin, 27% gum, and 9.5% essential oil (Önder et al. 2020).
Ethylcellulose (EC) is known as one of the most important and widely used ether cellulose derivatives, produced by replacing hydrogen in the final hydroxyl group with an ethylene molecule. This process results in hydrophobic molecules that are compatible with various organic solvents. EC is non-toxic, colorless, and resistant to sunlight and ultraviolet rays. It is also the only known polymer that directly affects the structure of edible oils. This polymer is one of the strongest and lightest cellulosic plastics and has the lowest water absorption properties (Moghtadaei et al. 2021). EC is often used in the solvent-casting method to produce films. EC films are known for their flexibility and thermoplastic behavior. The flexibility and barrier properties of EC films make them useful in packaging applications, providing protection against moisture and gases. EC is often blended with other polymers to achieve specific properties. Blending can enhance mechanical strength, flexibility, or other desired characteristics of the films. EC is mainly used as a plastic film. However, the addition of plasticizers, especially glycerol and polyethylene glycol, is necessary to improve the tensile and flexibility properties of the EC films (Kangarlou and Haririan 2007; Lin et al. 2021).
Despite using synthetic resins to produce films, there is no study, to our knowledge, about using natural resins for film preparation. Therefore, in this study, opopanax resin was used as a new, natural, and inexpensive source to evaluate its possibility for film formation. On the other hand, as high amounts of glycerol are needed for the film preparation from ethylcellulose, the possibility of using opopanax as a plasticizer was also investigated.
Materials and methods
Materials
Opopanax resin-gum was purchased from an herbal market in Isfahan. Ethyl alcohol (96%), glycerol (with 70% purity), and ethylcellulose (DS ~ 2.2 to 2.7, with a viscosity of 5400 cP and ethoxyl content of 47.9%) were provided by Sigma.
Opopanax resin extraction
Opopanax resin-gum was ground. Then, alcoholic extraction was used to separate gum and resin. Opopanax powder was refluxed with ethanol (96%) for seven h. After, the alcoholic solution containing resin was separated. The oven evaporated the solvent at 50 °C for 24 h. The obtained opopanax resin (OR) was collected and stored in a closed container in the refrigerator (4 °C) (Zhang et al. 2018). Oligosaccharides, phenolic compounds, part of colored compounds, etc., which are dissolved in ethanol, will be extracted with resin, and these impurities will affect resin properties. It is difficult to determine that each characteristic of the extracted is due to which compound; therefore, the sum of what was extracted in this way was called resin in this research.
Production of opopanax resin film
Opopanax resin was unable to produce film alone. Therefore, EC was used to prepare composite film. A certain weight of the resin was added to a glass container containing 20 ml ethanol. The mixture was heated at 50 °C by a heater to complete the desolation of resin in the alcohol. Then, a certain amount of ethylcellulose powder was added and stirred until complete dissolution. Then, the solution was cast on a glass plate and dried in an oven at 50 °C for 4 h. The glass plates were then placed in a desiccator for 48 h. After, the flexible films were easily separated. The minimum amount of EC required to make a resin film was 25%. So, 6 types of films with different percentages of OR (2.25, 2, 1.5, 1, 0.5, and 0.25 g) and EC (0.75, 1, 1.5, 2, 2.5, and 2.75 g) were prepared. Control film containing 3 g EC was also prepared. Because the EC film was so brittle, glycerol (1.5 g) was added to EC (3 g) solution as a plasticizer. Lower amounts of glycerol did not eliminate the fragility of the EC film.
Characterization of opopanax resin/ethylcellulose films
Thickness
The thickness of the films was calculated using a micrometer (DC-516, Germany) with an accuracy of 0.001 mm at three different locations of each film (Farajpour et al. 2020).
Moisture content (MC)
Film samples (4 × 4 cm2) were weighed (Mi), dried at 105 °C for 24 h, and weighed again (Mf). The MC of the films was determined through Eq. 1 (Greener and Fennema 1989; Liu et al. 2004).
| 1 |
Water solubility (WS)
The prepared film samples (4 × 4 cm2) were dried at 105 °C for 24 h and then weighed (Wi). The films were placed in Petri dishes containing distilled water. After 24, 48, and 72 h, the films were removed from distilled water, dried at 105 °C for 24 h, and weighed (Wf). The water solubility of the films was determined by Eq. 2 (Greener and Fennema 1989).
| 2 |
Shrinkage ratio (SR)
The thickness of film samples (4 × 4 cm2) was determined (Ti). Then the samples were dried at 105 °C for 24 h. Samples thickness was measured again after drying (Tf). The shrinkage ratio was determined by Eq. 3 (Liu et al. 2004).
| 3 |
Swelling index (SI)
Film samples (4 × 4 cm2) were weighed (Wi), placed in Petri dishes containing 30 mL distilled water, and stored at 25 °C for 24 h. After the samples were removed from the Petri dishes, their excess water was gently filtered through paper, and the final weight of the films was measured (Wf). The Swelling index was determined according to Eq. 4 (Farajpour et al. 2020).
| 4 |
Contact angle
The contact angle was determined to investigate the degree of hydrophobicity of the films. Five μL water droplets were randomly injected on 5 locations of the prepared films (1 × 1 cm2). The average angle of the water droplets with the surface of the film was immediately determined as the static contact angle (Farajpour et al. 2020).
