Abstract
Currently, there are four monoclonal antibodies (mAbs) that target the cluster of differentiation (CD) 20 receptor available to treat multiple sclerosis (MS): rituximab, ocrelizumab, ofatumumab, and ublituximab. B-cell depletion therapy has changed the therapeutic landscape of MS through robust efficacy on clinical manifestations and MRI lesion activity, and the currently available anti-CD20 mAb therapies for use in MS are a cornerstone of highly effective disease-modifying treatment. Ocrelizumab is currently the only therapy with regulatory approval for primary progressive MS. There are currently few data regarding the relative efficacy of these therapies, though several clinical trials are ongoing. Safety concerns applicable to this class of therapeutics relate primarily to immunogenicity and mechanism of action, and include infusion-related or injection-related reactions, development of hypogammaglobulinemia (leading to increased infection and malignancy risk), and decreased vaccine response. Exploration of alternative dose/dosing schedules might be an effective strategy for mitigating these risks. Future development of biosimilar medications might make these therapies more readily available. Although anti-CD20 mAb therapies have led to significant improvements in disease outcomes, CNS-penetrant therapies are still needed to more effectively address the compartmentalized inflammation thought to play an important role in disability progression.
Key Points
| The currently available anti-CD20 mAb therapies for use in multiple sclerosis—rituximab, ocrelizumab, ofatumumab, and ublituximab—have led to significant improvements in disease outcomes through robust efficacy on clinical manifestations and MRI lesion activity, and are a cornerstone of highly effective disease-modifying treatment. |
| Adaptations of how these medications are used to reduce adverse effects are under investigation. |
| CNS-penetrant therapies are still needed to more effectively address the compartmentalized inflammation that is thought to play an important role in disability progression. |
Introduction
The landscape of multiple sclerosis (MS) therapeutics has rapidly evolved over the past several decades, with more than 20 disease-modifying therapies (DMTs) currently available [1]. B-cell depletion therapy, which is achieved mainly through targeting of the cluster of differentiation (CD) 20 receptor, has historically been used in a variety of systemic autoimmune diseases with effects being exerted through a variety of key immunological mechanisms [2]. Currently available anti-CD20 monoclonal antibodies (mAbs) to treat MS include rituximab (used off-label), plus three with regulatory approval: ocrelizumab, ofatumumab, and ublituximab. All anti-CD20 mAbs are considered highly effective DMTs. Notably, ocrelizumab is the only regulatory-approved treatment for primary progressive (PP) MS. In this review, we aim to summarize the role of B cells in MS pathogenesis, molecular and pharmacological properties of anti-CD20 mAbs, monitoring and safety considerations for clinical use, and future directions.
Search Strategy
A literature search was conducted using the PubMed search engine, available through the National Library of Medicine. All available articles in the English language with different combinations of search terms “rituximab,” “ocrelizumab,” ofatumumab,” “ublituximab,” “CD20,” “multiple sclerosis,” and “B cells” were manually reviewed for suitability. The references of each eligible publication were also screened for any additional relevant papers. Criteria for inclusion consisted of access to the full published manuscript and appropriate scientific and clinical relevance, as determined by the authors. The final search was performed in December 2023.
Role of B Cells in Multiple Sclerosis (MS) Pathogenesis
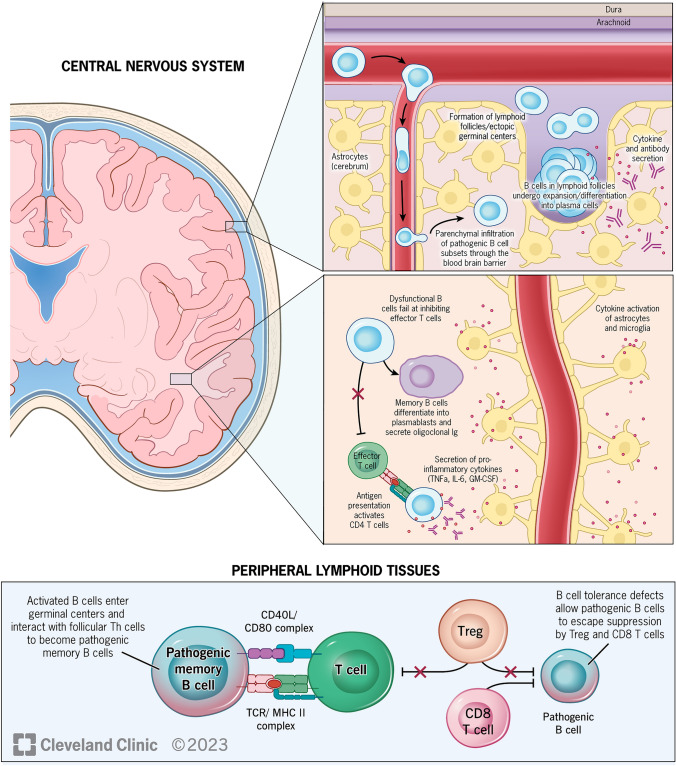
Historically, T-cell-mediated processes were thought to be the principal drivers of MS-related pathology, though mounting evidence over the past decade has shown that B-cell-related mechanisms (including interactions between B and T cells) are important (Fig. 1) [3, 4]. Several studies have shown co-localization of B- and T-cell infiltrates with active lesions and abundant plasma cells and CD20+ B cells within perivenular spaces in patients with progressive forms of MS [5, 6]. B-cell aggregates within the meninges, some of which resemble lymphoid follicle structures, correlate with areas of subpial demyelination, neuronal loss, and cortical atrophy [7]. Furthermore, small subsets of clonally related B cells (identified by deep sequencing of immunoglobulin G (IgG) heavy chain variable region genes) indicate peripheral B-cell activation, suggesting that B-cell maturation and immune responses occur in parallel in both the periphery and the central nervous system (CNS) [8].
Fig. 1.
B-cell targets in CNS and periphery [4]. B-cell populations in the periphery, which contain tolerance defects, escape suppression by Treg and CD8+ T cells and enter germinal centers, where they differentiate into pathogenic memory B cells through interactions with follicular Th cells. Subsets of these pathogenic memory B cells, which express chemokine receptors CXCR3 and CCR6, proinflammatory cytokines, and adhesion molecule VLA-4, then infiltrate CNS through the blood-brain barrier, where they encounter T cells in follicle-like structures, leading to clonal expansion. Once inside the CNS, memory B cells may become plasmablasts, which secrete antibodies. Pathogenic B cells also secrete pro-inflammatory cytokines, which leads to activation of astrocytes and microglia, failure of effector T-cell inhibition, and activation of CD4+ T cells. CD cluster of differentiation, CNS central nervous system, GM-CSF granulocyte-macrophage colony-stimulating factor, IL interleukin, Th T helper, TNF tumor necrosis factor, Treg T regulatory
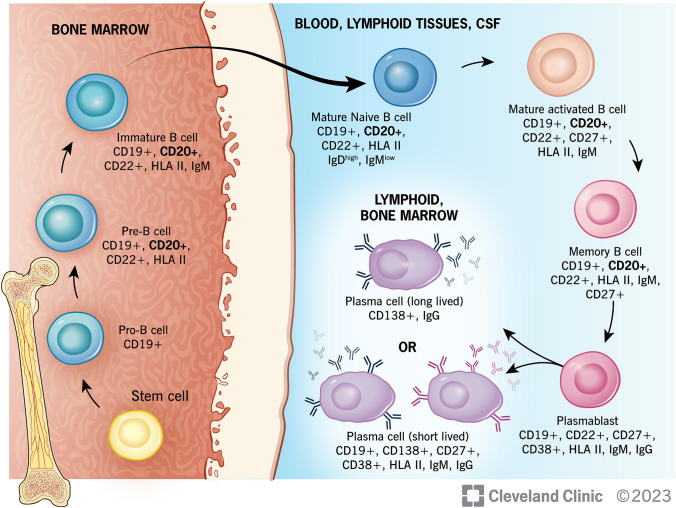
B-cell ontogeny involves antigen-independent maturation from hematopoietic stem cells in the bone marrow, followed by antigen-dependent maturation in the peripheral lymphoid tissues (Fig. 2) [9]. Pre-B cells (CD19+ and CD20+), originating from pro-B cells (CD19− and CD20−), develop into immature B cells in the bone marrow, at which point IgM is expressed. These cells then evolve into mature B cells following activation by their cognate antigen and co-stimulatory factors. Ig isotype switching after activation occurs in germinal centers, which stimulates B cells to migrate to various locations (including the bone marrow, brain, gut, spleen, and tonsils), where they differentiate into either memory B cells (CD27-low) or plasmablasts (both early/CD27-high and CD40L+ plasmablasts and late/CD27+ and CD38+ plasmablasts) [8]. Specific chemokines, including CXCL12, CCL25, and CCL28, subsequently direct these cells to become antibody-producing plasma cells. In these locations, B cells also function as antigen-presenting cells or produce proinflammatory cytokines, which enhance inflammatory processes [10].
Fig. 2.
B-cell maturation. B cells originate from common lymphoid progenitor (stem) cells in bone marrow. They first develop into pro-B cells, then differentiate into pre-B cells (at which point CD20 is expressed) and immature B cells through a process of rearrangements at the immunoglobulin locus which lead to surface expression of the pre-B cell receptor, and later a mature B-cell receptor capable of binding antigen. Immature B cells undergo a selection process to prevent development of self-reactivity. They then migrate out of the bone marrow into the periphery (lymph nodes and spleen), where they become mature naïve B cells. Binding to cognate antigen triggers development of antigen-specific mature activated B cells, which become either plasmablasts and antibody-secreting plasma cells (CD20-) or remain memory B cells (CD20+). CD cluster of differentiation, CSF cerebrospinal fluid, HLA human leukocyte antigen, Ig immunoglobulin
Activated B cells normally interact with T helper (Th) cells in germinal centers, where they differentiate into memory B cells and, in doing so, induce Th effector activation. It is thought that in MS, peripheral B cells escape the control of T regulatory cells, which are functionally impaired [8]. Highly pathogenic B (and T) cells can migrate through the blood-brain barrier by expressing distinct chemokine receptors, proinflammatory cytokines, and adhesion molecules, where they become reactivated and cause MS-related CNS pathology [8]. The precise mechanism(s) by which these cells contribute to the abnormal immune response in MS remains incompletely understood.
Molecular and Pharmacological Attributes of Anti-CD20 Monoclonal Antibodies
CD20 is postulated to function as either an ion channel and/or through indirect regulation of calcium release mediated by the B-cell antigen receptor [11, 12]. It is a transmembrane protein consisting of four helices and two extracellular loops [8], and is believed to have the capacity to assemble as a compact dimeric double barrel based on more recent structural analyses, which may argue against its potential function as an ion channel [13]. Depletion of B cells (both circulating B cells and subsets of CD20-expressing CD3+, CD4+, and CD8+ T cells) [14] by anti-CD20 mAbs occurs through several distinct mechanisms, including apoptosis, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC) [8]. Stem cells, pro-B cells, plasmablasts, and antibody-secreting plasma cells, which do not express CD20, are preserved.
