The elucidation of the conditions and mechanisms by which human FcR+ myeloid effectors mediate cancer cell resistance and killing during antibody treatment could help develop improved strategies for treating solid tumors.
Abstract
The clinical benefits of tumor-targeting antibodies (tAb) are modest in solid human tumors. The efficacy of many tAbs is dependent on Fc receptor (FcR)–expressing leukocytes that bind Fc fragments of tAb. Tumor-associated macrophages (TAM) and neutrophils (TAN) represent the majority of FcR+ effectors in solid tumors. A better understanding of the mechanisms by which TAMs and TANs regulate tAb response could help improve the efficacy of cancer treatments. Here, we found that myeloid effectors interacting with tAb-opsonized lung cancer cells used antibody-dependent trogocytosis (ADT) but not antibody-dependent phagocytosis. During this process, myeloid cells “nibbled off” tumor cell fragments containing tAb/targeted antigen (tAg) complexes. ADT was only tumoricidal when the tumor cells expressed high levels of tAg and the effectors were present at high effector-to-tumor ratios. If either of these conditions were not met, which is typical for solid tumors, ADT was sublethal. Sublethal ADT, mainly mediated by CD32hiCD64hi TAM, led to two outcomes: (i) removal of surface tAg/tAb complexes from the tumor that facilitated tumor cell escape from the tumoricidal effects of tAb; and (ii) acquisition of bystander tAgs by TAM with subsequent cross-presentation and stimulation of tumor-specific T-cell responses. CD89hiCD32loCD64lo peripheral blood neutrophils (PBN) and TAN stimulated tumor cell growth in the presence of the IgG1 anti-EGFR Ab cetuximab; however, IgA anti-EGFR Abs triggered the tumoricidal activity of PBN and negated the stimulatory effect of TAN. Overall, this study provides insights into the mechanisms by which myeloid effectors mediate tumor cell killing or resistance during tAb therapy.
Significance:
The elucidation of the conditions and mechanisms by which human FcR+ myeloid effectors mediate cancer cell resistance and killing during antibody treatment could help develop improved strategies for treating solid tumors.
Introduction
Antibodies that target tumor antigens (tAb) are widely used in the treatment of human tumors; however, the clinical benefits of these tAbs are modest in most solid tumors (1). A major challenge in improving their efficacy is a lack of complete understanding of tAbs effector mechanisms because most data has been derived from murine tumor models and human studies using peripheral blood cells. Mouse studies are suboptimal because of fundamental differences between mice and humans in tumor evolution, genetic heterogeneity, immune responses, intrinsic biology of effectors, biology of Fc receptors (FcR), and their affinity to immunoglobulin G (IgG) subclasses (2–5). Studies using peripheral blood cells of patients with cancer are also limited because they do not take into account the substantial effects of the tumor microenvironment (TME).
tAbs mediate tumor regression by multiple mechanisms involving the Fab and Fc fragments of the antibody (1, 6). The binding of the Fab fragment to a tumor surface receptor can interrupt oncogenic signals (1). A Fc fragment–dependent mechanism activates the complement cascade on the surface of opsonized tumor cells, resulting in cell lysis (7). Opsonized tumor cells also engage FcRs expressed on myeloid and natural killer (NK) cells to induce cytotoxicity (8). This FcR+cell–mediated effector mechanism has been defined as antibody-dependent cellular cytotoxicity (ADCC; refs. 1, 6). Of note, efficient ADCC has only been observed in models when tumor cell lines express high levels of the tumor antigen (tAg) and the effector cells are present at extremely high effector-to-tumor (E:T) cell ratios that are not physiologically relevant for solid tumors (9, 10). Finally, the induction of antitumor T-cell immunity is also important. In animal models, the efficacy of tAb is severely limited in mice with compromised adaptive immunity (11). In patients, the clinical response of tAbs occurs over weeks, not over days, suggesting that both FcR+ cells and ensuing adaptive T-cell immunity are essential for the full therapeutic effects of tAb (1). Although the induction of tumor-specific T cells has been observed in patients with cancer after tAb therapy (12, 13), the underlying mechanisms by which adaptive immunity is elicited in humans have not been elucidated.
Given that FcR+ effectors play a central role in regulating the clinical efficacy of tAbs (8, 14), it is important to consider the following key pertinent variables in solid human tumors: (i) targeted tAg density, (ii) availability of FcR+ cells, (iii) FcR repertoire and the level of their expression on effectors, and (iv) the functional status of tumor-infiltrating FcR+ effectors. Although the role of tumor NK cells in modulating the effector mechanisms of tAbs has been investigated (15, 16), there is little data elucidating the functional role of tumor-infiltrating myeloid cells in regulating tAb efficacy. In fact, it is still unknown whether these cells promote or prevent tumor cell resistance in the context of tAb therapy.
The effector mechanisms of tAbs in solid tumors are likely different than those in hematopoietic tumors for the several reasons. First, malignant epithelial cells in solid tumors are usually much larger than typical leukocyte-derived tumors. Second, in solid tumors, the malignant cells often form islands with tight junctions between cells (17). These physical constraints, combined with the large size of the tumor cells, could prevent myeloid effectors from mediating the antibody-dependent phagocytosis (ADP) of opsonized tumor cells that would result in their elimination. However, it is possible that under these conditions these effectors use an alternative mechanism recently described as “FcR-mediated trogocytosis” (18–20). During this process, FcR+ myeloid cells “nibble off” tumor cell fragments containing tAb/tAg complexes, resulting in the internalization of these fragments by effectors (19, 21). To date, tAb-triggered trogocytosis (ADT) has been studied only with blood effectors and there is still no consensus on whether this process results in inhibition of tumor cell growth, especially in solid tumors (18, 21, 22). Thus, a major goal of this study was to determine whether ADT is the primary effector mechanism of human tumor-infiltrating myeloid cells and to assess its role in the regulation of tAb efficacy in solid tumors.
Currently, antibodies directed to the EGFR are being used for patients with breast cancer, head and neck cancer, and non–small cell lung cancer (NSCLC). In NSCLC, however, the administration of anti-EGFR Abs (i.e., cetuximab) has had limited clinical response rates and marginal survival benefits (23, 24). Thus, to provide clinical relevance, we used anti-EGFR Abs and studied their FcR-mediated effector mechanisms in human lung tumors. In our study, we identified: (i) the relative contribution of tumor and blood FcR+ myeloid populations to efficacy of tAbs, (ii) the variables in the TME that favor FcR+ effector–mediated tumor cell killing versus tumor escape during tAbs treatment, (iii) mechanisms by which myeloid effectors mediate tumor cell killing or promote tumor cell resistance, and (iv) the mechanism of tAg uptake from live opsonized tumor cells with subsequent stimulation of tumor-specific T-cell responses.
Materials and Methods
Human subjects
A total of 208 random patients with stage I–IV lung cancer were selected for this study. Written informed consent for collection of a portion of tumor tissue and blood for research purposes was obtained from all patients. This study was approved by the University of Pennsylvania Institutional Review Board (protocol no. 805800) and conducted in accordance with recognized ethical guidelines. All patients met the following criteria: (i) histologically confirmed pulmonary squamous cell carcinoma (SCC) or adenocarcinoma (AC), (ii) no prior chemotherapy or radiotherapy within two years, and (iii) no other active malignancy. Detailed characteristics of the patients can be found in Supplementary Table S1A–S1F.
Reagents
The enzymatic cocktail for tumor digestion consisted of serum-free Leibovitz L-15 media (Thermo Fisher Scientific) supplemented with 1% penicillin–streptomycin, Collagenase type I and IV (170 mg/L = 45–60 U/mL), Collagenase type II (56 mg/L = 15–20 U/mL), DNase-I (25 mg/L), and Elastase (25 mg/L; all from Worthington Biochemical). The culture media DMEM/F-12 1:1 (HyClone, Thermo Fisher Scientific) was supplemented with 2.5 mmol/L l-glutamine, 15 mmol/L HEPES buffer, 10% of embryonic stem (ES) cell screened FBS (HyClone, Thermo Scientific), penicillin (100 U/mL) and streptomycin (100 μg/mL), hereafter referred to as complete cell culture media. HLA-A*0201–restricted NY-ESO-1 (157–165, SLLMWITQV) peptide was synthesized by AnaSpec, Inc. PKH67 Green Fluorescent Cell Linker, PKH26 Red Fluorescent Cell Linker, and LPS were purchased from Sigma. Fc-engineered IgA2 antibodies containing the variable regions of the EGFR antibody cetuximab were developed by Dr. T. Valerius and Dr. G. Georgiou (25). Clinical-grade, rituximab, and cetuximab were obtained from the Pharmacy at the Hospital of the University of Pennsylvania (Philadelphia, PA).
Human tumor cell lines
Human epidermoid carcinoma A431 cells (#CRL-1555), human lung adenocarcinoma cell line A549 (#CCL-185), H1975 (#CRL-5908), and Burkitt lymphoma cell line Daudi (#CCL-213) were purchased from ATCC and cultured in complete cell culture media. Authentication of cell lines was performed by ATCC using the short tandem repeat (STR) profiling. The cell lines were tested for the absence of Mycoplasma quarterly at the Cell Center Service of the University of Pennsylvania and used within 10 passages.
Preparation of a single-cell suspension from tumor lung tissue
Surgically resected fresh lung tumors were processed within 20 minutes of removal from the patient. We used an optimized disaggregation method for human lung tumors that preserves the phenotype and function of the immune cells as previously described (26–29). Briefly, under sterile conditions, all areas of tissue necrosis were trimmed away. The tumor lung tissues were sliced into 1 to 2 mm3 pieces with microdissecting scissors equipped with tungsten carbide insert blades. For enzymatic digestion, the pieces were incubated in a shaker for 45 minutes at 37°C in serum-free L-15 Leibovitz media containing enzymes (see specifics above) and 1% penicillin–streptomycin (Life Technologies). L-15 Leibovitz media was formulated for use in carbon dioxide–free systems. After 45 minutes, any visible tumor pieces were vigorously pipetted against the side of a 50-mL tube to enhance disaggregation and then further incubated for 30 to 50 minutes under the same conditions. Larger pieces of tumor tissue were permitted to settle to the bottom of the tube and the supernatant was passed through a 70-μm nylon cell strainer (BD Falcon). The remaining pieces in the tube underwent further pipetting before being passed through the same cell strainer. After filtration, the red blood cells were lysed using 1× Red Blood Cell Lysis Buffer (Santa Cruz Biotechnology). The remaining cells were washed twice in RPMI supplemented with 2% FBS and resuspended in complete cell culture media. Cell viability, as determined by Trypan blue exclusion or Fixable Viability Dye eFluor 450 staining, was typically >90%. If the viability of cells was less than 80%, dead cells were eliminated using a “dead cell removal kit” (130–090–101, Miltenyi Biotec Inc.).
Peripheral blood mononuclear cell isolation
Standard approaches were utilized. Peripheral blood mononuclear cells (PBMC) were separated by 1.077 g/mL Lymphoprep (StemCell Technologies) gradient density centrifugation of EDTA anticoagulated whole blood collected from patients with cancer and healthy donors. To account for any possible effect of tissue digestion enzymes on the function of blood FcR+ effectors, blood was incubated with the enzymatic cocktail before gradient separation.
Isolation of cells of macrophage/monocyte lineage
We used a combination of our tumor digestion protocol and either anti-CD14 microbeads (130–050–201, Miltenyi Biotec Inc.) or FACS sorting to isolate cells of monocyte/macrophage lineage from digested tumor and peripheral blood for functional studies as previously described (28). Monocytes from PBMC of EDTA anticoagulated peripheral blood were isolated in a similar fashion. Previously, we have demonstrated that the CD14+ cells isolated from tumor lung produced TNFα in response to LPS stimulation at levels similar to stimulated PBMC CD14+ cells, indicating that these cells are fully functional after isolation (28). We have shown that isolated macrophage/monocyte lineage CD14+ cells from the tumor are mostly represented by CD206+CD163+HLA-DRhi tumor-associated macrophages (TAM; ref. 28). Nevertheless, for experiments investigating the ability of TAM to cross-present tAg, a high purity of TAM is required and thus TAM were isolated by FACS based on the common phenotype of TAM as CD45+CD11b+CD14+HLA-DRhiCD206+ as described previously (28). Sterile cell sorting was performed on the FACSJazz (BD Biosciences) or MoFlo Astrios (Beckman Coulter).
Neutrophil isolation
Tumor-associated neutrophils (TAN) were isolated from tumor single-cell suspensions using positive selection with CD15 or CD66b antibody–conjugated magnetic microbeads (130–046–601 and 130–111–552, Miltenyi Biotec Inc.) according to the manufacturer's protocol as previously described (27, 29). Peripheral blood neutrophils (PBN) were obtained from EDTA anticoagulated peripheral blood collected from patients with lung cancer during surgery or from healthy donors. The PBNs were obtained from Lymphoprep (1.077 g/mL) density gradient centrifugation followed by erythrocyte lysis with 1× RBC Lysis Buffer. We have previously shown that PBNs and TANs isolated by this approach demonstrated high cell viability with minimal enzyme-induced premature cellular activation or cleavage of myeloid cell markers (27, 29). The purity of TANs and PBNs was typically higher than 94%.
Natural killer cell isolation
NK cells were isolated from PBMCs and single-cell suspension obtained from digested tumors using positive selection with CD56 antibody-conjugated magnetic microbeads (130–050–401, Miltenyi Biotec Inc.) according to the manufacturer's protocol with the following modification. Given that the CD56 molecule could also be expressed on T cells and monocyte/macrophage lineage cells, we first depleted PBMCs and tumor digest for these cells using CD3 and CD14 magnetic microbeads (130–050–101 and 130–050–201, Miltenyi Biotec Inc.) followed by isolation of NK cells. The purity of NK cells isolated from blood and tumor was typically higher than 95% and 90%, respectively. We used positive selection of NK cells because all methods using negative selection have been developed for the isolation of NK cells from blood and are not efficient for tumor tissue.
Generation of peripheral blood monocyte–derived dendritic cells
To differentiate blood monocytes into dendritic cells (DC), CD14+ cells were isolated from PBMCs as described above. Purified CD14+ cells were cultured in the presence of GM-CSF (25 ng/mL; 300–03, PeproTech) and IL4 (25 ng/mL; 200–04, PeproTech) for 7 days in complete cell culture media. LPS (100 ng/mL; L4516, Sigma), sOX40 L (50 ng/mL; 310–28, PeproTech), and TNFα 20 ng/mL (300–01A, PeproTech) were added at day 7 for additional 24 hours, as described in detail elsewhere (30).
Flow cytometry
Flow cytometric analysis was performed according to standard protocols. Matched isotype antibodies were used as controls. Negative gating was based on fluorescence minus one (FMO) strategy. To exclude dead cells from analysis, cells were stained with the LIVE/DEAD Fixable Dead Cell stains (L34966, Thermo Fisher Scientific).
For phenotypic and functional analysis of macrophage/monocyte lineage cells, these cells were gated on live CD11b+CD14hiCD66b− cells. TAMs and monocytes were gated on their definitive phenotypes CD11b+CD66b−CD14+HLA-DRhiCD206+ and CD11b+CD66b−CD14+HLA-DRintCD206−, respectively (28). TANs and PBNs were gated on their definitive phenotypes CD11b+CD66b+CD15hiCD14−HLA-DR−. The following cell surface antibodies were utilized: anti-CD11b (clone: ICRF44, BioLegend, 301340), anti-CD4 (clone: RPA-T4, BioLegend, 300518), anti-CD8 (clone: HIT8a, BioLegend, 300914), anti-CD3 (clone: OKT3, BioLegend, 317306), anti-CD14 (clone: HCD14, BioLegend, 325632), anti-CD66b (clone: G10F5, BioLegend, 305106), anti-HLA-DR (clone: L243, BioLegend, 307618), anti-EpCam (clone: 9C4, BioLegend, 324210), anti-CD15 (clone: HI98, BD Biosciences, 551376), anti-CD45 (clone: H130, BD Biosciences, 560367), anti-CD206 (clone: 15–2, BioLegend, 321104), anti-CD163 (clone: GHI/61, BioLegend, 333608), anti-CD64 (clone: 10.1, BioLegend, 305006), anti-CD32 (clone: FUN-2, BioLegend, 303208), anti-CD16 (clone: 3G8, BioLegend, 302021), anti-CD89 (clone: A59, BioLegend, 354108). Antibodies against Vβ13.1 TCR chain were purchased from (clone IMMU 222, Beckman Coulter, IM2292). Anti-human CD32b (FCGR2b) recombinant antibody (clone: 6G11, HPAB-0535YY) and anti-human CD32a (FCGR2a) antibody (clone: IV.3, NEUT-790CQ) were purchased from Creative Biolabs, Inc. Anti-human CD32b (FCGR2b) recombinant antibody (clone: 2B6) with modified silent Fc fragment were kindly provided by Dr. G. Georgiou.
For intracellular staining, fixed cells stained for surface markers were permeabilized with BD Perm/Wash Buffer (BD Biosciences, 554723) and then stained with FITC anti-human IFNγ (clone: 4S.B3, BioLegend, 502506) for 45 minutes at room temperature. All data were acquired using the BD LSRFortessa (BD Biosciences) flow cytometers or CytoFLEX S (Beckman Coulter) and analyzed using FlowJo software (TreeStar Inc.).
