Abstract
Introduction
Parkinson’s disease (PD) has a significant impact on a substantial number of individuals in China. Notably, 31% of patients with PD also grapple with the additional burden of anxiety. This dual challenge of managing both PD and anxiety underscores the complexity of the condition and the diverse range of symptoms patients may experience. Considering the circumstances, the cost and potential drawbacks associated with traditional antiparkinsonian drugs become increasingly relevant. Acupuncture emerges as a significant non-pharmacological adjunct therapy. Offering a potentially safer and more cost-effective option, acupuncture addresses the pressing need for holistic and complementary treatments that may alleviate both the motor symptoms of PD and the accompanying anxiety.
Methods and analysis
This is a multicentre, randomised controlled and assessor-blind trial. A total of 210 eligible patients with PD will be randomly assigned (1:1) to Jin’s three-needle (JTN) acupuncture group or waitlist (WL) group. Patients in the JTN group will receive acupuncture therapy three times per week for 4 weeks. Patients in the WL group will maintain their original dosage of antiparkinsonian drugs and receive acupuncture therapy after the observation period. The primary outcome measure will be the Unified Parkinson’s Disease Rating Scale score. The secondary outcome measures will be the scores of the Hoehn-Yahr Rating Scale, Unified Dyskinesia Rating Scale, Non-Motor Symptoms Scale, 39-item Parkinson’s Disease Questionnaire, Parkinson Anxiety Scale, Hamilton Anxiety Scale, Hamilton Depression Scale, Zarit burden interview and the level of cortisol and adrenocorticotropic hormone. The evaluation will be executed at baseline, the end of the treatment and a follow-up period.
Ethics and dissemination
The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (K[2023]014). All patients have to provide written, informed consent. The study will be disseminated through presentations in peer-reviewed international journals and at national and international conferences.
Trial registration number
Chinese Clinical Trial Registry; ChiCTR2300074675.
Keywords: Parkinson-s disease, Anxiety disorders, Protocols & guidelines, Clinical trials, COMPLEMENTARY MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study is a multicentre, randomised, controlled and assessor-blind trial, and the trial will be conducted in five healthcare centres.
This multicentre clinical study represents the first instance of using acupuncture as a treatment approach for addressing both motor symptoms and anxiety associated with Parkinson’s disease.
Our team first translated the Parkinson Anxiety Scale into Chinese and got permission to use it.
Spanning across the southern, eastern, northern and western regions of China, this study lays the groundwork for future research initiatives to expand upon.
Given the inherent nature of acupuncture, neither patients nor acupuncturists are blinded to the allocation.
Introduction
It is reported that the number of patients afflicted with Parkinson’s disease (PD) in China will exceed 4.94 million, constituting roughly half of the global PD patient population.1 A nationwide study indicated that the prevalence of PD in China was 1.37%, implying a substantial aggregate of 3.62 million patients with PD in 2021.2 Despite China’s expanding population, the prevalence of patients with PD in China has shown minimal alteration.3 Particularly noteworthy is the escalating total of patients with PD in China, driven by an ageing population. This phenomenon is poised to exert a huge burden on the Chinese healthcare system.3 Therefore, it becomes imperative to awaken public attention to the effective management of PD.
Antiparkinsonian drugs, including levodopa, pramipexole, rasagiline and others, were proven efficient in the treatment of PD.4 While these drugs effectively manage motor symptoms in patients with PD, they can lead to motor complications as their dosage increases.5 Apart from motor symptoms, approximately 31% of patients with PD appear to have mental illness, especially anxiety,6 which can worsen motor fluctuations and further contribute to PD progression.7 In a word, patients with PD with anxiety are closely bound up with their motor symptoms. To solve this problem, interventions such as antianxiety medications and psychotherapy have been proposed for managing anxiety in patients with PD.8 However, the use of additional medications may strain patients' liver and kidney functions—two major metabolic organs in the body. Furthermore, psychotherapy is not beneficial for most patients with PD in China because of the higher cost.9 Out of consideration of the above conditions in China, safe, efficient and rather cheaper adjunct treatments for PD are required.
Traditional Chinese Medicine (TCM) has been widely used for treating tremors, with a history spanning over 3000 years in China, and it has accumulated abundant and robust evidence.10 Acupuncture, an integral component of TCM and well-regarded globally,11 emerges as a significant non-pharmacological adjunct therapy. As a result, acupuncture is gaining widespread acceptance in China for treating PD. For many Chinese patients with PD, a combination of antiparkinsonian drugs for motor symptoms and acupuncture for mental symptoms proves to be a beneficial approach12 without exacerbating the physical or financial burden on patients. Jin’s three-needle (JTN) acupuncture therapy was established by Professor Jin Rui. JTN gained recognition as a result of its standardisation through a national research initiative and subsequent endorsement as a prominent acupuncture method in southern China.13
This study is a continuation of our previous study,14 which demonstrated the efficacy and safety of JTN in alleviating anxiety among patients with PD. In this study, we focus on the efficacy of JTN for both motor symptoms and anxiety among patients with PD to ascertain whether acupuncture can alleviate the motor symptoms and anxiety levels of patients with PD or not. We hypothesised that acupuncture presents an efficient and safe adjunct treatment option specifically targeted for early-stage patients with PD.
