Abstract
A recombinant Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) expressing the green fluorescence protein (GFP) under the control of the AcMNPV polyhedrin promoter was constructed to study the spatial and temporal regulation of baculovirus infection in a permissive host. Larvae that ingested AcMNPV-GFP showed localized expression of GFP in the midgut epithelial cells, as well as hemocytes, at 24 h postinfection. The presence of fluorescence in these tissues indicated not only that the virus was replicating but also that the very late viral proteins were being synthesized. Secondary infection occurred within the tracheal cells throughout the body cavity, confirming earlier reports, and these foci of infection allowed entry of the virus into other tissues, such as the epidermis and the fat body.
Nucleopolyhedroviruses (NPVs) are double-stranded, circular DNA viruses that are being studied for their potential as biological control agents for use against several lepidopteran pests that cause severe losses in agriculture and forestry. The type species of NPV is Autographa californica multicapsid NPV (AcMNPV). This virus is an atypical NPV because it not only has a wide host range but also kills the host rapidly, unlike most of the other NPVs currently being studied. AcMNPV has been well studied, and much is known about its genome, including the complete nucleotide sequence (1), gene organization, and temporal regulation of the genes involved in infection, replication (for a review of DNA replication, see reference 12), and pathogenesis (4, 5, 7–10). One aspect of AcMNPV infection that needs to be understood is the mechanism of virus entry and dissemination within the larval tissues.
One of the earliest models proposed to explain virus transmission suggested that while the ingested virus infected and replicated within the midgut epithelial cells, the primary route of transmission of the virus from the midgut was via the passage of the inoculating virus through the basal plasma membrane and the basal lamina of the midgut and into the hemocoel within 0.5 h postinfection (hpi) (8). Subsequent immunohistochemical studies established that midgut infection occurred simultaneously in both the columnar and regenerative cells of the midgut epithelium (9). As the infection progressed, the midgut recovered by sloughing off the infected cells (5, 9). It has also been suggested that infected hemocytes play a significant role in the systemic infection, but the exact mechanism is not readily apparent (9). More recently, it has been proposed that infection can also proceed via the tracheal system. Specifically, two epidermal structures including a trachea-specific lymph system and intercellular spaces (epidermal feet) of the tracheal epithelium could facilitate rapid spread of baculovirus infections (4). However, it is not clear if late viral proteins are synthesized within the midgut following replication or what the mechanism is by which the virus is transmitted from tissue to tissue within the insect.
The green fluorescence protein (GFP) was isolated from the bioluminescent jellyfish Aequorea victoria and has been used successfully as an effective reporter gene in many heterologous systems. When expressed in either prokaryotic or eukaryotic systems and exposed to either blue or UV light, GFP emits an intense green fluorescence. GFP expression is species independent, does not require any cofactors or substrates, and can be visualized in living tissues (2, 21). GFP has recently been expressed in baculoviruses (3, 20). In this paper, we report the construction of a modified AcMNPV containing GFP as a reporter gene, inserted into the polyhedrin (polh) locus, and its application to elucidate the process of infection in Trichoplusia ni larvae.
