Abstract
Diabetes is a chronic medical condition that may induce complications such as poor wound healing. Stem cell therapies have shown promise in treating diabetic wounds with pre‐clinical and clinical studies. However, little bibliometric analysis has been carried out on stem cells in the treatment of diabetic wounds. In this study, we retrieved relevant papers published from January 1, 2003, to December 31, 2023, from Chinese and English databases. CiteSpace software was used to analyze the authors, institutions, and keywords by standard bibliometric indicators. Our analysis findings indicated that publications on stem cells in the treatment of diabetic wounds kept increasing. The most prolific author was Qian Cai (n = 7) and Mohammad Bayat (n = 16) in Chinese and English databases, respectively. Institutions distribution analysis showed that Chinese institutions conducted most publications, and the most prolific institution was the Chinese People's Liberation Army General Hospital (n = 9) and Shahid Beheshti University of Medical Sciences (n = 17) in Chinese and English databases, respectively. The highest centrality keyword in Chinese and English databases was “wound healing” (0.54) and “in vitro” (0.13), respectively. There were 8 and 11 efficient and convincing keyword clusters produced by a log‐likelihood ratio in the Chinese and English databases, respectively. The strongest burst keyword was “exosome” (strength 3.57) and “endothelial progenitor cells” (strength 7.87) in the Chinese and English databases, respectively. These findings indicated a direction for future therapies and research on stem cells in the treatment of diabetic wounds.
Keywords: bibliometric analysis, CiteSpace, diabetic wound, stem cells
1. INTRODUCTION
Diabetes is a chronic disease affecting millions worldwide. 1 The mechanism behind diabetes is complex and involves several factors, including genetics, lifestyle choices, and environmental factors. 2 , 3 In people with diabetes, the pancreas either produces insufficient amounts of insulin or the body is unable to use it effectively, leading to high levels of glucose in the bloodstream. Furthermore, diabetes can lead to a variety of complications over time. One of the most common complications of diabetes is the development of chronic wounds, which are slow to heal and can lead to severe infections and amputations. Standard wound care typically involves cleaning the ulcer, removing dead tissue and debris, and applying a dressing. 4 , 5 However, even with meticulous wound care, ulcers in diabetic patients often fail to heal. Therefore, there is a need for new therapies that can promote healing and prevent complications.
In recent years, stem cells have been demonstrated to play a crucial role in tissue repair and regeneration, making them an attractive option in treating chronic wounds, including diabetic foot ulcers. 6 , 7 Stem cells are a type of cell that has the potential to differentiate into a variety of other cell types, and the classifications are based on their origin and differentiation potential. For example, mesenchymal stem cells (MSCs) are adult stem cells that can differentiate into various cell types involved in the healing process, such as endothelial cells, fibroblasts, and keratinocytes. 8 Adipose tissue‐derived MSCs (AMSCs) were able to promote wound healing in diabetic mice. 9 Induced pluripotent stem cells (iPSCs) are adult cells that have been reprogrammed to a pluripotent state, meaning they can differentiate into any cell type in the body and can be used in regenerative medicine without the ethical concerns associated with embryonic stem cells (ESCs). 10 Previous studies also found the applications and prospects of iPSCs in animal wound healing models, including diabetic ulcers and limb ischemia. 11
Bibliometrics is one of the essential quantitative index evaluation methods, mainly based on the quantity and quality of academic publications, and makes objective evaluations via the characteristics of content and structure. 12 , 13 With the development of computer technology, large professional databases, and various software, for example, CiteSpace, helped to realize the automation, intelligence, and visualization of bibliometrics and significantly improved the evaluation efficiency. CiteSpace software is run in a Java‐based environment and was first created by Chen MeiChao, a professor from Drexel University, United States. 13 , 14 The primary purpose of Citespace is to provide researchers with a graphical representation of the relationships between different scientific papers, authors, and institutions. This can be extremely useful for reviewing key areas of research and identifying current collaborators and research institutions. In this review, we use bibliometric analysis to summarize the advances in stem cell treatment of diabetic wounds.
2. METHODS
2.1. Data sources and search strategies
The Chinese publications were obtained through the China National Knowledge Infrastructure (CNKI) database (https://www.cnki.net/), Weipu database (http://www.cqvip.com/), and WanFang database (https://www.wanfangdata.com.cn/); and the English publications were obtained through the Web of Science Core Collection (WoSCC) database (https://www.webofscience.com/), PubMed database (https://pubmed.ncbi.nlm.nih.gov/) and Scopus database (http://www.scopus.com). The search strategy was “ALL = (stem cells) AND (ALL = (wound) OR ALL = (ulcer)) AND (ALL = (diabetes) OR ALL = (diabetic))” for relevant publications, and the reference type was “article or review.” The published year span was from 2003 to 2023. All data were acquired on January 1, 2024, to avoid the prejudice caused by the database update. The duplicated, unrelated, or editorial publications and case reports were removed.
2.2. Bibliometrics and visualization analysis
The method was described in our previous study. 15 Downloaded the information of Chinese publication and English publications from the Chinese database and English database, respectively; and saved them as Refwork format and plain text format, respectively. The presentation of institutions have been modified to improve accuracy: (a) the different departments of the same institution were considered as one institution; (b) the university and its affiliated hospitals were considered as two institutions; (c) the different names of the same institution (such as the previous one and the current one) were considered as one institution. The downloaded files were first renamed as download_*.txt, and then imported them into Citespace V6.2.R2 for bibliometric analysis. When mapping visualization knowledge figures, we followed the main process steps of CiteSpace, including time slicing, thresholding, modeling, pruning, merging, and mapping. The core concepts of CiteSpace include burst detection, betweenness centrality, and heterogeneous networks, enabling timely visualization of research status, hotspots, and frontiers. Nodes in different maps represent authors, institutions, or keywords, respectively; the larger node represents the more frequency. The connections between nodes represent close relationship in studies; the thicker line represents stronger relationship, the lighter line represents later relationship.
