Abstract
Recent studies have reported that direct antiglobulin test (DAT) results were negative in cases of alectinib-induced hemolytic anemia with abnormal red blood cell (RBC) morphology. We herein report the case of a 72-year-old female patient who was diagnosed with alectinib-induced hemolytic anemia who - in contrast to previous reports - showed a positive DAT result. After discontinuing famotidine and alectinib, the DAT results turned negative; however, when alectinib was resumed, hemolysis recurred. Although alectinib-induced hemolytic anemia has been previously thought to be associated with abnormal morphological changes of the RBCs, we suggest that alectinib-induced anemia may manifest as DAT-positive immune hemolytic anemia because of a complementary effect with other drugs.
Keywords: alectinib, lung cancer, hemolytic anemia, direct antiglobulin test, famotidine
Introduction
Alectinib is an anaplastic lymphoma kinase domain-targeted tyrosine kinase inhibitor (ALK-TKI) and is the standard treatment for ALK-positive lung adenocarcinoma (1). In a summary of adverse events in phase I/II and phase III clinical trials in Japan, the incidence rates of elevated blood bilirubin and anemia were 19.9% and 5.6%, respectively (2). However, the risk of hemolytic anemia has not been thoroughly investigated in clinical trials.
Recent studies have reported cases of alectinib-induced anemia with morphological changes of erythrocytes (3-6). The direct Coombs test [also known as the direct antiglobulin test (DAT)] was negative in these studies. We herein report a case of alectinib-induced hemolytic anemia with a positive DAT. Furthermore, possible interactions with other drugs that can induce immune hemolytic anemia are discussed.
Case Report
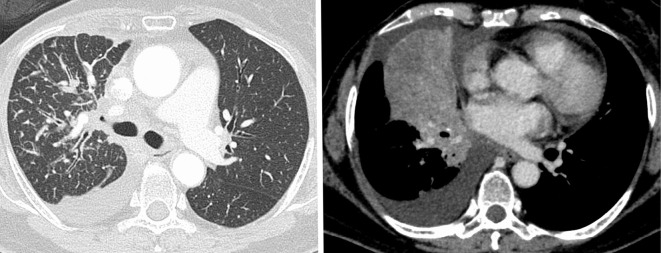
A 77-year-old female patient was referred to our hospital for further examination after experiencing dyspnea for several weeks with abnormal chest X-ray results. The patient presented with hypertension and gastroesophageal reflex, and had been taking candesartan, bisoprolol, and famotidine for several years. There was no family history of hematological disorder. Computed tomography revealed a right hilar tumor and right middle lobe atelectasis with pleural effusion (Fig. 1). After several tests, including transbronchial biopsy, cranial gadolinium-enhanced magnetic resonance imaging, and 18F-fluorodeoxyglucose-positron emission tomography, the patient was diagnosed with ALK-positive lung adenocarcinoma, cT4N3M1c Stage IVB, according to the Union for International Cancer Control, 8th edition. Treatment was started with alectinib (600 mg, daily). Before the initiation of alectinib treatment, the hemoglobin level was 11.7 g/dL.
Figure 1.
Chest computed tomography (before treatment) showed thickening of the interlobular septum and bronchovascular bundles in the right upper lobe and a mass in the right middle lobe. Right pleural effusion was also observed.
On the 42nd day of treatment, blood tests revealed normocytic anemia with a hemoglobin level of 7.6 g/dL. The number of reticulocytes and levels of lactic dehydrogenase (LDH) and bilirubin increased; however, there was no decrease in the levels of ferritin, vitamin B12, or folic acid. Although haptoglobin levels were not decreased, a direct Coombs test was positive (Table 1), and the patient was diagnosed with hemolytic anemia.
Table 1.
Laboratory Data on the 42nd Day of the Alectinib Therapy.