Recombinant AcMNPV-GFP was constructed by inserting gfp into the AcMNPV polyhedrin locus, thereby inactivating polyhedrin and resulting in production of a polyhedrin-negative mutant. We removed the 757-bp NheI/HindIII gfp coding region fragment from pS65T-C1 (Clontech Technologies, Palo Alto, Calif.) and cloned this region into the XbaI/HindIII site in the transfer vector pFastBac1 (Life Technologies Inc. [LTI], Burlington, Ontario, Canada). This clone, with gfp under the control of the AcMNPV polyhedrin promoter, was used to produce a recombinant virus via transposon-mediated recombination into the polyhedrin coding region of AcMNPV in DH10bac cells by using the Bac-to-Bac kit (LTI). The white colonies containing the recombinant were picked and cultured overnight, and the DNA was extracted by using Qiagen quick 8 kits (Qiagen Inc., Chatsworth, Calif.). Transfer of the gfp fragment was confirmed by PCR analysis using the universal forward and reverse sequencing primers. The recombinant was purified by transfection into SF-21 cells (17), seeded at a density of 6 × 105 cells/ml in Grace’s medium containing 10% fetal bovine serum. Two hours after the cells were seeded, the complete medium was removed and the monolayers were washed twice with serum-free Grace’s medium. Viral DNA (5 μl; approximately 0.5 μg) was added to 6 μl of CellFectin (LTI) and allowed to incubate at room temperature for 15 min in a polystyrene tube. The DNA-lipid complex was added to the cell monolayer and incubated at 28°C in a rocking incubator for 3 h. The transfection mixture was removed, 2 ml of complete medium was added, and the cells were incubated at 28°C. Three days posttransfection, the SF-21 cells became detached and the nuclei appeared swollen. An aliquot of the detached cells was collected and centrifuged, and the pellet was viewed under visible light for the characteristic yellow-green appearance. This pellet was then resuspended in a small volume of medium and placed under a binocular microscope equipped with an epifluorescence system, with a mercury lamp light source to provide the excitation wavelength and a filter to screen all light except 450 to 500 nm, the emission range of GFP.
AcMNPV-GFP-infected SF-21 cells were used as the inoculum. The virus-infected cells were collected, and the total numbers were determined on a hemocytometer. The number of cells that fluoresced under a fluorescence microscope was used to estimate the percentage of infected cells within the population. Aliquots equivalent to 0.5, 5.0, 150, and 400,000 infected cells were applied to either diet plugs or the surface of the diet, and larvae were then allowed to feed on the treated diet.
Larvae were examined at 12-h intervals. Larvae were then placed in Manduca saline solution (15), and various tissues were dissected and examined under a fluorescence microscope. Besides the overall fluorescence of the larvae, tissues such as the hemocytes, epidermis, tracheae, fat body, Malpighian tubules, brain, ganglia, reproductive organs, and both the lumen and hemolymph sides of the midgut were examined for GFP expression.
