Abstract
Background: Because depression is a major factor contributing to the global disease burden, we tried to analyze the effects and safety of Ginkgo biloba (GKB) on patients with depression.
Methods: We conducted a literature search for articles published between January 2002 and May 2022 in seven online databases (PubMed, Scopus, Embase, Google Scholar, Web of Sciences, Cochrane Library, and China National Knowledge Infrastructure). A systematic literature review and meta-analysis were performed to compare the effects and safety of GKB on patients with depression, including subjective and objective indicators of depression evaluation.
Results: In total, 21 eligible articles with nine indicators among 2074 patients were included. Several outcomes showed a difference, and the GKB group had better results than the control group, including the Hamilton Depression Scale (HAMD), after taking GKB for 4 weeks (MD = −2.86, 95%CI [−4.27, −1.46], p < 0.01), 6 weeks (mean difference (MD) = −3.36, 95%CI [−4.05, −2.67], p < 0.01), and 8 weeks (MD = −4.58, 95% CI [−6.11, −3.05], p < 0.01), modified Barthel index (MBI) (MD = 14.86, 95%CI [12.07, 17.64], p < 0.01), modified Edinburgh-Scandinavian stroke scale (MESSS) (MD = −4.57, 95%CI [−6.34, −2.79], p < 0.01), brain-derived neurotrophic factor (BDNF) (MD = 16.35, 95%CI [7.34, 25.36], p < 0.01), 5-hydroxytryptamine (5-HT) (MD = 4.57, 95%CI [3.08, 6.05], p < 0.01), and clinical efficacy (risk ratio, RR = 1.24, 95%CI [1.17, 1.32], p < 0.01). However, there were no differences in adverse events between GKB and controls.
Conclusion: In conclusion, the main finding was that patients treated with GKB had better MBI, MESSS, BDNF, 5-HT, and HAMD values after 4 weeks, 6 weeks, and 8 weeks than the control group. GKB might reduce the risk of depression or depressive symptoms with safe clinical efficacy.
Systematic Review Registration: identifier (INPLASY2023100052)
Keywords: Ginkgo biloba, GKB, depression, depressive symptoms, meta-analysis
Introduction
It is reported that a main factor contributing to the burden of disease throughout the world is mental illness (Liu et al., 2020). The two most incapacitating mental diseases, depression and anxiety disorders, are among the top 25 major causes of global disease burden in 2019, according to Global Burden of Disease (GBD) research (Collaborators, 2021). Of these, depression is a severe mood disorder characterized by a dearth of pleasure, diminished capacity for pleasure, sleepiness or insomnia, psychomotor agitation or retardation, exhaustion, feelings of worthlessness or guilt, trouble concentrating, and recurrent thoughts of suicide or death (Yuehua Li, 2006; Wang, 2010). According to WHO, depression is the presence of persistent sadness and the loss of interest in activities that one normally enjoys, accompanied by an inability to carry out daily activities for at least 2 weeks. It is different from the usual mood fluctuations or temporary sadness in response to challenges in everyday life (Collaborators, 2021).
After the COVID-19 pandemic, respiratory-transmitted illnesses continue to generate concern about the implications on mental health due to their immediate psychological effects and long-term economic and societal repercussions (Collaborators, 2021). Globally, economic activity is only slowly recovering, unemployment rates are rising, employment rates for young people—especially college students—are falling, and there are still widespread epidemics of respiratory diseases (Yueyi Kan and Rao, 2022). All factors have the potential to have a negative impact on people’s mental health. We must continue to identify effective ways to lessen the negative effects of the final phase of the COVID-19 pandemic on mental health, and we still need up-to-date data on the incidence and burden of mental illnesses across the world (Zhang et al., 2022).
There is evidence that ginkgo has favorable benefits in older persons with Alzheimer’s disease, multi-infarct dementia, and moderate cognitive impairment (Woelk et al., 2007; Dou, 2013). Ginkgo is used to treat cerebral insufficiency and intermittent claudication. Ginkgo biloba extract EGb761 has been utilized extensively in the treatment of central nervous system conditions such as age-related cognitive decline and dementia, vestibular and non-vestibular vertigo, tinnitus, and peripheral artery occlusive disease (Fournier et al., 2010; Yeung et al., 2018). Because EGb 761 is able to modify blood rheology to enhance blood flow as well as to promote neuroprotection and modulate neurotransmission, the mechanism of action behind these therapeutic benefits is complex (Kasper, 2015). Additionally, it has been discovered that people with mental impairment who take EGb 761 have less anxiety. Consequently, it is of significant clinical relevance to evaluate the efficacy and safety of GKB on mental health.
There is still some debate about evaluating the use of ginkgo for depression treatment because there is not enough research on the herb, and not all reports agree that it works well. In order to undertake a systematic analysis with high confidence, the current study attempted to incorporate all trials on ginkgo for depression conducted globally over a 20-year period. The main focus is to evaluate if GKB is effective and safe for depression, and we assume GKB is effective in ameliorating depression.
Methods
Research setup
Based on the PICO framework, we selected people with depression as patients. The intervention was GKB, and it was compared with placebo or traditional antipsychotics. The outcome included the Hamilton Depression Scale (HAMD), the modified Barthel index (MBI), the modified Edinburgh-Scandinavian stroke scale (MESSS), brain-derived neurotrophic factor (BDNF), 5-hydroxytryptamine (5-HT), and adverse events.
Literature search strategy
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram and checklist (Page et al., 2021), which were registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY2023100052), served as the foundation for this study. To find all randomized controlled trials evaluating GKB for depression, we searched seven internet databases (PubMed, Embase, Scopus, Web of Sciences, Google Scholar, Cochrane Library, and China National Knowledge Infrastructure) for pertinent articles. After a thorough screening, 21 publications, released without language restriction between January 2002 and May 2023, were included.
In the Boolean phrase ((Ginkgo biloba) OR (G. biloba) OR GKB) AND ((depression) OR (depressive symptoms)), the medical topic terms and keywords utilized for the search were “Ginkgo biloba,” “G. biloba,” “GKB,” “depression,” and “Depressive Symptoms.” The references’ related articles were also investigated. Studies qualified if they included the following data: observational studies reporting clinical effectiveness, adverse events, and random control trials reporting depression assessments. Evaluation of GKB’s impact on depression was the main goal.
Two authors (Jingya Lin Xiaojing Sun) extracted the data using a standardized data form after screening the titles, abstracts, and full texts of the publications identified by our search and assessing the risk of bias. Any discrepancies were discussed with a third author (Lingli Yang).
Inclusion and exclusion criteria
After the initial selection of the studies, the pertinent texts were examined, and the studies needed to fulfill the following criteria:
(1) Studies that compared GKB and a control for the treatment of depression;
(2) The design of research should be randomized control trials;
(3) Containing indicators evaluating the efficacy and safety of GKB on depression;
(4) Available in full text.
