Abstract
A 90-year-old man on maintenance hemodialysis was admitted due to severe symptomatic anemia. Biopsies under esophagogastroduodenoscopy demonstrated that the cause of anemia was intermittent blood oozing from multiple gastric hyperplastic polyps. Even after successful eradication of Helicobacter pylori, he showed hypergastrinemia (480 pg/mL) owing to esomeprazole (proton-pump inhibitor) therapy for the past 4.5 years to treat reflux esophagitis. Seven months after we switched esomeprazole to famotidine (H2-receptor antagonist), those gastric polyps and anemia were remarkably ameliorated with lowered gastrin levels. This case indicates that long-term use of a proton-pump inhibitor triggers chronic hypergastrinemia, leading to gastric hyperplastic polyps and subsequent severe anemia.
Keywords: hemodialysis, severe anemia, gastric bleeding, gastric hyperplastic polyp, proton-pump inhibitor, gastrin
Introduction
Chronic kidney disease (CKD) is an incurable and progressive disease that is characterized by persistent loss of kidney function (1). The number of patients with CKD is continuously growing with the increased prevalence of CKD risk factors, such as aging, diabetes, and hypertension (1,2). In approximately 1% of those patients, CKD progresses to end-stage kidney disease, the most severe form of CKD (2,3). Patients with end-stage kidney disease need dialysis therapy or kidney transplantation. In Japan, hemodialysis therapy is the most prevalent method for patients with end-stage kidney disease due to very low number of kidney transplants available. Thus, the number of hemodialysis patients is also growing in Japan (4).
Patients undergoing maintenance hemodialysis frequently experience anemia, not only because of renal anemia due to impaired renal erythropoietin synthesis but also because of a negative iron balance owing to reduced iron absorption and blood losses in dialyzers (5). In addition, the risk of upper gastrointestinal bleeding is increased among patients with end-stage kidney disease (6,7), and gastrointestinal bleeding elevates their in-hospital mortality (8).
Gastric and duodenal ulcers are the leading causes of upper gastrointestinal bleeding in patients with end-stage kidney disease (7). One of the risk factors for peptic ulcers is nonsteroidal anti-inflammatory drugs (NSAIDs), which are frequently prescribed to patients on hemodialysis. NSAIDs induce ulcers by interfering with the gastric ability to protect against gastric acids. Consequently, to prevent peptic ulcers, patients on hemodialysis commonly receive a prescription for acid suppressants such as histamine H2-receptor antagonists and proton-pump inhibitors (9). Proton-pump inhibitors suppress gastric acid secretion more potently than H2-receptor antagonists (10). Separately, those acid suppressants are prescribed to treat reflux esophagitis, which is also frequent in patients on hemodialysis (11).
Proton-pump inhibitors are a class of drugs that reduce gastric acid secretion by irreversibly binding to the H+/K+-adenosine triphosphatase (ATPase) enzyme in the parietal cells of the stomach. Proton-pump inhibitors are generally safe for long-term use without many complications (12,13).
We herein report a patient on maintenance hemodialysis who presented with severe anemia from multiple gastric hyperplastic polyps after long-term use of a proton-pump inhibitor for reflux esophagitis.
Case Report
A 90-year-old man was admitted to our hospital because of severe symptomatic anemia. He had started experiencing shortness of breath on exertion and orthostatic syncope one week before admission. He had been receiving maintenance hemodialysis therapy (dialysis modality: postdilution hemodiafiltration, three times a week, four hours per session) as an outpatient at our hospital for two years since developing end-stage kidney disease due to chronic glomerulonephritis and hypertensive nephrosclerosis.
His height and weight were 154 cm and 52.1 kg, respectively, and his body mass index was 21.9. With regard to activities of daily living, he did not have any problems transferring, using the toilet, or eating. He was able to independently walk with a cane. He had never smoked before but drank 20 g of whisky daily. He had not taken any antiplatelet agents or NSAIDs for the past 10 years. We intravenously administered 4,000 units (1,000 units bolus, followed by 750 units/h) of unfractionated heparin as an anticoagulant in each session of hemodialysis.
He had a medical history of hypertension, nephrotic syndrome, reflux esophagitis, and gastric hyperplastic polyps. To keep his systolic blood pressure <150 mmHg, we regularly prescribed multiple antihypertensive agents, including telmisartan (80 mg daily, angiotensin II receptor blocker), nifedipine controlled-release (20 mg daily, calcium channel blocker), amlodipine (5 mg daily, calcium channel blocker), carvedilol (10 mg daily, β-adrenergic blocking agent), and doxazosin (8 mg daily, α-adrenergic blocking agent). His other medications were calcium carbonate (1.5 g daily) for hyperphosphatemia and esomeprazole (20 mg daily) for reflux esophagitis.
Eight years ago, he had been diagnosed with atrophic gastritis and multiple gastric hyperplastic polyps in the body and antrum. At the same time, his rapid urease test had shown a positive result for Helicobacter pylori (H. pylori) infection. Immediately after that diagnosis, he had undergone successful eradication treatment for H. pylori using lansoprazole, amoxicillin, and clarithromycin. At that point, his atrophic gastritis gradually improved, and hyperplastic polyps became remarkably smaller. However, when he started to take a proton-pump inhibitor (esomeprazole, 20 mg daily) for reflux esophagitis (Los Angeles classification: grade C) 4.5 years ago, multiple gastric hyperplastic polyps (diameters: 2-10 mm) appeared in the body and antrum again and increased in number and size.
