Abstract
Background
Since the complication of diabetes mellitus (DM) is a risk for adverse cardiovascular outcomes in patients with coronary artery disease (CAD), appropriate risk estimation is needed in diabetic patients following percutaneous coronary intervention (PCI). However, there is no useful biomarker to predict outcomes in this population. Although stromal cell derived factor-1α (SDF-1α), a circulating chemokine, was shown to have cardioprotective roles, the prognostic impact of SDF-1α in diabetic patients with CAD is yet to be fully elucidated. Moreover, roles of SDF-1α isoforms in outcome prediction remain unclear. Therefore, this study aimed to assess the prognostic implication of three forms of SDF-1α including total, active, and inactive forms of SDF-1α in patients with DM and after PCI.
Methods
This single-center retrospective analysis involved consecutive patients with diabetes who underwent PCI for the first time between 2008 and 2018 (n = 849). Primary and secondary outcome measures were all-cause death and the composite of cardiovascular death, non-fatal myocardial infarction, and ischemic stroke (3P-MACE), respectively. For determining plasma levels of SDF-1α, we measured not only total, but also the active type of SDF-1α by ELISA. Inactive isoform of the SDF-1α was calculated by subtracting the active isoform from total SDF-1α.
Results
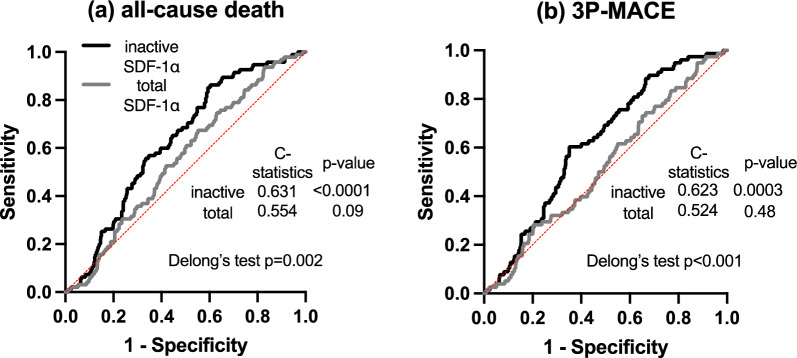
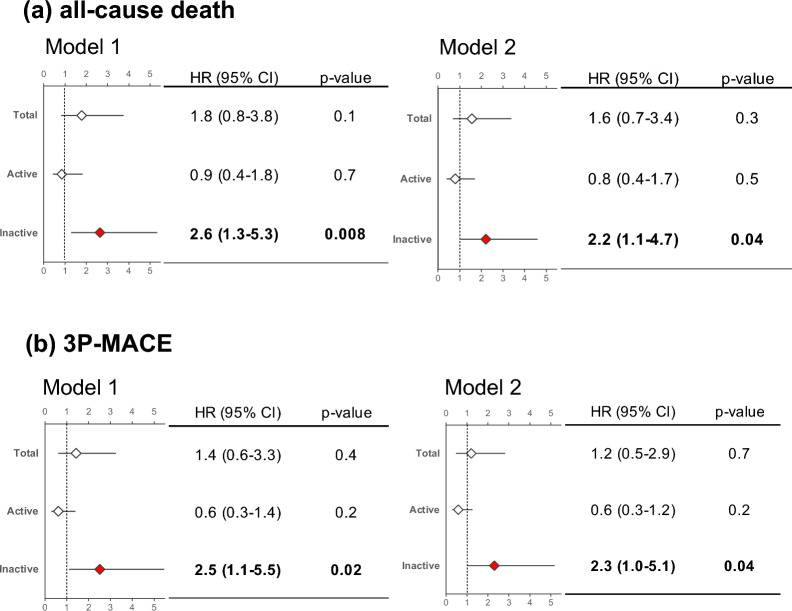
Unadjusted Kaplan–Meier analyses revealed increased risk of both all-cause death and 3P-MACE in patients with elevated levels of inactive SDF-1α. However, plasma levels of total and active SDF-1α were not associated with cumulative incidences of outcome measures. Multivariate Cox hazard analyses repeatedly indicated the 1 higher log-transformed inactive SDF-1α was significantly associated with increased risk of all-cause death (hazard ratio (HR): 2.64, 95% confidence interval (CI): 1.28–5.34, p = 0.008) and 3P-MACE (HR: 2.51, 95% CI: 1.12–5.46, p = 0.02). Moreover, the predictive performance of inactive SDF-1α was higher than that of total SDF-1α (C-statistics of inactive and total SDF-1α for all-cause death: 0.631 vs 0.554, for 3P-MACE: 0.623 vs 0.524, respectively).
Conclusion
The study results indicate that elevated levels of plasma inactive SDF-1α might be a useful indicator of poor long-term outcomes in diabetic patients following PCI.
Trial registration: This study describes a retrospective analysis of a prospective registry database of patients who underwent PCI at Juntendo University Hospital, Tokyo, Japan (Juntendo Physicians’ Alliance for Clinical Trials, J-PACT), which is publicly registered (University Medical Information Network Japan—Clinical Trials Registry, UMIN-CTR 000035587).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02197-z.
