Abstract
High-flow nasal oxygen (HFNO) therapy is extensively used in critical care units for spontaneously breathing patients. Trans-nasal humidified rapid insufflation ventilatory exchange (THRIVE) is a method of apnoeic oxygenation with continuous nasal delivery of warmed, humidified oxygen at high-flow rates up to 70L/min. THRIVE extends the apnoeic window before desaturation occurs so that tubeless anaesthesia is possible. The advent of THRIVE has had a monumental impact on anaesthetic practice, with a diverse range of clinical applications and it has been incorporated into difficult airway guidelines. THRIVE has many applications in otolaryngology and head and neck surgery. It is used as a pre-oxygenation tool during induction in both anticipated and unanticipated difficult airway scenarios and as a method of oxygenation for tubeless anaesthesia in elective laryngotracheal and hypopharyngeal surgeries and during emergence from anaesthesia. In this scoping review of the literature, we aim to provide an overview on the utility of THRIVE in otolaryngology, including the underlying physiologic principles, current indications and limitations, and its feasibility and safety in different surgical contexts and specific population groups.
Keywords: THRIVE, Apnoeic oxygenation, High-flow nasal oxygen, Otorhinolaryngology, Head and neck surgery
Introduction
High-flow nasal oxygen (HFNO) has been established in critical care for spontaneously breathing patients, with recognised indications in the management of acute respiratory failure and decompensated heart failure, the prevention of atelectasis, and to support oxygenation after weaning from mechanical ventilation [1]. Within the last decade, increasing research has focussed on the role of HFNO for apnoeic oxygenation and ventilation in the perioperative setting, including; preoxygenation prior to establishing a definitive airway in patients undergoing general anaesthesia [2–5], intraoperative ventilation during shared airway surgery, and post-extubation respiratory support [6]. Patel first described THRIVE in a case series of 25 patients with anticipated difficult airways [2]. It involves the continual delivery of warmed, humidified oxygen via wide-bore nasal cannulae with 100% FiO2 and high flow rates of upto 70L/min [2]. An optional gas blender can adjust the delivered oxygen concentration and positive end-expiratory pressures (PEEP) of up to 7 cm H20 can be achieved [2, 7].
The benefit in the perioperative period is the ability to extend the apnoeic window, defined as the timeframe between induction of anaesthesia and securement of a definitive airway, beyond that of conventional preoxygenation with facemask ventilation. This has been demonstrated in RCTs in adult [2, 4, 5, 8] and obese [3, 7, 8] populations. Extending the apnoeic window reduces the risk of hypoxia during induction and provides greater time to establish a definitive airway in a controlled setting –the advantage is evident in emergency and difficult airway situations [2, 9–11]. Recently, THRIVE has gained popularity and demonstrated advantages in shared airway procedures (Tables 1, 2) as an alternative to intubation or intermittent jet ventilation, as tubeless anaesthesia provides an unobstructed view of the surgical field [12–14]. Diverse clinical applications of THRIVE are described including diagnostic and therapeutic airway procedures (Table 3). The aim of this literature review is to contextualise the role of THRIVE in otolaryngology.
Table 1.
Summary of the advantages of THRIVE described in the literature
| Preoxygenation |
|
- Extends the safe apnoeic window - Airway management in patients with known difficult airway - Emergency surgery for airway obstruction |
| Apnoeic oxygenation |
| - Maintains tubeless anaesthesia for short, elective laryngeal and hypopharyngeal surgeries |
| Can be used throughout the perioperative period including for preoxygenation, intraoperative apnoeic oxygenation, and for postoperative support |
| Faster time to suspension and fewer suspension adjustments |
| Improved theatre efficiency and turnover from decreased operating and recovery time |
| Large evidence base from anaesthetic and critical care literature |
| Unobstructed field of view aids diagnostic assessment of the upper airway and provides more space for instrumentation in transoral endoscopic surgery |
| Avoids intubation and associated airway trauma |
| Reduced laryngospasm due to intubation |
| Maintains vocal cords in natural position, favourable for augmentation surgery |
| Higher surgeon satisfaction |
| Humidification of delivered gases prevents mucosal drying |
| Minimises need to interrupt surgery for jet ventilation |
| Decreased risk of barotrauma and vibrational tissue injury |
| Decreased risk of jetting specimen into lower airway |
| Maintains tissue alignment |
| Has been described in diverse clinical applications in otolaryngology |
Table 2.
Summary of the disadvantages of THRIVE described in the literature
| Requires specialised oxygen delivery equipment |
| Cost of machine and associated disposable equipment |
| Success is dependent of anaesthetist experience |
| Success is dependent on patient factors including age, BMI, OSA, cardiorespiratory status, smoking history, hypermetabolic states, and presence of upper airway pathology |
| No aspiration protection |
| End tidal CO2 is not monitored |
Table 3.
Summary of clinical applications of THRIVE in otolaryngologic surgery
| Diagnostic procedures |
| Microlaryngoscopy, bronchoscopy, and/or oesophagoscopy for examination under anaesthesia with or without biopsy of lesions [15–27] |
| Drug-induced sleep endoscopy and dynamic assessment of upper airway, e.g. in paediatric sleep disordered breathing, laryngomalacia, and other congenital airway pathology [18, 28, 29]** |
| Therapeutic procedures |
| Removal of benign and malignant lesions of the hypopharynx, larynx, and trachea—granuloma, vocal cord polyps and nodules, papillomas, cysts, webs, leukoplakia, carcinomas |
| Removal of airway foreign bodies [28, 30] |
| Balloon dilatations or resection of subglottic or tracheal stenosis [14, 15, 23, 24, 28, 31–37] |
| Vocal cord augmentation surgery including medialisation procedures [14, 15, 21, 23, 24, 36] |
| Stapling of pharyngeal pouches [23] |
| Oesophageal dilatation [23] |
| Paediatric airway surgery including supraglottoplasty [28, 29] |
| Laser airway surgery [15, 23, 28, 32, 35, 38–40] |
| Difficult airway management |
| Awake fibreoptic intubation [15, 18] |
| Surgical tracheostomy [15, 41] |
**Some authors refer to THRIVE in the context of apnoeic ventilation only, whilst others have used the terminology in spontaneously breathing patients under anaesthesia. The term “SponTaneous Respiration using IntraVEnous anaesthesia and Hi-flow nasal oxygen” or “STRIVE Hi” has been adopted by some instead to describe high-flow nasal oxygenation in non-apnoeic patients [32]
Methodology
A structured literature review was performed using Medline, PubMed, and Embase from inception until 01/04/2023, and restricted to the English language. The following search terms were used: “apnoeic oxygenation,” “trans-nasal humidified rapid insufflation ventilatory exchange,” “otolaryngology,” “head and neck surgery,” and “laryngeal surgery.” The titles and abstracts were assessed for relevance, and whole-text article screening was performed to determine eligibility for inclusion. Additional articles were found using Google Scholar, the reference lists of included articles, and the “similar articles” feature in PubMed.
