Abstract
Background
The evidence for whether ivermectin impacts recovery, hospital admissions, and longer-term outcomes in COVID-19 is contested. The WHO recommends its use only in the context of clinical trials.
Methods
In this multicentre, open-label, multi-arm, adaptive platform randomised controlled trial, we included participants aged ≥18 years in the community, with a positive SARS-CoV-2 test, and symptoms lasting ≤14 days. Participants were randomised to usual care, usual care plus ivermectin tablets (target 300–400 μg/kg per dose, once daily for 3 days), or usual care plus other interventions. Co-primary endpoints were time to first self-reported recovery, and COVID-19 related hospitalisation/death within 28 days, analysed using Bayesian models. Recovery at 6 months was the primary, longer term outcome.
Trial registration: ISRCTN86534580.
Findings
The primary analysis included 8811 SARS-CoV-2 positive participants (median symptom duration 5 days), randomised to ivermectin (n = 2157), usual care (n = 3256), and other treatments (n = 3398) from June 23, 2021 to July 1, 2022. Time to self-reported recovery was shorter in the ivermectin group compared with usual care (hazard ratio 1·15 [95% Bayesian credible interval, 1·07 to 1·23], median decrease 2.06 days [1·00 to 3·06]), probability of meaningful effect (pre-specified hazard ratio ≥1.2) 0·192). COVID-19-related hospitalisations/deaths (odds ratio 1·02 [0·63 to 1·62]; estimated percentage difference 0% [−1% to 0·6%]), serious adverse events (three and five respectively), and the proportion feeling fully recovered were similar in both groups at 6 months (74·3% and 71·2% respectively (RR = 1·05, [1·02 to 1·08]) and also at 3 and 12 months.
Interpretation
Ivermectin for COVID-19 is unlikely to provide clinically meaningful improvement in recovery, hospital admissions, or longer-term outcomes. Further trials of ivermectin for SARS-Cov-2 infection in vaccinated community populations appear unwarranted.
Funding
UKRI/National Institute of Health Research (MC_PC_19079).
Keywords: SARS-Cov2, COVID-19, Ivermectin, Clinical trial, Long-term follow up
Introduction
Ivermectin is a safe, cheap, well tolerated and widely used anti-parasitic drug that has been widely promoted and trialled as a treatment for COVID-19. There are various mechanisms by which ivermectin could have activity against SARS-CoV-2. Ivermectin may inhibit the binding and within-cell transport to the nucleus of viral proteins that normally suppress the cell’s anti-viral response, enhancing the host cell’s ability to respond to SARS-CoV-2 infection.1 In-vitro and animal studies have demonstrated that ivermectin significantly reduces the production of pro-inflammatory cytokines such as TNF-alpha, Interleukin–1 beta and Interleukin 62; and could therefore reduce the cytokine storm and ensuing cellular damage that occurs in some individuals with COVID-19. However, in-vitro studies have used concentrations of drug which are substantially higher than may be achievable in plasma using clinical dosing.3 In a molecular modelling study, ivermectin appeared to bind with high stability to RNA dependent RNA polymerase (RdRp), suggesting that ivermectin may inhibit SARS-CoV-2 activity through RdRp inhibition.4
Some early trials found that ivermectin reduced hospital admissions and death from COVID-19, but several of these trials were found to be flawed, for example due to inadequate or lack of description of randomisation, lack of allocation concealment, or evidence of differences between arms at baseline, requiring meta-analyses to be updated.5 Despite the controversial evidence base, prescriptions for the drug increased dramatically in the USA and many other parts of the world.6, 7
Recent systematic reviews have found insufficient evidence of benefit from ivermectin on recovery outcomes,8, 9, 10 hospital admission,11 ventilation,12 or mortality,5, 10, 11, 12 while one concluded there was insufficient evidence to make a firm recommendation.8 Shaifee and colleagues included 17 trials in a meta-analysis and concluded ivermectin should not be used outside of clinical trials for COVID-19,9 or should be used with caution.13 A Cochrane review found that ivermectin in addition to usual care versus usual care alone or placebo probably has little or no effect on outcomes including admission to hospital or death within 28 days, but that the certainty of these conclusions was very low for many outcomes including hospital admission and clinical improvement.11 Furthermore, none of the trials reporting duration of symptoms were eligible for meta-analysis.11 Some of the benefits from ivermectin treatment may be related to its antiparasitic effect in areas of high prevalence of parasite infection.14
The largest of the RCTs in outpatients, the TOGETHER trial, conducted in a largely unvaccinated Brazilian population, could not fully rule out potential for a modest benefit on the requirement for treatment escalation to secondary care.15 The ACTIV-6 and COVID-OUT placebo controlled trials of ivermectin at 400 ug/kg for 3 days16, 17 and 600 ug/kg for 6 days18 in largely vaccinated Americans, regardless of risk factors, found no clinically significant benefit in sustained recovery, hospital admissions or incidence of long COVID over 10 months,19 but were arguably underpowered to detect a difference in hospital admissions. Pragmatic trial evidence from high-income countries among a largely vaccinated population remains an important evidence gap, as does an understanding of whether ivermectin can reduce persistent symptoms or improve function in the longer term.
We aimed to determine whether ivermectin speeds recovery and reduces COVID-19 related hospital admission or death in people in the community, and in addition evaluated whether there was a longer-term effect on symptoms and function at 3, 6 and 12 months.
Methods
Trial design
We assessed the effectiveness of ivermectin in the UK national, multi-centre, primary care, open-label, multi-arm, prospective adaptive Platform Randomised trial of Treatments in the Community for Pandemic and Epidemic Illnesses (PRINCIPLE), which opened on April 2, 2020, and closed to recruitment on July 1, 2022. The protocol is available in the appendix (pp 6–103) and at the trial website, www.principletrial.org. A “platform trial” allows multiple interventions for the same disease to be tested simultaneously. A master protocol defines prospective decision criteria for dropping interventions for futility, declaring interventions superior, or adding new interventions.20 This allows interventions with little evidence of meaningful benefit to be rapidly dropped for futility and replaced by new interventions, thereby directing resources towards identifying community-based treatments for COVID-19. Interventions evaluated in PRINCIPLE include hydroxychloroquine, azithromycin,21 doxycycline,22 inhaled budesonide,23 colchicine,24 favipiravir (active arm from April 9 2021), and, reported here, ivermectin. The open trial design was chosen due to the urgency to determine whether prescribing repurposed medicines had an important beneficial impact, compared to not prescribing the drugs, meaning that the appropriate comparator condition was treatment without the study drug. Procuring matched placebo would also have delayed the opening of the study. Participants receiving a study intervention, regardless of whether it is active treatment or placebo, are likely to alter their health-seeking behaviour in response to this uncertainty,25 and effect sizes from open trials do not differ meaningfully from placebo controlled trials.26
The UK Medicines and Healthcare products Regulatory Agency and the South Central-Berkshire Research Ethics Committee (Ref: 20/SC/0158) approved the trial protocol. Online consent was obtained from all participants after person-to-person discussion and explanation. The authors vouch for the accuracy and completeness of the data and for fidelity to the protocol. An independent Trial Steering Committee and Data Monitoring and Safety Committee provided trial oversight.
Participants
When the ivermectin arm opened, people in the community were eligible if they were aged ≥65 years, or ≥18 years or above with co-morbidities, or breathlessness as part of their COVID-19 illness. They either had suspected COVID-19 using the syndromic definition available at the time or symptoms consistent with COVID-19 and a positive test for SARS-CoV-2 infection. Symptoms must have started within the previous 14 days and be ongoing. From 29th July 2021 onwards, amended eligibility criteria required all participants to have a positive test for SARS-CoV-2 infection and allowed recruitment of all adults regardless of co-morbidities (appendix pp16). People were ineligible to be randomised to ivermectin if they were already taking ivermectin or if ivermectin was contraindicated according to the British National Formulary. Additional exclusion criteria were: allergy to ivermectin/excipients; known or suspected pregnancy; breastfeeding; women of childbearing potential not prepared to use highly effective contraception for 28 days post enrolment; ever having travelled to countries endemic for Loa loa; bleeding diatheses; severe liver disease; and, grapefruit consumption. Initially, eligible people were recruited, screened and enrolled through participating general medical practices, but from May 17, 2020, people across the UK could also enrol online or by telephone. After patients completed a baseline and screening questionnaire, a clinician or trained research nurse confirmed eligibility using the patient’s primary care medical record or summary care record, accessed remotely where necessary, before randomisation. We implemented several community outreach strategies aiming to increase recruitment of people from ethnically diverse communities and socioeconomically deprived backgrounds, who have been disproportionally affected by COVID-19.27
Randomisation and masking
Eligible, consenting participants were randomised using a secure, in-house, web-based randomisation system (Sortition version 2.3). Randomisation was stratified by age (< 65 years/≥ 65 years), and presence of comorbidity (yes/no) and probabilities were determined using response adaptive randomisation via regular interim analyses, which allows allocation of more participants to interventions with better observed time to recovery outcomes, enabling the trial to demonstrate benefit sooner, or rapidly remove poorly performing intervention arms (appendix pp 160–163). The allocation probability for the Usual Care arm remained fixed at 1/Z throughout the trial, where Z is the number of active interventions studies in the platform. The trial team was blinded to randomisation probabilities.
