Purpose
There is an ongoing debate about the reasons behind the increasing incidence of thyroid cancer in the last two to three decades. Here, we investigate how thyroid nodules were detected in a large series of consultations for thyroid nodular pathology. Methods: In total, 576 patients were analyzed, with a total of 1014 nodules described. Results: In 347 (60.2%) cases, the diagnosis of a thyroid nodule was incidental, mostly during imaging tests for other reasons. Incidental diagnosis occurred among all ranges of nodule diameter and between palpable and non-palpable cases, even within a small proportion of symptomatic cases. In univariate analysis, incidental diagnosis was associated with smaller nodule diameter, non-palpable nodules, asymptomatic cases, older patient age, less advanced stages (T1–2), and conservative management. After multivariate analysis, older age, euthyroidism, and smaller diameter were statistically significant. Incidental diagnosis contributed to the diagnosis of 53.8% of the cases of cancer. Advanced T stages (T3–4) were more common in non-incidental diagnoses. Conclusion: Our results indicate that incidental diagnosis of thyroid nodules is a significant contributor to thyroid cancer diagnosis in all ranges of nodule diameter, especially at earlier stages.
Keywords: Thyroid Nodule; Thyroid Neoplasms; Incidence; Incidental Findings; Thyroid cancer, Papillary.
Introduction
The early 2000s were marked by a series of publications describing an increase in the incidence of thyroid cancer in different parts of the world [1–5]. Initially, no impact on disease mortality was observed, and most newly diagnosed cases were small nodules, which suggested the possibility that the occasional diagnosis of microcarcinomas could explain this phenomenon [6–9]. On the other hand, the increasing incidence of cases with larger diameters and tumors of more advanced stages [10–13] suggested that clinically apparent tumors were also being diagnosed more frequently. However, care must be taken to avoid data misinterpretation, as larger diameter does not mean, by itself, the presence of symptoms or of a palpable nodule. Supporting this, Iwata et al. observed that 44.2% of the non-palpable nodules in their study were larger than 2 cm in diameter [14].
The scenarios and definitions of incidental diagnosis vary in the literature [14–16], and we are not aware of associations between such forms of diagnosis and clinic-epidemiological or pathological variables being previously reported. The objectives of this study were to carefully examine the mode of diagnosis, refining definitions for thyroid incidentalomas, in a series of consultations for thyroid nodular pathology and to investigate which factors are associated with an increased risk of incidental diagnosis.
Materials and Methods
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (CAE: CAAE: 44857021.7.0000.5343). Patients who were evaluated for thyroid nodules in consultation with one of the surgeons (FMG) in our service between January 2011 and December 2020 were retrospectively selected and considered for this study. A cross survey according to the International Classification of Disease codes D34, C73, D44.0, and E04 and their variants was performed. All consecutive patients with thyroid nodule confirmation were included. Patients with only hormonal disease without evidence of a thyroid nodule were excluded. Patients were referred for consultations by other colleagues or services to our specialty or were identified on our own account in our service with symptomatic or self-perceived conditions. The method of detection of the nodule(s), age, sex, and education level of the patients, investigations, and surgical and pathological information were recorded and documented. All consultations for thyroid pathology in our service have followed a protocol since 2011, in which some information must always be present: mode of diagnosis of the thyroid nodule, related symptoms, past medical history, family history of thyroid pathology, clinical examination data, ultrasound information, thyroid-stimulating hormone (TSH) result, and cytopathology results (when indicated). The diagnostic process for thyroid nodules was performed in the qualitative phase of the study. At this stage, content analysis was completed after reading and interpreting the clinical data, admission notes, anamnesis, medical reviews, and clinical impressions described in the patients’ medical records. According to Bardin (1999), the analysis was divided into three stages: (1) pre-analysis, which included a “floating” reading and the delimitation of documents for further analysis; (2) evaluation and classification of the material and aggregation into the content analysis to allow for the emergence of hypotheses and the classification of units; and (3) interpretation of the obtained results [17]. The units and subunits derived from this stage of the study can be found in Table 1.