The parameters were set as follows: (1) time slicing at one year per slice; (2) the selection uses a modified g‐index in each slice: k = 25, which means that data were extracted on the top 25 results for each time slice; (3) the node type was set as author, institution, source, and keyword, respectively; (4) choosing “Pathfinder” and “Pruning the merged network” for Pruning parameters area to simplify the network and highlight its essential structural features of keywords, while choosing “Pruning the sliced network” for other information. The remaining parameters were the default settings.
3. RESULTS
3.1. Bibliometric analysis of the temporal distribution
According to the search strategy, 370, 101, and 292 publications were retrieved from CNKI, Weipu, and Wanfang databases, respectively. Meanwhile, 876, 1201, and 696 publications were retrieved from WoSCC, Pubmed, and Scopus, respectively. After removing the duplicates, 146 and 636 publications on stem cell treatment of diabetic wounds were retrieved from Chinese and English databases between 2003 and 2023. As shown in Figure 1, the number of publications in the English database showed a generally growing trend over the last decade. While in the Chinese database, the number of publications exceeded 10 for the first time in 2010, and the highest number was 27 in 2022.
FIGURE 1.
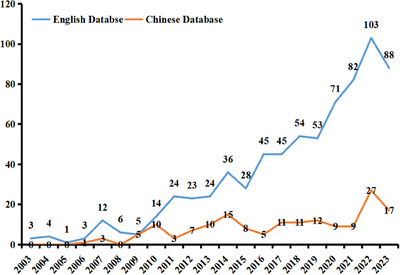
The number of publications on stem cell in the treatment of diabetic wounds from 2003 to 2023.
3.1.1. Bibliometric analysis of the author's distribution
The top five most prolific authors in both Chinese and English databases are shown in Table 1. The most prolific (n = 7) author in the Chinese database was Qian Cai from Lanzhou General Hospital of Lanzhou Military Command, China. He mainly focuses on the effect of stem cells on wound repair in diabetic rats. The most prolific (n = 16) author in the English database was Mohammad Bayat from Shahid Beheshti University of Medical Sciences, Iran. His primary focus is combining photobiomodulation therapy and stem cells to improve wound healing in diabetic rats. Co‐author analysis showed networks of authors who had co‐authorship in Chinese (Figure 2A) and English databases (Figure 2B); the larger spot indicated more publications, the thicker line indicated more co‐authorship, and the lighter line indicated later collaborative year. In Chinese databases, there are six major networks led by Qian Cai, Peisheng Jin, Xiaobing Fu, Dianbao Zhang, Tongbin Chu, and Meng Zhang, respectively. There are two major networks in English databases, one from China led by Xiaobing Fu; the other is an international group of researchers from the U.S. and Iran.
TABLE 1.
Top five prolific authors in the Chinese and English databases.
| Chinese database | English database | |||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Name | Publications | Been cited | Year of publications | Name | Publications | Been cited | Year of publications |
| 1 | Qian Cai | 7 | 111 | 2010‐2014 | Mohammad Bayat | 16 | 220 | 2016‐2023 |
| 2 | Yi Liu | 7 | 84 | 2010‐2023 | Sufan Chien | 13 | 188 | 2018‐2023 |
| 3 | Dewu Liu | 6 | 61 | 2010‐2019 | Abdollah Amini | 13 | 176 | 2016‐2023 |
| 4 | Peisheng Jin | 6 | 9 | 2017‐2023 | Seyed Kamran Ghoreishi | 7 | 168 | 2018‐2023 |
| 5 | Qiang Li | 5 | 8 | 2017‐2023 | Xiaobing Fu | 6 | 365 | 2012‐2023 |
FIGURE 2.
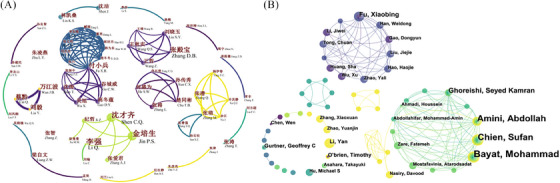
Bibliometric analysis of the authors of publications from the Chinese (A) and English (B) databases.
3.2. Bibliometric analysis of the institution's distribution
The top five most prolific institutions in both Chinese and English databases are shown in Table 2. The Chinese People's Liberation Army General Hospital was the most prolific (n = 9) institution in the Chinese database and the third (n = 13) in the English database. Shahid Beheshti University of Medical Sciences, Iran, was the most prolific (n = 17) institution in the English database. All institutions in the Chinese database were from China. In the English database, institutions from China had the most publications (n = 218), followed by the USA (n = 190) and Iran (n = 51) (Table 3). The degree score indicated the number of institutions that have collaborated with; the institutions from Italy and England collaborated with more institutions (n = 8) from other countries. The institutions from Germany had the highest centrality (0.78), followed by Iran (0.67) and England (0.65), which indicated that research of these institutions had a higher impact in this field. Co‐institution analysis showed networks of institutions that had collaborated in Chinese (Figure 3A) and English databases (Figure 3B). In the Chinese database, most of these collaborations were limited to medical universities and their affiliated hospitals. The Chinese People's Liberation Army General Hospital had the highest degree (n = 7) and centrality (0.01). In English databases, most of these are international collaborations; Harvard Medical School had the highest degree score (n = 12) and centrality (0.15).
TABLE 2.