Materials and methods
Study design
This study is designed as a multicentre, randomised controlled and assessor-blind trial. The trial will be conducted in five healthcare centres in China: the First Affiliated Hospital of Guangzhou University of Chinese Medicine, the Third Affiliated Hospital of Zhejiang Chinese Medical University, Qianxinan Autonomous Prefecture Hospital of Traditional Chinese Medicine, Chengdu Xindu District Hospital of Traditional Chinese Medicine and Liaoning Hospital of Traditional Chinese Medicine.
A total of 210 participants will be enrolled in the outpatient acupuncture and moxibustion department of the five healthcare centres. The flow chart is shown in figure 1. This trial protocol abides by the Standard Protocol Items: Recommendations for Interventional Trials checklist15 (online supplemental file 1) and the Declaration of Helsinki.16 The trial was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (K[2023]014) and has been registered at the Chinese Clinical Registry (ChiCTR2300074675).
Figure 1.
Flow chart of the study. ACTH, adrenocorticotropic hormone; CORT, cortisol; HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Scale; NMSS, Non-Motor Symptoms Scale; PAS, Parkinson Anxiety Scale; PD, Parkinson’s disease; PQD-39, 39-item Parkinson’s Disease Questionnaire; UDysRS, Unified Dyskinesia Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; ZBI, Zarit burden interview.
bmjopen-2023-081312supp001.pdf (109.2KB, pdf)
Participants
Patients who meet the diagnostic criteria for PD referred to the Movement Disorder Society Clinical Diagnostic Criteria for PD (2015)17 will be recruited from five centres.
The recruitment strategy encompasses various approaches, including, but not limited to, the display of posters within hospital premises and the utilisation of social media platforms such as WeChat. These methods are chosen to effectively reach potential patients who might be interested in participating. Eligible patients who meet the inclusion and exclusion criteria will be enrolled in the outpatient departments of each centre.
Diagnostic criteria
The diagnostic criteria for PD referred to the Movement Disorder Society Clinical Diagnostic Criteria for PD (2015),17 which verified a higher sensitivity of diagnosis in China.18
Inclusion criteria
Patients who meet all of the following criteria will be allowed to enrol:
Meet the criteria for idiopathic PD.17
Male or female, aged 40–80 years.
Hoehn-Yahr staging scale staged 1–3.
Received antiparkinsonian drugs for at least 6 months and received stable doses of antiparkinsonian drugs for at least 1 month.
Able to comprehend and willing to sign an informed consent form (online supplemental file 2).
bmjopen-2023-081312supp002.pdf (125.6KB, pdf)
Exclusion criteria
Patients who meet any of the following criteria will be excluded:
Accompanied by severe cognitive dysfunction and hallucinations, unable to match the scale assessment.
Participated in other clinical trials within 1 month.
Diagnosed with other severe illnesses such as heart failure, tumours, renal failure and so on.
Unable to regularly take antiparkinsonian drugs due to adverse reactions or other reasons.
Previous or planned to receive deep brain stimulation therapy or levodopa-carbidopa intestinal gel therapy during the study period.
Pregnancy or with the intention of pregnancy during the study period.
Breastfeeding females.
With childbearing potential.
Addicted to alcohol or drugs in the past 6 months.
Diagnosed with psychotic illnesses such as schizophrenia, agoraphobia and so on.
Afraid of acupuncture.
Dropout criteria
Patients who meet any of the following criteria will be excluded from the study:
The patient quits the study treatment by himself or herself.
Outbreaks of serious adverse events or adverse reactions.
Outbreaks of serious illness, including disability, cancer, organ failure and so on, urge patients to receive other vital treatments after evaluation by relevant specialists.
Sample size
Patients will be aware that they will be randomised into one of two groups. Group I will receive JTN acupuncture therapy, and Group II will retain their original antiparkinsonian drug dosages without JTN. After the observed period, patients in the WL group will subsequently receive the same acupuncture treatment as those in the JTN group.
Aligned with the study’s objective, we chose the Unified Parkinson’s Disease Rating Scale (UPDRS) score as the primary outcome to calculate the sample size. Drawing from the findings of our previous study,14 the mean UPDRS score of acupuncture was 31.28 with an SD of 12.59, while the mean UPDRS score of antiparkinsonian drugs was 38.84 with an SD of 14.70. We used PASS V.15.0 (NCSS, Kaysville, UT, USA) to calculate the sample size with a power level of 90% and a two-sided significance level of 5%. After calculation, we set the sample size of 81 patients in each group to observe the significant difference between the two groups. With a 20% withdrawal rate, we plan to enrol a total of 204 patients, with 102 patients in each group. Given the relatively uniform scale and stature of the healthcare centres, we will allocate an equitable number of patients to each centre, in accordance with the principles of multicenter sample size computation.19 As a means of accommodating potential variances across these centres, the sample size has been adjusted to encompass a total of 210 participants.
Randomisation and allocation concealment
According to the recommendation of the International Council of Harmonisation (ICH) stated in the E9 (ICH E9), each centre should have a separate random scheme.20 Therefore, we will allocate patients into the JTN acupuncture group or waitlist (WL) group in a 1:1 ratio, using stratified randomisation which is based on the healthcare centre of the trial. An independent researcher will set a random number as a seed and use IBM SPSS Statistics V.26 (IBM SPSS, Chicago, IL, USA) to generate a random allocation sequence, and each stratum will be generated from this unique random sequence. The researcher will then enclose cards bearing both random numbers and corresponding group assignments within sealed envelopes. It will be the responsibility of this researcher to manage the allocation assignment and subsequently communicate the group assignments to the coordinators stationed at each participating centre, given the eligibility of the patients.