Results
Eighty-four articles were identified and included; case report and case series (n = 8), editorials (n = 6), conceptual/modelling/physiologic studies (n = 11), literature reviews (n = 8), clinical audits (n = 1), retrospective (n = 10) and prospective (n = 17) cohort studies, systematic reviews (n = 4), clinical guidelines (n = 1), and RCTs (n = 18).
Discussion
Physiologic Mechanisms of Apnoeic Oxygenation and Ventilation in THRIVE
THRIVE has been shown to extend the safe apnoeic window before desaturation in multiple studies [2–5, 7, 8, 10]. Conceptual and animal models of apnoeic oxygenation since the early twentieth century have demonstrated that alveolar oxygen uptake occurs provided there is a continual supply of 100% oxygen [42–46]. This is limited by reduced CO2 clearance in the absence of ventilatory movements, resulting in hypercapnia and acidosis, which is why low-flow nasal oxygenation (LFNO) cannot be sustained in apnoeic patients. Patel demonstrated that high-flow rates of 70L/min extended apnoea times up to 65 min without desaturation and reduced the rate of CO2 accumulation from 0.35 to 0.45 kPa/min as reported in LFNO previous studies to 0.15 kPa/min. This suggests some degree of CO2 clearance [2].
Computational fluid dynamic models show HFNO delivery creates a highly turbulent supraglottic vortex that fills the upper airway with 100% oxygen. Continual insufflation provides flow-dependent deadspace flushing and washes out nitrogen and CO2 [47–49]. This creates a large pharyngeal oxygen reservoir that bypasses the high resistance of the upper airway and reduces work of breathing by allowing breathing to effectively occur from the level of the glottis. Studies in spontaneously breathing and apnoeic patients show that THRIVE generates a flow-dependent, positive airway pressure of 0.5–1.0 cm H2O per 10L increase in flow rate [16, 50–52]. Inter-patient variation is attributed to patient characteristics, fitting of the nasal cannula, and degree of mouth opening [50, 51, 53]. The physiological benefits include (1) providing a distending force and splinting the upper airway to maintain patency for airflow, particularly in patients with obesity, obstructive sleep apnoea, and structural upper airway obstruction, and (2) increasing end-expiratory lung volume and functional residual capacity (FRC) by preventing atelectasis—this translates to improved ventilation-perfusion match and reduced work of breathing [47].
Apnoeic oxygenation is a phenomenon where the differential rates of alveolar oxygen removal and of CO2 excretion provide a pressure gradient for a ventilatory mass flow of oxygen from the upper airways towards the alveoli. Alveolar oxygen extraction generates a subatmospheric alveolar pressure that drives the mass flow of oxygen into the alveoli [50]. Effective apnoeic oxygenation depends on continual insufflation for oxygen replenishment and deadspace washout to maximise the oxygen pressure gradient at the alveolar-capillary interface. CO2 excretion into the alveoli is comparatively slow due to high blood solubility and the presence of buffering systems. Once this buffering capacity is exceeded, alveolar CO2 accumulation will reduce the effectiveness of aventilatory mass flow and apnoeic oxygenation, leading to desaturations that require rescue ventilation. High FiO2 and flow rates prevents dilution of oxygen within the pharynx by renitrogenation, CO2 rebreathing, or entrainment of room air.
The mechanism underlying CO2 clearance in THRIVE is controversial. [50, 54, 55]. It is hypothesised to be an interaction of the supraglottic vortex with cardiogenic oscillations, which facilitate micro-ventilation and gas mixing within the deadspace [42, 43, 47–49]. During the cardiac cycle, rhythmic alterations in cardiac volume and associated changes in intrathoracic pressure create pulsatile gas flow, with estimated volumes between 6 and 40 mL. The heart compresses the lung parenchyma with each heartbeat, and pulmonary vascular flow stretches the small airways. This transports CO2 into the proximal trachea and distal pharynx, where entrained eddy currents from the supraglottic vortex facilitates gas mixing and washout. Higher flow rates improve CO2 clearance as the vortex extends further distally. Intraoperative blood gas CO2 monitoring suggests THRIVE may achieve up to 50% of gas exchange required for optimal ventilation [2, 16, 56].
Anaesthetic Set Up and Considerations
Earlier studies demonstrated that nasopharyngeal apnoeic preoxygenation with low-flow devices could delay the onset to and reduce the degree of desaturation during endotracheal intubation in ASA I-II adults undergoing elective surgery [57–59]. However, nasal delivery of 10-12L/min of cold, dry oxygen causes patient discomfort. Detrimental effects include reduced mucociliary clearance, drying of nasal secretions, mucosal trauma, and activation of protective nasopulmonary bronchoconstrictor responses that increase the upper airway resistance [60, 61]. HFNO and THRIVE overcome these limitations. THRIVE was first described for preoxygenation in patients with difficult airways undergoing hypopharyngeal or laryngotracheal surgery [2]. Preoxygenation achieves (1) denitrogenation of the lung volume to maximise oxygen storage, with studies showing an increase in alveolar oxygen content from 450 to 3000 mL and (2) maximising the oxygen carrying content of the blood by fully saturating haemoglobin [57]. This provides a reservoir of oxygen during efforts to secure an airway.
