Abstract
Human immunodeficiency virus type 1 (HIV-1) infection in mononuclear phagocyte lineage cells (monocytes, macrophages, and microglia) is a critical component in the pathogenesis of viral infection. Viral replication in macrophages serves as a reservoir, a site of dissemination, and an instigator for neurological sequelae during HIV-1 disease. Recent studies demonstrated that chemokine receptors are necessary coreceptors for HIV-1 entry which determine viral tropism for different cell types. To investigate the relative contribution of the β-chemokine receptors CCR3 and CCR5 to viral infection of mononuclear phagocytes we utilized a panel of macrophage-tropic HIV-1 strains (from blood and brain tissue) to infect highly purified populations of monocytes and microglia. Antibodies to CD4 (OKT4A) abrogated HIV-1 infection. The β chemokines and antibodies to CCR3 failed to affect viral infection of both macrophage cell types. Antibodies to CCR5 (3A9) prevented monocyte infection but only slowed HIV replication in microglia. Thus, CCR5, not CCR3, is an essential receptor for HIV-1 infection of monocytes. Microglia express both CCR5 and CCR3, but antibodies to them fail to inhibit viral entry, suggesting the presence of other chemokine receptors for infection of these cells. These studies demonstrate the importance of mononuclear phagocyte heterogeneity in establishing HIV-1 infection and persistence.
CD4 and chemokine receptors are necessary cofactors for human immunodeficiency virus type 1 (HIV-1) infection in its natural target cells (1, 3, 11, 14, 16, 23). Macrophage- and lymphocyte-tropic strains of HIV-1 utilize the chemokine receptors CCR5 (1, 3, 11, 14, 23) and CXCR4 (16), respectively, for viral entry. The importance of chemokine receptors in HIV-1 pathogenesis is demonstrated by the mutant allele of CCR5 with a 32-bp deletion, Δ32. Individuals homozygous for the mutant allele are highly resistant to HIV-1 infection, and peripheral blood mononuclear cells (PBMC) isolated from those individuals fail to support viral infection with macrophage-tropic strains (10, 25, 28, 32, 34, 36). Moreover, individuals heterozygous for this allele show slower progression to AIDS. CCR5 was shown recently to be an essential coreceptor for HIV-1 infection of monocytes, and resistance to macrophage-tropic HIV-1 infection was demonstrated with monocytes from Δ32-homozygous individuals (34).
Despite the rapid advances made in understanding the role of chemokine receptors for HIV-1 infection, the interactions between the virus and its principal host cells in the central nervous system (CNS), the microglia, remain incompletely defined. Infection and replication of HIV-1 in brain macrophages and microglia represent the principal reservoir and vehicle for viral dissemination in nonlymphoid tissues. The unique antigenic expression of chemokine receptors on microglia may render these cells susceptible to strains of HIV-1 that could evolve over time into neurotropic strains.
To investigate the role of β-chemokine receptors in HIV-1 infection of microglia, we utilized highly purified cell systems to analyze the early events of the viral life cycle after exposure of chemokines, their receptors, and virus to monocytes and microglia. The results demonstrated (i) that CCR5 and CCR3 are expressed by both monocytes and microglia, (ii) that these receptors are expressed in both normal and encephalitic brains, (iii) that CCR5, not CCR3, plays an essential role in viral infection of monocytes, (iv) that HIV-1 infection of microglia occurs through receptors other than CCR5 and CCR3, and (v) that the β chemokines themselves fail to alter viral infection of monocytes or microglia. Taken together, these data suggest that the two important macrophage-tropic HIV-1 receptors characterized to date, CCR5 and CCR3, are not essential for HIV-1 infection of microglia, the principal phagocyte within the CNS.
MATERIALS AND METHODS
Reagents.
Antibodies to mouse immunoglobulin G (IgG) F(ab′)2 and IgM F(ab′)2 fragments and rabbit IgG conjugated to fluorescein isothiocyanate, tetramethyl rhodamine isothiocyanate, and rhodamine, respectively, were utilized. These were purchased from Boehringer Mannheim Corp., Indianapolis, Ind. Antibodies to CD68, glial fibrillary acidic protein (GFAP), HIV-1 p24, von Willebrand factor, and HAM-56 were obtained from Dako Corp., Carpinteria, Calif. Chemokine peptides macrophage inflammatory protein 1 alpha (MIP-1α), MIP-1β, and regulated upon activation T-cell expressed and secreted (RANTES) were obtained from R & D Systems, Minneapolis, Minn. Eotaxin was purchased from PeproTech Inc., Rocky Hill, N.J. Antibodies to CCR5 and CCR3 (23) were supplied by LeukoSite Inc., Cambridge, Mass.
Viral isolates.
HIV-1JR-FL (30) was obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases. It was previously isolated from brain tissue of an HIV-1-infected individual with encephalitis. HIV-1ADA was isolated from PBMC of an individual with AIDS and was propagated as previously described (18). HIV-1YU-2 was obtained from genomic DNA from encephalitic brain tissue (27) and was rescued by transfecting the proviral DNA into a 293T packaging system (33). All isolates were prepared as viral stocks free of mycoplasma and endotoxin contamination. Standardized viral inocula (0.1 infectious viral particle/cell) were used for infections (18).
Brain autopsy material.
Brain tissue obtained by autopsy from patients who died of HIV-1 encephalitis and control (HIV-1-seronegative) tissue were used for immunological assessment of β-chemokine receptors (29). Pieces of brain tissue were removed from the frontal, temporal, and occipital lobes, the basal ganglia, the hippocampus, the cerebellum, and the medulla oblongata and were snap frozen at autopsy. Eight-micrometer-thick serial frozen sections were prepared for immunohistochemistry. Morphological features of HIV-1 encephalitis included the presence of multinucleated giant cells and perivascular macrophages, the formation of microglial nodules, and astrogliosis. Brain tissue specimens from two patients who died of carcinoma of the lung and hepatic failure (HIV-1 seronegative) were used as additional controls. These specimens have minimal neuropathological changes. Immunohistochemical evaluations for the levels of macrophage and microglia activation and viral infection were confirmed with antibodies to CD68, HAM-56, HLA-DR, and HIV-1 p24 antigens. The extent of astrogliosis (GFAP) and chemokine receptor antigen expression were assayed by indirect immunofluorescence (IF) assay as previously described (29). Double immunostaining was utilized to colocalize β-chemokine receptors and cellular antigens (HAM-56 and GFAP) in the human brain tissues.
