Abstract
目的
探究miR-515-5p抑制骨关节炎(osteoarthritis,OA)软骨细胞凋亡、缓解炎症反应的分子机制。
方法
体外培养人软骨细胞系C28/I2,使用10 ng/mL IL-1β处理细胞24 h构建体外OA模型;另外,分别采用miR mimics、mimics阴性对照(negative control,NC)、过表达(over expression,oe)-NC和oe-Toll样受体4(Toll-like receptor 4,TLR4)转染C28/I2细胞后,使用10 ng/mL IL-1β处理各组细胞24 h构建OA模型。采用细胞计数试剂盒8和EdU检测细胞增殖能力,流式细胞术检测细胞凋亡和细胞周期,Western blot检测B淋巴细胞瘤2蛋白(B-cell lymphoma 2 protion,Bcl-2)、Bcl-2相关X蛋白(Bcl-2-associated X protein,Bax)、裂解的半胱天冬酶3(cleaved-Caspase-3)、TLR4、髓样分化因子88(myeloid differentiation primary response gene 88,MyD88)、p65及磷酸化p65(phosphorylated p65,p-p65)蛋白的表达水平,实时荧光定量PCR检测miR-515-5p、TLR4 mRNA表达水平,ELISA检测细胞上清液中促炎因子前列腺素E2(prostaglandin E2,PGE2)、TNF-α、IL-6的水平。通过BiBiServ2数据库预测miR-515-5p和TLR4之间的潜在结合位点,并采用双荧光素酶报告实验验证miR-515-5p和TLR4的靶向关系。
结果
采用IL-1β处理C28/I2细胞后,miR-515-5p、Bcl-2蛋白的表达及细胞增殖能力均显著降低,Bax和cleaved-Caspase-3蛋白表达水平、细胞上清液中促炎因子(PGE2、TNF-α、IL-6)水平及细胞凋亡率均显著增加;此外,S期和G2期细胞比例显著降低,G1期细胞比例显著增加,提示IL-1β处理后细胞周期受到阻滞。而转染miR mimics后,细胞中miR-515-5p表达水平显著上调,部分逆转了IL-1β诱导的OA软骨细胞凋亡,缓解了OA软骨细胞的周期阻滞和炎症反应。采用IL-1β处理C28/I2细胞后,TLR4的mRNA和蛋白水平均显著升高;过表达miR-515-5p后,靶向抑制了TLR4的表达并且阻断了MyD88/NF-κB通路的激活。而过表达TLR4可部分逆转miR mimics对IL-1β诱导的OA软骨细胞凋亡及炎症的改善作用。
结论
miR-515-5p靶向负调控TLR4的表达,抑制了MyD88/NF-κB通路激活以及OA软骨细胞凋亡,并有效缓解了细胞炎症反应。
Keywords: 骨关节炎, 软骨细胞, 炎症反应, miR-515-5p, Toll样受体4, 细胞凋亡, 细胞周期
Abstract
Objective
To explore the molecular mechanism of miR-515-5p in inhibiting chondrocyte apoptosis and alleviating inflammatory response in osteoarthritis (OA).
Methods
Human cartilage cell line C28/I2 was cultured in vitro and treated with 10 ng/mL interleukin 1β (IL-1β) for 24 hours to construct an in vitro OA model. C28/I2 cells were transfected with miR mimics, mimics negative control (NC), over expression (oe)-NC, and oe-Toll-like receptor 4 (TLR4), respectively, and then treated with 10 ng/mL IL-1β for 24 hours to establish OA model. Cell proliferation capacity was detected by cell counting kit 8 and 5-Ethynyl-2’-deoxyuridine, cell apoptosis and cell cycle were detected by flow cytometry, and B-cell lymphoma 2 protion (Bcl-2), Bcl-2-associated X protein (Bax), cleaved-Caspase-3, TLR4, myeloid differentiation primary response gene 88 (MyD88), p65 and phosphorylated p65 (p-p65) protein expression levels were detected by Western blot. Real-time fluorescence quantitative PCR was used to detect mRNA expression levels of miR-515-5p and TLR4, and ELISA was used to detect pro-inflammatory factor prostaglandin E2 (PGE2), tumor necrosis factor α (TNF -α), and IL-6 levels in cell supernatant. The potential binding sites between miR-515-5p and TLR4 were predicted by BiBiServ2 database, and the targeting relationship between miR-515-5p and TLR4 was verified by dual luciferase reporting assay.