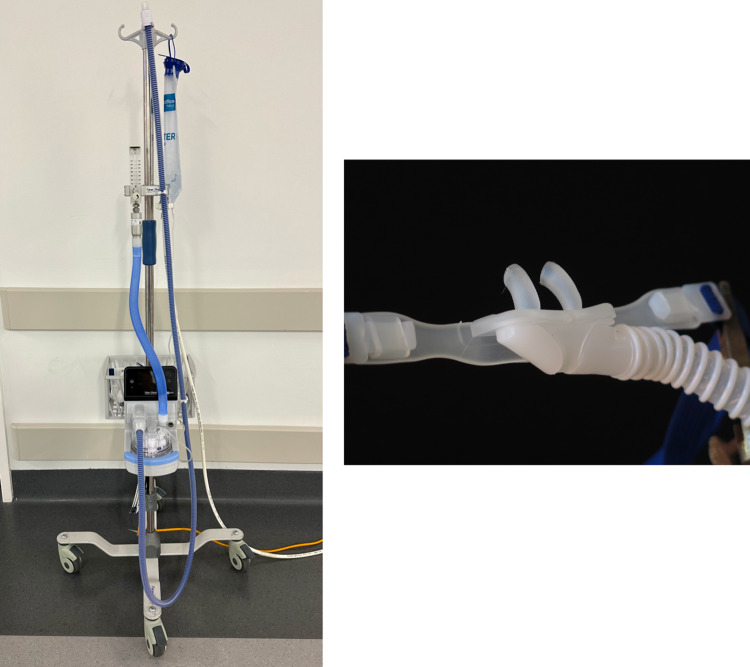
OptiFlow™ (Fisher and Paykel, New Zealand) is a commercially available trans-nasal humidified oxygen delivery system that is commonly used in THRIVE. The circuit comprises of a flow meter, heated humidifier, wide-bore nasal cannula, and an optional air/oxygen blender (Figs. 1, 2 and 3). Pre-oxygenation occurs with 100% FiO2 often in the head up position, which reduces basal atelectasis and increases FRC. The upper airway should be splinted with jaw thrust, or nasopharyngeal or oropharyngeal airway adjuncts in the obese patient, to maintain patency for adequate flow into the lower airway [62]. Intraoperatively, rigid laryngoscopy provides this splinting effect during tubeless anaesthesia. Patel commenced preoxygenation at 70L/min for 10 min [2], whilst other authors use lower flow rates of 30L/min for patient comfort whilst awake [15]. Total intravenous anaesthesia is often used, with propofol as an induction and maintenance agent and an opioid target-controlled infusion (remifentanil, alfentanil, or fentanyl). Whilst paralysis is not mandatory, a non-depolarising muscle relaxant is usually administered, though some have used succinylcholine [12]. Following induction, the flow rate is increased upto 70L/min, as required and intubation or tubeless anaesthesia can proceed.
Fig. 1.
The OptiFlow oxygen delivery system (left) and the wide-bore nasal cannulae used for trans-nasal high flow oxygen delivery during THRIVE (right)
Fig. 2.

Example of subject connected to THRIVE apparatus
Fig. 3.

Clinical photograph of apnoeic oxygenation using THRIVE during an elective microlaryngoscopy procedure
Close SpO2 monitoring is vital, with consideration of rescue facemask or jet ventilation for desaturations under 90%—though, in the stable, non-obese patient without cardiorespiratory comorbidities, desaturation may be tolerated to avoid interrupting critical portions of surgery. The need for intraoperative CO2 monitoring is controversial. End-tidal CO2 is not available during tubeless anaesthesia and the need for transcutaneous monitoring or blood sampling is not established. Evidence suggests that most THRIVE candidates tolerate short durations of mild hypercapnia and respiratory acidosis [12, 16]. A progressive increase in alveolar-arterial CO2 gradient is noted with time, however Forsberg showed that lung volume changes and atelectasis are not more common in THRIVE compared to mechanical ventilation and do not explain this finding [63]. Studies also show hyperventilation prior to preoxygenation had no clinically significant impact on lowering PaCO2 or extending the apnoeic window [17]. Piosik investigated intraoperative blood gas fluctuations during THRIVE in ASA I-II patients undergoing elective surgery, showing that, although oxygenation was maintained with a median apnoeic time of 25 min, all eventually developed significant respiratory acidosis (pH < 7.15) requiring discontinuation of THRIVE [64]. In a RCT comparing THRIVE with mechanical ventilation during short, elective laryngeal surgery, Forsberg no clinically relevant increase in inflammatory markers or biomarkers to suggest hypercapnia-related cerebral, cardiac, or renal insult [65].
RCTs have shown that THRIVE is safe and effective for preoxygenation in patients undergoing general anaesthesia and during intubation in critical care units [3–5, 7–9]. Subgroups that warrant special consideration should be discussed. Children have higher rates of oxygen consumption and CO2 production, reduced FRC, greater relative deadspace volume, and higher closing capacity, which results in faster onset to desaturation [56, 66–70]. The paediatric intensive care literature commonly describes flow rates of 1-2L/kg/min. Humphreys showed THRIVE prolonged apnoea time compared to jaw thrust alone prior to intubation [69]. Riva however showed THRIVE had no additive benefit in extending apnoeic window compared to LFNO [70]. Both studies failed to demonstrate effective CO2 clearance in children, with similar rise in transcutaneous CO2 in intervention and control arms. This may be due to technical factors in delivering THRIVE in children or anatomical and physiologic differences. Flow rates exceeding 2L/kg/min have not been investigated due to the uncertain safety profile. A systematic review suggested HFNO was not superior to LFNO for apnoeic oxygenation in elective and emergency paediatric intubation [68]. Obesity is associated with shortened apnoeic windows due to reduced FRC, expiratory reserve volume, chest wall compliance, and increased atelectasis during anaesthesia, and hence represents a cohort where THRIVE may be useful for preoxygenation. RCTs have shown that THRIVE prolongs the safe apnoea time in the obese patient undergoing general anaesthesia compared to LFNO and facemask ventilation [3, 7]. The window is shorter and they are more likely to desaturate than the non-obese patient, which must be considered when undergoing laryngeal surgery. The continuous positive pressure of approximately 7 cm H2O may be insufficient in this population to effectively prevent atelectasis [2]. Other medical conditions that may impact on the success of THRIVE include underlying lung disease (impaired gaseous exchange), cardiac disease (impaired cardiac output), anaemia (impaired oxygen carrying capacity), and pathophysiologic states of increased oxygen consumption, all of which can contribute to faster desaturation. Pregnant women warrant further attention due to reduced FRC. In one RCT, THRIVE performed slightly worse than facemask ventilation during preoxygenation for Caesarean section [71]. A case series showed THRIVE was successfully used during surgical resection of tracheal stenosis in pregnancy [31].