Isolation and cultivation of primary human monocytes and microglia.
PBMC obtained from HIV-1-, HIV-2-, and hepatitis B virus-seronegative donors by leukopheresis were purified by countercurrent centrifugal elutriation (18). Cells were >98% monocytes as determined by Wright and nonspecific esterase staining. Adherent monolayers were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% human serum and 1,000 U of highly purified recombinant macrophage colony stimulating factor (MCSF) per ml, a generous gift from Genetics Institute, Cambridge, Mass.
Fetal brain tissue (gestational age, 14 to 20 weeks) was obtained from elective abortions performed in full compliance with National Institutes of Health and University of Nebraska Medical Center ethical guidelines. Microglia were isolated and purified as previously described (2). Briefly, brain tissue was obtained and then dissociated with 0.25% trypsin for 30 min at 37°C. The resulting single-cell suspension was cultured in DMEM (Sigma Chemical Co.) supplemented with 10% fetal bovine serum and 1,000 U of MCSF per ml. After 14 days in culture, the nonadherent microglia cells were collected and purified by preferential adhesion. These procedures resulted in >98%-pure microglial cell populations (2).
HIV-1 infection.
Cells (monocytes and microglia) were cultured for 7 days prior to viral inoculation. Monocytes and microglia were cultivated at densities of 105 and 5 × 104 cells/well, respectively, in 96-well plates (Costar Corp., Cambridge, Mass.). Cells were inoculated with 0.1 infectious viral particle/cell. Cells were preincubated with antibodies to CCR5 (3A9; 100 μg/ml), CCR3 (20 μg/ml), or CD4 (OKT4A; 10 μg/ml) or with the β-chemokine peptides (500 ng/ml) for 1 h at 37°C and then exposed to the virus for an additional 4 h in the presence of the antibodies or peptides. After virus exposure, the medium was replaced with that supplemented with chemokine receptor antibodies and peptides at the exact concentrations used during the initial inoculations. Samples were obtained every 2 or 3 days for assay of reverse transcriptase (RT) activity. RT activity was determined by incubating 10 μl of sample with a reaction mixture consisting of 0.05% Nonidet P-40 (Sigma) and [3H]dTTP (2 Ci/mmol; Amersham Corp., Arlington Heights, Ill.) in Tris-HCl buffer (pH 7.9) for 24 h at 37°C. Radiolabeled nucleotides were precipitated on paper filters in an automatic cell harvester (Skatron, Sterling, Va.) by using cold 10% trichloroacetate and 95% ethanol. Incorporated activity was measured by liquid scintillation spectroscopy (26).
PCR analysis of viral DNA.
Monocytes and microglia were propagated and infected with virus in the presence or absence of β chemokines or their receptors as described above. Prior to infection, the HIV-1 stock virus was treated with DNase I for 30 min to eliminate contaminating viral DNA (44). At 8 and 96 h following infection, culture fluids were withdrawn for assay of RT activity and the cells were scraped in 1 ml of phosphate-buffered saline. Total cellular DNA was extracted from the cell pellet and resuspended at a concentration of 104 cell equivalents/μl. PCR was performed to quantitate the early, intermediate, and late events of proviral DNA synthesis with primers to long terminal repeat (LTR) U3/R, pol I and J, and LTR U3/gag regions of the viral genome. Standard HIV-1 DNAs for quantitation were prepared by amplification of serial twofold dilutions of DNA extracted from 8e5 cells (17). Tubulin was used as the internal standard. Amplified products of viral DNA were hybridized to radiolabeled oligonucleotide probes and quantified on a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
RNA PCR assays for β-chemokine receptor gene expression.
Total cellular RNA was isolated with TRIzol reagent (Life Technologies). Cells were lysed by repetitive pipetting in 1 ml of TRIzol reagent per 5 to 10 million cells. RNA was extracted with chloroform and precipitated with isopropanol (4, 5). DNA-free RNA was then precipitated with 3 M sodium acetate and ethanol. To verify the absence of DNA contamination, DNA PCR assays for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were performed on the cellular RNA preparations. Levels of chemokine mRNAs were examined after reverse transcription with antisense primers and PCR amplification of cDNA. Table 1 summarizes the primers used in this study. GAPDH RNA served as an internal standard for quantitation. The products of 28 cycles of amplification (30 s, 94°C; 30 s, 50°C; 60 s, 72°C) were analyzed by Southern blot hybridization with specific 32P-labeled oligonucleotides (35).
TABLE 1.
PCR primers used for assay of β-chemokine receptor expression
| Receptor or standarda | Nucleotides | Primer | Sequence |
|---|---|---|---|
| CCR5 (278) | 2785–2802 | Sense | TACCAGCCTCCGTATTTC |
| 3043–3063 | Antisense | TTACTATTCCCTCACCTTACC | |
| 2946–2964 | Probe | AAAAAACCTCTCTCTCTCC | |
| CCR3 (234) | 592–613 | Sense | CCCAGAGGATACAGTTATATATGC |
| 804–823 | Antisense | GATAGGAGAGAGAAGGATAGC | |
| 766–785 | Probe | AGAAAATGAAAAACACCGCC | |
| GAPDH (195) | 199–217 | Sense | CCATGGAGAAGGCTGGGG |
| 394–374 | Antisense | CAAAGTTGTCATGGATGACC | |
| 280–299 | Probe | CTGCACCACCACTGCTTAGC |
Numbers in parentheses are the base pairs in the associated cDNA.
Immunocytochemical assays.
Adherent monolayers of microglia or monocytes were cultured (as described above) in 8-mm Chamber-Tech slides. The cells were fixed in ice-cold acetone-methanol (1:1) for 20 min after 7 days of culture and then incubated with antibodies to CCR3, CCR5, HAM-56, GFAP, or von Willebrand factor at a dilution of 1:100, 1:50, 1:50, 1:200, or 1:200, respectively. Anti-mouse IgG F(ab′)2 fragments conjugated to fluorescein isothiocyanate were used for CCR5 and CCR3 detection. Anti-mouse IgM–tetramethyl rhodamine isothiocyanate conjugate was used for HAM-56, whereas rhodamine-conjugated anti-rabbit IgG was used for GFAP and von Willebrand factor detection. All secondary antibodies were purchased from Boehringer Mannheim Corp. Double immunostainings were performed to colocalize chemokine receptors with cell-specific antigens. Direct application of secondary antibodies was performed as a negative control for this assay.