The following predetermined exclusion criteria were used to disqualify studies from consideration:
(1) Research on other drugs;
(2) Other topics about depression;
(3) Study lacking available data;
(4) Review, abstract, or duplicate publication.
Data extraction and quality assessment
Two writers (Jingya Lin Xiaojing Sun) extracted the following information from each included study: the name of the first author, publication year, author’s country, groups based on intervention (GKB and control), research design, gender, average subject age, and research cycle. The outcome criteria for depression were GKB’s effectiveness and safety.
Using the ROB 2.0 scale (a revised Cochrane risk-of-bias instrument for randomized trials), two independent reviewers (Xiaojing Sun and Lingli Yang) assessed the caliber of the included studies.
Statistical analysis
We assessed heterogeneity using the Cochrane Q-statistic and Higgins and Thompson’s I2. Depending on its value, I2 was used to classify heterogeneity as low, moderate, or high: 25%, 50%, or 75%. When I2 was less than 50%, the fixed-effect model was used for the meta-analyses. If I2 was higher than 50%, the random-effect model was used in all other cases. For effect sizes of continuous outcomes, the mean difference (MD) with 95% CI (confidential interval) was precisely recalculated. Risk ratios (RRs) were computed for categorical outcomes. Our statistical analyses were conducted using R software version 4.2.1 (R Core Team, Vienna, Austria) and the R package meta (version 6.2.0). Statistical significance was defined as a p-value of 0.05 or less.
Publication bias was assessed for the Hamilton Depression Scale (HAMD) as the primary outcome using Egger’s regression test and a funnel plot. We ran a sensitivity analysis, deleting one included article at a time, to assess the robustness of the final results.
Results
Search process
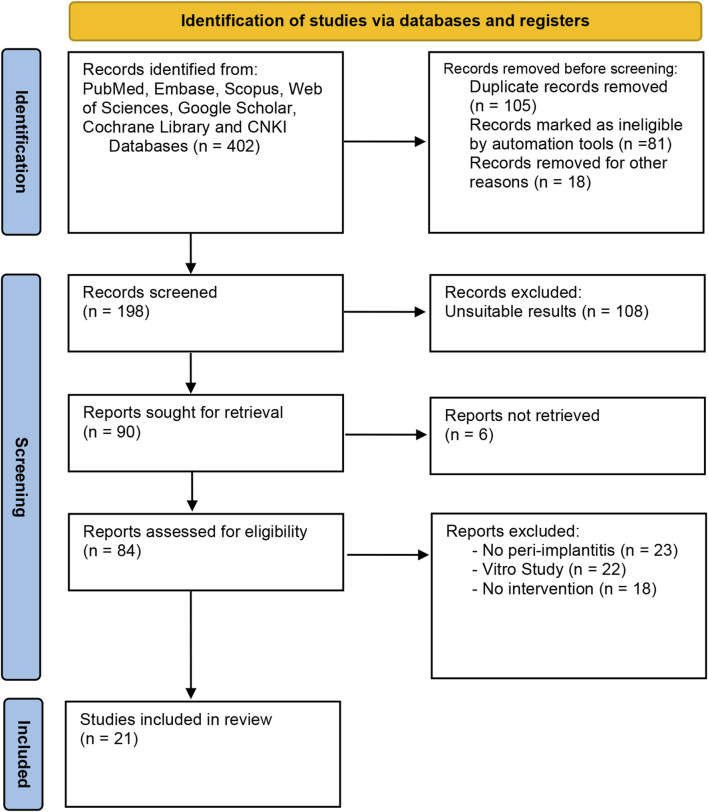
The systematic search of seven online databases identified 402 articles based on the PRISMA flow diagram and inclusion/exclusion criteria. Twenty-one publications (Lin, 2002; Fengqing Yu and Liu, 2004; Hartley et al., 2004; Kefeng Guo and Yan, 2006; Wei Chen, 2006; He, 2007; Binhua Chen and Su, 2011; Jia Yuan and Gan, 2011; Xiangdong Luo and Zhou, 2011; Xiangdong Luo and Zhou, 2012; Zhang, 2012; Gavrilova et al., 2014; Hao Song and Chen, 2014; Haixia Wu and Hao, 2016; Shichao and Chen, 2017; Dai et al., 2018; Ru Liu and Zhang, 2018; Junfeng Yin, 2019; Liang et al., 2019; Liu, 2020; Aijun Zhang and Yu, 2021) with 2074 patients were qualified for this meta-analysis after duplicate studies were excluded and eligible studies were screened [12–24]. The research selection flowchart is depicted in Figure 1, and the characteristics of the studies are listed in Table 1. The risk-of-bias evaluation for every included study is displayed in Supplementary Figures S1, S2. In addition, we list the chemical component or manufacturer of the GKB in Table 2 so we can make the evaluation of GKB clear.
FIGURE 1.
PRISMA diagram of data selection.
TABLE 1.
Main characteristics of the included studies.