Keywords: Stromal cell derived factor-1α, Long-term outcome, PCI, Diabetes mellitus
Background
Type 2 diabetes mellitus (T2DM) is a global health problem due to its high worldwide prevalence, high economic cost of treatment, and associated atherosclerotic cardiovascular (CV) mortality and morbidity [1–3]. The clinical outcome of coronary artery disease (CAD) concomitant with T2DM following percutaneous coronary intervention (PCI) has been reported to be twofold to fourfold worse than those without T2DM [4–6]. Therefore, precise risk stratification for predicting long-term outcomes following PCI is particularly important in those who are complicated by diabetes. Previously, several biomarkers, such as lipoprotein (a), high-sensitivity C-reactive protein and 1,5-anhydroglucitol have been raised as candidates of outcome predictors following PCI [7–9]. However, very limited data are available regarding the usefulness of any biomarkers as prognostic indicators in diabetic patients with CAD.
The chemokine stromal cell derived factor-1α (SDF-1α/CXCL12) is a CXC chemokine with chemotactic effects on CXCR4/CXCR7 (CXCR: CXC chemokine receptor) expressing progenitor cells [10] and is known to play beneficial roles in cardiomyocyte repair and ventricular remodelling [11]. The prognostic value of circulating SDF-1α levels has been previously evaluated in several studies in patients with CAD [12–14], most of which reported that an elevated level of total SDF-1α was significantly associated with increased risk of poor outcomes in patients with CAD. Moreover, previous studies have suggested that SDF-1α play a key role in the pathogenesis of T2DM [15, 16]. However, no study specifically evaluated the prognostic implication of SDF-1α in diabetic patients with CAD. Moreover, these previous studies did not consider cleavage of SDF-1α by exopeptidases [17, 18] and they did not clarify any potential roles of isoforms, active or inactive isoforms, of SDF-1α in this patient population. Therefore, in the present study, we evaluated and compared the prognostic implications of three forms of SDF-1α, including total (conventionally measured plasma level of SDF-1α), active, and inactive forms of SDF-1α, in patients with T2DM following PCI.
Methods
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Juntendo University (IRB-ID: 20-287). The single-center prospective all-comer registry database of patients who underwent any type of PCI at Juntendo University Hospital, Tokyo, Japan (Juntendo Physicians’ Alliance for Clinical Trial, J-PACT) since 1984 is publicly registered (University Medical Information Network Japan—Clinical Trials Registry, UMIN-CTR 000035587). Written informed consent was obtained from all participants for the J-PACT registry which had no exclusion criteria as far as written informed consent was achieved.
Participants, endpoints, follow-up, and follow-up duration
This study is a retrospective analysis of a portion of the J-PACT registry database involving consecutive 849 diabetic patients out of 4039 patients who underwent any type of PCI for CAD at Juntendo University Hospital between December 2008 and January 2018. Diabetes was defined as glycated hemoglobin (HbA1c-NG) ≥ 6.5% or if the patient was taking any diabetic medications at PCI procedure. Participants were divided into two groups according to the median of three forms of plasma SDF-1α levels (total SDF-1α: 2270 pg/mL, active SDF-1α: 686 pg/mL, inactive SDF-1α: 1537 pg/mL) at PCI procedure (high total, active, inactive SDF-1α group n = 425, low total, active, inactive SDF-1α group n = 424) and the incidence and risk of subsequent endpoints following PCI were assessed (Additional file 1: Fig. S1).
The primary and secondary outcome measures were all-cause death and the 3-point major adverse cardiovascular events (3P-MACE; a composite of CV death, non-fatal myocardial infarction and ischemic stroke), respectively. CV death was defined as a composite of the following types of death; sudden death in which non-cardiac death could not be excluded, and death due to myocardial infarction, heart failure, cardiogenic shock, a cerebrovascular event, or aortic diseases. In this prospective PCI registry database, patient follow-up was based on electronic chart review, as far as they were followed at Juntendo University Hospital. A prognosis survey questionnaire was mailed out every 5 years if they were transferred to other institutions. In cases in which no response was achieved, follow-up was terminated at the latest time point, at which their survival at our institution was confirmed, such as the last visit date to an outpatient clinic or the last day of any hospitalization. The median and range of the follow-up period were 4.1 and 0–10.4 years, respectively.
Blood sampling and the measurement of plasma levels of total and active form of SDF-1α
Blood samples were obtained immediately prior to PCI through an inserted blood access (5 to 8 French in diameter) and stored at − 80 ℃ until measurement of total and active SDF-1α concentrations (n = 849). Plasma levels of both total and active SDF-1α were determined using an enzyme-linked immunosorbent assay (ELISA) according to a previous protocol [19]. We calculated the levels of inactive SDF-1α by subtracting the active isoform level from the total SDF-1α level.
Statistical analysis
Quantitative variables are presented as the mean ± standard deviation or median with interquartile range (IQR) in accordance with the results of the Shapiro–Wilk normality test. Categorical variables are presented as the numbers and percentages. Quantitative data between groups were compared using Student’s t-test or the Wilcoxon rank sum test. Unadjusted Kaplan–Meier analysis evaluated the time to the cumulative incidence of endpoints followed by the log-rank test for comparisons. Univariate and multivariate analyses using the following models of Cox proportional hazards regression analyses (Models 1–3) calculated the hazard ratios (HRs) of total, active and inactive SDF-1α for all-cause death and 3P-MACE. Variables used in Models 1–3 were selected based on background demographics that were different between groups and associated with endpoints in univariate analyses. Moreover, the ratio of the number of the variables included in the occurrence of events for each model was set as approximately 1:10 [20, 21]. Model 1 included age (a continuous variable) and sex. Model 2 included age, sex, body mass index (BMI) (> 25), acute coronary syndrome (ACS), and chronic kidney disease (CKD) (≥ stage 3). Model 3 included age, sex, statins, multivessel disease, insulin, high-sensitivity C-reactive protein (hs-CRP) (a continuous variable), hemoglobin (a continuous variable), and B-type natriuretic peptide (BNP) (a continuous variable). Receiver operating characteristic (ROC) curves were obtained and the areas under the curve (C-statistic) were measured for all-cause death and 3P-MACE. We compared the two C-statistics. Furthermore, the net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) were calculated to examine the additive effects of total, active and inactive SDF-1α levels on the predictive value of the baseline model. Statistical significance was defined as a p-value < 0.05 and analyses were performed using statistical software (JMP Pro 16.0; SAS Institute Inc., Cary, NC, USA and IBM SPSS Statistics, Version 26.0. Armonk, NY, USA).