On admission, his hematological values demonstrated an extremely low level of hemoglobin (6.2 g/dL) and relatively low values for the mean corpuscular volume (MCV; average volume of a single erythrocyte) and mean corpuscular hemoglobin concentration (MCHC; average amount of hemoglobin in a single erythrocyte), as shown in Table. His hemoglobin level had persistently decreased for three months before admission, even though we had increased intravenous injection doses of darbepoetin alfa (erythropoiesis stimulating agent) and saccharated ferric oxide (iron supplementation) to stimulate erythropoiesis for renal anemia. One month before admission, his blood test results showed low levels of serum iron (22 μg/dL) and transferrin saturation (7.9%) with no obvious abnormalities in the levels of folic acid (6.1 ng/mL) or vitamin B-12 (469 pg/mL), as shown in Table. His fecal occult blood test was positive. These findings, combined with the low measurements of MCV and MCHC, suggested iron deficiency anemia due to gastrointestinal bleeding.
Table.
Hematological Data on Admission and One Month before Admission.
| Hematological data on admission | ||
|---|---|---|
| Reference value | Measured value | |
| Hemoglobin (g/dL) | 13.5-17.5 | 6.2 |
| Hematocrit (%) | 39.0-51.0 | 19.9 |
| Mean corpuscular volume (MCV) (femtoliter) | 85-102 | 87 |
| Mean corpuscular hemoglobin concentration (MCHC) (%) | 32.0-36.0 | 31.3 |
| Platelet count (×104/μL) | 12.0-36.0 | 15.9 |
| Hematological data one month before admission | ||
|---|---|---|
| Reference value | Measured value | |
| Serum iron (μg/dL) | 60-200 | 22 |
| Total iron binding capacity (TIBC) (μg/dL) | 250-380 | 277 |
| Transferrin saturation (%) | 20.0-50.0 | 7.9 |
| Serum ferritin (ng/mL) | 13.0-277.0 | 40.4 |
| Folic acid (ng/mL) | 2.4-10.0 | 6.1 |
| Vitamin B-12 (pg/mL) | 233-914 | 469 |
Measurements showed an extremely low level of hemoglobin and relatively low values for the mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) on admission. One month before admission, the serum iron levels and transferrin saturation had been decreased, but no abnormalities had been detected in the folic acid or vitamin B-12 levels. These data suggest that iron deficiency due to chronic gastric bleeding caused severe anemia in this case. Reference values were based on the laboratory data at our hospital.
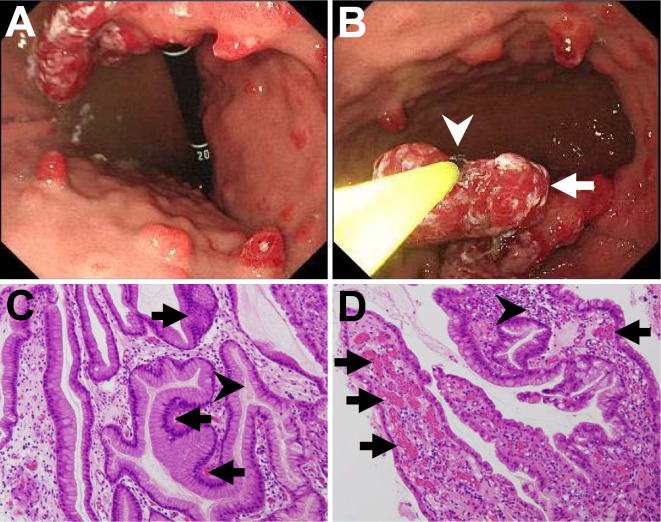
We switched unfractionated heparin to a short-acting anticoagulant, nafamostat methylate, for further hemodialysis therapy. We also performed the first esophagogastroduodenoscopy and total colonoscopy procedures to identify bleeding lesions one month before admission, which detected esophageal hiatal hernia, short segment Barrett's esophagus, atrophic gastritis, multiple gastric polyps, a single colon polyp (8 mm in diameter), and multiple colon diverticula. We found more than 20 gastric polyps (diameters: 5-15 mm) without any bleeding (Fig. 1A, B). Those polyps were mainly located at the gastric body and antrum, and most polyps had redness on their tops, indicating that they were rich in blood vessels. Histology of biopsy specimens from gastric polyps of the body and the antrum showed hyperplasia of the foveolar epithelium with capillary dilation and inflammatory cell infiltration (Fig. 1C, D), so the lesions were therefore diagnosed as multiple gastric hyperplastic polyps. We did not perform polypectomy or epinephrin injection into the polyps to treat the anemia because we did not detect any active bleeding from the polyps.
Figure 1.