Results
After the treatment of C28/I2 cells with IL-1β, the expressions of miR-515-5p and Bcl-2 protein and the proliferation ability of C28/I2 cells significantly reduced. The expression levels of Bax and cleaved-Caspase-3 protein, the levels of pro-inflammatory factors (PGE2, TNF-α, IL-6) in the supernatant of C28/I2 cells, and the apoptosis of C28/I2 cells significantly increased. In addition, the proportion of the cells at S phase and G2 phase decreased significantly, and the proportion of cells at G1 phase increased significantly, suggesting that the cell cycle was blocked after IL-1β treatment. After transfection with miR mimics, the expression level of miR-515-5p in the cells significantly up-regulated, partially reversing the apoptosis of OA chondrocytes induced by IL-1β, and alleviating the cycle arrest and inflammatory response of OA chondrocytes. After treating C28/I2 cells with IL-1β, the mRNA and protein levels of TLR4 significantly increased. Overexpression of miR-515-5p targeted inhibition of TLR4 expression and blocked activation of MyD88/nuclear factor κB (NF-κB) pathway. Overexpression of TLR4 could partially reverse the effect of miR mimics on IL-1β-induced apoptosis and inflammation of OA chondrocytes.
Conclusion
miR-515-5p negatively regulates the expression of TLR4, inhibits the activation of MyD88/NF-κB pathway and apoptosis of OA chondrocytes, and effectively alleviates the inflammatory response of the cells.
Keywords: Osteoarthritis, chondrocyte, inflammatory response, miR-515-5p, Toll-like receptor 4, cell apoptosis, cell cycle
骨关节炎(osteoarthritis,OA)是一种退行性关节疾病,主要表现为关节软骨的渐进性损伤、软骨细胞凋亡以及关节炎症,导致患者身体残疾,严重影响生活质量[1-2]。研究表明,软骨细胞凋亡以及促炎因子的产生与OA进展密切相关[3-4]。因此,抑制软骨细胞凋亡和缓解炎症反应是治疗OA的有效措施。然而,OA的具体发病机制尚不明确。
miRNA是一类非编码小RNA,其可通过靶向结合mRNA的特定互补序列,从而负调控基因表达[5],且与OA等多种疾病的发展紧密相关[6-7]。研究表明,在OA发生过程中,miR-515-5p可能作为内源性竞争RNA的中间因子,其表达缓解了IL-1β诱导的软骨细胞凋亡、炎症反应和细胞外基质降解[8]。Toll样受体4(Toll-like receptor 4,TLR4)是Toll样受体家族成员之一且与多种炎症性疾病密切相关,因其能够识别OA中微生物或宿主衍生配体而被广泛关注[9-11]。研究表明,TLR4在OA软骨和活化的滑膜细胞中表达[12],抑制TLR4的表达能够减少促炎因子产生,从而减轻OA[13]。TLR4通过激活髓样分化因子88(myeloid differentiation primary response gene 88,MyD88)/NF-κB信号通路释放促炎因子,从而促进炎症反应[14];而抑制MyD88/NF-κB信号通路能够有效缓解OA进展[15],提示TLR4/MyD88/NF-κB信号通路可能在OA进展中发挥重要作用。但目前关于miRNA与TLR4/MyD88/NF-κB信号通路在OA中作用机制的研究较少。本研究拟对此进行探究,为OA防治提供有效治疗策略。
1. 材料与方法
1.1. 主要试剂及仪器