Water vapor permeability (WVP)
Water vapor permeability was measured using the E96ASTM method. A piece of film was placed in the lid of a special glass container with a diameter of 2 cm containing a hole with a diameter of 8 mm. Calcium chloride was used to create zero relative humidity inside the containers. The glassware was weighed with its contents and placed inside a desiccator containing distilled water. Distilled water at 25 °C produces 100% relative humidity. The glass containers containing samples were weighed every 24 h for 7 days. The moisture content that was absorbed by the film but did not pass through it was determined based on the weight of the containers. So, water vapor transfer rate (WVTR) was calculated by dividing the slope of the weight change line over time, and then WVP was calculated through Eqs. 5 and 6 (Greener and Fennema 1989).
| 5 |
where Δm/Δt is water vapor transfer rate (g/s), X is the average film thickness, A is the surface area exposed to the film, and ∆p is the pressure difference between the two sides of the film.
| 6 |
where P0 is the saturated steam pressure at 25 °C, RH1 is the relative humidity inside the desiccator (%), and RH2 is the relative humidity inside the container (%).
Tensile strength (TS) and elongation at break (EB)
A texture analyzer (SANTAM-20, Iran) was used for the tensile test. Film samples (1 × 6 cm2) were placed between the grips of the device. The initial grip separation and cross-head speed were 50 mm and 10 mm/min, respectively. TS and EB were determined through Eqs. 7 and 8 (Chen et al. 2019).
| 7 |
| 8 |
Transparency
The films (4 × 4 cm2) were inserted into the cell of the spectrophotometer (T60 UV–Vis spectrophotometer, UK), and transmittance was determined at 600 nm. The transparency of the films was determined according to Eq. 9 (Galus and Lenart 2013).
| 9 |
where Tf is the film transmittance, Tc is the empty cell transmittance, and t is the film thickness.
Color
The colorimeter (ZE6000, Nippon-denshoku, Japan) was used to determine the color of the prepared films, and L* [black (0)/white (100)], a* [green (− 60)/red (+ 60)], and b* [blue (− 60)/yellow (+ 60)] factors were analyzed. The color values were calibrated with a standard white screen before the measurement (Liu et al. 2004).
Microstructure
Microscopic images of the surface and cross-section of the films were prepared by scanning electron microscopy (SEM) (Philips XL30, Philips, Netherlands) to investigate the microstructure of the films. Films were broken in liquid nitrogen to obtain cross-section images. For better conductivity during imaging, samples were covered with an ultrathin layer of gold (thickness of approximately 5–6 nm) for 500 s by a sputter coater (BAL-TEC) (Farajpour et al. 2020).
Chemical structure
Fourier transform infrared spectroscopy equipped with an attenuated total reflection system (FTIR-ATR) was used to investigate the chemical structure, functional groups, and bonds by examining the interaction of the electromagnetic wave in the infrared range with the sample. For this purpose, films were analyzed in the range of 400–4000 cm−1 with 32 spectra/scans at a resolution of 4 cm−1 (Meng et al. 2020).
Statistical analysis
All experiments were performed in three replications. A completely randomized design was used for the statistical analysis of the samples. In this study, a one-way analysis of variance (ANOVA) was performed using the least significant difference (LSD) at a 95% confidence level using SAS software to determine the significant difference between samples.
Results and discussion
Thickness
Thickness is an important parameter to investigate the mechanical and barrier properties of the film. The thickness of different prepared films is shown in Table 1. The highest thickness was related to the film containing 3 g EC and 1.5 g glycerol, and the lowest was related to the film containing 2.25 g OR and 0.75 g EC. The thickness of the films increased by resin decreasing and EC increasing. A high adhesion force between the resin molecules, which was due to the strong intermolecular bonds, caused the free space between the polymer chains to be less in the structure of films with high resin. Therefore, the thickness of those films reduced. In contrast, EC had a more free space between its polymers chains due to the replacement of hydrogen in the final group of hydroxyl with ethylene molecule. Therefore, the amount of EC effectively increased the thickness of the films. The presence of plasticizer in the EC film containing glycerol increased the thickness of this film significantly compared to other films. OR use as a plasticizer in the preparation of EC films caused thickness reduction, which could be attributed to the hydrophobic nature of the resin. Therefore, although plasticizers reduce the intermolecular forces of the polymer, their hydrophobic or hydrophilic nature determines the effect of plasticizers on the thickness of the films (Park et al. 1993). A hydrophilic plasticizer (such as glycerol) will absorb water in the free spaces between the polymer chains and increase the thickness. However, a hydrophobic plasticizer (such as resin) will not increase the thickness due to the lower water absorption than the hydrophilic composition.
Table 1.
