Abstract
Chronic liver disease is highly prevalent and often leads to fibrosis or cirrhosis and complications such as liver failure and hepatocellular carcinoma. The diagnosis and staging of liver fibrosis is crucial to determine management and mitigate complications. Liver biopsy for histologic assessment has limitations such as sampling bias and high interreader variability that reduce precision, which is particularly challenging in longitudinal monitoring. MR elastography (MRE) is considered the most accurate noninvasive technique for diagnosing and staging liver fibrosis. In MRE, low-frequency vibrations are applied to the abdomen, and the propagation of shear waves through the liver is analyzed to measure liver stiffness, a biomarker for the detection and staging of liver fibrosis. As MRE has become more widely used in clinical care and research, different contexts of use have emerged. This review focuses on the latest developments in the use of MRE for the assessment of liver fibrosis; provides guidance for image acquisition and interpretation; summarizes diagnostic performance, along with thresholds for diagnosis and staging of liver fibrosis; discusses current and emerging clinical applications; and describes the latest technical developments.
© RSNA, 2024
Summary
This review focuses on the clinical implementation of MR elastography for assessing liver fibrosis, simple thresholds for staging, current and emerging clinical applications, and latest technical developments.
Essentials
■ The Quantitative Imaging Biomarkers Alliance provides guidance to standardize imaging acquisition and interpretation for MR elastography (MRE).
■ The Liver Imaging Reporting and Data System Quantitative Imaging Working Group and the Society of Abdominal Radiology Liver Fibrosis Disease Focus Panel recommend a common set of simple thresholds applicable to most liver etiologies for the diagnosis and staging of liver fibrosis: 3.0 kPa for F1 or higher, 3.5 kPa for F2 or higher, 4.0 kPa for F3 or higher, and 5.0 kPa for F4.
■ Among noninvasive techniques, MRE provides the highest accuracy for detecting and staging liver fibrosis.
Introduction
In patients with chronic liver disease, the diagnosis and staging of liver fibrosis is crucial to inform management, with treatment more effective when initiated at early stages (1). While historically biopsy has been the reference standard for the diagnosis and staging of liver fibrosis, it is limited by risk of complications, high cost, sampling error, and high intra- and interobserver variability (1–3). Additionally, different histologic staging systems have been proposed, which can introduce staging discrepancies (4). To address these issues, minimally invasive or noninvasive techniques have been investigated.
As fibrosis develops, quantification of changes in liver stiffness can provide an important assessment of disease severity (5,6). Elastographic techniques indirectly measure tissue stiffness by assessing the mechanical properties of the tissue. Different elastographic techniques assess different stiffness-related parameters (eg, shear stiffness or Young, or elastic, modulus), although the underlying principle is the measurement of shear-wave propagation. In normal liver, the tissue is softer, resulting in shear waves with higher attenuation and slower propagation. As fibrosis develops, liver stiffness increases and wave propagation becomes faster, with longer wavelengths and lower attenuation (7). MR elastography (MRE) stiffness measurements provide the highest classification accuracy for noninvasive staging of liver fibrosis (8).
In this article, we explain general concepts of MRE, provide guidance on analysis and reporting, summarize the diagnostic performance of MRE and thresholds for diagnosis and staging of liver fibrosis, and discuss current and emerging clinical applications and the latest technical developments.
Introduction to MRE
General Concepts
MRE uses an external driver to produce and transmit mechanical low-frequency vibrations onto the abdominal wall over the liver. Phase-contrast sequences with motion-encoding gradients are used to image shear-wave propagations, and an inversion algorithm converts the wave propagation information into quantitative stiffness maps (ie, elastograms). The standard imaging marker measured is “shear stiffness,” expressed in kilopascals (7). Acquisition is performed using the end-expiration breath-hold technique. Clinical sequences and postprocessing steps are described in Appendix S1.
Interpretation and Reporting
To avoid sample bias, regions of interest covering as much liver parenchyma as possible are recommended (9). Regions of interest should be placed on the stiffness maps within areas that display parallel wave motion (on the wave images) and have valid pixel values (as indicated by the confidence map), while avoiding artifacts, large vessels, lesions, and the outer 1 cm below the capsule (Fig 1). Once the optimal regions of interest are drawn in areas of reliable data on each image section, the mean per-section stiffness is recorded, and the mean for four sections is calculated (10). Mean liver stiffness is reported as a continuous variable (in kilopascals), commonly with stiffness thresholds that relate to the stage of fibrosis (Fig 2).