| Study | Study | Year | Language | Country | Group | Intervention details | No. of patients (male/female) | Age range (mean) | n | Study design | Study date range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Binhua Chen | Chen | 2011 | Chinese | China | Intervention with GKB | Ginkgo biloba extract (40 mg/capsule)+Citalopram (20 mg/capsule) (1.36 ± 0.45 g/day) | 18/14 | 65.36 ± 5.27 | 32 | RCT | January 2008 through June 2010 |
| Control | Citalopram (20 mg/capsule) | 16/14 | 63.53 ± 6.17 | 30 | |||||||
| Wei Chen | Chen | 2006 | Chinese | China | Intervention with GKB | Ginkgo leaf extract + Prozac | - | - | 46 | RCT | March 2002 through November 2004 |
| Control | Prozac | - | - | 32 | |||||||
| Chunxiao Dai | Dai | 2018 | English | China | Intervention with GKB | Ginkgo biloba tablets + citalopram | 33/35 | 66.48 ± 4.12 | 68 | RCT | March 2015 through March 2017 |
| Control | Citalopram | 31/37 | 66.82 ± 3.35 | 68 | |||||||
| Gavrilova | Gavrilova | 2014 | English | Russia | Intervention with GKB | Ginkgo biloba extract | 22/58 | 65 ± 7 | 80 | RCT | - |
| Control | Placebo | 13/66 | 63 ± 7 | 79 | |||||||
| Kefeng Guo | Guo | 2006 | Chinese | China | Intervention with GKB | Ginkgo biloba extract (19.2 mg/capsule) + paroxetine (20 mg/capsule) (1.36 ± 0.45 g/day) | 17/23 | - | 40 | RCT | January 2003 through April 2004 |
| Control | Paroxetine (20 mg/capsule) | 16/22 | - | 38 | |||||||
| Hartley | Hartley | 2004 | English | United Kingdom | Intervention with GKB | Gincosan (120 mg Ginkgo biloba) | - | 58.4 ± 1.0 | 30 | RCT | - |
| Control | Placebo | - | 57.4 ± 0.7 | 27 | |||||||
| Jianzhong He | He | 2007 | Chinese | China | Intervention with GKB | Ginkgo leaf extract | 22/23 | 67.1 ± 2.5 | 45 | RCT | - |
| Control | Placebo | 24/21 | 68.1 ± 23 | 45 | |||||||
| Shichao Li | Li | 2017 | Chinese | China | Intervention with GKB | Ginkgo leaf extract | 30/20 | 53.3 ± 1.8 | 50 | RCT | January 2015 through January 2016 |
| Control | Placebo | 29/21 | 52.1 ± 1.6 | 50 | |||||||
| Zihong Liang | Liang | 2019 | English | China | Intervention with GKB | Ginkgo biloba extract + venlafaxine | 22/18 | 60.86 ± 8.63 | 40 | RCT | - |
| Control | Venlafaxine | 19/54 | 60.52 ± 8.68 | 40 | |||||||
| Chuan Lin | Lin | 2022 | Chinese | China | Intervention with GKB | Ginkgo biloba tablets + nimodipine | 26/16 | 57.2 ± 2.2 | 42 | RCT | November 19 through April 2001 |
| Controls | Nimodipine | 19/17 | 58.1 ± 3.2 | 36 | |||||||
| Ru Liu | Liu | 2018 | Chinese | China | Intervention with GKB | Ginkgo biloba tablets + butylphthalide | 27/32 | 68.2 ± 5.6 | 59 | RCT | March 2016 through August 2017 |
| Control | Butylphthalide | 28/31 | 68 ± 5.5 | 59 | |||||||
| Yajuan Liu | Liu | 2020 | Chinese | China | Intervention with GKB | Ginkgo biloba tablets + metformin | 27/24 | 63.4 ± 13.72 | 51 | RCT | January 2018 and through January 2019 |
| Control | Metformin | 28/23 | 64.11 ± 1.38 | 51 | |||||||
| Xiangdong Luo | Luo | 2011 | Chinese | China | Intervention with GKB | Ginkgo biloba extract (80 mg/capsule) + paroxetine (20 mg/capsule) (1.36 ± 0.45 g/day) | 22/25 | 69.37 ± 2.24 | 47 | RCT | February 2008 through February 2011 |
| Control | Paroxetine (20 mg/capsule) | 19/24 | 67.45 ± 4.19 | 43 | |||||||
| Xiangdong Luo | Luo | 2012 | Chinese | China | Intervention with GKB | Ginkgo biloba extract (80 mg/capsule) + paroxetine (20 mg/capsule) (1.36 ± 0.45 g/day) | 22/25 | 69.37 ± 2.24 | 47 | RCT | February 2008 through February 2011 |
| Control | Paroxetine (20 mg/capsule) | 19/24 | 67.45 ± 4.19 | 43 | |||||||
| Hao Song | Song | 2014 | Chinese | China | Intervention with GKB | Ginkgo leaf extract | 29/28 | 51.95 ± 9.04 | 57 | RCT | November 2010 through November 2013 |
| Control | Placebo | 26/22 | 52.08 ± 8.18 | 48 | |||||||
| Haixia Wu | Wu | 2016 | Chinese | China | Intervention with GKB | Ginkgo leaf extract (20 mg/capsule) + fluoxetine (20 mg/capsule) | 34/31 | 64.3 ± 3.4 | 65 | RCT | May 2014 through March 2015 |
| Control | Fluoxetine (20 mg/capsule) | 37/28 | 64.1 ± 3 | 65 | |||||||
| Junfeng Yin | Yin | 2019 | Chinese | China | Intervention with GKB | Ginkgo biloba tablets + sertraline | 27/23 | 68.93 ± 9.24 | 50 | RCT | February 2016 through February 2018 |
| Control | Sertraline | 26/24 | 67.29 ± 8.42 | 50 | |||||||
| Fengqing Yu | Yu | 2004 | Chinese | China | Intervention with GKB | Ginkgo leaf extract | 14/12 | 62 ± 5 | 26 | RCT | May 2001 through May 2003 |
| Control | Placebo | 10/11 | 60 ± 7 | 21 | |||||||
| Jia Yuan | Yuan | 2011 | Chinese | China | Intervention with GKB | Ginkgo leaf extract | 56/40 | - | 96 | RCT | February 2007 and May 2010 |
| Control | Placebo | 50/44 | - | 94 | |||||||
| Yujun Zhang | Zhang | 2012 | Chinese | China | Intervention with GKB | Ginkgo leaf extract | 23/29 | 67 ± 4 | 52 | RCT | January 2007 through June 2011 |
| Control | Placebo | 25/27 | 68 ± 5 | 52 | |||||||
| Aijun Zhang | Zhang | 2021 | Chinese | China | Intervention with GKB | Ginkgo biloba extract (80 mg/capsule) + paroxetine (20 mg/capsule) (1.36 ± 0.45 g/day) | 22/18 | 67.54 ± 7.85 | 40 | RCT | March 2018 through January 2019 |
| Control | Paroxetine (20 mg/capsule) | 24/16 | 65.26 ± 6.34 | 40 |
TABLE 2.
Chemical component of the included studies.