Providing anaesthesia and optimal surgical conditions for laryngotracheal surgery presents challenges with a higher risk of airway complications [72] and need for subspecialised airway management skills. Patient, anaesthetic/surgical, and institutional factors determine the success of THRIVE. Careful preoperative planning, patient optimisation and selection, familiarity with airway equipment, discussion of rescue ventilation strategies, and selection of an adequately trained staff is critical. Patients requiring airway procedures are likely elderly and with a history of tobacco or alcohol abuse, poor cardiopulmonary reserve, or airway symptoms. Shared airway surgery is a collective responsibility between the anaesthetist and proceduralist, and a multidisciplinary approach with clear and effective communication is fundamental. Providers must balance the length of apnoeic ventilation with the risk of hypercapnia, and know when to interrupt surgery for rescue ventilation. The options for rescue ventilation include face-mask ventilation, jet ventilation, intubation, or laryngeal mask.
Surgical Applications in Otolaryngology
THRIVE in otolaryngology is useful for (1) preoxygenation in the anticipated difficult airway and in elective and emergency airway management, (2) prolonging the apnoeic window between periods of positive ventilation, and (3) providing unimodal method of oxygenation for tubeless anaesthesia during short, elective surgeries of the airway. Most of the evidence base for THRIVE in otolaryngology comes from observational cohort studies and case series. THRIVE has been consistently shown to increase the apnoeic window during shared airway surgery. Mean apnoea times between 13 and 26 min are reported and the longest duration time reported is 65 min [2, 12]. It is likely that both the apnoea time and need for rescue ventilation will be underestimated by studies involving short laryngeal procedures.
THRIVE is an effective adjunct in the management of the difficult airway, extending the apnoeic window and timeframe to establish a definitive airway [2, 9, 18, 32, 73]. Patients with either unfavourable anatomy or cardiopulmonary status have reduced apnoeic windows as well. Multiple intubation attempts with cycles of facemask ventilation may further worsen intubation conditions due to laryngeal trauma and decreased pharyngeal tone from hypoxia. THRIVE generates positive pharyngeal pressures that distend the upper airway, which improves patency and visualisation for intubation. High flows can deliver oxygen through areas of critical obstruction and by bypassing upper airway resistance, providing PEEP, and meeting peak inspiratory flow demand, THRIVE reduces the respiratory effort required from the patient [47]. This improves the safety of conscious sedation during emergency and difficult airway management. Lau, Booth, and Badiger all demonstrated successful THRIVE for oxygenation in cases of elective and emergency awake fibreoptic intubations, tracheostomy, and laryngotracheal surgery in patients with structural airway pathology and cases of anticipated airway difficulty in head and neck procedures [15, 18, 32]
THRIVE offers advantages over conventional ventilation techniques in elective airway surgery including endotracheal intubation, intermittent intubation, intermittent bag mask ventilation, and intermittent jet ventilation (Tables 1 and 2). Diverse applications in elective otolaryngologic surgery are described in the literature (Table 3). No adverse patient safety events have been described during THRIVE in otolaryngologic surgery.
The literature in paediatric otolaryngology is limited but has been described in diagnostic airway assessment, drug-induced sleep study, removal of benign lesions, removal of foreign bodies, and laryngomalacia surgery [19, 28–30, 56, 74, 75]. Okland demonstrated that THRIVE significantly improved airway patency with flow-dependent increase in the anterior–posterior glottic view, which is useful for diagnostic assessment [74]. The HAMSTER trial is an ongoing RCT to determine the efficacy of THRIVE in paediatric airway surgery in reducing intraoperative desaturation and rescue ventilation compared to LFNO [76].
THRIVE during laser laryngeal surgery is controversial due to concerns regarding the risk of airway fires. Fire safety guidelines advocate for lowered FiO2 to reduce ignition risk, with most studies quoting 30%. Surgical airway fires involve a triad of oxygen (plentiful in THRIVE), heat (provided by diathermy or laser), and a fuel source. Modelling studies have investigated which laser and THRIVE settings are associated with increased combustion risk [77–79]. Recommendations to minimise airway fires include single operator control of the laser, using the lowest effective power setting, preferably using pulsed rather than continuous settings, minimising lasing time with short, intermittent application of laser, reducing FiO2, avoiding lasing adipose or charred tissue, removing any foreign endolaryngeal material, smoke evacuation of laser smog, and proper configuration of drapes to avoid oxygen pooling [15, 39, 77–81]. Evidence from retrospective case series supports that laser procedures can be safely performed with THRIVE within specific conditions [12, 15, 23, 28, 32, 38–40]. Lau and Huang interrupted oxygenation during lasing. However, Novakovic reduced the FiO2 from 100 to 30%, showing it is possible to deliver HFNO during lasing [39]. In a retrospective cohort study of 172 laser cases, Khan reported no safety events with continuous delivery of 100% FiO2 during lasing [40]. However, there is inadequate high-quality evidence in the literature to advocate the continued use of 100% FiO2 during lasing, with most studies focussing on single institution experiences. Given the potentially catastrophic consequences of airway fires, all possible measures should be implemented to reduce risk. There have been no descriptions of THRIVE laser fires. However, there is a report of inadvertent ignition from the monopolar diathermy during a palate biopsy in a patient with a dental implant, who suffered no burns [82].