Top five prolific institutions from the Chinese and English databases.
| Chinese database | English database | |||
|---|---|---|---|---|
| Rank | Institution | Publications | Institution | Publications |
| 1 | Chinese People's Liberation Army General Hospital | 9 | Shahid Beheshti University of Medical Sciences | 17 |
| 2 | Lanzhou General Hospital of Lanzhou Command | 8 | Shanghai Jiao Tong University | 14 |
| 3 | The First Affiliated Hospital of Nanchang University | 6 | Chinese People's Liberation Army General Hospital | 13 |
| 4 | Affiliated Hospital of Xuzhou Medical University | 6 | Central South University | 13 |
| 5 | Third Military Medical University | 4 | University of Louisville | 13 |
TABLE 3.
Top 10 prolific countries in the English databases.
| Rank | Publications | Degree | Centrality | Country | First publication year |
|---|---|---|---|---|---|
| 1 | 218 | 3 | 0.12 | China | 2003 |
| 2 | 190 | 3 | 0.18 | USA | 2003 |
| 3 | 51 | 4 | 0.67 | Iran | 2008 |
| 4 | 34 | 2 | 0.06 | Japan | 2004 |
| 5 | 33 | 1 | 0 | South Korea | 2005 |
| 6 | 29 | 8 | 0.48 | Italy | 2009 |
| 7 | 26 | 8 | 0.65 | England | 2009 |
| 8 | 24 | 1 | 0 | India | 2009 |
| 9 | 23 | 5 | 0.78 | Germany | 2007 |
| 10 | 13 | 3 | 0.29 | Ireland | 2012 |
FIGURE 3.
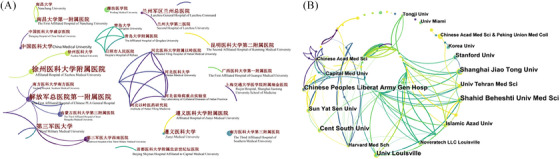
Bibliometric analysis of the institutions of publications from the Chinese (A) and English (B) databases.
3.3. Bibliometric analysis of co‐occurring keywords
The occurrence of high frequency and high centrality of keywords show the focus of most authors in a period, that is, the hot spots and frontiers of research. The top 10 keywords in both Chinese and English databases are shown in Table 4. In Chinese databases, the highest frequency, highest degree, and highest centrality keywords were “diabetic foot” (n = 43), “stem cells”(n = 17), and “wound healing” (0.54), respectively. In English databases, the highest frequency, highest degree, and highest centrality keywords were “wound healing” (n = 185), “in vitro”(n = 47), and “in vitro” (0.13), respectively. The network of co‐occurring keywords in Chinese and English databases is shown in Figure 4.
TABLE 4.
Top 10 high‐frequency keywords from the Chinese and English databases.
| Chinese database | English database | |||||||
|---|---|---|---|---|---|---|---|---|
| Rank | Frequency | Degree | Centrality | Keywords | Frequency | Degree | Centrality | Keywords |
| 1 | 43 | 15 | 0.34 | diabetic foot | 185 | 39 | 0.06 | wound healing |
| 2 | 39 | 11 | 0.23 | diabetes | 139 | 43 | 0.08 | mesenchymal stem cells |
| 3 | 24 | 15 | 0.54 | wound healing | 124 | 41 | 0.08 | differentiation |
| 4 | 22 | 17 | 0.47 | stem cells | 123 | 46 | 0.07 | angiogenesis |
| 5 | 13 | 6 | 0.13 | rats | 119 | 38 | 0.07 | stem cells |
| 6 | 12 | 6 | 0.09 | chronic wound | 84 | 41 | 0.06 | bone marrow |
| 7 | 11 | 7 | 0.05 | repair | 84 | 32 | 0.06 | therapy |
| 8 | 9 | 1 | 0 | exosome | 78 | 47 | 0.13 | in vitro |
| 9 | 9 | 6 | 0.07 | transplantation | 77 | 29 | 0.04 | stromal cells |
| 10 | 8 | 9 | 0.31 | angiogenesis | 75 | 31 | 0.05 | expression |
FIGURE 4.
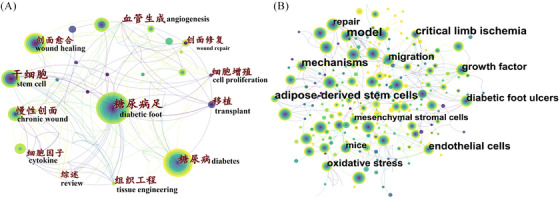
The keyword clusters produced from the Chinese (A) and English (B) databases.
The clustered network analysis functions to summarize the co‐occurring keywords by a scientific calculation method. The silhouette score above 0.7 is considered an efficient and convincing cluster. 16 There were 8 (silhouette score = 0.9111) and 11 (silhouette score = 0.8761) clusters produced by the log‐likelihood ratio in Chinese and English databases, respectively (Figure 5). The clusters comprised keywords with different colors; the #0 cluster contained the largest number of keywords, and the overlap indicated the keyword belonged to more clusters simultaneously. Therefore, the diabetic foot was the primary concern in the Chinese database, while the collagen scaffold was the primary concern in the English database.
FIGURE 5.
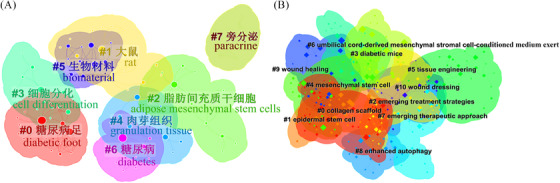
Bibliometric analysis of the keywords of publications from the Chinese (A) and English (B) databases.
