Abstract
Background
Asthma is a respiratory (airway) condition that affects an estimated 300 million people worldwide and is associated with significant morbidity and mortality. Omalizumab is a monoclonal antibody that binds and inhibits free serum immunoglobulin E (IgE). It is called an 'anti‐IgE' drug. IgE is an immune mediator involved in clinical manifestations of asthma. A recent update of National Institute for Health and Care Excellence (NICE) guidance in 2013 recommends omalizumab for use as add‐on therapy in adults and children over six years of age with inadequately controlled severe persistent allergic IgE‐mediated asthma who require continuous or frequent treatment with oral corticosteroids.
Objectives
To assess the effects of omalizumab versus placebo or conventional therapy for asthma in adults and children.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials for potentially relevant studies. The most recent search was performed in June 2013. We also checked the reference lists of included trials and searched online trial registries and drug company websites.
Selection criteria
Randomised controlled trials examining anti‐IgE administered in any manner for any duration. Trials with co‐interventions were included, as long as they were the same in each arm.
Data collection and analysis
Two review authors independently assessed study quality and extracted and entered data. Three modes of administration were identified from the published literature: inhaled, intravenous and subcutaneous injection. The main focus of the updated review is subcutaneous administration, as this route is currently used in clinical practice. Subgroup analysis was performed by asthma severity. Data were extracted from published and unpublished sources.
Main results
In all, 25 trials were included in the review, including 11 new studies since the last update, for a total of 19 that considered the efficacy of subcutaneous anti‐IgE treatment as an adjunct to treatment with corticosteroids.
For participants with moderate or severe asthma who were receiving background inhaled corticosteroid steroid (ICS) therapy, a significant advantage favoured subcutaneous omalizumab with regard to experiencing an asthma exacerbation (odds ratio (OR) 0.55, 95% confidence interval (CI) 0.42 to 0.60; ten studies, 3261 participants). This represents an absolute reduction from 26% for participants suffering an exacerbation on placebo to 16% on omalizumab, over 16 to 60 weeks. A significant benefit was noted for subcutaneous omalizumab versus placebo with regard to reducing hospitalisations (OR 0.16, 95% CI 0.06 to 0.42; four studies, 1824 participants), representing an absolute reduction in risk from 3% with placebo to 0.5% with omalizumab over 28 to 60 weeks. No separate data on hospitalisations were available for the severe asthma subgroup, and all of these data were reported for participants with the diagnosis of moderate to severe asthma. Participants treated with subcutaneous omalizumab were also significantly more likely to be able to withdraw their ICS completely than those treated with placebo (OR 2.50, 95% CI 2.00 to 3.13), and a small but statistically significant reduction in daily inhaled steroid dose was reported for omalizumab‐treated participants compared with those given placebo (weighted mean difference (WMD) ‐118 mcg beclomethasone dipropionate (BDP) equivalent per day, 95% CI ‐154 to ‐84). However, no significant difference between omalizumab and placebo treatment groups was seen in the number of participants who were able to withdraw from oral corticosteroid (OCS) therapy (OR 1.18, 95% CI 0.53 to 2.63).
Participants treated with subcutaneous omalizumab as an adjunct to treatment with corticosteroids required a small but significant reduction in rescue beta2‐agonist medication compared with placebo (mean difference (MD) ‐0.39 puffs per day, 95% CI ‐0.55 to ‐0.24; nine studies, 3524 participants). This benefit was observed in both the moderate to severe (MD ‐0.58, 95% CI ‐0.84 to ‐0.31) and severe (MD ‐0.30, 95% CI ‐0.49 to ‐0.10) asthma subgroups on a background therapy of inhaled corticosteroids; however, no significant difference between subcutaneous omalizumab and placebo was noted for this outcome in participants with severe asthma who were receiving a background therapy of inhaled plus oral corticosteroids. Significantly fewer serious adverse events were reported in participants assigned to subcutaneous omalizumab than in those receiving placebo (OR 0.72, 95% CI 0.57 to 0.91; 15 studies, 5713 participants), but more injection site reactions were observed (from 5.6% with placebo to 9.1% with omalizumab).
To reflect current clinical practice, discussion of the results is limited to subcutaneous use, and trials involving intravenous and inhaled routes have been archived.
Authors' conclusions
Omalizumab was effective in reducing asthma exacerbations and hospitalisations as an adjunctive therapy to inhaled steroids and during steroid tapering phases of clinical trials. Omalizumab was significantly more effective than placebo in increasing the numbers of participants who were able to reduce or withdraw their inhaled steroids. Omalizumab was generally well tolerated, although more injection site reactions were seen with omalizumab. Further assessment in paediatric populations is necessary, as is direct double‐dummy comparison with ICS. Although subgroup analyses suggest that participants receiving prednisolone had better asthma control when they received omalizumab, it remains to be tested prospectively whether the addition of omalizumab has a prednisolone‐sparing effect. It is also not clear whether there is a threshold level of baseline serum IgE for optimum efficacy of omalizumab. Given the high cost of the drug, identification of biomarkers predictive of response is of major importance for future research.
Keywords: Adult; Child; Humans; Adrenal Cortex Hormones; Adrenal Cortex Hormones/therapeutic use; Anti‐Asthmatic Agents; Anti‐Asthmatic Agents/administration & dosage; Anti‐Asthmatic Agents/therapeutic use; Antibodies, Anti‐Idiotypic; Antibodies, Anti‐Idiotypic/administration & dosage; Antibodies, Anti‐Idiotypic/therapeutic use; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/administration & dosage; Antibodies, Monoclonal, Humanized/therapeutic use; Asthma; Asthma/drug therapy; Asthma/immunology; Chronic Disease; Immunoglobulin E; Immunoglobulin E/blood; Immunoglobulin E/immunology; Injections, Subcutaneous; Omalizumab; Randomized Controlled Trials as Topic
Plain language summary
Omalizumab for chronic asthma in adults and children
Review question
We reviewed the evidence for the effect of omalizumab on people with asthma when compared with placebo. We focused on whether omalizumab is a beneficial but safe treatment for adults and children with asthma.
Background
Asthma is a respiratory condition that affects millions of people worldwide. It is thought that allergy may be an important part of the disease for many people with asthma. Omalizumab is a drug that targets a protein, called IgE, and removes it from free circulation in the body. IgE is centrally involved in allergy. Omalizumab is an expensive drug that is usually given by injection under the skin every two to four weeks. It is licenced for use in asthma sufferers who are not being adequately treated with standard therapy and who require frequent courses or continuous use of oral steroid tablets. We looked for evidence on whether administration of omalizumab is better or worse than giving placebo.
Study characteristics
Twenty‐five studies, involving 6382 people, were included in this review. These studies lasted between eight and 60 weeks. All of the people included in the studies had asthma, of different severity. Both men and women were included, and some of the studies included children and young people.
All studies compared omalizumab versus placebo. In keeping with current medical practice, most studies (21 of 25) used omalizumab given by injection under the skin. Some of the older studies used omalizumab injected into a vein or given by inhalation. The evidence presented here is current to June 2013. Most of the studies were sponsored by the pharmaceutical industry.
Key results
We found that people receiving omalizumab were less likely to have a flare‐up (‘exacerbation’) of their asthma. For example, on average, 26 of 100 people who were receiving placebo (over a 16 to 60‐week period) had an exacerbation compared with an average of 16 of 100 people receiving omalizumab.
People receiving omalizumab were also more likely to be able to reduce the doses of inhaled steroids. For example, on average, 21 of 100 people with moderate or severe asthma who were receiving placebo were able to completely stop their inhaled steroids (over a 28 to 32‐week period) compared with an average of 40 of 100 receiving omalizumab.
People receiving omalizumab also experienced improvement in their asthma symptoms and in their health‐related quality of life.
People receiving omalizumab were no more or less likely to have unwanted side effects overall. However, people receiving omalizumab were more likely to have skin reactions at the site of the injection.
Perhaps unfortunately, many of the trials in this review included participants with moderate asthma, and this drug is not licenced for this group. More trials need to focus on whether this drug is effective in people with the most severe asthma; evidence for efficacy in this group is poor, in spite of current guidelines.
Quality of the evidence
The evidence presented in this review is generally of moderate quality. Most of the studies did not clearly explain how investigators decided which people would receive omalizumab and which would receive placebo, and this decision is an important part of well‐conducted studies.
Summary of findings
Summary of findings for the main comparison. Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid) for asthma in adults and children.
| Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid) for asthma in adults and children | ||||||
| Patient or population: adults and children with asthma Settings: Intervention: subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Subcutaneous omalizumab+ steroid versus placebo + steroid (stable steroid) | |||||
|
Number of participants with at least one exacerbation All asthmatic participants (16 to 60 weeks) |
262 per 1000 | 163 per 1000 (130 to 176) | OR 0.55 (0.46 to 0.65) | 3261 (10 studies) |
⊕⊕⊕⊝ moderate1 | |
|
Number of participants with at least one exacerbation Moderate to severe asthma (16 to 60 weeks) |
274 per 1000 | 159 per 1000 (137 to 185) | OR 0.5 (0.42 to 0.6) | 2889 (7 studies) | ⊕⊕⊕⊝ moderate1 | |
|
Number of participants with at least one exacerbation Severe asthma (16 to 32 weeks) |
145 per 1000 | 145 per 1000 (78 to 252) | OR 1 (0.5 to 1.99) | 277 (2 studies) | ⊕⊕⊝⊝ low2 | |
|
Mortality 16 to 60 weeks |
2 per 1000 | 0 per 1000 (0 to 3) | OR 0.19 (0.02 to 1.67) | 4245 (9 studies) | ⊕⊕⊝⊝ low3,4 | |
|
Hospitalisations 28 to 60 weeks |
31 per 1000 | 5 per 1000 (2 to 13) | OR 0.16 (0.06 to 0.42) | 1824 (4 studies) | ⊕⊕⊕⊝ moderate5 | |
|
Adverse event—serious 16 to 60 weeks |
64 per 1000 | 47 per 1000 (37 to 58) | OR 0.72 (0.57 to 0.91) | 5713 (15 studies) | ⊕⊕⊕⊝ moderate6 | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1A point was deducted for risk of bias to reflect the fact that most studies scored UNCLEAR on both sequence generation and allocation concealment.
2A point was deducted for risk of bias to reflect the fact that only one of the two trials scored LOW on both sequence generation and allocation concealment. The remaining trial scored UNCLEAR on both sequence generation and allocation concealment. An additional point was deducted because of the imprecision of the results.
3A point was deducted for risk of bias to reflect the fact that only two of the nine trials scored LOW on both sequence generation and allocation concealment. Most (five) scored UNCLEAR on both sequence generation and allocation concealment.
4An additional point was deducted to reflect that a death occurred in only two of the nine trials; therefore, the contribution of most of the trials (seven) was non‐estimable. 5A point was deducted for risk of bias to reflect the fact that only one of the four trials scored LOW on both sequence generation and allocation concealment.
6A point was deducted for risk of bias to reflect the fact that only two of the 15 trials scored LOW on both sequence generation and allocation concealment. Most (10) scored UNCLEAR on both sequence generation and allocation concealment.
Summary of findings 2. Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction) for asthma in adults and children.
| Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction) for asthma in adults and children | ||||||
| Patient or population: adults and children with asthma Settings: Intervention: subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Subcutaneous omalizumab+ steroid versus placebo + steroid (steroid reduction) | |||||
|
Number of participants achieving complete inhaled steroid withdrawal 28 to 32 weeks |
212 per 1000 | 402 per 1000 (350 to 457) | OR 2.5 (2 to 3.13) | 1634 (4 studies) | ⊕⊕⊝⊝ low1 | |
|
>50% reduction in inhaled steroid usage 28 to 32 weeks |
560 per 1000 | 761 per 1000 (720 to 798) | OR 2.5 (2.02 to 3.1) | 1634 (4 studies) | ⊕⊕⊕⊝ moderate2 | |
|
Exacerbations requiring hospitalisation 28 weeks |
20 per 1000 | 3 per 1000 (1 to 11) | OR 0.11 (0.03 to 0.48) | 1405 (3 studies) | ⊕⊕⊕⊝ moderate3 | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Point deducted, as only two of the four included studies scored LOW on both sequence generation and allocation concealment in the risk of bas assessment. An additional point was deducted to reflect the level of heterogeneity (I2 = 35%).
2Point deducted as only two of the four included studies scored LOW on both sequence generation and allocation concealment in the risk of bas assessment. 3Point deducted as only one of the three included studies scored LOW on both sequence generation and allocation concealment in the risk of bas assessment.
Background
Description of the condition
Asthma is an airway disease that currently affects an estimated 300 million people worldwide and is associated with significant mortality and morbidity. It is a heterogenous disease characterised by recurrent dyspnoea, wheezing, cough and chest tightness and usually is associated with reversible airflow obstruction and airway hyperresponsiveness (Pelaia 2011). Active asthma accelerates the development of fixed airflow obstruction (Perret 2013). The mainstay of modern treatment has been the use of inhaled steroids and bronchodilator drugs. Although this approach has been useful in the management of mild and moderate forms of the disease, patients with severe asthma sometimes require oral steroids and other immunosuppressive regimens with their attendant side effects (Thomson 2012). In addition, people with poorly controlled asthma, even in spite of treatment, are at increased risk of hospitalisation and emergency room visits (Chipps 2012). Although this group accounts for only around 5% of people with asthma, it contributes to approximately 80% of the economic costs of asthma; therefore novel therapies have been developed for optimal treatment of these patients. It is estimated that more than 50% of people with poorly controlled asthma have allergic immunoglobulin E (IgE)‐mediated asthma and therefore may benefit from treatments targeted at IgE.
It is becoming increasingly apparent that ‘asthma’ is not a single condition but rather a collection of symptoms caused by different mechanisms (Haldar 2008). This heterogeneity in asthma expression appears to be multi‐dimensional, including variability in clinical, physiological, age of onset and pathological parameters. Confirmation of evidence of distinct and different combinations of symptom expression and underlying inflammation may require a different approach to therapeutic intervention that may clarify the role of omalizumab in clinical guidelines in the future.
Description of the intervention
Immunoglobulin E (IgE) plays a central role in the development of allergic diseases, including allergic asthma (Thomson 2012). In atopic (allergic) individuals, initial exposure/sensitisation to an allergen initiates a complex series of events, leading to the production of allergen‐specific IgE. The IgE becomes attached to inflammatory cells such as mast cells (in particular), basophils and macrophages via its Fc portion linking with Fc receptors. Further allergen exposure leads to cross‐bridging between allergen and IgE on the surface of these effector cells (Spector 1999; Wills‐Karp 1999). This results in degranulation of mast cells and basophils, leading to the release of proinflammatory mediators such as histamine, prostaglandins, leukotrienes, chemokines and cytokines. In some people with allergic asthma, higher than normal IgE levels may increase persistent airway inflammation and bronchial hyperresponsiveness (Burrows 1989; Sears 1991), presumably through ongoing chronic allergic activation of this complex system. Indeed, the level of circulating IgE to common allergens is a risk factor for emergency admissions with asthma (Thomson 2012). It is these people with high levels of IgE who have been featured in omalizumab studies conducted to date.
Omalizumab has been licenced for use since 2003 in the United States for people with moderate to severe asthma over the age of 12 years whose condition is inadequately controlled by inhaled corticosteroids. The European Medicines Agency followed suit in 2005 and more recently has approved the use of omalizumab in children six years of age and older (Pelaia 2011). In 2006, omalizumab was included as an add‐on treatment in Step 5 and above of the Global Initiative for Asthma (GiNA) guidelines (GiNA 2011). In April 2013, updated National Institute for Health and Care Excellence (NICE) guidance suggested that omalizumab can be used in adults and children over six years of age with inadequately controlled severe persistent allergic IgE‐mediated asthma who require continuous or frequent treatment with oral corticosteroids (usually accompanied by high‐dose inhaled corticosteroid) (NICE 2013). Omalizumab usually is considered only for those with allergic asthma who are sensitised to at least one aeroallergen and have circulating IgE levels within the specified range for determination of dosing.
Omalizumab is recommended to be administered as a subcutaneous injection. The dose and frequency of dosing are guided by a nomogram that is derived from the total serum IgE level and the body mass index. This is based on evidence that total serum IgE is a good predictor of clinical symptoms of asthma and correlates fairly well with the total number of immune cells in the body (tissue and circulation) that have functional cross‐linking Fc‐epsilon receptors. However, it is not well established whether at least some individuals with low or normal total serum IgE may also respond to treatment with omalizumab. In other words, it is not certain whether the level or the activity of IgE is the better determinant of the clinical efficacy of omalizumab in an individual.
How the intervention might work
Omalizumab (also referred to in the literature as rhuMAb‐E25, rhu‐Mab or Xolair) is a recombinant humanised IgG1 monoclonal antibody that recognises IgE at the same Fc site as the high‐affinity receptor binding site. This anti‐IgE antibody forms complexes with free IgE, thus blocking the interaction between IgE and effector cells. Omalizumab treatment also appears to down‐regulate the expression of high‐affinity IgE receptors on effector cells (Thomson 2012). The complexes of omalizumab and IgE formed as a result of treatment are small and are not thought to be able to trigger complement activation or to give rise to immune complex–mediated pathology. Omalizumab has been shown to reduce serum concentrations of free IgE after a single injection, resulting in significant reductions in early and late asthmatic responses following allergen inhalation and improved asthma symptom control (Milgrom 1997). Recent studies also suggest that treatment with omalizumab may reduce eosinophilic airway inflammation and IgE‐bearing cells, although effects on airway hyperresponsiveness and airway wall structural remodelling are less clear (Thomson 2012).
Why it is important to do this review
Omalizumab is a recent addition to the range of treatments available for asthma, but it is much more expensive than alternative asthma treatments. In the UK, national guidance (NICE 2013) states: "Omalizumab is recommended as an option for treating severe persistent confirmed allergic IgE‐mediated asthma as an add‐on to optimised standard therapy in people aged 6 years and older: who need continuous or frequent treatment with oral corticosteroids (defined as 4 or more courses in the previous year), and only if the manufacturer makes omalizumab available with the discount agreed in the patient access scheme." Little evidence has been found for this recommendation. Indeed, other international guidelines are less proscriptive and recommend this treatment for patients who remain suboptimally controlled after maximal therapy with a combination of inhaled corticosteroids and long‐acting bronchodilators and other add‐on therapies such as leukotriene antagonists, theophyllines or muscarinic antagonists.
To date, evidence is somewhat lacking about the efficacy of this drug in the more severe asthma population, as many trials include participants with mild or moderate disease. This review seeks to address whether omalizumab is safe and effective but with a particular emphasis on patients with more severe asthma—the group for whom the drug is licensed.
Objectives
To assess the effects of omalizumab versus placebo or conventional therapy for asthma in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
Only double‐blind randomised controlled trials (RCTs) were considered for inclusion. In view of the uncertain washout period for this form of treatment and our interest in inhaled steroid withdrawal and exacerbations, we elected to exclude cross‐over studies.
Types of participants
Adults and children with chronic asthma from all referral sources. We included studies in which populations were receiving maintenance therapy and those in which anti‐IgE was administered without background therapy. These study populations were analysed separately.
The definitions of chronic asthma varied; both doctor‐diagnosed cases and those identified with more objective criteria were considered. Distinctions were made among studies that differed in their definition, and when possible, subgroup analyses were performed on the basis of severity. We classified the studies according to the stepwise management plans recommended in CTS 2012, GiNA 2011 and BTS/SIGN 2012 guidelines.
Types of interventions
Anti‐IgE therapy at any dose or route versus placebo.
Types of outcome measures
Primary outcomes
Asthma exacerbations as defined by "events", i.e. hospital admissions, emergency room visits, days lost from work/school, unscheduled doctor visits, increase in medication.
Reduction or termination of steroid (inhaled, oral, both) use from baseline or run‐in period.
The order of the primary outcomes changed from protocol.
Secondary outcomes
Asthma symptoms.
Health‐related quality of life.
Rescue medication use.
Measures of lung function: forced expiratory volume in one second (FEV1), peak expiratory flow (PEF).
Adverse events.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group's Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and through handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy described in Appendix 2.
We searched the Register from its inception to June 2013 with no restriction on language of publication.
Searching other resources
To identify relevant randomised controlled trials (RCTs), we:
checked the reference lists of all identified RCTs to identify potentially relevant studies;
contacted all pharmaceutical companies producing anti‐IgE formulations and made enquiries about published and unpublished studies known to and/or supported by these companies;
examined the bibliographies of review articles and other selected articles;
sought data from online resources (e.g. www.fda.gov; www.clinicalstudyresults.org; http://www.novctrd.com; www.clinicaltrials.gov);
made personal contact with colleagues, collaborators and other trialists working in the field of asthma to identify other published and unpublished relevant studies; and
searched abstracts of studies presented at leading respiratory society meetings over the past three years to look for relevant studies.
Data collection and analysis
Selection of studies
Two review authors (SJM and PN) independently assessed abstracts and titles of references from search results. A list of potentially eligible references was agreed between the same two review authors, and these articles were retrieved. References were organised by study and were included or excluded on the basis of required prespecified characteristics.
Data extraction and management
Two independent review authors (SJM and either SW or RN) extracted data using a standard form developed before data were extracted. We sought missing information from study authors whenever possible.
Assessment of risk of bias in included studies
For the 2009 and 2013 updates of the review, we adopted the recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions for assessing the risk of bias in eligible studies (Cochrane Handbook). We judged the risk of bias (low, high or unclear) for each of the following potential sources of bias within each included study.
Allocation sequence generation.
Allocation concealment.
Blinding (all outcomes).
Handling of missing data (such as intention‐to‐treat analysis).
Selective reporting bias.
Other bias.
Previous methods are detailed in Differences between protocol and review.
Measures of treatment effect
For dichotomous variables, we calculated a fixed‐effect odds ratio (OR) with 95% confidence interval (CI) for individual studies. We pooled dichotomous data from similar studies using fixed‐effect ORs and 95% CIs. If significant heterogeneity (P < 0.1) was observed in continuous or dichotomous outcomes, we used random‐effects modelling. For statistically significant ORs, we pooled control group event rates to generate a baseline risk (%). Control event rates and corresponding expected rates with omalizumab are shown in Table 1 and Table 2 and are illustrated as Cates plots in Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 (generated by Visual Rx; www.nntonline.net).
1.
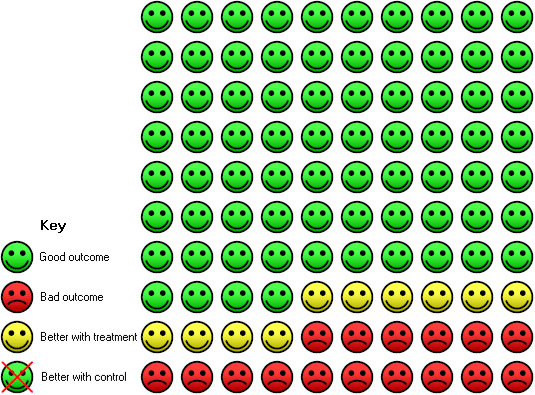
In the control group, 26 of 100 people with moderate to severe asthma had an asthma exacerbation over a 16‐ to 60‐week period, compared with 16 (95% CI 13 to 18) of 100 for the omalizumab group.
2.
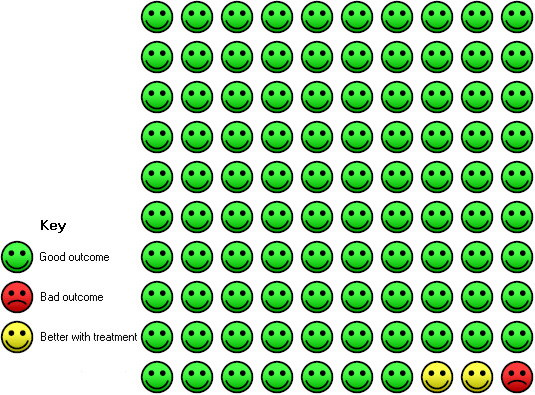
In the control group, three of 100 people with moderate to severe asthma had at least one hospitalisation over a 28‐ to 60‐week period, compared with one (95% CI 0 to 1) of 100 for the omalizumab group.
3.
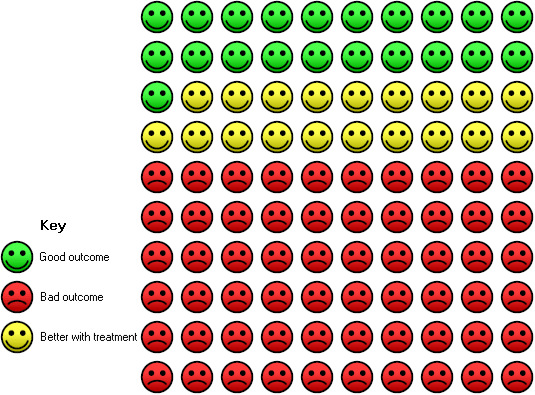
In the control group, 21 of 100 people with moderate or severe asthma were able to withdraw from treatment with inhaled corticosteroids completely (over a 28‐ to 32‐week period) compared with 40 (95% CI 35 to 46) of 100 for the omalizumab group with tapering corticosteroids.
4.
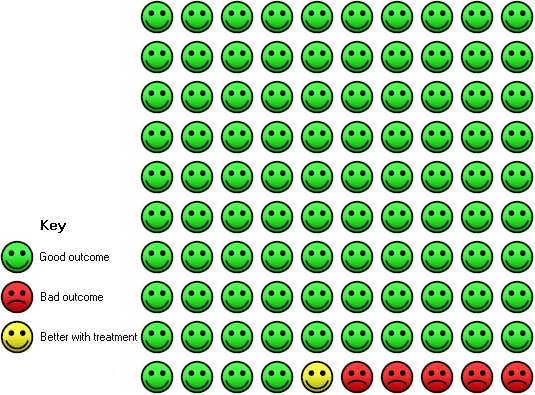
In the control group, six of 100 people with moderate to severe, or severe, asthma had at least one serious adverse event over a 16‐ to 60‐week period compared with five (95% CI 4 to 6) of 100 for the omalizumab group.
5.
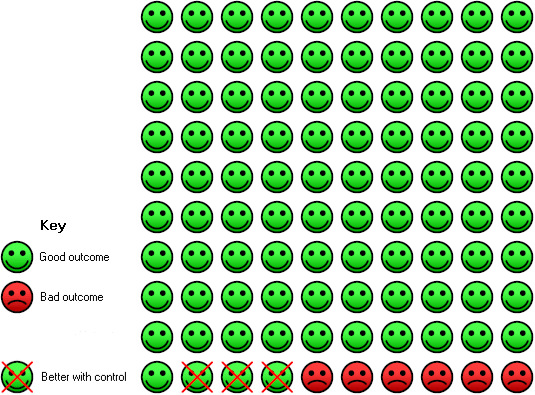
In the control group, six of 100 people with moderate to severe, or severe, asthma had an injection site reaction over a 16‐ to 60‐week period compared with nine (95% CI 7 to 12) of 100 for the omalizumab group.
We identified dichotomous and continuous outcome measures in the trial reports. If primary outcomes were reported as dichotomous variables, we sought from trialists continuous data as means and standard deviations (SDs) or as medians and ranges, and analysed these as appropriate. For the 2013 update of the review, we included analysis of exacerbation rates per participant and analysed these as rate ratios.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
If outcome data or information on trial design was missing, we attempted to contact authors for clarification.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of forest plots. I2 was considered and interpreted in relation to the following guidance.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: may represent considerable heterogeneity (Higgins 2011).
The Chi2 test was similarly considered (P value < 0.10). We regarded I2 as our primary measure of heterogeneity.
Assessment of reporting biases
We planned to perform funnel plots for our primary outcomes when the number of studies contributing data was greater than 10.
Data synthesis
We used RevMan 5.2 (RevMan 2012) to analyse data. For continuous variables, we calculated a fixed‐effect mean difference (MD) (for variables reported or transformed to the same scale) or standardised mean difference (SMD) (when different scales were pooled) with 95% CIs for each study. We pooled continuous data from similar studies using fixed‐effect MDs and 95% CIs.
Subgroup analysis and investigation of heterogeneity
We explored reasons for statistical heterogeneity. When I2 exceeded 50%, we undertook random‐effects modelling to assess whether adjustment for within‐ and between‐study variations impacted the summary estimate. A priori subgroup analyses consisted of:
age (children or adults);
trial medication;
asthma severity;
asthma diagnostic entry criteria; and
duration of treatment.
Sensitivity analysis
We planned to conduct sensitivity analyses, if necessary, based on methodological quality and fixed‐effect versus random‐effects modelling.
Results
Description of studies
Results of the search
A total of 147 references were identified through electronic searches conducted in June 2013, producing 11 new studies eligible for inclusion. In addition to studies identified in previous versions of the review, this brought the total number of included studies up to 25 randomised, placebo‐controlled clinical trials involving 6382 people with asthma (see Figure 6 for study flow diagram). Twenty‐one studies involved omalizumab given by the subcutaneous route. For details of previous search results, please see Table 3.
6.
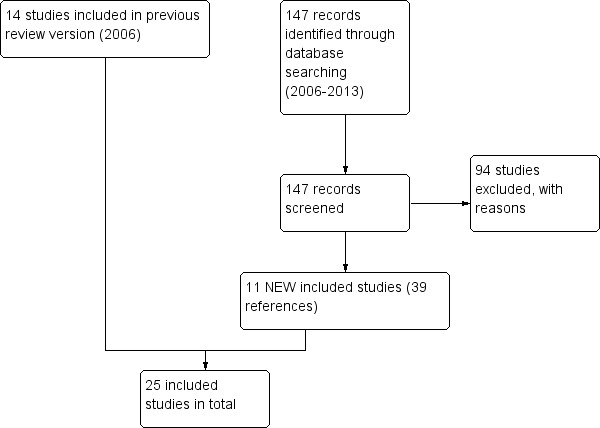
Study flow diagram.
1. Search history.
| Date | Search results |
| Initial version of the review (all years to January 2003) | From a total of 169 references identified by electronic searches and handsearching, we retrieved 20 papers and eight studies that met the inclusion criteria of the review. One study of subcutaneous anti‐IgE recruited adults with severe asthma (Holgate 2004a). Four studies examined intravenous or subcutaneous anti‐IgE in adults (Busse 2001; Milgrom 1999; Solèr 2001) and children (Milgrom 2001) with moderate to severe asthma, and three studies examined aerosolised or intravenous anti‐IgE in adults with mild asthma (Boulet 1997; Fahy 1997; Fahy 1999) |
| Update search results (January 2003 to 2004) | Publication of a critical review of the efficacy of omalizumab from published and unpublished clinical trials has prompted this update. For details of this publication, please see http://www.fda.gov. Two unpublished studies were identified from this report (ETOPA; Q2143G). One further unpublished study in people with co‐existing asthma and rhinitis is awaiting assessment (SOLAR) |
| Update search results (January 2004 to February 2006) | Electronic searches yielded a total of 48 new references from the Airways Group register. After exclusion of duplicates, a total of eight studies were retrieved for further scrutiny. Of these, five met the inclusion criteria (Prieto 2006; Djukanovic 2004; INNOVATE; van Rensen 2009; SOLAR). Details of studies that failed to meet the inclusion criteria are found in Characteristics of excluded studies |
| Update search results (June 2013) |
147 new references were identified by the Aiways Group register, producing an additional 11 studies that met the inclusion criteria: Bardelas 2012; Busse 2011; Chanez 2010; Garcia 2012; Gevaert 2012; Hanania 2011; Lanier 2009; Massanari 2010; NCT00096954; NCT01007149; Ohta 2009. Studies that did not meet the inclusion criteria are listed in Characteristics of excluded studies |
Included studies
Study design and duration
All trials were randomised and double‐blind and of parallel‐group design. Twenty‐two were reported in full; details of two of the remaining trials were available as clinical trial reports on the clinicaltrials.gov system (NCT00096954; NCT01007149), and one was reported as a conference abstract (Garcia 2012). Study duration ranged from eight to 60 weeks. The trials fall broadly into three categories: those in which the background medication (referred to as 'steroid stable' if the background medication included a steroid) was unchanged, those with a steroid stable period followed by an attempt to reduce the background steroid dose ('steroid reduction') and those that sought to demonstrate a reduction in airway responsiveness to allergens after treatment with omalizumab.
Nineteen studies examined the efficacy of subcutaneous anti‐IgE treatment as an adjunct to treatment with corticosteroids (Bardelas 2012; Busse 2001; Busse 2011; Chanez 2010; Garcia 2012; Gevaert 2012; Hanania 2011; Holgate 2004a; Holgate 2004b; INNOVATE; Lanier 2009; Massanari 2010; Milgrom 1999; Milgrom 2001; NCT00096954; NCT01007149; Ohta 2009; SOLAR; Solèr 2001). Beclomethasone dipropionate (BDP) was used as the background inhaled steroid in Busse 2001, Milgrom 2001 and Solèr 2001; two studies used high‐dose fluticasone propionate (FP) (Holgate 2004a; Holgate 2004b), and another used budesonide (BUD) (SOLAR). In Bardelas 2012; Busse 2011; Chanez 2010; Hanania 2011; INNOVATE; Lanier 2009; Massanari 2010; Milgrom 1999; NCT01007149 and Ohta 2009, participants remained on their current maintenance inhaled steroid. One study recruited a subgroup of oral steroid–dependent asthmatic participants (Holgate 2004b) for which data were published separately. Details of the background inhaled steroid used in Garcia 2012; Gevaert 2012 and NCT00096954 were not included in the trial report.
In ten studies, no changes were made to background inhaled corticosteroid (ICS) dosage (Bardelas 2012; Busse 2011; Chanez 2010; Hanania 2011; INNOVATE; Massanari 2010NCT00096954; NCT01007149; Ohta 2009; SOLAR).
In five studies, participants received a stable dose of oral or inhaled corticosteroids for between 12 and 28 weeks; this was followed by an attempt to reduce the corticosteroid dose (Busse 2001; Holgate 2004a; Lanier 2009; Milgrom 2001; Solèr 2001). In earlier updates, in which data on ICS usage were reported, values were transformed to BDP equivalent values. Data on oral corticosteroid (OCS) usage were sought as mean dose or as dichotomised data related to the number of participants who had succeeded in reducing OCS use. Holgate 2004b reported data on OCS tapering only.
Boulet 1997; Djukanovic 2004; Fahy 1997; Fahy 1999; Prieto 2006 and van Rensen 2009 assessed the effects of omalizumab in the absence of any need for background steroid therapy.
Individual steroid doses are detailed in the table Characteristics of included studies.
Most of the included studies were sponsored by the pharmaceutical industry.
Route of administration
Three routes of drug administration were identified: inhaled, one trial (Fahy 1999); intravenous, three trials (Boulet 1997; Fahy 1997; Milgrom 1999); and subcutaneous injection, 21 trials (Bardelas 2012; Busse 2001; Busse 2011; Chanez 2010; Djukanovic 2004; Garcia 2012; Gevaert 2012; Hanania 2011; Holgate 2004a; Holgate 2004b; INNOVATE; Lanier 2009; Massanari 2010; Milgrom 2001; NCT00096954; NCT01007149; Ohta 2009; Prieto 2006; SOLAR; Solèr 2001; van Rensen 2009).
In all studies, anti‐IgE was compared with placebo, although doses of omalizumab differed. The study using intravenous omalizumab in moderate to severe asthma compared high (5.8 mcg/kg/ng IgE/mL) and low (2.5 mcg/kg/ng IgE/mL) doses versus placebo (Milgrom 1999); in the two studies that considered intravenous omalizumab in mild asthma, the comparison was 1.0 mg/kg versus placebo (Boulet 1997) and 0.5 mg/kg versus placebo (Fahy 1997). Inhaled omalizumab was given at doses of 1 mg or 10 mg, and subcutaneous omalizumab at doses of 0.016 mg/kg/IU/mL every two to four weeks (Fahy 1999).
To reflect current clinical practice, discussion of the results is limited to subcutaneous use; trials involving intravenous and inhaled routes have been archived and can be found in Appendix 3.
Asthma severity and type
Participants with a diagnosis of allergic asthma were recruited in all trials, with the exception of Garcia 2012 (in which participants with severe non‐allergic asthma were studied).
Adult and adolescent populations were assessed in Bardelas 2012; Busse 2001; Busse 2011; Hanania 2011; Holgate 2004a; Holgate 2004b; INNOVATE; Milgrom 1999; NCT00096954; Solèr 2001 and SOLAR, whereas Lanier 2009 and Milgrom 2001 recruited paediatric participants. Only adult participants were involved in Chanez 2010; Gevaert 2012; Massanari 2010; NCT01007149 and Ohta 2009. In Prieto 2006 and van Rensen 2009, the age of participants was unclear. SOLAR recruited participants with co‐existing asthma and rhinitis. Adults with mild asthma were recruited to Boulet 1997; Djukanovic 2004; Fahy 1997 and Fahy 1999. Allergic and non‐allergic patients with nasal polyps and asthma participated in Gevaert 2012.
Asthma severity varied within and between studies. Subgroup analyses were performed according to asthma severity (severe, moderate/severe and mild asthma) as defined by the review authors (Table 4). Data on asthma severity according to author and review author classification are shown in Table 4. Primary indicators of asthma severity were FEV1 and baseline therapy. Our classification of severity is based on the stepwise guide to asthma management recommended in the BTS 2005 and BTS/SIGN 2012 guidelines. We examined baseline steroid requirements and FEV1 (percentage predicted) to determine whether participants were largely mildly (step one of BTS 2005 and BTS/SIGN 2012), moderately (step two), moderately/severely (mixed population samples, step two/three) or severely asthmatic (step four and above). Following analysis of one participant population (Busse 2001), the review authors reclassified severity as moderate to severe (step two/three of BTS/SIGN 2012).