An incidental thyroid nodule was defined when it was found during a physical examination (thyroid palpation) or imaging study of a patient when the symptoms that initiated the consultation were not clearly related to the thyroid gland or during unrelated investigations (such as during an imaging test requested for reasons unrelated to a thyroid disorder or symptom). All patients were asked for compressive symptoms. When the answer was positive in the context of an incidentally found nodule (for example, in an imaging test for another reason), the case was still considered incidental. Thyroid cancer diagnosis was considered incidental when identified in the histological examination of a thyroid gland removed with the hypothesis of it being a benign condition. For all cases, the mode of diagnosis was recorded as part of the routine protocol for first consultations in thyroid nodule pathology. The routine of investigation of thyroid nodules was carried out in the usual way, usually with ultrasonography, TSH and, in the indicated cases, FNAB, regardless of the way the nodule was diagnosed.
The data were summarized using descriptive analysis. Continuous variables with normal distribution were expressed as mean and standard deviation. Ranges were reported for variables with non-parametric distributions. Categorical variables were expressed as absolute and relative frequencies. The chi-square test was used for categorical variables. The stepwise backward method was used to achieve a final model, and the maintenance of variables was according to the potential to predict incidental diagnosis, as determined by p < 0.20. Variables were assessed using logistic regression to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) in a multivariate model. Information about physical examinations and symptoms was excluded from multivariate analysis due to collinearity.
The outcome was defined as alive, lost to follow-up, deceased due to the tumor, or deceased due to other causes. Lost to follow-up was defined as a period of > 12 months since last contact. The date of events (local recurrence, nodal recurrence, death) and the date of the last consultation were retrieved. Survival analysis for thyroid cancer cases was performed based on disease-free survival (DFS) time, which was defined as the time between diagnosis and loco-regional recurrence or death associated with disease versus censured cases (alive, lost to follow-up, deceased due to other causes). Univariable DFS analysis was made through Cox proportional hazard models. The results were expressed in terms of hazard ratios (HRs) and relative 95% CIs. The Kaplan–Meier method was used to graphically represent DFS according to the significant variables. The curves were compared using the log-rank test, and the estimated survival time with the relative 95% CI was presented. The statistical analyses were performed using SPSS Statistics, Version 20.0 (IBM Corp., Armonk, NY, USA). All tests were assigned a significance level of 5%.
Results
In total, 576 patients were analyzed, with 1014 nodules described. The mean age was 53.45 years (range 6–95 years), with a predominance of Caucasians (532; 92.3%) over Afro-descendants (44; 7.6%). The male to female ratio was 1:7.3; 304 (52.7%) patients had education up to elementary school. In 380 (65.9%) cases, there were palpable nodules, and 149 (25.8%) were symptomatic for thyroid pathology (see Table 2). The majority had no associated hormonal diseases (450; 78.1%). In 347 (60.2%) cases, the diagnosis of a thyroid nodule occurred incidentally, mostly in imaging tests for other reasons (239; 41.4%). We did not identify any statistically significant variation in the occurrence of incidental diagnosis of nodules between the two halves of the study period (2011–2015 vs. 2016–2020) (57.4% vs. 61.7%; p = 0.309). We identified an association between incidental diagnosis and smaller nodule diameter on ultrasound (p < 0.001), non-palpable nodules (p < 0.001), asymptomatic cases (p < 0.001), older age (p < 0.001), and conservative management (p < 0.001).
Table 1.
Mode of diagnosis of thyroid nodules
| Mode of diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total Number of Cases | Cancer Cases | Incidental diagnosis | ||||||
| 183 | 14 | In US for other causes or vague or unspecific complaints* | ||||||
| 1 | 1 | In PET-CT for other causes | ||||||
| 8 | 1 | In MRI for other causes | ||||||
| 6 | 0 | In X-ray for other causes | ||||||
| 41 | 7 | In CT for other causes | ||||||
| 1 | 1 | In a pathologic fracture due to an unknown thyroid cancer** | ||||||
| 75 | 9 | In neck palpation in medical consult for other reasons | ||||||
| 32 | 5 | In US in the context of thyroid hormonal disease without palpable neck nodules | ||||||
| Non-incidental diagnosis | ||||||||
| 174 | 25 | Nodule or neck volume self-perceived | ||||||
| 33 | 7 | In US in the context of thyroid hormonal disease with palpable neck nodules | ||||||
| 22 | 1 | With compressive symptoms | ||||||
Legend: *vague or unspecific symptoms occurred in 22 cases, with oscillatory dysphagia, dysphonia, odynophagia, pyrosis, neck pain, globus pharyngeous (symptoms not relieved after treatment); **thyroidectomy was not performed
Diagnosis occurred incidentally in 94.4%, 71.5%, 60.2%, 42.3%, and 31.4% of patients with nodules measuring < 1 cm, 1.1–2 cm, 2.1–3 cm, 3.1–4 cm, and > 4 cm, respectively. We did not observe an association between incidental diagnosis and gender (p = 0.524) or education level (p = 0.827). After multivariate analysis, older age (p < 0.001), euthyroidism (p = 0.025), and smaller diameter (p < 0.001) were statistically significant. The mode of diagnosis of thyroid nodules is detailed in Table 1.
