Abstract
Background
Chronic pelvic pain is a common and debilitating condition; its aetiology is multifactorial, involving social, psychological and biological factors. The management of chronic pelvic pain is challenging, as despite interventions involving surgery, many women remain in pain without a firm gynaecological diagnosis.
Objectives
To assess the effectiveness and safety of non‐surgical interventions for women with chronic pelvic pain.
Search methods
We searched the Menstrual Disorders and Subfertility Group Specialised Register. We also searched (from inception to 5 February 2014) AMED, CENTRAL, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS. We handsearched sources such as citation lists, trial registers and conference proceedings.
Selection criteria
Randomised controlled trials (RCTs) on non‐surgical management of chronic pelvic pain were eligible for inclusion. We included studies of women with a diagnosis of pelvic congestion syndrome or adhesions but excluded those with pain known to be caused by endometriosis, primary dysmenorrhoea (period pain), active chronic pelvic inflammatory disease or irritable bowel syndrome. We considered studies of any non‐surgical intervention, including lifestyle, physical, medical and psychological treatments.
Data collection and analysis
Study selection, quality assessment and data extraction were performed independently by two review authors. Meta‐analysis was performed using the Peto odds ratio (Peto OR) for dichotomous outcomes and the mean difference (MD) for continuous outcomes, with 95% confidence intervals (CIs). The primary outcome measure was pain relief, and secondary outcome measures were psychological outcomes, quality of life, requirement for analgesia and adverse effects. The quality of the evidence was assessed by using GRADE methods.
Main results
Twenty‐one RCTs were identified that involved non‐surgical management of chronic pelvic pain: 13 trials were included in the review, and eight were excluded. The studies included a total of 750 women—406 women in the intervention groups and 344 in the control groups. Included studies had high attrition rates, and investigators often did not blind adequately or did not clearly describe randomisation procedures.
Medical treatment versus placebo
Progestogen (medroxyprogesterone acetate (MPA)) was more effective than placebo at the end of treatment in terms of the number of women achieving a greater than 50% reduction in visual analogue scale (VAS) pain score immediately after treatment (Peto OR 3.00, 95% CI 1.70 to 5.31, two studies, n = 204, I2 = 22%, moderate‐quality evidence). Evidence of benefit was maintained up to nine months after treatment (Peto OR 2.09, 95% CI 1.18 to 3.71, two studies, n = 204, I2 = 0%, moderate‐quality evidence). Women treated with progestogen reported more adverse effects (e.g. weight gain, bloatedness) than those given placebo (high‐quality evidence). The estimated effect of lofexidine on pain outcomes when compared with placebo was compatible with benefit and harm (Peto OR 0.42, 95% CI 0.11 to 1.61, one study, 39 women, low‐quality evidence). Women in the lofexidine group reported more adverse effects (including drowsiness and dry mouth) than women given placebo (moderate‐quality evidence).
Head‐to‐head comparisons of medical treatments
Head‐to‐head comparisons showed that women taking goserelin had greater improvement in pelvic pain score (MD 3, 95% CI 2.08 to 3.92, one study, n = 47, moderate‐quality evidence) at one year than those taking progestogen. Women taking gabapentin had a lower VAS pain score than those taking amytriptyline (MD ‐1.50, 95% CI ‐2.06 to ‐0.94, n = 40, low‐quality evidence). Study authors reported that no statistically significant difference was observed in the rate of adverse effects among women taking gabapentin compared with women given amytriptyline. The study comparing goserelin versus progestogen did not report on adverse effects.
Psychological treatment
Women who underwent reassurance ultrasound scans and received counselling were more likely to report improved pain than those treated with a standard 'wait and see' policy (Peto OR 6.77, 95% CI 2.83 to 16.19, n = 90, low‐quality evidence). Significantly more women who had writing therapy as a disclosure reported improvement in pain than those in the non‐disclosure group (Peto OR 4.47, 95% CI 1.41 to 14.13, n = 48, very low‐quality evidence). No difference between groups in pain outcomes was noted when other psychological therapies were compared with standard care or placebo (quality of evidence ranged from very low to low). Studies did not report on adverse effects.
Complementary therapy
Distension of painful pelvic structures was more effective for pain when compared with counselling (MD 35.8, 95% CI 23.08 to 48.52 on a zero to 100 scale, one study, n = 48, moderate‐quality evidence). No difference in pain levels was observed when magnetic therapy was compared with use of a control magnet (very low‐quality evidence). Studies did not report on adverse effects.
The results of studies examining psychological and complementary therapies could not be combined to yield meaningful results.
Authors' conclusions
Evidence of moderate quality supports progestogen as an option for chronic pelvic pain, with efficacy reported during treatment. In practice, this option may be most acceptable among women unconcerned about progestogenic adverse effects (e.g. weight gain, bloatedness—the most common adverse effects). Although some evidence suggests possible benefit of goserelin when compared with progestogen, gabapentin as compared with amytriptyline, ultrasound versus 'wait and see' and writing therapy versus non‐disclosure, the quality of evidence is generally low, and evidence is drawn from single studies.
Given the prevalence and healthcare costs associated with chronic pelvic pain in women, RCTs of other medical, lifestyle and psychological interventions are urgently required.
Keywords: Female; Humans; Amines; Amines/therapeutic use; Amitriptyline; Amitriptyline/therapeutic use; Analgesics; Analgesics/adverse effects; Analgesics/therapeutic use; Chronic Pain; Chronic Pain/therapy; Clonidine; Clonidine/adverse effects; Clonidine/analogs & derivatives; Clonidine/therapeutic use; Contraceptive Agents, Female; Contraceptive Agents, Female/adverse effects; Contraceptive Agents, Female/therapeutic use; Cyclohexanecarboxylic Acids; Cyclohexanecarboxylic Acids/therapeutic use; Gabapentin; Goserelin; Goserelin/therapeutic use; Medroxyprogesterone Acetate; Medroxyprogesterone Acetate/adverse effects; Medroxyprogesterone Acetate/therapeutic use; Pain Measurement; Pelvic Pain; Pelvic Pain/therapy; Psychotherapy; Randomized Controlled Trials as Topic; gamma‐Aminobutyric Acid; gamma‐Aminobutyric Acid/therapeutic use
Plain language summary
Non‐surgical interventions for the management of chronic pelvic pain
Review question
Cochrane authors considered the evidence for effectiveness and safety of non‐surgical treatrments for managing chronic pelvic pain in women.
Background
Chronic pelvic pain in women is a common problem. Specific causes are often difficult to identify, even after investigation with ultrasound and inspection of the pelvis with key hole surgery. Treatment is frequently limited to relief of symptoms obtained with a concoction of medicines. Cochrane review authors examined the evidence about non‐surgical interventions for the management of chronic pelvic pain.
Study characteristics
Twenty‐one randomised controlled studies were identified, of which 13 were included. Eight studies were excluded. The studies included a total of 750 women—406 women in the intervention groups and 344 women in the control groups. The interventions assessed included medical treatment and psychological, cognitive, behavioural, complementary and physical therapies. The evidence is current to February 2014.
Key results
The review concludes that evidence shows improvement of pain in women given a high dose of progestogen (50 mg medroxyprogesterone acetate) immediately post‐treatment and for up to nine months after treatment. However, progestogen was associated with adverse effects such as weight gain and bloating. Women who underwent reassurance ultrasound scans and who received counselling were more likely to report improved pain than those whose treatment involved a 'wait and see' policy. Some evidence of benefit was seen with writing disclosure therapy and with distension of painful pelvic structures. No good evidence of benefit was noted with other interventions when compared with standard care or placebo.
The quality of the evidence was low or moderate for most comparisons, and in most cases evidence was derived from single small studies. Moreover, we were unable to draw meaningful conclusions on quality of life and physical and functional outcomes because of the large variation in outcome measures used by the included studies. Many interventions identified in this review involved only single studies with small sample sizes. Additional studies will be required in the future to replicate results obtained with the use of specific medical interventions.
Summary of findings
Summary of findings for the main comparison. Medical treatment compared with placebo for the management of chronic pelvic pain.
| Medical treatment compared with placebo for the management of chronic pelvic pain | ||||||
|
Population: women with chronic pelvic pain Setting: any Intervention: medical treatment Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Medical treatment | |||||
| Improvement in pain score at end of treatment: progesterone versus placebo | 24/85 | 541 per 1000 (401 to 676) | Peto OR 3.00 (1.70 to 5.31) | 204 (two studies) | ⊕⊕⊕⊝ moderate1,2 | Improvement defined as ≥ 50% reduction in VAS pain score |
| Improvement in pain score (up to nine months after treatment): progesterone versus placebo | 27/85 | 493 per 1000 (355 to 633) | Peto OR 2.09 (1.18 to 3.71) | 204 (two studies) | ⊕⊕⊕⊝ moderate1 | Improvement defined as ≥ 50% reduction in VAS pain score |
| Weight gain: progesterone versus placebo | 14/40 | 808 per 1000 (638 to 909) | Peto OR 7.82 (3.28 to 18.65) | 85 (one study) | ⊕⊕⊕⊕ high | |
| Bloatedness: progesterone versus placebo | 14/40 | 633 per 1000 (425 to 801) | Peto OR 3.20 (1.37 to 7.47) | 85 (one study) | ⊕⊕⊕⊕ high | |
| Improvement in pain score: lofexidine versus placebo | eight/20 | 219 per 1000 (68 to 518) | Peto OR 0.42 (0.11 to 1.61) | 39 (one study) | ⊕⊕⊝⊝ low1,2,3 | Improvement defined as ≥ 50% reduction in VAS pain score |
| Drowsiness/lethargy: lofexidine versus placebo | eight/20 | 717 per 1000 (421 to 898) | Peto OR 3.80 (1.09 to 13.26) | 39 (one study) | ⊕⊕⊕⊝ moderate2 | |
| Dry mouth: lofexidine versus placebo | three/20 | 603 per 1000 (301 to 842) | Peto OR 8.60 (2.44 to 30.31) | 39 (one study) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
140% dropout rate in one of the two studies.
2> 10% dropout rate.
3Wide confidence intervals compatible with no effect or with higher rates of improvement in placebo group.
Summary of findings 2. Medical treatment of chronic pelvic pain: head‐to‐head comparisons.
| Medical treatment of chronic pelvic pain: head‐to‐head comparisons | ||||
| Patient or population: management of chronic pelvic pain Settings: any Intervention: medical treatment: head‐to‐head comparison | ||||
| Outcomes | Medical treatment: head‐to‐head comparison | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Improvement in pelvic pain at one year: goserelin versus progestogen | Improvement in mean pelvic pain score in the goserelin group was three points greater than in the progestogen group (95% CI 2.08 higher to 3.92 higher) | 47 (one study) | ⊕⊕⊕⊝ moderate1 | Improvement in pelvic pain score measured on a scale of one to 100 |
| Pain at 24 months: gabapentin versus amytriptyline | Mean pain score in the gabapentin group was 1.5 points lower than in the amytriptyline group (95% CI 2.06 lower to 0.94 lower) | 40 (one study) | ⊕⊕⊝⊝ low1,2 | Pain score measured on a VAS scale of zero to 10 |
| CI: Confidence interval. | ||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1Participants not blinded.
2> 10% attrition.
Summary of findings 3. Psychological therapy compared with control interventions for the management of chronic pelvic pain.
| Psychological therapy compared with control interventions for the management of chronic pelvic pain | ||||||
|
Population: women with chronic pelvic pain Setting: any Intervention: psychological therapy Comparison: control intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Psychological therapy | |||||
| Improvement in pain scores—Ultrasound scan and counselling session versus 'wait and see' | 113 per 1000 | 465 per 1000 (266 to 675) | Peto OR 6.77 (2.83 to 16.19 ) | 90 (one study) | ⊕⊕⊕⊝ low1,2,6 | Improvement defined as a change of five or more points on McGill Pain Questionnaire |
| Improvement in pain scores—Somatocognitive therapy (Mensendieck) versus standard gynaecological clinical care | 250 per 1000 | 530 per 1000 (244 to 797) | Peto OR 3.38 (0.97 to 11.80 ) | 40 (one study) | ⊕⊝⊝⊝ very low3,4,5 | Improvement defined as ≥ 50% reduction in VAS pain score |
| Improvement in pain scores—Integrated approach (somatic, psychological, dietary, environmental and physiotherapeutic factors) versus standard care | 510 per 1000 | 613 per 1000 (425 to 773) | Peto OR 1.52 (0.71 to 3.27 ) | 106 (one study) | ⊕⊕⊝⊝ low2,5,6 | Improvement defined as ≥ 50% reduction in VAS pain score |
| Improvement in pain scores—Writing therapy (disclosure of pain) versus non‐disclosure | 200 per 1000 | 528 per 1000 (261 to 779) | Peto OR 4.47 (1.41 to 14.13 ) | 48 (one study) | ⊕⊕⊝⊝ very low2,7,8,9 | Improvement considered an improvement of at least one point on a zero to five pain scale |
| Improvement in pain scores—Psychotherapy versus placebo, measured at end of treatment | 320 per 1000 | 271 per 1000 (101 to 549) | Peto OR 0.79 (0.24 to 2.59 ) | 51 (one study) | ⊕⊕⊕⊝ low10,11 | Improvement defined as ≥ 50% reduction in VAS pain score |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
110% attrition.
