Abstract
Objectives
Though the concomitant occurrence of non-severe aortic stenosis (AS) and mitral regurgitation (MR) is highly prevalent, there are limited data to guide clinical decision-making in this condition. Here, we attempt to determine an aortic valve area (AVA) cut-off value associated with worse clinical outcomes in patients with combined non-severe AS and MR.
Methods
Single-centre, retrospective analysis of consecutive patients who underwent echocardiography examination between 2010 and 2021 with evidence of combined non-severe AS and MR. We excluded patients with ≥moderate aortic valve regurgitation or mitral stenosis, as well as patients who underwent any aortic or mitral intervention either prior or following our assessment (n=372).
Results
The final cohort consisted of 2933 patients with non-severe AS, 506 of them with >mild MR. Patients with both pathologies had lower cardiac output and worse diastolic function.
Patients with an AVA ≤1.35 cm² in the presence of >mild MR had the highest rates of heart failure (HF) hospitalisations (HR 3.1, IQR 2.4–4, p<0.001) or mortality (HR 2, IQR 1.8–2.4, p<0.001), which remained significant after adjusting for clinical and echocardiographic parameters.
Conclusion
Patients with combined non-severe AS and MR have a higher rate of HF hospitalisations and mortality. An AVA≤1.35 cm² in the presence of >mild MR is associated with worse clinical outcomes.
Keywords: Heart failure, Adult cardiology, Echocardiography, Valvular heart disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Single-centre retrospective analysis of patients who underwent echocardiography examination between 2010 and 2021 which demonstrated combined non-severe aortic stenosis and mitral regurgitation.
Patients with other significant left-sided valvular abnormalities and those in whom an aortic or mitral valve intervention was done were excluded from the analysis.
Classification and regression trees modelling was used to identify the optimal aortic valve area cut-off value predictive of heart failure hospitalisation or all-cause mortality.
Further studies are warranted to validate this cut-off value.
Introduction
Multiple valvular heart disease (mVHD) is defined as the combination of stenotic or regurgitant lesions occurring in ≥2 cardiac valves.1 The presence of mVHD may significantly affect the evaluation of each valvular lesion severity by affecting left ventricular (LV) filling pressures, preload and afterload. Moreover, mVHD was associated with worse outcomes. In the Euro Heart Survey, mVHD was observed in 20% of the patients with native VHD,2 whereas in a Swedish nationwide study, mVHD was present in 11% of patients, with high prevalence of combined aortic stenosis (AS) and mitral regurgitation (MR).3 Notably, definition and specific cut-off values for mVHD currently lack and are based on local practice or registries.
As the impact of combined non-severe mVHD has not been appropriately defined or evaluated, contemporary guideline documents4 5 focus mainly on mVHD in which at least one of the lesions involved is defined as severe. Therefore, in this study, we chose to evaluate the presence and the impact of non-severe mVHD on patients’ outcomes in a large tertiary centre and seek an aortic valve area (AVA) cut-off value associated with worse clinical outcomes.
Material and methods
We used a retrospective analysis performed in a single university-affiliated large tertiary care hospital.
Study population
Adult patients who underwent an echocardiography at our centre between January 2010 and March 2021, with evidence of less than severe AS combined with less than severe MR, were included in the initial cohort.
Patients with ≥moderate aortic valve regurgitation (AR) or ≥moderate mitral stenosis and those in whom an aortic or mitral valve intervention was done (n=372) were excluded from the analysis.
Doppler echocardiography
To evaluate the presence of mVHD, all patients underwent a comprehensive two-dimensional (2D) and Doppler echocardiographic study with multiple windows during the same examination. Echocardiography was performed according to contemporary European Society of Cardiology (ESC) guideline.6 All measurements were retrieved from the echocardiography reporting system.
Stroke volume was calculated as the product of LV outflow tract (LVOT) area and the time-velocity integral of the aortic flow velocity. Cardiac output (CO) measured as stroke volume multiplied by heart rate.
AVA was calculated using continuity equation from the flow through the LVOT with respect to the flow through the aortic valve. Multiple windows were used for the highest velocity. Severe AS was defined as a peak velocity >4 m/s, mean gradient >40 mm Hg or estimated AVA<1 cm2. Both classical low-flow-low gradient and paradoxical low-flow low gradient AS were not included in the current study.
MR severity was determined by an integrative, semiquantitative and quantitative approach, including assessment of vena contracta width, valve morphology, chamber size, jet area, jet density and contour, and when available, effective orifice area (ERO) and regurgitant volume. After excluding those defined as severe MR, we grouped those patients into MR≤mild and MR>mild.