Nucleotide sequence accession numbers.
The cDNA sequences used for the design of RT-PCR primers were obtained from GenBank. For CCR5, the complete cDNA sequence of human chemokine receptor 5 mRNA was listed under accession no. U54994 and that of human eosinophil chemokine receptor 3 mRNA was listed under accession no. U28694.
RESULTS
Expression of CCR5 and CCR3 on primary human monocytes and microglia.
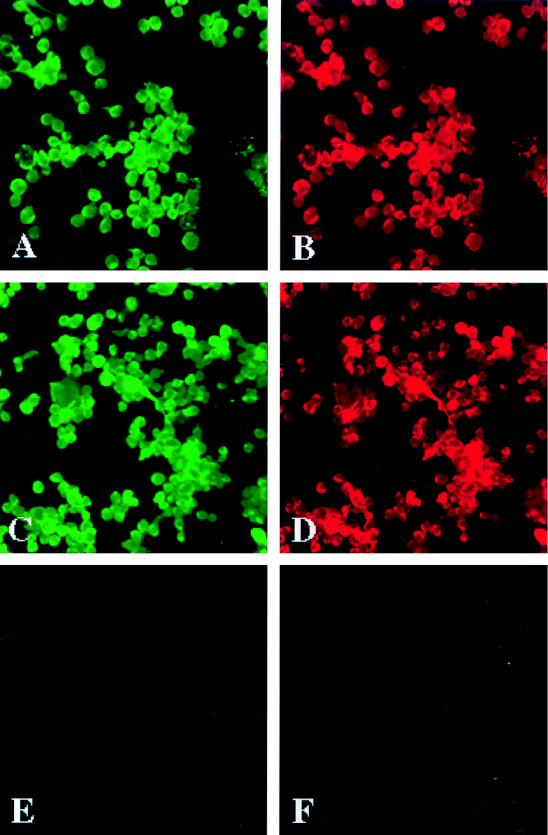
To investigate the role of CCR5 and CCR3 as receptors for HIV-1 infection, we examined the expression of the β-chemokine receptors in monocytes and microglia. Monocytes were recovered from PBMC by centrifugal elutriation, and total cellular RNA was isolated after initial cell isolation and after tissue culture propagation. CCR3 and CCR5 mRNAs were identified by RT-PCR assays of monocytes from the time of cell isolation through cell differentiation (during a 7-day cultivation period). The levels of both CCR5 and CCR3 transcripts increased with cellular differentiation (data not shown). To substantiate the mRNA results, we examined the surface expression of CCR3 and CCR5 on cells cultured for different time periods (1, 5, 7, and 10 days). The β-chemokine receptor antigens were localized in association with HAM-56, a macrophage-specific marker, by day 5 after cell culture. CCR3 immunoreactivity was observed both on the cell surface and in the cytoplasm of HAM-56 antigen-positive cells (Fig. 1A and B). A similar distribution of CCR5 was also seen (Fig. 1C and D). Thus, cultured monocytes expressed both CCR5 and CCR3 (Fig. 1). Fluorescence-activated cell sorter analysis of freshly isolated monocytes (data not shown) showed only weak staining for CCR5 and little CCR3 expression, consistent with previous findings (24, 43). Intense staining for CCR3 was observed on lymph node macrophages by immunohistochemistry (data not shown), similar to the pattern reported previously for CCR5 (43).
FIG. 1.
Expression of chemokine receptors on human monocytes. Monocytes were recovered from PBMC of HIV- and hepatitis B virus-seronegative donors after leukopheresis, purified by countercurrent centrifugal elutriation, and cultured for 7 days before immunocytochemical evaluation. Cell suspensions were >98% monocytes by the criteria of cell morphology in the Wright-stained cytosmears and by CD68 and HAM-56 immunostaining (macrophage markers). Expression of CCR3 was detected on the cell membranes and in the cytoplasm of HAM-56-positive monocytes (A and B). A similar distribution of CCR5 immunostaining was found on human monocytes double immunolabeled for HAM-56 (C and D). The application of the secondary antibodies (Abs) only (negative control) did not produce detectable signals on the replicate cells (E and F). Panels A and B show double immunostaining with anti-CCR3 (7B11) and HAM-56 Abs; panels C and D show double immunostaining with anti-CCR5 (5C7) and HAM-56 Abs; panels E and F display negative controls for which primary antibodies were omitted. Original magnification, ×200. The cellular antigens are visualized by IF assay. The results are representative of three independent experiments performed with three separate donors.
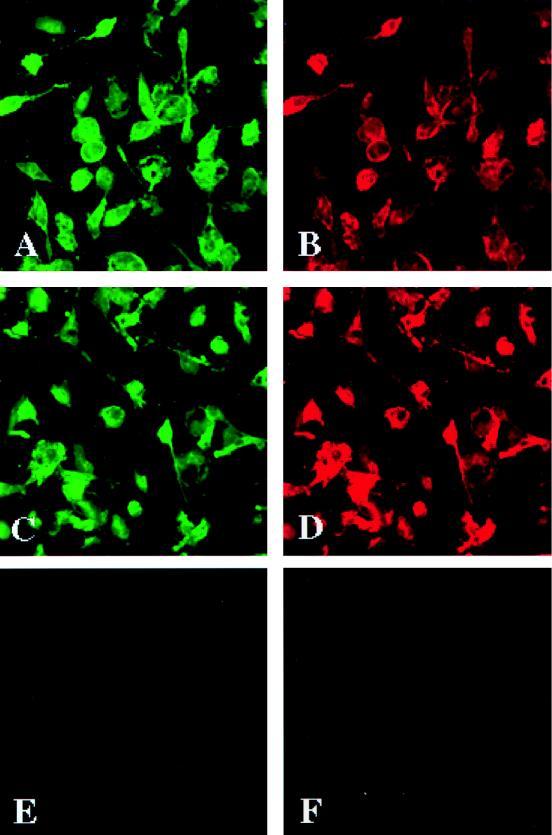
To analyze the distribution of CCR3 and CCR5 in primary human microglia, we isolated pure (>98%) populations of the cells from fetal sources (2). CCR3 antigens were found predominantly on the cell membranes of the HAM-56-immunoreactive microglia (Fig. 2C and D). CCR5 was present both on the cell membrane and in the microglial cytoplasm (Fig. 2A and B). Thus, both monocytes and microglia express β-chemokine receptors CCR5 and CCR3.