2Sequence generation method not described.
3No attrition figures stated, but at one year, only 13 women in each group had completed the questionnaires.
4Unblinded.
5Wide confidence intervals compatible with no effect or with benefit from intervention.
6Participants not blinded.
720% loss to follow‐up.
8Allocation concealment methods not described.
9Unclear whether participants blinded.
10Wide confidence intervals compatible with no effect or with increased pain from intervention.
1118% attrition.
Summary of findings 4. Complementary therapy compared with control interventions for the management of chronic pelvic pain.
| Complementary therapy compared with control interventions for the management of chronic pelvic pain | ||||||
| Population: women with chronic pelvic pain Settings: any Intervention: complementary therapy Comparison: control intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Complementary therapy | |||||
| Reduction in pain score: distension of painful pelvic structures versus counselling | 23 | Mean reduction in pain score in the intervention group was 35.8 higher (23.08 higher to 48.52 higher) | ‐ | 48 (one study) | ⊕⊕⊕⊝ moderate1 | Reduction in pain score, measured on a self assessed one to 100 VAS scale |
| Pain score after treatment: magnetic therapy versus control magnet | 11 | Mean pelvic pain score in the intervention group was 0.5 lower (1.92 lower to 0.92 higher) | ‐ | 19 (one study) | ⊕⊝⊝⊝ very low2,3 | Post‐treatment pelvic pain score on a zero to five‐point scale, where 0 = no pain and 5 = excruciating pain |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Unclear whether blinded, 12% attrition.
241% dropout rate at two to four weeks.
3Wide confidence interval compatible with no effect or benefit from magnetic therapy.
Background
Description of the condition
The management of women with chronic pelvic pain (CPP) has for many years posed a challenge for healthcare professionals. Among women in the reproductive age group, the estimated prevalence of CPP varies widely, depending on study definitions, from 2.1% to 24% of the female population worldwide (Latthe 2006), with a notably high prevalence in the USA and the UK (14.7% and 24%, respectively) (Daniels 2010; Mathias 1996; Zondervan 1999). The reported incidence of CPP in primary care of 38 per 1000 women is comparable with the incidence of back pain (41 per 1000) and asthma (37 per 1000) (Zondervan 1999). Up to 20% of visits to gynaecologists, 40% of laparoscopies and 15% of hysterectomies in gynaecology are attributed to CPP (Gelbaya 2001; Howard 1993). At laparoscopy, a significant proportion of women with CPP (up to 55%) have no obvious pathological cause for their pain (Daniels 2009; Howard 1993). As the pathophysiology of CPP is not well understood, its treatment is often unsatisfactory and limited to symptom relief. Treatment options, ranging from conservative management to opioid analgesia and surgical intervention, vary accordingly, as do treatment outcomes (Cheong 2006). In a large proportion of women, treatment does not necessarily result in relief of pain. Thus, living with CPP carries a significant mental, social and physical burden for the sufferer, and its chronic nature puts a heavy burden on healthcare systems worldwide (Daniels 2010; Latthe 2006).
Description of the intervention
Diagnosis and treatment of CPP is complex and may be complicated by psychosocial circumstances. Non‐surgical management often includes an entire concoction of treatments, such as analgesics, adjunctive agents such as anticonvulsants and antidepressants, hormonal drugs (medroxyprogesterone acetate, goserelin), α2‐adrenoceptor agonists (lofexidine hydrochloride), venoconstrictor drugs (ergotamine) and venomimetics (daflon). The non‐surgical approach also encompasses psychological drug interventions, which have been suggested to be beneficial in the treatment of CPP (Williams 2012). Other alternatives to medical management of CPP include psychological therapy, cognitive therapy, physiotherapy and various forms of complementary therapies (Cheong 2006). Multidisciplinary management, which is a common approach to many chronic conditions such as asthma and diabetes, still is not commonly available in gynaecology because of cost factors and the limited availability of interested specialists (Cheong 2007).
How the intervention might work
The aetiology of chronic pelvic pain is complex. Pelvic congestion, adhesions, musculoskeletal nerve‐related disorders and psychosomatic factors have been suggested as causes of CPP. Interventions targeting these factors have been used in the management of CPP.
It is suggested that pelvic pain in women can be of visceral and/or neuropathic origin. If treatment using anti‐nociceptive medication (e.g. Tramadol) for visceral pain fails, second‐line treatment generally consists of anti‐convulsants targeting neuropathic pain (Sator‐Katzenschlager 2005). Women with CPP, similar to those with other chronic pain syndromes, often have co‐existing conditions such as depression. Although anti‐depressants may indirectly improve the pain experience by enhancing mood, they may also have a direct analgesic effect, as both depressive symptoms and pain are modulated by the neurotransmitters serotonin and norepinephrine (Brown 2008).
A possible vascular basis for CPP has prompted the evaluation of vasoactive agents for treatment, by analogy with cerebral migraine (Stones 2001). It has been suggested that pelvic congestion syndrome, which is responsible for CPP in a large proportion of women with no detectable organic pathology, is the result of ovarian dysfunction, and that inducing a hypo‐oestrogenic state or antagonising the effects of oestrogens by using progesterone results in resolution of symptoms, hence the trials of medroxyprogesterone acetate versus placebo (Farquhar 1989).
Evidence suggests that up to 85% of women with CPP have dysfunction of the musculoskeletal system, including postural changes, as well as changes in the pelvic muscles, such as spasm of the levator ani (Baker 1993; Prendergast 2003). Such chronic pain caused by spasm of the pelvic floor muscles has been treated by various modalities such as local anaesthetic blockade (Langford 2007). Another technique that is being studied increasingly is the injection of type A botulinum toxin into affected muscles of the pelvic floor. This agent is thought to act selectively on the endings of cholinergic peripheral nerves, thus inhibiting the presynaptic release of acetylcholine and reducing excessive muscle tone (Gupta 2006; Thompson 2005). Other possibilities that have been explored in the treatment of high‐tone dysfunction of the pelvic floor in women with CPP include transvaginal manual therapy and transvaginal electrostimulation of pelvic floor musculature. Electrical stimulation is thought to promote analgesia through the counterirritative effect that results in activation of the pain suppression system, thus offering pain relief at low cost and with few side effects (Duleba 1996).
Diagnosis and treatment of CPP is complex and may be complicated by psychosocial circumstances. Apart from medical and surgical approaches, it has been suggested that psychological treatments are helpful in reducing the frequency and severity of symptoms in all sorts of chronic pain, including unexplained pelvic pain (Williams 2012).
Why it is important to do this review
This review is important because it will inform women with chronic pelvic pain and healthcare professionals about available evidence on management of this disease.
Objectives
To assess the effectiveness and safety of non‐surgical interventions for women with chronic pelvic pain.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs). We excluded trials that were not properly randomised. We included cross‐over studies if first‐phase data were valid.
Types of participants
Women with CPP, defined as intermittent or constant pain of at least three to six months' duration localised in the abdomen or pelvis, not limited to the period of menstruation or intercourse and not associated with pregnancy. We excluded studies examining specific cohorts of women known to have solely endometriosis, primary dysmenorrhoea and/or pain due to active chronic pelvic inflammatory disease.
Types of interventions
We considered comparisons made within the following intervention groups.
Medical interventions versus placebo/no treatment or other types of interventions.
Medical interventions included interventions such as non‐steroidal anti‐inflammatory drugs (NSAIDs), oral contraceptive pills (OCPs), oral and non‐oral progestogen, danazol, gonadotropin‐releasing hormone (GnRH) analogues (alone or with ‘add‐back’ oestrogen), progestogen‐releasing intrauterine devices (IUCDs), drugs affecting blood vessels, anticholinergic drugs, antidepressants, anticonvulsants, analgesics, combined analgesic and caffeine preparations and local anaesthetic infiltration alone or in combination with corticosteroids.
Psychological/behavioural/cognitive treatments versus no treatment/placebo/other non‐surgical treatments or other types of interventions.
Interventions in this category included investigations for reassurance, written or oral emotional disclosures, psychotherapy, counselling, cognitive and biofeedback therapy and lifestyle interventions.
Physical and complementary treatments versus no treatment/sham or placebo or other types of interventions.
Interventions such as magnetic field therapy, therapies encompassed in traditional Chinese medicine (TCM) (acupuncture, herbal therapy, moxibustion), transcutaneous nerve stimulation and transcranial current stimulation and physical and massage therapy were considered.
Types of outcome measures
Primary outcomes
Effectiveness of treatment: pain measured by validated pain scales, for example, visual analogue pain scale (VAS) scores, the McGill Pain Questionnaire (MPQ), a pain improvement rating scale, general pain experience and a gynaecological pain questionnaire.
Secondary outcomes
Psychological outcomes indicated by scores such as depression scores (Hamilton Depression Rating Scale (HAM‐D) score, Hospital Anxiety Depression Scale) and mood scores.
Quality of life: indicated by, for example, the Medical Outcomes Study Short Form 36 (SF‐36), the Social Adjustment Survey (SAS‐WR), the Sickness Impact Profile (SIP), a general health questionnaire (GHQ), the revised Sabbatsberg Sexual Rating Scale (rSSRS) and EuroQOL‐5D (EQ‐5D).
Requirement for analgesia.
Adverse outcomes (e.g. treatment intolerance, side effects of intervention).
Search methods for identification of studies
We searched all published and unpublished RCTs to 5 February 2014 with no language restriction and in consultation with the Menstrual Disorders and Subfertility Group (MDSG)Trials Search Co‐ordinator.
Electronic searches
We searched the following electronic databases, trial registers and websites.
The Menstrual Disorders and Subfertility Group (MDSG) Specialised Register of Controlled Trials.
The Cochrane Central Register of Controlled Trials (CENTRAL).
MEDLINE.
EMBASE.
PsycINFO.
CINAHL.
Other electronic sources of trials included the following.
Trial registers for ongoing and registered trials (http://www.controlled‐trials.com, http://clinicaltrials.gov/ct2/home, http://www.who.int/trialsearch/Default.aspx).
Citation indexes (http://scientific.thomson.com/products/sci/).
Conference abstracts in the Web of Knowledge (http://wokinfo.com/).
LILACS database, for trials from the Portuguese‐ and Spanish‐speaking world (http://bases.bireme.br/cgibin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F).
PubMed (http://www.ncbi.nlm.nih.gov/pubmed/).
OpenSIGLE database (http://opensigle.inist.fr/ and Google for grey literature).
See Appendix 1,Appendix 2,Appendix 3,Appendix 4,Appendix 5 and Appendix 6.
Searching other resources
We searched the citation lists of relevant publications, review articles and included studies.
We handsearched relevant journals, abstracts and conference proceedings and several key grey literature sources.
We personally contacted study authors to request further information, if needed.
We approached drug/device manufacturers and experts in the field to ask for references.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search conducted by the MDSG Trials Search Co‐ordinator, the full texts of all potentially eligible studies were retrieved. Two review authors (GS and YCC) reviewed the titles and abstracts independently, examining these articles for compliance with the inclusion criteria and selecting studies eligible for inclusion in the review. We corresponded with study investigators as required. Disagreements as to study eligibility were resolved by discussion or by a third review author (AW). The selection process has been documented with a PRISMA flow chart Figure 1. See also Figure 2 and Figure 3.
1.
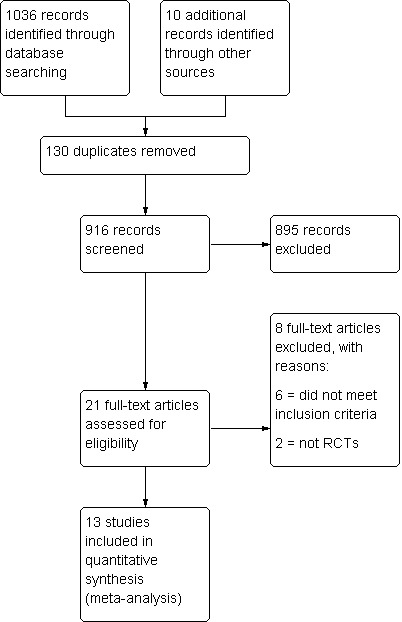
Study flow diagram.
2.