Figure 1:
RSNA Quantitative Imaging Biomarkers Alliance recommendations for region of interest placement and measurement of shear-wave propagation in MR elastography. A region of interest (outline) is drawn on the (A) axial source magnitude image, with simultaneous visualization of the (B) wave image and the (C) elastogram with overlaid confidence map (hatching), in such a way as to include the largest portion of liver tissue. The region of interest should be placed on individual image sections excluding areas of low wave propagation, large vessels, focal lesions, marginal liver tissues (to avoid edge effect), and areas identified by the inversion algorithm as invalid (hatching in C). The mean liver stiffness is reported by recording the mean stiffness value of each region of interest (ie, each section) and then calculating the mean value for all sections, weighted by the size of the region of interest. In the wave image (B), red indicates peaks and blue indicates troughs. Highly saturated colors indicate high wave amplitude, and black indicates low wave amplitude. Color bar in the elastogram (C) indicates stiffness range.
Figure 2:
Example axial T2-weighted (T2 W) MRI scans (left), wave images (middle), and elastograms (right) illustrating the contexts of use of MR elastography in patients with advanced fibrosis with different liver disease etiologies. The reported stiffness threshold for fibrosis stages may vary based on differences in study population and reference standard (ie, histologic examination) sampling variability. Based on consensus interpretation of multiple studies and meta-analysis, and to simplify the interpretation of MR elastographic stiffness in clinical care, a common set of simple thresholds is recommended regardless of the underlying etiology of chronic liver disease: 3.0 kPa for stage F1 or higher, 3.5 kPa for stage F2 or higher, 4.0 kPa for stage F3 or higher, and 5.0 kPa for stage F4. HBV = hepatitis B virus infection, HCV = hepatitis C virus infection, MASH = metabolic dysfunction–associated steatohepatitis, PSC = primary sclerosing cholangitis.
Variations in published thresholds likely stem from differences in study populations, inclusion of different etiologies, variability in interpretation of biopsy as reference standard, and other confounders (Fig 3). To address this issue and simplify the interpretation of MRE stiffness in clinical care, the Liver Imaging Reporting and Data System Quantitative Imaging Working Group and the Society of Abdominal Radiology Liver Fibrosis Disease Focus Panel recommend a common set of simple thresholds applicable to most etiologies: 3.0 kPa for stage F1 or higher, 3.5 kPa for stage F2 or higher, 4.0 kPa for stage F3 or higher, and 5.0 kPa for stage F4 (9,11). These thresholds were derived from consensus interpretation of multiple studies and meta-analysis and were endorsed by 88% (22 of 25) of the members in the working group.
Figure 3:
Graph shows reported MR elastographic (MRE) stiffness thresholds (in kilopascals) for fibrosis staging. Dotted lines represent the thresholds recommended by the Liver Imaging Reporting and Data System Quantitative Imaging Working Group: 3.0 kPa or higher for stage F1 (mild fibrosis), 3.5 kPa or higher for stage F2 (significant fibrosis), 4.0 kPa or higher for stage F3 (advanced fibrosis), and 5.0 kPa or higher for stage F4 (cirrhosis). * = These studies report results for different sequences, frequencies, readers, or stiffness thresholds investigated. † = “Various liver diseases” includes hepatitis B virus infection, hepatitis C virus infection, metabolic dysfunction–associated steatohepatitis (MASH), and autoimmune hepatitis. MAFLD = metabolic dysfunction–associated fatty liver disease.