The top 10 keywords with the strongest citation bursts are presented in Figure 6. The light blue line indicates the time interval, the blue line indicates the period when a keyword appeared, and the red line indicates the period when a keyword had a burst. These burst keywords were detected based on the increase in the frequency of the publications in that year, regardless of the total usage. The strongest (strength 3.57) and latest (from 2020 till now) burst of keywords in the Chinese database was the “exosome.” Exosomes can promote cell proliferation, reduce the inflammatory response, and promote new angiogenesis and wound healing; therefore, they may play an essential synergistic role in stem cells treatment of diabetic wounds. In English databases, the strongest and latest burst of keywords were “endothelial progenitor cells” (strength 7.87) and “diabetic wound” (from 2019 till now), respectively. Endothelial progenitor cells (EPCs) are a type of stem cell that can differentiate into endothelial cells, promote angiogenesis, and accelerate wound healing in diabetic patients.
FIGURE 6.
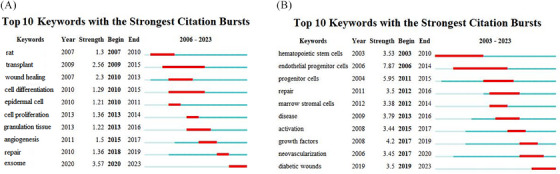
Top 10 keywords with the strongest citation bursts from the Chinese (A) and English (B) databases.
4. DISCUSSION
4.1. Summary of findings
Between 2003 and 2023, 146 and 636 qualified Chinese and English publications were included in the final analysis, respectively. Overall, the number of publications in this field has risen annually. Researchers who had published more studies were more highly represented, suggesting their high reputation in the field and the likelihood that they will generate in‐depth discoveries regarding stem cells in the treatment of diabetic wounds. The most prolific authors were Qian Cai (n = 7) and Mohammad Bayat (n = 16) in Chinese and English databases, respectively. Institutions distribution analysis showed that most publications were conducted by Chinese institutions, and the most prolific institutions were the Chinese People's Liberation Army General Hospital (n = 9) and Shahid Beheshti University of Medical Sciences (n = 17) in Chinese and English databases, respectively. As collaboration among these institutions was relatively limited, efforts to establish more robust relationships may help further advance this field. The highest centrality keyword in Chinese and English databases was “wound healing” (0.54) and “in vitro” (0.13), respectively. There were 8 and 11 efficient and convincing keyword clusters produced by a log‐likelihood ratio in the Chinese and English databases, respectively. The strongest burst keywords were “exosome” (strength 3.57) and “endothelial progenitor cells” (strength 7.87) in the Chinese and English databases, respectively.
4.2. Hot spots and frontiers
4.2.1. Mechanism studies
In recent decades, the use of stem cells for the treatment of diabetic wounds has gained significant attention. Regarding the mechanisms of action, different types and sources of stem cells have been shown to work through different pathways. For example, MSCs can secrete various substances such as growth factors and cytokines, participate in the body's regulation of immune response and promote angiogenesis; in contrast, ESCs and iPSCs are pluripotent and self‐renewing, have the potential to differentiate into various cell types in the body.
MSCs can be found in various tissues, such as bone marrow, cord blood, and cord tissue, placental tissue, adipose tissue, etc. Therefore, MSCs can differentiate into osteoblasts, chondroblasts, and adipocytes under appropriate conditions. 8 MSCs are nutrient factories that produce a wide variety of cytokines and growth factors, such as vascular endothelial growth factor (VEGF), platelet‐derived growth factor (PDGF), basic fibroblast growth factor (bFGF), transforming growth factor‐β (TGF‐β), Angiopoietin‐1 (Ang‐1), and stroma‐derived factors‐1 (SDF‐1). 17 , 18 Wu et al. found that in db/db mice treated with bone marrow MSCs (BM‐MSCs), VEGF, Ang‐1, and keratinocyte‐specific protein keratin were higher in wounds, and the growth of keratinocytes increased at the wound site. 19 Hou et al. found that the MSC‐conditioned medium accelerated the migration and proliferation of human umbilical vein endothelial cells by regulating the chemokine receptors‐related signaling pathways such as protein‐serine‐threonine kinase (AKT) signaling pathway and extracellular signal‐regulated kinases (ERK) signaling pathway in both in vivo and in vitro studies. 20 Furthermore, MSCs also have immunomodulatory functions, such as increasing the levels of anti‐inflammatory factors IL‐10 and IL‐4 and decreasing the levels of TNF‐α and IFN‐γ. 21 In vitro studies have shown that MSCs can promote keratinocytes to participate in wound epidermis formation and regulate the local microenvironment.