2. Asthma severity.
| Study ID | FEV1 (incl criteria) | B/line FEV1 mean | Symptom freq | OCS rx | ICS rx | Author opinion | BTS step |
| Bardelas 2012 | ≤ 80% pred (or symptoms > 2 days/wk, ≥1 night‐time wakening/wk or > 2 SABA use/wk | 75.5% pred ± 17.25 | Asthma control test mean score = 13.8 | No | Yes—at least 250 mcg fluticasone bd or 320 mcg budesonide bd | Severe | Step 4 and above |
| Boulet 1997 | > 70% pred | 91.89 ± 11.03 (range 83 to 106) | No indication reported | No | No | Mild | Step 1 |
| Busse 2001 | ≥ 40% to ≤ 80% | 67.95 ± 14.59 | Puffs of medication per day 4.85 ± 2.6; asthma score: 4.27 ± 1.17 (scale 0 to 9, with 9 indicating most severe). Limited physical activity in 482/525 participants | No | Yes—mean BDP dose: 569 mcg/d (range 336 to 1008) | Severe | Step 2. Range in baseline FEV1 extends to above 80% predicted, and range of BDP extends below stated criteria |
| Busse 2011 | Not stated | 92.1% pred ± 17.1 | Astham control test mean score = 19 or less Asthma‐related symptoms—number of days in two weeks preceding visit = 3.1 ± 3.6 (placebo group); 3.0 ± 3.5 (treatment group) |
No | Yes—at least 180 μg budesonide once a day | Mild, moderate and severe | Steps 1 to 6 (26.5% steps 1 and 2, 54% steps 4 to 6) |
| Chanez 2010 | FEV1 < 80% pred | 63.2% pred ± 13.75 | Absenteeism from school or work in previous year (days): mean 33.8 ± 100.22 | Yes; seven (22%) participants receiving maintenance OCS | Yes—at least 1000 mcg beclometasone dipropionate or equivalent daily mean dose/d 3556 mcg ± 1157.8 BDP equivalent/d | Severe | Step 4 and above |
| Djukanovic 2004 | Not stated | 85% | Not stated | No | No | Mild to moderate | Step 1 |
| Fahy 1997 | ≥ 70% pred | 94.5 ± 10.72 | No indication reported | No | No | Mild | Step 1 |
| Fahy 1999 | ≥ 70% pred | 82.74 ± 16.09 | No indication reported | OCS rx excluded | No | Mild | Step 1 |
| Garcia 2012 | Not stated | Not stated | Not stated | Not stated | Not stated | Severe | Not specifically stated but likely step 4 and above |
| Gevaert 2012 | Not stated | FEV1 (% predicted), median (IQR) OMA 88.5 (71.0 to 114.8); placebo 99.5 (73.5 to 110.3) | Not stated | During the study, participants were not permitted to use systemic corticosteroids | During the study, participants were not permitted to use an inhaled corticosteroid (doses of greater than 1000 mg/d beclomethasone dipropionate or equivalent) | Total serum IgE levels between 30 and 700 kU/mL | Not stated |
| Hanania 2011 | FEV1 40% to 80% pred | 64.9% pred ± 14.6 | Mean total asthma symptom severity score = 3.9 ± 1.8 Mean AQLQ(S) score 4.0 ± 1.1 Mean puffs of rescue medication per day 4.0 ± 2.9 (treatment group) and 4.1 ± 3.2 (placebo group) |
Yes, 60 (7.1%) of participants using long‐term OCS at baseline | Yes, minimum dose of 500 mcg of fluticasone dry powder inhaler (or its equivalent) twice daily | Severe | Step 4 and above |
| Holgate 2004 (ICS) | Not stated | 64.41% (no range given) | Not stated | No | Optimal control on 1000 to 2000 mcg/d FP ± OCS, and long‐acting β‐agonist. Mean FP dose: 1368.9 | Severe | Step 4 |
| Holgate (ICS & OCS) | Not stated | 59% | Not stated | Yes | Optimal control on 1000 to 2000 mcg/d; mean prednisolone dose: 10.2 mcg/d | Severe | Step 5 |
| INNOVATE | Not stated | 61% | Not stated | Yes—22% receiving maintenance OCS | Yes—2400 mcg/d BDP equivalent | Severe | Step 4 |
| Lanier 2009 | Not stated | 86.4% pred ± 18.0 | Mean normal number of daily puffs of short‐acting β2‐agonist at baseline 2.8 ± 2.6 | Yes, 1.3% of participants were using maintenance oral steroids at baseline | Yes, mean ICS dose, mg/d (fluticasone propionate equivalent): 515.1 ± 285.4 | Intermittent to severe persistent (99% of participants moderate to severe) | Steps 1 to 6 |
| Massanari 2010 | FEV1 ≥ 75% pred | 87.1% pred ± 11.43 | Average total asthma symptom score = 1.16 ± 0.90 Average daily number of rescue puffs of β‐agonist = 0.98 ± 1.12 |
No | Yes, all participants receiving ICS at baseline; no further details given | Moderate to severe (however, participants with unstable asthma excluded) | Steps 2 to 4 |
| Milgrom 1999 | 50% to 90% pred | 71 (range 29 to 129) | Use of β‐agonist: 8.6 puffs per day (range 2 to 37.7), mean symptom score: 4 (range 1.5 to 6.5). Inclusion criteria at least 2.5 on each of seven days before randomisation | Yes—35 participants (median: 10 mg per day, range: 2.5 to 40) | Yes—282 participants, median dose: 800 mcg per day (range 200 to 4000) | Moderate persistent to severe persistent, defined as: mean FEV1 71% pred value, daily symptom score 4 (0 to 7 scale, 7 indicating most severe), daily β‐agonist use | Step 2. Although range of FEV1 and symptom scores outside the inclusion criteria suggest that this was a heterogenous population that included some mild persistent participants |
| Milgrom 2001 | FEV1 ≥ 60% pred | 84.33% (range 43 to 129) | Mean albuterol use: 1.2 puffs per day, mean daytime symptom score: 0.54, mean nocturnal symptom score: 0.22, mean am score: 0.17 (daytime scale: 0 to 4, nocturnal scale: 0 to 4 and am scale: 0/1) | Not reported | Mean dose of BDP: 278.45 mcg/d (range 168 to 672) | Moderate to severe | Step 2 |
| NCT00096954 | FEV1 ≥ 80% predicted | Not stated | "Evidence of inadequate asthma symptom control despite inhaled corticosteroids with or without other controller asthma medications" | No | Yes, fluticasone dry powder inhaler (DPI) ≥ 200 μg/d or equivalent | Mild to severe | Step 2 and above |
| NCT01007149 | FEV1 < 80% pred | Not stated | "Uncontrolled according to Global Initiative for Asthma (GINA) 2007 guidelines and at least 2 exacerbations having required systemic corticosteroid and/or at least 1 hospitalisation or emergency room visit in the past year" | Yes, but no details of numbers receiving maintenance OCS | Yes, > 1000 µg beclometasone dipropionate equivalent per day | Severe | Step 4 and above |
| Ohta 2009 | FEV1 or mean PEF 40% to 80% pred (or another marker of poor control; see paper for details) | Treatment group = 74.06% ± 19.912; placebo group = 75.81% ± 20.888 | Hospitalisation due to asthma in previous year = 10.1% of participants; ER visits due to asthma in previous year = 19.7% of participants |
Yes; 9.5% receiving maintenance OCS at baseline | Yes, ≥ 800 mcg/d beclomethasone (or equivalent). Mean dose = 1169 mcg/d | Moderate to severe | Step 3 and above |
| Solèr 2001 | Off bronchodilator, ≥ 40% pred to ≤ 80% pred | 69.85 (range 22 to 112) | β‐Agonist on as‐needed or regular basis. Mean symptom score > 3, maximum 9 | No | 770.54 (range 200 to 2000 mcg/d). Inclusion criteria stated inclusion of participants on 500 to 1200 mcg BDP/d | Moderate to severe. Severe participants: 60 in treatment group and 59 in placebo group defined as baseline FEV1 ≤ 65% pred and mean total symptom score < 4 during last 14 days of run‐in period | Step 2. Most participants fall into this category, but judging by baseline FEV1 and BDP dose, some milder participants may be included |
| SOLAR | Not stated | 78.1 (SD 16.61) | QoL scores indicating at least mild symptoms. Mean baseline puffs/d: 2.8 | No | 870 mcg BUD | Moderate to severe | Step 2 |
Studies deemed to include participants with mild asthma were Boulet 1997; Djukanovic 2004; Fahy 1997; Fahy 1999; Prieto 2006 and van Rensen 2009; with moderate/severe (step two/three) disease: Busse 2001; Busse 2011; Lanier 2009; Massanari 2010; Milgrom 1999; Milgrom 2001; NCT00096954; Ohta 2009; Solèr 2001 and SOLAR; and with severe (step four) disease: Bardelas 2012; Chanez 2010; Garcia 2012; Hanania 2011; Holgate 2004a; INNOVATE and NCT01007149. All of these studies recruited severe high‐dose inhaled steroid–dependent participants. Holgate 2004b recruited participants who required high‐dose ICS plus OCS to maintain asthma control and were classified as most severe (step five). We have undertaken analyses of exacerbations that both include and exclude the Holgate 2004b study. Allergic and non‐allergic patients with nasal polyps and asthma participated in Gevaert 2012, but details of participant severity were not reported.
Entry criteria for all studies included positive skin tests to common aeroallergens. Threshold ranges of IgE levels were a stated inclusion criterion in all studies with the exception of Boulet 1997; Garcia 2012; Milgrom 1999 and NCT01007149. Baseline IgE levels are presented in Table 5.
3. Baseline IgE levels.
| Study | IgE level (mean) |
| Bardelas 2012 | 180 IU/mL ± 130.5 |
| Boulet 1997 | 1152.4 IU/mL (data skewed: SD 2304.5) |
| Busse 2001 | 179.26 IU/mL |
| Busse 2011 | Unclear |
| Chanez 2010 | 220.2 IU/mL ± 151.96 |
| Djukanovic 2004 | Median: omalizumab group: 155.5; placebo group: 141 |
| Fahy 1997 | 141.5 IU/mL |
| Fahy 1999 | 230.1 IU/mL |
| Garcia 2012 | Not stated |
| Gevaert 2012 | Not stated |
| Hanania 2011 | 176.9 IU/mL |
| Holgate 2004 | 266.26 IU/mL |
| INNOVATE | Between 30 and 1300 IU/mL (no mean given) |
| Lanier 2009 | 469.7 IU/mL ± 338.0 |
| Massanari 2010 | 176.63 IU/mL ± 138.018 |
| Milgrom 1999 | 441.7 IU/mL |
| Milgrom 2001 | 339.85 IU/mL |
| NCT00096954 | ≥ 30 to ≤ 1300 IU/mL; no mean given |
| NCT01007149 | Unclear |
| Ohta 2009 | 508.1 IU/mL |
| Prieto 2006 | 199.2 IU/mL |
| SOLAR | 193.6 IU/mL |
| Solèr 2001 | 214.38 IU/mL |
| van Rensen 2009 | Unclear |
Outcome measures
Outcome measures reported ICS or OCS withdrawal, mortality, asthma exacerbations, rescue medication use, lung function, quality of life, global evaluation of treatment effectiveness and adverse events. For each outcome, results are presented separately for any steroid stable phase (omalizumab given as adjunctive therapy to inhaled corticosteroids) and steroid reduction phase (omalizumab given during steroid reduction).
Subgroup analysis
It was not possible to use the a priori subgroups as planned. Many studies included adults and children over 12 years of age but did not present results separately for the children. Only three studies focused exclusively on a paediatric or adolescent population (Lanier 2009 and Milgrom 2001 included children six to 12 years of age, and Busse 2011 included participants six to 20 years of age). Subgroups were analysed separately for route of delivery of the trial medication, but to reflect clinical practice, we have moved the results of the intravenous and inhaled subgroups to Appendix 3. An attempt was made to analyse the results according to asthma severity; this is discussed under each outcome, when possible. Asthma diagnostic entry criteria did not prove to be a useful subgroup, as all studies, with the exception of Garcia 2012, enrolled only participants with proven allergic asthma and IgE levels within the specified range. We did not attempt subgroup analysis for duration of treatment.
Excluded studies
One hundred ten studies failed to meet the eligibility criteria for our review. They are listed in Characteristics of excluded studies.
Forty‐two (38%) of the excluded studies did not compare omalizumab versus placebo, a further 24 (22%) were non‐randomised, 13 (12%) were not focused on participants with asthma, 10 (9%) were pooled analyses of trials, nine (8%) were review articles, six (5%) were open‐label studies, three (3%) were not completed, two (2%) were cross‐over trials and one (1%) was a letter.
Risk of bias in included studies
Allocation
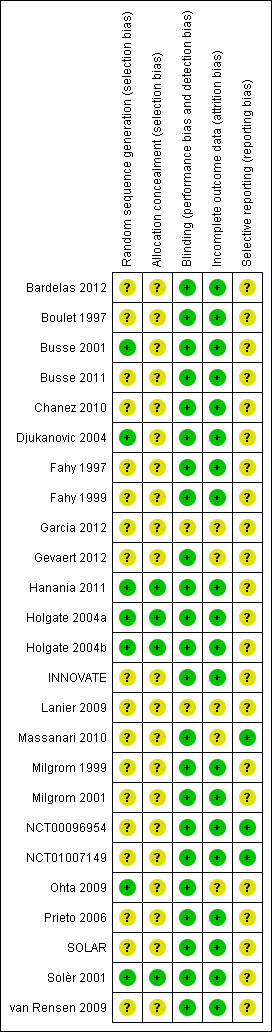
Seven studies (29%) (Busse 2001; Djukanovic 2004; Hanania 2011; Holgate 2004a; Holgate 2004b; Ohta 2009; Solèr 2001) were assessed as having low risk of selection bias. The remaining 18 studies were categorised as having unclear risk (Figure 7).
7.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
Only four studies (17%) (Hanania 2011; Holgate 2004a; Holgate 2004b; Solèr 2001) were judged as having low risk of performance and detection bias. The remaining 21 studies were assessed as having unclear risk.
Incomplete outcome data
Twenty‐three studies (96%) were viewed as having low risk of attrition bias, and only Lanier 2009 and Garcia 2012 were judged to be in the unclear category
Selective reporting
Three studies (13%) (Massanari 2010; NCT00096954; NCT01007149) were assessed as having low risk of reporting bias. The remaining 22 studies were categorised as having unclear risk.
Effects of interventions
Primary outcomes
1. Asthma exacerbations
Treatment with omalizumab resulted in fewer exacerbations overall. This effect was maintained during the steroid stable and steroid reduction phases of the included trials but with much greater uncertainty when only participants with severe disease were considered.
Steroid stable phase
Odds ratio of having one or more exacerbations
Overall, treatment with subcutaneous omalizumab resulted in a significant reduction in the odds of having one or more exacerbations when compared with placebo in the steroid stable trials (OR 0.55, 95% CI 0.46 to 0.65; ten studies, 3261 participants). This represents an absolute reduction from 26% for participants suffering an exacerbation with placebo to 16% with omalizumab, over 16 to 60 weeks, as shown in Figure 1.
In analyses based on asthma severity, we found that in participants with moderate/severe asthma and in those who were receiving background inhaled steroid therapy, a significant reduction in the odds of having an asthma exacerbation favoured subcutaneous omalizumab (OR 0.50, 95% CI 0.42 to 0.60; seven studies, 1889 participants; Analysis 1.1).
1.1. Analysis.
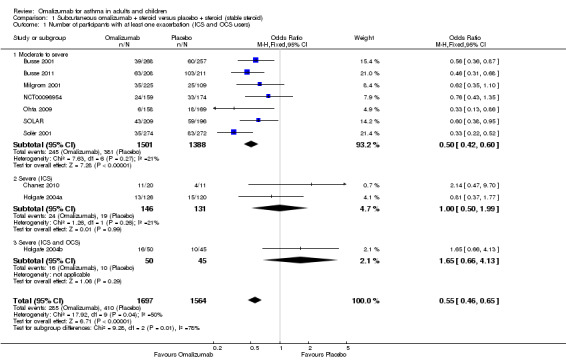
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 1 Number of participants with at least one exacerbation (ICS and OCS users).
However, little effect, but with wide confidence intervals, was noted for omalizumab versus placebo in participants who were diagnosed with severe asthma and who were receiving background inhaled steroid therapy (OR 1.00, 95% CI 0.50 to 1.99; two studies, 277 participants; Analysis 1.1), nor for those who were diagnosed with severe asthma who were receiving background inhaled plus oral steroid therapy (OR 1.65, 95% CI 0.66 to 4.13; one study, 95 participants; Analysis 1.1). We are therefore much less certain of any positive impact of omalizumab on exacerbations in patients with more severe asthma.
Exacerbation rate ratio
With regard to exacerbations requiring oral steroids, the clearest benefit in favour of subcutaneous omalizumab was again observed in participants with moderate/severe asthma (rate ratio 0.52, 95% CI 0.37 to 0.73; two studies, 1038 participants; Analysis 1.2). For participants with severe asthma, only one study (Hanania 2011) found significant benefit in favour of subcutaneous omalizumab for those who were receiving background therapy of both inhaled corticosteroids and long‐acting beta2‐agonists, but again, more uncertainty surrounds those receiving a background therapy of inhaled plus oral corticosteroids. However, it should be noted that these findings are drawn from a single study, and no significant differences were noted between these subgroups.
1.2. Analysis.
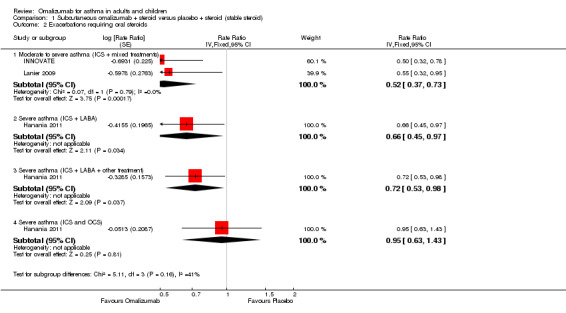
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 2 Exacerbations requiring oral steroids.
Hospitalisations
Significant benefit was seen for omalizumab versus placebo with regard to reducing the number of people experiencing one or more hospitalisation (OR 0.16, 95% CI 0.06 to 0.42; four studies, 1824 participants; Analysis 1.3), representing an absolute reduction in risk from 3% with placebo to 0.5% with omalizumab (Figure 2). No data were available for the severe asthma subgroup; data were reported for all participants with the diagnosis of moderate to severe asthma.
1.3. Analysis.
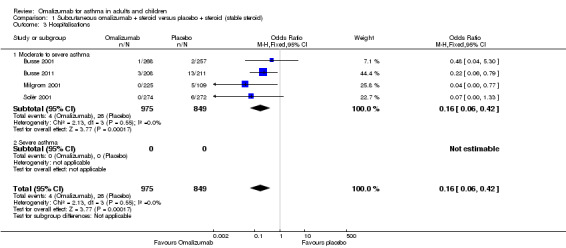
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 3 Hospitalisations.
Steroid tapering phase
Odds ratio of having one or more exacerbations
During the steroid tapering phase, participants treated with subcutaneous omalizumab were less likely to experience an asthma exacerbation compared with those treated with placebo (OR 0.46, 95% CI 0.36 to 0.59; four trials, 1631 participants). With data added for the subgroup of oral steroid users, the OR was 0.49 (95% 0.39 to 0.62; five trials, 1726 participants; Analysis 2.1). Again, we were less certain of the benefit of omalizumab when the data from participants with severe asthma were considered alone (OR 0.59, 95% CI 0.30 to 1.16).
2.1. Analysis.
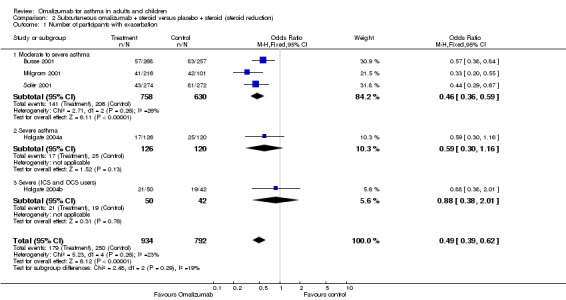
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 1 Number of participants with exacerbation.
Hospitalisations
A significant reduction was observed in the odds of hospitalisation in participants with moderate asthma treated with omalizumab compared with those treated with placebo (OR 0.11, 95% CI 0.03 to 0.48; three studies, 1408 participants; Analysis 2.2). This represents an absolute reduction from 20% with placebo to 3% with omalizumab, as shown in Table 2. No trials included participants with severe asthma that contributed to this outcome.
2.2. Analysis.
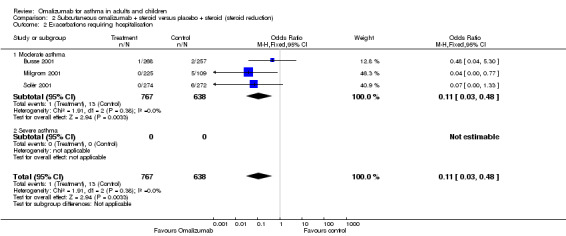
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 2 Exacerbations requiring hospitalisation.
2. Steroid withdrawal/reduction
Participants treated with omalizumab were significantly more likely to be able to reduce and completely withdraw their inhaled corticosteroids. For the subset of participants receiving oral corticosteroids, we remain uncertain whether benefit is derived from omalizumab over placebo for those withdrawing or reducing their steroid treatment.
Inhaled steroid withdrawal
Participants treated with subcutaneous omalizumab were significantly more likely to be able to withdraw their ICS completely than those treated with placebo (OR 2.50, 95% CI 2.00 to 3.13; four trials, 529 participants; Analysis 2.3). This represents an absolute reduction from 40% in the placebo group to 21% in the omalizumab group, as shown in Figure 3. Most of the evidence comes from trials in participants with moderate to severe asthma, and considerable uncertainty remains about whether benefit is seen in the severe asthma subgroup (OR 1.55, 95% CI 0.80 to 2.98; one trial, 45 participants).
2.3. Analysis.
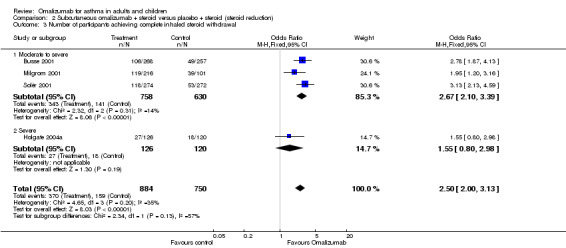
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 3 Number of participants achieving complete inhaled steroid withdrawal.
In a trial extension of 32 weeks, 34% (85/254) of moderate to severe participants in the omalizumab‐treated group were able to achieve complete steroid withdrawal compared with 14% (31/229) in the control group (Solèr 2001; P < 0.001).
Inhaled steroid reduction
Change from baseline in ICS dose
A small but statistically significant reduction in daily steroid dose was seen among omalizumab‐treated participants compared with those given placebo (WMD ‐118 mcg BDP equivalent per day, 95%CI ‐154 to ‐84; three studies, 1188 participants; Analysis 2.4). Although a high degree of heterogeneity was observed (I2 = 67.2%), random‐effects modelling did not alter the direction of the effect but widened the confidence interval (MD ‐141.24 mcg, 95% CI ‐221 to ‐61). The reduction in ICS dose was greater in the trial with severe asthma than in the two trials with moderate to severe asthma, although this difference did not reach statistical significance (test for subgroup differences: Chi² = 3.33, df = 1 (P = 0.07), I² = 70.0%).
2.4. Analysis.
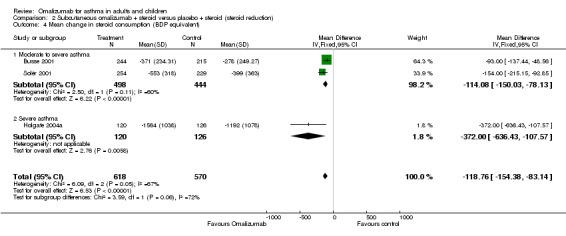
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 4 Mean change in steroid consumption (BDP equivalent).
In paediatric participants (Milgrom 2001), median BDP dose reduction was 100% in the omalizumab‐treated group compared with 66.7% in the placebo group (P = 0.001).
Likelihood of achieving 50% reduction in ICS dose
Participants treated with omalizumab were significantly more likely to be able to reduce their inhaled steroid dose by greater than 50% (OR 2.50, 95% CI 2.02 to 3.10; four studies, 1098 participants; Analysis 2.5).
2.5. Analysis.
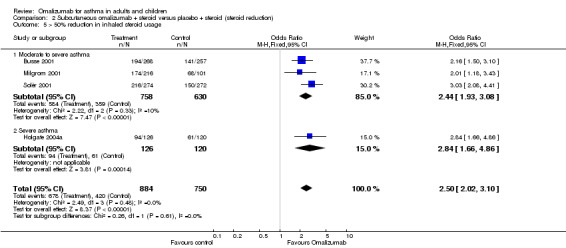
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 5 > 50% reduction in inhaled steroid usage.
Oral steroid withdrawal
No significant difference was noted in the number of participants who were able to withdraw from oral steroid therapy between omalizumab and placebo treatment (OR 1.18, 95% CI 0.53 to 2.63; one study, 95 participants; Analysis 3.1).
3.1. Analysis.
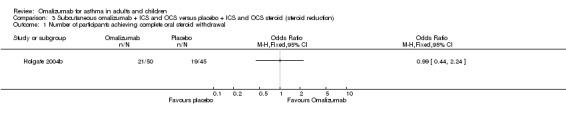
Comparison 3 Subcutaneous omalizumab + ICS and OCS versus placebo + ICS and OCS steroid (steroid reduction), Outcome 1 Number of participants achieving complete oral steroid withdrawal.
Oral steroid reduction
No significant difference in the median reduction of daily oral steroid dose was noted between omalizumab‐ and placebo‐treated participants in Holgate 2004b (69% vs 75%; P = 0.675).
Secondary outcomes
1. Asthma symptoms
Treatment with omalizumab generally improved asthma symptom scores in both steroid stable and steroid reduction phases.
Steroid stable phase
End of treatment symptom scores
A significant difference favouring omalizumab was observed with regard to symptom scores for moderate to severe participants in four of the seven studies reporting data on this outcome (Busse 2001; Busse 2011; Lanier 2009; Solèr 2001), and a significant difference favouring omalizumab was reported for severe participants in two out of four studies (Hanania 2011 and Holgate 2004a). In view of the heterogeneity among different approaches to assessing symptom scores, we have avoided statistical aggregation of these data (Analysis 1.10).
1.10. Analysis.
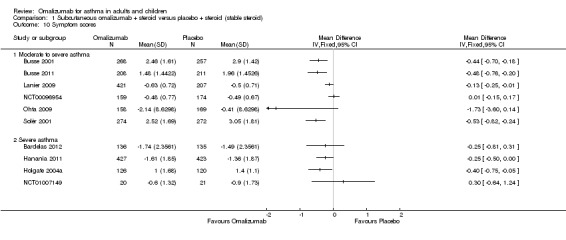
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 10 Symptom scores.
Change from baseline in symptom scores
Significant reductions in symptom scores from baseline in favour of omalizumab were reported in two trials (SOLAR, ‐1.8, P = 0.023; INNOVATE, P = 0.039, no mean scores presented).
Steroid reduction phase
Change from baseline in symptom scores
Busse 2001 reported that mean change in symptom scores between baseline and the end of steroid reduction was greater in the omalizumab group than in the placebo group (‐1.93 vs ‐1.44, respectively; P < 0.001), and Milgrom 2001 reported that median nocturnal symptom scores were unchanged in either treatment group for the duration of the study, although mean scores were lower in the treatment group at all evaluations (no P values reported). No difference between groups in daytime symptom scores was detected until week 22 during steroid reduction phase: median value 0.36 versus 0.54 for the treatment and control groups, respectively; P value not reported); this reduction in daytime symptom scores then persisted until the end of the study.
2. Health‐related quality of life
In most trials reporting quality of life, a significant benefit of omalizumab over placebo was reported during both steroid stable and steroid reduction phases.
Steroid stable phase
Change from baseline in quality of life scores
Significantly greater improvement in the overall Asthma Quality of Life Questionnaire (AQLQ) favoured omalizumab (MD 0.31, 95% CI 0.23 to 0.39; six studies, 2981 participants; Analysis 1.12), but this finding did not reach the validated clinically relevant effect size of 0.5 (Juniper 1994).
1.12. Analysis.
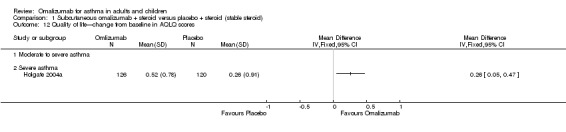
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 12 Quality of life—change from baseline in AQLQ scores.
Assessment of asthma control
Participants' global asthma control was significantly better when taking omalizumab than placebo (OR 2.12, 95% CI 1.67 to 2.68; four studies, 1136 participants; Analysis 1.13); however, the very high degree of heterogeneity in this analysis (I2 = 69%) indicates that findings warrant especially careful interpretation, although it is clear that a significant advantage for subcutaneous omalizumab versus placebo was observed in the moderate to severe (OR 3.32, 95% CI 2.19 to 5.05) and to a lesser extent in the severe (OR 1.69, 95% CI 1.26 to 2.26) subgroup.
1.13. Analysis.
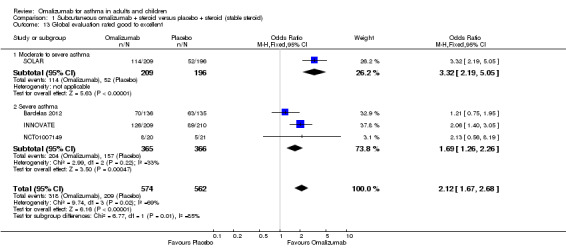
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 13 Global evaluation rated good to excellent.
Steroid reduction phase
Change from baseline in quality of life scores
Unpublished data were obtained from Holgate 2004a: Overall change was 0.68 (SD 1.02) for omalizumab versus 0.26 (SD 0.96) for placebo (no P values available). In severe participants, a significant difference can be seen in the numbers of participants who achieved clinically relevant improvement in their overall quality of life (an increase of at least 0.5 above baseline) in the omalizumab group (57.5%) compared with the placebo group (38.6%; P < 0.01). A greater number of participants in the omalizumab group (16%) than in the placebo group (5.9%) also reported clinically relevant improvement in their overall quality of life (P < 0.05).
Assessment of asthma control
Moderate to severe participants in two studies were more likely to rate treatment as good or excellent when treated with omalizumab than with placebo (OR 2.72, 95% CI 2.04 to 3.62; two studies, 842 participants).
3. Rescue medication use
Participants were more likely to be able to reduce their rescue medication when using omalizumab.
Steroid stable phase
Participants treated with subcutaneous omalizumab required significantly less rescue beta2‐agonist medication compared with those given placebo (nine studies, 3524 participants; Analysis 1.14). This benefit was observed in both moderate to severe (MD ‐0.58, 95% CI ‐0.84 to ‐0.31) and severe (MD ‐0.30, 95% CI ‐0.49 to ‐0.10) asthma subgroups, with the latter receiving a background therapy of inhaled corticosteroids; however, much more uncertainty remains about the difference between subcutaneous omalizumab and placebo for this outcome in severe asthma participants who were receiving a background therapy of inhaled plus oral corticosteroids. No statistically significant difference was seen in results from the three subgroups.
1.14. Analysis.
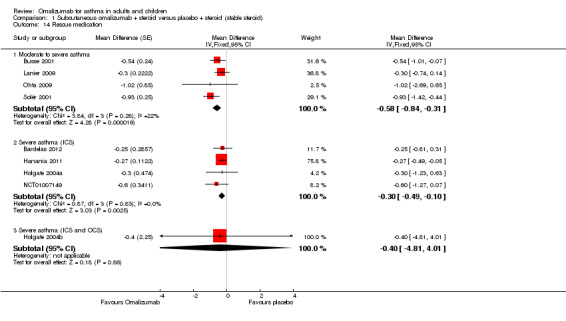
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 14 Rescue medication.
Steroid reduction phase
Change from baseline in rescue medication use
Omalizumab treatment enabled participants to use significantly less rescue medication than placebo (WMD ‐0.74 puffs per day, 95% CI ‐1.05 to ‐0.43; four studies, 1373 participants; Analysis 2.10). Baseline levels were approximately 4.5 puffs per day for these studies, so the effect size was quite small.
2.10. Analysis.
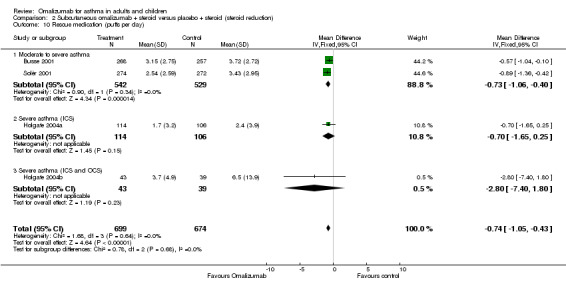
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 10 Rescue medication (puffs per day).
4. Measures of lung function
Improvements in lung function were inconsistent across the trials analysed, and the range of different measures presented in the trials prevented meaningful meta‐analysis.
End of treatment AM PEF
Differences were very small, and no overall significant difference was reported between participants treated with subcutaneous omalizumab and those given placebo (MD 3.56 L/min, 95% CI ‐5.05 to 12.18; four studies, 1651 participants; Analysis 1.5).
1.5. Analysis.
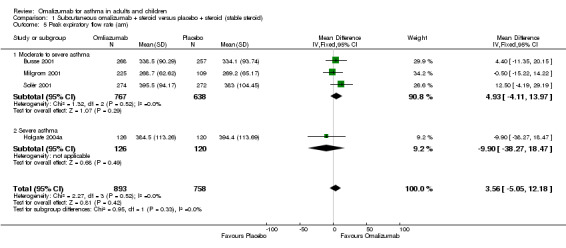
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 5 Peak expiratory flow rate (am).
Change from baseline in AM PEF
A small but statistically significant benefit for subcutaneous omalizumab versus placebo was observed in participants with moderate to severe asthma (MD 11.00 L/min, 95% CI 4.51 to 17.49; one study, 405 participants; Analysis 1.6), and no benefit was observed in severe participants (MD ‐0.60 L/min, 95%CI ‐29.77 to 28.57; one study, 31 participants; Analysis 1.6). Given the small effect size and the small numbers of studies and participants contributing to this analysis (especially in the severe asthma subgroup), we recommend that any interpretation of these data be reserved until additional study findings become available.
1.6. Analysis.
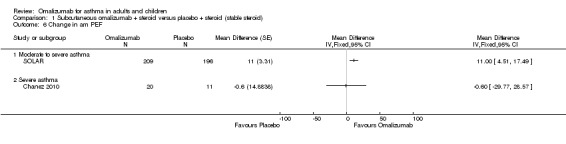
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 6 Change in am PEF.
End of treatment FEV1 (mL)
No significant difference in FEV1 was noted in moderate to severe adolescent and adult participants (MD 68.31 mL, 95% CI ‐23.45 to 160.07; two studies, 1071 participants; Analysis 1.7).
1.7. Analysis.
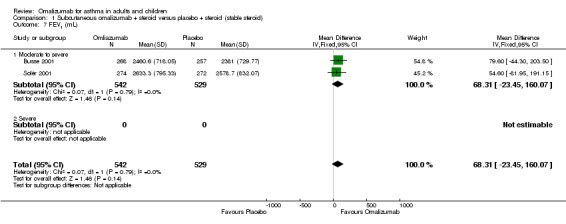
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 7 FEV1 (mL).
Change from baseline in FEV1 (mL)
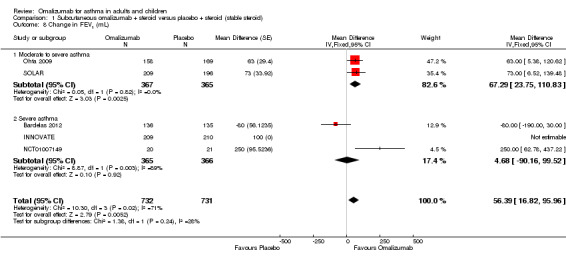
Small but significant improvements from baseline were observed in the moderate to severe subgroup (MD 67.29 mL, 95% CI 23.75 to 110.83; two studies, 732 participants; Analysis 1.8). Considerable heterogeneity was seen between the two studies in the severe subgroup in this analysis (I² = 89%); in particular, uncertainties are described regarding the data from NCT01007149, for which baseline values were unavailable in the study report, and the change score in the placebo group was reported as 0.00 L; we have not received clarification on this point from the pharmaceutical company sponsoring this study. In Garcia 2012, the abstract reported that the placebo‐adjusted absolute change in FEV1 with omalizumab was larger, at +250 mL (P = 0.032).
1.8. Analysis.
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 8 Change in FEV1 (mL).