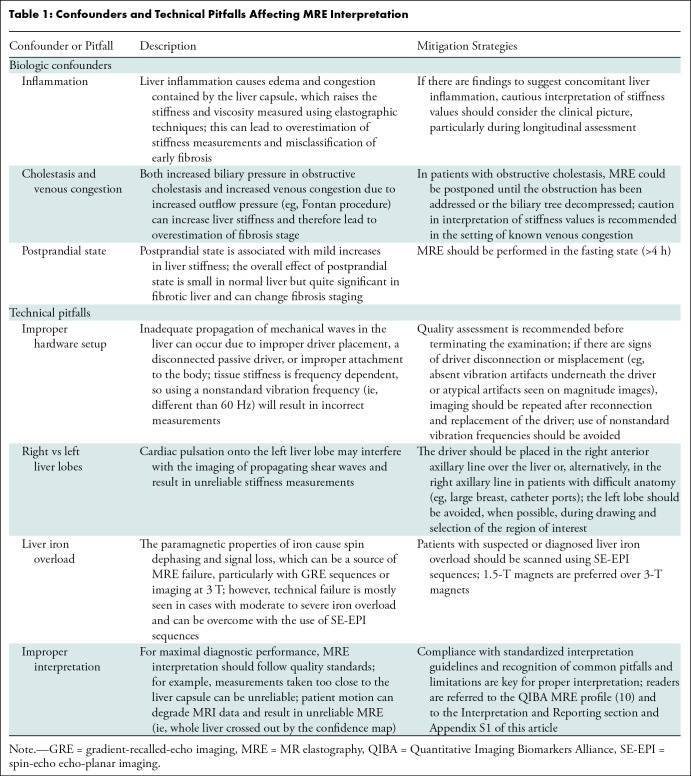
In longitudinal studies, the relative change (expressed as a percentage) in the elastographic stiffness value should be reported since a change exceeding 20% between examinations can be interpreted as a true biologic change (10,12), whereas smaller changes may be attributable to measurement variability. Caution is required when interpreting change in patients with viral hepatitis undergoing antiviral therapy, because a rapid drop in stiffness values may reflect a reduction in inflammation rather than fibrosis reversal (13). Fluctuations in liver stiffness due to inflammation may also be observed in other types of hepatitis during treatment (eg, autoimmune hepatitis) (14), though further research is needed to fully understand this process. Other potential pitfalls and confounders in interpretation are summarized in Table 1.
Table 1:
Confounders and Technical Pitfalls Affecting MRE Interpretation
Diagnostic Performance
Early literature focused on the overall feasibility and accuracy of MRE to diagnose liver fibrosis, and since 2006, more than 500 articles have been published, with several focusing on the diagnostic performance of different stiffness thresholds for staging liver fibrosis. The diagnostic performance of MRE for advanced (≥F3) fibrosis is excellent, with most studies reporting greater than 80% sensitivity and greater than 90% specificity. More granular discriminations (ie, F1 vs F2 vs F3 vs F4) also show high performance (15–18). Table 2 summarizes the diagnostic performance of MRE for staging liver fibrosis reported in the literature. In a large meta-analysis by Singh et al (8) including 12 studies and 697 individual patients with different chronic liver diseases from Europe and the United States, the area under the receiver operating characteristic curve values for discriminating any (≥F1), significant (≥F2), or advanced fibrosis (≥F3) or cirrhosis (≥F4) were 0.84, 0.88, 0.93, and 0.92, respectively. High reproducibility—with low bias (−0.03 to 0.11 kPa) and narrow limits of agreement—has been reported, with accurate fibrosis classification using different MRI scanners and at different imaging centers (19,20).
Table 2:
Diagnostic Performance of MR Elastography for Staging Liver Fibrosis Reported in the Literature
Current and Emerging Clinical Applications of MRE
Liver Fibrosis Diagnosis, Staging, and Monitoring
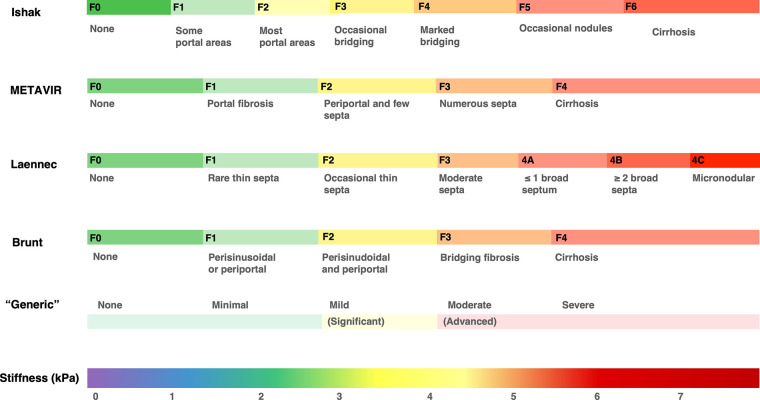
Several histologic staging systems for liver fibrosis have been proposed, most of which are etiology specific. For simplicity and improved communication in clinical practice, two terms are widely accepted for evaluating the utility of noninvasive markers of liver fibrosis: Significant fibrosis refers to stage F2 or higher (F3 for Ishak score), and advanced fibrosis refers to stage F3 or higher (F4 for Ishak score). However, translating stiffness values to categorical fibrosis stages may ignore the biologic complexity and continuous spectrum of liver disease. This is particularly important once cirrhosis is established, as patients with cirrhosis are grouped in a single category in most histologic systems, without further categorization that may relate to severity or outcome prediction. Thus, interpreting liver stiffness as a continuous variable to inform clinical management may provide a better prognostic correlation (Fig 4) (21).
