Abstract
Oxidative stress has been implicated in the uteroplacental ischemia characteristic of preeclampsia and small-for-gestational-age (SGA) birth, both of which are more common at high (>2500 m) vs low altitude. Since Andeans are protected relative to Europeans from the altitude-associated rise in SGA, we asked whether alterations in maternal antioxidant status or oxidative stress contributed to their protection. Enzymatic antioxidant (erythrocyte catalase and superoxide dismutase [SOD]) activity and a plasma marker of lipid peroxidation (8-iso-PGF2α) were measured during pregnancy and in the non-pregnant state in Andean or European residents of low (400 m) or high altitude (3600–4100 m). Pregnancy and altitude increased catalase and/or SOD activity to a greater extent in Andeans than Europeans. 8-iso-PGF2α levels were independent of altitude and pregnancy. SOD was lower in mothers of SGA infants at weeks 20 and 36. Our findings are consistent with the possibility that elevated enzymatic antioxidant activity contributes to Andean protection against altitude-associated SGA.
Keywords: Adaptation, fetal growth restriction, hypoxia, oxidative stress
Introduction
One of the most potent environmental factors raising the risk of a newborn being small for gestational age (SGA) is the chronic hypoxia of residence at altitudes greater than 2500 m (8250 ft [1,2]). The incidence of preeclampsia is also greater at high altitude, an effect which contributes to but does not explain the extent to which fetal growth is compromised [3]. The effect of high altitude on birth weight is independent of maternal age, parity, prenatal care or other factors known to influence fetal growth [4,5], but is heavily influenced by population ancestry. Specifically, infants of multigenerational highland populations (Tibetans or Andeans) weigh more at birth and are less likely to be SGA than infants born to high-altitude residents of lowland ancestry (European or Han “Chinese [2,6]”). Physiologic studies indicate that such effects are likely due to protection against hypoxia-related impairment of maternal vascular responses to pregnancy [7–9]. Here we consider the possibility that maternal antioxidant or oxidant status contributes to Andean protection from hypoxia-associated reductions in fetal growth.
Oxidative stress is characterized by a rate of oxidant production that outpaces antioxidant defense systems. Pregnancy itself increases markers of oxidative damage [10] and reduces levels of circulating antioxidants, with the pro-oxidant state being exaggerated in cases of preeclampsia or fetal growth restriction [11–13]. While recent studies, indicate that supplementation with dietary antioxidants exerts no protective effect against the development of these pregnancy complications (e.g [14,15]), current literature overwhelmingly indicates that oxidative stress remains important in their etiology. Since hypoxia also induces oxidative stress [16], we asked whether the chronic hypoxia of residence at high altitude amplified the pro-oxidative effects of pregnancy so as to compromise fetal growth. Given Andean protection from hypoxia-associated fetal growth reduction, we also asked whether Andeans had lower levels of oxidative stress and/or greater antioxidant capacity during pregnancy than Europeans at high altitude and, if so, whether such differences were associated with fetal growth. To address these questions, we measured maternal superoxide dismutase and catalase activity as indices of endogenous antioxidant status and plasma isoprostane (8-iso-PGF2α) levels as an index of oxidative stress in the non-pregnant state as well as at weeks 20 and 36 of pregnancy in Andean and European residents of low or high altitude in Bolivia. Birth weight and other indices of fetal growth were obtained at delivery. We considered that such studies would enable us to determine whether oxidant status was involved in Andean protection from hypoxia-associated SGA and aid in our understanding of these still incompletely understood conditions.
Materials and methods
Subjects
A total of 160 women were studied, comprising 54 residents of low altitude (Santa Cruz, 400 m) and 106 residents of high altitude (La Paz or El Alto, 3600– 4100 m) in Bolivia. Most (n = 78) of them were participants in a larger study [9]. At low altitude, 22 Andeans and 32 Europeans were studied at weeks 20 and 36 of pregnancy as well as 3–4 months postpartum for a measurement in the non-pregnant state. At high altitude, 24 Europeans were studied at the same time points but prospective studies in Andean women participating in the larger study had been completed by the time these oxidative stress studies began. For this reason, additional cross-sectional studies in Andean women at high altitude were required to complete the aims of this study [n = 83; 14 at 20 weeks, 47 at 36 weeks and 16 in the non-pregnant state (7 nulliparous and 9 ≥ 3 months postpartum)].
Women were recruited through their prenatal care providers at private or public clinics after obtaining their informed consent. Inclusion criteria were that women self-identify as being Andean or European ancestry; had resided at the altitude of study for ≥1 year; were receiving prenatal care, of good general health, having a singleton pregnancy, and free of known risk factors for adverse pregnancy outcome (e.g., diabetes). None of the women included here developed preeclampsia. High-altitude studies were conducted at the Instituto Boliviano de Biología de Altura (Bolivian High-Altitude Biology Institute) and the Clinica del Sur (Southern Clinic) in La Paz (PB = 495 mmHg) and low-altitude studies at Clinica Siraní in Santa Cruz (PB = 725 mm Hg). Because maternal age, gene markers indicative of ancestry group, and infant birth weights were similar between the prospectively-studied women reported elsewhere [9] and these cross-sectional subjects, we felt the inclusion of the cross-sectional data in this otherwise prospective study design was justified. All recruiting procedures and study protocols were approved by the Colorado Multiple Institutional Review Board (COMIRB) and the Colegio Médico, the equivalent ethical review group in Bolivia, and adhered to the principles described in the Declaration of Helsinki.
Protocol, variables and definitions
On the first visit, each woman completed a questionnaire administered in the subject’s spoken language to determine her self-identified ancestry, parents’ and grandparents’ surnames; altitude of birth, childhood and current residence; medical and reproductive history; and personal characteristics that could influence fetal growth or pregnancy outcome (e.g., body weight before pregnancy, prenatal care, income and educational status). Maternal height was measured at the first visit and a general clinical assessment conducted at each visit that included measurements of blood pressure, heart rate, weight and skinfold thickness, and collection of the blood sample.