MSC‐derived exosomes (MSC‐Exos), the major secretion products of the paracrine pathway of MSCs, are membranous vesicles released into the extracellular matrix after the fusion of intracellular vesicles with cell membranes. 22 Accumulated studies have shown that MSC‐Exos can not only show biotherapeutic efficacy similar to MSCs in the treatment of diabetic wounds but also have extra advantages. First, exosomes act as paracrine forms of stem cells to avoid immune rejection, oncogenesis, and other side effects. It has been reported that exosomes can directly inhibit the activity of effector T cells, thus suppressing the immune response. 23 , 24 Second, exosomes can pass the blood‐brain barrier and placental barrier and play a role in the microenvironment that stem cells cannot reach, which indicates that exosomes have a more comprehensive application range than stem cell therapy alone, and can directly reach the target area through the barrier. 25 , 26 Third, the dose of MSC‐Exos can be adjusted, which can easily be manually controlled during application. Exosomes can be personalized, adjusted dose and composition to deliver drugs to patients. 27 Fourth, MSC‐Exos can enhance the therapeutic effect through gene modification, such as modifying cells through gene overexpression and using exosomes derived from the cells to provide potential drug delivery systems, or overexpressing some bioactive components in exosomes to promote wound healing. 28 Fifth, the regulation of MSC‐Exos is more extensive, and can repair or prevent the damage caused by diabetic complications through multiple effects. 29 Human umbilical cord MSC‐Exos can also improve type 2 diabetes by reversing peripheral insulin resistance and alleviating the destruction of islet cells. 30
IPSCs were first reported in 2006, they can differentiate into all cell types in the three dermal layers, and terminally differentiated cells may promote diabetic wound healing through paracrine or direct cellular effects. 31 , 32 Kashpur et al. injected iPSC‐derived fibroblasts into 3D tissues and found that they could grow into diabetic wounds and promote wound healing, indicating that iPSCs have the potential to treat diabetic foot ulcers. 33 Recently, Gorecka et al. found that differentiation of neonatal fibroblast iPSCs into smooth muscle cells (experimental group) and delivery via collagen matrix can accelerate wound healing and wound bed neovascularization in mice with diabetes. Compared with AMSCs in the control group, smooth muscle cells in the experimental group remained on the wound bed 7 days after implantation and secreted higher levels of angiogenic cytokines to accelerate wound closure. In addition, the number of pro‐inflammatory M1 macrophages decreased in the wound area of the experimental group, while the number of pro‐regenerative M2 macrophages increased. 34 Itoh et al. showed that human iPSC‐differentiated smooth muscle cells significantly improved in mice with lower limb ischemia. 35 These studies indicate that iPSCs are one of the ideal cell types for wound treatment.
4.2.2. Application studies
Stem cell manipulation can help host cells homing to the wound site, mainly including increasing stem cell circulation, enhancing cell binding to the wound site, promoting stem cell differentiation into target cells, and increasing angiogenesis. 36 Promoting wound neovascularization is by far the most important goal of wound healing because it is the limiting factor in promoting the wound healing process. Various advances in stem cell manipulation to achieve this goal are discussed below.
A scaffold matrix can manipulate the stem cell microenvironment to enhance its ability to promote regeneration. Dash et al. found that scaffold collagen fiber density could improve cell regeneration by regulating the secretion function of iPSC‐SMC. 37 Dense fibrous collagen increases the production of pro‐healing cytokines, such as VEGF, IL‐8, IL‐10, TGF‐β, and keratinocyte growth factor (KGF), as well as endothelial cell proliferation and migration. Surface modification of scaffolds with bioactive polymers also affects the activity of stem cells. For example, adding laminin and hyaluronic acid increased cell survival and promoted VEGF production in implanted cells. 38 In addition, intermolecular bonds in the scaffold microenvironment regulate cell proliferation and differentiation through physical actions. For example, MSC can differentiate into neurons, myoblasts, or osteoblasts when cultured in polyacrylamide hydrogels of varying hardness (0.1–25 kPa); The “softer” matrix (0.1–1 kPa) was favorable for differentiation into neurogenic cells, while the “harder” matrix (8–17 kPa) was favorable for differentiation into myogenic cells. 39 Recent studies in nanotechnology also emphasize the importance of the physical properties of scaffolds, such as manipulating cell fate by regulating porosity to accelerate wound healing. 40 Therefore, changing the mechanical properties of the matrix helps the implanted stem cells to perceive mechanical, environmental factors, thus promoting cell migration and differentiation and accelerating wound healing at different stages.
Hypoxic preconditioning can enhance the ability of MSC to promote angiogenesis and thus promote wound healing. Rosova et al. proposed that cells should be preconditioned with hypoxia before transplantation to resist apoptotic stimulation when transplanted into an internal environment with severe hypoxia, such as ischemic tissue at chronic wounds or other injury sites. 41 Another advantage of hypoxic preconditioning of MSC is to maintain cell dryness and pluripotency, which is very beneficial in areas requiring tissue regeneration.
Stem cells can be genetically modified to improve their regenerative and therapeutic potential. Using iPSCs creates opportunities for further genetic modification to correct congenital defects. Teo et al. proposed to use ZFN, TALEN, CRISPR, and other technologies to repair diabetes‐related mutant gene sequences in vitro and then transplant them back into diabetic patients. 42 Genetically correct iPSCs of elderly diabetic patients remove defective genes related to diabetes and aging and enhance the effect of autologous cell therapy. Song et al. found that the introduction of v‐Myc into human AMSCs by lentivirus transfection could promote VEGF secretion and in vitro angiogenesis. 43 The preclinical study also confirmed that MSC could be safely genetically modified by lentiviral vectors, produced high levels of VEGF, and promoted angiogenesis in an animal model of lower limb ischemia. 44
Nanotechnology can enhance the potential and differentiation of stem cells. Adipose stem cells implanted into photosensitive and biomimetic core‐shell nanofiber scaffolds can differentiate into epidermal keratinocytes under light stimulation. 45 Nanoparticles can also be used to deliver genetic material to cells. Yang et al. developed a non‐viral, biodegradable polymer nanoparticle that had VEGF gene to MSC and ESC‐derived cells; the treated stem cells significantly enhanced the generative ability of VEGF, cell viability, and ability to bind target tissues. In a mouse model of lower limb ischemia, it was further confirmed that the vascular density of MSC transmutation into the VEGF genome by biodegradable polymer nanoparticles was significantly higher than that of the control group, and muscle degeneration and tissue fibrosis were reduced. 46 These results suggest that engineered stem cells based on biodegradable polymer nanoparticles effectively treat ischemic diseases and show potential for wound treatment.