Trial procedures
Participants were followed up through an online, daily symptom diary for 28 days after randomisation, supplemented with telephone calls to non-responders on days 7, 14 and 28. The diary included questions about illness recovery (ascertained by answering the question, “Do you feel recovered today? (i.e. symptoms associated with illness are no longer a problem) Yes/No”), overall illness severity (a rating of how well they are feeling on a scale of 1–10 [1 being the worst and 10 being the best]), individual symptom severity on a four-point scale (0 = no problem to 3 = major problem), and healthcare service utilisation. Participants could nominate a trial partner to help provide follow-up data. We obtained consent to ascertain healthcare use outcome data from general practice and hospital records.
Long term follow up
All participants were contacted via email or phone call at 3, 6 and 12 months after randomisation, accepting responses (3 months [range: 2·7–6·5]; 6 months [range: 6·0–9·3]; 12 months [range: 12·0–14·2]) up to 3 months after each date, and requested to complete a questionnaire.
Trial interventions
Participants received usual care plus ivermectin according to weight bands to target 300–400 μg/kg, taken as one dose daily for 3 days [18 mg daily (6 ×3 mg tablets) for weight 45–64 kg, 24 mg daily (8 ×3 mg tablets) for weight 65–84 kg, and 30 mg daily (10 ×3 mg tablets) for weight ≥84 kg]. Participants were advised not to eat two hours before or after taking ivermectin. Medication and study packs were delivered to the participant by urgent courier. Usual care in the UK National Health Service (NHS) for suspected COVID-19 in the community is largely conservative and focused on symptom management.28 From 16 December 2021, a minority of extremely clinically vulnerable patients, could also access antiviral treatment or a monoclonal antibody infusion.29, 30
Primary outcomes
The trial commenced with the primary outcome of suspected COVID-19 related hospitalisation or death within 28 days. However, hospitalisation rates in the UK were lower than initially expected.31, 32 Therefore, the Trial Management Group and Trial Steering Committee recommended amending the primary outcome to also include illness duration,33, 34 which is an important outcome for patients and has substantial economic and social impacts. This received ethical approval on September 16, 2020, and was implemented before performing any interim analyses. Thus, the trial has two co-primary endpoints measured within 28 days of randomisation: 1) time to first reported recovery defined as the first instance that a participant reports feeling recovered; and 2) hospitalisation or death related to COVID-19. Decisions about COVID-19 relatedness were made after independent review of available data by two clinicians blinded to treatment allocation and study identifiers.
Secondary outcomes
Secondary outcomes (defined in section 3.3. of the Master Statistical Analysis Plan, appendix pp 126–133) included: a binary outcome of early, sustained recovery (recovered by day 14 and remains recovered until day 28), time to sustained recovery (date participant first reported recovery and subsequently remained well until 28 days), daily rating from 1–10 of how well participants felt, time to initial alleviation of symptoms (date symptoms first reported as minor or none), time to sustained alleviation of symptoms (date symptoms first reported as minor or none and remained minor or none until 28 days), time to initial reduction of the severity of symptoms (among people with the symptom at baseline, the date symptom severity was reported at least one scale lower), worsening of symptoms (worsening symptom by one grade from mild to moderate/major, or from moderate to major, and excluding individuals reporting symptom severity as major at baseline), contacts with healthcare services, hospital assessment without admission, duration of hospital admission, oxygen administration, Intensive Care Unit admission, mechanical ventilation, WHO ordinal scale of clinical progression, adherence to study treatment, WHO-5 Well-Being Index,35 serious adverse events, all cause death or non/elective or urgent hospitalisation and reports of new household infections. All time to event analyses used date of randomisation as baseline. We included secondary outcomes that captured sustained recovery due to the often recurrent and relapsing nature of COVID-19 symptoms.
Long term follow-up outcomes
To assess the impact of ivermectin in reducing the risk of long terms effects of COVID-19 we collected data at 3, 6 and 12 months post-randomisation. This was assessed by the patient reported primary outcome of feeling fully recovered at 6 months, with full recovery at 3 and 12 months follow up as a secondary outcome. Other secondary outcomes collected at 3, 6 and 12 months included: number of unwell days (in the past 2 weeks) if participant reported partial or no recovery from their original COVID-19 illness (range from 0 to 14 days), rating from 1–10 of how well participants felt on the day of questionnaire completion, WHO-5 Well-Being Index,35 ongoing persistent COVID-19 pre-specified symptoms (feverish, cough, shortness of breath, chest pain, loss of smell, loss of taste, nausea/vomiting, diarrhoea, headache, muscle ache, generally unwell, fatigue) defined as participants reporting the same symptoms and severity repeatedly at 3, 6 and 12 months and relatedness reported as yes or unsure. Impact of COVID-19 on work/studies at any point during follow-up was assessed at 12 months, and participant reported contacts with healthcare services from 28 days after randomisation up until 12 months.
Statistical analysis
Sample size calculation and statistical analysis are detailed in the Adaptive Design Report (appendix pp 158–329), the Master Statistical Analysis Plan (appendix pp 103–157), and Statistical Analysis Plan for Long Term Follow-up (appendix pp 330–344). In the Adaptive Design Report we justify sample sizes by simulating the operating characteristics of the adaptive design in multiple scenarios, which explicitly account for response adaptive randomisation, early stopping for futility/success and multiple interventions. In brief, for the primary outcome analyses, assuming a hazard ratio of 1.3 (median time to recovery of nine days in the usual care group and seven for ivermectin), approximately 400 participants per group would provide 90% power to demonstrate superiority of ivermectin versus usual care. Assuming 5% hospitalisation in the usual care group at the time of designing the study, approximately 1500 participants per group would provide 90% power to detect a 50% reduction in the relative risk of hospitalisation/death for ivermectin versus usual care.
The first co-primary outcome, time to first self-reported recovery, was analysed using a Bayesian piecewise exponential model. The second co-primary outcome, hospitalisation/death, was analysed using a Bayesian logistic regression model. Both models were regressed on treatment group and stratification covariates (age < 65 years /≥ 65 years and comorbidity yes/no), and vaccination status. These primary outcomes were evaluated using a “gatekeeping” strategy to preserve the overall Type I error without additional adjustments for multiple hypotheses. The hypothesis for the time-to-first-recovery endpoint was evaluated first, and if the null hypothesis was rejected, the hypothesis for the second co-primary endpoint of hospitalisation/death was evaluated. In the context of multiple interim analyses, the master protocol specifies that each null hypothesis is rejected if the Bayesian posterior probability of superiority exceeded 0·99 for the time to recovery endpoint and 0·975 (via gatekeeping) for the hospitalisation/death endpoint. For the purposes of defining futility rules, we pre-specified a clinically meaningful hazard ratio for time to first reported recovery as 1·2 or larger (equating to approximately 1·5 days difference in median time to recovery, assuming 9 days recovery in the usual care arm), and a clinically meaningful odds ratio as 0·80 or smaller for hospitalisations/deaths (equating to approximately a 1% decrease in the hospitalisation rate, assuming a rate of 5% in the usual care arm). However, due to larger sample size as the trial continued, it became apparent that the futility rule for hospitalisation/death was too conservative. With the approval of the Trial Steering Committee, the futility rule was made more aggressive by increasing the futility threshold for the probability of meaningful benefit on hospitalisation from 0.01 to 0.25, a change dated June 1, 2022 and described in detail in Section 4.1.2 of the Adaptive Design Report version 5.0 (appendix, pp 168).
If there was insufficient evidence of a clinically meaningful benefit in time to recovery, futility was declared and randomisation to that intervention would be stopped, meaning other interventions could be evaluated more rapidly in the trial. For each primary outcome endpoint (time to recovery and hospitalisation/death), a pre-specified model-based estimate of absolute benefit (days and percent, respectively) was obtained by applying the model-based estimate of treatment benefit (hazard ratio or odds ratio, respectively) to a bootstrap sample of the concurrent and eligible usual care population.
At the beginning of the trial, due to initial difficulties with community SARS-CoV-2 PCR testing in the UK, participants with suspected COVID-19 were included in the primary analysis population, irrespective of confirmatory testing. When testing became more accessible, the Trial Steering Committee recommended restricting the primary analysis population to those with confirmed COVID-19. This change was included in protocol version 7·1 on February 22, 2021 and approved on March 15, 2021, before the introduction of ivermectin to the trial platform. Therefore, the pre-specified primary analysis population includes all eligible SARS-CoV-2 positive participants randomised to ivermectin, usual care, and other interventions, from the start of the platform trial until the ivermectin arm was closed, on 1 July, 2022. This population includes participants randomised to usual care before the ivermectin group opened. The primary analysis models include parameters to adjust for potential temporal drift in the trial population, by estimating the primary endpoint in the usual care group across time via Bayesian hierarchical modelling.36
We also conducted a key pre-specified sensitivity analysis of the primary outcomes using the concurrent randomised population; defined as all SARS-CoV-2 positive participants randomised during the time period when the ivermectin arm was active. To determine the applicability of our results to situations where PCR testing may not be readily available, we also conducted secondary analyses of time to recovery and COVID-19 related hospitalisation/death among the overall study population, irrespective of SARS-CoV-2 status.