Flow cytometry–based killing and trogocytosis assay
To assess the tumoricidal activity of FcR+ effectors toward A431 and A549 tumor cell lines in the presence or absence of anti-EGFR Abs, target cells were labeled with a cell membrane dye PKH67 Green Fluorescent Cell Linker (Sigma, PKH67GL) according to the manufacturer's protocol. PKH67-labeled tumor cells were vigorously washed with PBS and plated at 50,000 total cells/well (24-well cell culture plate). After tumor cells were allowed to adhere for at least 2 hours, the IgG1 isotype of anti-EGFR Ab (cetuximab) or IgA isotype of anti-EGFR Ab were added at concentration 1 μg/mL. The nonspecific human IgG1 or IgA isotype Abs were used as a control Abs. Fifteen minutes later, purified FcR+ effectors were added to the opsonized tumor cells at specified E:T ratios in complete cell culture media. Cocultures without anti-EGFR Abs were used as a negative control. Twelve hours later, all floating cells within the well were collected by vigorous pipetting. Adherent cells were lifted using Accutase cell detachment solution (Sigma, SCR005) by vigorous pipetting. The pooled collected cells were stained with cell-impermeant nucleic acid stain TO-PRO-3 Iodide (Thermo Fisher Scientific, T3605) at 4°C. Cell death of PKH67-labeled target cells was determined by flow cytometry as a percent of PKH-67+TO-PRO-3+ cells.
Summary graphs represent the total tumoricidal activity of effectors that includes the percent of dead/dying intact tumor cells determined as PKH-67+TO-PRO-3+ targets and tumor cells that lost their cellular integrity at the moment of measurement determined as the difference in total PKH+ tumor cells between control and treated groups. Specifically, we first calculated the total number (#) of the remaining live targets in treated group and control group by using the following formula: # live targets = (total # added effectors + total # added targets) × (decimal remaining PKH+ targets) × (decimal TO-PRO-3−PKH+ live targets). Next, we calculated the percent of total dead cells in treated group by using the following formula: % dead targets = 100% − (# live targets (treated) /÷ # live targets (control)) × 100%.
To quantify ADT, pooled cells were collected at indicated time points and stained with fluorochrome-conjugated anti-CD14 Abs and anti-CD66b Abs to identify macrophages/monocytes and granulocytes, respectively. Anti-EGFR Ab-triggered trogocytosis was evaluated by flow cytometry as a percentage of double-positive CD66b+PKH67+ or CD14+PKH67+ TO-PRO-3neg cells having acquired PKH67+ fragments from PKH-labeled A549 or A431 tumor cells. Some experiments were performed with blocking anti-CD64 F(ab')2 (clone 10.1, Ancell, 216–520) and anti-CD32 F(ab')2 Abs (clone 7.3, Ancell, 181–520).
IncuCyte live tumor cell growth analysis
The growth of A431 and A549 tumor cell line cocultured with indicated effectors in the presence or absence of anti-EGFR Ab was quantified over time in the IncuCyte S3 Live-Cell Analysis System (Essen BioScience, Inc.) according to the manufacturer's protocol. Briefly, A431 and A549 cell lines that were transduced to stably express GFP fluorescent protein. GFPhi A431 and GFPhi A549 tumor cells were sorted by flow cytometry and expanded in cell culture. For the Incucyte assay, these GFPhi tumor cell lines were plated at 2,500 total cells/well (96-flat bottom well plate). Tumor cells were allowed to adhere for at least 2 hours. Once tumor cells become adherent, cetuximab or IgA anti-EGFR Abs were added at a concentration of 1 μg/mL. The nonspecific human IgG1 or IgA isotype Abs were used as a control Abs. Fifteen minutes later, purified FcR+ effectors were added to opsonized tumor cells at different E:T ratios in the complete cell culture media. Cells were coincubated in the IncuCyte Live-Cell imaging system for the indicated time. Tumor cell growth was quantified over time by measuring integrated fluorescence intensity (FI) of the remaining cell-associated GFP fluorescence of adherent A549 and A431 cells. Percentage of tumor cell growth inhibition and/or stimulation in the absence of anti-EGFR Abs is calculated at 48 hours using the formula: (FI)(A431)-FI(A431+effectors)/FI (A431) × 100%, and in the presence of anti-EGFR Abs: (FI)(A431+Abs)-FI(A431+effectors+Abs)/FI(A431+Abs) × 100%. The data were analyzed, and tumor cell growth curves were generated using GraphPad Prism using GraphPad Prism 9 software.
Time-lapse microscopy and live cell imaging
Daudi and A431 tumor cells were labeled with cell membrane dye PKH67 Green Fluorescent Cell Linker (Sigma) and FcR+ effectors were labeled with cell membrane dye PKH26 Red Fluorescent Cell Linker (Sigma), according to the manufacturer's protocol. The PKH-labeled effectors and targets were plated into a poly-D-lysine-coated 8-well chamber μ-slide (Ibidi) in AIMV cell culture media with AlbuMAX Supplement (Thermo Fisher Scientific). Then, the chamber slide was centrifuged at 300 × g for 30 seconds to facilitate cell-to-cell contact. The slides were placed in a Leica DMI4000 inverted microscope chamber at 37°C with 5% CO2. Rituximab was added into cell cocultures with Daudi cells and cetuximab were added into cell cocultures with A431 tumor cells. All antibodies were used at a concentration of 1 μg/mL. Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope that was equipped with a Yokogawa CSU-X1 spinning disk confocal attachment. The images were taken every 5 or 15 minutes for 2 hours with Hamamatsu ImagEM 16-bit cooled EMCCD camera at ×20 magnification. The videos were assembled with MetaMorph 7 software (Molecular Devices Corporation).
Generation of NY-ESO-1–specific Ly95 T cells and A549/A2-NY-ESO-1 target lung cancer cell line
The NY-ESO-1–reactive Ly95 TCR construct is an affinity-enhanced variant of the wild-type IG4 TCR identified from T cells recognizing the HLA-A2 restricted NY-ESO-1:157–165 peptide antigen. The generation of this Ly95 TCR construct and its packaging into a lentiviral vector has been described in detail previously (31). Human T cells were isolated from PBMCs of healthy volunteer donors by negative selection using RosetteSep kit (StemCell Technologies). Isolated T cells were stimulated with magnetic beads coated with anti-CD3/anti-CD28 Abs at a 1:3 cell-to-bead ratio. T cells were transduced with lentiviral vectors at a multiplicity of infection (MOI) of approximately 5. Cells were counted and fed with complete cell culture medium every 2 days. A small portion of expanded cells was stained for flow cytometry confirmation of successful Ly95 transduction using the Vβ13.1 TCR chain antibody. Transduction of human T cells undergoing anti-CD3/CD28 mAb-coated bead activation with high titer lentivirus that encodes the Ly95 TCR recognizing NY-ESO-1 resulted in approximately 50% of TCRVb13.1+ CD8+ cells.
For target cells, we genetically modified the A549 human lung adenocarcinoma cell line to express both NY-ESO-1 protein and HLA-A*02 as described earlier (31). Briefly, the A549 cell line was transduced by a retroviral vector encoding NY-ESO-1-T2A-HLA-A*02. The transduced A549 cells were subjected to limiting dilution at 0.5 cell per well in 96-well plates. The resulting clones were tested by flow cytometry for HLA-A*02 expression using anti-HLA-A2 Abs (clone: bb7.2, BioLegend, 343306). HLA-A2–positive clones were selected and tested in coculture with T cells expressing the NY-ESO-1 Ly95 TCR. The clones expressing HLA-A2 that could stimulate NY-ESO-1 Ly95 TCR-expressing T cells to secrete IFNγ were pooled to generate the A549-NY-ESO-1-A2 (A549-A2-ESO) cell line. Flow sorting was performed to enrich for high HLA-A2–expressing tumor cells. The expression of intracellular NY-ESO was analyzed by flow cytometry using NY-ESO-1 (D1Q2U) mAb (Cell Signaling Technology) that recognizes endogenous levels of total NY-ESO-1 protein.
Antigen-presenting cell functions of TAMs
To assess whether the TAMs perform antigen-presenting cells (APC) functions, freshly sorted from tumor HLA-A2+CD14+CD206+HLA-DRhi TAMs were preloaded with HLA-A2–restricted NY-ESO-1 (157–165, SLLMWITQV) peptide (2 μg/mL) for 1 hour. TAMs were washed three times with cell culture medium and mixed with Ly95 T cells at concentration 1.5 × 105 cells/well (96-well U-bottom plate) in ratio 1:1 in the complete cell culture media. The Ly95 T cells cocultured with NY-ESO-1–positive (A549-A2-ESO) and -negative A549 (A549) tumor cells at the same ratio were used as a positive and negative control, respectively. Eighteen hours later, NY-ESO–specific activation of the Ly95 cells was assessed by measuring intracellular IFNγ in gated CD8+TCRVβ13.1+ cells. Specifically, to accumulate intracellular IFNγ, BD GolgiStop (BD Biosciences, 554724) were added into the cell cultures during the last 6 hours. The cells were collected, washed in Stain Buffer (BD Biosciences, 554656) and stained for CD8 and Ly95 TCR surface markers using anti-CD8 and anti-TCRVβ13.1 antibodies. Surface-stained cells were fixed with BD Cytofix Fixation Buffer (BD Biosciences, 554655) for 20 minutes. The fixed cells were permeabilized with BD Perm/Wash Buffer (BD Biosciences, 554723) and then stained with the anti-human IFNγ. The production of IFNγ was analyzed in gated live CD8+TCRVβ13.1+ cells by flow cytometry.
To assess the ADT-dependent ability of TAM to uptake and cross-present bystander tAg from live opsonized targets, we mixed CD14 bead-sorted TAM from HLA-A2+ donors with EGFR+A549/HLA-A2+NY-ESO tumor cells in the presence or absence of cetuximab (1 μg/mL) at a ratio of 1:1 in 6-well Ultra-Low Attachment plates (Corning). The same cell coculture conditions without cetuximab were used as negative controls. Monocyte-derived DCs were used as positive controls. Four hours after coincubation, TAMs were sorted from this coculture by flow cytometry and additionally exposed to Ly95 cells at a concentration of 1.5 × 105 cells/well (96-well U-bottom plate) in a ratio of 1:1 for 12 hours in the complete cell culture media. NY-ESO–specific activation of the Ly95 cells was assessed by measuring intracellular IFNγ in gated live CD8+TCRVβ13.1+ cells as described above.
Statistical analysis
All data were tested for normal distribution of variables. Comparisons between two groups were assessed with a two-tailed Student t test for paired and unpaired data if data were normally distributed. Nonparametric Wilcoxon matched-pairs test and Mann–Whitney unpaired test were used when the populations were not normally distributed. Likewise, multiple groups were analyzed by one-way ANOVA with corresponding Tukey multiple comparison test if normally distributed, or by the Kruskal–Wallis with Dunn multiple comparison test if not normally distributed. Nonparametric Spearman or parametric Pearson tests were used for correlation analysis. All statistical analyses were performed with GraphPad Prism 9. A P <0.05 was considered statistically significant.
Data availability
The data generated in this study are available within the article and its Supplementary Data files or upon request from the corresponding author.
Results
TANs and TAMs comprise the majority of FcR+ effectors in human lung tumors, but differentially express FcRs
To determine subsets of FcR+ effectors residing in lung cancers, fresh lung tumor tissue was digested using a previously described optimized approach (26). We characterized TANs as CD11b+CD15hiCD66b+MPO+Arg1+IL5Ra−cells (27, 29) and TAM/monocyte lineage cells as CD11b+CD14+HLA-DR+CD66b− cells that were mostly represented by CD206+CD163+HLA-DRhi TAMs (28). We phenotypically defined tumor-infiltrating NK cells as CD45+CD56+CD94+CD3−CD14−CD19−cells (Supplementary Fig. S1A) and DCs as IRF8hiHLA-DRhiCD11c+CD141+CD14− cells (Supplementary Fig. S1B). We found that TANs and TAMs comprised the majority of all FcR+ effectors in lung tumors (Fig. 1A). The total frequencies of TANs and TAMs in lung tumor digests varied from 1% to 25% of all live cells (10.1 ± 6.7%, n = 73, and 8.5±5.9%, n = 89, respectively). The accumulation of NK cells in lung tumors was significantly lower (3.2 ± 2.4%, n = 92, among all live cells) and less heterogeneous compared with TANs and TAMs (Fig. 1A). DCs were minimally present (0.9 ± 0.9%, n = 21; Fig. 1A) and thus not analyzed further. We found no significant differences in the accumulation of FcR+ effectors in tumors of patients with early versus advanced staged disease (Supplementary Fig. S1C). Detailed characteristics of patients involved in flow cytometric phenotyping are shown in Supplementary Table S1A.
Figure 1.
Accumulation, FcR expression, and tumoricidal activity of tumor-infiltrating myeloid cells. A, Frequencies of indicated FcR+ effectors in lung tumors analyzed by flow cytometry in a single-cell suspension obtained from digested lung tumors. B–D, Representative dot plots and cumulative flow cytometry results showing the expression of the indicated FcRs on myeloid effectors in blood and tumors. MFI, mean fluorescence intensity. E, Representative dot plots and cumulative flow cytometry results showing the expression of CD32a and CD32b molecules on the surface of gated CD14+HLA-DRintCD206− blood monocytes and CD14+HLA-DRhiCD206+ TAMs. Cells stained with isotype control Abs were used to set the gates. Wilcoxon matched paired test. F and G, Representative dot plots and cumulative flow cytometry results showing the ability of blood and tumor FcR+ effectors to kill PKH-67+A431 tumor cells in the presence of cetuximab (1 μg/mL) at a 50:1 E:T ratio in a 12-hour assay. Dead A431 tumor cells were defined as TO-PRO-3+PKH67+ cells. Summary graphs represent the total tumoricidal activity of effectors calculated as described in Materials and Methods. H and I, Representative images of GFP+A431 tumor cells cocultured with indicated blood effectors (patient LC441) and cetuximab for 48 hours in the IncuCyte Live Cell Analysis System. Scale bar, 400 μm. Representative experiment (H) and summary results (I) showing the kinetics of GFP+A431 tumor cell growth during coculturing with blood and tumor FcR+ effectors at a 50:1 E:T ratio in the presence of cetuximab (1 μg/mL) and in the presence of IgA anti-EGFR Ab (1 μg/mL; J and K) in the IncuCyte Live Cell Analysis System. Percentage of tumor cell growth inhibition/stimulation in the presence of anti-EGFR Abs was calculated at 48 hours using the formula: (FI)(A431+Ab)-FI(A431+effectors+Ab)/FI(A431+Ab) × 100%. FI, Integrated fluorescence intensity. The number of patients included in each analysis is indicated on the graphs. All data are represented as mean ± SEM. All comparisons used one-way ANOVA with Tukey multiple comparisons tests. FcR+ effectors were freshly isolated for all experiments.
We next evaluated the types and expression of FcRs, including CD64 (FcγRI), CD32 (FcγRII), CD16 (FcγRIII), and CD89 (FcαRI) on TAN and TAM in 20 patients with NSCLC. In tumors, there was a substantial upregulation of CD89 on TANs and CD32 and CD64 on TAMs compared with their counterparts in blood (Fig. 1B–D). TAMs were also able to express CD89 but at a lower level than TANs (Fig. 1C and D). TANs did not express CD32 and CD64 (Fig. 1B and D) and dramatically downregulated CD16 that is highly expressed on PBN (Fig. 1C and D).
Next, we analyzed the expression of activating CD32a and inhibitory CD32b receptors on the surface of TAMs and blood monocytes using anti-CD32a Ab (clone IV.3) and anti-CD32b Ab (clone 6G11) that have been demonstrated to bind these FcRs with high specificity (32–34). We found that blood monocytes express CD32a at a moderate level and CD32b at a low level, whereas TAM significantly upregulated CD32b and CD32a on their surface (Fig. 1E). Thus, CD32hiCD32bhiTAM coexpress inhibitory and activating CD32 receptors at significantly higher levels than CD32+CD32blo monocytes in blood (Fig. 1E).
On the basis of this phenotype, CD16−CD32hiCD64hiCD89+ TAMs have the most potential to respond to tumor cells opsonized with an IgG Ab isotype. CD16loCD32−/loCD64−/loCD89hi TANs are unlikely to mediate isotype IgG FcγR–mediated effector functions but could have effector functions if their CD89 was engaged by tAb of isotype IgA.
Cetuximab-triggered tumoricidal activity of tumor-infiltrating FcR+ effectors is impaired
Our current knowledge about the role of myeloid effectors in regulating the tAb-driven tumor cell cytotoxicity in humans is largely based on studies using peripheral blood. Thus, in this study, we evaluated the tumoricidal ability of myeloid cells in tumor in comparison with their counterparts in blood of patients with lung cancer.