Endoscopic images and histologic assessments of polyps at the first esophagogastroduodenoscopy examination performed one month before admission. (A) The endoscopic assessment revealed multiple gastric polyps located at the gastric body. Many polyps showed redness on their tops, indicating their vascular-rich structure. Some polyps were pedunculated. (B) We performed multiple biopsies from those polyps, including a large pedunculated polyp (arrow), using biopsy forceps (arrowhead). (C, D) Histology of a biopsy sample from gastric hyperplastic polyps at the gastric body showed (C) hyperplasia of foveolar epithelium (arrows) and mucus accumulation in the lumens of the elongated gastric glands (arrowhead) and (D) dilated capillaries (arrows) and inflammatory cell infiltration (arrowhead). Mechanical damage to polyps, uremia-related acid injury, use of heparin, and regular alcohol consumption could have caused prolonged oozing of blood from the capillaries in this case. C, D: Hematoxylin and Eosin staining. Magnification ×100.
Clinical course after admission and onward
After admission, an emergent esophagogastroduodenoscopy examination was suspended because he tested positive for coronavirus antigen. For the next 5 days after admission, he was not allowed to eat and received a total of 6 units of packed red blood cells, as his hemoglobin level was <7 g/dL (14). After transfusion, his hemoglobin level increased to 8.9 g/dL (Fig. 2A). Serum quantification of anti-H. pylori immunoglobulin G (IgG) using a latex agglutination test was negative (9 U/mL, positive if the value is >10), denying currently active H. pylori infection.
Figure 2.
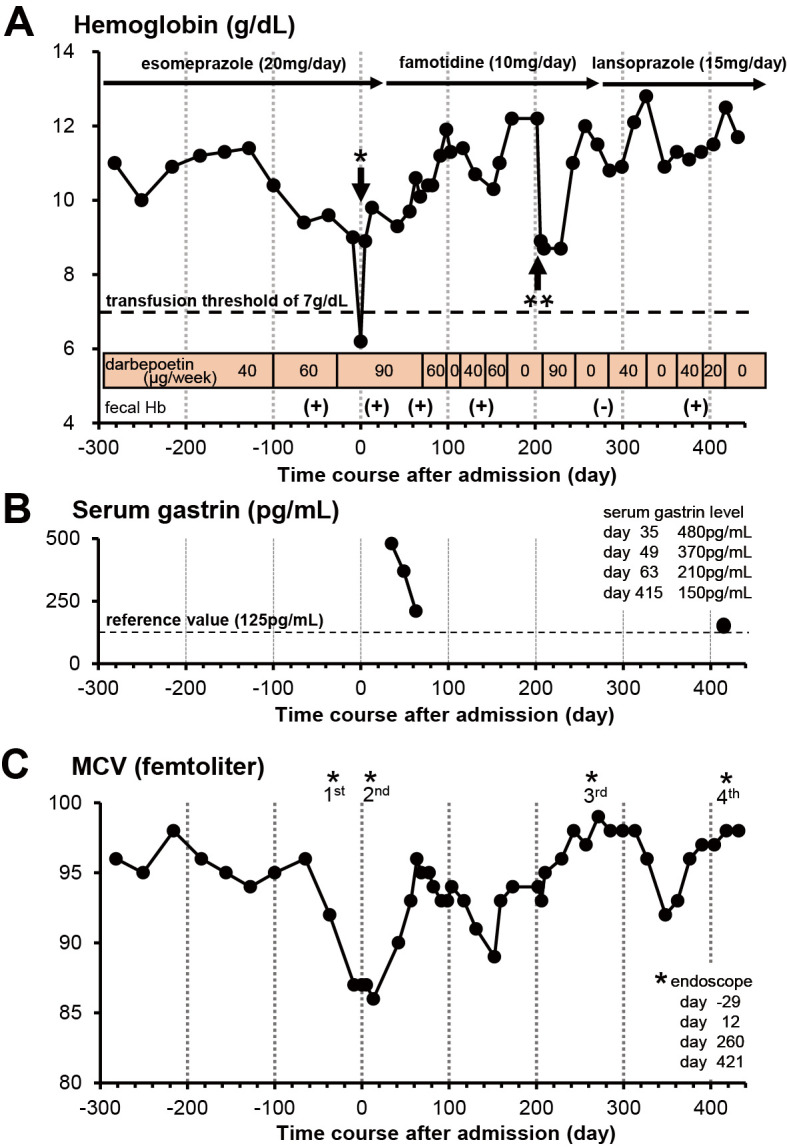
Measurements of hemoglobin, serum gastrin, and mean corpuscular volume (MCV) along the time course before and after admission. (A) Measurements of the hemoglobin level (g/dL). Day 0 denotes the day of admission to our hospital. After we changed esomeprazole (20 mg daily) to famotidine (10 mg daily) (35 days after admission), his hemoglobin level trended upward to 12 g/dL. Accordingly, we were able to decrease the dose of darbepoetin alfa (erythropoiesis-stimulating agent) from 90 to 40 μg/week. However, bleeding from the hyperplastic polyps continued, as indicated by positive findings of fecal occult blood tests (fecal Hb). Although his fecal occult blood test was negative at day 271, it became positive after he started to take a low dose of lansoprazole for 5 months. A single asterisk indicates the transfusion of 6 units of packed red blood cells at day 5. A double asterisk indicates the progression of anemia due to bipolar hip arthroplasty for his left femoral neck fracture at day 205. A horizontal dotted line indicates the hemoglobin level (7 g/dL), below which blood transfusion is necessary (14). (B) Measurements of the serum gastrin levels (pg/mL). Day 0 denotes the day of admission to our hospital. Serum gastrin levels quickly decreased within 30 days after esomeprazole was switched to famotidine. Subsequently, the hemoglobin levels increased to 11 g/dL, as shown in (A). The serum gastrin level was still close to the upper limit of our reference value (125 pg/mL) at day 415 postadmission, even though we had prescribed a low dose of lansoprazole. A horizontal dotted line indicates the upper limit of our reference value (125 pg/mL). (C) Measurements of the mean corpuscular volume (MCV) (femtoliter). Day 0 denotes the day of admission to our hospital. In our case, low MCV values imply iron deficiency anemia due to bleeding from gastric hyperplastic polyps. MCV values decreased at day 152, probably because some gastric hyperplastic polyps had incompletely healed. MCV values decreased again at day 348, probably because bleeding from hyperplastic polyps recurred with a low dose of lansoprazole (15 mg daily). Asterisks indicate the examination time points of esophagogastroduodenoscopy (days -29, 12, 260, and 421).