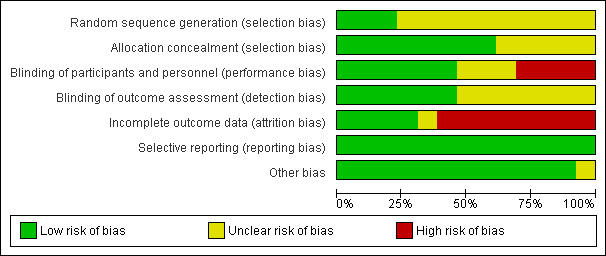
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
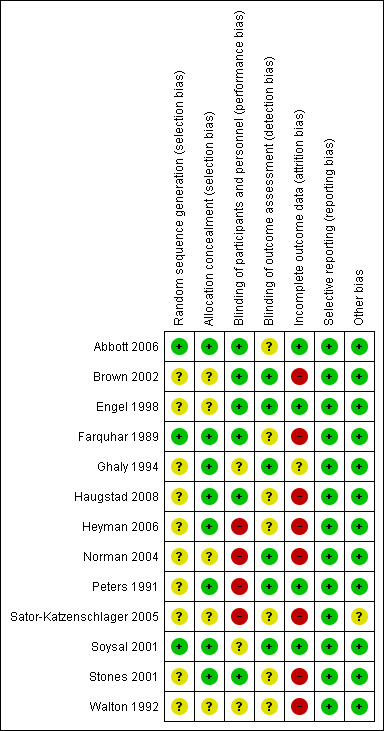
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Data extraction and management
GS and YCC independently extracted the information for each included trial, using a data extraction form designed and pilot tested by the review authors. Any disagreements were resolved by discussion or by a third review author (AW). When studies had multiple publications, the main trial report was used as the reference, and additional details were derived from secondary papers. We corresponded with study investigators to request further data on methods and/or results, as required.
Assessment of risk of bias in included studies
Two review authors (GS and YCC) independently assessed the risk of bias of included studies. The included studies were assessed using the Cochrane risk of bias assessment tool (Version 5.1) to assess the following domains: selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other bias. We resolved disagreements by discussion.
Measures of treatment effect
For dichotomous data, we used the numbers of events in the control and intervention groups of each study to calculate Peto odds ratios (ORs). For continuous data, we calculated the mean difference (MD) between treatment groups. We presented 95% confidence intervals (CIs) for all treatment outcomes.
Unit of analysis issues
No unit of analysis issues were noted, as women were the unit of randomisation.
Dealing with missing data
Data were analysed on an intention‐to‐treat basis as far as possible, and attempts were made to obtain missing data from the original trialists. When these could not be obtained, only the available data were analysed. If studies reported sufficient detail for calculation of MDs but no information on associated standard deviation (SD), we planned to assume that the outcome had an SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. Heterogeneity assessment was performed by measuring I2. An I2 measurement greater than 50% was taken to indicate substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we minimised their potential impact by ensuring a comprehensive search for eligible studies and duplication of data. When appropriate, we planned to use a funnel plot to assess the possibility of small‐study effects. We planned to construct a funnel plot to assess potential publication bias if sufficient studies reported the same comparison.
Data synthesis
When continuous measurements were used to assess the effects of interventions, we calculated the mean difference or, alternatively, the standardised mean difference if different scales were used. We combined the data of studies that were sufficiently similar using a fixed‐effect model for the following comparisons. For binary (or dichotomous) outcomes, we expressed results for each study as Peto ORs with 95% CIs. We applied a fixed‐effect model to assess outcomes if heterogeneity was minor; otherwise we planned to use a random‐effects Mantel‐Haenszel model. We performed statistical analysis using Review Manager software (RevMan 2012). When data were skewed or SDs were not calculable, the data were entered in "Other data" tables.
Medical interventions
Medical interventions versus placebo/no treatment or other interventions, specifically:
Progestogen versus placebo;
Sertaline versus placebo; or
Lofexidine versus placebo.
Medical interventions: head‐to‐head comparisons.
Goserelin versus progestogen.
Gabapentin versus amytriptyline.
Psychological treatments versus placebo/no treatment, or other interventions, specifically:
Psychotherapy versus no treatment;
Ultrasound versus traditional treatment;
Somatocognitive therapy versus no treatment;
Somatic, psychological or dietary therapy or physiotherapy versus no treatment; or
Written disclosure versus no treatment.
Complementary treatments versus no treatment/sham or placebo or other interventions.
Magnetic field therapy versus placebo magnet.
Physical therapy versus standard treatment (counselling).
Subgroup analysis and investigation of heterogeneity
If substantial heterogeneity was noted, the review authors planned to reassess the data and perform a random‐effects meta‐analysis. We also planned to consider doing meta‐regression analysis or subgroup analysis for the following variables.
-
Intervention type.
Physical therapy.
Hormonal therapy.
Non‐hormonal therapy.
Multi‐disciplinary treatment.
Duration of intervention: one session versus repeated sessions.
Duration of follow‐up: immediately after treatment versus post‐treatment, for example, two weeks, four weeks, three months, six months, 12 months.
Sensitivity analysis
If sufficient studies were making the same comparison, the review authors planned to conduct sensitivity analyses for the primary outcome while considering the following issues: risk of bias, assessment of inclusion or exclusion of a study, nature of the data analysed and methods of analysis used.
Overall quality of the body of evidence: summary of findings table
We prepared a summary of findings table using Guideline Development Tool software. This table evaluates the overall quality of the body of evidence for outcomes of pain and adverse effects, using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate or low) have been incorporated into the reporting of results for each outcome.
Results
Description of studies
Results of the search
The search revealed 21 studies that were potentially eligible and were retrieved in full text. A total of 13 studies met our inclusion criteria. Eight studies were excluded. See the study tables Characteristics of included studies and Characteristics of excluded studies.
Included studies
Study design and setting
A total of 13 RCTs were included in the review. Besides Walton 1992, which was a multicentre study, all other studies were single‐centre studies. Four were conducted in the UK, three in the USA, one in Sweden, one in the Netherlands, one in Turkey, one in Austria (Sator‐Katzenschlager 2005), one in Australia (Abbott 2006) and one in Norway (Haugstad 2008).
Participants
The studies included 406 women in the intervention group and 344 women in the control group.
Study populations had a similar age distribution, with mean ages of 27 to 35 years. All studies had comparable control and treatment groups with respect to parity except for Stones 2001, in which the intervention group had higher parity. All study populations had similar chronicity of pain, except in Soysal 2001, in which chronicity was not stated. All participants were women with chronic pelvic pain.
Interventions
Medical interventions versus placebo/no treatment or other medical interventions (seven studies).
Two of seven studies compared medroxyprogesterone against placebo (Farquhar 1989; Walton 1992).
One of seven studies compared medroxyprogesterone versus goserelin (Soysal 2001).
One of seven studies compared sertraline versus placebo (Engel 1998).
One of seven studies compared lofexidine versus placebo (Stones 2001).
One of seven studies compared gabapentin versus amytriptyline versus both (Sator‐Katzenschlager 2005).
One of seven studies compared injection of botulinum toxin A versus placebo (Abbott 2006).
Complementary treatments versus no treatment/sham or placebo or other medical interventions (two studies).
One of two studies compared static magnetic fields versus placebo magnets (Brown 2002).
One of two studies compared physical treatment (distension of painful pelvic structures) versus control (usual care with counselling) (Heyman 2006).
Psychological/behavioural/cognitive treatments versus no treatment/placebo/other interventions (five studies).
One of five studies compared writing therapy (disclosure about their pain) versus control (non‐disclosure) (Norman 2004).
One of five studies compared ultrasound scan and counselling session versus 'wait and see' (Ghaly 1994).
One of five studies examined an integrated approach (somatic, psychological, dietary, environmental and physiotherapeutic factors) versus standard care (Peters 1991).
One of five studies compared somatocognitive therapy (Mensendieck) versus standard care (Haugstad 2008).
One of five studies compared psychotherapy versus placebo (Farquhar 1989).
Outcomes
Primary outcomes.
12 of 13 studies reported pain scores (e.g. VAS, MPQ, pain improvement rating scale, general pain experience, gynaecological pain questionnaire, pain beliefs and perceptions inventory (PBPI), composite pain score, Pain Disability Index) (Abbott 2006; Brown 2002; Engel 1998; Farquhar 1989; Ghaly 1994; Haugstad 2008; Heyman 2006; Norman 2004; Peters 1991; Sator‐Katzenschlager 2005; Stones 2001; Walton 1992). When results are presented in dichotomous data, the improvement in pain is taken to be improvement in pain scores > 50% as defined by the studies.
Secondary outcomes.
Four of 13 studies reported depression scores (e.g. HAM‐D, Hospital Anxiety and Depression Scale (HADS)) (Engel 1998; Ghaly 1994; Heyman 2006; Soysal 2001).
Six of 13 studies reported overall effect (e.g. disturbance of daily activities, Clinical Global Impression Scale, social adjustment survey, SF‐36, SIP, GHQ and EQ‐5D) (Abbott 2006; Brown 2002; Engel 1998; Haugstad 2008; Norman 2004; Peters 1991).
Two of 13 studies checked sexual variables (e.g. rSSRS) (Heyman 2006; Soysal 2001).
Farquhar 1989, Sator‐Katzenschlager 2005, Stones 2001 and Walton 1992 reported the side effects of treatments.
The above outcomes were reported over the following follow‐up duration.
Four weeks to two months: Brown 2002; Heyman 2006 (four weeks); Norman 2004; Stones 2001 (two months).
Three to six months: Abbott 2006; Engel 1998; Soysal 2001; Walton 1992.
Nine to 12 months: Farquhar 1989; Ghaly 1994; Haugstad 2008; Peters 1991.
Excluded studies
Eight studies were excluded from the review for the following reasons.
Two of eight studies were randomised cross‐over trials with no washout period (Fenton 2008;Simsek 2007).
One of eight studies was an open‐label uncontrolled trial (Brown 2008).
One of eight studies was excluded as it was an abstract with insufficient available information (Pearce 1986).
One of eight studies was excluded as this pilot study showed very inconsistent findings, leading the authors to not undertake analysis of outcomes (Hawk 2002).
One of eight studies had participants entered and evaluated at different time points in control and active groups, making comparison between groups difficult (Elcombe 1997).
One of eight studies was excluded as it included a surgical treatment (Onwude 2004).
One of eight studies was excluded as it was not an RCT (Reginald 1987).
Risk of bias in included studies
Allocation
Five studies were at low risk of selection bias related to sequence generation, as they used computer randomisation or a random numbers table (Abbott 2006; Farquhar 1989; Norman 2004; Soysal 2001; Stones 2001). The other eight studies did not describe the method used and were at unclear risk of this bias (Brown 2002; Engel 1998; Ghaly 1994; Haugstad 2008; Heyman 2006;Peters 1991; Sator‐Katzenschlager 2005; Walton 1992).
Seven studies were at low risk of selection bias related to allocation concealment (Abbott 2006; Farquhar 1989; Haugstad 2008; Norman 2004; Peters 1991; Soysal 2001; Stones 2001). The other six studies were at unclear risk of this bias (Brown 2002; Engel 1998; Ghaly 1994; Heyman 2006; Sator‐Katzenschlager 2005; Walton 1992).
Blinding
We considered blinding to influence the findings of the outcome.
Six studies were at low risk for blinding of participants and personnel (Abbott 2006; Brown 2002; Engel 1998; Farquhar 1989; Haugstad 2008; Stones 2001). Seven studies were at low risk for blinding of outcome assessors (Abbott 2006; Brown 2002; Engel 1998; Ghaly 1994; Norman 2004; Peters 1991; Soysal 2001). Blinding was not stated in one study (Walton 1992). Blinding of participants was not fully possible in four studies (Heyman 2006; Norman 2004; Peters 1991; Sator‐Katzenschlager 2005).
Incomplete outcome data
Four studies analysed most (> 90%) of the randomly assigned women, and we judged them as low risk (Abbott 2006; Engel 1998; Peters 1991; Soysal 2001). One had 10% loss to follow‐up and was deemed to be at unclear risk (Ghaly 1994).
Eight studies were considered at high risk of attrition bias (Brown 2002; Farquhar 1989; Haugstad 2008; Heyman 2006; Norman 2004; Sator‐Katzenschlager 2005; Stones 2001; Walton 1992).
Selective reporting
Protocols were available for all studies.
Other potential sources of bias
None were identified.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
1) Medical interventions versus placebo/no treatment
1.1 Progesterone versus placebo/no treatment
Primary outcome: pain
Medroxyprogesterone acetate versus placebo
Progestogen (MPA) was effective at the end of treatment, as denoted by the rate of women achieving a > 50% reduction in VAS pain score (Peto OR 3.00, 95% CI 1.70 to 5.31, two studies, n = 204, I2 = 22%). Evidence of benefit was maintained up to nine months after treatment (Peto OR 2.09, 95% CI 1.18 to 3.71, two studies, n = 204, I2 = 0%) (Analysis 1.1).
1.1. Analysis.
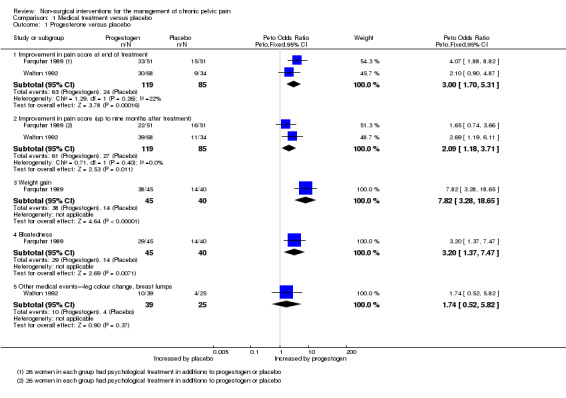
Comparison 1 Medical treatment versus placebo, Outcome 1 Progesterone versus placebo.
