Highlights
-
•
IL-1β could affect hMPV replication by regulating its expression in vitro.
-
•
The cGAS-STING signaling pathway involved in hMPV infection.
-
•
IL-1β could regulate hMPV replication by activating the cGAS-STING pathway.
Keywords: Human metapneumovirus, IL-1β, cGAS-STING signaling pathway
Abstract
Background
Human metapneumovirus(hMPV) is one of the most common viruses that cause acute lower respiratory tract infections. Interleukin-1β (IL-1β) has been reported to play an important role in multiple virus replication. Patients with hMPV infection have increased levels of IL-1β which reminds IL-1β is associated with hMPV infection. However, the mechanism by which IL-1β affects hMPV replication remains unclear. In this study, we explore the effect of IL-1β on hMPV replication and investigate its specific mechanism of action.
Methods
We established an hMPV infection model through Human bronchial epithelial cells (16HBE). qRT-PCR and Western Blot were used to detect the expression levels of IL-1β, cyclic GMP-AMP synthase (cGAS), and interferon stimulating factor (STING). Regulating IL-1β expression by small interfering RNA (siRNA) or exogenous supplementary to study the influence of hMPV replication. The selective cGAS inhibitor RU.521, G150, and STING inhibitor H-151 were utilized to detect hMPV replication in 16HBE cells.
Results
The level of IL-1β protein increased in a time-dependent and dose-dependent manner after hMPV infection. The mRNA and protein levels of cGAS and STING were significantly up-regulated. Knockdown of IL-1β could contribute to the decreased viral loads of hMPV. While the exogenous supplement of recombinant human IL-1β in cells, replication of hMPV was significantly increased. Additionally, the level of cGAS-STING protein expression would be affected by regulating IL-1β expression. Inhibitors of the cGAS-STING pathway led to a lower level of hMPV replication.
Conclusion
This study found that IL-1β could promote hMPV replication through the cGAS-STING pathway, which has the potential to serve as a candidate to fight against hMPV infection, targeting IL-1β may be an effective new strategy to restrain virus replication.
1. Introduction
Human metapneumovirus (hMPV) is a prevalent cause of respiratory infections, first discovered by van den Hoogen in 2001, although it has been circulating for over six decades (van den Hoogen et al., 2001). hMPV accounts for substantial hospitalization and economic burden in high-risk ages (Wang et al., 2021). The clinical symptoms of hMPV infection, such as coughing, fever, and sore throat are similar to those caused by other respiratory viruses (Bai et al., 2021; Haddadin et al., 2021; Behnood et al., 2022). In some areas, the incidence rate of hMPV infection even reached up to 43 % (Rafiefard et al., 2008). Unfortunately, there are currently no antiviral medications or vaccines available for the management or prevention of hMPV infection.
Interleukin-1β (IL-1β) has received considerable attention in the field of viral infection (Ainouze et al., 2018; Delphin et al., 2021; Yaseen et al., 2023). Many studies have shown that IL-1β could affect the replication of various viruses. As for hMPV infection, clinical studies found that children infected with hMPV had significantly elevated levels of IL-1β (Malmo et al., 2016; Park et al., 2017). The mortality of IL-1β-deficient (IL-1β−/−) mice infected with hMPV was lower than that of wild-type mice infected, indicating that IL-1β played an important role in hMPV infection (Le et al., 2019). Indeed, IL-1β has been linked to worse severity and longer hospitalization time (Cioccarelli et al., 2021). It can mediate multiple inflammatory responses promoting infiltration into tissues by immune activity (Malmo et al., 2016; Bent et al., 2018). However, it remains unclear whether IL-1β affects hMPV replication and the specific mechanism by which it functions.
IL-1β can activate the cyclic guanosine monophosphate (GMP)-AMP synthetase (cGAS) and stimulants of interferon genes (STING) pathways, directing an effective innate immune response that limits pathogen infection (Wu et al., 2023). This pathway acts as a crucial link between the detection of viruses and the immune defense mechanism of the host (Ma and Damania, 2016; Erttmann et al., 2022). There is growing evidence that the pathway has a significant role in RNA viruses (Ding et al., 2018; Reinert et al., 2021; Domizio et al., 2022). Interestingly, the role of IL-1β in virus replication remains controversial (Ramos et al., 2012). It is unknown whether IL-1β could regulate the cGAS-STING signaling pathway to play a role in the replication of hMPV. In this study, we aim to explore the effect of IL-1β on hMPV replication and elaborate its mechanism of action.
2. Materials and methods
2.1. Cells and virus
16HBE and Vero-E6 cells were obtained from the Chinese Classic Culture Treasure Center (CCTCC) and maintained in the Key Laboratory of Childhood Infection Immunity. The cells were cultured in a complete culture medium consisting of 10 % fetal bovine serum (FBS, NTC, USA), 1 % penicillin-streptomycin (Hyclone, USA), and 90 % Dulbecco's modified Eagle's medium (DMEM, Gibco, USA), and then incubated at 37 °C with 5 % CO2. Cell viability was measured by using the CCK8 kit (AbMole Bioscience, USA). The status of 16HBE cells was observed and recorded at different time points with hMPV infection or not under microscope. hMPV was reconstructed by reverse genetic technique in our laboratory, and the virus genotype belongs to type A (hMPV NL/1/00). Vero-E6 cells were employed for virus propagation, and hMPV was amplified in a medium containing 3 % FBS, 1 % penicillin-streptomycin, 0.1 % pancreatic enzyme, and 97 % DMEM. Once the hMPV amplification reached 80 %, the cells and supernatants were collected and frozen at −80 °C. The samples were subjected to three cycles of freezing and thawing, followed by centrifugation at 4 °C and 5000 rpm for 30 min. The resulting supernatant was then filtered using a 0.22um filter.
