Abstract
Purpose:
We explored the efficacy of PARP inhibition with or without programmed death ligand-1 (PD-L1) blockade as chemotherapy-free maintenance therapy for advanced triple-negative breast cancer (aTNBC) sensitive to platinum-based chemotherapy.
Patients and Methods:
In the phase II non-comparative DORA trial (NCT03167619), patients with ongoing stable disease (SD) or complete/partial response (CR/PR) to first- or second-line platinum-based chemotherapy for TNBC (≤10% estrogen/progesterone receptor expression) were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1,500 mg on day 1 every 4 weeks. The primary objective was to compare progression-free survival (PFS) versus a historical control of continued platinum-based therapy.
Results:
45 patients were randomized (23 to olaparib alone, 22 to the combination; 3 with estrogen/progesterone receptor expression 1%–10%). At 9.8 months’ median follow-up, median PFS from randomization was 4.0 [95% confidence interval (CI), 2.6–6.1] months with olaparib and 6.1 (95% CI, 3.7–10.1) months with the combination, both significantly longer than the historical control (P = 0.0023 and P < 0.0001, respectively). Clinical benefit rates (SD ≥24 weeks or CR/PR) were 44% (95% CI, 23%–66%) and 36% (95% CI, 17%–59%) in the monotherapy and combination arms, respectively. Sustained clinical benefit was seen irrespective of germline BRCA mutation or PD-L1 status, but tended to be associated with CR/PR to prior platinum, particularly in the olaparib-alone arm. No new safety signals were reported.
Conclusions:
PFS was longer than expected with both regimens. A patient subset with wild-type BRCA platinum-sensitive aTNBC had durable disease control with chemotherapy-free maintenance.
Translational Relevance.
PARP inhibition is an established maintenance strategy in some tumor types but evidence is limited in advanced triple-negative breast cancer (TNBC). The non-comparative randomized phase II DORA trial evaluated olaparib with or without the PD-L1 inhibitor durvalumab as a chemotherapy-free maintenance regimen. Sustained clinical benefit was seen irrespective of germline BRCA mutation or PD-L1 status but tended to be associated with response to prior platinum, particularly in the olaparib-alone arm. Maintenance PARP inhibition showed sustained disease control in a subset of patients with neither germline nor somatic BRCA mutations. These data provide new information on the role of maintenance therapy for advanced TNBC, offering the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens.
Introduction
First-line standard therapy for advanced triple-negative breast cancer (aTNBC) generally comprises a backbone of taxane- or platinum-based chemotherapy. In the past 10 years, targeted treatment options for aTNBC have become standard of care in biomarker-selected populations following demonstration of significantly improved progression-free survival (PFS) and, in some cases, overall survival (OS) with the addition of immune checkpoint inhibitors (atezolizumab or pembrolizumab) to first-line chemotherapy in patients with programmed death ligand-1 (PD-L1)–positive aTNBC (1–3) and with PARP inhibitors (olaparib and talazoparib) instead of chemotherapy in patients with HER2-negative tumors associated with germline BRCA (gBRCA) pathogenic variants (4–7). Furthermore, the BROCADE3 trial demonstrated significant benefit from the addition of veliparib to platinum-based chemotherapy, with continuation of single-agent maintenance PARP inhibition if chemotherapy was discontinued (8). However, only approximately 40%–45% of patients presenting with aTNBC have PD-L1–positive tumors (1, 2), and approximately 11% have tumors harboring gBRCA mutations (9). For patients without these molecular markers, there remains a need for more effective treatment strategies.
Continuous chemotherapy via traditional systemic delivery or newer antibody–drug conjugates with targeted payload delivery is recommended in aTNBC, but toxicities are challenging for patients from a tolerability standpoint. Chemotherapy-free maintenance strategies are attractive if they can provide adequate disease control and superior quality of life. Experience in ovarian cancer, where PARP inhibitors are an established maintenance therapy after response to platinum-based chemotherapy, has demonstrated that a broader population of patients beyond those whose tumors harbor gBRCA mutations may benefit from maintenance PARP inhibition (10–14). Although benefit from treatment appears greatest in patients with gBRCA mutations, patients without gBRCA mutations also derive benefit. Homologous recombination deficiency (HRD), frequently caused by loss-of-function mutations in the BRCA genes, is a defect in the mechanism used to repair double-stranded DNA breaks. HRD is characterized by sensitivity to PARP inhibitors and/or platinum salts. A substantial proportion of sporadic TNBC tumors without gBRCA mutations have HRD (15), which may play a role in predicting sensitivity to DNA-damaging agents and PARP inhibitors in early-stage TNBC (16–19). However, the clinical utility of testing for HRD is unclear in aTNBC (20, 21) and technical challenges, genomic scarring, and reversion mutations highlight the limitations of many existing HRD testing approaches. In BRCA-like TNBC, the addition of veliparib to cisplatin significantly improved PFS in the randomized phase II S1416 trial but no significant benefit was seen in the cohort with non-BRCA-like TNBC (22).