Secondary outcomes
No studies reported psychological outcomes, quality of life or requirement for analgesia.
Adverse outcomes
Farquhar 1989 reported a higher risk of weight gain and bloating in the MPA group than in the placebo group (weight gain 7.82, 95% CI 3.28 to 18.65, n = 85; bloatedness Peto OR 3.20, 95% CI 1.37 to 7.47, n = 85). No significant difference was noted between MPA and placebo groups in other reported medical events (e.g. leg colour change, benign breast lumps (Peto OR 1.74, 95% CI 0.52 to 5.82, n = 64)) (Walton 1992) (Analysis 1.1; Figure 4).
4.
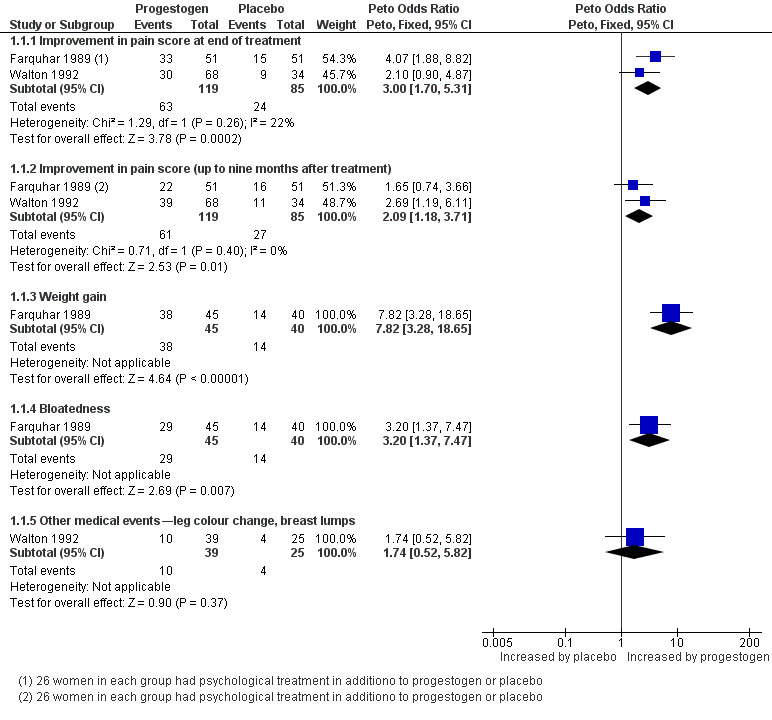
Forest plot of comparison: 1 Medical treatment versus placebo, outcome: 1.1 Progesterone versus placebo.
1.2 Lofexidine hydrochloride versus placebo
Primary outcome: pain
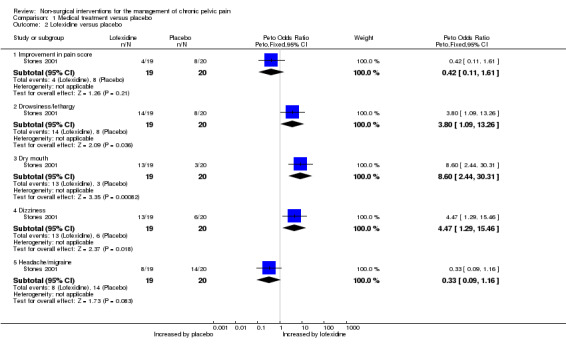
No evidence showed a significant difference between lofexidine hydrochloride and placebo in the rate of women achieving a > 50% reduction in VAS pain score (Peto OR 0.42, 95% CI 0.11 to 1.61, one study, n = 39) (Analysis 1.2).
1.2. Analysis.
Comparison 1 Medical treatment versus placebo, Outcome 2 Lofexidine versus placebo.
Secondary outcomes
No studies reported psychological outcomes, quality of life or requirement for analgesia.
Adverse outcomes
Women taking lofexidine reported significantly higher rates of dry mouth (Peto OR 8.6, 95% CI 2.44 to 30.31, one study, n = 39), drowsiness (Peto OR 3.8, 95% CI 1.09 to 13.26, one study, n = 39) and dizziness (Peto OR 4.77, 95% CI 1.29 to 15.46, one study, n = 39) than women who took placebo. No significant difference between groups was noted in the incidence of headache or migraine (Peto OR 0.33, 95% CI 0.09 to 1.16, one study, n = 39) (Analysis 1.2).
1.3 Sertraline versus placebo
Primary outcome: pain
No analysable data were available for this outcome, but one study reported no evidence of a significant difference between sertraline and placebo in pain scores on a zero to ten scale (MD ‐0.02, 95% CI ‐0.6 to ‐0.6, one study, n = 25) (Table 5).
1. Change in pain, depression, somatisation and functional status.
| Engel 1998 | ||
| Composite pain intensity | SF‐36 functioning‐emotional subscale | Health perception |
| ‐0.02, 95% CI ‐0.6 to 0.6 | ‐30.4, 95% CI ‐50.3 to ‐10.6 | 3.0, 95% CI 0.3 to 5.7 |
Secondary outcomes
Psychological outcomes
No studies reported psychological outcomes.
Quality of life
Sertraline versus placebo
No analysable data were available for this outcome, but one study reported that the SF‐36 subscale for health perception (range zero to ten) showed a small but statistically significant improvement in the sertraline arm (MD 3.0, 95% CI 0.3 to 5.7, one study, n = 25), and the functioning‐emotional subscale (range not specified) showed a large, statistically significant decrement in the sertraline arm (MD ‐30.4, 95% CI ‐50.3 to ‐10.6, one study, n = 25) (Engel 1998) (Table 5).
Requirement for analgesia
No studies reported this outcome.
Adverse outcomes
No studies reported this outcome.
2) Medical treatment versus other medical treatment
2.1 Goserelin versus progestogen
Primary outcome: pain
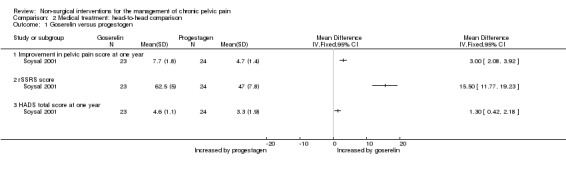
Women taking goserelin showed greater improvement in pelvic pain score at one year (as measured on a scale of one to 100) than those taking progesterone (MD 3, 95% CI 2.08 to 3.92, one study, n = 47) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Medical treatment: head‐to‐head comparison, Outcome 1 Goserelin versus progestogen.
Secondary outcomes
Psychological outcomes
Mood and sexual function were improved to a greater extent one year after treatment among women taking goserelin than among those taking progestogen (Soysal 2001) (HADS total score, MD 1.3, 95% CI 0.42 to 2.18, n = 47; rSSRS score (revised Sabbatsberg Sexual Rating scale), MD 15.5, 95% CI 11.7 to 19.23, n = 47). HADS is a self assessment mood scale that is designed specifically for use in non‐psychiatric hospital outpatients to assess anxiety and depression (Zigmond 1983). The score is out of a total of 42, and the higher the score, the greater the anxiety or depression. The rSSRS is a 12‐item questionnaire of sexual functioning (Garrat 1995). A higher score (scale of zero to 100) represents greater sexual satisfaction (Analysis 2.1).
This study did not report on our other secondary outcomes (quality of life, requirement for analgesia or adverse outcomes).
2.2 Gabapentin versus amytriptyline
Primary outcome: pain
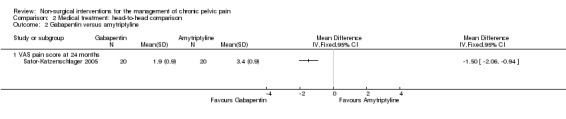
The VAS pain score (on a one to ten scale) favoured gabapentin compared with amytriptyline at 24 months' follow‐up (MD ‐1.50, 95% CI ‐2.06 to ‐0.94, n = 40) (Sator‐Katzenschlager 2005) (Analysis 2.2).
2.2. Analysis.
Comparison 2 Medical treatment: head‐to‐head comparison, Outcome 2 Gabapentin versus amytriptyline.
Secondary outcomes
This study did not report on our other secondary outcomes (psychological outcomes, quality of life or requirement for analgesia).
Adverse outcomes
Study authors reported no significant difference in the rate of side effects among women taking gabapentin compared with women who took amytriptyline (no extractable data).
3) Psychological treatment versus placebo/no treatment or other interventions
3.1 Improvement in pain scores
Primary outcome: pain
Ultrasound scan and counselling session versus 'wait and see'
At four to nine months' follow‐up, women who had ultrasound reassurance and counselling were more likely to have improved pain than those given a 'wait and see' policy (Peto OR 6.77, 95% CI 2.83 to 16.19, n = 90) (Ghaly 1994) (Analysis 3.1; Figure 5). Assessment was based on a 40‐point scale on the McGill Pain Questionnaire, with a change in score of five points deemed a meaningful improvement.
3.1. Analysis.
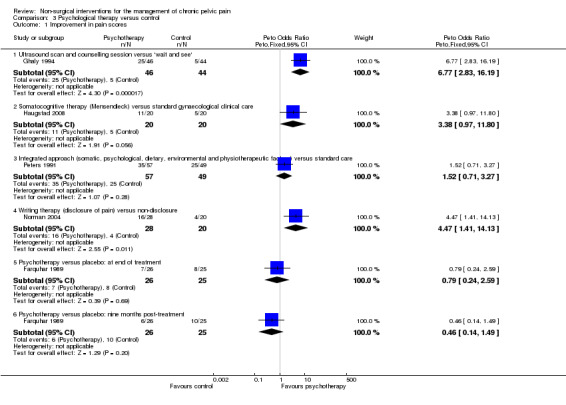
Comparison 3 Psychological therapy versus control, Outcome 1 Improvement in pain scores.
5.
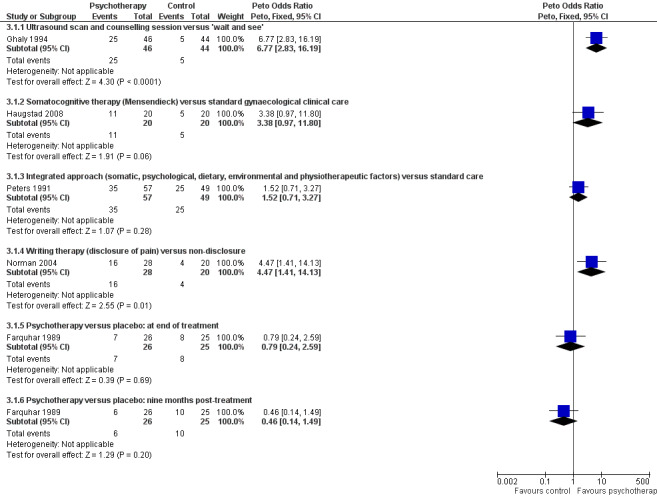
Forest plot of comparison: 3 Psychological therapy versus control, outcome: 3.1 Improvement in pain scores.
Somatocognitive therapy (Mensendieck) versus standard gynaecological clinical care
Rates of improvement in pain (≥ 50% reduction in VAS score) did not significantly differ between women who underwent somatocognitive therapy and those who underwent standard gynaecological treatment (Peto OR 3.38, 95% CI 0.97 to 11.80, n = 40) (Haugstad 2008) (Analysis 3.1).
Integrated approach (somatic, psychological, dietary, environmental and physiotherapeutic factors) versus standard care
Rates of improvement in pain (≥ 50% reduction in VAS score) did not significantly differ between women who underwent the integrated approach and those who underwent standard care (Peto OR 1.52, 95% CI 0.71 to 3.27, n = 106) (Peters 1991) (Analysis 3.1).
Writing therapy (disclosure of pain) versus non‐disclosure
Rates of improvement in pain (measured on a pain scale of zero to five, with improvement considered an improvement of at least one point) showed that a significantly higher number of 'disclosure patients' improved compared with 'non‐disclosure patients' (Peto OR 4.47, 95% CI 1.41 to 14.13, n = 48) (Norman 2004) (Analysis 3.1).
Psychotherapy versus placebo
No significant difference was noted between the psychotherapy group and the placebo group in rates of pain improvement (≥ 50% reduction in VAS score) immediately after treatment (Peto OR 0.79, 95% CI 0.24 to 2.59, n = 51) or at nine months' follow‐up (Peto OR 0.46, 95% 0.14 to 1.49, n = 51) (Farquhar 1989) (Analysis 3.1).
Secondary outcomes
3.2 Psychological outcomes
Physiotherapy and psychotherapy versus standard gynaecological advice
Depression scores (as measured on the depression scale of the GHQ‐30) did not differ significantly between women who received psychological treatment (physiotherapy and psychotherapy) and those who received standard gynaecological advice (MD ‐0.30, 95% CI ‐0.80 to 0.20, n = 26) (Haugstad 2008) (Analysis 3.2).