FNAB was performed on 278 of a total of 676 nodules among cases with incidental diagnosis, with a Bethesda I-VI distribution of 16 (5.7%), 125 (44.9%), 69 (24.8%), 53 (19.0%), 13 (4.6%) and 2 (0.7%), respectively. Among the cases of non-incidental diagnosis, FNAB was performed in 160 of a total of 338 nodules, with a Bethesda I-VI distribution of 14 (8.7%), 63 (39.3%), 48 (30%), 28 (17.5%), 3 (1.8%) and 3 (1.8%), respectively. We did not observe statistically significant differences between the two groups (p = 0.5045).
Of the total sample, 280 (48.6%) patients underwent surgery in our service, and malignancy was confirmed in 71 (12.3%) cases. The majority consisted of papillary carcinomas (63; 88.7%). The incidental diagnosis of nodules contributed to the diagnosis in 38 cases of cancer (53.5% of the malignant cases). An incidental diagnosis of thyroid cancer was made in five (0.8%) cases. No differences in cancer rates were observed between incidental and non-incidental cases (p = 0.300), even when comparing incidental non-palpable vs. non-incidental nodules (p = 0.131). Advanced T stages (T3–4) were more common in the non-incidental diagnosis group (p = 0.003). Although high American Thyroid Association (ATA) risk was also more common in this group (four vs. two cases; 12.1% and 5.2%, respectively), no statistically significant difference was observed (p = 0.532).
The median follow-up time was 31.66 months (range 0–121 months). In 16 cases (22.5%), there was no contact in the last 12 months (considered lost to follow-up). Death due to the tumor occurred in three cases (4.2%): two with distant metastasis at presentation and the other with an anaplastic carcinoma. Six cases presented with recurrence within a mean of 22.2 months after diagnosis: four on neck lymph nodes and two on distant sites. Patients who experienced recurrence were all alive after a median follow-up of 66.76 months.
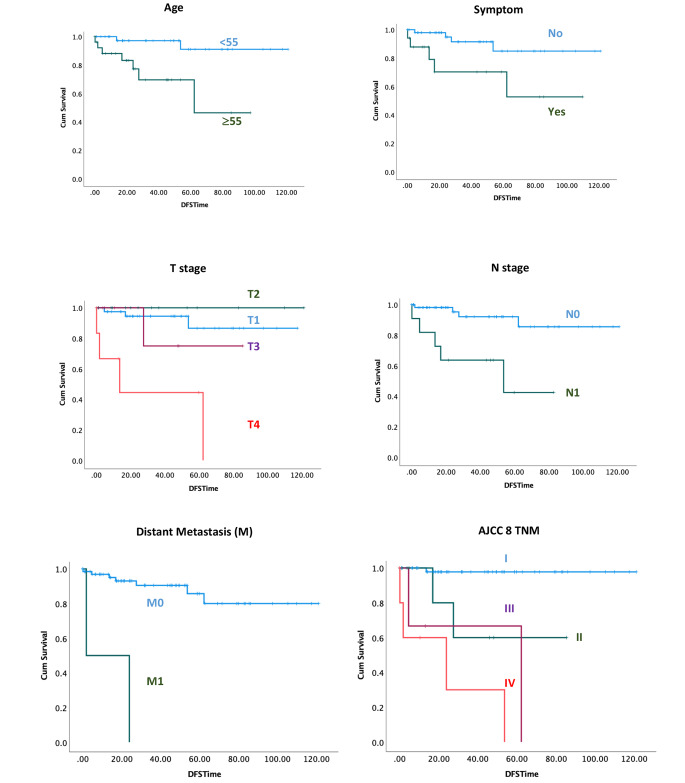
Factors associated with an increased risk of recurrence of malignant cases during follow-up included older age (HR: 8.3, p = 0.009), presence of symptoms (HR: 4.45, p = 0.02), poorly differentiated histology (HR: 10.54, p = 0.004), T4 vs. T1 (HR: 12.71, p < 0.001), presence of nodal metastasis (HR: 7.29, p = 0.003), and presence of distant metastasis (HR: 21.21, p < 0.001) (Table 3). Stages II, III, and IV were also associated with poor DFS compared to stage I (HR: 17.99, 42.32, and 85.59; p = 0.01, 0.002, and < 0.001, respectively) (Table 3). No association between incidental diagnosis and DFS was observed (p = 0.53). Survival curves are presented in Fig. 1.