Analyses of all secondary outcomes, and pre-specified sub-group analyses, were conducted using SARS-CoV-2 positive participants eligible for ivermectin, and concurrently randomised to ivermectin or usual care; the concurrently randomised and eligible SARS-CoV-2 positive population, using frequentist approach. Secondary time-to-event outcomes were analysed using Cox proportional hazard models, and binary outcomes were analysed using logistic regression, adjusting for comorbidity, age, duration of illness and vaccination status. Due to the high proportion contributing to the analysis of primary outcomes (92.7%), we did not explore the potential impact of missing data.
Analyses of long-term follow-up outcomes were conducted using SARS-CoV-2 positive participants eligible for ivermectin, and concurrently randomised to ivermectin or usual care who contributed to the primary analysis of day 1- 28 outcomes. A sensitivity analyses of the primary outcome included all SARS-CoV-2 positive participants eligible for ivermectin, and concurrently randomised to ivermectin or usual care regardless of whether they contributed to the primary analysis of day 1–28 outcomes. Generalised linear models for the long term follow-up outcomes were fitted using a frequentist approach adjusting for the same covariates in the main analyses.
All model assumptions were evaluated. Analyses were conducted using R (version 4.0.3) and Stata (versions 16.1 and 18.0).
Role of the funding source
The funder had no role in the study design, data collection, analysis, interpretation nor writing of the paper, nor decision to submit for publication. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Results
Population
The first participant was randomised into PRINCIPLE on April 2, 2020. Enrolment into the ivermectin group started on June 23, 2021. On July 1, 2022, the Trial Steering Committee advised the Trial Management Group to stop randomisation to ivermectin because the update, pre-specified futility criterion had been met on hospitalisation/death. All participants were followed up for the full 28 days. Participants taking ivermectin were asked to stop taking treatment as futility had been demonstrated.
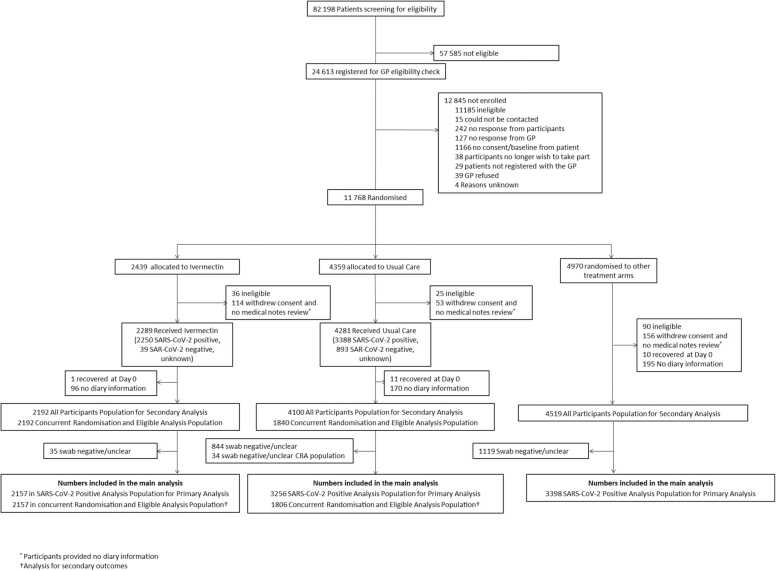
11768 participants had been randomised of whom 2439 were allocated to ivermectin, 4359 to usual care alone, and 4970 to other treatments (Fig. 1 and S1). The Bayesian primary analysis model includes data from 8811 of 9577 (92%) SARS-CoV-2 positive participants who provided follow up data and were randomised to ivermectin (n = 2157), usual care alone (n = 3256), and other treatment groups (n = 3398). To protect the integrity of the platform trial and other interventions, we only provide descriptive summaries of participants randomised to ivermectin and usual care. The average age (range) of participants was 53·8 (18–100) years, 5331 (95%) were White and 3391 (71%) had comorbidities. At randomisation, median time from symptom onset was 5 (interquartile range 3–7) days. Baseline characteristics were similar between the comparison groups (Tables 1, S1 and S2). Data regarding inhaled corticosteroid was not consistently recorded early in the trial, but in the concurrent randomisation analysis population, 399/2157 (19%) of the ivermectin arm and 338/1806 (19%) of the usual care arm reported taking inhaled corticosteroids at randomisation or during follow-up.
Fig. 1.
Participant flow diagram.
Table 1.
Baseline characteristics of SARS-CoV-2 positive participants by treatment group.
| Primary Analysis Populationa |
Concurrently and Eligible randomised population |
|||
|---|---|---|---|---|
| Ivermectin (N = 2250) | Usual Care (N = 3388) | Ivermectin (N = 2250) | Usual Care (N = 1869) | |
| Age, years | ||||
| mean(SD) | 51·2 (13·0) | 55·6 (12·9) | 51·2 (13·0) | 51·8 (12·7) |
| 18–49 | 932 (41%) | 912 (27%) | 932 (41%) | 734 (39%) |
| 50–64 | 959 (43%) | 1460 (43%) | 959 (43%) | 828 (44%) |
| 65 and over | 359 (16%) | 1016 (30%) | 359 (16%) | 307 (16%) |
| Sex, n(%) | ||||
| Female | 1315 (58%) | 1972 (58%) | 1315 (58%) | 1149 (61%) |
| Male | 933 (41%) | 1412 (42%) | 933 (41%) | 719 (38%) |
| Other | 0 | 3 (<1%) | 0 | 1 (<1%) |
| Missing, n(%) | 2 (<1%) | 1 (<1%) | 2 (<1%) | 0 |
| Ethnicity, n(%)b | ||||
| White | 2140 (95%) | 3191 (94%) | 2140 (95%) | 1792 (96%) |
| Mixed background | 35 (2%) | 35 (1%) | 35 (2%) | 26 (1%) |
| South Asian | 30 (1%) | 110 (3%) | 30 (1%) | 26 (1%) |
| Black | 11 (<1%) | 13 (<1%) | 11 (0%) | 7 (0%) |
| Other | 34 (2%) | 38 (1%) | 34 (2%) | 18 (1%) |
| Missing, n(%) | 0 | 1 (<1%) | 0 | 0 |
| IMD quintile, n(%) | ||||
| 1 (Most deprived) | 253 (11%) | 460 (14%) | 253 (11%) | 221 (12%) |
| 2 | 324 (14%) | 528 (16%) | 324 (14%) | 280 (15%) |
| 3 | 460 (20%) | 646 (19%) | 460 (20%) | 355 (19%) |
| 4 | 531 (24%) | 779 (23%) | 531 (24%) | 429 (23%) |
| 5 (Least deprived) | 682 (30%) | 975 (29%) | 682 (30%) | 584 (31%) |
| Duration of illness prior to randomisation, median(IQR) | 4·0 (3·0 to 7·0) | 5·0 (3·0 to 8·0) | 4·0 (3·0 to 7·0) | 4·0 (3·0 to 7·0) |
| Smoking status, n(%) | ||||
| Current smoker | 147 (7%) | 223 (7%) | 147 (7%) | 127 (7%) |
| Former smoker | 755 (34%) | 1199 (35%) | 755 (34%) | 609 (33%) |
| Never smoker | 1333 (59%) | 1931 (57%) | 1333 (59%) | 1114 (60%) |
| Missing, n(%) | 15 (1%) | 35 (1%) | 15 (1%) | 19 (1%) |
| Received SARS-CoV-2 vaccination, n(%) | 2110 (94%) | 2227 (66%) | 2110 (94%) | 1764 (94%) |
| Comorbidity, n(%) | 1524 (68%) | 2467 (73%) | 1524 (68%) | 1269 (68%) |
| Have you taken antibiotics since your illness started, n(%)d | 83 (4%) | 186 (5%) | 83 (4%) | 81 (4%) |
| Missing, n(%) | 0 | 2 (<1%) | 0 | 0 |
| Use of healthcare services at baseline | ||||
| GP, n(%) | 338 (15%) | 630 (19%) | 338 (15%) | 266 (14%) |
| Other primary care services, n(%) | 72 (3%) | 163 (5%) | 72 (3%) | 58 (3%) |
| NHS 111, n(%) | 139 (6%) | 258 (8%) | 139 (6%) | 111 (6%) |
| A&E, n(%) | 29 (1%) | 45 (1%) | 29 (1%) | 21 (1%) |
| Other healthcare services, n(%) | 63 (3%) | 79 (2%) | 63 (3%) | 47 (3%) |
| Baseline wellbeing score, mean(SD) | 4·9 (1·4) | 4·9 (1·4) | 4·9 (1·4) | 4·9 (1·4) |
| Missing, n(%) | 0 | 1075 (32%) | 0 | 0 |
| Day 1 wellbeing score, mean(SD) | 5·1 (1·4) | 5·2 (1·5) | 5·1 (1·4) | 5·2 (1·4) |
| Missing, n(%) | 143 (6%) | 370 (11%) | 143 (6%) | 119 (6%) |
| Well-being (WHO5 Questionnaire)e, mean(SD) | 57·1 (23·3) | 52·8 (24·8) | 57·1 (23·3) | 56·3 (23·7) |
| Missing, n(%) | 0 | 2 (<1%) | 0 | 0 |
3 E.g. angina, heart attack, heart failure, atrial fibrillation, valve problems.
Includes participants randomised before the ivermectin arm was open.