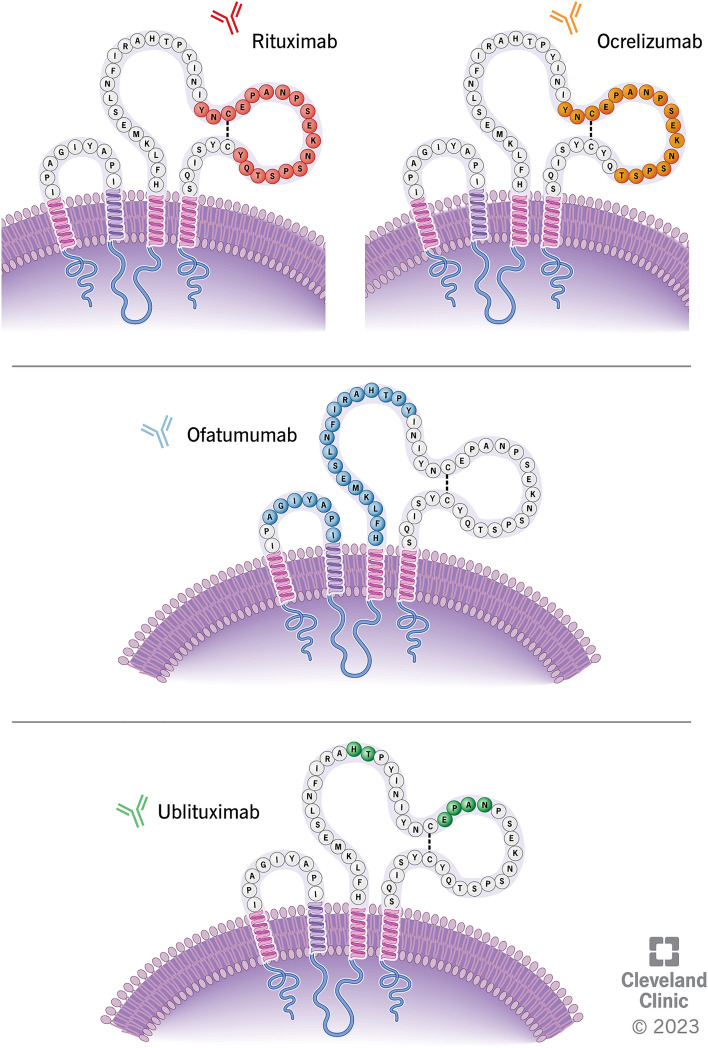
Currently four anti-CD20 mAbs are utilized to treat MS: rituximab, ocrelizumab, ofatumumab, and ublituximab. These treatments have distinct molecular structures, target epitopes, and pharmacological features (including route of administration, dosing regimens, mechanisms of B-cell depletion, reconstitution patterns, risks, and immunogenicity) [8, 14]. The degree of CDC versus ADCC elicited upon binding of anti-CD20 mAbs varies between therapies. Key characteristics are summarized in Fig. 3 and Table 1.
Fig. 3.
Anti-CD20 monoclonal antibody target epitopes (minor variability exists in published data). Ofatumumab binds to discontinuous sequences of the small (residues 74–80) and large extracellular loops (residues 145–161) of CD20 [137]. Rituximab binds to amino acid residues 165-182 on large extracellular loop of CD20 while ocrelizumab binds to amino acid residues 165–180 on large extracellular loop of CD20 [137]. Ublituximab binds to residues 158–159 and 168–171 on large extracellular loop of CD20 [138]. CD cluster of differentiation
Table 1.
Comparison of target epitope, molecular structure, effector mechanism, dose and dosing schedule, and pharmacokinetic characteristics of Anti-CD20 monoclonal antibody therapies for multiple sclerosis
| Rituximab [16] | Ocrelizumab [18] | Ofatumumab [32] | Ublituximab [74] | |
|---|---|---|---|---|
| CD20 target epitope | Binds to amino acid residues 168-175 on large extracellular loop | Binds to amino acid residues 165–180 on large extracellular loop | Binds to discontinuous sequences of the small (residues 74–80) and large extracellular loops (residues 145–161) | Binds to residues 168–171 and 158–159 on large extracellular loop |
| Degree of humanization | Chimeric murine/human IgG1κ anti-CD20 mAb | Recombinant humanized glycosylated IgG1VκI anti-CD20 mAb | Fully human IgG1κ anti-CD20 mAb | Chimeric IgG1κ anti-CD20 mAb with glycoengineered Fc segment that enhances affinity for FCγRIIIa receptors |
| Effector mechanism |
CDC > ADCC apoptosis + |
ADCC > CDC apoptosis ++ |
CDC = ADCC apoptosis ++ |
ADCC > CDC |
| Route of administration | IV infusion | IV infusion | SC injection | IV infusion |
| Dosing schedule |
Initial dose 1000 mg Second dose 1000 mg at week 2 Subsequent dosing 1000 mg Q6 months |
Initial dose 300 mg over ≥ 2.5 h Second dose 300 mg over ≥ 2.5 h at week 2 Subsequent dosing 600 mg over ≥ 3.5 h or over ≥ 2 h if no prior infusion reaction Q6 months |
Initial dose 20 mg Second dose 20 mg at weeks 1 and 2 Subsequent dosing 20 mg Q4 weeks starting at week 4 |
Initial dose 150 mg over 4 h Second dose 450 mg over 1 h at week 2 Subsequent dosing 450 mg over 1 h at week 24 |
| Terminal half-life | 22 days | 33 days | 16 days | 22 days |
| Immunogenicity | Most | Less | Least | Less |
ADCC antibody-dependent cellular cytotoxicity, CD20 cluster of differentiation-20, CDC compliment-dependent cytotoxicity, Ig immunoglobulin, IV intravenous, mAb monoclonal antibody, MS multiple sclerosis, SC subcutaneous
Rituximab
Rituximab is a chimeric murine-human IgG1 kappa mAb that binds to the large extracellular loop of CD20 amino acid residues 168-175 (Fig. 3) [15]. It has a molecular weight of approximately 145 kilodaltons (kDa) and elicits greater CDC than ADCC [14]. Rituximab is administered via intravenous (IV) infusion, in MS typically starting with an initial dose of 1000 mg on day 1 followed by a second 1000 mg dose on day 15. Subsequent doses of 1000 mg are administered every 6 months. Premedication with diphenhydramine, acetaminophen, and corticosteroids is often administered to reduce infusion-related adverse effects. In studies evaluating pharmacodynamics of rituximab in patients with rheumatoid arthritis, most patients demonstrated near-complete depletion of circulating CD19+ B cells within 2 weeks following the first infusion, with reconstitution occurring after 6 months, with a small proportion showing reconstitution delayed up to beyond 3 years [16].
Ocrelizumab
Ocrelizumab is a recombinant humanized glycosylated anti-CD20 IgG1 kappa mAb, which targets the large extracellular loop of CD20 binding to amino-acid residues 165–180 (overlapping the epitope bound by rituximab) (Fig. 3) [15]. The molecular weight of ocrelizumab is approximately 145 kDa. Ocrelizumab elicits greater ADCC than CDC [14]. Ocrelizumab is administered via IV infusion at an initial dose of 300 mg over a minimum of 2.5 h on day 1 followed by a second 300 mg dose at day 15. Subsequent doses of 600 mg are administered every 6 months thereafter over at least 3.5 h (or 2 h in cases where no previous serious infusion reaction(s) were observed). Premedication with IV methylprednisolone (100 mg, or alternative corticosteroid at an equivalent dose) and antihistamine is recommended 30–60 min prior to each infusion. Near-complete depletion of B cells within 2 weeks of treatment was seen in the ocrelizumab phase III clinical trials [17]. Repletion of B cells occurred within 2.5 years of the last infusion in 90% of patients, with a median time of 72 weeks [18].
Ofatumumab
Ofatumumab is a fully human IgG1 kappa anti-CD20 mAb that binds to discontinuous sequences of both small and large extracellular loops of CD20, at amino-acid residues 74–80 and 145–161, respectively (Fig. 3) [14]. It has a molecular weight of approximately 146 kDa and elicits greater CDC than ADCC [14]. Ofatumumab is administered via subcutaneous (SC) injection at a dose of 20 mg weekly for three doses then 20 mg monthly starting at week 4. Premedication generally is not needed. In the phase III clinical trials of ofatumumab in MS, near-complete depletion of B cells resulted in 77.0–78.8% of patients within 1 week and 95.0–95.8% 2 weeks after treatment initiation [19]. The median time to repletion of B cells to either the lower limit of normal (LLN) or baseline value after treatment discontinuation was 24.6 weeks.
Ublituximab
The most recently approved anti-CD20 therapy, ublituximab, is a chimeric anti-CD20 IgG1 kappa mAb, which binds to the large extracellular loop of CD20 at amino-acid residues 158–159 and 168–171 (Fig. 3) [20]. It has a glycosylated/glycoengineered fragment crystallizable (Fc) segment designed to enhance affinity for FCγRIIIa receptors and elicits greater ADCC than CDC [14]. Ublituximab is administered via IV infusion at an initial dose of 150 mg over 4 h on day 1 followed by a 450 mg dose over 1 h on day 15. Subsequent doses of 450 mg are administered over 1 h every 24 weeks thereafter. Premedication consists of oral antihistamine and corticosteroid administered 30 min prior to each infusion. In the phase III clinical trials of ublituximab, treatment reduced CD19 counts by the first measured timepoint 24 h after the initial infusion, and the median time to repletion of B cells to either LLN or baseline was 70.3 weeks after the last infusion [21].
Clinical Trials
The following section aims to summarize key clinical trials that have led to approval of these therapies. This list is not exhaustive, as ongoing interest in relative efficacy and alternative dosing regimens for these therapies has led to additional studies that are currently ongoing.
Rituximab
Rituximab frequently is used off-label to treat MS but currently does not have regulatory approval for this indication. It has been studied in both relapsing remitting (RR) MS and PPMS. A 48-week phase II double-blind placebo-controlled clinical trial of rituximab in 104 RRMS patients evaluated the total count of brain MRI gadolinium-enhancing (GdE) lesions at weeks 12, 16, 20, and 24 following a single course of treatment with 1000 mg of IV rituximab on days 1 and 15 [22]. There were reduced numbers of total and total new GdE lesions and a lower proportion of patients with relapses at week 24 (14.5%) compared to those receiving placebo (34.3%). Another multicenter, rater-blinded phase III trial of rituximab in 200 patients with either RRMS or CIS randomized to IV rituximab 1000 mg followed by 500 mg every 6 months or oral dimethyl fumarate 240 mg twice daily (RIFUND-MS) demonstrated superiority of rituximab in preventing protocol-defined relapses over 24 months (3% in the rituximab group compared to 16% in the dimethyl fumarate group) [23].
In a trial investigating the effect of four courses of IV rituximab (two 1000 mg infusions every 24 weeks through 96 weeks) on time to confirmed disease worsening measured by increase in EDSS maintained for 12 weeks in a cohort of 439 PPMS patients (OLYMPUS), differences between rituximab and placebo did not reach statistical significance [24]. However, rituximab-treated patients had less increase in T2 lesion volume, and in a subgroup analysis, individuals < 51 years of age and those with GdE lesions at baseline showed delayed time to confirmed EDSS (Expanded Disability Status Scale) worsening compared to those treated with placebo.