Blinding
Given the inherent nature of acupuncture, neither patients nor acupuncturists are blinded to the allocation. However, stringent measures have been implemented to mitigate any potential bias. Throughout the trial’s duration, the investigators responsible for assessing the scales remain in charge of the allocation. Additionally, the assessors who analyse the data are unaware of group assignments to further safeguard against introducing bias. In addition, patients and acupuncturists are instructed not to disclose the allocation during any interactions with the evaluators conducting the scale assessments or at any other conceivable point in time.
Interventions
JTN acupuncture (JTN group)
All acupuncturists possess a minimum of 5 years of formal acupuncture education, supplemented by clinical experience spanning at least 1 year within a hospital. They will collectively undergo training to ensure a comprehensive understanding of the established acupuncture therapy standards.
Patients in the JTN group will receive a 12-session acupuncture treatment (4 weeks, three times a week). Each acupuncture session will take 30 min. Throughout the duration of the study, patients will maintain their original antiparkinsonian drug dosages. If the dosages of drugs need to be adjusted in accordance with an individual patient’s conditions, the changes will be recorded in the case report forms (CRFs). To provide a standardised baseline measurement of medication, drugs will be converted into levodopa equivalent doses (LEDs).
The acupoints of the JTN group (figure 2) include Sishenzhen (the main acupoints of JTN21), Shenting (DU24), Yintang (DU29), Shenmen (HT7) and Sanyinjiao (SP6). The acupoint locations are according to the ‘2021 People’s Republic of China National Standard’ (GB/T1236-2021).22 The disposable, sterile and stainless steel needles (φ0.30 mm×40 mm, produced by Huanqiu Co., China) will be used for the acupuncture procedure. Needle insertion depth is estimated at approximately 25–30 mm. The Deqi sensation, a critical aspect of acupuncture,23 will be elicited through acupuncturist manipulation. Once the patients experience the Deqi sensation, the acupuncturist will cease manipulation and maintain the inserted needle for a duration of 30 min.
Figure 2.
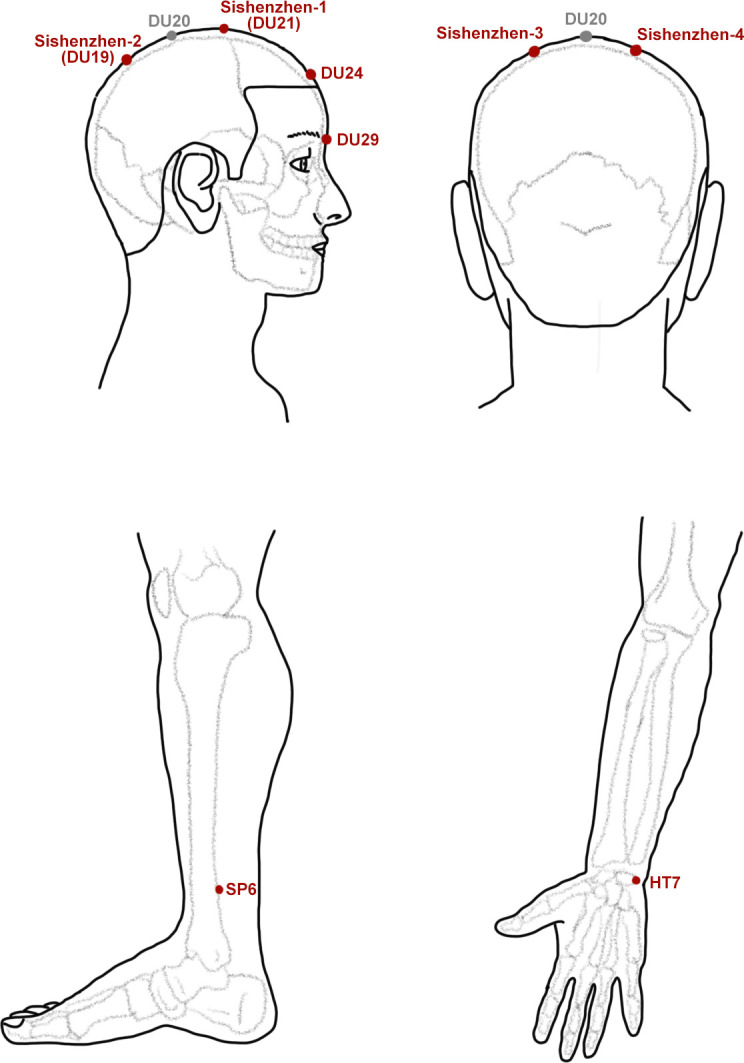
Acupoints.
WL control (WL group)
Patients in the WL group will retain their original antiparkinsonian drug dosages throughout the entirety of the observation period. Any adjustments to drug dosages will be recorded in the CRFs. Similarly, the dosages of drugs will be converted into LEDs to measure the baseline of medication. After the observed period, patients in the WL group will subsequently receive the same acupuncture treatment as those in the JTN group.