3.2. Analysis.
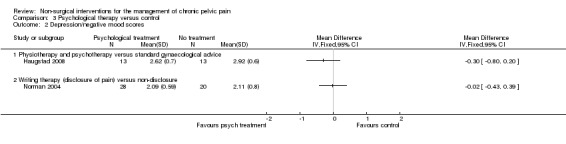
Comparison 3 Psychological therapy versus control, Outcome 2 Depression/negative mood scores.
Writing therapy (disclosure of pain) versus non‐disclosure
Mood at the end of two months (as measured on the negative affect scale of the Positive Affect Negative Affect Scale (PANAS)) did not differ significantly between women who underwent writing therapy (disclosure of pain) and controls (non‐disclosure) (MD ‐0.02, 95% CI ‐0.43 to 0.39, n = 48) (Norman 2004) (Analysis 3.2).
Studies of psychological treatment did not report other secondary outcomes (quality of life, requirement for analgesia or adverse effects).
4) Complementary treatments versus no treatment/sham or placebo or other interventions
Physical treatment versus standard treatment
Primary outcome: pain
Physical treatment (distension of painful pelvic structures) was found to be significantly better than standard treatment (counselling), as reflected by a greater reduction in self rating scores on a VAS one to 100 scale for pain intensity (MD 35.80, 95% CI 23.08 to 48.52) and for pain during intercourse (MD 19.13, 95% CI 3.61 to 34.65) (Heyman 2006) (Analysis 4.1).
4.1. Analysis.
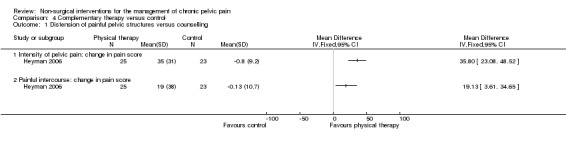
Comparison 4 Complementary therapy versus control, Outcome 1 Distension of painful pelvic structures versus counselling.
Magnetic therapy versus placebo magnet
Primary outcome: pain
No evidence of benefit was found in women receiving active magnets who completed four weeks of double‐blind treatment compared with those having placebo magnets in terms of the Pelvic Pain Index (MD 0.50, 95% CI ‐1.92 to 0.92, one study, n = 19), Clinical Global Impressions‐Severity (MD ‐0.90, 95% CI ‐2.05 to 0.25, one study, n = 19) and McGill Pain Disability scores (MD ‐16.70, 95% CI ‐34.05 to 0.65, one study, n = 19) (Brown 2002) (Analysis 4.2).
4.2. Analysis.
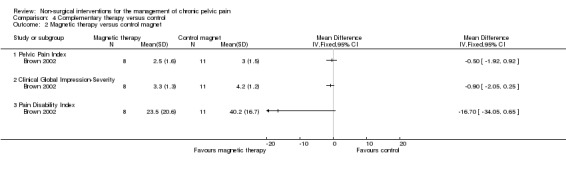
Comparison 4 Complementary therapy versus control, Outcome 2 Magnetic therapy versus control magnet.
Studies of physical treatment did not report secondary outcomes (quality of life, requirement for analgesia or adverse effects).
Other analyses
Too few studies reported the same comparison for planned subgroup and sensitivity analyses to be conducted, or for a funnel plot to be constructed.
Discussion
Summary of main results
Progestogen (MPA) was more effective than placebo at the end of treatment in terms of the number of women achieving a > 50% reduction in VAS pain score immediately after treatment (Peto OR 3.00, 95% CI 1.70 to 5.31, two studies, n = 204, I2 = 22%). Evidence of benefit was maintained up to nine months after treatment (Peto OR 2.09, 95% CI 1.18 to 3.71, two studies, n = 204, I2 = 0%). Women receiving medical treatment with progestogen reported more adverse effects (weight gain and bloating) than those given placebo.
Head‐to‐head comparisons showed that women taking goserelin had greater improvement in pelvic pain score (MD 3, 95% CI 2.08 to 3.92, one study, n = 47), mood (HADS total score, MD 1.3, 95% CI 0.42 to 2.18, n = 47) and sexual function at one year than those taking progestogen (rSSRS score (revised Sabbatsberg Sexual Rating Scale), MD 15.5, 95% CI 11.7 to 19.23, n = 47). Women taking gabapentin had a more favourable VAS pain score than those taking amytriptyline (MD ‐1.50, 95% CI ‐2.06 to ‐0.94, n = 40). Women who underwent reassurance ultrasound scans and counselling were more likely to have improved pain than those given a standard 'wait and see' policy (Peto OR 6.77, 95% CI 2.83 to 16.19, n = 90). Significantly more women who had writing therapy as a disclosure had improvement in pain compared with those in the non‐disclosure group (MD ‐0.02, 95% CI ‐0.43 to 0.39, n = 48).
Because of the heterogeneity of the included studies, we were not able to combine the results of studies examining the effects of various forms of psychological and complementary treatment on pain and quality of life in women with CPP.
Overall completeness and applicability of evidence
Many of the interventions were single randomised controlled studies, and this limited the available evidence on which current clinical practice can be based. Progestogen appears to be an effective treatment for chronic pelvic pain, but its efficacy beyond 12 months and in older women has not been studied. High‐dose progestogen treatments are often limited by side effects and are contraindicated in women attempting to conceive. The use of neuromodulators such as gabapentin has attracted much interest, and undoubtedly, results from the feasibility study of the efficacy of action of gabapentin (Horne 2012), which is currently under way, will help shed some light on the question of whether neuromodulators are effective in the management of CPP.
We were unable to combine different treatments that involve psychological interventions to provide meaningful analysis. Although in principle, each of the interventions could be tested more rigorously in RCTs, it makes more sense in clinical terms to apply effective methods of cognitive and behavioural pain management (Williams 2012) to pelvic pain because it shares so much of the impact and problems of chronic pain at other sites (Daniels 2010).
This review is not able to conclude on the effect of non‐surgical interventions on quality of life because of diversity or absence of outcome measures in the studies reviewed. It would be helpful if trialists observed the recommendations of the IMMPACT initiative on outcome measurement in pain trials (Dworkin 2005; Dworkin 2010).
Quality of the evidence
Thirteen RCTs were included in this review. These studies included 406 women in the intervention groups and 344 women in the control groups. The conclusion of evidence of benefit drawn mainly from improvement in pain assessment scores (VAS specifically) cannot be directly translated to improvement in functional outcomes and quality of life, the latter being key contributors to the morbidity associated with chronic pelvic pain. RCTs included in this review also suffered from high attrition rates, which contributed to incomplete outcome data. A wide range of follow‐up was provided (range four weeks to 12 months); studies with shorter follow‐up data lend themselves to confounding effects of placebo, although studies with longer follow‐up are associated with higher attrition rates.
The quality of the evidence, which was rated using GRADE methods, ranged from very low to high. The main reasons for downgrading evidence quality were high attrition rates, lack of blinding, failure to clearly describe randomisation procedures and lack of precision.
Potential biases in the review process
Both YCC and GS independently screened and identified relevant studies; furthermore, a final search was performed at the completion of the review to ensure that no new studies had been published during preparation of the manuscript. However, despite all efforts, studies in press may have been missed.
Agreements and disagreements with other studies or reviews
None known.
Authors' conclusions
Implications for practice.
Evidence of moderate quality supports progestogen as an option for chronic pelvic pain, with efficacy during treatment. In practice, it may be most acceptable among women unconcerned about progestogenic side effects such as weight gain and bloating. No evidence indicates that lofexidine or sertraline was more effective than placebo, although data were very scanty.
Head‐to‐head comparisons revealed that women taking goserelin showed greater improvement in pelvic pain score, mood and sexual function at one year compared with those taking progestogen; women taking gabapentin had a more favourable VAS pain score than those taking amytriptyline, although these results were based on single studies and therefore have to be interpreted with caution.
Women who underwent reassurance ultrasound scans and counselling were more likely to have improved pain compared with those having a standard 'wait and see' policy. Significantly more women who had writing therapy as a disclosure had improvement in pain compared with those in the non‐disclosure group. Again, as these findings are based on single studies, more studies will be required to confirm the results.
The quality of the evidence was low or moderate for most comparisons, and in most cases, evidence was derived from single small studies at a high or unclear risk of bias
Implications for research.
This update of the review has shown that a wide range of interventions have been tried for the non‐surgical management of chronic pelvic pain. But the fact that many of these therapies were single randomised controlled studies greatly limits the available evidence on which current clinical practice can be based. Hence, more studies are required to be replicated on these individual interventions to confirm the findings of existing studies.
Future trials should employ recommended objective and patient‐reported measures that capture not just pain measures but outcome measures focused on the biopsychosocial aspects of women with CPP (Dworkin 2005; Dworkin 2010).
Future researchers in this area should consider taking advantage of advancements in technology to design studies that can capture more accurate activity and quality of life data than the limited snap‐shot data that questionnaires can generate.
As chronic pelvic pain has a multifactorial aetiology, shifting the research paradigm from single‐intervention RCTs to those for which the main objective is to develop and deliver effective integrated multidisciplinary care pathways will be critical to the successful management of this disorder.
History
Protocol first published: Issue 11, 2010 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 30 October 2009 | New search has been performed | This review was previously part of the review 'Interventions for treating chronic pelvic pain in women'. Due to the increase in the total number of studies, this review will now focus only on the non‐surgical management of chronic pelvic pain. Another review on surgical management is also planned. |
Acknowledgements
Marian Showell (Trial Search Co‐ordinator of the Cochrane Menstrual Disorders and Subfertility Group) for designing and running the search. Helen Nagels and Jane Marjoribanks for methodological and editorial help.
Appendices
Appendix 1. Ovid MEDLINE
1 exp Life Style/ (55316) 2 exp exercise/ or exp exercise therapy/ or exp relaxation techniques/ or exp walking/ or exp yoga/ (119058) 3 Life Style$.tw. (8185) 4 exercis$.tw. (172356) 5 (walk$ or jog or run or yoga).tw. (108651) 6 exp Diet/ (169662) 7 diet$.tw. (334694) 8 (treatment$ or therap$).tw. (3515613) 9 exp psychology/ or exp cognitive science/ or exp psychology, medical/ (55140) 10 psycholog$.tw. (141613) 11 (cognitive adj5 therap$).tw. (9657) 12 psychotherap$.tw. (28610) 13 meditation.tw. (1882) 14 (biofeedback or hypnosis or reassur$).tw. (17256) 15 ultraso$.tw. (213950) 16 exp analgesics/ or exp clonidine/ or exp analgesics, non‐narcotic/ or exp anti‐inflammatory agents, non‐steroidal/ or exp diclofenac/ or exp ibuprofen/ or exp naproxen/ or exp cyclooxygenase inhibitors/ (398830) 17 non‐steroidal anti‐inflammator$.tw. (9925) 18 NSAIDS.tw. (11889) 19 exp Contraceptives, Oral/ (39927) 20 (oral contracept$ or OCP).tw. (22244) 21 exp Progestins/ (58187) 22 (progestin$ or progestogen$).tw. (13525) 23 (danazol or GnRH analogue$).tw. (3231) 24 exp Antidepressive Agents/ (112330) 25 Antidepress$.tw. (41592) 26 anticonvuls$.tw. (18010) 27 analges$.tw. (77720) 28 caffeine.tw. (20048) 29 local anaesth$.tw. (9304) 30 corticosteroid$.tw. (67567) 31 (counselling or counseling).tw. (50580) 32 iucd.tw. (414) 33 transcutaneous nerve stimulation.tw. (248) 34 exp complementary therapies/ or exp acupressure/ or exp acupuncture therapy/ or exp electroacupuncture/ or exp moxibustion/ or exp holistic health/ or exp medicine, traditional/ or exp mind‐body therapies/ or exp "biofeedback (psychology)"/ or exp hypnosis/ or exp "imagery (psychotherapy)"/ or exp meditation/ or exp relaxation therapy/ or exp yoga/ or exp phytotherapy/ or exp reflexotherapy/ (157318) 35 exp anesthetics, local/ or exp lidocaine/ (84838) 36 local anesthetic$.tw. (12217) 37 referral$.tw. (56399) 38 magnetic field$.tw. (22048) 39 sertraline.tw. (2747) 40 medroxyprogesterone$.tw. (5030) 41 emotional disclosure.tw. (102) 42 reinforcement.tw. (20607) 43 goserelin.tw. (696) 44 dihydroergotamine.tw. (1274) 45 lofexidine.tw. (146) 46 (chinese medicine$ or chinese herb$).tw. (12418) 47 pain relief.tw. (18775) 48 gonadotropin‐releasing hormone analogue$.tw. (367) 49 or/1‐48 (5112093) 50 exp Pelvic Pain/ (5690) 51 (Pelv$ adj1 Pain$).tw. (5141) 52 (Pelv$ adj1 congest$).tw. (203) 53 abdomin$ pain$.tw. (31974) 54 or/50‐53 (40525) 55 49 and 54 (22265) 56 randomized controlled trial.pt. (322734) 57 controlled clinical trial.pt. (83763) 58 randomized.ab. (238641) 59 placebo.tw. (137933) 60 clinical trials as topic.sh. (158570) 61 randomly.ab. (175416) 62 trial.ti. (102055) 63 (crossover or cross‐over or cross over).tw. (52722) 64 or/56‐63 (791047) 65 (animals not (humans and animals)).sh. (3594930) 66 64 not 65 (730147) 67 66 and 55 (2640) 68 (200909$ or 200910$ or 200911$ or 200912$).ed. (242918) 69 (2011$ or 2012$).ed. (1175160) 70 68 or 69 (1418078) 71 67 and 70 (293)
This search was updated on 15 May 2013, and again on 5 February 2014.