MSCs and iPSCs exert paracrine effects by secreting some nutrients to promote the therapeutic effect of transplanted cells, which consists of soluble factors (e.g., cytokines, chemokines, and growth factors) and insoluble extracellular vesicles (EVs). More and more research has focused on using secretions in cellular supernatants, especially soluble factors isolated from insoluble EVs. EVs contain nucleic acids, proteins, and lipids derived from cells, and function as important mediators of intracellular interactions. 47 The production of EVs was affected by the microenvironment, cell density, cell age, culture system geometry, hypoxia, and mechanical stress. 48 Many studies have shown that EVs from different cell sources can promote skin wound healing by promoting collagen synthesis and angiogenesis. 49 , 50 , 51 In addition, EVs have also been loaded onto tissue engineering scaffolds as bioactive molecules for wound healing. 52 A recent meta‐analysis showed that EVs rich in non‐coding RNA or microRNAs significantly affected diabetic wound healing and positive improvements in vascular density and number, scar width, and reepithelialization. 53 These results help promote the clinical transformation of MSC‐EV. Furthermore, the EVs can modify therapeutic materials to produce a predictable dose‐response effect in vivo and remain active after a long time of storage.
4.2.3. Clinical studies
Previous studies showed that stem cell transplantation is even better than surgery in improving wound healing and reducing amputation rates in patients with diabetic foot ulcers. 54 In the treatment of diabetic wounds, the main stem cell transplantation methods include systemic injection and local injection. Systemic intravenous infusion of stem cells helps relieve some systemic complications of diabetes but may lead to embolization of MSCs in the lung, while arterial injection may improve the survival rate of circulating MSCs, but increases the risk of invasiveness and complications. 55 , 56 Although direct local injection is relatively simple, this method is not so efficient. 19 Scaffolds containing biomaterials can improve the efficiency of stem cell transplantation, meanwhile, promote wound healing directly. 57
On the other hand, depending on the source of stem cells, transplantation can be divided into autologous and allogeneic in clinical practice. Autologous stem cells include peripheral blood mononuclear cells (PBMNCs), bone marrow mononuclear cells (BMMNCs), BM‐MSCs, AMSCs, and an adipose tissue‐derived stromal vascular fraction (SVF). Allogeneic stem cells are isolated from allogeneic sources such as the placenta, umbilical cord, and amniotic membrane. 58 Compared with animal studies, the clinical studies also showed that both autologous and allogeneic stem cells can promote angiogenesis, immune regulation, matrix remodeling, and the production of regenerative cytokines without observing adverse effects. 59 , 60 Overall, stem cells may be effective and safe for treating diabetic wounds in clinical studies. However, due to the small sample size in each study, the clinical diagnosis and treatment standard of stem cells in the treatment of diabetic wounds has not been established yet, which is also one of the limiting factors restricting its clinical application. To solve this problem, it is necessary to conduct in‐depth basic research and clinical trials on the application in this field and establish a set of clinical standards through perfect experimental design and strict and meticulous observation. 61
4.3. Strengths and limitations
This scientometrics study is the first to our knowledge to have explored research trends focused on stem cells in the treatment of diabetic wounds from both Chinese and English databases, which helps researchers quickly understand the research hot spots and future trends in this field. Since Chinese researchers conducted most studies, the literature in the Chinese and English databases covered a substantial amount of data in this field, which ensured the results were highly reliable. However, this study also had some limitations. First, due to the limitation of the language and format that the bibliometrics software can recognize, the relevant publications might not be exhaustively identified. Second, there was a time limit for our literature search. Stem cell therapy was first introduced to wound healing in the late 20th century, and the number of relevant publications was small. 31 , 62 Third, recently published studies may have been largely overlooked concerning their significance owing to the low citation frequency inevitably observed during the immediate period following publication.
5. CONCLUSION
In the present study, we utilized scientometrics to analyze research on stem cells in the treatment of diabetic wounds in both Chinese and English databases. Our results revealed this field's high‐impact authors, institutions, journals, keywords, and hot spots. Following the increasing incidences of DM and the health strategies in response to the growing burden of DM, publications on DF have increased remarkably since 2016. China leads the research on stem cells in the treatment of diabetic wounds. The recent studies focus on the mechanism, application, and clinical studies. The hotspots of this field include MSCs, exosomes, and biomaterials. Our results indicated a method for future therapies and research on stem cells in the treatment of diabetic wounds.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest regarding the publication of this paper.
ACKNOWLEDGMENTS
This study was supported by grants from the Open Guangxi Natural Science Foundation, No. (2023JJA140982);“Medical Excellence Award” Funded by the Creative Research Development Grant from the First Afiliated Hospital of Guangxi Medical University, No. (6); Guangxi University’s young and middle‐aged teachers’ basic scientific research ability enhancement project, No. (2022KY0104); Open Research Fund from Guangxi Key Laboratory of Regenerative Medicine, Guangxi Medical University, No. (Gui Zai Zhong Kai202003).
Ma K, Luo C, Du M, et al. Advances in stem cells treatment of diabetic wounds: A bibliometric analysis via CiteSpace. Skin Res Technol. 2024;30:e13665. 10.1111/srt.13665
Ke Ma and Chao Luo contributed equally.
Contributor Information
Li Zheng, Email: zhengli224@163.com.
Mingde Liao, Email: gxlmd@126.com.
DATA AVAILABILITY STATEMENT
Data available on request.
