Abstract
Cancer testis antigen (CTA) Melanoma Antigen Gene A3 (MAGEA3) were overexpressed in multiple tumor types, but the expression pattern of MAGEA3 in the serum of lung adenocarcinoma (LUAD) remains unclear. Clinically derived serum and serum exosome samples were used to assess the mRNA expression of MAGEA3 and MAGEA4 by qRT-PCR, and serum MAGEA3 and MAGEA4 protein expression were evaluated by ELISA in total 133 healthy volunteers’ and 289 LUAD patients’ serum samples. An analysis of the relationship of the mRNA and protein expression of MAGEA3 and MAGEA4 with clinicopathologic parameters was performed and the diagnostic value of MAGEA3 and MAGEA4 was plotted on an ROC curve. In addition, the correlation of MAGEA3 mRNA with infiltrating immune cells was investigated through TIMER, the CIBERSORT algorithm and the TISIDB database. Expression of serum and serum exosome MAGEA3 and MAGEA4 mRNA were significantly higher in LUAD patients than in healthy donors. MAGEA3 mRNA associated with tumor diameter, TMN stage, and NSE in LUAD serum samples, and MAGEA3 mRNA correlated with N stage in serum-derived exosomes, possessing areas under the curve (AUC) of 0.721 and 0.832, respectively. Besides, serum MAGEA3 protein levels were elevated in LUAD patients, and were closely related to stage and NSE levels, possessing AUC of 0.781. Further analysis signified that the expression of MAGEA3 mRNA was positive correlation with neutrophil, macrophages M2, dendritic cells resting, and eosinophilic, but negatively correlated with B cells, plasma cells, CD8 + T cells, CD4 + T cells, Th17 cells, macrophages and dendritic cells. Collectively, our results suggested that the MAGEA3 expression in mRNA and protein were upregulated in LUAD, and MAGEA3 could be used as a diagnostic biomarker and immunotherapy target for LUAD patients.
Keywords: MAGEA3, Lung adenocarcinoma, Cancer-testis antigen, Exosome, Diagnosis, Tumor immune infiltration
Subject terms: Cancer, Immunology, Biomarkers
Introduction
Lung cancer is one of the most frequently diagnosed tumors worldwide with approximately 2.2 million new cases and 1.79 million deaths annually1. Among them, lung adenocarcinoma (LUAD) is the leading subtype of lung cancer, accounting for 40% to 50% of lung cancer patients2. The majority of lung cancer patients were diagnosed at intermediate to advanced stages, losing time for optimal treatment3. Conventional screening method X-ray and low-dose chest-computed tomography scans have false positives, which may prompt patients to be over-treated4. Further, traditional tumor biomarkers like Neuron-Specific Enolase (NSE), Cytokeratin 19 Fragment (CYFRA21-1) and Carcinoembryonic Antigen (CEA) have no desired specificity and sensitivity4,5. Accordingly, a non-invasive biomarker for early diagnosis of LUAD is imperative.
Cancer testis antigens (CTAs) is a class of proteins that are restricted expression in germ cells of the testis and placenta but not expressed or under-expressed in other normal somatic cells6. Emerging evidence has shown that CTAs has abnormal expression when various oncogenesis6,7. The first identified members of the CTAs, Melanoma Antigen Gene (MAGE) family, is classified into MAGE-I and II according to their location and specific expression. MAGE-I located on the X chromosome includes MAGE-A, B and C subfamilies, while MAGE-II ubiquitous exist in healthy person includes others MAGE subfamily8. The MAGE-A family express abnormally in various tumors, including melanoma, lung cancer, breast cancer and pancreatic cancer9–11. Currently, growing studies concentrated on the MAGE-A family as a tumor biomarker, carcinogenesis and tumor immunotherapy target in multiple neoplasms12,13. MAGEA3 immunogenicity and carcinogenicity ranked eighth out of 75 tumor antigens in a National Cancer Institute study and ranked first in CTAs14. MAGEA4 is a prognostic biomarker in salivary gland carcinomas related to tumor grading15.The expression level of MAGEA 1–4, 6 and 12 increased when malignant transformation occurs in oral leucoplakia16. Besides, MAGE-A family can predict the effect of cytotoxic T lymphocyte-associated protein-4(CTLA-4) blockers in metastatic melanoma17. Nonetheless, the comprehensive analysis of the MAGE-A family in serum mRNA and protein level of LUAD remains unknown.
Exosomes are a subset of extracellular vesicles with a diameter range of 40-150 nm and involve in cell-to-cell communication by transporting their contents (nucleic acids, lipids, proteins) to target cells18. Exosomes can protect their "cargo" from RNase degradation in multiple body fluids due to their unique bilayer membranes, their contents have higher stability and a powerful potential as a diagnostic biomarker than serum19,20. MenXD et al. observed elevated levels of PLA2G10 mRNA and protein in serum exosomes, which can be a diagnostic marker to distinguish healthy from non-small cell lung cancer21. Thus, exploring particular mRNA in serum exosomes as a biomarker for early diagnosis of LUAD is worthwhile.
In the present study, we firstly screened potential biomarkers in serum MAGEA1-6, and then the database-derived tissues and clinic-derived serum expression levels of MAGEA3 and MAGEA4 were investigated in LUAD. Finally, the correlation between MAGEA3 expression and immune cells infiltrating levels were explored. Our results uncovered the significant function of MAGEA3 in LUAD, as well as proposed potential connection between MAGEA3 and immune infiltration of LUAD patients.
Materials and methods
Clinical samples
Serums were collected from 289 patients with lung adenocarcinoma and 133 healthy volunteers from the Clinical Laboratory, Fujian Provincial Hospital (Fuzhou, China) between January 2020 and March 2023. Lung adenocarcinoma was diagnosed via pathological analysis. All the donors had signed the informed consent, and none was treated with radiotherapy or chemotherapy prior to collection. Clinicopathological characteristics were recorded, including age, gender, tumor diameter, TNM classification, stage etc. The present study was conducted with the approval of the ethics committee of Fujian Provincial Hospital and complied with the ethical standards of the Helsinki Declaration.