Data on ethnicity were collected retrospectively via notes review before July 2020.
Includes Ramipril, Lisinopril, Perindopril, Captopril or Enalapril.
Includes five items relating to wellbeing measured on a five-point scale; a total score is computed by summing the scores to the five individual questions to give a raw score of 0–25, which is then multiplied by 4 to give the final score from 0, representing the worst imaginable wellbeing, to 100, representing the best imaginable wellbeing.
Of 2157 participants randomised to ivermectin who provided medication use information, 1917 (89%) reported initiating ivermectin and 1889 (88%) reported taking it on all three days.
Primary outcomes
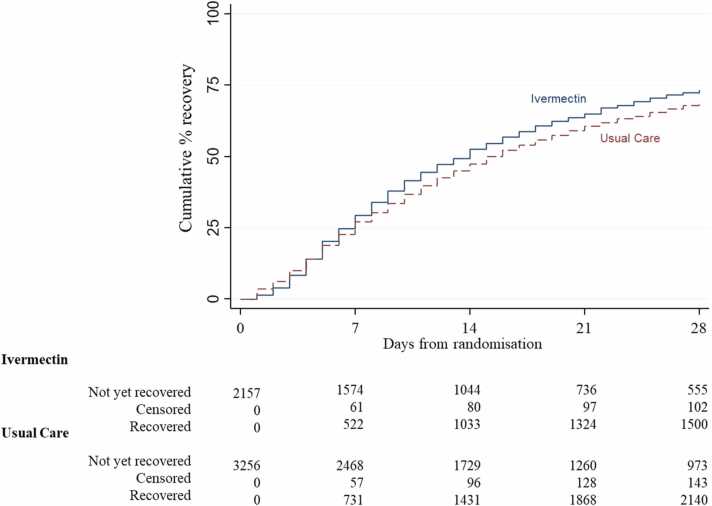
In the SARS-CoV-2 positive primary analysis population, the observed median time to first recovery was 14 days in the ivermectin group compared to 15 in the usual care group (Fig. 2). In the concurrent randomisation analysis population, (excluding participants randomised to usual care before the ivermectin arm opened) the observed median time to first recovery was 14 in the ivermectin group and 16 in the usual care group. Based on the Bayesian primary analysis model which adjusts for temporal drift, there was evidence of a benefit in time-to-first-recovery in the ivermectin group versus usual care (hazard ratio 1·145, 95% Bayesian credible interval [1·066 to 1·231]. Based on a bootstrap estimated median time to recovery of 16 days in the concurrent and eligible usual care SARS-CoV-2 positive population, the model-based estimated hazards ratio corresponds to an estimated 2·055 (0·999 to 3·06) fewer days in median time to first reported recovery for ivermectin relative to usual care. The probability that time to recovery was shorter in the ivermectin group versus usual care (i.e. probability of superiority) was >0·9999, which met the pre-specified superiority threshold of 0.99. The probability of meaningful effect (pre-specified as a hazard ratio ≥1.2 for the purpose of evaluating futility) was 0·192 (Table 2). This treatment effect was consistent in the concurrent randomisation and overall study population (Table 2).
Fig. 2.
Summary and results of the time to first self-reported recovery (Primary Population Analysis - SARS-CoV-2 positive analysis population).
Table 2.
Primary and secondary outcomes.
| Ivermectin | Usual Care | Estimated difference median TTR or hospitalisation/death rate (95% BCI) | Hazard Ratio/ Odds Ratio (95% BCI) | Pr (Superiority) | Pr (Meaningful) | |
|---|---|---|---|---|---|---|
| Primary outcomes (Primary analysis: SARS-CoV-2 positive population) | (N = 2157) | (N = 3256) | ||||
| Time to first reported recovery, days | 14 (7 to not reached)a | 15 (7 to not reached)a | 2·06 (1·00 to 3·06)b | 1·15 (1·07 to 1·23)b | >0·999b | 0·19b |
| Hospitalisation/death at 28 days | 34/2157 (1.6%)a | 144/3256 (4.4%)a | 0% (−1% to 0·6%)c | 1·02 (0·63 to 1·62) | 0·472c | 0·22c |
| Primary outcomes (Secondary analysis: all participants population) | (N = 2192) | (N = 4099) | ||||
| Time to first reported recovery, days | 14 (7 to not reached)a | 14 (6 to not reached)a | 2·07 (1·02 to 3·06)b | 1·15 (1·07 to 1·23)b | >0·999b | 0·20b |
| Hospitalisation/death at 28 days | 34/2192 (1.6%)a | 162/4100 (4.0%)a | 0% (−0·9% to 0·6%)c | 1·02 (0·64 to 1·62) | 0·472c | 0·22c |
| Primary outcomes (Sensitivity analysis: concurrent and eligible analysis population) | (N = 2157) | (N = 1806) | ||||
| Time to first reported recovery, days | 14 (7 to not reached)a | 16 (8 to not reached)a | 2·07 (0·97 to 3·11)b | 1.15 (1·06 to 1·24)b | >0·999b | 0·21b |
| Hospitalisation/death at 28 days | 34/2157 (1.6%)a | 27/1806 (1.5%)a | 0% (−1% to 0·6%)c | 1·01 (0·61 to 1·68)c | 0·481c | 0·24c |
| Secondary outcomesd | Ivermectin (N = 2157) | Usual Care (N = 1806) | Estimated treatment effect (95% CI) | P-value | ||
| Early sustained recovery, n/N (%)e | 454/2154 (21.1%) | 293/1805 (16.2%) | 1·30 (1·14 to 1·48)f | <0·0001 | ||
| Sustained recovery, n/N (%) | 1228/2157 (56.9%) | 936/1806 (51.8%) | ||||
| Time to sustained recovery (days), median (IQR) | 26 (15 to not reached) | 27 (18 to not reached) | 1·19 (1·09 to 1·29)g | <0·0001 | ||
| Alleviation of all symptoms, n/N (%) | 1677/1826 (91.8%) | 1374/1536 (89.5%) | ||||
| Time to alleviations of all symptoms (days), median (IQR) | 4 (2 to 8) | 5 (2 to 10) | 1.19 (1.11 to 1.27)g | <0·0001 | ||
| Sustained alleviation of all symptoms, n/N (%) | 1460/1826 (80.0%) | 1158/1536 (75.4%) | ||||
| Time to sustained alleviation of all symptoms (days), median (IQR) | 12 (4 to 25) | 16 (6 to 27) | 1·21 (1·12 to 1·31)g | <0·0001 | ||
| Initial reduction of severity of symptoms, n/N (%) | 1796/2157 (83.3%) | 1445/1805 (80.1%) | ||||
| Time to initial reduction of severity of symptoms (days), median (IQR) | 7 (4 to 17) | 9 (5 to 19) | 1·16 (1·09 to 1·25)g | <0·0001 | ||
| Rating of how well participant feels (1 worst, 10 best), mean (SD) [n] | ||||||
| Day 7 | 7·0 (1·6) [2041] | 6·7 (1·7) [1719] | 0·31 (0·21 to 0·41)h | <0·0001 | ||
| Day 14 | 7·8 (1·6) [1999] | 7·5 (1·7) [1666] | 0·25 (0·14 to 0·37)h | <0·0001 | ||
| Day 21 | 8·0 (1·6) [1923] | 7·8 (1·7) [1572] | 0·21 (0·08 to 0·34)h | 0·0012 | ||
| Day 28 | 8·3 (1·6) [1979] | 8·2 (1·6) [1623] | 0·15 (−0·00 to 0·30)h | 0·053 | ||
| Well-being (WHO5 Questionnaire), mean (SD)[n] | ||||||
| Day 14 | 46·7 (21·8) [1179] | 46·2 (22·9) [2024] | 2·21 (0·94 to 3·49)h | 0·0007 | ||
| Day 28 | 58·8 (22·2) [1173] | 59·0 (22·5) [2009] | 2·86 (1·59 to 4·14)h | <0·0001 | ||
| Self-reported contact with ≥ 1 healthcare service, n/N (%) | 958/2153 (44.5%) | 858/1805 (47.5%) | 0·89 (0·78 to 1·01)f | 0·0605 | ||
| GP reported contact with ≥ 1 healthcare service, n/N (%) | 737/1840 (40.1%) | 622/1576 (39.5%) | 1·03 (0·89 to 1·18)f | 0·72 | ||
| New infections in household, n/N (%) | 613/2151 (28.5%) | 536/1803 (29.7%) | 0·94 (0·82 to 1·08)i | 0·40 | ||
| Prescription of antibiotics, n/N (%) | 111/1789 (6.2%) | 88/1526 (5.8%) | 1·08 (0·80 to 1·46)i | 0·61 | ||
| Hospital assessment without admission, n/N (%) | 37/2157 (1.7%) | 34/1806 (1.9%) | 0·91 (0·55 to 1·50)i | 0·72 | ||
| Oxygen Administration, n/N (%) | 17/2150 (0.8%) | 10/1800 (0.6%) | 1·43 (0·62 to 3·50)i | 0·44 | ||
| Mechanical ventilation, n/N (%) | 3/2149 (0.1%) | 1/1801 (0.06%) | 2·52 (0·20 to 32·15)i | 0·63 | ||
| ICU admission, n/N (%) | 5/2149 (0.2%) | 1/1800 (0.06%) | 4·20 (0·47 to 198·5)i | 0·23 |
BCI – Bayesian credible interval
Observed median (interquartile range) time to recovery or observed number (%) hospitalisation/death. These values have not been adjusted for the temporal drift. The usual care group in the primary and secondary analysis populations included participant randomised before the ivermectin arm opened and therefore direct comparisons may reflect temporal differences in the underlying outcome rather than a treatment effect.