Results
Superiority of inactive SDF-1α as an accurate prognostic indicator compared to total and active SDF-1α
The C-statistics of total and inactive SDF-1α for both endpoints were determined by ROC analysis. We found that the C-statistics of inactive SDF-1α for both endpoints were significantly higher than those of total SDF-1α (C-statistics of inactive and total SDF-1α for all-cause death: 0.631 vs 0.554, p = 0.002, for 3P-MACE: 0.623 vs 0.524, p < 0.001, respectively) (Fig. 1). Based on these findings, to assess the usefulness of each type of SDF-1α as a prognostic indicator, NRI and IDI were calculated by using 2 models in multivariate Cox proportional hazard analysis for all-cause death and 3P-MACE. Consequently, NRI and IDI for all-cause death were significantly improved by the addition of inactive SDF-1α to Model 1 and Model 2, while adding total and active SDF-1α did not improve them. Similar findings were obtained in the analyses for 3P-MACE (Table 1).
Fig. 1.
Predictive performance of inactive and total SDF-1α for all-cause death and 3P-MACE. Receiver operating characteristic (ROC) curves of inactive and total SDF-1α with reference line for all-cause death (a) and 3P-MACE (b)
Table 1.
Effects on the predictive performance by adding isoforms of SDF-1α on the baseline models
| NRI | p-value | IDI | p-value | |
|---|---|---|---|---|
| All-cause death | ||||
| Model 1 | ||||
| + Total | 0.11 | 0.33 | 0.0009 | 0.58 |
| + Active | 0.15 | 0.30 | 0.003 | 0.28 |
| + Inactive | 0.41 | 0.0002 | 0.01 | 0.007 |
| Model 2 | ||||
| + Total | 0.15 | 0.17 | 0.0002 | 0.85 |
| + Active | 0.12 | 0.40 | 0.004 | 0.26 |
| + Inactive | 0.29 | 0.009 | 0.007 | 0.03 |
| 3P-MACE | ||||
| Model 1 | ||||
| + Total | 0.01 | 0.93 | 0.0002 | 0.72 |
| + Active | 0.18 | 0.27 | 0.007 | 0.16 |
| + Inactive | 0.45 | 0.0001 | 0.008 | 0.01 |
| Model 2 | ||||
| + Total | − 0.08 | 1.48 | 0.0000 | 0.92 |
| + Active | 0.16 | 0.33 | 0.01 | 0.12 |
| + Inactive | 0.38 | 0.001 | 0.007 | 0.01 |
Model 1 included age and sex
Model 2 included age, sex, body mass index > 25, acute coronary syndrome and chronic kidney disease
Baseline characteristics in 2 groups divided by the median of plasma inactive SDF-1α levels at PCI procedure
To clarify the similarities and differences in background demographics between patients with and without high inactive SDF-1α, the background demographics, comorbidities, and medications were compared. As shown in Table 2, most of the background demographics, including age, sex, left ventricular ejection fraction (LVEF), and number of diseased vessels were similar in both groups. Disease duration of diabetes was significantly longer in the high inactive SDF-1α group while HbA1c levels were similar in both groups. Hemoglobin levels and BMI were significantly lower in the high inactive SDF-1α group. Furthermore, in high inactive SDF-1α group, the proportions of patients with CKD and chronic hemodialysis were substantially higher, consistent with those having elevated serum creatinine and decreased estimated glomerular filtration rate (eGFR), compared to low inactive SDF-1α group. The proportion of patients with dyslipidemia and statin use was lower in the high inactive SDF-1α group. Additionally, the ratio of dipeptidyl peptidase-4 (DPP4) inhibitor use was significantly lower in the high inactive SDF-1α group, while there were no significant differences in other diabetic medications, including insulin.
Table 2.