Thickness, moisture content, shrinkage ratio, and swelling index of films prepared with different amounts of OR and EC
| Films | Thickness (mm) | Moisture content (%) | Shrinkage ratio | Swelling index (%) |
|---|---|---|---|---|
| 2.25 OR + 0.75 EC | 0.335 ± 0.012e | 15.23 ± 3.94b | 0.122 ± 0.389a | 19.88 ± 5.88a |
| 2 OR + 1 EC | 0.354 ± 0.008d | 9.75 ± 2.47c | 0.087 ± 0.022bc | 16.21 ± 2.68ab |
| 1.5 OR + 1.5 EC | 0.357 ± 0.012d | 9.24 ± 0.52c | 0.074 ± 0.021bc | 11.71 ± 3.80bc |
| 1 OR + 2 EC | 0.372 ± 0.017c | 7.00 ± 0.26d | 0.063 ± 0.013c | 15.27 ± 8.19ab |
| 0.5 OR + 2.5 EC | 0.372 ± 0.012c | 3.43 ± 0.0.28e | 0.063 ± 0.014c | 3.93 ± 1.32d |
| 0.25 OR + 2.75 EC | 0.361 ± 0.010cd | 2.02 ± 0.21ef | 0.063 ± 0.013c | 7.18 ± 0.76cd |
| 3 EC | 0.385 ± 0.016b | 1.20 ± 0.90f | 0.063 ± 0.024bc | 6.30 ± 0.48d |
| 3 EC + 1.5 glycerol | 0.448 ± 0.014a | 40.28 ± 1.04a | 0.099 ± 0.028ab | 7.38 ± 4.19cd |
Different letters show significant differences at p < 0.05 in each column
Moisture content
The moisture content of the films is related to the volume of free space occupied by water molecules in the microstructure of the film network. Table 1 shows the MC of prepared films. The highest amount of MC was related to EC film containing glycerol, which had a significantly higher MC than other films due to the hydrophilic nature of glycerol and the protective role of this plasticizer from the moisture of the prepared film. The next film with a high MC was 2.25 g OR + 0.75 g EC film. The MC of the films decreased by decreasing the amount of resin and increasing the amount of EC. So, the film without resin had the lowest MC. It seems that although opopanax resin and ethylcellulose are both hydrophobic polymers, ethylcellulose is more hydrophobe. Since OR was extracted by ethanol and no other purification steps were performed, other compounds soluble in alcohol were also present in the extracted resin, which had increased the hydrophilicity of the resin. As a result, more water molecules can form hydrogen bonds with the resin, and the MC of the film increases by increasing the resin content. Unlike glycerol, which significantly increased the MC of EC films, the use of resin as a plasticizer to prepare EC-based composite films (films containing 1, 0.5, and 0.25 g OR) slightly increased the MC of EC films. So, the film with 0.25 g resin did not cause a significant difference in the MC of the EC film, which was an advantage for using the resin as a plasticizer (He and Yan 2005).
Water solubility
WS is one of the important features of edible films and is considered to determine the resistance of film samples to water. WS of prepared films is shown in Table 2. It was observed that the WS of the films increased by increasing the exposure time of films to water due to higher penetration of water into the structure over time. EC film containing glycerol had significantly higher WS than the other films due to the hydrophilic nature of glycerol. In the case of OR-containing films, it was observed that the higher OR content, the higher WS due to the hydrophilic impurities in the extracted resin. Glycerol significantly increased the WS of EC films. However, the use of OR as a plasticizer to prepare EC-based composite films caused no significant difference in films WS, which was an advantage of using resin as a plasticizer. Luangtana‐Anan et al. (2017) investigated the characteristics of a composite film containing pectin, shellac, and plasticizer. They found that the water solubility of the composite film decreased (from 90 to 45%) as the amount of shellac increased (from 10 to 50%) due to the low polarity of the composite films, which caused a lower affinity with water.
Table 2.
Water solubility of films prepared with different amounts of OR and EC at 24, 48 and 72 h
| Films | WS (24 h) | WS (48 h) | WS (72 h) |
|---|---|---|---|
| 2.25 OR + 0.75 EC | 6.09 ± 1.24Ab | 6.05 ± 0.67Ab | 6.37 ± 0.06Ab |
| 2 OR + 1 EC | 4.26 ± 0.44Cbc | 5.18 ± 0.19Bbc | 5.67 ± 0.070Ab |
| 1.5 OR + 1.5 EC | 2.56 ± 0.22Bcd | 3.02 ± 0.22Bcd | 3.73 ± 0.15Ac |
| 1 OR + 2 EC | 0.43 ± 0.18Ce | 0.84 ± 0.15Bd | 1.13 ± 0.15Ad |
| 0.5 OR + 2.5 EC | 0.56 ± 0.21Be | 0.78 ± 0.10Abd | 0.99 ± 0.01Ad |
| 0.25 OR + 2.75 EC | 0.47 ± 0.05Ce | 0.63 ± 0.10Bd | 0.93 ± 0.07Ad |
| 3 EC | 0.74 ± 0.13Bde | 1.032 ± 0.28Bd | 1.69 ± 0.09Ad |
| 3 EC + 1.5 glycerol | 10.84 ± 2.32Aa | 13.02 ± 1.60Aa | 14.78 ± 1.33Aa |
Different capital letters show significant differences at p < 0.05 between samples in each row
Different small letters show significant differences at p < 0.05 between samples in each column
It should also be noticed that the WS of resin-containing films (EC-based composite films containing 1, 0.5, and 0.25 g OR) was much lower than those of natural polysaccharide and protein films (mostly have higher than 20% water solubility), which is an advantage of using resin films in food packaging (Duggal et al. 2020; Omar-Aziz et al. 2021).