| Study | Year | Country | GKB chemical component |
|---|---|---|---|
| Chen | 2011 | China | There is no chemical component, but we report the manufacturer: EGb Tablets (produced by Zhejiang Kangenbei Pharmaceutical Co., Ltd.) |
| Chen | 2006 | China | Not available |
| Dai | 2018 | China | There is no chemical component, but we report the manufacturer: EGb Tablets (Harbin HaoBo Pharmaceutical Co., Ltd.). |
| Gavrilova | 2014 | Russia | EGb 761 is a dry extract from G. biloba leaves (35–67:1); extraction solvent: acetone 60% (w/w). The extract is adjusted to 22.0%–27.0% ginkgo flavonoids calculated as ginkgo flavone glycosides and 5.0%–7.0% terpene lactones consisting of 2.8%–3.4% ginkgolides A, B, and C and 2.6%–3.2% bilobalide and contains less than 5 ppm ginkgolic acids |
| Guo | 2006 | China | There is no chemical component, but we report the manufacturer: Ginkgo Leaf (produced by Guizhou Xinbang Pharmaceutical Co., Ltd., Chinese medicine: Z20028023) |
| Hartley | 2004 | United Kingdom | Gincosan capsules (Pharmaton SA, Switzerland). These capsules contain the standard ginkgo extract GK501 (Pharmaton SA, Switzerland) and the ginseng extract G115 (Pharmaton SA, Switzerland) |
| He | 2007 | China | Not available |
| Li | 2017 | China | There is no chemical component, but we report the manufacturer: Ginkgo biloba (approval number: national medicine standard H14023515, Shanxi Pude Pharmaceutical Co., Ltd.) |
| Liang | 2019 | China | Not available |
| Lin | 2022 | China | There is no chemical component, but we report the manufacturer: GBE761 produced by German Willmar Schwabe Pharmaceutical Co., Ltd. |
| Liu | 2018 | China | There is no chemical component, but we report the manufacturer: Ginkgo biloba capsule (Chinese medicine: Z20040100) |
| Liu | 2020 | China | There is no chemical component, but we report the manufacturer: Ginkgo folic acid capsule (Beijing Maidihai Pharmaceutical Co., Ltd., Chinese medicine: H11020317) |
| Luo | 2011 | China | There is no chemical component, but we report the manufacturer: Ginkgo biloba leaves (trade name: Danakang, produced by Bofu-Ipson Company) |
| Luo | 2012 | China | There is no chemical component, but we report the manufacturer: Ginkgo biloba leaves (trade name: Danakang, produced by Bofu-Ipson Company) |
| Song | 2014 | China | There is no chemical component, but we report the manufacturer: Ginkgo biloba capsule (Hunan Hansen Pharmaceutical Co., Ltd., batch number: 20100819) |
| Wu | 2016 | China | There is no chemical component, but we report the manufacturer: Ginkgo biloba extract (Chinese medicine Z20055358) |
| Yin | 2019 | China | Not available |
| Yu | 2004 | China | There is no chemical component, but we report the manufacturer: Ginkgo biloba leaves produced by Shanghai Xingling Pharmaceutical Factory |
| Yuan | 2011 | China | Not available |
| Zhang | 2012 | China | There is no chemical component, but we report the manufacturer: Ginkgo biloba (produced by Shenzhou Pharmaceutical Industry) |
| Zhang | 2021 | China | Ginkgo honey ring oral liquid (national medicine standard: H20013079, produced by Qionglai Tianyin Pharmaceutical Co., Ltd.) |
Based on the ROB 2.0, it was determined that the overall quality of the included studies was sufficient in terms of selection bias, comparability quality, and outcome quality.
Characteristics of included studies
Of all the studies, 10 had more than 100 patients, while 11 had less than 100. The duration of the study varied from 1 to 4 years. All the research designs used in the included articles were randomized control trials (RCTs). The median ages of the patients studied in the included articles ranged from 51.95 to 69.37. There were nearly equal numbers of men and women (952 men and 1,020 women). The specific traits of the included articles are displayed in Table 1.
Results of quality assessment
The Supplementary Figures S1, S2 show the ROB 2.0 summary and quality assessment details, respectively. More than 50% of the articles indicated a low risk of bias, while less than 20% exhibited a high risk. Only three articles had a high overall bias risk, six had a medium bias risk, and twelve had a low bias risk.
Results of the heterogeneity test
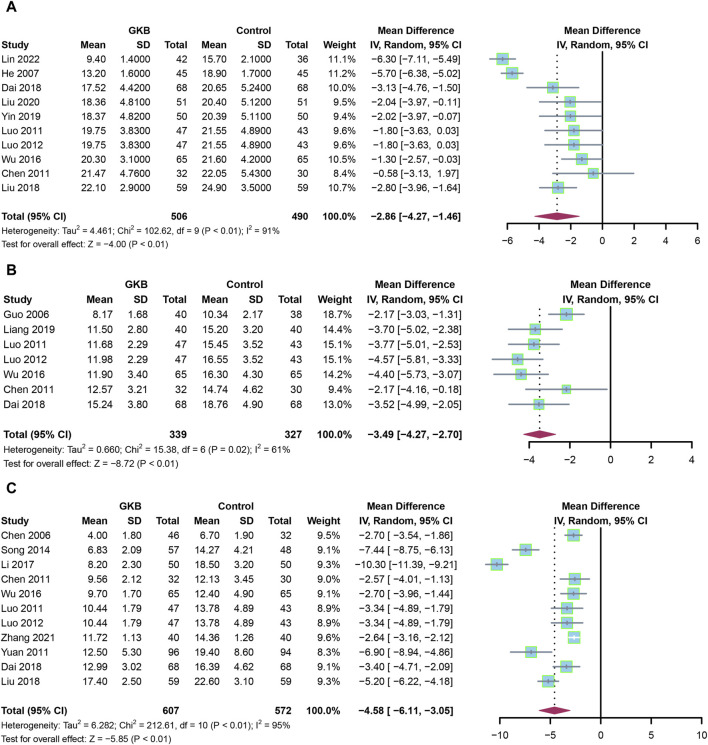
First, we evaluated the HAMD scale after giving GKB for 4 weeks, 6 weeks, and 8 weeks. Pooled estimates from 10 studies showed that patients receiving GKB had substantially lower 4-week HAMD scores than the control group (MD = −2.86, 95%CI [−4.27, −1.46], p < 0.01, I2 = 91%) (Figure 2A). The 6-week HAMD score was documented in seven studies. The results showed that patients receiving GKB also had lower 6-week HAMD scores than the control group (MD = −3.36, 95%CI [−4.05, −2.67], p < 0.01, I2 = 50%) (Figure 2B). After taking GKB for 8 weeks, the patients receiving GKB had lower HAMD scores than the control group, according to a meta-analysis of 11 publications (MD = −4.58, 95% CI [−6.11, −3.05], p < 0.01, I2 = 95%) (Figure 2C).
FIGURE 2.
HAMD scales of GKB on patients with depression after taking GKB: (A) HAMD scale after taking GKB for 4 weeks. (B) HAMD scale after taking GKB for 6 weeks. (C) HAMD scale after taking GKB for 8 weeks. Abbreviations: SD: standard deviation; 95% CI: 95% confidence interval; Chi2: chi-squared test; Tau2: tau-squared; I2: I-squared; P: probability.
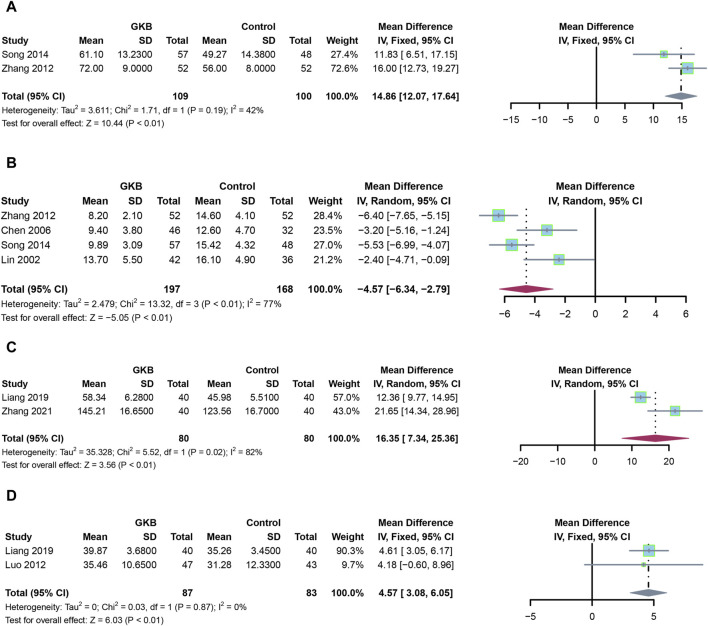
In addition to GKB therapy, we further investigate the serum indices and other depression scales. Patients receiving GKB had a higher MBI than those in the control group (Figure 3A, MD = 14.86, 95%CI [12.07, 17.64], p < 0.01, I2 = 42%). Meanwhile, patients receiving GKB had lower MESSS values than those in the control groups, according to a pooled analysis (Figure 3B, MD = −4.57, 95%CI [−6.34, −2.79], p < 0.01, I2 = 77%). Serum levels of BDNF (MD = 16.35, 95%CI [7.34, 25.36], p < 0.01, I2 = 82%, Figure 3D), and 5-HT revealed that patients receiving GKB had greater values than those in the control groups (MD = 4.57, 95%CI [3.08, 6.05], p < 0.01, I2 = 0%, Figure 3C).