We hypothesized that sensitivity to previous platinum-containing therapy may help to identify a subgroup of patients with aTNBC likely to benefit from maintenance PARP inhibition. Furthermore, we hypothesized that dual blockade of PARP and PD-L1 may be synergistic in aTNBC. PARP inhibitors can enhance the immune response via activation of genes in the cyclic GMP–AMP synthase–stimulator of IFN pathway and upregulation of immune checkpoints (23). In the clinical setting, the combination of olaparib and the anti–PD-L1 agent durvalumab demonstrated activity in gBRCA-mutated metastatic breast cancer (mBC) in the MEDIOLA study, without overlapping toxicities (24). In addition, combining olaparib and durvalumab with standard neoadjuvant weekly paclitaxel increased the pathologic response rate compared with paclitaxel alone in HER2-negative and triple-negative early breast cancers in the I-SPY2 trial (25).
The DORA trial was designed to evaluate a chemotherapy-free maintenance regimen of olaparib, with or without durvalumab, in patients with aTNBC showing ongoing clinical benefit from platinum therapy.
Patients and Methods
Study design, patient population, randomization, and treatment
DORA (NCT03167619) was a non-comparative multicenter randomized phase II trial conducted at five sites in the Republic of Korea, the US, and Singapore.
Eligible patients had histologically confirmed triple-negative or estrogen/progesterone receptor-low (≤10% tumor cells positive) disease that was inoperable locally advanced or metastatic and not amenable to curative resection. Before randomization on the DORA trial, patients had to have ongoing investigator-determined stable disease (SD) or complete/partial response (CR/PR) after at least three 3- or 6-weekly cycles (including bi-weekly or days 1 and 8 every 21 days) of first- or second-line platinum-based chemotherapy (monotherapy or combination therapy). Additional eligibility criteria included age ≥21 years and adequate hematologic, renal, and hepatic function. Patients previously treated with a PARP inhibitor were ineligible. Following the FDA accelerated approval of atezolizumab in combination with first-line nab-paclitaxel for PD-L1–positive aTNBC, and with inclusion of immunotherapy in clinical trials conducted in early TNBC, the protocol was amended in March 2020 to allow prior immune checkpoint inhibitors in any setting, except if patients had required discontinuation of a PD-1, PD-L1, or CTLA-4 inhibitor because of treatment-related toxicities, or if patients had previously experienced an immune-related grade 3 or 4 adverse event. No washout period or treatment-free interval was specified as it was expected that patients would have received the minimum period of induction platinum-based chemotherapy before randomization in this trial.
The aim of randomization was to reduce bias due to patient selection in either treatment arm. The trial was not designed to determine the relative efficacy of the two treatment arms or to determine potential differences between them; comparison of the two treatment arms was an exploratory objective. Randomization was stratified by site and treatment line (first- vs. second-line therapy for aTNBC). Eligible patients were allocated in a 1:1 ratio to receive olaparib maintenance therapy either alone or in combination with durvalumab using stratified permuted block randomization. In both treatment arms, platinum-based chemotherapy was discontinued after randomization, and maintenance therapy was to begin within 4 weeks after the last dose of chemotherapy. Maintenance therapy was administered in 28-day cycles comprising oral olaparib 300-mg tablets twice daily every day in the single-agent arm and the same regimen in combination with intravenous durvalumab 1,500 mg on day 1 every 28 days in the combination arm. Maintenance therapy was continued until objective disease progression according to RECIST version 1.1, providing the investigator still considered the patient to be benefiting from treatment.
Objectives and outcomes
The primary objective was to determine investigator-assessed PFS of the two regimens. PFS was defined as the interval between randomization and first reported disease progression (according to RECIST version 1.1) or death from any cause within 30 days of the last dose of study treatment. Secondary endpoints included OS (defined as the interval between randomization and death from any cause), clinical benefit rate (defined as SD for ≥24 weeks or CR/PR according to RECIST version 1.1), tolerability, and safety. Objective response rate was a predefined secondary endpoint but, in line with published trials of maintenance PARP inhibition (10–12), this endpoint was not considered to be relevant in the maintenance setting given the possible confounding effect of prior platinum response. Exploratory objectives included characterization of the molecular epidemiology of biomarkers in aTNBC through next-generation sequencing (NGS), exploration of the tumor microenvironment, and analysis of epigenetic changes (to be reported separately).
Sample size determination and statistical analysis
Median PFS from the time of randomization (after platinum-based induction therapy) was assumed to be approximately 2 months without maintenance therapy (26). An improvement to 4 months with maintenance therapy would be considered clinically meaningful. To test the null hypothesis of median PFS of 2 months against an alternative hypothesis of median PFS of 4 months with investigational maintenance therapy, assuming exponential PFS distribution and with 90% power at a 2-sided 5% significance level, 25 patients were required in each treatment arm. Allowing for a 20% drop-out rate, the planned sample was 60 patients overall.
Study assessments
Before randomization, tissue samples (archival or fresh) were collected from all patients. Tumors were sequenced using the NGS Tempus xT assay version 4 (Tempus Laboratories, Inc.), which is a custom testing panel consisting of 648 genes with single-nucleotide variants, indels, and translocation measured by hybrid capture NGS. gBRCA mutation testing was not mandatory, but the total number of patients known to be carriers of gBRCA mutations was limited to 10. PD-L1 status was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies), with combined positive score (CPS) ≥10 defined as PD-L1 positive.