Change from baseline in FEV1 % predicted
A significant benefit for subcutaneous omalizumab versus placebo was observed (MD 2.15, 95% CI 1.01 to 3.30; four studies, 1079 participants; Analysis 1.9).
1.9. Analysis.
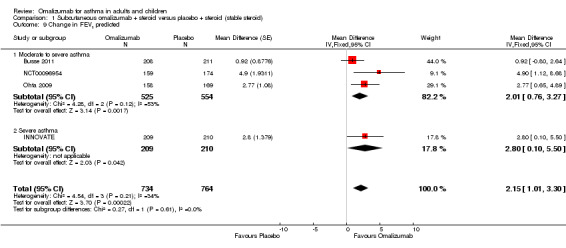
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 9 Change in FEV1 predicted.
Subcutaneous omalizumab (in participants not receiving ICS)
No significant differences between placebo and omalizumab were reported in terms of FEV1 % predicted. Baseline imbalances between groups at baseline meant that data on FEV1 could not be reliably analysed. Prieto 2006 and van Rensen 2009 reported no significant differences in the mean change in methacholine responsiveness between omalizumab and placebo. van Rensen 2009 reported a significant difference in late asthmatic response (LAR) in favour of omalizumab (P < 0.05).
5. Adverse events including withdrawals and mortality
Participants receiving subcutaneous omalizumab experienced significantly fewer serious adverse events compared with those given placebo. However, they also experienced significantly more injection site reactions. No significant difference in mortality was detected.
Mortality
No significant difference between subcutaneous omalizumab and placebo with respect to mortality was observed (OR 0.19, 95% CI 0.02 to 1.67; Analysis 1.4). In the nine studies contributing data to this analysis, among 4245 participants, only four deaths were reported—all in the placebo group. Two deaths occurred during the study period and two more than six weeks after discontinuation of the study. None were reported to be asthma‐related. Three of the four deaths occurred in the severe asthma subgroup.
1.4. Analysis.
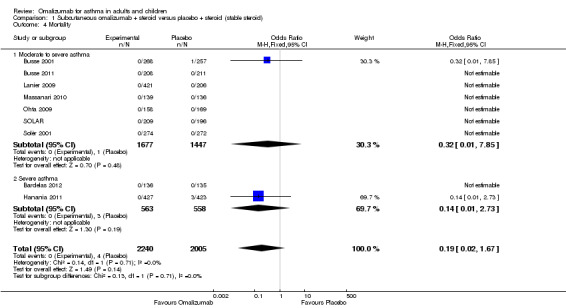
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 4 Mortality.
Adverse event—serious
Significantly fewer serious adverse events occurred in participants assigned to subcutaneous omalizumab than in those given placebo (OR 0.72, 95% CI 0.57 to 0.91; 15 studies, 5713 participants; Analysis 1.16), and the level of heterogeneity among these studies (I2 = 7%) was very low. This represents an absolute reduction from 6% receiving placebo to 4% taking omalizumab, as shown in Figure 4.
1.16. Analysis.
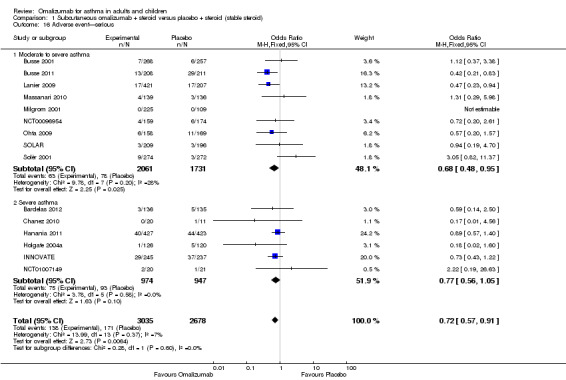
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 16 Adverse event—serious.
Adverse event—any
In terms of all adverse events, no significant difference was seen between subcutaneous omalizumab and placebo (OR 0.92, 95% CI 0.81 to 1.06; 14 studies, 5167 participants; Analysis 1.15). However, the level of heterogeneity among these studies (I2 = 22%) was pronounced.
1.15. Analysis.
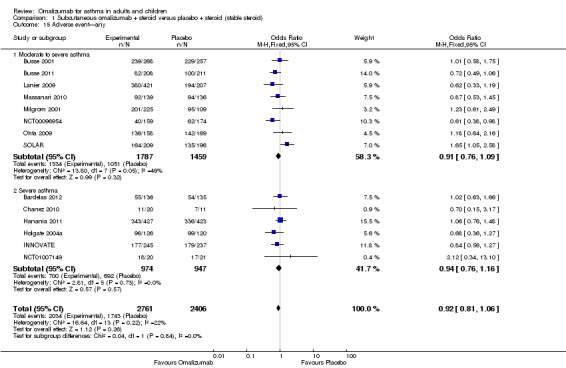
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 15 Adverse event—any.
Adverse event—injection site reactions
Significantly more injection site reactions were reported among participants assigned to subcutaneous omalizumab than among those receiving placebo (OR 1.72, 95% CI 1.33 to 2.24; nine studies, 3577 participants; Analysis 1.17), and the level of heterogeneity among these studies (I2 = 42%) was considerable. This represents an absolute increase from 6% on placebo to 9% on omalizumab, as shown in Figure 5.
1.17. Analysis.
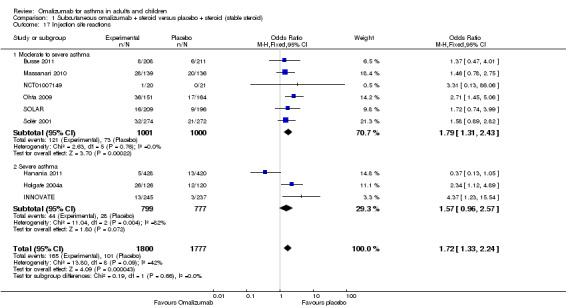
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 17 Injection site reactions.
No differences were reported in headache, urticaria, number of participants with any adverse events or number of withdrawals due to adverse events.
Withdrawals
Withdrawals were infrequent in studies using subcutaneous omalizumab. Among adult participants, Busse 2001 reported two withdrawals from the treatment group due to adverse events. Neither was considered drug‐related. Solèr 2001 reported five withdrawals from that study—all were from the placebo group. In the paediatric study (Milgrom 2001), five of 225 (2.2%) treated children withdrew from the trial—four because of pain or fear of injection and one because of mild to moderate urticaria on two occasions. In the study placebo group, two of 109 (1.8%) children withdrew because of pain and/or fear of injection, and one child was withdrawn because of prolonged hospitalisation for hip fracture. Two participants withdrew from the severe adult population (Holgate 2004a), both from the placebo group.
Discussion
Summary of main results
We have reviewed the use of omalizumab in 25 randomised, placebo‐controlled clinical trials involving 6382 people with differing asthma severity, with most suffering from moderate to severe disease. The trials reviewed varied in design, as described earlier. Treatment duration ranged between 8 and 60 weeks, and some studies included a steroid reduction phase between 8 and 16 weeks in duration. Most of the studies (21, n = 5975) used a subcutaneous route to deliver the drug. Currently, omalizumab is delivered exclusively by the subcutaneous route in clinical practice; for this reason, discussion of results from older studies of inhaled and intravenous administration has been moved to the appendices.
Primary outcomes
Exacerbations
Omalizumab reduced exacerbations when assessed both as an adjunctive treatment and as a steroid‐sparing agent in moderate to severe asthma. However, in the subgroup of participants with more severe asthma, including those requiring oral steroids, omalizumab had no significant effect on asthma exacerbations. The results presented here also suggest a reduction in hospitalisations for participants with moderate to severe asthma using omalizumab compared with those given placebo, but no data in this area are available for the more severe subgroup independently.
Steroid sparing effects
Some people with more severe asthma depend on high doses of inhaled or oral corticosteroids to control their disease. Long‐term oral steroid use is associated with many unwanted side effects, including hypertension, reduction in bone density, bruising, immune suppression, cataracts, growth failure and hyperglycaemia, among many others. Agents that allow asthma sufferers to reduce their daily steroid dose are therefore of great interest.
The reduction in daily inhaled steroid dose following treatment with omalizumab was clinically modest but statistically significant. The amount of variation in this outcome could be attributable to the higher doses of BDP equivalent inhaled steroid in Holgate 2004a in relation to Busse 2001 and Solèr 2001. It is noteworthy that participants treated with placebo were also able to reduce their intake of ICS by a significant amount, probably because of better adherence to the prescribed doses. The presentation of dichotomous data in the published studies and of unpublished continuous data in the FDA report has enabled us to look at the significance of both methods of measuring effects (Table 6). Treatment with omalizumab increased the likelihood of steroid reduction, but variable baseline steroid doses and a modest mean outcome difference in steroid consumption between treatment and placebo groups bring into question the true size of the steroid sparing effect of omalizumab.
4. Corticosteroid use during steroid‐tapering phase.
| Solèr 2001 | Busse 2001 | Milgrom 2001 | Holgate 2004 | Pooled estimates | |
| Baseline mean ICS dose (omalizumab/placebo) | 766/777 | 564/522 | 284/267 | 1407/1376 | N/A |
| Length of study (weeks) | 52 | 28 | 28 | 32 | N/A |
| Length of tapering phase | 12 | 12 | 12 | 16 | N/A |
| Mean daily ICS dose at end of tapering phase (omalizumab/placebo) | 213/378 Source: FDA website | 193/274 Source: FDA website | N/A | 506/690 Source: unpublished data (Acumed) | N/A |
| Change in CS dose (omalizumab/placebo), mcg | Mean change (ICS): ‐553/‐399 Source: FDA website | Mean change (ICS): ‐371/‐278 Source: FDA website | N/A | Mean change (ICS): ‐782/‐596 Source: published paper | Busse 2001/Solèr 2001/Holgate 2004: WMD ‐119 mcg/d (95% CI ‐153.72 to ‐84.34) |
| Median (95% CI) daily ICS dose at end of tapering (omalizumab/placebo) | 100 (0 to 400)/300 (100 to 600) | N/A | N/A | N/A | N/A |
| Change (%) (omalizumab/placebo) | 72/51 | 66/50 | Median change (ICS): 100/67 P = 0.001 | Median change (ICS): 60/50 P = 0.003 Source: FDA website Median change (OCS): 69%/75% P = 0.7 |
N/A |
| Number of participants achieving > 50% reduction in ICS dose (n/N) (omalizumab/placebo) | 216/274 150/272 | 194/268 141/257 | 174/216 68/101 | 94/126 61/120 | OR 2.5 (95% CI 2.02 to 3.10) NNTB five to seven |
| Number of participants achieving complete ICS withdrawal (omalizumab/placebo) | 118/274 53/272 | 106/268 49/257 | 119/216 39/101 | 27/126 18/120 | OR 2.5 (95% CI 2.0 to 3.13) NNTB five to eight |
Not all participants across the studies benefited from omalizumab treatment. Approximately 16% of severe participants achieved less than 25% reduction in daily inhaled steroid use over the steroid reduction phase. In the study involving paediatric participants, nine of 225 omalizumab‐treated participants appeared to have needed the same amount or an increased amount of steroid therapy (reported as < 0% reduction in steroid use) (Milgrom 2001). It was not obvious whether these children had more severe asthma. These results confirm the need to better define which patients will benefit most from omalizumab treatment. It is important to note that not all asthmatic patients at the severe end of the spectrum who may benefit most from steroid reduction will respond to omalizumab treatment; this may reflect the heterogeneity of asthma aetiology and pathology, especially in this group (Walker 2006).
Secondary outcomes
Asthma symptoms
Asthma symptom scores were not reported by all included studies. Participants with moderate to severe and severe asthma receiving omalizumab were more likely to experience an improvement in their asthma symptoms, but this difference only reached significance in seven out of the eleven studies reporting this outcome.The heterogeneity of methods for assessing asthma severity prevented meaningful meta‐analysis.
Health‐related quality of life
Significant improvements in health‐related quality of life were observed with omalizumab compared with placebo.
A statistically significant improvement was noted in steroid stable studies of subcutaneous omalizumab for AQLQ scores in the treatment group compared with the placebo group, but this difference did not reach the validated clinically relevant effect size. In addition, Global Asthma Control scores improved significantly in the treatment group compared with the placebo group, but with a high level of heterogeneity.
Further evidence of positive participant perception of omalizumab treatment was ascertained from pooled analysis of the results for global effectiveness of treatment from two trials involving subcutaneous omalizumab. Once again, improvements in global treatment efficacy and overall quality of life noted among control participants suggest that the basic trial design, which included close medical monitoring, might have contributed to a large placebo or more likely Hawthorne effect, mediated through improved adherence to medication.
Rescue medication use
Participants receiving omalizumab were significantly more likely to be able to reduce their use of short‐acting bronchodilators or ‘rescue medication’. This was true for participants with both moderate and severe disease but was not found for the relatively small number of more severely affected participants receiving oral as well as inhaled corticosteroid as background therapy.
Measures of lung function
Pooled results showed consistent but very modest improvements in the treatment group when compared with the placebo group for the change from baseline FEV1 predicted and morning PEF.
This is consistent with studies reporting no relationship between reduced hospital admissions and improved lung function (Qureshi 1998) and a poor association between lung function and health‐related quality of life (Wijnhoven 2001). It is not yet clear how such small improvements in lung function may equate to clinically relevant findings. The relationship between asthma disease severity and lung function requires further investigation. Given the context in which omalizumab has been assessed in this review, optimising background adherence to inhaled therapy may be more important than adding extra medication to achieve these modest improvements in lung function.
Adverse events
It appears that participants receiving omalizumab were less likely to experience a serious adverse event than those receiving placebo, with a low level of heterogeneity (I2 = 7%). Injection site reactions were more common in the omalizumab group, but this result also had a considerable degree of heterogeneity (I2 = 42%). Although no significant difference was seen between groups in the low mortality rates encountered, any advantage favoured omalizumab, as all four deaths occurred among participants receiving placebo (at least two not asthma‐related, with no details provided regarding the other two).
Use of a humanised anti‐IgE antibody has raised theoretical concerns about immune complex–mediated pathology and abnormal immune responses to parasitic infection. Administration of parenteral anti‐IgE results in the formation of small immune complexes (< 10 KDa), which are cleared through the kidney (Arshad 2001). No reports have described immune complex–mediated side effects over up to 60 weeks of administration. Additionally, antibodies to omalizumab did not develop in participants treated with subcutaneous or intravenous omalizumab, although they occurred transiently in one participant who received inhaled anti‐IgE therapy.
Further information is needed on the safety profile of the drug after long‐term use and in different populations such as those with endemic parasitism. There is a theoretical potential that anti‐IgE therapy may lead to increased risk of cancer because of the role of IgE in the immune response to neoplasia; again, longer studies are needed to further explore this possibility.
Overall completeness and applicability of evidence
An important question is the place of omalizumab in the treatment of asthma according to current guidelines. NICE guidance recommends use only in patients with inadequately controlled severe persistent allergic IgE‐mediated asthma who require continuous or frequent treatment with oral corticosteroids (i.e. step five of the BTS guidelines for asthma management in children and adults) (BTS/SIGN 2012; NICE 2013). However, this is not strongly supported by the evidence. Few studies recruited only participants with severe disease, and thus our subgroups may not have been adequately powered to detect different responses. Moreover, the current data available in relation to participants on oral steroids (Holgate 2004b; 95 participants) are not sufficient to justify the extrapolation of our main findings to this group. Additional trials limited to this severe oral steroid–dependent population are required to determine whether they would benefit from omalizumab therapy.
Our own classification of the studies included in this review failed to identify a consistently different response to therapy for participants with differing disease severity, with the exception of participants experiencing one or more exacerbations in the steroid stable treatment group who were treated with subcutaneous omalizumab. In this important analysis, it appears that participants with more severe disease actually benefit less from omalizumab treatment, with a significant difference detected between the subgroups.
In addition, the steroid sparing effects of omalizumab, which could be important in severe asthmatic patients, who are at risk of serious side effects from daily use of high‐dose inhaled steroids or oral steroids, were generally small. Such modest steroid sparing effects of omalizumab in moderately severe asthmatic patients have to be balanced against the cost of anti‐IgE treatment. Studies with a steroid sparing phase of considerably longer than 16 weeks will be required to answer this question more definitively. Discontinuation of omalizumab treatment is associated with increases in circulating free IgE to prebaseline values within eight weeks (Casale 1997). This implies that treatment would need to be continued long term for efficacy to persist, which has significant cost implications.
The cost of omalizumab ranges from approximately £1665 per patient per year for a 75‐mg dose administered every four weeks to approximately £26,640 per patient per year for a 600‐mg dose administered every two weeks (NICE 2013). Given the time‐consuming and costly nature of the treatment and importance of targeting patients who are most likely to benefit, it is essential that future studies focus on methods of identifying potential responders. One of the challenges when treatment with omalizumab is considered is that to date, there is no reliable way of identifying those people before treatment is started. Four responder analyses with varying definitions of 'at risk' or 'severe' asthmatic participants have been conducted (Babu 2001; Bousquet 2004; Holgate 2001; Wenzel 2002). Bousquet 2004 reported that participants were more likely to respond to treatment if characterised by one or more of the following: low FEV1, frequent hospitalisation and high ICS dose (for additional information on these analyses, see Table 7).
5. Responder analyses.
| Study | Type of asthma | Definition | Trials analysed | Severe participants (n) | Response |
| Babu 2001 | Severe | Three definitions of severe asthma explored: 1. ≤ 60% baseline FEV1 predicted 2. ≤ 65% baseline FEV1 predicted 3. ≤ 65% baseline FEV1 + symptom score > 4 (out of 9) | Busse 2001; Milgrom 2001; Solèr 2001 | 22% adult participants; 9% paediatric participants taking omalizumab and 6% given placebo | Median BDP reduction: Severe participants reduced consumption by 60% to 67% versus 80% to 83% in moderate asthmatic participants. (These numbers vary depending upon the 'severe' criteria applied.) |
| Bousquet 2004 | Moderate to severe | 1. BDP dose ≥ 800 mcg/d 2. FEV1 ≤ 65% predicted 3. History of emergency treatment | Busse 2001; Solèr 2001 | BDP dose ≥ 800 mcg/d: 432 FEV1 ≤ 65% predicted: 379 History of emergency treatment: 733 | Response to therapy defined as (1) reduction in symptoms of at least one, with no increase in SABA; reduced use of rescue medication; (2) reduced usage of SABA (≥one puff per day and no increase in symptoms; (3) improved lung function (increase in am PEF ≥ 15%); (4) improvement in QoL (increase in AQLQ of 1 in overall score); (5) composite of at least one four responses with no asthma exacerbation Odds ratio of composite response according to baseline characteristic indicated that participants more likely to respond with two or more variables |
| Holgate 2001 | 'At‐risk' asthmatic participants | Intubation at some point prior to screening/ hospitalised in the past year | Busse 2001; Soler 2001; Chung 2002 | 254 | N = 34 experienced exacerbations in omalizumab treated group versus N = 42 in placebo |
| Wenzel 2002 | Severe | High dose BDP, poor lung function, history of emergency asthma treatment in the last year. | Busse 2001; Soler 2001 | This sensitivity analysis was conducted in order to determine baseline predictors of efficacy | Participants who experienced a reduction in symptom scores, reduction in use of rescue medication, improvement in lung function, improvement in quality of life. |
A recent logistic regression analysis from the EXTRA (Hanania 2011) trial did not entirely support this previous work on clinical predictions of efficacy. In contrast, these trial authors suggested that preserved FEV1 (> 65% predicted) with no intubations for asthma in the preceding year but higher numbers of exacerbations requiring oral steroids in the preceding year was predictive of a better response. These authors speculate that this may indicate that omalizumab is most effective in individuals with uncontrolled disease but in whom irreversible airway remodelling has not yet occurred. Further work in this area would be justified. Hanania 2011 also attempted to identify biomarkers that would allow prospective prediction of likely response to omalizumab and suggested that elevated fractional exhaled nitric oxide (FeNO), blood eosinophilia and serum periostin may predict a better response to omalizumab when compared with placebo.
Another study included in this review (Busse 2011) carried out a rather different prespecified subgroup analysis to investigate potential responders. This study showed that omalizumab had a significant impact on asthma control and exacerbations in an inner city population of children and young adults with severe asthma but also suggested that it was more effective in those who are both sensitized and exposed to cockroach allergen. Compared with those who were neither sensitised nor exposed to cockroach allergen, people receiving omalizumab had bigger reductions in inhaled corticosteroid dose (P = 0.03) and asthma exacerbations (P = 0.06) and increased odds of not having an asthma exacerbation (P = 0.06). This may represent the beginning of an alternative approach to selecting patients for treatment with omalizumab that allows prior identification of likely responders, although, it is important to note, it is not clear from the paper or the supplementary appendices how many people in this study were actually both sensitised and exposed to cockroach allergen (Walker 2011). Further validation of this approach is required.
Entry criteria for the studies in our review required evidence of sensitivity to aeroallergens and raised levels of serum IgE, which may not be representative of the asthmatic population in general. To date, most trials have excluded patients who do not have proven sensitivity to aeroallergens (i.e. non‐atopic individuals), thereby raising questions about the generalisability of study findings to the asthmatic population as a whole. It is estimated that up to 50% of severe asthmatic patients are non‐atopic. However, a recent small study (Garcia 2012; n = 41) that enrolled non‐atopic participants with severe asthma suggests that omalizumab may be effective in this population; larger studies are now required to confirm this finding,
The high number of screening failures in several studies is also noteworthy (e.g. Busse 2001: 1117 screened, 525 enrolled; Lanier 2009: 1433 screened, 628 enrolled; Solèr 2001: 1356 screened, 546 enrolled). IgE levels outside the range of those set as entry criteria were the most common reason for screening failure. Even within this selected stratum, there were participants who demonstrated little or no response to omalizumab. It is still not clear from studies published so far why some patients respond and others do not; it is therefore difficult to extrapolate some of the positive findings of this review to the general asthmatic population. Gevaert 2012 suggested that 'local tissue' IgE may have a significant role in mediating airway symptoms in asthma, and therefore serum IgE, or sensitivity to aeroallergens, may not be a good predictor of response to treatment.
Most participants were adults or adolescents, with only two trials (Lanier 2009 and Milgrom 2001; n = 962) recruiting paediatric participants between five and 12 years of age. Most of the 'adult' studies included some participants from 12 to 17 years of age, although this group of participants represented only a small percentage of the overall sample (between 6% and 8%; see Table 8). Confirmation of these effects in paediatric populations is also required, especially because compliance with monthly injections of medication may prove more challenging in paediatric patients. Milgrom 2001 reported a small number of withdrawals in children due to pain and fear of injection.
6. Paediatric populations.
| Trial | Omalizumab | Placebo | % |
| Bardelas 2012 | Footnote1 | ||
| Busse 2001 | 20/268 | 21/257 | 7.8% |
| Busse 2011 | Footnote2 | ||
| Hanania 2011 | 23/427 | 16/421 | 4.6% |
| Holgate 2004 and Holgate 2004a (N between 12 and 17 years) | 12/176 | 9/165 | 6.5% |
| Lanier 2009 | 421/421 | 206/206 | 100% |
| Milgrom 2001 | 225/225 | 109/109 | 100% |
| NCT00096954 | Footnote3 | ||
| Solèr 2001 | 18/274 | 17/272 | 6.4% |
1Bardelas 2012 included participants from 12 years of age. However, no details are provided in the study report on the proportion of paediatric participants in the sample (mean ages: omalizumab 41.9 ± 14.60 and placebo 40.7 ± 14.85).
2Busse 2011 included participants from six to 20 years of age. However, no details are provided in the study report on the proportion of paediatric participants in the sample (mean ages: omalizumab 10.8 ± 3.4 and placebo 10.9 ± 3.6).
3NCT00096954 included participants from 12 years of age. However, no details are provided in the study report on the proportion of paediatric participants in the sample (mean ages: omalizumab 36.0 ± 14.7 and placebo 38.1 ± 15.1).
Future clinical studies should have more realistic clinical designs that could be more readily generalised to a routine asthma clinic and the licenced indication for omalizumab. One such observational 'real‐life' study, EXCELS, is currently under way (Chen 2012) and may go some way toward reducing the strong Hawthorne effect that we have seen in the current analyses.
Quality of the evidence
On the whole, the quality of the included studies, with respect to our risk of bias assessment, was variable. Only seven studies (29%) were categorised as low in relation to risk of selection bias, whereas the risk of selection bias in the remaining 18 studies was viewed as unclear. Only four studies (17%) were evaluated as having low risk of performance and detection bias, and the remaining 21 studies were assessed as unclear. The risk of reporting bias was also viewed as a concern, as just three studies (13%) were assessed as having low risk of reporting bias, with the remaining 24 studies in the unclear category. However, 23 studies (96%) were viewed as having low risk of attrition bias, and only Lanier 2009 and Garcia 2012 were judged to be in the unclear category.
Potential biases in the review process
We believe that we have identified a very significant proportion of the research addressing this clinical question through the use of a comprehensive systematic search, conducted by a highly experienced information specialist who has also provided considerable support in the identification of unpublished studies. At the same time, we acknowledge that there is a possibility of publication bias in this review, in that through failure to identify unpublished negative trials, the positive effects of omalizumab may be overestimated and, conversely, any failure to have identified unpublished positive trials may have reduced our estimate of the therapeutic benefit. However, these are concerns with most systematic reviews.
In addition, we acknowledge the possibility of study selection bias; however, all studies were independently evaluated by two review authors, and we are confident that studies excluded from the analyses were assessed on the basis of consistent and appropriate criteria.
Agreements and disagreements with other studies or reviews
We have added 11 new clinical trials to the 14 reported in Walker 2006. Our emphasis has shifted to primarily the subcutaneous route of administration of omalizumab (18 studies in total) to maintain consistency between the focus of the review and current clinical treatment. The 11 new clinical trials added to the review all focus on subcutaneous omalizumab versus placebo; therefore the conclusions pertaining to inhaled and intravenous omalizumab are unchanged, although our assessment of the quality, with respect to risk of bias, of the inhaled and intravenous omalizumab trials has been updated to reflect developments in Cochrane methodology. In broad terms, our conclusions related to subcutaneous omalizumab are similar to those of Walker 2006, although we have added to the update outcomes that consider mortality, hospitalisations and adverse events to sharpen the applicability of the review. The most consistent finding from Walker 2006 that subcutaneous omalizumab reduces the likelihood of asthma exacerbations, when compared with placebo, is strengthened by this current update.
Authors' conclusions
Implications for practice.
Data from the included trials have shown that omalizumab is both effective and safe in patients with moderate to severe asthma that is uncontrolled on moderate to high doses of inhaled steroids with or without long‐acting beta2‐agonists. Insufficient evidence of benefit has been found in participants specifically with severe OCS‐dependent asthma. Very few studies have explored efficacy in children with moderate to severe asthma. Although the drug does enable a modest reduction in the dose of inhaled steroids, it is not clear whether this outcome would justify the cost of this expensive treatment. Because omalizumab is an expensive treatment option, it will be important to determine which people would benefit most from its use. To justify treatment with anti‐IgE, the amount of steroid that asthma sufferers are able to forego as a result of therapy would need to result in meaningful advantages in terms of lower risk derived from reduced exposure to steroids. The effect of the drug in participants with extreme values of serum IgE have not been evaluated. Finally, direct comparisons with other controller medications such as leukotriene antagonists or the novel anti‐interleukin (IL)‐5 therapies or biomarker‐based (e.g. sputum eosinophil count) treatment strategies have not been evaluated. Thus, omalizumab remains one of the therapeutic options in patients with atopic asthma whose condition remains uncontrolled despite optimum therapy with high levels of inhaled corticosteroids and long‐acting bronchodilators, and in those for whom this therapy can be afforded.
Implications for research.
Further research is needed to examine the role of omalizumab as a treatment for chronic asthma. Specifically, the following are needed.
Clinical trials that assess the long‐term use of omalizumab, how and when to reduce or stop treatment and whether benefits continue after discontinuation.
More studies assessing the steroid sparing effect in the most severe asthma group.
Further clinical assessment in the paediatric population.
Clinical assessment of the effects of omalizumab in participants with multiple allergic diseases (e.g. allergic asthma and eczema).
Efficacy in participants with extremes of serum IgE (i.e. very high IgE, as in conditions such as allergic bronchopulmonary aspergillosis (ABPA)) and with low IgE (modest atopy or non‐atopic individuals).
Direct comparison with other newer controller medications and management strategies.
Research to identify clinical, biochemical and genetic markers predictive of response.
What's new
| Date | Event | Description |
|---|---|---|
| 13 June 2013 | New citation required and conclusions have changed | Title changed and inclusion criteria limited to omalizumab. 11 new trials of subcutaneous omalizumab included: Bardelas 2012; Busse 2011; Chanez 2010; Garcia 2012; Gevaert 2012; Hanania 2011; Lanier 2009; Massanari 2010; NCT00096954; NCT01007149; Ohta 2009. New risk of bias, summary of findings table added, new author team and complete re‐write of text. |
| 13 June 2013 | New search has been performed | New literature search run |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 10 February 2010 | New search has been performed | Literature search re‐run |
| 30 June 2008 | Amended | Converted to new review format. |
| 21 February 2006 | New citation required and conclusions have changed | This review includes data from six new trials. Two of these were conducted in large samples of inhaled steroid‐dependent asthma patients (SOLAR; INNOVATE) and the remainder were conducted in mild, non‐steroid dependent asthma patients (Djukanovic 2004; Bruno 2005; van Rensen 2005; Hanf 2005). One of these studies assessed the effects of treatment in particularly severe adult and adolescent asthma patients. The data from these studies have improved the precision of our summary effect estimates. Assessment of this drug in children remains a priority. |
| 30 January 2004 | New search has been performed | This review has been updated with additional data that were not available when the initial version of the review was published. These data were obtained from the Food and Drug Administration's medical officer's review of clinical trial data. One unpublished study has since been published as a full article (Holgate 2004). The outcomes enhanced by these new data were exacerbations (for steroid stable and tapering phases), and also mean reduction in inhaled steroid consumption for three studies (Busse 2001; Solèr 2001; Holgate 2004). One study published in abstract form has come to the attention of the reviews (SOLAR 2003). This placebo‐controlled study recruited people with co‐existent asthma and rhinitis. |
Acknowledgements
The authors are extremely grateful for the Overseas Researcher bursary made available by the Thriplow Charitable Trust for completion of the first version of this review. This contribution enabled Dr Michele Monteil to travel to the Airways Group Editorial Base and work on the review for three weeks. A grant from Nederlands Astma Fonds enabled us to update this review in 2006. We are grateful to Dr Ken MacRitchie from Novartis, who provided abstracts for some of the trials, and who assisted in making contact with study authors. We are also very grateful to Steve Cook of Acumed, who provided us with unpublished data. Very many thanks to Karen Blackhall, Bettina Reuben and Steve Milan, who provided extensive clerical, technical and methodological support. Thanks to Donna‐Marie Sugden for checking the synopsis and review from a consumer's perspective. The editorial input of Dr Chris Cates and Professor Paul Jones was gratefully received.
In 2012 the authors responsible for the update of this review would particularly like to acknowledge the excellent support and assistance provided by Emma Welsh, Liz Stovold and Emma Jackson of the Cochrane Airways Review Group, together with the greatly appreciated guidance received from Chris Cates (Cochrane Airways Review Group Co‐ordinating Editor). We are also grateful to Dr Marc Vaillancourt from Novartis for providing clarification on the identification of two trials. The support provided by librarians Judith Scammel, Jane Appleton and Hilary Garrett at St Georges, University of London, is also greatly appreciated. As well, we would like to thank the corresponding authors Makoto Hoshino and Chris Corrigan for providing further information about their studies.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy for the Cochrane Airways Group Register of Trials
Strategy used for 2013 update
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 anti‐IgE
#6 "anti‐immunoglobulin E"
#7 omalizumab
#8 rhuMAb‐E25
#9 Xolair
#10 "monoclonal antibody"
#11 #5 or #6 or #7 or #8 or #9 or #10
#12 #4 and #11
[In search line #1, MISC1 denotes the field where the reference has been coded for condition, in this case, asthma]
Strategy used for previous versions
anti‐IgE OR "anti‐immunoglobulin E" OR "anti‐IgE antibody" OR "anti‐immunoglobulin E antibody" OR Omalizumab OR rhuMAb‐E25 or Xolair
[Limited to asthma records in the register]
Appendix 3. Archived results from trials of intravenous and inhaled omalizumab
Primary outcomes
1. Exacerbations
Intravenous omalizumab
Odds ratio of having one or more exacerbations
Fewer participants had exacerbations compared with placebo treatment in Milgrom 1999 (omalizumab: 32/106 vs placebo: 47/105; P = 0.01) during the steroid stable phase.
During the steroid reduction phase, asthma exacerbations were also reduced following treatment with intravenous omalizumab, with 30.2% of participants in the actively treated group having at least one exacerbation versus 44.8% of controls (P = 0.03) (Milgrom 1999).
2. Steroid reduction/withdrawal
Intravenous omalizumab
Following IV omalizumab, no significant difference was noted between the numbers of participants in treated (18.6%, 18/97) and control (11.8%, 11/93) groups who achieved complete withdrawal of daily ICS (Milgrom 1999).
Intravenous high‐dose omalizumab also resulted in more treated participants (50/97, 51.6%) achieving a greater than 50% reduction than control participants (35/93, 37.6%) (Milgrom 1999; P = 0.05).
Secondary outcomes
1. Symptom scores
Intravenous omalizumab
Asthma symptom scores were significantly lower in the active group compared with the placebo group during the steroid stable phase. Mean asthma scores at 12 weeks were 2.8 (SD 1.01) in the high‐dose treatment group compared with 3.1 (SD 1.02) in the control group (P = 0.008) (Milgrom 1999).
A small (but statistically significant) reduction in mean asthma symptom scores was noted in participants treated with IV omalizumab at the end of 20 weeks, after the steroid reduction phase (2.7 (SD 1.01)) compared with placebo 2.9 (SD 1.0; P < 0.05) (Milgrom 1999).
Intravenous omalizumab versus placebo (in participants not receiving ICS)
Pooled analysis of symptom scores in mild asthmatic participants who received intravenous omalizumab did not show a treatment effect in favour of omalizumab (SMD ‐0.33, 95% CI ‐0.96 to 0.31).
2. Health‐related quality of life
Intravenous omalizumab
In participants treated with IV omalizumab, one study (Milgrom 1999) showed a mean increase of 1.4 in the high‐dose group versus 0.8 in the placebo group on the Asthma Quality of Life Questionnaire for adults (scale 1 to 7) (omalizumab group vs own baseline P < 0.001; placebo vs own baseline P value not published). Paediatric results were similar (values not presented).
3. Rescue medication use
Intravenous omalizumab
A significant reduction in rescue medication use was noted following treatment with IV high‐dose omalizumab. At the end of the stable steroid phase, participants reduced their albuterol use by 14% (1.2 puffs per day) from baseline in the actively treated group compared with 10% (0.8 puffs per day) in the placebo group (P = 0.02) (Milgrom 1999).
Statistically significant changes in rescue medication usage in favour of omalizumab achieved at 12 weeks apparently continued during the steroid reduction phase (P values not available; Milgrom 1999).
Intravenous omalizumab versus placebo (in patients not receiving ICS)
No significant difference in rescue medication was observed between treatment with intravenous omalizumab or placebo (Boulet 1997; Fahy 1997) (P = 0.67).
4. Lung function
Intravenous omalizumab
Change from baseline in am PEF
A significant increase in morning PEF of 30.7 L/min was reported in actively treated participants compared with 11.3 L/min in the control group (P = 0.007; Milgrom 1999).
End of treatment FEV1
In participants who received intravenous IV omalizumab, no significant difference in FEV1 was reported (Milgrom 1999).
Aerosolised omalizumab
No significant differences were found in FEV1 or morning PEF between omalizumab‐treated and placebo‐treated participants (FEV1: P = 0.12; PEF: P = 0.3).
Intravenous omalizumab (in participants not receiving ICS)
No statistically significant differences were detected in FEV1 or PEF at the end of study protocols (no P values reported in published papers). A pooled analysis of FEV1 was non‐significant (SMD 0.51, 95% CI ‐0.13 to 1.15). Pooled analysis of PEF was also non‐significant (SMD 0.35, 95% CI ‐0.29 to 1.00). This represents a difference of 32 mL (95% CI ‐26.53 to 91.5).
5. Adverse events
Aerosolised omalizumab
More complaints of headache were seen among aerosolised omalizumab‐treated participants compared with placebo participants (nine of 12 participants receiving low‐dose omalizumab, eight of 10 participants receiving high‐dose omalizumab and three of 11 placebo‐treated participants). However, these differences did not achieve statistical significance. One participant developed IgG and IgA anti‐omalizumab antibodies during the treatment phase of the trial. These antibodies were not detected at follow‐up 11 weeks after completion of the study.
Intravenous omalizumab (in participants not receiving ICS)
Few adverse events were noted among participants with mild asthma who received intravenous omalizumab, and these events were not significantly different from side effects observed in placebo‐treated participants.
In the intravenous study in moderate to severe participants, withdrawals were similar in the actively treated population (three of 106 (omalizumab) vs five of 105 (placebo); no reported P values, but obviously not significant) (Milgrom 1999).