RNA isolation and quantitative real-time polymerase chain reaction (qRT‑PCR)
We used TRIzol™ LS Reagent (Invitrogen, Carlsbad, CA, USA) to extract the total RNA from 254 serum samples based on the manufacturer’s instructions. Then, RNA was dissolved in enzyme-free water and the concentration was quantified by NanoDrop One/Onec Spectrophotometer (Thermo Scientific). The PrimeScript™ RT reagent kit (RR037A; TAKARA, Dalian, China) was used for reversing transcription under 37 °C for 15 min, followed by 85 °C for 5 s. Finally, cDNA was amplified by TB Green® Premix Ex Taq™ II Kit (RR820A; TAKARA, Dalian, China), following the manufacturer’s instruction in 40 cycles of denaturation at 95 °C for 10 min, 95 °C for 15 s, with extension at 60 °C for 1 min using LightCycler 480 System. The 2−ΔΔCT method indicated the relative expression level, and GAPDH served as a reference gene. QRT-PCR’s primer sequences are shown in Table S1.
Enzyme linked immunosorbent assay (ELISA)
According to the manufacturer’s protocol, the serum protein of MAGEA3 and MAGEA4 was detected by the Human ELISA Kit (MLBio, Shanghai, China). Diluting standards at the concentrations indicated in the manufacturer’s instructions for determination of samples’ concentration. Serum samples were diluted at 1:5, which added 10μL of serum to 40μL of diluent and incubated at 37 °C for 30 min. After washing the plate with phosphate-buffered saline with Tween 20 (PBST), 50μL of HRP conjugated reagent was added and incubated at 37 °C for 30 min. 50μL of chromogen solution A and B were then added to each well in the dark and incubated for 10 min at 37 °C. Finally, 50μL of the stopping solution was added, and OD values were measured within 15 min at 450 nm wavelength using an enzyme-labelled instrument (Bio-Rad).
The Cancer Genome Atlas (TCGA) database
The gene expression profiles of LUAD patients were obtained from the TCGA database (http://portal.gdc.cancer.gov/). We analyzed the expression of MAGEA3 and MAGEA4 mRNA between 541 LUAD samples and 59 adjacent para-cancerous lung tissues. Besides, the expression levels of MAGEA3 and MAGEA4 were further compared between 59 LUAD tissues and matched normal tissues using TCGA database.
The Kaplan–Meier plotter database
The Kaplan–Meier plotter database contained survival information for 865 patients with LUAD (http://kmplot.com)22. LUAD patients were divided into high expression group and low expression group according to the median expression level of MAGEA3 or MAGEA4. The prognostic value of MAGEA3 and MAGEA4 (progression free survival) was assessed by Kaplan–Meier plotter.
PrognoScan database
The correlation between MAGEA3 and MAGEA4 expression and overall survival (OS) was explored by PrognoScan database, which is a freely available resource was collected from Gene Expression Omnibus (GEO), ArrayExpress, and individual laboratory websites (http://www.prognoscan.org/). Cox P value < 0.05 was considered statistically significant.
TIMER database
TIMER is a user-friendly, comprehensive database containing over 10,000 tumor samples from TCGA for 32 cancer types (https://cistrome.shinyapps.io/timer/)23. In the present study, we used TIMER to estimate the relationship between MAGEA3 expression and immune infiltrates of B cells, CD4 + T cells, CD8 + T cells, neutrophils, macrophages, and dendritic cells in LUAD.
CIBERSORT algorithm
CIBERSORT (https://www.biostars.org/p/428905/) is an analytical tool, which aids in estimating the proportions of 22 types of infiltrating immune cells through gene expression data24. We explored the relationship between MAGEA3 and tumor-infiltrating immune cells using CIBERSORT algorithm.
TISIDB database
TISIDB (http://cis.hku.hk/TISIDB/) is an online portal for tumor and immune system interaction25. TISIDB was used to examine MAGEA3 and tumor-infiltrating cells expression in LUAD.
Statistical analysis
R software package was used to implement TCGA database analysis. The statistical analyses were performed with the SPSS 25.0 software package (SPSS Inc. Chicago, USA) and GraphPad Prism 9.0 (GraphPad Software, USA). Survival curves were generated using Kaplan–Meier plots. QRT-PCR and ELISA were analyzed using the unpaired Student’s t-test. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to analyze the diagnostic efficiency. Spearman test was used to measure the correlation between MAGEA3 and Tumor infiltrating lymphocytes (TILs). When P < 0.05, the data is statistically significant.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Fujian Provincial Hospital.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Results
MAGEA1-6 expression in LUAD
We first conducted a preliminary experiment using qRT-PCR to explore potential targets of MAGEA1-6 in lung adenocarcinoma by 60 samples. Due to MAGEA5 from house mouse, it was except (https://www.ncbi.nlm.nih.gov/gene/17141). The results showed that MAGEA3 and MAGEA4 had a statistical significance (P < 0.05), but MAGEA1, MAGEA2 and MAGEA6 had no statistical significance (P > 0.05) (Table S2). Based on this, we chose MAGEA3 and MAGEA4 for further exploration.