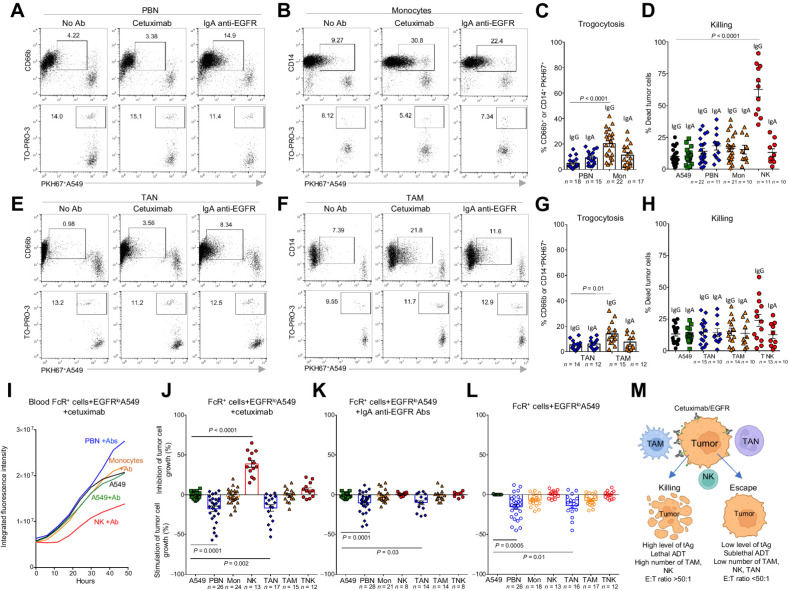
First, we performed a flow cytometry–based cytotoxic assay under commonly used “optimal conditions,” in which a tumor cell line with a high level of the targeted tAg (EGFRhiA431 cells) are mixed with effector cells at a high 50:1 (E:T) ratio in the presence of the anti-EGFR Ab cetuximab. Given that NK cells are the principal effector cells in the blood that efficiently kill cetuximab-opsonized tumor cells (35), we used these cells as positive controls. Under these optimized conditions, blood NK cells killed an average of 75% of opsonized A431 tumor cells (Fig. 1F and G). We performed analysis of 27 patients with NSCLC and found that blood monocytes were also able to kill opsonized tumor cells, although at a much lower efficiency (∼30%) compared with NK cells (Fig. 1F and G). In contrast, PBN were not capable of mediating cetuximab-triggered A431 tumor cell cytotoxicity. In tumors, TANs remained unable to kill opsonized A431 cells and TAMs failed to significantly kill opsonized targets (Fig. 1F and G). Tumor NK cells still significantly killed cetuximab-opsonized A431 cells but their tumoricidal ability was dramatically decreased compared with the blood NK cells (Fig. 1F and G). There was no tumoricidal activity detected with any of the effectors in the presence of IgG1 isotype control Abs (Supplementary Fig. S1D). Detailed characteristics of patients involved in the evaluation of killing ability of effectors in flow cytometry–based killing assay are shown in Supplementary Table S1B.
Next, we investigated the effect of FcR+ effectors on GFP+ A431 tumor cell growth in the presence of cetuximab over time using the IncuCyte Live Cell Analysis System. This analysis showed that blood NK cells efficiently eradicated tumor cells, while monocytes were able to inhibit tumor cell growth but to a lesser degree (Fig. 1H and I). TAM and NK cells from tumors (n = 28) had substantially decreased ability to inhibit tumor cell growth in the presence of cetuximab than their blood counterparts (Fig. 1H and I). Interestingly, despite the inability of TAM to directly kill the opsonized A431 tumor cells in the flow cytometry–based assay (Fig. 1G), TAMs from many patients could exert a tumoristatic effect during prolonged coincubation with opsonized A431 tumor cells at a 50:1 (E:T) ratio (Fig. 1I). There was no tumor cell growth inhibition observed with blood and tumor effectors in the absence of cetuximab (Supplementary Fig. S1E). We have previously demonstrated that tumor cells increase the survival of PBNs and TANs in vitro up to 48 hours (27, 29); however, PBNs and TANs were unable to inhibit A431 tumor cell growth in this assay. In contrast, we found that these potentially cytotoxic cells actually promoted the growth of A431 tumor cells in the presence and even in the absence of cetuximab (Fig. 1H and I; Supplementary Fig. S1E). PBNs isolated from patients with lung cancer and heathy donors did not differ in their ability to stimulate the A431 tumor cell growth (Supplementary Fig. S1F). Detailed characteristics of patients involved in the evaluation of killing ability of effectors in IncuCyte killing assays are shown in Supplementary Table S1C.
Our correlation analyses revealed that the tumoristatic effect of TAMs is significantly decreased in advanced stage of lung tumors (Supplementary Fig. S2A). There were no other significant correlations between the ability of FcR+ effectors to inhibit or stimulate the tumor cell growth in the presence of cetuximab and key clinical parameters of patients with lung cancer (Supplementary Fig. S2A–S2D). Also, we did not find a significant correlation between the expression of FcR on myeloid effectors and their tumoricidal activity under “favorable for killing” conditions (Supplementary Fig. S3).
Anti-EGFR Abs of the IgA isotype trigger the tumoricidal activity of PBNs and negate TAN's stimulatory effect on the tumor cell growth
Given, that among all FcRs, TAN highly express only CD89 (FcαRI; Fig. 1D), we asked whether FcαRI ligation could trigger the tumoricidal potential of TAN against opsonized tumor cells. We thus performed an IncuCyte assay where PBNs (n = 49) and TANs (n = 28) were cocultured with EGFRhiA431 tumor cell line in the presence of anti-EGFR Abs of the IgA isotype. We found that PBNs began to significantly inhibit the growth of A431 tumor cells (on average more than 50% killing) in the presence of IgA anti-EGFR Ab (Fig. 1J and K; Supplementary Fig. S4A). Noteworthy, PBNs stimulated with IgA anti-EGFR Ab were able to inhibit the growth of A431 tumor cells as efficiently as blood NK cells inhibited the cetuximab-opsonized A431 targets (Fig. 1H–K). NK cells did not respond to A431 cell opsonized with IgA anti-EGFR Ab, because the expression of CD89 is restricted to cells of the myeloid lineage (36). In contrast to PBNs, TANs did not acquire the ability to significantly inhibit A431 tumor cell growth in the presence of IgA anti-EGFR Ab (Fig. 1J and K). However, the stimulatory effect of TANs, as well as PBN, on A431 tumor cells was significantly reduced in the presence of IgA anti-EGFR Ab (Fig. 1K; Supplementary Fig. S4A). Monocytes (n = 27) and TAMs (n = 24) were able to inhibit the A431 cell growth in the presence of IgA anti-EGFR Ab as efficiently as in the presence of cetuximab (Fig. 1I and K). There was no tumoricidal activity detected with any of the effectors in the presence of IgA2 isotype control Abs (Supplementary Fig. S4B).
Therefore, our data show that even when appropriate FcRs are expressed, additional tumor-induced suppressive mechanisms limit the ability of TANs and TAMs to kill opsonized targets.
tAb-triggered tumoricidal activity of myeloid cells depends on the level of FcR-mediated trogocytosis
During interactions with opsonized targets, myeloid effectors could use several killing mechanisms including antibody-dependent phagocytosis (ADP) and antibody-dependent trogocytosis (ADT; refs. 18, 37). However, whether, and to what extent myeloid effectors use these mechanisms in solid human tumors has been largely unexplored. We thus quantitatively distinguished between ADP and ADT in cell culture while taking into account the size of the target cell. TAMs were cocultured with either “small” CD20+Daudi lymphoma cells in the presence of rituximab (anti-CD20 Abs) or with “large” EGFR+A431 epithelial tumor cells in the presence of cetuximab (anti-EGFR Abs). Using time-lapse microscopy, we determined that TAMs were able to perform classical ADP and engulf rituximab-opsonized Daudi cells (Fig. 2A; Supplementary Movie S1), whereas the larger A431 cells could not be phagocytosed, but were subject only to ADT where TAMs were able to only “gnaw off” tumor cell fragments (Fig. 2B; Supplementary Movie S2).
Figure 2.
Anti-EGFR Ab-triggered trogocytosis and tumoricidal activity mediated by blood and tumor myeloid cells. A and B, Representative images (taken from time-lapse microscopy; Supplementary Movies S1 and S2 at the 30 minutes after recording) showing the TAM-mediated phagocytosis of rituximab-opsonized Daudi tumor cells (A) and trogocytosis of cetuximab-opsonized A431 tumor cells (B). PKH26-labeled TAMs (red) were incubated with opsonized PKH67-labeled targets (green) at a 3:1 E:T ratio. Scale bar, 10 μm. C and D, Representative dot plots demonstrating TAM-mediated phagocytosis of Daudi tumor cells in the presence or absence of rituximab (1 μg/mL; C) and trogocytosis of A431 tumor cells in the presence or absence of cetuximab (1 μg/mL; D). TAMs were cocultured with PKH67-labeled targets at 3:1 E:T ratio for 2 hours. E–L, Representative dot plots showing the different levels of trogocytosis and killing activity mediated by indicated blood and tumor myeloid effectors cocultured with PKH67+A431 cells at a 50:1 E:T ratio in the presence or absence of cetuximab (IgG1) or IgA anti-EGFR Abs (IgA) for 12 hours. F, H, J, and L, Summary results of experiments described above in E–L. Unpaired t test. The number of patients included in each analysis is indicated on the graphs. All data represented as mean ± SEM. FcR+ effectors were freshly isolated for all experiments.
To distinguish and quantify ADP and ADT, we labeled Daudi or A431 tumor cells with the green fluorescent dye PKH-67 and cocultured them with TAMs in the presence or absence of the appropriate tAbs (Fig. 2C and D). Two hours later, cells were collected and additionally stained with Abs for CD14 to identify TAM. Flow cytometry demonstrated that both TAM-mediated ADP (with Daudi cells) and ADT (with A431 cells) could be observed as double-positive CD14+PKH67+ cells (Fig. 2C and D). ADT could be distinguished from ADP by the following characteristics: (i) the fluorescence of the CD14+PKH67+ effectors (Fig. 2D, red box) is much lower than the fluorescence of PKH-67hi tumor cells (Fig. 2D, blue box); (ii) the percent of CD14+PKH67+ effectors substantially exceeds the original number of PKH-67hi tumor cells (Fig. 2D, red box); and (iii) the frequency of the remaining PKH-67hi tumor cells is not markedly changed (Fig. 2D, blue box). Importantly, in these coculture experiments, pretreatment of TAMs with cytochalasin D (an actin depolymerizing agent) or adding blocking anti-CD64 and anti-CD32 F(ab')2 fragments inhibited the formation of double-positive PKH-67+CD14+cells (Supplementary Fig. S4C). These data indicate that trogocytosis reflected the uptake of tumor cell fragments mediated by the FcR and actin cytoskeleton and was not a result of PKH dye bleeding during the effector/tumor cell contact.
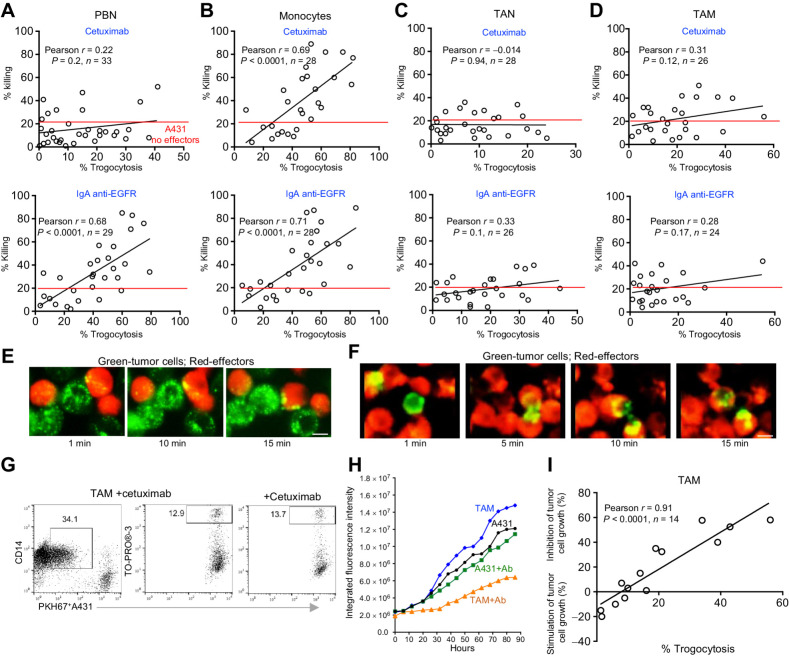
Next, we evaluated the ability of blood and tumor-derived myeloid effectors to mediate ADT or ADP in parallel with their ability to kill opsonized tumor cells in a large cohort of patients with lung cancer (n = 43; Fig. 2E–L). First, we performed these experiments under optimal killing conditions in which EGFRhiA431 cells were mixed with effector cells at a high 50:1 (E:T) ratio in the presence of the IgG1- or IgA-isotype anti-EGFR Abs for 12 hours. Under these conditions, none of the effectors were able to mediate ADP of A431 cells. Instead, they performed ADT, but at strikingly different levels. The different levels of ADT depended on (i) the type of myeloid cells, (ii) the isotypes of tAb and involved FcR, and (iii) the origin (blood vs. tumor) of effectors (Fig. 2E–L).
Specifically, we found that PBNs perform ADT in the presence of cetuximab at a much lower level than blood monocytes. The data show a lower percent and mean fluorescence intensity of double-positive CD66b+PKH67+ PBN (Fig. 2E, red box and F) compared with CD14+PKH67+ monocytes (Fig. 2G, red box and H). However, we found that PBN can significantly increase the ADT in the presence of IgA isotype anti-EGFR Abs (Fig. 2E, blue box and F). Monocytes mediated ADT at a maximum level in the presence of either cetuximab or IgA anti-EGFR Abs (Fig. 2G and H). TANs were not able to perform ADT in the presence of cetuximab (Fig. 2I, red box and J), most likely due to dramatic downregulation of FcγR in tumor (Fig. 1D). However, TANs retained high expression of CD89 (Fig. 1D) and thus performed ADT triggered by IgA anti-EGFR Ab, although at a much lower level than PBNs (Fig. 2I, blue box and J). The magnitude of TAM-mediated trogocytosis was decreased in the presence of cetuximab compared with blood monocytes (Fig. 2K, red box and L) despite the marked upregulation of FcγR (CD32, CD64) on TAM (Fig. 1D). TAM-mediated trogocytosis triggered with the IgA anti-EGFR Abs was also reduced compared with monocytes (Fig. 2K, blue box and L). These data indicate that the TME limits the ability of TAMs and TANs to perform ADT, even if the FcRs are highly expressed. ADT was not observed with any of the effectors in the presence of IgG1 and IgA2 isotype control Abs (Supplementary Fig. S4D). Detailed characteristics of patients involved in the evaluation of effectors ability to perform ADT are shown in Supplementary Table S1D.
Next, we compared the extent of ADT related to the ability of effectors to kill opsonized EGFRhiA431 tumor cells at a high 50:1 (E:T) ratio. The level of ADT where tumor cell death began to significantly exceed the tumor cell death in control cell cultures without effectors was termed “tumoricidal” or “lethal” ADT. Analysis revealed a strong association between the magnitude of ADT performed by PBNs (n = 33), monocytes (n = 28), TANs (n = 28), and TAMs (n = 26) and their ability to kill opsonized A431 tumor cells, once trogocytosis exceeded a threshold (Fig. 3A–D). Specifically, a significant correlation between ADT and killing of tumor cells was found in cocultures of PBN stimulated with IgA anti-EGFR Ab (Fig. 3A) and with monocytes stimulated with either IgG or IgA anti-EGFR Ab (Fig. 3B). In these cocultures, killing occurred when the level of ADT exceeded 40% on average (Figs. 2F and H, and 3A and B). PBNs were not able to kill tumor cells in the presence of cetuximab because they performed ADT at sublethal levels (<40%; Figs. 2F and 3A). The cetuximab-triggered sublethal ADT mediated by PBN (Fig. 3E; Supplementary Movie S3) and lethal ADT by monocytes (Fig. 3F; Supplementary Movie S4) could be also visualized by time-lapse microscopy. TANs and TAMs also performed ADT triggered by IgA anti-EGFR Ab and cetuximab, although at sublethal levels (Fig. 2I–L) that did not result in significant tumor cell killing (Fig. 3C and D).
Figure 3.
The lethal and sublethal effects of ADT on A431 tumor cells mediated by blood and tumor myeloid cells in the presence of anti-EGFR Abs. A–D, Correlation of the ability of indicated blood and tumor myeloid effectors to mediate ADT with their ability to kill A431 tumor cells in the presence of anti-EGFR Abs determined by flow cytometry. Pearson test. E and F, Images (taken from time-lapse microscopy; Supplementary Movies S3 and S4 at the indicated time points after recording) showing the different amount of cetuximab-triggered trogocytic uptake of tumor cell fragments by PKH26-labeled effectors (red) PBNs (E) and monocytes (F) during their interaction with PKH67-labeled A431 targets (green). Scale bar, 10 μm. G, Representative dot plots demonstrating trogocytosis and killing activity mediated by TAMs cocultured with PKH67-labeled A431 cells at a 50:1 E:T ratio in the presence of cetuximab for 12 hours. H, Representative experiment showing the kinetics of GFP+A431 tumor cell growth with TAM at a 50:1 E:T ratio in the presence or absence of cetuximab in the IncuCyte Live Cell Analysis System. I, Summary results showing the correlation between the abilities of TAMs to mediate ADT and regulate A431 tumor cell growth in the presence of cetuximab in the IncuCyte Live Cell Analysis System. Pearson test. The number of patients included in each analysis is indicated on the graphs. FcR+ effectors were freshly isolated for all experiments
Next, we determined whether the upregulated inhibitory CD32b (Fig. 1E) is involved in the reduced ability of TAM to perform ADT and killing of cetuximab-opsonized A431 tumor cells under “favorable for killing” conditions. Flow cytometry short-term and IncuCyte long-term killing assays revealed no significant differences in the ability of TAMs and blood monocytes to perform ADT (Supplementary Fig. S5A and S5B) and killing in the presence or absence of two clones (2B6 and 6G11) of anti-human CD32b Abs (Supplementary Fig. S5C and S5CD) that have previously been shown to have an antagonistic effect on CD32b (32, 34). These data indicate that other more dominant tumor-induced mechanisms limit the tumoricidal ability of TAM.