FIG. 2.
Expression of chemokine receptors on primary human microglia. Human fetal microglia (>98% pure) were cultured for 7 days in 8-mm Chamber-Tech slides at a density of 50,000 cells/well. CCR3 expression was shown predominantly on cell membranes of HAM-56-immunoreactive microglia (A and D). Application of the secondary antibodies (Abs) only (negative control) did not produce detectable staining on the replicate cells (E and F). Panels A and B show double immunostaining with anti-CCR3 (7B11) and HAM-56 Abs; panels C and D show double immunostaining with anti-CCR5 (5C7) and HAM-56 Abs; panels E and F display negative controls for which primary Abs were omitted. Original magnification, ×200. The cellular antigens are visualized by IF assay. The results are representative of two independent experiments performed with two separate tissue donors.
Expression of β-chemokine receptors in HIV-1-encephalitic and control brain tissues.
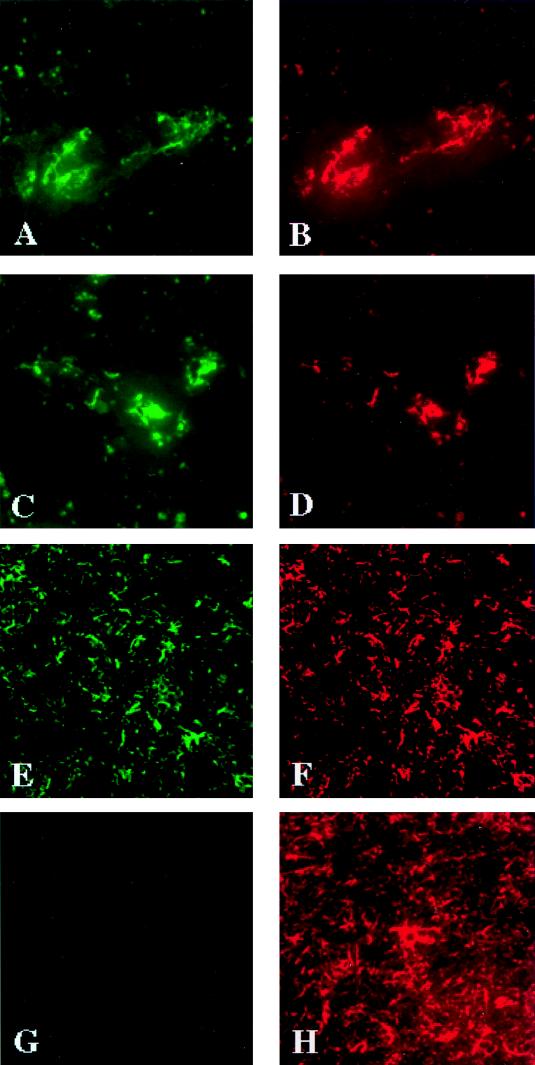
In order to determine whether the tissue culture analyses of CCR3 and CCR5 expression were indicative of what is present in vivo, we analyzed CCR3 and CCR5 production in HIV-1-infected and control brain tissues. To assess the distribution of the chemokine receptors in the human brain, 8-μm-thick serial frozen sections were cut from the frontal cortex and basal ganglia of tissue obtained at autopsy from patients with encephalitis (three cases) and HIV-1-seronegative controls (two cases). In all HIV-1-infected tissues, neuropathological features of HIV-1 encephalitis, including macrophage infiltration, the presence of multinucleated giant cells, microglial nodules, and astrogliosis, were found. Double immunostainings (for cell and chemokine receptor antigens) were performed to identify which brain cells expressed CCR3 and CCR5. The majority of the identified microglial cells and perivascular macrophages (identified by HAM-56 immunoreactivity) expressed CCR3 (7B11) (Fig. 3A and B). The identical cells expressed significant levels of cytoplasmic and membrane CCR5 antigen (5C7) (Fig. 3C and D). Reactive astrocytes, identified by GFAP immunostaining, also expressed CCR3 (Fig. 3E and F). Interestingly, the astrocytes were uniformly CCR5 negative (Fig. 3G and H). The results were reproduced in all five cases regardless of the presence of HIV-1 infection or morphological signs of encephalitis. Primary human fetal astrocytes also expressed CCR3 transcripts but not CCR5, consistent with the brain tissue findings (data not shown).
FIG. 3.
Distribution of chemokine receptors on cells in human brain tissue. Eight-micrometer-thick serial frozen sections were cut from human brain tissue obtained at autopsy from patients with HIV-1 encephalitis (three cases) or seronegative controls (two cases). Double immunostaining was performed in order to identify the cells expressing chemokine receptors. Microglial cells were double immunopositive for CCR3 (A) and HAM-56 (B). These cells also expressed CCR5 (C) and HAM-56 (D). Astrocytes showed CCR3 (E) and GFAP antigens (F) but were negative for CCR5 (G and H). Panels A and B show double immunostaining with antibodies to CCR5 (5C7) and HAM-56; panels E and F show double immunostaining with antibodies to CCR3 (7B11) and GFAP; panels G and H show double immunostaining with antibodies to CCR5 (5C7) and GFAP. Original magnifications, ×400 (panels A to D and G and H) and ×200 (panels E and F). The cellular antigens are visualized by IF assay. The results are representative for the five brain tissue specimens examined. Identical results were found for both control and encephalitic brains.
Analysis of the effects of β chemokines and their receptors on viral infection. (i) PCR analysis of HIV-1 DNA synthesis.
Confirmation of the expression of CCR3 and CCR5 on monocytes and microglia confirmed the notion that these cells could use either or both of the β-chemokine receptors for viral entry. To investigate this idea we utilized the β chemokines and their receptor antibodies to assess if viral infection could be inhibited in monocytes inoculated with macrophage-tropic strains HIV-1ADA and HIV-1YU-2. The β-chemokine receptor antibodies and chemokine peptides were examined for whether they could inhibit reverse transcription, the first step in establishment of HIV-1 infection. Antibodies to CD4 (OKT4A) and the antiretroviral drug zidovudine (AZT), both known inhibitors of viral infection, were used as controls for the assay. MIP-1α, MIP-1β, RANTES, and antibodies to CCR3 (7B11) did not signficantly affect HIV-1ADA nucleic acid production. Moreover, none of the chemokine peptides or receptor antibodies produced any significant or sustained inhibition of HIV-1 reverse transcription as observed by a comparison to results with CD4 antibodies or with AZT (data not shown).