For the midgut study, groups of eight 10-day-old larvae were allowed to feed on diet that had been treated on the surface with 4 × 105 infected SF-21 cells. The time at which the larvae were placed on the diet was counted as 0 hpi. We examined midguts every 4 hpi until fluorescence was observed. Starting at 12 hpi, larvae were collected at 6-h intervals and both the hemocytes and midguts were examined for the presence of fluorescence.
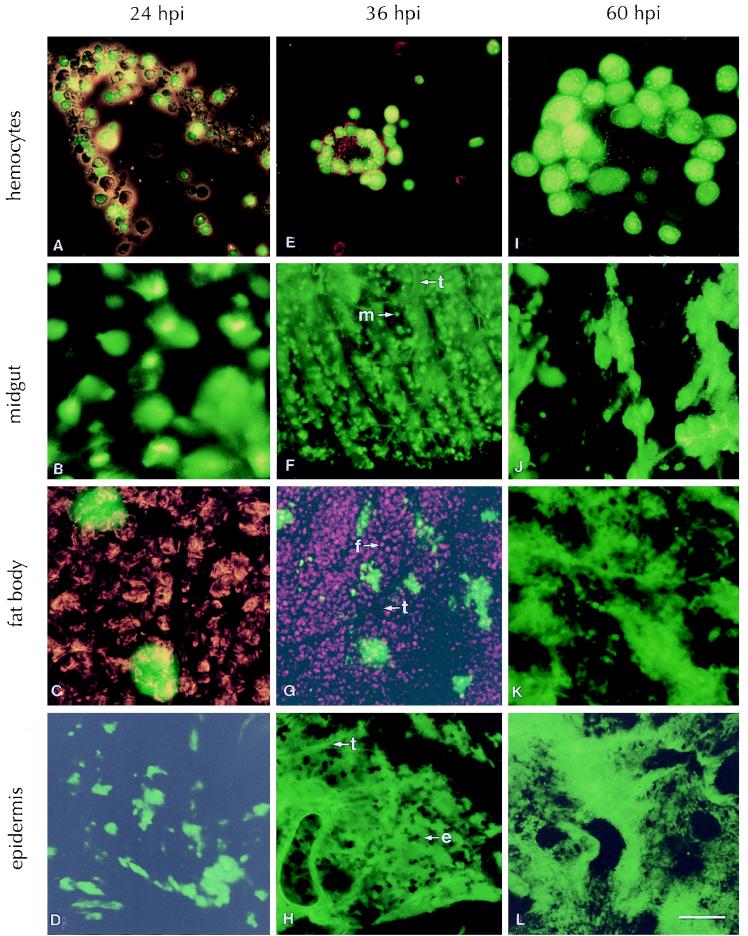
T. ni larvae that were infected by feeding on AcMNPV-GFP did not exhibit external signs of whole-body fluorescence until 24 hpi. However, the first signs of fluorescence occurred internally in the midgut epithelial cells and the hemocytes as early as 12 hpi (data not shown). The appearance of fluorescence in the hemocytes and midgut epithelial cells indicated that the virus had infected both of these tissues, had undergone replication, and was expressing GFP under the control of the very late polyhedrin promoter. By 24 hpi, more hemocytes (Fig. 1A) and midgut epithelial cells (Fig. 1B) showed fluorescence. The proportion of fluorescent hemocytes and the intensity of fluorescence increased with time (Fig. 1E and I). At 36 hpi, the fluorescence was decreasing in the midgut epithelial cells whereas the tracheae associated with the midgut appeared to fluoresce (Fig. 1F), and by 60 hpi the fluorescence in the midgut epithelial cells disappeared while the fluorescence in the epithelium of the tracheae attached to the midgut increased (Fig. 1J).
FIG. 1.
Observation of GFP fluorescence in selected tissues of T. ni infected with AcMNPV-GFP at several time points. Tissues were dissected out of larvae at 24 (A, B, C, and D), 36 (E, F, G, and H), or 60 (I, J, K, and L) hpi and photographed with an Olympus BX50 fluorescence microscope sporting a fluoresein cube. The 1-cm bar in panel L represents 24 μm for panels B, C, D, F, G, I, and J and 48 μm for panels A, E, H, K, and L. The following representative cell types are indicated: tracheae (t), a midgut epithelial cell (m), a fat body cell (f), and an epidermal cell (e).
Beginning at 24 hpi, we observed isolated pockets of fluorescence within the fat body. This fluorescence was limited to cells associated with the tracheae invading the fat body (Fig. 1C). The proportion of these localized areas of fluorescent tracheae within the fat body increased through 36 hpi (Fig. 1G), and by 60 hpi, the fluorescence had spread to the entire fat body tissue (Fig. 1K), indicating massive infection by the virus.
The earliest appearance of fluorescence observed within the epidermis was again associated with the tracheae supplying the epidermal tissue at 24 hpi (Fig. 1D). However, by 36 hpi, the epidermal cells became strongly fluorescent (Fig. 1H), indicating virus infection, viral DNA replication, and very late gene expression within epidermal tissues, and reached peak levels by 60 hpi (Fig. 1L).