Aberrant expression and prognostic value of MAGEA3 and MAGEA4 in LUAD
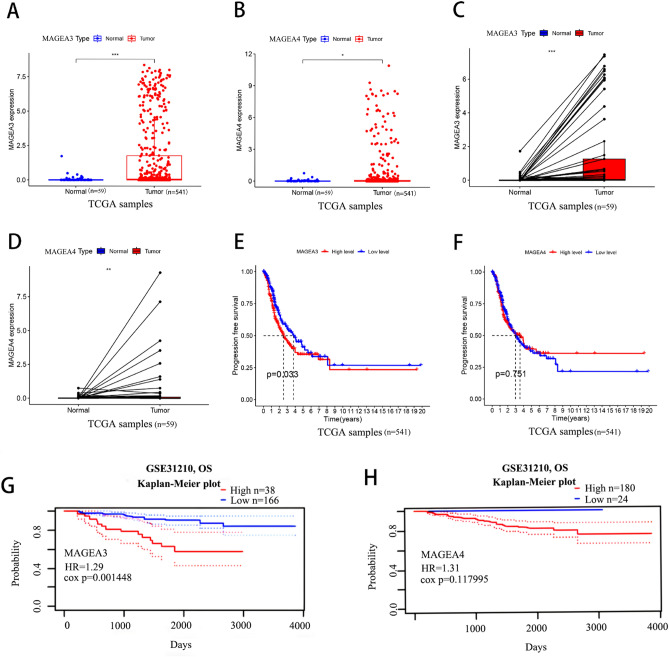
To further corroborate the preliminary experimental results, we analyzed the gene expression profiles of 541 LUAD patients’ samples and 59 normal samples from the TCGA database. The analysis showed that MAGEA3 had a remarkable enhanced in LUAD samples than the adjacent normal lung tissues (Fig. 1A). The expression level of MAGEA4 was elevated in LUAD samples, too (Fig. 1B). The results were in line with the 59 matched tissue samples from the LUAD patients (Fig. 1C,D). After that, we analyzed the relationship between the expression level of MAGEA3 and MAGEA4 and prognosis in LUAD by Kaplan–Meier plotter and PrognoScan database. As show in Fig. 1E–H, high MAGEA3 expression was associated with a poor progression free survival (PFS) and overall survival (OS), while MAGEA4 had no correlation with prognosis(P > 0.05).
Figure 1.
Expression levels and prognosis of MAGEA3 and MAGEA4 in LUAD patients. The expression levels of MAGEA3 and MAGEA4 in LUAD and para-cancerous lung tissues by TCGA database (A,B). MAGEA3 and MAGEA4 expression in LUAD and matched para-carcinoma tissue by TCGA database (C,D). The progression free survival (PFS) rate of MAGEA3 and MAGEA4 expression in TCGA LUAD samples (E,F). The overall survival (OS) of MAGEA3 and MAGEA4 expression in GSE31210 cohorts of PrognoScan database (G,H).
Serum and serum exosome expression of MAGEA3 and MAGEA4 in LUAD
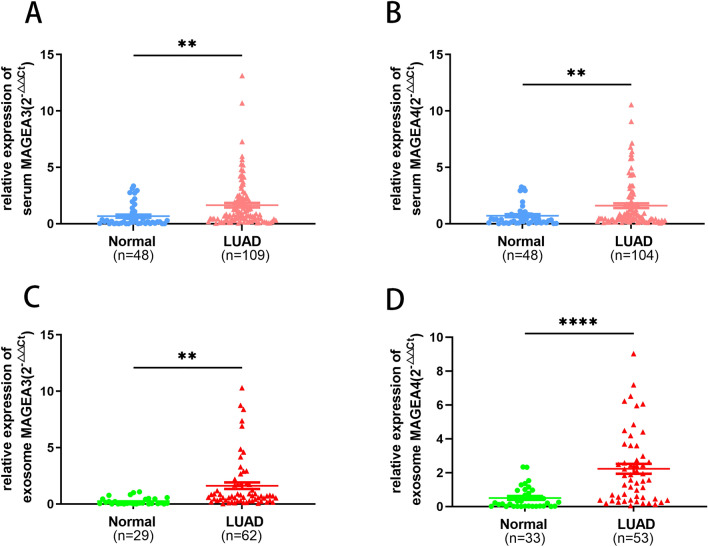
We expanded the sample size for exploration to verify the preliminary experimental results. The qRT-PCR results indicated that MAGEA3 was higher in 109 LUAD patients than in 48 healthy volunteers (P = 0.0024), and MAGEA4 increased in serum from 104 LUAD patients compared to 48 healthy volunteers (P = 0.0052) (Fig. 2A,B). The clinicopathological feature shed light into a solid relationship between MAGEA3 and TNM classification, stage and tumor diameter (P < 0.05) (Table 1).
Figure 2.
The expression of MAGEA3 and MAGEA4 in serum and serum-derived exosome with patients of LUAD patients using qRT-PCR. Serum mRNA expression of MAGEA3 (A) and MAGEA4 (B), and serum-derived exosome expression of MAGEA3 (C) and MAGEA4 (D). (**P < 0.01, ****P < 0.0001).
Table 1.
Correlation of serum MAGEA3 and MAGEA4 mRNA expression and clinicopathological parameters in LUAD patients.
| Clinicopathological factor | n | MAGEA3 | n | MAGEA4 | ||
|---|---|---|---|---|---|---|
| Mean ± SEM | P | Mean ± SEM | P | |||
| Age (years) | 0.2448 | 0.5942 | ||||
| < 60 | 65 | 1.450 ± 0.1868 | 49 | 1.701 ± 0.2941 | ||
| ≥ 60 | 44 | 1.924 ± 0.4083 | 45 | 1.478 ± 0.2822 | ||
| Gender | 0.9123 | 0.7437 | ||||
| Male | 41 | 1.670 ± 0.3602 | 41 | 1.689 ± 0.3287 | ||
| Female | 68 | 1.624 ± 0.2359 | 63 | 1.550 ± 0.2664 | ||
| Diameter (cm) | 0.0337 | 0.2076 | ||||
| < 2 | 83 | 1.353 ± 0.1818 | 76 | 1.621 ± 0.2418 | ||
| ≥ 2 | 13 | 2.344 ± 0.6649 | 13 | 2.466 ± 0.7765 | ||
| Stage | 0.0421 | 0.058 | ||||
| < II | 91 | 1.462 ± 0.1827 | 84 | 1.795 ± 0.2477 | ||
| ≥ II | 18 | 2.548 ± 0.9686 | 20 | 0.806 ± 0.1714 | ||
| T | 0.0004 | 0.0694 | ||||
| Tis + 1 | 90 | 1.391 ± 0.1500 | 79 | 1.815 ± 0.2544 | ||
| ≥ 2 | 14 | 3.505 ± 1.1140 | 25 | 0.941 ± 0.2627 | ||
| N | 0.0009 | 0.1353 | ||||
| 0 | 93 | 1.443 ± 0.1595 | 86 | 1.783 ± 0.2428 | ||
| ≥ 1 | 11 | 3.637 ± 1.3330 | 14 | 0.865 ± 0.1959 | ||
| M | 0.0124 | 0.1100 | ||||
| 0 | 97 | 1.467 ± 0.1585 | 91 | 1.730 ± 0.2307 | ||
| ≥ 1 | 12 | 3.046 ± 1.2490 | 13 | 0.733 ± 0.2216 | ||
| CEA | 0.3195 | 0.1556 | ||||
| < 5 ng/ml | 87 | 1.590 ± 0.1997 | 83 | 1.737 ± 0.2459 | ||
| ≥ 5 ng/ml | 16 | 2.169 ± 0.8130 | 17 | 0.938 ± 0.2694 | ||
| NSE | 0.0628 | 0.4731 | ||||
| < 16.3 ng/ml | 63 | 1.471 ± 0.2057 | 59 | 1.481 ± 0.2960 | ||
| ≥ 16.3 ng/ml | 8 | 2.924 ± 1.4890 | 11 | 0.974 ± 0.3303 | ||
Significant values are in bold.