Shrinkage ratio
The shrinkage ratio of prepared films is shown in Table 1. The highest SR was related to the film containing 2.25 g OR and 0.75 g EC. The SR decreased as the amount of resin decreased. Impurities in the extracted resin increased the hydrophilicity of the resin. Accordingly, the higher the amount of resin, the higher the film moisture content, removed after heating in the oven to calculate the SR. So, the SR of films with higher OR was higher than the others. When the OR was less than 1 g, its effect on the SR was insignificant, so even the SR of a film with no resin was equal to the SR of films containing 1, 0.5, and 0.25 g resin. It could be explained by the fact that EC was the main matrix in these films, and the OR had only a plasticizing role, and therefore its small amount in the films did not affect the SR. The SR was increased when glycerol was used as a plasticizer for EC film. As a hydrophilic plasticizer, glycerol can form more hydrogen bonds with water and increase the moisture content of the film. This moisture will release during the drying of the film, and the SR of the film will increase. On the other hand, it was observed that glycerol-containing EC film did not have the highest SR. Glycerol has water-retaining properties. So, higher water remains in the glycerol-containing EC film after drying (Zhao et al. 2018).
Swelling index
The solvent penetrates the free space of the film polymer network when it comes in contact with and causes the film to swell. According to Table 1, which shows the swelling index of the prepared films, the highest SI was obtained at 2.25 g OR + 0.75 g EC film. The SI decreased as the amount of OR decreased. It was also observed that EC film without any plasticizer had a low SI, which could be attributed to its highly cohesive network, inflexible structure, and its hydrophobic nature. The SI of EC film containing glycerol was not significantly different from EC film without a plasticizer. On the other hand, glycerol is a hydrophilic compound and is expected to increase film SI. But no increase in the SI of this film was related to the moisture retention property of glycerol during drying (Omar-Aziz et al. 2021).
Contact angle
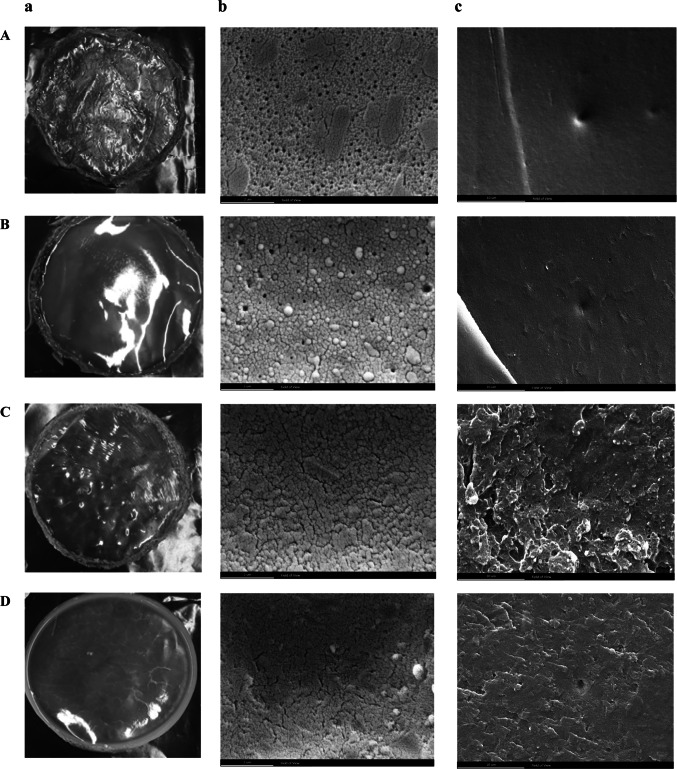
When a water droplet is injected on a solid surface, it disperses based on intermolecular interactions between the solid and the water. The contact angle is related to the microstructure and chemical properties of the film. Other surface properties such as wetness, smoothness, porosity, and pore size can also affect the contact angle. (Table 3) shows the contact angle of different films. The maximum contact angle, which represented the highest hydrophobicity, was related to the film with the maximum amount of OR. The increased contact angle of high-resin films could be attributed to the denser and more compact structure of resin films, which caused a smoother surface with lower porosity. SEM images also confirmed it (Fig. 3). The contact angle of films decreased in the resin-based composite films (OR contained the main film matrix) by OR decreasing. EC reduced the contact angle of resin films by reducing the dense resin network. While the contact angle results showed that resin films were more hydrophobic than EC films, all previous tests showed that the sensitivity of resin films to water was higher. The contact angle depends not only on the chemical properties of the sample but also on the surface properties of the film. Although EC had a higher hydrophobicity than OR, according to SEM results, the EC film surface was more uneven (aggregated parts were observed along with deep cracks), which caused a lower contact angle of the water droplet with the EC films. Adding plasticizer to EC film reduced the contact angle of the films due to the disruption of the network structure of EC films by the plasticizers.
Table 3.