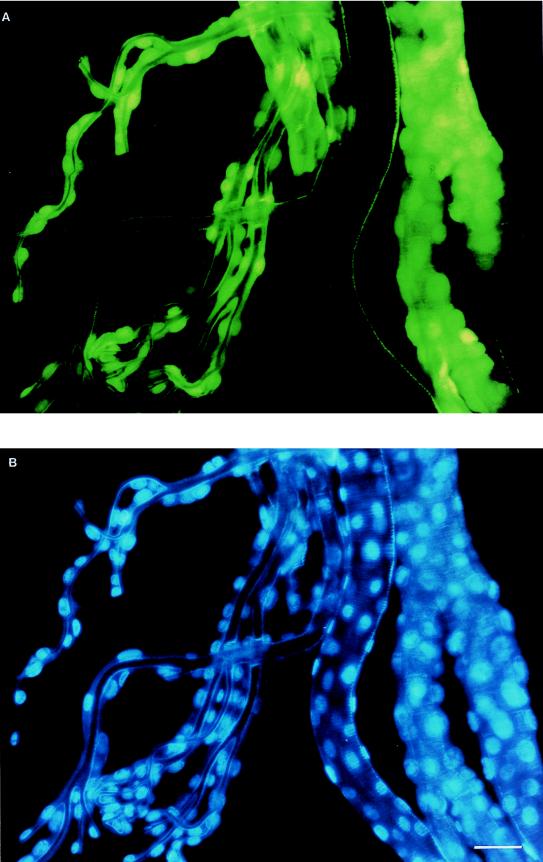
In numerous situations, we observed that fluorescence was confined to the tracheal trunks, with no visible fluorescence in the tracheoles. To determine whether these fluorescent regions were tracheal epithelial cells or, perhaps, hemocytes adhering to tracheal trunks, we stained the tracheal tissue with a nucleus-specific stain, DAPI (4,6-diamidine-2′-phenylindole dihydrochloride [Boehringer Mannheim]). We found that DAPI stained the epithelial cells of the tracheae (Fig. 2B), and when the fluorescing cells were observed under the GFP-specific fluorescence filter, it was clear that they were tracheal epithelial cells (Fig. 2A). As well, we observed that fluorescence in the tracheal tissue was initially localized and not continuous. This observation suggested that infection occurs at specific points and spreads from these locations. These foci of infection are likely tracheoblast cells already identified as possible sites of secondary infection (4).
FIG. 2.
(A) Tracheae collected 60 hpi from a T. ni larva infected with AcMNPV-GFP and observed under an Olympus BX50 fluorescence microscope with a fluoresein cube. (B) Same sample as that shown above but stained with DAPI and observed with an Olympus BX50 fluorescence microscope utilizing a DAPI cube. The 1-cm bar in panel B represents 48 μm for both panels.
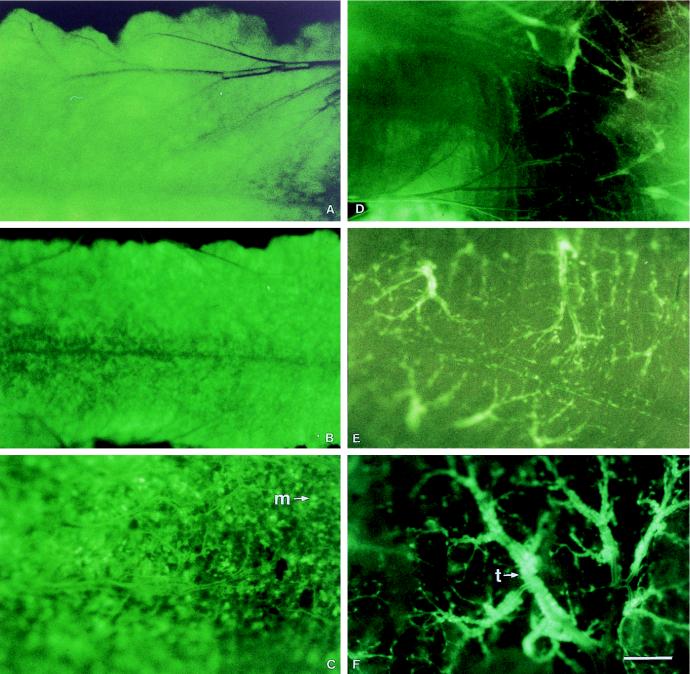
The appearance of GFP fluorescence within the midgut was the result of infection of both the midgut epithelium and the tracheae supplying the midgut. To study this phenomenon, we examined the midguts of T. ni larvae every 4 hpi until fluorescence was first observed and then every 6 h thereafter. Pictures taken at selected time points are shown in Fig. 3. No fluorescence was observed at 4 or 8 hpi; however, beginning at approximately 12 hpi (Fig. 3A), GFP fluorescence was observed within the cells of the midgut but at that time there was no evidence of infection, as expressed by fluorescence, in the tracheae attached to the midgut. The level and intensity of fluorescence in the midgut epithelium increased until approximately 36 hpi (Fig. 3B and C), after which the intensity of fluorescence decreased. By 48 hpi, very low levels of fluorescence were seen in midgut epithelial cells but the first signs of fluorescence in the tracheae attached to the midgut could be seen (Fig. 3D). The number of fluorescent tracheae progressively increased over time until it reached a maximum by 60 to 72 hpi (Fig. 3E and F).