THRIVE’s limitations warrant discussion. The lack of definitive airway increases the risk of aspiration of blood and laryngeal secretions. This may be addressed by supine positioning, regular suctioning of the airway, and precise haemostasis. Surgical factors such as size, number, location, and vascularity of lesions for biopsy or resection, and estimated bleeding risk, must be considered when choosing candidates for tubeless anaesthesia. Positive pharyngeal pressures pose a theoretical risk of gastric insufflation, with regurgitation and aspiration of gastric contents. However, studies in awake and anaesthetised patients do not find increased gastric volumes during HFNO—this can be due to mouth opening, which is required for microlaryngeal surgery [10, 83, 84]. Some suggest that patients with significant gastroesophageal reflux or oesophageal sphincter incompetence be reconsidered as THRIVE candidates [15]. The flow rates and PEEP are dependent on proper fitting of the nasal cannula, mouth opening, and the absence of structural nasal abnormalities. Nasal mucosal trauma, rhinorrhoea, and epistaxis are potential complications, though delivering humidified, warmed air should reduce this risk. THRIVE is contraindicated in patients with suspected or known pneumothorax, base of skull defects, and mid-facial fractures, due to the risk of tension pneumothorax, pneumocephalus, and subcutaneous emphysema. Finally, given the physiologic mechanisms of THRIVE, with sufficient time the patient will desaturate or develop hypercapnia, with requirement for rescue ventilation. Obese patients are more likely to desaturate due to upper airway collapse and require supportive manouevres, airway adjuncts, or rescue strategies. The need for rescue ventilation does not represent a failure of THRIVE as advanced airway management relies on familiarity with and incorporation of a variety of techniques.
Conclusion
THRIVE is a feasible and effective technique for preoxygenation and providing apnoeic ventilation in otolaryngologic surgeries with diverse clinical applications in both emergency and elective settings. Future considerations and areas of further research in this field may include improving anaesthetist and surgeon familiarity with incorporating THRIVE in perioperative airway management; identifying factors that predict failure of THRIVE and need for rescue ventilation, particularly in at-risk populations; and establishing guidelines for safe use during laser laryngeal surgery, that balances the risk of airway fires with the necessity of maintaining apnoeic oxygenation.
Abbreviations
- CO2
Carbon dioxide
- FiO2
Fraction of inspired oxygen
- FRC
Functional residual capacity
- HFNO
High-flow nasal oxygen
- PEEP
Positive end-expiratory pressure
- KTP
Potassium titanyl phosphate
- RCTs
Randomised controlled trials
- THRIVE
Trans-nasal humidified rapid insufflation ventilatory exchange
Funding
No funding was required for this project.
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
Ethical approval was not required for this project – it is a literature review and does not include any patient data. This project complies with the ethical standards of the institution’s research ethics committee.
Informed Consent
Informed consent was not required for this project as no patient data is collected or reported.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arora G, Arshad Z, Prakash R, et al. High-flow nasal cannula as an alternative weaning strategy: a randomized controlled trial. Cureus. 2023;15(3):e36511. doi: 10.7759/cureus.36511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A, Nouraei S. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70(3):323–329. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong D, Dallaire A, Singh K, et al. High-flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: a randomized controlled trial. Anesth Analg. 2019;129(4):1130–1136. doi: 10.1213/ANE.0000000000003966. [DOI] [PubMed] [Google Scholar]
- 4.Hua Z, Liu Z, Li Y, et al. Transnasal humidified rapid insufflation ventilatory exchange vs. facemask oxygenation in elderly patients undergoing general anaesthesia: a randomized controlled trial. Sci Rep. 2020;10(1):5745. doi: 10.1038/s41598-020-62716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng I, Krieser R, Mezzavia P, et al. The use of Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) for pre-oxygenation in neurosurgical patients: a randomised controlled trial. Anaesth Intensive Care. 2018;46(4):360–367. doi: 10.1177/0310057X1804600403. [DOI] [PubMed] [Google Scholar]
- 6.Burra V, Putta G, Prasad S, et al. A prospective study on use of thrive (transnasal humidified rapid insufflation ventilatory exchange) versus conventional nasal oxygenation following extubation of adult cardiac surgical patients. Ann Card Anaesth. 2021;24(3):353–357. doi: 10.4103/aca.ACA_16_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy L, Christensen R, Dodd B, et al. The effect of transnasal humidified rapid-insufflation ventilator exchange (THRIVE) versus nasal prongs on safe apnoea time in paralysed obese patients: a randomised controlled trial. Br J Anaesth. 2022;128(2):375–381. doi: 10.1016/j.bja.2021.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Rajan S, Joseph N, Tosh P, et al. Effectiveness of transnasal humidified rapid-insufflation ventilatory exchange versus traditional preoxygenation followed by apnoeic oxygenation in delaying desaturation during apnoea: a preliminary study. Indian J Anaesth. 2018;62(3):202–207. doi: 10.4103/ija.IJA_717_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binks M, Holyoak R, Melhuish T, et al. Apnoeic oxygenation during intubation in the intensive care unit: a systematic review and meta-analysis. Heart Lung. 2017;46(6):452–457. doi: 10.1016/j.hrtlng.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Lodenius A, Piehl J, Östlund A, et al. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) vs. facemask breathing pre-oxygenation for rapid sequence induction in adults: a prospective randomised non-blinded clinical trial. Anaesthesia. 2018;73(5):564–571. doi: 10.1111/anae.14215. [DOI] [PubMed] [Google Scholar]