Ancestry was assessed by self-identification as either Andean (i.e., Aymara or Quechua) or European, examination of her parents’ and grandparents’ surnames, and a panel of 100 ancestry informative genetic markers (AIMs) that had been selected in order to quantify the proportion of an individual’s ancestry that can be ascribed to African, European or Indigenous American origin [17]. Since AIMs cannot presently distinguish between Andean vs. Amazonian or other low-altitude Indigenous American groups, parental and grandparental surnames and residential history were examined in cases where self-identification and AIMs did not match [18]. A woman was classified as Andean if at least three of her parents’ four surnames were Aymara or Quechua, she self-identified as Andean and had no known non-Andean parentage, and >60% of her AIMs were of Indigenous American origin. Women were classified as of low-altitude origin if she self-identified as European or other low-altitude populations and had <50% Indigenous American AIMs. The principal population of low-altitude origin was European, this group is referred to as “European” here. Women who did not fit these criteria were excluded.
Maternal weight was measured by balance scale, height by stadiometer, and skinfolds as the bilateral average of the triceps and subscapular skinfold-thickness (Lange calipers; Beta Technology Inc., Santa Cruz, CA). Blood pressure was measured by arm cuff sphygmomanometer and heart rate by auscultation.
For measuring markers of oxidative stress and antioxidant status, blood (14 ml) was withdrawn from the antecubital vein at each study time, prepared as required for the particular assay, and stored at −80°C until analysis. Oxidative stress was assessed by measuring plasma 8-iso-PGF2α concentrations as quantified by HPLC-MS [19]. Antioxidant status was measured as total erythrocyte superoxide dismutase (SOD) activity (Mn-SOD; Cu, Zn-SOD and EC-SOD) using the xanthine oxidase/xanthine/cytochrome c method [20] and erythrocyte catalase activity using the method of Beers and Sizer which determines the rate of disappearance of hydrogen peroxide (H2O2) by catalase [21].
Birth weight, ponderal index, head and abdominal circumferences, and gestational age were obtained from labor and delivery records. Gestational age was calculated using the elapsed weeks from the last menstrual period as verified by fetal biometry at 20 weeks. Infants were considered to be SGA when the birth weight for gestational age and sex was less than the 10th percentile of published sea-level values [22]. Ponderal index was calculated as 100 × birth weight (gm)/crown-heel length (cm)3. Infants born at ≤37 weeks of gestation were considered preterm. All the low-altitude and nearly all [94% (Andean) and 96% (European)] the high-altitude infants were born in hospitals; the remainder were born at home and birth weights were obtained at the hospital within 24 hours of birth.
Statistical Analyses
Maternal attributes, fetal biometry and newborn characteristics were compared within altitude and ancestry groups using ANOVA or χ2 tests as appropriate. Two-way ANOVA was used to detect interaction between the effects of altitude, time and/or ancestry on the primary variables of interest. Categorical variables were dichotomized using a biologically-relevant threshold (e.g., prematurity) or were self-dichotomized (e.g., prenatal vitamin use, yes/no). Scheffé’s post-hoc tests were used to determine the source of significant differences observed across time for continuous variables. Differences were considered significant when p < 0.05 and trends were considered when 0.05 < p <0.10. Values are expressed as mean ± standard error of the mean (SEM) or as percentages with 95% confidence intervals (CI) as appropriate.
Results
Maternal characteristics
Lowland Andeans and Europeans did not differ in age, primiparity, height, body weight (non-pregnant and week 36), skin-folds, non-pregnant blood pressures, reported smoking, prenatal vitamin use, monthly income, educational status (all reported having secondary school education or greater), or years of high-altitude residence (2.6 ± 1.3 and 1.1 ± 0.8 years, respectively) (Table I). Lowland Andeans were of largely Indigenous American ancestry (71.8 ± 2.6%) with some European (18.2 ± 2.6%) or West African (10.0 ± 1.0%) admixture. Lowland Europeans were largely of European (49.5 ± 3.5%) or Indigenous American origin (40.1 ± 2.8%), with all of the latter being from Central American, Caribbean, Guaraní, or other low-altitude regions. In other words, the Indigenous American origin in the lowland European group was attributed to lowland rather than highland Indigenous American populations.
Table I.
Maternal attributes.
| Variable | Ancestry | Low altitude | High altitude | p-altitude |
|---|---|---|---|---|
|
| ||||
| Age, years | European | 26.9 ± 6.0 (18) | 30.9 ± 1.1 (11) | <0.05 |
| Andean | 27.9 ± 5.6 (23) | 25.9 ± 0.7 (82) | NS | |
| p-ancestry | NS | <0.01 | ||
| Height, cm | European | 160.7 ± 1.0 (18) | 161.9 ± 1.6 (11) | NS |
| Andean | 158.1 ± 1.0 (24) | 150.5 ± 0.6 (82) | <0.001 | |
| p-ancestry | NS | <0.01 | ||
| Primiparous, % | European | 38.9 [20.3, 61.4] | 45.8 [27.9, 64.9] | NS |
| Andean | 54.5 [28.0, 78.7] | 36.6 [27.0, 47.4] | NS | |
| p-ancestry | NS | NS | ||
| Weight, non-preg, kg | European | 69.1 ± 2.3 (18) | 64.0 ± 2.0 (21) | NS |
| Andean | 66.6 ± 1.8 (11) | 55.5 ± 1.7 (82) | <0.001 | |
| p-ancestry | NS | <0.01 | ||
| Weight, 36 weeks, kg | European | 77.1 ± 2.3 (18) | 73.1 ± 1.8 (22) | NS |
| Andean | 74.3 ± 2.1 (11) | 62.7 ± 1.7 (46) | <0.01 | |
| p-ancestry | NS | <0.001 | ||
| Skinfolds, non-preg mm | European | 66.1 ± 3.0 (18) | 48.6 ± 5.7 (14) | <0.01 |
| Andean | 64.8 ± 3.2 (11) | 23.9 ± 1.1 (82) | <0.001 | |
| p-ancestry | NS | <0.001 | ||
| MAP, non-preg mmHg | European | 77.3 ± 1.0 (18) | 78.7 ± 1.7 (21) | NS |
| Andean | 77.8 ± 2.6 (11) | 71.9 ± 1.4 (81) | NS† | |
| p-ancestry | NS | <0.01 | ||
| Smoke, % yes | European | 6.3 [1.7, 20.2] (18) | 7.7 [2.1, 24.1] (23) | NS |
| Andean | 4.6 [0.8, 21.8] (11) | 2.3 [0.6, 8.1] (82) | NS | |
| p-ancestry | NS | <0.001 | ||
| Prenatal vitamins, % yes | European | 78.1 [61.3, 89.0] (18) | 88.9 [71.9, 96.2] (23) | NS |
| Andean | 86.3 [66.7, 95.2] (11) | 47.7 [37.5, 58.1] (68) | <0.05 | |
| p-ancestry | NS | <0.05 | ||
| Income, $/mo. | European | 379 ± 56 (15) | 1250 ± 386 (13) | <0.001 |
| Andean | 282 ± 53 (8) | 84 ± 6 (82) | <0.001 | |
| p-ancestry | NS | <0.001 | ||
Values are reported as means ± SEM or as percentages with 95% CI in brackets []. One-way ANOVA was used for comparisons between continuous variables; chi-squared tests were used for comparisons between categorical variables.