Tumors were evaluated according to RECIST version 1.1 at baseline and every 8 weeks thereafter. Safety was assessed on an ongoing basis, with adverse events graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
The trial was conducted in compliance with Good Clinical Practice guidelines (International Conference on Harmonisation E6: Good Clinical Practice or Singapore Guideline for Good Clinical Practice) and applicable national and local regulatory requirements, and in accordance with the ethical principles of the Declaration of Helsinki. The protocol, any amendments, and all patient materials were approved by the Institutional Review Board or Ethics Committee at each participating site before initiation of the trial. All patients provided written informed consent before undergoing any study-specific procedures.
Data availability
Data availability is subject to local rules and regulations. Clinical trial and sequencing data presented in this article are not publicly available because subjects did not provide consent for their data to be made available. Every reasonable effort will, however, be made to promptly satisfy scientifically valid requests. Requests for data should be made to the corresponding author together with a detailed study plan and a commitment not to use the data and their derivatives for commercial purposes. The proposal will require approval by the SingHealth Centralized Institutional Review Board, National Cancer Centre Singapore and the Principal Investigators of the study. Requesting researchers will be required to sign a data access agreement with the relevant parties.
Results
Patient population and treatment exposure
Between February 4, 2019 and December 24, 2020, 45 patients were randomly assigned to maintenance therapy: 23 to olaparib alone and 22 to olaparib plus durvalumab combination therapy. Baseline characteristics and the representativeness of study participants are shown in Table 1 and Supplementary Tables S1 and S2. Eight patients had known gBRCA mutations as a medical history (1 with BRCA2 pathogenic variant in the single-agent arm, 7 with BRCA1 pathogenic variants in the combination arm). Most patients (76%) were Asian and approximately 60% had CR/PR to previous platinum therapy.
Table 1.
Baseline characteristicsa.
| Characteristic | Olaparib alone (n = 23) | Olaparib plus durvalumab (n = 22) |
|---|---|---|
| Median (range) age, years | 48 (35–77) | 51.5 (25–72) |
| Age, years | ||
| ≤65 | 19 (83) | 21 (95) |
| >65 | 4 (17) | 1 (5) |
| Race | ||
| Asian | 16 (70) | 18 (82) |
| White | 5 (22) | 4 (18) |
| Other/missing | 2 (9) | 0 |
| ECOG performance status | ||
| 0 | 16 (70) | 13 (59) |
| 1 | 7 (30) | 8 (36) |
| 2 | 0 | 1 (5) |
| Most recent platinum regimen | ||
| 1st line | 18 (78) | 19 (86) |
| 2nd line | 5 (22) | 3 (14) |
| Median (range) duration of prior platinum, months | 2.9 (1.4–5.9) | 2.7 (1.4–22.3) |
| Best response to prior platinum | ||
| CR/PR | 14 (61) | 13 (59) |
| SD | 9 (39) | 9 (41) |
| Germline BRCA status | ||
| Deleterious mutation | 1 (4)b | 7 (32)c |
| No mutation detected/variant of unknown significance | 13 (57) | 6 (27) |
| Not testedd | 9 (39) | 9 (41) |
| Tumor cells positive for estrogen receptor | ||
| <1% | 21 (91) | 21 (95) |
| ≥1%–≤10% | 2 (9) | 0 |
| Missing | 0 | 1 (5) |
| Tumor cells positive for progesterone receptor | ||
| <1% | 23 (100) | 0 |
| ≥1%–≤10% | 0 | 1 (5) |
| DFI from initial diagnosis to advanced/metastatic TNBC | ||
| De novo | 7 (30) | 4 (18) |
| ≤1 year | 3 (13) | 2 (9) |
| >1 year | 13 (57) | 16 (73) |
| Median (range) interval between metastatic diagnosis and randomization, months | 5.3 (2.7–61.2) | 4.9 (2.5–14.6) |
Note: Data are n (%) unless otherwise specified.
Abbreviations: CR/PR, complete response/partial response; DFI, disease-free interval; ECOG, Eastern Cooperative Oncology Group; SD, stable disease; TNBC, triple-negative breast cancer.
aPlease see the full table in the Supplementary Table S2.
b BRCA2.
cAll BRCA1.
d BRCA testing is less readily available at Asian sites.
At the data cutoff date (June 30, 2021), median follow-up was 9.8 months (range, 2.1–26.1 months; median 13.6 months in the single-agent group and 8.8 months in the combination group). The median number of olaparib cycles was 5 (range, 2–19) in the olaparib-alone arm and 5.5 (range, 1–24) in the combination arm (Table 2). The median number of durvalumab cycles was 4 (range, 1–24). At the data cutoff date, 20% of patients were still on treatment. Among those who had discontinued study treatment permanently, the reason was disease progression in all but 1 patient, in whom combination treatment was discontinued by the investigator for non-compliance with the study procedure before the first tumor assessment.
Table 2.