Outcome measure
The schedule of the whole procedure is shown in table 1. Before the onset of the trial, all centre leaders had already discussed and agreed on the protocol of this trial, and all acupuncturists would be trained to ensure consistency of acupuncture intervention. The comprehensive training provided to investigators involved in scale assessment, including the utilisation of official videos and learning modules,24 25 further ensures the accuracy and reliability of data collection. Each centre has an independent assessor who, unaware of the group assignments, will collect outcome data for analysis. These assessors will assess scales in a separate, isolated room. Importantly, the assessor will have access solely to the pertinent content of each assessment during the respective evaluation time. This access will not extend to the results of previous evaluations. All scales will be assessed on the first day and the 28th day of the trial.
Table 1.
Schedule of enrollment, intervention, assessment and safety
| Study period | |||||||
| Time point | Week −1 | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 8 |
| Enrollment | |||||||
| Eligibility screen | × | ||||||
| Informed consent | × | ||||||
| Randomisation | × | ||||||
| Allocation | × | ||||||
| Intervention | |||||||
| JTN group |

|
||||||
| WL group |

|
||||||
| Assessments | |||||||
| UPDRS | × | × | × | × | |||
| Hoehn-Yahr rating scale | × | × | × | × | |||
| UDysRS | × | × | × | × | |||
| NMSS | × | × | × | ||||
| PDQ-39 | × | × | × | ||||
| PAS | × | × | × | ||||
| HAMA | × | × | × | ||||
| HAMD | × | × | × | ||||
| ZBI | × | × | × | ||||
| ACTH | × | × | |||||
| CORT | × | × | |||||
| Safety | |||||||
| Vital signs | × | × | × | × | |||
| Adverse events | × | × | × | × | × | ||
ACTH, adrenocorticotropic hormone; CORT, cortisol; HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Scale; NMSS, Non-Motor Symptoms Scale; PAS, Parkinson Anxiety Scale; PQD-39, 39-item Parkinson’s Disease Questionnaire; UDysRS, Unified Dyskinesia Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; ZBI, Zarit burden interview.
Primary outcome
The UPDRS
UPDRS26 serves as a quintessential assessment tool for evaluating the symptoms of PD. Comprising four distinct sections, the UPDRS encompasses mentation, behaviour and mood, activities of daily living, motor examination and complications of therapy. The scale comprises a total of 42 items, each rated on a four-point scale, ultimately contributing to an overall score that ranges from 0 to 199.
Secondary outcomes
Hoehn-Yahr rating scale
The Hoehn-Yahr rating scale measures the severity of patients with PD and stages them into five degrees.27 In Stage I, patients have unilateral involvement only with no or minimal functional impairment. Stage II signifies bilateral or midline involvement, yet balance impairment is not evident. In Stage III, patients experience balance dysfunction. Stage IV denotes the presence of notable dyskinesia; however, patients retain the capability to walk or stand with assistance. Stage V captures a state of autonomous mobility loss, rendering patients reliant on a wheelchair or bed for mobility.
The Unified Dyskinesia Rating Scale (UDysRS)
As PD advances, some patients may experience a deterioration in their condition, potentially leading to the development of dyskinesia.28 The UDysRS aimed to evaluate the dyskinesia of patients with PD and was divided into four parts, including the historical disability of on-dyskinesia impact (44 points), off-dystonia impact (16 points), objective impairment and type (28 points) and objective disability based on part III activities (16 points).29
The Non-Motor Symptoms Scale (NMSS)
There is a view that fluctuations in motor symptoms are interconnected with fluctuations in non-motor symptoms. The NMSS,30 derived from NMSQuest,31 serves as a tool for measuring the severity of non-motor symptoms in patients with PD. Comprising a total of 30 items, the scale employs a four-point rating system, ultimately contributing to an overall score that ranges from 0 to 120.
The 39-item Parkinson’s Disease Questionnaire (PDQ-39)
The PDQ-3932 was designed to evaluate the life quality of patients with PD. It contains 39 items and covers eight dimensions, including mobility, activities of daily living, emotional well-being, stigma, social support, cognitions, communication and bodily discomfort. A higher score corresponds to a lower quality of life experienced by the patient.
The Parkinson Anxiety Scale (PAS)
The PAS33 was developed in 2015 for measuring the severity of anxiety in patients with PD, not for screening or diagnosis. It contains 12 items, including persisting anxiety (five items), episodic anxiety (four items) and avoidance behaviour (three items). The PAS is rated on a four-point scale with a total score ranging from 0 to 48. Our team first translated the PAS into Chinese and got permission to use it.
Hamilton Anxiety Scale (HAMA)
Given the fact that the PAS is not specifically designed for diagnosis, it would indeed be valuable to include a scale that serves this purpose. The HAMA34 is an appropriate choice. It is designed to facilitate the diagnosis of anxiety and is structured into two parts: psychic and somatic. It is a 14-item scale rated on a four-point scale with a total score ranging from 0 to 56. HAMA is evaluated as no anxiety if HAMA<7, probable anxiety if 7≤HAMA<14, mild anxiety if 14≤HAMA<21, moderate anxiety if 21≤HAMA<28 and severe anxiety if HAMA≥28.