Estimated difference (ivermectin-usual care) in median time to recovery derived from a Bayesian piecewise exponential model adjusted for age, comorbidity and vaccination status at baseline, with 95% BCI. A positive value in estimated median time to recovery (or HR < 1) corresponds to an increase in time to recovery in days in ivermectin compared to Usual Care. Pr(Superiority) is the probability of superiority and treatment superiority is declared if Pr(superiority) ≥0.99 versus usual care. Pr(Meaningful) is probability that the hazards ratio for Ivermectin versus usual care is 1.2 or larger.
Estimated absolute percentage difference (ivermectin-usual care) in hospitalisation/death derived from a Bayesian logistic regression model adjusted for age, comorbidity and vaccination status at baseline, with 95% Bayesian credible interval. A positive value in the estimated difference percentage (OR < 1) favours ivermectin. Pr(Superiority) is the probability of superiority and treatment superiority is declared if Pr(superiority) ≥ 0·975 versus usual care. Pr(Meaningful) is the probability that the odds ratio for Ivermectin versus usual care is 0.80 or smaller.
All secondary outcome analyses were conducted on the concurrent and eligible randomisation SARS-CoV-2 positive population, but restricted to those who are in the ivermectin and usual care group only. Secondary outcomes were analysed using frequentist statistics.
Defined as recovered within 14 days and reports feeling recovered for the next 14 days (or recovered at 14 and 28 days if only call data available)
Relative risks adjusted for age, comorbidity at baseline, duration of illness, and vaccination status at baseline.
Estimated hazard ratio derived from a Cox proportional hazard model adjusted for age, comorbidity at baseline, duration of illness, and vaccination status at baseline, with 95% confidence interval.
Mixed effect model adjusting age, comorbidity, duration of illness, vaccination status at baseline, and time. Participant was fitted as a random effect. WHO well-being score was also adjusted for the score at baseline
Unadjusted relative risks due to low event rate.
In the SARS-CoV-2 positive primary analysis population, there were 34/2157 (1.6%) COVID-19 related hospitalisations/deaths in the ivermectin group (33 hospitalisations, of whom 2 died, 1 death without hospitalisation), and 144/3256 (4.4%) in the usual care group (143 hospitalisations, of whom 11 died, 1 death without hospitalisation). The high levels of hospitalisations/deaths in the usual care group in the primary analysis population were driven by the higher event rate before the ivermectin arm opened. In the usual care group in concurrent randomisation analysis population, which excluded participants randomised to usual care before the ivermectin arm opened, there were 27/1806 (1.5%) COVID-19 related hospitalisations/deaths. In the Bayesian primary analysis model, which takes into account the temporal change in event rates, COVID-19 related hospitalisation/deaths in the ivermectin group compared to usual care were similar, with an estimated odds ratio of 1·017 (95% credible interval 0·633 to 1·622). Based on a bootstrap estimated hospitalisation rate of 1·5% in the concurrent and eligible usual care population, the model-based estimated odds ratio corresponds to an estimated difference in the hospitalisation rate of 0% [−1·0% to 0·6%]) (Table 2). The probability that COVID-19 related hospitalisations/deaths were lower in the ivermectin arm versus usual care (i.e. probability of superiority) was 0·472. The probability that there was a meaningful reduction in COVID-19 related hospitalisations/deaths (predefined as an odds ratio of 0·80 or smaller) was 0·223 which is below the 0·25 threshold indicating enrolment should stop for futility.
Secondary outcomes
Analyses of secondary outcomes, using the concurrent randomisation and eligible SARS-CoV-2 positive population, are presented in Table 2, Figs. S1 to S4 (appendix pp345—349). There was statistical evidence of benefit of ivermectin for the proportion of participants self-reporting early sustained recovery, sustained recovery, sustained alleviation of symptoms and initial reduction of severity of symptoms. There were also small benefits in terms of participant self-rating of how well they felt at days 7, 14 and 21 and WHO wellbeing scale ratings at days 14 and 28. There was no evidence of benefit for all other secondary outcomes assessed including measures of healthcare usage, antibiotic usage and escalation of care.
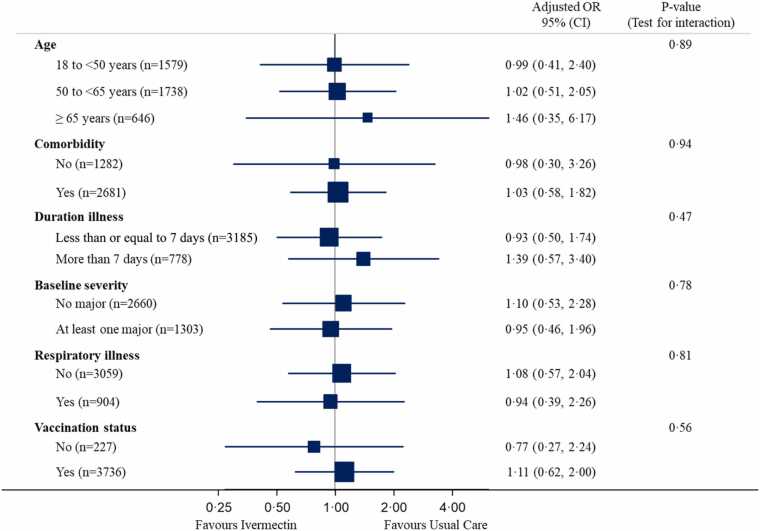
In the pre-specified subgroup analyses, there was no statistical evidence that symptom duration prior to randomisation, baseline illness severity score, inhaled corticosteroid use, age or comorbidity modified the effect of ivermectin on time to first reported recovery (Fig. 3 and S2, appendix pp346), although numbers were small. In post-hoc sub-group analyses, there was no evidence that ivermectin effects differed by vaccination status (Fig. 3), although numbers were small. Regarding serious adverse events, there were five hospitalisations unrelated to COVID-19 in the ivermectin group and three in usual care.
Fig. 3.
Forest plot of subgroup analysis of hospitalisation/death (concurrent randomisation and eligible SARS-CoV-2 positive population).
Long term follow-up outcomes
Baseline characteristics were comparable for individuals included in the long-term follow-up analyses (Fig. S1, appendix pp345). Primary and secondary analyses are presented in Table 3. The primary outcome was available for 1750 /2157 (81%) of those receiving ivermectin and 1455/1806 (81%) receiving usual care. At 6 months 1301/1750 (74%) of respondents in the ivermectin group and 1037/1455 (71%) in the usual care group reported feeling fully recovered from the original COVID-19 illness (RR 1·05, 95% CI [1·02 to 1·08] p = 0·0035). Results remained consistent in the sensitivity analysis and a similar small difference was evident at 3 and 12 months. The proportion of participants experiencing ongoing persistent COVID-19 pre-specified symptoms, ratings of how well the participant felt on the day of completion, and WHO-5 Well-Being Index were improved in the ivermectin group versus usual care at all 3 timepoints. No difference was observed at any timepoint on the number of days participants felt unwell in the previous 2 weeks There was no difference in impact on work or studies and healthcare use over the 12 month follow-up. Results of follow-up outcomes are additionally reported in Tables S3 to S39 (appendix pp 357–393).
Table 3.