Data and analyses
Comparison 1. Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with at least one exacerbation (ICS and OCS users) | 10 | 3261 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.46, 0.65] |
| 1.1 Moderate to severe | 7 | 2889 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.42, 0.60] |
| 1.2 Severe (ICS) | 2 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.50, 1.99] |
| 1.3 Severe (ICS and OCS) | 1 | 95 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.66, 4.13] |
| 2 Exacerbations requiring oral steroids | 3 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Moderate to severe asthma (ICS + mixed treatments) | 2 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.37, 0.73] | |
| 2.2 Severe asthma (ICS + LABA) | 1 | Rate Ratio (Fixed, 95% CI) | 0.66 [0.45, 0.97] | |
| 2.3 Severe asthma (ICS + LABA + other treatment) | 1 | Rate Ratio (Fixed, 95% CI) | 0.72 [0.53, 0.98] | |
| 2.4 Severe asthma (ICS and OCS) | 1 | Rate Ratio (Fixed, 95% CI) | 0.95 [0.63, 1.43] | |
| 3 Hospitalisations | 4 | 1824 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 3.1 Moderate to severe asthma | 4 | 1824 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.06, 0.42] |
| 3.2 Severe asthma | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mortality | 9 | 4245 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.67] |
| 4.1 Moderate to severe asthma | 7 | 3124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.85] |
| 4.2 Severe asthma | 2 | 1121 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.73] |
| 5 Peak expiratory flow rate (am) | 4 | 1651 | Mean Difference (IV, Fixed, 95% CI) | 3.56 [‐5.05, 12.18] |
| 5.1 Moderate to severe asthma | 3 | 1405 | Mean Difference (IV, Fixed, 95% CI) | 4.93 [‐4.11, 13.97] |
| 5.2 Severe asthma | 1 | 246 | Mean Difference (IV, Fixed, 95% CI) | ‐9.90 [‐38.27, 18.47] |
| 6 Change in am PEF | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6.1 Moderate to severe asthma | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Severe asthma | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 FEV1 (mL) | 2 | 1071 | Mean Difference (IV, Fixed, 95% CI) | 68.31 [‐23.45, 160.07] |
| 7.1 Moderate to severe | 2 | 1071 | Mean Difference (IV, Fixed, 95% CI) | 68.31 [‐23.45, 160.07] |
| 7.2 Severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Change in FEV1 (mL) | 5 | 1463 | Mean Difference (Fixed, 95% CI) | 56.39 [16.82, 95.96] |
| 8.1 Moderate to severe asthma | 2 | 732 | Mean Difference (Fixed, 95% CI) | 67.29 [23.75, 110.83] |
| 8.2 Severe asthma | 3 | 731 | Mean Difference (Fixed, 95% CI) | 4.68 [‐90.16, 99.52] |
| 9 Change in FEV1 predicted | 4 | 1498 | Mean Difference (Fixed, 95% CI) | 2.15 [1.01, 3.30] |
| 9.1 Moderate to severe asthma | 3 | 1079 | Mean Difference (Fixed, 95% CI) | 2.01 [0.76, 3.27] |
| 9.2 Severe asthma | 1 | 419 | Mean Difference (Fixed, 95% CI) | 2.8 [0.10, 5.50] |
| 10 Symptom scores | 10 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Moderate to severe asthma | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Severe asthma | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Mean change in Wasserfallen asthma score | 1 | Symptoms (Fixed, 95% CI) | Totals not selected | |
| 11.1 Moderate to severe asthma | 1 | Symptoms (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Quality of life—change from baseline in AQLQ scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Moderate to severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Severe asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Global evaluation rated good to excellent | 4 | 1136 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.67, 2.68] |
| 13.1 Moderate to severe asthma | 1 | 405 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.32 [2.19, 5.05] |
| 13.2 Severe asthma | 3 | 731 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.26, 2.26] |
| 14 Rescue medication | 9 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 14.1 Moderate to severe asthma | 4 | Mean Difference (Fixed, 95% CI) | ‐0.58 [‐0.84, ‐0.31] | |
| 14.2 Severe asthma (ICS) | 4 | Mean Difference (Fixed, 95% CI) | ‐0.30 [‐0.49, ‐0.10] | |
| 14.3 Severe asthma (ICS and OCS) | 1 | Mean Difference (Fixed, 95% CI) | ‐0.4 [‐4.81, 4.01] | |
| 15 Adverse event—any | 14 | 5167 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.06] |
| 15.1 Moderate to severe asthma | 8 | 3246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.09] |
| 15.2 Severe asthma | 6 | 1921 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.76, 1.16] |
| 16 Adverse event—serious | 15 | 5713 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.91] |
| 16.1 Moderate to severe asthma | 9 | 3792 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.48, 0.95] |
| 16.2 Severe asthma | 6 | 1921 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.05] |
| 17 Injection site reactions | 9 | 3577 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.33, 2.24] |
| 17.1 Moderate to severe asthma | 6 | 2001 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.31, 2.43] |
| 17.2 Severe asthma | 3 | 1576 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.96, 2.57] |
1.11. Analysis.
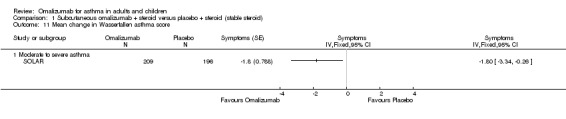
Comparison 1 Subcutaneous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 11 Mean change in Wasserfallen asthma score.
Comparison 2. Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with exacerbation | 5 | 1726 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.39, 0.62] |
| 1.1 Moderate to severe asthma | 3 | 1388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.36, 0.59] |
| 1.2 Severe asthma | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.30, 1.16] |
| 1.3 Severe (ICS and OCS users) | 1 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.38, 2.01] |
| 2 Exacerbations requiring hospitalisation | 3 | 1405 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.48] |
| 2.1 Moderate asthma | 3 | 1405 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.48] |
| 2.2 Severe asthma | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number of participants achieving complete inhaled steroid withdrawal | 4 | 1634 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [2.00, 3.13] |
| 3.1 Moderate to severe | 3 | 1388 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [2.10, 3.39] |
| 3.2 Severe | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.80, 2.98] |
| 4 Mean change in steroid consumption (BDP equivalent) | 3 | 1188 | Mean Difference (IV, Fixed, 95% CI) | ‐118.76 [‐154.38, ‐83.14] |
| 4.1 Moderate to severe asthma | 2 | 942 | Mean Difference (IV, Fixed, 95% CI) | ‐114.08 [‐150.03, ‐78.13] |
| 4.2 Severe asthma | 1 | 246 | Mean Difference (IV, Fixed, 95% CI) | ‐372.0 [‐636.43, ‐107.57] |
| 5 > 50% reduction in inhaled steroid usage | 4 | 1634 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [2.02, 3.10] |
| 5.1 Moderate to severe asthma | 3 | 1388 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.93, 3.08] |
| 5.2 Severe asthma | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.84 [1.66, 4.86] |
| 6 Mean steroid dose at end of reduction phase | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Moderate to severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Severe asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life—change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Moderate to severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Severe asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Numbers of participants achieving clinically relevant improvement in quality of life (> 0.5) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Severe asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Global evaluation rated good to excellent | 2 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.72 [2.04, 3.62] |
| 9.1 Moderate to severe asthma | 2 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.72 [2.04, 3.62] |
| 9.2 Severe asthma | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Rescue medication (puffs per day) | 4 | 1373 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.05, ‐0.43] |
| 10.1 Moderate to severe asthma | 2 | 1071 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.06, ‐0.40] |
| 10.2 Severe asthma (ICS) | 1 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.65, 0.25] |
| 10.3 Severe asthma (ICS and OCS) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐7.40, 1.80] |
2.6. Analysis.
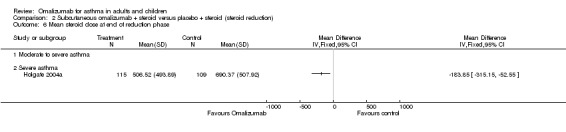
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 6 Mean steroid dose at end of reduction phase.
2.7. Analysis.
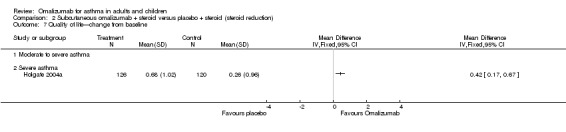
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 7 Quality of life—change from baseline.
2.8. Analysis.
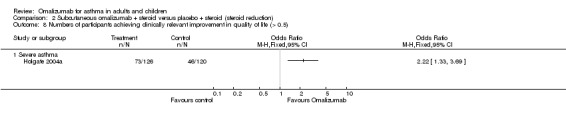
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 8 Numbers of participants achieving clinically relevant improvement in quality of life (> 0.5).
2.9. Analysis.
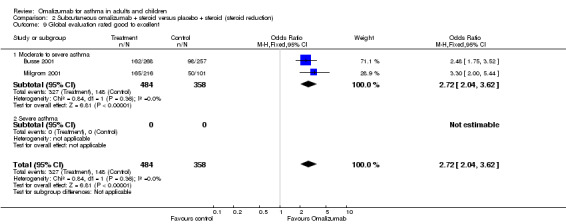
Comparison 2 Subcutaneous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 9 Global evaluation rated good to excellent.
Comparison 3. Subcutaneous omalizumab + ICS and OCS versus placebo + ICS and OCS steroid (steroid reduction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants achieving complete oral steroid withdrawal | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of participants with exacerbation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in AQLQ scores | 6 | Mean Difference (Fixed, 95% CI) | 0.31 [0.23, 0.39] | |
| 3.1 Moderate to severe asthma | 3 | Mean Difference (Fixed, 95% CI) | 0.30 [0.17, 0.42] | |
| 3.2 Severe asthma | 3 | Mean Difference (Fixed, 95% CI) | 0.32 [0.21, 0.43] |
3.2. Analysis.
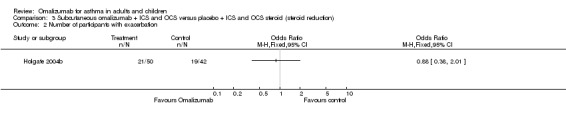
Comparison 3 Subcutaneous omalizumab + ICS and OCS versus placebo + ICS and OCS steroid (steroid reduction), Outcome 2 Number of participants with exacerbation.
3.3. Analysis.
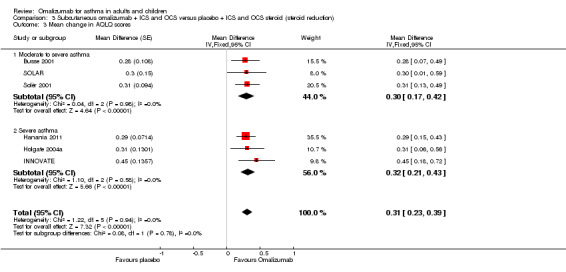
Comparison 3 Subcutaneous omalizumab + ICS and OCS versus placebo + ICS and OCS steroid (steroid reduction), Outcome 3 Mean change in AQLQ scores.
Comparison 4. Subcutaneous omalizumab versus placebo (without inhaled corticosteroids).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in PC20 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
Comparison 4 Subcutaneous omalizumab versus placebo (without inhaled corticosteroids), Outcome 1 FEV1 (litres).
4.2. Analysis.
Comparison 4 Subcutaneous omalizumab versus placebo (without inhaled corticosteroids), Outcome 2 FEV1 (% predicted).
4.3. Analysis.
Comparison 4 Subcutaneous omalizumab versus placebo (without inhaled corticosteroids), Outcome 3 Change in PC20.
Comparison 5. Subcutaneous omalizumab + steroid versus placebo + steroid (trial extension).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants achieving complete inhaled steroid withdrawal | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Moderate to severe | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Severe | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Participants with one or more exacerbation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Moderate to severe asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Severe asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Hospitalisations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Moderate to severe asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Severe asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants with any adverse event | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Moderate to severe asthma | 1 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.39] |
| 4.2 Severe asthma | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
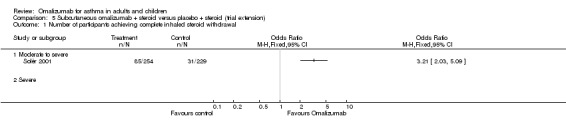
Comparison 5 Subcutaneous omalizumab + steroid versus placebo + steroid (trial extension), Outcome 1 Number of participants achieving complete inhaled steroid withdrawal.
5.2. Analysis.
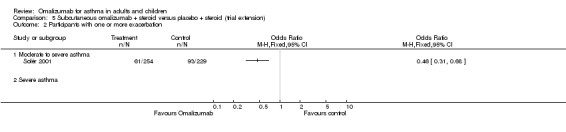
Comparison 5 Subcutaneous omalizumab + steroid versus placebo + steroid (trial extension), Outcome 2 Participants with one or more exacerbation.
5.3. Analysis.
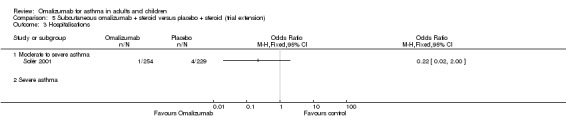
Comparison 5 Subcutaneous omalizumab + steroid versus placebo + steroid (trial extension), Outcome 3 Hospitalisations.
5.4. Analysis.
Comparison 5 Subcutaneous omalizumab + steroid versus placebo + steroid (trial extension), Outcome 4 Number of participants with any adverse event.
Comparison 6. High‐dose intravenous omalizumab + steroid versus placebo + steroid (stable steroid).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rescue medication usage | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mild asthma | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Moderate to severe asthma | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Severe asthma | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Morning PEF | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mild asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Moderate to severe asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Moderate to severe | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Symptom scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Moderate to severe asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of life | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mild asthma | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Moderate to severe asthma | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Severe asthma | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of participants with > 50% reduction in symptom score | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Moderate to severe asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Severe asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
Comparison 6 High‐dose intravenous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 1 Rescue medication usage.
6.2. Analysis.
Comparison 6 High‐dose intravenous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 2 Morning PEF.
6.3. Analysis.
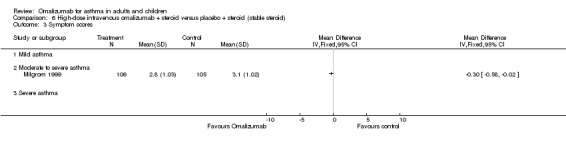
Comparison 6 High‐dose intravenous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 3 Symptom scores.
6.4. Analysis.
Comparison 6 High‐dose intravenous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 4 Quality of life.
6.5. Analysis.
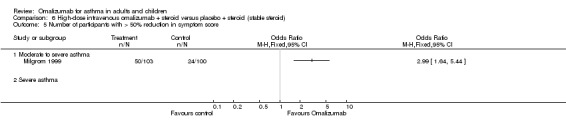
Comparison 6 High‐dose intravenous omalizumab + steroid versus placebo + steroid (stable steroid), Outcome 5 Number of participants with > 50% reduction in symptom score.
Comparison 7. High‐dose intravenous omalizumab + steroid versus placebo + steroid (steroid reduction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants achieving complete inhaled steroid withdrawal | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mild asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Moderate to severe | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Severe | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 > 50% reduction in inhaled steroid usage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mild asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Moderate to severe asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Severe asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Moderate to severe asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants with > 50% reduction in symptom scores | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mild asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Moderate to severe asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Severe asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of participants with exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Mild asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Moderate to severe asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Severe asthma | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
Comparison 7 High‐dose intravenous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 1 Number of participants achieving complete inhaled steroid withdrawal.
7.2. Analysis.
Comparison 7 High‐dose intravenous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 2 > 50% reduction in inhaled steroid usage.
7.3. Analysis.
Comparison 7 High‐dose intravenous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 3 Symptom score.
7.4. Analysis.
Comparison 7 High‐dose intravenous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 4 Number of participants with > 50% reduction in symptom scores.
7.5. Analysis.
Comparison 7 High‐dose intravenous omalizumab + steroid versus placebo + steroid (steroid reduction), Outcome 5 Number of participants with exacerbations.
Comparison 8. Intravenous omalizumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rescue medication use (one week after end of treatment) | 2 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.50, 0.77] |
| 1.1 Mild asthma | 2 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.50, 0.77] |
| 1.2 Moderate asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 2 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.13, 1.15] |
| 2.1 Mild asthma | 2 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.13, 1.15] |
| 2.2 Moderate asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Fall in FEV1 after allergen challenge (%) (zero to one hour) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Fall in FEV1 after allergen challenge (%) (two to seven hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Peak expiratory flow (am) | 2 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.29, 1.00] |
| 5.1 Mild asthma | 2 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.29, 1.00] |
| 5.2 Moderate asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Severe | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Symptom scores | 2 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.96, 0.31] |
| 6.1 Mild asthma | 2 | 39 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.96, 0.31] |
| 6.2 Moderate asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
Comparison 8 Intravenous omalizumab versus placebo, Outcome 1 Rescue medication use (one week after end of treatment).
8.2. Analysis.
Comparison 8 Intravenous omalizumab versus placebo, Outcome 2 FEV1 (litres).
8.3. Analysis.
Comparison 8 Intravenous omalizumab versus placebo, Outcome 3 Fall in FEV1 after allergen challenge (%) (zero to one hour).
8.4. Analysis.
Comparison 8 Intravenous omalizumab versus placebo, Outcome 4 Fall in FEV1 after allergen challenge (%) (two to seven hours).
8.5. Analysis.
Comparison 8 Intravenous omalizumab versus placebo, Outcome 5 Peak expiratory flow (am).
8.6. Analysis.
Comparison 8 Intravenous omalizumab versus placebo, Outcome 6 Symptom scores.
Comparison 9. High‐dose aerosolised omalizumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Area under the curve for % fall in FEV1 (early response: zero to one hour) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Severe | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Area under the curve for % fall in FEV1 (late response: three to seven hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Peak expiratory flow (am) (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Severe | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
Comparison 9 High‐dose aerosolised omalizumab versus placebo, Outcome 1 FEV1 (litres).
9.2. Analysis.
Comparison 9 High‐dose aerosolised omalizumab versus placebo, Outcome 2 Area under the curve for % fall in FEV1 (early response: zero to one hour).
9.3. Analysis.
Comparison 9 High‐dose aerosolised omalizumab versus placebo, Outcome 3 Area under the curve for % fall in FEV1 (late response: three to seven hours).
9.4. Analysis.
Comparison 9 High‐dose aerosolised omalizumab versus placebo, Outcome 4 Peak expiratory flow (am) (L/min).
Comparison 10. Low‐dose aerosolised omalizumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Area under curve for fall in FEV1 (% × minutes)—early response (zero to one hour) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Area under curve for fall in FEV1 (% × minutes)—late response (three to seven hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Peak expiratory flow (am) (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FEV1 (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mild asthma | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Moderate asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Severe asthma | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
Comparison 10 Low‐dose aerosolised omalizumab versus placebo, Outcome 1 Area under curve for fall in FEV1 (% × minutes)—early response (zero to one hour).
10.2. Analysis.
Comparison 10 Low‐dose aerosolised omalizumab versus placebo, Outcome 2 Area under curve for fall in FEV1 (% × minutes)—late response (three to seven hours).
10.3. Analysis.
Comparison 10 Low‐dose aerosolised omalizumab versus placebo, Outcome 3 Peak expiratory flow (am) (L/min).
10.4. Analysis.
Comparison 10 Low‐dose aerosolised omalizumab versus placebo, Outcome 4 FEV1 (litres).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bardelas 2012.
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled study | |
| Participants | Treatment group: 136. Age: 41.9 (14.6). Males: 43 (31.6%). Baseline lung function: mean % predicted FEV1 (SD): 74.4 (17.5) Control group: 135. Age: 40.7 (14.9). Males: 48 (35.6%). Baseline lung function: mean % predicted FEV1 (SD): 76.5 (17.0) Inclusion criteria stated as: males and females; 12 years or over; inadequately controlled persistent allergic asthma (ACT score equal to or less than 19) and positive skin prick test; on step 4 or above of NHLBI maintenance treatment (ICS + LABA/leukotriene receptor antagonist/theophylline/zileuton); total serum IgE 30 to 700 IU/mL. One or more of the following with four weeks of screening phase: symptoms > 2 days/wk; night‐time awakenings ≥ 1 time/wk; use of SABA > 2 days/wk; FEV1 ≤ 80% predicted Exclusion criteria stated as: body weight > 150 kg; current smoker or ex‐smoker within last year, or pack‐year history ≥ 10 years; history of intubation for asthma or anaphylaxis; systemic steroids within last four weeks; active lung disease other than asthma; current or anticipated use of beta‐blockers or methotrexate, gold, cyclosporine or troleandomycin within three months of enrolment; elevated serum IgE levels for reasons other than atopy or a combination of serum IgE levels and weight requiring doses of omalizumab greater than 750 mg per four weeks Location(s): USA |
|
| Interventions | Omalizumab subcutaneous based on body weight and serum IgE; 150 or 300 mg every four weeks or 225, 300 or 375 mg every two weeks versus placebo with same inactive ingredients as study drug Background inhaled steroid dose: at least 250 mcg fluticasone twice daily or 320 mcg budesonide twice daily |
|
| Outcomes | Change from baseline in ACT scores, Investigator’s Global Evaluation of Treatment Effectiveness (IGETE), Work productivity and activity impairment questionnaire–asthma (WPAI‐A), e‐diaries, spirometric measurements, use of rescue corticosteroids, safety assessment |
|
| Notes | Two‐week screening period. 24 weeks Co‐medication: background asthma maintenance therapy continued unchanged (e.g. step four or above of NHLBI maintenance treatment (ICS + LABA/leukotriene receptor antagonist/theophylline/zileuton). Oral or IV rescue steroids were allowed if required for an exacerbation This study is identified as NCT00267202 in the Methods section of Bardelas 2012. However, it has been confirmed by Dr Marc Vaillancourt at Novartis that NCT00267202 relates to Massanari 2010, and that the Bardelas 2012 study is in fact NCT00870584 Authors are employees/stockholders of sponsoring pharmaceutical company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled—placebo contained same inactive ingredients as active preparation. Most outcome data recorded by participants themselves, but not specified whether those analysis data were also blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced dropout from groups, all participants clearly accounted for in flow chart |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures reported However, subgroup analysis was ad hoc and produced the only significant results |
Boulet 1997.
| Methods | Randomised, double‐blind, parallel‐group placebo‐controlled trial | |
| Participants | N = 20. Mean age: 27 ± 8.06. Eight females. Mild asthmatic participants were recruited Inclusion criteria: stable, mild asthma, requiring only an inhaled beta2‐agonist on demand to control; at least one highly positive allergy skin prick test to at least one aeroallergen; early asthmatic response; FEV1 > 70%; methacholine provocative concentration causing 20% fall in FEV1 Exclusion criteria: history of anaphylaxis; recently unstable asthma (ER visit in previous six weeks); respiratory infection or aeroallergen exposure (other than HDM) within four weeks; smoking within 12 months; women of child‐bearing age and lacking effective contraception. Baseline characteristics: FEV1: 92 ± 11; IgE: placebo: 1808 ± 3382, omalizumab: 616 ± 487 |
|
| Interventions | Intravenous rhuMAb‐E25 (2.0 mg/kg) or placebo at day 0. Six subsequent injections of 1.0 mg per kg versus placebo. Treatment lasted for 10 weeks | |
| Outcomes | Tolerance and safety, allergen PC15, methacholine responsiveness, serum rhuMab‐E25 and IgE levels, respiratory symptoms and pulmonary function, cutaneous responses to allergen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not included in study report |
| Allocation concealment (selection bias) | Unclear risk | Details not included in study report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One of the 20 participants did not complete the trial |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Busse 2001.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial. Randomisation by computer‐generated random number sequences | |
| Participants | N = 525. Age range: 12 to 75, 215 males. Treatment group: N = 268. Control group: N = 257. Participants with moderate to severe asthma were recruited Inclusion criteria: asthma diagnosed for longer than one year; positive response to skin prick to one common allergen; total IgE serum > 30 IU/mL and < 700 IU/mL; FEV1 reversibility of 12% |
|
| Interventions | Subcutaneous omalizumab (0.016 mg/kg IgE (IU/mL) per four weeks). Participants received 150 or 300 mg every four weeks or 225, 300 or 375 mg every two weeks, or placebo. Initial phase of the trial was a stable steroid phase of 16 weeks' duration, followed by a 12‐week steroid reduction phase | |
| Outcomes | Number of participants with exacerbations, mean number of exacerbations per participant, mean number of days per exacerbation, adverse events, reduction in ICS, rescue medication usage, global evaluation, serum IgE levels | |
| Notes | Jadad score: 4 Trial 008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated random number sequences |
| Allocation concealment (selection bias) | Unclear risk | Details not included in study report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details of participants not completing are included in trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Busse 2011.
| Methods | Randomised, double‐blind, parallel‐group trial | |
| Participants | Treatment group: 208. Age: 10.9 ± 3.6. Males: 122 (59%). Baseline lung function: mean % predicted FEV1 (SD): 92.9 ± 18.7 Control group: 211. Age: 10.8 ± 3.4. Males: 120 (57%). Baseline lung function: mean % predicted FEV1 (SD): 92.2 ± 17.6 73% of participants had moderate/severe asthma according to NAEPP guidelines Inclusion criteria stated as: males and females between the ages of 6 and 20 years; both body weight and total serum IgE suitable for omalizumab dosing (more information about this criterion can be found in the protocol); diagnosis of asthma made by a physician more than one year before study entry OR diagnosis of asthma made less than one year before study entry but asthma symptoms for longer than 1 year before study entry; receiving long‐term asthma control therapy OR symptoms consistent with persistent asthma OR evidence of uncontrolled disease; positive prick skin test to at least one perennial allergen (e.g. dust mite, cockroach, mold, cat, dog, rat, mouse); live in a preselected zip code area; able to perform spirometry measurements; willing to sign informed consent or parent or guardian willing to provide informed consent; previously had chicken pox or received varicella (chicken pox) vaccine; some form of healthcare insurance that covers costs of medications Exclusion criteria stated as: if participant meets any of these criteria, not eligible at that time but may be reassessed: systemic prednisolone (or equivalent) during the two weeks before visit two; systemic prednisolone (or equivalent) for more than 30 of the 60 days before study entry; pregnancy or breast‐feeding; acute sinusitis or chest infection requiring antibiotics within one month of study screening; currently participating in another asthma‐related clinical trial or previously participated in an another asthma‐related trial within one month of study entry; does not sleep at least four nights per week in one home; lives with a foster parent; does not have access to a phone; plans to move during the study; previously treated with anti‐IgE therapy within one year of study entry; currently receiving or received hyposensitisation therapy to any allergen in the year before study entry; previously received hyposensitisation therapy to dust mite, Alternaria or cockroach for longer than six months in the three years before study entry. If participant meets any of these criteria, he or she is not eligible for the study and may not be reassessed: significant medical illness. More information on this criterion can be found in the protocol: certain medications within four weeks of study screening. More information on this criterion can be found in the protocol: known hypersensitivity to any ingredients of omalizumab or related drugs; diagnosis of cancer; being investigated for possible cancer, or history of cancer; will not allow study physician to manage asthma; does not primarily speak English (or Spanish at centres with Spanish‐speaking staff); history of severe anaphylactoid or anaphylactic reaction(s) Location(s): eight centres in USA |
|
| Interventions | Stated as: Subcutaneous injections of omalizumab will be administered every two or four weeks, along with standardised asthma care for 60 weeks, beginning with the randomisation visit. Dosage is dependent on participant's individual characteristics. Injection dose of omalizumab (75 to 375 mg) was calculated on the basis of individual weight and total serum IgE level to ensure a minimum monthly dose of 0.016 mg per kilogram of body weight per international unit of IgE per mL versus placebo Background inhaled corticosteroid dose: at least 180 μg budesonide once a day |
|
| Outcomes | Primary outcome measures stated as: maximum number of asthma symptom days (recorded monthly throughout study) Secondary outcome measures stated as: economic outcomes (recorded monthly throughout study); asthma‐related medical care resource utilisation (recorded monthly throughout study); asthma exacerbations (recorded monthly throughout study); pulmonary function and exhaled nitric oxide (recorded at various visits throughout the study); asthma control test or childhood asthma control test (recorded monthly throughout study); number of missed school/work days (recorded monthly throughout study); asthma‐specific quality of life (QOL) (recorded at various visits throughout study); asthma medication use, rescue beta‐agonist and inhaled corticosteroid (ICS) use (recorded at various visits throughout the study); safety (recorded at every study visit) |
|
| Notes | 60‐Week trial Co‐medication: oral prednisolone for exacerbations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not included in trial report |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not included in trial report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported as double‐blind. Nurses giving Rx aware of Rx allocation; all other staff and participants blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90% (386) included in primary outcome analysis 272 missed, 25% of Rx visits |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Chanez 2010.
| Methods | Randomised, double‐blind, placebo‐controlled study | |
| Participants | Treatment group: 20 (17 completed). Age: 45.7 ± 13.30. Males: 6 (30%). Baseline lung function: mean % predicted FEV1 (SD): 61.3 (14.83) Control group: 11 (8 completed). Age: 50.6 ± 16.31. Males: 6 (54.5%). Baseline lung function: mean % predicted FEV1 (SD): 66.6 (11.38) Inclusion criteria stated as: adults aged ≥ 18 years; participants with severe persistent allergic asthma with the following characteristics: FEV1 < 80% of predicted; frequent daily symptoms (≥ four days/wk on average) or nocturnal awakening (≥ one/wk on average); multiple severe asthma exacerbations: either ≥ two severe asthma exacerbations requiring an unscheduled medical intervention with systemic corticosteroid in the past year, or hospitalisation (including emergency room treatment) for an asthma exacerbation in the past year, despite a high‐dose inhaled corticosteroid > 1000 mg beclomethasone dipropionate or equivalent and inhaled long‐acting beta2‐agonist; an allergy to a perennial allergen demonstrated with convincing criteria (i.e. positive prick skin test or in vitro reactivity to a perennial aeroallergen (RAST)); total serum IgE level ≥ 30 to ≤ 700 IU/mL and suitable serum total IgE level; weight according to Xolair dosing tablets Exclusion criteria stated as: age < 18 years; smoking history > 20 pack‐years; asthma exacerbation during the four weeks before randomisation; history of food‐ or drug‐related severe anaphylactoid or anaphylactic reaction; elevated serum IgE levels for reasons other than allergy (e.g. parasite infections, hyperimmunoglobulin E syndrome, Wiskott‐Aldrich Syndrome, allergic bronchopulmonary aspergillosis); patients with active cancer, suspicion of cancer or any history of cancer; pregnant women; known hypersensitivity to omalizumab or to one of its components; previous treatment with omalizumab (indeed previous treatment with omalizumab could have modified the FceRI expression); participated in a clinical trial in the past three months Location(s): France |
|
| Interventions | Omalizumab injected subcutaneously every two weeks or every four weeks for 16 weeks (dose and dosing interval determined on the basis of participant body weight and pretreatment serum IgE level) versus placebo Background inhaled corticosteroid dose: at least 1000 mcg beclomethasone dipropionate or equivalent daily. Mean dose/d 3556 mcg ± 1157.8 BDP equivalent/d Participants receiving maintenance OCS at baseline = 7 (22%) |
|
| Outcomes | Primary outcome measures stated as: change (%) from baseline in FcϵRI (high‐affinity IgE receptor) expression on blood basophils and dendritic cells after 16 weeks of treatment with omalizumab as compared with placebo (time frame: baseline and week 16); change (%) from baseline in mean fluorescence intensity of FcϵRI after 16 weeks of treatment with omalizumab as compared with placebo (time frame: baseline and week 16) Secondary outcome measures stated as: change (%) from baseline in percent of basophils and dendritic cells expressing FcϵRI after 4, 8, 12 and 16 weeks of treatment (time frame: baseline, weeks 4, 8, 12 and 16); change (%) from baseline in mean fluorescence intensity of FcϵRI after 4, 8, 12 and 16 weeks of treatment (time frame: baseline, weeks 4, 8, 12 and 16); change from baseline in the number of days with asthma symptoms per week (time frame: baseline (four‐week screening period before randomisation) and end of study (weeks 12 to 16)); change from baseline in the number of puffs of rescue medication per week (time frame: baseline (four‐week screening period before randomisation) and end of study (weeks 12 to 16)); change from baseline in the number of nights with awakenings per week (time frame: baseline (four‐week screening period before randomisation) and end of study (weeks 12 to 16)); change from baseline in the number of days with impairment in daily activities per week (time frame: baseline (four‐week screening period before randomisation) and end of study (weeks 12 to 16)); change from baseline in the number of days with absence from school or work due to asthma symptoms (time frame: baseline (four‐week screening period before randomisation) and end of study (weeks 12 to 16)); change from baseline in the number of days with hospitalisations (time frame: baseline (four‐week screening period before randomisation) and end of study (weeks 12 to 16)); change from baseline in the number of unscheduled clinic visits (time frame: baseline (four‐week screening period before randomisation) and end of study (weeks 12 to 16)); change from baseline in morning daily peak expiratory flow (PEF) (time frame: baseline (four‐week screening period before randomisation) and end of study (weeks 12 to 16)); physician's overall assessment of treatment effectiveness (time frame: after 16 weeks of treatment) |
|
| Notes | 16‐Week trial Co‐medication: steroids and LABA (all), oral corticosteroids three and four, theophylline one and one, montelukast eight and four, anticholinergics six and six |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by centre and ratio of 2:1. Details of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six participants did not complete the trial (three in each group); reasons for their withdrawal are included in trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Djukanovic 2004.
| Methods | Randomised, double‐blind, parallel‐group placebo‐controlled trial Methods of allocation/blinding not reported |
|
| Participants | N = 46. Median age: 26 (range 19 to 48), Treatment group: 22; control group: 23. Gender: M/F: 22/24, FEV1 (% predicted): omalizumab: 84; placebo: 86 Inclusion criteria: stable, mild to moderate asthma (NHLBI definition); treatment with inhaled beta‐agonists only; exacerbation‐free six weeks before study entry; age 18 to 50 years; total serum IgE 30 to 700 IU/mL; +ve skin prick test to ≥ one allergen; airway hyperresponsiveness as defined by PC20 ≤ 8 mg/mL; sputum eosinophilia 2% or more of nonsquamous cells |
|
| Interventions | Sucutaneous omalizumab (0.016 mg/kg per IgE (IU/mL)) versus placebo Study duration: 16 weeks |
|
| Outcomes | Methacholine challenge; FEV1; serum free IgE levels | |
| Notes | Jadad score: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Details not included in study report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants were withdrawn, and details are included in trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Fahy 1997.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial | |
| Participants | N = 19. Mean age: 31.5 ± 4.87. Gender not reported. FEV1: 94.5% predicted ± 16.72; PC20 mg/mL: 0.62 ± 0.77; IgE IU/L: 141 ± 119. Mild asthmatics were recruited Inclusion criteria: FEV1 > 70%, bronchial hyperreactivity to methacholine, positive skin prick test to HDM, cat pelt/rye grass; serum IgE level < 500 IU/mL. Exclusion criteria: use of corticosteroids in previous six weeks, U/L RTI in previous six weeks, tobacco use/history of significant medical illness |
|
| Interventions | Intravenous infusion of rhuMAb‐E25 5 mg/mL (0.5 mg/kg) for nine visits versus placebo. Treatment lasted nine weeks | |
| Outcomes | FEV1, PEF (am and pm), asthma symptoms, albuterol use, total serum IgE, induced sputum, PC20, percentage fall in FEV1 during early and late response, blood eosinophil percentage | |
| Notes | Jadad score: 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not included in study report |
| Allocation concealment (selection bias) | Unclear risk | Details not included in study report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant did not complete the trial and was withdrawn after week four |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Fahy 1999.
| Methods | Randomised, multi‐centre, double‐blind, parallel‐group, placebo‐controlled study with identical matching placebo | |
| Participants | N = 33. Mean age: 28.64 ± 6.6; 21 male participants. Twelve participants were randomly assigned to receive E25 1 mg, 10 participants were randomly assigned to receive E25 10 mg and 11 were randomly assigned to receive placebo. Two participants dropped out of the placebo group. All had mild asthma Inclusion criteria: FEV1 > 70% predicted; bronchial hyperreactivity to methacholine; serum IgE < 300 IU/mL; positive skin prick test to aeroallergen Exclusion criteria: corticosteroids in previous six weeks; symptoms of upper/lower RTI in previous six weeks; history of tobacco use. Baseline characteristics: FEV1 81.96% predicted ± 15.92; IgE 243.61 ± 149.5 |
|
| Interventions | Aerosolised rhuMAb‐E25 (1 mg or 10 mg) versus placebo via inhaler device. Treatment lasted for eight weeks with a four‐week follow‐up period | |
| Outcomes | FEV1, PEF (am), PC20, serum IgE levels | |
| Notes | Jadad score: 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified according to late‐phase response to allergen during the screening phase. Details of sequence generation not included in study report |
| Allocation concealment (selection bias) | Unclear risk | Details not included in study report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants did not complete the trial; reasons for their withdrawal are included in trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Garcia 2012.