We performed the second esophagogastroduodenoscopy procedure 12 days after admission. We still detected more than 20 gastric hyperplastic polyps, although most of the gastric hyperplastic polyps had slightly decreased in size, probably due to the lowered serum gastrin levels without food intake. He continued to take esomeprazole 20 mg daily.
He was discharged from our hospital 26 days after admission. However, his hemoglobin level was still <10 g/dL even though we continued to administer a high dose of darbepoetin alfa (90 μg/week) (Fig. 2A). Although we performed chest and abdominal CT, no other bleeding sources were detected. Therefore, we concluded that the cause of the severe anemia had been intermittent blood oozing from the multiple gastric hyperplastic polyps. Based on findings of multiple gastric hyperplastic polyps and atrophic gastritis after H. pylori eradication, we speculated that autoimmune gastritis or long-term use of a proton-pump inhibitor had induced chronic hypergastrinemia through severely impaired gastric acid secretion, leading to remarkable proliferation of gastric epithelial cells of the foveolar mucosa (15-17).
Although we did not measure anti-parietal cell antibody levels to screen for autoimmune gastritis, we did not detect anti-thyroglobulin antibody or anti-thyroid microsome antibody, negating the possibility of autoimmune thyroid diseases, which are frequently detected in patients with autoimmune gastritis (18). The serum level of fasting gastrin increased to 480 pg/mL (reference value at our hospital: 20-125 pg/mL).
Thirty-five days after admission, we changed a proton-pump inhibitor (esomeprazole, 20 mg daily) to an H2 receptor antagonist (famotidine, 10 mg daily) and rebamipide (300 mg daily) to test whether or not long-term use of a proton-pump inhibitor triggered the development of multiple gastric hyperplastic polyps. His hypergastrinemia gradually ameliorated, and his serum gastrin levels decreased to 210 pg/mL over the next 30 days after cessation of esomeprazole (Fig. 2B). Furthermore, his hemoglobin levels slowly increased to 11 g/dL, and doses of darbepoetin alfa were consequently reduced to 40-60 μg/week during the next 2 months, although fecal occult blood tests were still positive (Fig. 2A).
The possibility of current H. pylori infection was denied again by negative results of the urea breath test and stool H. pylori antigen test. Although his hemoglobin level decreased temporarily to 8.9 g/dL due to bipolar hip arthroplasty for his left femoral neck fracture, we were able to manage his anemia by increasing the dose of darbepoetin alfa (Fig. 2A).
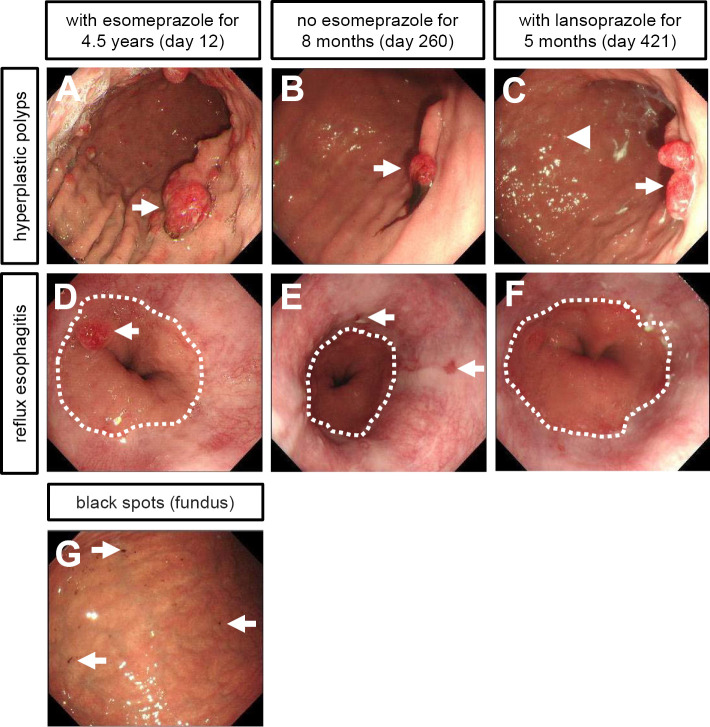
Seven months after we stopped prescription of esomeprazole, we confirmed that all gastric hyperplastic polyps had greatly decreased in size and number at the third esophagogastroduodenoscopy examination (Fig. 3B). There was no active blood oozing from those contracted gastric hyperplastic polyps. Finally, his fecal occult blood test turned negative 271 days after admission (8 months after cessation of esomeprazole) (Fig. 2A).