Hamilton Depression Scale (HAMD)
The HAMD35 is a scale for the diagnosis of depression. Given the prevalence of co-occurring anxiety and depression among patients with PD,36 it is indeed valuable to incorporate HAMD to gauge the severity of depression. This approach allows you to explore potential correlations between anxiety, depression and the effectiveness of acupuncture. The HAMD is evaluated as no anxiety if HAMD≤7, mild anxiety if 8≤HAMD<17, moderate anxiety if 17≤HAMD<24 and severe anxiety if HAMD≥24.37
The Zarit burden interview (ZBI)
The ZBI,38 designed to assess the burden experienced by caregivers of patients with dementia, has found wide application in measuring the burden encountered by caregivers of elderly patients.39 The majority of patients with PD experience onset after the age of 50,40 and motor disability and cognitive impairment are prominent complications of PD.41 Therefore, these patients with PD with motor disorders or cognitive impairments need caregivers to take care of their daily activities. This caregiving burden extends beyond the caregivers themselves, affecting patients and even broader society.42 The ZBI is a 22-item scale rated on a four-point scale with an overall score ranging from 0 to 88.
Blood serum index
In this study, we will use ELISA to quantify the levels of cortisol (CORT) and adrenocorticotropin hormone (ACTH) within the serum samples obtained from patients on the first day and 28th day of the trial. These related tests will be carried out by the clinical laboratory of the First Affiliated Hospital of Guangzhou University of Chinese Medicine.
Safety outcome
Researchers will record vital signs, including body temperature, respiratory rate, pulse rate and blood pressure, on the first day and the 28th day of the trial. Any adverse events (AEs) that happened in the observation period will be promptly and accurately recorded in CRFs and immediately reported to the primary centre (the First Affiliated Hospital of Guangzhou University of Chinese Medicine). In addition, intervenors should treat patients with AEs as soon as possible not only to alleviate their discomfort but also to mitigate the AEs. Meanwhile, we will inform AEs to the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine. In cases where AEs have been addressed and treated, the researchers will respectfully engage with the patients, inquiring about their willingness to continue participating in the trial. Their preferences will be honoured and respected.
Follow-up
After the completion of the observed period, patients will be requested to return to the hospital for the purpose of scoring the scales and getting the blood sample within the first month following the trial. This follow-up assessment aims to ascertain whether the efficacy of acupuncture treatment remains consistent over time. By evaluating the sustainability of acupuncture’s therapeutic effects, this step contributes to a comprehensive understanding of its long-term impact on patients’ well-being.
Data monitoring and statistical analysis
In this study, the clinical trial electronic data acquisition system will be used for data entry and data management. Following the completion of the clinical raw data collection, assessors at each centre will provide a duplicate version to the First Affiliated Hospital of Guangzhou University of Chinese Medicine. In addition, physical copies of the data will be archived in the medical record room of the First Affiliated Hospital of Guangzhou University of Chinese Medicine for a duration of 3 years.
To ensure the integrity of the data collection process, an independent assistant will oversee the data collection procedures. Furthermore, the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine will conduct periodic audits to ensure adherence to established protocols. Two independent study assistants will carry out data input and subsequent self-checks. In the event of discrepancies, these will be rectified. In addition, quality inspectors will check the CRFs randomly to ensure that there are no recorded errors.
IBM SPSS Statistics V.26 (IBM SPSS, Chicago, IL, USA) will be used to analyse the data. The scale scores assessed in each group will be subjected to normality tests. If the scores conform to a normal distribution, a one-way analysis of variance will be performed. If not, the Kruskal-Wallis rank-sum test will be performed. If the significance of the F-test of the univariate linear model exceeds 0.05, CORT and ACTH levels will be subjected to covariance analysis. If not, the Kruskal-Wallis rank-sum test will be performed. All statistical tests will be two-sided, and the significance level will be set at 0.05.
A technical appendix, statistical code and dataset could be provided by email to scientific researchers who need them.
Discussion
Antiparkinsonian drugs such as levodopa, dopamine agonists and monoamine oxidase-B (MAO-B) inhibitors are commonly recommended as initial medical therapy to alleviate motor symptoms in the early stages of patients with PD. However, these drugs may lead to increased motor fluctuations and even motor complications,42 thereby exacerbating the challenges faced by patients. Compounding these difficulties, the progression of PD often gives rise to mental health issues, such as anxiety. In situations where such complications arise, the suggested approach involves adjusting drug dosages or introducing additional medications.4
In China, a substantial number of antiparkinsonian drugs need to be imported, contributing to their relatively high cost. The prospect of increasing medication dosages or trying different drug options translates into heightened financial burdens for Chinese patients with PD. Therefore, Chinese patients with PD may face dual pressure from the disease itself and finance. Acupuncture, known as a safe and efficient therapy in China, emerges as a potential solution to address these challenges. JTN, characterised by a unique combination of acupoints,43 holds particular significance as a well-regarded and widely promoted acupuncture treatment in southern China. However, most acupuncture clinical trials focus solely on motor symptoms or non-motor symptoms of PD.44 Recognising the intricate interplay between motor and non-motor aspects, such as anxiety, is pivotal. Motor symptoms often interact with non-motor symptoms, like anxiety, in a bidirectional manner.45 The emergence of anxiety can intensify motor symptoms, potentially leading to the onset of motor complications in patients with PD.46 Given this complex relationship, a more holistic approach that considers both motor symptoms and associated mental health aspects is necessary.