MAP, mean arterial pressure; pntl, prenatal; p-ancestry = significance values between ancestry groups within altitude groups; p-altitude: significance values between altitude groups, within ancestry groups
= 0.05< p < 0.10.
Sample sizes are in parentheses ().
Highland Andeans had lived at altitudes ~2500 m an average of 11.6 years longer than their European high-altitude counterparts (25.0 ± 0.7 vs. 13. 8 ± 2.5 years, respectively; p < 0.001). Highland Andeans were younger, shorter and had lower body weight, subcutaneous adiposity and mean arterial pressure in the non-pregnant state than Europeans (Table I). Primiparity was equally prevalent in highland Europeans and Andeans. Highland Europeans compared to Andeans reported greater monthly incomes (Table I) as well as a higher frequency of smoking, prenatal vitamin use, and secondary school education (100% [87.1, 100] and 72% [61.4, 80.2], respectively, p < 0.05). Highland Andean AIMs were also largely of Indigenous American origin (82.9 ± 1.4%), with less than 10% being of European or West African origin (9.6 ± 1.3% and 7.5 ± 0.6%, respectively). Highland Europeans AIMs were largely of European origin (72.9 ± 3.5%) with some Indigenous American (19.6 ± 3.1%) or West African (7.4 ± 1.4%) origin.
Antioxidant activity and oxidative stress
As shown in Figure 1, catalase was unaffected by pregnancy and did not differ between ancestry groups at low altitude. At high altitude, catalase declined with pregnancy in Europeans (p < 0.01) but remained constant in Andeans. Comparing altitudes, non-pregnant catalase values were greater at high than low altitude in both groups (p < 0.05), whereas only Andeans showed elevated catalase at high compared to low altitude during pregnancy (20 or 36 weeks, p < 0.01). In other words, Andean ancestry appeared to defend against the pregnancy associated decline in catalase that was seen in the Europeans at high altitude (interaction of time, altitude and ancestry; p < 0.05) with the result that catalase was elevated during pregnancy (20 and 36 weeks) in Andeans compared to Europeans.
Figure 1.
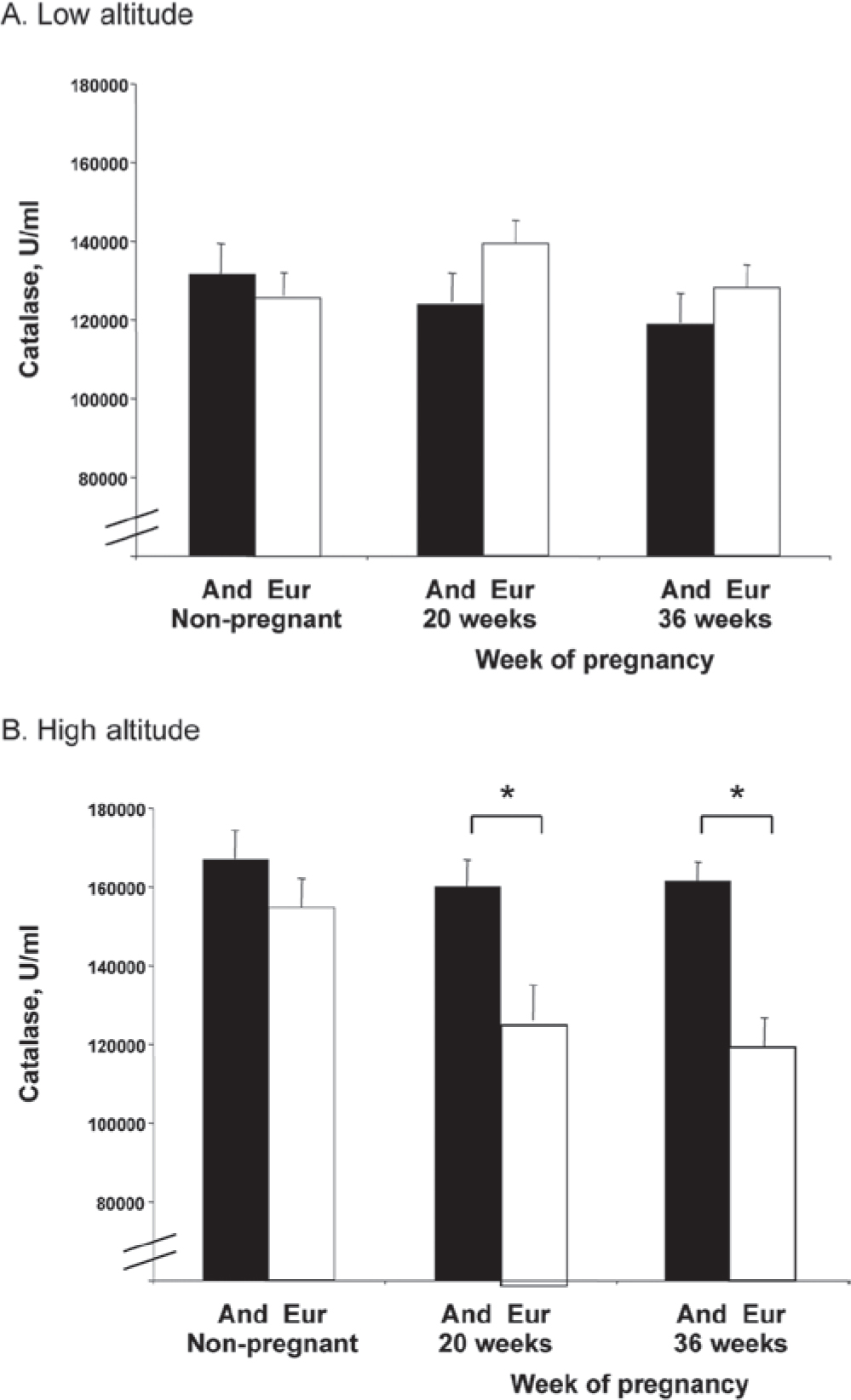
At low altitude catalase activity did not vary by ancestry or across time. At high altitude catalase was greater in Andeans than Europeans at 20 or 36 weeks (both p < 0.05) due to a pregnancy-related decline among Europeans (p < 0.01). Catalase activity was greater at high than low altitude in the non-pregnant (NP) state in Europeans (p < 0.01) and at all times in Andeans (NP, p < 0.05; 20 or 36 weeks, p < 0.01). The effect of altitude on catalase levels across time differed between ancestry groups (time x altitude x ancestry, p < 0.05). Significant differences (p < 0.05) between ancestry groups are marked with an asterisk (★).