2.2. Nucleic acid extraction and quantitative real-time pcr (qRT-PCR)
Cell lysates infected with hMPV at different times (0, 24, 48, and 72 h) were collected, and RNA was extracted using an RNA extraction kit (Magen, China) according to the manufacturer's instructions. mRNA was reverse-translated into cDNA using an RT kit (ABclonal Technology, China). The TaqMan probe method is used for absolute quantification of virus titers. The probe sequence is 5′- TTGCCAACACACGAACTCCATTCCC - 3′; The reaction conditions were 95 °C for 30 s, 95 °C for 5 s, 65 °C for 30 s, and 40 cycles. Cytoplasmic mitochondrial DNA (mtDNA) and total DNA during hMPV replication were extracted in the presence of 3# or IL-1β protein treatment. The level of DNA containing specific mitochondrial (MT-ATP6) and nuclear (RPL13A) genes was quantitatively detected by qRT-PCR. SYBR Green Dye was used to detect the relative expression of IL-1β, cGAS, and STING, Type I interferons (IFN-Is, such as IFN-α, IFN-β) genes, with GAPDH as the internal reference. The reaction process was 95 °C 3 min, 95 °C 5 s, 63 °C 30 s, and 40 cycles. The reaction was performed in a CFX96 fluorescent thermal circulator (Bio-Rad, Hercules, CA, USA) and the data was analyzed using CFX Manager software (Bio-Rad). The relative quantification of target gene expression was analyzed by the 2−△△ct method. The specific primer sequences are shown in Supplementary Table 1.
2.3. Western Blot
The 16HBE cells were lysed using RIPA lysis buffer containing protease inhibitor and phosphatase inhibitor on ice for 30 min. Following centrifugation, the supernatant was collected and the protein concentration was determined using the BCA (Beyotime, China) method. Mixed the samples and protein loading buffer in a ratio of 1:4. Then boiled for 10 min. The protein samples were separated using sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis and subsequently transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was then enclosed in a rapid sealing solution (New Cell Molecular Biotech, China) at room temperature for 15 min. Specific primary antibodies were incubated with the membrane overnight at a temperature of 4 °C, each at a dilution of 1:1000. The antibodies we used are listed as follows: hMPV (Abcam, USA), IL-1β (HUABIO, China), cGAS (HUABIO, China), p-STING (ABMART, China), STING (HUABIO, China), and GAPDH (HUABIO, China). Following three washes with TBS with Tween-20 (Beyotime, China), the membrane was incubated with Sheep anti-rabbit or Goat anti-mouse IgG labeled with HRP (Cell Signaling Technology, USA) at room temperature for 1 h. Subsequently, the membrane was washed three times with TBST. Finally, an ECL Luminescent Liquid (New Cell Molecular Biotech, China) was used for exposure and development, and the resulting gray values were analyzed using Image J software.
2.4. Immunofluorescence
The 16HBE cells were rinsed once with phosphate-buffered saline (PBS), fixed with 4 % paraformaldehyde at room temperature for 15 min. Rinsed three times with PBS, and subsequently incubated with a fluorescent sealing solution (Beyotime, China) at room temperature for 1 h. The cells were then exposed to mouse anti-hMPV N protein (diluted 1:500) at 4 °C overnight. After being washed three times with PBS, the cells were treated with Goat anti-mouse IgG H&L Alexa Fluor 488 (diluted 1:200, Abcam, USA) and incubated at 37 °C for 1 h. Following another three washes with PBS, the cells were incubated at room temperature with DAPI for 1 h, washed three times with PBS, and then 10ul of an anti-fluorescence quenching sealing solution containing 4, 6-diamino-2-styryl alcohol (Beyotime, China) was added to prepare cell slides on anti-slip slides. Finally, the fluorescence was observed under a fluorescence microscope.
2.5. Small interfering RNA (siRNA)
For the experiment, we used small interfering RNA for IL-1β and its negative control (siRNA-NC) from Tsingke Biotechnology (Beijing, China). The specific sequences are listed in Supplementary Table 2. Before the transfection experiment, we inoculated 2 × 105 cells into a 24-well plate and 8 × 105 cells into a 6-well plate one day in advance. The transfection experiment was performed when the cell culture density of the pore plate reached 70–80 %. According to the instructions of the reagent manufacturer, we transfected 1.6ul Lipofectamine 8000 (Beyotime, China) and 40 pmol si-IL-1β into the 24-well plate for 24 h to detect IL-1β mRNA level. At the same time, we transfected 5ul Lipofectamine 8000 (Beyotime, China) and 100 pmol si-IL-1β into 6-well plates. We detected the level of IL-1β protein 48 h after transfection. NC was compared with the experimental group as a blank control.
2.6. Exogenous supplementation of recombinant human IL-1β
We inoculated 8 × 105 cells into a 6-well plate in advance. When the cells reached 70–80 % confluence, then were treated with 30 ng/ml recombinant human IL-1β (Novoprotein, China) for 24 h, and finally infected the cells with hMPV (MOI=10) for 2 h, and then replaced 3 % viral maintenance fluid for 48 h. Cell lysate was collected to measure protein expression levels.
2.7. Treatment with inhibitor
For the experiment, 16HBE cells were inoculated into 6-well plates and then treated with different types of inhibitors such as cGAS inhibitor RU.521(Selleck, China), G150(Selleck, China), STING inhibitor H-151(Selleck, China) or Dimethylsulfoxide (DMSO). When the cells reached 70–80 %, we treated cells with different concentrations of RU.521, G150, or H-151 and Dimethylsulfoxide (DMSO) for 24 h. After the cells were infected with hMPV (MOI=10), the virus maintenance solution was replaced with 3 % and the cells were further cultured in an incubator for 48 h. Finally, the cell lysates were collected to detect the hMPV N protein.
2.8. ELISA assay
Cell culture supernatants of hMPV (MOI=10) infected 6-well plates at different time points was collected and frozen for cytokine IL-1β detection. Human IL-1β ELISA Kit (JIANGLAI, China) was used to detect the concentrations of mature IL-1β in cell culture supernatants. The experiments were performed according to the kit instructions. Finally, the plate was detected at 450 nm and the results were calculated based upon a standard curve.
2.9. Statistical analyses
The experimental data were subjected to statistical analysis using GraphPad prism8.0. The mean of the two groups was compared using a group t-test. For the comparison of multiple groups, a one-way analysis of variance was employed. Further pairwise comparisons between groups were conducted using the LSD-t-test. Statistical significance was determined based on the following criteria: P < 0.05*, P < 0.001 **, 0.05 < P < 0.001 ***, and P < 0.0001 ****. These statistical tests were used to determine if there were significant differences between the groups.