人软骨细胞C28/I2、HEK293T细胞(上海雅吉生物科技有限公司)。mimics阴性对照(negative control,NC)和mimics miR(MedChemExpress公司,美国);pcDNA-TLR4和pcDNA3.1质粒(上海吉玛制药技术有限公司);pGL3-TLR4-MUT、pGL3-TLR4-WT和pGL3质粒(湖南丰晖生物科技有限公司);LipofectamineTM2000(Thermo Fisher公司,美国);细胞计数试剂盒8(cell counting kit 8,CCK-8;Dojindo Molecular Technologies公司,日本);Annexin Ⅴ-FITC/碘化丙啶(propidium iodide,PI)细胞凋亡检测试剂盒(武汉伊莱瑞特生物科技股份有限公司);SYBR® Premix Ex TaqTM Ⅱ(Takara公司,日本);总蛋白提取试剂盒(沈阳万类生物科技有限公司);人TNF-α ELISA试剂盒、人IL-6 ELISA试剂盒、抗Ⅱ型胶原抗体、抗B淋巴细胞瘤2蛋白(B-cell lymphoma 2 protein,Bcl-2)抗体、抗Bcl-2相关X蛋白(Bcl-2-associated X protein,Bax)抗体、抗裂解的半胱天冬酶3(cleaved-Caspase-3)抗体、抗TLR4蛋白抗体、抗MyD88蛋白抗体、抗p65蛋白抗体、抗磷酸化p65(phosphorylated p65,p-p65)蛋白抗体、GAPDH、辣根过氧化物酶标记的山羊抗兔IgG、前列腺素E2(prostaglandin E2,PGE2)ELISA试剂盒、EdU增殖试剂盒(Abcam公司,美国);TRIzol试剂盒、PrimeScript RT试剂盒(Invitrogen公司,美国)。
Bio-Rad 680酶标仪(Bio-Rad公司,美国);流式细胞仪(Aceabio公司,美国);Image J软件(National Institutes of Health,美国);ABI 7900HT快速PCR实时系统(Applied Biosystems公司,美国);GraphPad Prism 8.01软件(GraphPad Software公司,美国);荧光显微镜(Leica公司,德国)。
1.2. 细胞培养及分组
取人软骨细胞C28/I2接种于含10%FBS的DMEM培养基中,于37℃、5%CO2、95%湿度培养箱中培养[16],待细胞附着90%后行传代培养,取第3代以后的对数期细胞经免疫荧光染色鉴定[17]后进行后续实验。
取上述C28/I2细胞进行以下分组及处理:对照组(A组,细胞在上述培养条件下培养不作任何处理),OA组(B组,细胞采用10 ng/mL IL-1β处理24 h)[18],OA+mimics NC组(C组,mimics NC转染细胞24 h后,10 ng/mL IL-1β处理细胞24 h),OA+mimics miR组(D组,mimics miR转染细胞24 h后,10 ng/mL IL-1β处理细胞24 h),OA+mimics miR+过表达(over expression,oe)-NC组(E组,mimics miR与oe-NC共转染细胞24 h后,10 ng/mL IL-1β处理细胞24 h),OA+mimics miR+oe-TLR4组(F组,mimics miR与oe-TLR4共转染细胞24 h后,10 ng/mL IL-1β处理细胞24 h)。转染方法:采用LipofectamineTM2000试剂将oe-TLR4和oe-NC、mimics NC和mimics miR转染C28/I2细胞,转染终浓度为50 nmol/L。
1.3. miR-515-5p抑制OA软骨细胞凋亡
1.3.1. 免疫荧光染色观察
将培养的C28/I2细胞用4%多聚甲醛固定20 min,PBS洗3次,免疫染色通透液处理5 min,1%牛血清白蛋白封闭1 h;加入抗Ⅱ型胶原抗体4℃下孵育过夜;PBS洗3次,加入二抗孵育30 min;PBS洗3次,DAPI染色,荧光显微镜观察。采用Image J软件分析细胞内Ⅱ型胶原阳性率,鉴定细胞纯度[17]。
1.3.2. CCK-8检测细胞增殖
取A~D组细胞于37℃、5%CO2、95%湿度条件下分别培养0、24、48、72 h,加入25 μL CCK-8试剂孵育2 h,用酶标仪测定450 nm波长处吸光度(A)值;实验重复3次[8]。
1.3.3. EdU检测细胞增殖
取A~D组细胞,按照EdU增殖试剂盒说明书方法检测细胞增殖,荧光显微镜下观察EdU染色细胞并计数,以相对荧光表达量表示各组荧光素酶活性并反映细胞增殖活力[19]。
1.3.4. 流式细胞术检测细胞凋亡
取A~D组细胞,以0.25%胰蛋白酶消化离心后,PBS洗3次,结合缓冲液重悬细胞;根据Annexin Ⅴ-FITC/PI细胞凋亡检测试剂盒说明书方法,将Annexin Ⅴ-FITC和PI于室温避光条件下孵育细胞15~20 min,1 h内用流式细胞仪检测细胞凋亡情况[20]。
1.3.5. Western blot检测
取A~D组细胞,采用总蛋白提取试剂盒提取细胞总蛋白,BCA蛋白检测试剂盒检测蛋白浓度;行电泳、转膜、封闭处理后,加入一抗(Bcl2、Bax、cleaved-Caspase-3)于4℃孵育过夜;洗膜,加入二抗37℃孵育1 h。采用化学发光试剂盒检测蛋白条带,使用Image J软件进行灰度分析。
1.3.6. ELISA检测
取A~D组细胞,采用ELISA试剂盒检测细胞上清液中促炎因子(PGE2、TNF-α、IL-6)的表达水平。
1.3.7. 实时荧光定量PCR(real-time fluorescence quantitative PCR,RT-qPCR)检测
取A~D组细胞,使用TRIzol regent提取样本总RNA,以PrimeScript RT试剂盒转录成cDNA;然后于ABI 7900HT快速PCR实时系统上使用SYBR® Premix Ex TaqTM Ⅱ进行qPCR。以U6为内参,采用2−ΔΔCt法计算miR-515-5p mRNA相对表达量。引物序列见表1。
表 1.