Measurements of mitral inflow included the peak early filling (E wave) and late diastolic filling (A wave) velocities, the E/A ratio and deceleration time (DT) of early filling velocity. Early diastolic mitral annular velocities (e') were measured from both septal and lateral annulus. Left atrium volume was calculated by tracing the endocardial borders at end-systole in the apical four-chamber and two-chamber views, with LA volume index calculated by adjusting to the patient’s body surface area.
Systolic pulmonary arterial pressure (sPAP) was determined by the maximal tricuspid regurgitant velocity and an estimation of right atrial pressure according to the vena cava width and responsiveness.
LV diameters including left ventricle end systolic and diastolic diameter (LVESd, LVEDd) were measured using lineal 2D echocardiography or M-mode parallel to the mitral valve annulus.
Right ventricular (RV) size and function assessment was based on multiple views of the RV. An integrative qualitative grading of RV function was formulated by a specialised imaging cardiologist responsible for the echocardiographic study.
Clinical data and outcome measures
Baseline characteristics including age, sex and major comorbidities were extracted from the electronic health record (EMR). Hospitalisation for heart failure (HF) which occurred at our medical centre alone was retrieved from the electronic health record. The date of mortality (if occurred) was automatically updated in the hospital records via the Ministry of Health.
All the data obtained in the study were retrieved from the hospital anonymised database that includes all clinical and echocardiographic information.
Statistical analysis
Categorical variables are reported as numbers and percentages, and continuous variables are reported as means and SD or medians and IQRs, as appropriate. Continuous variables were tested for normal distribution using histograms, Q-Q plots and normality tests (Kolmogorov-Smirnov and Shapiro-Wilk). Continuous variables were compared between groups using independent Mann-Whitney U test, post hoc Bonferroni correction applied to analyse subgroup comparison. Categorical variables were compared using χ2 test or Fisher’s exact test, post hoc Bonferroni correction applied to analyse subgroup comparison.
The AVA was divided into categories by means of a classification and regression trees model for the prediction of HF hospitalisation, with a minimum of 100 cases in parent node and minimum of 50 cases in child node. The analysis selects the best predictor for splitting the data into child nodes. A p value is given for each branch.
Long-term outcome (all-cause mortality or HF hospitalisation) assessed using a Cox regression model, also adjusted for clinical and echocardiographic parameters. The following variables were included:
Clinical variables: Age, sex, chronic renal failure (CRF), hypertension, ischaemic heart disease (IHD), AF, HF and chronic obstructive pulmonary disease (COPD).
Echocardiographic variables: Ejection fraction (EF), LVEDd, LVESd, degree of AR, RV function and RV size. Of note, due to the expected effect of mVHD on LV filling indices and forward flow (stroke volume), as the major haemodynamic consequences leading to HF hospitalisation, these parameters we evaluated in the Cox regression model separately.
All statistical tests were two sided, and a p<0.05 was considered statistically significant. SPSS software was used for all statistical analysis (IBM SPSS statistics, V.25).
Results
Patient clinical characteristics
The study cohort included 2933 patients with non-severe AS. Of whom, 2427 had ≤mild MR and 506>mild MR. Data regarding the aetiology of >MR were available in 59% (299 patients), in whom 22 secondary and 277 with primary MR. Table 1 provides the patients’ clinical characteristics.
Table 1.
Patients’ clinical characteristics in the entire cohort and according to severity of mitral regurgitation (MR)
| All patients (n=2933) | Patients with up to mild MR (n=2427) | Patients with greater than mild MR (n=506) | P value | |
| Age (years)* | 80.64 (73.16–86.7) | 80.11 (72.42–86.24) | 83.15 (76.3–88.57) | <0.001 |
| Follow-up (days)* | 1127.54 (392.45–1998.65) | 1227.27 (488.60–2100.26) | 721.52 (150.39–1471.61) | <0.001 |
| Sex (female) | 1379 (47) | 1111 (45.8) | 268 (53) | 0.03 |
| Deceased during Follow-up | 1571 (53.6) | 1236 (50.9) | 335 (66.2) | <0.001 |
| Heart failure admission | 435 (14.8) | 314 (12.9) | 121 (23.9) | <0.001 |
| AF | 657 (22.4) | 471 (19.4) | 186 (36.8) | <0.001 |
| CRF | 423 (14.4) | 313 (12.9) | 110 (21.7) | <0.001 |
| Malignancy | 642 (21.9) | 528 (21.8) | 114 (22.5) | 0.702 |
| Hypertension | 1877 (64) | 1516 (62.5) | 361 (71.3) | <0.001 |
| DM | 965 (32.9) | 801 (33) | 164 (32.4) | 0.796 |
| CVA/TIA | 379 (12.9) | 305 (12.6) | 74 (14.6) | 0.209 |
| IHD | 1131 (38.6) | 901 (37.1) | 230 (45.5) | <0.001 |
| COPD | 269 (9.2) | 223 (9.2) | 46 (9.1) | 0.945 |
*Median and IQR. All other values represent the number of patients and percentages.