Figure 3.
Endoscopic images of multiple gastric hyperplastic polyps and reflux esophagitis at the 2nd, 3rd, and 4th esophagogastroduodenoscopy examinations. The 2nd, 3rd, and 4th endoscopic tests were performed 12, 260, and 421 days after admission, respectively. (A-C) Time-course changes in gastric hyperplastic polyps in the gastric body. (A) 2nd endoscopic test. Multiple gastric hyperplastic polyps had formed due to the long-term use of esomeprazole. Some polyps were pedunculated (arrow). (B) 3rd endoscopic test. Most gastric hyperplastic polyps had disappeared or regressed without esomeprazole for eight months. One pedunculated polyp remained (arrow). (C) 4th endoscopic test. Small gastric hyperplastic polyps (arrowhead) had relapsed with a low dose of lansoprazole for five months. One pedunculated polyp increased in size (arrow). (D-F) Time-course changes in reflux esophagitis. A dotted line denotes the gastroesophageal junction. (D) 2nd endoscopic test. No esophagitis was detected with the long-term use of esomeprazole, whereas esophageal hiatus hernia and one hyperplastic polyp (arrow) in the junctional mucosa were detected. (E) 3rd endoscopic test. Reflux esophagitis had developed without esomeprazole for eight months. Arrows indicate multiple mucosal breaks (Los Angeles classification grade B). (F) 4th endoscopic test. Reflux esophagitis had been ameliorated with a low dose of lansoprazole for five months. (G) The 3rd endoscopic test identified black spots in the gastric mucosa of the fundus, a typical finding in patients treated with a proton-pump inhibitor.
However, the third esophagogastroduodenoscopy examination also revealed recurrence of reflux esophagitis (Los Angeles classification: grade B) (Fig. 3E). His symptom of upper abdominal discomfort recurred and worsened. Therefore, we replaced famotidine with a low dose of proton-pump inhibitor (lansoprazole 15 mg daily). Apart from these findings, we found black spots in the gastric mucosa at the gastric fundus (Fig. 3G), which are typically detected in patients taking proton-pump inhibitors or after H. pylori eradication (19). Four months after he started to take lansoprazole, his fecal occult blood test was positive again. The measurements of MCV were also lowered (Fig. 2C), suggesting relapse of iron deficiency due to gastrointestinal bleeding.
The fourth esophagogastroduodenoscopy examination revealed that one pedunculated hyperplastic polyp had increased in size, showing redness on its top (Fig. 3C). We found a few hyperplastic polyps and no recurrence of reflux esophagitis (Los Angels classification: grade M) (Fig. 3C, F). His serum gastrin level was slightly elevated (150 pg/mL) above the upper limit of our reference value (125 pg/mL) with lansoprazole therapy. To prevent deterioration of reflux esophagitis, we decided to continuously prescribe a low dose of lansoprazole and periodically monitor the formation of gastric hyperplastic polyps by follow-up esophagogastroduodenoscopy.
Discussion
We encountered a rare case of a hemodialysis patient presenting with severe anemia due to intermittent bleeding from gastric hyperplastic polyps. Discontinuation of a proton-pump inhibitor (esomeprazole) decreased serum gastrin levels, followed by shrinkage of gastric hyperplastic polyps and amelioration of bleeding from those polyps. When this patient restarted a low dose of proton-pump inhibitor (lansoprazole), small hyperplastic polyps relapsed with a slightly elevated serum gastrin level. To our knowledge, this is the first case to demonstrate that discontinuation of proton-pump inhibitor treatment improves hypergastrinemia, hyperplastic polyp formation, and bleeding from those polyps in a hemodialysis patient.
Generally, peptic ulcers and gastric antral vascular ectasia are the most frequent causes of gastric bleeding in patients with advanced CKD (6,20), implying that gastric hyperplastic polyps are not a major cause of gastric bleeding. The present patient was prescribed a proton-pump inhibitor to treat reflux esophagitis for 4.5 years, which persistently promoted the formation of multiple gastric hyperplastic polyps through chronic hypergastrinemia. End-stage kidney disease can worsen hypergastrinemia because diseased kidneys cannot efficiently metabolize or inactivate serum gastrin (21). Experimental data using canine nephrectomy models demonstrated that the kidneys are an important organ for the metabolic clearance of exogenous gastrin (22). In our case, hyperplastic polyps were histologically demonstrated to be rich in small blood vessels (Fig. 1D). Repeated food intake mechanically damages the fragile mucosal membrane of hyperplastic polyps and periodically triggers blood oozing from gastric polyps. Chronic uremia due to end-stage kidney disease can render the gastric mucosa more susceptible to acid injury by impairment of the gastric mucosal barrier (23), although long-term use of a proton-pump inhibitor suppressed gastric acid secretion in our case. Furthermore, the use of heparin per hemodialysis session and regular alcohol consumption (20 g/day) can prolong such oozing by compromising coagulation systems (24,25). These conditions may have contributed to the severe anemia from hyperplastic polyps in this case.