MAGEA3 melanoma-associated antigen family A3, MAGEA4 melanoma-associated antigen family A4, LUAD lung adenocarcinoma, Tis tumor in situ, TNM tumor node metastasis, CEA carcinoembryonic antigen, NSE neuron-specific enolase.
Exosomes as a more stable cargo, we further extracted serum exosome successfully and investigated the expression levels of MAGEA3 and MAGEA4 in serum-derived exosomes by qRT-PCR. As shown in Fig. 2C and D, the expression of MAGEA3 (P = 0.0021) and MAGEA4 (P < 0.0001) in serum exosomes were high expression and MAGEA3 level was positively correlated with lymph node metastasis of LUAD patients (Table 2). Of note, the levels of MAGEA3 and MAGEA4 in serum exosomes were statistically higher than those in serum samples. Nevertheless, there were nonsignificant correlations between MAGEA4 of serum and serum-derived exosome and clinicopathological factor (Tables 1, 2). Thus, MAGEA3 and MAGEA4 were all abundant in LUAD patients’ serum and serum-derived exosome, and MAGEA3 was more clinically significant.
Table 2.
Correlation of serum exosomal MAGEA3 and MAGEA4 expression and clinicopathological parameters.
| Clinicopathological factor | n | MAGEA3 serum exosome | n | MAGEA4 serum exosome | ||
|---|---|---|---|---|---|---|
| Mean ± SEM | P | Mean ± SEM | P | |||
| Age(year) | 0.1414 | 0.8863 | ||||
| < 60 | 35 | 1.153 ± 0.1895 | 30 | 2.138 ± 0.3521 | ||
| ≥ 60 | 27 | 1.960 ± 0.5666 | 23 | 2.221 ± 0.4727 | ||
| Gender | 0.1476 | 0.3117 | ||||
| Male | 23 | 2.017 ± 0.5765 | 20 | 1.802 ± 0.4019 | ||
| Female | 39 | 1.202 ± 0.2600 | 33 | 2.399 ± 0.3833 | ||
| Diameter | 0.7723 | 0.6400 | ||||
| < 2 | 39 | 1.567 ± 0.3479 | 34 | 2.200 ± 0.3720 | ||
| ≥ 2 | 15 | 1.768 ± 0.6551 | 13 | 2.529 ± 0.5716 | ||
| Stage | 0.1492 | 0.8776 | ||||
| < II | 42 | 1.233 ± 0.2610 | 39 | 2.200 ± 0.3499 | ||
| ≥ II | 20 | 2.073 ± 0.6307 | 14 | 2.100 ± 0.4659 | ||
| T | 0.6924 | 0.2933 | ||||
| Tis + 1 | 45 | 1.472 ± 0.2919 | 40 | 2.374 ± 0.3567 | ||
| ≥ 2 | 15 | 1.730 ± 0.7122 | 12 | 1.652 ± 0.3335 | ||
| N | 0.018 | 0.8856 | ||||
| 0 | 48 | 1.210 ± 0.2401 | 42 | 2.228 ± 0.3506 | ||
| ≥ 1 | 12 | 2.842 ± 0.9551 | 10 | 2.121 ± 0.2623 | ||
| M | 0.9807 | 0.6519 | ||||
| 0 | 49 | 1.508 ± 0.3147 | 43 | 2.112 ± 0.3194 | ||
| ≥ 1 | 13 | 1.491 ± 0.5380 | 10 | 2.442 ± 0.6310 | ||
| CEA | 0.1858 | 0.6929 | ||||
| < 5 ng/ml | 44 | 1.135 ± 0.2388 | 23 | 2.214 ± 0.4702 | ||
| ≥ 5 ng/ml | 13 | 1.868 ± 0.6309 | 25 | 1.972 ± 0.3949 | ||
| NSE | 0.8648 | 0.9833 | ||||
| < 16.3 ng/ml | 36 | 1.388 ± 0.3599 | 34 | 2.116 ± 0.4057 | ||
| ≥ 16.3 ng/ml | 7 | 1.245 ± 0.3410 | 5 | 2.093 ± 0.4284 | ||
Significant values are in bold.