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; CVA, cerebrovascular accident; DM, diabetes mellitus; IHD, ischaemic heart disease; TIA, transient ischaemic attack.
The median follow-up time of the entire cohort was 1127 days (IQR 392–1999), during which 1572 patients (53.6%) had died and 435 patients (14.8%) had experienced an HF hospitalisation.
Compared with patients with ≤mild MR, patients with >mild MR were older (80.1 years, IQR 72.4–86.2 vs 83.2 years, IQR 76.3–88.6, p<0.001), with a predominance female population (45.8% vs 53%, p=0.03), respectively.
In addition, patients with >mild MR were more likely to have a history of AF (36.8% vs 22.4%, p<0.001), CRF (21.7% vs 12.9, p<0.001), hypertension (71.3% vs 62.5%, p<0.001) and IHD (45.5% vs 37.1%, p<0.001).
Examining outcomes, patients with >mild MR experienced a higher rate of HF hospitalisations (23.9% vs 12.9%, p<0.001) and increased all-cause mortality (66.2% vs 53.6%, p<0.001).
Patient echocardiographic measurements
Patients’ echocardiographic measurements in the entire cohort and according to severity of MR are presented in table 2.
Table 2.
Patients’ echocardiographic measurements in the entire cohort and according to severity of mitral regurgitation (MR)
| All patients (n=2933) | Patients with up to mild MR (n=2427) | Patients with greater than mild MR (n=506) | P value | ||
| Ejection fraction* | 60 (55–60) | 60 (55–60) | 55 (45–60) | <0.001 | |
| Cardiac output (liter/min)* | 5.56 (4.67–6.53) | 5.64 (4.78–6.61) | 5.03 (4.29–6.18) | <0.001 | |
| LVEDd (mm)* | 47 (43–51) | 47 (43–51) | 49 (45–54) | <0.001 | |
| LVESd (mm)* | 29 (25–34) | 28 (25–33) | 31 (26–38) | <0.001 | |
| Aortic valve area (cm2)* | 1.4 (1.2–1.6) | 1.4 (1.2–1.7) | 1.3 (1.1–1.5) | <0.001 | |
| Peak aortic gradient (mm Hg)* | 26 (21–34) | 27 (22–35) | 26 (21–33) | 0.045 | |
| Mean aortic gradient (mm Hg)* | 15 (12–20) | 15 (12–20) | 15 (11–19) | 0.018 | |
| LAVI (mL/m2)* | 42.7 (33.5–53.5) | 40.3 (32.2–50.8) | 53.1 (44–65.7) | <0.001 | |
| Deceleration time (ms)* | 219 (174–274) | 225 (180–275) | 187 (153–241) | <0.001 | |
| E/e'* | 14.02 (10.97–18.34) | 13.62 (10.54–17.7) | 17.05 (13.18–22.39) | <0.001 | |
| Average e’* | 6 (4.93–7.21) | 6 (4.96–7.2) | 6 (4.73–7.35) | 0.452 | |
| E/A ratio* | 0.8 (0.7–1.1) | 0.8 (0.6–1.1) | 1.1 (0.9–1.6) | <0.001 | |
| sPAP (mm Hg)* | 36 (30–47) | 34 (29–44) | 46 (37–58) | <0.001 | |
| Aortic valve regurgitation | None | 1485 (50.6) | 1288 (53.1) | 197 (38.9) | <0.001 |
| Minimal | 577 (19.7) | 478 (19.7) | 99 (19.6) | ||
| Mild | 685 (23.4) | 532 (21.9) | 153 (30.2) | ||
| Mild to moderate | 186 (6.3) | 129 (5.3) | 57 (11.3) | ||
| Right ventricle function | Normal | 2668 (91) | 2264 (93.3) | 404 (79.8) | <0.001 |
| Mild dysfunction | 207 (7.1) | 131 (5.4) | 76 (15) | ||
| Moderate dysfunction | 51 (1.7) | 29 (1.2) | 22 (4.3) | ||
| Severe dysfunction | 7 (0.2) | 3 (0.1) | 4 (0.8) | ||
| Right ventricle size | Normal | 2593 (88.4) | 2208 (91) | 385 (76.1) | <0.001 |
| Mild dilatation | 257 (8.8) | 165 (6.8) | 92 (18.2) | ||
| Moderate dilatation | 63 (2.1) | 41 (1.7) | 22 (4.3) | ||
| Severe dilatation | 20 (0.7) | 13 (0.5) | 7 (1.4) | ||
*Median and IQR. All other values represent the number of patients and percentages.