Primer sequences of each gene in RT-qPCR
RT-qPCR各基因引物序列
| 基因 Gene |
引物序列(5'→3') Primer sequence (5'→3') |
| miR-515-5p | 上游TTCTCCAAAAGAAAGCACTTTCTG |
| 下游CTCGCTTCGGCAGCACA | |
| TLR4 | 上游GATCTACTCACTTTACCATA |
| 下游GCTAATCGAGGCTACGACT | |
| U6 | 上游GCGCGTCGTGAAGCGTTC |
| 下游GTGCAGGGTCCGAGG | |
| GAPDH | 上游CACATGGCCTCCAAGGAGTAA |
| 下游GAGGGTCTCTCTCTTCCTCTTGT |
1.4. miR-515-5p促进OA软骨细胞周期
为研究miR-515-5p对IL-1β诱导的OA软骨细胞周期的调控作用,采用流式细胞术检测OA软骨细胞的细胞周期情况。取A~D组细胞,使用无水乙醇4℃固定过夜,加入PI染色液孵育30 min,通过流式细胞仪检测染色后的细胞并记录不同细胞周期的细胞比例。
1.5. miR-515-5p靶向调控TLR4
为研究miR-515-5p与TLR4之间的靶向关系,采用BiBiServ2数据库(https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/RNAhybrid)预测miR-515-5p和TLR4之间的潜在结合位点,并采用双荧光素酶报告实验验证miR-515-5p和TLR4的靶向关系。将miR-515-5p与TLR4的互补结合序列及其突变序列进行扩增,克隆至pGL3载体上,构建野生型质粒TLR4-WT和对应的突变型质粒TLR4-MUT。使用Lipofectamine 2000转染试剂将TLR4-MUT+mimics NC、TLR4-MUT+mimics miR、TLR4-WT+mimics NC和TLR4-WT+mimics miR共转染HEK293T细胞,双荧光素酶报告实验检测荧光素酶活性。
然后,同1.3.5和1.3.7方法分别采用Western blot和RT-qPCR检测各组TLR4蛋白和mRNA相对表达量,以GAPDH为内参,引物序列见表1。
1.6. TLR4逆转miR-515-5p对OA软骨细胞凋亡抑制及炎症改善作用
取D~F组细胞,同1.3.2和1.3.3方法采用CCK-8法和EdU检测细胞增殖能力,同1.3.4方法采用流式细胞术检测细胞凋亡情况及细胞周期,ELISA法检测细胞促炎因子(PGE2、TNF-α和IL-6)表达水平,同1.3.5方法采用Western blot检测TLR4蛋白及凋亡相关蛋白(Bcl-2、Bax和cleaved-Caspase-3)相对表达量。
1.7. miR-515-5p靶向TLR4阻断MyD88/NF-κB信号通路激活
为进一步研究miR-515-5p靶向负调控TLR4对MyD88/NF-κB信号通路的调控作用,同1.3.5方法采用Western blot检测A~F组细胞中MyD88、p-p65、p65蛋白相对表达量。
1.8. 统计学方法
采用GraphPad Prism 8.01统计软件进行分析。计量资料经Shapiro-Wilk正态性检验,均符合正态分布,数据以均数±标准差表示,CCK-8检测结果多组间比较采用双因素方差分析,其余指标多组间比较采用单因素方差分析,两两比较采用Tukey多重比较检验;检验水准取双侧α=0.05。
2. 结果
2.1. miR-515-5p抑制OA软骨细胞凋亡
免疫荧光染色检测示,C28/I2细胞的纯度高于90%。见图1a。
图 1.
Inhibitory effect of overexpression of miR-515-5p on IL-1β-induced apoptosis of OA chondrocytes
过表达miR-515-5p对IL-1β诱导的OA软骨细胞凋亡的抑制作用
*P<0.05 a. 免疫荧光染色鉴定C28/I2细胞纯度(荧光显微镜×200);b. CCK-8法检测细胞增殖;c. EdU检测细胞增殖 从左至右依次为A~D组荧光显微镜观察(×200)和相对荧光表达量定量检测;d. 流式细胞术检测细胞凋亡 从左至右依次为A~D组流式细胞术检测和细胞凋亡率;e. Western blot检测凋亡相关蛋白表达电泳图 Mr:相对分子质量 1:A组 2:B组 3:C组 4:D组;f. Western blot检测凋亡相关蛋白相对表达量;g. ELISA检测炎症因子表达水平;h. RT-qPCR检测miR-515-5p基因相对表达量
*P<0.05 a. Identification of the purity of C28/I2 cells by immunofluorescence staining (Fluorescence microscopy×200); b. Cell proliferation was detected by CCK-8 method; c. EdU was used to detect cell proliferation From left to right for fluorescence microscopy observation (×200) in groups A-D respectively and quantitatively detected relative fluorescence expression; d. Flow cytometry was used to detect cell apoptosis From left to right for flow cytometry detection in groups A-D respectively and quantitatively detected cell apoptosis rates; e. Electrophoresis of apoptosis-related protein expressions detected by Western blot Mr: Relative molecular mass 1: Group A 2: Group B 3: Group C 4: Group D; f. The relative expressions of apoptosis-related proteins detected by Western blot; g. The expression levels of inflammatory factors detected by ELISA; h. The relative mRNA expression of miR-515-5p was detected by RT-qPCR