Table 2.
Descriptive, univariate and multivariate analyses comparing incidental and non-incidental diagnoses
| Variables | Incidental Diagnosis (n = 347) | Non-incidental diagnosis (n = 229) | n = 576 (100%) | univariate p value | multivariate p value |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 44 (12.6%) | 25 (10.9%) | 69 (11.9%) | 0.524 | |
| Female | 303 (87.3%) | 204 (89.0%) | 507 (88.0%) | ||
| Age, in Years | |||||
| Mean ± SD | 56.10 ± 14.39 | 49.44 ± 15.87 | 53.45 ± 15.33 | 0.001 | < 0.001 |
| Range | 06–95 | 13–89 | 06–95 | ||
| Education level, n (%) | |||||
| Illiterate | 3 (0.8%) | 1 (0.4%) | 4 (0.6%) | 0.827 | |
| Until 1st grade | 183 (52.7%) | 121 (52.8%) | 304 (52.7%) | ||
| Until 2nd grade | 63 (18.1%) | 42 (18.3%) | 105 (18.2%) | ||
| ≥ 3rd grade | 71 (20.4%) | 42 (18.3%) | 113 (19.6%) | ||
| NI | 27 (7.7%) | 23 (10.0%) | 50 (8.6%) | ||
| Race, n (%) | |||||
| White | 326 (93.9%) | 206 (89.9%) | 532 (92.3%) | 0.078 | 0.082 |
| Afrodescendant | 21 (6.0%) | 23 (10.0%) | 44 (7.6%) | ||
| Hormonal status | |||||
| Euthyroidism | 229 (65.9%) | 140 (61.1%) | 369 (64.0%)) | 0.076 | 0.025 |
| Hyperthyroidism | 18 (5.1%) | 25 (10.9%) | 43 (7.4%) | ||
| Hypothyroidism | 49 (14.1%) | 34 (14.8%) | 83 (14.4%) | ||
| NI | 51 (14.6%) | 30 (13.1%) | 81 (14.0%) | ||
| Diameter, in cm | |||||
| Mean ± SD | 2.2 ± 1.36 | 3.6 ± 2.00 | 2.7 ± 1.78 | < 0.001 | < 0.001 |
| Range | 0.3–9.3 | 0.7–16 | 0.3–16 | ||
| Symptomatic, n (%) | |||||
| Yes | 52 (14.9%) | 97 (42.3%) | 149 (25.8%) | < 0.001 | |
| No | 295 (85.0%) | 128 (55.8%) | 423 (73.4%) | ||
| Missing | 0 | 4 (1.7%) | 4 (0.6%) | ||
| Palpable, n (%) | |||||
| Yes | 158 (45.5%) | 222 (96.9%) | 380 (65.9%) | < 0.001 | |
| No | 183 (52.7%) | 7 (3.0%) | 190 (32.9%) | ||
| Missing | 6 (1.7%) | 0 | 6 (1.0%) | ||
| Surgical treatment, n (%) | |||||
| Yes | 134 (38.6%) | 147 (64.1%) | 281 (48.7%) | < 0.001 | |
| No | 213 (61.3%) | 82 (35.8%) | 295 (51.2%) | ||
| Pathological result, n (%) | |||||
| Benign | 96 (71.6%) | 114 (77.5%) | 210 (74.7%) | 0.300 | |
| Malignant | 38 (28.3%) | 33 (22.4%) | 71 (25.2%)* |
NI: not informed; *in one of confirmed cancer cases, there was no thyroidectomy performed. The thyroid cancer diagnosis was through bone metastasis
Fig. 1.
Kaplan-Meier cumulative disease-free survival curves of statically significant results
Table 3.