Diagnostic value of MAGEA3 and MAGEA4 of LUAD in serum and serum exosome
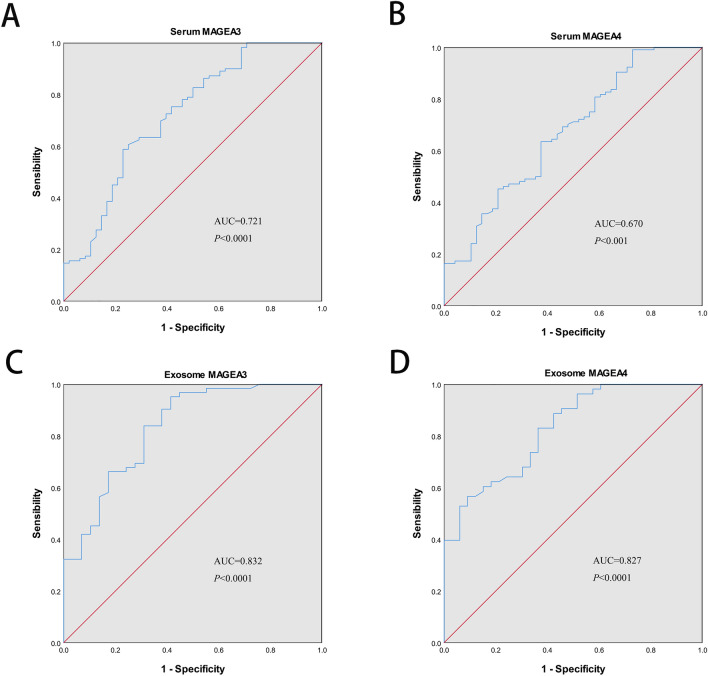
Next, we assessed the diagnostic significance of MAGEA3 and MAGEA4 in serum and serum exosomes. For MAGEA3 mRNA expression, ROC curves analysis revealed that AUC of serum MAGEA3 was 0.721 with a sensitivity of 58.70%, specificity of 77.10%, and the AUC of serum exosome of MAGEA3 was 0.832 with a sensitivity of 95.20%, specificity of 58.60% (Fig. 3A,C and Table 3). Meanwhile, the ROC curves analysis yielded an AUC of 0.670 with sensitivity of 99.00% against specificity of 27.10% for the serum MAGEA4, and an AUC of 0.827 with sensitivity of 56.60% against specificity of 90.00% for the serum exosome MAGEA4 (Fig. 3B,D and Table 3). The MAGEA3 had superior AUCs than MAGEA4 regardless of serum or serum-derived exosome.
Figure 3.
Diagnostic value of MAGEA3 and MAGEA4 in serum (A,B) and serum-derived exosome (C,D) of patients with LUAD by plotting receiver operating characteristic (ROC) curves.
Table 3.
Diagnostic efficacy of MAGEA3 and MAGEA4 mRNA in serum and serum exosome and diagnostic efficacy of MAGEA3 protein in serum.
| Variables | AUC (95% CI) | Cut off | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| mRNA expression | Serum MAGEA3 | 0.721 (0.631–0.812) | 0.358 | 58.70 | 77.10 |
| Serum MAGEA4 | 0.670 (0.577–0.764) | 0.261 | 99.00 | 27.10 | |
| Exosome MAGEA3 | 0.832 (0.741–0.923) | 0.538 | 95.20 | 58.60 | |
| Exosome MAGEA4 | 0.827 (0.741–0.913) | 0.475 | 56.60 | 90.00 | |
| Protein expression | Serum MAGEA3 | 0.781 (0.686–0.876) | 0.533 | 82.10 | 72.10 |
Serum protein expression of MAGEA3 and MAGEA4 in LUAD
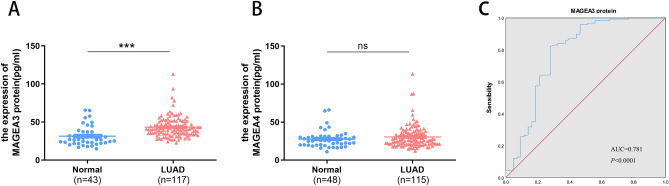
We then evaluated the expression level of serous MAGEA3 and MAGEA4 proteins in LUAD by ELISA. The results revealed that serous MAGEA3 protein was remarkably elevated in 117 LUAD patients (P < 0.0001). The sensitivity, specificity, and the AUC of MAGEA3 protein were estimated to be of 82.10%, 72.10%, and 0.781 (p < 0.0001), respectively (Fig. 4A,C and Table 3). Furthermore, MAGEA3 had significant differences in the clinical stage and traditional tumor biomarker NSE (Table 4). Unfortunately, there was no difference in MAGEA4 protein expression between 115 LUAD patients and 48 healthy controls (P > 0.05) (Fig. 4B).
Figure 4.
The expression of MAGEA3 and MAGEA4 protein in serum with patients of LUAD by ELISA. Protein expression of MAGEA3 (A) and MAGEA4 (B) in serum. Diagnostic performance of MAGEA3 protein (C) in serum by ROC curves. (ns P ≥ 0.05, ***P < 0.001).
Table 4.
Correlation of MAGEA3 protein expression and clinicopathological parameters.
| Clinicopathological factor | n | MAGEA3 protein | |
|---|---|---|---|
| Mean ± SEM | P | ||
| Age (year) | 0.816 | ||
| < 60 | 72 | 43.05 ± 1.569 | |
| ≥ 60 | 45 | 43.66 ± 2.137 | |
| Gender | 0.101 | ||
| Male | 47 | 45.82 ± 2.611 | |
| Female | 70 | 41.58 ± 1.151 | |
| Diameter | 0.6 | ||
| < 2 | 75 | 43.74 ± 1.635 | |
| ≥ 2 | 19 | 41.88 ± 2.771 | |
| Stage | 0.031 | ||
| < II | 83 | 41.55 ± 1.187 | |
| ≥ II | 34 | 47.52 ± 3.159 | |
| T | 0.11 | ||
| Tis + 1 | 82 | 42.41 ± 1.468 | |
| ≥ 2 | 28 | 47.28 ± 2.879 | |
| N | 0.283 | ||
| 0 | 81 | 42.38 ± 1.503 | |
| ≥ 1 | 36 | 45.33 ± 2.322 | |
| M | 0.863 | ||
| 0 | 97 | 43.18 ± 1.384 | |
| ≥ 1 | 20 | 43.77 ± 3.167 | |
| CEA | 0.922 | ||
| < 5 ng/ml | 85 | 43.51 ± 1.503 | |
| ≥ 5 ng/ml | 27 | 43.21 ± 2.685 | |
| NSE | 0.013 | ||
| < 16.3 ng/ml | 75 | 42.94 ± 1.455 | |
| ≥ 16.3 ng/ml | 9 | 55.52 ± 7.764 | |
Significant values are in bold.