CCK-8和EdU检测示,B组细胞增殖能力显著低于A组,D组显著高于C组,培养24、48、72 h A值及EdU相对荧光表达量组间比较差异均有统计学意义(P<0.05)。见图1b、c。
流式细胞术检测示,B组细胞凋亡率显著高于A组,D组显著低于C组,差异均有统计学意义(P<0.05)。见图1d。
Western blot检测示,B组Bcl-2蛋白相对表达量显著低于A组,D组显著高于C组;B组Bax和cleaved-Caspase-3蛋白相对表达量显著高于A组,D组显著低于C组;差异均有统计学意义(P<0.05)。见图1e、f。
ELISA检测示,B组PGE2、TNF-α、IL-6表达水平显著高于A组,D组显著低于C组,差异均有统计学意义(P<0.05)。见图1g。
RT-qPCR检测示,B组miR-515-5p mRNA相对表达量显著低于A组,D组显著高于C组,差异均有统计学意义(P<0.05)。见图1h。
2.2. miR-515-5p促进OA软骨细胞周期
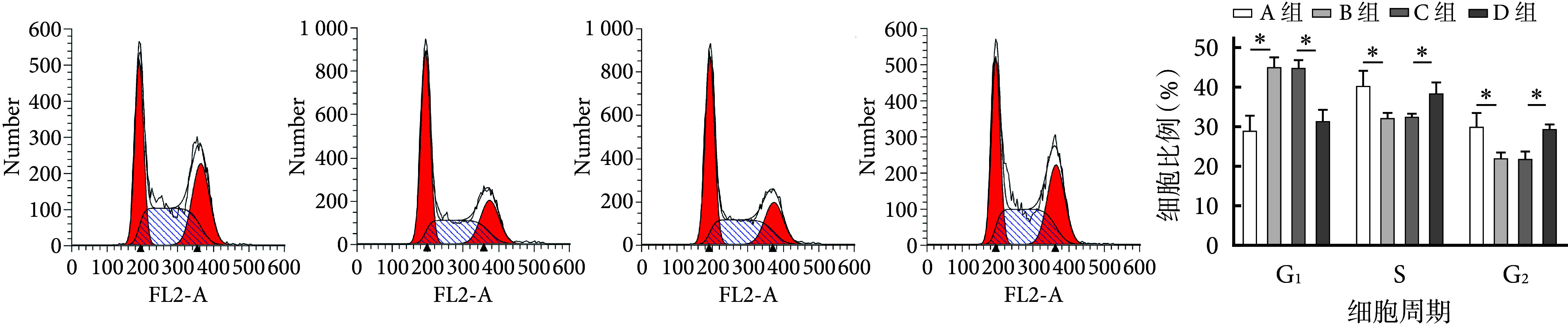
流式细胞术检测示,与A组相比,B组G1期细胞比例显著增加,而S期和G2期显著减小;与C组相比,D组G1期细胞比例显著减小,S期和G2期显著增加;差异均有统计学意义(P<0.05)。见图2。
图 2.
The promoting effect of overexpression of miR-515-5p on IL-1β-induced chondrocyte cycle in OA detected by flow cytometry
流式细胞术检测过表达miR-515-5p对IL-1β诱导的OA软骨细胞周期的促进作用
*P<0.05 从左至右依次为A~D组流式细胞术检测和各周期细胞比例
*P<0.05 From left to right for flow cytometry detection in groups A-D and the proportion of cells in each phase
2.3. miR-515-5p靶向调控TLR4
BiBiServ2数据库预测示,miR-515-5p与TLR4之间存在结合位点。双荧光素酶报告实验验证示,在突变后的MUT组中,转染mimics NC或mimics miR两者之间的荧光素酶活性差异无统计学意义(P>0.05);但在野生型的WT组中,转染mimics miR后其荧光素酶活性显著降低(P<0.05),表明miR-515-5p能够与TLR4靶向结合。RT-qPCR和Western blot检测示,B组TLR4 mRNA和蛋白相对表达量显著高于A组,D组均显著低于C组,差异均有统计学意义(P<0.05)。见图3。
图 3.
miR-515-5p targeting negative regulatory TLR4 detection
miR-515-5p靶向负调控TLR4相关检测
*P<0.05 a. BiBiServ2数据库预测miR-515-5p与TLR4结合位点;b. 荧光素酶报告实验验证miR-515-5p与TLR4的靶向关系;c. RT-qPCR检测TLR4 mRNA相对表达量;d. Western blot检测TLR4蛋白表达电泳图 Mr:相对分子质量 1:A组 2:B组 3:C组 4:D组;e. Western blot检测TLR4蛋白相对表达量
*P<0.05 a. BiBiServ2 database predicted the binding sites of miR-515-5p and TLR4; b. Luciferase reporting experiment verified the targeting relationship between miR-515-5p and TLR4; c. The relative expression of TLR4 mRNA detected by RT-qPCR; d. Electrophoresis of TLR4 protein expression detected by Western blot Mr: Relative molecular mass 1: Group A 2: Group B 3: Group C 4: Group D; e. The relative expression of TLR4 protein detected by Western blot
2.4. 上调TLR4部分逆转过表达miR-515-5p对IL-1β诱导的OA软骨细胞凋亡的抑制作用及炎症改善作用
与D、E组相比,F组细胞的增殖能力明显降低,培养24、48、72 h A值及EdU相对荧光表达量比较差异均有统计学意义(P<0.05);细胞凋亡率显著增加,G1期细胞比例显著增加,S期和G2期细胞比例显著减少;细胞上清液中促炎因子PGE2、TNF-α、IL-6表达水平均显著升高;TLR4、Bax和cleaved-Caspase-3蛋白相对表达量显著升高,Bcl-2蛋白相对表达量显著降低;差异均有统计学意义(P<0.05)。以上指标D、E组间差异均无统计学意义(P>0.05)。见图4。
图 4.