E/A, Early diastolic flow velocity (E velocity) divided by late diastolic transmitral flow velocity (A velocity); LAVI, Left Atrial Volume Index; LVEDd, left ventricle end diastolic diameter; LVESd, left ventricle end systolic diameter; sPAP, Systolic pulmonary artery pressure.
Patients with >mild MR had slightly lower CO values (5.03 mL/m2, IQR 4.29–6.18 vs 5.64 (IQR 4.78–6.61, p<0.001) and a greater left ventricle end-systolic (31 mm, IQR 26–38, vs 28, IQR 25–33, p<0.001) and end-diastolic diameters (49 mm, IQR 45–54 vs 47, IQR 43–51, p<0.001).
Proximal isovelocity hemispheric surface area data were available only in a portion of patients with >mild MR. These patients had an ERO area of 0.1 cm² (IQR 0.1–0.2, n=184/514) with a regurgitant volume of 26 mL (IQR 17–35 mL, n=105/330).
As expected, patients with >mild MR had an overall worse diastolic indices with a larger LA volume index, shorter DT, higher E/A ratio and elevated SPAP compared with patients with ≤mild MR. The average e’ for the entire cohort was mildly reduced (6, IQR 4.93–7.21), with no difference between MR severity groups.
Higher rates of RV dysfunction and RV dilatation were found in patients with >mild MR (table 2).
AVA optimal cut-off value
In patients with >mild MR, a classification tree analysis revealed a cut-off value of 1.35 cm² to be predictive for HF hospitalisations. Accordingly, we further divided both MR groups according to the suggested AS cut-off value. Patients’ clinical and echocardiographic measurements in these four subgroups are presented in table 3.
Table 3.
Patients’ clinical and echocardiographic measurements according to MR severity and aortic valve area (AVA) of 1.35 cm²
| MR≤mild | MR>mild | ||||||||
| AVA>1.35 | AVA≤1.35 | AVA>1.35 | AVA≤1.35 | ||||||
| Group 1 N=1333 |
Group 2 N=1094 |
P value | Group 3 N=211 |
Group 4 N=295 |
P value | P value Group 2–4 |
P value Group 1–3 |
||
| Age (years)* | 79.3 (70.7–85.6) | 81.46 (74.5–86.7) | <0.001 | 81.2 (73.6–87.4) | 84.4 (77.5–89.2) | 0.002 | <0.001 | 0.027 | |
| Follow-up (days)* | 1393 (541–2178) | 1107 (432–1955) | 0.002 | 1006 (242–1751) | 574 (112–1249) | 0.003 | <0.001 | <0.001 | |
| Sex (female) | 527 (39.5) | 584 (53.4) | <0.001 | 103 (48.8) | 165 (55.9) | NS | NS | NS | |
| Deceased during Follow-up | 647 (48.4) | 589 (53.8) | <0.001 | 124 (58.8) | 211 (71.5) | 0.017 | <0.001 | 0.035 | |
| Heart failure admission | 176 (13.2) | 138 (12.6) | NS | 38 (18) | 83 (28.1) | 0.024 | <0.001 | NS | |
| AF | 257 (19.3) | 214 (19.6) | NS | 78 (37) | 108 (36.6) | NS | 0.012 | <0.001 | |
| CRF | 172 (12.9) | 141 (12.9) | NS | 51 (24.2) | 59 (20) | NS | 0.012 | <0.001 | |
| Malignancy | 295 (22.1) | 233 (21.3) | NS | 45 (21.3) | 69 (23.4) | NS | NS | NS | |
| HTN | 853 (64) | 663 (60.6) | NS | 145 (68.7) | 216 (73.2) | NS | <0.001 | NS | |
| DM | 439 (32.9) | 362 (33.1) | NS | 70 (33.2) | 94 (31.9) | NS | NS | NS | |
| CVA/TIA | 167 (12.5) | 138 (12.6) | NS | 26 (12.3) | 48 (16.3) | NS | NS | NS | |
| IHD | 512 (38.4) | 389 (35.6) | NS | 99 (46.9) | 131 (44.4) | NS | 0.032 | NS | |
| COPD | 139 (10.4) | 84 (7.7) | NS | 15 (7.1) | 31 (10.5) | NS | NS | NS | |
| LV EF* | 60 (55–60) | 60 (55–60) | 1 | 60 (45–60) | 55 (45–60) | 0.514 | <0.001 | 0.001 | |
| Cardiac output (L/min)* | 6.05 (5.1–7) | 5.01 (4.3–5.9) | <0.001 | 5.9 (4.9–6.6) | 4.8 (4.0–5.7) | <0.001 | 0.058 | 0.001 | |