As far as our current understanding goes, this multicentre clinical study represents the first instance of using acupuncture as a treatment approach for addressing both motor symptoms and anxiety associated with PD. In this study, we have placed a strong emphasis on comprehensively investigating the efficacy of acupuncture in the early stages of patients with PD from multiple dimensions. By targeting both motor symptoms and anxiety, our goal is to provide a well-rounded understanding of the potential benefits of acupuncture. To achieve this goal, we have designed this protocol and selected a range of outcomes and scales that capture various aspects of PD and its impact on patients’ lives. We designed UPDRS as the primary outcome, which can evaluate both motor symptoms and certain elements of anxious conditions simultaneously. To ensure that our study focuses on early stage patients with PD, we have included the Hoehn-Yahr rating scale as a secondary outcome measure. This staging assessment helps confirm that participants fall within the intended stage range (stages I–III) and ensures the relevance of our findings to the early PD population. The reason why we do not enrol in the late stage of patients with PD is that they rely on wheelchairs and beds, and they need deep brain stimulation therapy or levodopa-carbidopa intestine gel therapy to manage their symptoms and improve their quality of life.5 Levodopa is the main drug in antiparkinsonian drugs, though patients taking levodopa may increase the risk of motor complications. Thus, we added UDysRS as a secondary outcome to measure the severity of dyskinesia in patients with PD. By including this outcome, we can assess how acupuncture might impact motor complications associated with levodopa treatment. In addition, we aim to explore whether acupuncture could enhance the overall quality of life for patients with PD. The PDQ-39 has been selected as an outcome to evaluate the life quality of patients with PD to investigate whether acupuncture can help patients with PD improve their quality of life and make their lives easier. Besides motor symptoms, we note that non-motor symptoms may also reduce the life quality of patients with PD. Therefore, we added NMSS as the supplemental part of UPDRS to measure the non-motor symptoms fluctuation of patients with PD. This addition allows us to capture non-motor symptom fluctuations alongside motor symptoms. As a representative and vital part of non-motor symptoms, anxiety is one of the key points in this study. A professional scale designed for PD anxiety is urgently needed. We are glad to contact the author of PAS, a specialised scale targeted at PD anxiety, and get permission for its first usage in China. Therefore, we added PAS as a secondary outcome. Besides, we added HAMA, a long-used scale for anxiety, to supplement PAS. For the sake of eliminating the influence of depression levels, we added HAMD as a secondary outcome to investigate whether patients with PD have anxiety and depression or not. We will simultaneously analyse the level of anxiety and depression to eliminate the bias, bringing depression as close as possible. It is reported that the prevalence of Chinese patients with PD aged over 60 years was as high as 1.73%.2 These elderly patients need better care. On the one hand, their caregivers were burdened. On the other hand, too much burden leads to caregivers being overwhelmed, eventually resulting in negligence or even accidents such as falls.47 Thus, it is important to pay attention to caregivers’ burden. The ZBI will help us assess the impact of caregiving on caregivers’ lives and the potential benefits of acupuncture in this aspect. The hypothalamic–pituitary–adrenal (HPA) axis is a vital endocrine system for life. If patients with PD experience anxiety, their hypothalamus responds by secreting corticotropin-releasing hormone, which in turn stimulates the anterior pituitary gland to release ACTH.48 ACTH then triggers the adrenal cortex to produce and release CORT.49 Research has demonstrated that patients suffering from anxiety tend to exhibit elevated levels of both ACTH and CORT.50 In addition, patients who have persistent neuroendocrine abnormalities are at a higher risk of experiencing relapses or showing resistance to treatment.50 This emphasises the significance of the functional HPA axis in relation to anxiety and its potential impact on the efficacy of antiparkinsonian drugs. Dysregulated HPA axis functioning not only reflects the anxiety levels of patients but could also negatively influence the response to treatment for anxiety. To gain a more comprehensive understanding of the anxious state in patients with PD, we have incorporated the measurement of CORT and ACTH levels in serum as an additional method to assess the level of anxiety.
This study aims to provide comprehensive insights into the potential benefits of acupuncture for patients dealing with these challenging aspects of PD. However, it is important to acknowledge that, despite the innovative approach, there are certain limitations inherent to the study. One notable limitation is the geographic distribution of the participating subcentres. Our research centres are distributed across various regions of China, providing valuable insights into a substantial portion of the PD population in the country. However, given the expansive size of China, it is important to note that our results may not comprehensively represent the entire nation. Despite this limitation, this study provides a foundation for future research endeavours to build upon, potentially involving a more diverse geographic representation to enhance the generalisability of the results to a wider spectrum of the PD population in China.
Ethics and dissemination
The protocol was amended, and the trial was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (K[2023]014). Before being included in the study, all patients have to provide written informed consent.
The study will be disseminated through presentations in peer-reviewed international journals and at national and international conferences.
Patient and public involvement
Patients and the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research. The study results will be disseminated to participants and the public in the form of booklets or flyers and published in open access, peer-reviewed journals.
Trial status
Currently, the trial is in the recruitment stage, with the first patient being recruited on 4 September 2023. The anticipated completion of the trial is set for 31 August 2025.