Baseline characteristics of study patients
| Overall | High inactive SDF-1α | Low inactive SDF-1α | p-value | |
|---|---|---|---|---|
| n = 849 | n = 425 | n = 424 | ||
| Baseline characteristics | ||||
| Age, years | 67.8 ± 10.1 | 67.9 ± 10.7 | 67.7 ± 9.4 | 0.86 |
| Male, n (%) | 701 (82.6) | 349 (82.1) | 352 (83.0) | 0.73 |
| Body mass index, kg/m2 | 24.8 ± 3.8 | 24.4 ± 3.8 | 25.1 ± 3.7 | 0.01 |
| Hypertension, n (%) | 665 (78.3) | 342 (80.5) | 323 (76.2) | 0.13 |
| Dyslipidemia, n (%) | 665 (78.3) | 316 (74.4) | 349 (82.3) | 0.005 |
| Current smoker, n (%) | 206 (24.3) | 97 (22.8) | 109 (25.7) | 0.33 |
| Chronic kidney disease, n (%) | 254 (29.9) | 151 (35.5) | 103 (24.3) | 0.0003 |
| Hemodialysis, n (%) | 70 (8.2) | 54 (12.7) | 16 (3.8) | < 0.0001 |
| Acute coronary syndrome, n (%) | 193 (22.7) | 92 (21.7) | 101 (23.8) | 0.45 |
| LVEF, % | 60.6 ± 12.7 | 59.6 ± 13.7 | 61.7 ± 11.5 | 0.06 |
| Diabetes duration, years | 14 (6, 21) | 15 (7, 24) | 13 (5, 20) | 0.04 |
| Number of diseased vessels | 2.0 ± 0.8 | 2.0 ± 0.8 | 1.9 ± 0.8 | 0.46 |
| Vessel location | ||||
| RCA, n (%) | 253 (29.8) | 126 (29.7) | 127 (30.0) | 0.92 |
| LAD, n (%) | 433 (51.0) | 215 (50.6) | 218 (51.4) | 0.81 |
| LCX, n (%) | 165 (19.4) | 86 (20.2) | 79 (18.6) | 0.56 |
| Laboratory findings | ||||
| TC, mg/dL | 166.4 ± 38.7 | 163.3 ± 40.7 | 169.6 ± 36.3 | 0.02 |
| LDL-C, mg/dL (Friedewald) | 95.8 ± 30.2 | 94.6 ± 31.7 | 97.0 ± 28.5 | 0.25 |
| HDL-C, mg/dL | 43.2 ± 13.2 | 42.1 ± 11.9 | 44.2 ± 14.3 | 0.02 |
| TG, mg/dL | 120 (88, 163) | 113 (85, 158) | 123 (91, 169) | 0.02 |
| FBG, mg/dL | 133.4 ± 53.1 | 132.4 ± 55.3 | 134.5 ± 50.7 | 0.56 |
| HbA1c, % | 7.2 ± 1.1 | 7.2 ± 1.1 | 7.2 ± 1.0 | 0.57 |
| hs-CRP, mg/L | 0.09 (0.03, 0.28) | 0.09 (0.03, 0.33) | 0.08 (0.04, 0.22) | 0.49 |
| Hemoglobin, g/dL | 13.3 ± 1.9 | 13.1 ± 2.0 | 13.5 ± 1.8 | 0.009 |
| Creatinine, mg/dL | 0.80 (0.67, 0.98) | 0.81 (0.68, 1.06) | 0.79 (0.67, 0.93) | 0.01 |
| eGFR, ml/min/1.73m2 | 69.4 ± 28.2 | 66.4 ± 32.1 | 72.4 ± 23.4 | 0.002 |
| BNP, pg/mL | 48.7 (21.3, 126.5) | 63.3 (25.2, 173.3) | 40.3 (18.7, 90.5) | < 0.0001 |
| Medication | ||||
| Sulfonylurea, n (%) | 228 (26.9) | 123 (28.9) | 105 (24.8) | 0.18 |
| Metformin, n (%) | 161 (19.0) | 79 (18.6) | 82 (19.4) | 0.77 |
| Thiazolidinedione, n (%) | 94 (11.1) | 40 (9.4) | 54 (12.8) | 0.12 |
| SGLT-2 inhibitor, n (%) | 17 (2.0) | 9 (2.1) | 8 (1.9) | 0.81 |
| DPP4 inhibitor, n (%) | 310 (36.6) | 137 (32.2) | 173 (40.9) | 0.009 |
| GLP-1 receptor agonist, n (%) | 6 (0.7) | 2 (0.5) | 4 (1.0) | 0.41 |
| α-Glucosidase inhibitor, n (%) | 182 (21.5) | 92 (21.7) | 90 (21.3) | 0.90 |
| Glinide, n (%) | 71 (8.4) | 31 (7.3) | 40 (9.5) | 0.26 |
| Insulin, n (%) | 213 (25.1) | 117 (27.5) | 96 (22.7) | 0.10 |
| ACE-I/ ARB, n (%) | 468 (55.1) | 232 (54.6) | 236 (55.7) | 0.75 |
| β-Blocker, n (%) | 391 (46.1) | 191 (44.9) | 200 (47.2) | 0.51 |
| Statin, n (%) | 581 (68.6) | 272 (64.0) | 309 (73.2) | 0.004 |
| Ezetimibe, n (%) | 50 (5.9) | 22 (5.2) | 28 (6.6) | 0.37 |
| Fibrate, n (%) | 34 (4.0) | 17 (4.0) | 17 (4.0) | 0.99 |
LVEF left ventricular ejection fraction, RCA right coronary artery, LAD left anterior descending artery, LCX left circumflex artery, TC total cholesterol, LDL-C low-density lipoprotein, HDL-C high-density lipoprotein, TG triglycerides, FBG fasting blood glucose, HbA1c glycated hemoglobin, hs-CRP high-sensitivity C-reactive protein, eGFR estimated glomerular filtration rate, BNP B-type natriuretic peptide, SGLT-2 sodium-glucose co-transporter-2, DPP4 Dipeptidyl peptidase-4, GLP-1 glucagon-like peptide-1, ACE-I angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blockers
Prognostic impact of inactive SDF-1α in diabetic patients after PCI
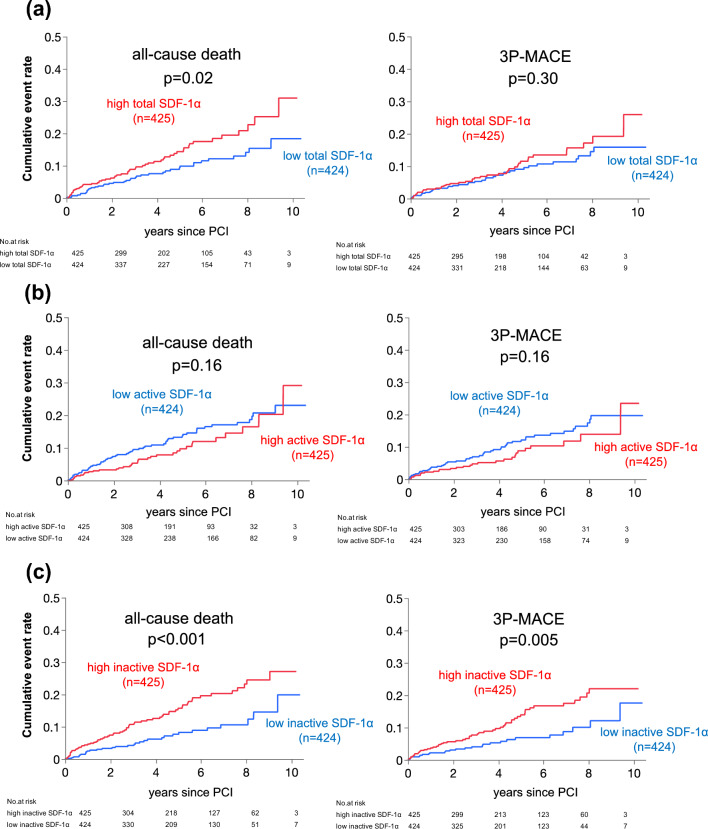