Contact angle, WVP, EB, and TS of films prepared with different amounts of OR and EC
| Films | Contact angle | WVP (g m/Pa s m2) × 10–9 | EB (%) | TS (MPa) |
|---|---|---|---|---|
| 2.25 OR + 0.75 EC | 59.16 ± 1.50a | 0.22 ± 0.41 | 66.59 ± 18.48a | 0.40 ± 0.01d |
| 2 OR + 1 EC | 56.97 ± 0.93b | 0.31 ± 0.14c | 59.56 ± 17.61ab | 1.67 ± 0.38bc |
| 1.5 OR + 1.5 EC | 55.32 ± 2.18c | 0.80 ± 0.69c | 3.26 ± 0.46d | 2.73 ± 0.14a |
| 1 OR + 2 EC | 43.59 ± 1.00g | 2.50 ± 1.71b | 2.08 ± 1.09d | 2.09 ± 0.38ab |
| 0.5 OR + 2.5 EC | 43.07 ± 2.10g | 2.43 ± 0.18b | 31.05 ± 0.84c | 1.05 ± 0.91cd |
| 0.25 OR + 2.75 EC | 46.74 ± 3.12e | 2.18 ± 0.91b | 32.50 ± 0.51bc | 0.82 ± 1.18cd |
| 3 EC | 52.40 ± 2.17d | 2.75 ± 0.45ab | 24.93 ± 0.07c | 2.67 ± 0.43ab |
| 3 EC + 1.5 glycerol EC | 45.20 ± 1.87f | 3.61 ± 0.43a | 0.28 ± 0.01d | 0.31 ± 0.06d |
Different letters show significant differences at p < 0.05 in each column
Fig. 3.
Images (a) and SEM images at ×6500 magnification of surface (b) and cross-section (c) of A: 2.25 OR + 0.75 EC, B: 2 OR + 1 EC, C: 1.5 OR + 1.5 EC, D: 1 OR + 2 EC, E: 0.5 OR + 2.5 EC, F: 0.25 OR + 2.75 EC, G: 3 EC, and H: 3 EC + 1.5 GLY films
Siracusa et al. (2018) investigated the properties of active edible films based on citral essential oil, alginate, and pectin. Contact angle measurement showed a high wettability of sodium alginate/pectin film due to its large amount of ether bonds and free hydroxyl groups. The addition of citral essential oil increased the contact angle and decreased the wettability of the edible film due to its hydrophobic nature.
Water vapor permeability
Low water vapor permeability is one of the most important characteristics of edible films. Table 3 shows the results of the WVP of the prepared films. The highest WVP was related to the glycerol-containing EC film due to the hydrophilic nature of glycerol and the reduction of intermolecular bonds between the EC chains, which caused a very porous film structure and therefore the easy transfer of water vapor from this film. The results showed that the use of OR in film preparation reduced the WVP of different films significantly. The OR-based composite films (containing 2.25, 2, and 1.5 g OR) had the lowest WVP. However, the EC-based composite films (containing 1, 0.5, and 0.25 g OR) with resin as the plasticizer had higher WVP. The lower WVP of high-resin films was attributed to the denser and more compact structure of OR, which created a film structure with lower porosity. The WVP of resin-containing films was lower than that of glycerol-containing films, indicating the advantage of using resin as the plasticizer.
Luangtana‐Anan et al. (2017) used shellac as a hydrophobic polymer in pectin/shellac composite film. They found that adding 50% shellac to the composite film caused the lowest water vapor permeability (0.56 × 10–11 g m/Pa s m2), showing a better effect of shellac in WVP reduction of pectin/shellac composite film than OR in OR/EC composite films.
Mechanical properties
Elongation at break and tensile strength are important mechanical properties of edible films. The results of EB and TS of different films are shown in Table 3. The results showed that the highest EB was related to the film with the highest amount of OR in its formulation. EB decreased by reducing the amount of OR-based composite films. Since the OR was very adhesive, the resulting films also had higher elasticity. A similar trend was observed in the EC-based composite films (resin was the plasticizer). EB was decreased by reducing the main matrix material. It seems that EB increases when the amount of EC or OR decreases due to the formation of stronger intermolecular bonds between the main matrix molecules. The results of Table 3 also show that the addition of glycerol to the EC film reduced EB significantly so that the lowest EB was related to it. Severe network destruction of EC and the creation of extensive cavities by glycerol addition caused decreasing film stiffness. However, the lowest amount of OR as the plasticizer for EC film did not significantly change the EB of EC film, which was an advantage of resin compared to glycerol. In practice, the elasticity of EC film containing glycerol was observed to be very low. However, the EC film was elastic with the addition of resin to EC. Resin seems to be able to weaken the non-covalent bonds present in EC but not to the extent that the network is destroyed, thus improving the flexibility of EC films.
Results of TS (Table 3) show that TS increased by resin decreasing in the OR-based composite films. In other words, the film with the highest OR content had the lowest TS. The TS also increased as the content of EC decreased in the EC-based composite films. These results are consistent with the results of EB. The EC film, which had no plasticizer, had a high TS. Adding plasticizer to EC film reduced the TS of the film. Comparing the TS of EC films containing resin or glycerol, it was observed that these two plasticizers did not have a significant difference in reducing the TS of EC films. Plasticizers are placed between the polymer chains of EC and reduce TS.
Altogether, considering the mechanical properties of prepared films, it could be said that the OR-based composite films had a high EB and a low TS, which resulted in soft and flexible films. EC alone had a low EB and a high TS and was therefore very brittle. However, OR addition to EC film caused a soft and flexible film, indicating the advantage of OR as a plasticizer.