Long term follow-up: Primary and secondary outcomes.
| Ivermectin |
Usual Care |
Estimated treatment effect [95% CI] | P-value | |
|---|---|---|---|---|
| (N = 2157) | (N = 1806) | |||
| Primary outcome: | ||||
| Feeling fully recovereda, n/N(%) | ||||
| 3 months | 1265/1766 (71.6%) | 993/1486 (66.8%) | 1·06 [1·03 to 1·10] | 0·0002 |
| 6 monthsb | 1301/1750 (74.3%) | 1037/1455 (71.3%) | 1·05 [1·02 to 1·08] | 0·0035 |
| 12 months | 1431/1848 (77.4%) | 1113/1533 (72.6%) | 1·06 [1·03 to 1·09] | 0·0001 |
| Primary outcome: sensitivity analysis | (N = 2250) | (N = 1869) | ||
| Feeling fully recovereda, n/N(%) | ||||
| 3 months | 1267/1769 (71.6%) | 996/1490 (66.8%) | 1·06 [1·03 to 1·09] | 0·0002 |
| 6 monthsb | 1304/1753 (74.4%) | 1042/1461 (71.3%) | 1·05 [1·01 to 1·08] | 0·0039 |
| 12 months | 1436/1853 (77.5%) | 1120/1540 (72.7%) | 1·06 [1·03 to 1·09] | 0·0001 |
| Secondary outcomes: | ||||
| Number of unwell days in the past 2 weeksc, mean (SD) [n] | ||||
| 3 months | 10.5 (4·4) [501] | 10.2 (4·5) [493] | 0·19 [−0·38 to 0·77] | 0·51 |
| 6 months | 10.3 (4·7) [449] | 9.9 (4·7) [418] | 0·34 [−0·26 to 0·95] | 0·27 |
| 12 months | 9.6 (5·0) [417] | 9.1 (5·0) [420] | 0·42 [−0·20 to 1·03] | 0·18 |
| Rating of how well participant feels on day of completion (1 worst, 10 best)d, mean (SD) [n] | ||||
| 3 months | 8·2 (1·6) [1765] | 8·0 (1·7) [1486] | 0·18 [0·07 to 0·29] | 0·0017 |
| 6 months | 8·0 (1·7) [1750] | 7·8 (1·8) [1455] | 0·15 [0·04 to 0·27] | 0·0081 |
| 12 months | 7·9 (1·8) [1847] | 7·7 (1·7) [1533] | 0·14 [0·03 to 0·25] | 0·0145 |
| Well-being (WHO-5)d, mean (SD) [n] | ||||
| 3 months | 62·1 (22·3) [1765] | 59·5 (22·5) [1485] | 2·49 [1·20 to 3·79] | 0·0002 |
| 6 months | 61·3 (22·4) [1750] | 58·9 (22·8) [1455] | 2·33 [1·03 to 3·63] | 0·0005 |
| 12 months | 61·6 (22·4) [1845] | 59·3 (22·5) [1533] | 2·41 [1·13 to 3·69] | 0·0002 |
| Ongoing persistent COVID-19 symptoms at 3, 6 and 12 monthse,f, n/N (%) | 94/1941 (4·8%) | 109/1624 (6·7%) | 0·72 [0·55 to 0·94] | 0·0153 |
| Impact of COVID-19 on work/studies,h n/N (%) and median (IQR) [n] | ||||
| Stopping work/studiesf | 149/1998 (7.5%) | 134/1671 (8.0%) | 0·92 [0·73 to 1·14] | 0·44 |
| Having time off work/studyingf | 322/1998 (16.1%) | 267/1671 (16.0%) | 0·98 [0·85 to 1·14] | 0·83 |
| Total time off work/studying (days)g | 14 (7 to 34) [322] | 15 (7 to 35) [266] | -1·43 [−5·06 to 2·20] | 0·44 |
| Change job/studies | 116/1998 (5.8%) | 87/1671 (5.2%) | 1.10 [0.84 to 1.43] | 0.50 |
| Healthcare service utilisationh, n/N (%) and median (IQR) [n] | ||||
| Any contactf | 430/1998 (21.5%) | 375/1671 (22.4%) | 0·95 [0·84 to 1·07] | 0·42 |
| Number of contacts | 4 (2 to 7) [429] | 4 (2 to 8) [375] | – | – |
Ivermectin versus concurrent and eligible usual care.
Relative risks (RR), derived from frequentist approach mixed effect logistic regression model, adjusted for assessment time point, age, presence of comorbidity, duration of illness at randomisation, vaccination status, and an interaction between randomised group and assessment time point as fixed effects, and participant as a random effect. RR < 1 favours ivermectin. P < 0.05 indicates statistical significance.
Long term follow-up primary outcome.
Number of unwell days in the past 2 weeks, if participant reported partial or no recovery from their original COVID-19 illness (range from 0 to 14 days). Estimated mean difference, derived from frequentist approach linear mixed model adjusted for randomised group, assessment time point, age, presence of comorbidity, duration of illness at randomisation, vaccination status, baseline score (if applicable), and an interaction between randomised group and assessment time point as fixed effects, and participant as a random effect. Mean difference < 0 favours ivermectin. P < 0.05 indicates statistical significance.
Mean difference, derived from frequentist approach linear mixed model adjusted for randomised group, assessment time point, age, presence of comorbidity, duration of illness at randomisation, vaccination status, baseline score (if applicable), and an interaction between randomised group and assessment time point as fixed effects, and participant as a random effect. Mean difference > 0 favours ivermectin. P < 0.05 indicates statistical significance.
Pre-specified long COVID-19 symptoms (feverish, cough, shortness of breath, chest pain, loss of smell, loss of taste, nausea/vomiting, diarrhoea, headache, muscle ache, generally unwell and fatigue), defined as participants reporting the same symptoms, severity, and relatedness in all 3 timepoints.
Relative risks, derived from frequentist approach mixed effect logistic regression model, adjusted for assessment time point, age, presence of comorbidity, duration of illness at randomisation, vaccination status, and an interaction between randomised group and assessment time point as fixed effects, and participant as a random effect. RR < 1 favours ivermectin. P < 0.05 indicates statistical significance.
Median difference, derived from quintile regression adjusted for randomised group, age, presence of comorbidity, duration of illness at randomisation, and vaccination status. Median difference < 0 favours ivermectin. P < 0.05 indicates statistical significance.
Assessed at 12 months, healthcare service utilisation from 28 days after randomisation.
Discussion
Summary
This analysis from an open-label platform, randomised controlled trial of ivermectin for COVID-19 in the community suggests clinically meaningful improvements in recovery time are unlikely, with no reduction in hospital admissions, little difference in symptoms and no difference in days unwell, or impact on work and studies, at one, three, six and 12 months.
Ivermectin reduced the time to first reported recovery by about two days from the median of 16 days in the usual care group. This result was statistically significant (HR = 1·14, 95% Interval= 1·07 – 1·23), but the estimated hazard ratio was less than the pre-specified meaningful effect of 1·2. Given that the proportion of illness duration reduced is the most meaningful assessment of benefit, rather than the absolute number of days with illness saved, and that mean illness duration varies over time with COVID-19, our blind prior was that a benefit with an HR of less than 1·2 (approximately 1·5 days difference in median time to recovery, assuming 9 days to recovery in the usual care arm) would not be considered clinically meaningful. There was no evidence that ivermectin reduced the need for hospital admission. Findings were similar in the primary analysis population, which included all SARS-CoV-2 positive participants, and in the sensitivity analyses that include only those controls randomised concurrently with ivermectin. We found a small benefit of ivermectin in terms of the proportion of participants feeling fully recovered at 3, 6 and 12 months, on a range of measures of recovery and time to recovery, and on ratings of wellbeing. There were no differences in number of days participants felt unwell in the previous 2 weeks, or impact on work or studies and healthcare usage at 3, 6 and 12 months. Overall, these findings, while evidencing a small benefit in symptom duration, do not support the use of ivermectin as treatment for COVID-19 in the community among a largely vaccinated population at the dose and duration we used, which is in keeping with current NICE guidance.29
Comparison with current evidence
PRINCIPLE is the first UK randomised trial to evaluate the effect of ivermectin on time to recovery and hospital admission for mostly vaccinated people with COVID-19 in the community, and with longer term follow to assess persisting symptoms and function.