Treatment exposure.
| Treatment exposure | Olaparib alone (n = 23) | Olaparib plus durvalumab (n = 22) |
|---|---|---|
| Olaparib | ||
| Median (range) of olaparib cycles | 5 (2–19) | 5.5 (1–24) |
| Patients with olaparib dose interruption | 18 (78) | 18 (82) |
| Due to AE | 9 (39) | 10 (45) |
| Lasting ≥3 days | 8 (35) | 9 (41) |
| Lasting ≥14 days | 3 (13) | 1 (5) |
| Patients with olaparib dose reductiona | 4 (17) | 2 (9) |
| Due to AE | 3 (13) | 2 (9) |
| Due to physician decision | 2 (9) | 0 |
| Patients with olaparib permanently discontinued | 20 (87) | 16 (73) |
| Olaparib treatment ongoing | 3 (13) | 6 (27) |
| Durvalumab | ||
| Median (range) of durvalumab cycles | — | 4 (1–24) |
| Patients with ≥1 durvalumab cycle omitted | — | 2 (9) |
| Patients with durvalumab permanently discontinued | — | 16 (73) |
| Durvalumab treatment ongoing | — | 6 (27) |
Note: Data are n (%) unless otherwise specified.
Abbreviation: AE, adverse event.
aMore than one reason possible.
PFS
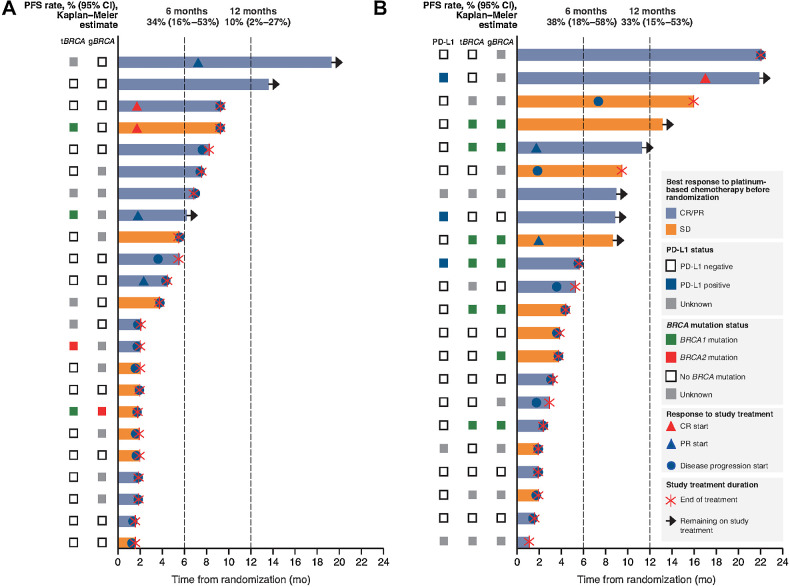
By the data cutoff date, PFS events had been recorded in 20 patients (87%) in the olaparib-alone arm and 15 (68%) in the combination arm. In all patients, the first PFS event was disease progression. In the primary PFS analysis, PFS in both treatment groups was longer than for the historical control. Median PFS was 4.0 months [95% confidence interval (CI), 2.6–6.1] with olaparib alone (P = 0.0023 vs. historical control) and 6.1 months (95% CI, 3.7–10.1) with the combination (P < 0.0001 vs. historical control). Kaplan–Meier estimates of 1-year PFS rates were 10% (95% CI, 2%–27%) with olaparib alone and 33% (95% CI, 15%–53%) with the combination (Fig. 1).
Figure 1.
Treatment exposure and response according to tumor characteristics. A, Olaparib alone. B, Olaparib plus durvalumab. One patient in the olaparib plus durvalumab arm had a germline pathogenic variant in BRCA1 confirmed by the site but reported as a BRCA1 variant of unknown significance and pathogenic PALB2 variant on tumor testing. Each bar represents an individual patient. tBRCA, tumor BRCA.
In subgroup analyses of the primary endpoint, PFS was longer in patients with CR/PR than with SD to prior platinum-based chemotherapy. In the olaparib arm, median PFS was 5.4 (95% CI, 3.0–9.7) months in patients with CR/PR to prior platinum and 2.2 (95% CI, 1.2–4.3) months in patients with SD to prior platinum. Corresponding values in the combination arm were 7.6 (95% CI, 3.8–15.1) months and 4.4 (95% CI, 2.1–9.3) months, respectively. Exploratory analyses in the subgroup of 37 patients treated in the first-line setting showed median PFS of 4.1 (95% CI, 2.5–6.7) months with olaparib and 7.0 (95% CI, 4.0–12.3) months with the combination.