- 11.Higgs A, McGrath B, Goddard C, et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120(2):323–352. doi: 10.1016/j.bja.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Dharmawardana N, Badenoch A, et al. A review of the use of transnasal humidified rapid insufflation ventilatory exchange for patients undergoing surgery in the shared airway setting. J Anesth. 2020;34(1):134–143. doi: 10.1007/s00540-019-02697-3. [DOI] [PubMed] [Google Scholar]
- 13.Pearson K, McGuire B. Anaesthesia for laryngo-tracheal surgery, including tubeless field techniques. BJA Education. 2017;17(7):242–248. doi: 10.1093/bjaed/mkx004. [DOI] [Google Scholar]
- 14.Benninger M, Zhang E, Chen B, et al. Utility of transnasal humidified rapid insufflation ventilatory exchange for microlaryngeal surgery. Laryngoscope. 2021;131(3):587–591. doi: 10.1002/lary.28776. [DOI] [PubMed] [Google Scholar]
- 15.Lau J, Loizou P, Riffat F, et al. The use of THRIVE in otolaryngology: our experiences in two Australian tertiary facilities. Aust J Otolaryngol. 2019;2:22. doi: 10.21037/ajo.2019.07.02. [DOI] [Google Scholar]
- 16.Lyons C, Callaghan M. Apnoeic oxygenation with high-flow nasal oxygen for laryngeal surgery: a case series. Anaesthesia. 2017;72(11):1379–1387. doi: 10.1111/anae.14036. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson I, Lodenius A, Tunelli J, et al. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) – a physiological study. Br J Anaesth. 2017;118(4):610–617. doi: 10.1093/bja/aex036. [DOI] [PubMed] [Google Scholar]
- 18.Badiger S, John M, Fearnley R, et al. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. Br J Anaesth. 2015;115(4):629–632. doi: 10.1093/bja/aev262. [DOI] [PubMed] [Google Scholar]
- 19.Caruso T, Sidell D, Lenning M, et al. Transnasal Humidified Rapid Insufflation Ventilatory Exchange (THRIVE) augments oxygenation in to children with cyanotic heart disease during microdirect laryngoscopy and bronchoscopy. J Clin Anesth. 2019;56:53–54. doi: 10.1016/j.jclinane.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Rudolf B, Hohenhorst W. Use of apneic oxygenation for the performance of pan-endoscopy. Otolaryngol Head Neck Surg. 2013;149(2):235–239. doi: 10.1177/0194599813486248. [DOI] [PubMed] [Google Scholar]
- 21.Waters E, Kellner M, Milligan P, et al. The use of Transnasal Humidified Rapid-insufflation Ventilatory Exchange (THRIVE) in one hundred and five upper airway endoscopies. A case series. Clin Otolaryngol. 2019;44(6):1115–1119. doi: 10.1111/coa.13408. [DOI] [PubMed] [Google Scholar]
- 22.Stewart K, McNarry A, Nixon I. Transnasal humidified rapid insufflation ventilatory exchange (THRIVE) in Elective Laryngopharyngoscopy. Clin Otolaryngol. 2019;44(6):1239–1241. doi: 10.1111/coa.13447. [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Athanasiadis T, Woods C, et al. The use of transnasal humidified rapid insufflation ventilatory exchange in laryngeal and pharyngeal surgery: flinders case series. Aust J Otolaryngol. 2019;2:17. doi: 10.21037/ajo.2019.05.02. [DOI] [Google Scholar]
- 24.Nekhendzy V, Saxena A, Mittal B, et al. The safety and efficacy of transnasal humidified rapid-insufflation ventilatory exchange for laryngologic surgery. Laryngoscope. 2020;130(12):E874–881. doi: 10.1002/lary.28562. [DOI] [PubMed] [Google Scholar]
- 25.Ma B, Liu F, Wang D, et al. High-flow nasal cannula in nonlaser microlaryngoscopic surgery: a prospective study of 19 cases in a Chinese population. BMC Anesthesiol. 2022;22(1):81. doi: 10.1186/s12871-022-01627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Wu C, Tseng W, et al. Nonintubated laryngomicrosurgery with transnasal humidified rapid-insufflation ventilatory exchange: a case series. J Formos Med Assoc. 2019;118(7):1138–1143. doi: 10.1016/j.jfma.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Huh G, Min S, Cho S, et al. Application and efficiency of transnasal humidified rapid-insufflation ventilatory exchange in laryngeal microsurgery. Laryngoscope. 2022;132(5):1061–1068. doi: 10.1002/lary.29848. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava R, Agarwal A, Agarwal S, et al. High flow nasal cannula: a game changer in airway surgery. Indian J Otolaryngol Head Neck Surg. 2019;71(3):299–303. doi: 10.1007/s12070-019-01717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridgway R, Dumbarton T, Brown Z. Update on ENT anaesthesia in children. Anaesth Intensive Care Med. 2019;20(1):56–60. doi: 10.1016/j.mpaic.2018.11.003. [DOI] [Google Scholar]
- 30.Srivastava R, Pathak M, Mallan D. THIRVE in foreign body bronchus removal–a novel approach. Indian J Otolaryngol Head Neck Surg. 2021;73(3):356–359. doi: 10.1007/s12070-021-02577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castano-Ramirez D, Zamudio-Castilla L, Tintinago-Londono L, et al. Transnasal humidified rapid insufflation ventilatory exchange in laryngotracheal resection and reconstruction: a report of two pregnant cases. Anesth Pain Med. 2022;12(3):e123829. doi: 10.5812/aapm-123829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booth A, Vidhani K, Lee P, et al. SponTaneous Respiration using IntraVEnous anaesthesia and Hi-flow nasal oxygen (STRIVE Hi) maintains oxygenation and airway patency during management of the obstructed airway: an observational study. Br J Anaesth. 2017;118(3):444–451. doi: 10.1093/bja/aew468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemay F, Cooper J, Thompson S, et al. Combination of transnasal humidified rapid-insufflation ventilatory exchange with high frequency jet ventilation for shared airway surgery. Can J Anaesth. 2020;67(9):1264–1265. doi: 10.1007/s12630-020-01635-2. [DOI] [PubMed] [Google Scholar]