To more closely simulate the in vivo situation, we asked whether blood and tumor-derived FcR+ effectors were able to mediate ADT and inhibit the growth of opsonized A431 cells cultured as three-dimensional tumor spheroids. Coculturing of these FcR+ effectors with cetuximab-opsonized A431 tumor cell spheroids (Supplementary Fig. S6A–S6C) revealed similar results obtained with A431 cells cultured as a monolayer. PBNs and TANs did not mediate ADT of A431 spheroids in the presence of cetuximab (Supplementary Fig. S6C) and significantly stimulated the spheroid growth (Supplementary Fig. S6A and S6B). However, IgA anti-EGFR Abs triggered PBNs, but not TANs, to mediate lethal ADT and inhibit the spheroid growth (Supplementary Fig. S6D–S6F).
Overall, these data demonstrate that Ab-triggered tumoricidal activity of blood monocytes PBNs, TANs, and TAMs toward tumor cells that express high levels of tAg depends on their ability to perform FcR-mediated ADT.
Persistent sublethal trogocytosis mediated by TAMs inhibit tumor cell growth
Because TAMs are not able to execute tumoricidal ADT (Fig. 2L) and thus directly kill tumor cells in the flow cytometry–based assay (Figs. 1F and 3D), we asked why TAMs from some patients are able to inhibit the A431 tumor cell growth during more prolonged coincubation (Fig. 1I). We hypothesized that persistent sublethal ADT caused cumulative damage to the tumor cell membrane over time, resulting in the observed late tumoristatic effect. To address this question, we cocultured TAMs with EGFRhiA431 tumor cells in the presence of cetuximab at a 50:1 (E:T) ratio in the IncuCyte assay. In parallel, we quantified the level of ADT mediated by TAMs by flow cytometry. Similar to the results described above, we discovered that TAM-mediated sublethal ADT and no direct tumor cell killing was observed in the flow cytometry–based assay (Fig. 3D and G). However, there was a marked tumoristatic effect in the IncuCyte long-term assay (Fig. 3H). Correlation analysis revealed that the inhibition of tumor growth was significantly associated with the sublethal ADT mediated by TAMs (Fig. 3I).
Blood and tumor-derived myeloid effectors lose the ability to kill and inhibit tumor cell growth under conditions representing the solid human tumors
The data above show the potential of blood and tumor FcR+ effectors to kill opsonized tumor cells and inhibit their growth under “favorable for killing” conditions (tAghi and E:T ratiohi) that may exist during tAb therapy of hematologic cancers. However, solid tumors likely express differing and lower levels of tAg and are heterogeneously infiltrated with TANs and TAMs (Fig. 1A). In fact, our flow cytometric analysis of lung tumors demonstrated that the numbers of TAMs or TANs do not exceed the numbers of malignant EpCam+ cells (Fig. 4A), making it unlikely that they could achieve high E:T ratios. Therefore, we first sought to determine whether the myeloid cells retain their trogocytic, tumoricidal, and/or tumoristatic activity toward opsonized EGFRhiA431 tumor cells at reduced E:T ratios.
Figure 4.
The ability of blood and tumor myeloid cells to perform ADT and kill EGFRhi A431 cells in the presence of anti-EGFR Abs under conditions representing solid human tumors. A, The frequencies of CD45−EpCam+cells, CD14+CD206+HLA-DRhiTAM, and CD11b+CD66b+CD14−TAN were analyzed by flow cytometry in digested lung tumors. Paired t test. B and C, Representative dot plots showing the levels of trogocytosis and killing activity mediated by blood monocytes (B) and PBNs (C) cocultured with PKH67+ A431 cells at the indicated E:T ratios in the presence of cetuximab and IgA anti-EGFR Abs, respectively, for 12 hours. Nonspecific human IgG1 or IgA2 isotype control Abs were used as a control. D and E, Cumulative flow cytometry results showing the ability of indicated FcR+ effectors to kill PKH67+ A431 tumor cells in the presence of cetuximab (IgG) or IgA anti-EGFR (IgA) Abs at a 2:1 E:T ratio in a 12-hour FACS-based assay. One-way ANOVA with Tukey multiple comparisons tests. Summary graphs represent the total tumoricidal activity of effectors calculated as described in Materials and Methods. F–H, Representative images of GFP+A431 tumor cells cocultured with blood monocytes (patient LC441) at different E:T ratios and cetuximab for 48 hours in the IncuCyte Live Cell Analysis System. Image of A431+Ab, where Ab is a cetuximab, is intentionally the same in both Figs. 1H and 4F. Scale bar, 400 μm. Representative experiments showing the kinetics of A431 tumor cell growth when cocultured with blood monocytes (F), PBNs (G), and TAMs (H) in the presence of anti-EGFR Abs at the indicated E:T ratios in the IncuCyte Live Cell Analysis System. I and J, Summary results showing the kinetics of GFP+A431 tumor cell growth when cocultured with FcR+ effectors in the presence of cetuximab or IgA anti-EGFR Ab at 2:1 E:T ratio in the IncuCyte Live Cell System. The percentage of tumor cell growth inhibition/stimulation was calculated at 48 hours. One-way ANOVA with Tukey multiple comparisons tests. K, Representative dot plots and cumulative flow cytometry data demonstrating the expression of EGFR on the surface of EpCam+ cells in tumor and distant lung tissue. Paired t test. MFI, mean fluorescence intensity. L, Representative flow cytometry histograms showing the expression of EGFR on the surface of EpCam+ cells in tumor and distant lung tissue in comparison with A431 and A549 tumor cells. Number of patients is indicated on the graphs. All data represented as mean ± SEM. FcR+ effectors were freshly isolated for all experiments.
We found that regardless of E:T ratio, monocytes interacting with EGFRhiA431 cells in the presence of cetuximab were still able to perform high amounts trogocytosis (>50%; Fig. 4B). However, despite this high level of trogocytosis, monocytes completely lost the ability to kill cetuximab-opsonized tumor cells at a E:T ratio 2:1 (a ratio that could be realistically achieved in lung tumors). Similarly, although PBNs showed high levels of trogocytosis in the presence of IgA anti-EGFR Ab, they lost their tumoricidal activity at an E:T ratio 2:1 (Fig. 4C). Cumulative data obtained from 14 patients showed that neither blood nor tumor myeloid cells were able to directly kill EGFRhiA431tumor cells at low E:T ratio in the presence of either cetuximab or IgA anti-EGFR Abs in the FACS-based killing assay (Fig. 4D and E). Of note, blood, but not tumor NK cells were still able to kill even at the 2:1 ratio (Fig. 4D). Similarly, the IncuCyte assay demonstrated that monocytes (Fig. 4F), PBNs (Fig. 4G), and TAMs (Fig. 4H) failed to exert tumoristatic effects on EGFRhiA431 tumor cells at a 2:1 E:T ratio in the presence of either cetuximab or IgA anti-EGFR abs (Fig. 4I and J), although this effect was obvious at a 50:1 ratio (Fig. 4F–H). However, PBNs (n = 20) and TANs (n = 20) retained the ability to significantly stimulate A431 tumor cell growth at the low E:T ratio in the presence of cetuximab, but not with IgA anti-EGFR Abs (Fig. 4I and J). Tumoricidal activity was not observed with any of the effectors at a E:T ratio 2:1 in the presence of IgG1 and IgA2 isotype control Abs in FACS-based and IncuCyte assays (Supplementary Fig. S7A–S7C).
Together, these data demonstrate that both a tumoricidal level of ADT and a high number of myeloid effectors are requisite for successful killing of opsonized tumors.
It has been documented that EGFR is heterogeneously expressed in human lung tumors and the therapeutic efficacy of cetuximab depends on the density of EGFR (23, 38). Indeed, our flow cytometry analysis of EGFR expression on malignant EpCam+ cells demonstrated wide variability in the expression of EGFR in lung tumors (n = 31), which is consistently higher than in a distant lung tissue (Fig. 4K). Thus, different levels of the targeted tAg could be another variable affecting the success of Ab-triggered tumoricidal activity of myeloid effectors in tumors. To address this issue, we utilized the human NSCLC cell line A549 because the level of the EGFR expression was comparable to that in most lung tumors (Fig. 4L). Also, A549 cells are known to be insensitive to the blockade of EGFR signaling due to the presence of a KRAS mutation (39) and are thus resistant to the Fab-mediated effect of cetuximab. Therefore, the tumoricidal mechanism of anti-EGFR Abs toward A549 cells directly depends on the FcR effectors.
Having analyzed 28 patients with NSCLC, we found that PBNs and monocytes cocultured with EGFRloA549 cells even at a high 50:1 E:T ratio lost their ability to perform tumoricidal ADT in the presence of cetuximab or IgA anti-EGFR Abs (Fig. 5A–C) when compared with EGFRhiA431 cells (Fig. 2E–H). This dramatic reduction in trogocytosis from the lethal to sublethal levels (or even to complete loss) resulted in the failure of monocytes and PBNs to kill EGFRloA549 cells in the presence of cetuximab or IgA anti-EGFR Abs, respectively, even at “favorable for killing” 50:1 E:T ratio (Fig. 5A–D). Noteworthy, blood NK cells were still able to kill EGFRloA549 cells in the presence of cetuximab (Fig. 5D), indicating that this tumor cell line is sensitive to FcR-dependent tumoricidal mechanisms. Likewise, TANs were unable to perform ADT in coculture with EGFRloA549 cells in the presence of either cetuximab or IgA anti-EGFR Abs, while TAMs performed ADT at only sublethal level (Fig. 5E–G). As a result, no killing of EGFRloA549 cells by TANs and TAMs was detected in these cocultures (Fig. 5E–H). The IncuCyte assay also confirmed the inability of blood and tumor myeloid effectors to inhibit EGFRloA549 cell growth in the presence of cetuximab or IgA anti-EGFR Abs even at a “favorable for killing” 50:1 E:T ratio (Fig. 5I–K). Similar to EGFRhiA431 tumor cell line, PBNs and TANs retained their ability to stimulate EGFRloA549 cell growth in the presence of cetuximab and IgA anti-EGFR Abs (Fig. 5J and K), or even without Abs (Fig. 5L). There was no tumoricidal activity mediated by any of the effectors in the presence of IgG1 and IgA2 isotype control Abs in flow cytometry–based and IncuCyte-based killing assays (Supplementary Fig. S7D–S7F). Coculturing of blood and tumor-derived FcR+ effectors with cetuximab-opsonized A549 tumor cell spheroids (Supplementary Fig. S8A and S8B) revealed similar results obtained with A549 cells cultured as a monolayer.
Figure 5.
The ability of blood and tumor myeloid cells to perform ADT and kill EGFRlo A549 cells in the presence of anti-EGFR Abs under conditions representing solid human tumors. A–H, Representative dot plots and cumulative flow cytometry results showing the different levels of ADT and killing activity mediated by PBNs (A, C, and D), blood monocytes (B, C, and D), TANs (E, G, and H), and TAMs (F, G, and H) cocultured with PKH67+A549 cells at a 50:1 E:T ratio in the presence or absence of cetuximab (IgG) or IgA anti-EGFR Abs (IgA) for 12 hours. Summary graphs represent the total tumoricidal activity of effectors calculated as described in Materials and Methods. I, J, and K, Representative experiment and summary results showing the kinetics of A549 tumor cell growth when cocultured with indicated blood and tumor FcR+ effectors in the presence of cetuximab or IgA anti-EGFR Abs at a 50:1 E:T ratio in the IncuCyte Live Cell Analysis System. The percentage of tumor cell growth inhibition/stimulation was calculated at 48 hours. L, Cumulative results showing the kinetics of A549 tumor cell growth cocultured with blood and tumor FcR+ effectors at a 50:1 E:T ratio in the presence of human IgG1 isotype control Abs in the IncuCyte Live Cell Analysis System. The number of patients included in each analysis is indicated on the graphs. All data represented as mean ± SEM. All comparisons used one-way ANOVA with Tukey multiple comparisons tests. FcR+ effectors were freshly isolated for all experiments. M, The schematic representation of requisite conditions in TME that are essential for efficient killing of opsonized tumors by FcR+ effectors or tumor escape from tAbs. (M, Created with BioRender.com.)
Similar results were obtained with another EGFRlo tumor cell line, H-1975 (Supplementary Fig. S8C–S8E). That is, blood and tumor myeloid effectors were not able to perform cetuximab-triggered trogocytosis at the lethal level and thus kill targets even at a “favorable for killing” 50:1 E:T ratio (Supplementary Fig. S8D and S8E).
In summary, our data demonstrate that myeloid effectors have the potential to kill opsonized tumor cells and inhibit their growth only under these three conditions: (i) the tumor cells express a high level of targeted tAg, (ii) the effector cells are present at high E:T ratios, and (iii) the effector cells mediate ADT at a tumoricidal level. If these conditions are unsatisfied, the myeloid cells fail to kill sufficient numbers of opsonized tumor cells to inhibit their growth (Fig. 5M; Supplementary Table S2).
Sublethal trogocytosis facilitates tumor cells to escape from tAb due to the removal of tAb/tAg complexes from the target's surface
Next, we asked what might be the functional consequences of the sublethal ADT mediated by TAMs under the conditions typical for solid human tumors. We hypothesized that one effect might be that persistent sublethal trogocytosis removes tAb/tAg complexes (cetuximab/EGFR in our case) from the surface of tumor cells that might then confer resistance to subsequent attacks by NK cells due to loss of tAb/tAg complexes, thereby further reducing tAb efficacy (Fig. 6A, scheme).
Figure 6.
Role of TAM-mediated ADT in facilitating tumor cell escape from tAbs. A, Scheme proposing the role of TAM-mediated ADT in the downmodulation of cetuximab/EGFR complexes on the surface of opsonized tumor cells that could lead to resistance of targets to subsequent attacks by NK cells. B, Representative flow cytometry histograms showing the expression of cetuximab/EGFR complexes on the surface of cetuximab-opsonized A431 cells cocultured in the presence or absence of TAMs at a 1:1 E:T ratio for 2 hours. C, Cumulative results showing the expression of cetuximab/EGFR complexes on the surface of cetuximab-opsonized A431 and A549 cells cocultured in the presence or absence TAMs at a 1:1 E:T ratio for 2 hours. Wilcoxon matched-pairs signed rank test. D and E, Representative dot plots and cumulative flow cytometry data demonstrating the reduced ability of blood NK cells to kill cetuximab-opsonized A431 and A549 tumor cells in the presence of TAMs. Tumor cell lines were preopsonized with cetuximab and mixed with TAMs at a 1:1 E:T ratio; two hours later, NK cells were added at a 10:1 E:T ratio for additional 12 hours. Wilcoxon matched pairs signed rank test. Some experiments were performed with blocking anti-CD64 F(ab')2 and anti-CD32 F(ab')2 Abs (5 μg/mL) and representative dot plots from one of three experiments are shown. F, The kinetics of A431 tumor cell growth when cocultured with TAMs and blood NK cells in the presence of cetuximab in the IncuCyte Live Cell Analysis System. A431 cells were preopsonized with cetuximab and mixed with TAMs at a 3:1 E:T ratio; two hours later, NK cells were added at a 10:1 E:T ratio. The ability of TAMs to mediate ADT was assessed by flow cytometry in the cocultures of TAMs and cetuximab-opsonized PKH67+A431 cells as described earlier. Two patients (LC#643 and LC#649) are shown. All data represented as mean ± SEM. FcR+ effectors were freshly isolated for all experiments. (A, Created with BioRender.com.)
We first determined whether the density of cetuximab/EGFR complexes on the target's surface was reduced during sublethal ADT observed in the coculture of TAMs with opsonized tumor cells. EGFRhiA431 or EGFRloA549 tumor cells were preincubated with cetuximab and then mixed with TAMs at a ratio 1:1 for 2 hours. The levels of cetuximab/EGFR complexes were then analyzed on the surface of gated EpCam+ tumor cells and compared with opsonized targets cultured without effectors where only the natural internalization of cetuximab/EGFR could occur (40). Given that cetuximab is a humanized IgG1 anti-EGFR Ab, we used an Ab specific for the human Fc fragment of IgG that allowed us to analyze the level of cetuximab/EGFR complexes on the target. We found that the density of cetuximab/EGFR complexes on the surface of opsonized EGFRhiA431 cells was significantly decreased after co-culture with TAM (Fig. 6B and C). Coincubation of TAM with EGFRloA549 cells also downmodulated cetuximab/EGFR complexes (Fig. 6C), although to a much lesser extent due to the lower level of TAM-mediated ADT of EGFRloA549 cells compared to EGFRhiA431cells (Figs. 2K and L and 5F and G). Thus, TAM-mediated ADT results in the decrease of tAb/tAg complexes on the target's surface.