DFS Univariable Cox Regression Models
| HR | 95% CI | p value | |
|---|---|---|---|
| Gender | |||
| Male | Ref | 0.75 | |
| Female | 0.77 | 0.16–3.47 | |
| Age | |||
| <55 | Ref | 0.009 | |
| ≥55 | 8.32 | 1.68–41.17 | |
| Palpable node | |||
| No | Ref | 0.79 | |
| Yes | 1.23 | 0.25–5.93 | |
| Symptoms | |||
| No | Ref | 0.02 | |
| Yes | 4.45 | 1.19–16.69 | |
| Incidental diagnosis | |||
| No | Ref | 0.53 | |
| Yes | 0.66 | 1.77–2.47 | |
| TSH | |||
| Normal | Ref | ||
| Hypo | 2.18 | 0.51–9.16 | 0.28 |
| Hyper | 3.90 | 0.43–35.24 | 0.22 |
| Pathology | |||
| Well differentiated | Ref | 0.004 | |
| Poorly differentiated | 10.54 | 2.08–53.19 | |
| T | |||
| 1 | Ref |
0.44 < 0.001 |
|
| 2 | - | - | |
| 3 | 2.40 | 0.24–23.25 | |
| 4 | 12.71 | 2.80–57.66 | |
| N | |||
| 0 | Ref | 0.003 | |
| 1 | 7.29 | 1.92–27.59 | |
| M | |||
| 0 | Ref | < 0.001 | |
| 1 | 21.21 | 3.84–117.15 | |
| AJCC | |||
| I | Ref |
0.01 0.002 < 0.001 |
|
| II | 17.99 | 1.62–199.57 | |
| III | 42.32 | 3.75–477.51 | |
| IV | 85.59 | 9.15–799.95 |
Discussion
From the observation that the majority of new diagnoses of thyroid cancer were microcarcinomas [3] and that the presence of microcarcinomas was frequent in autopsy studies [18, 19], it became apparent to researchers that incidental diagnosis plays an important role in the increasing incidence of disease. However, the degree of importance of incidental diagnosis in the disease is unknown, and the observation that more advanced tumors were also showing an increase in incidence [10–13] suggests the possibility that other factors could also be influencing this phenomenon.
We believe a better way to explore this topic is through an analysis of the mode of diagnosis. The majority of the published literature on this issue fails to reproduce a real-world scenario, falling on biased sample collections by analyzing only surgical patients [20] or only cases that underwent fine-needle aspiration biopsies [14] or imaging studies [15]. We collected a consecutive series of consultations, similar to studies by Kroeker et al., Marina et al., and Rothberger et al. [16, 21, 22], with similar rates of incidental diagnosis, despite the benefit of having no losses because of previous protocol organization. The rate of incidental diagnosis in our sample was 60.2%, occurring among all ranges of nodule diameter and between palpable and non-palpable cases, even within a small proportion of symptomatic cases, without significant variations over the last decade.
Furthermore, in any case, it was important not to stifle the criteria of incidentality and to consider that all fortuitous diagnoses would be incidental. Differences in incidentaloma definition and sample collection are probably the reasons for the disparities observed between studies. The rate of incidentalomas ranges from 6.6 to 37.4% in the literature [14, 15, 20, 21], although it might be underestimated by losses of documented histories [21] or group subdivision [16]. According to Haugen et al., incidentalomas are “Nonpalpable nodules detected on anatomic imaging studies” [23]. For Russ et al., an incidentaloma is a broad entity: “an unexpected, asymptomatic thyroid tumor fortuitously discovered during the investigation of an unrelated condition” [24]. Kang et al. defined incidentally detected nodules as impalpable thyroid nodules 1.5 cm in diameter discovered by imaging [25]. For Iwata et al., all incidental nodules were non-palpable [14]. We chose to scrutinize the mode of detection and better understand the process, as shown in Table 1, which is in agreement with the Merriam-Webster dictionary, in which “incidental” is defined as something that is “…occurring merely by chance or without intention or calculation”. Overall, 45.5% of the incidentalomas in our sample were palpable, and 14.9% were in symptomatic patients, which indicates that the terms “incidental”, “asymptomatic”, and “non-palpable” should not be used as equivalents.
We observed that there was no difference in the cancer rate between incidental and non-incidental cases, which shows that a self-perceived nodule is not correlated with oncological diagnosis but only with higher staging. A recently published systematic review also found no difference in cancer risk when comparing incidental and non-incidental groups, with risks ranging from 4 to 23.5% and 3.8–28.7%, respectively [26]. Overall, incidentally detected thyroid cancer was significantly less likely to have a recurrence/residual disease and had higher 5-year recurrence‐free and overall survival rates [26]. In our sample, symptomatic nodules were associated with worse DFS but not incidental detection. Higher age, advanced pathological staging, nodal and distant metastases, poor differentiation, and larger tumors were also associated with worse DFS; these are all features that are classically related to poorer outcomes in thyroid cancer.