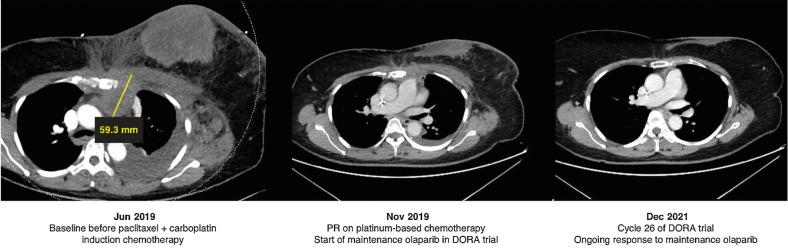
Focusing on BRCA status, in the single-agent olaparib arm, only 1 patient had a known gBRCA mutation, but maintenance PARP inhibition showed sustained disease control in some patients with neither germline nor somatic BRCA mutations (Fig. 1). For example, one 50-year-old female diagnosed with de novo metastatic TNBC (pleura, liver, peritoneal, cutaneous nodules, bone, and lymph nodes) in June 2019, who had wild-type (WT) gBRCA with tumor expressing PD-L1 on 2% of immune cells, achieved a PR for 4 months on weekly paclitaxel and carboplatin. She was randomized to single-agent olaparib in the DORA trial, and still had an ongoing response 2 years later after 26 cycles of maintenance olaparib (Fig. 2).
Figure 2.
Case study: maintenance olaparib in wild-type germline BRCA metastatic triple-negative breast cancer. PR, partial response.
Treatment outcomes according to prior platinum sensitivity, BRCA mutation status, and PD-L1 status are shown at the patient level in Fig. 1. Among patients with durable (>6 months) clinical benefit, 7 of 8 in the olaparib-alone arm had CR/PR to prior platinum, none of the 8 had known gBRCA mutation a priori, and 2 were identified to have tumor BRCA1 mutations. In the olaparib plus durvalumab combination arm, 5 of 9 patients with durable clinical benefit had CR/PR to prior platinum and 4 had SD; 3 of the 9 had known gBRCA mutations (all BRCA1), 2 had PD-L1–positive tumors (both WT BRCA), and 2 did not have sufficient tumor samples for NGS.
Secondary efficacy endpoints
The clinical benefit rate was 44% (95% CI, 23%–66%) with olaparib alone and 36% (95% CI, 17%–59%) with olaparib plus durvalumab. Overall response rate is shown in Supplementary Table S3 together with details of best overall response.
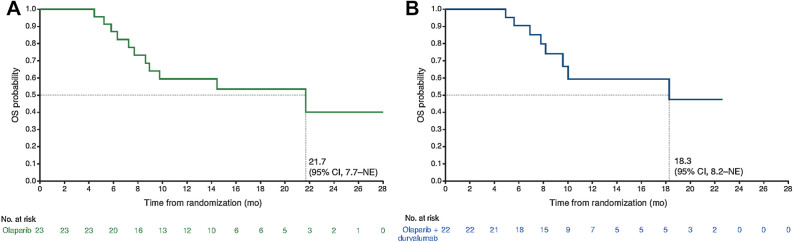
By the data cutoff date, after deaths in 11 patients (48%) in the olaparib arm and 8 (36%) in the combination arm, median OS was 21.7 months with olaparib alone and 18.3 months with the combination regimen (Fig. 3).
Figure 3.
Overall survival (OS). A, Olaparib alone. B, Olaparib plus durvalumab. NE, not estimable.
Safety
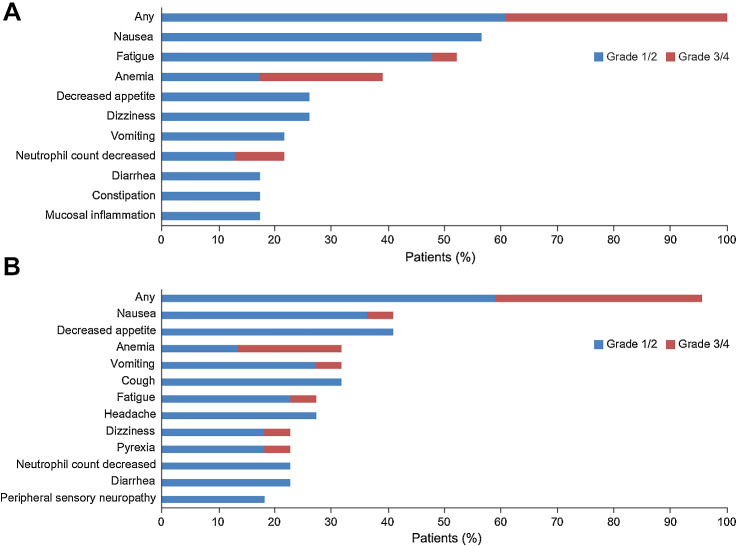
The most common adverse events (reported in >30% of patients) were nausea, fatigue, and anemia in the olaparib-alone arm and nausea, decreased appetite, anemia, vomiting, and cough in the olaparib plus durvalumab combination arm (Fig. 4). Grade 3/4 adverse events were reported in 9 patients (39%) in the olaparib arm and 8 patients (36%) in the combination arm. Three patients (14%) in the combination arm experienced immune-related adverse events. Generally, adverse events were manageable with dose interruptions or reductions. In the olaparib-alone arm, 1 patient (4%) discontinued olaparib because of tumor-associated fever (not considered treatment related). In the olaparib plus durvalumab combination arm, 2 patients (9%) discontinued durvalumab because of pneumonitis and thyroiditis (one case each) but none discontinued olaparib.
Figure 4.