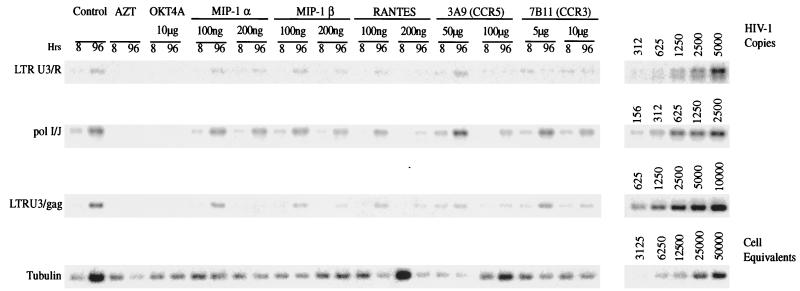
To substantiate these findings, we repeated the PCR analyses with a viral isolate obtained from direct cloning of genomic DNA from encephalitic brain tissue, HIV-1YU-2. Interestingly, RANTES resulted in higher levels of late HIV-1 DNA products 96 h following monocyte inoculation with HIV-1YU-2 (Fig. 4). MIP-1α and MIP-1β peptides produced only a partial inhibition of viral DNA production. Antibodies to CCR3 did not affect (to any significant degree) the levels of late products of viral reverse transcription. However, antibodies to CCR5 (3A9) produced a marked inhibition of viral DNA, comparable to that observed with antibodies to OKT4A and AZT. In summary, although previous reports demonstrated an inhibition of macrophage-tropic HIV-1 infection by β chemokines (31, 42), we found complete synthesis of HIV-1 proviral DNA after such cell treatments. Antibodies to CCR5 alone were able to inhibit viral infection. Because of the limitations in the numbers of microglia recovered from fetal tissue, analysis of viral infection could not be determined by viral DNA PCR and was limited to the RT assays shown below.
FIG. 4.
Synthesis of viral nucleic acids in HIV-1-infected monocytes. Adherent monolayers of >98%-pure monocytes (3 × 106 cells/well in a six-well plate) were infected with cell-free stocks of HIV-1YU-2 in the presence of chemokine peptides or antibodies as described in Materials and Methods. AZT (5 μM) was used as a negative control. Control infected and uninfected cells were maintained in parallel. Chemokine peptides were used at 100 and 200 ng/ml postinfection. At 8 and 96 h postinfection, samples were withdrawn for RT analysis and the cells were used for extraction of cellular DNA. PCR was performed to identify early (primers to LTR U3/R), intermediate (primers to pol I and J), and late (primers to LTR U3/gag regions of viral genome) products of reverse transcription. Standard HIV-1 cDNA was extracted from 8e5 cells that harbor a defective HIV-1 provirus. Tubulin was used as an internal standard. Amplified products of viral nucleic acids synthesized in HIV-1-infected cultured monocytes were hybridized to radiolabeled oligonucleotide probes and quantified on a PhosphorImager (Molecular Dynamics). Data for reverse transcription products for HIV-1YU-2 in the presence of the panel of β-chemokine receptor and CD4 antibodies and β-chemokine peptides are shown.
(ii) RT assays for detection of progeny virions.
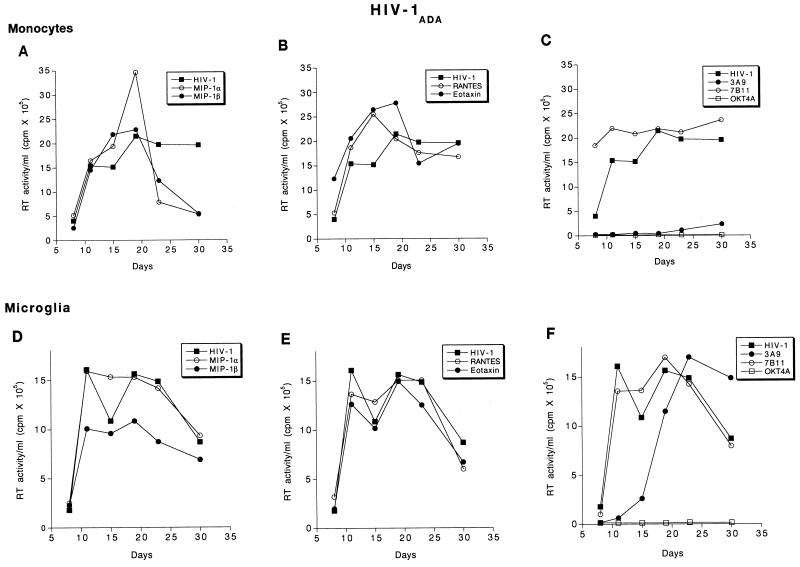
To further assess the role of antibodies to β chemokines and their receptors for HIV-1 infection of monocytes and microglia, we assayed the levels of progeny virion production by reverse transcriptase activity from infected cells. The assays were done following exposure of cells to the β-chemokine peptides, receptor antibodies, and virus. As previous reports demonstrated that the use of specific chemokine receptors is dependent on the viral strain and target cells, a panel of viral isolates was studied, including HIV-1ADA (derived from AIDS patient PBMC), HIV-1JR-FL, and HIV-1YU-2 (derived from virus-infected brain tissue). Antibodies to OKT4A and AZT inhibited HIV-1 infection of both monocytes and microglia by all viral isolates (Fig. 5C and F, Fig. 6C and F, and Fig. 7C and F). Interestingly, the β chemokines studied, MIP-1α, MIP-1β, RANTES, and eotaxin, produced a minimal enhancement of HIV-1ADA infection of monocytes (Fig. 5A and B). When HIV-1ADA was inoculated into microglia, MIP-1β produced a partial inhibition of reverse transcription (Fig. 5D), whereas MIP-1α, RANTES, and eotaxin did not affect viral production. Antibodies to CCR3 (7B11) increased HIV-1ADA replication in monocytes but did not alter microglial infection (Fig. 5C). In contrast to the observations described above, antibodies to CCR5 (3A9) abrogated HIV-1ADA infection of monocytes. However, in microglia, 3A9 only delayed the onset of productive viral replication. The peak RT values of microglia exposed to virus and 3A9 were comparable to those of microglia exposed to virus alone (Fig. 5C and F).