Constitutive elevation of serum gastrin levels induces hyperplasia of parietal cells, enterochromaffin-like cells, and foveolar epithelial cells in transgenic mice (26,27). When the number or function of parietal cells is impaired by H. pylori infection (H. pylori-related atrophic gastritis), autoimmune disease (autoimmune gastritis), or proton-pump inhibitors, serum gastrin levels are increased to compensate for impaired acid secretion from parietal cells (15-17). Immunohistochemical staining of human hyperplastic polyps in the stomach revealed that the gastrin receptor is abundantly expressed in proliferating foveolar epithelial cells and that its expression is upregulated in regenerating injured mucosa after H. pylori infection (28,29). Mucosal H. pylori infection itself promotes gastrin synthesis in gastrin-producing G cells and accelerates hyperplasia of foveolar epithelial cells (30). Consistent with this finding, one clinical study of patients with H. pylori infection demonstrated that H. pylori eradication therapy causes the gradual disappearance of gastric hyperplastic polyps with reduced serum gastrin levels (31). In our case, cessation of esomeprazole ameliorated hypergastrinemia and polyp formation. Therefore, we concluded that chronic hypergastrinemia played a central role in hyperplastic polyp formation in our case.
Proton-pump inhibitors produce greater increases in serum gastrin levels than H2-receptor antagonists as a consequence of a greater degree of acid suppression by proton-pump inhibitors (32). Therefore, we were able to ameliorate the hypergastrinemia and hyperplastic polyps after switching esomeprazole to famotidine. Similarly, when a more potent proton-pump inhibitor, vonoprazan (also called a potassium-competitive acid blocker or P-CAB), was prescribed to patients with erosive esophagitis, it increased serum gastrin levels more than lansoprazole (33), suggesting that vonoprazan may be a potent inducer of hyperplastic polyps after its long-term use. We prescribed rebamipide in our case because long-term rebamipide therapy counteracted hypergastrinemia by repairing gastric mucosal damage in patients with H. pylori infection (34). In our case, hyperplastic polyps were slightly shrunken by abstinence from food after admission, probably because food intake stimulates the formation of polyps via an increase in serum gastrin levels (35).
Although proton-pump inhibitors are one of the most frequently used classes of medications among patients receiving hemodialysis therapy, not all patients develop gastric hyperplastic polyps after long-term use of proton-pump inhibitors. Hyperplastic or fundic polyps were found only in 7-9% of nondialysis patients taking proton-pump inhibitors for long-term treatment of gastroesophageal reflux diseases (17,36). It took more than 30 months to form gastric hyperplastic polyps since patients started to take a proton-pump inhibitor (36). However, hypergastrinemia was rapidly induced within a few weeks after proton-pump inhibitor treatment was initiated (37). To our knowledge, there have been no reports demonstrating the formation of multiple gastric hyperplastic polyps in patients with gastrin-secreting tumors where serum gastrin levels were markedly elevated. These findings suggest that the formation of hyperplastic polyps occurs at a slow speed and that hypergastrinemia itself is not enough to trigger polyp formation. Previous studies suggest that gastric mucosal injury is a critical trigger of hyperplastic polyp formation because hyperplastic polyps result from excessive mucosal healing (38). Indeed, gastric hyperplastic polyps arise from damaged mucosa due to peptic ulcers (39,40), partial gastrectomy (41), or argon plasma laser photocoagulation for gastric antral vascular ectasia (42,43). Most likely, long-term use of a proton-pump inhibitor promotes hyperplastic polyp formation, particularly in gastric mucosa with chronic injury. Similar to our case, there were multiple cases where proton-pump inhibitor-related hyperplastic polyps were detected in gastric mucosa without H. pylori infection (28,44,45). Further research is necessary to identify risk factors for hyperplastic polyp formation.
At the third esophagogastroduodenoscopy examination in our patient, we found multiple black spots in the gastric mucosa of the fundus (Fig. 3G). As we mentioned above, hypergastrinemia induces the proliferation of parietal cells and foveolar epithelial cells, which causes parietal cell protrusion and plugging by mucus secreted from proliferating foveolar cells. This results in outflow obstruction and cystic dilation of fundic glands (46). Our study also detected that mucus permeates the lumens of elongated gastric glands (Fig. 1C). Histologically, black spots were often identified as brownish pigmentation in fundic gland cysts of patients taking proton-pump inhibitors or H. pylori eradication therapy (19). Thus, it is possible that the mucosal black spots in our case developed due to the long-term use of a proton-pump inhibitor.
Long-term use of a proton-pump inhibitor may increase gastric cancer development in patients with H. pylori-related chronic gastritis (47,48) or advanced autoimmune gastritis (49), where gastric mucosal tissues are persistently injured. Those patients are likely to develop gastric hyperplastic polyps. Thus, when we detect hyperplastic polyps in patients with severe gastritis and proton-pump inhibitor treatment, we need to monitor them for cancer development.