| LVEDd (mm)* | 47 (43–51) | 46 (42–51) | 0.019 | 50 (46–55) | 48 (44–54) | 0.18 | <0.001 | <0.001 | |
| LVESd (mm)* | 29 (25–33) | 28 (25–33) | 0.334 | 31 (27–39) | 31 (26–38) | 1 | <0.001 | <0.001 | |
| Aortic valve area (cm²)* | 1.6 (1.5–1.9) | 1.2 (1.1–1.3) | <0.001 | 1.6 (1.4–1.8) | 1.13 (1.08–1.26) | <0.001 | 1 | 0.725 | |
| Peak aortic gradient (mm Hg)* | 24 (20–30) | 31 (24–40) | <0.001 | 23 (19–29) | 29 (22–38) | <0.001 | 0.001 | 0.491 | |
| Mean aortic gradient (mm Hg)* | 14 (11–17) | 18 (14–24) | <0.001 | 13 (11–16) | 17 (13–21) | <0.001 | <0.001 | 0.294 | |
| LAVI (mL/m²)* | 40 (32.1–50.6) | 40.8 (32.4–51) | 1 | 54.1 (44.66.2) | 52.1 (44.2–65) | 1 | <0.001 | <0.001 | |
| Deceleration time (ms)* | 225 (182–275) | 224 (180–277) | 1 | 208 (162–254) | 180 (148–229) | 0.056 | <0.001 | 0.001 | |
| E/e'* | 13.2 (10.2–17.1) | 14 (11–18.2) | 0.001 | 16.3 (12.6–22) | 17.6 (14–22.6) | 0.425 | <0.001 | <0.001 | |
| Average e’* | 6.2 (5.1–7.4) | 5.8 (4.7–7) | <0.001 | 6 (4.7–7.5) | 6 (4.7–7.2) | 1 | 1 | 1 | |
| E/A ratio* | 0.8 (0.7–1.1) | 0.8 (0.6–1.1) | 1 | 1.1 (0.8–1.5) | 1.2 (0.9–1.7) | 0.451 | <0.001 | <0.001 | |
| sPAP (mm Hg)* | 34 (28–43) | 35 (29–45) | 0.089 | 42 (34–54) | 49 (39–59) | <0.001 | <0.001 | <0.001 | |
| MR ERO (cm²)* | NA | NA | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 0.148 | ||||
| MR Rvol (mL)* | NA | NA | 25 (17–35) | 26 (17–34) | 0.893 | ||||
| AR | None | 739 (55.4) | 549 (50.2) | NS | 82 (38.9) | 115 (39) | NS | 0.005 | <0.001 |
| Minimal | 253 (19) | 225 (20.6) | 30 (14.2) | 69 (23.4) | |||||
| Mild | 266 (20) | 266 (24.3) | 72 (34.1) | 81 (27.5) | |||||
| Mild to moderate | 75 (5.6) | 54 (4.9) | 27 (12.8) | 30 (10.2) | |||||
| RV function | Normal | 1245 (93.4) | 1019 (93.1) | NS | 173 (82) | 231 (78.3) | NS | <0.001 | <0.001 |
| Mild | 72 (5.4) | 59 (5.4) | 31 (14.7) | 45 (15.3) | |||||
| Moderate | 15 (1.1) | 14 (1.3) | 6 (2.8) | 16 (5.4) | |||||
| Severe | 1 (0.1) | 2 (0.2) | 1 (0.5) | 3 (1) | |||||
| RV size | Normal | 1221 (91.6) | 987 (90.2) | NS | 167 (79.1) | 218 (73.9) | NS | <0.001 | <0.001 |
| Mild | 83 (6.2) | 82 (7.5) | 33 (15.6) | 59 (20) | |||||
| Moderate | 24 (1.8) | 17 (1.6) | 9 (4.3) | 13 (4.4) | |||||
| Severe | 5 (0.4) | 8 (0.7) | 2 (0.9) | 5 (1.7) | |||||
*Median and IQR. All other values represent the number of patients and percentages.
AF, atrial fibrillation; AR, aortic regurgitation; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; CVA, cerebrovascular accident; DM, diabetes mellitus; E/A, Early diastolic flow velocity (E velocity) divided by late diastolic transmitral flow velocity (A velocity); ERO, effective orifice area; IHD, ischaemic heart disease; LAVI, Left Atrial Volume Index; LVEDd, left ventricle end diastolic diameter; LV EF, left ventricle ejection fraction; LVESd, left ventricle end systolic diameter; MR, mitral regurgitation; NA, not available; NS, not significant; RV, rigth ventricle; sPAP, systolic pulmonary artery pressure; TIA, transient ischaemic attack.
Haemodynamic impact of AVA in patients with >mild MR
Among patients with >mild MR, those with AVA≤1.35 cm² were older compared with patients with AVA>1.35 cm² (84.4 years, IQR 77.5–89.2 vs 81.2 years, IQR 73.6–87.3, respectively, p=0.002). There were no other statistically significant differences in baseline clinical characteristics between these two subgroups.