- 34.To K, Harding F, Scott M, et al. The use of transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in 17 cases of subglottic stenosis. Clin Otolaryngol. 2017;42(6):1407–1410. doi: 10.1111/coa.12921. [DOI] [PubMed] [Google Scholar]
- 35.Tam K, Jeffery C, Sung C. Surgical management of supraglottic stenosis using intubationless optiflow. Ann Otol Rhinol Laryngol. 2017;126(9):669–672. doi: 10.1177/0003489417720220. [DOI] [PubMed] [Google Scholar]
- 36.Maupeu L, Raguin T, Hengen M, et al. Indications of Transnasal Humidified Rapid- Insufflation Ventilatory Exchange (THRIVE) in laryngoscopy, a prospective study of 19 cases. Clin Otolaryngol. 2019;44(2):182–186. doi: 10.1111/coa.13252. [DOI] [PubMed] [Google Scholar]
- 37.Youssef D, Paddle P. Tubeless anesthesia in subglottic stenosis: comparative review of apneic low-flow oxygenation with THRIVE. Laryngoscope. 2021;132(6):1231–1236. doi: 10.1002/lary.29885. [DOI] [PubMed] [Google Scholar]
- 38.Inglis D, Gilhooly M, Patel A. The simultaneous use of three ventilatory techniques to maintain oxygenation in a patient undergoing tracheal laser resection of tumour. Anaesth Rep. 2019;7(2):70–72. doi: 10.1002/anr3.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novakovic D, Sheth M, Fellner A, et al. Microlaryngeal laser surgery using high-flow nasal ventilation at two oxygen concentration deliveries. Laryngoscope. 2023;133(3):634–639. doi: 10.1002/lary.30271. [DOI] [PubMed] [Google Scholar]
- 40.Khan N, Vukkadala N, Saxena A, et al. Safety and utility of transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) for laser laryngeal surgery. Otolaryngol Head Neck Surg. 2023 doi: 10.1002/ohn.324. [DOI] [PubMed] [Google Scholar]
- 41.Bharathi M, Kumar M, Prakash B, et al. New visionary in upper airway surgeries–THRIVE, a tubeless ventilation. Indian J Otolaryngol Head Neck Surg. 2021;73(2):246–251. doi: 10.1007/s12070-021-02491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slutsky A. Gas mixing by cardiogenic oscillations: a theoretical quantitative analysis. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(5):1287–1293. doi: 10.1152/jappl.1981.51.5.1287. [DOI] [PubMed] [Google Scholar]
- 43.Slutsky A, Brown R. Cardiogenic oscillations: a potential mechanism enhancing oxygenation during apneic respiration. Med Hypotheses. 1982;8(4):393–400. doi: 10.1016/0306-9877(82)90032-9. [DOI] [PubMed] [Google Scholar]
- 44.Meltzer S, Auer J. Continuous respiration without respiratory movements. J Exp Med. 1909;11:622–625. doi: 10.1084/jem.11.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volhard F. Uber kunstliche Atmung durch Ventilation der Trachea und eine einfache Vorrichtung zur hytmischen kunstlichen Atmung. München Medizinische Wochenschrift. 1908;55:209–211. [Google Scholar]
- 46.Draper W, Whitehead R, Spencer J. Studies on diffusion respiration: alveolar gases and venous blood pH of dogs during diffusion respiration. Anesthesiology. 1947;8(5):524–533. doi: 10.1097/00000542-194709000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Nouraei R, Shorthouse J, Keegan J et al (2018) What is Transnasal Humidified Rapid- Insufflation Ventilatory Exchange (THRIVE)? ENT & Audiology News 27(2). Accessed from https://www.entandaudiologynews.com/development/spotlight-on-innovation/post/what-is-transnasal-humidified-rapid-insufflation-ventilatory-exchange-thrive
- 48.Laviola M, Das A, Chikhani M, et al. Computer simulation clarifies mechanisms of carbon dioxide clearance during apnoea. Br J Anaesth. 2019;122(3):395–401. doi: 10.1016/j.bja.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Hermez L, Spence C, Payton M, et al. A physiological study to determine the mechanism of carbon dioxide clearance during apnoea when using transnasal humidified rapid insufflation ventilatory exchange (THRIVE) Anaesthesia. 2019;74(4):441–449. doi: 10.1111/anae.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyons C, Callaghan M. Uses and mechanisms of apnoeic oxygenation: a narrative review. Anaesthesia. 2019;74(4):497–507. doi: 10.1111/anae.14565. [DOI] [PubMed] [Google Scholar]
- 51.Parke R, Eccleston M, McGuinness S. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56(8):1151–1155. doi: 10.4187/respcare.01106. [DOI] [PubMed] [Google Scholar]
- 52.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103(6):886–890. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riva T, Meyer J, Theiler L, et al. Measurement of airway pressure during high-flow nasal therapy in apnoeic oxygenation: a randomised controlled crossover trial. Anaesthesia. 2021;76(1):27–35. doi: 10.1111/anae.15224. [DOI] [PubMed] [Google Scholar]
- 54.Weingart S, Levitan R. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59(3):165–175. doi: 10.1016/j.annemergmed.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Toner A. Carbon dioxide clearance during apnoea with high-flow nasal oxygen: Epiphenomenon or a failure to THRIVE? Anaesthesia. 2020;75(5):580–582. doi: 10.1111/anae.14848. [DOI] [PubMed] [Google Scholar]
- 56.Lyons C. Carbon dioxide clearance during apnoea. Anaesthesia. 2019;74(6):816–817. doi: 10.1111/anae.14689. [DOI] [PubMed] [Google Scholar]
- 57.Lyons C, Callaghan M. Apnoeic oxygenation in paediatric anaesthesia: a narrative review. Anaesthesia. 2021;76(1):118–127. doi: 10.1111/anae.15107. [DOI] [PubMed] [Google Scholar]
- 58.Grude O, Solli H, Andersen C, et al. Effect of nasal or nasopharyngeal apneic oxygenation on desaturation T during induction of anesthesia and endotracheal intubation in the operating room: a narrative review of randomized controlled trials. J Clin Anesth. 2018;51:1–7. doi: 10.1016/j.jclinane.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Ramachandran S, Cosnowski A, Shanks A, et al. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, controlled trial of nasal oxygen administration. J Clin Anesth. 2010;22(3):164–168. doi: 10.1016/j.jclinane.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Salah B, Xuan A, Fouilladieu J, et al. Nasal mucociliary transport in healthy subjects is slower when breathing dry air. Eur Respir J. 1988;1:852–855. doi: 10.1183/09031936.93.01090852. [DOI] [PubMed] [Google Scholar]