FIG. 5.
Effect of β-chemokine peptides and receptor antibodies on replication profiles of HIV-1ADA. Adherent monolayers of microglia (5 × 104 cells/well in a 96-well plate) and monocytes (105 cells/well) were cultured for 7 days before infection with cell-free stocks of HIV-1ADA. Prior to infection, cells were incubated with antibodies to CCR3 (7B11; 20 μg/ml), CCR5 (3A9; 100 μg/ml), CD4 (OKT4A; 10 μg/ml), or the β-chemokine peptides MIP-1α, MIP-1β, RANTES, and eotaxin (500 ng/ml each) for 1 h at 37°C. Control infected and uninfected cells were maintained simultaneously. The infection proceeded for 4 h after which the virus was washed off and the cells were maintained with media supplemented with appropriate concentrations of antibodies as described in Materials and Methods. Culture supernatant samples were collected twice weekly over a period of 4 weeks postinfection. Each experimental condition was assayed in triplicate, and RT activity was measured independently for each obtained sample.
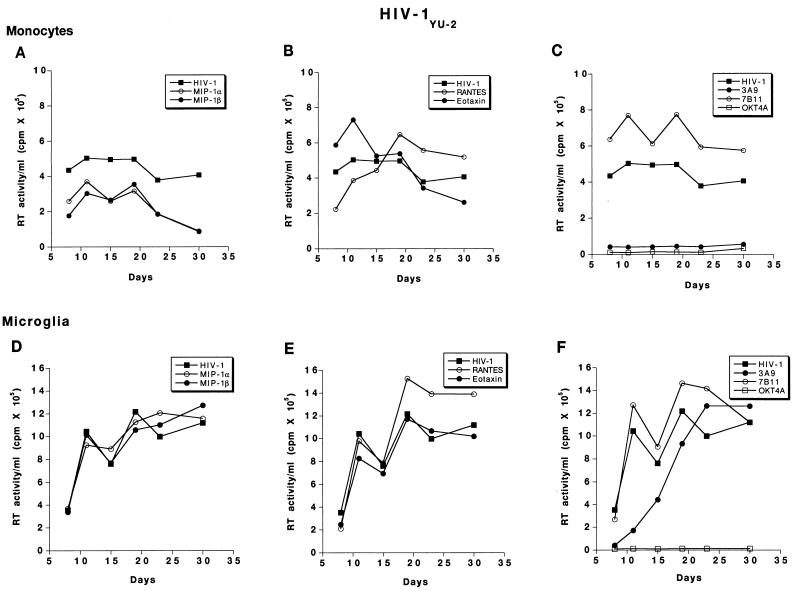
FIG. 6.
Effect of β-chemokine peptides and receptor antibodies on replication profiles of HIV-1YU-2. Adherent monolayers of microglia (5 × 104 cells/well in a 96-well plate) and monocytes (105 cells/well) were cultured for 7 days before infection with cell-free stocks of HIV-1YU-2. Prior to infection, cells were incubated with antibodies to CCR3 (7B11; 20 μg/ml), CCR5 (3A9; 100 μg/ml), CD4 (OKT4A; 10 μg/ml), or the β-chemokine peptides MIP-1α, MIP-1β, RANTES, and eotaxin (500 ng/ml each) for 1 h at 37°C. Control infected and uninfected cells were maintained simultaneously. The infection proceeded for 4 h after which the virus was washed off and the cells were maintained with media supplemented with appropriate concentrations of antibodies as described in Materials and Methods. Culture supernatant samples were collected twice weekly over a period of 4 weeks postinfection. Each experimental condition was assayed in triplicate, and RT activity was measured independently for each obtained sample.
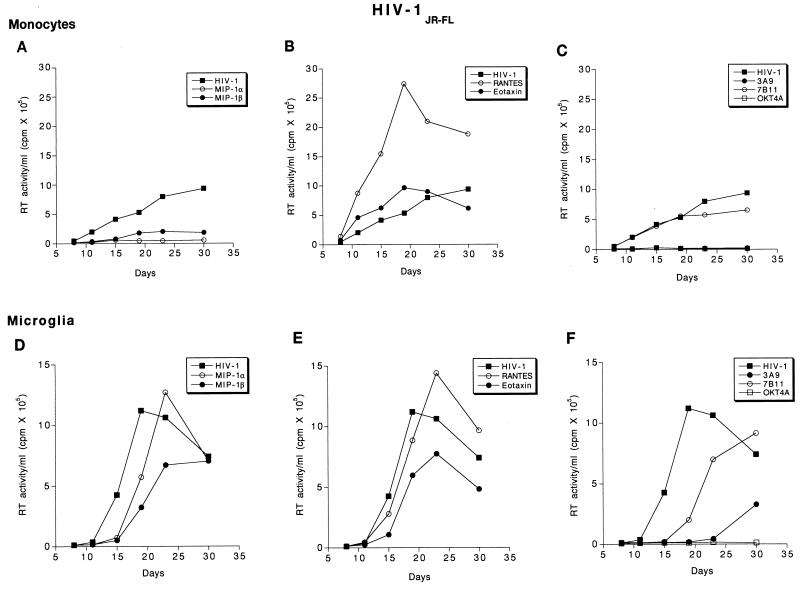
FIG. 7.
Effect of β-chemokine peptides and receptor antibodies on replication profiles of HIV-1JR-FL. Adherent monolayers of microglia (5 × 104 cells/well in a 96-well plate) and monocytes (105 cells/well) were cultured for 7 days before infection with cell-free stocks of HIV-1JR-FL. Prior to infection, cells were incubated with antibodies to CCR3 (7B11; 20 μg/ml), CCR5 (3A9; 100 μg/ml), CD4 (OKT4A; 10 μg/ml), or the β-chemokine peptides MIP-1α, MIP-1β, RANTES, and eotaxin (500 ng/ml each) for 1 h at 37°C. Control infected and uninfected cells were maintained simultaneously. The infection proceeded for 4 h after which the virus was removed and the cells were maintained with media supplemented with antibodies as described above. Culture supernatant samples were collected twice weekly over a period of 4 weeks following infection. Each experimental condition was assayed in triplicate, and RT activity was measured independently for each obtained sample.