Ocrelizumab
Ocrelizumab was approved by the US Food and Drug Administration (FDA) in March 2017 and by the European Medicines Agency (EMA) in January 2018 for use in adults with RRMS (including clinically isolated syndrome (CIS)), active secondary progressive (SP) MS, and PPMS) [18]. It is currently being investigated in phase II/III trials in pediatric MS. The phase II placebo-controlled clinical trial of ocrelizumab evaluated the number of GdE T1 lesions and annualized relapse rate (ARR) over 24 weeks amongst 218 patients included in the intention-to-treat analysis [25]. In patients treated with 600 mg of ocrelizumab, the number of GdE lesions was reduced by 89% and ARR was reduced by 80% compared to placebo. The phase III clinical trials of ocrelizumab, OPERA I/II [17] and ORATORIO [26] demonstrated efficacy and safety in the treatment of patients with RRMS and PPMS, respectively. In the OPERA I/II trials, which compared ocrelizumab to interferon beta 1a (IFN β-1a) in patients with RRMS, ARR was reduced by 46–47% (ARR 0.16 vs. 0.29) at 96 weeks. Ocrelizumab performed better than IFN β-1a on secondary endpoints including 3-month confirmed EDSS worsening (9.1–6.9% vs. 13.6–10.5%), total and mean number of GdE lesions (94–95% fewer lesions), and total number of new or newly enlarged T2 lesions (77–83% fewer lesions). The percentage of patients who achieved no evidence of disease activity and percentage brain volume loss favored ocrelizumab, but based on the hierarchical statistical testing algorithm were not considered significant. The ORATORIO study, which compared ocrelizumab to placebo in patients with PPMS, demonstrated a relative risk reduction in 12-week confirmed EDSS worsening of 23% (32.9% vs. 39.3%) and in 24-week EDSS confirmed worsening of 25% (29.6% vs. 35.7%). Measures of total T2 lesion volume, number of new or enlarging T2 lesions, and brain volume loss favored ocrelizumab.
Ofatumumab
Ofatumumab was approved for use in adults with RRMS (including CIS) and active SPMS by the FDA in August 2020 and by the EMA in March 2021. Phase III clinical trials in pediatric MS are ongoing. The phase III clinical trials of ofatumumab, ASCLEPIOS I/II [19], compared ofatumumab to teriflunomide in 1,882 and 955 patients with RRMS, respectively. The primary outcome measure ARR was 0.11 in those treated with ofatumumab versus 0.22 in those treated with teriflunomide in ASCLEPIOS I (difference of − 0.11; 95% confidence interval (95% CI) − 0.16, − 0.06), and 0.10 and 0.25, respectively, in ASCLEPIOS II (difference of − 0.15; 95% CI 0.20, − 0.09). Secondary endpoints included clinical outcomes (3- and 6-month confirmed EDSS worsening, 6-month confirmed EDSS improvement), MRI metrics (number of GdE lesions, number of new or enlarging lesions, and annual rate of brain volume loss), and a fluid biomarker endpoint (serum neurofilament light chain concentration at 3 months and beyond). Ofatumumab outperformed teriflunomide on clinical endpoints (3-month confirmed EDSS worsening 10.9% vs. 15.0%, 6-month confirmed EDSS worsening 8.1% vs. 12.0%, and 6-month confirmed EDSS improvement 11.0% vs. 8.1%), MRI and biomarker measures except change in brain volume, which favored teriflunomide, and reduction in serum neurofilament light chain concentration.
Ublituximab
Ublituximab was approved by the FDA in December 2022 and by the EMA in May 2023. It is currently approved for use in adults with RRMS (including CIS) and active SPMS. The phase III clinical trials of ublituximab, ULTIMATE I/II [21], compared ublituximab to teriflunomide in 549 and 545 patients with RRMS, respectively, using ARR as the primary outcome measure. ARR was 0.08 in those treated with ublituximab compared to 0.19 in those treated with teriflunomide in ULTIMATE I (rate ratio 0.41; 95% CI 0.27, 0.62), and 0.09 and 0.18, respectively, in ULTIMATE II (rate ratio 0.51; 95% CI 0.33, 0.78). On secondary outcomes, there was a mean number of GdE lesions of 0.02 in the ublituximab group and 0.49 in the teriflunomide group/0.01 in the ublituximab group and 0.25 in the teriflunomide group in ULTIMATE I/II, respectively. Three-month confirmed EDSS worsening occurred in 5.2% of patients in the ublituximab group and 5.9% in the teriflunomide group in the pooled analysis. This difference did not reach statistical significance (HR 0.84; 95% CI 0.50, 1.41).
Relative Efficacy
There have only been a few studies investigating the relative efficacy of the clinically available anti-CD20 mAbs. A recent observational cohort study evaluated the effectiveness of rituximab versus ocrelizumab in RRMS over a minimum of 6 months using a propensity-matched non-inferiority approach [27]. A total of 710 ocrelizumab-treated patients were matched to 186 rituximab-treated patients. The ARR was higher in patients treated with rituximab compared to those on ocrelizumab, with a rate ratio of 1.8 (95% CI 1.4, 2.4), and cumulative hazard of relapse (hazard ratio) 2.1 (95% CI 1.5, 3.0) over a pairwise censored mean follow-up of 1.4 years. This study did not demonstrate the expected noninferiority of rituximab compared to ocrelizumab. However, there were several potential limitations of this study, including the observational design, differences in disease duration and exposure to prior therapies, inclusion of both brand and biosimilar rituximab products, and variability of dosing schedules. Currently, there are three ongoing clinical trials, TRIO (NCT05758831) OVERLORD-MS (NCT04578639), and DanNORMS (NCT04688788), comparing the efficacy of rituximab and ocrelizumab.
Immunogenicity of Anti-CD20 Monoclonal Antibodies
Antibodies reacting with therapeutic mAbs can develop, possibly interfering with drug function or leading to adverse events. Although it is generally expected that immunogenicity would decrease with humanization of mAbs (fully human < humanized < chimeric), this is not always seen in practice, and immunogenicity is still observed even with fully human mAbs [28–30].
In a cross-sectional study, anti-drug antibodies (ADAs) to rituximab were detected in 37% of relapsing MS and 26% of progressive MS patients, and their presence was associated with incomplete B-cell depletion but not with adverse events or altered clinical outcomes [31]. In the two ASCLEPIOS trials, two out of 914 (0.2%) patients with RRMS who received ofatumumab tested positive for ADAs. No neutralizing antibodies were detected. The number of patients was insufficient to study the impact of ADAs on safety or efficacy [32].
There are limited data available regarding immunogenicity of ocrelizumab and ublituximab. Development of ADAs to ocrelizumab has been observed in a small proportion of patients. Over a period of 6.5 years, 1% of relapsing MS and 1.6% of progressive MS patients developed ADAs [33]. The clinical significance of these antibodies is not well understood.
Mechanism of Action and Adverse Events
Depletion of B cells by mAb administration can produce infusion-related reactions (IRRs) and injection-related reactions. CDC is believed to be a more potent driver of infusion- or injection-related reactions, favoring ocrelizumab and ublituximab compared to rituximab and ofatumumab due to their relatively less potent CDC activity [34]. Although often thought of as two separate processes, CDC and ADCC are both important drivers of mAb efficacy, and their function is affected by multiple complex interactions. CDC is more sensitive to target antigen expression, and ADCC may be more effective with a lower level of antigen expression and availability [35, 36]. Another factor may be the availability of complement, as CDC is thought to be more effective in the vascular compartment where complement components may be more readily available, while ADCC may be more effective in extravascular tissues [37].
B-cell function is critical for immune protection against infection and surveillance for malignancy through antibody-dependent and -independent mechanisms. Although plasma cells do not express CD20, hypogammaglobulinemia is a potential long-term complication of anti-CD20 therapy. Given the importance of Igs in neutralizing, targeting, and removing foreign antigens, the risk of infections is thought to be increased with hypogammaglobulinemia, particularly when sustained for a prolonged period [38]. Although, in general, bacterial respiratory tract infections are thought to be most commonly associated with hypogammaglobulinemia, there may be variability in specific infection types observed depending on concurrent comorbidities [39–41]. IgG comprises 70–80% of total serum Igs and has the longest half-life amongst all Ig classes [38]. Although all Ig classes play important roles in maintenance of immunity, deficiencies in IgG are most frequently implicated in infection susceptibility, whereas some individuals with IgM deficiency may remain asymptomatic in the presence of normal levels of IgG and IgA [38].
There have been only a few head-to-head comparisons of the risk of hypogammaglobulinemia and infection among different anti-CD20 mAbs, although the risk is reported to be lower with ofatumumab compared to rituximab and ocrelizumab [42]. This difference could be due to differences in affinity for the target epitope or route of administration, as SC antibody administration induces less B-cell depletion in the spleen as opposed to IV administration [34]. In cases where hypogammaglobulinemia occurs, various strategies for management are possible, including reducing the dose or increasing the dosing interval of anti-CD20 mAb, supplementation with IV or SC Ig, or change in DMT. In a recent retrospective review, treatment with IV or SC Ig led to the largest increase in Ig levels [43].
Rituximab
Although there are limited randomized clinical trial data for use of rituximab in MS, in the open-label phase I trial of rituximab in 26 patients with RRMS, 65.4% of patients experienced IRRs, which were all mild to moderate in severity and decreased with subsequent infusions [44]. In the phase II trial of rituximab in 104 patients with RRMS, IRRs were reported in 40% of patients [22]. In the randomized controlled trial of rituximab in 439 patients with progressive MS, 67.1% of patients experienced IRRs [24]. Infections were reported in 61.5% of patients in the phase I trial, were mild-to-moderate in severity, and most commonly included nasopharyngitis, bronchitis, upper respiratory tract infection, and urinary tract infection [44]. In the phase II RRMS trial, infections were noted in 71.4% of patients receiving rituximab versus 69.6% of patients receiving placebo [22]. In the progressive MS trial, 68.2% of patients receiving rituximab experienced infections while 65.3% of patients receiving placebo experienced infections [24]. In the phase II trial evaluating rituximab in patients with relapsing MS, one patient receiving rituximab developed malignant thyroid neoplasm [22]. In a retrospective review of patients receiving rituximab for MS, 44% of patients who received therapy for a median of five cycles had IgG levels below LLN (vs. 17% prior to starting rituximab), and 35.8% of patients had IgM levels below LLN (vs. 14.1% prior to starting rituximab) [45]. In this study, reduced levels of IgG but not IgM were associated with increased risk of infections when accounting for EDSS, age, and sex, and older age was associated with increased risk of developing reduced IgG when accounting for sex and history of immunosuppression [45].