3. Results
3.1. hMPV successfully infected 16HBE cells
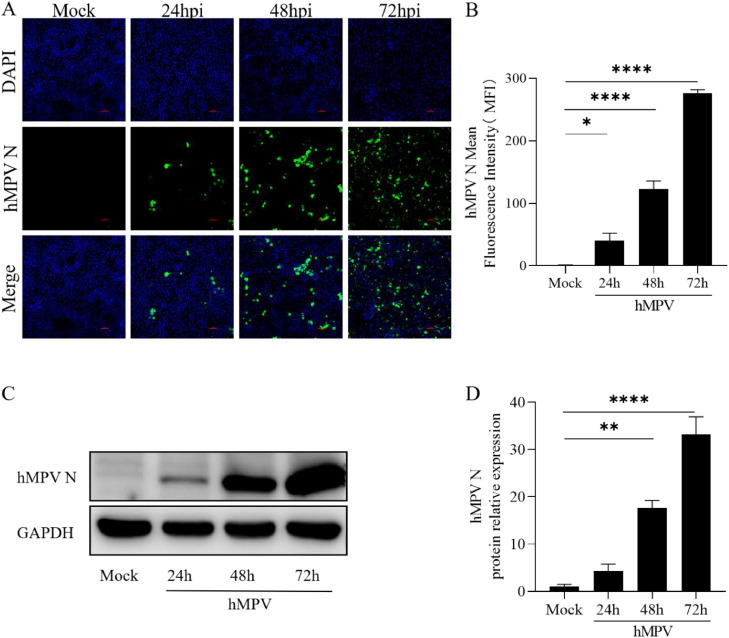
Epithelial cells serve as the initial barrier against viral infections in the host. Thus, we first investigated whether hMPV could be replicated in 16HBE cells. We detected the Mean Fluorescence Intensity (MFI) of hMPV N protein in 16HBE cells infected with hMPV (MOI=10) at different times of 0, 24, 48, and 72 h. Three fluorescence images with different fields of view were selected for statistical analysis and the data shown are mean ± SEM (n = 3) (Fig. 1A and B). At the same time, Western Blot was used to detect the expression of hMPV N protein, and three independent experiments were measured by Image J (Fig. 1C and D). These findings provide evidence that hMPV could replicate in 16HBE cells and indicate that hMPV was replicated at a time-dependent increase.
Fig. 1.
hMPV successfully infected 16HBE cells
16HBE cells infected with hMPV (MOI=10) for 0, 24, 48, 72 h. A. The expression of the hMPV N protein was detected by Immunofluorescence. B. The mean fluorescence intensity of the hMPV N protein was analyzed by Image J. C. Cell lysates were collected to detect hMPV N protein expression through Western Blot. D. The relative expression of hMPV N was quantitatively analyzed by Image J. MOI, the multiplicity of infection, P < 0.05*, P < 0.001**, 0.05<P < 0.001***, P < 0.0001****.
3.2. Cell viability and cell death
To exclude the influence of cell death on the experimental results, CCK8 was used to measure the percentage of cell viability (Supplementary Fig. 1A). At the same time, we observed and recorded the status of cells infected or not infected with hMPV at different time points (Supplementary Fig. 1B). There was no significant difference.
3.3. Expression of mature IL-1β increased after hMPV infected 16HBE cells
Following hMPV infection, the expression of IL-1β protein (17 kDa) was assessed at different time points (Fig. 2A and C). We demonstrated the expression of IL-1β protein increased at a time-dependent upon hMPV (MOI=10) infection, with a statistically significant difference. This indicates that the expression of IL-1β is activated and gradually upregulated during hMPV infection. Subsequently, we examined the changes in IL-1β protein after infecting cells with hMPV at different MOI for 0.1, 1, 10 (Fig. 2B and D). The expression level of IL-1β showed a positive correlation with the viral load of hMPV infection, indicating that as the viral load increased, the expression of IL-1β also increased. Meantime, we detected the concentration of secreted IL-1β in cell culture supernatant at different time points (Fig. 2E). This suggests that the expression of IL-1β is regulated by the dose effect of hMPV infection. Consequently, these findings suggest that hMPV infection can stimulate the expression of IL-1β in 16HBE cells.
Fig. 2.
The expression of IL-1β increased after hMPV infected 16HBE cells
The expression of IL-1β (17 kDa) in 16HBE cells infected hMPV (MOI=10) for 0, 24, 48, 72 h or different MOI (0.1, 1, 10) for 72 h. A. Western Blot was used to measure the expression of IL-1β at times of 0, 24, 48, and 72 h with hMPV (MOI=10) infected. B. The expression of IL-1β in 16HBE cells at MOI of 0.1, 1, and 10 for 72 h was detected by Western Blotting. C-D. The analysis of IL-1β (17 kd) protein relative quantitative was measured by Image J. E. The concentrations of secreted IL-1β in cell culture supernatant at different infection times were detected by ELISA.
3.4. Knock-down IL-1β by small interfering RNA (siRNA) in 16HBE cells
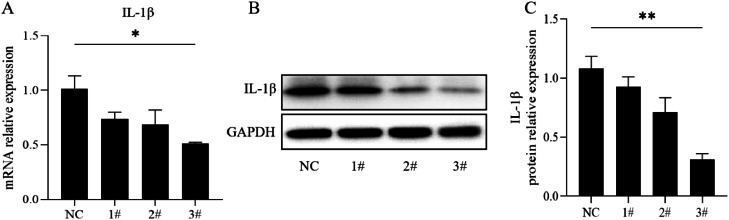
To further study the impact of IL-1β on hMPV replication, we transfected siRNA targeting IL-1β in 16HBE cells. The siRNA with the highest efficacy in the knockdown of IL-1β was assessed through qRT-PCR and Western Blot. The level of pro-IL-1β mRNA levels was analyzed after transfecting NC, siRNA 1#, siRNA 2#, and siRNA 3# into 16HBE cells for 24 h (Fig. 3A). Additionally, IL-1β protein expression changes were evaluated by transfecting NC, 1# siRNA, 2# siRNA, and 3# siRNA into 16HBE cells for 48 h (Fig. 3B, C). Notably, 3# siRNA exhibited the most effective silencing of IL-1β. Consequently, the sequences of 3# siRNA were chosen for subsequent experiments.
Fig. 3.