Next, we determined whether this TAM-mediated downmodulation of cetuximab/EGFR complexes by ADT affected the ability of NK cells to perform classical ADCC (Fig. 6A, scheme). We opsonized EGFRhiA431 or EGFRloA549 cells with cetuximab and then preincubated them with freshly isolated TAMs at E:T ratio 1:1 for 2 hours. Under these conditions, TAMs are only able to mediate the sublethal ADT but not tumor cell killing (Figs. 4E and 5F and H). Two hours later, autologous blood NK cells were added to these cocultures for additional 12 hours. We found that NK cell tumoricidal activity was significantly decreased in the presence of TAMs (Fig. 6D and E). To further evaluate the role of TAM-mediated ADT in the inhibition of NK ADCC, we performed these experiments in the presence of blocking anti-CD64 F(ab')2 and anti-CD32 F(ab')2 Abs. The blockade of these FcγRs that are highly expressed only on TAMs (Fig. 1D), did not trigger tumoricidal activity of TAMs (Supplementary Fig. S9A and S9B), but substantially restored the ability of NK cells to kill opsonized A431 and A549 targets, indicating that FcR-mediated trogocytosis contributed to the inhibition of NK-mediated ADCC (Fig. 6D). The inability of FcR blockade to fully restore the tumoricidal ability of NK cells suggests the presence of other non–FcR-dependent suppressive mechanisms mediated by TAMs, as described elsewhere (41). The TAM-mediated suppression of blood NK cell tumoricidal activity was more pronounced in coculture with opsonized EGFRhiA431 cells compared with EGFRloA549 cells (Fig. 6D and E), most likely due to the ability of TAMs to perform a higher level of ADT of EGFRhiA431 (Figs. 2K and L and 5F and G). The IncuCyte assay also revealed that NK cell tumoricidal activity toward cetuximab-opsonized A431 cells was more reduced in cocultures where TAMs mediated higher levels of ADT (Fig. 6F).
These results support the hypothesis that TAM-mediated sublethal ADT represents a mechanism of tumor cell escape from being killed by subsequent FcR+ effectors (i.e., NK cells) during tAb treatment of solid tumors.
TAM-mediated ADT represents a new mechanism of tAg acquisition from live targets that can stimulate cognate T cells
There is compelling evidence that T-cell immunity is necessary for the therapeutic effect of tAb in mice and humans (11, 42). The currently accepted mechanism (derived from murine studies) suggests that first, the opsonized tumor cells should be killed by FcR+ effectors (8). This tumor cell killing then results in the release of tAgs that are up-taken and cross-presented by APC to cognate T cells. Importantly, in all these murine studies, tumor cell lines overexpressing tAg were used. However, as we demonstrated above, human lung tumors do not usually express tAgs at high levels (Fig. 4K) and tumor-infiltrating effectors are not able to directly kill opsonized tumor cells (Figs. 4E and 5H). Nevertheless, the ability of tAb (including cetuximab) to induce tumor-specific T cells has been reported in humans (12, 13, 43). The mechanisms underlying this process in humans are unknown and were thus explored.
We hypothesized that TAMs could acquire different tAgs during sublethal ADT where they can “nibble off” tumor cell membranes containing the cetuximab/EGFR with accompanying HLA class I/peptide membrane complexes and the surrounding tumor cell cytoplasmic fragments with intracellular “bystander” tAgs. These “antigen-loaded” TAM could then either directly present freshly captured intact tumor membrane–derived HLA–peptide complexes (cross-dressing pathway) or internalize and cross-present “bystander” tAg, resulting in stimulation of cognate T-cell responses (cross-presentation pathway).
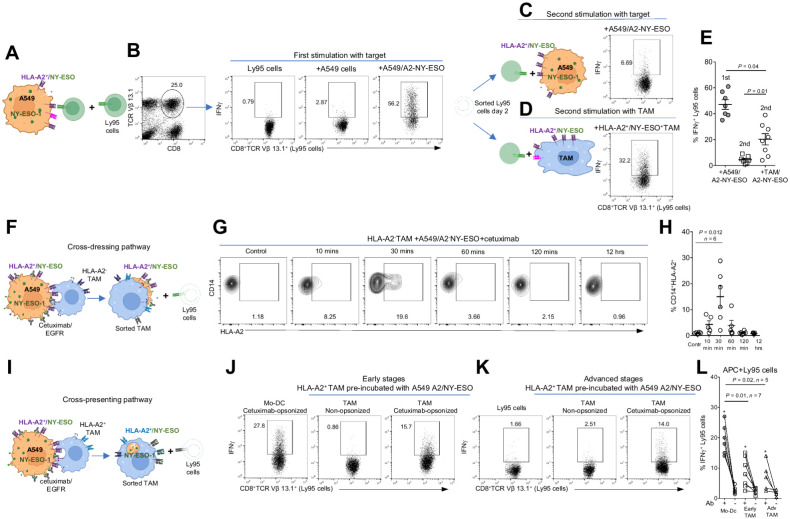
To test our hypothesis, we used our published in vitro model allowing us to investigate human tumor antigen–specific T-cell effector responses (28, 31). We transduced human T cells with a high-affinity transgenic T-cell receptor (TCR) called Ly95 that recognizes an HLA-A*0201–restricted peptide sequence from the human cancer testis antigen NY-ESO-1. We also genetically modified EGFRloA549 human lung adenocarcinoma cells to express the NY-ESO-1 protein and HLA-A*0201 (A549/A2–NY-ESO cells) (Fig. 7A, scheme). When we cocultured Ly95 cells with A549 or A549/A2–NY-ESO cells for 24 hours and measured the production of IFN-γ by the Ly95 cells (identified by an Ab against the Vβ13.1 portion of the TCR), we found that CD8+TCR Vβ 13.1+ Ly95 cells cultured with A549/A2–NY-ESO cells, but not with parental A549 cells, showed robust production of IFNγ (Fig. 7B). These data demonstrated the specificity of the Ly95 cells and showed that activation is not the result of allostimulation. Importantly, the restimulation of purified Ly95 cells with fresh A549/A2–NY-ESO cells led to unresponsive Ly95 cells (Fig. 7C).
Figure 7.
Role of TAM-mediated ADT in tAg uptake from live opsonized targets following stimulation of tumor-specific T cells. A, Scheme showing the in vitro model of human tumor antigen–specific T-cell responses (A549/HLA-A2+NY-ESO tumor cells interacting with NY-ESO–specific Ly95 T cells). B, Representative dot plots demonstrating the Ly95 (CD8+TCR Vβ13.1+) cells and their NY-ESO–specific IFNγ production after exposure to A549/HLA-A2+NY-ESO tumor cells compared with control A549 cells after the first round of stimulation. C and D, Schemes and representative dot plots showing the second round of stimulation of purified Ly95 cells with A549/HLA-A2+NY-ESO tumor cells (C) or HLA-A2+TAM preloaded with NY-ESO157–165 peptide (D) and the production of IFNγ by Ly95 cells in response to the second round of the stimulation. E, Cumulative data of the experiments described in B–D demonstrating the Ly95 cell responses after the first and second round of stimulation (intracellular IFNγ production). Kruskal–Wallis multiple comparison test. F, Scheme showing the ADT-dependent mechanism of Ly95 cell stimulation by HLA-A2neg TAMs that trogocytosed and displayed on their surface the A549 tumor cell membranes containing the surface cetuximab/EGFR and bystander HLA-A2/NY-ESO complexes (cross-dressing pathway). G and H, Representative dot plots and summary results demonstrating the acquisition and display of HLA-A2 molecules on the surface of HLA-A2neg TAMs during their interaction with cetuximab-opsonized A549/HLA-A2+NY-ESO cells at a 1:1 E:T ratio at the indicated time points. Kruskal–Wallis multiple comparisons test. Data represented as mean ± SEM. I, Scheme showing the ADT-dependent mechanism of Ly95 cell stimulation by HLA-A2+ TAM that trogocytosed A549 tumor cell fragments containing cetuximab/EGFR and bystander intact NY-ESO Ag followed by the cross-presentation to Ly95 cells (cross-presentation pathway). J–L, Representative dot plots and cumulative data showing the ability of HLA-A2+ TAMs (from early and advanced stage lung cancers) to stimulate Ly95 cells responses (intracellular IFNγ) after preincubation with A549/HLA-A2+NY-ESO tumor cells in the presence or absence of cetuximab for 4 hours. Mo-DCs were used as a professional APC. Wilcoxon matched pairs test for groups with (+) or without (−) Ab. *, P < 0.01. Kruskal–Wallis test for groups with Mo-DCs, early-stage TAMs, and advanced stage TAMs. FcR+ effectors were freshly isolated for all experiments. (A,C,D,F, and I, Created with BioRender.com.)
Given that the majority of effector T cells are hypofunctional in tumors (44), we first determined whether TAMs presenting a relevant tumor-specific antigenic peptide were able to reinvigorate these hypofunctional T cells. We isolated TAMs from patients with HLA-A2+ lung cancer, pulsed them with NY-ESO-1157–165 peptide, and cocultured them with the hypofunctional Ly95 cells purified after the first round of stimulation with A549/A2–NY-ESO cells. We found that in contrast to the second ineffective stimulation with A549/A2–NY-ESO tumor cells (Fig. 7C and E), HLA-A2+ TAMs preloaded with NY-ESO157–165 peptide were able to reinvigorate these Ly95 cells to produce IFNγ (Fig. 7D and E), although less efficiently when compared with the first round of stimulation with A549/A2–NY-ESO cells (Fig. 7B and E). Thus, TAMs expressing the relevant MHC class I/tumor peptide complexes were able to activate the tumor-specific T cells.
Next, we asked whether TAM could acquire tAg from live opsonized targets. First, we explored the “cross-dressing pathway” (Fig. 7F, scheme), where during sublethal cetuximab-triggered trogocytosis, TAMs could remove EGFR/cetuximab complexes from the opsonized EGFRloA549/A2-NY-ESO targets with accompanying tumor cell membranes containing nearby cell-surface proteins, including HLA-A2/NY-ESO peptide complexes. Via this mechanism, TAMs would display freshly captured tumor cell membranes containing intact HLA-A2/NY-ESO complexes on their surface and activate T cells (Fig. 7F, scheme). To determine whether this process was operative, we used HLA-A2–negative TAMs that would not be able to present processed NY-ESO to the Ly95 T cells. HLA-A2neg TAM were thus cocultured with cetuximab preopsonized A549/A2-NY-ESO tumor cells and the presence of tumor-derived HLA-A2 complexes was analyzed on the HLA-A2neg TAMs by flow cytometry using an anti-HLA-A2 Ab at 10, 30, 60, 120 minutes, and 12 hours of cell coculturing. We found that the highest density of tumor-derived HLA-A2 molecules could be detected on the surface of HLA-A2neg TAMs during the initial 30 minute of coincubation with cetuximab-opsonized A549/A2-NY-ESO cells (Fig. 7G). However, these “trogocytosed” membrane HLA-A2+ molecules disappeared within 2 hours of coincubation (Fig. 7G and H), most likely due to internalization.
Next, we determined whether these HLA-A2neg TAMs with freshly captured tumor cell membranes were capable of directly stimulating Ly95 cells (Fig. 7F, scheme). We opsonized EGFRloA549/HLA-A2+-NY-ESO tumor cells with cetuximab and coincubated with HLA-A2neg TAMs. TAMs were purified from this coculture at 30 minutes, 120 minutes, and 12 hours of coincubation and then exposed to Ly95 T cells overnight. The same cell cocultures without cetuximab were used as negative controls. We found that some TAMs isolated from cocultures at 30 minutes, but not at 120 minutes, were able to stimulate Ly95 cells, although at a relatively low level (Supplementary Fig. S9C). The cumulative data showed a trend but did not reach the statistical significance compared with the negative control (Supplementary Fig. S9D).
We next explored the cross-presentation pathway (Fig. 7I, scheme). We have previously demonstrated the ability of TAMs isolated from some patients with lung cancer to cross-present NY-ESO intact protein and activate tumor-specific effector Ly95 T-cell responses (28). In this study, we determined whether cross-presentation pathway takes place following interactions of TAMs with opsonized tumor cells. Specifically, during sublethal ADT, TAMs could “nibble off” tumor cell membranes along with surrounding tumor cell cytoplasmic fragments containing intracellular “bystander” tAgs (NY-ESO in our case; Fig. 7I, scheme). To address this hypothesis, we isolated TAMs from patients with HLA-A2+ lung cancer and cocultured them with the cetuximab-opsonized EGFR+A549/A2-NY-ESO targets at ratio 1:1 (E:T) for 4 hours to allow the processing and cross-presentation of NY-ESO captured from A549/A2-NY-ESO tumor cells. Under these cell culture conditions, TAMs perform ADT, but do not cause the tumor cell death (Fig. 5F–H). The same cell coculture conditions without cetuximab were used as negative controls. Monocyte-derived DCs (Mo-DC) were used as professional APC controls. Four hours after coincubation, TAMs were purified from this coculture and exposed to Ly95 cells overnight (Fig. 7J). Of note, cross-dressing pathway described above is not operative in this setting because the freshly captured tumor cell-surface HLA-A2+/NY-ESO complexes disappear from the TAMs’ surface within 2 hours of coincubation with opsonized targets (Fig. 7G and H). We found that TAM isolated from some, but not all, patients were able to directly stimulate Ly95 cells, although at a lower level compared with Mo-DCs (Fig. 7J–L). As a key control, we did not observe this Ly95 stimulatory effect of TAMs incubated with nonopsonized tumor cells (Fig. 7J–L). Also, we were able to perform the experiments with TAMs isolated from several patients with advanced stage (III–IV) lung cancer. We found that some of TAMs from patients with advanced stage disease were also able to stimulate Ly95 cells (Fig. 7K and L).
Next, we asked whether the ability of TAMs to cross present NY-ESO depended on the level of its expression in opsonized tumor cells. To address this question, we cocultured cetuximab-opsonized NY-ESOloA549 cells or NY-ESOhiA549 cells (Supplementary Fig. S9E) with HLA-A2+ TAMs at ratio 1:1. Four hours after coincubation, TAMs were purified from this coculture and exposed to Ly95 cells overnight. We found that TAMs cocultured with cetuximab-opsonized NY-ESOhiA549 cells, but not NY-ESOloA549 cells, were able to stimulate NY-ESO–specific Ly95 T cells (Supplementary Fig. S9F–S9H). These data indicate that the high expression of bystander tAg being trogocytosed with cetuximab/EGFR complexes by TAMs is required for successful tAg cross-presentation and the stimulation of cognate T cells. Detailed characteristics of patients involved in the evaluation of ability TAMs to downregulate tAb/tAg complexes and cross-present tAg are shown in Supplementary Table S1E.
These data suggest that even though TAMs are unable to directly kill the opsonized tumor cells, they can, under the correct conditions, acquire tAg from live opsonized targets and stimulate cognate antitumor T cells that have the potential to eventually kill targets.
Discussion
Therapeutic Abs targeting tumor antigens are at the forefront of cancer therapeutics. However, our knowledge about the mechanism of action of these Abs is primarily limited to mechanisms mediated by the blocking ability of the Fab fragment of Abs. Therefore, in this study, we investigated the FcR-mediated effector mechanisms of tAbs mediated by tumor-infiltrating myeloid effectors isolated from lung cancers.
Our data demonstrated that after cetuximab treatment in vitro, blood monocytes, but not PBNs, were able to kill opsonized tumor cells. In tumors, TAMs did not have tumoricidal ability, but were able to exert tumoristatic effects. However, the tumoricidal/tumoristatic activity of monocytes and TAMs could only be observed under specific conditions (tAghi and E:T ratiohi). PBNs and TANs were unable to kill the cetuximab-opsonized tumor cells even under optimized conditions. The difference in cetuximab-triggered tumoricidal activity between monocytes/macrophages and PBNs could be, in part, explained by differences in the expression and function of FcγRs. Monocytes express a high level of the intermediate to high-affinity FcγRIIa (CD32a) and FcγRI (CD64), which are the most potent FcRs for triggering tumoricidal activity (45). In contrast, PBNs express lower levels of CD32 than monocytes, but abundant levels of the low-affinity FcγRIIIb receptor (CD16b). Importantly, in tumors, neutrophils dramatically downregulated all types of FcγRs and were thus unable to respond to cetuximab, while TAMs upregulated CD32 and CD64 and exerted tumoristatic effects on the opsonized target. In addition, CD16b is thought to be a nonsignaling FcγR in neutrophils, and, thus, could actually redirect IgG away from CD32, resulting in insufficient activation of PBNs to deploy their formidable tumoricidal potential (46, 47). It should be noted that the low tumoricidal activity of PBNs on cetuximab-opsonized tumor cells that has been reported in the literature, occurred only at an extremely high 80:1 E:T ratio (9) or after stimulation of the PBNs with G(M)-CSF that dramatically upregulated the high affinity receptor CD64, thus supporting the importance of the high affinity FcγRs in the activation of tumoricidal activity. In our study, resting PBNs could become highly tumoricidal, but only when their highly expressed FcαRI (CD89) was engaged with an anti-EGFR Ab of isotype IgA. Our data are in concordance with other studies demonstrating that CD89 ligation with IgA isotype tAbs triggers tumoricidal activity of neutrophils in vitro toward tumor cell lines expressing various tumor-associated antigens, including EGFR, Her-2/neu, and CD20 (25, 48–50). In contrast to PBNs, stimulation of TANs with IgA anti-EGFR Ab did not result in significant tumor cell killing, despite the fact that CD89 was highly upregulated on TANs, indicating tumor-induced suppression. Noteworthy, the stimulatory effect of TANs on tumor cell growth was overridden by ligation with IgA anti-EGFR Abs, suggesting that TAN killing machinery was engaged but not sufficient, most likely due to effect of suppressive TME.