For HIV-1YU-2-exposed monocytes, MIP-1α and MIP-1β produced only a partial inhibition of virus production; however, no alterations in RT production were observed in similarly exposed microglia (Fig. 6A and D). RANTES produced minimal increases in the levels of HIV-1YU-2 replication in monocytes and microglia, whereas eotaxin did not alter RT levels in either cell type (Fig. 6B and E). Antibodies to CCR3 (7B11) increased RT levels in both monocytes and microglia exposed to HIV-1YU-2, whereas 3A9 inhibited infection in monocytes but not microglia. In microglia exposed to HIV-1YU-2, antibodies to CCR5 delayed the onset of viral replication only. Peak RT values were similar in 3A9- and control HIV-1YU-2-inoculated microglial cultures (Fig. 6C and F).
For HIV-1JR-FL, MIP-1α and -β produced a partial inhibition of viral replication in monocytes, whereas in microglia MIP-1α produced a partial inhibition and MIP-1β merely delayed the onset of RT production (Fig. 7A and D). Interestingly, RANTES increased RT levels in monocyte and microglial cultures infected with HIV-1JR-FL, whereas eotaxin, the CCR3 ligand, produced a partial inhibition (Fig. 7B and E). Consistently, antibodies to CCR3 minimally inhibited virus production in both monocytes and microglia. In contrast to the other viral strains, antibodies to CCR5 (3A9) markedly reduced RT production in both monocytes and microglia exposed to HIV-1JR-FL (Fig. 7C and F).
DISCUSSION
Mononuclear phagocytes are a major reservoir for HIV-1 in its infected human host (19). Of all tissue macrophages, microglia remain one of the most common nonlymphoid reservoirs for HIV-1 (13). Long-term effective antiviral therapies, especially those that target viral entry, will need to take into account the importance of such cells as long-term reservoirs for persistent virus production. Importantly, macrophage-tropic strains easily infect microglia. Indeed, we have shown, in the companion paper, that a panel of divergent HIV-1 strains infect monocytes and microglia at equal efficiencies (20). Whether the two types of cells utilize identical coreceptors for HIV-1 remained in question. To research this question, we examined, side by side, the role of β-chemokine receptors for HIV-1 infection of monocytes and microglia. A panel of neurotropic and macrophage-tropic viral isolates was used to limit questions of HIV-1 heterogeneity and specific target cell tropism. Surprisingly, our results demonstrated that the chemokine receptors used for HIV-1 entry into monocytes and microglia are distinct. Whereas CCR5 was utilized as the predominant coreceptor by HIV-1 for monocytes, this was not demonstrated for microglial infection. Of the panel of isolates used for viral inoculation, antibodies to CCR5 restricted only HIV-1JR-FL infection of microglia.
In a previous report by He and colleagues (23) the authors suggested that both CCR5 and CCR3 facilitated HIV-1 infection of microglia and that CCR5 was critical for viral infection in monocytes. The study was distinct from what was performed in this report in several respects. First, the investigators used mixed cultures of brain cells where microglial cells were only a minor fraction (<5%) of the total evaluated. Second, the levels of viral production in the control unmanipulated cells were at or near the levels of detection by RT assays. Third, heterologous reporter gene systems used in the analyses permitted the evaluation of only single cycles of viral replication. Fourth, we demonstrated in this study that astrocytes express CCR3; such cells can support limited viral replication and were the predominant cell type in the mixed glial cultures (40) used in the He study (23). These cells were absent in our system because of the high level of microglial purity. This provides several likely explanations for the discrepant results. Clearly, in pure cultures of microglia, as used in this study, CCR3 is not an obligatory coreceptor for infection by macrophage-tropic HIV-1 strains. Indeed, the blocking CCR3 antibodies utilized in our culture systems were ineffective at affecting viral entry into both monocytes and microglia.
Interestingly, all chemokine peptides tested failed to inhibit viral infection. This was an unexpected finding given that these molecules were identified as suppressive factors for virus (6). They appear to act through the HIV-1 V3 domain rather than the regular signal transduction pathways of the viral receptors (7). Nonetheless, there is controversy regarding the competitive inhibition of HIV-1 infection in monocytes by the chemokine receptor ligands and peptides. Schmidtmayerova et al. (37) found that MIP-1α, MIP-1β, and RANTES led to higher levels of replication in monocytes infected with viral isolates 92US657 and 92US660 than in untreated controls. Dragic et al. (15) also reported a lack of inhibition of infection by chemokines in heterologous systems on the basis of single-cycle infection analysis. However, Verani et al. (42) reported that RANTES (the most potent of the chemokine peptides) produced an inhibition of infection of monocytes by HIV-1BAL. Thus, there is a lack of consistency regarding the inhibition of HIV-1 infection by chemokine peptides. On balance, by using a highly purified set of cell isolation systems, two independent cell types for viral inoculation (monocytes and microglia), and three different HIV-1 isolates, it was found that the β-chemokine peptides consistently failed to affect viral infection in mononuclear phagocytes. Moreover, the specific HIV-1 isolate may have played a role in such interactions, as in this report, MIP-1α produced an enhancement of the synthesis of viral cDNA when monocytes were infected with HIV-1ADA but not with HIV-1YU-2.
There is overwhelming evidence that suggests that the chemokine receptors play an important role in HIV-1 disease progression (8–10, 25, 28, 32, 34, 36). However, the exact mechanism by which they do so remains uncertain. It was reported that a RANTES derivative produced potent inhibition of HIV-1 infectivity in monocytes (39). Given the resistance of individuals homozygous for CCR5 deletion to disease progression (28, 36), the refractory nature of cells isolated from such individuals to infection in vitro (34), the synthesis of chemokines as suppressor factors for HIV-1 infection (6, 7), the changes in coreceptor usage with disease progression (9), and the discrepant data regarding the extent of competitive inhibition of cellular infection by chemokine receptor antibodies and receptor ligands (15, 31, 37, 42), the complexity of the issue is further demonstrated. It may be that the HIV-1 infection of microglia proceeds through other receptors. Recently, it has been reported that there are at least six members of the chemokine receptor family that function in HIV-1 entry: CCR5, CXCR4, CCR2b, CCR3, Bonzo, and BOB (12, 38). The role of these other receptors in microglia infection remains to be elucidated. Differences in fetal (as described in this report) and adult microglia may also be important for HIV-1 coreceptor usage.