Supplementary Material
Footnotes
Contributors: LZ conceptualised and designed the study. XL drafted the manuscript. JF was in charge of the data analysis. IIL and WL will help to analyse the data. YZ and MG polished the draft. LZ provided methodological recommendations and will manage the research. All authors have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Funding: This work was supported by the State Natural Science Fund projects (8217150722) and the National Administration of Traditional Chinese Medicine (2022ZD04). The funding body had no role in the study design, data collection, statistical analysis or interpretation or preparation of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007;68:384–6. 10.1212/01.wnl.0000247740.47667.03 [DOI] [PubMed] [Google Scholar]
- 2. Qi S, Yin P, Wang L, et al. Prevalence of Parkinson’s disease: a community-based study in China. Mov Disord 2021;36:2940–4. 10.1002/mds.28762 [DOI] [PubMed] [Google Scholar]
- 3. Li G, Ma J, Cui S, et al. Parkinson’s disease in China: a forty-year growing track of bedside work. Transl Neurodegener 2019;8:22. 10.1186/s40035-019-0162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA 2020;323:548–60. 10.1001/jama.2019.22360 [DOI] [PubMed] [Google Scholar]
- 5. Antonini A, Moro E, Godeiro C, et al. Medical and surgical management of advanced Parkinson’s disease. Mov Disord 2018;33:900–8. 10.1002/mds.27340 [DOI] [PubMed] [Google Scholar]
- 6. Broen MPG, Narayen NE, Kuijf ML, et al. Prevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 2016;31:1125–33. 10.1002/mds.26643 [DOI] [PubMed] [Google Scholar]
- 7. Zhu K, van Hilten JJ, Marinus J. Onset and evolution of anxiety in Parkinson’s disease. Eur J Neurol 2017;24:404–11. 10.1111/ene.13217 [DOI] [PubMed] [Google Scholar]
- 8. Dissanayaka NNW, White E, O’Sullivan JD, et al. Characteristics and treatment of anxiety disorders in Parkinson’s disease. Mov Disord Clin Pract 2015;2:155–62. 10.1002/mdc3.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heuzenroeder L, Donnelly M, Haby MM, et al. Cost-effectiveness of psychological and pharmacological interventions for generalized anxiety disorder and panic disorder. Aust N Z J Psychiatry 2004;38:602–12. 10.1080/j.1440-1614.2004.01423.x [DOI] [PubMed] [Google Scholar]
- 10. Fan J-Q, Lu W-J, Tan W-Q, et al. Acupuncture for Parkinson’s disease: from theory to practice. Biomedicine & Pharmacotherapy 2022;149:112907. 10.1016/j.biopha.2022.112907 [DOI] [PubMed] [Google Scholar]
- 11. Liang Y, Zhou J, Du J, et al. Prosepects for the development of Acupuncture analgesia from an international perspective. World Journal of Acupuncture - Moxibustion 2023;33:6–8. 10.1016/j.wjam.2022.11.002 [DOI] [Google Scholar]
- 12. Chen W, Xu Z-M, Wang G, et al. Non-motor symptoms of Parkinson’s disease in China: a review of the literature. Parkinsonism & Related Disorders 2012;18:446–52. 10.1016/j.parkreldis.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 13. Meichen L, Lixing Z. Jin’s three-Neddle treatment of mental illness. Journal of Traditional Chinese Medicine 2022;63:2388–92. [Google Scholar]
- 14. Fan J-Q, Lu W-J, Tan W-Q, et al. Effectiveness of Acupuncture for anxiety among patients with Parkinson disease: A randomized clinical trial. JAMA Netw Open 2022;5:e2232133. 10.1001/jamanetworkopen.2022.32133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Medical Association . World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 18. Li J, Jin M, Wang L, et al. MDS clinical diagnostic criteria for Parkinson’s disease in China. J Neurol 2017;264:476–81. 10.1007/s00415-016-8370-2 [DOI] [PubMed] [Google Scholar]
- 19. Harden M, Friede T. Sample size calculation in multi-centre clinical trials. BMC Med Res Methodol 2018;18:156. 10.1186/s12874-018-0602-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hilgers R-D, Manolov M, Heussen N, et al. Design and analysis of stratified clinical trials in the presence of bias. Stat Methods Med Res 2020;29:1715–27. 10.1177/0962280219846146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Wang Y, Li K, et al. The efficacy and safety of jin’s three-needle therapy vs. placebo Acupuncture on anxiety symptoms in patients with post-stroke anxiety: A study protocol for a randomized controlled trial. Front Psychiatry 2022;13:941566. 10.3389/fpsyt.2022.941566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiaodong W, Longxiang H, Jingsheng Z. Interpretation of China National standard nomenclature and location of Meridian points (GB/T 12346–2021). Chinese Acupuncture & Moxibustion 2022;42. [DOI] [PubMed] [Google Scholar]
- 23. Jung W-M, Shim W, Lee T, et al. More than Deqi: spatial patterns of Acupuncture-induced bodily sensations. Front Neurosci 2016;10:462. 10.3389/fnins.2016.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goetz CG, Stebbins GT, Chmura TA, et al. Teaching program for the movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale: (MDS-UPDRS). Mov Disord 2010;25:1190–4. 10.1002/mds.23096 [DOI] [PubMed] [Google Scholar]