Importantly, with regard to cancer therapy, we found that in many lung tumors (where the number of effectors is relatively low compared with the number of tumor cells, and tumor cells do not express tAg at high levels), blood and tumor-derived myeloid effectors completely lost their ability to directly kill and inhibit tumor cell growth in the presence of either anti-EGFR Abs of isotype IgA or IgG. Our results could provide some mechanistic explanation of the large FLEX clinical trial that revealed that adding cetuximab to chemotherapy of patients with NSCLC produced a clinical benefit only in individuals with high EGFR levels (23). However, EGFR expression level was only a weak predictor for cetuximab benefit in that trial. Our data suggest that the frequency of TAMs in tumors should also be considered.
One of the key questions of our study was to determine mechanisms by which FcR+ myeloid effectors killed opsonized tumor cells and how tumors cells escaped this killing. Unlike the antibody-dependent phagocytosis of opsonized “small-size” lymphocytic tumors, the interaction of myeloid effectors with opsonized “large-size” epithelial tumor cell lines led to an alternative process termed antibody-dependent trogocytosis. The preponderance of ADT over ADP is likely explained by the physical constraints of the effector being unable to engulf a large-sized epithelial tumor cell. Although the process of ADT has been only studied with peripheral blood myeloid effectors, the functional consequences of ADT have been controversial. For instance, ADT has recently been described as a dominant tumoricidal mechanism by which PBNs kill SK-BR-3 cells or A431 tumor cell lines in the presence of anti-HER2/neu or anti-EGFR Ab of isotype IgG1 or IgA (18, 51). It is important to emphasize that in these studies, prestimulation of PBN with G-CSF and IFNγ was required to achieve the tumoricidal effect, and the tumoricidal effect of resting, non-primed PBN was not studied. Another study has shown that macrophage-mediated trogocytosis resulted in killing of tumor cell lines overexpressing HER-2 in the presence of the anti-HER2/neu Ab trastuzumab (52). In contrast, there have been several studies demonstrating that opsonized tumor cells are resistant to ADT performed by PBNs or monocytes (22, 53, 54). In our study, we found that the effect of ADT on tumor cells could either be lethal or sublethal depending on conditions. ADT was lethal only if (i) the myeloid cells “trogocytosed” a large amount of tumor cell fragments, (ii) the myeloid cells were present at a high E:T ratio (50:1), and (iii) the tumor cells expressed a high density of the targeted tAg. If any of these conditions were not met (which is typical for solid tumors), ADT still occurred; however, its magnitude was insufficient to cause tumor cell death.
Given these observations, it was important to determine the functional outcome of the sublethal ADT that likely occurs in most lung tumors. We found that although sublethal amounts of ADT do not cause immediate tumor cell death, this process appeared to damage the tumor cell membranes, resulting in a tumoristatic effect if the cocultures had a high number of TAMs and the targets expressed high levels of tAg. Under all other conditions, it appeared that the strength of trogocytic attacks were not powerful enough to cause the inhibition of tumor cell growth and, in fact, sublethal trogocytosis began to exert a protumoral effect. Specifically, TAM-mediated sublethal trogocytosis substantially decreased the density of cetuximab/EGFR complexes on the target's surface, resulting in the reduced ability of NK cells to mediate classical ADCC and thereby compromising the therapeutic efficacy of cetuximab. These data are supported by clinical studies demonstrating the downmodulation of different targeted Ags in hematologic cancers after tAb therapy (22, 55, 56). It has also been demonstrated that during the coculture of rituximab-opsonized Daudi lymphoma cells and PBMCs, there is an ongoing competition between monocyte-mediated trogocytosis and NK cell–mediated ADCC that eventually leads to the loss of bound rituximab and lower NK killing ability (57). These results are consistent with our data showing that a similar FcR-dependent process operates in solid tumors during the interaction of TAMs with cetuximab-opsonized targets. Thus, TAM-mediated sublethal trogocytosis represents one of the resistance mechanisms to tAb treatment. However, on the other hand, it has recently been suggested that trogocytosis-mediated downmodulation of tAg could also directly cause the death of tumor cells whose survival depends on the trogocytosis-targeted receptor for signaling (58).
In humans, tAbs do not induce massive direct tumor cell death but appear to activate tumor-specific T-cell responses, that are believed to be an important antitumoral effector mechanism of tAb therapy (12, 13). We discovered a trogocytosis-dependent mechanism that could at least partially explain the activation of tumor-specific T-cell responses in tumors where tAb do not directly kill tumors and thus the massive release of tAg to be up-taken and presented by APC is highly unlikely. During cetuximab-triggered sublethal trogocytosis, TAMs acquire tumor cell fragments and membranes containing cetuximab/EGFR complexes with accompanying other “bystander” tAgs, thus potentially enabling them to present freshly captured tumor membrane-derived HLA/peptide complexes to T cells. This mechanism of acquisition and display of intact tumor-derived HLA/peptide complexes has been previously described and termed as “MHC-I cross-dressing” (59, 60). Several studies have demonstrated that MHC-I cross-dressing may play an important role for antitumor CD8+ T-cell priming by DCs (61, 62). In our experimental setting, the stimulation of tumor-specific T cells by “cross-dressed” human TAMs was present, but weak, due to quick disappearance of MHC-I/peptide complexes from the TAMs’ surface. Of perhaps more importance, we also observed that during sublethal trogocytosis, TAMs could acquire tumor cell fragments with bystander tumor proteins and then process them to allow cross-presentation of these tAg to cognate T cells causing the stimulation of T-cell responses. These stimulated tumor-specific T cells would presumably then kill targets and activate the more efficient classical mechanism of stimulated T-cell responses when released tAgs from dying tumor cells are cross-presented to cognate T cells by professional APC.
There is an ongoing discussion about the ability of APC to efficiently trigger tumor-specific T-cell responses, especially in human tumors. On the one hand, the poor immunogenicity of tAg and the presence of immunosuppressive macrophages and tolerogenic DCs present major barriers for the induction of effective adaptive antitumor immunity. Conversely, there is growing evidence indicating the ability of specialized subsets of DCs and macrophages in tissues and lymph nodes to cross-present tAg (63, 64). Furthermore, there is compelling evidence for the stimulatory role of tAb and FcR in the function of APC. For example, tAbs are able to trigger: (i) FcR-induced amelioration of suppressive APC in patients with cancer (65), (ii) FcR-mediated increases in the expression of MHC II molecules and T-cell costimulatory molecules on APC (66), (iii) FcR-mediated augmentation of the cross-priming of CD8 cells (67, 68), and (iv) the immunogenicity of opsonized tumor cells (69). These studies strongly support our findings about the acquired ability of FcR-stimulated TAMs to cross-present tAg and stimulate cognate T-cell response during the tAb treatment.
This study has certain limitations. We investigated the role of human tumor FcR effectors in the regulation of tAb efficacy using in vitro and ex vivo models that may not faithfully reflect events in tumors of patients with cancer. However, there is no perfect in vivo approach to model the tumoricidal and T-cell responses mediated by human FcR+ effectors in mouse models due to various crucial differences between human and mice including Ab cross-reactivity, immunogenicity, pharmacokinetics, biology of FcR, and myeloid cell effectors, and affinity to immunoglobulin G subclasses (2–5, 70–72). To date, immunodeficient mouse models are being actively developed to overcome these limitations (73) and thus could have been suitable for evaluating direct effects of human FcR effectors on targets. However, given that human tumor-infiltrating myeloid effectors do not mediate the direct cytotoxic effect on opsonized tumor cells even in vitro, and that adaptive T-cell immunity appears to be potent in mediating the tumoricidal effect of tAbs, these immunodeficient mice were not useful for this study. Therefore, we are unaware of any murine experimental system that can faithfully represent the biology of human FcR+ effectors and their effects on T-cell responses during tAbs therapy to yield sound and clinically relevant insights. Another limitation is that we were not able to evaluate the tAb-triggered effect of FcR effectors on primary tumor cells in our assays due to their rapid cell death in cell culture in vitro. Also, we could not assess the role of tumor-infiltrating DC in the regulation of tAbs efficacy because the human lung tumors accumulate a very low number of DCs. However, given our data obtained with monocyte-derived DC (Fig. 7J and L), we speculate that tumor-infiltrating DC activated through their FcRs could also play an important role in stimulating antitumor T-cell responses during tAb therapy. Finally, we studied only one type of tumor and only one tAg due to the limitation in accessing and acquisition of the freshly resected tumors from a large cohort of patients with other types of cancer.
In summary, we found that TANs and TAMs comprise the majority of FcR+ effectors in human lung tumors. However, these effectors are unable to directly kill opsonized tumor cells, but instead, could facilitate tumor cell escape from tAb therapy due to the removal of surface tAg/Ab complexes. Although there are multiple suppressive mechanisms in the TME (i.e., presence of PGE2, TGFβ, suppressive protumoral TAMs and TANs), an important reason for the inability of myeloid effector cells to use their tumoricidal potential in the tumor is the absence of the requisite conditions (E:T ratiohi and tAghi) that are essential for efficient killing by myeloid cells. Even fully functional blood monocytes and PBNs lose their ability to kill tAbs-opsonized targets under the conditions recapitulating the TME (E:T ratiolo and tAglo). To date, many different approaches have been developed to improve FcR-mediated effector mechanisms of tAbs. These approaches aimed at enhancing the Ab-binding affinity to activating FcR receptors or decreasing the Ab binding to the inhibitor CD32b receptor using various types of Fc engineering to change glycosylation and amino acid residues, engineering bispecific antibodies that target two tumor Ags simultaneously, and designing Ab cross-isotype or cross-subclass exchanges (37, 74). Unfortunately, these strategies have yet not shown significant clinical improvements, most likely due to inability to overcome the presence of the “unfavorable for killing” conditions (E:T ratiolo and tAglo) in human solid tumors. Thus, our data suggest that therapeutic approaches to enhance the efficacy of tAbs by stimulation of the tumoricidal activity of myeloid effectors need to be combined with strategies aimed at generating more “favorable for killing” conditions and weakening the tumor-induced suppressive mechanisms. Also, given the ability of TAMs (and possibly DCs) to uptake tAg from opsonized live targets, cross-present it, and subsequently stimulate the cognate T-cell responses, therapeutic approaches directed to enhancing adaptive immunity could be another fruitful strategy to improve the FcR-mediated efficacy of tAbs in human solid tumors. Indeed, our results potentially provide some new mechanistic explanations for the recently observed promising clinical activity of cetuximab combined with checkpoint inhibitors in clinical trials (75).
Supplementary Material
Suppl movie 2, trogocytosis Mph A431
Suppl movie 3, Neutrophil sublethal trogocytosis
Suppl Movie 4, monocyte lethal trogocytosis
Supplementary Figure S1. (A and B) Flow cytometric analysis of indicated markers of NK and DC in the single cell suspension obtained from digested lung tumor. (C) The comparison among the frequencies of tumor FcR+ effectors and lung tumor stages; All data represented as mean ± SEM. One-way ANOVA with Turkey’s multiple comparisons test. (D) Cumulative flow cytometry results showing the ability of blood and tumor FcR+ effectors to kill PKH-67+A431 tumor cells in the presence of nonspecific human IgG1 isotype control Abs (1ug/ml) at a 50:1 E:T ratio in a 12 hrs FACS-based assay. (E) Cumulative results showing the kinetics of GFP+A431 tumor cell growth when co-cultured with blood and tumor FcR+ effectors for 48 hrs at 50:1 E:T ratio in the presence of IgG1 isotype control Abs in the IncuCyte® Live Cell Analysis System. One-way ANOVA with Dunnett’s multiple comparisons test. (F) Cumulative results showing the kinetics of GFP+A431 tumor cell growth co-cultured with PBN isolated from lung cancer patients and healthy volunteers for 48 hrs at a 50:1 E:T ratio in the presence of human IgG1 isotype control Abs in the IncuCyte® Live Cell Analysis System.
Supplementary Table S2. Heatmap showing comparisons of the abilities of different types of blood and tumor FcR+ effectors to affect tumor cell growth depending on targeted tAg density, E:T ratios, and anti-tumor Ab isotypes. The indicated FcR+ effectors were added to EGFRhiA431 (high tAg density) or EGFRloA549 (low tAg density) tumor cells at different E:T ratios in the presence of cetuximab (IgG) or IgA anti-EGFR Ab (IgA). The effect on the tumor cell growth was determined at 48 hours using the IncuCyte Live Cell Analysis System. The numbers on the heatmap represent the percent of inhibition of tumor growth. A positive value indicates the inhibition of tumor growth (degrees of red color). A negative value indicates the stimulation of tumor cell growth (degrees of green color). The numbers represent the mean values obtained from experiments shown in Fig.1I, 1K, 4I, and 4J.
Supplementary Table S1. Patient characteristics
Supplementary Figure S2. (A-D) The correlation analyses of the ability of blood and tumor-derived FcR+ effectors to regulate A431 tumor cell growth in the presence of anti-EGFR Abs with key clinical parameters of lung cancer patients including tumor type, stage, size, and smoking history. The effect of FcR+ effectors on A431 tumor cell growth was evaluated in the IncuCyte® Live Cell Analysis System. AC-adenocarcinoma, SCC-squamous cell carcinoma. Pearson test. Number of patients included in each analysis is indicated on the graphs.
Supplementary Figure S3. The correlation analyses between the ability of blood and tumor FcR+ myeloid effectors to regulate A431 tumor cell growth in the presence of anti-EGFR Abs and the expression of indicated FcR on the surface of these effectors. The effect of FcR+ effectors on A431 tumor cell growth was evaluated in IncuCyte® Live Cell Analysis System. The expression of FcR was analyzed in a single-cell suspension obtained from digested tumors by flow cytometry. Spearman test. Number of patients included in each analysis is indicated on the graphs
Supplementary Figure S4. (A) Cumulative results showing the kinetics of GFP+A431 tumor cell growth when co-cultured with PBN isolated from lung cancer patients at 50:1 E:T ratio in the presence of cetuximab (IgG) and IgA anti-EGFR Ab (IgA) using the IncuCyte® Live Cell Analysis System. One-way ANOVA with Turkey’s multiple comparisons test. Number of patients included in each analysis is indicated on the graphs. All data represented as mean ± SEM. (B) Cumulative results showing the kinetics of GFP+A431 tumor cell growth when co-cultured with blood and tumor FcR+ effectors for 48 hrs at 50:1 E:T ratio in the presence of IgA2 isotype control Abs in the IncuCyte® Live Cell Analysis System. One-way ANOVA with Dunnett's multiple comparisons test. All data represented as mean ± SEM. (C) Representative dotplots showing ADT mediated by TAM pretreated with cytohalasin D (1uM) or blocking anti-CD64 F(ab')2 and anti-CD32 F(ab')2 fragments (5ug/ml). TAM were co-cultured with PKH67+ A431 cells at a ratio 3:1 in the presence of cetuximab (1ug/ml) for 2 hrs. (D) Cumulative flow cytometry results showing the trogocytosis mediated by indicated blood and tumor myeloid effectors co-cultured with PKH67+A431 cells for 12 hrs at a 50:1 E:T ratio in the presence of IgG1 and IgA2 isotype control Abs (1ug/ml). Indicated FcR+ effectors were freshly isolated for all experiments.
Supplementary Figure S5. (A) Representative dotplots showing the levels of trogocytosis and killing activity mediated by TAM co-cultured with cetuximab-opsonized PKH67+A431 cells at a 50:1 E:T ratio for 12 hrs in the presence of blocking anti-CD32a Ab (clone 2B6) or blocking anti-CD32a Ab (clone 6G11). (B and C) Summary results showing the levels of trogocytosis (B) and killing activity (C) mediated by blood monocytes and TAM co-cultured with cetuximab-opsonized PKH67+A431 cells at a 50:1 E:T ratio for 12 hrs in the presence of blocking anti-CD32a Ab (clone2B6) or anti-CD32a Ab (clone 6G11). Blocking anti-CD32a Ab were added at concentration (10ug/ml). Summary graphs represent the total tumoricidal activity of effectors that was calculated as described in material and methods. IC Ab-isotype control Abs. (D) Representative experiment and cumulative results showing the kinetics of GFP+A431 tumor cell growth with blood monocytes or TAM at a 50:1 E:T ratio in the presence of cetuximab and blocking anti-CD32a Ab (clone2B6) or blocking anti-CD32a Ab (clone 6G11) in the IncuCyte® Live Cell Analysis System. Percentage of tumor cell growth inhibition/stimulation was calculated at 60 hrs as described earlier. Number of patients included in each analysis is indicated on the graphs. One-way ANOVA with Friedman multiple comparisons test. All data represented as mean ± SEM.