Knock-down IL-1β by small interfering RNA (siRNA) in 16HBE cells
Select the best sequence of knock-down IL-1β (17 kDa). A. Transfected NC, 1# siRNA, 2# siRNA, 3# siRNA into 16HBE cells for 24 h. Detection of IL-1β mRNA expression levels by qRT-PCR. B. Transfected NC, 1# siRNA, 2# siRNA, 3# siRNA into 16HBE cells for 48 h. The level of IL-1β protein expression was measured by Western Blotting. C. A histogram of IL-1β relative expression was obtained from three independent experiments. NC, negative control, siRNA, small interfering RNA.
3.5. IL-1β promotes hMPV replication in vitro
The impact of IL-1β on the replication of hMPV was investigated in the presence of 3# siRNA sequence. The expression of hMPV N protein was assessed through Immunofluorescence and Western Blot, and statistical analysis was conducted by Image J with three times independent experiments (Fig. 4A, C, and E). The findings indicated a decrease in hMPV replication following the knockdown of IL-1β. To further validate the influence of IL-1β on hMPV, 16HBE cells were cultivated with an exogenous supplementary 30 ng/ml IL-1β for 24 h, followed by hMPV (MOI=10) infection. The expression of the hMPV N protein was then examined through Immunofluorescence and Western Blot (Fig. 4B, D, and F). The results demonstrated an increase in hMPV replication after the exogenous addition of IL-1β compared to the group infected with hMPV alone. We repeated the experiment of extrinsic addition of IL-1β protein in the presence of 3# siRNA by Western Blot (Fig. 4G). hMPV N protein was reduced after 3#siRNA knockdown of IL-1β, while was increased after exogenous supplementation of IL-1β. Those findings provide evidence that IL-1β can regulate the replication of hMPV in 16HBE cells.
Fig. 4.
IL-1β promotes hMPV replication in vitro
IL-1β promotes hMPV replication in 16 HBE cells. We used siRNA to knock down the level of IL-1β expression for 48 h and exogenous supplementation IL-1β for 24 h, then infected hMPV (MOI=10) for 48 h. A–D. The level of hMPV N protein expression was detected by Immunofluorescence, and the mean fluorescence was analyzed by image J. E-F. Western Blot detected the expression of hMPV N protein after transfecting siRNA targeted rIL-1β or treating with IL-1β protein, its relative expression histogram. G. The expression of hMPV N protein in 16HBE cells treated with an additional 30 ng/ml rIL-1β in the presence of 3# siRNA was detected by Western Blot. The experiment data were independently repeated three times for statistical analysis. rIL-1β, recombinant IL-1β.
3.6. cGAS-STING pathway is involved in hMPV infection
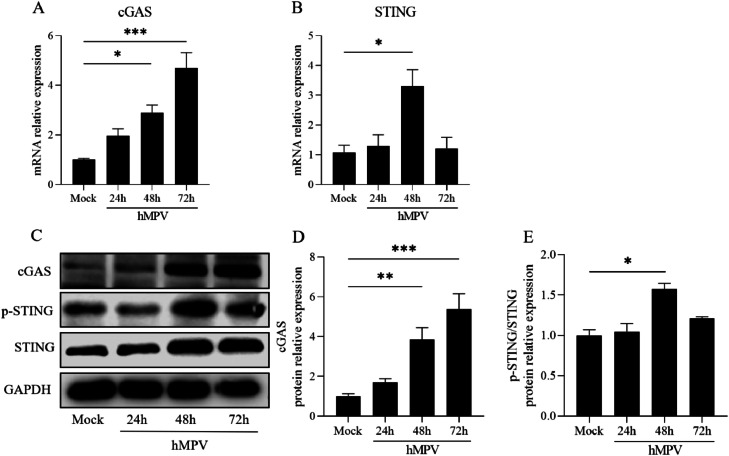
IL-1β has been identified as a key regulator of the innate immune response by activating the cGAS-STING pathway, which is known to play a crucial role in the defense against various DNA and RNA viruses. However, the involvement of this pathway in hMPV infection has not been fully understood. Then, we conducted an experiment where cells were infected with hMPV (MOI=10) for different time points (0, 24, 48, and 72 h). We then examined the expression levels of cGAS, STING mRNA (Fig. 5A, B), and protein components of the cGAS-STING pathway (Fig. 5C–E). The mRNA and protein levels of cGAS and STING were up-regulated after hMPV infection. Notably, STING phosphorylation exhibited the most significant increase at 48 h post-infection, as confirmed by Western Blotting (referred to as "48hpi" for subsequent experiments).
Fig. 5.
cGAS-STING pathway is involved in hMPV infection
Expression of cGAS-STING in 16HBE cells infected with hMPV (MOI=10) for different times (0, 24, 48, 72 h). A, B. The lever of cGAS and STING mRNA was measured by RT-PCR and analyzed by 2−△△ct methods. C–E. The protein expression levels cGAS and p-STING/STING were detected by Western Blotting after 0 h, 24 h, 48 h, and 72 h of hMPV infection. The relative quantification of cGAS and p-STING/STING were measured by Image J.
3.7. Effect of IL-1β on the cGAS-STING pathway
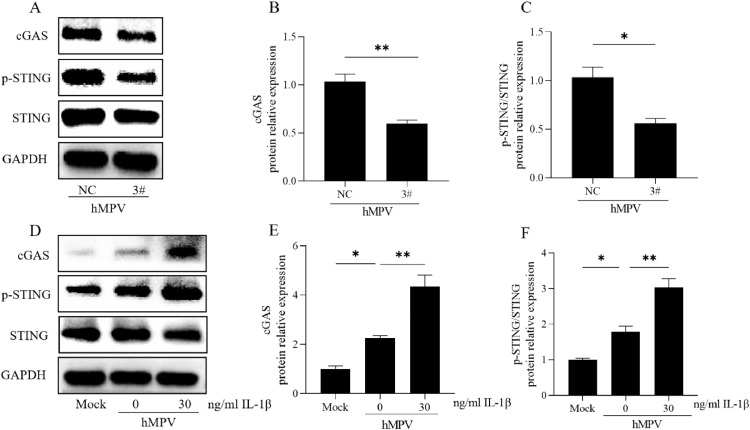
NC and 3# siRNA were transfected into 16HBE cells, followed by infection with hMPV (MOI=10). After incubating for an additional 48 h, the levels of cGAS and p-STING/STING proteins were assessed for statistical analysis (Fig. 6A–C). The transfection of 3# siRNA led to a decrease in the expression levels of cGAS and p-STING/STING proteins. To make sure the expression of IL-1β was suppressed throughout infection, we measured the mature IL-1β secretion by ELISA (Supplementary Fig. 2A). The level of pro-IL-1β mRNA was detected by qRT-PCR and the mature IL-1β protein (17 kDa) was measured by Western Blot (Supplementary Fig. 2B, C). Subsequently, the cells were stimulated with the exogenous addition of IL-1β (30 ng/ml) before hMPV (MOI=10). Changes in the levels of cGAS and p-STING/STING proteins were evaluated after another 48 h for statistical analysis (Fig. 6D–F). Upon the exogenous addition of IL-1β, there was an increase in the expressions of cGAS and p-STING/STING proteins during hMPV infection. These findings suggest that IL-1β modulates the expression of proteins involved in the cGAS-STING signaling pathway during hMPV infection.