SOD activity at low altitude was higher in non-pregnant Andeans than Europeans, but equivalent between groups during pregnancy (Figure 2). Pregnancy lowered SOD in Andeans at low altitude, whereas values were unchanged in Europeans (interaction of time and ancestry, p < 0.05). At high altitude, SOD was similar between ancestry groups in the non-pregnant state, but greater in Andeans than Europeans during pregnancy (36 weeks; p < 0.05). In other words, SOD was higher in Andeans than European during pregnancy due to ancestry-dependent differences in the effects of pregnancy on antioxidant status (interaction of time and ancestry, p < 0.05) rather than preexisting differences between groups. Comparing altitudes, non-pregnant SOD was reduced at high relative to low altitude in both ancestry groups (both, p < 0.05; Figure 2).
Figure 2.
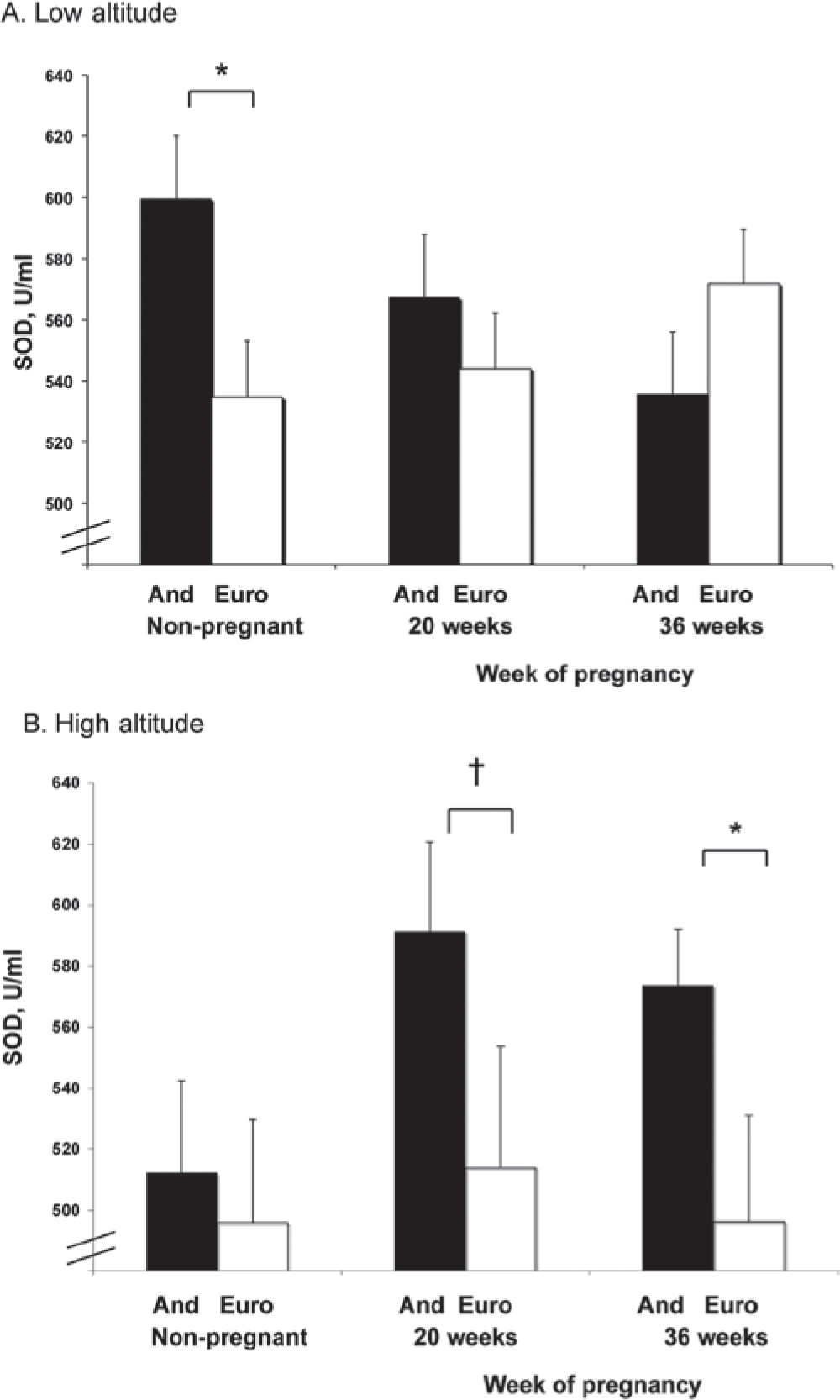
Superoxide dismutase (SOD) activity was unaffected by pregnancy in either ancestry group at low (A) or high altitude (B). Non-pregnant SOD activity was reduced at high relative to low altitude in both ancestry groups (both, p < 0.05). During pregnancy, SOD activity was reduced at high relative to low altitude in Europeans but not Andeans. Andeans had greater SOD activity than Europeans at high altitude at 36 weeks (p < 0.05). Significant differences (p < 0.05) between ancestry groups are marked with an asterisk (★); trends (0.05 < p < 0.10) are marked with a cross symbol (†).