Patients with AVA≤1.35 cm² had lower CO compared with patients with an AVA>1.35 cm² (4.77 L/min, IQR 4.03–5.7 vs 5.93 L/min, IQR 4.85–6.62, respectively, p<0.001) and had elevated sPAP values (49 mm Hg, IQR 39–59 compared with 42 mm Hg, IQR 34–54, p<0.001), whereas other diastolic or RV function indices did not significantly differ between the two groups (table 3).
Effect of AVA and MR severity on clinical outcomes
The impact of MR grade and AVA on HF hospitalisations within each subgroup is presented in table 4.
Table 4.
Impact of MR grade and aortic valve area (AVA) on heart failure hospitalisation
| HR | 95% CI | P value | |
| Up to mild MR+AVA≤1.35 cm² vs AVA>1.35 cm² | |||
| Univariate analysis | 1.036 | 0.829 to 1.295 | 0.754 |
| Greater than mild MR+ AVA≤1.35 cm² vs AVA>1.35 cm² |
|||
| Univariate | 1.893 | 1.288 to 2.781 | 0.001 |
| Adjusted for all clinical* | 1.941 | 1.309 to 2.880 | <0.001 |
| Adjusted for all echocardiographic† | 1.672 | 1.097 to 2.548 | 0.017 |
| Adjusted for both*† | 1.774 | 1.157 to 2.72 | 0.009 |
| Adjusted for diastolic parameter # +cardiac output | 1.555 | 0.833 to 2.904 | 0.166 |
| AVA>1.35 cm²+ MR up to mild vs greater than mild |
|||
| Univariate analysis | 1.624 | 1.143 to 2.308 | 0.007 |
| Adjusted for all clinical* | 1.249 | 0.873 to 1.788 | 0.223 |
| Adjusted for all echocardiographic† | 0.992 | 0.652 to 1.508 | 0.969 |
| Adjusted for both*† | 0.881 | 0.572 to 1.358 | 0.567 |
| Adjusted for diastolic parameter # +cardiac output | 0.645 | 0.356 to 1.168 | 0.148 |
| AVA≤1.35 cm²+ MR greater than mild vs up to mild |
|||
| Univariate analysis | 3.056 | 2.324 to 4.018 | <0.001 |
| Adjusted for all clinical* | 2.241 | 1.689 to 2.973 | <0.001 |
| Adjusted for all echocardiographic† | 2.162 | 1.545 to 3.025 | <0.001 |
| Adjusted for both*† | 1.625 | 1.163 to 2.271 | 0.004 |
| Adjusted for diastolic parameter # +cardiac output | 1.816 | 1.135 to 2.906 | 0.013 |
| Greater than mild MR+AVA ≤1.35 cm² vs Up to mild MR+AVA>1.35 cm² |
|||
| Univariate analysis | 3.089 | 2.374 to 4.019 | <0.001 |
| Adjusted for all clinical* | 2.164 | 1.641 to 2.852 | <0.001 |
| Adjusted for all echocardiographic† | 1.67 | 1.205 to 2.314 | 0.002 |
| Adjusted for both*† | 1.296 | 0.941 to 1.784 | 0.112 |
| Adjusted for diastolic parameter # +cardiac output | 1.175 | 0.708 to 1.948 | 0.533 |
*For clinical variables—age, sex, atrial fibrillation, chronic renal failure hypertension, ischaemic heart disease, COPD.
†For echocardiographic variables—ejection fraction, left ventricle end diastolic diameter, left ventricle end systolic diameter, aortic valve regurgitation grade, right ventricle size, right ventricle function # For diastolic parameter—LAVI, DT, average E/e’, E/A ratio, sPAP.
COPD, chronic obstructive pulmonary disease; DT, deceleration time; E/A, Early diastolic flow velocity (E velocity) divided by late diastolic transmitral flow velocity (A velocity); LAVI, Left Atrial Volume Index; MR, mitral regurgitation.
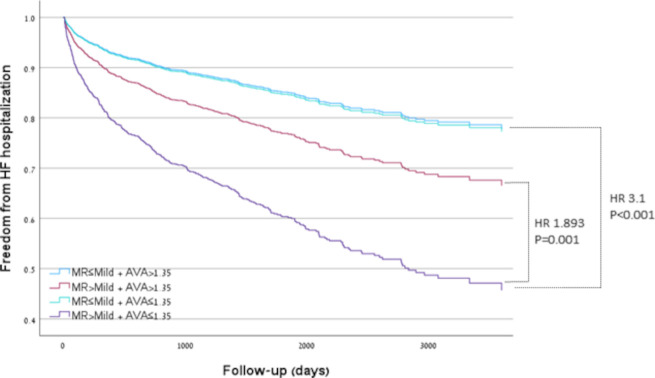
In univariate Cox regression analysis (figure 1), patients with >mild MR and an AVA≤1.35 cm² had the highest rate of HF hospitalisations compared with patients ≤mild MR and an AVA>1.35 cm² (HR 3.1, IQR 2.4–4, p<0.001).