| WBC | 7,180 | /μL | T-Bil | 1.85 | mg/dL | Fe | 48 | μg/dL | |||||
| RBC | 2.56×106 | /μL | LDH | 351 | U/L | ferritin | 879 | ng/mL | |||||
| Hb | 7.6 | g/dL | Alb | 2.2 | g/dL | UIBC | 122 | μg/dL | |||||
| MCV | 88.3 | % | BUN | 17.1 | mg/dL | CH50 | 36.8 | U/mL | |||||
| Ret | 47 | ‰ | Cre | 0.84 | mg/dL | C3 | 133 | mg/dL | |||||
| PLT | 42.3×104 | /μL | Na | 139 | mEq/L | C4 | 28 | mg/dL | |||||
| K | 3.3 | mEq/L | IgG | 1,678 | mg/dL | ||||||||
| AST | 21 | U/L | Cl | 93 | mEq/L | VB12 | 608 | pg/mL | |||||
| ALT | 12 | U/L | Ca | 8.4 | mg/dL | Folic acid | 6.6 | ng/mL | |||||
| ALP (JS) | 263 | U/L | CRP | 7.8 | mg/dL | Hpt | 86 | mg/dL | |||||
| γ-GTP | 13 | U/L | DAT | Positive | |||||||||
Alb: albumin, ALP: alkaline phosphatase, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BUN: blood urea nitrogen, C3: complement 3, C4: complement 4, Ca: calcium, CH50: 50% hemolytic complement activity, Cl: chloride, Cre: creatinine, CRP: C-reactive protein, DAT: direct antiglobulin test, Fe: serum iron, Hb: hemoglobin, Hpt: haptogulobin, IgG: immunoglobulin G, JS: Japan Society of Clinical Chemistry, K: potassium, LDH: lactate dehydrogenase, MCV: mean corpuscular volume, Na: sodium, PLT: platelet, RBC: red blood cell, Ret: reticulocyte, T-Bil: total bilirubin, UIBC: unsaturated iron binding capacity, VB12: vitamin B12, WBC: white blood cell, γ-GTP: γ-glutamyl transpeptidase
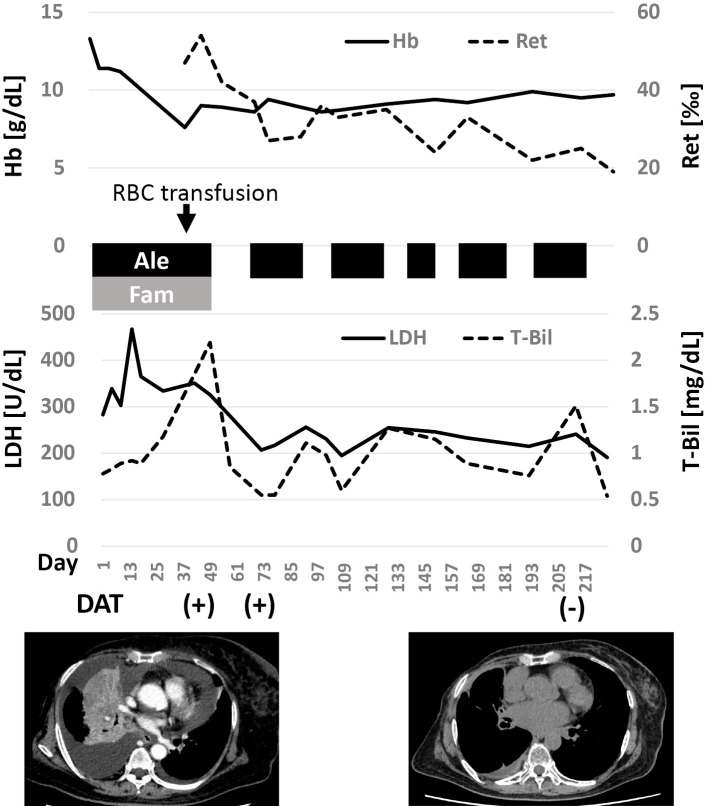
The possibility of drug-induced hemolytic anemia could not be ruled out, so alectinib was discontinued. Famotidine (10 mg twice daily, orally) was also discontinued because of reports of histamine H2 receptor blocker-induced hemolytic anemia (7). The discontinuation of both drugs resulted in a decrease in LDH and bilirubin as well as a decrease in reticulocytes (Fig. 2). The patient required a blood transfusion once, but the anemia did not worsen without alectinib and famotidine. However, when alectinib treatment was resumed, the hemolysis-related test results, such as increased LDH and bilirubin, recurred. The tumor had shrunk by then, and the dyspnea had improved. Therefore, we continued alectinib therapy with a drug withdrawal period. Alectinib (600 mg daily, orally) was continued for 2-4 weeks, pending blood test results related to hemolysis (e.g., hemoglobin, LDH and bilirubin), followed by approximately 2 weeks off the drug. The resumption of alectinib repeatedly increased the LDH and bilirubin levels, but anemia progressed much more slowly than in the first case with famotidine. On the 213th day, a DAT revealed negative results. There has been no tumor regrowth to date. At one year after the initiation of treatment, a peripheral blood smear revealed a change in erythrocyte morphology, but the anemia remained mild (9.1 g/dL) (Fig. 3).
Figure 2.
Changes in laboratory test results after the initiation of alectinib treatment. Alectinib repeatedly induced reticulocytosis and the elevation of LDH and bilirubin, while the degree of anemia was greatest when the patient was first treated with famotidine. Ale: alectinib, Fam: famotidine, DAT: direct antiglobulin test, Hb: hemoglobin, LDH: lactate dehydrogenase, Ret: reticulocyte, T-Bil: total bilirubin
Figure 3.