8-iso-PGF2α levels at low altitude were unchanged by pregnancy in Europeans but tended to decline in Andeans, resulting in lower 8-iso-PGF2α Andean levels at weeks 20 or 36 (Figure 3). At high altitude, pregnancy reduced 8-iso-PGF2α levels in both ancestry groups. Comparing altitudes, 8-iso-PGF2α values were similar in Andeans but lower at 36 weeks in the European women at high compared to low altitude (p < 0.001).
Figure 3.
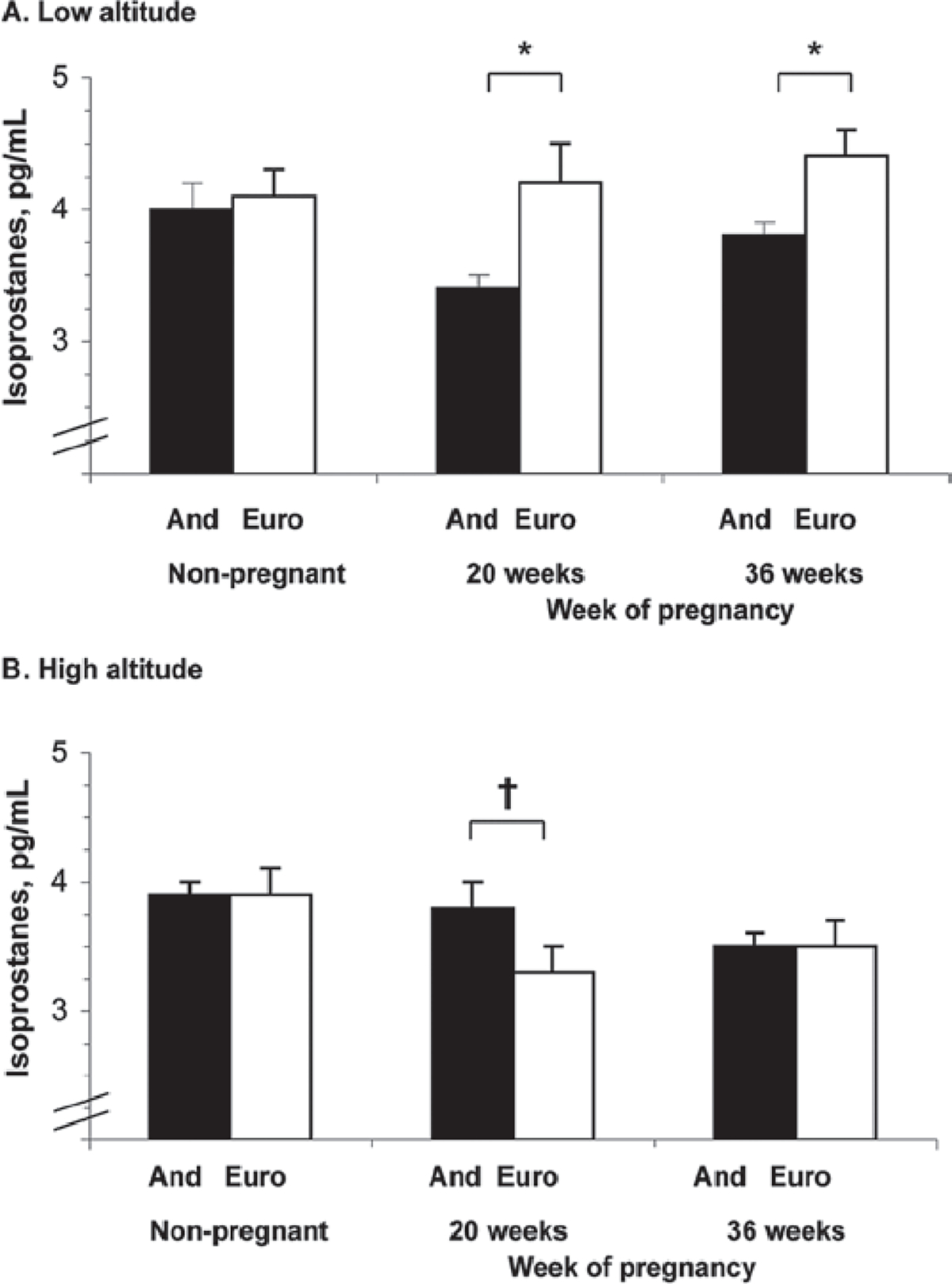
Isoprostane levels tended to decrease with pregnancy at low (A) or high (B) altitude in Andeans but not Europeans. At low altitude isoprostanes were higher in European than Andean women at 20 and 36 weeks of pregnancy. Altitude had no effect on isoprostane levels in Andeans at any time but levels were lower at high than low altitude in Europeans at 20 and 36 weeks (both p < 0.05). Significant differences (p < 0.05) between ancestry groups are marked with an asterisk (*); trends (0.05 < p < 0.10) are marked with a cross symbol (†).
Delivery and newborn characteristics
As shown in Table II, delivery and newborn characteristics were similar between ancestry groups at low altitude. At high altitude, the frequency of preterm deliveries tended to be greater in the European than Andean groups due to the high proportion of elective c-sections (71% vs. 30%, respectively; p < 0.05), which were generally scheduled 1 week earlier than vaginal deliveries. Absolute birth weights tended to be greater in Andeans than Europeans (Table II). After adjusting for gestational age, Andean infants weighed 208 gm more than Europeans (p < 0.05, 1-tailed) and twice as many Europeans were SGA. All other newborn characteristics were similar between ancestry groups at high altitude.
Table II.