FIG. 3.
Time course of fluorescence within the midguts of AcMNPV-GFP-infected T. ni larvae. Midguts were dissected at 12 (A), 24 (B), 36 (C), 48 (D), 60 (E), and 72 (F) hpi and photographed with a Leica MZ12 dissecting microscope with a fluorescence module and a Leica MPS60 camera with Kodak Ektachrome 160T slide film. The 1-cm bar in panel F represents 100 μm for panels A, B, and C and 88 μm for panels D, E, and F. A representative midgut epithelial cell (m) and tracheae (t) are indicated.
We found that at around 40 hpi, the fluorescence within the midgut cells was no longer observed. We attribute this loss to the sloughing of infected midgut cells which has previously been reported (5, 9). By 48 hpi, the midgut looked healthy and normal again. A healthy gut would allow the infected larvae to continue feeding while the virus continued to replicate and increase in numbers in other tissues. By 48 hpi, the tracheae surrounding the midgut began to fluoresce intensely. The fluorescence, however, was restricted to the tracheal elements and not to the midgut cells. Electron microscopic examination indicated that the midgut cells no longer contained any virus particles, but the nuclei of the tracheal epithelium were heavily infected (data not shown).
To confirm that our observations were not the result of extreme viral doses, we fed T. ni larvae diet plugs containing the equivalent of 0.5, 5.0, 15, or 150 fluorescent cells and repeated our observations at 12-h intervals. At these doses, most of the larvae pupated by 84 hpi, indicating that these were sublethal doses. The time when the larva was added to the tube containing the diet plug was considered 0 hpi. Larvae given 150 fluorescent cells did not exhibit any fluorescence until 36 hpi, at which time both the hemocytes and the tracheae associated with the midgut epithelial cells were fluorescent, indicating expression of GFP. We found fluorescent hemocytes in larvae fed the lower dose of 15 fluorescent cells at 36 hpi. Five of the six larvae examined at this point contained isolated patches of fluorescent tracheae on the midgut. The remaining one larva exhibited fluorescent hemocytes and fluorescent midgut epithelial cells. No fluorescence was observed in larvae fed on diet plugs containing 0.5 or 5.0 infected cells. Therefore, we conclude that at sublethal doses, the infection process proceeds in a manner similar to that illustrated with higher doses. However, the time of appearance of fluorescence was delayed in both the midgut and the hemocytes and it was dose dependent.
Based on our study of AcMNPV-GFP infection of T. ni larvae, we propose that the infection occurs in a stepwise fashion. Upon ingestion and release of nucleocapsids, a proportion of the virus enters the nuclei of the columnar cells, where the virus replicates, synthesizing late and very late proteins, resulting in GFP expression. Simultaneously, some of the parental nucleocapsids pass directly through the midgut via the plasma membrane reticular system (PMRS) and directly infect hemocytes, which then undergo replication and expression of the late and very late viral genes, resulting in production of GFP. The PMRS is an infolding of the basal membrane of the cells of several tissues, including the midgut, and contains many open channels (13, 14). Electron microscopy observations showed the presence of virus within the midgut PMRS (data not shown). These events occur within the first 12 h after the larvae are placed on a surface-treated diet. As an extension of this process, infected midgut cells are sloughed off and eliminated from the system. This clearing of the midgut infection results in a loss of fluorescence, indicating the return of the midgut to a healthy stage. Passage of AcMNPV through the midgut within 0.5 hpi and simultaneous infection of T. ni midgut cells and hemocytes, as well as detection of occlusion bodies within hemocytes as early as 16 hpi, have been reported (8). More recently, it has been suggested that AcMNPV infection is restricted to infection and replication in the midgut as the primary site of infection (5, 9, 18).