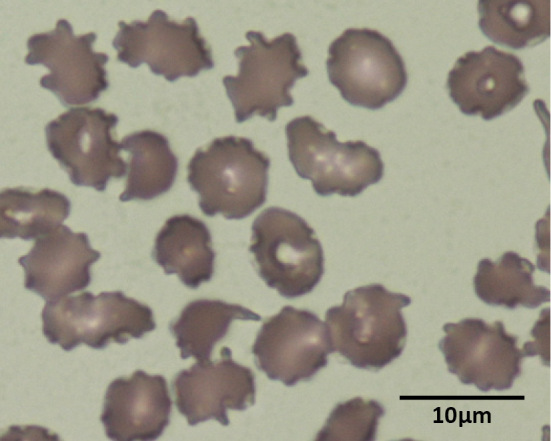
Peripheral blood smears approximately one year after the initiation of alectinib showed the change in erythrocyte morphology. Famotidine was discontinued at this time.
Discussion
Several reports of alectinib-induced hemolytic anemia with abnormal erythrocyte morphology have been published in recent years (Table 2) (3-6). DATs were found to be negative in all of these reports, and peripheral blood smears showed abnormal erythrocyte morphology. The U.S. Food and Drug Administration's adverse event reporting system reported 31 cases of alectinib-induced hemolytic anemia between 2015 and 2021, 11 of which were DAT-negative (8). In a small prospective study, 13 of 19 alectinib-treated patients had anemia, and of the 11 patients (with and without anemia) who were selected for further testing, all had negative DAT results, and all showed morphological changes in erythrocytes after peripheral blood smears (9). Erythrocyte membrane abnormalities have been suggested as a cause of such hemolysis, and a reduction in eosin-5-maleimide binding - as seen in hereditary spherocytosis - has been frequently reported (10). These morphological changes may reduce erythrocyte deformability and lead to splenic entrapment and extravascular hemolysis, similar to the hypothesis in acanthocytosis syndromes (11). Thus, unlike most drug-induced hemolytic anemias, alectinib-induced hemolysis likely results from a non-immunological mechanism.
Table 2.
Case Reports of Alectinib-related Anemia.
| Reference number | Age/ Gender |
Disesases | DAT | Erythrocyte morphological change | Decrease of Hpt |
|---|---|---|---|---|---|
| (3) | 85/M | NSCLC | Negative | (+) | (+) |
| (4) | 62/M | NSCLC, α-thalassaemia | Negative | (+) | (+) |
| (5) | 66/M | NSCLC, β-thalassaemia | Negative | (+) | (+) |
| 77/F | NSCLC | Negative | (+) | (-) | |
| (6) | 79/F | NSCLC | No mention | (+) | No mention |
| 66/M | NSCLC | No mention | (+) | No mention | |
| 14/F | NSCLC | Negative | (+) | (-) | |
| 30/F | Neuroblastoma | No mention | (+) | (-) | |
| 66/M | NSCLC, β-thalassaemia | Negative | (+) | (+) | |
| 77/F | NSCLC | Negative | (+) | (+) |
DAT: direct antiglublin test, Hpt: haptoglobin, NSCLC: non-small cell lung carcinoma
In our case, the patient developed subacute anemia following the initiation of alectinib treatment, and hemolytic anemia was diagnosed based on reticulocytosis, increased LDH, and bilirubin levels. Alectinib was suspected to be involved because the hemolytic findings flared up after the drug was resumed. A decrease in haptoglobin was not observed; however, a reduced haptoglobin level was only seen in 37% of the alectinib-treated patients according to a previous report (9). This low frequency of a reduced haptoglobin level is thought to be due to the hemolysis occurring in the extravascular component. Although peripheral blood smear images could not be adequately evaluated during the diagnosis of hemolytic anemia due to the laboratory system in our hospital, erythrocyte morphology abnormality was later revealed, confirming the diagnosis. However, the initial positive DAT result suggested an immunologic mechanism and was identified as atypical for the type of case.
The patient had been taking famotidine for several years before starting alectinib treatment. Histamine H2 receptor blockers are listed as one of the causes of drug-induced immune hemolytic anemia in the reference guide issued by a research group funded by the Ministry of Health, Labour and Welfare in Japan (7) Therefore, famotidine was withdrawn alongside alectinib. Following the resumption of alectinib, the DAT was negative, and the positive DAT was attributed to famotidine.