Care must be taken to not misunderstand these results, as there are no safety and observation criteria based solely on the fact that the diagnosis was incidental, and these cases should not be managed differently [27]. We found nodules of all sizes diagnosed incidentally, including cases of more advanced stages. Our results suggest incidental diagnosis is the main reason for the increase in incidence observed for thyroid cancer in recent years, regardless of staging.
One limitation of this study is that it was a single-center retrospective study. In addition, our definition of incidentaloma may differ from other studies. Another point is that although this cohort was larger, the low number of cancer cases does not allow for a highly powered calculation of differences among categories.
One major point of strength of this study is that despite using data from medical records to determine the method of detection of thyroid nodules, there were no missing data, as protocols for first consultation were adjusted 10 years ago. Moreover, the incidental causes of referral were deeply detailed in our study, providing a close scenario to the real world.
Conclusion
Our results indicate that there is a significant contribution of incidentalomas to thyroid nodule diagnosis, as well as to new thyroid cancer cases, in all ranges of nodule diameter, especially at earlier stages. Older age, euthyroidism, and smaller diameter were statistically associated with incidental cases. The majority of incidentalomas was diagnosed in US for other causes, mainly vague or unspecific complaints.
Acknowledgements
The authors are grateful to Objetiva Pathology Laboratory for helping with data review. Manoela Domingues Martins is a research fellow funded by the Brazilian National Council for Scientific and Technological Development (CNPq).
Authors’ Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Fábio Muradás Girardi and Vivian Petersen Wagner. The first draft of the manuscript was written by Fábio Muradás Girardi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors have no funding or financial relationships.
Declarations
Conflicts of interest
The authors have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burgess JR (2002) Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982–1997). Thyroid 12(2):141-9 [DOI] [PubMed]
- 2.Enewold L, Zhu1 Z, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS (2009) Rising thyroid cancer incidence in the United States by demographic and Tumor characteristics, 1980–2005. Cancer Epidemiol Biomark Prev 18(3):784–791 [DOI] [PMC free article] [PubMed]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Leenhardt L, Bernier MO, Boin-Pineau MH, Conte Devolx B, Maréchaud R, Niccoli-Sire P, Nocaudie M, Orgiazzi J, Schlumberger M, Wémeau JL, Chérie-Challine L, De Vathaire F. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur J Endocrinol. 2004;150(2):133–139. doi: 10.1530/eje.0.1500133. [DOI] [PubMed] [Google Scholar]
- 5.Verkooijen HM, Fioretta G, Pache J-C, Franceschi S, Raymond L, Schubert H, Bouchardy C. Diagnostic changes as a reason for the increase in papillary thyroid cancer incidence in Geneva, Switzerland. Cancer Causes Control. 2003;14(1):13–17. doi: 10.1023/A:1022593923603. [DOI] [PubMed] [Google Scholar]
- 6.Heymart MR, Banerjee M, Reyes-Gastelum D, Caoili C, Norton EC. Thyroid ultrasound and the increase in diagnosis of low-risk thyroid cancer. J Clin Endocrinol Metab. 2019;104(3):785–792. doi: 10.1210/jc.2018-01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 8.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 9.Welch GH. Cancer screening, overdiagnosis, and regulatory capture. JAMA Intern Med. 2017;177(7):915–916. doi: 10.1001/jamainternmed.2017.1198. [DOI] [PubMed] [Google Scholar]
- 10.Burgess JR, Tucker P. Incidence trends for papillary thyroid carcinoma and their correlation with thyroid Surgery and thyroid fine-needle aspirate cytology. Thyroid. 2006;16(1):47–53. doi: 10.1089/thy.2006.16.47. [DOI] [PubMed] [Google Scholar]