Although proton-pump inhibitors are generally safe for long-term use without many complications, clinicians need to assess whether or not patients benefit from medication with proton-pump inhibitors and whether or not they require current doses of these drugs based on clinical findings to avoid adverse effects of proton-pump inhibitors. For example, proton-pump inhibitors do not prevent NSAID- or aspirin-related lower gastrointestinal bleeding (50); in fact, it has been debated whether or not proton-pump inhibitors increase small bowel injuries related to NSAID or low-dose aspirin treatment by inducing changes in the intestinal microbiota (50-52). It has been demonstrated that a certain proportion of proton-pump inhibitor users on long-term therapy are able to discontinue this medication without adverse effects (53,54).
In conclusion, our findings in the present hemodialysis patient indicate that long-term use of a proton-pump inhibitor triggers chronic hypergastrinemia, leading to multiple gastric hyperplastic polyps and subsequent severe anemia. To our knowledge, this is the first case of a hemodialysis patient in whom discontinuation of a proton-pump inhibitor ultimately improved severe anemia due to upper gastrointestinal bleeding. Discontinuation or dose reduction of proton-pump inhibitors should be considered when gastric hyperplastic polyps are detected by an endoscopic assessment.
We obtained written informed consent from the patient before we started preparation of our manuscript.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Yuka Endoh and Hiroshi Itoh (Department of Gastroenterology, Takashimadaira Chūō General Hospital, Tokyo, Japan) for endoscopic examination and Miho Matsukawa (Department of Gastrointestinal Endoscopy, Tokyo Metropolitan Geriatric Medical Center, Tokyo, Japan) for providing the past endoscopic information of this case. The authors acknowledge the utility of the microscope image acquisition system of the Department of Pathology, Funabashi Futawa Hospital (Chiba, Japan). Takashimadaira Chūō General Hospital (Medical Affairs Division) bore part of the cost for the serum gastrin measurement.
References
- 1.Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen B, Perkovic V. Chronic kidney disease. Lancet 398: 786-802, 2021. [DOI] [PubMed] [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 382: 260-272, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Anderson S, Halter JB, Hazzard WR, et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 20: 1199-1209, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Nitta K, Goto S, Masakane N, et al. Annual dialysis date report for 2018, JSDT Renal Data Registry: survey methods, facility data, incidence, prevalence, and mortality. Ren Replace Ther 6: 41, 2020. [Google Scholar]
- 5.Macdougall IC, White C, Anker SD, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 380: 447-458, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Wasse H, Gillen DL, Ball AM, et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int 64: 1455-1461, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Kuo CC, Kuo HW, Lee IM, Lee CT, Yang CY. The risk of upper gastrointestinal bleeding in patients treated with hemodialysis: a population-based cohort study. BMC Nephrol 14: 15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sood P, Kumar G, Nanchal R, et al. Chronic kidney disease and end-stage renal disease predict higher risk of mortality in patients with primary upper gastrointestinal bleeding. Am J Nephrol 35: 216-224, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saran R, Robinson B, Abbott KC, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 71: A7, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt RH. Importance of pH control in the management of GERD. Arch Intern Med 159: 649-657, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Karahan D, Sahin I. Comparison of gastrointestinal symptoms and findings in renal replacement therapy modalities. BMC Nephrol 23: 261, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology 153: 35-48, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto K, Hongo M; Maintenance Study Group. Safety and efficacy of long-term maintenance therapy with oral dose of rabeprazole 10 mg once daily in Japanese patients with reflux esophagitis. Intern Med 50: 179-188, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 368: 11-21, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol 25: 500-507, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Haruma K, Yoshihara M, Sumii K, et al. Gastric acid secretion, serum pepsinogen I, and serum gastrin in Japanese with gastric hyperplastic polyps or polypoid-type early gastric carcinoma. Scand J Gastroenterol 28: 633-637, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Hongo M, Fujimoto K, Gastric Polyps Study Group.. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol 45: 618-624, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Lahner E, Centanni M, Agnello G, et al. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am J Med 121: 136-141, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Hatano Y, Haruma K, Ayaki M, et al. Black spot, a novel gastric finding potentially induced by proton pump inhibitors. Intern Med 55: 3079-3084, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuckerman GR, Cornette GL, Clouse RE, Harter HR. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med 102: 588-592, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Wesdorp RI, Falco HA, Banks PB, Martino J, Fischer JE. Gastrin and gastric acid secretion in renal failure. Am J Surg 141: 334-338, 1981. [DOI] [PubMed] [Google Scholar]