Newborn and delivery characteristics.
| Altitude | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Ancestry | Low 400 m | High 3600 m–4100 m | p-alt |
|
| ||||
| C-section, % | European | 75.0 [57.9, 86.8] (18) | 71.4 [50.0, 86.2] (24) | NS |
| Andean | 85.7 [65.4, 95.0] (11) | 29.8 [19.5, 42.7] (82) | <0.05 | |
| p-ancestry | NS | <0.05 | ||
| Hospital delivery, % | European | 100 [89.3, 100] (18) | 96.0 [80.5, 99.3] (22) | NS |
| Andean | 100 [85.1, 100] (11) | 94.0 [85.6, 97.7] (66) | NS | |
| p-ancestry | NS | NS | ||
| Birth weight, gm | European | 3337 ± 88 (18) | 2928 ± 87 (23) | <0.01 |
| Andean | 3396 ± 112 (10) | 3135 ± 65 (59) | <0.05 | |
| p-ancestry | NS | NSt | ||
| Birth weight, gm adjusted* | European | 3396 ± 84 (18) | 2949 ± 84 (18) | <0.05 |
| Andean | 3325 ± 53 (10) | 3157 ± 53 (10) | NS | |
| p-ancestry | NS | <0.05 | ||
| PI, kg/m3 | European | 27.1 ± 0.5 (18) | 26.3 ± 0.7 (22) | NS |
| Andean | 27.4 ± 0.7 (10) | 26.3 ± 0.1 (54) | NS | |
| p-ancestry | NS | NS | ||
| PI, kg/m3 adjusted* | European | 27.9 ± 0.7 | 26.0 ± 0.3 | NS |
| Andean | 27.6 ± 1.0 | 26.3 ± 0.1 | NS | |
| p-ancestry | NS | NS | ||
| Head circ, cm | European | 35.0 ± 0.2 (17) | 33.8 ± 0.4 (18) | <0.01 |
| Andean | 35.3 ± 0.2 (10) | 34.3 ± 0.6 (53) | NS | |
| p-ancestry | NS | NS | ||
| Gest age, wks | European | 38.1 ± 0.5 (18) | 38.9 ± 0.3 (23) | NS |
| Andean | 38.8 ± 0.2 (10) | 38.4 ± 0.4 (59) | NS | |
| p-ancestry | NS | NS | ||
| Preterm, % | European | 32.2 [28.6, 49.9] (18) | 26.9 [13.7, 46.1] (24) | NS |
| Andean | 27.2 [13.2, 48.2] (11) | 13.6 [7.0, 24.5] (82) | NS† | |
| p-ancestry | NS | NSt | ||
| Male, % | European | 41.9 [26.4, 59.2] (18) | 46.2 [28.8, 64.5] (24) | NS |
| Andean | 57.1 [36.6, 75.5] (11) | 47.5 [35.5, 59.8] (82) | NS | |
| p-ancestry | NS | NS | ||
| SGA, % | European | 9.7 [3.4, 24.9] (18) | 25.9 [13.2.2, 44.7] (23) | NS† |
| Andean | 14.3 [5.0, 34.6] (10) | 13.3 [6.9, 24.2] (59) | NS | |
| p-ancestry | NS | NS† | ||
Values for continuous variables are reported as means ± SEM and as percentages with the 95% CI in brackets []. One-way ANOVA was used for comparisons across altitude or ancestry groups for continuous variables. Chi-squared tests were used for comparisons across altitude or ancestry groups for categorical variables.
PI, ponderal index; SGA, small-for-gestational age
birth weight adjusted for gestational age
p-alt = significance values between altitude groups, within ancestry groups; p-ancestry = significance values between ancestry groups, within altitude groups
= 0.05 < p < 0.10. Sample sizes are in parentheses ().
Comparing altitudes, European infants weighed less at birth at high compared to low altitude (adjusted or unadjusted for gestational age). The unadjusted Andean values were also lower, but this was due to modest differences in gestational age as the adjusted Andean values were similar at the two altitudes (Table II). Head circumference was reduced at high vs. low altitude in the Europeans but not Andeans.
Relationship between antioxidant status, oxidative stress and birth weight
Antioxidant activity or 8-iso-PGF2α levels were unrelated to infant birth weight (adjusted or unadjusted for gestational age) in either altitude or ancestry group. However, women who delivered SGA infants had lower levels of SOD at week 20 and 36 compared to those who delivered infants of average size for their gestational age at high altitude (p < 0.05) (Table III). The same tendency was apparent at 20 weeks when both altitudes were considered together, largely due to lower SOD activity in Europeans at high altitude. European women delivering SGA vs. AGA babies at high altitude also tended to have lower catalase at 20 weeks. 8-iso-PGF2α levels were unrelated to SGA at either altitude or in either ancestry group.
Table III.
Comparison of maternal antioxidant status between normal and SGA deliveries.
| High altitude | Gestational week | p-value | ||||
|---|---|---|---|---|---|---|
| Week 20 SGA status | Week 36 SGA status | |||||
| Normal | SGA | p-value | Normal | SGA | ||
| Catalase, U/mL | 135845 ± 14912 (7) | 148740 ± 8207 (22) | NS | 149246 ± 5388 (51) | 15396 ± 10815 (10) | NS |
| SOD, U/mL | 567 ± 24 (22) | 434 ± 82 (5) | <0.05 | 564 ± 19 (47) | 474 ± 46 (9) | <0.10 |
| All altitudes | Gestational week | |||||
| Week 20 SGA status | Week 36 SGA status | p-value | ||||
| Normal | SGA | p-value | Normal | SGA | ||
| Catalase, U/mL | 141427 ± 6298 (10) | 130872 ± 12017 (44) | NS | 143203 ± 4434 (75) | 148518 ± 8969 (13) | NS |
| SOD, U/mL | 558 ± 106 (44) | 480 ± 57 (8) | <0.10 | 562 ± 14 (72) | 501 ± 136 (11) | NS |
Since there were few cases of SGA at low altitude, we elected to include only high altitude and both altitudes combined.
Discussion
Our findings indicate that endogenous antioxidant activity was markedly greater in Andean than European women during pregnancy at high but not low altitude. Strengthening our observations, SOD and catalase followed a similar pattern across time at high-altitude. In each case, non-pregnant values at high altitude were equivalent between Andeans and Europeans and diverged with the additional stress of pregnancy such that antioxidant activity was greater in Andeans than Europeans during pregnancy. The chief difference being that Andeans did not exhibit the pregnancy-related decline in catalase or the altitude-related decline in SOD that was apparent in Europeans. While no differences in oxidative stress (using 8-iso-PGF2α as a marker of lipid peroxidation) were identified, and no relationships between antioxidants or oxidative stress markers and birth weight were present, women who delivered SGA infants at high altitude had lower SOD activity than those who delivered infants of normal birth weight for a given gestational age. We therefore concluded that antioxidant capacity may contribute to the protective effect of Andean ancestry against altitude-associated SGA.