The median and range of the follow-up period were 4.1 and 0–10.4 years, respectively. During the follow-up period following PCI, the total numbers of all-cause death and 3P-MACE were 95 (11.2%) and 78 (9.2%) out of 849 participants, respectively. Unadjusted Kaplan–Meier analysis followed by the log-rank comparison test demonstrated that the cumulative incidences of both all-cause death and 3P-MACE were significantly higher in the high inactive SDF-1α group compared to the low inactive SDF-1α group (Fig. 2c). Moreover, a similar result was observed in the cumulative incidence of all-cause death between the high and low total SDF-1α groups, however, there was no difference in that of 3P-MACE (Fig. 2a). In contrast, the cumulative incidences of both endpoints were slightly lower in the high active SDF-1α group compared to the low active SDF-1α group (Fig. 2b).
Fig. 2.
Cumulative incidences of adverse events in groups divided by median levels of total, active and inactive plasma SDF-1α. a Cumulative incidences of all-cause death and 3P-MACE in patients with high and low plasma total SDF-1α levels. b Cumulative incidences of all-cause death and 3P-MACE in patients with high and low plasma active SDF-1α levels. c Cumulative incidences of all-cause death and 3P-MACE in patients with high and low plasma inactive SDF-1α levels
Univariate Cox proportional hazard analyses for calculating hazard ratios for subsequent all-cause death and 3P-MACE following PCI revealed that an elevated level of plasma inactive SDF-1α at procedure was significantly associated with increased risk of both endpoints (Additional file 1: Tables S1 and S2). Multivariate Cox proportional analyses using 3 models in which covariates were selected based on background demographics and univariate analyses (Additional file 1: Tables S1 and S2) indicated that elevated levels of plasma inactive SDF-1α were significantly associated with increased risk of all-cause death and 3P-MACE (Fig. 3 and Additional file 1: Table S3). However, elevated levels of plasma total SDF-1α were not significantly associated with increased risk of both endpoints. Consistent with the Kaplan–Meier analysis, elevated levels of plasma active SDF-1α tended to be associated with decreased risk of both endpoints (Fig. 3 and Additional file 1: Table S3).
Fig. 3.
Prognostic impact of each isoform of SDF-1α in 2 Cox proportional hazard models. Hazard ratios were calculated by multivariate Cox proportional hazard analyses by using 2 different models. Model 1: age and sex, Model 2: included age, sex, body mass index (BMI) (> 25), acute coronary syndrome (ACS), and chronic kidney disease (CKD) (≥ stage 3) HR hazard ratio, 95%CI confidence interval
Discussion
This single-center observational study of a prospective PCI registry database evaluated and compared the prognostic implications of three forms of SDF-1α (total, active, and inactive) in high CV risk patients for the first time. The cumulative incidences and risk for all-cause death and 3P-MACE were assessed in two patient groups divided according to the median of the plasma levels of each isoform of SDF-1α at PCI procedure. The primary findings are as follows. (1) Predictive performance of inactive SDF-1α for all-cause death and 3P-MACE was higher compared to those of total SDF-1α. Additionally, the predictability of all-cause death and 3P-MACE was significantly improved when inactive SDF-1α was added to the Cox proportional hazard models compared to total and active SDF-1α. (2) Unadjusted Kaplan–Meier analyses showed the higher cumulative incidences of both endpoints in patients with high inactive SDF-1α. (3) Multivariate Cox proportional hazard analyses using 3 models continuously showed that an elevated plasma inactive SDF-1α level was significantly associated with increased risk of all-cause death and 3P-MACE.