Transparency and color
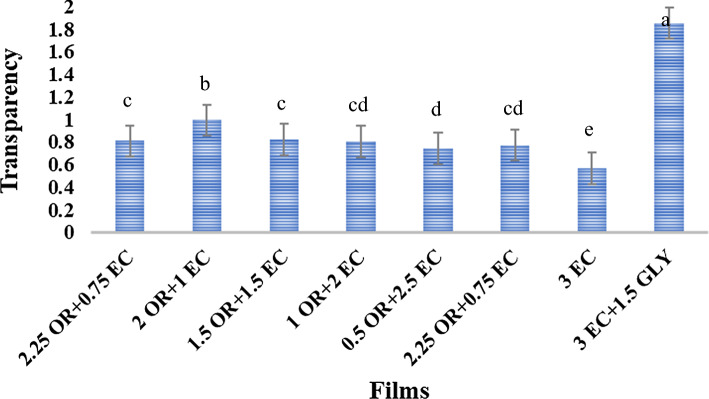
One of the most important features of films used as food coatings or packaging is transparency. The transparency of the different films is shown in Fig. 1. The lowest transparency was related to EC film. The compact structure of EC film prevented light transmission, and therefore the transparency of the film was low. Adding plasticizer to EC film reduced the turbidity of EC film. Plasticizers weaken the EC network, so pathways are created for the passage of light, which increases the transparency of the film. The effect of glycerol on the transparency increase of EC film was greater than that of OR, probably due to its greater impact on the weakening of the EC structure. The OR-based composite films were more transparent than EC-based composite films.
Fig. 1.
Transparency of films prepared with different amounts of OR and EC
The color of the edible films affects the appearance of the products. The highest L* was associated with EC film containing glycerol due to the white color of EC and glycerol. The whiteness of films decreased by OR increasing (results not shown). The results also show that a* and b* of the films increased by OR increasing due to the presence of color compounds in the extracted resin, which caused yellowness and redness of the prepared films.
Chemical structure
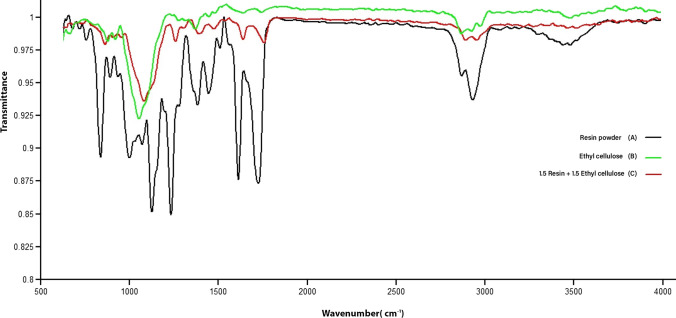
Fourier transform infrared spectroscopy was used to investigate the chemical structure of the prepared films. Figure 2 shows the infrared spectra of OR, EC film, and 1.5 g OR + 1.5 g EC film. The band at 3600–3100 cm−1 was related to the tensile vibrations of NH and OH in OR. The absorption bands at 2926, 2937, and 2885 cm−1 were attributed to the tensile vibration –CH2–. The band at 1667 cm−1 was represented the tensile vibration of the carbonyl of the urea amide. The band at 1630 cm−1 was reflected the tensile vibration of N–H of urea. The band at 1421 cm−1 was related to the bending vibration of CH in –N–CH2 and –CH2–O–. The peak at 1029 cm−1 was attributed to the C-O asymmetric tensile vibration (Trovati et al. 2010; Zorba et al. 2008).
Fig. 2.
FTIR spectra of (A) OR, (B) EC film, and (C) 1.5 OR + 1.5 EC film
The EC spectra showed a band at 3485 cm−1 due to the tensile vibrations of –OH. The bands at 2974 and 2869 cm−1 were related to tensile vibrations of C–H. Other important bands at 1091 and 1373 cm−1 were related to C–O–C tension and C–H bending, respectively (Pineda and Hechenleitner 2004; Xiao et al. 2016).
Significant bands at 3485 and 2873, and 2976 cm−1 in OR + EC film were associated with -OH tension, and CH tension, respectively. The absorption bands at 1066 and 1375 cm−1 were due to C–O–C tension and C–H bending, respectively (Singh 2004).
The C–O–C tension in EC film at 1091 cm −1 was slightly shifted forward (1124 cm−1) in the spectrum of OR + EC film. Therefore, it can be said that the bond strength and the C–O–C bond strength constant in OR + EC film increased compared to the EC film (Ahammed et al. 2020). On the other hand, the band at 2873 cm−1 in EC film, which was related to CH tension, was shifted forward (2890 cm−1) in the spectra of OR + EC film. It could be due to the increase of CH bond strength and the bond strength constant in the OR + EC film compared to EC film due to the presence of resin (Singh 2004). The compact structure of resin and the formation of physical bonds between the OR and EC resulted in more strength with fewer empty pores in the structure of OR + EC film (Duggal et al. 2020).
Microstructure
SEM images from the surface and cross-section of different films are shown in Fig. 3. It was observed that the smoother and softer films were produced by increasing the amounts of resin in the OR-based composite films. But at the same time, there were fine surface pores in these films, which could be attributed to the high adhesion of the resin. The images of the EC-based composite films were more uneven than previous films due to the nature of EC. Also, films porosity increased with increasing the amount of EC and decreasing the amount of OR entering the EC film network. EC films with no plasticizer showed a very uneven structure with high pores. Glycerol addition to EC film increased the film porosity due to weakening the intermolecular bonds in the EC chain (De-Castro et al. 2020).