Association between MAGEA3 and tumor infiltrating immune cells in LUAD
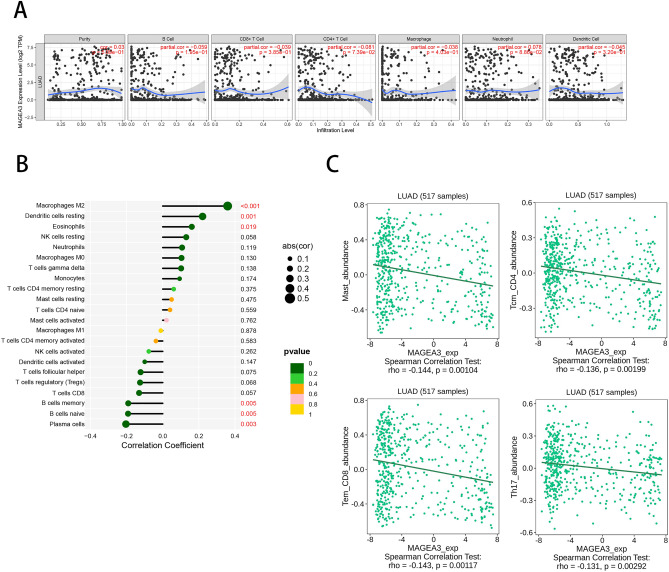
CTA is suitable targets for cancer immunotherapy due to its unique immune-privileged characteristic6. Moreover, the ELISA results suggest that MAGEA3 is conspicuous associated with humoral immunity in LUAD. Therefore, we further explored the relationship between MAGEA3 and tumor immune cell infiltration. The TIMER analysis results showed that MAGEA3 expression had a positive correlation with neutrophil, and a negative correlation with B cells, CD8 + T cells, CD4 + T cells, macrophages and dendritic cells (Fig. 5A). Furthermore, high expression of MAGEA3 is associated with infiltration of macrophages M2, dendritic cells resting, and eosinophilic, as well as decreased expression of B cells memory, B cells naive, and plasma cells by CIBERSORT algorithm (Fig. 5B). This negative correlation between the expression level of MAGEA3 and central CD4 + T cells, CD8 + T cells and Th17 cells using TISIDB database (Fig. 5C).These results indicated that MAGEA3 might have an important effect on tumor immune infiltration in LUAD.
Figure 5.
Correlation between MAGEA3 expression and immune infiltration in LUAD. The association between MAGEA3 and infiltration of purity, B cells, CD8 + T cells, CD4 + T cells, macrophage, neutrophil, dendritic cells was analyzed using TIMER (A). The relationship between MAGEA3 and the infiltrating abundance of different immune cells was investigated using CIBERSORT (B). The association between MAGEA3 and immune cells was analyzed by TISIDB (C).
Discussion
The Melanoma-Associated Antigen Family A (MAGE-A), which is the earliest Cancer-Testis Antigens (CTAs) identified in melanoma as tumor specific antigens, abnormally expressed in a variety of malignant tumors, including lung cancer26. Several studies have investigated the expression of MAGE-A family in NSCLC tissues and lung cancer cell lines using IHC or RT-PCR27–29. Considering that these serum markers have the advantage of being readily obtained, calculated and inexpensive, they may become of considerable value. Blood have been chosen as compartment for the prediction of broadly disseminated disease and systemic tumor load. However, to the best of our knowledge, the mRNA and protein expression levels of MAGE-A family in serum of LUAD patients have not yet been investigated.
It was reported that different members of the MAGE-A gene family are abnormally expressed in lung cancer tissues and those studies that do exist are each individually focused on a small subset of MAGE-A genes30. Our result showed that in the comprehensive analysis of MAGE-A1-6 expression, MAGEA3 and MAGEA4 were significant differentially expressed in serum of LUAD patients. And we further validated their expression and prognosis by analyzing LUAD tissues from databases derived. In the study of the differential expressed profile of the different MAGE genes subclass in NSCLC tumor tissue, 70% of samples expressed MAGE-A1 and 85% expressed MAGEA331. Lina et al. qualitatively analyzed the expression of MAGEA1-6 in the peripheral blood of 150 lung cancer patients using multiplex semi-nested PCR, and the positive rate was MAGEA2(15.3%) > MAGEA6 > MAGEA4 > MAGEA3 > MAGEA127. In fact, the frequency of CTAs expression was variable depend on histologic subtypes, which might account for the difference frequency of expression of MAGE-A family between our study and previous studies32,33.
We analyzed the expression of MAGEA3 and MAGEA4 in the serum and serum exosome of LUAD patients using qRT-PCR. MAGEA3 and MAGEA4 were up-regulated in LUAD. These results paralleled similar findings in previous studies, indicated MAGEA3 and MAGEA4 may serve as a diagnostic and prognostic biomarker for patients with NSCLC28,29. It’s not surprising that the diagnostic efficacy of serum exosome MAGEA3 and MAGEA4 was better than that of serum, and the AUC was higher than 0.8. As one of the media of information communication between cells, exosomes present a lipid bilayer, which gives them stability in the bloodstream or during bulk storage, preserving their content against degradation18. To investigate if MAGE-A mRNA expression is translated into MAGE-A protein, our study was the first time to analyze the serum protein level of MAGEA3 and MAGEA4 using ELISA. The interesting thing is that the expression level of serum MAGEA3 protein was elevated in LUAD patients than healthy people, but MAGEA4 was meaningless. Furthermore, the protein expression of MAGEA3 correlated with stage and tumor biomarker NSE, and the diagnostic efficacy of MAGEA3 serum protein is better than that of serum. In Cai et al. study, 8 autoantibodies targeting tumor-associated antigens (TAAs) were obtained via liquid chip technique, including MAGEA4 but no MAGEA334. We hypothesized that autoantibody production is sufficient to neutralize detection of the MAGEA4 protein in serum. As we known, clinical pathological parameters, especially TNM staging, are closely related to the prognosis of patients. In contrast to MAGEA3, MAGEA4 was not associated with the clinical pathological parameters of LUAD patients. This suggests that there is little correlation between MAGEA4 and clinical progression in patients with LUAD. In addition, several studies in MAGEA3 peptide vaccines have been used in patients with NSCLC35,36. These results suggest that MAGEA3 is the best biomarker of MAGEA1-6 in LUAD.