SDF-1α, also referred to as C-X-C motif chemokine 12 (CXCL12), is a small peptide consisting of 89 amino acids, which is originally produced at the platelet membrane, and plays a prominent role in hematopoiesis, angiogenesis, immunogenesis, stem cell mobilization, and tissue repair through its receptors CXCR4 and CXCR7 [10, 22]. Previous studies have reported that SDF-1α and its receptor, CXCR4, or SDF-1/CXCR4 axis was cardioprotective after myocardial infarction, including attenuation of adverse ventricular remodelling and preservation of ventricular function [23, 24]. SDF-1 has emerged as one of the key regulators in cardiac tissue repair [25], which have been shown to induce mobilization of bone marrow-derived and cardiac premature cells to the injured cardiac tissues, promote angiogenesis, inhibit cardiac myocyte cell apoptosis, and finally lead to the inhibition of cardiac remodelling [26, 27]. Clinically, a placebo-controlled trial evaluated the safety and efficacy of plasmid-induced SDF-1 gene overexpression, which was delivered via endomyocardial injection into patients with ischemic heart failure. The study described the potential for attenuating LV remodelling and improving LVEF in high-risk ischemic cardiomyopathy [28]. Furthermore, several experimental studies have indicated that SDF-1α/CXCR4 axis has both beneficial and detrimental effects on glycometabolism. It has been reported that the SDF-1α/CXCR4 axis improve glycemic control by inhibiting dedifferentiation of islet β cells, but exacerbate it by reducing insulin effectiveness on adipocytes [15, 16]. Therefore, the overall association between the progression of diabetes and circulating total SDF-1α level remains inconclusive.
Myocardial ischemia results in an elevated plasma level of SDF-1α, which was reported to associate with increased risk of heart failure and all-cause mortality in patients with CAD [29]. When cardiomyocytes are under ischemic conditions, hypoxia inducible factor 1α (HIF-1α) activates the SDF-1α/CXCR4 pathway [30, 31]. While the functional roles of the CXCR7 pathway have yet to be clarified, the SDF-1α/CXCR4 pathway has been intensively studied and found to exert cardiovascular protective effects by Erk and Akt activation [32]. Moreover, SDF-1α/CXCR4 signalling promotes angiogenesis in ischemic tissues by enhancing local angiogenic mechanisms and by the recruitment or mobilization of endothelial progenitor cells (EPCs) [17, 23, 33]. Additionally, accumulating evidence has suggested that SDF-1α plays a role in ischemic preconditioning of the cardiac tissue, which is known to be associated with the reduction of infarct size in patients with myocardial infarction [34] and with better clinical outcomes in patients following PCI and cardiac surgery [19, 35–37].
SDF-1α is biologically active only before the cleavage (active SDF1α) by various exopeptidases, such as DPP4 and the family of matrix metalloproteinases (MMP) [17, 18]. After shedding amino acids from the active form, SDF-1α (inactive SDF1α) was suggested not only to lose its chemotactic functions, but also to be a potential antagonist of CXCR4 pathways [18]. The nonfunctional or even potential antagonistic properties of inactive SDF-1α may interfere with or attenuate the beneficial cardioprotective properties of SDF-1α.
Previous studies assessing circulating SDF-1α levels as a prognostic indicator in CAD patients have shown that its elevation was associated with increased risk of adverse cardiovascular events, including myocardial infarction and CV mortality in chronic coronary syndrome patients and ACS patients [12–14]. Conversely, other studies have reported a reduction in circulating SDF-1α in patients with myocardial infarction [38], and with stable and unstable angina compared to healthy controls [39]. As all these studies utilized ELISA antibodies recognizing SDF-1α without considering cleavage, the conjugated plasma levels of both active plus inactive SDF-1α (total SDF-1α) were measured, which might be responsible for the inconsistency regarding the change in ischemic heart disease and the prognostic impact of plasma SDF-1α. Therefore, the separate measurement of these two isoforms of SDF-1α might be very helpful for the clarification of its biological roles. Moreover, it allows us to assess the usefulness of SDF-1α as a biomarker to predict outcomes in patients with ischemic heart disease. In the present study, we found that elevation of inactive SDF-1α which was cleaved from the active form was more closely associated with increased risk of all-cause mortality and 3P-MACE compared to the active form and total SDF-1α. Based on these novel findings in this study, we can speculate that disturbance of the beneficial effects of the SDF-1α/CXCR4 pathway by the inactivated form of SDF-1α itself may lead to adverse outcomes in diabetic patients with CAD. Accordingly, an elevated level of inactive SDF-1α may be a more accurate prognostic indicator compared to the active form or total SDF-1α, in diabetic patients following PCI.
Limitations
There are several limitations to this study. First, it was a single center, retrospective observational study involving almost solely Japanese patients and therefore did not compare findings amongst different ethnicities. Further large-scale clinical studies are warranted to confirm our results. Second, the possibility that unknown confounders might have had an influence on the results could not be completely eliminated, even though multivariate analyses adjusted for baseline characteristics and known prognostic factors. Third, plasma levels of inactive SDF-1α were not measured directly but rather were estimated by the differences between total SDF-1α levels and active SDF-1α levels. Fourth, approximately 30% of participants were prescribed DPP4 inhibitors. As DPP4 is one of the exopeptidases which cleave SDF-1α, the potential effects of taking DPP4 inhibitors on the prognostic implication of SDF-1α may need to be considered, although the findings of our sub-analysis with DPP4 inhibitors (36% of study participants) were very similar to those for total participants and without DPP4 inhibitors (data not shown). Fifth, previous studies suggested possible associations between the SDF-1α/CXCR4 axis and development of various clinical conditions such as immunological diseases and cancers [40, 41]. However, as this study did not assess the immunological diseases and cancers, their potential influence on the patient outcomes cannot be completely excluded. Despite these major limitations, this study is strengthened by providing novel evidence regarding the clinical utility of inactive SDF-1α.