| Methods | Randomised parallel‐group placebo‐controlled study | |
| Participants | Reported as 41 participants with severe non‐atopic asthma uncontrolled despite daily high‐dose inhaled corticosteroids (with or without maintenance oral corticosteroids) plus a long‐acting beta2‐agonist | |
| Interventions | Randomly assigned to receive omalizumab or placebo in a 1:1 ratio 16‐Week study |
|
| Outcomes | Reported as the following: The primary endpoint was change in expression of high‐affinity IgE receptor FceRI on blood basophils and plasmacytoid dendritic cells (pDC2) after 16 weeks. Impact on lung function and clinical parameters was also assessed | |
| Notes | Funded by Novartis Pharma SAS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not provided (conference abstract) |
| Allocation concealment (selection bias) | Unclear risk | Details not provided (conference abstract) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Details not provided (conference abstract) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not provided (conference abstract) |
| Selective reporting (reporting bias) | Unclear risk | Not possible to assess from conference abstract |
Gevaert 2012.
| Methods | Randomised double‐blind placebo‐controlled study | |
| Participants | Treatment group: 16. Control group: eight. Details of trial are very limited—reported as conference abstract Allergic and non‐allergic participants with nasal polyps and asthma |
|
| Interventions | Stated as: Participants received four to eight (subcutaneous) doses of omalizumab or placebo, depending on serum IgE concentrations (30 to 700 kU/L) and body weight No details given of background steroid dose |
|
| Outcomes | Stated as: Primary endpoint was reduction in total nasal endoscopic polyp score after 16 weeks. Secondary endpoints included a change in the following: sinus CT scan, nasal and asthma symptoms, validated questionnaires (SF‐36, RSOM‐31 and AQLQ) and serum/nasal secretion biomarkers | |
| Notes | Limited details reported (conference abstract) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not provided (conference abstract) |
| Allocation concealment (selection bias) | Unclear risk | Details not provided (conference abstract) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not provided (conference abstract) |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Hanania 2011.
| Methods | Randomised, multi‐centre, parallel‐group, double‐blind, placebo‐controlled trial | |
| Participants | Treatment group: 427 (427 completed). Age: 43.7 (14.3). Males: 165 (38.6%). Baseline lung function: mean % predicted FEV1 (SD): 65.4 (15.2) Control group: 423 (421 completed). Age: 45.3 (13.9). Males: 126 (29.9%). Baseline lung function: mean % predicted FEV1 (SD): 64.4 (13.9) Inclusion criteria stated as: The study included participants 12 to 75 years of age with a history of severe allergic asthma for at least one year before screening. Participants received a diagnosis of asthma from physician investigators at each site on the basis of criteria specified by the NAEPP guidelines. Patients whose asthma was not well controlled despite treatment with high‐dose ICS and LABAs with or without other controllers (including OCS) were enrolled. Asthma was considered not well controlled if participants had persistent asthma symptoms with current therapy, defined as an average of one or more night‐time awakenings per week and daytime asthma symptoms requiring the use of rescue medication for two or more days per week during the four weeks before screening and for two consecutive weeks up to four weeks before randomisation. In addition, participants were required to have at least one documented asthma exacerbation during the previous 12 months, defined as increased asthma symptoms requiring treatment with systemic corticosteroid rescue therapy. High‐dose ICS was given at a minimum dose of 500 mcg of fluticasone dry powder inhaler twice daily or its similar ex‐valve dose for at least eight weeks before screening. Long‐acting beta2‐agonist treatment could consist of salmeterol 50 mcg twice daily or formoterol 12 mcg twice daily for at least eight weeks before screening. Patients were also required to have objective evidence of allergy to a relevant perennial aeroallergen, defined as a positive skin test result or in vitro response (radioallergosorbent test) to dog, cat, cockroach, Dermatophagoides farinae (dust mite) or D. pteronyssinus documented in the 12 months before screening. Consistent with earlier pivotal studies, participants were also required to have baseline pre‐bronchodilator FEV1 of 40% to 80% of predicted values, serum IgE level of 30 to 700 IU/mL and body weight of 30 to 150 kg Exclusion criteria stated as: Persons were excluded if they had an asthma exacerbation requiring intubation in the 12 months before screening or an exacerbation requiring treatment with systemic corticosteroids (or an increase in the baseline dose of OCS) in the 30 days before screening. Other exclusion criteria included active lung disease other than asthma, treatment with omalizumab in the 12 months before screening, elevated serum IgE levels for reasons other than allergy (e.g. parasite infections, hyperimmunoglobulin E syndrome, Wiskott–Aldrich syndrome, bronchopulmonary aspergillosis) or smoking history of 10 or more pack‐years Location(s): 193 sites in the United States and four sites in Canada |
|
| Interventions | Minimum dose of 0.008 mg/kg of body weight per IgE (IU/mL) every two weeks or 0.016 mg/kg per IgE (IU/mL) every four weeks versus placebo Background inhaled corticosteroid dose—at least 500 mcg of fluticasone dry powder inhaler (or its equivalent) twice daily Participant using long‐term OCS at baseline = 60 (7.1%) |
|
| Outcomes | Stated as: The primary endpoint was the rate of protocol‐defined exacerbations over the study period. Secondary efficacy endpoints included the change from baseline to week 48 in mean daily number of puffs of albuterol, mean total asthma symptom score and mean overall score on the standardised version of the Asthma Quality of Life Questionnaire (AQLQ[S]). Safety endpoints included the frequency and severity of treatment‐emergent adverse events | |
| Notes | 48‐Week trial Co‐medication stated as: All participants received albuterol as rescue medication throughout the study. In addition, one or more of the following controller medications were allowed: leukotriene modifiers, including montelukast and zafirlukast; zileuton; oral, inhaled or nasal anticholinergic therapy; mast cell stabilisers, including cromolyn and nedocromil; specific immunotherapy; theophylline; and long‐term maintenance OCS. Long‐term OCS use consisted of a minimum dose of oral prednisolone (or comparable dose of another corticosteroid) of two to 40 mg/d or five to 80 mg every other day for at least four weeks immediately before the screening visit. Participants were classified in the M3 subgroup if they were long‐term OCS users at baseline or had at least four asthma exacerbations during the previous year requiring treatment with OCS. Participants were not permitted to receive levalbuterol, gold salts, macrolide antibiotics, methotrexate, cyclosporine, intravenous immunoglobulin or immunosuppressants during the run‐in and treatment periods In trial report, NCT number is provided as 00314575. However, on inspection of NCT registry, it appears to be NCT00314574 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated as: Randomisation was stratified by using a generalisation of the hierarchical dynamic randomisation scheme to achieve approximate overall balance between treatment groups and within each stratum by using the following hierarchy: overall balance, study drug dosing regimens, baseline asthma controller medication group and centre |
| Allocation concealment (selection bias) | Low risk | Stated as: Only the interactive voice response system provider and the unbinding statistician had access to the unbinding code during the study, for randomisation and safety purposes; neither was involved in adjudication of study outcomes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83 discontinued in omalizumab group 94 discontinued in placebo group |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Holgate 2004a.
| Methods | Randomised, double‐blind, parallel‐group multi‐centre placebo‐controlled trial. Randomisation by computer‐generated randomisation after run‐in. Allocation by independent personnel. Scratch cards given to investigators to be broken in case of emergency | |
| Participants | N = 246. Treatment group: 126; control group: 120 (two withdrawals due to keratitis and dysphonia—communication from Acumed). Mean age (placebo): 40.5 (12 to 71); treatment group: 41.1 (12 to 75). Female/Male percentage: placebo: 57.5/42.5; treatment: 64.3/35.7. Severe asthmatic participants optimally controlled, requiring high‐dose fluticasone. FP dose: between 1000 and 2000 mcg/d Inclusion criteria stated as: male/females 12 to 75 years of age, severe asthma according to ATS guidelines, allergic response (> one positive skin prick test to one or more aeroallergens, mean total daily symptom score ≥ four over seven days before randomisation, ≥ 12% reversibility, FEV1 within 30 minutes of salbutamol in 12 months before or at randomisation, stable medication four weeks before randomisation, IgE between 30 and 700 IU/mL Exclusion criteria stated as: females for whom current or future pregnancy could not be excluded, evidence/history of drug or alcohol abuse, history of non‐compliance with medical regimens, those considered potentially unreliable, known sensitivity to study drugs (omalizumab, corticosteroids, salbutamol and terbutaline), those using theophylline, those suffering from live/kidney disease, haematological abnormality, anaphylaxis, near‐fatal asthma exacerbation in last three years, elevated serum IgE for reasons other than atopy (parasitic infections, etc). Baseline data: mean duration of disease: placebo: 22.3 years; treatment: 22.6 years. Never smoked/ex‐smokers: placebo: 91/29; treatment: 99/27. Mean serum total IgE levels (IU/mL): placebo: 265.7 (±190.2); treatment: 266.8 (±218.0). Mean fluticasone dose (mcg/d): placebo: 1362.5 (±359.2); treatment: 1375 (±361.6). Participants taking LABA: placebo: 52 (43%); treatment: 62 (49%). Mean FEV1 (percentage predicted): placebo: 66 (±20.2); treatment: 62.9 (±17.5). Mean FEV1 reversibility: placebo: 20.6; treatment: 18.6. PEFR: placebo: 385.2; treatment: 371.9 |
|
| Interventions | Subcutaneous omalizumab (0.016 mg/kg/IgE (IU/mL) at two‐ or four‐weekly intervals depending on body weight versus placebo. Four‐phase study. SIx‐ to 10‐week run‐in phase, 16‐week steroid stable phase, 16‐week steroid reduction phase, 12‐week follow‐up | |
| Outcomes | Percentage reduction from baseline in inhaled FP, number of participants achieving > 50% reduction in inhaled fluticasone (subgroup according to LABA consumption), exacerbations, PEFR, QoL | |
| Notes | Jadad score: 5 Trial 011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated randomisation after run‐in |
| Allocation concealment (selection bias) | Low risk | Allocation by independent personnel |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 participants did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Holgate 2004b.
| Methods | Identical to Holgate 2004 (ICS) | |
| Participants | N = 95 (treatment: 50; control: 45). Mean age: not specified (likely to be similar to Holgate 2004). FEV1 (% predicted): treatment: 60; control: 57. Overnight hospital admission in last year: treatment: 23%; placebo: 23%; prednisolone dose (mg/d): treatment: 10; control: 10.6; ICS dose: (mcg/d): treatment: 1490; control: 1411 Inclusion criteria: identical to Holgate 2004 (ICS) |
|
| Interventions | Identical to Holgate 2004 (ICS) | |
| Outcomes | Identical to Holgate 2004 (ICS) | |
| Notes | Unpublished data on oral corticosteroid users from Holgate 2004a (source: FDA report) Trial 011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated randomisation after run‐in |
| Allocation concealment (selection bias) | Low risk | Allocation by independent personnel |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 participants did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
INNOVATE.
| Methods | Randomised, double‐blind, parallel‐group, multi‐centre, placebo‐controlled trial. Blinding: matched placebo. Methods of allocation not reported. Randomisation stratified by concomitant asthma treatment and country of origin | |
| Participants | N = 482. Mean age: omalizumab: 43.4; placebo: 43.3. FEV1: omalizumab: 61; placebo: 61.6; rescue medication usage: omalizumab: 6.6; placebo: 5.5. Overall AQLQ: 3.9 (both groups); serum total IgE: omalizumab: 197.6; placebo: 189.6; ICS dose (BDP equivalent, mcg/d): omalizumab: 2359; placebo: 2301. All participants receiving high‐dose ICS + LABA. 22% receiving maintenance oral steroids Inclusion criteria: +ve skin prick test to ≥ one aeroallergen; serum IgE: 30 to 700 IU/mL; severe persistent asthma requiring > 1000 BDP or equivalent and LABA treatment; FEV1 40% to 80%; FEV1 reversibility ≥ 12% post SABA; ≥ two exacerbations requiring OCS in previous 12 months or one severe exacerbation resulting in hospitalisation Exclusion criteria: smokers/smoking history of ≥ 10 pack‐years; treatment for exacerbation four weeks before randomisation; use of methotrexate/gold salts/troleandomycin/cyclosporin within three months of first visit; prior omalizumab treatment |
|
| Interventions | Subcutaneous omalizumab (0.016 mg/kg per IU/mL) (plus usual care) versus placebo (plus usual care). Study duration: 28 weeks; run‐in phase: seven‐day screening period; eight‐week run‐in phase. Follow‐up: 16‐week (data not presented). During initial four weeks of run‐in phase, medicines adjusted to achieve best control. No further adjustments permitted in last four weeks of run‐in | |
| Outcomes | Exacerbatrions (requiring OCS); hospitalisation; emergency room treatment; lung function; AQLQ; adverse events | |
| Notes | Jadad score: 4. Imbalance between groups at baseline for primary outcome in the trial. Greater instance of exacerbations requiring oral steroids in omalizumab group compared with placebo group. Adjusted data were extracted and entered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stratified by concomitant asthma treatment and country of origin |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 52 participants did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Lanier 2009.
| Methods | Randomised, multi‐centre, double‐blind, parallel‐group, placebo‐controlled study | |
| Participants | Treatment group: 421 (352 completed). Age: 8.7 ± 1.7. Males: 287 (68.2%). Baseline lung function: mean % predicted FEV1 (SD): 86.0 (17.8) Control group: 206 (175 completed). Age: 8.4 ± 1.7. Males: 138 (66.7%). Baseline lung function: mean % predicted FEV1 (SD): 87.2 (18.4) Inclusion criteria stated as: Parent or legal guardian was informed of the study procedures and medications and gave written informed consent. Outpatient males and females aged 6 to less than 12 years on study entry, with body weight between 20 and 150 kg. Total serum IgE level ≥ 30 to ≤ 1300 IU. Diagnosis of allergic asthma ≥ one year's duration, according to American Thoracic Society (ATS) criteria, and a screening history consistent with clinical features of moderate or severe persistent asthma according to National Heart Lung and Blood Institute (NHLBI) guidelines. Positive prick skin test to at least one perennial allergen, documented within the past two years or taken at screening. A radioallergosorbent test (RAST) could have been performed for participants with a borderline skin prick test result after consultation with Novartis clinical personnel. Patients with ≥ 12% increase in forced expiratory volume in one second (FEV1) over starting value within 30 minutes of taking up to four puffs (4 × 100 µg) salbutamol (albuterol) or nebulised salbutamol up to 5 mg (or equivalent of alternative β2‐agonist) documented within the past year, at screening, during the run‐in period or before randomisation. Patients were not to take their long‐acting β2‐agonist (LABA) medication within 12 hours of reversibility testing. Clinical features of moderate or severe persistent asthma (at least step three) despite therapy at step three or four (at least medium‐dose inhaled corticosteroid (ICS) fluticasone dry powder inhaler (DPI) ≥ 200 mg/d or equivalent with or without other controller medications) Documented history of experiencing asthma exacerbations and demonstrated inadequate symptom control during the past four weeks of run‐in despite receiving an equivalent dose of fluticasone DPI ≥ 200 mg/d total daily ex‐valve dose Exclusion criteria stated as: patients who received systemic corticosteroids for reasons other than asthma, beta‐adrenergic antagonists by any route, anticholinergics within 24 hours of screening, methotrexate, gold salts, cyclosporin or troleandomycin, or had received desensitisation therapy with less than three months of stable maintenance doses before screening. Patients with a history of food‐ or drug‐related severe anaphylactoid or anaphylactic reaction, a history of allergy to antibiotics, with aspirin‐ or other non‐steroidal anti‐inflammatory drug (NSAID)‐related asthma (unless the NSAID could be avoided), with active lung disease or acute sinusitis/chest infection, elevated serum IgE levels for other reasons, presence/history of a clinically significant uncontrolled systemic disease, cancer, abnormal electrocardiogram (ECG) in the previous month, or platelets ≤ 100 × 109/L or clinically significant laboratory abnormalities at screening Location(s): 87 centres in seven countries: Argentina (eight), Brazil (three), Canada (six), Colombia (five), Poland (six), USA (58) and South Africa (one) |
|
| Interventions | Stated as: Participants received omalizumab administered by subcutaneous injection every two or four weeks for a duration of 52 weeks. Omalizumab dose was based on participant's body weight and total serum IgE level at screening. The first 24 weeks of the treatment period was a fixed steroid phase, where the steroid dose was maintained constant; in the following 28 weeks, the steroid dose was adjustable, depending on the participant's condition. Following the 52‐week treatment period, participants were followed up for an additional 16 weeks Placebo was administered by subcutaneous injection every two or four weeks, depending on the dosing schedule in the protocol for a total of 52 weeks. The first 24 weeks of the treatment period was a fixed steroid phase, where the steroid dose was maintained constant; in the following 28 weeks, the steroid dose was adjustable, depending on the participant's condition. Following the 52‐week treatment period, participants were followed up for an additional 16 weeks Matched vials of placebo supplied as sterile powder in a 5‐mL vial designed to deliver 150 mg of placebo for s/c administration upon reconstitution with 1.4 mL sterile water Backgound inhaled corticosteroid dose: at least 200 mg/d fluticasone propionate via dry powder inhaler or equivalent, mean ICS dose 515.1 ± 285.4 mcg/d (fluticasone propionate equivalent) Participants using maintenance oral steroids at baseline = 8 (1.3%) |
|
| Outcomes | Primary outcome measures stated as: rate of clinically significant asthma exacerbations per participant in the 24‐week fixed‐dose steroid treatment period (time frame: baseline to end of the fixed‐dose steroid treatment period (week 24). Percentage of participants with at least one adverse event (time frame: baseline to end of the study (week 68)) Secondary outcome measures stated as: change in mean nocturnal asthma symptom score from baseline to the end (last four weeks) of the 24‐week fixed‐dose steroid treatment period (time frame: baseline to the end (last four weeks) of the 24‐week fixed‐dose steroid treatment period). Rate of clinically significant asthma exacerbations per participant in the 52‐week treatment period (time frame: baseline to end of treatment period (week 52)). Change in mean daily number of puffs of asthma rescue medication from baseline to end (last four weeks) of 24‐week fixed‐dose steroid treatment period (time frame: baseline to the end (last four weeks) of 24‐week fixed‐dose steroid treatment period). Change in Pediatric Asthma Quality of Life Questionnaire (Standardised) (PAQLQ(S)) scores from baseline to end of 24‐week fixed‐dose steroid treatment period (week 24) (time frame: baseline to end of 24‐week fixed‐dose steroid treatment period (week 24)) |
|
| Notes | One‐year trial Co‐medication: inhaled corticosteroids 24‐week fixed‐dose and 28‐week adjustable‐dose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned (two:one) to receive omalizumab or placebo by a randomisation card system |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details of 101 participants who did not complete the trial are reported |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Massanari 2010.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study | |
| Participants | Treatment group: 139. Age: 38.2 (9.89). Males: 51 (37%). Baseline lung function: mean % predicted FEV1 (SD): 86.1 (11.18) (n = 134) Control group: 136. Age: 38.2 (10.02). Males: 37 (27%). Baseline lung function: mean % predicted FEV1 (SD): 88.1 (11.64) (n = 131) Inclusion criteria stated as: male or female, any race, ages 18 to 55 years, body weight ≥ 20 kg and ≤ 150 kg, total serum IgE concentration ≥ 30 and ≤ 700 IU/mL at visit 0. History of at least moderate persistent allergic asthma of ≥ one year in duration, on a stable asthma treatment regimen including inhaled corticosteroids for the preceding four weeks, an FEV1 while withholding short‐acting beta‐agonists for at least six hours and long‐acting beta‐agonists for at least 12 hours, of ≥ 75% of predicted value at visit 0, reversibility (increase in FEV1 of ≥ 12% between 20 and 30 minutes after four puffs), positive skin test to at least one perennial allergen (house dust mite, cat or dog), average PEFR variability ≤ 20%, prespecified level of nocturnal asthma symptoms, non‐smoker for at least one year before visit 1, with a smoking history of no more than 10 pack‐years, good physical and mental health Exclusion criteria stated as: history of intubation for asthma or requiring systemic steroids in last three months, asthma requiring ED visit on admission in the preceding six months, URTI or sinusitis within the preceding four weeks, history of an anaphylactic allergic reaction (except to stinging insects, foods or drugs other than omalizumab), history of treatment with immunotherapy to any allergen within past three years, history of aspirin‐ or non‐steroidal anti‐inflammatory drug (NSAID)‐related asthma, history of or current malignancy, any clinically significant uncontrolled systemic disease or a history of such disease within the previous three months, clinically significant laboratory abnormalities at visit 1, platelet levels ≤ 130 × 109/L at visit one, pregnant or breast‐feeding women or women using inadequate contraception, history of hypersensitivity to the study medication or drugs related to omalizumab (e.g. monoclonal antibodies, polyclonal gammaglobulin), Previous treatment with omalizumab within one year of screening, Considered by investigator to be potentially unreliable or who may not have reliably attended study visits, history of drug or alcohol abuse Location(s): USA |
|
| Interventions | At least 0.016 mg/kg/IgE (IU/mL) omalizumab subcutaneous per four weeks. Study drug administered by subcutaneous injection every two or four weeks according to weight and baseline IgE versus placebo Background inhaled corticosteroid dose—all participants receiving ICS at baseline; no further details given |
|
| Outcomes | Primary: systemic allergic reaction to participant‐specific allergen. Secondary: severity of the first SAR to SIT, achievement of target maintenance SIT dose, number of visits required to complete the cluster SIT regimen, number of doses of rescue medications for managing SIT reactions | |
| Notes | 26‐Week study that consisted of four periods: screening (two weeks), treatment with omalizumab or placebo (16 weeks), cluster SIT (four weeks, including three weeks of overlap with omalizumab/placebo) and maintenance SIT (seven weeks) Co‐medication: Patients remained on usual asthma treatment. After 13 weeks of omalizumab treatment (or placebo), they were challenged with their specific allergens (SIT) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of sequence generation. Stratified by allergens |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Because reconstituted placebo vials did not exactly match those containing omalizumab, a study technician not involved with any participant study assessments was responsible for preparing and administering all injections (providing this technician did not divulge information to participants or study staff) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants accounted for but higher drop out in the placebo group (75% vs 61%) |
| Selective reporting (reporting bias) | Low risk | All stated outcome measures reported |
Milgrom 1999.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial | |
| Participants | N = 317 (569 screened). Mean age 30 years, 133 male. High‐dose group: N = 106; low‐dose group: N = 106; placebo group: N = 105. FEV1 71% predicted. ICS dose: 800 mcg, OCS dose: 10 mg/d (35 participants), inhaled beta‐agonist dose: seven puffs/d, IgE 273 to 374 IU/mL. Five participants dropped out of the placebo group, and two participants withdrew from each of the two treatment groups Inclusion criteria: inhaled triamcinolone, flunisolide or beclomethasone (200 mcg/d), positive skin prick tests, < 1785 IU/mL serum IgE Exclusion criteria: symptom score < 2.5, poorly reversible airway obstruction, treatment doses projected to be < 1 mL, negative skin prick tests, active disease other than asthma, lack of compliance |
|
| Interventions | Twice‐weekly intravenous low‐/high‐dose omalizumab versus placebo. Low dose: 2.5 mcg/kg/ng IgE/mL, high dose: 5.8 mcg/kg/ng IgE/mL. Treatment during stable steroid phase lasted for 12 weeks, followed by eight weeks of steroid reduction. Follow‐up was 10 weeks | |
| Outcomes | FEV1, PEF, QoL, withdrawals, asthma exacerbations, daily total symptom score, beta‐agonist use, mean decrease in CS use, adverse effects | |
| Notes | Jadad score: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not included in trial report |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not included in trial report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34 participants did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Milgrom 2001.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial | |
| Participants | N = 334 (501 screened). Age range: six to 12 years. Treatment group: N = 225; control group: N = 109. 231 males. Mean PEFR (L/min): treatment group: 261 (101 to 408); control group: 264 (140 to 407). Mean FEV1 (percentage predicted) treatment group: 84 (49 to 129); control group: 85 (43 to 116). Number hospitalised for asthma in past year: treatment group: N = 18; control group: N = 9. Mean BDP dose: treatment group: 284 (168 to 672); control group: 267 (168 to 504). Mean albuterol use (per day): treatment group: 1.1, control group: 1.4 Inclusion criteria: diagnosis of allergic asthma of at least one year's duration; positive skin prick test to one of: Dermatophagoides farinae, Dermatophagoides pteronyssinus, cockroach, dog or cat; total serum IgE level between 30 and 1300 IU/mL; body weight < 90 kg; baseline FEV1 > 60% of predicted normal value; at least 12% increase in FEV1 over baseline within 30 minutes of taking one or two puffs of albuterol (90 mcg/puff); stable asthma, defined as no significant change in regular asthma medication and no acute asthma exacerbation requiring corticosteroid rescue for at least four weeks before enrolment Exclusion criteria: previous treatment with omalizumab; known hypersensitivity to any study drug; history of acute infectious sinusitis or respiratory tract infection or active lung disease other than allergic asthma within one month or any other significant systemic disease within three months of visit one; clinically significant abnormalities in electrocardiogram, chest x‐ray or lab values, or elevated serum IgE levels for reasons other than atopy; children requiring doses greater than 750 mg per four weeks, based on total serum IgE and body weight consideration (0.016 mg/IgE in IU/mL × body weight in kg) |
|
| Interventions | Subcutaneous administration of omalizumab (0.016 mg/kg/IgE (IU/mL), equivalent to 150 or 300 mg every four weeks, or 225, 300 or 375 mg every two weeks, depending on participant's body weight. Run‐in phase lasted four to six weeks with stabilisation on BDP, followed by a stable steroid phase (16 weeks) and a steroid reduction phase (12 weeks) | |
| Outcomes | BDP dose, asthma symptom score, asthma exacerbation rate, rescue beta‐agonist use, pulmonary function—FEV1 + PEFR, global evaluation of treatment, pharmacoeconomics, pharmacodynamics, adverse events, withdrawals | |
| Notes | Jadad score: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not included in trial report |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not included in trial report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight participants did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
NCT00096954.
| Methods | Randomised, multi‐centre, parallel‐group, double‐blind, placebo‐controlled study | |
| Participants | Treatment group: 159. Age: 36.0 (14.7). Males: 47 (30%). Baseline lung function: mean % predicted FEV1 (SD): not stated Control group: 174. Age: 38.1 (15.1). Males: 55 (32%). Baseline lung function: mean % predicted FEV1 (SD): not stated Patients with 'difficult to treat atopic asthma' Inclusion criteria stated as: documented history of asthma as well as evidence of ≥ 12% reversibility of FEV1; baseline FEV1 ≥ 80% predicted normal value before randomisation; positive skin test (diameter of wheal ≥ 3 mm vs control) or in vitro radioallergosorbent test (RAST(R)) or ImmunoCap(R) to one relevant perennial aeroallergen such as cat or house dust mites documented within the previous year; receiving at least an inhaled corticosteroid dosage of fluticasone dry powder inhaler (DPI) ≥ 200 μg/d or equivalent; during four‐week run‐in period before randomisation demonstrate evidence of inadequate asthma symptom control; inadequate asthma symptom control defined as at least one of the following reported on the participant diary card during four‐week run‐in period: daytime asthma symptoms as a score of ≥ one (scale of zero to four) on at least 20 of 28 days (missing data to be treated as a day with no symptoms) and mean symptom score ≥ 1.5 or night‐time awakening because of asthma symptoms (more than four times during four‐week run‐in period); meet study drug‐dosing table eligibility criteria (serum baseline IgE level ≥ 30 to ≤ 1300 IU/mL and body weight ≥ 20 to ≤ 150 kg); if female of child‐bearing potential, using an effective method of contraception Exclusion criteria stated as: received long‐term systemic corticosteroids (oral or intravenous) within three months or received a burst of oral corticosteroids within the last two weeks before screening; received Xolair therapy at any time within 12 months before screening; pregnant or lactating; known hypersensitivity to any ingredients of Xolair, including excipients (sucrose, histidine, polysorbate 20); lifetime history of smoking > 10 pack‐years; active lung disease other than asthma (e.g. chronic bronchitis, emphysema, cystic fibrosis, chronic obstructive pulmonary disease); history of upper respiratory infection or lower respiratory infection within 30 days before randomisation; diagnosis of aspirin‐ or nonsteroidal anti‐inflammatory drug‐induced asthma; immunosuppressants or other investigational drugs within 30 days before screening; significant medical illness other than asthma Location(s): unclear |
|
| Interventions | Omalizumab (Xolair) was administered subcutaneously every two or four weeks. Dose (mg) and dosing frequency were determined by serum total IgE level (IU/mL), measured before the start of treatment, and body weight (kg). Dose of placebo consisting of sucrose, L‐histidine, L‐histidine hydrochloride monohydrate and polysorbate 20 administered by subcutaneous injection every two or four weeks Background inhaled corticosteroid dose—fluticasone dry powder inhaler (DPI) ≥ 200 μg/d or equivalent |
|
| Outcomes | Rate of asthma exacerbations over 24‐week treatment period; number of participants experiencing one or more protocol‐defined asthma exacerbations during the treatment period; change from baseline in nocturnal and daytime asthma symptom scores at week 24; relative percentage change from baseline in forced expiratory volume in one second (FEV1) at week 24 | |
| Notes | 24‐Week study (four‐week monitoring run‐in; baseline therapy not changed) Co‐medication too: usual asthma regimen (immunotherapy not allowed) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on participants failing to complete included in report. 157 (99%) completed in intervention group and 171 (99%) in control |
| Selective reporting (reporting bias) | Low risk | All outcome measures clearly reported |
NCT01007149.
| Methods | Randomised, multi‐centre, double‐blind, placebo‐controlled, parallel‐group | |
| Participants | Treatment group: 20. Age:55.0 (9.67). Males: seven (35%). Baseline lung function: mean % predicted FEV1 (SD): not stated Control group: 21. Age: 54.6 (12.78). Males: 8 (38%). Baseline lung function: mean % predicted FEV1 (SD): not stated Participants with severe persistent asthma (GINA criteria) Inclusion criteria stated as: severe persistent asthma with the following characteristics: uncontrolled according to Global Initiative for Asthma (GINA) 2007 guidelines and at least two exacerbations having required systemic corticosteroid and/or at least one hospitalisation or emergency room visit in the past year; treated with high‐dose inhaled corticosteroid (i.e. > 1000 µg beclometasone dipropionate equivalent per day) plus inhaled long‐acting β2‐agonist (with or without maintenance oral corticosteroid); non‐atopic (i.e. negative blood multi‐allergic testing and negative Aspergillus‐specific IgE‐radioallergosorbent blood test and negative skin prick tests to a battery of common aeroallergens Exclusion criteria stated as: current smoker or smoking history stopped for less than three years or > 10 pack‐years; asthma exacerbation during the four weeks before randomisation; active lung disease other than non‐atopic asthma; patients with an active cancer, a suspicion of cancer or any history of cancer with less than five disease‐free years; pregnant or nursing (lactating) women; treatment with omalizumab Location(s): 10 centres in France |
|
| Interventions | Intervention: Participants received subcutaneous injections of omalizumab every two weeks or every four weeks; dosage dependent on IgE level and body weight Control: Participants received subcutaneous injections of placebo to omalizumab every two weeks or every four weeks Background inhaled corticosteroid dose > 1000 µg beclometasone dipropionate equivalent per day Patients using oral corticosteroids were included, but no further details were given |
|
| Outcomes | Change from baseline in expression of FcϵRI receptors of blood basophils; change from baseline in expression of FcϵRI receptors of dendritic cells; change in fractional exhaled nitric oxide; change from baseline in induced sputum eosinophil count; change from baseline in score of the shortened version of the Asthma Control Questionnaire; change from baseline in nasal symptom global score and individual components; physician and participant global evaluation of treatment effectiveness; change in forced expiratory volume in one second (FEV1) from baseline to 16 weeks; number of participants with at least one asthma‐related event over 16 weeks | |
| Notes | 16‐Week trial. Two‐week ‘screening’ period; no run‐in described Co‐medication: not specifically stated, but all participants had to be using high‐dose inhaled steroids plus LABA to be eligible for inclusion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High completion rate. 100% in intervention group and 20 (95%) in control group |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
Ohta 2009.