Most common adverse events (AE; grade ≥3 in any patient or any grade in ≥15% of patients). A, Olaparib alone. B, Olaparib plus durvalumab. Additional grade 3/4 AEs in the olaparib-alone arm comprised lymphocyte count decreased, cough, hypophosphatemia, and neutropenia, each in 1 patient (4%). Additional grade 3/4 AEs in the olaparib plus durvalumab combination arm comprised non-cardiac chest pain, neutropenia, pneumonia, upper abdominal pain, amylase increased, white blood cell count decreased, and lipase increased, each in only 1 patient (5%).
There were no treatment-related deaths during the study and no new safety signals were reported.
Targeted panel sequencing
Archival tumor samples were available for 42 of the 45 enrolled patients, of which 6 were of insufficient DNA quality or quantity to complete sequencing. The most frequent genetic alterations were in the TP53 gene (67%), followed by BRCA1 (20%), PIK3CA (13%), and RB1 (9%; Supplementary Fig. S1). Specific to homologous recombination-related genes, we identified alterations in BRCA1 in 9 tumor samples, PALB2 in 2 samples, and BRCA2 in 1 sample.
Discussion
The DORA trial evaluated a novel, chemotherapy-free maintenance approach for patients with aTNBC after induction platinum-containing therapy. The primary objective of DORA was met in both treatment arms with a median PFS that was statistically significantly superior to the historical control reference. The median PFS with first-line chemotherapy regimens in aTNBC is 3–6 months, including in the most recent KEYNOTE-355 clinical trial, in which patients unselected for PD-L1 who received platinum- or taxane-based chemotherapy with placebo achieved a median PFS of 5.6 months (2). Given the 2- to 3-month platinum induction required for entry into the DORA trial, an added PFS of 4 months or more with a maintenance strategy was considered to be clinically meaningful. More than one third of patients achieved disease control for ≥6 months. Furthermore, maintenance therapy ongoing in 20% of patients at data cutoff date and study closure indicates sustained tumor control with the chemotherapy-free regimens evaluated in DORA. Together with the good safety profile, these findings suggest that maintenance olaparib with or without durvalumab deserves further investigation in platinum-responsive aTNBC, offering patients a favorable balance of disease control and tolerability.
Potential clinical and genomic biomarkers of prolonged disease control with this strategy were evaluated. Small patient numbers in planned subgroup analyses according to BRCA mutation status or platinum response preclude firm conclusions; however, encouraging signals were seen in true responders with CR/PR to the prior platinum regimen.
In terms of the contribution of durvalumab, it is not possible to interpret the relative efficacy of the two regimens, as the trial was not designed to compare the two experimental regimens and there were marked imbalances between treatment arms with regard to BRCA mutation status (not only the presence/absence of BRCA mutations, but also the type of BRCA mutation). Furthermore, no conclusions can be made on the true synergistic impact of PD-L1 inhibition on PARP inhibition. However, a recently reported randomized phase II trial in patients with BRCA-mutated advanced breast cancer showed no benefit from the addition of the PD-L1 inhibitor atezolizumab to olaparib, either in the overall population or in the aTNBC subgroup (27).
Maintenance PARP inhibition is standard of care in platinum-sensitive high-grade serous ovarian cancer (28) but there is a paucity of data in TNBC (29). Maintenance regimens are rarely used in aTNBC, but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer. Several trials in breast cancer have evaluated “switch maintenance” strategies, in which patients receive an intensive induction therapy and then, after response, switch to an alternative, more tolerable non-cross-resistant regimen. For example, in HER2-negative mBC, the IMELDA phase III trial demonstrated PFS and OS benefit from a switch to capecitabine and bevacizumab maintenance treatment versus bevacizumab alone after induction therapy with docetaxel and bevacizumab (30). In BRCA-mutated HER2-negative mBC, subgroup analyses of the randomized phase III BROCADE3 trial mentioned above (8) suggested a benefit from maintenance veliparib after stopping chemotherapy (29), although the trial was not designed to compare maintenance versus no maintenance therapy. Finally, the randomized phase II SAFIR-02 BREAST IMMUNO trial compared switch maintenance therapy to durvalumab versus continuation of chemotherapy after induction chemotherapy for HER2-negative mBC. Neither PFS nor OS was improved with the switch maintenance strategy, but an exploratory analysis suggested improved OS with durvalumab in the subgroup of patients with TNBC (31).
The main limitations of the present trial are the relatively small sample size and the lack of a standard control arm, which prevents assessment of the relative contribution of each drug. Designing and conducting a trial in the pure maintenance setting for TNBC is challenging not only because of the evolving standards of care, but also because the few maintenance regimens evaluated in clinical trials (30, 32) have not been adopted uniformly across healthcare systems. For patients with PD-L1–positive aTNBC, the combination of pembrolizumab and chemotherapy demonstrated median PFS of almost 10 months in the KEYNOTE-522 trial (2). However, access to these regimens and the testing capabilities required to select eligible patients varies between countries. Furthermore, most patients with aTNBC have PD-L1–negative tumors [only 3 (16%) of 19 patients in DORA with known PD-L1 status had CPS ≥10]. Therefore, alternative maintenance strategies, particularly those offering the convenience of oral instead of intravenous administration, are worthy of consideration.