Conclusions
The results of this retrospective observational study indicate that elevated plasma levels of inactive SDF-1α were significantly associated with poor long-term outcomes in diabetic patients following PCI. Moreover, the predictive performance of inactive SDF-1α was more accurate than that of conventionally used plasma total SDF-1α. Preprocedural plasma inactive SDF-1α levels may be a useful prognostic indicator in diabetic patients following PCI.
Supplementary Information
Additional file 1: Table S1. Univariate Cox proportional hazard analyses for predictors of all-cause death following PCI. Table S2. Univariate Cox proportional hazard analyses for predictors of 3P-MACE following PCI. Table S3. Multivariate cox proportional hazard analyses using 3 models to assess the hazard ratios of total/active/inactive SDF-1α for all-cause death and 3P-MACE. Figure S1. Flow diagram of the study participants.
Acknowledgements
The authors wish to thank Ms. Yumi Nozawa and Ms. Emiko Nakamura for their significant contributions to this study. The authors also appreciate Dr. Sean M. Davidson for kindly providing active SDF-1α specific ELISA antibody.
Abbreviations
- DM
Diabetes mellitus
- CAD
Coronary artery disease
- PCI
Percutaneous coronary intervention
- SDF-1α
Stromal cell-derived factor-1α
- 3P-MACE
3-Point major adverse cardiovascular events
- ELISA
Enzyme-linked immunosorbent assay
- HRs
Hazard ratios
- CI
Confidence interval
- T2DM
Type 2 diabetes mellitus
- CV
Cardiovascular
- CXCR
CXC chemokine receptor
- IRB
Institutional Review Board
- HbA1c
Glycated hemoglobin
- IQR
Interquartile range
- BMI
Body mass index
- ACS
Acute coronary syndrome
- CKD
Chronic kidney disease
- NRI
Net reclassification improvement
- IDI
Integrated discrimination improvement
- LVEF
Left ventricular ejection fraction
- eGFR
Estimated glomerular filtration rate
- CXCL12
C-X-C motif chemokine
- HIF1α
Hypoxia inducible factor 1α
- EPCs
Endothelial progenitor cells
- MMP
Matrix metalloproteinases
Author contributions
Y.C.: Data Curation, Measurement of plasma biomarkers, Formal analysis, Writing-Original Draft. H.I.: Formal analysis, Writing-Review and Editing, Conceptualization, Methodology and Supervision. K.M.: Measurement of plasma biomarkers, Formal analysis, Supervision. R.N.: Formal analysis, Data Curation, Project administration. T.K., S.M., H.Y.: Data Curation. T.F., S.D., H.E., H.W., M.O., T.D., T.K.: Data Curation, Project administration. K.I., S.O., K.M., T.M.: Writing-Review and Editing, Supervision. All authors read and approved the final manuscript.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (18K08049).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Juntendo University (IRB-ID: 20-287), and all patients gave their written informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
One of authors, Katsutoshi Miyosawa (KM) is employed by a commercial company, Kowa Company, Ltd. The funder provided support in the form of salaries for an author [KM], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Others have no competing interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 2.Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, Vollmer S. The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017;5:423–430. doi: 10.1016/s2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- 3.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Elezi S, Kastrati A, Pache J, Wehinger A, Hadamitzky M, Dirschinger J, et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998;32:1866–1873. doi: 10.1016/s0735-1097(98)00467-7. [DOI] [PubMed] [Google Scholar]
- 5.von Birgelen C, Kok MM, Sattar N, Zocca P, Doelman C, Kant GD, et al. "Silent" diabetes and clinical outcome after treatment with contemporary drug-eluting stents: the BIO-RESORT Silent Diabetes Study. JACC Cardiovasc Interv. 2018;11:448–459. doi: 10.1016/j.jcin.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Lee CH, Choi SW, Jun SW, Hwang J, Kim IC, Cho YK, et al. Clinical impact of diabetes mellitus on 2-year clinical outcomes following PCI with second-generation drug-eluting stents; landmark analysis findings from patient registry: pooled analysis of the Korean multicenter drug-eluting stent registry. PLoS ONE. 2020;15:e0234362. doi: 10.1371/journal.pone.0234362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konishi H, Miyauchi K, Kasai T, Tsuboi S, Ogita M, Naito R, et al. Impact of lipoprotein(a) as residual risk on long-term outcomes in patients after percutaneous coronary intervention. Am J Cardiol. 2015;115:157–160. doi: 10.1016/j.amjcard.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Wada H, Dohi T, Miyauchi K, Shitara J, Endo H, Doi S, et al. Preprocedural high-sensitivity C-reactive protein predicts long-term outcome of percutaneous coronary intervention. Circ J. 2016;81:90–95. doi: 10.1253/circj.CJ-16-0790. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi S, Shimada K, Miyauchi K, Miyazaki T, Sai E, Ogita M, et al. Low and exacerbated levels of 1,5-anhydroglucitol are associated with cardiovascular events in patients after first-time elective percutaneous coronary intervention. Cardiovasc Diabetol. 2016;15:145. doi: 10.1186/s12933-016-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rollins BJ. Chemokines. Blood. 1997;90:909–928. doi: 10.1182/blood.V90.3.909. [DOI] [PubMed] [Google Scholar]