| Methods | Randomised, double‐blind, parallel‐group, multi‐centre study | |
| Participants | Treatment group: 158. Age: 48.8 (14.88). Males: 74 (46.8%). Baseline lung function: mean % predicted FEV1 (SD): 74.06 (19.91) Control group: 169. Age: 49.2 (14.42). Males: 70 (42.7%). Baseline lung function: mean % predicted FEV1 (SD): 75.81 (20.89) Inclusion criteria stated as: males and females with inadequately controlled allergic asthma for > one year (positive skin prick test), 20 to 75 years, weighing 30 to 150 kg, with allergic asthma, IgE level 30 to 700 IU/mL, taking inhaled corticosteroids at a dosage of BDP 800 μg/d (or equivalent) and at least one more drug for managing their asthma at least three months before trial observation (e.g. oral corticosteroids, β2‐agonists (oral, inhaled or patch‐type) theophylline, leukotriene‐3 antagonists or a thromboxane A2 inhibitor/antagonist) Exclusion criteria stated as: pregnant or breast‐feeding, history of severe anaphylactic reaction or anaphylactoid reaction, patients taking unacceptable medications (e.g. > 10 mg of prednisolone‐equivalent oral corticosteroids, immunosuppressants), significant underlying medical conditions that could impact interpretation of results Location(s): 73 centres in Japan |
|
| Interventions | Subcutaneous dose of omalizumab was based on participant's body weight and total serum IgE level at visit 1 and was at least 0.016 mg/kg/IgE (IU/mL) every four weeks or 0.008 mg/kg/IgE (IU/mL) every two weeks versus placebo Background inhaled corticosteroid dose > 1000 µg beclometasone dipropionate equivalent per day Participants using oral corticosteroids at baseline included but no further details given |
|
| Outcomes | Morning peak expiratory flow (PEF) at baseline and at end of treatment, pulmonary function parameters measured by spirometer, frequency of rescue medication use, symptoms score, activities of daily living score, night‐time sleep score, adverse events | |
| Notes | Two‐week pretreatment phase, 16‐week treatment phase and 12‐week follow‐up Co‐medication: doses of ICS and other concomitant asthma medications were kept constant for one month before pretreatment phase and were maintained during treatment phase. Participants were permitted to use rescue medication as needed. If any worsening of asthma occurred, which required additional treatment with a systemic corticosteroid, the participant was discontinued from the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated as: randomised and allocated to receive either omalizumab or placebo using a third party’s central registration system |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind but unclear whether blinding continued beyond end of 16‐week treatment phase |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unbalanced withdrawals from groups (8.2% from treatment group vs 16.6% from placebo group) |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures reported with exception of serum IgE levels and correlation with efficacy and other pharmacological measurements |
Prieto 2006.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial | |
| Participants | N = 34. Mean age unclear. Treatment group: 18; control group: 16 Inclusion criteria: mild to moderate allergic asthma |
|
| Interventions | Subcutaneous omalizumab versus placebo (dosage unclear) Treatment lasted for 12 weeks |
|
| Outcomes | Airway responsiveness | |
| Notes | Unpublished conference abstract: Jadad score: 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not included in trial report |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not included in trial report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
SOLAR.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial. Method of allocation: not reported | |
| Participants | N = 405 (no data on N screened). Treatment: 209; control: 196. Age range: 12 to 75 years. Mean steroid dose (BUD equivalent mcg/d): treatment: 842; control: 901. Mean exacerbations requiring OCS in past year: treatment: 2.1; control: 2.1 Inclusion criteria: FEV1 reversibility ≥ 12%; IgE level ≥ 30 to ≤ 1300 IU/mL; +ve skin prick test to one or more indoor allergen; co‐existing moderate to severe perennial rhinitis; ≥ 400 mch/d ICS; ≥ two unscheduled medical visits for asthma in past year; score ≥ 64/192 on AQLQ Exclusion criteria: patients taking systemic steroids; long‐acting antihistamines; cromolyn sodium, oral beta‐agonists; theophylline; leukotriene antagonists; inhaled anticholinergics; methotrexate; gold salts; cyclosporin; allergen‐specific immunotherapy; non‐allergic rhinitis; pregnancy; platelet count ≤ 130 × 10(9)/one |
|
| Interventions | Subcutaneous omalizumab 0.016 mg/kg/IgE (IU/mL) every four weeks versus placebo, in addition to ICS therapy and other stable preexisting drug regimens (e.g. LABAs, nasal steroids). Four‐week run‐in phase; participants switched to BUD equivalent Turbuhaler; dose kept stable for at least four weeks before study entry Study duration: 28 weeks |
|
| Outcomes | Asthma exacerbations (defined as worsening of asthma symptoms necessitating treatment with oral steroids/doubling dose of baseline ICS); AQLQ, RQLQ; rescue medication usage; symptoms; lung function (FEV1, FVC am PEF), ICS use | |
| Notes | Jadad score: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not included in trial report |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not included in trial report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 participants did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
Solèr 2001.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial. Randomisation by random number sequences. Participants randomly assigned at visit three. Independent personnel were responsible for allocation | |
| Participants | N = 546 (1356 screened). Age range: 12 to 75, 268 male participants. Asthma diagnosed according to ATS guidelines Inclusion criteria: asthma diagnosed for longer than one year, positive skin prick test to at least one allergen, serum total IgE level > 30 and < 700 IU/mL‐1, body weight < 150 kg, baseline FEV1 > 40% and < 80% predicted, increasing by > 12% within 30 minutes of taking salbutamol, mean total daily symptom score > 3 (max 9) during 14 days before randomisation, treatment with ICS 200 mcg BDP per day for > three months before randomisation, use of beta‐agonists on an as‐needed/regular basis Exclusion criteria: unstable asthma, significant alteration to regular medication and acute exacerbation requiring additional corticosteroid treatment > one month before screening visit, oral steroids. 59 participants withdrew from the study (placebo: n = 40; omalizumab: n = 19). Reasons cited were withdrawal of consent (placebo: n = 14; omalizumab: n = 3), unsatisfactory therapeutic effect (placebo: n = 11; omalizumab: n = 8), adverse events (placebo: n = 5; omalizumab: n = 0) |
|
| Interventions | Subcutaneous omalizumab ≥ 0.016 mg/kg/IgE (IU/mL) versus placebo over a core 28‐week period. Run‐in phase was four to six weeks with stabilisation of BDP. Stable and reduction phases of BDP followed randomisation. Trial extension phase lasted 32 weeks | |
| Outcomes | Number of exacerbations, change in serum free IgE, reduction in BDP, symptom score, rescue medication use, morning PEF, safety and tolerability | |
| Notes | Jadad score: 5 Trial 009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random number sequences. Participants randomly assigned at visit three |
| Allocation concealment (selection bias) | Low risk | Independent personnel responsible for allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 participants did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
van Rensen 2009.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial | |
| Participants | N = 25. Mean age: unclear Inclusion criteria: mild persistent asthma |
|
| Interventions | Subcutaneous omalizumab versus placebo (dosing levels unclear) Study duration: 12 weeks |
|
| Outcomes | PC20 methacholine, sputum and allergen challenge followed by bronchoscopy at 24 hours; changes in PC20, sputum eosinophils, max fall in FEV1 during late asthmatic response (LAR), post‐allergen eosinophils (EG2) and mast cells (AA1) | |
| Notes | Unpublished conference abstract. Jadad score: 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of sequence generation not included in trial report |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not included in trial report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant did not complete the trial, and reasons for withdrawal are included in the trial report |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of selective reporting bias |
ATS: American Thoracic Society; BDP: Beclomethasone; CS: Corticosteroid; E25/rhu‐MAb E25/Xolair/omalizumab: anti‐IgE; FEV1: forced expiratory volume in one second; HDM: House dust mite; ICS: Inhaled CS; IgE: Immunoglobulin E; LABA: Long‐acting beta‐agonists; OCS: Oral corticosteroid; PC20: Bronchial challenge; PEFR: peak flow; QoL: Quality of life; RTI: respiratory tract infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 2000 | Review article reporting data from Milgrom 1999. |
| Anonymous 2000b | German language review article with summary of Milgrom 1999. |
| Anonymous 2003 | Not a randomised study |
| Ayars 2011 | Trial focuses on mepolizumab rather than omalizumab. |
| Ayars 2013 | Not Omalizumab Added June 2013 |
| Babu 2001 | Review article. |
| Beeh 2006 | Pooled analysis from 4 studies (not identified in conference abstract). |
| Berger 2002 | Review article. |
| Bisberg 1996 | Single blind placebo controlled study. No randomisation reported ‐ pharmacokinetic and pharmacodynamic profiles were analysed. |
| Blanken 2013 | Respiratory syncytial virus and recurrent wheeze in healthy preterm infants Added June 2013 |
| Bousquet 2010 | A comparison between different clinical measures |
| Bousquet 2011 | Open label study |
| Bruselle 2009 | Observational study |
| Buhl 2001 | Meta‐analysis of data drawn from included studies. |
| Busse 2013 | Not Omalizumab Added June 2013 |
| Castro 2011 | Evaluation of bronchial thermoplasty |
| Chipps 2009 | Pooled analysis from 2 studies (not identified in conference abstract). |
| CIGE025A1305 | Study in population with seasonal allergic rhinitis |
| CIGE025A1306 | Study in population with seasonal allergic rhinitis |
| CIGE025A1307 | Non‐randomised study |
| CIGE025A2208 | Non‐randomized study |
| CIGE025A2303 | Mixed population of patients with asthma and allergic rhinitis |
| CIGE025AUS23 | Study focuses on impact of Xolair on immunotherapy outcomes |
| Corren 2010 | Phase 2 study of AMG 317, an IL‐4Ralpha antagonist |
| Corren 2011 | Trial focuses on lebrikizumab rather than omalizumab. |
| Corren 2011a | This trial focuses on the effect of omalizumab on cat‐allergen induced bronchospasm. |
| Demoly 1997 | Review article. |
| Eckman 2010 | Diagnosis is cat‐induced allergic rhinitis rather than asthma |
| Emmrich 2001 | German language summary of the Milgrom 1999 study. |
| ETOPA | Inadequate control (best standard care without placebo) |
| Fernandez 2005 | Non‐randomised safety study |
| Frew 1998 | Review article. |
| Gauvreau 2012 | Not Omalizumab Added June 2013 |
| Gauvreau 2012a | Not Omalizumab Added June 2013 |
| Gober 2008 | Trial focuses on chronic idiopathic urticaria rather than asthma |
| Gossage 2010 | Trial focuses on subcutaneous (SC) dose study of MEDI‐563, a monoclonal antibody |
| Gossage 2012 | Not Omalizumab Added June 2013 |
| Hanania 2011a | Trial focuses on lebrikizumab rather than omalizumab. |
| Hendeles 2007 | Crossover study |
| Hodsman 2013 | Not Omalizumab Added June 2013 |
| Holgate 2001 | Review article. |
| Hoshino 2011 | Open label study |
| Hughes 2000 | Review article. |
| Johansson 2009 | Diagnosis is cat‐induced allergy rather than asthma |
| Kamin 2010 | Main focus of trial is on allergic rhinitis |
| Karpel 2010 | Pooled analysis from two trials Busse 2001 and Solèr 2001 |
| Kenyon 2011 | Trial focuses on L‐arginine supplementation |
| Kopp 2009 | Study focuses on impact of Xolair on immunotherapy outcomes |
| Lanier 2010 | Non‐randomized study |
| Leynadier 2004 | Target population with latex allergy. |
| Lobo 2007 | Assessment of a quality of life instrument rather than a comparison between two groups |
| Massanari 2008 | Pooled analysis of two studies (not identified in conference abstract). |
| Massanari 2009 | Pooled analysis from two trials Busse 2001 and Solèr 2001 |
| Maykut 2008 | Pooled analysis from five trials (not identified in conference abstract) |
| McClintock 2012 | Not Omalizumab Added June 2013 |
| Milgrom 2007 | Pooled analysis from four trials (not identified in conference abstract) |
| Milgrom 2009 | Pooled analysis from two trials (not identified in conference abstract) |
| Milgrom 2011 | Pooled analysis from two trials Milgrom 2001 and Lanier 2009 |
| Molfino 2013 | Not Omalizumab Added June 2013 |
| Moulton 2000 | Journal letter. |
| NCT00109187 | Non‐randomized study |
| NCT00109200 | Non‐randomized study |
| NCT00133042 | Crossover study |
| NCT00180011 | Non‐randomized study |
| NCT00189228 | Non‐randomized study |
| NCT00201097 | Open label study |
| NCT00219323 | Non‐randomized study |
| NCT00242359 | The study was withdrawn due to problems identifying the target population. |
| NCT00283504 | Non‐randomized study |
| NCT00287378 | Study terminated due to difficulties with enrolment |
| NCT00401596 | Open label study |
| NCT00434434 | Trial focuses on the effect of omalizumab on allergen‐induced airway responsiveness |
| NCT00482248 | Non‐randomized study |
| NCT00482508 | Non‐randomized study |
| NCT00500539 | Non‐randomized study |
| NCT00546143 | Non‐randomized study |
| NCT00567476 | Open label study |
| NCT00624832 | This trial focuses on the effect of omalizumab on allergen‐induced bronchospasm |
| NCT00639691 | Non‐randomized study |
| NCT00777764 | Non‐randomized study |
| NCT00784485 | Terminated due to difficulties with enrolment |
| NCT00829179 | Non‐randomized study |
| NCT01155700 | Non‐randomized study |
| NCT01219036 | Non‐randomized study |
| Nopp 2010 | Non‐randomized study |
| Oh 2010 | Trial focuses on Interleukin‐9 Monoclonal Antibody (MEDI‐528) |
| Oh 2012 | Not Omalizumab Added June 2013 |
| Ong 2005 | Study not concerned with asthma; volunteers with atopy |
| Parker 2010 | Trial focuses on Interleukin‐9 Monoclonal Antibody (MEDI‐528) |
| Parker 2011 | Trial focusing on brain magnetic resonance imaging in adults with asthma |
| Parker 2011a | Trial focusing on multiple subcutaneous doses of MEDI‐528, a humanized anti‐interleukin‐9 monoclonal antibody |
| Patel 2009 | This trial focuses on the effect of omalizumab on airway hyperresponsiveness |
| Pavord 2012 | Not Omalizumab Added June 2013 |
| Piper 2011 | Phase 2 study of tralokinumab |
| Piper 2013 | Not Omalizumab Added June 2013 |
| Q2143G | Inadequate control (best standard care without placebo) |
| Riviere 2008 | Assessment of different preparations of Anti‐IgE |
| Riviere 2009 | Bioequivalence study without placebo arm |
| Riviere 2011 | Bioequivalence study without placebo arm |
| Rubin 2012 | Open label study |
| Scheerens 2011 | Trial focuses on lebrikizumab rather than omalizumab. |
| Stallings 2009 | Study focuses on rhinovirus colds |
| Tajiri 2013 | Not asthma Added June 2013 |
| Townley 2011 | This trial focuses on the effects of omalizumab on bronchial and alveolar airway inflammation as measured by exhaled nitric oxide |
| Wenzel 2013 | Not Omalizumab Added June 2013 |
| Wilson 2008 | Bronchial biopsy from patients included in studies already in the review. |
| Yalcin 2011 | Not an RCT and no comparison with placebo |
| Zaidi 2009 | Study focuses on changes in the Fc‐epsilonRI‐Beta: Fc‐epsilonRI‐alpha Ratio |
| Zielen 2009 | This trial focuses on the effect of omalizumab on allergen‐induced bronchospasm |
| Zielen 2013 | Allergen‐induced bronchoconstriction Added June 2013 |
Characteristics of studies awaiting assessment [ordered by study ID]
Creticos 2010.
| Methods | We have been unable to obtain confirmation that this study is the same trial as NCT00162773 (which is reported as randomised, but for which no data are reported). It is unclear from the trial report (a conference abstract) whether Creticos 2010 was randomised. It is hoped that this issue will be clarified by the next update of this review |
| Participants | Eight participants in total, with five allocated to intervention and three to control. Details in conference abstract are very limited. Moderate to severe non‐allergic asthma |
| Interventions | Omalizumab versus placebo |
| Outcomes | Please see Notes below |
| Notes | Conference abstract results and conclusions reported as: database/advertising screen of 870+ adult asthmatic patients yielded 85 participants (< 10%) meeting initial criteria, 29 of whom completed screening, with eight (1%) qualifying for enrolment. All participants had negative primary puncture skin test sensitivity/RASTs to perennial allergens (dust mite/cat/ dog/cockroach); two demonstrated secondary reactivity to dust mites. Mechanistically, an expected rise in serum IgE (1.5‐/6.4‐fold) was observed in two or four subjects four months taking omalizumab. DC and basophils from these participants showed a > 50% decrease in FceRIa. However, low serum IgE levels (<two of eight IU) were not increased in the other two omalizumab participants, nor were changes in FceRIa expression discernible. Placebo participants (n = 53) showed no drop in FceRIa. Finally, no discernible changes were observed in basophil/DC function in any of the omalizumab‐treated participants. Conclusions: This study demonstrates the difficulty in identifying a true subset of participants with the disease entity of NAA. Although expected shifts in cellular FceRIa expression were evident, we observed no evidence of clinical benefit (symptoms/lung function/med usage/QOL) in NAA participants treated with omalizumab |
NCT00046748.
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Adults and adolescents with severe persistent asthma |
| Interventions | Subcutaneous omalizumab versus placebo |
| Outcomes | Clinically significant asthma exacerbation Medical resource utilisation Time to first asthma exacerbation Quality of life assessment at baseline, last visit Frequency of asthma rescue medication use Safety/tolerability of omalizumab |
| Notes | No data available at time of completion of 2012 update of this review |
NCT00226200.
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Moderate to severe allergic or non‐allergic asthmatic patients |
| Interventions | Omalizumab versus placebo |
| Outcomes | Measure of sCD23 in plasma CD23 expression on T cell correlated with spirometry, AQLQ and RQLQ |
| Notes | No data available at time of completion of 2012 update of this review |
NCT00329381.
| Methods | Randomised, parallel‐group, placebo‐controlled, double‐blind, multi‐centre |
| Participants | Treatment group: 139. Age: 38.2 (9.89). Males: 51 (36.7%). Baseline lung function: mean FEV1 L (SD): 2.93 (0.69) Control group: 136. Age: 38.2 (10.02). Males: 37 (27.2%). Baseline lung function: mean FEV1 L (SD): 2.84 (0.62) Inclusion criteria stated as: men and women 18 years to 55 years; clinical diagnosis and history of moderate persistent allergic asthma > one year's duration (GINA guidelines); body weight ≥ 20 kg and ≤ 150 kg; total serum IgE ≥ 30 and ≤ 700 IU/mL; on stable asthma treatment including corticosteroids for the preceding four weeks; non‐smoker for at least one year before visit one; prebronchodilation FEV1 at least 75% predicted at first visit; documented (positive skin prick test) sensitivity to at least one of three perennial aeroallergens (house dust mite, dog, cat); judged to be in good physical and mental health and capable of completing the trial Exclusion criteria stated as: history of intubation for asthma or exacerbation requiring systemic steroids within preceding three months or exacerbation requiring hospital treatment within preceding six months; history of immunotherapy to any allergen within the past three years; history of anaphylactic allergic reaction; upper respiratory tract infection within preceding four weeks; history of NSAID‐ or aspirin‐related asthma; history of or current malignancy or other clinically significant uncontrolled systemic medical condition within preceding three months; platelets < 130 × 109/L at first visit; pregnant or breast‐feeding women, or women not practicing a medically approved contraceptive method; allergy to any of the study medications or components; history of drug or alcohol abuse; inability to complete diary or perform lung function tests; oral, IM, IV or intra‐articular steroids within preceding four weeks; beta‐agonists within preceding one week; antihistamines within one week before skin prick testing; could then be used for remainder of study; IV gammaglobulin or immunosuppressants within preceding four weeks; tricyclic antidepressants within preceding one week; investigational drugs within preceding four weeks Location(s): 70 centres in the USA |
| Interventions | Omalizumab 150 to 375 mg SQ every two or four weeks based on body weight and pretreatment IgE level (to ensure receipt of at least 0.016 mg/kg/IgE per four weeks) versus matching placebo administered subcutaneously |
| Outcomes | Effect of omalizumab on systemic allergic reactions to specific immunotherapy (SIT) in participants with persistent allergic asthma who require treatment with inhaled steroids Severity of first SAR to SIT (grade one to four), achievement of target maintenance SIT dose Number of visits required to complete cluster SIT dosing regimen, number of doses of rescue medication (epinephrine, oral steroids or antihistamines), adverse events |
| Notes | 26‐Week trial (period one: screening (two weeks); period two: study drug treatment (16 weeks including three‐week overlap with period three); period three: cluster immunotherapy (four weeks); period four: maintenance immunotherapy (seven weeks) Co‐medication: oral steroids, epinephrine, antihistamines, beta2‐agonists, h2‐agonists and ‘other rescue medications’ as required |
NCT00367016.
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Mild or moderate persistent asthma |
| Interventions | Subcutaneous omalizumab versus placebo |
| Outcomes | Reduction in FcϵRI level on basophils and to examine whether this occurs at a transcriptional level Suppression of IgE production, in addition to sequestration of IgE |
| Notes | No data available at time of completion of 2013 update of this review |
NCT00495612.
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Participants eight to 65 years of age with history of cat dander–induced asthma in the three years before randomisation |
| Interventions | Subcutaneous omalizumab versus placebo |
| Outcomes | Percentage change in FEV1 Percentage decrease in FEV1 Maximum percentage change in FEV1 Duration of cat chamber exposure Change in chest symptom score Change in nasal and ocular symptom scores |
| Notes | Above change scores are from baseline to week 16 No data available at time of completion of 2013 update of this review |
NCT00670930.
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Participants 18 to 60 years of age with moderate to severe persistent allergic asthma |
| Interventions | Omalizumab at a dose of 0.016 mg/kg/IU/mL versus placebo |
| Outcomes | Number of subepithelial eosinophils following 78 weeks of treatment, as assessed by biopsy samples Number of subepithelial mast cells following 78 weeks of treatment, as assessed by biopsy samples Number of subepithelial CD4+ T lymphocytes following 78 weeks of treatment, as assessed by biopsy samples Thickness of the lamina reticularis following 78 weeks of treatment, as assessed by biopsy samples Safety and tolerability of 78 weeks of therapy |
| Notes | Background therapy: inhaled corticosteroids and long‐acting beta‐agonists. No data available at time of completion of 2013 update of this review |
NCT00691873.
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Patients with at least moderate persistent allergic asthma inadequately controlled with inhaled corticosteroids |
| Interventions | Xolair 150 to 375 mg SQ every two or four weeks based on body weight and pretreatment IgE level versus placebo |
| Outcomes | Effect of omalizumab on systemic allergic reactions to specific immunotherapy (SIT) in participants with persistent allergic asthma who require treatment with inhaled steroids |
| Notes | No data available at time of completion of 2013 update of this review |
NCT01393340.
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Patients at least 18 years of age with a diagnosis of asthma for longer than two years |
| Interventions | Xolair(R) will be administered subcutaneously in a dose of 75 to 375 mg every two to four weeks. Doses (mg) and dosing frequency are determined by total serum IgE level (IU/mL) measured at the start of treatment and body weight (kg). During this 20‐week trial, participants will receive four or eight doses of omalizumab |
| Outcomes | Effect of omalizumab on nasal polyp size and evolution of nasal polyps Nasal examination at all visits by endoscopy of each nasal fossa Daytime and night‐time symptom scores Morning and evening peak flows Exhaled nitric oxide Total dosage of rescue beta2‐agonists Total symptom‐free days Quality of life scores Markers of airway remodelling and inflammation Local IgE synthesis in the bronchial mucosa and its expression |
| Notes | No data available at time of completion of 2013 update of this review |
Scripps 2009.
| Methods | Double‐blind, randomised, parallel‐group trial |
| Participants | Inclusion criteria: aspirin‐exacerbated respiratory disease, allergic asthma Exclusion criteria: pregnant females, starting immunotherapy in the past three months, prior treatment with Xolair, negative allergy skin tests, unable to participate in lung function tests, unable to complete data forms, low platelets, serum IgE greater than 700 IU, cancer, another uncontrolled medical condition, unacceptable concomitant medication, younger than 18 years of age Protocol: '40/60 patients will receive omalizumab injections every month for the next 4 months and the other 20 patients, via a random program, will receive placebo injections' and '60 patients with aspirin‐exacerbated respiratory disease will be screened to determine if they also have allergic respiratory tract disease as a co‐morbid complication. This will involve history, allergy skin tests and a serum IgE level. They must also have been desensitised to aspirin and be taking aspirin 325 or 650 mg morning and night'. |
| Interventions | Omalizumab versus placebo |
| Outcomes | Primary: respiratory index score. Secondary: FEV1, nasal flow rates, nasal smell scores, quality of life scores for rhinitis and asthma |
| Notes | Four‐month trial. No data available at time of completion of 2013 update of this review |
Characteristics of ongoing studies [ordered by study ID]
NCT00139152.
| Trial name or title | Non‐invasive ways to evaluate lung disease after treatment with Xolair |
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | People with stable asthma. 12 years of age and older |
| Interventions | Xolair 0.016 mg/kg IgE, SQ versus saline placebo |
| Outcomes | Levels of pH, nitrate/nitrite in exhaled breath condensate before and after four months of treatment |
| Starting date | September 2005 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT00139152 |
| Notes |
NCT00208234.
| Trial name or title | Effect of Xolair on airway hyperresponsiveness |
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Steroid‐naive allergic asthma patients 19 to 50 years |
| Interventions | Omalizumab 0.016 mg/kg IgE versus placebo |
| Outcomes | Changes in PC20 values to methacholine bronchoprovocation challenges and/or PC15 values to hypertonic saline‐induced bronchoprovocation challenges in a time‐dependent manner Exhaled NO and sputum eosinophilia |
| Starting date | January 2004 |
| Contact information | http://www.clinicaltrials.gov/ct2/show/NCT00208234 |
| Notes |
NCT00555971.
| Trial name or title | Therapeutic utility of Xolair in patients undergoing aspirin desensitisation |
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Patients with aspirin‐exacerbated respiratory disease 18 years of age or older |
| Interventions | Dosage (150 to 375 mg) based on IgE levels; administered subcutaneously every two to four weeks for 16 weeks versus placebo |
| Outcomes | FEV1 and changes in serum and urinary markers of eosinophil activation during desensitisation and change in urinary LTE4 during bronchospasm |
| Starting date | May 2006 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT00555971 |
| Notes |
NCT01113437.
| Trial name or title | Omalizumab in non‐atopic asthma |
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Patients 18 to 60 years of age with moderate or severe non‐atopic asthma |
| Interventions | Omalizumab or placebo by subcutaneous injections, at four‐weekly or two‐weekly intervals versus placebo |
| Outcomes | Prebronchodilator FEV1 Before reduction of existing antiasthma therapy (first 12 weeks of study): pre‐bronchodilator FEV1 Disease exacerbation |
| Starting date | April 2010 |
| Contact information | http://clinicaltrials.gov/ct2/show/record/NCT01113437 |
| Notes | Dr Chris Corrigan (PI) has confirmed that trial is still blinded and will be until April 2013 at the earliest |
NCT01125748.
| Trial name or title | A study evaluating the persistency of response with or without Xolair after long‐term therapy (XPORT) |
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Patients with allergic asthma 17 to 70 years of age |
| Interventions | Omalizumab versus placebo |
| Outcomes | Severe exacerbation |
| Starting date | May 2010 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT01125748 |
| Notes |
NCT01202903.
| Trial name or title | Omalizumab in patients with moderate to severe persistent allergic asthma not adequately controlled despite GINA (2009) step four therapy |
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | 18‐ to 75‐year‐old Chinese patients with moderate to severe persistent allergic asthma |
| Interventions | Omalizumab versus placebo |
| Outcomes | Change from baseline in mean morning peak expiratory flow (PEF) following 24‐week treatment period FEV1 percent predicted, PEF, overall score of the standardised AQLQ, ACQ, investigators' and participants' GETE, total nocturnal, daytime and morning asthma symptom scores |
| Starting date | September 2010 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT01202903 |
| Notes |
NCT01430403.
| Trial name or title | Preventative omalizumab or step‐up therapy for severe fall exacerbations (PROSE) |
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Children with asthma six to 17 years |
| Interventions | Three arms: omalizumab, fluticasone and placebo |
| Outcomes | Exacerbation requiring systemic corticosteroid therapy or hospitalisation |
| Starting date | September 2011 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT01430403 |
| Notes |
NCT01544348.
| Trial name or title | Phase 1, randomised, placebo‐controlled, dose escalation safety study of MEDI4212 in allergic subjects (MEDI42121085) |
| Methods | Randomised, double‐blind, parallel‐group study |
| Participants | Allergic asthma Allergic dermatitis Allegic rhinitis |
| Interventions | Omalizumab, MEDI4212 and placebo |
| Outcomes | Safety and tolerability |
| Starting date | January 2012 |
| Contact information | http://clinicaltrials.gov/ct2/show/NCT01544348 |
| Notes |
Differences between protocol and review
1. Study assessment
We have adopted the risk of bias assessment tool as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook). The previous method of assessing study quality was not applied to the studies in this updated review. Our original method for assessing study quality was as follows.
Two review authors independently assessed the methodological quality of eligible RCTs using the five‐point scoring instrument proposed by Jadad 1996. This instrument evaluated the reported quality of randomisation, blinding and description of withdrawals and dropouts. Each study was scored according to the following criteria.
Was the study described as randomised? (1 = Yes, 0 = No).
Was the study described as double‐blind? (1 = Yes, 0 = No).
Was there a description of withdrawals and dropouts? (1 = Yes, 0 = No).
Was the method of randomisation well described and appropriate? (1 = Yes, 0 = No).
Was the method of double‐blinding well described and appropriate? (1 = Yes, 0 = No).
Deduct one point if methods used for randomisation or blinding were inappropriate.
We resolved any disagreements by consensus.
Two review authors also independently ranked quality of allocation concealment using the Cochrane approach.
Grade A: adequate concealment.
Grade B: uncertain concealment.
Grade C: clearly inadequate concealment.
2. NNT calculations
We have used a Summary of findings table to express the results of the meta‐analysis in absolute terms and to provide a summary assessment of the overall quality of the evidence. The number needed to treat for an additional beneficial outcome (NNTB) results have been replaced by natural frequencies of events on control treatment and omalizumab, which is in keeping with the Summary of findings tables.
3. 2013 update
In the 2013 update, we have brought greater clarity to the decision that only double‐blind trials should be included in the review. All previously included studies were double‐blind, but the inclusion criteria had not explicitly addressed this point in earlier versions of the review. The order of the primary outcomes changed from protocol.
4. Generic inverse variance
This method has been used to carry out meta‐analyses of adjusted outcomes (such as exacerbation rates) in the 2013 update.
The Jadad scoring system used in previous versions of the review has been replaced by a Cochrane risk of bias assessment for each included trial.
Contributions of authors
Original version of review: SW developed the protocol with input from K Phelan (KP) and M Monteil (MM). Editorial support was given by EHW. Studies were selected and appraised by SW and MM. Data were extracted by MM, T Lasserson (TL) and SW, and then were entered by MM and TL. MM and TL developed the analysis with input from SW, KP and EHW. MM and SW developed the discussion, with guidance from KP and EHW.
TL and SW wrote the update of the review, with additional input from EHW, MM and KP.
In the 2013 update, SW and PN updated the Background section with input from EHW, and SJM updated the Methods section. Studies were selected and appraised by SW and PN, and data were extracted by SJM, SW, and RN, and then were entered by SJM. The risk of bias of the included studies was assessed by SJM, RN, SW and PN. SJM conducted the analysis with input from SW, PN and RN. The Results section was written by SJM with input from SW, PN and RN. The summary of findings tables were completed by SJM and RN. The Discussion and Conclusions sections were written by SW and PN with input from RN, SJM and EHW.
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
Nederlands Astma Fonds, Netherlands.
The Thriplow Charitable Trust, UK.
Declarations of interest
2013 update:
SW has received travel grants from AstraZeneca, GlaxoSmithKline, Schering‐Plough, Aventis Pharma and 3M.
SM has received support with travel costs and conference attendance from Novartis.
EHW has received research support from GlaxoSmithKline, AstraZeneca, Novartis, Boehringer and Schering‐Plough in the past but none in recent years.
PN has received research grants from GSK, AZ and Schering, and honoraria or travel grants from GSK, AZ, Merck, Novartis, Teva, BI and Cipla.He is listed on a patent for a sputum filtration device and has provided scientific advice for a university spin‐off company, Cellometrics Inc.
RN: none known.
Original version of the review: Michele Monteil has received travel grants from GlaxoSmithKline and Merck Sharpe & Dohme.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bardelas 2012 {published data only}
- Bardelas J, Figliomeni M, Kianifard F, Meng X. A 26‐week, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. Journal of Asthma 2012;49(2):144‐52. [DOI] [PubMed] [Google Scholar]
- Figliomeni M, Kianifard F, Meng X. A 26‐week, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the effect of omalizumab on markers of asthma impairment in patients with persistent allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB84. [DOI] [PubMed] [Google Scholar]
- NCT00870584. A 26‐Week Randomized, Double‐blind, Placebo‐Controlled, Multi‐Center Study to Evaluate the Effect of Omalizumab on Markers of Asthma Impairment in Patients With Persistent Allergic Asthma. www.clinicaltrials.gov/show/NCT00870584 (accessed 7 January 2013). []
Boulet 1997 {published data only}
- Boulet L‐P, Chapman KR, Côté J, Kalra S, Bhagat R, Swystun VA, et al. Inhibitory effects of an anti‐IgE antibody E25 on allergen‐induced early asthmatic response. American Journal of Respiratory and Critical Care Medicine 1997;155:1835‐40. [DOI] [PubMed] [Google Scholar]
Busse 2001 {published and unpublished data}
- Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti‐IgE antibody, in patients with allergic asthma. Chest 2004;125(4):1378‐86. [DOI] [PubMed] [Google Scholar]
- Busse W, Corren J, Lanier BQ, Mcalary M, Fowler‐Taylor A, Della Cioppa G, et al. Anti‐IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. Journal of Allergy and Clinical Immunology 2001;108(2):184‐90. [DOI] [PubMed] [Google Scholar]
- Finn A, Gross G, Bavel J, Lee T, Windom H, Everhard F, et al. Omalizumab improves asthma‐related quality of life in patients with severe allergic asthma. Journal of Allergy and Clinical Immunology 2003;111(2):278‐84. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm113459.pdf (accessed 7 January 2013):26‐78.
- Lanier RQ, Busse W, Corren J, Chervinsky P, Bernstein J, McAlary M, et al. Long‐term improvement in asthma control and exacerbation frequency is achieved with omalizumab (Xolair) in patients with moderate‐severe asthma. ATS International Conference; May 18‐23; San Francisco. 2001:185.
- Luskin AT, Kosinski M, Bresnahan BW, Ashby M, Wong DA. Symptom control and improved functioning: the effect of omalizumab on Asthma‐Related Quality of Life (ARQL). Journal of Asthma 2005;42(10):823‐7. [DOI] [PubMed] [Google Scholar]
- Massanari M, Deniz Y, Lee J, Kianifard F, Blogg M, Reisner C, Geba GP. Omalizumab improved asthma control and reduced rescue steroid bursts in moderate to severe allergic asthma [Abstract]. XIX World Allergy Organization Congress; Jun 26‐Jul 1; Munich. 2005:Abstract 308.
- Zeldin R, Massanari M, Blogg M, Jimenez P, Geba G. Treatment of moderate severe asthma with omalizumab is associated with a decrease in peripheral blood eosinophils [Abstract]. European Respiratory Journal 2007;30(Suppl 51):353s. [Google Scholar]
Busse 2011 {published data only}
- Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti‐IgE) for asthma in inner‐city children. New England Journal of Medicine 2011;364(11):1005‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00377572. Inner‐City Anti‐IgE Therapy for Asthma. www.clinicaltrials.gov/show/NCT00377572 (accessed 7 January 2013). []
Chanez 2010 {published data only}
- CIGE025AFR02. Double blind placebo controlled study to assess the expression of Fcϵ1 on blood basophils and dendritic cells in patients with uncontrolled severe persistent allergic asthma after a 16‐week omalizumab treatment. www.novctrd.com (accessed 2 October 2010).
- Chanez P, Contin‐Bordes C, Garcia G, Verkindre C, Didier A, Blay F, et al. Omalizumab‐induced decrease of FcRI expression in patients with severe allergic asthma. Respiratory Medicine 2010; Vol. 104, issue 11:1608‐17. [DOI] [PubMed]
- NCT00454051. Double Blind Placebo Controlled Study to Assess the Expression of IgE on Basophils and Dendritic Cells During Omalizumab Treatment. www.clinicaltrials.gov/show/NCT00454051 (accessed 7 January 2013). []
Djukanovic 2004 {unpublished data only}
- Djukanovic R, Wilson SJ, Kraft M, Jarjour N, Steel M, Chung KF, et al. Effect of treatment with anti‐IgE antibody (omalizumab) on airway inflammation in mild atopic asthma [abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle C082.
- Djukanovic R, Wilson SJ, Kraft M, Jarjour N, Steel M, Chung KF, et al. Omalizumab, an anti‐IgE antibody, suppresses airway inflammation in mild allergic asthma via a reduction in mast cell surface‐associated interlukin‐4 [Abstract]. Allergy & Clinical Immunology International 2003, issue Suppl 1:Abstract No: 0‐17‐3.
- Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti‐immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. American Journal of Respiratory & Critical Care Medicine 2004;170(6):583‐93. [DOI] [PubMed] [Google Scholar]
Fahy 1997 {published data only}
- Fahy JV, Flemming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti‐IgE monoclonal antibody on the early‐ and late‐phase responses to allergen inhalation in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1828‐34. [DOI] [PubMed] [Google Scholar]
Fahy 1999 {published data only}
- Fahy JV, Cochroft DW, Boulet LP, Wong HH, Deschesnes F, Davis EE, et al. Effect of aerosolized anti‐IgE (E25) on airways responses to inhaled allergen in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine 1999;160(3):1023‐7. [DOI] [PubMed] [Google Scholar]
Garcia 2012 {published data only}
- Garcia G, Magnan A, Chiron R, Cecile C‐B, Berger P, Taille C. A randomized‐controlled trial of omalizumab in patients with severe difficult to control nonatopic asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A6764. [] [Google Scholar]
- Garcia G, Magnan A, Chiron R, Girodet P‐O, Gros VMH. A proof‐of‐concept randomized‐controlled trial of omalizumab in patients with severe difficult to control nonatopic asthma [Abstract]. European Respiratory Journal 2012;40(Suppl 56):856s [4692]. [] [DOI] [PubMed] [Google Scholar]
Gevaert 2012 {published data only}
- Gevaert P, Calus L, Zele T, Blomme K, Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and non‐allergic patients with nasal polyps and asthma [Abstract]. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl):AB69 [258]. [DOI] [PubMed] [Google Scholar]
- Gevaert P, Calus L, Zele T, Blomme K, Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients. Journal of Allergy and Clinical Immunology 2013;131(1):110‐116e1. [] [DOI] [PubMed] [Google Scholar]
Hanania 2011 {published data only}
- Condemi JJ, Hamilos DL, Hanania NA, Reyes‐Rivera I, Rosen KE, Wong D, et al. Efficacy and safety of omalizumab in patients with moderate‐to‐severe persistent asthma poorly controlled on high‐dose inhaled corticosteroids and long‐acting beta‐agonists: results of a phase III randomized controlled trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A6840. [Google Scholar]
- Dorenbaum A, Trzaskoma B, Haselkorn T, Mink D, Chen H, Solari P. Patient characteristics predictive of omalizumab response in EXTRA. Annals of Allergy, Asthma & Immunology 2012;109(Suppl):A54. [] [Google Scholar]
- Hanania N, Condemi J, Hamilos D, Reyes‐Rivera I, Rosen KE, Wong D, et al. Omalizumab in patients with moderate‐to‐severe persistent asthma poorly controlled on high‐dose inhaled corticosteroids and long‐acting beta‐agonists: results of a phase IIIb randomized controlled trial [Abstract]. European Respiratory Society Annual Congress; September 18‐22; Barcelona. 2010:[E5487].
- Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes‐Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Annals of Internal Medicine 2011;154(4):573‐82. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Wenzel S, Rosen K, Hsieh H‐J, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma. American Journal of Respiratory and Critical Care Medicine 2013;187(8):804‐11. [] [DOI] [PubMed] [Google Scholar]
- NCT00314574. A Phase IIIb Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Study of Xolair in Subjects With Moderate to Severe Persistent Asthma Who Are Inadequately Controlled With High‐Dose Inhaled Corticosteroids and Long‐Acting Beta‐Agonists. www.clinicaltrials.gov/show/NCT00314574 (accessed 7 January 2013). []
Holgate 2004a {published and unpublished data}
- CIGE0250011E1. An open‐label extension to assess long‐term safety and tolerability of omalizumab treatment in adolescents and adults with severe allergic asthma who participated in the 32‐week core study. www.novctrd.com (accessed 10 February 2010).
- CIGE0250011E3. An open‐label extension study to assess long term safety and tolerability of omalizumab treat‐ment in adults and adolescents with severe allergic asthma who participated in the 52 week CIGE025 0011E2 study. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2314 (accessed 7 January 2013).
- Chuchalin AG, Herbert J, Rolli M, Gao J, Resiner C. Long‐term safety and tolerability of omalizumab an anti‐IgE monoclonal antibody in patients with severe allergic asthma. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 421. [Google Scholar]
- Chung F, Holgate S, O'Brien J, Fox H, Thirlwell J. Inhaled corticosteroid dose reducing effect of omalizumab in patients with controlled severe asthma, according to usage of inhaled long acting beta‐agonist. American Academy of Allergy Asthma and Immunology 58th annual meeting. New York, New York, USA. March 1‐6. 2002:[726].
- Hebert J, Chuchalin A, Rolli M, Fox H. Long‐term safety and tolerability of omalizumab in adults with severe allergic asthma. American Thoracic Society Meeting 100th International Conference; 2004 May 21‐26; Orlando. 2004.
- Hebert J, Rolli M, Gao J, Reisner C. Omalizumab an anti‐IgE monoclonal antibody demonstrates long‐term asthma control safety and tolerability in patients with severe allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S9. [Google Scholar]
- Holgate ST, Chuchalin A, Herbert J, Lotvall J, Chung KF, Bousquet J, et al. Omalizumab (Xolair, rhumab‐e25) a novel therapy for severe allergic asthma. Proceedings of the ATS 97th International Conference; 2001 May 18‐23; San Francisco. 2001:159.
- Holgate ST, Chuchalin A, Herbert J, Persson G, Chung F, Bousquet J, et al. Omalizumab (rhumab‐e25) improves asthma‐specific quality of life in patients with severe allergic asthma. Proceedings of the ATS 97th International Conference; 2001 May 18‐23; San Francisco. 2001:184; [D31] [Poster: K13].