Figure 1.
Univariate Cox regression analysis for heart failure (HF) hospitalisation according to severity of MR and AVA. AVA, aortic valve area; MR, mitral regurgitation.
AVA had more impact on patients’ outcomes since the presence of significant MR in patients with an AVA>1.35 cm² was associated with increased rates of HF hospitalisations in univariate analysis (group 1 vs group 3, HR 1.6, IQR 1.1–2.3, p=0.007), this effect was lost after adjusting for echocardiographic parameters and/or clinical parameters. Furthermore, following adjustment for either clinical comorbidities or echocardiographic parameters only patients with a combination of >mild MR and AVA≤1.35 cm² had a higher HF hospitalisations rate.
Analysis concerning all-cause mortality is available in online supplemental table S1, figure S1. Patients with >mild MR and AVA≤1.35 cm² had higher mortality rates compared with patients with ≤mild MR and AVA>1.35 cm², even after adjusting for clinical and/or echocardiographic parameters
bmjopen-2023-080914supp001.pdf (1.1MB, pdf)
The effect of diastolic function on outcome is presented in table 4.
The effect of surgical AV replacement in patients with >mild MR and AVA≤1.35 cm² (n=10, one patient with concomitant mitral valve intervention) on outcomes is presented in online supplemental tables S2,S3 and figure S2.
Discussion
This study investigated the clinical outcomes of patients with combined non-severe AS and low-grade MR. We found two key findings:
Patients with combined non-severe AS and low-grade MR had lower CO and impaired diastolic function compared with those without these conditions.
AVA between 1.0 and 1.35 cm² in the presence of more than mild MR was associated with worse clinical outcomes, even after accounting for other relevant factors. Conversely, patients with an AVA greater than 1.35 cm² had clinical outcomes comparable to those without AS, regardless of the degree of non-severe MR.
AS and MR are the most prevalent VHDs in high-income countries.7 However, unless the patient is planned for an aortic or coronary surgery, current guidelines recommend intervention only when these valvular lesions are severe4 5 and limited recommendations exist for the management of patients with combined non-severe AS and MR.
The haemodynamic effects of AS result from chronic increased afterload that leads to LV hypertrophy, diastolic dysfunction and increased systolic intraventricular pressures. MR, on the other hand, reduces afterload, SV and CO but increases preload. The net effect of both lesions will reduce the net forward flow with augmentation of diastolic pressures,8 9 a finding compatible with our results.
While previous studies demonstrated increased mortality risk in moderate AS compared with no or mild AS,10–12 the impact of combined non-severe AS and low-grade MR remained less explored. Similar to our finding, smaller studies found predictors of poor outcome in this population, including ≥moderate MR, as well as lower range AVA13 or stage 2 cardiac structural abnormalities such as either LA enlargement or >mild MR (only nine patients in total).14 15 Notably, Benfari et al 16 showed that in patients with transaortic velocity >2.5 m/s and AVA>1cm2, an MR ERO area >0.1 cm² was associated with a higher rate of HF hospitalisations or death. Our study adds to this evidence by highlighting the specific association between AVA size and clinical outcomes in the context of non-severe AS and low-grade MR.
Our cohort’s all-cause mortality rate was higher compared with existing studies on severe17 or moderate AS.18 While baseline comorbidities and the presence of MR in our cohort might contribute to this finding, the most likely explanation is the older age of our study population (80.1 vs 77.8 years in severe AS and 74 years in moderate AS cohorts).
In clinical practice, it is challenging to determine the optimal timing for valvular correction of mVHD. Our data, encompassing almost 3000 patients with comprehensive echocardiographic evaluation and valid clinical outcomes, suggest that patients with combined >mild MR and AVA≤1.35 cm² have worse clinical outcomes and as such could benefit from close follow-up visits and frequent serial evaluation by a multidisciplinary heart valve team. It remains to be seen, however, whether early interventions could improve the clinical outcome of these patients.
Several important limitations should be addressed. First, this is a single-centre retrospective study; thus, prospective data are needed to further establish its findings. Second, due to relatively small number of patient with combined non-severe AS and MR, we did not divide our cohort into a learning and validation groups, consequently reducing the internal validity of the study. Third, due to the observational nature of the design, we cannot definitively prove a causal relationship between the valvular abnormalities or their individual impact on outcomes. Last, as we excluded patients with other left-sided valvular abnormalities, the current finding should not be applied to other mVHD.