This study was designed to test the hypotheses that enzymatic antioxidant status during pregnancy was diminished and oxidative stress augmented at high compared to low altitude, and that Andeans were protected relative to Europeans from such effects. Our hypotheses were based on two lines of evidence. First, hypoxia and pregnancy are independently recognized to increase oxidative stress [10,16] and therefore it is likely that the hypoxia of chronic exposure to high altitude amplifies the pro-oxidant effect that accompanies pregnancy. Second, oxidative stress is implicated in the maternal endothelial dysfunction characteristic of preeclampsia and/or SGA [11,13,23] at low altitude. For these reasons, we considered that maternal antioxidant status may be of even greater importance during pregnancy at high compared to low altitude if altitude and pregnancy act synergistically to increase oxidative stress. Although dietary antioxidant supplementation does not appear to prevent these conditions [14,15] we considered that endogenous antioxidant capacity might protect against hypoxia-associated SGA. The reason for this is that enzymatic antioxidants act as catalysts of dismutation reactions and therefore have greater and more prolonged antioxidant effects than dietary sources that are stoichiometrically consumed. We also considered that Andean protection against SGA relative to European populations at high altitude may be the result, in part, of greater antioxidant activity and reduced oxidative stress.
It was surprising that 8-iso-PGF2α levels were unaffected by altitude or pregnancy given the variation in antioxidant activity observed. While it may be that oxidant production is no greater during pregnancy at high relative to low altitude and may not vary between ancestry groups there are several other possible explanations for our observations. In particular, the direct quantification of oxidant production is notoriously difficult given the unstable nature and extremely short half-life of oxidant species. For this reason we chose to use circulating levels of 8-iso-PGF2α as a marker of oxidative stress based on the chemical stability, specificity (i.e., non-enzymatic free radical-induced peroxidation of arachidonic acid) and prior indications of reliability as an indicator of lipid peroxidation resulting from free radical production and/or antioxidant insufficiency [24]. Since we were not able to assess oxidant production directly or in multiple tissues it is possible that we were simply unable to detect differences by assessing 8-iso-PGF2α in the peripheral circulation. It may also be that lipids were not the primary oxidation targets. However, Zamudio et al. report that protein carbonylation products were equivalent in villous core placental tissue at high (3100 m) compared to lower altitude (1600 m [25]), suggesting that markers of protein oxidation would likely also be similar between altitudes in our study. Lastly, we recognize that measurements of oxidative stress in the systemic circulation are not equivalent to those of other tissues that play a more direct role in fetal growth (e.g., maternal vessels supplying the uteroplacental circulation). We were unable to obtain such measurements of oxidative stress given that access to such samples during pregnancy is limited by ethical considerations.
Antioxidants might influence pregnancy outcomes through one or more of several mechanisms. One is the underlying concept that maternal antioxidant status serves to protect against the oxidative stress that accompanies pregnancy. Several clinical trials that sought to determine whether dietary antioxidant supplementation could decrease the frequency of preeclampsia and/or SGA were thus far ineffective (e.g., [14]). However, enzymatic antioxidants may still play an important role in reducing oxidative damage during pregnancy and thereby protect against preeclampsia and SGA. A second possibility is that oxidative stress or antioxidant factors act as signaling mechanisms for triggering changes in other important substances. In particular, it has been suggested that enhanced oxidant production increases the transcription of hypoxia-dependent genes [26], whose products influence vasoconstriction or vasodilation (e.g., endothelin 1), vascular growth (e.g., VEGF) and inflammation (e.g., IL-6). Antioxidants such as catalase also inhibit the effect of certain agonists (e.g., angiotensin II) to increase the expression of HIF-1 α regulated genes [27]. For these reasons, we considered it likely that redox status during pregnancy at high altitude may be of importance as a mediator of HIF-1α expression.
Given the purported role of oxidative stress in the etiology of preeclampsia or SGA, we asked whether antioxidant activity or oxidative stress markers influenced fetal growth at high altitude. As has been reported previously, Andean infants were relatively protected from the hypoxia-associated fetal growth restriction compared to Europeans [1,2]. Mothers of SGA infants had lower levels of antioxidant activity at high altitude at either 20 or 36 weeks of pregnancy compared to women who delivered infants of normal birthweight. Given the length of the erythrocyte life cycle enzymatic antioxidant values obtained at 20 weeks reflect events occuring between approximately 8 and 20 weeks of gestation, a critical timeframe for protecting against oxidative insults that occur during the establishment of the feto-placental circulation. Oxygen tensions are extremely low in the very early stages of human pregnancy [28]. At approximately 12 weeks gestation the intervillous space is gradually exposed to higher O2 tensions of the maternal blood [28], and enzymatic antioxidant activity rises in parallel [29]. We therefore speculated that increased antioxidant status may reflect earlier vascular events during pregnancy that confer benefits in terms of placentation, the establishment of uteroplacental blood flow, and subsequently protection from hypoxia-associated SGA. If so, it would appear that such influences operated via a threshold effect, rather than affecting fetal growth across the birth-weight continuum.
In summary, we concluded that greater enzymatic antioxidant activity may contribute to the protection against altitude-associated SGA afforded by Andean ancestry. The ancestry-associated variation in antioxidant status identified was only revealed by the combined effects of pregnancy and high altitude, suggesting the importance of environmental conditions for the expression of Andean-specific attributes or, possibly, gene-environment interaction. Given that our genome scan and gene marker studies indicate that native Andean highlanders have undergone genetic adaptation to high altitude (e.g., [30]) it may be that the antioxidant differences observed here may stem from be genetic or epigenetic variations in HIF-1α regulated or regulatory genes, given the role of redox status in oxygen sensing. High altitude provides a unique opportunity to identify genetic and environmental factors contributing to reduced fetal growth, whose pivotal role not only for neonatal health, but also for the development of disease in later life.