Appendix 2. PsycINFO
1 (Pelv$ adj1 Pain$).tw. (344) 2 (Pelv$ adj1 congest$).tw. (7) 3 1 or 2 (348) 4 random.tw. (34819) 5 control.tw. (271098) 6 double‐blind.tw. (15805) 7 clinical trials/ (5895) 8 placebo/ (3166) 9 exp Treatment/ (511206) 10 or/4‐9 (774043) 11 3 and 10 (158) 12 limit 11 to yr="2009 ‐Current" (37)
This search was updated on 15 May 2013, and again on 5 February 2014.
Appendix 3. EMBASE
1 Life Style$.tw. (10557) 2 exercis$.tw. (206770) 3 (walk$ or jog or run or yoga).tw. (129681) 4 exp Diet/ (162989) 5 diet$.tw. (385363) 6 psycholog$.tw. (200082) 7 (cognitive adj5 therap$).tw. (14563) 8 psychotherap$.tw. (40897) 9 meditation.tw. (2356) 10 (biofeedback or hypnosis or reassur$).tw. (21541) 11 ultraso$.tw. (269667) 12 non‐steroidal anti‐inflammator$.tw. (12612) 13 NSAIDS.tw. (16871) 14 (oral contracept$ or OCP).tw. (22315) 15 (progestin$ or progestogen$).tw. (14259) 16 (danazol or GnRH analogue$).tw. (4102) 17 Antidepress$.tw. (55468) 18 anticonvuls$.tw. (22015) 19 analges$.tw. (98950) 20 caffeine.tw. (23068) 21 local anaesth$.tw. (11695) 22 corticosteroid$.tw. (84150) 23 (counselling or counseling).tw. (62272) 24 iucd.tw. (464) 25 transcutaneous nerve stimulation.tw. (286) 26 local anesthetic$.tw. (14301) 27 referral$.tw. (71060) 28 magnetic field$.tw. (19054) 29 sertraline.tw. (3769) 30 medroxyprogesterone$.tw. (5409) 31 emotional disclosure.tw. (132) 32 reinforcement.tw. (21250) 33 goserelin.tw. (937) 34 dihydroergotamine.tw. (1486) 35 lofexidine.tw. (195) 36 (chinese medicine$ or chinese herb$).tw. (17182) 37 pain relief.tw. (25378) 38 gonadotropin‐releasing hormone analogue$.tw. (435) 39 nerve block.tw. (4772) 40 exp nerve block/ (21291) 41 exp lifestyle/ (59627) 42 exp exercise/ (169176) 43 exp psychology/ (143266) 44 exp analgesic agent/ (555528) 45 exp nonsteroid antiinflammatory agent/ (370296) 46 exp oral contraceptive agent/ (48771) 47 exp antidepressant agent/ (270421) 48 exp gestagen/ (126786) 49 exp alternative medicine/ (29258) 50 exp local anesthetic agent/ (158486) 51 or/1‐50 (2794136) 52 Pelv$ Pain$.tw. (6765) 53 Pelv$ congest$.tw. (252) 54 abdomin$ pain$.tw. (42822) 55 exp pelvis pain syndrome/ (8090) 56 CPP.tw. (6602) 57 or/52‐56 (58811) 58 Clinical Trial/ (862803) 59 Randomized Controlled Trial/ (318508) 60 exp randomization/ (57568) 61 Single Blind Procedure/ (15595) 62 Double Blind Procedure/ (107813) 63 Crossover Procedure/ (33346) 64 Placebo/ (194847) 65 Randomi?ed controlled trial$.tw. (72598) 66 Rct.tw. (8838) 67 random allocation.tw. (1124) 68 randomly allocated.tw. (16791) 69 allocated randomly.tw. (1783) 70 (allocated adj2 random).tw. (703) 71 Single blind$.tw. (11911) 72 Double blind$.tw. (125667) 73 ((treble or triple) adj blind$).tw. (263) 74 placebo$.tw. (171422) 75 prospective study/ (199004) 76 or/58‐75 (1231050) 77 case study/ (14928) 78 case report.tw. (221515) 79 abstract report/ or letter/ (824756) 80 or/77‐79 (1056765) 81 76 not 80 (1196494) 82 51 and 57 and 81 (2878) 83 (2011$ or 2012$).em. (1317175) 84 82 and 83 (336)
This search was updated on 15 May 2013, and again on 5 February 2014.
Appendix 4. Cochrane Central Register of Controlled Trials
1 exp Life Style/ (1848) 2 exp exercise/ or exp exercise therapy/ or exp relaxation techniques/ or exp walking/ or exp yoga/ (13798) 3 Life Style$.tw. (230) 4 exercis$.tw. (25842) 5 (walk$ or jog or run or yoga).tw. (11305) 6 exp Diet/ (9692) 7 diet$.tw. (20782) 8 (treatment$ or therap$).tw. (287111) 9 exp psychology/ or exp cognitive science/ or exp psychology, medical/ (731) 10 psycholog$.tw. (8177) 11 (cognitive adj5 therap$).tw. (3502) 12 psychotherap$.tw. (2268) 13 meditation.tw. (367) 14 (biofeedback or hypnosis or reassur$).tw. (2025) 15 ultraso$.tw. (9144) 16 exp analgesics/ or exp clonidine/ or exp analgesics, non‐narcotic/ or exp anti‐inflammatory agents, non‐steroidal/ or exp diclofenac/ or exp ibuprofen/ or exp naproxen/ or exp cyclooxygenase inhibitors/ (31219) 17 non‐steroidal anti‐inflammator$.tw. (1089) 18 NSAIDS.tw. (1154) 19 exp Contraceptives, Oral/ (2830) 20 (oral contracept$ or OCP).tw. (1584) 21 exp Progestins/ (1708) 22 (progestin$ or progestogen$).tw. (1379) 23 (danazol or GnRH analogue$).tw. (451) 24 exp Antidepressive Agents/ (8730) 25 Antidepress$.tw. (5618) 26 anticonvuls$.tw. (527) 27 analges$.tw. (19712) 28 caffeine.tw. (1704) 29 local anaesth$.tw. (1915) 30 corticosteroid$.tw. (6894) 31 (counselling or counseling).tw. (4514) 32 iucd.tw. (21) 33 transcutaneous nerve stimulation.tw. (73) 34 exp complementary therapies/ or exp acupressure/ or exp acupuncture therapy/ or exp electroacupuncture/ or exp moxibustion/ or exp holistic health/ or exp medicine, traditional/ or exp mind‐body therapies/ or exp "biofeedback (psychology)"/ or exp hypnosis/ or exp "imagery (psychotherapy)"/ or exp meditation/ or exp relaxation therapy/ or exp yoga/ or exp phytotherapy/ or exp reflexotherapy/ (10237) 35 exp anesthetics, local/ or exp lidocaine/ (8493) 36 local anesthetic$.tw. (2318) 37 referral$.tw. (2888) 38 magnetic field$.tw. (275) 39 sertraline.tw. (1089) 40 medroxyprogesterone$.tw. (1246) 41 emotional disclosure.tw. (62) 42 reinforcement.tw. (1178) 43 goserelin.tw. (333) 44 dihydroergotamine.tw. (294) 45 lofexidine.tw. (62) 46 (chinese medicine$ or chinese herb$).tw. (1599) 47 pain relief.tw. (5639) 48 gonadotropin‐releasing hormone analogue$.tw. (64) 49 or/1‐48 (370766) 50 exp Pelvic Pain/ (507) 51 (Pelv$ adj1 Pain$).tw. (390) 52 (Pelv$ adj1 congest$).tw. (11) 53 abdomin$ pain$.tw. (1687) 54 or/50‐53 (2412) 55 49 and 54 (2000) 56 limit 55 to yr="2009 ‐Current" (275)
This search was updated on 15 May 2013, and again on 5 February 2014.
Appendix 5. AMED (Allied and Complementary Medicine)
1 (Pelv$ adj1 Pain$).tw. (105) 2 (Pelv$ adj1 congest$).tw. (4) 3 1 or 2 (109) 4 random.tw. (1984) 5 control.tw. (18120) 6 double‐blind.tw. (1482) 7 clinical trials/ (1669) 8 placebo/ (517) 9 or/4‐8 (22380) 10 3 and 9 (14)
This search was updated on 15 May 2013, and again on 5 February 2014.
Appendix 6. Search strategy
YC816 Search string 09.09.09
Keywords CONTAINS "pelvic congestion" or "pelvic venous congestion" or "pelvic pain" or "chronic pain" or "chronic pelvic pain" or "pain‐pelvic" or Title CONTAINS "pelvic congestion" or "pelvic venous congestion" or "pelvic pain" or "chronic pain" or "chronic pelvic pain" or "pain‐pelvic"
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomized trials which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.0.2 chapter 6, 6.4.11) The EMBASE search is combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) http://www.sign.ac.uk/methodology/filters.html#random There is no language restriction in these searches.
Data and analyses
Comparison 1. Medical treatment versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progesterone versus placebo | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Improvement in pain score at end of treatment | 2 | 204 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.00 [1.70, 5.31] |
| 1.2 Improvement in pain score (up to nine months after treatment) | 2 | 204 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [1.18, 3.71] |
| 1.3 Weight gain | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.82 [3.28, 18.65] |
| 1.4 Bloatedness | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.20 [1.37, 7.47] |
| 1.5 Other medical events—leg colour change, breast lumps | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.52, 5.82] |
| 2 Lofexidine versus placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Improvement in pain score | 1 | 39 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.42 [0.11, 1.61] |
| 2.2 Drowsiness/lethargy | 1 | 39 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.80 [1.09, 13.26] |
| 2.3 Dry mouth | 1 | 39 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.60 [2.44, 30.31] |
| 2.4 Dizziness | 1 | 39 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.47 [1.29, 15.46] |
| 2.5 Headache/migraine | 1 | 39 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.09, 1.16] |
Comparison 2. Medical treatment: head‐to‐head comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Goserelin versus progestogen | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Improvement in pelvic pain score at one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 rSSRS score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 HADS total score at one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Gabapentin versus amytriptyline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 VAS pain score at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Psychological therapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in pain scores | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Ultrasound scan and counselling session versus 'wait and see' | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.77 [2.83, 16.19] |
| 1.2 Somatocognitive therapy (Mensendieck) versus standard gynaecological clinical care | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.38 [0.97, 11.80] |
| 1.3 Integrated approach (somatic, psychological, dietary, environmental and physiotherapeutic factors) versus standard care | 1 | 106 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.71, 3.27] |
| 1.4 Writing therapy (disclosure of pain) versus non‐disclosure | 1 | 48 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.47 [1.41, 14.13] |
| 1.5 Psychotherapy versus placebo: at end of treatment | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.24, 2.59] |
| 1.6 Psychotherapy versus placebo: nine months post‐treatment | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.14, 1.49] |
| 2 Depression/negative mood scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Physiotherapy and psychotherapy versus standard gynaecological advice | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Writing therapy (disclosure of pain) versus non‐disclosure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Complementary therapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Distension of painful pelvic structures versus counselling | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Intensity of pelvic pain: change in pain score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Painful intercourse: change in pain score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Magnetic therapy versus control magnet | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Pelvic Pain Index | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clinical Global Impression‐Severity | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Pain Disability Index | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbott 2006.
| Methods | Randomised controlled trial | |
| Participants | N = 60 (30 each group) Women aged 18 to 55 years who had more than two years of chronic pelvic pain that caused disruption to their daily activities Women were excluded if they were breastfeeding, pregnant or desiring pregnancy during the study period, were unwilling to use contraception during the study period or had previously received botulinum toxin type A injections to the pelvic floor. Palpable pelvic pathology, current use of aminoglycoside antibiotics, history of neurological or bleeding disorders and known sensitivity to the formulation of botulinum toxin type A were also reasons for exclusion |
|
| Interventions | Women were randomly assigned to receive 80 units of botulinum toxin type A at a concentration of 20 units/mL or saline injections (placebo group) FU 26 weeks after injections |
|
| Outcomes | VAS, EuroQOL‐5D (EQ‐5D), SF‐12, Sexual Activities Questionnaire | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo injection given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% dropout Two from placebo; one from botulinum group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Brown 2002.