Ocrelizumab
The most common adverse events reported in patients treated with ocrelizumab are IRRs. In the OPERA I/II (821 and 835 patients respectively) and ORATORIO phase III (732 patients) trials, the incidence of IRRs was 34.3% and 39.9%, respectively [17, 26]. Most IRRs were mild to moderate, were reported at the first infusion of the first cycle, and were managed with temporarily stopping and/or slowing the rate of infusion and treatment of symptoms. The most frequent symptoms of IRRs with ocrelizumab included pruritus, rash, throat irritation, and flushing. In practice, pre-medication with a combination of corticosteroids, antihistamines, and antipyretics generally is effective to mitigate these effects. In the OPERA I/II trials, the incidence of infection was 56.9–60.2% compared to 52.5–54.3% in the IFN β-1a group, while serious infections occurred with an incidence of 1.3% in the ocrelizumab group compared to 2.9% in the IFN β-1a group. The most common infections (reported in ≥ 10% of the patients) were upper respiratory tract infections, nasopharyngitis, and urinary tract infections. Among ocrelizumab-treated patients in the ORATORIO trial, the incidence of infection was 71.4% compared to 69.9% among patients on placebo (with 6.2% and 5.9% having serious infections, respectively). Again, upper respiratory tract infections, nasopharyngitis, and urinary tract infections were the most often reported infections.
There were four neoplasms reported in the ocrelizumab-treated cohort in the OPERA I/II trials (compared to two in the IFN β-1a cohort), which included two ductal breast carcinomas, one renal cancer, and one malignant melanoma. In the ORATORIO trial, there were 11 patients with malignancy in the ocrelizumab treatment arm (compared to two in the placebo arm), which included four breast cancers, three basal cell carcinomas, one lymphoma, one endometrial adenocarcinoma, one malignant histiocytoma, and one pancreatic carcinoma. Although there was imbalance in the incidence of breast cancer in these trials, the overall incidence of neoplasms was comparable to that of the general population. Subsequent analysis of post-marketing data and of clinical trial participants receiving long-term, continuous treatment with ocrelizumab (up to 7 years) demonstrated no increased risk of malignancy (including female breast cancer) in ocrelizumab-treated patients compared to matched controls from a reference MS population and the general population [33].
Ocrelizumab is known to variably decrease Ig levels [46]. Over a period of up to 7 years of ocrelizumab treatment, there was a mean absolute reduction in serum IgM levels of − 0.78 g/L (mean relative reduction of 55.8%) and serum IgG levels decreased at an average rate of − 0.33 g/L per year (− 2.99% per year) in the OPERA population, with similar patterns noted in the ORATORIO population [33]. When combining data from the clinical trials and open-label extension studies, an association between decreased IgG levels < 5.65 g/L and increased rates of serious infections was observed and among 2092 patients continuously treated with ocrelizumab for up to 7 years, in which 15 (0.7%) patients experienced a serious infection while IgG was below the LLN [33].
In a study of patients receiving rituximab or ocrelizumab for MS and other neurological disorders, 12% of patients developed hypogammaglobulinemia (9% in the ocrelizumab group and 25% in the rituximab group, p = 0.0123), with the difference persisting after matching based on time on treatment [47]. In this study older age (> 50 years), White race and lower baseline IgG and IgA levels were predictors of hypogammaglobulinemia on treatment, while lymphopenia and total time on therapy were found to be predictors of serious infection [47].
Ofatumumab
In the phase III clinical trials of ofatumumab in RRMS, 16.1% of the 927 patients in ASCLEPIOS I and 24.1% of the 955 patients in ASCLEPIOS II experienced injection-related reactions [19]. Infections were reported in 49.2% (vs. 51.5% in the teriflunomide arm) in ASCLEPIOS I, and in 53.8% (vs. 53.8% in the teriflunomide arm) in ASCLEPIOS II [19]. Serious infections occurred in 2.6% (vs. 1.5% in the teriflunomide arm) in ASCLEPIOS I and in 2.5% (vs. 2.1% in the teriflunomide arm) in ASCLEPIOS II [19]. Neoplasms occurred in five patients, including one melanoma, one breast carcinoma, one non-Hodgkin’s lymphoma, and two basal cell carcinomas. In the pooled analysis of several trials of ofatumumab, 54.3% of 1969 patients reported an infection, with 2.9% being serious infections [48]. Mean serum levels of IgG remained stable throughout the treatment period, and only 1.5% of patients had IgG values below the LLN [48]. Mean serum levels of IgM decreased in patients treated with ofatumumab, and 23.1% had IgM values below the LLN [48].
Ublituximab
In phase III clinical trials of ublituximab in RRMS, IRRs occurred in 44.0% of the 549 patients and 51.5% of the 545 patients in ULTIMATE I and II trials, respectively [21]. Most were mild-to-moderate in severity and decreased in frequency with subsequent doses [21]. Infections were reported in 49.5% (vs. 48.4% in the teriflunomide arm) in ULTIMATE I, and in 62.1% (vs. 60.4% in the teriflunomide arm) in ULTIMATE II [21]. The most common infections were respiratory tract related and were mild-to-moderate in severity [21]. Serious infections occurred in 5.5% (vs. 2.2% in the teriflunomide arm) in ULTIMATE I, and in 4.4% (vs. 3.7% in the teriflunomide arm) in ULTIMATE II [21]. Two neoplasms occurred in the clinical trials, one each of endometrial and uterine cancers [21]. The overall portion of patients treated with ublituximab with IgG levels below the LLN remained relatively stable (baseline 6.3%, 48 weeks 5.3%, and 96 weeks 6.5%), while the proportion with IgM levels below the LLN increased with time (baseline 1.1%, 48 weeks 12.8%, and 96 weeks 20.9%) [21].
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy is a potential serious complication of immunotherapy. Based on cases collectively reported from the Genentech, Novartis, and other drug registry data in patients with MS, ten on rituximab (two carry-over cases with prior natalizumab exposure) [16, 49], nine on ocrelizumab (seven carry-over cases with prior exposure to natalizumab (6) or fingolimod (1)) [18, 50, 51], and none on ofatumumab or ublituximab have been reported to have developed treatment-related progressive multifocal leukoencephalopathy through October 2022 [52].
Immune-Mediated Colitis
Based on post-marketing data, the FDA updated the prescribing information for ocrelizumab to include immune-mediated colitis as a possible complication of treatment. In a recent review of the experience in MS, there have been 11 cases of colitis associated with ocrelizumab and rituximab, with onset ranging from 1 week up to 18 months after initiation of therapy [53]. Hypothesized mechanisms include dysregulation of intestinal mucosal immunity with increased infiltration of T cells triggering inflammation and decreased level of interleukin 10 [53].
COVID-19
In the cross-sectional analysis of the registry of patients with MS and Corona Virus Disease 2019 (COVID-19), rituximab and ocrelizumab treatment were associated with higher odds of hospitalization (OR 4.56 and 1.63, respectively) when compared to a reference category of no DMT, although no associations with intensive care admission or death were identified [54]. In the ALITHIOS open-label extension study of 1703 patients treated with ofatumumab, 14.4% of patients reported a COVID-19-related adverse event (of whom 44.1% had a mild course, 46.5% a moderate course, and 9% a severe or life-threatening course of whom two died) [55]. Prior to COVID-19, IgG levels were within the normal range in all COVID-19-affected patients, while IgM was < 0.4 g/L in 23 (9.4%) patients.
Tuberculosis
Although data on reactivation of tuberculosis (TB) in the MS population are limited, this topic has been further evaluated in the rheumatology literature with rituximab showing that, overall, there seems to be a very low risk of TB reactivation in patients treated with rituximab, and that rituximab use appears to be safe even in patients concurrently being treated for active TB [56–59]. This observation is consistent with our current understanding regarding human immune defense mechanisms against TB mainly being driven by cellular immunity, with little contribution from the humoral immune response that is impacted by B-cell-directed therapy. Testing for latent TB was not required in the phase III trials for ocrelizumab and ublituximab, and no cases of TB infection were reported [17, 21, 26]. However, some patients may meet criteria for screening regardless of immunotherapy decision, based on country-specific guidelines or risk factors.
Vaccine Response
Although there are various types of vaccines, apart from the Bacillus Calmette-Guerin vaccine, all others are thought to confer protection primarily through stimulation of antibody production and induction of memory B cells, although there also is considerable induction of T-cell responses [60]. Therefore, there is concern for impairment of vaccine response with B-cell depletion.
In a study evaluating patients treated with rituximab for autoimmune diseases, there was a reduced antibody response compared to healthy controls following two doses of mRNA COVID-19 vaccine (29% developing neutralizing antibodies in the rituximab cohort vs. 92% in the healthy control group) [61]. Time since last infusion was an important factor in determining response. Notably, T-cell responses were preserved. In another study evaluating rituximab-treated MS patients, B-cell count was also determined to be a critical factor impacting vaccine response [62].
Vaccination response with ocrelizumab treatment was studied in the VELOCE phase III clinical trial [63], in which patients were randomized to ocrelizumab or control (no immunotherapy or IFN β therapy). Vaccination in the ocrelizumab group was given 12 weeks after the infusion and response to various antigens, including tetanus toxoid, pneumococcal polysaccharide vaccine (PPSV-23), and influenza, were measured. Patients who had received tetanus toxoid containing vaccine within 2 years, PPSV-23 within 5 years, or seasonal influenza vaccine during the enrollment periods were excluded. The proportion of patients with a positive response to tetanus toxoid 8 weeks after vaccination was 23.9% in the ocrelizumab group compared to 54.5% in the control group, with a treatment difference of − 30.7% (95% CI − 10.8, − 50.5). Humoral responses to both T-cell-dependent and -independent antigens were attenuated in ocrelizumab-treated patients with peripheral B-cell depletion. Nevertheless, either seroprotection or a marked increase in antibody levels was achieved after vaccination in ocrelizumab-treated patients.
In one study, only 25% of patients treated with ocrelizumab (25%) generated detectable protective IgG levels 8 weeks following COVID-19 mRNA vaccination, and this response was not maintained 24 or 36 weeks post vaccination [64]. However, in another study, a sequential increase in the proportion of patients with antibody response after each booster dose was found, and, after booster vaccinations with four doses, 90% of patients treated with ocrelizumab developed an antibody response [65]. In a study comparing antibody response on rituximab and ocrelizumab, both groups demonstrated reduced antibody levels compared to untreated patients (ocrelizumab 201-fold decrease and rituximab 20-fold decrease) [66]. In this study, comparison between ocrelizumab and rituximab was complicated by variability in intervals between last infusion and vaccination between groups and presence of a correlation between time since last infusion and antibody response. In a study of MS patients enrolled in the MS-PATHS registry in the USA, Germany, and Spain, 40% of patients treated with anti-CD20 mAbs achieved post-vaccination IgG response [67]. Similar reduced responses to COVID-19 vaccination were reported with ofatumumab treatment, although a robust T-cell response has also been observed [68, 69].
Extended interval dosing is one possible strategy to improve vaccination response [70]. The current expert consensus recommendations include administration of COVID-19 vaccination 6 weeks prior to starting treatment and at least 3 months following the last infusion [71]. There is accumulating evidence that T-cell responses may be preserved or even augmented with anti-CD20 mAb treatment, potentially mitigating the consequences of humoral vaccine response [72, 73].