Conclusion
This study investigated opopanax resin as a biosource to prepare the edible film. The opopanax resin could not produce a film. So, ethylcellulose was used to produce OR/EC composite films. Various films with different amounts of OR and EC were made, and their characteristics were investigated. It was interesting that OR had plasticizing effect for EC films. EC film is very brittle, and plasticizers such as glycerol are necessary to improve its mechanical properties. Results showed that the film moisture content, shrinkage ratio, water solubility, and swelling index were reduced by resin decreasing, which indicates higher water sensitivity of resin. However, water vapor permeability of the films increased, and the contact angle decreased by resin decreasing due to smoother and more homogeneous structures of OR-based composite films with lower porosity, confirmed by SEM images. Mechanical properties showed that OR-based composite films were flexible, while EC-based composite films were brittle. The color and transparency of the films were affected by the color compounds present in the OR. Eventually, OR could be used to prepare hydrophobic films with desirable mechanical properties and, at the same time, could be a plasticizer for EC films.
Acknowledgements
Not applicable.
Abbreviations
- OR
Opopanax resin
- EC
Ethylcellulose
- WVP
Water vapor permeability
- SEM
Scanning electron microscopy
- FTIR
Fourier transform infrared spectroscopy
Authors' contributions
FA: Conceptualization, Formal analysis, Writing-original draft. HS: Supervision, Conceptualization, Methodology, Investigation, Validation, writing- review and editing.
Funding
This research received no specific grant from any funding agency.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
Ethics approval was not required for this research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahammed S, Liu F, Khin MN, Yokoyama WH, Zhong F. Improvement of the water resistance and ductility of gelatin film by zein. Food Hydrocoll. 2020;105:105804–105814. doi: 10.1016/j.foodhyd.2020.105804. [DOI] [Google Scholar]
- Auda SH, Salem-Bekhit MM, Alanazi FK, Alsarra IA, Shakeel F. Antimicrobial evaluation of novel buccoadhesive films containing Myrrh extract. Polym Bull. 2017;74:4041–4054. doi: 10.1007/s00289-017-1946-x. [DOI] [Google Scholar]
- Chen H, Chen Y, Zhan H, Wu G, Xu JM, Wang JN. Preparation of carbon nanotube/epoxy composite films with high tensile strength and electrical conductivity by impregnation under pressure. Front Mater Sci. 2019;13(2):165–173. doi: 10.1007/s11706-019-0460-5. [DOI] [Google Scholar]
- Cho BG, Lee JE, Jeon SY, Chae HG. A study on miscibility properties of polyacrylonitrile blending films with biodegradable polymer, shellac. Polym Test. 2023;121:107983. doi: 10.1016/j.polymertesting.2023.107983. [DOI] [Google Scholar]
- De-Castro EF, Azevedo VLB, Nima G, de Andrade OS, Dias CTDS, Giannini M. Adhesion, mechanical properties, and microstructure of resin-matrix CAD-CAM ceramics. J Adhes Dent. 2020;22(4):421–431. doi: 10.3290/j.jad.a44874. [DOI] [PubMed] [Google Scholar]
- Duggal S, Sharma S, Verma R, Kumar D. Prepration of different zein films from corn gluten meal and their use in coating of different model foods. J Food Sci. 2020;13:13–45. [Google Scholar]
- Farajpour R, Emam Djomeh Z, Moeini S, Tavahkolipour H, Safayan S. Structural and physico-mechanical properties of potato starch-olive oil edible films reinforced with Zein nanoparticles. Int J Biol Macromol. 2020;149:941–950. doi: 10.1016/j.ijbiomac.2020.01.175. [DOI] [PubMed] [Google Scholar]
- Galus S, Lenart A. Development and characterization of composite edible films based on sodium alginate and pectin. J Food Eng. 2013;115(4):459–465. doi: 10.1016/j.jfoodeng.2012.03.006. [DOI] [Google Scholar]
- Greener IK, Fennema O. Evaluation of edible, bilayer films for use as moisture barriers for food. J Food Sci. 1989;54(6):1400–1406. doi: 10.1111/j.1365-2621.1989.tb05121.x. [DOI] [Google Scholar]
- He G, Yan N. Effect of moisture content on curing kinetics of pMDI resin and wood mixtures. Int J Adhes Adhes. 2005;25(5):450–455. doi: 10.1016/j.ijadhadh.2004.12.002. [DOI] [Google Scholar]
- Kangarlou S, Haririan I. Physico-mechanical analysis of free ethylcellulose films plasticized with incremental weight percents of dibutyl sebacate. Iran J Pharm Sci. 2007;3(3):135–142. [Google Scholar]
- Lin Y, Asante FO, Xu X, Li S, Ding H, Xu L, Li M. A naturally tailored small molecule for the preparation of ethyl cellulose supramolecular composite film. Cellulose. 2021;28(1):289–300. doi: 10.1007/s10570-020-03532-9. [DOI] [Google Scholar]
- Liu CC, Tellez-Garay AM, Castell-Perez ME. Physical and mechanical properties of peanut protein films. LWT Food Sci Technol. 2004;37(7):731–738. doi: 10.1016/j.lwt.2004.02.012. [DOI] [Google Scholar]