Our findings are in broad agreement with findings from recent systematic reviews,9, 10, 11, 12, 13 and from large, placebo controlled outpatient trials conducted in the USA (COVID-OUT and ACTIV-6 (two trials, both evaluating a similar dose to this study of 400 ug/kg for 3 days16, 17, and ACTIV-6 additionally evaluating 600 ug/kg for 6 days18) and Brazil (TOGETHER, 400 ug/kg for 3 days,) 15. The participant age in these trials was similar, with a median age of 46 years,17 48 years,16 48 years, 18 and 49 years,15 respectively. All participants in the TOGETHER trial were aged ≥18 years with a comorbidity, or were aged over 50 years.15 For inclusion in COVID-OUT, participants had to be overweight or obese.17 Whilst having a comorbidity was not a prerequisite for participation in the ACTIV-6 trials, the authors noted that many participants had additional risk factors for a worse outcome from COVID-19, such as a BMI >30 kg/m² hypertension and asthma. In PRINCIPLE, the majority of participants had comorbidities (71%). Whilst 94% of PRINCIPLE participants were vaccinated during the recruitment of ivermectin phase, 47%, 84% and 52% of participants in ACTIV-6 lower and higher dose and COVID-OUT trials were vaccinated, respectively. The vaccination status of participants in the TOGETHER trial was not reported.15
The TOGETHER investigators found no significant difference between ivermectin and placebo groups in the risk of hospitalisation or an ED visit lasting >6 h due to COVID-19 within 28 days of randomisation (relative risk, 0.90; 95% Bayesian credible interval, 0·70–1·16).15 ACTIV-6 (400 ug/kg for 3 days) found no evidence of a significant benefit with ivermectin compared to placebo on time to sustained recovery, defined as three consecutive days without symptoms (hazard ratio 1·07, 95% credible interval 0·96–1·17.16 Ivermectin reduced the amount of time spent feeling unwell, measured at day 14, by 0·49 days, which was deemed clinically unimportant (95% credible interval 0·15 to 0·82 days).16 There was no beneficial impact of ivermectin on symptom severity, nor the primary composite outcome of hypoxaemia, emergency department visits, hospitalisations or death (adjusted odds ratio 1·05; 95% CI: 0·76–1·45; P = 0·78).16 The placebo controlled ACTIV-6 trial of a higher dose and duration of ivermectin (600 ug/kg for 6 days) did not find a benefit in terms of sustained recovery or the composite of hypoxaemia, emergency department visits, hospitalisations or death. The COVID-OUT study similarly found no difference in the same composite outcome (adjusted OR 1·05 (95% CI, 0·76 to 1·45; P = 0·78)) and no impact upon symptom duration.17 None of the trials reported safety concerns with ivermectin. These findings are broadly in keeping with PRINCIPLE, in which ivermectin did not reduce hospitalisations, and produced a modest benefit in time to recovery that is unlikely to be clinically significant.
Only one other trial, COVID-OUT, has reported follow-up over the longer term, namely at 10 months from study entry. There was no benefit in terms of the cumulative incidence of long COVID medical diagnoses up to and including day 300 (7·7% ivermectin and 8·1% placebo (HR 0·95, 95% CI 0·57–1·59).19
Strengths and limitations
The PRINCIPLE trial adopted a pragmatic, adaptive platform design, which allowed for efficient evaluation of the effectiveness of ivermectin as an early, standalone intervention as it might be used in the community. The use of a shared control group added to the efficiency of the trial, reducing the overall number of participants required to determine ivermectin’s effectiveness, compared with traditional trial designs. Although concerns have been raised that use of a common control group in platform trials may lead to an inflation of type I error, the overall type I error rate has been shown to be smaller in platform trials with a common control compared to a trial with individual controls. 37 Furthermore, the Bayesian primary analysis model leveraged previous enrolments in the usual care arm to increase the precision of estimates while also adjusting for potential temporal change in previous usual care enrolments. Interim analyses allowed ivermectin to be discontinued (due to futility on the hospitalisation/ death outcome). We focused on patients at increased risk of complications and used routine electronic health records to confirm hospitalisation/death, obtaining primary outcome data on over 92% of participants. The median time from symptom onset to stating treatment was five days. We are unique in evaluating a number of long-term follow-up outcomes including symptoms and healthcare usage.
We used time to first-reported recovery as a primary outcome as it was of greatest interest to our patient and public involvement (PPI) contributors and is best ascertained by direct patient report, rather than by the use of surrogate measures.
We used an open label design, similar to other large COVID-19 platform trials,38, 39 with the aim of rapidly assessing clinically meaningful benefit of ivermectin in addition to usual care. This is in keeping with pragmatic trial design, which is more closely reflective of usual clinical practice, and consequently the results are more likely to reflect real-world outcomes.40 Whilst we are unable to estimate the effect of placebo on our findings, we found no evidence of a benefit in time to recovery for other open evaluations of interventions in the PRINCIPLE trial.21, 22, 24 The findings of small differences in self-reported recovery outcomes may have been influenced by high expectations of benefit from ivermectin treatment as the drug had been prominently featured in the media. The direction of any bias from a placebo effect is likely to favour a drug rather than usual care alone, hence we pre-specified a threshold for clinically meaningful benefit in our patient-reported recovery outcome. We did not consider that hospital admission, decided upon by independent clinicians according to clinical criteria, could be meaningfully influenced by the open-label design. Our findings for ivermectin are broadly in keeping with conclusions from three, large placebo-controlled trials.15, 16, 17, 18
PCR testing as well as lateral flow testing was accepted as confirmation of SARS-CoV-2 infection. Accepting positive lateral flow tests is, again, in keeping with pragmatic trial design and reflective of current clinical practice in many countries. Furthermore, lateral flow tests have been found to be an accurate alternative to PCR tests when used in symptomatic patients in primary care, with a positive predictive value of over 97% 41.
Conclusion
The most recent Cochrane review on ivermectin for COVID-19 identified low certainty evidence that ivermectin treatment for outpatients does not reduce death or hospital admission over 28 days, and low certainty evidence of no improvement on symptom resolution up to 14 days. The results from our trial add to the certainty to findings on these outcomes and support the position that ivermectin should not be used to treat SARS-Cov-2 infection in the community in high-income countries with a largely vaccinated population. Furthermore, given our findings in an open label trial of no differences in hospital admission, a modest reduction in first-reported time to recovery, and no impact work or studies at three, six and 12 months, we consider that additional studies of ivermectin in this population should not be a priority for research.
Writing group contributions
CCB and FRDH had full access to all of the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis, and decided to publish the paper. BS, NB, L-MY, CCB, FDRH, GH, MS, OVH, OAG, JD, DR, PL contributed to trial design. SdeL, PHE, NT and MPG helped plan the trial and ongoing recruitment. EO, NT, SdeL, were responsible for acquisition of data. CCB, L-MY, GH, JD, BS, MB, FDRH, OVH, PL and OAG drafted the manuscript. BS, NB, L-MY, MAD, MF, CS, VH, and MS contributed to statistical analysis. All members of the PRINCIPLE writing group critically revised the manuscript. The members of the PRINCIPLE Collaborative Group and their roles in the conduct of the trial are listed at the end of the manuscript.
Funding
The PRINCIPLE trial is funded by a grant to the University of Oxford from UK Research and Innovation and the Department of Health and Social Care through the National Institute for Health Research as part of the UK Government’s rapid research response fund. The views expressed are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Declaration of Competing Interest
Drs. Saville, Berry, Detry, Fitzgerald and Saunders report grants from The University of Oxford, for the Sponsor's grant from the UK NIHR, for statistical design and analyses for the PRINCIPLE trial during the conduct of the study. Prof de Lusignan is Director of the Oxford-RCGP Research and Surveillance Centre and reports that through his University he has had grants outside the submitted work from AstraZeneca, GSK, Sanofi, Seqirus and Takeda for vaccine related research, and membership of advisory boards for AstraZeneca, Sanofi and Seqirus. Profs Hobbs and Butler report grants from UKRI, during the conduct of the study. All other authors have no competing interests to declare.
Acknowledgements
We thank the patients who participated in this study. We also thank the many health and social care professionals and who contributed. We thank the TSC and DMEC members for their significant contributions to trial oversight. The PRINCIPLE trial platform was led from the Primary Care and Vaccines Collaborative Clinical Trials Unit at the University of Oxford’s Nuffield Department of Primary Care Health Sciences. We gratefully acknowledge the advice we received from Dr Carlos Chaccour, MD, about including ivermectin in the PRINCIPLE Trial while planning this study arm, and for putting us in contact with Edenbridge Pharmaceuticals, LLC. PRINCIPLE was supported by a large network of care homes, pharmacies, NHS 111 Hubs, hospitals, and 1,401 GP practices across England, Wales, Scotland, and Northern Ireland. The trial was integrated with the Oxford-Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) ORCHID digital platform. PRINCIPLE has been supported by the NIHR and its Clinical Research Network, NHS DigiTrials, Public Health England, Health and Care Research Wales, NHS Research Scotland, the Health and Social Care Board in Northern Ireland, and the Therapeutics Task Force. We gratefully acknowledge Edenbridge Pharmaceuticals, LLC for supplying ivermectin for use in the PRINCIPLE Trial without cost to the trial.