The appearance of fluorescent tracheae indicates the second step of the infection process. It is reasonable to infer that the infected hemocytes, circulating in the hemolymph, are releasing budded virus, as shown by Granados and Lawler (8). These virus particles infect the tracheal system at numerous locations within the body, and the infection proceeds from these foci of infection. Our results illustrate the usefulness of GFP as a marker in studies of viral pathogenesis in lepidopteran larvae and confirm the utilization by the virus of the tracheal system to gain access to the tissues involved in secondary infection. We found that, with the exception of the midgut cells and hemocytes, the fluorescence always appears first in the tracheae that infuse a particular tissue and that fluorescence appears later in cells surrounding tracheal attachment within that tissue. We suggest that the virus can penetrate and infect specific areas of the tracheal cells that have a different, thinner, or weaker layer of protection. These susceptible cells are probably the tracheoblasts. According to Engelhard et al. (4), AcMNPV can circumvent the basal lamina because tracheoblasts penetrate the basal lamina and have direct contact with the midgut epithelial cells. Infection would then spread from these tracheoblasts, from one cell to another, possibly in a manner described earlier (16). As the infection spreads along the tracheae from the various foci of infection, the virus gains access to other regions, such as the epidermis and the fat body. This stepwise infection would result in the infection appearing at different but distinct times postingestion of the inoculum in the various tissues. Based on the sequential appearance of GFP in different tissues, this is precisely what we observed. Initially, GFP appears in the midgut and hemocytes (12 hpi), followed by fluorescence in the tracheae (24 hpi), after which the fluorescence reaches the epidermis (36 hpi) and, finally, the fat body (50 hpi).
The baculovirus infection process in insects is relatively poorly understood. Recent works from Volkman’s (4, 9, 10, 18, 19) and Vlak’s (5–7, 11) laboratories have advanced the subject considerably. The current study, employing a very sensitive reporter gene, has provided new information to advance our understanding of the baculovirus infection process further. Simultaneous replication of virus in the midgut and hemocytes and additional evidence for the clearance of the virus from the midgut by 48 h after infection and multiple foci of infection of the tracheal system to gain entrance into the fat body and epidermis tissue are some of the contributions of this study.
Acknowledgments
We thank A. Retnakaran, B. M. Arif, and K. Jamieson for critically reading an earlier version of the manuscript. Thanks also to P. Jakibchuk for preparing the color plates and the Insect Production Unit of CFS-SSM for providing the T. ni larvae.
This research was funded by the National Biotechnology Strategy Fund and the Canadian Forest Service.