Pregnancy and Family Planning Considerations
Administration of anti-CD20 mAbs is not recommended during pregnancy. All women treated with anti-CD20 mAbs should be appropriately counselled on contraceptive use, and decision-making regarding anti-CD20 therapy and pregnancy planning should consider the patient’s disease characteristics and family planning wishes. The FDA-approved prescribing information recommends that women continue contraception for 12 months following the last treatment of rituximab, 6 months following the last treatment of ocrelizumab or ublituximab, and 3 months following the last treatment of ofatumumab [16, 18, 32, 74]. In select cases of women with highly active disease, a shorter interval may be considered to reduce time off therapy. These patients may discontinue contraception to attempt to become pregnant 1–3 months after rituximab or ocrelizumab, as they are eliminated by 3.5–4.5 months after dosing based on half-lives [75]. Because there is minimal placental transfer of IgG during the first trimester [76], if a woman conceives 1–3 months after the last dose of therapy, the risk of fetal exposure is low [77]. In instances where the fetus has been exposed to B-cell-depleting therapy in either the second or third trimesters, the neonate should be screened for B-cell depletion and/or pancytopenia, and administration of live or live-attenuated vaccines should be delayed until B-cell recovery has been confirmed. In pregnancies specifically exposed to rituximab, there also is a risk of congenital malformations in the fetus and neonatal infections per the prescribing information [16], which should prompt consideration of referral to a high-risk obstetrician. Although the pregnancy registries for rituximab and ocrelizumab have been completed, it is recommended that exposed pregnancies for all B-cell-depleting therapies be reported directly to the respective drug companies. Notably, practices may vary from the recommendations outlined above to align with regional standards and professional society recommendations (example [78]).
Anti-CD20 mAb therapy also is not recommended to be routinely used during breastfeeding [16, 18, 32, 74]. Because anti-CD20 mAbs are large molecules, modest transfer to breast milk does occur. However, the low oral bioavailability is likely to limit the absorption by the newborn further (relative infant dose of less than 10%) [79]. Therefore, postpartum anti-CD20 mAb therapy can be considered in select cases in women with highly active MS who desire to breastfeed.
Pre-Treatment Testing and Safety Monitoring
Pre-treatment testing is similar for the anti-CD20 mAb therapies [16, 18, 32, 74]. General recommendations are summarized in Table 2. Although the prescribing information for ocrelizumab, ofatumumab, and ublituximab only recommend serum testing for hepatitis B serologies and Ig levels, at our center, we recommend that all patients also be screened with a complete blood count (CBC) with differential, complete metabolic panel (CMP), Quantiferon/tuberculosis screen, chronic hepatitis panel, varicella zoster virus (VZV) IgG (to confirm immunity), and a urine or serum pregnancy test when appropriate prior to treatment initiation. We also typically perform a baseline brain MRI in all patients. It is recommended that all necessary vaccinations be completed > 4 weeks prior to the first dose of treatment. Contraindications to treatment include active hepatitis B infection and history of life-threatening IRRs (for rituximab, ocrelizumab, and ublituximab). Notably, practices may vary from the recommendations outlined above to align with regional standards and professional society recommendations (example [80]).
Table 2.
Pre-treatment testing and safety monitoring for anti-CD20 monoclonal antibody therapy
| Contraindications | Active hepatitis B infectiona |
| History of life-threatening infusion reaction to medicationb | |
| Recommended pretreatment testinga | Complete blood count with differential |
| Comprehensive metabolic panel | |
| Quantiferon/tuberculosis screen | |
| Hepatitis panel | |
| Varicella zoster virus IgG (to ensure immunity) | |
| Quantitative Ig levels | |
| Urine or serum beta human chorionic gonadotropin (where appropriate) | |
| Brain MRI | |
| All necessary vaccinations > 4 weeks prior to first dose | |
| Recommended monitoring schedulea | Complete blood count with differential (every 6 months) |
| Comprehensive metabolic panel (every 6 months) | |
| CD19 count (every 6 months) | |
| Quantitative Ig levels (every 6 months) | |
| Monitor for recurrent or serious infection | |
| Monitor for malignancy/ensure all age-appropriate cancer screening up to date | |
| Pregnancy and family planning | Recommend contraception during treatment and for 6 months aftera |
After therapy is initiated, monitoring at our center consists of CBC with differential, CMP, CD19 count, and quantitative Ig measures every 6 months. Patients should also be regularly monitored for recurrent/serious infections and maintain up-to-date age-appropriate cancer screening.
Future Directions
Dose and Dosing Schedule
Currently, there are two ongoing clinical trials in relapsing and progressive MS (NCT04544436 and NCT04548999) evaluating the efficacy and safety of ocrelizumab doses higher than those currently approved based on the results of post hoc analyses from the phase III studies suggesting that participants with higher exposures have greater benefit on disability progression with similar safety outcomes [81].
A 6-month interval dosing is most common for rituximab, ocrelizumab, and ublituximab, based on median time for B-cell repopulation. There is interest in evaluating the efficacy and safety of these agents at > 6-month dosing intervals, which might be associated with several potential advantages, including reduced cost, increased convenience, reduced risk of infections, improved vaccine response, and reduced risk of hypogammaglobulinemia [82]. For example, one ongoing study, WINDCORE (NCT05999604), is evaluating annual versus semi-annual infusions of ocrelizumab in patients with active MS. The initial evaluation of rituximab [22, 44] demonstrated that efficacy lasts beyond 6 months after administration (and possibly up to 12 months). A more recent prospective cohort study demonstrated no difference between clinical or neuroradiologic disease activity in patients stratified into four dosing intervals based on time since last infusion (< 8, ≥ 8–12, ≥ 12–18, and 18 months). Median total B-cell count reconstitution occurred after 12 months and median memory B-cell reconstitution occurred after 16 months [83]. In the initial phase II trials of ocrelizumab, progression and disease activity in the ocrelizumab cohort remained low for up to 18 months after the last infusion, suggesting longer-term efficacy [84–86].
Currently, there is no FDA-approved dosing schedule for rituximab in MS [87]. In one study evaluating use of rituximab in neuromyelitis spectrum disorders (NMOSD), patients initially were retreated based on reappearance of B cells but later followed at fixed-interval dosing of 6–9 months) [88]. Relapses were effectively prevented during periods of B-cell depletion, but relapses tended to recur after B-cell repopulation, emphasizing the need to monitor CD19+ counts with extended interval dosing [88]. An interval of 6–9 months has been recommended to account for the typical time until reappearance of B cells [89]. Other studies in MS [90] and NMOSD [91] demonstrated that reconstitution may occur earlier than 6 months in some patients. In one recent study at a single center, two distinct rituximab administration regimens were evaluated including fixed 6-month interval dosing and extended-interval dosing up to 24 months [92]. The study demonstrated that disease activity did not differ between groups. In another study evaluating patients with MS and NMOSD treated with rituximab, low B-cell counts were associated with reduced ARR and GdE lesions, serving as an effective marker for individualized dosing schedules [93].
Prompted by the COVID-19 pandemic, several studies evaluated ocrelizumab extended-interval dosing. Acknowledging the limitations of the majority being retrospective and utilizing variable dosing protocols, in aggregate, the results suggested that extended-interval dosing could have comparable efficacy in both relapsing and progressive forms of MS, although the association with B-cell repopulation was variable [94–103]. There is an ongoing randomized clinical trial comparing 6- and 12-month dosing intervals for rituximab in MS (NCT03979456).
Monitoring of B-Cell Subsets
Inflammatory disease activity has been known to occur despite lack of evidence for B-cell reconstitution based on measurement of CD19+ B-cell counts and, conversely, reappearance of CD19+ B cells predicts a return of MS disease activity only approximately [88, 90–92]. Therefore, there have been attempts to assess whether specific B cell subsets, particularly memory B cells, might be a more predictive biomarker, given their association with MS disease activity [104, 105]. Monitoring levels of CD27+ memory B cells in NMOSD has been done through redosing maintenance rituximab therapy when CD27+ memory B cells reached 0.05% in peripheral blood, with a significant reduction of relapses [87]. However, in a study in MS, disease stability was maintained despite CD27+ subset reappearance [92], which may reflect differences in the pathogenesis between MS and NMOSD.
Treatment De-Escalation
De-escalation approaches with CD20 mAbs include dose reduction, increasing dosing intervals, switching to low or medium efficacy DMT, and treatment discontinuation. As discussed above, extended-interval dosing might be a reasonable approach to de-escalation. Dose reduction is another approach to reduce cost and mitigate risk. Two studies evaluating the use of rituximab in RRMS found that a reduced dose of rituximab (< 1000 mg) had comparable efficacy to the standard dose [106, 107]. De-escalation to a low or medium efficacy DMT specifically may be relevant in older patients. In a retrospective review of a real-world MS cohort, although high-efficacy DMTs including natalizumab and rituximab were found to be more effective in suppressing disease activity in younger patients (< 45 years) compared to oral agents, this advantage was not observed in older patients [108]. Although infection mitigation strategies should be considered in all patients starting or continuing a DMT [109], de-escalation is particularly relevant in populations at high risk for infection [110, 111].
The ultimate de-escalation approach is discontinuation of therapy. As with all other DMTs for MS, the decision when to stop treatment remains challenging. A recent clinical trial DISCO-MS failed to show non-inferiority of discontinuing treatment in older patients with previously stable disease [112]. The overall risk of rebound disease after stopping CD20 mAbs appears low due to the long-standing effects of these treatments that gradually dissipate [113, 114], but there are no uniformly accepted consensus guidelines regarding discontinuation of treatment. A trial evaluating discontinuation of ocrelizumab currently is ongoing (NCT05285891).
Biosimilars
Drug costs account for approximately half the total cost of MS care, and while MS drugs consist of < 0.1% of prescriptions in the USA, they account for 3.1% of total US drug costs [115–117]. Follow-on medications may offer a cost-effective alternative to treat MS. In the context of anti-CD20 mAbs, since these treatments are considered biologic drugs (large and complex molecules derived from living organisms), the follow-on drugs are referred to as biosimilars [118]. To be accepted as a biosimilar, a drug’s quality, safety, efficacy, and immunogenicity must be comparable to the reference drug, and the market protection period of the reference medicine must be expired [119]. Currently there are several biosimilars of rituximab with regulatory approval, though with some regional variations.
Persistent/Compartmentalized Inflammation
An area of recent focus in MS is the concept of persistent/compartmentalized inflammation, which is highlighted pathologically by mixed lesions with an inactive core and active microglial rim, paramagnetic rim lesions (PRLs) on MRI, and meningeal inflammatory nodules, and manifested clinically as progression independent of relapses (PIRA) [120, 121]. Although B cells likely contribute to PIRA through modulating the activity of microglia in CALs [122], current DMTs, and particularly B-cell-directed therapies, are thought to have less capability of targeting compartmentalized inflammation due to the high-molecular weight of mAbs and their inability to enter the CNS (except when there is blood-brain barrier disruption) [121]. This phenomenon has been observed in practice in a study evaluating PIRA and PRLs longitudinally in patients treated with CD20 mAbs [121]. This study found that over an average period of 22 months after initiation of therapy, PIRA was experienced by 20% of patients, consistent with previously pooled clinical trials and real-world data [123, 124], and PRLs did not disappear in any of the treated patients.