- 25. Goetz CG, Nutt JG, Stebbins GT, et al. Teaching program for the unified dyskinesia rating scale. Movement Disorders 2009;24:1296–8. 10.1002/mds.22563 [DOI] [PubMed] [Google Scholar]
- 26. S, F., E. R, and M.o.t.U.D. Committee . Recent Developments in Parkinson’s Disease. Macmillan Health Care Information, 1987: 293–304. [Google Scholar]
- 27. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–42. 10.1212/wnl.17.5.427 [DOI] [PubMed] [Google Scholar]
- 28. Okun MS. Management of Parkinson disease in 2017: personalized approaches for patient-specific needs. JAMA 2017;318:791–2. 10.1001/jama.2017.7914 [DOI] [PubMed] [Google Scholar]
- 29. Goetz CG, Nutt JG, Stebbins GT. The unified dyskinesia rating scale: presentation and Clinimetric profile. Mov Disord 2008;23:2398–403. 10.1002/mds.22341 [DOI] [PubMed] [Google Scholar]
- 30. Chaudhuri KR, Martinez-Martin P, Brown RG, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord 2007;22:1901–11. 10.1002/mds.21596 [DOI] [PubMed] [Google Scholar]
- 31. Chaudhuri KR, Martinez-Martin P, Schapira AHV, et al. International multicenter pilot study of the first comprehensive self-completed Nonmotor symptoms questionnaire for Parkinson’s disease: the Nmsquest study. Mov Disord 2006;21:916–23. 10.1002/mds.20844 [DOI] [PubMed] [Google Scholar]
- 32. Jenkinson C, Fitzpatrick R, Peto V, et al. The Parkinson’s disease questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 1997;26:353–7. 10.1093/ageing/26.5.353 [DOI] [PubMed] [Google Scholar]
- 33. Leentjens AFG, Dujardin K, Pontone GM, et al. The Parkinson anxiety scale (PAS): development and validation of a new anxiety scale. Mov Disord 2014;29:1035–43. 10.1002/mds.25919 [DOI] [PubMed] [Google Scholar]
- 34. HAMILTON M. The assessment of anxiety States by rating. Br J Med Psychol 1959;32:50–5. 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- 35. HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang T, Yang R, Pan J, et al. Parkinson’s disease related depression and anxiety: A 22-year Bibliometric analysis (2000-2022). Neuropsychiatr Dis Treat 2023;19:1477–89. 10.2147/NDT.S403002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zimmerman M, Martinez JH, Young D, et al. Severity classification on the Hamilton depression rating scale. J Affect Disord 2013;150:384–8. 10.1016/j.jad.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 38. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649–55. 10.1093/geront/20.6.649 [DOI] [PubMed] [Google Scholar]
- 39. Lu L, Wang L, Yang X, et al. Zarit Caregiver burden interview: development, Reliability and validity of the Chinese version. Psychiatry Clin Neurosci 2009;63:730–4. 10.1111/j.1440-1819.2009.02019.x [DOI] [PubMed] [Google Scholar]
- 40. Zhou X, Liu Z, Zhou X, et al. The Chinese Parkinson’s disease Registry (CPDR): study design and baseline patient characteristics. Mov Disord 2022;37:1335–45. 10.1002/mds.29037 [DOI] [PubMed] [Google Scholar]
- 41. Koros C, Stefanis L, Scarmeas N. Parkinsonism and dementia. J Neurol Sci 2022;433:120015. 10.1016/j.jns.2021.120015 [DOI] [PubMed] [Google Scholar]
- 42. Rogers G, Davies D, Pink J, et al. Parkinson’s disease: summary of updated NICE guidance [BMJ (Clinical]. BMJ 2017;358:j1951. 10.1136/bmj.j1951 [DOI] [PubMed] [Google Scholar]
- 43. Qing Y, Longlin L, Xiujin S, et al. On connotation of jin’s three-Neddle technique. Chinese Acupuncture & Moxibustion 2014;34:701–4. [PubMed] [Google Scholar]
- 44. Liu H, Chen L, Zhang Z, et al. Effectiveness and safety of Acupuncture combined with Madopar for Parkinson’s disease: a systematic review with meta-analysis. Acupunct Med 2017;35:404–12. 10.1136/acupmed-2016-011342 [DOI] [PubMed] [Google Scholar]
- 45. Ray S, Agarwal P. Depression and anxiety in Parkinson disease. Clin Geriatr Med 2020;36:93–104. 10.1016/j.cger.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 46. Lintel H, Corpuz T, Paracha S-U-R, et al. Mood disorders and anxiety in Parkinson’s disease: Current concepts. J Geriatr Psychiatry Neurol 2021;34:280–8. 10.1177/08919887211018267 [DOI] [PubMed] [Google Scholar]
- 47. Gazibara T, Pekmezovic T, Kisic-Tepavcevic D, et al. Incidence and prediction of falls in Parkinson’s disease: a prospective cohort study. Eur J Epidemiol 2015;30:349–52. 10.1007/s10654-015-0019-4 [DOI] [PubMed] [Google Scholar]
- 48. Carey G, Görmezoğlu M, de Jong JJA, et al. Neuroimaging of anxiety in Parkinson’s disease: A systematic review. Mov Disord 2021;36:327–39. 10.1002/mds.28404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. part II: neurochemistry, Neurophysiology and Neurocognition. World J Biol Psychiatry 2017;18:162–214. 10.1080/15622975.2016.1190867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lightman SL, Birnie MT, Conway-Campbell BL. Dynamics of ACTH and Cortisol secretion and implications for disease. Endocr Rev 2020;41:bnaa002. 10.1210/endrev/bnaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-081312supp001.pdf (109.2KB, pdf)
bmjopen-2023-081312supp002.pdf (125.6KB, pdf)