An important strength is the observation that, although existing biomarkers may miss a significant proportion of patients who could benefit from a maintenance PARP inhibitor strategy, prior platinum response may serve as a biomarker for benefit from maintenance PARP inhibitor therapy, with or without immunotherapy (33). Information on response to early platinum cycles is readily available and may help in patient selection for maintenance therapy. The opportunity to taper chemotherapy to a more tolerable chemotherapy-free maintenance regimen may be attractive to patients, enabling them to avoid prolonged toxicity from platinum-containing therapy. Further evaluation of this approach is ongoing in the phase II/III KEYLYNK-009 trial (NCT04191135) evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC. Extensive ongoing analyses of the DORA trial aim to explore methylation status, markers of resistance, and other potential associations with clinical benefit to inform future research of this promising strategy.
Supplementary Material
Supplement
Acknowledgments
We thank the patients, their families, and the study site teams for their participation. This investigator-initiated study was supported by AstraZeneca Pharmaceuticals LP. Medical writing support was provided by Jennifer Kelly (Medi-Kelsey Ltd.), funded by Duke Cancer Institute. Translational work was supported by funding from Duke/Duke-NUS Collaboration Pilot Project Award, National Medical Research Council Singapore, and Tempus Laboratories, Inc. We thank Pang Menyuan for her technical contribution to this study. We also thank Puay Hoon Tan and Jason Chan and their laboratories at Singapore General Hospital and National Cancer Centre Singapore, respectively, for their assistance in biospecimen sample management.
This article is featured in Highlights of This Issue, p. 1215
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
T.J. Tan reports grants and other support from AstraZeneca, non-financial support from Tempus, and grants from National Medical Research Council Singapore during the conduct of the study. T.J. Tan also reports grants, personal fees, and non-financial support from AstraZeneca; grants and personal fees from Novartis, Lilly, and Daiichi Sankyo; personal fees from MSD Oncology, Pillar Biosciences, Everest Medicine, and DKSH; personal fees and non-financial support from Pfizer; and grants from Bayer, Odonate Therapeutics, Seagen, Sanofi, and Roche/Genentech outside the submitted work. S. Sammons reports grants and personal fees from AstraZeneca, Relay Therapeutics, Seagen, and Sermonix, as well as personal fees from Daiichi Sankyo, Gilead, Eli Lilly, Incyclix, Pfizer, and Novartis outside the submitted work. L. She reports other support from AstraZeneca during the conduct of the study. R. Bigelow reports grants from AstraZeneca during the conduct of the study, as well as stock holdings in Merck, Johnson & Johnson, Covidien, Pfizer, Sanofi, McKesson, Viatris and Organon. T.A. Traina reports other support from AstraZeneca during the conduct of the study. T.A. Traina also reports grants and personal fees from Genentech/Roche, Pfizer, AstraZeneca, and Daiichi Sankyo; personal fees from Gilead Sciences, Novartis, GlaxoSmithKline, GE Healthcare, bioTheranostics, Hengrui Pharmaceutical, G1 Therapeutics, TerSera, and Stemline Therapeutics; and grants from Exact Sciences and Astellas Pharma outside the submitted work. C. Anders reports other support from Puma, Lilly, Seattle Genetics, Nektar, Tesaro, G1 Therapeutics, Zion, Novartis, Pfizer, AstraZeneca, Elucida, Caris, Incyclix, Genentech, Eisai, Ipsen, Immunomedics, Athenex, Roche, UptoDate, and Jones and Bartlett during the conduct of the study. E. Renzulli reports other support from Tempus Laboratories, Inc. during the conduct of the study, as well as other support from Tempus Laboratories, Inc. outside the submitted work. R. Dent reports grants from AstraZeneca during the conduct of the study, as well as other support from AstraZeneca, Merck, Pfizer, Roche, DKSH, and Novartis outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
T.J. Tan: Conceptualization, resources, data curation, supervision, funding acquisition, methodology, writing–review and editing. S. Sammons: Conceptualization, resources, data curation, supervision, funding acquisition, methodology, writing–review and editing. Y.-H. Im: Resources, writing–review and editing. L. She: Software, formal analysis, validation, visualization, methodology, writing–review and editing. K. Mundy: Project administration, writing–review and editing. R. Bigelow: Software, formal analysis, validation, visualization, writing–review and editing. T.A. Traina: Resources, writing–review and editing. C. Anders: Resources, writing–review and editing. J. Yeong: Resources, data curation, methodology, writing–review and editing. E. Renzulli: Resources, data curation, methodology, writing–review and editing. S.-B. Kim: Conceptualization, resources, methodology, writing–review and editing. R. Dent: Conceptualization, resources, methodology, writing–review and editing.