In order to better address the mechanisms involved in development and perpetuation of compartmentalized inflammation, several additional drugs with novel mechanisms of action are being investigated for use in MS. These approaches include therapies such as atacicept [125–127], belimumab (NCT04767698) [128, 129], CD19-directed therapies [130] including inebilizumab [131], Bruton tyrosine kinase (BTK) inhibitors [132–134], and chimeric antigen receptor (CAR)-T cells [135, 136]. Detailed discussion of these therapies is beyond the scope of this review.
Conclusions
Anti-CD20 mAbs have changed the MS treatment landscape through their robust efficacy in preventing clinical relapses and MRI lesion activity, and contribution to delaying disability worsening. Currently, there are four available anti-CD20 mAb therapies for use in MS: rituximab, ocrelizumab, ofatumumab, and ublituximab, which as a class are a cornerstone of the highly effective treatment strategy and are becoming increasingly more utilized as first-line therapies in the management of newly diagnosed MS. As a consequence of target antigen specificity and affinity, relative potencies of cell-depletion effector functions, dose and dosing schedule, and route of administration, differences between these medications would not be unexpected. However, there are few data comparing the efficacy and safety of these therapies head-to-head. As a result, currently, clinical considerations for selecting a specific B-cell-depleting mAb include patient-level comorbidities, route of administration and patient preference for IV or SC, and cost and payor constraints. Adaptations of how these medications are used to reduce adverse effects are under investigation. Although anti-CD20 mAb therapies have led to significant improvements in disease outcomes, CNS-penetrant therapies are still needed to more effectively address the mechanisms that drive disability progression.
Declarations
Funding
No funding was received for this article.
Conflict of interest
Alise K. Carlson reports personal compensation for consulting for Sanofi, Novartis, Bristol-Myers Squibb, and Vigil Neuro. Moein Amin reports fellowship grants from Novartis (NGC44741) and Biogen (23046PFEL). Jeffrey A. Cohen reports personal compensation for consulting for Astoria, Bristol-Myers Squibb, Convelo, EMD Serono, FiND Therapeutics, INMune, and Sandoz; and serving as an Editor of Multiple Sclerosis Journal.
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
AKC, MA, and JAC contributed to the concept and design and revised the draft. AKC and MA performed the search strategy and contributed to acquisition of resources and drafting the manuscript. All authors participated in reviewing and approving the final manuscript, and agree to be accountable for the work.
Footnotes
Alise K. Carlson and Moein Amin contributed equally.
References
- 1.Amin M, Hersh CM. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegener Dis Manag. 2023;13(1):47–70. doi: 10.2217/nmt-2021-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DSW, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20(3):179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arneth BM. Impact of B cells to the pathophysiology of multiple sclerosis. J Neuroinflammation. 2019;16(1):128. doi: 10.1186/s12974-019-1517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Langelaar J, Rijvers L, Smolders J, van Luijn MM. B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers. Front Immunol. 2020;11:760. doi: 10.3389/fimmu.2020.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado-Santos J, Saji E, Troscher AR, Paunovic M, Liblau R, Gabriely G, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141(7):2066–2082. doi: 10.1093/brain/awy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(Pt 4):1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 8.de Sèze J, Maillart E, Gueguen A, Laplaud DA, Michel L, Thouvenot E, et al. Anti-CD20 therapies in multiple sclerosis: from pathology to the clinic. Front Immunol. 2023;14:1004795. doi: 10.3389/fimmu.2023.1004795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crickx E, Weill JC, Reynaud CA, Mahevas M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: successes, failures and future perspectives. Kidney Int. 2020;97(5):885–893. doi: 10.1016/j.kint.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97(4):742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol. 1993;121(5):1121–1132. doi: 10.1083/jcb.121.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walshe CA, Beers SA, French RR, Chan CHT, Johnson PW, Packham GK, et al. Induction of cytosolic calcium flux by CD20 is dependent upon B cell antigen receptor signaling. J Biol Chem. 2008;283(25):16971–16984. doi: 10.1074/jbc.M708459200. [DOI] [PubMed] [Google Scholar]
- 13.Rougé L, Chiang N, Steffek M, Kugel C, Croll TI, Tam C, et al. Structure of CD20 in complex with the therapeutic monoclonal antibody rituximab. Science. 2020;367(6483):1224–1230. doi: 10.1126/science.aaz9356. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Or A, O'Brien SM, Sweeney ML, Fox EJ, Cohen JA. Clinical perspectives on the molecular and pharmacological attributes of anti-CD20 therapies for multiple sclerosis. CNS Drugs. 2021;35(9):985–997. doi: 10.1007/s40263-021-00843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein C, Lammens A, Schafer W, Georges G, Schwaiger M, Mossner E, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013;5(1):22–33. doi: 10.4161/mabs.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RITUXAN® (rituximab) [package insert]. 2020. https://www.gene.com/download/pdf/rituxan_prescribing.pdf. Accessed 14 Oct 2023.
- 17.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 18.OCREVUS® (ocrelizumab) [package insert]. 2021. https://www.gene.com/download/pdf/ocrevus_prescribing.pdf. Accessed 14 Oct 2023.
- 19.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 20.Fox E, Lovett-Racke AE, Gormley M, Liu Y, Petracca M, Cocozza S, et al. A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult Scler. 2021;27(3):420–429. doi: 10.1177/1352458520918375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinman L, Fox E, Hartung HP, Alvarez E, Qian P, Wray S, et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N Engl J Med. 2022;387(8):704–714. doi: 10.1056/NEJMoa2201904. [DOI] [PubMed] [Google Scholar]
- 22.Hauser SL, Waubant E, Aronld DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 23.Svenningsson A, Frisell T, Burman J, Salzer J, Fink K, Hallberg S, et al. Safety and efficacy of rituximab versus dimethyl fumarate in patients with relapsing-remitting multiple sclerosis or clinically isolated syndrome in Sweden: a rater-blinded, phase 3, randomised controlled trial. Lancet Neurol. 2022;21(8):693–703. doi: 10.1016/S1474-4422(22)00209-5. [DOI] [PubMed] [Google Scholar]
- 24.Hawker K, O'Connor P, Freeman MS, Calabresi PA, Antal J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 25.Kappos L, Li D, Calabresi PA, O'Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 26.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 27.Roos I, Hughes S, McDonnell G, Malpas CB, Sharmin S, Boz C, et al. Rituximab vs ocrelizumab in relapsing-remitting multiple sclerosis. JAMA Neurol. 2023;80(8):789–797. doi: 10.1001/jamaneurol.2023.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark M. Antibody humanization: a case of the ‘emperor’s new clothes’? Immunol Today. 2000;21(8):397–402. doi: 10.1016/s0167-5699(00)01680-7. [DOI] [PubMed] [Google Scholar]
- 29.Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36(1):3–10. doi: 10.1016/j.ymeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Doevendans E, Schellekens H. Immunogenicity of innovative and biosimilar monoclonal antibodies. Antibodies (Basel). 2019;8(1):21. doi: 10.3390/antib8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn N, Jut A, Ryner M, Manouchehrinia A, Piccoli L, Fink K, et al. Rituximab in multiple sclerosis: frequency and clinical relevance of anti-drug antibodies. Mult Scler. 2018;24(9):1224–1233. doi: 10.1177/1352458517720044. [DOI] [PubMed] [Google Scholar]
- 32.KESIMPTA® (ofatumumab) [package insert]. 2020. https://www.novartis.us/sites/www.novartis.us/files/kesimpta.pdf. Accessed 14 Oct 2023.
- 33.Hauser SL, Kappos L, Montalban X, Craveiro L, Chognot C, Hughes R, et al. Safety of ocrelizumab in patients with relapsing and primary progressive multiple sclerosis. Neurology. 2021;97(16):e1546–e1559. doi: 10.1212/WNL.0000000000012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotchett KR, Dittel BN, Obeidat AZ. Comparison of the efficacy and safety of anti-CD20 B cells depleting drugs in multiple sclerosis. Mult Scler Relat Disord. 2021;49:102787. doi: 10.1016/j.msard.2021.102787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Meerten T, van Rijn RS, Hol S, Hagenbeek A, Ebeling SB. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res. 2006;12(13):4027–4035. doi: 10.1158/1078-0432.CCR-06-0066. [DOI] [PubMed] [Google Scholar]
- 36.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15(24):7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers LM, Veeramani S, Weiner GJ. Complement in monoclonal antibody therapy of cancer. Immunol Res. 2014;59(1–3):203–210. doi: 10.1007/s12026-014-8542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furst DE. Serum immunoglobulins and risk of infection: how low can you go? Semin Arthritis Rheum. 2009;39(1):18–29. doi: 10.1016/j.semarthrit.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Doron S, Ruthazer R, Werner BG, Rabson A, Snydman DR. Hypogammaglobulinemia in liver transplant recipients: incidence, timing, risk factors, and outcomes. Transplantation. 2006;81(5):697–703. doi: 10.1097/01.tp.0000180531.66518.9e. [DOI] [PubMed] [Google Scholar]
- 40.Roberts DM, Jones RB, Smith RM, Alberici F, Kumaratne DS, Burns S, et al. Immunoglobulin G replacement for the treatment of infective complications of rituximab-associated hypogammaglobulinemia in autoimmune disease: a case series. J Autoimmun. 2015;57:24–29. doi: 10.1016/j.jaut.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Goldfarb NS, Avery RK, Goormastic M, Mehta AC, Schilz R, Smedira N, et al. Hypogammaglobulinemia in lung transplant recipients. Transplantation. 2001;71(2):242–246. doi: 10.1097/00007890-200101270-00013. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez E, Longbrake EE, Rammohan KW, Stankiewicz J, Hersh CM. Secondary hypogammaglobulinemia in patients with multiple sclerosis on anti-CD20 therapy: pathogenesis, risk of infection, and disease management. Mult Scler Relat Disord. 2023;79:105009. doi: 10.1016/j.msard.2023.105009. [DOI] [PubMed] [Google Scholar]
- 43.Kelly H, Vishnevetsky A, Chibnik LB, Levy M. Hypogammaglobulinemia secondary to B-cell depleting therapies in neuroimmunology: comparing management strategies. Mult Scler J Exp Transl Clin. 2023;9(2):20552173231182534. doi: 10.1177/20552173231182534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bar-Or A, Calabresi PAJ, Darnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 45.Perriguey M, Maarouf A, Stellmann JP, Rico A, Boutiere C, Demortiere S, et al. Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with rituximab. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1115. doi: 10.1212/NXI.0000000000001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evertsson B, Hoyt T, Christensen A, Nimer FA, Foley J, Piehl F. A comparative study of tolerability and effects on immunoglobulin levels and CD19 cell counts with ocrelizumab vs low dose of rituximab in multiple sclerosis. Mult Scler J Exp Transl Clin. 2020;6(4):2055217320964505. doi: 10.1177/2055217320964505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mears V, Jakubecz C, Seeco C, Woodson S, Serra A, Abboud H. Predictors of hypogammaglobulinemia and serious infections among patients receiving ocrelizumab or rituximab for treatment of MS and NMOSD. J Neuroimmunol. 2023;377:578066. doi: 10.1016/j.jneuroim.2023.578066. [DOI] [PubMed] [Google Scholar]
- 48.Hauser SL, Cross AH, Winthrop K, Wiendl H, Nicholas J, Meuth SG, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler. 2022;28(10):1576–1590. doi: 10.1177/13524585221079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oshima Y, Tanimoto T, Yuji K, Tojo A. Drug-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult Scler. 2019;25(8):1141–1149. doi: 10.1177/1352458518786075. [DOI] [PubMed] [Google Scholar]
- 50.Patel A, Sul J, Gordon M, Steinklein J, Sanguinetti S, Pramanik B, et al. Progressive multifocal leukoencephalopathy in a patient with progressive multiple sclerosis treated with ocrelizumab monotherapy. JAMA Neurol. 2021;78(6):736–740. doi: 10.1001/jamaneurol.2021.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clifford DB, Gass A, Richert N, Tornatore C, Vermersch P, Hughes R. Cases reported as progressive multifocal leukoencephalopathy in Ocrelizumab-treated patients with multiple sclerosis. In: 2019: poster session presented at the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) in Stockholm.