- 11.Bromage DI, Davidson SM, Yellon DM. Stromal derived factor 1α: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther. 2014;143:305–315. doi: 10.1016/j.pharmthera.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuoka S, Uematsu M, Nakamura T, Shimizu T, Futamata M, Obata JE, et al. High levels of stromal cell-derived factor-1α predict secondary cardiac events in stable patients with a history of myocardial infarction. J Cardiol. 2017;69:320–325. doi: 10.1016/j.jjcc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Ghasemzadeh N, Hritani AW, De Staercke C, Eapen DJ, Veledar E, Al Kassem H, et al. Plasma stromal cell-derived factor 1α/CXCL12 level predicts long-term adverse cardiovascular outcomes in patients with coronary artery disease. Atherosclerosis. 2015;238:113–118. doi: 10.1016/j.atherosclerosis.2014.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peiró ÓM, Farré N, Cediel G, Bonet G, Rojas S, Quintern V, Bardají A. Stromal cell derived factor-1 and long-term prognosis in acute coronary syndrome. Biomark Med. 2019;13:1187–1198. doi: 10.2217/bmm-2019-0133. [DOI] [PubMed] [Google Scholar]
- 15.Chen XY, Shi YX, Huang YP, Ding M, Shen QL, Li CJ, Lin JN. SDF-1 inhibits the dedifferentiation of islet β cells in hyperglycaemia by up-regulating FoxO1 via binding to CXCR4. J Cell Mol Med. 2022;26:750–763. doi: 10.1111/jcmm.17110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin J, Fukuhara A, Onodera T, Kita S, Yokoyama C, Otsuki M, Shimomura I. SDF-1 is an autocrine insulin-desensitizing factor in adipocytes. Diabetes. 2018;67:1068–1078. doi: 10.2337/db17-0706. [DOI] [PubMed] [Google Scholar]
- 17.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/circulationaha.107.718718. [DOI] [PubMed] [Google Scholar]
- 18.Baerts L, Waumans Y, Brandt I, Jungraithmayr W, Van der Veken P, Vanderheyden M, De Meester I. Circulating stromal cell-derived factor 1α levels in heart failure: a matter of proper sampling. PLoS ONE. 2015;10:e0141408. doi: 10.1371/journal.pone.0141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bromage DI, Taferner S, Pillai M, Yellon DM, Davidson SM. A novel recombinant antibody specific to full-length stromal derived factor-1 for potential application in biomarker studies. PLoS ONE. 2017;12:e0174447. doi: 10.1371/journal.pone.0174447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 21.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 22.Ghadge SK, Mühlstedt S, Ozcelik C, Bader M. SDF-1α as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–2231. doi: 10.1161/circulationaha.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Gu H, Zhang W, Manukyan MC, Shou W, Wang M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;301:H1496–1505. doi: 10.1152/ajpheart.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/s0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 26.Dong F, Harvey J, Finan A, Weber K, Agarwal U, Penn MS. Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation. 2012;126:314–324. doi: 10.1161/circulationaha.111.082453. [DOI] [PubMed] [Google Scholar]
- 27.Cheng K, Malliaras K, Smith RR, Shen D, Sun B, Blusztajn A, et al. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Fail. 2014;2:49–61. doi: 10.1016/j.jchf.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung ES, Miller L, Patel AN, Anderson RD, Mendelsohn FO, Traverse J, et al. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. Eur Heart J. 2015;36:2228–2238. doi: 10.1093/eurheartj/ehv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian S, Liu C, Aviv A, Ho JE, Courchesne P, Muntendam P, et al. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arterioscler Thromb Vasc Biol. 2014;34:2100–2105. doi: 10.1161/atvbaha.114.303579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 31.Esencay M, Sarfraz Y, Zagzag D. CXCR7 is induced by hypoxia and mediates glioma cell migration towards SDF-1α. BMC Cancer. 2013;13:347. doi: 10.1186/1471-2407-13-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/circulationaha.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. Faseb J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 34.Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/s0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 35.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/circulationaha.108.809723. [DOI] [PubMed] [Google Scholar]
- 36.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/s0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 37.Günaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493–496. doi: 10.1006/phrs.1999.0611. [DOI] [PubMed] [Google Scholar]
- 38.Fortunato O, Spinetti G, Specchia C, Cangiano E, Valgimigli M, Madeddu P. Migratory activity of circulating progenitor cells and serum SDF-1α predict adverse events in patients with myocardial infarction. Cardiovasc Res. 2013;100:192–200. doi: 10.1093/cvr/cvt153. [DOI] [PubMed] [Google Scholar]
- 39.Damås JK, Waehre T, Yndestad A, Ueland T, Müller F, Eiken HG, et al. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002;106:36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]
- 40.García-Cuesta EM, Santiago CA, Vallejo-Díaz J, Juarranz Y, Rodríguez-Frade JM, Mellado M. The role of the CXCL12/CXCR4/ACKR3 axis in autoimmune diseases. Front Endocrinol (Lausanne) 2019;10:585. doi: 10.3389/fendo.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Univariate Cox proportional hazard analyses for predictors of all-cause death following PCI. Table S2. Univariate Cox proportional hazard analyses for predictors of 3P-MACE following PCI. Table S3. Multivariate cox proportional hazard analyses using 3 models to assess the hazard ratios of total/active/inactive SDF-1α for all-cause death and 3P-MACE. Figure S1. Flow diagram of the study participants.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.