REFERENCES
- 1. IDF Atlas‐10th edition. 2021. www. diabetesatlas.org. Accessed April 12, 2023
- 2. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- 3. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–390. doi: 10.1038/s41581-020-0278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang M, Nguyen TT. Strategy for treatment of infected diabetic foot ulcers. Accounts Chem Res. 2021;54(5):1080–1093. doi: 10.1021/acs.accounts.0c00864 [DOI] [PubMed] [Google Scholar]
- 5. Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Transl Med. 2021;13(585):eabe4839. doi: 10.1126/scitranslmed.abe4839 [DOI] [PubMed] [Google Scholar]
- 6. An T, Chen Y, Tu Y, Lin P. Mesenchymal stromal cell‐derived extracellular vesicles in the treatment of diabetic foot ulcers: application and challenges. Stem Cell Rev Rep. 2021;17(2):369–378. doi: 10.1007/s12015-020-10014-9 [DOI] [PubMed] [Google Scholar]
- 7. Krasilnikova OA, Baranovskii DS, Lyundup AV, Shegay PV, Kaprin AD, Klabukov ID. Stem and somatic cell monotherapy for the treatment of diabetic foot ulcers: review of clinical studies and mechanisms of action. Stem Cell Rev Rep. 2022;18(6):1974–1985. doi: 10.1007/s12015-022-10379-z [DOI] [PubMed] [Google Scholar]
- 8. Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassanshahi A, Hassanshahi M, Khabbazi S, et al. Adipose‐derived stem cells for wound healing. J Cell Physiol. 2019;234(6):7903–7914. doi: 10.1002/jcp.27922 [DOI] [PubMed] [Google Scholar]
- 10. Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16(2):115–130. doi: 10.1038/nrd.2016.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin PE, O'Shaughnessy EM, Wright CS, Graham A. The potential of human induced pluripotent stem cells for modelling diabetic wound healing in vitro. Clin Sci. 2018;132(15):1629–1643. doi: 10.1042/CS20171483 [DOI] [PubMed] [Google Scholar]
- 12. Shen JM, Chen J, Feng L, Feng C. A scientometrics analysis and visualisation of diabetic foot research from 1955 to 2022. Int Wound J. 2023;20(4):1072–1087. doi: 10.1111/iwj.13964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Zheng X, Lv H, et al. A bibliometrics study on the status quo and hot topics of pathogenesis of psoriasis based on Web of Science. Skin Res Technol. 2024;30(1):e13538. doi: 10.1111/srt.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101, Suppl 1(Suppl 1):5303–5310. doi: 10.1073/pnas.0307513100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masson R, Ma E, Park S, et al. Top cited articles in dissecting cellulitis of the scalp: a bibliometric analysis. Skin Res Technol. 2023;29(11):e13509. doi: 10.1111/srt.13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tu SJ, Jin C, Chen BT, Xu AY, Luo C, Wang XH. Study on the fusion of sports and medicine in China from 2012 to 2021: a bibliometric analysis via CiteSpace. Front Public Health. 2022;10:939557. doi: 10.3389/fpubh.2022.939557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow‐derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57 [DOI] [PubMed] [Google Scholar]
- 18. English K, Mahon BP. Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem. 2011;112(8):1963–1968. doi: 10.1002/jcb.23119 [DOI] [PubMed] [Google Scholar]
- 19. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226 [DOI] [PubMed] [Google Scholar]
- 20. Hou C, Shen L, Huang Q, et al. The effect of heme oxygenase‐1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials. 2013;34(1):112–120. doi: 10.1016/j.biomaterials.2012.09.022 [DOI] [PubMed] [Google Scholar]
- 21. Al‐Massri KF, Ahmed LA, El‐Abhar HS. Mesenchymal stem cells therapy enhances the efficacy of pregabalin and prevents its motor impairment in paclitaxel‐induced neuropathy in rats: role of Notch1 receptor and JAK/STAT signaling pathway. Behav Brain Res. 2019;360:303–311. doi: 10.1016/j.bbr.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 22. Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of mesenchymal stem cell‐derived exosomes on autoimmune diseases. Front Immunol. 2021;12:749192. doi: 10.3389/fimmu.2021.749192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126(4):1173–1180. doi: 10.1172/JCI81131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szwedowicz U, Łapińska Z, Gajewska‐Naryniecka A, Choromańska A. Exosomes and other extracellular vesicles with high therapeutic potential: their applications in oncology, neurology, and dermatology. Molecules. 2022;27(4):1303. doi: 10.3390/molecules27041303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi: 10.1124/pr.112.005983 [DOI] [PubMed] [Google Scholar]
- 26. Nair S, Salomon C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin Immunopathol. 2018;40(5):425–437. doi: 10.1007/s00281-018-0680-2 [DOI] [PubMed] [Google Scholar]
- 27. Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B, Rev. 2015;21(1):45–54. doi: 10.1089/ten.TEB.2014.0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan wound dressings incorporating exosomes derived from microRNA‐126‐overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full‐thickness skin defects in a diabetic rat model. Stem Cells Trans Med. 2017;6(3):736–747. doi: 10.5966/sctm.2016-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newton WC, Kim JW, Luo JZQ, Luo L. Stem cell‐derived exosomes: a novel vector for tissue repair and diabetic therapy. J Mol Endocrinol. 2017;59(4):R155–R165. doi: 10.1530/JME-17-0080 [DOI] [PubMed] [Google Scholar]
- 30. Sun Y, Shi H, Yin S, et al. Human mesenchymal stem cell derived exosomes alleviate Type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β‐cell destruction. ACS Nano. 2018;12(8):7613–7628. doi: 10.1021/acsnano.7b07643 [DOI] [PubMed] [Google Scholar]