- 52.Sharma K, Tolaymat S, Yu H, Elkhooly M, Jaiswal S, Jena A, et al. Progressive multifocal leukoencephalopathy in anti-CD20 and other monoclonal antibody (mAb) therapies used in multiple sclerosis: a review. J Neurol Sci. 2022;443:120459. doi: 10.1016/j.jns.2022.120459. [DOI] [PubMed] [Google Scholar]
- 53.Tolaymat S, Sharma K, Kagzi Y, Sriwastava S. Anti-CD20 monoclonal antibody (mAb) therapy and colitis: a case series and review. Mult Scler Relat Disord. 2023;75:104763. doi: 10.1016/j.msard.2023.104763. [DOI] [PubMed] [Google Scholar]
- 54.Salter A, Fox RJ, Newsome SD, Halper J, Li DKB, Kanellis P, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cross AH, Delgado S, Habek M, Davydovskaya M, Ward BJ, Cree BAC, et al. COVID-19 outcomes and vaccination in people with relapsing multiple sclerosis treated with ofatumumab. Neurol Ther. 2022;11(2):741–758. doi: 10.1007/s40120-022-00341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pehlivan Y, Kisacik B, Bosnak VK, Ontat AM. Rituximab seems to be a safer alternative in patients with active rheumatoid arthritis with tuberculosis. BMJ Case Rep. 2013;bcr2012006585. 10.1136/bcr-2012-006585. [DOI] [PMC free article] [PubMed]
- 57.Alkadi A, Alduaiji N, Alrehaily A. Risk of tuberculosis reactivation with rituximab therapy. Int J Health Sci (Qassim). 2017;11(2):41–44. [PMC free article] [PubMed] [Google Scholar]
- 58.Nixon A, Ogden L, Woywodt A, Dhaygude A. Infectious complications of rituximab therapy in renal disease. Clin Kidney J. 2017;10(4):455–460. doi: 10.1093/ckj/sfx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao TL, Lin CH, Chen YM, Chang CL, Chen HH, Chen DY. Different risk of tuberculosis and efficacy of isoniazid prophylaxis in rheumatoid arthritis patients with biologic therapy: a nationwide retrospective cohort study in Taiwan. PLoS ONE. 2016;11(4):e0153217. doi: 10.1371/journal.pone.0153217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bitoun S, Henry J, Desjardins D, Vauloup-Fellous C, Dib N, Belkhir R, et al. Rituximab impairs B cell response but not T cell response to COVID-19 vaccine in autoimmune diseases. Arthritis Rheumatol. 2022;74(6):927–933. doi: 10.1002/art.42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tolf A, Wiberg A, Muller M, Nazir FH, Pavlovic I, Lauren I, et al. Factors associated with serological response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with rituximab. JAMA Netw Open. 2022;5(5):e2211497. doi: 10.1001/jamanetworkopen.2022.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bar-Or A, Calkwood JC, Chognot C, Evershed J, Fox EJ, Herman A, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaber A, Patel M, Sylvester A, Yarussi M, Kalina JT, Mendoza JP, et al. COVID-19 vaccine response in people with multiple sclerosis treated with dimethyl fumarate, diroximel fumarate, natalizumab, ocrelizumab, or interferon beta therapy. Neurol Ther. 2023;12(2):687–700. doi: 10.1007/s40120-023-00448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Räuber S, Willison A, Korsen M, Kolsche T, Golombeck KS, Plaack B, et al. Vaccine-based clinical protection against SARS-CoV-2 infection and the humoral immune response: a 1-year follow-up study of patients with multiple sclerosis receiving ocrelizumab. Front Immunol. 2022;13:1037214. doi: 10.3389/fimmu.2022.1037214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sormani MP, Inglese M, Schiavetti I, Carmisciano L, Laroni A, Lapucci C, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581. doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen JA, Bermel RA, Grossman CI, Hersh CM, Hyland M, Mowry EM, et al. Immunoglobulin G immune response to SARS-CoV-2 vaccination in people living with multiple sclerosis within multiple sclerosis partners advancing technology and health solutions. Mult Scler. 2022;28(7):1131–1137. doi: 10.1177/13524585211061343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faissner S, Heitmann N, Plaza-Sirvent C, Trendelenburg P, Ceylan U, Motte J, et al. Immune response in ofatumumab treated multiple sclerosis patients after SARS-CoV-2 vaccination. Front Immunol. 2022;13:980526. doi: 10.3389/fimmu.2022.980526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziemssen T, Groth M, Ettle B, Bopp T. Immune response to SARS-CoV-2 mRNA vaccines in an open-label multicenter study in participants with relapsing multiple sclerosis treated with ofatumumab. Vaccines (Basel). 2022 doi: 10.3390/vaccines10122167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 71.Centonze D, Rocca MA, Gasperini C, Kappos L, Hartung HP, Magyari M, et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J Neurol. 2021;268(11):3961–3968. doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brill L, Rechtman A, Zveik O, Haham N, Oiknine-Djian E, Wolf DG, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78(12):1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.BRIUMVI® (ublituximab-xiiy) [package insert]. 2022. https://www.tgtherapeutics.com/label-prescribing-info/uspi-briumvi.pdf. Accessed 14 Oct 2023.
- 75.Carlson AK, Ontaneda D, Rensel MR, Cohen JA, Kunchok A. Reproductive issues and multiple sclerosis: 20 questions. Cleve Clin J Med. 2023;90(4):235–243. doi: 10.3949/ccjm.90a.22066. [DOI] [PubMed] [Google Scholar]
- 76.Pels SG, Paidas MJ. Microangiopathic disorders in pregnancy. Hematol Oncol Clin N Am. 2011;25(2):311–22, viii. doi: 10.1016/j.hoc.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Dobson R, Hellwig K. Use of disease-modifying drugs during pregnancy and breastfeeding. Curr Opin Neurol. 2021;34(3):303–311. doi: 10.1097/WCO.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 78.Vukusic S, Carra-Dalliere C, Ciron J, Maillart E, Micheal L, Leray E, et al. Pregnancy and multiple sclerosis: 2022 recommendations from the French multiple sclerosis society. Mult Scler. 2023;29(1):11–36. doi: 10.1177/13524585221129472. [DOI] [PubMed] [Google Scholar]
- 79.Bove RM, Houtchens MK. Pregnancy management in multiple sclerosis and other demyelinating diseases. Continuum (Minneap Minn). 2022;28(1):12–33. doi: 10.1212/CON.0000000000001108. [DOI] [PubMed] [Google Scholar]
- 80.Papeix C, Donze C, Lebrun-Frenay C. Infections and multiple sclerosis: Recommendations from the French Multiple Sclerosis Society. Rev Neurol (Paris). 2021;177(8):980–994. doi: 10.1016/j.neurol.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Hauser SL, Bar-Or A, Weber MS, Kletzl H, Gunther A, Manfrini M, et al. Association of higher ocrelizumab exposure with reduced disability progression in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10(2):e200094. doi: 10.1212/NXI.0000000000200094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Kempen ZL, Toorop AA, Sellebjerg F, Giovannoni G, Killestein J. Extended dosing of monoclonal antibodies in multiple sclerosis. Mult Scler. 2022;28(13):2001–2009. doi: 10.1177/13524585211065711. [DOI] [PubMed] [Google Scholar]
- 83.Starvaggi Cucuzza C, Longinetti E, Ruffin N, Evertsson B, Kockum I, Jagodic M, et al. Sustained low relapse rate with highly variable B-cell repopulation dynamics with extended rituximab dosing intervals in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200056. doi: 10.1212/NXI.0000000000200056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kappos L, et al. Long-term safety and efficacy of ocrelizumab in patients with relapsing-remitting multiple sclerosis: week 144 results of a phase II, randomised, multicentre trial. In: 2012: 28th congress of the European Committee for Treatment and Research in Multiple Sclerosis 2012 Oct 10, p. 362.
- 85.Hauser S, et al. Week 144 results of a phase II, randomized, multicenter trial assessing the safety and efficacy of ocrelizumab in patients with relapsing–remitting multiple sclerosis (RRMS). (S31. 004).