FIGURE 3.
Effects of GKB on patients with depression, including other scales and serum indexes: (A) MBI, (B) MESSS, (C) 5-HT, and (D) BDNF. Abbreviations: SD: standard deviation; 95% CI: 95% confidence interval; Chi2: chi-squared test; Tau2: tau-squared; I2: I-squared; P: probability.
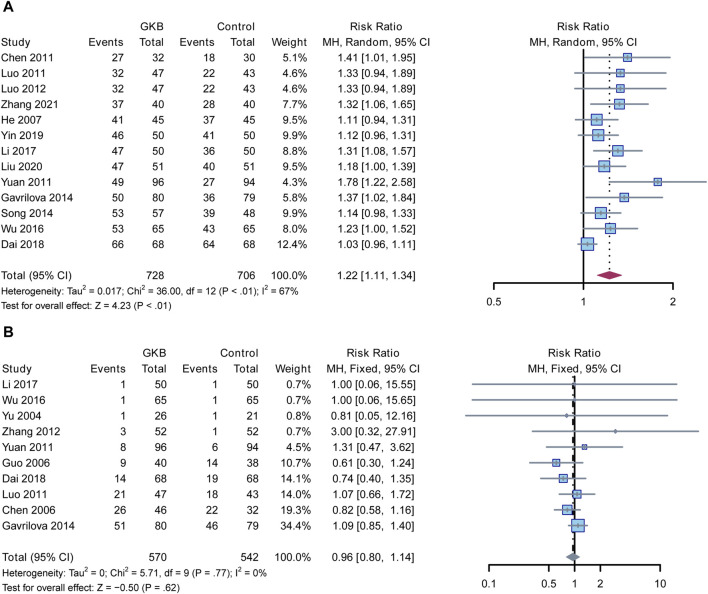
Finally, we assessed the clinical effectiveness and adverse events in the GKB and control groups. In a meta-analysis of clinical efficacy, the GKD groups outperformed the control groups in terms of clinical efficacy (RR = 1.22, 95%CI [1.11, 1.34], p < 0.01, I2 = 67%) (Figure 4A), whereas there was no difference between the GKB and the control group in terms of adverse events (RR = 0.96, 95%CI [0.8, 1.14], p = 0.62, I2 = 0%) (Figure 4B).
FIGURE 4.
linical efficacy and adverse events reported about the effects of GKB on patients with depression: (A) Clinical efficacy and (B) Adverse events. Abbreviations: 95% CI: 95% confidence interval; Chi2: chi-squared test; Tau2: tau-squared; I2: I-squared; P: probability.
Results of sensitivity analysis and publication bias
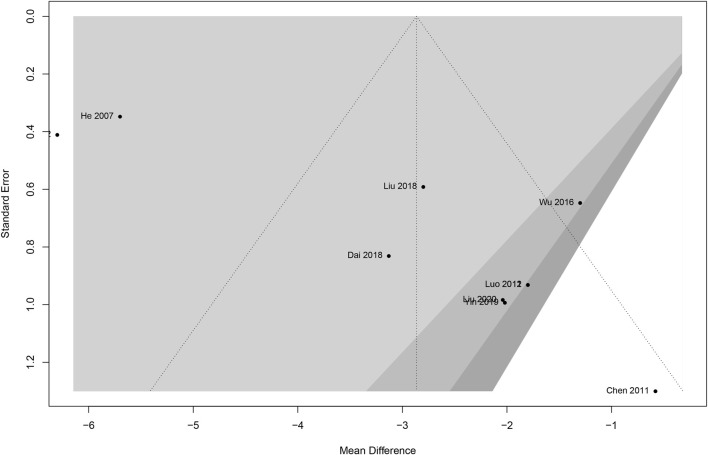
Begg funnel plots for the meta-analysis of adverse events were created to examine the publication bias. The funnel plots (Figure 5) demonstrated visual symmetry and revealed a low level of publication bias in this study. The Egger regression test for adverse events further indicated there was no publication bias (z = 0.23; p = 0.19).
FIGURE 5.
Funnel plot for potential publication bias.
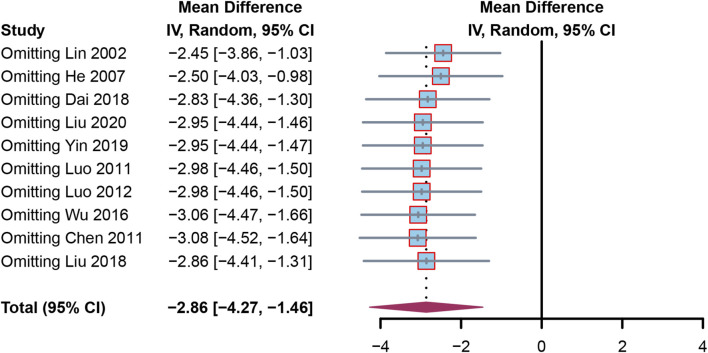
Sensitivity analysis was done to verify that the results were reliable. We removed each article in turn from the sensitivity forest plot (Figure 6). Each included article in the adverse events meta-analysis exhibited a comparable RR in terms of robustness, with Lin 2002 having the greatest RR at −2.45 [−3.86, −1.03] and Chen 2011 having the lowest RR at −3.08, 95%CI [−4.52, −1.64]. These findings suggested that the conclusions were sound.
FIGURE 6.
Sensitivity analysis by omitting each article in turn.
Discussion
The HAMD is a scale developed by Max Hamilton in 1960 to assess the symptoms of patients diagnosed with depressive states (Hamilton, 1960). Although the Patient Health Questionnaire-9 (PHQ-9) scale has been used more often in recent years to assess depressive states, the HAMD is still an accepted scale for the assessment of depression (Xiaonan Zhang, 2014; Zheng et al., 2020; Zhang et al., 2023). Using HAMD as an assessment outcome, the Ginkgo biloba extract group had lower HAMD values than the control group at 4 weeks, 6 weeks, and 8 weeks after taking the medication. The efficacy of adding Ginkgo biloba extract to the traditional treatment regimen for depression may be better than the traditional treatment regimen. In the results of other scales about stroke in patients with depression, the GKB group had a higher MBI than the control group. Also, the GKB group had lower MESSS scores than the control group. In addition to this, we also performed an analysis of serum biomarkers, including 5-HT and BDNF, and the results showed that the GKB group had higher levels of 5-HT and BDNF than the control groups. Again, these findings supported that the GKB groups had better CNS co-functional activity than patients treated with traditional depression medicine.