Fig. 6.
Effect of IL-1β on the cGAS-STING pathway
IL-1β activated cGAS-STING pathway to affect hMPV replication in 16 HBE cells. Knockdown IL-1β for 48 h or exogenous supplementation of 30 ng/ml IL-1β for 24 h in 16HBE cells, then infected hMPV (MOI=10) for 48 h. A–C. Western Blot detected the expression of cGAS or p-STING/STING protein in 16HBE cells knocked down IL-1β. The data of three times independent experiments were measured by Image J. E, F. The expression of cGAS or p-STING/STING protein in 16HBE cells cultivated with 30 ng/ml rIL-1β was detected by Western Blotting. Data were obtained from three independent experiments and were expressed as mean ± standard deviation.
3.8. Detection of cytoplasmic mtDNA
We detected the cytoplasmic mtDNA during hMPV replication in the presence of #3 or IL-1β protein treatment. Cytosolic and nuclear fractions were used to quantify the level of DNA containing specific mitochondrial (MT-ATP6) and nuclear (RPL13A) genes by qRT-PCR (Supplementary Fig. 3A, B). It was found that the expression of MT-ATP6 was down-regulated in the presence of 3# siRNA. However, IL-1β-treated cells demonstrated significant enrichment of mtDNA in the cytosolic fraction compared to virus-treated cells. Therefore, we confirmed that IL-1β would play a role in hMPV infection by promoting the cGAS-STING pathway through mtDNA.
3.9. Effect of inhibitors on hMPV virus replication
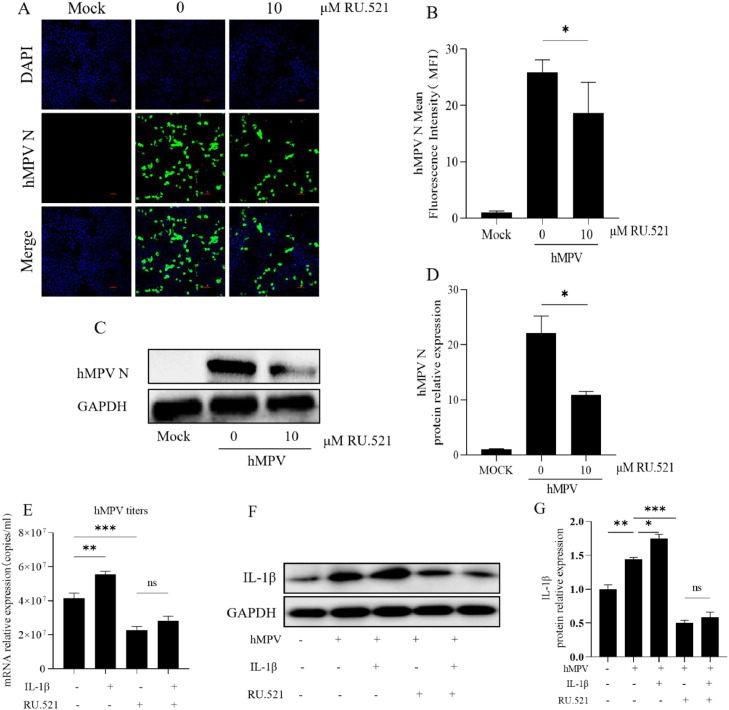
To investigate the role of the cGAS-STING pathway in hMPV replication, 16HBE cells were pretreated by cGAS inhibitor RU.521 and G150, or STING inhibitor H-151 at 1uM, 10uM, 20uM, and DMSO for 24 h, then infected hMPV (MOI=10) for 48 h. hMPV replication decreased significantly after 16HBE cells were treated with different concentrations of inhibitors, and 10uM RU.521 had a significant inhibitory effect (Supplementary Fig. 4A–G). We detected the expression of hMPV N protein by Immunofluorescence and Western Blot which showed that 10 uM RU.521 inhibited hMPV replication in 16HBE cells (Fig. 7A–D). Therefore, we chose RU.521 for the subsequent research. In the presence of RU.521, the copies of hMPV titer after exogenous addition of IL-1β were detected by PCR (Fig. 7E). The effect of IL-1β on hMPV replication was weakened when the cGAS-STING pathway was blocked. The expression of IL-1β was also detected by Western Blot and PCR using different inhibitors and concentrations (Supplementary Fig. 5A–F). The results showed that the expression of IL-1β was also suppressed by cGAS-STING inhibitors. There was no difference in IL-1β expression after exogenous addition of IL-1β in the presence of RU.521 (Fig. 7F–G).
Fig. 7.
Effect of cGAS inhibitor RU.521 on hMPV virus replication
RU.521 was used to inhibit cGAS in 16HBE cells. 16HBE cells cultivated with 10 μM DMSO or RU.521 in advance for 24 h, then infected with hMPV (MOI=10) for 48 h. A, B. The level of hMPV N protein expression was detected by Immunofluorescence and the mean fluorescence intensity of hMPV N protein was measured by Image J. C, D. Western Blot was used to detect the expression of hMPV N protein. The data are relative expression values compared with GAPDH protein. E. hMPV titers were detected by PCR in the presence or absence of exogenous IL-1β (30 ng/ml) and RU.521 (10uM) inhibitors. F, G. Protein expression levels of IL-1β (17 kDa) were detected by Western Blot in the presence or absence of exogenous IL-1β or RU.521 inhibitors. Image J was used to detect the results of three independent repeated tests.