MAGEA3 are antigens encoded by cancer-germline genes, and have been identified as a potential prognostic biomarker and pro-survival factor in multiple types of cancer37–39. The expression of CT genes is known to be regulated epigenetically by promoter methylation. MAGEA3 expression levels were closely associated with markers of active histone modifications in breast cancer cell40. Brother of the regulator of imprinted Sites (BORIS) was reported that it bound to the promoters of MAGEA3 genes and was associated with their transcriptional activation in lung cancer29. These above reports might explain the phenomena we observed here at high expression levels of MAGEA3 in serum and serum exosomes of LUAD patients and also promised that MAGEA3 is the most important tumor antigen target of MAGEA1-6 in LUAD. As CTAs are known to be immunogenic, we finally explored the relationship between the high level of MAGEA3 and immune cell infiltration in LUAD. In our study, we found that MAGEA3 was negative correlated with CD8 + cells, CD4 + cells, Th17, B cell, plasma cells and dendritic cell, which are related to humoral and cellular immunity. Kim et al. also reported that the high expression of MAGEA3 lead to infiltrate of less dendritic cells, which lead to tumor cells escape the immune surveillance of a host41. Macrophages M2, a cancer-promoting phenotype of macrophages, was positive correlated with MAGEA3, which indicated that with the increase of MAGEA3, it contributes to the formation of tumor microenvironment promoting cancer. In a word, the correlation between MAGEA3 and immune cells further demonstrated that MAGEA3 seemed to play an important role in oncogenesis. The immunotherapy related to MAGEA3 in lung cancer has entered the clinical trial stage, and the immunogenicity and safety are good42,43. Along this line, it is perhaps more plausible that the improved immunotherapy response in anti-MAGEA3 immunization trials were mediated through humoral and cellular immunological mechanisms.
To sum up, the mRNA expression levels of MAGEA3 and MAGEA4 in tissues, serum and serum exosomes of LUAD patients were elevated and correlated with poor prognosis. The expression of MAGEA3 in serum and serum exosome were closely related to clinicopathological parameters. MAGEA3 is still highly expressed in serum proteins of LUAD patients, which is related to stage and NSE. In addition, the expression of MAGEA3 correlated with the infiltration of immune lymphocytes. Our results suggested that the mRNA and protein expression of MAGEA3 were upregulated in LUAD, and MAGEA3 could be used as a diagnostic biomarker and immunotherapy target for LUAD patients. However, the immune mechanism needs further studied.
Supplementary Information
Author contributions
CL and CJ: designed the study. GY and KL: performed the experiments. ZR, YQ, CL and LL: analyzed the data. CL and GY: The first draft of the manuscript was written. All authors have read and approved the final manuscript. The authors affirm that human research participants provided informed consent for publication of the images in Figs. 2, 3, 4.
Funding
The study was supported by Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2023Y9279); Startup Fund for scientific research of Fujian Medical University (Grant number: 2021XH1277).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuhan Gan and Yanli Kang.
Contributor Information
Jinhua Chen, Email: Cjh1257@163.com.
Liangyuan Chen, Email: liangyuan039083@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-58003-z.
References
- 1.Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398(10299):535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022;20(5):497–530. doi: 10.6004/jnccn.2022.0025. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch FR, Franklin WA, Gazdar AF, Bunn PA., Jr Early detection of lung cancer: Clinical perspectives of recent advances in biology and radiology. Clin. Cancer Res. 2001;7(1):5–22. [PubMed] [Google Scholar]
- 4.Duffy MJ, O'Byrne K. Tissue and blood biomarkers in lung cancer: A review. Adv. Clin. Chem. 2018;86:1–21. doi: 10.1016/bs.acc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Patz EF, Jr, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: A retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol. 2016;17(5):590–599. doi: 10.1016/S1470-2045(15)00621-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng X, Sun X, Liu Z, He Y. A novel era of cancer/testis antigen in cancer immunotherapy. Int. Immunopharmacol. 2021;98:107889. doi: 10.1016/j.intimp.2021.107889. [DOI] [PubMed] [Google Scholar]
- 7.Yang P, Meng M, Zhou Q. Oncogenic cancer/testis antigens are a hallmarker of cancer and a sensible target for cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer. 2021;1876(1):188558. doi: 10.1016/j.bbcan.2021.188558. [DOI] [PubMed] [Google Scholar]
- 8.Schooten E, Di Maggio A, van Bergen E, Henegouwen PMP, Kijanka MM. MAGE-A antigens as targets for cancer immunotherapy. Cancer Treat. Rev. 2018;67:54–62. doi: 10.1016/j.ctrv.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Tsang YH, Mills GB. The roles of MAGEA6 variants in pancreatic cancer development and their potential impact on cancer immunotherapy. Autophagy. 2020;16(10):1923–1924. doi: 10.1080/15548627.2020.1802091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Õunap K, Kurg K, Võsa L, Maiväli Ü, Teras M, Planken A. Antibody response against cancer-testis antigens MAGEA4 and MAGEA10 in patients with melanoma. Oncol. Lett. 2018;16(1):211–218. doi: 10.3892/ol.2018.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann S, Meyer TJ, Brands RC, Haubitz IR, Linz C, Seher A, et al. MAGE-A expression clusters and antineoplastic treatment in head and neck cancer. Int. J. Mol. Med. 2015;35(6):1675–1682. doi: 10.3892/ijmm.2015.2174. [DOI] [PubMed] [Google Scholar]
- 12.Lee TB, Lim SC, Moon YS, Choi CH. Melanoma antigen gene family A as a molecular marker of gastric and colorectal cancers. Oncol. Rep. 2013;30(1):234–238. doi: 10.3892/or.2013.2458. [DOI] [PubMed] [Google Scholar]