It is generally accepted that the clinical manifestations of HIV dementia are accompanied pathologically by an infiltration and recruitment of monocytes through the blood-brain barrier (21). These cells, together with the resident microglia, are the cellular targets for HIV-1 infection of the brain (19). The lack of a cause-and-effect relationship between the levels of virus and the progression of disease in the CNS has further supported the notion that it is the inflammatory neurotoxins produced from immunologically activated mononuclear phagocytes that produce clinical neurological disease. Thus, the greater the numbers of these neurotoxin-producing cells the faster the disease progression (21). Chemokines likely play an important role in the recruitment and infiltration of circulating blood monocytes into the CNS. Given the possibility that virus is transmitted in tissues through monocytes moving throughout the body yet undetected by the immune system, the Trojan horse hypothesis (22), a reduced recruitment of monocytes in the brain could facilitate the reduction of resident microglial infection.
Individuals homozygous for the CCR5 Δ32 allele may be resistant to neurological disease progression due to reduced infection of brain macrophages, to alternative (less efficient) chemokine receptors for viral entry being utilized, or perhaps to alterations in monocyte recruitment into the brain (41). These events clearly are not mutually exclusive. The concept of alternative chemokine receptors for microglial infection may be significant, as HIV-1-neurovirulent strains may utilize receptors other than CCR3 and CCR5 as viral coreceptors for microglial infection. Indeed, our data suggest that both monocytes and microglia are capable of supporting HIV-1 infection with strains that do not utilize CCR3. We, therefore, hypothesize that other chemokine receptors may be responsible for the evolution of microglia-tropic strains of HIV-1 leading to progressive neurological impairments seen during advanced clinical disease.
ACKNOWLEDGMENTS
We thank Chun Chao and Shuxian Hu of the Hennepin County Medical Center in Minneapolis, Minn., for advice and guidance in establishing the microglia isolation and culture at the University of Nebraska Medical Center and Karen Spiegel for excellent editorial and graphic support.
This work was supported in part by NIH grants P01 NS31492-05, R01 NS34239-04, 1 R01 NS36126-01, and 1 P01 MH57556-01, the Charles A. Dana Foundation, and the University Biotechnology start-up funds. Adeline Nukuna is a Nicholas B. Badami Fellow.
REFERENCES
- 1.Alkhatib F, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Chao C C, Gekker G, Hu S, Peterson P K. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- 3.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. [PubMed] [Google Scholar]
- 6.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 8.Connor R I, Paxton W A, Sheridan K E, Koup R A. Macrophages and CD4+ T lymphocytes from two multiply exposed, uninfected individuals resist infection with primary non-syncytium-inducing isolates of human immunodeficiency virus type 1. J Virol. 1996;70:8758–8764. doi: 10.1128/jvi.70.12.8758-8764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor R I, Sheriden K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. . (Comments.) [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Sutton R E, Hill C, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Deng H, Unutmaz D, Kewalramani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 13.Dickson D W, Mattiace L A, Kure K, Huchins K, Lyman W D, Bronsan C F. Biology of disease. Microglia in human disease with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991;64:135–156. [PubMed] [Google Scholar]
- 14.Doranz B J, Rucker J, Yi Y, Smith R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that used fusin and the β-chemokine receptors CCR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 15.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Broder C, Kennedy P, Berger E. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 17.Folks T M, Powell D, Lightfoote M, Koenig S, Fauci A S, Benn S, Rabson A, Daugherty D, Gendelman H E, Hoggan M D, Venkaesan S, Martin M A. Biological and biochemical characterization of a clone Leu-3 cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gendelman H E, Orenstein J M, Martin M A, Ferrua C, Mitra R, Phipps T, Wahl L A, Lane H C, Fauci A J, Burke D S, Skillman D R, Meltzer M S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1498–1506. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gendelman H E, Lipton S, Tardieu M, Genis P, Jett M, Zhai J, Nottet H. The neuropathogenesis of HIV-1 infection. J Leukocyte Biol. 1994;56:389–399. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- 20.Ghorpade A, Nukuna A, Che M, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L, Gendelman H E. Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in microglia and monocytes. J Virol. 1998;72:3340–3350. doi: 10.1128/jvi.72.4.3340-3350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glass J D, Fedor H, Wesselingh S L, McArthur J C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 22.Haase A T. Pathogenesis of lentivirus infections. Nature. 1986;322:130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- 23.He J, Che Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabudzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 24.Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath P D, Mackay C R. Chemokine receptor usage by human eosinophils. J Clin Invest. 1997;99:1–7. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstaman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 26.Kalter D C, Nakamura M, Turpin J A, Baca L M, Dieffenbach C, Ralph P, Gendelman H E, Meltzer M S. Enhanced HIV replication in MCSF-treated monocytes. J Immunol. 1991;146:298–307. [PubMed] [Google Scholar]
- 27.Li Y, Kappes J C, Conway J A, Price R W, Shaw G M, Hanh B H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 29.Nottet H S L M, Persidsky Y, Sasseville V G, Nukuna A N, Bock P, Zhai Q H, Sharer L R, McComb R D, Swindells S, Soderland C, Gendelman H E. Mechanism for the transendothelial migration of HIV-1 infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- 30.O’Brien W A, Koyanagi Y, Namazi A, Zhao J-Q, Diagne A, Idler K, Zack J A, Chen I S-Y. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD-4 binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 31.Oravecz T, Pall M, Norcross M A. β-Chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 32.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDevanter N L, Pandian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 33.Pear W S, Nolan G P, Scott M S, Baltimore D. Production of high-titre helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rana S, Besson G, Cook D G, Rucker J, Smyth R J, Yi Y, Turner J D, Guo H-H, Du J-G, Peiper S C, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms R W, Parmentier M, Collman R G. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the Δccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 11.3–11.55. [Google Scholar]
- 36.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection of Caucasian individuals bearing mutant alleles of the CCR5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 37.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 38.Sica A, Saccani A, Borsatti A, Power C A, Wells T N, Luini W, Polentarutti N, Sozzani S, Mantovani A. Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocytes. J Exp Med. 1997;185:969–974. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 40.Tornatore C, Chandra R, Berger J R, Major E O. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- 41.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 42.Verani A, Scarlatti G, Comar M, Tresoldi E, Polo S, Giacca M, Lusso P, Siccardi A G, Vercelli D. C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophage and T cells. J Exp Med. 1997;185:805–816. doi: 10.1084/jem.185.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]