3.10. Expression of type Ⅰ interferons (IFN-Ⅰs)
IFN-Ⅰs are the primary antiviral molecule of the innate immune system. cGAS-STING pathway activation can induce IFN-Ⅰs production. To investigate the role of IFN-Ⅰs in hMPV infection, we utilized qRT-PCR to detect the expression of IFN-Ⅰs at different times of 0, 24, 48, and 72 h. IFN-α increased significantly only at 72 h, and IFN-β increased after 48 h (Supplementary Fig. 6A, B).
4. Discussion
hMPV is a single negative-stranded RNA virus that is widespread globally, and it remains a potential epidemic threat due to the rapid evolution of RNA viruses (Uddin and Thomas, 2023). IL-1β, a crucial mediator of intercellular communication and viral clearance within the immune system, has been found to be associated with the severity of hMPV infection (Zhang et al., 2020; Cioccarelli et al., 2021). However, the mechanism by which IL-1β affects hMPV pathogenicity remains unclear. In this study, we found that IL-1β could promote hMPV replication by activating the cGAS-STING signaling pathway, thus the activity of the cGAS-STING signaling pathway could be necessary for IL-1β expression.
IL-1β is a pro-inflammatory cytokine produced primarily by monocytes and endothelial cells which is a common host response to viral infections (Pan et al., 2021; Vu et al., 2022). Reports indicate that patients with severe hMPV infection have higher levels of IL-1β, and the concentration of IL-1β in hMPV-infected patients is more obvious than that in respiratory syncytial virus (RSV) (Malmo et al., 2016; Park et al., 2017). IL-1β has become increasingly important in viral replication, but its roles depend on the specific type of virus. The report proposes that the release of IL-1β initiates an inflammatory positive feedback loop that promotes retroviral replication (Browne, 2015). Additionally, IL-1β increases the sensitivity of mouse immune cells to human immunodeficiency virus (HIV) infection and promotes HIV replication (Lawson et al., 2020). However, exogenous supplementation of IL-1β could reduce HBV and HDV replication (Delphin et al., 2021). Animals that lack the IL-1β receptor has an increased susceptibility to West Nile virus (Ramos et al., 2012). In this study, we observe that the expression of IL-1β is regulated in a time-dependent and dose-dependent manner. hMPV replication is reduced after transfection of siRNA targeting the IL-1β gene, and increased when exogenous supplement of rIL-1β. IL-1β expression is positively correlated with hMPV replication, and its underlying mechanism deserves further investigation.
IL-1β can induce mtDNA release and activate innate immune signaling by releasing cGAS-STING pathway proteins to combat viral and microbial infections (Aarreberg et al., 2019; Wu et al., 2023). cGAS is known to catalyze the reaction between guanosine triphosphate (GTP) and adenosine triphosphate (ATP) and activate the interferon key adaptation protein stimulator interferon gene (STING) in response to viral infection (Decout et al., 2021). Studies have shown that IL-1β mediated cGAS-STING pathway may suppress DENV or Rabies virus (RABV) replication (Aarreberg et al., 2019; Wu et al., 2023). This signaling pathway has been shown to play a role in the replication of RNA viruses (Liu et al., 2022; Su et al., 2023). In this paper, we observe that knockdown of IL-1β infected with hMPV leads to reduced cGAS expression and STING phosphorylation. Conversely, the addition of exogenous IL-1β led to increased expression of cGAS and phosphorylation of STING. We found that the expression of MT-ATP6 was down-regulated in the presence of 3# siRNA compared to virus-treated cells. However, the expression of MT-ATP6 increased in IL-1β protein treated cells, indicating that IL-1β induced enrichment of mtDNA in the cytosolic fraction. Our study identified that IL-1β is dependent upon cytosolic detection of mtDNA to drive cGAS-STING activation. Additionally, we utilized a cGAS inhibitor RU.521 to investigate the effect of the pathway on hMPV replication. As expected, RU.521 suppressed the replication of hMPV. In the presence of RU.521, the exogenous addition of IL-1β could not affect hMPV replication. Thus, IL-1β promotes hMPV replication primarily by activating the cGAS-STING pathway, which is similar to PCV2 and influenza viruses (Huang et al., 2018; Tang et al., 2023).
Type I interferons (IFN-Ⅰs) are known to be against virus replication (Hastings et al., 2015; Lei et al., 2020; Li et al., 2020). Studies have shown that the cGAS-STING pathway can trigger the production of IFN-Ⅰs, which plays a crucial role in host defense against invading pathogens (Domizio et al., 2022). According to our previous research findings (Li et al., 2023; Zhang et al., 2023), with the extension of infection time, hMPV replication was significantly reduced after infecting for 96 h. By detecting the expression of IFN-Ⅰs, we found that IFN-α increased significantly only at 72 h. In this paper, our experiments were conducted at 48 h, so IFN-Ⅰs may play a role in the late stage of hMPV infection. Early activation of IL-1β-cGAS-STING may promote hMPV replication through some mechanism of action to avoid activating type I interferon production and antiviral signaling.
One limitation of our study is that we study the effect of hMPV infection by establishing 16HBE cells model in vitro. However, epithelial cells are the main protective barrier against viruses and are the main sites of viral replication (Johnston et al., 2021). Most studies of respiratory viruses focus on epithelial cells which is essential for initiating the regulatory function of the immune system (Schultz et al., 2020). Research reported that IL-1β plays a major role in the communication between epithelial cells and monocytes, as well as to the inflammatory reaction to viral infections (Stokes et al., 2011). In this study, we explore the specific effect and mechanism of IL-1β in hMPV replication in vitro. Although the in vitro study may not truly reflect a systemic immunomodulatory process in the host after hMPV infection, they provide an approximate state of initial viral infection.
In conclusion, we confirmed that IL-1β could activate the cGAS-STING pathway to promote hMPV replication. Enhancing the host antiviral pathway to prevent or limit viral infection is a promising strategy, thus indicating that IL-1β has valuable insights for future studies to block viral replication and develop targeted drugs.
CRediT authorship contribution statement
Guojin Wu: Writing – original draft, Formal analysis, Data curation, Methodology, Software, Investigation. Yueyan Zhang: Resources, Methodology, Investigation. Linlin Niu: Supervision, Project administration, Methodology. Yuan Hu: Writing – review & editing, Supervision. Yuting Yang: Writing – review & editing, Methodology, Conceptualization, Project administration, Funding acquisition, Visualization. Yao Zhao: Funding acquisition, Conceptualization, Project administration, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Clinical Research Center for Child Health and Disorders General Project (No. NCRCCHD-2019-GP-04) and Youth Project (No. NCRCCHD-2021-YP-03); Chongqing Talent Program (CQYC20210303393); Program for Youth Innovation in Future Medicine, Chongqing Medical University.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2024.199344.