Inhibitory effect of up-regulated TLR4 partially reversing overexpression of miR-515-5p on IL-1β-induced apoptosis of OA chondrocytes and amelioration of inflammation
上调TLR4部分逆转过表达miR-515-5p对IL-1β诱导的OA软骨细胞凋亡的抑制作用及炎症改善作用
*P<0.05 a. Western blot检测TLR4和凋亡相关蛋白表达电泳图 Mr:相对分子质量 1:D组 2:E组 3:F组;b. Western blot检测TLR4和凋亡相关蛋白相对表达量;c. CCK-8法检测细胞增殖;d. EdU检测细胞增殖 从左至右依次为D~F组荧光显微镜观察(×200)和相对荧光表达量定量检测;e. 流式细胞术检测细胞凋亡 从左至右依次为D~F组流式细胞术检测和细胞凋亡率;f. 流式细胞术检测细胞周期 从左至右依次为D~F组流式细胞术检测和各周期细胞比例;g. ELISA检测炎症相关因子表达水平
*P<0.05 a. Electrophoresis of TLR4 and apoptosis-related proteins expressions detected by Western blot Mr: Relative molecular mass 1: Group D 2: Group E 3: Group F; b. The relative expressions of TLR4 and apoptosis-related proteins detected by Western blot; c. Cell proliferation detected by CCK-8 method; d. EdU was used to detect cell proliferation From left to right for fluorescence microscopy observation (×200) in groups D-F respectively and quantitatively detected relative fluorescence expression; e. Flow cytometry was used to detect cell apoptosis From left to right for flow cytometry detection in groups D-F respectively and quantitatively detected cell apoptosis rates; f. Flow cytometry was used to detect cell cycle From left to right for flow cytometry detection in groups D-F respectively and the proportion of cells in each phase; g. The expression levels of inflammatory factors detected by ELISA

2.5. miR-515-5p靶向TLR4阻断MyD88/NF-κB信号通路激活
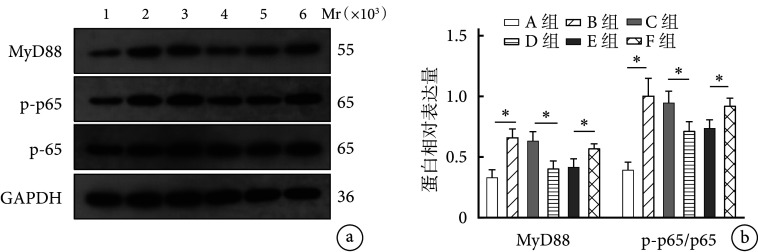
Western blot检测示,B组细胞中MyD88及p-p65/p65蛋白相对表达量显著高于A组,D组显著低于C组,F组显著高于E组,差异均有统计学意义(P<0.05)。见图5。
图 5.