Supplementary Figure S6. (A and B) Representative experiment and summary results showing the effect of indicated blood and tumor FcR+ effectors on the growth of A431 tumor cell spheroids in the presence of cetuximab (1ug/ml). GFP+A431 cells were seeded at 500 cells/well in a round bottom ultra-low adhesion cell culture plate designed to form spheroids. Indicated FcR+ effectors were added at E:T ratio 50:1 into the spheroid cultures. The growth of tumor spheroids was monitored in the IncuCyte® Live Cell Analysis System for 48 hrs. Representative images of GFP+A431 tumor cell spheroids co-cultured with indicated effectors and cetuximab for 48 hrs taken from IncuCyte® Live Cell Analysis System (original magnification x10). Scale bar, 400 µm. Percentage of tumor cell growth inhibition/stimulation in the presence of anti-EGFR Abs was calculated at 48 hrs using the formula: (FI)(A431+Ab)-FI(A431+effectors+Ab)/FI(A431+Ab)x100%, FI-Integrated fluorescence intensity. Data are represented as mean ± SEM. Wilcoxon matched pairs test (n=7). (C) Representative dotplots showing the different levels of ADT mediated by indicated FcR+ effectors co-cultured with spheroids formed with PKH67-labeled A431 cells. FcR+ effectors were co-cultured at a E:T ratio 50:1 in the presence or absence of cetuximab for 12 hrs. Representative results from 1 of 3 experiments are shown. (D and E) Representative experiment and summary results showing the effect of PBN and TAN on the growth of GFP+A431 tumor cell spheroids in the presence of IgA anti-EGFR Ab (IgA) (1ug/ml). Experiments were performed as described in (A and B). Original magnification x10. Scale bar, 400 µm. Data are represented as mean ± SEM. Wilcoxon matched pairs test (n=5). (F) Representative dotplots showing the ADT mediated by PBN and TAN co-cultured with spheroids formed with PKH67-lableld A431 cells. PBN and TAN were co-cultured at a E:T ratio 50:1 in the presence or absence of IgA anti-EGFR Ab for 12 hrs. Representative results from 1 of 3 experiments are shown.
Supplementary Figure S7. (A) Cumulative flow cytometry results showing the ability of blood and tumor FcR+ effectors to kill PKH-67+A431 tumor cells in the presence of nonspecific human IgG1 or IgA2 isotype control Abs (1ug/ml) at a 2:1 E:T ratio in a 12 hrs FACS-based assay. (B and C) Cumulative results showing the kinetics of GFP+A431 tumor cell growth when co-cultured with blood and tumor FcR+ effectors for 48 hrs at a 2:1 E:T ratio in the presence of IgG1 or IgA2 isotype control Abs (1ug/ml) in the IncuCyte® Live Cell Analysis System. (D) Cumulative flow cytometry results showing the ability of blood and tumor FcR+ effectors to kill PKH-67+A549 tumor cells in the presence of nonspecific human IgG1 or IgA2 isotype control Abs (1ug/ml) at a 50:1 E:T ratio in a 12 hrs FACS-based assay. (E and F) Cumulative results showing the kinetics of GFP+A549 tumor cell growth when co-cultured with blood and tumor FcR+ effectors for 48 hrs at a 50:1 E:T ratio in the presence of IgG1 or IgA2 isotype control Abs (1ug/ml) in the IncuCyte® Live Cell Analysis System. All comparisons used one-way ANOVA with Dunnett’s multiple comparisons test. All Data are represented as mean ± SEM. Indicated FcR+ effectors were freshly isolated for all experiments.
Supplementary Figure S8. (A and B) Representative experiment and summary results showing the effect of blood and tumor FcR+ effectors on the growth of GFP+A549 tumor cell spheroids in the presence of cetuximab (1ug/ml). Experiments were performed as described in supplemental figure 5 (A and B). Original magnification x10. Scale bar, 400 µm. Data are represented as mean ± SEM. Wilcoxon matched pairs test (n=6). Indicated FcR+ effectors were freshly isolated for all experiments. (C) Flow cytometry histogram showing the expression of EGFR on the surface of H1975 tumor cell line in comparison with A431 and A549 tumor cells. (D and E) Cumulative flow cytometry results showing the different levels of trogocytosis and killing activity mediated by blood and tumor FcR+ effectors co-cultured with PKH67+ H1975 tumor cells at a 50:1 E:T ratio in the presence of cetuximab or isotype control Abs for 12 hrs. All comparisons used one-way ANOVA with Turkey’s multiple comparisons tests. All data represented as mean ± SEM. FcR+ effectors were freshly isolated for all experiments. IC-isotype control Ab.
Supplementary Figure S9. (A and B) Representative dotplots and summary results showing the ability of TAM pretreated with blocking anti-CD64 F(ab')2 and anti-CD32 F(ab')2 fragments (5ug/ml) to kill PKH67+ A431 cells at a 1:1 E:T ratio in the presence of cetuximab (1ug/ml) in a 12 hrs FACS-based assay. Dead A431 tumor cells were defined as TO-PRO-3+PKH67+ cells. (C and D) Representative dotplots and summary results demonstrating the ability of HLA-A2neg TAM to stimulate of Ly95 cells responses (intracellular IFN-γ) after their preincubation with cetuximab-opsonized A549/HLA-A2+NY-ESO tumor cells at the indicated time points. Kruskal-Wallis multiple comparisons test. Data represented as mean ± SEM. (E) The expression of NY-ESO-1 protein in different batches of transduced A549 tumor cells. The expression of intracellular NY-ESO was evaluated by flow cytometry using NY-ESO-1 mAb (clone D1Q2U) that recognizes endogenous levels of total NY-ESO-1 protein. Non transduced A549 cells were used as a control. (F-H) Representative dotplots and cumulative data showing the ability of HLA-A2+TAM to stimulate of Ly95 cells responses (intracellular IFN-γ) after preincubation with A549/HLA-A2+NY-ESOhi (F) and A549/HLA-A2+NY-ESOlo tumor cells (G) in the presence or absence of cetuximab for 4 hrs. Wilcoxon matched pairs test for groups with (+) or without (-) Ab. * p<0,01. Kruskal-Wallis test for TAM groups co-cultured with A549/HLA-A2+NY-ESOhi or A549/HLA-A2+NY-ESOlo. Data represented as mean ± SEM. FcR+ effectors were freshly isolated for all experiments.
Supplementary Movie S1. TAM-mediated phagocytosis of rituximab-opsonized Daudi tumor cells. PKH26-labeled TAM (red) were incubated with PKH67-labeled Daudi tumor cells (green) at a 3:1 E:T ratio for 2 hrs in the presence of rituximab (1ug/ml). Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope. The images were taken every 5 min with Hamamatsu ImagEM 16-bit cooled EMCCD camera at 20x magnification. Supplementary Movie S2. TAM-mediated trogocytosis of cetuximab-opsonized A431 tumor cells. PKH26-labeled TAM (red) were incubated with PKH67-labeled A431 tumor cells (green) at a 3:1 E:T ratio for 2 hrs in the presence of cetuximab (1ug/ml). Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope. The images were taken every 5 min with Hamamatsu ImagEM 16-bit cooled EMCCD camera at 20x magnification. Supplementary Movie S3. Sublethal trogocytosis performed by PBN during their interaction with cetuximab–opsonized A431 tumor cells. PKH26-labeled PBN (red) were incubated with PKH67-labeled A431 targets (green) at a 10:1 E:T ratio for 2 hrs in the presence of cetuximab (1ug/ml). Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope. The images were taken every 5 min with Hamamatsu ImagEM 16-bit cooled EMCCD camera at 20x magnification. Supplementary Movie S4. Lethal trogocytosis performed by blood monocytes during their interaction with cetuximab–opsonized A431 tumor cells. PKH26-labeled monocytes (red) were incubated with PKH67-labeled A431 targets (green) at a 10:1 E:T ratio for 2 hrs in the presence of cetuximab (1ug/ml). Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope. The images were taken every 5 min with Hamamatsu ImagEM 16-bit cooled EMCCD camera at 20x magnification.
Suppl movie 1, Mph Daudi phagocytosis
Acknowledgments
This work was supported by the NIH (RO1 CA187392–01 to E. Eruslanov) and Janssen Pharmaceuticals Inc. (to E. Eruslanov). N.T. Sullivan was supported by the NIH T32 training grant (CA009140). G. Georgiou acknowledges support from Clayton Foundation. We thank F. Tuluc and other members of Flow Cytometry Core Lab (Children's Hospital of Philadelphia) for technical support.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Authors' Disclosures
N.T. Sullivan reports grants from NIH T32 during the conduct of the study. G. Georgiou reports compensation from Asher Bio and Amgen and has received royalties for technologies unrelated to this work from Grifols, Cell Signaling Technology, and Abcam. E.B. Eruslanov reports grants from NIH NCI, NIH T32, and Janssen Pharmaceuticals during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
S. Singhal: Data curation, formal analysis, funding acquisition, investigation, methodology, writing–review and editing. A.S. Rao: Data curation, formal analysis, investigation, methodology. J. Stadanlick: Data curation, formal analysis, investigation. K. Bruns: Data curation, formal analysis, investigation. N.T. Sullivan: Data curation, formal analysis. A. Bermudez: Data curation, formal analysis. A. Honig-Frand: Data curation, formal analysis. R. Krouse: Data curation, formal analysis. S. Arambepola: Data curation, formal analysis. E. Guo: Data curation, formal analysis. E.K. Moon: Resources, formal analysis, methodology. G. Georgiou: Resources, data curation, investigation, writing–review and editing. T. Valerius: Resources, methodology, writing–review and editing. S.M. Albelda: Conceptualization, formal analysis, supervision, writing–review and editing. E.B. Eruslanov: Conceptualization, data curation, formal analysis, supervision, funding acquisition, investigation, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol 2010;28:4390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731–8. [DOI] [PubMed] [Google Scholar]
- 3. Eruslanov EB, Singhal S, SM A. Mouse versus human neutrophils in cancer: a major knowledge gap. Trends Cancer 2017;3:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev 2015;268:25–51. [DOI] [PubMed] [Google Scholar]
- 5. Loisel S, Ohresser M, Pallardy M, Dayde D, Berthou C, Cartron G, et al. Relevance, advantages and limitations of animal models used in the development of monoclonal antibodies for cancer treatment. Crit Rev Oncol Hematol 2007;62:34–42. [DOI] [PubMed] [Google Scholar]
- 6. Nimmerjahn F, Gordan S, Lux A. FcgammaR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol 2015;36:325–36. [DOI] [PubMed] [Google Scholar]
- 7. Dechant M, Weisner W, Berger S, Peipp M, Beyer T, Schneider-Merck T, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res 2008;68:4998–5003. [DOI] [PubMed] [Google Scholar]
- 8. DiLillo DJ, Ravetch JV. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell 2015;161:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trivedi S, Srivastava RM, Concha-Benavente F, Ferrone S, Garcia-Bates TM, Li J, et al. Anti-EGFR targeted monoclonal antibody isotype influences antitumor cellular immunity in head and neck cancer patients. Clin Cancer Res 2016;22:5229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, Derer S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol 2010;184:512–20. [DOI] [PubMed] [Google Scholar]
- 11. Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010;18:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, et al. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a “vaccinal effect” of rituximab. Blood 2009;113:3809–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kansy BA, Shayan G, Jie HB, Gibson SP, Lei YL, Brandau S, et al. T cell receptor richness in peripheral blood increases after cetuximab therapy and correlates with therapeutic response. Oncoimmunology 2018;7:e1494112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Egmond M, Bakema JE. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin Cancer Biol 2013;23:190–9. [DOI] [PubMed] [Google Scholar]
- 15. Andre P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 2018;175:1731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol 2015;6:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onozato ML, Kovach AE, Yeap BY, Morales-Oyarvide V, Klepeis VE, Tammireddy S, et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am J Surg Pathol 2013;37:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep 2018;23:3946–59. [DOI] [PubMed] [Google Scholar]
- 19. Pham T, Mero P, Booth JW. Dynamics of macrophage trogocytosis of rituximab-coated B cells. PLoS One 2011;6:e14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beum PV, Mack DA, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J Immunol 2008;181:8120–32. [DOI] [PubMed] [Google Scholar]
- 21. Taylor RP, MA L. Fcgamma-receptor-mediated trogocytosis impacts mAb-based therapies: historical precedence and recent developments. Blood 2015;125:762–6. [DOI] [PubMed] [Google Scholar]
- 22. Jones JD, Hamilton BJ, Rigby WF. Rituximab mediates loss of CD19 on B cells in the absence of cell death. Arthritis Rheum 2012;64:3111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 2012;13:33–42. [DOI] [PubMed] [Google Scholar]
- 24. Lynch TJ, Patel T, Dreisbach L, McCleod M, Heim WJ, Hermann RC, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010;28:911–7. [DOI] [PubMed] [Google Scholar]
- 25. Lohse S, Meyer S, Meulenbroek LA, Jansen JH, Nederend M, Kretschmer A, et al. An anti-EGFR IgA that displays improved pharmacokinetics and myeloid effector cell engagement in vivo. Cancer Res 2016;76:403–17. [DOI] [PubMed] [Google Scholar]
- 26. Quatromoni JG, Singhal S, Bhojnagarwala P, Hancock WW, Albelda SM, Eruslanov E. An optimized disaggregation method for human lung tumors that preserves the phenotype and function of the immune cells. J Leukoc Biol 2015;97:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 2014;124:5466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singhal S, Stadanlick J, Annunziata MJ, Rao AS, Bhojnagarwala PS, O'Brien S, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med 2019;11:eaat1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singhal S, Bhojnagarwala PS, O'Brien S, Moon EK, Garfall AL, Rao AS, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 2016;30:120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 1999;223:77–92. [DOI] [PubMed] [Google Scholar]
- 31. Moon EK, Ranganathan R, Eruslanov E, Kim S, Newick K, O'Brien S, et al. Blockade of programmed death 1 augments the ability of human T cells engineered to target NY-ESO-1 to control tumor growth after adoptive transfer. Clin Cancer Res 2016;22:436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roghanian A, Teige I, Martensson L, Cox KL, Kovacek M, Ljungars A, et al. Antagonistic human FcgammaRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer Cell 2015;27:473–88. [DOI] [PubMed] [Google Scholar]
- 33. Meyer T, Robles-Carrillo L, Davila M, Brodie M, Desai H, Rivera-Amaya M, et al. CD32a antibodies induce thrombocytopenia and type II hypersensitivity reactions in FCGR2A mice. Blood 2015;126:2230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, et al. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology 2007;121:392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res 2007;13:1552–61. [DOI] [PubMed] [Google Scholar]
- 36. Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol 2003;21:177–204. [DOI] [PubMed] [Google Scholar]
- 37. Gul N, van Egmond M. Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res 2015;75:5008–13. [DOI] [PubMed] [Google Scholar]
- 38. Seo Y, Ishii Y, Ochiai H, Fukuda K, Akimoto S, Hayashida T, et al. Cetuximab-mediated ADCC activity is correlated with the cell surface expression level of EGFR but not with the KRAS/BRAF mutational status in colorectal cancer. Oncol Rep 2014;31:2115–22. [DOI] [PubMed] [Google Scholar]
- 39. Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, Lindeman N, et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst 2005;97:1185–94. [DOI] [PubMed] [Google Scholar]
- 40. Sunada H, Magun BE, Mendelsohn J, MacLeod CL. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc Natl Acad Sci USA 1986;83:3825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Molgora M, Supino D, Mavilio D, Santoni A, Moretta L, Mantovani A, et al. The yin-yang of the interaction between myelomonocytic cells and NK cells. Scand J Immunol 2018;88:e12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med 2016;22:1402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferris RL, Lenz HJ, Trotta AM, Garcia-Foncillas J, Schulten J, Audhuy F, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev 2018;63:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell 2018;33:547–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Valerius T, Repp R, de Wit TP, Berthold S, Platzer E, Kalden JR, et al. Involvement of the high-affinity receptor for IgG (Fc gamma RI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy. Blood 1993;82:931–9. [PubMed] [Google Scholar]
- 46. Treffers LW, van Houdt M, Bruggeman CW, Heineke MH, Zhao XW, van der Heijden J, et al. FcgammaRIIIb restricts antibody-dependent destruction of cancer cells by human neutrophils. Front Immunol 2018;9:3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nagarajan S, Venkiteswaran K, Anderson M, Sayed U, Zhu C, Selvaraj P. Cell-specific, activation-dependent regulation of neutrophil CD32A ligand-binding function. Blood 2000;95:1069–77. [PubMed] [Google Scholar]
- 48. Brandsma AM, Bondza S, Evers M, Koutstaal R, Nederend M, Jansen JHM, et al. Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to IgG. Front Immunol 2019;10:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rouwendal GJ, van der Lee MM, Meyer S, Reiding KR, Schouten J, de Roo G, et al. A comparison of anti-HER2 IgA and IgG1 in vivo efficacy is facilitated by high N-glycan sialylation of the IgA. MAbs 2016;8:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pascal V, Laffleur B, Debin A, Cuvillier A, van Egmond M, Drocourt D, et al. Anti-CD20 IgA can protect mice against lymphoma development: evaluation of the direct impact of IgA and cytotoxic effector recruitment on CD20 target cells. Haematologica 2012;97:1686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Treffers LW, Ten Broeke T, Rosner T, Jansen JHM, van Houdt M, Kahle S, et al. IgA-mediated killing of tumor cells by neutrophils is enhanced by CD47-SIRPalpha checkpoint inhibition. Cancer Immunol Res 2020;8:120–30. [DOI] [PubMed] [Google Scholar]
- 52. Velmurugan R, Challa DK, Ram S, Ober RJ, Ward ES. Macrophage-mediated trogocytosis leads to death of antibody-opsonized tumor cells. Mol Cancer Ther 2016;15:1879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Valgardsdottir R, Cattaneo I, Klein C, Introna M, Figliuzzi M, Golay J. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood 2017;129:2636–44. [DOI] [PubMed] [Google Scholar]
- 54. Beum PV, Kennedy AD, Williams ME, Lindorfer MA, RP T. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol 2006;176:2600–9. [DOI] [PubMed] [Google Scholar]
- 55. Rossi EA, Goldenberg DM, Michel R, Rossi DL, Wallace DJ, Chang CH. Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab. Blood 2013;122:3020–9. [DOI] [PubMed] [Google Scholar]
- 56. Krejcik J, Frerichs KA, Nijhof IS, van Kessel B, van Velzen JF, Bloem AC, et al. Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin Cancer Res 2017;23:7498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beum PV, Lindorfer MA, Taylor RP. Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J Immunol 2008;181:2916–24. [DOI] [PubMed] [Google Scholar]
- 58. Vijayaraghavan S, Lipfert L, Chevalier K, Bushey BS, Henley B, Lenhart R, et al. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Ther 2020;19:2044–56. [DOI] [PubMed] [Google Scholar]
- 59. Dolan BP, Gibbs KD Jr., Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol 2006;177:6018–24. [DOI] [PubMed] [Google Scholar]
- 60. Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 2011;471:629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Duong E, Fessenden TB, Lutz E, Dinter T, Yim L, Blatt S, et al. Type I interferon activates MHC class I-dressed CD11b(+) conventional dendritic cells to promote protective anti-tumor CD8(+) T cell immunity. Immunity 2022;55:308–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. MacNabb BW, Tumuluru S, Chen X, Godfrey J, Kasal DN, Yu J, et al. Dendritic cells can prime anti-tumor CD8(+) T cell responses through major histocompatibility complex cross-dressing. Immunity 2022;55:982–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of t cell immunity in melanoma. Cancer Cell 2016;30:324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity 2011;34:85–95. [DOI] [PubMed] [Google Scholar]
- 65. Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer 2015;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Junker F, Gordon J, Qureshi O. Fc gamma receptors and their role in antigen uptake, presentation, and T cell activation. Front Immunol 2020;11:1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation ofcCellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med 2002;195:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med 2002;195:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pozzi C, Cuomo A, Spadoni I, Magni E, Silvola A, Conte A, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med 2016;22:624–31. [DOI] [PubMed] [Google Scholar]
- 70. Masopust D, Sivula CP, Jameson SC. Of mice, dirty mice, and men: using mice to understand human immunology. J Immunol 2017;199:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol 2014;34:433–54. [PubMed] [Google Scholar]
- 72. Nauseef WM. Human neutrophils ≠ murine neutrophils: Does it matter? Immunol Rev 2022;314:442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chuprin J, Buettner H, Seedhom MO, Greiner DL, Keck JG, Ishikawa F, et al. Humanized mouse models for immuno-oncology research. Nat Rev Clin Oncol 2023;20:192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dixon KJ, Wu J, Walcheck B. Engineering anti-tumor monoclonal antibodies and Fc receptors to enhance ADCC by human NK cells. Cancers (Basel) 2021;13;312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sacco AG, Chen R, Worden FP, Wong DJL, Adkins D, Swiecicki P, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol 2021;22:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl movie 2, trogocytosis Mph A431
Suppl movie 3, Neutrophil sublethal trogocytosis
Suppl Movie 4, monocyte lethal trogocytosis
Supplementary Figure S1. (A and B) Flow cytometric analysis of indicated markers of NK and DC in the single cell suspension obtained from digested lung tumor. (C) The comparison among the frequencies of tumor FcR+ effectors and lung tumor stages; All data represented as mean ± SEM. One-way ANOVA with Turkey’s multiple comparisons test. (D) Cumulative flow cytometry results showing the ability of blood and tumor FcR+ effectors to kill PKH-67+A431 tumor cells in the presence of nonspecific human IgG1 isotype control Abs (1ug/ml) at a 50:1 E:T ratio in a 12 hrs FACS-based assay. (E) Cumulative results showing the kinetics of GFP+A431 tumor cell growth when co-cultured with blood and tumor FcR+ effectors for 48 hrs at 50:1 E:T ratio in the presence of IgG1 isotype control Abs in the IncuCyte® Live Cell Analysis System. One-way ANOVA with Dunnett’s multiple comparisons test. (F) Cumulative results showing the kinetics of GFP+A431 tumor cell growth co-cultured with PBN isolated from lung cancer patients and healthy volunteers for 48 hrs at a 50:1 E:T ratio in the presence of human IgG1 isotype control Abs in the IncuCyte® Live Cell Analysis System.