- Holgate ST, Chuchalin AG, Hébert J, Lötvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant anti‐immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clinical Experimental Allergy 2004;34(4):632‐8. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. http://www.fda.gov (accessed 7 January 2013):99‐122.
Holgate 2004b {published data only}
- CIGE0250011E1. An open‐label extension to assess long‐term safety and tolerability of omalizumab treatment in adolescents and adults with severe allergic asthma who participated in the 32‐week core study. www.novctrd.com (accessed 10 February 2010).
- CIGE0250011E3. An open‐label extension study to assess long term safety and tolerability of omalizumab treatment in adults and adolescents with severe allergic asthma who participated in the 52 week CIGE025 0011E2 study. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2314 (accessed 7 January 2013).
- Chuchalin AG, Herbert J, Rolli M, Gao J, Resiner C. Long‐term safety and tolerability of omalizumab an anti‐IgE monoclonal antibody in patients with severe allergic asthma. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 421. [Google Scholar]
- Chung F, Holgate S, O'Brien J, Fox H, Thirlwell J. Inhaled corticosteroid dose reducing effect of omalizumab in patients with controlled severe asthma, according to usage of inhaled long acting beta‐agonist. American Academy of Allergy Asthma and Immunology 58th Annual Meeting. New York, New York, USA. March 1‐6, 2002 2002:[726].
- Hebert J, Chuchalin A, Rolli M, Fox H. Long‐term safety and tolerability of omalizumab in adults with severe allergic asthma. American Thoracic Society Meeting 100th International Conference; 2004 May 21 ‐ 26; Orlando. 2004.
- Hebert J, Rolli M, Gao J, Reisner C. Omalizumab an anti‐IgE monoclonal antibody demonstrates long‐term asthma control safety and tolerability in patients with severe allergic asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S9. [Google Scholar]
- Holgate ST, Chuchalin A, Herbert J, Lotvall J, Chung KF, Bousquet J, et al. Omalizumab (Xolair, rhumab‐e25) a novel therapy for severe allergic asthma. Proceedings of the ATS 97th International Conference; 2001 May 18‐23; San Francisco. 2001:159.
- Holgate ST, Chuchalin A, Herbert J, Persson G, Chung F, Bousquet J, et al. Omalizumab (rhumab‐e25) improves asthma‐specific quality of life in patients with severe allergic asthma. Proceedings of the ATS 97th International Conference; 2001 May 18‐23; San Francisco. 2001:184.
- Holgate ST, Chuchalin AG, Hébert J, Lötvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant anti‐immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clinical Experimental Allergy 2004;34(4):632‐8. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. www.fda.gov (accessed 7 January 2013):99‐122.
INNOVATE {unpublished data only}
- Berger W, Humbert M, Leighton T, Turk F, Hedgecock S, Ayre G, et al. Asthma patients judged by the physician to have responded to add‐on omalizumab therapy have a greater percentage of symptom‐free days. Proceedings of the American Thoracic Society; 2006 May 19‐24; San Diego. 2006:A591.
- Bleecker E, Rubinfield A, Hedgecock S, Fox H, Surrey K, Reisner C. Add‐on omalizumab therapy significantly improves symptom control and reduces exacerbations in patients with inadequately controlled severe persistent asthma despite GINA 2002 Step 4 therapy irrespective of maintenance oral corticosteroid (OCS) use: INNOVATE. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. 2005:B36; Poster: G51.
- Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respiratory Medicine 2007;101(7):1483‐92. [DOI] [PubMed] [Google Scholar]
- Brown R, Turk F, Groot M, Dale P. Cost effectiveness of omalizumab in patients with severe persistent allergic (IgE‐mediated) asthma adaptation of INNOVATE and ETOPA data to the Netherlands. European Respiratory Journal 2007;30(Suppl 51):194s. [Google Scholar]
- Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add‐on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60(3):309‐16. [DOI] [PubMed] [Google Scholar]
- Humbert M, Berger W, Rapatz G, Turk F. Add‐on omalizumab improves day‐to‐day symptoms in inadequately controlled severe persistent allergic asthma. Allergy 2008;63(5):592‐6. [DOI] [PubMed] [Google Scholar]
- Korenblat P, Levy R, Slavin R, Hedgecock S, Fox H, Surrey K. Omalizumab add‐on therapy significantly reduces asthma exacerbations in patients with inadequately controlled severe persistent asthma despite GINA 2002 step 4 therapy: INNOVATE [Abstract]. Journal of Allergy and Clinical Immunology 2005;115(Suppl 2):S76. [Google Scholar]
- Korenblat P, Levy R, Slavin R, Hedgecock S, Fox H, Surrey K, Reisner C. Add‐on omalizumab therapy significantly reduces severe asthma exacerbations and emergency visits in patients with inadequately controlled severe persistent asthma despite GINA 2002 step 4 therapy INNOVATE [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005.
- Korenblat PE, Hegecock S, Surrey K, Fox H. Omalizumab in patients with severe persistent allergic asthma inadequately controlled by GINA step 4 therapy [Abstract]. American Thoracic Society 100th International Conference; May 21‐6; Orlando 2004:B37; G88.
- Lowe P, Jaffe J, Martin C, Brookman L, Fox H. The effect of discontinuing omalizumab therapy on free lgE concentration [Abstract]. Journal of Allergy and Clinical Immunology 2007;119(Suppl 1):S6. [Google Scholar]
- Martin C, Freeman P, Blogg M. Pre‐treatment specific IgE levels are not useful in predicting a response to omalizumab therapy [Abstract]. Journal of Allergy and Clinical Immunology 2008;21(2 Suppl 1):S171. [Google Scholar]
- Massanari M, Kianifard F, Maykut R, Zeldin R, Hedgecock S, Reisner C, et al. Omalizumab reduced need for steroid bursts and improved treatment effectiveness in asthmatics on inhaled salmeterol and fluticasone combination therapy. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):s10. [Google Scholar]
- Massanari M, Maykut RJ, Kianifard F, Zeldin RK, Geba GP. Addition of omalizumab improves quality of life in moderate to severe asthmatics receiving fluticasone 500 ug/salmeterol 50ug [Abstract]. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl):S4. [Google Scholar]
- Massanari M, Maykut RJ, Zeldin RK, Kianifard F, Geba GP. Addition of omalizumab improves lung function and treatment effectiveness in patients with moderate‐severe persistent allergic asthma inadequately controlled with inhaled steroids and long‐acting‐beta‐agonists. Chest 2006;130(109s):4. [Google Scholar]
- Massanari M, Zeldin R, Maykut R, Kianifard F, Geba G. Omalizumab improves lung function and treatment effectiveness in patients with moderate‐severe asthma receiving fluticasone 500mcg/salmeterol 50mcg. Proceedings of the American Thoracic Society; 19‐24 May; San Diego. 2006:A590.
- Matz J, Melamed I, Ledford D, Hedgecock S, Fox H, Surrey K, Resiner C. Add‐on omalizumab therapy significantly improves quality of life in patients with inadequately controlled severe persistent asthma despite GINA 2002 step 4 treatment, INNOVATE [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:[B36; Poster G47].
- Novartis. Study number 2306. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=1601 (accessed 7 January 2013).
- Slavin RG, Ferioli C, Tannenbaum SJ, Martin C, Blogg M, Lowe PJ. Asthma symptom re‐emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. Journal of Allergy and Clinical Immunology 2009;123(1):107‐13. [DOI] [PubMed] [Google Scholar]
- Sthoeger ZM, Eliraz A, Asher I, Berkman N, Elbirt D. The beneficial effects of Xolair (omalizumab) as add‐on therapy in patients with severe persistent asthma who are inadequately controlled despite best available treatment (GINA 2002 step IV—the Israeli arm of the INNOVATE study. Israel Medical Association Journal 2007;9(6):472‐5. [PubMed] [Google Scholar]
- Wahn U, Martin C, Freeman P, Blogg M, Jimenez P. Relationship between pre‐treatment specific IgE and the response to omalizumab therapy [Abstract]. European Respiratory Society Annual Congress; 2008 October 4‐8; Berlin. 2008.
- Wahn U, Martin C, Freeman P, Blogg M, Jimenez P. Relationship between pretreatment specific IgE and the response to omalizumab therapy. Allergy 2009;64(12):1780‐7. [DOI] [PubMed] [Google Scholar]
Lanier 2009 {published data only}
- Bousquet J, Kulus M, Fox H, Blogg M, Fowler‐Taylor A, Fernandez‐Vidaurre C. Omalizumab therapy reduces asthma exacerbations in children with severe allergic (ige‐mediated) asthma irrespective of lung function at baseline [Abstract].. European Respiratory Society Annual Congress; September 12‐16; Vienna. 2009:3281.
- Bridges T, Hebert J, Fowler‐Taylor A, Fernandez‐Vidaurre C, Berhane I. Omalizumab reduces asthma exacerbations in children (6‐<12 years) with moderate‐to‐severe allergic (IgE‐mediated) asthma irrespective of baseline LABA use [Abstract]. European Respiratory Society Annual Congress; September 12‐16; Vienna, Austria. 2009:P1219.
- CIGE025AIA05. A 1 year, randomized, double blind, parallel‐group, placebo‐controlled, multicenter evaluation of efficacy, safety, pharmacokinetics and pharmacodynamics of omalizumab in children (6 ‐ <12 years) with moderate‐severe, persistent, inadequately controlled allergic asthma. www.novctrd.com (accessed 10 February 2010).
- Kulus M, Bridges T, Fowler‐Taylor A, Blogg M, Jimenez P. A randomized controlled study of omalizumab in children with moderate to severe persistent allergic asthma [Abstract]. European Respiratory Society Annual Congress; 2008 October 4‐8; Berlin.
- Kulus M, Hébert J, Garcia E, Fowler Taylor A, Fernandez Vidaurre C, Blogg M. Omalizumab in children with inadequately controlled severe allergic (IgE‐mediated) asthma. Current Medical Research and Opinion 2010;26(6):1285‐93. [DOI] [PubMed] [Google Scholar]
- Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE‐mediated) asthma. Journal of Allergy and Clinical Immunology 2009;124(6):1210‐6. [DOI] [PubMed] [Google Scholar]
- Milgrom H, Fink J, Fowler‐Taylor A, Fernandez Vidaurre C, Blogg M, Fox H. Safety of omalizumab in children with inadequately controlled moderate‐severe allergic (IgE‐mediated) asthma [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A2809 [Poster #J36].
- Milgrom H, Fowler Taylor A, Blogg M, Fernandez Vidaurre C. Systemic steroid use in children with allergic (IgE‐mediated) asthma receiving omalizumab [Abstract]. European Respiratory Society Annual Congress; Sept 18‐22; Barcelona. 2010:[P2642].
- Milgrom H, Fowler‐Taylor A, Fernandez‐Vidaurre C, Jayawardene S. Safety of omalizumab therapy in children with allergic asthma [Abstract]. European Respiratory Society Annual Congress; Sept 12‐16; Vienna, Austria. 2009:P1214.
- Milgrom H, Wasserman RL, Fowler‐Taylor A, Fernandez Vidaurre C, Blogg M, Fox H. Add‐on omalizumab significantly reduces exacerbation rates in children with inadequately controlled moderate‐severe allergic (IgE mediated) asthma. American Thoracic Society International Conference; May 15‐20, San Diego. 2009:A2767.
- NCT00079937. A 1 Year, Randomized, Double‐Blind, Parallel‐Group, Placebo‐Controlled, Multicenter Evaluation of Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of Omalizumab in Children (6‐< 12 Years) With Moderate‐Severe, Persistent, Inadequately Controlled Allergic Asthma. www.clinicaltrials.gov/show/NCT00079937 (accessed 7 January 2013). []
Massanari 2010 {published data only}
- CIGE025A US23. A 26‐week, randomized, double‐blind, parallel‐group, placebo‐controlled, multi‐center study to evaluate the effect of omalizumab on improving the tolerability of specific immunotherapy in patients with at least moderate persistent allergic asthma inadequately controlled with inhaled corticosteroids. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=1601 (accessed 7 January 2013).
- Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. Journal of Allergy and Clinical Immunology 2010;125(2):383‐9. [DOI] [PubMed] [Google Scholar]
- NCT00267202. A 26‐Week, Randomized, Double‐Blind, Parallel‐Group, Placebo‐Controlled, Multi‐Center Study to Evaluate the Effect of Omalizumab on Improving the Tolerability of Specific Immunotherapy in Patients With at Least Moderate Persistent Allergic Asthma Inadequately Controlled With Inhaled Corticosteroids. www.clinicaltrials.gov/show/NCT00267202 (accessed 7 January 2013). []
Milgrom 1999 {published data only}
- Metzger WJ, Fick RB, Bush RK, Busse W, Casale T, Chodosh S, et al. Corticosteroid (CS) withdrawal in a study of recombinant humanized monoclonal antibody to IgE (rhu MAbE25). Journal of Allergy and Clinical Immunology 1998;101(1):231. [Google Scholar]
- Milgrom H, Fick RB, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti‐IgE antibody. New England Journal of Medicine 1999;341(26):1966‐73. [DOI] [PubMed] [Google Scholar]
Milgrom 2001 {published data only}
- Berger W, Gupta N, McAlary M, Fowler‐Taylor A. Evaluation of long‐term safety of the anti‐IgE antibody, omalizumab, in children with allergic asthma. Annals of Allergy Asthma & Immunology 2003;91(2):182‐8. [DOI] [PubMed] [Google Scholar]
- Buhl R, Soler M, Fox H, Ashby M, McAlary M, Cooper J, et al. Omalizumab (Xolair, rhumab‐e25) decreases hospitalisations due to serious asthma exacerbations. Proceedings of the ATS 97th International conference; 2001 May 18‐23; San Francisco. 2001:186.
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. www.fda.gov (accessed 7 January 2013):79‐98.
- Lemanske RF, Nayak A, McAlary M, Everhard F, Fowler‐Taylor A, Gupta N. Omalizumab improves asthma‐related quality of life in children with allergic asthma. Pediatrics 2002;110(5):e55. [DOI] [PubMed] [Google Scholar]
- Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti‐immunoglobulin E antibody (omalizumab). Pediatrics 2001;108:36. [DOI] [PubMed] [Google Scholar]
- Milgrom H, Miller SD, Lanier BQ, Fowler‐Taylor A, Chen H, Gupta N. Long‐term omalizumab therapy is well tolerated in children with moderate‐to‐severe IgE‐medicated asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:B36; Poster: G46.
- Nayak A, Milgrom H, Berger W, Pollard S, Watrous M, Doyle J, et al. Rhumab‐E25 (E25) improves quality of life (QOL) in children with allergic asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A504. [Google Scholar]
NCT00096954 {published data only}
- NCT00096954. A Prospective, Randomized, Double‐Blind Study of the Efficacy of Omalizumab (Xolair) in Atopic Asthmatics With Good Lung Capacity Who Remain Difficult to Treat (EXACT). Http://clinicaltrials.gov/show/NCT00096954 (accessed 7 January 2013). []
NCT01007149 {published data only}
- CIGE025AFR05 A 16‐Week Treatment, Multicenter, Randomized, Double Blind, Placebo‐Controlled, Parallel‐Group Study to Assess the Effect of Omalizumab on the Expression of FcεRI Receptors of Blood Basophils and Dendritic Cells in Patients With Severe Persistent Non‐Atopic Asthma, Uncontrolled Despite Optimal Therapy. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=5323 (accessed 7 January 2013).
- NCT01007149. A 16‐Week Treatment, Multicenter, Randomized, Double Blind, Placebo‐Controlled, Parallel‐Group Study to Assess the Effect of Omalizumab on the Expression of FcεRI Receptors of Blood Basophils and Dendritic Cells in Patients With Severe Persistent Non‐Atopic Asthma, Uncontrolled Despite Optimal Therapy. www.clinicaltrials.gov/show/NCT01007149 (accessed 7 January 2013). []
Ohta 2009 {published data only}
- CIGE025A1304. Multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled study with a 16‐weektreatment phase to determine the efficacy, safety and tolerability of subcutaneous omalizumab at a dose of at least 0.016mg/kg/IgE[IU/ml] every 2 or 4 weeks for the treatment of patients with moderate to severe bronchial asthma. The trial consisted of 3 periods: a 2‐week screening period, a16‐week treatment period and a 24‐week post‐treatment follow‐up period. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2197 (accessed 7 January 2013).
- NCT00232050. Study of Omalizumab in Moderate to Severe Bronchial Asthma. www.clinicaltrials.gov/show/NCT00232050 (accessed 7 January 2013). []
- Ohta K, Miyamoto T, Amagasaki T, Yamamoto M. Efficacy and safety of omalizumab in an Asian population with moderate‐to‐severe persistent asthma. Respirology 2009;14(8):1156‐65. [DOI] [PubMed] [Google Scholar]
- Ohta K, Miyamoto T, Yamamoto M, Fox H, Blogg M. Omalizumab improves lung function in asthmatic smokers with severe persistent allergic asthma. American Thoracic Society International Conference; 18‐23 May; San Francisco. 2007:Poster F76.
Prieto 2006 {published data only}
- Bruno L, Prieto L, Gutierrez V, Colas C, Tabar AI, Perez‐Frances, et al. Effect of omalizumab on adenosine 5'‐monophosphate responsiveness in allergic asthma [Abstract]. XIX World Allergy Organization Congress; Munich. 2005:Abstract 306.
- CIGE025AES01. Effect of omalizumab on bronchial responsiveness to adenosine 5'‐monophosphate (AMP) in patients with asthma. www.novctrd.com (accessed 2 October 2010).
- Prieto L, Gutierrez V, Colas C, Tabar A, Perez‐Frances C, Bruno L, et al. Effect of omalizumab on adenosine 5'‐monophosphate responsiveness in subjects with allergic asthma. International Archives of Allergy and Immunology 2006;139(2):122‐31. [DOI] [PubMed] [Google Scholar]
SOLAR {published data only}
- Boulet LP, Canonica GW, Dahl R, Hedgecock S, Blogg M, Surrey K, et al. Omalizumab, an Anti‐IgE antibody, provides parallel improvements in symptoms of allergic asthma and perennial allergic rhinitis in patients with both diseases: the SOLAR study. Chest 2003;124(4):105s. [Google Scholar]
- CIGE025A2304. A phase IIIb, multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled study with a 28‐week treatment phase to determine the efficacy, safety and tolerability of subcutaneous omalizumab for the treatment of 12‐75 year‐old patients with comorbid moderate‐to‐severe allergic asthma and perennial allergic rhinitis. www.novctrd.com (accessed 10 February 2010).
- Dahl R, Ayres J, Hedgecock S, Blogg M, Surrey K, Fox H. Efficacy of omalizumab, an anti‐IgE antibody, in patients with concomitant moderate‐severe allergic asthma and persistent allergic rhinitis [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S37. [Google Scholar]
- Hanf G, Noga O, Brachmann I, Kleine‐Tebbe J, Rosseau S, Suttorp N, et al. Omalizumab (rhuMAb‐E25) inhibits the IgE in vitro release of stimulated PBMC of allergic asthmatics. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:B36; Poster: G58.
- Harnest U, Boulet L, Hedgecock S, Blogg M, Surrey K, Fox H. Omalizumab, an anti‐IgE antibody, improves both asthma and rhinitis‐related quality of life in patients with concomitant moderate‐severe disease [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S175. [Google Scholar]
- Humbert M, Boulet LP, Niven RM, Panahloo Z, Blogg M, Ayre G. Omalizumab therapy: patients who achieve greatest benefit for their asthma experience greatest benefit for rhinitis. Allergy 2009;64(1):81‐4. [DOI] [PubMed] [Google Scholar]
- Noga O, Hanf G, Brachmann I, Klucken AC, Kleine‐Tebbe J, Rosseau S, et al. Effect of omalizumab treatment on peripheral eosinophil and T‐lymphocyte function in patients with allergic asthma. Journal of Allergy & Clinical Immunology 2006;117(6):1493‐9. [DOI] [PubMed] [Google Scholar]
- Noga O, Hanf G, Brachmann I, Rosseau S, Suttorp N, Kunkel G. Omalizumab (rhuMAb‐E25) induced apoptosis of eosinophils in allergic asthmatics [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:B36; Poster: G59.
- Vignola AM, Humbert M, Bousquet J, Boulet LP, Hedgecock S, Blogg M, et al. Efficacy and tolerability of anti‐immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy 2004;59(7):709‐17. [DOI] [PubMed] [Google Scholar]
- Vignola M, Bousquet J, Maspero J, Fox H, Hedgecock S, Blogg M. Treatment of co‐morbid allergic asthma and perennial allergic rhinitis with the anti IgE agent omalizumab [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: [1388]. [Google Scholar]
Solèr 2001 {published data only}
- Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti‐IgE antibody, in patients with allergic asthma. Chest 2004;125(4):1378‐86. [DOI] [PubMed] [Google Scholar]
- Buhl R, Hanf G, Soler M, Bensch G, Wolfe J, Everhard F, et al. The anti‐IgE antibody omalizumab improves asthma‐related quality of life in patients with allergic asthma. European Respiratory Journal 2002;20(5):1088‐94. [DOI] [PubMed] [Google Scholar]
- Buhl R, Solèr M, Matz J, Townley R, O'Brien J, Noga O, et al. Omaliziumab provides long‐term control in patients with moderate‐to‐severe allergic asthma. European Respiratory Journal 2002;20:73‐8. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. www.fda.gov (accessed 7 January 2013):26‐78.
- Luskin AT, Kosinski M, Bresnahan BW, Ashby M, Wong DA. Symptom control and improved functioning: the effect of omalizumab on Asthma‐Related Quality of Life (ARQL). Journal of Asthma 2005;42(10):823‐7. [DOI] [PubMed] [Google Scholar]
- Massanari M, Deniz Y, Lee J, Kianifard F, Blogg M, Reisner C, et al. Omalizumab improved asthma control and reduced rescue steroid bursts in moderate to severe allergic asthma [Abstract]. XIX World Allergy Organization Congress, June 26‐July 1, Munich, Germany. 2005:308.
- Soler M, Buhl R, Bensch O, Noga O, O'Brien J, Champain K, et al. Omalizumab (Xolair rhuMAb‐E25) treatment reduces inhaled corticosteroid use in moderate/severe allergic asthma. Proceedings of the ATS 97th International Conference; 18‐23 May; San Francisco. 2001:183; [D31] [Poster: K11].
- Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti‐IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. European Respiratory Journal 2001;18:254‐61. [DOI] [PubMed] [Google Scholar]
- Solèr M, Matz J, Townley RG, Buhl R, O'Brien J, Fox HG, et al. rhuMAb‐E25, a novel therapy for the treatment of allergic asthma (AA). European Respiratory Journal 2000;16(Suppl 31):10s. [Google Scholar]
- Zeldin R, Massanari M, Blogg M, Jimenez P, Geba G. Treatment of moderate severe asthma with omalizumab is associated with a decrease in peripheral blood eosinophils [Abstract]. European Respiratory Journal 2007;30(Suppl 51):353s. [Google Scholar]
van Rensen 2009 {published data only}
- Rabe KF, Rensen ELJ, Evertse CE, Schadewijk WAA, Wijngaarden S, Ayre G, et al. Anti‐IgE induced changes in bronchial high affinity IgE receptor expressing cells and eosinophils in biopsies in patients with asthma role of DC‐SIGN [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A569.
- Rensen EL, Evertse CE, Gauw SA, Ayre G, Sterk PJ, Rabe KF. Anti‐IgE treatment improves lung function in patients with mild persistent allergic asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S9. [Google Scholar]
- Rensen EL, Evertse CE, Schadewijk WA, Wijngaarden S, Ayre G, et al. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti‐IgE treatment. Allergy 2009;64(1):72‐80. [DOI] [PubMed] [Google Scholar]
- Rensen ELJ, Evertse CE, Schadewijk WAA, Veen H, Timmers MC. Anti‐IgE omalizumab treatment reduces allergen‐induced eosinophilia in biopsies and sputum in patients with asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:C12.
References to studies excluded from this review
Anonymous 2000 {published data only}
- Anonymous. Anti‐IgE for allergic asthma. Hospital Practice 2000;24(2):27‐8. [Google Scholar]
Anonymous 2000b {published data only}
- Anonymous. Anti‐IgE antibody reduces need for glucocorticosteroids [Anti‐IgE Antikörper: Bedarf an Glucocorticoiden sinkt]. Deutsche Apotheker Zeitung 2000;140(12):1289‐92. [Google Scholar]
Anonymous 2003 {published data only}
- Anonymous. Omalizumab appears effective in patients with poorly controlled allergic asthma. Formulary 2003;38(4):197, 203. [Google Scholar]
Ayars 2011 {published data only}
- Ayars AG, Altman LC, Potter‐Perigo S, Wight TN, Nair P. Sputum hyaluronan as a biomarker of airway remodeling in severe asthma [Abstract]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB8. [Google Scholar]
Ayars 2013 {published data only}
- Ayars AG, Altman LC, Potter‐Perigo S, Radford K, Wight TN, Nair P. Sputum hyaluronan and versican in severe eosinophilic asthma. International Archives of Allergy and Immunology 2013;161(1):65‐73. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Babu 2001 {published data only}
- Babu KS, Arshad SH, Holgate ST. Anti‐IgE treatment: an update. Allergy 2001;56:1121‐8. [DOI] [PubMed] [Google Scholar]
Beeh 2006 {published data only}
- Beeh K‐M, Pereno R, Chen H, Jimenez P. Adding omalizumab to high dose ICS and LABA significantly improves quality of life in patients with severe persistent allergic asthma [Abstract]. European Respiratory Journal 2006;28(Suppl 50):440s. [Google Scholar]
Berger 2002 {published data only}
- Berger WE. Monoclonal anti‐IgE antibody: a novel therapy for allergic airways disease. Annals of Allergy and Immunology 2002;88:152‐60. [DOI] [PubMed] [Google Scholar]
Bisberg 1996 {unpublished data only}
- Bisberg D, Froehlich J, Schoenhoff M, Mendelson J. Multiple administrations of the Anti‐IgE recombinant humanized monoclonal antibody E25 (rhuMAb‐E25) reduces free IgE levels in a dose dependent manner in adolescents and children with moderate to severe allergic asthma. Journal of Clinical Pharmacology 1996;36:859. [Google Scholar]
Blanken 2013 {published data only}
- Blanken MO, Rovers MM, Molenaar JM, Winkler‐Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy. New England Journal of Medicine 2013;368(19):1791‐9. [] [DOI] [PubMed] [Google Scholar]
Bousquet 2010 {published data only}
- Bousquet J, Smith N, Peckitt C, Maykut R, Peachey G. The effectiveness of different clinical measures in evaluating response to omalizumab. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:E3764.
Bousquet 2011 {published data only}
- Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE‐mediated) asthma. Allergy 2011;66(5):671‐8. [DOI] [PubMed] [Google Scholar]
- Magyar P, Peckitt C, Maykut R, Peachey G. Persistency of treatment response to omalizumab in patients with severe allergic (ige‐mediated) asthma [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna, Austria. 2009:[E1870].
- NCT00264849. A Randomized, Open Label, Parallel‐group, International, Multicenter Study Evaluating Persistency of Response to Omalizumab During 32 Weeks Treatment Given as Add on to Optimized Asthma Therapy in Adult and Adolescent Patients With Severe Persistent Allergic Asthma, Who Remain Inadequately Controlled Despite GINA (2004) Step 4 Therapy. www.clinicaltrials.gov/show/NCT00264849 (accessed 7 January 2013). []
- Siergiejko Z, Swiebocka E, Peckitt C, Maykut R, Peachey G. Omalizumab improves quality of life in adults and adolescents (≥12 years) with uncontrolled severe allergic asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A6651. [Google Scholar]
- Siergiejko Z, Swiebocka E, Peckitt C, Maykut R, Peachey G. Omalizumab reduces healthcare resource utilization in adults and adolescents (≥12 years) with uncontrolled severe allergic asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A5404. [Google Scholar]
- Siergiejko Z, Swiebocka E, Smith N, Peckitt C, Leo J, Peachey G, Maykut R. Oral corticosteroid sparing with omalizumab in severe allergic (IgE‐mediated) asthma patients. Current Medical Research and Opinion 2011;27(11):2223‐8. [DOI] [PubMed] [Google Scholar]
Bruselle 2009 {published data only}
- Brusselle G, Michils A, Louis R, Dupont L, Maele B, Delobbe A, et al. "Real‐life" effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respiratory Medicine 2009;103(11):1633‐42. [DOI] [PubMed] [Google Scholar]
Buhl 2001 {unpublished data only}
- Buhl R, Soler M, Fox H, Ashby M, McAlary M, Cooper J, et al. Omalizumab (XOLAIR, rhu‐MAb‐E25), decreases hospitalisations die to serious asthma exacerbations. Proceedings of the Annual Thoracic Society 97th International Conference, May 18‐23; San Francisco. 2001:186.
Busse 2013 {published data only}
- Busse WW, Holgate ST, Kerwin EM, Chon Y, Feng JY, Lin JH, et al. A randomized, double‐blind, placebo‐controlled, multiple‐dose study to evaluate the safety, tolerability, and efficacy of brodalumab (AMG 827) in subjects with moderate to severe asthma. Journal of Allergy and Clinical Immunology 2013;131(2):AB230 [817]. [] [Google Scholar]
Castro 2011 {published data only}
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Annals of Allergy Asthma and Immunology 2011;107(1):65‐70. [DOI] [PubMed] [Google Scholar]
Chipps 2009 {published data only}
- Chipps B, Kianifard F, Fernandez VC, Massanari M. Effect of omalizumab on peripheral blood eosinophils in children with moderate‐severe persistent allergic asthma [Abstract]. Chest 2009;136(4):35S. [Google Scholar]
CIGE025A1305 {unpublished data only}
- CIGE025A1305. Comparative study of IGE025 with suplatast tosilate (IPD) in patient with Japanese cedar pollen‐induced seasonal allergic rhinitis (SAR). www.novctrd.com (accessed 10 February 2010).
CIGE025A1306 {published data only}
- CIGE025A1306. Open‐label study of IGE025 in patients with Japanese cedar pollen‐induced seasonal allergic rhinitis (SAR) for second season administration. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2041 (accessed 7 January 2013).
CIGE025A1307 {unpublished data only}
- CIGE025A1307. Long‐term study of IGE025 in moderate to severe bronchial asthma. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2266 (accessed 7 January 2013).
CIGE025A2208 {published data only}
- CIGE025A2208. Multi‐center, open‐label, multiple dose study in mild to moderate asthmatics (with IgE/body weight combinations above that in the SmPC dosing table) to determine safety, tolerability, pharmacokinetics, and pharmacodynamics of omalizumab. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2739 (accessed 7 January 2013).
CIGE025A2303 {unpublished data only}
- CIGE025A2303. A 52 week treatment, multicenter, randomized, double‐blind, parallel‐group, placebo controlled study to investigate the effect of omalizumab (rhuMAb‐E25) on intestinal geohelminth reinfection in adolescent/ adult patients with allergic asthma and/or perennial allergic rhinitis previously treated with an anti intestinal geohelminth treatment regimen. www.novctrd.com (accessed 10 February 2010).
CIGE025AUS23 {unpublished data only}
- CIGE025AUS23. A 26‐week, randomized, double‐blind, parallel‐group, placebo‐controlled, multi‐center study to evaluate the effect of omalizumab on improving the tolerability of specific immunotherapy in patients with at least moderate persistent allergic asthma inadequately controlled with inhaled corticosteroids. www.novartisclinicaltrials.com (accessed 12 March 2009).
Corren 2010 {published data only}
- Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL‐4Ralpha antagonist, in patients with asthma. American Journal of Respiratory and Critical Care Medicine 2010;181(8):788‐96. [DOI] [PubMed] [Google Scholar]
Corren 2011 {published data only}
- Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. New England Journal of Medicine 2011;365(12):1088‐98. [DOI] [PubMed] [Google Scholar]
Corren 2011a {published data only}
- Corren J, Wood R, Patel D, Zhu J, Yegin A, Dhillon G, et al. Omalizumab efficacy following cat allergen exposure: Rresults from a randomized, double‐blind, placebo‐controlled, parallel‐group study in patients with moderate asthma. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[E3962].
- Corren J, Wood RA, Patel D, Zhu J, Fish J E. A randomized, double‐blind, placebo‐controlled, parallel‐group study of the efficacy of omalizumab in prevention of bronchoconstriction following environmental aeroallergen exposure [Abstract]. Journal of Allergy and Clinical Immunology 2010;125(2 Suppl 1):AB72. [Google Scholar]
- Corren J, Wood RA, Patel D, Zhu J, Yegin A, Dhillon G, et al. Effects of omalizumab on changes in pulmonary function induced by controlled cat room challenge. Journal of Allergy and Clinical Immunology 2011;127(2):398‐405. [DOI] [PubMed] [Google Scholar]
- NCT00495612. A Phase IV, Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group Study of the Efficacy of Omalizumab in Preventing Bronchoconstriction Following Environmental Cat Dander Exposure in Patients With Cat Dander‐Induced Asthma. www.clinicaltrials.gov/show/NCT00495612 (accessed 7 January 2013). []
Demoly 1997 {published data only}
- Demoly P, Bousquet J. Rhu‐MAb‐E25 reduces but does not abrogate the early and late phase reaction following allergen bronchial challenge. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1825‐7. [DOI] [PubMed] [Google Scholar]
Eckman 2010 {published data only}
- Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. Journal of Allergy and Clinical Immunology 2010;125(4):889‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmrich 2001 {published data only}
- Emmrich P, Kruse K, Reinhardt D. Anti‐IgE antibodies in the treatment of acute asthma [Asthmatherapie mit Anti‐IgE Antikörpern]. Monatsschrift für Kinderheilkunde 2001;149(5):506. [Google Scholar]
ETOPA {published and unpublished data}
- Ayres G, Anthonissen C, Martin C, Turk F, Thomas K. Assessment of a responder identification treatment algorithm for omalizumab is a naturalistic setting. European Respiratory Journal 2007;30(Suppl 51):623s. [Google Scholar]
- Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti‐immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate‐to‐severe) allergic asthma. Allergy 2004;59(7):701‐8. [DOI] [PubMed] [Google Scholar]
- Ayres JG, Niven R, Ayre G, Blogg M, Fox H. Omalizumab reduces the rate asthma deterioration related incidents in patients with poorly controlled allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S202. [Google Scholar]
- Bousquet J, Ayres G, Blogg M. Omalizumab added to best standard care reduces exacerbations in patients with severe persistent asthma according to GINA 2002 classification [Abstract]. European Respiratory Journal 2004;24(Suppl 48):220s. [Google Scholar]
- Bousquet J, Niven R, Ayre G, Fox H, Bogg M. Efficacy of omalizumab in patients with moderate to severe allergic asthma that is poorly controlled on GINA (1998) treatment step 3 or 4 [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: 1389. [Google Scholar]
- Brown R, Turk F, Dale P, Bousquet J. Cost‐effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy 2007;62(2):149‐53. [DOI] [PubMed] [Google Scholar]
- Brown R, Turk F, Groot M, Dale P. Cost effectiveness of omalizumab in patients with severe persistent allergic (IgE‐mediated) asthma adaptation of INNOVATE and ETOPA data to the Netherlands. European Respiratory Journal 2007;30(Suppl 51):194s. [Google Scholar]
- CIGE025IA04E. A 52‐week randomized, open‐label, controlled, multi‐center study to evaluate efficacy and tolerability of subcutaneous administration of omalizumab in patients with poorly controlled moderate‐to‐severe allergic asthma in a naturalistic setting. www.novctrd.com (accessed 10 February 2010).
- CIGE025IA04E2. A two year open‐label extension study to assess long term safety and tolerability of omalizumab treatment in poorly controlled moderate to severe allergic asthma patients who participated in the 52‐week CIGE025IA04E1 study. www.novctrd.com (accessed 10 February 2010).
- Chilvers E, Howes T, Izquierdo JL, Blogg M, Oshinyemi K, Ayre G, et al. Anti‐IgE therapy with omalizumab Improves lung function in patients with poorly controlled allergic asthma [abstract]. American Thoracic Society 99th International Conference; May 16‐21; Seattle. 2003:C104; Poster D38.
- Chipps B, Kim K, Korenblat P, Deniz Y, Zberg B, Caroll A. Effect of omalizumab on healthcare utilization in patients with moderate to severe allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S144. [Google Scholar]
- Chung F, Kunkel G, Ramos S, Ayre G, Fox H, Blogg M. Anti IgE therapy with omalizumab decreases exacerbations in patients with poorly controlled moderate to severe allergic asthma [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: 1390. [Google Scholar]
- Higgins B, Britton M, Carrillo T, Oshinyemi K, Blogg M. Anti‐IgE therapy with omalizumab improves asthma related quality of life of patients with poorly controlled allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S144. [DOI] [PubMed] [Google Scholar]
- Howes T, Izquierdo JL, Chilvers E, Blogg M, Oshinyemi K, Ayre G, et al. Omalizumab, an anti‐IgE antibody, decreases exacerbations in patients with poorly controlled allergic asthma. American Thoracic Society 99th International Conference; May 16‐21; Seattle. 2003:C104; Poster D39.
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0 (Study IA04). www.fda.gov (accessed 7 January 2013):136‐45.
- Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open‐label study. 2008411524. Respiratory Medicine 2008;102(10):1371‐8. [DOI] [PubMed] [Google Scholar]
- Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Efficacy of omalizumab in patients with inadequately controlled severe persistent allergic (IgE‐mediated) asthma: a subgroup analysis of an open label trial. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:414.
Fernandez 2005 {published data only}
- Fernandez C, Busse W, Resiner C, Gupta N. Clinical data do not suggest a casual relationship between omalizumab therapy and cancer. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:[B36; Poster: G50].
Frew 1998 {published data only}
- Frew AJ. Effects of anti‐IgE in asthmatic subjects. Thorax 1998;53(Suppl 2):52‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gauvreau 2012 {published data only}
- Gauvreau G, Boulet L‐P, Cockcroft D, Davis B, Fitzgerald M, Leigh R, et al. Effects of anti‐M1 prime monoclonal antibody, MEMP1972A following allergen challenge in patients with mild asthma [Abstract]. European Respiratory Journal 2012;40(Suppl 56):547s [3087]. [] [Google Scholar]
Gauvreau 2012a {published data only}
- Gauvreau G, Boulet L‐P, Cockcroft DW, Davis B, Fitzgerald MJ, Leigh R. Effect of an anti‐M1 prime monoclonal antibody, MEMP1972A, in a phase II proof‐of‐activity allergen challenge study in patients with mild asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012; Vol. 185, issue Meeting Abstracts:A6793. []
Gober 2008 {published data only}
- Gober LM, Sterba PM, Eckman JA, Saini SS. Effect of anti‐IgE (omalizumab) in chronic idiopathic urticaria (CIU) patients [Abstract]. Journal of Allergy and Clinical Immunology 2008;21(2 Suppl 1):S147. [Google Scholar]
Gossage 2010 {published data only}
- Gossage D, Geba G, Gillen A, Le C, Molfino N. A multiple ascending subcutaneous (SC) dose study of MEDI‐563, a humanized anti‐IL‐5R? monoclonal antibody, in adult asthmatics. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[P1177].
Gossage 2012 {published data only}
- Gossage DL, Laviolette M, Gauvreau GM, Leigh R, Kolbeck R, Wu Y. Depletion of airway eosinophils by benralizumab an anti‐IL5 receptor alpha monoclonal antibody [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A3961. [] [Google Scholar]
Hanania 2011a {published data only}
- Hanania NA, Lemanske RFJr, Korenblat PE, Arron JR, Harris JM, Su Z, et al. Efficacy of an anti‐IL13 monoclonal antibody, lebrikizumab, in adults with inadequately controlled asthma is enhanced in those with high periostin levels. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:608s [3426].
Hendeles 2007 {unpublished data only}
- Hendeles L, Khan Y, Massanari M, Spencer T, Shuster J, Chesrown S. The effect of omalizumab on airway responsiveness to adenosine in asthma patients with poor adherence to inhaled steroids. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster #418.
- NCT00133042. The Effect of Omalizumab on Airway Responsiveness to Adenosine in Patients With Poorly Controlled Asthma. www.clinicaltrials.gov (accessed 10 February 2010).
Hodsman 2013 {published data only}
- Hodsman P, Ashman C, Cahn A, Boever E, Locantore N, Serone A, et al. A phase 1, randomized, placebo‐controlled, dose‐escalation study of an anti‐IL‐13 monoclonal antibody in healthy subjects and mild asthmatics. British Journal of Clinical Pharmacology 2013;75(1):118‐28. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holgate 2001 {published data only}
- Holgate S, Bousquet J, Wenzel S, Fox H, Liu J, Castellsague J. Efficacy of omalizumab, an anti‐immunoglobulin E antibody, in patients at high risk of serious asthma‐related morbidity and mortality. Current Medical Research and Opinion 2001;17(4):233‐40. [PubMed] [Google Scholar]
Hoshino 2011 {published data only}
- Hoshino M. Effects of add‐on omalizumab therapy on airway wall thickening in severe persistent asthma. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam 2011;38(55):23s [P267]. [Google Scholar]
- Hoshino M, Ohtawa J. Effects of adding omalizumab, an anti‐immunoglobulin E antibody, on airway wall thickening in asthma.. Respiration 2012;83:520‐8. [DOI] [PubMed] [Google Scholar]
Hughes 2000 {published data only}
- Hughes TD. Anti‐IgE antibody may help treat some asthma patients. Journal of the American Medical Association 2000;284(22):2859‐60. [PubMed] [Google Scholar]
Johansson 2009 {published data only}
- Johansson SG, Nopp A, Oman H, Ankerst J, Cardell LO, Gronneberg R, et al. The size of the disease relevant IgE antibody fraction in relation to 'total‐IgE' predicts the efficacy of anti‐IgE (Xolair) treatment. Allergy 2009;64(10):1472‐7. [DOI] [PubMed] [Google Scholar]
Kamin 2010 {published data only}
- Kamin W, Kopp M V, Erdnuess F, Schauer U, Zielen S, Wahn U. Safety of anti‐IgE treatment with omalizumab in children with seasonal allergic rhinitis undergoing specific immunotherapy simultaneously. Pediatric Allergy and Immunology 2010;21(1 Pt 2):e160‐5. [DOI] [PubMed] [Google Scholar]
Karpel 2010 {published data only}
- Karpel J, Massanari M, Geba GP, Kianifard F, Inhaber N, Zeldin RK. Effectiveness of omalizumab in reducing corticosteroid burden in patients with moderate to severe persistent allergic asthma. Annals of Allergy Asthma & Immunology 2010;105(6):465‐70. [DOI] [PubMed] [Google Scholar]
Kenyon 2011 {published data only}
- Kenyon NJ, Last MA, Bratt JM, Kwan VW, O'Roark E, Linderholm A. L‐arginine supplementation and metabolism in asthma. Pharmaceuticals 2011;4(1):187‐201. [Google Scholar]
Kopp 2009 {published data only}
- CIGE025ADE03. A randomized, 20 week, double‐blind, placebo‐controlled, parallel‐group, multiple‐dose, multicenter study to assess the efficacy and safety of omalizumab in combination with Depigoid, versus Depigoid only, in adult and adolescent patients with seasonal allergic asthma and comorbid seasonal allergic rhinoconjunctivitis—open‐label Depigoid monotherapy extension periods 2007and 2008. www.novctrd.com (accessed 10 February 2010).
- Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann KC, Sieder C, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co‐morbid seasonal allergic asthma. Clinical and Experimental Allergy 2009;39(2):271‐9. [DOI] [PubMed] [Google Scholar]
- NCT00396409. A Randomized, 20 Week, Double‐blind, Placebo‐controlled, Parallel‐group, Multiple‐dose, Multicenter Study to Assess the Efficacy and Safety of Omalizumab in Combination With Depigoid, Versus Depigoid Only, in Adult and Adolescent Patients With Seasonal Allergic Asthma and Comorbid Seasonal Allergic Rhinoconjunctivitis—Open‐label Depigoid Monotherapy Extension Periods 2007 and 2008. www.clinicaltrials.gov/show/NCT00396409 (accessed 7 January 2013). []
Lanier 2010 {published data only}
- Lanier B, Fowler Taylor A, Vidaurre CF, Blogg M. Number needed to treat (NNT) to prevent one exacerbation per year with omalizumab (OMA) In children with inadequately controlled allergic (IgE‐mediated) asthma. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[P2640].
Leynadier 2004 {published data only}
- Leynadier F, Doudou O, Gaouar H, Gros V, Bourdeix I, Guyomarch‐Cocco L, et al. Effect of omalizumab in health care workers with occupational latex allergy. Journal of Allergy and Clinical Immunology 2004;113(2):360‐1. [DOI] [PubMed] [Google Scholar]
Lobo 2007 {published data only}
- Lobo ES, Revicki D, Grant W, Turk F, Massanari M. Assessment of the psychometric properties of the paediatric asthma quality of life questionnaire (PAQLQ) in moderate to severe pediatric asthma patients [Abstract]. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl):S151. [Google Scholar]
Massanari 2008 {published data only}
- Massanari M, Jimenez P, Kianifard F, Maykut R, Zeldin R. The omalizumab associated decrease in peripheral blood eosinophils in moderate severe IgE mediated asthma is sustained following inhaled steroid dose reduction [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A105.
Massanari 2009 {published data only}
- Massanari M, Kianifard F, Zeldin RK, Geba GP. Efficacy of omalizumab in cat‐allergic patients with moderate‐to‐severe persistent asthma. Allergy and Asthma Proceedings 2009;30(5):534‐9. [DOI] [PubMed] [Google Scholar]
- Massanari M, Sacco P, Kianifard F, Maykut R, Zeldin R. Addition of omalizumab improved functional health status in patients with impaired quality of life associated with moderate to severe persistent allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2008;21(2 Suppl 1):S154. [Google Scholar]
Maykut 2008 {published data only}
- Maykut R, Massanari M, Kianifard F, Zeldin R. Effect of omalizumab on asthma control and quality of life in patients with moderate severe persistent IgE‐mediated asthma and allergy to house dust mite [Abstract]. Journal of Allergy and Clinical Immunology 2008;21(2 Suppl 1):S157. [Google Scholar]
McClintock 2012 {published data only}
- McClintock D, Corren J, Hanania NA, Mosesova S, Lal P, Arron JR. Lebrikizumab, an anti‐IL‐13 monoclonal antibody, reduces severe asthma exacerbations over 32 weeks in adults with inadequately controlled asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A3959. [] [Google Scholar]
Milgrom 2007 {published data only}
- Milgrom H, Massanari M, Maykut RJ, Kianifard F, Zeldin RK, Geba GP. Addition of omalizumab reduces school absenteeism in children with moderate to severe persistent asthma [Abstract]. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl):S150. [Google Scholar]
Milgrom 2009 {published data only}
- Milgrom H, Fink J, Fowler‐Taylor A, Fernandez Vidaurre C, Blogg M. Safety and tolerability of omalizumab in children with inadequately controlled moderate‐to‐severe allergic (IgE‐mediated) asthma [Abstract]. Thorax 2009;64(Suppl IV):A18 [S31]. [Google Scholar]
Milgrom 2011 {published data only}
- Milgrom H, Fowler‐Taylor A, Vidaurre CF, Jayawardene S. Safety and tolerability of omalizumab in children with allergic (IgE‐mediated) asthma. Current Medical Research and Opinion 2011;27(1):163‐9. [DOI] [PubMed] [Google Scholar]
Molfino 2013 {published data only}
- Molfino NA, Bardin PG, Thompson PJ, Luckey A, Yarranton G. A randomized placebo‐controlled safety and pharmacodynamic study of KB002, a chimeric anti‐GM‐CSF monoclonal antibody, in patients with asthma. Journal of Allergy and Clinical Immunology 2013;131(2):AB229 [814]. [] [Google Scholar]
Moulton 2000 {published data only}
- Moulton D. Anti‐IgE asthma treatment reduces corticosteroid use. Canadian Medical Association 2000;162(6):864. [Google Scholar]
NCT00109187 {published data only}
- NCT00109187. Open‐Label Extension Study II of Xolair (Omalizumab) in Moderate to Severe, Persistent Asthma Subjects Who Completed Study Q2143g (ALTO). www.clinicaltrials.gov/show/NCT00109187 (accessed 7 January 2013). []
NCT00109200 {published data only}
- NCT00109200. A Continued Access Protocol to Provide Xolair® (Omalizumab) to Subjects With Severe Allergic Asthma Who Have Received Xolair Treatment in a Previous Investigational Study. www.clinicaltrials.gov/show/NCT00109200 (accessed 7 January 2013). []
NCT00133042 {published data only}
- The Effect of Omalizumab on Airway Responsiveness to Adenosine in Patients With Poorly Controlled Asthma. http://ClinicalTrials.gov/show/NCT00133042 (accessed 7 January 2013).
NCT00180011 {published data only}
- NCT00180011. Efficacy of Omalizumab as Add on Therapy for Minority Patients With Moderate to Severe Asthma. www.clinicaltrials.gov/show/NCT00180011 (accessed 7 January 2013). []
NCT00189228 {published data only}
- NCT00189228. Not a Drug Trial. We Are Using Anti‐IgE to Examine the Role of Pulmonary Mast Cells in Asthma. www.clinicaltrials.gov/show/NCT00189228 (accessed 7 January 2013). []
NCT00201097 {published data only}
- NCT00201097. Immune Dysregulation in Allergic Asthma. www.clinicaltrials.gov/show/NCT00201097 (accessed 7 January 2013). []
NCT00219323 {published data only}
- NCT00219323. Long‐Term Study of IGE025 in Moderate to Severe Bronchial Asthma. www.clinicaltrials.gov/show/NCT00219323 (accessed 7 January 2013). []
NCT00242359 {unpublished data only}
- Bernstein JA. A Pilot Study Investigating the Effect of Omalizumab (Xolair) in Work‐Related Animal Induced Asthma. http://clinicaltrials.gov/show/NCT00242359 (accessed 7 January 2013).
NCT00283504 {published data only}
- NCT00283504. A Description of Inflammatory Cell Types in Moderate to Severe Pediatric Asthma: Eosinophilic and Non Eosinophilic Sputum Markers While on Anti‐IgE Therapy (Xolair). www.clinicaltrials.gov/show/NCT00283504 (accessed 7 January 2013). []
NCT00287378 {published data only}
- NCT00287378. Effect of Ozone on Airway Inflammation in Allergic Asthmatics Treated With Omalizumab. www.clinicaltrials.gov/show/NCT00287378 (accessed 7 January 2013). []
NCT00401596 {published data only}
- NCT00401596. A Multicenter, Randomized, Controlled, Open‐Label Study to Evaluate the Safety of Xolair in Moderate to Severe Persistent Asthma Subjects Already Treated With Other Therapies (ALTO). www.clinicaltrials.gov/show/NCT00401596 (accessed 7 January 2013). []
NCT00434434 {published data only}
- NCT00434434. A Phase II, Multicenter, Randomized, Double‐Blind, Parallel‐Group, Placebo‐Controlled Study to Evaluate the Efficacy and Safety of Lyophilized and Aged Liquid Omalizumab in the Prevention of Allergen‐Induced Airway Obstruction in Adults With Mild Allergic Asthma. www.clinicaltrials.gov/show/NCT00434434 (accessed 7 January 2013). []
NCT00482248 {published data only}
- NCT00482248. An Open‐label Extension Study to Assess Long Term Safety and Tolerability of Omalizumab Treatment in Adults and Adolescents With Severe Allergic Asthma Who Participated in the 52 Week CIGE250011E2 Study. www.clinicaltrials.gov/show/NCT00482248 (accessed 7 January 2013). []
NCT00482508 {published data only}
- NCT00482508. A One Year Open‐label Extension Study to Assess Long Term Safety and Tolerability of Omalizumab Treatment in Poorly Controlled Moderate to Severe Allergic Asthma Patients Who Participated in the 52‐week CIGE24IA04E1 Study. www.clinicaltrials.gov/show/NCT00482508 (accessed 7 January 2013). []
NCT00500539 {published data only}
- NCT00500539. An Open Label, Single Arm Study to Assess the Safety and Immunogenicity of Omalizumab Liquid Administered Subcutaneously to Male and Female Adolescents and Adults With Persistent Allergic Asthma. www.clinicaltrials.gov/show/NCT00500539 (accessed 7 January 2013). []
NCT00546143 {published data only}
- NCT00546143. Multi‐Center, Open‐Label, Multiple Dose Study in Mild to Moderate Asthmatics (With IgE/Body Weight Combinations Above That in the SmPC Dosing Table) to Determine Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Omalizumab. www.clinicaltrials.gov/show/NCT00546143 (accessed on 7 January 2013). []
NCT00567476 {published data only}
- NCT00567476. A Randomized, Open‐Label, Multicenter Study to Evaluate the Effect of Xolair (Omalizumab) as Add‐on Therapy to Inhaled Corticosteroid + Long‐Acting Beta Agonist in Fixed or Flexible Dosing Compared to Isolated Inhaled Corticosteroid + Long‐Acting Beta Agonist in Fixed or Flexible Dosing in the Asthma‐Related Quality of Life in Patients With Severe Persistent Allergic Asthma. www.clinicaltrials.gov/show/NCT00567476 (accessed 7 January 2013). []
NCT00624832 {unpublished data only}
- CIGE025A2210. A Randomized, Double‐Blind, Placebo‐Controlled Study to Demonstrate the Efficacy of Xolair in an Allergen Bronchoprovocation Study in Asthmatic Populations Defined by Serum IgE Concentrations. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2758 (accessed 2 October 2010).
- NCT00624832. A Randomized, Double‐Blind, Placebo‐Controlled Study to Demonstrate the Efficacy of Xolair in an Allergen Bronchoprovocation Study in Asthmatic Populations Defined by Serum IgE Concentrations. www.clinicaltrials.gov/show/NCT00624832 (accessed 8 February 2013). []
NCT00639691 {published data only}
- NCT00639691. A Compassionate Access Protocol to Assess the Safety of XolairTM (Omalizumab)in Patients (≥ 6 Years Old) With Severe Allergic Asthma Who Remain Symptomatic Despite Optimal Therapy. www.clinicaltrials.gov/show/NCT00639691 (accessed 7 February 2013). []
NCT00777764 {published data only}
- NCT00777764. The Safety and Utility of Skin Testing With XOLAIR® (Omalizumab) and Placebo Omalizumab (Formulation Excipients). www.clinicaltrials.gov/show/NCT00777764 (accessed 7 February 2013). []
NCT00784485 {published data only}
- NCT00784485. Non‐Invasive Measures of Distal Lung Disease in Asthmatics Before and After Treatment With Omalizumab. www.clinicaltrials.gov/show/NCT00784485 (accessed 7 February 2013). []
NCT00829179 {published data only}
- NCT00829179. Role of RhuMab‐E25 in Reducing Exhaled NO in Allergic Asthma. www.clinicaltrials.gov/show/NCT00829179 (accessed 7 February 2013). []
NCT01155700 {published data only}
- NCT01155700. A 24 Week, Open Label, Multi‐center Evaluation of Pharmacokinetics and Pharmacodynamics, Efficacy and Safety of Omalizumab in Japanese Children (6 ‐ 15 Years) With Inadequately Controlled Allergic Asthma Despite Current Recommended Treatment. www.clinicaltrials.gov/show/NCT01155700 (accessed 7 February 2013). []
NCT01219036 {published data only}
- NCT01219036. The Use of Fractional Exhaled Nitric Oxide (FeNO) and Induced Sputum in the Identification of Non‐adherence in Difficult to Control Asthma. www.clinicaltrials.gov/show/NCT01219036 (accessed 7 February 2013). []
Nopp 2010 {published data only}
- Nopp A, Johansson SG, Adedoyin J, Ankerst J, Palmqvist M, Oman H, et al. After 6 years with Xolair; a 3‐year withdrawal follow‐up. Allergy 2010;65(1):56‐60. [DOI] [PubMed] [Google Scholar]
Oh 2010 {published data only}
- Oh C, Parker J, Geba G, Molfino N. Safety profile and clinical activity of multiple subcutaneous (SC) doses of MEDI‐528, a humanized anti‐interleukin‐9 monoclonal antibody, in subjects with asthma. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[377].
Oh 2012 {published data only}
- Oh CK, McLaurin KK, Kim K, Hultquist M, Molfino NA. A phase 2b, randomized study to evaluate the clinical activity and safety profile of subcutaneous MEDI‐528, an anti‐IL‐9 monoclonal antibody, In adults with uncontrolled asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2760. [] [Google Scholar]
Ong 2005 {published data only}
- Ong YE, Menzies‐Gow A, Barkans J, Benyahia F, Ou TT, Ying S, et al. Anti‐IgE (omalizumab) inhibits late‐phase reactions and inflammatory cells after repeat skin allergen challenge. Journal of Allergy and Clinical Immunology 2005;116(3):558‐64. [DOI] [PubMed] [Google Scholar]
Parker 2010 {published data only}
- Parker J, Brazinsky S, Miller DS, Nayak A, Korenblat PE, Sari S, et al. Randomized, double‐blind, placebo‐controlled, multicenter phase 2A study to evaluate the effect of a humanized interleukin‐9 monoclonal antibody (MEDI‐528) on exercise‐induced bronchospasm [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A5394. [Google Scholar]
Parker 2011 {published data only}
- Parker J, Wolansky LJ, Khatry D, Geba GP, Molfino NA. Brain magnetic resonance imaging in adults with asthma. Contemporary Clinical Trials 2011;32(1):86‐9. [DOI] [PubMed] [Google Scholar]
Parker 2011a {published data only}
- Parker JM, Oh CK, LaForce C, Miller SD, Pearlman DS, Le C, et al. Safety profile and clinical activity of multiple subcutaneous doses of MEDI‐528, a humanized anti‐interleukin‐9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulmonary Medicine 2011;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2009 {published data only}
- Patel BM, Chiang DT, Clark JP, Romero FA, Casale TB. Effects of omalizumab (Xolair®) on airway hyperresponsiveness [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S263. [Google Scholar]
Pavord 2012 {published data only}
Piper 2011 {published data only}
- Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. Phase 2 randomized, double‐blind, placebo‐controlled study of tralokinumab, an anti‐IL‐13 monoclonal antibody, in moderate to severe asthma. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:608s [3425].
Piper 2013 {published data only}
- Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. A phase II placebo‐controlled study of tralokinumab in. European Respiratory Journal 2013;41(2):330‐8. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Q2143G {unpublished data only}
- Israel E, Cohn J, Meltzer E, McCarty J, Zheng B, Carroll A. Omalizumab does not induce thrombocytopenia in treatment of asthma [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S146. [Google Scholar]
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. www.fda.gov (accessed 7 February 2013):123‐36.
Riviere 2008 {published data only}
- Riviere GJ, Kuebler P, Jaffe JS, Yeh CM, Reynolds C, Brookman L. A liquid formulation of omalizumab is bioequivalent to the current lyophilized formulation [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A613.
Riviere 2009 {published data only}
- Riviere GJ, Abbi S, Koehne‐Voss S, Kim K, Jaffe JS. Bioequivalence of a new formulation of omalizumab, solution for injection in prefilled syringe, to the current lyophilized formulation [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[E1873].
Riviere 2011 {published data only}
- Riviere GJ, Yeh CM, Reynolds CV, Brookman L, Kaiser G. Bioequivalence of a novel omalizumab solution for injection compared with the standard lyophilized powder formulation. Journal of Bioequivalence and Bioavailability 2011;3(6):144‐50. [Google Scholar]
Rubin 2012 {published data only}
- Rubin AS, Souza‐Machado A, Andradre‐Lima M, Ferreira F, Honda A, Matozo TM. Effect of omalizumab as add‐on therapy on asthma‐related quality of life in severe allergic asthma: a Brazilian study (QUALITX). Journal of Asthma 2012;49(3):288‐93. [DOI] [PubMed] [Google Scholar]
Scheerens 2011 {published data only}
- Scheerens H, Arron JR, Su Z, Zheng Y, Putnam W, Erickson RW, et al. Predictive and pharmacodynamic biomarkers of interleukin‐13 blockade: effect of lebrikizumab on late phase asthmatic response to allergen challenge [Abstract]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB164. [Google Scholar]
Stallings 2009 {published data only}
- Stallings A, McLaughlin A, Murphy D, Carper H, Platts‐Mills T, Heymann P. A surveillance study of natural rhinovirus colds in young adults with mild asthma [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S56. [Google Scholar]
Tajiri 2013 {published data only}
- Tajiri T, Matsumoto H, Hiraumi H, Ikeda H, Morita K, Izuhara K, et al. Efficacy of omalizumab in eosinophilic chronic rhinosinusitis. Annals of Allergy Asthma & Immunology 2013;110(5):387‐8. [] [DOI] [PubMed] [Google Scholar]
Townley 2011 {published data only}
- Townley R, Jourdheuil D, Jourdheuil F, DeMeyere‐Coursey K, Mahon L, Romero T, et al. The effects of omalizumab on bronchial and alveolar airway inflammation as measured by exhaled nitric oxide (ENO) in moderate to severe asthmatics [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A4478. [Google Scholar]
Wenzel 2013 {published data only}
Wilson 2008 {published data only}
- Wilson SJ, Ward JA, Jarjoiur NN, Kraft M, Chung KF, Fahy JV, et al. Omalizumab reduces mast cell numbers in airway smooth muscle [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A499.
Yalcin 2011 {published data only}
- Yalcin AD, Kargi A, Kose S, Terzioglu E, Gorczynski RM. Efficacy of omalizumab and specific subcutaneous immunotherapy in allergic asthma. Respirology 2011;16(Suppl 2):Abstracts (pages 1‐326). [Google Scholar]
Zaidi 2009 {published data only}
- Zaidi AK, Saini SS, MacGlashan DW Jr. Changes in the Fc‐epsilonRI‐beta: Fc‐epsilonRI‐alpha ratio during treatment with omalizumab [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S193. [Google Scholar]
Zielen 2009 {published data only}
- Zielen S, Lieb A, Monchy J, Motte S, Wagner F, Fuhr R, et al. Omalizumab protects against allergen‐induced bronchoconstriction in patients with allergic (IgE‐mediated) asthma and high baseline IgE levels [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[E1869].
Zielen 2013 {published data only}
- Zielen S, Lieb A, Motte S, Wagner F, Monchy J, Fuhr R, et al. Omalizumab protects against allergen‐induced bronchoconstriction in allergic (immunoglobulin E‐‐mediated) asthma. International Archives of Allergy and Immunology 2013;160(1):102‐10. [] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Creticos 2010 {published data only}
- Creticos PS, Saini SS, Scarupa MD, Balcer‐Whaley SL, Bieneman AP, Schroeder JT. Effects of omalizumab in non‐allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2010;125(2 Suppl 1):AB197. [Google Scholar]
- NCT00162773. Effect of Anti‐IgE in Non‐Allergic Asthma. www.clinicaltrials.gov/show/NCT00162773 (accessed 7 February 2013). []
NCT00046748 {published data only}
- CIGE025A2306 28‐Wk, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group Study to Assess Efficacy, Safety, Tolerability of Subcutaneous Omalizumab in Adults and Adolescents With Severe Persistent Allergic Asthma Who Remain Inadequately Controlled Despite GINA (2002) Step 4 Therapy. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=1601 (accessed 7 February 2013).
- NCT00046748. Ph III, 28‐Wk, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group Study to Assess Efficacy, Safety, Tolerability of SC Omalizumab in Adults and Adolescents w/ Severe Persist. Allergic Asthma & Are Inadequately Controlled Despite GINA (2002) Step 4 Tx. www.clinicaltrials.gov/show/NCT00046748 (accessed 7 February 2013). []
NCT00226200 {published data only}
- NCT00226200. Soluble CD23 Expression as a Marker of Immunomodulation and Clinical Response in Asthma Patients Treated With Omalizumab. www.clinicaltrials.gov/show/NCT00226200 (accessed 7 February 2013). []
NCT00329381 {published data only}
- NCT00329381. A 26‐Wk, Randomized, Double‐Blinded,Parallel‐Group, Placebo‐Controlled,Multi‐Centered Study to Evaluate the Effect of Xolair (Omalizumab) on Improving the Tolerability of Spec. Immunotherapy in Patients With at Least Mod. Persistent Allergic Asthma Inadequately Controlled with Inhaled Corticosteroids. www.clinicaltrials.gov/show/NCT00329381 (accessed 7 February 2013). []
NCT00367016 {published data only}
- NCT00367016. Immunologic Basis of Anti‐IgE Therapy (Study II: On Patients With Asthma). www.clinicaltrials.gov/show/NCT00367016 (accessed 7 February 2013). []
NCT00495612 {published data only}
- NCT00495612. A Study of Omalizumab in Preventing Bronchoconstriction Following Environmental Cat Dander Exposure in Patients With Cat Dander‐Induced Asthma (AERO). www.clinicaltrials.gov/ct2/show/NCT00495612 (accessed 7 February 2013).
NCT00670930 {published data only}
- CIGE025A2432 Efficacy of Omalizumab in Adults (18‐60 Years of Age) With Moderate‐Severe, Persistent Allergic Asthma, Despite Receiving Inhaled Corticosteroids and Long Acting Beta‐Agonists. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=7463 (accessed 7 February 2013).
- NCT00670930. A Randomized, Multi‐Center, Double‐Blind, Placebo‐Controlled, Parallel‐Group Trial to Explore the Effects of 78 Weeks Omalizumab Treatment Given as Add on Therapy on Markers of Airway Inflammation and Remodeling in Patients With Moderate to Severe Persistent Allergic Asthma Receiving Inhaled Corticosteroids and Long Acting Beta‐agonists. www.clinicaltrials.gov/show/NCT00670930 (accessed 7 February 2013). []
NCT00691873 {published data only}
- NCT00691873. A 26‐Wk, Randomized, Dble‐Blinded, Parallel‐Grp, Placebo‐Controlled, Multi‐Centered Study to Eval the Effect of Xolair (Omalizumab) on Improving the Tolerability of Spec. Immunotherapy in Patients With at Least Mod. Persistent Allergic Asthma Inadequately Controlled w/Inhaled Corticosteroids. www.clinicaltrials.gov/show/NCT00691873 (accessed 7 February 2013). []
NCT01393340 {published data only}
- NCT01393340. Clinical and Biological Effects of Anti‐IgE (Omalizumab) in Patients With Bilateral Nasal Polyposis and Asthma. www.clinicaltrials.gov/show/NCT01393340 (accessed 7 February 2013). []
Scripps 2009 {published data only}
- NCT00286416. Double Blind Study to Determine Effect of Omalizumab Treatment in Patients With the Co‐Morbid Conditions of Aspirin Exacerbated Respiratory Disease(AERD) and Allergic Asthma and Rhinitis. www.clinicaltrials.gov/show/NCT00286416 (accessed 7 February 2013). []
- Scripps Clinic. Double Blind Study to Determine Effect of Omalizumab Treatment in Patients With the Co‐morbid Conditions of Aspirin Exacerbated Respiratory Disease (AERD) and Allergic Asthma and Rhinitis (completed). NCT00286416 2009:ClinicalTrials.gov ID: NCT00286416.
References to ongoing studies
NCT00139152 {published data only}
- NCT00139152. Exhaled Breath Condensate and Nitric Oxide: Non‐Invasive Evaluation of Lung Disease After Treatment With Xolair. www.clinicaltrials.gov/show/NCT00139152 (accessed 7 February 2013). []
NCT00208234 {published data only}
- NCT00208234. The Effects of Xolair (Omalizumab) on Airway Hyperresponsiveness. www.clinicaltrials.gov/show/NCT00208234 (accessed 7 February 2013). []
NCT00555971 {published data only}
- NCT00555971. A Placebo Controlled, Double‐Blind Investigation of the Therapeutic Utility of Xolair (Omalizumab) for Attenuating Aspirin Induced Bronchospasm in Patients With Aspirin Exacerbated Respiratory Disease (AERD) Undergoing Aspirin Desentization. www.clinicaltrials.gov/show/NCT00555971 (accessed 7 February 2013). []
NCT01113437 {published data only}
- NCT01113437. The Effect of a Humanised Monoclonal Anti‐IgE Antibody, Omalizumab, on Disease Control and Bronchial Mucosal Inflammation in Non‐Atopic Asthma. www.clinicaltrials.gov/show/NCT01113437 (accessed 7 February 2013). []
NCT01125748 {published data only}
- NCT01125748. A Phase IV, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Study Evaluating the Persistency of Response With or Without Xolair After Long‐Term Therapy. www.clinicaltrials.gov/show/NCT01125748 (accessed 7 February 2013). []
NCT01202903 {published data only}
- NCT01202903. A 24‐Week, Phase III Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group, Multicenter Study of Xolair® (Omalizumab) in Patients With Moderate to Severe Persistent Allergic Asthma Who Remain Not Adequately Controlled Despite GINA (2009) Step 4 Therapy. www.clinicaltrials.gov/show/NCT01202903 (accessed 7 February 2013). []
NCT01430403 {published data only}
- NCT01430403. Preventative Omalizumab or Step‐Up Therapy for Severe Fall Exacerbations (ICAC‐20). www.clinicaltrials.gov/show/NCT01430403 (accessed 7 February 2013). []
NCT01544348 {published data only}
- NCT01544348. A Phase 1, Randomized, Placebo‐Controlled, Dose‐Escalation Study to Evaluate the Safety of MEDI4212 in Allergic Subjects. www.clinicaltrials.gov/show/NCT01544348 (accessed 7 February 2013). []
Additional references
Arshad 2001
- Arshad SH, Babu KS, Holgate ST. Anti‐IgE Therapy in Asthma and Allergy. 1st Edition. London: Martin Dunitz, 2001. [Google Scholar]
Bousquet 2004
- Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti‐IgE antibody, in patients with allergic asthma. Chest 2004;125(4):1378‐86. [DOI] [PubMed] [Google Scholar]
BTS 2005
- British Thoracic Society. British guideline on the management of asthma. www.sign.ac.uk/pdf/sign101.pdf (accessed 7 February 2013).
BTS/SIGN 2012
- British Guideline on the Management of Asthma. A National Clinical Guideline. www.sign.ac.uk/guidelines/fulltext/101/ (accessed 7 February 2013).
Burrows 1989
- Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE and skin test reactivity to allergens. New England Journal of Medicine 1989;320:271‐7. [DOI] [PubMed] [Google Scholar]
Casale 1997
- Casale TB, Bernstein IL, Busse WW, et al. Use of an anti‐IgE humanised monoclonal antibody in ragweed‐induced allergic rhinitis. Journal of Allergy and Clinical Immunology 1997;100:110‐21. [DOI] [PubMed] [Google Scholar]
Chen 2012
Chipps 2012
- Chipps BE, Figliomeni M, Spector S. Omalizumab: an update on efficacy and safety in moderate‐to‐severe allergic asthma. Allergy Asthma Proceedings 2012;33(5):377‐85. [DOI] [PubMed] [Google Scholar]
CTS 2012
- 2012 CTS Guideline Update: diagnosis and management of asthma in preschoolers, children and adults. www.respiratoryguidelines.ca/2012‐cts‐guideline‐asthma‐update (accessed 7 February 2013).
GiNA 2011
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2011. www.ginasthma.org/guidelines‐gina‐report‐global‐strategy‐for‐asthma.html (accessed 16 September 2012).
Haldar 2008
- Haldar P, Pavord I, Shaw D, Berry M, Thomas M, Brightling C, et al. Cluster analysis and clinical asthma phenotypes. American Journal of Respiratory and Critical Care Medicine 2008;178(3):218‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Juniper 1994
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease‐specific quality of life questionnaire. Journal of Clinical Epidemiology 1994;47:81‐7. [DOI] [PubMed] [Google Scholar]
Milgrom 1997
- Milgrom H, Fick RB, Su JQ, et al. Treatment of allergic asthma with monoclonal anti‐IgE antibody. New England Journal of Medicine 1999;341(26):1966‐73. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Omalizumab for the treatment of severe persistent allergic asthma in children aged 6 and over and adults (review of TA133 and TA201). http://www.nice.org.uk/nicemedia/live/14157/63689/63689.pdf (accessed 25 June 2013).
Pelaia 2011
- Pelaia G, Gallelli L, Renda T, Romeo P, Busceti MT, Grembiale RD, et al. Update on optimal use of omalizumab in management of asthma. Journal of Asthma and Allergy 2011;4:49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perret 2013
- Perret JL, Dharmage SC, Matheson MC, Johns DP, Gurrin LC, et al. The interplay between the effects of lifetime asthma, smoking, and atopy on fixed airflow obstruction in middle age. American Journal of Respiratory and Critical Care Medicine 2013;187(1):42‐8. [DOI] [PubMed] [Google Scholar]
Qureshi 1998
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339:1030‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sears 1991
- Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relationship between airways responsiveness and serum IgE in children with asthma and in apparently normal children. New England Journal of Medicine 1991;325:1067‐71. [DOI] [PubMed] [Google Scholar]
Spector 1999
- Spector SL. Allergic inflammation in upper and lower airways. Annals of Allergy Asthma & Immunology 1999;83:435‐44. [DOI] [PubMed] [Google Scholar]
Thomson 2012
- Thomson NC, Chaudhuri R. Omalizumab: clinical use for the management of asthma. Clinical Medical Insights. Circulatory, Respiratory and Pulmonary Medicine 2012;6:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2011
- Walker S. Omalizumab reduces frequency of asthma exacerbations in children. Journal of Pediatrics 2011;159(3):512‐3. [DOI] [PubMed] [Google Scholar]
Wenzel 2002
- Wenzel S, Bousquet J, Holgate S, Freeman P, Fox H. Patients with more severe allergic asthma gain greatest relative benefit from omalizumab therapy. American Journal of Respiratory & Critical Care Medicine. 2002; Vol. 165:A215.
Wijnhoven 2001
- Wijnhoven HAH, Kriegsman DMW, Hesselink AE, Penninx BWJH, Haan M. Determinants of different dimensions of disease severity in asthma and COPD: pulmonary function and quality of life. Chest 2001;119(4):1034‐42. [DOI] [PubMed] [Google Scholar]
Wills‐Karp 1999
- Wills‐Karp M. Immunologic basis of antigen‐induced airway hyperresponsiveness. Annual Review of Immunology 1999;17:255‐81. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Walker 2006
- Walker S, Monteil M, Phelan K, Lasserson TJ, Walters EH. Anti‐IgE for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003559.pub3] [DOI] [PubMed] [Google Scholar]