- 13.Oh C, Kim HR, Oh S, Ko JY, Kim Y, Kang K, et al. Epigenetic upregulation of MAGE-A isoforms promotes breast cancer cell aggressiveness. Cancers (Basel) 2021;13(13):3176. doi: 10.3390/cancers13133176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vital D, Ikenberg K, Moch H, Roessle M, Huber GF. The expression of the cancer testis antigen MAGE A4: A favorable prognostic biomarker in salivary gland carcinomas related to low tumor grading. Laryngoscope Investig. Otolaryngol. 2018;3(3):182–190. doi: 10.1002/lio2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baran CA, Agaimy A, Wehrhan F, Weber M, Hille V, Brunner K, et al. MAGE-A expression in oral and laryngeal leukoplakia predicts malignant transformation. Mod. Pathol. 2019;32(8):1068–1081. doi: 10.1038/s41379-019-0253-5. [DOI] [PubMed] [Google Scholar]
- 17.Shukla SA, Bachireddy P, Schilling B, Galonska C, Zhan Q, Bango C, et al. Cancer-germline antigen expression discriminates clinical outcome to CTLA-4 blockade. Cell. 2018;173(3):624–633.e8. doi: 10.1016/j.cell.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin. Chim. Acta. 2019;488:165–171. doi: 10.1016/j.cca.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022;15(1):83. doi: 10.1186/s13045-022-01305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Ma X, Lou C, Zhou C, Zhao X, Li N, et al. PLA2G10 incorporated in exosomes could be diagnostic and prognostic biomarker for non-small cell lung cancer. Clin. Chim. Acta. 2022;530:55–65. doi: 10.1016/j.cca.2022.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Lánczky A, Nagy Á, Bottai G, Munkácsy G, Szabó A, Santarpia L, et al. miRpower: A web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat. 2016;160(3):439–446. doi: 10.1007/s10549-016-4013-7. [DOI] [PubMed] [Google Scholar]
- 23.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le T, Aronow RA, Kirshtein A, Shahriyari L. A review of digital cytometry methods: Estimating the relative abundance of cell types in a bulk of cells. Brief. Bioinform. 2021;22(4):bbaa219. doi: 10.1093/bib/bbaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, et al. TISIDB: An integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35(20):4200–4202. doi: 10.1093/bioinformatics/btz210. [DOI] [PubMed] [Google Scholar]
- 26.Lucas S, De Smet C, Arden KC, Viars CS, Lethé B, Lurquin C, et al. Identification of a new MAGE gene with tumor-specific expression by representational difference analysis. Cancer Res. 1998;58(4):743–752. [PubMed] [Google Scholar]
- 27.Gu L, Sang M, Yin D, Liu F, Wu Y, Liu S, et al. MAGE-A gene expression in peripheral blood serves as a poor prognostic marker for patients with lung cancer. Thorac. Cancer. 2018;9(4):431–438. doi: 10.1111/1759-7714.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Wang L, Liu J, Huang L, Yang L, Gao Q, et al. Expression and prognostic relevance of MAGE-A3 and MAGE-C2 in non-small cell lung cancer. Oncol. Lett. 2017;13(3):1609–1618. doi: 10.3892/ol.2017.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhan S, Negi SS, Shao C, Glazer CA, Chuang A, Gaykalova DA, et al. BORIS binding to the promoters of cancer testis antigens, MAGEA2, MAGEA3, and MAGEA4, is associated with their transcriptional activation in lung cancer. Clin. Cancer Res. 2011;17(13):4267–4276. doi: 10.1158/1078-0432.CCR-11-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sang M, Gu L, Yin D, Liu F, Lian Y, Zhang X, et al. MAGE-A family expression is correlated with poor survival of patients with lung adenocarcinoma: A retrospective clinical study based on tissue microarray. J. Clin. Pathol. 2017;70(6):533–540. doi: 10.1136/jclinpath-2016-203718. [DOI] [PubMed] [Google Scholar]
- 31.Karimi S, Mohammadi F, Porabdollah M, Mohajerani SA, Khodadad K, Nadji SA. Characterization of melanoma-associated antigen-a genes family differential expression in non-small-cell lung cancers. Clin. Lung Cancer. 2012;13(3):214–219. doi: 10.1016/j.cllc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Ayyoub M, Memeo L, Alvarez-Fernández E, Colarossi C, Costanzo R, Aiello E, et al. Assessment of MAGE-A expression in resected non-small cell lung cancer in relation to clinicopathologic features and mutational status of EGFR and KRAS. Cancer Immunol. Res. 2014;2(10):943–948. doi: 10.1158/2326-6066.CIR-13-0211. [DOI] [PubMed] [Google Scholar]
- 33.Zou C, Shen J, Tang Q, Yang Z, Yin J, Li Z, et al. Cancer-testis antigens expressed in osteosarcoma identified by gene microarray correlate with a poor patient prognosis. Cancer. 2012;118(7):1845–1855. doi: 10.1002/cncr.26486. [DOI] [PubMed] [Google Scholar]
- 34.Cai R, Zhao F, Zhou H, Wang Z, Lin D, Huang L, et al. A tumor-associated autoantibody panel for the detection of non-small cell lung cancer. Front. Oncol. 2022;12:1056572. doi: 10.3389/fonc.2022.1056572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother.36(2), 133–151 (2013). [DOI] [PMC free article] [PubMed]
- 36.Hege, K. M. & Carbone, D. P. Lung cancer vaccines and gene therapy. Lung Cancer41(1), S103–S113 (2003). [DOI] [PubMed]
- 37.Zhang S, Zhou X, Yu H, Yu Y. Expression of tumor-specific antigen MAGE, GAGE and BAGE in ovarian cancer tissues and cell lines. BMC Cancer. 2010;10:163. doi: 10.1186/1471-2407-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng JR, Chen HS, Mou DC, Cao J, Cong X, Qin LL, et al. Expression of cancer/testis (CT) antigens in Chinese hepatocellular carcinoma and its correlation with clinical parameters. Cancer Lett. 2005;219(2):223–232. doi: 10.1016/j.canlet.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Dyrskjøt L, Zieger K, Kissow Lildal T, Reinert T, Gruselle O, Coche T, et al. Expression of MAGE-A3, NY-ESO-1, LAGE-1 and PRAME in urothelial carcinoma. Br. J. Cancer. 2012;107(1):116–122. doi: 10.1038/bjc.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borchers A, Pieler T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes (Basel) 2010;1(3):413–426. doi: 10.3390/genes1030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim, S. H. et al. Expression of cancer-testis antigens MAGE-A3/6 and NY-ESO-1 in non-small -cell lung carcinomas and their relationship with immune cell infiltration. Lung187(6), 401–411 (2009). [DOI] [PubMed]
- 42.Vansteenkiste, J. et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J. Clin. Oncol.31(19), 2396–2403 (2013). [DOI] [PubMed]
- 43.Vansteenkiste, J. F. et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non- small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.17(6), 822–835 (2016). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.