| Methods | Method of allocation: unclear Power calculation: yes Exclusion postrandomisation: zero Losses to follow‐up: one Intention‐to‐treat analysis: no Source of funding: BIOflex Medical Magnets |
|
| Participants | Country: USA Number of participants: 32 Inclusion criteria: pelvic pain > six months despite other treatment, impaired social function, trigger/circumscribed tender point on examination, normal pelvic examination, normal cervical smears Age: 18 to 50 years Source of participants: gynaecology clinic Exclusion criteria: pregnancy, breastfeeding, medical disorders, metal/electronic device, BMI > 35 |
|
| Interventions | Treatment: static magnetic fields (n = 16) Control: no treatment (n = 17) Duration: two to four weeks |
|
| Outcomes | The McGill Pain Questionnaire, Pain Disability Index, Clinical Global Impression Scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Variable duration of study. Subgroup analysis of women who continued to have four weeks of treatment showed significance. High discontinuation between two and four weeks (41% attrition rate) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Engel 1998.
| Methods | Randomisation: unclear Method of allocation: unclear Double‐blinded Exclusions postrandomisation: two Losses to follow‐up: zero Unusual study design: cross‐over design |
|
| Participants | Country: USA Number of participants: 25 Age: mean 29 Sex: F Inclusion criteria: pelvic pain for longer than three months, no psychoactive medication for previous two weeks Exclusion criteria: laparoscopy within three months, failed seven to 10‐day placebo run‐in phase |
|
| Interventions | Treatments: sertaline 50 mg twice daily Control: placebo Duration: six weeks Follow‐up: end of treatment |
|
| Outcomes | Composite pain score SF‐36 Hamilton Depression Rating Scale Social Adjustment Survey Hopkins Symptoms Checklist Somatization items Pin |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not clearly specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25 randomly assigned, 23 completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Farquhar 1989.
| Methods | Randomisation: blocks of 12 Method of allocation: sealed envelopes Exclusion postrandomisation: zero Losses to follow‐up: seven Unusual study design (e.g. factorial) |
|
| Participants | Country: England Number of participants: 102 Age: mean 29.8 Sex: F Inclusion criteria: pelvic pain for longer than six months, no pathology on laparoscopy, venogram score of five or higher Exclusion criteria: recent psychiatric disease, history of thromboembolism Postmenopausal, previous hysterectomy |
|
| Interventions | Treatments: medroxyprogesterone acetate (MPA) 50 mg daily (n = 25) MPA and psychotherapy (n = 26) Control: placebo (n = 25) Placebo and psychotherapy (n = 26) Duration: four months Follow‐up: nine months |
|
| Outcomes | Visual analogue scale pain score Pain improvement rating scale Side effects |
|
| Notes | Not blinded to psychotherapy. Quality score A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random numbers table |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes for medications |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was recognised that the women might know whether they were receiving MPA because of its effect on their menstrual cycle. Therefore, all were told that both treatments— MPA and placebo—could induce amenorrhoea or irregular vaginal bleeding or have no obvious effect. In addition, women were asked to stop all forms of hormonal or intrauterine contraception and to use barrier methods throughout the 13 months of the trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 102 women, 84 completed the full study (18% attrition). Of the other 18 women, 12 withdrew during the treatment period and six during the follow‐up. Reasons cited were pregnancy, failure to attend the clinic and inability to maintain trial protocol |
| Selective reporting (reporting bias) | Low risk | Less than 10% loss to follow‐up |
| Other bias | Low risk | Nil |
Ghaly 1994.
| Methods | Closed envelope system Losses to follow‐up: 10 |
|
| Participants | Country: Scotland Number of participants: 100 (50 each group) Age: 21 to 55 Sex: F Inclusion criteria: > six months' pain, negative laparoscopy Exclusion criteria: previous malignant disease, mental retardation, medical treatment for pelvic pain at first visit, suspicion of malignant disease on pelvic examination, abnormal pelvic examination |
|
| Interventions | Treatments: US scan and education/counselling sessions Control: 'wait and see' policy (standard treatment) Duration: four to nine months' reassessment |
|
| Outcomes | McGill Pain Scale score: at least five‐point improvement Hospital Anxiety Depression Scale: improvement in category (normal, borderline, depressed) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified, quote: 'random allocation to groups' |
| Allocation concealment (selection bias) | Low risk | Closed envelope system |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible because of the nature of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessed blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 100 randomly assigned, 10 failed to attend for follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Haugstad 2008.
| Methods | Randomised trial | |
| Participants | Women between the ages of 20 and 50 years with deep pelvic pain lasting between one and 10 years | |
| Interventions | Treatment group received 10 treatment sessions with the Mensendieck therapist (Haugstad 2007) of one hour’s duration over 90 days, and the control group received standard gynaecological advice at inclusion and again during the treatment period. This therapeutic approach can be seen as a mixture of physiotherapy and psychotherapy. It teaches patients to change their posture, breathing patterns and the way they move to reduce their pain. It uses a cognitive approach to allow women to have an improved understanding and experience of their body Treatment period: three months FU: one year 16 study groups, 18 controls |
|
| Outcomes | VAS, GHQ‐30 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred by drawing a folded piece of paper with the participant's name from a jar, thus allocating the name to a previously chosen treatment group. Randomisation was performed by a person external to the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not possible in this case, unclear whether assessors blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No attrition figures stated, but at one year, only 13 women in each group had completed the questionnaires |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | Nil |
Heyman 2006.
| Methods | Allocation concealment: sealed envelope system, blocks of four (balanced) Blinding: no Exclusion postrandomisation: zero Losses to follow‐up: five (three/two) Power calculation: yes Intention‐to‐treat analysis: none |
|
| Participants | Country: Sweden No. of participants: 50 (25 each group) Age: median 33 (19 to 54) Sex: F Inclusion criteria: pelvic pain in fertile women > 19 years of age with a duration of at least six months, continuous or intermittent pain at least two days/wk Exclusion criteria: known disease of abdomen, pelvis or lumbar spine; pregnancy; STI; severe mental illness; substance abuse; previous treatment with distension of painful structures in the pelvic floor |
|
| Interventions | Treatment: The participant lay in a prone position; the physician placed his index finger deep into the participant's rectum, and previously identified painful structures were treated in the following order: At a point two finger‐widths lateral of the sacrum, the physician used his index finger to exert strong pressure against the sacrotuberous/spinal ligaments for 15 s to elicit pain. Thereafter, the musculature of the pelvic floor and the joint between the coccyx and the sacrum were concurrently forcefully distended dorsally for 60 s with the index finger. This procedure was repeated after two to three weeks Control: standard care (counselling) Duration: four to six weeks |
|
| Outcomes | VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated |
| Allocation concealment (selection bias) | Low risk | By drawing an envelope, assignment to treatment or control group was performed in blocks of four (i.e. the number of participants in each group was balanced after each fourth participant) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible, unclear whether assessors blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three of 25 (> 10%) dropped out from each group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Norman 2004.
| Methods | Method of allocation: randomly assigned in blocks of two Power calculation: no Intention‐to‐treat analysis: no |
|
| Participants | Country: United States Number of participants: 48 (28 disclosure, 20 control) Age: 18 to 64 Sex: F |
|
| Interventions | Treatments: Two groups of women wrote about their positive (control group) and their negative (disclosure group) experience of pain and had their health status assessed at the end of two months | |
| Outcomes | McGill Pain Questionnaire | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised in blocks of 2' into sealed packets |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: 'interviewer was blind to group assignment' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 60 randomly assigned, 12 dropped out (four intervention, eight control group). Reasons obtained; > 10% attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Peters 1991.
| Methods | Methods of allocation: sealed envelope Outcome assessor blind to allocation Losses to follow‐up: six unsuitable for laparoscopy |
|
| Participants | Country: Netherlands Participants: 106 (57 treatment, 49 control) Age: 16 to 58 Sex: F Inclusion criteria: chronic pelvic pain > three months, no problem with Dutch, no mental retardation Exclusion criteria: no malignancy/disease requiring prompt gynaecological intervention, no history of psychiatric/psychotherapeutic treatment for abdominal pain, no elaborate medical analysis re abdominal pain in the past two years |
|
| Interventions | Treatment: integrated (guided by pain model by Loeser 1980, which comprises four components: nociception, pain sensation, pain suffering and pain behaviour; equal attention was devoted to possible organic, psychological, dietary and environmental causes of pain) Control: standard treatment (includes laparoscopy) Duration of treatment: six months Follow‐up: one year |
|
| Outcomes | General pain experience Disturbance of daily activities Associated symptoms McGill VAS score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Quote: 'randomisation was done by means of closed envelope procedure' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible, but unclear whether assessors blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Sator‐Katzenschlager 2005.
| Methods | Randomisation methods not specified | |
| Participants | CPP longer than six months | |
| Interventions | Gabapentin (n = 20), amytriptyline (n = 20) or combination (n = 16) Dose of amytriptyline was increased from an initial dose of 25 mg per day up to a maximum dose of 150 mg per day in 25‐mg increments each week until sufficient pain relief or the occurrence of side effects such as somnolence, dizziness, orthostatic hypotension, palpitations, dry mouth and weight gain. The dose of gabapentin was increased from 300 mg per day up to a maximum dose of 3600 mg per day in 300‐mg increments each week until sufficient pain relief or the occurrence of side effects |
|
| Outcomes | VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded as open‐label trial, unclear whether assessors blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven participants dropped out because of side effects; > 10% dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Seven participants excluded after randomisation, so not analysed by intention‐to‐treat, but same number of dropouts from gabapentin (three) or amytriptyline group (three) |
Soysal 2001.
| Methods | Method of allocation: computer‐generated numbered opaque sealed envelopes | |
| Participants | Country: Turkey 47 women with pelvic pain and venographically demonstrated pelvic congestion |
|
| Interventions | Goserelin 3.6 mg subcutaneous implant monthly for six months versus medroxyprogesterone acetate tablets 30 mg daily for six months | |
| Outcomes | Venography score; pelvic symptom and physical examination score (modified from Biberoglu and Behrman); Hospital Anxiety, Depression and Total scores; revised Sabbatsberg Sexual Rating Scale | |
| Notes | Note: NO dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding: some outcomes assessed double‐blind. Participants not blind owing to modes of drug administration |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At final assessment of periuterine venography, operators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion postrandomisation: zero Losses to follow‐up: zero |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Stones 2001.
| Methods | Sealed envelope system. Double‐blinded Power calculation: yes Number of participants randomly assigned: 39 |
|
| Participants | Country: England Number of participants: 39 Age: 25 to 35 Sex: F Inclusion criteria: pelvic pain > six months, laparoscopy identified no pathology Exclusion criteria: hysterectomised women |
|
| Interventions | Treatment: lofexidine 200 mcg twice daily, increasing to 600 mcg twice daily (first three weeks) Control: placebo tablets Duration: eight weeks Follow‐up: until the end of treatment |
|
| Outcomes | Visual analogue scale pain score. Participant's self rating of pain as worst, unchanged, somewhat relieved, considerably relieved or completely relieved | |
| Notes | Note high dropout rate in treatment group (nine of 19 completed eight weeks of treatment) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Women were randomised using a sealed envelope system to receive lofexidine hydrochloride or placebo'' |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, unclear whether assessors blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate, > 10% dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Walton 1992.
| Methods | Methods of allocation: not stated. Blinding: not stated Exclusion postrandomisation: zero |
|
| Participants | Country: UK Number of participants: 165 Age: not given Sex: F Inclusion criteria: pelvic pain for longer than six months No pathology on laparoscopy Exclusion criteria not given |
|
| Interventions | Treatment: medroxyprogesterone acetate 50 mg daily Control: placebo tablets Duration: four months Follow‐up: only until end of treatment |
|
| Outcomes | Visual analogue scale pain score Pain improvement rating: better/not better |
|
| Notes | Note very high dropout rate in MPA and placebo groups. Published report does not give SD for mean VAS, so data entered in Table are the numbers reporting 50% reduction in VAS at completion of the study. This allowed comparison with Farquhar 1989, as the drug, dose and duration of therapy are the same. Complete study report obtained by the review authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Two patients were allocated, at random, to the treatment group for each one given placebo" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate, reasons obtained; commonest is non‐compliance; losses to follow‐up: 64% of those taking active drug and 57% of those taking placebo completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Nil |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brown 2008 | Open‐label trial. Lack of placebo control group, lack of randomisation |
| Elcombe 1997 | Participants entered and evaluated at different time points in control and active groups, making comparison between groups difficult |
| Fenton 2008 | Cross‐over trial with no washout period |
| Onwude 2004 | Includes surgery |
| Pearce 1986 | Abstract only. No data available |
| Reginald 1987 | Not an RCT |
| Simsek 2007 | Randomised cross‐over trial without washout period |
Characteristics of studies awaiting assessment [ordered by study ID]
Horne 2012.
| Methods | Randomised controlled trial (pilot feasibility study) |
| Participants | n = 60 women recruited from NHS UK hospital |
| Interventions | Women randomly assigned to gabapentin versus placebo |
| Outcomes | Primary objective is to assess recruitment and retention rates. Secondary objectives are to determine the effectiveness and acceptability to participants of proposed methods of recruitment and randomisation, drug treatments and assessment tools and to perform a pretrial cost‐effectiveness assessment of treatment with gabapentin |
| Notes |
Differences between protocol and review
This review revises and updates the previous review entitled 'Interventions for women with chronic pelvic pain', which included only studies involving non‐surgical interventions. Surgical interventions will be covered in a separate review.