5-HT is a key excitatory neurotransmitter in the central nervous system that is found throughout the brain (Tao et al., 2023; Wu et al., 2023; Jinyi et al., 2024). Previous research has shown that 5-HT abnormalities are connected to the etiology of depression (Jaster et al., 2022; Jastrzebska et al., 2023). BDNF and 5-HT have a strong association in the typical human brain. 5-HT promotes BDNF production, and BDNF improves 5-HT signaling (Popova et al., 2022; Marcinkowska et al., 2023). However, this link is disturbed when the brain is ischemic and hypoxic, which may result in decreased production of BDNF and 5-HT (Kumar et al., 2018; Yadav et al., 2019; Kumar et al., 2020; Panigrahi et al., 2021; Mishra et al., 2022).
BDNF is broadly distributed throughout the brain, including the cerebral cortex and hippocampus (Kumar and Singh, 2016; Kumar and Singh, 2018; Kumar et al., 2022). It influences the release of neurotransmitters and trophic factors and encourages the differentiation and regeneration of injured neurons (Singh et al., 2023; Vyas et al., 2023). According to earlier research, BNDF is essential for preserving and fostering nerve fiber regeneration in monkey stroke models. Previous research revealed reduced serum BDNF levels in depressed people (Gabryelska et al., 2022; Gliwinska et al., 2023). According to a comprehensive study, antidepressant therapy may help depressed individuals’ serum BDNF levels rise (Kumar and Singh, 2015; Kumar and Sarveshanand, 2017; Kumar, 2021). Earlier research reported that BDNF plays a crucial role in the antidepressant effects of venlafaxine (Brammanathan et al., 2023).
Finally, the GKB group was also superior to the control group in terms of overall clinical efficacy. In the safety analysis results, no differences could be seen between the two groups. Regarding the comparative results in terms of adverse events, consistent with previous studies, there were no differences between the GKB and the control groups in terms of adverse events when treating depression and anxiety (Zhang et al., 2022). The clinical efficacy and safety of GKB on depression was consistent with previous research (Sarris et al., 2011).
We list various potential causes for the heterogeneity in some of our results. First, the variability in GKB dose may have an impact. Second, the different study sites of the collected publications unquestionably add to the variability. Third, there is a chance that personnel and research measurement variations will contribute to heterogeneity. Overall, this study suggested that GKB could lower the risk of depression or depressed symptoms based on a sizable sample and several indications. GKB was safe for patients with depression at the same time.
The current study had certain limitations. First, it was impossible to have a larger sample for each indication due to the variety of indicators reported in the included research. Second, comprehensive results for numerous populations were lacking because trials of GKB for patients with depression were not found in the United States or Europe. Last but not least, future indicators should contain more contemporary measurements like PHQ-9 and other biomarkers.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
JL: writing–original draft, methodology, formal analysis, and data curation. XS: writing–original draft, validation, and methodology. LY: writing–review and editing, validation, supervision, formal analysis, and conceptualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1364030/full#supplementary-material
Abbreviations
GKB, Ginkgo biloba; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; INPLASY, International Platform of Registered Systematic Review and Meta-analysis Protocols; MD, mean difference; ORs, odds ratio; 95%CI, 95% confidence interval; BDNF, brain-derived neurotrophic factor; 5-HT, 5-hydroxytryptamine; HADS, Hospital anxiety depression Scale; MESSS, modified Edinburgh-Scandinavian Stroke Scale; PHQ-9, Patient Health Questionnaire-9; RCTs, randomized controlled trials; ROB 2.0, version 2 of the Cochrane risk-of-bias tool for randomized trials; HAMD, Hamilton Depression Scale.
References
- Aijun Zhang F., Yu K. (2021). Effect of ginkgo honey ring oral liquid on colonization resistance of Gut microbiota and serum 5-HT in patients with depression after PCI of coronary heart disease. J. Integr. Traditional Chin. West. Med. Cardiovasc. Cerebrovasc. Dis. 19 (12), 2118–2120. 10.12102/j.issn.1672-1349.2021.12.039 [DOI] [Google Scholar]
- Binhua Chen R. Q., Su X. (2011). Contrast study of folium ginkgo preparation and citalopram combined treatment in vascular depression. J. Zhejiang Univ. Traditional Chin. Med. 35 (4), 513–515. [Google Scholar]
- Brammanathan S., Jain R., Sarkar S., Raghav R., Sagar R. (2023). Serum BDNF levels among patients with alcohol dependence, depression and alcohol dependence with comorbid depression - a comparative study. J. Psychoact. Drugs, 1–9. 10.1080/02791072.2023.2192985 [DOI] [PubMed] [Google Scholar]
- Collaborators C.-M. D. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398 (10312), 1700–1712. 10.1016/S0140-6736(21)02143-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C. X., Hu C. C., Shang Y. S., Xie J. (2018). Role of Ginkgo biloba extract as an adjunctive treatment of elderly patients with depression and on the expression of serum S100B. Med. Baltim. 97 (39), e12421. 10.1097/MD.0000000000012421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou L. (2013). The application of cecropram combined with ginkgo biloba extract in elderly coronary heart disease patients with depression. China Pract. Med. 8 (26), 41–42. 10.3969/j.issn.1673-7555.2013.26.025 [DOI] [Google Scholar]
- Fengqing Yu A. W., Liu X. (2004). Clinical observation on ginkgo biloba leaf combined with prozac in the treatment of poststroke depression. Chin. Tradit. Pat. Med. 266 (9), 23–24. 10.3969/j.issn.1001-1528.2004.09.045 [DOI] [Google Scholar]
- Fournier J. C., DeRubeis R. J., Hollon S. D., Dimidjian S., Amsterdam J. D., Shelton R. C., et al. (2010). Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303 (1), 47–53. 10.1001/jama.2009.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryelska A., Turkiewicz S., Ditmer M., Karuga F. F., Strzelecki D., Bialasiewicz P., et al. (2022). BDNF and proBDNF serum protein levels in obstructive sleep apnea patients and their involvement in insomnia and depression symptoms. J. Clin. Med. 11 (23), 7135. 10.3390/jcm11237135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova S. I., Preuss U. W., Wong J. W., Hoerr R., Kaschel R., Bachinskaya N., et al. (2014). Efficacy and safety of Ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo-controlled, double-blind, multi-center trial. Int. J. Geriatr. Psychiatry 29 (10), 1087–1095. 10.1002/gps.4103 [DOI] [PubMed] [Google Scholar]