References
- 1. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- 2. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020;396:1817–28. [DOI] [PubMed] [Google Scholar]
- 3. Cortes J, Rugo HS, Cescon DW, Im S-A, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med 2022;387:217–26. [DOI] [PubMed] [Google Scholar]
- 4. Robson M, Im S-A, Senkus Eż, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523–33. [DOI] [PubMed] [Google Scholar]
- 5. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018;379:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robson ME, Tung N, Conte P, Im S-A, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019;30:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee K-H, Gonçalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol 2020;31:1526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diéras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub J-P, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:1269–82. [DOI] [PubMed] [Google Scholar]
- 9. Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 2015;33:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154–64. [DOI] [PubMed] [Google Scholar]
- 11. González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019;381:2391–402. [DOI] [PubMed] [Google Scholar]
- 12. Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 2019;381:2416–28. [DOI] [PubMed] [Google Scholar]
- 13. Monk BJ, Parkinson C, Lim MC, O'Malley DM, Oaknin A, Wilson MK, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol 2022;40:3952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 2019;381:2403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma P, Barlow WE, Godwin AK, Pathak H, Isakova K, Williams D, et al. Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple-negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313). Ann Oncol 2018;29:654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L, Wu Z, Li J, Zhu D, Yang L, Shao Y, et al. Impact of homologous recombination deficiency on outcomes in patients with triple-negative breast cancer treated with carboplatin-based neoadjuvant chemotherapy: secondary analysis of the NeoCART randomized clinical trial. JCO Precis Oncol 2023;7:e2200337. [DOI] [PubMed] [Google Scholar]
- 17. Telli ML, Hellyer J, Audeh W, Jensen KC, Bose S, Timms KM, et al. Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer. Breast Cancer Res Treat 2018;168:625–30. [DOI] [PubMed] [Google Scholar]
- 18. Loibl S, Weber KE, Timms KM, Elkin EP, Hahnen E, Fasching PA, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol 2018;29:2341–7. [DOI] [PubMed] [Google Scholar]
- 19. Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res 2016;22:3764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galland L, Roussot N, Desmoulins I, Mayeur D, Kaderbhai C, Ilie S, et al. Clinical utility of genomic tests evaluating homologous recombination repair deficiency (HRD) for treatment decisions in early and metastatic breast cancer. Cancers 2023;15:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodgson D, Lai Z, Dearden S, Barrett JC, Harrington EA, Timms K, et al. Analysis of mutation status and homologous recombination deficiency in tumors of patients with germline BRCA1 or BRCA2 mutations and metastatic breast cancer: OlympiAD. Ann Oncol 2021;32:1582–9. [DOI] [PubMed] [Google Scholar]
- 22. Rodler E, Sharma P, Barlow WE, Gralow JR, Puhalla SL, Anders CK, et al. Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2023;24:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bound NT, Vandenberg CJ, Kartikasari AER, Plebanski M, Scott CL. Improving PARP inhibitor efficacy in high-grade serous ovarian carcinoma: a focus on the immune system. Front Genet 2022;13:886170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Domchek SM, Postel-Vinay S, Im S-A, Park YH, Delord J-P, Italiano A, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol 2020;21:1155–64. [DOI] [PubMed] [Google Scholar]
- 25. Pusztai L, Yau C, Wolf DM, Han HS, Du L, Wallace AM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell 2021;39:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med 2018;24:628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fanucci KA, Pilat MJ, Shyr D, Shyr Y, Boerner SA, Durecki D, et al. Abstract CT145: olaparib ± atezolizumab in patients with BRCA-mutated (BRCAmt) locally advanced unresectable or metastatic (advanced) breast cancer: an open-label, multicenter, randomized phase II trial. Cancer Res 2023;83:CT145. [Google Scholar]
- 28. Tew WP, Lacchetti C, Kohn EC. Poly(ADP-ribose) polymerase inhibitors in the management of ovarian cancer: ASCO guideline rapid recommendation update. J Clin Oncol 2022;40:3878–81. [DOI] [PubMed] [Google Scholar]
- 29. Han HS, Arun BK, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, et al. Veliparib monotherapy following carboplatin/paclitaxel plus veliparib combination therapy in patients with germline BRCA-associated advanced breast cancer: results of exploratory analyses from the phase III BROCADE3 trial. Ann Oncol 2022;33:299–309. [DOI] [PubMed] [Google Scholar]
- 30. Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga J-Y, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1351–60. [DOI] [PubMed] [Google Scholar]
- 31. Bachelot T, Filleron T, Bieche I, Arnedos M, Campone M, Dalenc F, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med 2021;27:250–5. [DOI] [PubMed] [Google Scholar]
- 32. Park YH, Jung KH, Im S-A, Sohn JH, Ro J, Ahn J-H, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07–02. J Clin Oncol 2013;31:1732–9. [DOI] [PubMed] [Google Scholar]
- 33. Marchetti C, Fagotti A, Scambia G. Rucaparib maintenance in upfront ovarian cancer: the long-lasting challenge of predicting response to poly (ADP-ribose) polymerase inhibitors. J Clin Oncol 2023;41:935–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement
Data Availability Statement
Data availability is subject to local rules and regulations. Clinical trial and sequencing data presented in this article are not publicly available because subjects did not provide consent for their data to be made available. Every reasonable effort will, however, be made to promptly satisfy scientifically valid requests. Requests for data should be made to the corresponding author together with a detailed study plan and a commitment not to use the data and their derivatives for commercial purposes. The proposal will require approval by the SingHealth Centralized Institutional Review Board, National Cancer Centre Singapore and the Principal Investigators of the study. Requesting researchers will be required to sign a data access agreement with the relevant parties.