Cases of drug-induced immune hemolytic anemia induced by the histamine H2 receptor blocker cimetidine with the appearance of drug-specific and non-specific antibodies have been reported (12,13), and famotidine may also induce the production of these antibodies. In general, two types of antibodies are associated with the pathogenesis of drug-induced immune hemolytic anemia (7,14). Drug-dependent antibodies are antibodies to the drug itself, which is covalently bound to erythrocytes or to a complex of erythrocytes and the drug. Some antibodies of this type lead to fragment crystallizable regions-mediated extravascular hemolysis, and others activate complement and can induce acute severe intravascular hemolysis. Drug-independent antibodies are autoantibodies induced by drugs to erythrocytes themselves. In our case, the relatively gradual or subacute progression of anemia suggested extravascular hemolysis, and the antibodies in this case did not appear to strongly activate complement. That the DAT was still positive at 1 month after the discontinuation of famotidine suggests that drug-independent antibodies may have been primarily involved (Fig. 2).
In our case, the DAT results before alectinib treatment were unknown, and it could not be determined whether antibodies related to the positive DAT were latent before the administration of alectinib or whether they emerged afterward. However, the degree of alectinib-induced anemia decreased after the discontinuation of famotidine, indicating that the complementary effect of these drugs contributed to the manifestation of hemolytic anemia. Although the alectinib-induced hemolysis is likely to be non-immune erythrocyte membranopathy and alectinib alone is unlikely to produce antibodies, as previously discussed (8,10), the erythrocyte morphological change caused by alectinib may have induced the production of anti-RBC antibodies by famotidine or enhanced the affinity of antibodies to erythrocytes that were already being produced. However, the mechanism by which some drugs affect the immune system to produce erythrocyte-associated antibodies remains controversial (14), and further studies are required to elucidate the detailed pathophysiology.
In conclusion, we observed hemolytic anemia attributable to alectinib and a positive DAT. Although abnormal erythrocyte morphology has been previously reported, this case report suggests the involvement of an immunologic mechanism that takes place when other drugs are administered.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 390: 29-39, 2017. [DOI] [PubMed] [Google Scholar]
- 2.ALECENSAⓇ Interview form, 11th ed. 2020. Chugai Pharmaceutical, Ed [Internet]. [cited 2022 Oct 30]. Available from: https://chugai-pharm.jp/content/dam/chugai/product/alc/cap/if/doc/alc_if.pdf (in Japanese).
- 3.Okumoto J, Sakamoto S, Masuda T, et al. Alectinib-induced immune hemolytic anemia in a patient with lung adenocarcinoma. Intern Med 60: 611-615, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li THS, Chik YKJ, Wong WS. Alectinib-induced red cell morphological changes in a patient with underlying α-thalassaemia trait. Int J Lab Hematol 44: 65-66, 2022. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Y, Mapp S, Xu W. Two cases of marked red cell anisopoikilocytosis and haemolysis with alectinib, an anaplastic lymphoma kinase inhibitor. Br J Haematol 190: 642, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Gullapalli V, Xu W, Lewis CR, Anazodo A, Gerber GK. A multi-centre case series of alectinib-related erythrocyte membrane changes and associated haemolysis. J Hematopathol 14: 131-136, 2021. [Google Scholar]
- 7.Grant-in-Aid for Research Committee on the Idiopathic Disorders of Hematopoietic Organs from the Ministry of Health, Labour and Welfare of Japan. Reference guide of autoimmune hemolytic anemia [Internet]. 2022 [cited 2022 Oct 30]. Available from: http://zoketsushogaihan.umin.jp/file/2020/09.pdf (in Japanese).
- 8.Dores GM, Nayernama A, Cheng C, Moureaud C, Jones SC. Hemolytic anemia following alectinib reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Am J Hematol 97: E129-E132, 2022. [DOI] [PubMed] [Google Scholar]
- 9.Kunz J, Wiedemann C, Grosch H, et al. Early development of ubiquitous acanthocytosis and extravascular hemolysis in lung cancer patients receiving alectinib. Cancers (Basel) 14: 2720, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzich JA, Heynemann S, Geoghegan N, et al. Alectinib induces marked red cell spheroacanthocytosis in a near-ubiquitous fashion and is associated with reduced eosin-5-maleimide binding. Pathology 53: 608-612, 2021. [DOI] [PubMed] [Google Scholar]
- 11.Morse EE. Mechanisms of hemolysis in liver disease. Ann Clin Lab Sci 20: 169-174, 1990. [PubMed] [Google Scholar]
- 12.Wu Y, Wu Y, Ji Y, et al. Case report: oral cimetidine administration causes drug-induced immune hemolytic anemia by eliciting the production of cimetidine-dependent antibodies and drug-independent non-specific antibodies. Front Med (Lausanne) 8: 723167, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arndt PA, Garratty G, Brasfield FM, Vemuri SL, Asuncion DJ. Immune hemolytic anemia due to cimetidine: the first example of a cimetidine antibody. Transfusion 50: 302-307, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Garratty G. Drug-induced hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73-79, 2009. [DOI] [PubMed] [Google Scholar]