- Gliwinska A., Czubilinska-Lada J., Wieckiewicz G., Swietochowska E., Badenski A., Dworak M., et al. (2023). The role of brain-derived neurotrophic factor (BDNF) in diagnosis and treatment of epilepsy, depression, schizophrenia, anorexia nervosa and Alzheimer's disease as highly drug-resistant diseases: a narrative review. Brain Sci. 13 (2), 163. 10.3390/brainsci13020163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haixia Wu M. G., Hao F. (2016). Evaluation of therapeutic effect of ginkgo biloba extract combined with Fluoxetine on depression after acute cerebral infarction. J. Int. PSYCHIATRY 43 (3), 474–476. [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23 (1), 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Song J. M., Chen Y. (2014). Analysis of the therapeutic effect of jieyu pill combined with ginkgo biloba capsule on post-stroke depression. Chin. J. Traditional Med. Sci. Technol. 21 (6), 671–672. [Google Scholar]
- Hartley D. E., Elsabagh S., File S. E. (2004). Gincosan (a combination of Ginkgo biloba and Panax ginseng): the effects on mood and cognition of 6 and 12 weeks' treatment in post-menopausal women. Nutr. Neurosci. 7 (5-6), 325–333. 10.1080/10284150400015557 [DOI] [PubMed] [Google Scholar]
- He Z. Y. (2007). Observation on the therapeutic effect of psychological intervention mode combined with ginkgo biloba leaves on post-stroke depression. J. Med. reseach 36 (8), 130. [Google Scholar]
- Jaster A. M., Elder H., Marsh S. A., de la Fuente Revenga M., Negus S. S., Gonzalez-Maeso J. (2022). Effects of the 5-HT(2A) receptor antagonist volinanserin on head-twitch response and intracranial self-stimulation depression induced by different structural classes of psychedelics in rodents. Psychopharmacol. Berl. 239 (6), 1665–1677. 10.1007/s00213-022-06092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebska J., Frankowska M., Smaga I., Hubalewska-Mazgaj M., Suder A., Pieniazek R., et al. (2023). Evaluation of the 5-HT(2C) receptor drugs RO 60-0175, WAY 161503 and mirtazepine in a preclinical model of comorbidity of depression and cocaine addiction. Pharmacol. Rep. 75 (1), 99–118. 10.1007/s43440-022-00428-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Yuan L. Z., Gan L. (2011). Clinical study of Ginkgo biloba combined with interpersonal psychological intervention in the treatment of coronary heart disease with depression. Chin. J. New Drugs Clin. Pract. 30 (12), 920–924. [Google Scholar]
- Jinyi W., Zhang Y., Wang K., Peng P. (2024). Global, regional, and national mortality of tuberculosis attributable to alcohol and tobacco from 1990 to 2019: a modelling study based on the Global Burden of Disease study 2019. J. Glob. health 14, 04023. 10.7189/jogh.14.04023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junfeng Yin Z. Z. (2019). Efficacy of butylphthalide combined with ginkgo biloba capsule in the treatment of Parkinson's disease with depression and Sleep disorder. Clin. reserach 27 (2), 108–109. [Google Scholar]
- Kasper S. (2015). Phytopharmaceutical treatment of anxiety, depression, and dementia in the elderly: evidence from randomized, controlled clinical trials. Wien Med. Wochenschr 165 (11-12), 217–228. 10.1007/s10354-015-0360-y [DOI] [PubMed] [Google Scholar]
- Kefeng Guo S. G., Yan K. (2006). Clinical observation of ginkgo biloba leaves combined with Paroxetine in the treatment of depression. Chin. J. Clin. Rehabilitation 10 (2), 43–45. 10.3321/j.issn:1673-8225.2006.02.012 [DOI] [Google Scholar]
- Kumar D. (2021). Radiation effect on magnetohydrodynamic flow with induced magnetic field and Newtonian heating/cooling: an analytic approach. Propuls. Power Res. 10 (3), 303–313. 10.1016/j.jppr.2021.07.001 [DOI] [Google Scholar]
- Kumar D., Sarveshanand A. (2017). Effect of Hall current and wall conductance on hydromagnetic natural convective flow between vertical walls. Int. J. Industrial Math. 36, 289–299. [Google Scholar]
- Kumar D., Singh A. K. (2015). Effect of induced magnetic field on natural convection with Newtonian heating/cooling in vertical concentric annuli. Procedia Eng. 127, 568–574. 10.1016/j.proeng.2015.11.346 [DOI] [Google Scholar]
- Kumar D., Singh A. K. (2016). Effects of heat source/sink and induced magnetic field on natural convective flow in vertical concentric annuli. Alexandria Eng. J. 55 (4), 3125–3133. 10.1016/j.aej.2016.08.019 [DOI] [Google Scholar]
- Kumar D., Singh A. K. (2018). Effect of Newtonian heating/cooling on hydromagnetic free convection in alternate conducting vertical concentric annuli. Applications of fluid dynamics (Singapore: Springer Singapore; ). [Google Scholar]
- Kumar D., Singh A. K., Bhattacharyya K., Banerjee A. (2022). Effects of Hall current on MHD natural convection in between two vertical flat walls with induced magnetic field and heat source/sink. Int. J. Ambient Energy 43 (1), 4075–4088. 10.1080/01430750.2021.1874516 [DOI] [Google Scholar]
- Kumar D., Singh A. K., Kumar D. (2018). Effect of Hall current on the magnetohydrodynamic free convective flow between vertical walls with induced magnetic field. Eur. Phys. J. Plus 133, 207–210. 10.1140/epjp/i2018-12012-4 [DOI] [Google Scholar]
- Kumar D., Singh A. K., Kumar D. (2020). Influence of heat source/sink on MHD flow between vertical alternate conducting walls with Hall effect. Phys. A Stat. Mech. its Appl. 544, 123562. 10.1016/j.physa.2019.123562 [DOI] [Google Scholar]
- Liang Z. H., Jia Y. B., Wang M. L., Li Z. R., Li M., Yun Y. L., et al. (2019). Efficacy of ginkgo biloba extract as augmentation of venlafaxine in treating post-stroke depression. Neuropsychiatr. Dis. Treat. 15, 2551–2557. 10.2147/NDT.S215191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. (2002). Effects of Ginkgo biloba extract on depression and neurological function after acute cerebral infarction. WEST CHINA Med. J. 17 (1). 10.3969/j.issn.1002-0179.2002.01.075 [DOI] [Google Scholar]
- Liu Q., He H., Yang J., Feng X., Zhao F., Lyu J. (2020). Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J. Psychiatr. Res. 126, 134–140. 10.1016/j.jpsychires.2019.08.002 [DOI] [PubMed] [Google Scholar]