Supplementary Table S2. Heatmap showing comparisons of the abilities of different types of blood and tumor FcR+ effectors to affect tumor cell growth depending on targeted tAg density, E:T ratios, and anti-tumor Ab isotypes. The indicated FcR+ effectors were added to EGFRhiA431 (high tAg density) or EGFRloA549 (low tAg density) tumor cells at different E:T ratios in the presence of cetuximab (IgG) or IgA anti-EGFR Ab (IgA). The effect on the tumor cell growth was determined at 48 hours using the IncuCyte Live Cell Analysis System. The numbers on the heatmap represent the percent of inhibition of tumor growth. A positive value indicates the inhibition of tumor growth (degrees of red color). A negative value indicates the stimulation of tumor cell growth (degrees of green color). The numbers represent the mean values obtained from experiments shown in Fig.1I, 1K, 4I, and 4J.
Supplementary Table S1. Patient characteristics
Supplementary Figure S2. (A-D) The correlation analyses of the ability of blood and tumor-derived FcR+ effectors to regulate A431 tumor cell growth in the presence of anti-EGFR Abs with key clinical parameters of lung cancer patients including tumor type, stage, size, and smoking history. The effect of FcR+ effectors on A431 tumor cell growth was evaluated in the IncuCyte® Live Cell Analysis System. AC-adenocarcinoma, SCC-squamous cell carcinoma. Pearson test. Number of patients included in each analysis is indicated on the graphs.
Supplementary Figure S3. The correlation analyses between the ability of blood and tumor FcR+ myeloid effectors to regulate A431 tumor cell growth in the presence of anti-EGFR Abs and the expression of indicated FcR on the surface of these effectors. The effect of FcR+ effectors on A431 tumor cell growth was evaluated in IncuCyte® Live Cell Analysis System. The expression of FcR was analyzed in a single-cell suspension obtained from digested tumors by flow cytometry. Spearman test. Number of patients included in each analysis is indicated on the graphs
Supplementary Figure S4. (A) Cumulative results showing the kinetics of GFP+A431 tumor cell growth when co-cultured with PBN isolated from lung cancer patients at 50:1 E:T ratio in the presence of cetuximab (IgG) and IgA anti-EGFR Ab (IgA) using the IncuCyte® Live Cell Analysis System. One-way ANOVA with Turkey’s multiple comparisons test. Number of patients included in each analysis is indicated on the graphs. All data represented as mean ± SEM. (B) Cumulative results showing the kinetics of GFP+A431 tumor cell growth when co-cultured with blood and tumor FcR+ effectors for 48 hrs at 50:1 E:T ratio in the presence of IgA2 isotype control Abs in the IncuCyte® Live Cell Analysis System. One-way ANOVA with Dunnett's multiple comparisons test. All data represented as mean ± SEM. (C) Representative dotplots showing ADT mediated by TAM pretreated with cytohalasin D (1uM) or blocking anti-CD64 F(ab')2 and anti-CD32 F(ab')2 fragments (5ug/ml). TAM were co-cultured with PKH67+ A431 cells at a ratio 3:1 in the presence of cetuximab (1ug/ml) for 2 hrs. (D) Cumulative flow cytometry results showing the trogocytosis mediated by indicated blood and tumor myeloid effectors co-cultured with PKH67+A431 cells for 12 hrs at a 50:1 E:T ratio in the presence of IgG1 and IgA2 isotype control Abs (1ug/ml). Indicated FcR+ effectors were freshly isolated for all experiments.
Supplementary Figure S5. (A) Representative dotplots showing the levels of trogocytosis and killing activity mediated by TAM co-cultured with cetuximab-opsonized PKH67+A431 cells at a 50:1 E:T ratio for 12 hrs in the presence of blocking anti-CD32a Ab (clone 2B6) or blocking anti-CD32a Ab (clone 6G11). (B and C) Summary results showing the levels of trogocytosis (B) and killing activity (C) mediated by blood monocytes and TAM co-cultured with cetuximab-opsonized PKH67+A431 cells at a 50:1 E:T ratio for 12 hrs in the presence of blocking anti-CD32a Ab (clone2B6) or anti-CD32a Ab (clone 6G11). Blocking anti-CD32a Ab were added at concentration (10ug/ml). Summary graphs represent the total tumoricidal activity of effectors that was calculated as described in material and methods. IC Ab-isotype control Abs. (D) Representative experiment and cumulative results showing the kinetics of GFP+A431 tumor cell growth with blood monocytes or TAM at a 50:1 E:T ratio in the presence of cetuximab and blocking anti-CD32a Ab (clone2B6) or blocking anti-CD32a Ab (clone 6G11) in the IncuCyte® Live Cell Analysis System. Percentage of tumor cell growth inhibition/stimulation was calculated at 60 hrs as described earlier. Number of patients included in each analysis is indicated on the graphs. One-way ANOVA with Friedman multiple comparisons test. All data represented as mean ± SEM.
Supplementary Figure S6. (A and B) Representative experiment and summary results showing the effect of indicated blood and tumor FcR+ effectors on the growth of A431 tumor cell spheroids in the presence of cetuximab (1ug/ml). GFP+A431 cells were seeded at 500 cells/well in a round bottom ultra-low adhesion cell culture plate designed to form spheroids. Indicated FcR+ effectors were added at E:T ratio 50:1 into the spheroid cultures. The growth of tumor spheroids was monitored in the IncuCyte® Live Cell Analysis System for 48 hrs. Representative images of GFP+A431 tumor cell spheroids co-cultured with indicated effectors and cetuximab for 48 hrs taken from IncuCyte® Live Cell Analysis System (original magnification x10). Scale bar, 400 µm. Percentage of tumor cell growth inhibition/stimulation in the presence of anti-EGFR Abs was calculated at 48 hrs using the formula: (FI)(A431+Ab)-FI(A431+effectors+Ab)/FI(A431+Ab)x100%, FI-Integrated fluorescence intensity. Data are represented as mean ± SEM. Wilcoxon matched pairs test (n=7). (C) Representative dotplots showing the different levels of ADT mediated by indicated FcR+ effectors co-cultured with spheroids formed with PKH67-labeled A431 cells. FcR+ effectors were co-cultured at a E:T ratio 50:1 in the presence or absence of cetuximab for 12 hrs. Representative results from 1 of 3 experiments are shown. (D and E) Representative experiment and summary results showing the effect of PBN and TAN on the growth of GFP+A431 tumor cell spheroids in the presence of IgA anti-EGFR Ab (IgA) (1ug/ml). Experiments were performed as described in (A and B). Original magnification x10. Scale bar, 400 µm. Data are represented as mean ± SEM. Wilcoxon matched pairs test (n=5). (F) Representative dotplots showing the ADT mediated by PBN and TAN co-cultured with spheroids formed with PKH67-lableld A431 cells. PBN and TAN were co-cultured at a E:T ratio 50:1 in the presence or absence of IgA anti-EGFR Ab for 12 hrs. Representative results from 1 of 3 experiments are shown.
Supplementary Figure S7. (A) Cumulative flow cytometry results showing the ability of blood and tumor FcR+ effectors to kill PKH-67+A431 tumor cells in the presence of nonspecific human IgG1 or IgA2 isotype control Abs (1ug/ml) at a 2:1 E:T ratio in a 12 hrs FACS-based assay. (B and C) Cumulative results showing the kinetics of GFP+A431 tumor cell growth when co-cultured with blood and tumor FcR+ effectors for 48 hrs at a 2:1 E:T ratio in the presence of IgG1 or IgA2 isotype control Abs (1ug/ml) in the IncuCyte® Live Cell Analysis System. (D) Cumulative flow cytometry results showing the ability of blood and tumor FcR+ effectors to kill PKH-67+A549 tumor cells in the presence of nonspecific human IgG1 or IgA2 isotype control Abs (1ug/ml) at a 50:1 E:T ratio in a 12 hrs FACS-based assay. (E and F) Cumulative results showing the kinetics of GFP+A549 tumor cell growth when co-cultured with blood and tumor FcR+ effectors for 48 hrs at a 50:1 E:T ratio in the presence of IgG1 or IgA2 isotype control Abs (1ug/ml) in the IncuCyte® Live Cell Analysis System. All comparisons used one-way ANOVA with Dunnett’s multiple comparisons test. All Data are represented as mean ± SEM. Indicated FcR+ effectors were freshly isolated for all experiments.
Supplementary Figure S8. (A and B) Representative experiment and summary results showing the effect of blood and tumor FcR+ effectors on the growth of GFP+A549 tumor cell spheroids in the presence of cetuximab (1ug/ml). Experiments were performed as described in supplemental figure 5 (A and B). Original magnification x10. Scale bar, 400 µm. Data are represented as mean ± SEM. Wilcoxon matched pairs test (n=6). Indicated FcR+ effectors were freshly isolated for all experiments. (C) Flow cytometry histogram showing the expression of EGFR on the surface of H1975 tumor cell line in comparison with A431 and A549 tumor cells. (D and E) Cumulative flow cytometry results showing the different levels of trogocytosis and killing activity mediated by blood and tumor FcR+ effectors co-cultured with PKH67+ H1975 tumor cells at a 50:1 E:T ratio in the presence of cetuximab or isotype control Abs for 12 hrs. All comparisons used one-way ANOVA with Turkey’s multiple comparisons tests. All data represented as mean ± SEM. FcR+ effectors were freshly isolated for all experiments. IC-isotype control Ab.
Supplementary Figure S9. (A and B) Representative dotplots and summary results showing the ability of TAM pretreated with blocking anti-CD64 F(ab')2 and anti-CD32 F(ab')2 fragments (5ug/ml) to kill PKH67+ A431 cells at a 1:1 E:T ratio in the presence of cetuximab (1ug/ml) in a 12 hrs FACS-based assay. Dead A431 tumor cells were defined as TO-PRO-3+PKH67+ cells. (C and D) Representative dotplots and summary results demonstrating the ability of HLA-A2neg TAM to stimulate of Ly95 cells responses (intracellular IFN-γ) after their preincubation with cetuximab-opsonized A549/HLA-A2+NY-ESO tumor cells at the indicated time points. Kruskal-Wallis multiple comparisons test. Data represented as mean ± SEM. (E) The expression of NY-ESO-1 protein in different batches of transduced A549 tumor cells. The expression of intracellular NY-ESO was evaluated by flow cytometry using NY-ESO-1 mAb (clone D1Q2U) that recognizes endogenous levels of total NY-ESO-1 protein. Non transduced A549 cells were used as a control. (F-H) Representative dotplots and cumulative data showing the ability of HLA-A2+TAM to stimulate of Ly95 cells responses (intracellular IFN-γ) after preincubation with A549/HLA-A2+NY-ESOhi (F) and A549/HLA-A2+NY-ESOlo tumor cells (G) in the presence or absence of cetuximab for 4 hrs. Wilcoxon matched pairs test for groups with (+) or without (-) Ab. * p<0,01. Kruskal-Wallis test for TAM groups co-cultured with A549/HLA-A2+NY-ESOhi or A549/HLA-A2+NY-ESOlo. Data represented as mean ± SEM. FcR+ effectors were freshly isolated for all experiments.
Supplementary Movie S1. TAM-mediated phagocytosis of rituximab-opsonized Daudi tumor cells. PKH26-labeled TAM (red) were incubated with PKH67-labeled Daudi tumor cells (green) at a 3:1 E:T ratio for 2 hrs in the presence of rituximab (1ug/ml). Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope. The images were taken every 5 min with Hamamatsu ImagEM 16-bit cooled EMCCD camera at 20x magnification. Supplementary Movie S2. TAM-mediated trogocytosis of cetuximab-opsonized A431 tumor cells. PKH26-labeled TAM (red) were incubated with PKH67-labeled A431 tumor cells (green) at a 3:1 E:T ratio for 2 hrs in the presence of cetuximab (1ug/ml). Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope. The images were taken every 5 min with Hamamatsu ImagEM 16-bit cooled EMCCD camera at 20x magnification. Supplementary Movie S3. Sublethal trogocytosis performed by PBN during their interaction with cetuximab–opsonized A431 tumor cells. PKH26-labeled PBN (red) were incubated with PKH67-labeled A431 targets (green) at a 10:1 E:T ratio for 2 hrs in the presence of cetuximab (1ug/ml). Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope. The images were taken every 5 min with Hamamatsu ImagEM 16-bit cooled EMCCD camera at 20x magnification. Supplementary Movie S4. Lethal trogocytosis performed by blood monocytes during their interaction with cetuximab–opsonized A431 tumor cells. PKH26-labeled monocytes (red) were incubated with PKH67-labeled A431 targets (green) at a 10:1 E:T ratio for 2 hrs in the presence of cetuximab (1ug/ml). Time-lapse microscopy was performed using a Leica DMI4000 inverted microscope. The images were taken every 5 min with Hamamatsu ImagEM 16-bit cooled EMCCD camera at 20x magnification.
Suppl movie 1, Mph Daudi phagocytosis
Data Availability Statement
The data generated in this study are available within the article and its Supplementary Data files or upon request from the corresponding author.