- 11.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115(16):3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 12.Colonna M, Guizard AV, Schvartz C, Velten M, Raverdy N, Molinie F, Delafosse P, Franc P, Grosclaude P. A time trend analysis of papillary and follicular cancers as a function of tumour size: a study of data from six cancer registries in France (1983–2000) Eur J Cancer. 2007;43(5):891–900. doi: 10.1016/j.ejca.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Enewold LR, Zhou J, Devesa SS, de Gonzalez AB, Anderson WF, Zahm SH, Stojadinovic A, Peoples GE, Marrogi AJ, Potter JF, McGlynn KA, Zhu K. Thyroid cancer incidence among active duty U.S. military personnel, 1990–2004. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2369–2376. doi: 10.1158/1055-9965.EPI-11-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata AJ, Bhan A, Lahiri S, Williams AM, Taylor AR, Chang SS, Singer MC. Comparison of incidental versus palpable thyroid nodules presenting for fine-needle aspiration biopsy. Head Neck. 2018;40(7):1508–1514. doi: 10.1002/hed.25132. [DOI] [PubMed] [Google Scholar]
- 15.Uppal A, White MG, Nagar S, Aschebrook-Kilfoy B, Chang PJ, Angelos P, Edwin L, Kaplan EL, Grogan RH. Benign and malignant thyroid incidentalomas are rare in routine clinical practice: a review of 97,908 imaging studies. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1327–1331. doi: 10.1158/1055-9965.EPI-15-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothberger GD, Cohen M, Sahay P, Szczepanczyk PT, Islam S. Method of detection of thyroid nodules: correlation with frequency of fine-needle aspiration and malignancy rate. Head Heck. 2020;42(2):210–216. doi: 10.1002/hed.25984. [DOI] [PubMed] [Google Scholar]
- 17.Bardin L. Análise de conteúdo. In: Chiesa AM, Ciampone MHT, editors. Princípios gerais para a abordagem de variáveis qualitativas e o emprego da metodologia de grupos focais. A classificação internacional das práticas de enfermagem em saúde coletiva. Brasília: ABEN; 1999. pp. 306–324. [Google Scholar]
- 18.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid: a normal finding in Finland. Cancer. 1985;56(3):531–538. doi: 10.1002/1097-0142(19850801)56:3<531::AID-CNCR2820560321>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Mohorea IS, Socea B, Serban D, Ceausu Z, Tulin A, Melinte V, Ceausu M. Incidence of thyroid carcinomas in an extended retrospective study of 526 autopsies. Exp Ther Med. 2021;21(6):607. doi: 10.3892/etm.2021.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrá JC, Picado O, Liu S, Ouyang W, Teo R, Franco AM, Lew JI. Clinically significant cancer rates in incidentally discovered thyroid nodules by routine imaging. J Surg Res. 2017;219:341–346. doi: 10.1016/j.jss.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Kroeker TR, le Nobel G, Merdad M, Freeman JL. Outcomes of incidentally discovered thyroid nodules referred to a high-volume head and neck surgeon. Head Neck. 2014;36(1):126–129. doi: 10.1002/hed.23273. [DOI] [PubMed] [Google Scholar]
- 22.Marina M, Ceda GP, Aldigeri R, Ceresini G. Causes of referral to the first endocrine visit of patients with thyroid carcinoma in a mildly iodine-deficient area. Endocrine. 2017;57(2):247–255. doi: 10.1007/s12020-016-1140-1. [DOI] [PubMed] [Google Scholar]
- 23.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid Cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russ G, Leboulleux S, Leenhardt L, Hegedüs L. Thyroid incidentalomas: epidemiology, risk stratification with ultrasound and workup. Eur Thyroid J. 2014;3(3):154–163. doi: 10.1159/000365289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang HW, No JH, Chung JH, Min YK, Lee MS, Lee MK, Yang JH, Kim KW. Prevalence, clinical and ultrasonographic characteristics of thyroid incidentalomas. Thyroid. 2004;14(1):29–33. doi: 10.1089/105072504322783812. [DOI] [PubMed] [Google Scholar]
- 26.Chooi JE, Ravindiran A, Balasubramanian SP. The influence of Incidental detection of thyroid nodule on thyroid Cancer risk and prognosis - a systematic review. J Endocr Soc. 2021;5(Suppl 1):A871. doi: 10.1210/jendso/bvab048.1780. [DOI] [PubMed] [Google Scholar]
- 27.Tufano RP, Noureldine SI, Angelos P. Incidental thyroid nodules and thyroid cancer: considerations before determining management. JAMA Otolaryngol Head Neck Surg. 2015;141(6):566–572. doi: 10.1001/jamaoto.2015.0647. [DOI] [PubMed] [Google Scholar]