Observation of miR-515-5p targeting TLR4 to block activation of MyD88/NF-κB signaling pathway
miR-515-5p靶向TLR4阻断MyD88/NF-κB信号通路激活观测
*P<0.05 a. Western blot检测MyD88及p-p65/p65蛋白表达凝胶电泳图 Mr:相对分子质量 1:A组 2:B组 3:C组 4:D组 5:E组 6:F组;b. Western blot检测MyD88及p-p65/p65蛋白相对表达量
*P<0.05 a. Electrophoresis of MyD88 and p-p65/p65 protein expressions detected by Western blot Mr: Relative molecular mass 1: Group A 2: Group B 3: Group C 4: Group D 5: Group E 6: Group F; b. The relative expressions of MyD88 and p-p65/p65 proteins detected by Western blot
3. 讨论
OA是由严重关节变形引起的关节疼痛和功能障碍综合征[21],其发生、进展与炎症紧密相关。在OA发生过程中,滑膜细胞、关节软骨细胞以及其他细胞均会表达炎症介质[22]。为此,通过靶向调控相关炎症途径是治疗OA的潜在策略。本研究中,我们首先使用IL-1β诱导C28/I2细胞模拟OA软骨炎症损伤,结果显示,IL-1β可以显著诱导PGE2、TNF-α和IL-6促炎因子的产生,这与既往研究报道一致[23]。
据报道,miRNA可通过调节软骨细胞增殖和凋亡、细胞外基质代谢和炎症反应参与OA的发生、发展[24-26]。研究表明,miR-181-5p能靶向负调控DDX3X(DEAD-box RNA解旋酶亚家族中研究最广泛且进化保守的成员之一,被认为是癌症和病毒的治疗靶点)的表达,从而调控NF-κB信号通路的活化,进而减少炎症因子TNF-α和IL-6的释放[27]。与上述结果一致,本研究中我们使用IL-1β诱导软骨细胞OA软骨炎症损伤模型,结果示miR-515-5p在OA中的表达水平下调;随后,使用miR mimics转染后诱导OA模型,结果显示过表达miR-515-5p显著减少了软骨细胞PGE2、TNF-α和IL-6促炎因子的产生,缓解了细胞的炎症反应。TLR4/MyD88/NF-κB信号通路是经典的炎症反应信号通路,通过上调TLR4、p65等蛋白的表达能够诱发炎症反应,释放TNF-α和IL-6促炎因子并参与OA的发展[14]。本研究诱导OA模型后发现,B组细胞中TLR4、MyD88及p-p65蛋白的表达水平均显著升高,TNF-α、PGE2和IL-6促炎因子的释放也显著增加。TLR4是Toll样受体家族成员之一,是NF-κB的上游靶标,能够有效识别机体中某些损伤或病原体相关分子,从而激活信号,诱发炎症反应,促进多种炎症因子的产生和释放[28]。MyD88是TLR4/NF-κB信号通路中重要的衔接蛋白。TLR4通过激活依赖性MyD88通路,促进MyD88与TNF受体相关因子6结合,从而激活下游NF-κB信号通路,进而激活细胞内信号转录表达,促进TNF-α和IL-6等促炎因子的释放并产生信号级联反应诱发OA[29]。因此,TLR4/MyD88/NF-κB信号通路是探究OA发病机制和治疗机制的一个有效靶点。研究表明,miR-93可通过靶向负调控TLR4,抑制NF-κB信号通路的活化,减少TNF-α和IL-6等促炎因子的释放从而缓解OA[15]。而在本研究中,IL-1β的诱导激活了TLR4/MyD88/NF-κB信号通路,促进了PGE2、TNF-α和IL-6促炎因子的释放;而过表达miR-515-5p后,miR-515-5p通过靶向负调控TLR4抑制NF-κB信号通路的活化,减少了PGE2、TNF-α和IL-6促炎因子的释放,从而缓解OA;上调TLR4的表达后,部分逆转了过表达miR-515-5p对OA的抑制作用,进一步证实了miR-515-5p可通过靶向负调控TLR4抑制NF-κB信号通路的活化,从而影响OA的进展。
综上述,本研究首次发现miR-515-5p通过靶向负调控TLR4阻断MyD88/NF-κB信号通路的活化,从而抑制OA软骨细胞凋亡,进而减轻炎症反应,为治疗OA提供了新思路。然而,本研究仅在人的软骨细胞系C28/I2中进行了体外实验,尚需结合动物实验进一步验证,并在未来研究中进一步探究其他潜在的调控机制。
利益冲突 在课题研究和文章撰写过程中不存在利益冲突;经费支持没有影响文章观点和对研究数据客观结果的统计分析及其报道
作者贡献声明 蔡东峰:研究设计、文章撰写;杨子肖、钟超:研究实施、数据收集整理;张靖:统计分析;洪嵩:对文章的知识性内容作批评性审阅,行政支持
Funding Statement
贵州省科技厅基础研究计划项目(黔科合基础-ZK[2021]一般392)
Basic Research Program of Guizhou Science and Technology Department (Guizhou Science and Technology Foundation-ZK [2021] General 392)
References
- 1.Taruc-Uy RL, Lynch SA Diagnosis and treatment of osteoarthritis. Prim Care. 2013;40(4):821–836. doi: 10.1016/j.pop.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, et al The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3.Aigner T, Söder S, Gebhard PM, et al. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis—structure, chaos and senescence. Nat Clin Pract Rheumatol, 2007, 3(7): 391-399.