CCB acknowledges part support as Senior Investigator of the National Institute of Health Research, the NIHR Community Healthcare Medtech and In-Vitro Diagnostics Co-operative (MIC), and the NIHR Health Protection Research Unit on Health Care Associated Infections and Antimicrobial Resistance. FDRH acknowledges his part support as an NIHR Senior Investigator and as Director, NIHR Applied Research Collaboration (ARC) Oxford Thames Valley, JD is funded by the NIHR (CL-2022–13-005). OAG receives funding from the European Clinical Research Alliance on Infectious Diseases (project number 101046109). GH is funded by an NIHR Advanced Fellowship and by the NIHR Community Healthcare Medtech and In-Vitro Diagnostics Co-operative (MIC). For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Data sharing
Data can be shared with qualifying researchers who submit a proposal with a valuable research question as assessed by a committee formed from the TMG including senior statistical and clinical representation. A contract should be signed.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jinf.2024.106130.
Contributor Information
FD Richard Hobbs, Email: principle@phc.ox.ac.uk.
Christopher C. Butler, Email: principle@phc.ox.ac.uk.
Appendix A. Supplementary material
Supplementary material
Supplementary material
.
References
- 1.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X., Song Y., Ci X., An N., Ju Y., Li H., et al. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res. 2008;57(11):524–529. doi: 10.1007/s00011-008-8007-8. [DOI] [PubMed] [Google Scholar]
- 3.Eastman R.T., Rusinova R., Herold K.F., Huang X.P., Dranchak P., Voss T.C., et al. Nonspecific membrane bilayer perturbations by ivermectin underlie SARS-CoV-2 in vitro activity. bioRxiv. 2023 [Google Scholar]
- 4.Sen Gupta P.S., Biswal S., Panda S.K., Ray A.K., Rana M.K. Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with in-vitro effective drug ivermectin. J Biomol Struct Dyn. 2022;40(5):2217–2226. doi: 10.1080/07391102.2020.1839564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izcovich A., Peiris S., Ragusa M., Tortosa F., Rada G., Aldighieri S., et al. Bias as a source of inconsistency in ivermectin trials for COVID-19: A systematic review. Ivermectin's suggested benefits are mainly based on potentially biased results. J Clin Epidemiol. 2022;144:43–55. doi: 10.1016/j.jclinepi.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lind J.N., Lovegrove M.C., Geller A.I., Uyeki T.M., Datta S.D., Budnitz D.S. Increase in outpatient ivermectin dispensing in the US during the COVID-19 pandemic: a cross-sectional analysis. J Gen Intern Med. 2021;36(9):2909–2911. doi: 10.1007/s11606-021-06948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latin America’s embrace of an unproven COVID treatment is hindering drug trials Unchecked ivermectin use in the region is making it difficult to test the anti-parasite drug’s effectiveness against the coronavirus. Nature. 2020;586:481–482. doi: 10.1038/d41586-020-02958-2. [DOI] [PubMed] [Google Scholar]
- 8.Murchu E.O., Spillane S., Byrne P., O'Neill M., Harrington P., Ryan M. Interventions in an ambulatory setting to prevent progression to severe disease in patients with covid-19: a systematic review. Ann Pharm. 2022;56(3):309–318. doi: 10.1177/10600280211028242. [DOI] [PubMed] [Google Scholar]
- 9.Shafiee A., Teymouri Athar M.M., Kohandel Gargari O., Jafarabady K., Siahvoshi S., Mozhgani S.H. Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients. Virol J. 2022;19(1):102. doi: 10.1186/s12985-022-01829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roman Y.M., Burela P.A., Pasupuleti V., Piscoya A., Vidal J.E., Hernandez A.V. Ivermectin for the treatment of coronavirus disease 2019: a systematic review and meta-analysis of randomized controlled trials. Clin Infect Dis. 2022;74(6):1022–1029. doi: 10.1093/cid/ciab591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popp M., Reis S., Schießer S., Hausinger R.I., Stegemann M., Metzendorf M.I., et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev. 2022;6(6) doi: 10.1002/14651858.CD015017.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcolino M.S., Meira K.C., Guimaraes N.S., Motta P.P., Chagas V.S., Kelles S.M.B., et al. Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype. BMC Infect Dis. 2022;22(1):639. doi: 10.1186/s12879-022-07589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S., Shen S., Hou N. Is ivermectin effective in treating COVID-19? Front Pharm. 2022;13 doi: 10.3389/fphar.2022.858693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bitterman A., Martins C.P., Cices A., Nadendla M.P. Comparison of trials using ivermectin for COVID-19 between regions with high and low prevalence of strongyloidiasis: a meta-analysis. JAMA Netw Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reis G., Silva E., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., et al. Effect of early treatment with ivermectin among patients with COVID-19. N Engl J Med. 2022;386(18):1721–1731. doi: 10.1056/NEJMoa2115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naggie S., Boulware D.R., Lindsell C.J., Stewart T.G., Gentile N., Collins S., et al. Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;328(16):1595–1603. doi: 10.1001/jama.2022.18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bramante C.T., Huling J.D., Tignanelli C.J., Buse J.B., Liebovitz D.M., Nicklas J.M., et al. Randomized trial of metformin, ivermectin, and fluvoxamine for COVID-19. N Engl J Med. 2022;387(7):599–610. doi: 10.1056/NEJMoa2201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naggie S., Boulware D.R., Lindsell C.J., Stewart T.G., Slandzicki A.J., Lim S.C., et al. Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial. JAMA. 2023;329(11):888–897. doi: 10.1001/jama.2023.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bramante C.T., Buse J.B., Liebovitz D.M., Nicklas J.M., Puskarich M.A., Cohen K., et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. 2023;23(10):1119–1129. doi: 10.1016/S1473-3099(23)00299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodcock J., LaVange L.M. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N Engl J Med. 2017;377(1):62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 21.Butler C.C., Dorward J., Yu L.-M., Gbinigie O., Hayward G., Saville B.R., et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397(10279):1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler C.C., Yu L.M., Dorward J., Gbinigie O., Hayward G., Saville B.R., et al. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir Med. 2021;9(9):1010–1020. doi: 10.1016/S2213-2600(21)00310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L.-M., Bafadhel M., Dorward J., Hayward G., Saville B.R., Gbinigie O., et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398(10303):843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorward J., Yu L.M., Hayward G., Saville B.R., Gbinigie O., Van Hecke O., et al. Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial. Br J Gen Pr. 2022;72(720):446–455. doi: 10.3399/BJGP.2022.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand R., Norrie J., Bradley J.M., McAuley D.F., Clarke M. Fool's gold? Why blinded trials are not always best. BMJ. 2020;368:l6228. doi: 10.1136/bmj.l6228. [DOI] [PubMed] [Google Scholar]
- 26.Moustgaard H., Clayton G.L., Jones H.E., Boutron I., Jorgensen L., Laursen D.R.T., et al. Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study. BMJ. 2020;368:l6802. doi: 10.1136/bmj.l6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel M.G., Dorward J., Yu L.-M., Hobbs F.D.R., Butler C.C. Inclusion and diversity in the PRINCIPLE trial. Lancet. 2021;397(10291):2251–2252. doi: 10.1016/S0140-6736(21)00945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence (NICE) COVID-19 Rapid Guideline: Managing Symptoms (including at the end of life) in the Community. National Institute for Health and Care Excellence; London: 2020. [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence. COVID-19 rapid guideline: Managing COVID-19. 2022. Accessed August 16, 2022. https://app.magicapp.org/#/guideline/L4Qb5n/section/nBMk69. [PubMed]
- 30.NHS England 2023. Accessed March 2nd 2024 https://www.england.nhs.uk/statistics/statistical-work-areas/covid-therapeutics-antivirals-and-neutralising-monoclonal-antibodies/.
- 31.The United Kingdom Government. Coronavirus (COVID-19) in the UK 2020 (updated February 12, 2021) https://coronavirus.data.gov.uk/.
- 32.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skipper C.P., Pastick K.A., Engen N.W., Bangdiwala A.S., Abassi M., Lofgren S.M., et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173(8):623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topp C.W., Østergaard S.D., Søndergaard S., Bech P. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom. 2015;84(3):167–176. doi: 10.1159/000376585. [DOI] [PubMed] [Google Scholar]
- 36.Saville B.R., Berry D.A., Berry N.S., Viele K., Berry S.M. The Bayesian Time Machine: accounting for temporal drift in multi-arm platform trials. Clin Trials. 2022;19(5):490–501. doi: 10.1177/17407745221112013. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen Q., Hees K., Hofner B. Adaptive platform trials: the impact of common controls on type one error and power. J Biopharm Stat. 2023:1–18. doi: 10.1080/10543406.2023.2275765. [DOI] [PubMed] [Google Scholar]
- 38.Recovery Collaborative Group, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.REMAP-CAP Investigators, Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacPherson H. Pragmatic clinical trials. Complement Ther Med. 2004;12(2-3):136–140. doi: 10.1016/j.ctim.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 41.Leber W., Lammel O., Siebenhofer A., Redlberger-Fritz M., Panovska-Griffiths J., Czypionka T. Comparing the diagnostic accuracy of point-of-care lateral flow antigen testing for SARS-CoV-2 with RT-PCR in primary care (REAP-2) EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material