- Liu Y. (2020). To explore the clinical efficacy of butylphthalide combined with ginkgo folic acid capsule in the treatment of Parkinson's disease with depression and Sleep disorder. Psychol. Mon. 15 (14), 85. [Google Scholar]
- Marcinkowska M., Mordyl B., Siwek A., Gluch-Lutwin M., Karcz T., Gawalska A., et al. (2023). Dual molecules targeting 5-HT(6) and GABA-A receptors as a new approach to combat depression associated with neuroinflammation. ACS Chem. Neurosci. 14 (8), 1474–1489. 10.1021/acschemneuro.3c00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Panda J. P., Kumar D., Sahoo S. S. (2022). Thermal radiation and Soret effects on boundary layer flow past a vertical surface embedded in porous medium with induced magnetic field with reference to aluminum industry. J. Therm. Analysis Calorim. 147 (23), 13829–13845. 10.1007/s10973-022-11644-6 [DOI] [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. Engl. Ed. 74 (9), 790–799. 10.1016/j.rec.2021.07.010 [DOI] [PubMed] [Google Scholar]
- Panigrahi L., Kumar D., Panda J. P. (2021). Impact of chemical reaction, Hall current, and radiation on MHD flow between vertical walls. J. Eng. Thermophys. 30 (1), 122–144. 10.1134/s1810232821010100 [DOI] [Google Scholar]
- Popova N. K., Tsybko A. S., Naumenko V. S. (2022). The implication of 5-HT receptor family members in aggression, depression and suicide: similarity and difference. Int. J. Mol. Sci. 23 (15), 8814. 10.3390/ijms23158814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru Liu H. W., Zhang S. (2018). Efficacy evaluation of butylphthalide combined with ginkgo biloba capsule in the treatment of Parkinson's disease with depression and Sleep disorder. J. Int. PSYCHIATRY 45 (3), 522–524. [Google Scholar]
- Sarris J., Panossian A., Schweitzer I., Stough C., Scholey A. (2011). Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 21 (12), 841–860. 10.1016/j.euroneuro.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Shichao J. Y., Chen H. (2017). Clinical efficacy of Ginkgo biloba and Damo injection as adjunctive therapy for depression disorder. Chin. J. Pract. Nerv. Dis. 20 (8). [Google Scholar]
- Singh S., Fereshetyan K., Shorter S., Paliokha R., Dremencov E., Yenkoyan K., et al. (2023). Brain-derived neurotrophic factor (BDNF) in perinatal depression: side show or pivotal factor? Drug Discov. Today 28 (2), 103467. 10.1016/j.drudis.2022.103467 [DOI] [PubMed] [Google Scholar]
- Tao Q., Mu L., Wu J., Wang X. (2023). Investigating the factors affecting the competence of a traditional Chinese medicine practitioner using structural equation model. Ann. Transl. Med. 11 (6), 256. 10.21037/atm-23-888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas C. M., Mischoulon D., Chang G., Reynolds C. F., 3rd, Cook N. R., Weinberg A., et al. (2023). Relation of serum BDNF to major depression and exploration of mechanistic roles of serum BDNF in a study of vitamin D3 and omega-3 supplements for late-life depression prevention. J. Psychiatr. Res. 163, 357–364. 10.1016/j.jpsychires.2023.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. (2010). Disease burden of depressive disorders in Beijing. Cap. J. Public Health 12 (1), 34–48. [Google Scholar]
- Wei Chen M. W. (2006). Clinical observation of Fluoxetine hydrochloride combined with ginkgo biloba leaves in the treatment of post-stroke depression. Mod. J. Integr. Traditional Chin. West. Med. 15 (2). 10.3969/j.issn.1008-8849.2006.02.034 [DOI] [Google Scholar]
- Woelk H., Arnoldt K. H., Kieser M., Hoerr R. (2007). Ginkgo biloba special extract EGb 761 in generalized anxiety disorder and adjustment disorder with anxious mood: a randomized, double-blind, placebo-controlled trial. J. Psychiatr. Res. 41 (6), 472–480. 10.1016/j.jpsychires.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Wu J., Wang K., Tao F., Li Q., Luo X., Xia F. (2023). The association of blood metals with latent tuberculosis infection among adults and adolescents. Front. Nutr. 10, 1259902. 10.3389/fnut.2023.1259902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiangdong Luo J. W., Zhou Bo. (2011). The efficacy of Paroxetine combined with ginkgo biloba leaves in the treatment of vascular depression. Med. J. West China 23 (9), 1644–1646. 10.3969/j.issn.1672-3511.2011.09.009 [DOI] [Google Scholar]
- Xiangdong Luo J. W., Zhou Bo. (2012). Effect of Paroxetine combined with ginkgo biloba leaves on serum BDNF in patients with vascular depression. Sichuan Med. J. 33 (1), 4–6. 10.3969/j.issn.1004-0501.2012.01.002 [DOI] [Google Scholar]
- Xiaonan Zhang S. H. (2014). Anti depression effect of ginkgo. LIAONING J. TRADITIONAL Chin. Med. 41 (9), 2023–2026. [Google Scholar]
- Yadav S. L., Kumar D., Singh A. K. (2019). Magnetohydrodynamic flow in horizontal concentric cylinders. Int. J. Industrial Math. 11, 89–98. [Google Scholar]
- Yeung K. S., Hernandez M., Mao J. J., Haviland I., Gubili J. (2018). Herbal medicine for depression and anxiety: a systematic review with assessment of potential psycho-oncologic relevance. Phytother. Res. 32 (5), 865–891. 10.1002/ptr.6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuehua Li F. Z. (2006). Current status and future research objectives of depression research. Chin. J. Inf. Traditional Chin. Med. 13 (10), 1–3. [Google Scholar]
- Yueyi Kan M. Z., Rao M. (2022). Research progress on antidepressant effects of Ginkgo biloba and its active ingredients. J. psychiatry 35 (3), 245–249. [Google Scholar]
- Zhang W., Yan Y., Wu Y., Yang H., Zhu P., Yan F., et al. (2022). Medicinal herbs for the treatment of anxiety: a systematic review and network meta-analysis. Pharmacol. Res. 179, 106204. 10.1016/j.phrs.2022.106204 [DOI] [PubMed] [Google Scholar]
- Zhang Y. (2012). Clinical study of ginkgo biloba and Eleutherococcus senticosus injection in the treatment of depression after cerebral infarction. SHANXI Med. J. 41 (4), 396–397. 10.3969/j.issn.0253-9926.2012.04.051 [DOI] [Google Scholar]
- Zhang Y., Wang K., Zhu J., Wu J. (2023). A network suspected infectious disease model for the development of syphilis transmission from 2015 to 2021 in Hubei province, China. J. Appl. Microbiol. 134 (12), lxad311. 10.1093/jambio/lxad311 [DOI] [PubMed] [Google Scholar]
- Zheng X., Shi J., Wu J. (2020). Analysis of factors and corresponding interactions influencing clinical management assistant ability using competency model in China. Medicine 99 (51), e23516. 10.1097/MD.0000000000023516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.