Acknowledgements
We would like to acknowledge all the women who generously donated their time for this project. Our appreciation is also extended to the many physicians who helped with numerous aspects of the study: Fernando Armaza, Diva Bellido, José L. Casanova, Pilar Garcia, Jose Luis Pablo Lopez, Jessica Pardo, Julio Roca, Jessika Schaymann, Lilian Toledo, Mercedes Villena, and Elizabeth Zelada. We are also grateful for the technical help provided by Mabel Agramonte, Ana-Maria Alarcón, Marta Aguilar, Esteban Alanoca, Hugo Aquino, Ruliana Arce, Marta Cardenas, Dolly Condori, Maria Elena Gira, Cristina Gonzáles, Ida Gonzales, Jennifer Hageman, Jost Klawitter, Freddy Lamachi, Damon Maes, Zaida Martinez, Lourdes Mavrich, Gene and Rosann McCullough, Caterina Romero, Tita Sanchez, and Wilmar Velasquez. We would also like to thank the following hospitals or clinics for their participation: CEMES, Clinica Siraní, Caja Nacional de Salud, Clinica Centro Integral de Educación Sexual and Clinica Grupo Médico Solidario.
Footnotes
Declaration of Interest: NIH HLBI-079647, HLBI-060131 and TW-01188 (LGM); as well as an AHA pre-doctoral fellowship (CGJ), an NSF doctoral dissertation improvement grant (CGJ) and an NSF graduate research fellowship (MJW).
References
- 1.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res 2003;54:20–25. [DOI] [PubMed] [Google Scholar]
- 2.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed 2007;92:F372–F377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 1999;180:1161–1168. [DOI] [PubMed] [Google Scholar]
- 4.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: Independent or interactive effects? Am J Public Health 1997;87:1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 2001;49:490–494. [DOI] [PubMed] [Google Scholar]
- 6.Moore LG, Young D, McCullough RE, Droma T, Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol 2001;13:635–644. [DOI] [PubMed] [Google Scholar]
- 7.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol 1995;79:7–14. [DOI] [PubMed] [Google Scholar]
- 8.Wilson MJ, Lopez M, Vargas M, Julian C, Tellez W, Rodriguez A, Bigham A, et al. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol 2007;293:R1313–R1324. [DOI] [PubMed] [Google Scholar]
- 9.Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, et al. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol 2009;296:R1564–R1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raijmakers MT, Roes EM, Poston L, Steegers EA, Peters WH. The transient increase of oxidative stress during normal pregnancy is higher and persists after delivery in women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 2008;138:39–44. [DOI] [PubMed] [Google Scholar]
- 11.Vanderlelie J, Venardos K, Clifton VL, Gude NM, Clarke FM, Perkins AV. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005;26:53–58. [DOI] [PubMed] [Google Scholar]
- 12.Poston L Intrauterine vascular development: Programming effects of nutrition on vascular function in the new born and adult. Nutr Health 2001;15:207–212. [DOI] [PubMed] [Google Scholar]
- 13.Longini M, Perrone S, Kenanidis A, Vezzosi P, Marzocchi B, Petraglia F, Centini G, Buonocore G. Isoprostanes in amniotic fluid: A predictive marker for fetal growth restriction in pregnancy. Free Radic Biol Med 2005;38:1537–1541. [DOI] [PubMed] [Google Scholar]
- 14.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet 2006;367:1145–1154. [DOI] [PubMed] [Google Scholar]
- 15.Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, De Greeff A, et al. ; WHO Vitamin C and Vitamin E trial group. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG 2009;116:780–788. [DOI] [PubMed] [Google Scholar]
- 16.Magalhães J, Ascensão A, Viscor G, Soares J, Oliveira J, Marques F, Duarte J. Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat Space Environ Med 2004;75:16–22. [PubMed] [Google Scholar]
- 17.Shriver MD, Mei R, Parra EJ, Sonpar V, Halder I, Tishkoff SA, Schurr TG, et al. Large-scale SNP analysis reveals clustered and continuous patterns of human genetic variation. Hum Genomics 2005;2:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty R, Barton SA, Ferrell RE, Schull WJ. Ethnicity determination by names among the Aymara of Chile and Bolivia. Hum Biol 1989;61:159–177. [PubMed] [Google Scholar]
- 19.Haschke M, Zhang YL, Kahle C, Klawitter J, Korecka M, Shaw LM, Christians U. HPLC-atmospheric pressure chemical ionization MS/MS for quantification of 15-F2t-isoprostane in human urine and plasma. Clin Chem 2007;53:489–497. [DOI] [PubMed] [Google Scholar]
- 20.McCord JM. Assessment of the Activity of Antioxidant Enzymes, in Current Protocols in Toxicology. 1999; pp. 7.3.1–7.3.9. [Google Scholar]
- 21.Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 1952;195:133–140. [PubMed] [Google Scholar]
- 22.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol 1982;59:624–632. [PubMed] [Google Scholar]
- 23.Atamer Y, Koçyigit Y, Yokus B, Atamer A, Erden AC. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur J Obstet Gynecol Reprod Biol 2005;119:60–66. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Morrow JD, Yin H. Quantification of F2-isoprostanes as a reliable index of oxidative stress in vivo using gas chromatography-mass spectrometry (GC-MS) method. Free Radic Biol Med 2009;47:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamudio S, Kovalenko O, Vanderlelie J, Illsley NP, Heller D, Belliappa S, Perkins AV. Chronic hypoxia in vivo reduces placental oxidative stress. Placenta 2007;28:846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med 2007;42:165–174. [DOI] [PubMed] [Google Scholar]
- 27.BelAiba RS, Djordjevic T, Bonello S, Flügel D, Hess J, Kietzmann T, Görlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol Chem 2004;385:249–257. [DOI] [PubMed] [Google Scholar]
- 28.Jauniaux E, Gulbis B, Burton GJ. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus–a review. Placenta 2003;24 Suppl A:S86–S93. [DOI] [PubMed] [Google Scholar]
- 29.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update 2006;12:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigham AW, Mao X, Mei R, Brutsaert T, Wilson MJ, Julian CG, Parra EJ, et al. Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Hum Genomics 2009; 4:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