- 86.Baker D, Pryce G, James LK, Marta M, Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord. 2020;44:102279. doi: 10.1016/j.msard.2020.102279. [DOI] [PubMed] [Google Scholar]
- 87.Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68(11):1412–1420. doi: 10.1001/archneurol.2011.154. [DOI] [PubMed] [Google Scholar]
- 88.Pellkofer HL, Krumbholz M, Berthele A, Hemmer B, Gerdes LA, Havla J, et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology. 2011;76(15):1310–1315. doi: 10.1212/WNL.0b013e3182152881. [DOI] [PubMed] [Google Scholar]
- 89.Stüve O, Leussink VI, Frohlich R, Hemmer B, Hartung HP, Menge T, et al. Long-term B-lymphocyte depletion with rituximab in patients with relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66(2):259–261. doi: 10.1001/archneurol.2008.551. [DOI] [PubMed] [Google Scholar]
- 90.Barra ME, Soni D, Vo KH, Chitnis T, Stankiewicz JM. Experience with long-term rituximab use in a multiple sclerosis clinic. Mult Scler J Exp Transl Clin. 2016;2:2055217316672100. doi: 10.1177/2055217316672100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greenberg BM, Graves D, Remington G, Hardeman P, Mann M, Karandikar N, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler. 2012;18(7):1022–1026. doi: 10.1177/1352458511432896. [DOI] [PubMed] [Google Scholar]
- 92.Claverie R, Perriguey M, Rico A, Boutiere C, Demortiere S, Durozard P, et al. Efficacy of rituximab outlasts B-cell repopulation in multiple sclerosis: time to rethink dosing? Neurol Neuroimmunol Neuroinflamm. 2023;10(5):e200152. doi: 10.1212/NXI.0000000000200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ellrichmann G, Bolz J, Peschke M, Duscha A, Hellwig K, Lee D, et al. Peripheral CD19+ B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J Neurol. 2019;266(1):57–67. doi: 10.1007/s00415-018-9092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tazza F, Lapucci C, Cellerino M, Boffa G, Novi G, Poire I, et al. Personalizing ocrelizumab treatment in multiple sclerosis: what can we learn from Sars-Cov2 pandemic? J Neurol Sci. 2021;427:117501. doi: 10.1016/j.jns.2021.117501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guerrieri S, Bucca C, Nozzolillo A, GEnchi A, Zanetta C, Cetta I, et al. Ocrelizumab extended-interval dosing in multiple sclerosis during SARS-CoV-2 pandemic: a real-world experience. Eur J Neurol. 2023;30(9):2859–2864. doi: 10.1111/ene.15891. [DOI] [PubMed] [Google Scholar]
- 96.Rolfes L, Pawlitzki M, Pfeuffer S, Nelke C, Lux A, Pul R, et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1035. doi: 10.1212/NXI.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schuckmann A, Steffen F, Zipp F, Bittner S, Pape K, et al. Impact of extended interval dosing of ocrelizumab on immunoglobulin levels in multiple sclerosis. Med. 2023;4(6):361–372.e3. doi: 10.1016/j.medj.2023.05.001. [DOI] [PubMed] [Google Scholar]
- 98.Zanghì A, Ferraro D, Callari G, Valentino P, Granella F, Patti F, et al. Ocrelizumab extended interval dosing in primary progressive multiple sclerosis: an Italian experience. Curr Neuropharmacol. 2023 doi: 10.2174/1570159X22666231002142709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodriguez-Mogeda C, van Lierop ZYGJ, van der Pol SMA, Coenen L, Hogenboom L, Kamermans A, et al. Extended interval dosing of ocrelizumab modifies the repopulation of B cells without altering the clinical efficacy in multiple sclerosis. J Neuroinflamm. 2023;20(1):215. doi: 10.1186/s12974-023-02900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bou Rjeily N, Fitzgerald KC, Mowry EM. Extended interval dosing of ocrelizumab in patients with multiple sclerosis is not associated with meaningful differences in disease activity. Mult Scler. 2023;13524585231208311. 10.1177/13524585231208311. [DOI] [PubMed]
- 101.Sahi NK, Abidi SMA, Salim O, Abraham R, Kalra S, Al-Araji A. Clinical impact of Ocrelizumab extended interval dosing during the COVID-19 pandemic and associations with CD19+B-cell repopulation. Mult Scler Relat Disord. 2021;56:103287. doi: 10.1016/j.msard.2021.103287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rempe T, Elfasi A, Rodriguez E, Vasquez M, Graves J, Kinkel R. Ocrelizumab B-cell repopulation-guided extended interval dosing versus standard dosing—similar clinical efficacy with decreased immunoglobulin M deficiency rates. Mult Scler Relat Disord. 2023;79:105028. doi: 10.1016/j.msard.2023.105028. [DOI] [PubMed] [Google Scholar]
- 103.van Lierop ZY, Toorop AA, van Ballegoij WJ, Olde Dubbelink TB, Strijbis EM, de Jong BA, et al. Personalized B-cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID-19 pandemic. Mult Scler. 2022;28(7):1121–1125. doi: 10.1177/13524585211028833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine. 2017;16:41–50. doi: 10.1016/j.ebiom.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El Mahdaoui S, Refstrup Husted S, Bredahl Hansen M, Cobanovic S, Reith Mahler M, Buhelt S, et al. Cerebrospinal fluid soluble CD27 is associated with CD8. J Neuroimmunol. 2023;381:578128. doi: 10.1016/j.jneuroim.2023.578128. [DOI] [PubMed] [Google Scholar]
- 106.Boremalm M, Sundström P, Salzer J. Discontinuation and dose reduction of rituximab in relapsing-remitting multiple sclerosis. J Neurol. 2021;268(6):2161–2168. doi: 10.1007/s00415-021-10399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Disanto G, Ripellino P, Riccitelli GC, Sacco R, Scotti B, Fucili A, et al. De-escalating rituximab dose results in stability of clinical, radiological, and serum neurofilament levels in multiple sclerosis. Mult Scler. 2021;27(8):1230–1239. doi: 10.1177/1352458520952036. [DOI] [PubMed] [Google Scholar]
- 108.Vollmer BL, Wolf AB, Sillau S, Corboy JR, Alvarez E. Evolution of disease modifying therapy benefits and risks: an argument for de-escalation as a treatment paradigm for patients with multiple sclerosis. Front Neurol. 2021;12:799138. doi: 10.3389/fneur.2021.799138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tur C, Dubessy AL, Otero-Romero S, Amato MP, Derfuss T, Di Pauli F, et al. The risk of infections for multiple sclerosis and neuromyelitis optica spectrum disorder disease-modifying treatments: eighth European Committee for Treatment and Research in Multiple Sclerosis focused workshop review. April 2021. Mult Scler. 2022;28(9):1424–1456. doi: 10.1177/13524585211069068. [DOI] [PubMed] [Google Scholar]
- 110.Chen C, Zhang E, Zhu C, Wei R, Ma L, Dong X, et al. Comparative efficacy and safety of disease-modifying therapies in patients with relapsing multiple sclerosis: a systematic review and network meta-analysis. J Am Pharm Assoc (2003) 2023;63(1):8–22. doi: 10.1016/j.japh.2022.07.009. [DOI] [PubMed] [Google Scholar]
- 111.Smith TE, Madhavan M, Gratch D, Patel A, Saha V, Sammarco C, et al. Risk of COVID-19 infection and severe disease in MS patients on different disease-modifying therapies. Mult Scler Relat Disord. 2022;60:103735. doi: 10.1016/j.msard.2022.103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corboy JR, Fox RJ, Kister I, Cutter GR, Morgan CJ, Seale R, et al. Risk of new disease activity in patients with multiple sclerosis who continue or discontinue disease-modifying therapies (DISCOMS): a multicentre, randomised, single-blind, phase 4, non-inferiority trial. Lancet Neurol. 2023;22(7):568–577. doi: 10.1016/S1474-4422(23)00154-0. [DOI] [PubMed] [Google Scholar]
- 113.Juto A, Fink K, Al Nimer F, Piehl F. Interrupting rituximab treatment in relapsing-remitting multiple sclerosis; no evidence of rebound disease activity. Mult Scler Relat Disord. 2020;37:101468. doi: 10.1016/j.msard.2019.101468. [DOI] [PubMed] [Google Scholar]
- 114.Chappuis M, Rousseau C, Bajeux E, Wiertlewski S, Laplaud D, Le Page E. Discontinuation of second- versus first-line disease-modifying treatment in middle-aged patients with multiple sclerosis. J Neurol. 2023;270(1):413–422. doi: 10.1007/s00415-022-11341-2. [DOI] [PubMed] [Google Scholar]
- 115.Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? Neurology. 2015;84(21):2185–2192. doi: 10.1212/WNL.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hauser SL, Johnston SC. Multiple sclerosis drugs: sticker shock. Ann Neurol. 2012;71(5):A5–6. doi: 10.1002/ana.23608. [DOI] [PubMed] [Google Scholar]
- 117.Giovannoni G, Hawkes C, Levy M, Waubant E. Are the high-costs of MS disease-modifying therapies justified? Mult Scler Relat Disord. 2018;20:A3–A5. doi: 10.1016/j.msard.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 118.Greenberg B, Giovannoni G. A place for biosimilars in the changing multiple sclerosis treatment landscape. Mult Scler Relat Disord. 2023;77:104841. doi: 10.1016/j.msard.2023.104841. [DOI] [PubMed] [Google Scholar]
- 119.Lim S. Overview of the regulatory framework and FDA’s guidance for the development and approval of biosimilar products in the US. FDA, Editor. Silver Springs; 2018.
- 120.Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J, et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597(7878):709–714. doi: 10.1038/s41586-021-03892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yong HYF, Yong VW. Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis. Nat Rev Neurol. 2022;18(1):40–55. doi: 10.1038/s41582-021-00581-x. [DOI] [PubMed] [Google Scholar]
- 122.Maggi P, Vanden Bulcke C, Pedrini E, Bugil C, Sellimi A, Wynen M, et al. B cell depletion therapy does not resolve chronic active multiple sclerosis lesions. EBioMedicine. 2023;94:104701. doi: 10.1016/j.ebiom.2023.104701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140. doi: 10.1001/jamaneurol.2020.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ingwersen J, Masanneck L, Pawlitzki M, Samadzadeh S, Weise M, Aktas O, et al. Real-world evidence of ocrelizumab-treated relapsing multiple sclerosis cohort shows changes in progression independent of relapse activity mirroring phase 3 trials. Sci Rep. 2023;13(1):15003. doi: 10.1038/s41598-023-40940-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kappos L, Hartung HP, Freedman MS, Boyko A, Wilhelm Radu E, Mikol DD, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. 2014;13(4):353–363. doi: 10.1016/S1474-4422(14)70028-6. [DOI] [PubMed] [Google Scholar]
- 126.Sergott RC, Bennett JL, Rieckmann P, Montalban X, Mikol D, Freudensprung U, et al. ATON: results from a phase II randomized trial of the B-cell-targeting agent atacicept in patients with optic neuritis. J Neurol Sci. 2015;351(1–2):174–178. doi: 10.1016/j.jns.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 127.Baker D, Pryce G, James LK, Schmierer K, Giovannoni G. Failed B cell survival factor trials support the importance of memory B cells in multiple sclerosis. Eur J Neurol. 2020;27(2):221–228. doi: 10.1111/ene.14105. [DOI] [PubMed] [Google Scholar]
- 128.Moore PA, Belvedere O, Bar-Or A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 129.Dubey AK, Handu SS, Dubey S, Sharma P, Sharma KK, Ahmed QM. Belimumab: first targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. 2011;2(4):317–319. doi: 10.4103/0976-500X.85930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen D, Gallagher S, Monson NL, Herbst R, Wang Y. Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: insights from preclinical studies. J Clin Med. 2016;5(12):107. doi: 10.3390/jcm5120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Agius MA, Klodowska-Duda G, Maciejowski M, Potemkowski A, Li J, Patra K, et al. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler. 2019;25(2):235–245. doi: 10.1177/1352458517740641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krämer J, Bar-Or A, Turner TJ, Wiendl H. Bruton tyrosine kinase inhibitors for multiple sclerosis. Nat Rev Neurol. 2023;19(5):289–304. doi: 10.1038/s41582-023-00800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Montalban X, Arnold DL, Weber MS, Staikov I, Piasecka-Stryczynska K, Willmer J, et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N Engl J Med. 2019;380(25):2406–2417. doi: 10.1056/NEJMoa1901981. [DOI] [PubMed] [Google Scholar]
- 134.Reich DS, Arnold DL, Vermersch P, Bar-Or A, Fox RJ, Matta A, et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021;20(9):729–738. doi: 10.1016/S1474-4422(21)00237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gupta S, Simic M, Sagan SA, Shepherd C, Duecker J, Sobel RA, et al. CAR-T cell-mediated B-cell depletion in central nervous system autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2023;10(2):e200080. doi: 10.1212/NXI.0000000000200080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 138.Sawas A, Farber CM, Schreeder MT, Khalil MY, Mahadevan D, Deng C, et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br J Haematol. 2017;177(2):243–253. doi: 10.1111/bjh.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]