- 31. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 32. Maxwell KG, Millman JR. Applications of iPSC‐derived beta cells from patients with diabetes. Cell Rep Med. 2021;2(4):100238. doi: 10.1016/j.xcrm.2021.100238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kashpur O, Smith A, Gerami‐Naini B, et al. Differentiation of diabetic foot ulcer‐derived induced pluripotent stem cells reveals distinct cellular and tissue phenotypes. FASEB J. 2019;33(1):1262–1277. doi: 10.1096/fj.201801059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gorecka J, Gao X, Fereydooni A, et al. Induced pluripotent stem cell‐derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen Med. 2020;15(2):1277–1293. doi: 10.2217/rme-2019-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao X, Gao M, Gorecka J, et al. Human‐induced pluripotent stem‐cell‐derived smooth muscle cells increase angiogenesis to treat hindlimb ischemia. Cells. 2021;10(4):792. doi: 10.3390/cells10040792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liesveld JL, Sharma N, Aljitawi OS. Stem cell homing: from physiology to therapeutics. Stem Cells. 2020;38(10):1241–1253. doi: 10.1002/stem.3242 [DOI] [PubMed] [Google Scholar]
- 37. Dash BC, Setia O, Gorecka J, et al. A dense fibrillar collagen scaffold differentially modulates secretory function of iPSC‐derived vascular smooth muscle cells to promote wound healing. Cells. 2020;9(4):966. doi: 10.3390/cells9040966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cerqueira MT, da Silva LP, Santos TC, et al. Gellan gum‐hyaluronic acid spongy‐like hydrogels and cells from adipose tissue synergize promoting neoskin vascularization. ACS Appl Mater Interfaces. 2014;6(22):19668–19679. doi: 10.1021/am504520j [DOI] [PubMed] [Google Scholar]
- 39. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- 40. Jiang S, Li SC, Huang C, Chan BP, Du Y. Physical properties of implanted porous bioscaffolds regulate skin repair: focusing on mechanical and structural features. Adv Healthc Mater. 2018;7(6):e1700894. doi: 10.1002/adhm.201700894 [DOI] [PubMed] [Google Scholar]
- 41. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26(8):2173–2182. doi: 10.1634/stemcells.2007-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Teo AK, Wagers AJ, Kulkarni RN. New opportunities: harnessing induced pluripotency for discovery in diabetes and metabolism. Cell Metab. 2013;18(6):775–791. doi: 10.1016/j.cmet.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song SH, Lee MO, Lee JS, et al. Genetic modification of human adipose‐derived stem cells for promoting wound healing. J Dermatol Sci. 2012;66(2):98–107. doi: 10.1016/j.jdermsci.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 44. Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011;29(11):1727–1737. doi: 10.1002/stem.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin G, Prabhakaran MP, Ramakrishna S. Photosensitive and biomimetic core‐shell nanofibrous scaffolds as wound dressing. Photochem Photobiol. 2014;90(3):673–681. doi: 10.1111/php.12238 [DOI] [PubMed] [Google Scholar]
- 46. Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. 2010;107(8):3317–3322. doi: 10.1073/pnas.0905432106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell‐derived extracellular vesicles: toward cell‐free therapeutic applications. Mol Ther. 2015;23(5):812–823. doi: 10.1038/mt.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patel DB, Santoro M, Born LJ, Fisher JP, Jay SM. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv. 2018;36(8):2051–2059. doi: 10.1016/j.biotechadv.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells‐derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hu Y, Rao SS, Wang ZX, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR‐21‐3p‐mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8(1):169–184. doi: 10.7150/thno.21234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet‐rich plasma promote the re‐epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7(1):81–96. doi: 10.7150/thno.16803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang C, Wang M, Xu T, et al. Engineering bioactive self‐healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi: 10.7150/thno.29766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bailey AJM, Li H, Kirkham AM, et al. MSC‐derived extracellular vesicles to heal diabetic wounds: a systematic review and meta‐analysis of preclinical animal studies. Stem Cell Rev Rep. 2022;18(3):968–979. doi: 10.1007/s12015-021-10164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vu NB, Nguyen HT, Palumbo R, Pellicano R, Fagoonee S, Pham PV. Stem cell‐derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112(3):384–400. doi: 10.23736/S0026-4806.20.07205-5 [DOI] [PubMed] [Google Scholar]
- 55. Eggenhofer E, Benseler V, Kroemer A, et al. Mesenchymal stem cells are short‐lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Namestnikova DD, Gubskiy IL, Revkova VA, et al. Intra‐arterial stem cell transplantation in experimental stroke in rats: real‐time MR visualization of transplanted cells starting with their first pass through the brain with regard to the therapeutic action. Front Neurosci. 2021;15:641970. doi: 10.3389/fnins.2021.641970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther. 2016;7:37. doi: 10.1186/s13287-016-0303-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rustad KC, Gurtner GC. Mesenchymal stem cells home to sites of injury and inflammation. Adv Wound Care. 2012;1(4):147–152. doi: 10.1089/wound.2011.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carstens MH, Quintana FJ, Calderwood ST, et al. Treatment of chronic diabetic foot ulcers with adipose‐derived stromal vascular fraction cell injections: Safety and evidence of efficacy at 1 year. Stem Cells Transl Med. 2021;10(8):1138–1147. doi: 10.1002/sctm.20-0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arango‐Rodríguez ML, Solarte‐David VA, Becerra‐Bayona SM, et al. Role of mesenchymal stromal cells derivatives in diabetic foot ulcers: a controlled randomized phase 1/2 clinical trial. Cytotherapy. 2022;24(10):1035–1048. doi: 10.1016/j.jcyt.2022.04.002 [DOI] [PubMed] [Google Scholar]
- 61. Yu X, Liu P, Li Z, Zhang Z. Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front Endocrinol. 2023;14:1099310. doi: 10.3389/fendo.2023.1099310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoang DM, Pham PT, Bach TQ, et al. Stem cell‐based therapy for human diseases. Signal Trans Target Ther. 2022;7(1):272. doi: 10.1038/s41392-022-01134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request.