Contributor Information
Yuting Yang, Email: 412384223@qq.com.cn.
Yao Zhao, Email: Zhaoy@cqmu.edu.cn.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Aarreberg L.D., Esser-Nobis K., Driscoll C., Shuvarikov A., Roby J.A., Gale M., Jr. Interleukin-1beta induces mtDNA Release to Activate Innate Immune Signaling via cGAS-STING. Mol. Cell. 2019;74:801–815. doi: 10.1016/j.molcel.2019.02.038. e806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainouze M., Rochefort P., Parroche P., Roblot G., Tout I., Briat F., Zannetti C., Marotel M., Goutagny N., Auron P., Traverse-Glehen A., Lunel-Potencier A., Golfier F., Masson M., Robitaille A., Tommasino M., Carreira C., Walzer T., Henry T., Zanier K., Trave G., Hasan U.A. Human papillomavirus type 16 antagonizes IRF6 regulation of IL-1beta. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Zhao Y., Dong J., Liang S., Guo M., Liu X., Wang X., Huang Z., Sun X., Zhang Z., Dong L., Liu Q., Zheng Y., Niu D., Xiang M., Song K., Ye J., Zheng W., Tang Z., Tang M., Zhou Y., Shen C., Dai M., Zhou L., Chen Y., Yan H., Lan K., Xu K. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021;31:395–403. doi: 10.1038/s41422-021-00473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnood S.A., Shafran R., Bennett S.D., Zhang A.X.D., O'Mahoney L.L., Stephenson T.J., Ladhani S.N., De Stavola B.L., Viner R.M., Swann O.V. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J. Infect. 2022;84:158–170. doi: 10.1016/j.jinf.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent R., Moll L., Grabbe S., Bros M. Interleukin-1 Beta-A friend or foe in malignancies? Int. J. Mol. Sci. 2018;19:2155. doi: 10.3390/ijms19082155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne E.P. An interleukin-1 beta-encoding retrovirus exhibits enhanced replication in vivo. J. Virol. 2015;89:155–164. doi: 10.1128/JVI.02314-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioccarelli C., Sanchez-Rodriguez R., Angioni R., Venegas F.C., Bertoldi N., Munari F., Cattelan A., Molon B., Viola A. IL1beta promotes TMPRSS2 expression and SARS-CoV-2 cell entry through the p38 MAPK-GATA2 axis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.781352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decout A., Katz J.D., Venkatraman S., Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021;21:548–569. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delphin M., Faure-Dupuy S., Isorce N., Rivoire M., Salvetti A., Durantel D., Lucifora J. Inhibitory effect of IL-1beta on HBV and HDV replication and HBs antigen-dependent modulation of its secretion by macrophages. Viruses. 2021;14:65. doi: 10.3390/v14010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q., Gaska J.M., Douam F., Wei L., Kim D., Balev M., Heller B., Ploss A. Species-specific disruption of STING-dependent antiviral cellular defenses by the Zika virus NS2B3 protease. Proc. Natl. Acad. Sci. USA. 2018;115:E6310–E6318. doi: 10.1073/pnas.1803406115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domizio J.D., Gulen M.F., Saidoune F., Thacker V.V., Yatim A., Sharma K., Nass T., Guenova E., Schaller M., Conrad C., Goepfert C., de Leval L., Garnier C.V., Berezowska S., Dubois A., Gilliet M., Ablasser A. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;603:145–151. doi: 10.1038/s41586-022-04421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erttmann S.F., Swacha P., Aung K.M., Brindefalk B., Jiang H., Hartlova A., Uhlin B.E., Wai S.N., Gekara N.O. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity. 2022;55:847–861. doi: 10.1016/j.immuni.2022.04.006. e810. [DOI] [PubMed] [Google Scholar]
- Haddadin Z., Beveridge S., Fernandez K., Rankin D.A., Probst V., Spieker A.J., Markus T.M., Stewart L.S., Schaffner W., Lindegren M.L., Halasa N. Respiratory syncytial virus disease severity in young children. Clin. Infect. Dis. 2021;73:e4384–e4391. doi: 10.1093/cid/ciaa1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings A.K., Erickson J.J., Schuster J.E., Boyd K.L., Tollefson S.J., Johnson M., Gilchuk P., Joyce S., Williams J.V. Role of type I interferon signaling in human metapneumovirus pathogenesis and control of viral replication. J. Virol. 2015;89:4405–4420. doi: 10.1128/JVI.03275-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Zhang L., Lu M., Li J., Lv Y. PCV2 infection activates the cGAS/STING signaling pathway to promote IFN-beta production and viral replication in PK-15 cells. Vet. Microbiol. 2018;227:34–40. doi: 10.1016/j.vetmic.2018.10.027. [DOI] [PubMed] [Google Scholar]
- Johnston S.L., Goldblatt D.L., Evans S.E., Tuvim M.J., Dickey B.F. Airway epithelial innate immunity. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.749077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K.S., Prasad A., Groopman J.E. Methamphetamine enhances HIV-1 replication in CD4(+) T-cells via a novel IL-1beta auto-regulatory loop. Front. Immunol. 2020;11:136. doi: 10.3389/fimmu.2020.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le V.B., Dubois J., Couture C., Cavanagh M.H., Uyar O., Pizzorno A., Rosa-Calatrava M., Hamelin M.E., Boivin G. Human metapneumovirus activates NOD-like receptor protein 3 inflammasome via its small hydrophobic protein which plays a detrimental role during infection in mice. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., Guo F., Zhao Z., Zhou Z., Xiang Z., Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang T., Zhang Y., Wei F. Evasion mechanisms of the type I interferons responses by influenza A virus. Crit. Rev. Microbiol. 2020;46:420–432. doi: 10.1080/1040841X.2020.1794791. [DOI] [PubMed] [Google Scholar]