In the published protocol, we planned to consider the following six intervention groups.
Medical interventions.
Alternative/complementary therapies (such as magnetic field therapy, therapies encompassed in traditional Chinese medicine (acupuncture, herbal therapy, moxibustion), transcutaneous nerve stimulation and transcranial current stimulation).
Psychological therapies (such as ultrasonography as a reassurance tool, written or oral emotional disclosures, counselling, cognitive therapy and biofeedback).
Physical therapy (such as massaging and stretching).
Lifestyle (such as exercise and diet).
Multidisciplinary/integrated approach: interventions combining two or more of the approaches described in the categories above.
In the review, we considered the following three intervention groups.
Medical interventions.
Psychological treatments versus placebo/no treatment, or other interventions.
Complementary treatments versus no treatment/sham or placebo or other interventions.
Rationale for change
Interventions were regrouped using broader principles than those used in the protocol. Complementary and physical therapies were grouped together, as they aim to make a physical change to reduce pain, thereby achieving improved quality of life; psychological, educational and rehabilitative treatments were grouped together, as they aim to enable the person with CPP to manage the pain better, thereby achieving a better quality of life.
This regrouping is consistent with handling of physical, psychological and rehabilitative therapies in the Pain, Palliative and Supportive Care CRG (PaPaS) and with the rationales of the interventions. It allows some combination of studies, which otherwise would all be single studies, from which we could learn little. It is rare if not impossible for these interventions, which rely much more on the skills, training and orientation of the therapist than on a highly quality‐controlled substance, to resemble one another much in practice.
Contributions of authors
YCC performed the analysis and was the lead author in the writing of the review. GS contributed to the methodology and content of the review. AW contributed to the content and acted as a moderator.
Sources of support
Internal sources
University of Southampton, UK.
University of Auckland, New Zealand.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Abbott 2006 {published data only}
- Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women. American College of Obstetricians and Gynecologists 2006;108(4):915‐23. [DOI] [PubMed] [Google Scholar]
Brown 2002 {published data only}
- Brown CS, Ling FW, Wan JY, Pilla AA. Efficacy of static magnetic field therapy in chronic pelvic pain: a double‐blind pilot study. American Journal of Obstetrics and Gynaecology 2002;187:1581‐7. [DOI] [PubMed] [Google Scholar]
Engel 1998 {published data only}
- Engel CC Jr, Walker EA, Engel AL, Bullis J, Armstrong A. A randomized double‐blind crossover trial of sertraline in women with chronic pelvic pain. Journal of Psychosomatic Research 1998;44(2):203‐7. [DOI] [PubMed] [Google Scholar]
Farquhar 1989 {published data only}
- Farquhar CM, Rogers V, Franks S, Pearce S, Wadsworth J, Beard RW. A randomized controlled trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestion. British Journal of Obstetrics and Gynaecology 1989;96:1153‐62. [DOI] [PubMed] [Google Scholar]
Ghaly 1994 {published data only}
- Ghaly AFF. The psychological and physical benefits of pelvic ultrasonography in patients with chronic pelvic pain and negative laparoscopy. A random allocation trial. Journal of Obstetrics and Gynaecology 1994;14:269‐71. [Google Scholar]
Haugstad 2008 {published data only}
- Haugstad GK, Haugstad TS, Kirste UM, Leganger S, Wojniusz S, Klemmetsen I, et al. Continuing improvement of chronic pelvic pain in women after short‐term Mensendieck somatocognitive therapy: results of a 1‐year follow‐up study. American Journal Obstetrics and Gynaecology 2008;199:199:615.e1‐615.e8. [DOI] [PubMed] [Google Scholar]
Heyman 2006 {published data only}
- Heyman J, Ohrvik J, Leppert J. Distension of painful structures in the treatment for chronic pelvic pain in women. Acta Obstetricia et Gynaecologica Scandinavica 2006;85(5):599‐603. [DOI] [PubMed] [Google Scholar]
Norman 2004 {published data only}
- Norman SA, Lumley MA, Dooley JA, Diamond MP. For whom does it work? Moderators of the effects of written emotional disclosure in a randomised trial among women with chronic pelvic pain. Psychosomatic Medicine 2004;66:174‐83. [DOI] [PubMed] [Google Scholar]
Peters 1991 {published data only}
- Peters AAW, Dorst E, Jellis B, Zuuren E, Hermans J, Trimbos JB. A randomized clinical trial to compare two different approaches in women with chronic pelvic pain. Obstetrics and Gynecology 1991;77(5):740‐4. [PubMed] [Google Scholar]
Sator‐Katzenschlager 2005 {published data only}
- Sator‐katzenschlager SM, Scharbert G, Kress GH, Frickey N, Ellend A, Gleiss A, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien klin Wochenschr 2005;117(21‐22):761‐8. [DOI] [PubMed] [Google Scholar]
Soysal 2001 {published data only}
- Soysal ME, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Human Reproduction 2001;16(5):931‐9. [DOI] [PubMed] [Google Scholar]
Stones 2001 {published data only}
- Stones RW, Bradbury L, Anderson D. Randomized placebo controlled trial of lofexidine hydrochloride for chronic pelvic pain in women. Human Reproduction 2001;16(8):1719‐21. [DOI] [PubMed] [Google Scholar]
Walton 1992 {published data only}
- Walton SM, Batra HK. The use of medroxyprogesterone acetate 50mg in the treatment of painful pelvic conditions: preliminary results from a multicentre trial. Journal of Obstetrics and Gynaecology 1992;12(suppl 2):s50‐3. [Google Scholar]
References to studies excluded from this review
Brown 2008 {published data only}
- Brown CS, Franks AS, Wan J, Ling FW. Citalopram in the treatment of women with chronic pelvic pain. Journal of Reproductive Medicine 2008;53:191‐5. [PubMed] [Google Scholar]
Elcombe 1997 {published data only}
- Elcombe S, Gath D, Day A. The psychological effects of laparoscopy on women with chronic pelvic pain. Psychological Medicine 1997;27:1041‐50. [DOI] [PubMed] [Google Scholar]
Fenton 2008 {published data only}
- Fenton BW, Palmieri PA, Boggio P, Fanning J, Fregni F. A preliminary study of transcranial direct current stimulation for the treatment of refractory chronic pelvic pain. Brain Stimulation 2009;2:103‐7. [DOI] [PubMed] [Google Scholar]
Onwude 2004 {published data only}
- Onwude JL, Thornton JG, Morley S, Lilleyman J, Currie I, Lilford RJ. A randomised trial of photographic reinforcement during postoperative counselling after diagnostic laparoscopy for pelvic pain. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;112(1):89‐94. [DOI] [PubMed] [Google Scholar]
Pearce 1986 {published data only}
- Pearce S, Beard RW, Botwood V, Greenwood H, Knight C, Reginald PW. A randomised controlled trial of psychological methods in the management of chronic pelvic pain in women. Abstracts of the 24th British Congress of Obstetrics and Gynaecology. Royal College of Obstetrics and Gynaecology 1986;1:88. [Google Scholar]
Reginald 1987 {published data only}
- Reginald PW, Beard RW, Kooner JS, Mathias CJ, Samarage SU, Sutherland IA, et al. Intravenous dihydroergotamine to relieve pelvic congestion with pain in young women. Lancet 1987;ii:351‐3. [DOI] [PubMed] [Google Scholar]
Simsek 2007 {published data only}
- Simsek M, Burak F, Taskin O. Effects of micronized purified flavonoid fraction (Daflon) on pelvic pain in women with laparoscopically diagnosed pelvic congestion syndrome: a randomized crossover trial. Clinical & Experimental Obstetrics & Gynecology 2007;34(2):96‐8. [PubMed] [Google Scholar]
References to studies awaiting assessment
Horne 2012 {published data only}
- Horne AW, Critchley HOD, Doust A, Fehr D, Wilson J, Wu O, et al. GaPP: a pilot randomised controlled trial of the efficacy of action of gabapentin for the management of chronic pelvic pain in women: study protocol. BMJ Open 2012; Vol. 2, issue 3. [DOI] [PMC free article] [PubMed]
Additional references
Baker 1993
- Baker PK. Musculoskeletal origins of chronic pelvic pain. Diagnosis and treatment. Obstetrics and Gynecology Clinics of North America 1993;20:719‐42. [PubMed] [Google Scholar]
Cheong 2006
- Cheong Y, Stones RW. Chronic pelvic pain: aetiology and therapy. Best Practice & Research Clinical Obstetrics and Gynaecology 2006;20(5):695‐711. [DOI] [PubMed] [Google Scholar]
Cheong 2007
- Cheong Y, Stones RW. Doctors and the chronic pelvic pain patient. Minerva Ginecology 2007;59(6):613‐8. [PubMed] [Google Scholar]
Daniels 2009
- Daniels J, Gray R, Hills RK, Latthe P, Buckley L, Gupta J, et al. Laparoscopic uterosacral nerve ablation for alleviating chronic pelvic pain: a randomized controlled trial. Journal of the American Medical Association 2009;302(9):955‐61. [DOI] [PubMed] [Google Scholar]
Daniels 2010
- Daniels JP, Khan KS. Chronic pelvic pain in women. BMJ 2010;341:4834. [DOI] [PubMed] [Google Scholar]
Duleba 1996
- Duleba A, Keltz M, Olive D. Evaluation and management of chronic pelvic pain. Journal of the American Association of Gynecologic Laparoscopists 1996;3:205‐27. [DOI] [PubMed] [Google Scholar]
Dworkin 2005
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT guidelines. Pain 2005;113:9‐19. [DOI] [PubMed] [Google Scholar]
Dworkin 2010
- Dworkin RH, Turk DC, Peirce‐Sandner S, Baron R, Bellamy N, Burke LB, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149:177‐93. [DOI] [PubMed] [Google Scholar]
Garrat 1995
- Garrat KF, Torgesson DJ, Wyness J, et al. Measuring sexual functioning in premenopausal women. British Journal of Obstetrics & Gynaecology 1995;102:311‐6. [DOI] [PubMed] [Google Scholar]
Gelbaya 2001
- Gelbaya TA, El‐Halwagy HE. Focus on primary care: chronic pelvic pain in women. Obstetrical & Gynecological Survey Dec 2001;56(12):757‐64. [DOI] [PubMed] [Google Scholar]
Gupta 2006
- Gupta VK. Botulinum toxin—a treatment for migraine? A systematic review. Pain Medicine 2006;7:386‐94. [DOI] [PubMed] [Google Scholar]
Haugstad 2007
- Haugstad GK, Haugstad TS, Kirste U, Leganger S, Hammel B, Klemmetsen I, et al. Reliability and validity of a standardized Mensendieck physiotherapy test (SMT). Physiotherapy Theory & Practice 2006;22(4):189‐205. [DOI] [PubMed] [Google Scholar]
Hawk 2002
- Hawk C, Long C, Reiter R, Davis C, Cambron J, Evans R. Issues in planning a placebo‐controlled trial of manual methods: results of a pilot study. Journal of Alternative and Complementary Medicine 2002;8(1):21‐32. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. [Google Scholar]
Horne 2012
- Horne AW, Skubisz MM, Doust A, Duncan WC, Wallace E, Critchley HO, et al. Phase II single arm open label multicentre clinical trial to evaluate the efficacy and side effects of a combination of gefitinib and methotrexate to treat tubal ectopic pregnancies (GEM II): study protocol. BMJ Open 2013;3(7):e002902. [DOI: 10.1136/bmjopen-2013-002902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 1993
- Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstetrical & Gynecological Survey 1993;48:357‐87. [DOI] [PubMed] [Google Scholar]
Langford 2007
- Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilised treatment for chronic pelvic pain. Neurourology & Urodynamics 2007;26:59‐62. [DOI] [PubMed] [Google Scholar]
Latthe 2006
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006;6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loeser 1980
- Loeser JD. The definition of pain. Medicine 1980;7:3‐9. [Google Scholar]
Mathias 1996
- Mathias S, Kuppermann M, Liberman R, Lipschutz R, Steege J. Chronic pelvic pain: prevalence, health‐related quality of life, and economic correlates. Obstetrics & Gynecology 1996;87(3):321‐7. [DOI] [PubMed] [Google Scholar]
Prendergast 2003
- Prendergast SA, Weiss JM. Screening for musculoskeletal causes of pelvic pain. Clinical Obstetrics and Gynecology 2003;46:773‐82. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Thompson 2005
- Thompson AJ, Jarvis SK, Lenart M, Abbott JA, Vancaillie TG. The use of botulinum toxin type A (BOTOX) as treatment for intractable chronic pelvic pain associated with spasm of the levator ani muscles. BJOG 2005;112:247‐9. [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams ACDC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007407.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
Zondervan 1999
- Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. Prevalence and incidence of chronic pelvic pain in primary care: evidence from a national general practice database.. British Journal of Obstetrics and Gynaecology 1999;106:1149‐55. [DOI] [PubMed] [Google Scholar]