- 4.Le LT, Swingler TE, Clark IM Review: the role of microRNAs in osteoarthritis and chondrogenesis. Arthritis Rheum. 2013;65(8):1963–1974. doi: 10.1002/art.37990. [DOI] [PubMed] [Google Scholar]
- 5.Lagos-Quintana M, Rauhut R, Lendeckel W, et al Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 6.Nugent M MicroRNAs: exploring new horizons in osteoarthritis. Osteoarthritis Cartilage. 2016;24(4):573–580. doi: 10.1016/j.joca.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Vicente R, Noël D, Pers YM, et al Deregulation and therapeutic potential of microRNAs in arthritic diseases. Nat Rev Rheumatol. 2016;12(4):211–220. doi: 10.1038/nrrheum.2015.162. [DOI] [PubMed] [Google Scholar]
- 8.Wu R, Zhang F, Cai Y, et al Circ_0134111 knockdown relieves IL-1β-induced apoptosis, inflammation and extracellular matrix degradation in human chondrocytes through the circ_0134111-miR-515-5p-SOCS1 network. Int Immunopharmacol. 2021;95:107495. doi: 10.1016/j.intimp.2021.107495. [DOI] [PubMed] [Google Scholar]
- 9.Abella V, Scotece M, Conde J, et al The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1. Sci Rep. 2016;6:20356. doi: 10.1038/srep20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Zhu F, Tong Z, et al Response of chondrocytes to shear stress: antagonistic effects of the binding partners Toll-like receptor 4 and caveolin-1. FASEB J. 2011;25(10):3401–3415. doi: 10.1096/fj.11-184861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.吴文娟, 曾妮, 王硕莹, 等 黄芩苷对支原体肺炎小鼠TLR4/NF-κB信号通路的抗炎及肺功能保护作用. 中华医院感染学杂志. 2023;33(23):3521–3526. [Google Scholar]
- 12.Kim HA, Cho ML, Choi HY, et al The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54(7):2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 13.Qi W, Chen Y, Sun S, et al Inhibiting TLR4 signaling by linarin for preventing inflammatory response in osteoarthritis. Aging (Albany NY) 2021;13(4):5369–5382. doi: 10.18632/aging.202469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Li N, Wu Y, et al Zhuifeng tougu capsules inhibit the TLR4/MyD88/NF-κB signaling pathway and alleviate knee osteoarthritis: In vitro and in vivo experiments. Front Pharmacol. 2022;13:951860. doi: 10.3389/fphar.2022.951860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y, Wang L, Zhao Q, et al MicroRNA-93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF-κB signaling pathway. Int J Mol Med. 2019;43(2):779–790. doi: 10.3892/ijmm.2018.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Ji ML, Zhang XJ, et al MicroRNA-218-5p as a potential target for the treatment of human osteoarthritis. Mol Ther. 2017;25(12):2676–2688. doi: 10.1016/j.ymthe.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.李永福. 淫羊藿苷介导PI3K-Akt-mTOR信号通路调控关节软骨细胞自噬对骨关节炎的作用及机制研究. 杭州: 浙江中医药大学, 2022.
- 18.Lin J, Huang Y, Lin X, et al Bauhinia championii alleviates extracellular matrix degradation in IL-1β induced chondrocytes via miRNA-145-5p/TLR4/NF-κB axis. Heliyon. 2023;9(8):e19138. doi: 10.1016/j.heliyon.2023.e19138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi Y, Yang N, Yang Z, et al LncRNA TM1-3P regulates proliferation, apoptosis and inflammation of fibroblasts in osteoarthritis through miR-144-3p/ONECUT2 axis. Orthop Surg. 2022;14(11):3078–3091. doi: 10.1111/os.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou H, Li J, Wang J, et al ITGA9 inhibits proliferation and migration of dermal microvascular endothelial cells in psoriasis. Clin Cosmet Investig Dermatol. 2022;15:2795–2806. doi: 10.2147/CCID.S394398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Li Y, Hou L, et al Forsythoside B alleviates osteoarthritis through the HMGB1/TLR4/NF-κB and Keap1/Nrf2/HO-1 pathways. J Biochem Mol Toxicol. 2024;38(1):e23569. doi: 10.1002/jbt.23569. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Abu-Amer Y, O’Keefe RJ, et al Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017;58(1):49–63. doi: 10.1080/03008207.2016.1208655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang H, Ren X, Jiang F, et al Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis. Mol Med. 2023;29(1):17. doi: 10.1186/s10020-023-00614-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malemud CJ MicroRNAs and osteoarthritis. Cells. 2018;7(8):92. doi: 10.3390/cells7080092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Tang S, Nie X, et al Decreased miR-214-3p activates NF-κB pathway and aggravates osteoarthritis progression. EBioMedicine. 2021;65:103283. doi: 10.1016/j.ebiom.2021.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Wang L, Guan X, et al MiR-4303 relieves chondrocyte inflammation by targeting ASPN in osteoarthritis. J Orthop Surg Res. 2021;16(1):618. doi: 10.1186/s13018-021-02731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao P, Ma G, Ma L miR-181a-5p targets DDX3X to inhibit the progression of osteoarthritis via NF-ΚB signaling pathway. J Orthop Surg Res. 2023;18(1):606. doi: 10.1186/s13018-023-04073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.庄丽华, 詹松华, 杨烁慧, 等 电针通过调节TLR4/MyD88/NF-κB信号通路改善缺血性脑卒中损伤研究现状. 中华中医药学刊. 2019;37(9):2182–2185. [Google Scholar]
- 29.刘姣. 推拿对大鼠膝骨关节炎模型的TLR4/MyD88信号转导通路的影响. 广州: 广州中医药大学, 2019.