REFERENCES
- 1.Ayres M D, Howard S C, Kuzio J, Lopez-Ferber M, Possee R D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- 2.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 3.Chao Y-C, Chen S-L, Li C-F. Pest control by fluorescence. Nature. 1996;380:396–397. [Google Scholar]
- 4.Engelhard E K, Kam-Morgan L N W, Washburn J O, Volkman L E. The insect tracheal system: a conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc Natl Acad Sci USA. 1994;91:3224–3227. doi: 10.1073/pnas.91.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flipsen J T M, van Lent J W M, Goldbach R W, Vlak J M. Expression of polyhedrin and p10 in the midgut of AcMNPV-infected Spodoptera exigua larvae: an immunoelectron microscopic investigation. J Invertebr Pathol. 1993;61:17–23. [Google Scholar]
- 6.Flipsen J T M, Mans R M W, Kleefsman A W F, Knebel-Mörsdorf D, Vlak J M. Deletion of the baculovirus ecdysteroid UDP-glucosyltransferase gene induces early degeneration of Malpighian tubules in infected insects. J Virol. 1995;69:4529–4532. doi: 10.1128/jvi.69.7.4529-4532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flipsen J T M, Martens J W M, Van Oers M M, Vlak J M, van Lent J W M. Passage of Autographa californica nuclear polyhedrosis virus through the midgut epithelium of Spodoptera exigua larvae. Virology. 1995;208:328–335. doi: 10.1006/viro.1995.1156. [DOI] [PubMed] [Google Scholar]
- 8.Granados R R, Lawler K A. In vivo pathway of Autographa californica baculovirus invasion and infection. Virology. 1981;108:297–308. doi: 10.1016/0042-6822(81)90438-4. [DOI] [PubMed] [Google Scholar]
- 9.Keddie B A, Aponte G W, Volkman L E. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science. 1989;243:1728–1730. doi: 10.1126/science.2648574. [DOI] [PubMed] [Google Scholar]
- 10.Kirkpatrick B A, Washburn J O, Engelhard E K, Volkman L E. Primary infection of insect tracheae by Autographa californica M nuclear polyhedrosis virus. Virology. 1994;203:184–186. doi: 10.1006/viro.1994.1472. [DOI] [PubMed] [Google Scholar]
- 11.Knebel-Mörsdorf D, Flipsen J T M, Roncarati R, Jahnal F, Kleefsman A W F, Vlak J M. Baculovirus infection of Spodoptera exigua larvae: lacZ expression driven by promoters of early genes pe38 and me53 in larval tissue. J Gen Virol. 1996;77:815–824. doi: 10.1099/0022-1317-77-5-815. [DOI] [PubMed] [Google Scholar]
- 12.Kool M, Ahrens C H, Vlak J M, Rohrmann G F. Replication of baculovirus DNA. J Gen Virol. 1995;76:2103–2118. doi: 10.1099/0022-1317-76-9-2103. [DOI] [PubMed] [Google Scholar]
- 13.Locke M. A structural analysis of postembryonic development. In: Kerkut G A, Gilbert L I, editors. Comprehensive insect physiology, biochemistry and pharmacology. 2. Postembryonic development. Toronto, Ontario, Canada: Pergamon Press; 1985. pp. 87–149. [Google Scholar]
- 14.Locke M, Huie P. A function for plasma membrane reticular systems. Tissue Cell. 1983;15:885–902. doi: 10.1016/0040-8166(83)90056-3. [DOI] [PubMed] [Google Scholar]
- 15.Riddiford L M, Curtis A T, Kiguchi K. Culture of the epidermis of the tobacco hornworm, Manduca sexta. Tissue Cult Assoc Man. 1979;5:975–985. [Google Scholar]
- 16.Ritter K S, Tanada Y, Hess R T, Omi E M. Eclipse period of baculovirus infection in larvae of the armyworm, Pseudaletia unipuncta. J Invertebr Pathol. 1982;39:203–209. [Google Scholar]
- 17.Vaughn J L, Goodwin R H, Tompkins G J, McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera:Noctuidae) In Vitro. 1977;13:213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- 18.Volkman L E. Baculovirus bounty. Science. 1995;269:1834. doi: 10.1126/science.7569919. [DOI] [PubMed] [Google Scholar]
- 19.Washburn J O, Kirkpatrick B A, Volkman L E. Comparative pathogenesis of Autographa californica M nuclear polyhedrosis virus in larvae of Trichoplusia ni and Heliothis virescens. Virology. 1995;209:561–568. doi: 10.1006/viro.1995.1288. [DOI] [PubMed] [Google Scholar]
- 20.Wilson L E, Wilkinson N, Marlow S A, Possee R D, King L A. Identification of recombinant baculoviruses using green fluorescent protein as a selectable marker. BioTechniques. 1997;22:674–681. doi: 10.2144/97224st02. [DOI] [PubMed] [Google Scholar]
- 21.Yeh E, Gustafson K, Boulianne G L. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci USA. 1995;92:7036–7040. doi: 10.1073/pnas.92.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]