- Luangtana-Anan M, Soradech S, Saengsod S, Nunthanid J, Limmatvapirat S. Enhancement of moisture protective properties and stability of pectin through formation of a composite film: effects of shellac and plasticizer. J Food Sci. 2017;82(12):2915–2925. doi: 10.1111/1750-3841.13956. [DOI] [PubMed] [Google Scholar]
- Meng Q, Kenelak V, Chand A, Kang H, Han S, Liu T. A highly flexible, electrically conductive, and mechanically robust graphene/epoxy composite film for its self-damage detection. J Appl Polym Sci. 2020;137(34):48991–49021. doi: 10.1002/app.48991. [DOI] [Google Scholar]
- Moghtadaei M, Soltanizadeh N, Goli SAH, Sharifimehr S. Physicochemical properties of beef burger after partial incorporation of ethylcellulose oleogel instead of animal fat. J Food Sci Technol. 2021;58(12):4775–4784. doi: 10.1007/s13197-021-04970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olewnik-Kruszkowska E, Gierszewska M, Wrona M, Nerin C, Grabska-Zielińska S. Polylactide-based films with the addition of poly(ethylene glycol) and extract of propolis—physico-chemical and storage properties. Foods. 2022;11:1488. doi: 10.3390/foods11101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar-Aziz M, Gharaghani M, Hosseini SS, Khodaiyan F, Mousavi M, Askari G, Kennedy JF. Effect of octenylsuccination of pullulan on mechanical and barrier properties of pullulan-chickpea protein isolate composite film. Food Hydrocoll. 2021;121:107047–107071. doi: 10.1016/j.foodhyd.2021.107047. [DOI] [Google Scholar]
- Önder A, Nahar L, Nath S, Sarker SD. Phytochemistry, traditional uses and pharmacological properties of the genus opopanax WDJ Koch: a mini-review. Pharm Sci. 2020;26(2):99–106. doi: 10.34172/PS.2020.8. [DOI] [Google Scholar]
- Pak ES, Ghaghelestani SN, Najafi MA. Preparation and characterization of a new edible film based on Persian gum with glycerol plasticizer. J Food Sci Technol. 2020;57(9):3284–3294. doi: 10.1007/s13197-020-04361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Weller CL, Vergano PJ, Testin RF. Permeability and mechanical properties of cellulose-based edible films. J Food Sci. 1993;58(6):1361–1364. doi: 10.1111/j.1365-2621.1993.tb06183.x. [DOI] [Google Scholar]
- Pineda A, Hechenleitner A. Characterization of ethylcellulose films containing natural polysaccharides by thermal analysis and FTIR spectroscopy. Acta Farm Bonaer. 2004;23(1):53–57. [Google Scholar]
- Purwanti N, Yunus F, Darmawati E. Synthesis and characterization of aloe vera-based edible film incorporated with shellac resin and hydrocolloids. In IOP Conf Ser Mater Sci Eng. 2019;557:12–20. doi: 10.1088/1757-899X/557/1/012076. [DOI] [Google Scholar]
- Senarathna S, Navaratne S, Wickramasinghe I, Coorey R. Use of fenugreek seed gum in edible film formation: major drawbacks and applicable methods to overcome. J Food Sci Technol. 2022;60:1860–1869. doi: 10.1007/s13197-022-05465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Preparation and structural characterization of melamine–methylurea–formaldehyde resin and its blends separately with ethyl cellulose, starch, teakwood, and almond shell powders by 13C NMR, IR, TGA, and SEM techniques. J Appl Polym Sci. 2004;92(6):3437–3446. doi: 10.1002/app.20279. [DOI] [Google Scholar]
- Siracusa V, Romani S, Gigli M, Mannozzi C, Cecchini JP, Tylewicz U, Lotti N. Characterization of active edible films based on citral essential oil, alginate and pectin. Materials. 2018;11(10):19–80. doi: 10.3390/ma11101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovati G, Sanches EA, Neto SC, Mascarenhas YP, Chierice GO. Characterization of polyurethane resins by FTIR, TGA, and XRD. J Appl Polym Sci. 2010;115(1):263–268. doi: 10.1002/app.31096. [DOI] [Google Scholar]
- Xiao M, Wan L, Corke H, Yan W, Ni X, Fang Y, Jiang F. Characterization of konjac glucomannan-ethyl cellulose film formation via microscopy. Int J Biol Macromol. 2016;85:434–441. doi: 10.1016/j.ijbiomac.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Yong H, Liu J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: a review. Compr Rev Food Sci Food Saf. 2021;20:2106–2145. doi: 10.1111/1541-4337.12697. [DOI] [PubMed] [Google Scholar]
- Zhang Q-W, Lin L-G, Ye W-C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13:20–30. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao SM, Yao S, Yang T. Influence of resin flow on shrinkage of additive manufacturing coated sand molds. China Foundry. 2018;15(4):291–298. doi: 10.1007/s41230-018-7184-5. [DOI] [Google Scholar]
- Zorba T, Papadopoulou E, Hatjiissaak A, Paraskevopoulos K, Chrissafis K. Urea-formaldehyde resins characterized by thermal analysis and FTIR method. J Therm Anal Calorim. 2008;92(1):29–33. doi: 10.1007/s10973-007-8731-2. [DOI] [Google Scholar]
- Isfran D, Chacon WDC, Alves MJDS, Monteiro AR, Ayala Valencia G (2023) Active films and coatings based on propolis extract and chitosan: physicochemical characterization and potential application in refrigerated shrimps (Litopenaeus vannamei). Starch (in press)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Not applicable.