Our study suggests that combined non-severe AS and low-grade MR may be associated with worse clinical outcomes, particularly when the AVA falls below 1.35 cm². This finding highlights the need for further investigation into the potential benefits of early intervention for these patients. Future studies could explore whether early intervention strategies, such as valve replacement or repair, can improve patients outcomes in this specific population.
Supplementary Material
Footnotes
Contributors: YNG accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. All authors took responsibility for all aspects of the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was reviewed and approved by the institutional review board with a waiver of informed consent.
References
- 1. Unger P, Pibarot P, Tribouilloy C, et al. European society of cardiology council on valvular heart disease. Multiple and Mixed Valvular Heart Diseases Circ Cardiovasc Imaging 2018. 10.1161/CIRCIMAGING.118.007862 [DOI] [PubMed] [Google Scholar]
- 2. Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro heart survey on valvular heart disease. Eur Heart J 2003;24:1231–43. 10.1016/s0195-668x(03)00201-x [DOI] [PubMed] [Google Scholar]
- 3. Andell P, Li X, Martinsson A, et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart 2017;103:1696–703. 10.1136/heartjnl-2016-310894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otto CM, Nishimura RA, Bonow RO, et al. ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: A report of the American college of cardiology/American heart Association joint committee on clinical practice guidelines. Circulation 2021;143:e35–71. 10.1161/CIR.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 5. Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 6. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr 2005;18:1440–63. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 7. Aluru JS, Barsouk A, Saginala K, et al. Valvular heart disease epidemiology. Med Sci (Basel) 2022;10:32. 10.3390/medsci10020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Unger P, Tribouilloy C. Aortic stenosis with other concomitant valvular disease: aortic regurgitation, mitral regurgitation, mitral stenosis, or tricuspid regurgitation. Cardiol Clin 2020;38:33–46. 10.1016/j.ccl.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 9. Mantovani F, Fanti D, Tafciu E, et al. When aortic stenosis is not alone: epidemiology, pathophysiology diagnosis and management in mixed and combined valvular disease. Front Cardiovasc Med 2021;8:744497. 10.3389/fcvm.2021.744497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mann TD, Loewenstein I, Ben Assa E, et al. Natural history of moderate aortic stenosis with preserved and low ejection fraction. J Am Soc Echocardiogr 2021;34:735–43. 10.1016/j.echo.2021.02.014 [DOI] [PubMed] [Google Scholar]
- 11. Pankayatselvan V, Raber I, Playford D, et al. Moderate aortic stenosis: culprit or bystander Open Heart 2022;9:e001743. 10.1136/openhrt-2021-001743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coisne A, Scotti A, Latib A, et al. Impact of moderate aortic stenosis on long-term clinical outcomes: a systematic review and meta-analysis. JACC Cardiovasc Interv 2022;15:1664–74. 10.1016/j.jcin.2022.06.022 [DOI] [PubMed] [Google Scholar]
- 13. Bae HJ, Hwang J, Han S, et al. Long term clinical outcomes in patients with moderate aortic stenosis. Heart Surg Forum 2020;23:E358–65. 10.1532/hsf.2971 [DOI] [PubMed] [Google Scholar]
- 14. Tastet L, Tribouilloy C, Maréchaux S, et al. Staging cardiac damage in patients with asymptomatic aortic valve stenosis. J Am Coll Cardiol 2019;74:550–63. 10.1016/j.jacc.2019.04.065 [DOI] [PubMed] [Google Scholar]
- 15. Amanullah MR, Pio SM, Ng ACT, et al. Prognostic implications of associated cardiac abnormalities detected on echocardiography in patients with moderate aortic stenosis. JACC Cardiovasc Imaging 2021;14:1724–37. 10.1016/j.jcmg.2021.04.009 [DOI] [PubMed] [Google Scholar]
- 16. Benfari G, Setti M, Nistri S, et al. Relevance of functional mitral regurgitation in aortic valve stenosis. Am J Cardiol 2020;136:115–21. 10.1016/j.amjcard.2020.09.016 [DOI] [PubMed] [Google Scholar]
- 17. Minamino-Muta E, Kato T, Morimoto T, et al. Causes of death in patients with severe aortic stenosis: an observational study. Sci Rep 2017;7:14723. 10.1038/s41598-017-15316-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strange G, Stewart S, Celermajer D, et al. National echocardiography database of Australia contributing sites. poor long-term survival in patients with moderate aortic stenosis. J Am Coll Cardiol 2019:1851–63. 10.1016/j.jacc.2019.08.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-080914supp001.pdf (1.1MB, pdf)
Data Availability Statement
Data are available on reasonable request. The data that support the findings of this study are available from the corresponding author on reasonable request.