- 61.Fontanari P, Burnet H, Zattara-Hartmann C, et al. Changes in airway resistance induced by nasal inhalation of cold ddry, dry, or moist air in normal individuals. J Appl Physiol. 1996;81(4):1739–1743. doi: 10.1152/jappl.1996.81.4.1739. [DOI] [PubMed] [Google Scholar]
- 62.Chen L, Yang L, Tian W, et al. Transnasal humidified rapid insufflation ventilatory exchange with nasopharyngeal airway facilitates apneic oxygenation: a randomized clinical noninferiority trial. Front Med (Lausanne) 2020;7:577891. doi: 10.3389/fmed.2020.577891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forsberg I, Ullman J, Hoffman A, et al. Lung volume changes in Apnoeic Oxygenation using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) compared to mechanical ventilation in adults undergoing laryngeal surgery. Acta Anaesthesiol Scand. 2020;64(10):1491–1498. doi: 10.1111/aas.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piosik Z, Dirks J, Rasmussen L, et al. Exploring the limits of prolonged apnoea with high-flow nasal oxygen: an observational study. Anaesthesia. 2021;76(6):798–804. doi: 10.1111/anae.15277. [DOI] [PubMed] [Google Scholar]
- 65.Forsberg I, Mkrtchian S, Ebberyd A, et al. Biomarkers for oxidative stress and organ injury during THRIVE compared to mechanical ventilation in adults undergoing microlaryngoscopy - a randomised controlled study. Acta Anaesthesiol Scan. 2021;65(9):1276–1284. doi: 10.1111/aas.13927. [DOI] [PubMed] [Google Scholar]
- 66.Jagannathan N, Burjek N. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a step forward in apnoeic oxygenation, paradigm-shift in ventilation, or both? Br J Anaesth. 2017;118(2):150–152. doi: 10.1093/bja/aew432. [DOI] [PubMed] [Google Scholar]
- 67.Kleine-Brueggeney M, Grosshauser M, Greif R. Apneic oxygenation in pediatric anesthesia. Curr Opin Anaesthesiol. 2022;35(3):361–366. doi: 10.1097/ACO.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 68.George S, Wilson M, Humphreys S, et al. Apnoeic oxygenation during paediatric intubation: a systematic review. Front Pediatr. 2022;10:918148. doi: 10.3389/fped.2022.918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Humphreys S, Lee-Archer P, Reyne G, et al. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. 2017;118(2):232–238. doi: 10.1093/bja/aew401. [DOI] [PubMed] [Google Scholar]
- 70.Riva T, Pedersen T, Seiler S, et al. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth. 2018;120(3):592–599. doi: 10.1016/j.bja.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 71.Shippam W, Preston R, Douglas J, et al. High-flow nasal oxygen vs. standard flow-rate facemask pre-oxygenation in pregnant patients: a randomised physiological study. Anaesthesia. 2019;74(4):450–456. doi: 10.1111/anae.14567. [DOI] [PubMed] [Google Scholar]
- 72.Cook T, Woodall N, Frerk C, et al. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011;106(5):617–631. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 73.Mir F, Patel A, Iqbal R, et al. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. 2017;72(4):439–443. doi: 10.1111/anae.13799. [DOI] [PubMed] [Google Scholar]
- 74.Okland T, Liu G, Caruso T, et al. Prospective evaluation of the safety and efficacy of THRIVE for children undergoing airway evaluation. Pediatr Qual Saf. 2020;5(5):e348. doi: 10.1097/pq9.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caruso T, Gupta A, Sidell D, et al. The successful application of high flow nasal oxygen during microdirect laryngoscopy and T bronchoscopy in patients under 7 kg. J Clin Anesth. 2019;52:27–28. doi: 10.1016/j.jclinane.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 76.Humphreys S, Ungern-Sternberg B, Skowno J, et al. High-flow oxygen for children’s airway surgery: randomised controlled trial protocol (HAMSTER) BMJ Open. 2019;9(10):e031873. doi: 10.1136/bmjopen-2019-031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baudouin R, Rigal T, Circiu M, et al. Feasibility and safety of THRIVE in transoral laser microsurgery. Am J Otolaryngol. 2022;43(5):103605. doi: 10.1016/j.amjoto.2022.103605. [DOI] [PubMed] [Google Scholar]
- 78.Huang L, Badenoch A, Vermeulen M, et al. Risk of airway fire with the use of KTP laser and high flow humidified oxygen delivery in a laryngeal surgery model. Sci Rep. 2022;12(1):543. doi: 10.1038/s41598-021-04636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang M, Chen J, Lin S, et al. Fire safety study on high-flow nasal oxygen in shared-airway surgeries with diathermy and laser: simulation based on a physical model. J Clin Monit Comput. 2022;36(3):649–655. doi: 10.1007/s10877-021-00690-4. [DOI] [PubMed] [Google Scholar]
- 80.Stuermer K, Ayachi S, Gostian A, et al. Hazard of CO2 laser-induced airway fire in laryngeal surgery: experimental data of contributing factors. Eur Arch Otorhinolaryngol. 2013;270(10):2701–2707. doi: 10.1007/s00405-013-2521-1. [DOI] [PubMed] [Google Scholar]
- 81.Ward P. THRIVE and airway fires. Anaesthesia. 2017;72(8):1035. doi: 10.1111/anae.13993. [DOI] [PubMed] [Google Scholar]
- 82.Onwochei D, El-Boghdadly K, Oakley R, et al. Intra-oral ignition of monopolar diathermy during transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) Anaesthesia. 2017;72(6):781–783. doi: 10.1111/anae.13873. [DOI] [PubMed] [Google Scholar]
- 83.Chang M, Kwak H, Kim J, et al. Effect of high-flow nasal oxygenation on gastric insufflation in patients undergoing laryngeal microsurgery under tubeless general anesthesia with neuromuscular blockade. J Clin Med. 2023;12(5):1800. doi: 10.3390/jcm12051800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLellan E, Lam K, Behringer E, et al. High-flow nasal oxygen does not increase the volume of gastric secretions during spontaneous ventilation. Br J Anaesth. 2020;125(1):e75–e80. doi: 10.1016/j.bja.2020.02.023. [DOI] [PubMed] [Google Scholar]