- Li J., Zhao Y., Dai Y., Zhao J. Identification of gamma-Fagarine as a novel antiviral agent against respiratory virus (hMPV) infection. Virus Res. 2023;336 doi: 10.1016/j.virusres.2023.199223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.N., Li L.W., Gao F., Jiang Y.F., Yuan W.Z., Li G.X., Yu L.X., Zhou Y.J., Tong G.Z., Zhao K. cGAS restricts PRRSV replication by sensing the mtDNA to increase the cGAMP activity. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.887054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Damania B. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe. 2016;19:150–158. doi: 10.1016/j.chom.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmo J., Moe N., Krokstad S., Ryan L., Loevenich S., Johnsen I.B., Espevik T., Nordbo S.A., Dollner H., Anthonsen M.W. Cytokine profiles in human metapneumovirus infected children: identification of genes involved in the antiviral response and pathogenesis. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0155484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P., Shen M., Yu Z., Ge W., Chen K., Tian M., Xiao F., Wang Z., Wang J., Jia Y., Wang W., Wan P., Zhang J., Chen W., Lei Z., Chen X., Luo Z., Zhang Q., Xu M., Li G., Li Y., Wu J. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021;12:4664. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.S., Kim Y.H., Kwon E., Callaway Z., Fujisawa T., Kim C.K. Comparison of nasal cytokine profiles of human metapneumovirus and respiratory syncytial virus. Asia Pac. Allergy. 2017;7:206–212. doi: 10.5415/apallergy.2017.7.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiefard F., Yun Z., Orvell C. Epidemiologic characteristics and seasonal distribution of human metapneumovirus infections in five epidemic seasons in Stockholm, Sweden, 2002-2006. J. Med. Virol. 2008;80:1631–1638. doi: 10.1002/jmv.21242. [DOI] [PubMed] [Google Scholar]
- Ramos H.J., Lanteri M.C., Blahnik G., Negash A., Suthar M.S., Brassil M.M., Sodhi K., Treuting P.M., Busch M.P., Norris P.J., Gale M., Jr. IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert L.S., Rashidi A.S., Tran D.N., Katzilieris-Petras G., Hvidt A.K., Gohr M., Fruhwurth S., Bodda C., Thomsen M.K., Vendelbo M.H., Khan A.R., Hansen B., Bergstrom P., Agholme L., Mogensen T.H., Christensen M.H., Nyengaard J.R., Sen G.C., Zetterberg H., Verjans G.M., Paludan S.R. Brain immune cells undergo cGAS/STING-dependent apoptosis during herpes simplex virus type 1 infection to limit type I IFN production. J. Clin. Investig. 2021;131:e136824. doi: 10.1172/JCI136824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D., Surabhi S., Stelling N., Rothe M., Group K.S., Methling K., Hammerschmidt S., Siemens N., Lalk M. 16HBE cell lipid mediator responses to mono and co-infections with respiratory pathogens. Metabolites. 2020;10:113. doi: 10.3390/metabo10030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C.A., Ismail S., Dick E.P., Bennett J.A., Johnston S.L., Edwards M.R., Sabroe I., Parker L.C. Role of interleukin-1 and MyD88-dependent signaling in rhinovirus infection. J. Virol. 2011;85:7912–7921. doi: 10.1128/JVI.02649-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Shen S., Hu Y., Chen S., Cheng L., Cai Y., Wei W., Wang Y., Rui Y., Yu X.F. SARS-CoV-2 ORF3a inhibits cGAS-STING-mediated autophagy flux and antiviral function. J. Med. Virol. 2023;95:e28175. doi: 10.1002/jmv.28175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Liu X., Wang C., Shu C. USP18 promotes innate immune responses and apoptosis in influenza A virus-infected A549 cells via cGAS-STING pathway. Virology. 2023;585:240–247. doi: 10.1016/j.virol.2023.06.012. [DOI] [PubMed] [Google Scholar]
- Uddin S., Thomas M. StatPearls; Treasure Island (FL): 2023. Human Metapneumovirus. [PubMed] [Google Scholar]
- van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A., Osterhaus A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu L.D., Phan A.T.Q., Hijano D.R., Siefker D.T., Tillman H., Cormier S.A. IL-1beta promotes expansion of IL-33(+) lung epithelial stem cells after respiratory syncytial virus infection during infancy. Am. J. Respir. Cell Mol. Biol. 2022;66:312–322. doi: 10.1165/rcmb.2021-0313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li Y., Deloria-Knoll M., Madhi S.A., Cohen C., Ali A., Basnet S., Bassat Q., Brooks W.A., Chittaganpitch M., Echavarria M., Fasce R.A., Goswami D., Hirve S., Homaira N., Howie S.R.C., Kotloff K.L., Khuri-Bulos N., Krishnan A., Lucero M.G., Lupisan S., Mira-Iglesias A., Moore D.P., Moraleda C., Nunes M., Oshitani H., Owor B.E., Polack F.P., O'Brien K.L., Rasmussen Z.A., Rath B.A., Salimi V., Scott J.A.G., Simoes E.A.F., Strand T.A., Thea D.M., Treurnicht F.K., Vaccari L.C., Yoshida L.M., Zar H.J., Campbell H., Nair H., Respiratory Virus Global Epidemiology Network Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob. Health. 2021;9:e33–e43. doi: 10.1016/S2214-109X(20)30393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wang J., Huang C., Zhao J., Fu Z.F., Zhao L., Zhou M. Interleukin-1beta suppresses rabies virus infection by activating cGAS-STING pathway and compromising the blood-brain barrier integrity in mice. Vet. Microbiol. 2023;280 doi: 10.1016/j.vetmic.2023.109708. [DOI] [PubMed] [Google Scholar]
- Yaseen M.M., Abuharfeil N.M., Darmani H. The role of IL-1beta during human immunodeficiency virus type 1 infection. Rev. Med. Virol. 2023;33:e2400. doi: 10.1002/rmv.2400. [DOI] [PubMed] [Google Scholar]
- Zhang W., Huang Z., Huang M., Zeng J. Predicting severe enterovirus 71-infected hand, foot, and mouth disease: cytokines and chemokines. Mediat. Inflamm. 2020;2020 doi: 10.1155/2020/9273241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu G., Yang Y., Niu L., Zhao Y. Interleukin-4 promotes human metapneumovirus replication through the JAK/STAT6 pathway. Viral Immunol. 2023;36:449–457. doi: 10.1089/vim.2023.0027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.