- 22.Clendinnen BG, Reeder DD, Brandt EN Jr, Thompson JC. Effect of nephrectomy on the rate and pattern of the disappearance of exogenous gastrin in dogs. Gut 14: 462-467, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintero E, Kaunitz J, Nishizaki Y, De Giorgio R, Sternini C, Guth PH. Uremia increases gastric mucosal permeability and acid back-diffusion injury in the rat. Gastroenterology 103: 1762-1768, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Shen JI, Winkelmayer WC. Use and safety of unfractionated heparin for anticoagulation during maintenance hemodialysis. Am J Kidney Dis 60: 473-486, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukamal KJ, Jadhav PP, D'Agostino RB, et al. Alcohol consumption and hemostatic factors: analysis of the Framingham Offspring cohort. Circulation 104: 1367-1373, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918-1929, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118: 36-47, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto S, Kato M, Matsuda K, et al. Gastric hyperplastic polyps associated with proton pump inhibitor use in a case without a history of Helicobacter pylori infection. Intern Med 56: 1825-1829, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takamura A, Ito M, Boda T, et al. High expression of gastrin receptor protein in injured mucosa of Helicobacter pylori-positive gastritis. Dig Dis Sci 58: 634-640, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Gunawardhana N, Jang S, Choi YH, et al. Helicobacter pylori-induced HB-EGF upregulates gastrin expression via the EGF receptor, c-Raf, Mek1, and Erk2 in the MAPK pathway. Front Cell Infect Microbiol 7: 541, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohkusa T, Takashimizu I, Fujiki K, et al. Disappearance of hyperplastic polyps in the stomach after eradication of Helicobacter pylori. A randomized, controlled trial. Ann Intern Med 129: 712-715, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Lanzon-Miller S, Pounder RE, Hamilton MR, et al. Twenty-four-hour intragastric acidity and plasma gastrin concentration before and during treatment with either ranitidine or omeprazole. Aliment Pharmacol Ther 1: 239-251, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Laine L, DeVault K, Katz P, et al. Vonoprazan versus lansoprazole for healing and maintenance of healing of erosive esophagitis: a randomized trial. Gastroenterology 164: 61-71, 2023. [DOI] [PubMed] [Google Scholar]
- 34.Haruma K, Ito M, Kido S, et al. Long-term rebamipide therapy improves Helicobacter pylori-associated chronic gastritis. Dig Dis Sci 47: 862-867, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Korman MG, Soveny C, Hansky J. Effect of food on serum gastrin evaluated by radioimmunoassay. Gut 12: 619-624, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choudhry U, Boyce HW, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol 110: 615-621, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas P. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther 42: 649-663, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Jain R, Chetty R. Gastric hyperplastic polyps: a review. Dig Dis Sci 54: 1839-1846, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka J, Fujimoto K, Iwakiri R, et al. Hyperplastic polyps following treatment of acute gastric ulcers. Intern Med 33: 366-368, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Chang WH, Shih SC, Wang HY, Chang CW, Chen CJ, Chen MJ. Acquired hyperplastic gastric polyps after treatment of ulcer. J Formos Med Assoc 109: 567-573, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Stemmermann GN, Hayashi T. Hyperplastic polyps of the gastric mucosa adjacent to gastroenterostomy stomas. Am J Clin Pathol 71: 341-345, 1979. [DOI] [PubMed] [Google Scholar]
- 42.Shah N, Cavanagh Y, Kaswala DH, Shaikh S. Development of hyperplastic polyps following argon plasma coagulation of gastric antral vascular ectasia. J Nat Sci Biol Med 6: 479-482, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishino K, Kawanaka M, Suehiro M, et al. Gastric hyperplastic polyps after argon plasma coagulation for gastric antral vascular ectasia in patients with liver cirrhosis: a case suggesting the “gastrin link theory”. Intern Med 60: 1019-1025, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasugi K, Haruma K, Kawanaka H, et al. Disappearance of gastric hyperplastic polyps after the discontinuation of proton pump inhibitor in a patient with liver cirrhosis. Case Rep Gastroenterol 15: 202-209, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubo K, Kimura N, Maiya N, et al. Proton pump inhibitor-associated large hyperplastic polyp in non-Helicobacter pylori-infected stomach. Case Rep Gastroenterol 15: 539-544, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Synnerstad I, Holm L. Omeprazole induces high intraglandular pressure in the rat gastric mucosa. Gastroenterology 112: 1221-1230, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Hagiwara T, Mukaisho KI, Nakayama T, Sugihara H, Hattori T. Long-term proton pump inhibitor administration worsens atrophic corpus gastritis and promotes adenocarcinoma development in Mongolian gerbils infected with Helicobacter pylori. Gut 60: 624-630, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 67: 28-35, 2018. [DOI] [PubMed] [Google Scholar]
- 49.Dilaghi E, Bellisario M, Esposito G, Carabotti M, Annibale B, Lahner E. The impact of proton pump inhibitors on the development of gastric neoplastic lesions in patients with autoimmune atrophic gastritis. Front Immunol 13: 910077, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lué A, Lanas A. Proton pump inhibitor treatment and lower gastrointestinal bleeding: balancing risks and benefits. World J Gastroenterol 22: 10477-10481, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 141: 1314-1322, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Nagata N, Niikura R, Aoki T, et al. Effect of proton-pump inhibitors on the risk of lower gastrointestinal bleeding associated with NSAIDs, aspirin, clopidogrel, and warfarin. J Gastroenterol 50: 1079-1086, 2015. [DOI] [PubMed] [Google Scholar]
- 53.Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 121: 1095-1100, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Björnsson E, Abrahamsson H, Simrén M, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther 15: 945-954, 2006. [DOI] [PubMed] [Google Scholar]