Figure 4:
Graph shows multiple scoring systems that have been proposed for staging liver fibrosis. These systems differ in their purpose, definitions of fibrosis stages, and subgroups. The use of liver stiffness as a continuous variable has advantages over ordinal categories. It may provide better clinical and prognostic correlation, especially given that most staging systems group patients with cirrhosis in a single category, without taking into consideration the severity of cirrhosis or any longitudinal histologic progression or regression. Brunt = Brunt scoring system, F = fibrosis stage, Ishak = Ishak score, Laennec = Laennec staging system, METAVIR = Meta-analysis of Histological Data in Viral Hepatitis scoring system.
Imaging-based elastographic techniques to measure liver stiffness have been implemented using US and MRI technology. US-based elastographic methods, discussed in detail elsewhere (7,22), have the advantage of higher accessibility, with satisfactory accuracy in the clinical setting. Limitations include reduced accuracy in patients with large body habitus or narrow intercostal spaces, and limited intra- and interreader agreement (23). MRE provides a larger field of view than US-based elastographic methods, reducing sampling variability, with overall higher accuracy for staging liver fibrosis, particularly at early stages (24). Further, the high interdevice and interoperator reproducibility of MRE is advantageous for longitudinal monitoring and assessment of treatment response in clinical care and clinical trials (25).
Improved Participant Selection for Clinical Trials
Noninvasive identification of patients with early-stage fibrosis who could potentially benefit from pharmacologic therapy is currently a major unmet clinical gap (26). For inclusion in clinical trials of therapies for metabolic dysfunction–associated fatty liver disease (MAFLD), the U.S. Food and Drug Administration and European guidelines require a diagnosis of significant (≥F2) liver fibrosis stage, but selecting potential candidates without liver biopsy can be challenging. Among noninvasive tests, MRE provides the highest accuracy for detecting and staging liver fibrosis (ie, F1 vs F2) (24,27). Therefore, MRE-determined liver stiffness may provide a biomarker for treatment selection and enrollment in phase II clinical trials, and disease-specific cutoffs can be considered. For phase III trials, regulatory agencies currently continue to require histologic confirmation of fibrosis staging. In this setting, MRE may be used for cohort enrichment by excluding patients who otherwise would undergo biopsy without the required stage of fibrosis.
Ruling in or Ruling out Liver Fibrosis
The American Association for the Study of Liver Diseases recommends noninvasive tests to help identify advanced fibrosis risk, optimizing the benefit-to-risk ratio for invasive procedures like biopsy. MRE, with its high specificity and positive predictive value, is an ideal method to help identify high-risk patients who should undergo liver biopsy. Conversely, the high sensitivity and negative predictive value of MRE mean that it can help rule out disease, which is crucial for determining which patients can be treated by primary care physicians and which patients need hepatologist referral. Unlike many other noninvasive tests, MRE excels in both ruling out and ruling in liver fibrosis, making it an ideal choice for clinical practice (28).
Risk Stratification of Liver-related Complications
Several studies highlight the potential utility of MRE in predicting disease progression and liver-related complications. In patients with hepatitis C virus infection, liver stiffness is an independent risk factor for cirrhosis development, with negligible 1-year progression risk when liver stiffness is less than 3.3 kPa (29). For patients with MAFLD, MRE may help differentiate those at risk for progressing to advanced stages from those with simple steatosis (30). In a prospective MAFLD study, a 15% increase in elastography-derived liver stiffness was associated with histologic progression to advanced fibrosis or cirrhosis (31).
Decompensation and death were more common in patients with MAFLD-related advanced fibrosis or cirrhosis who had serially increasing liver stiffness compared with those who had unchanged stiffness (30,32). MRE has also shown promise in stratifying the risk of variceal bleeding and the development of hepatocellular carcinoma in patients with advanced liver disease (33,34). In a study of patients with sustained hepatitis C virologic response, the risk of hepatocellular carcinoma development at 1, 2, and 3 years was 6.6%, 11.9%, and 14.5%, respectively, for patients with liver stiffness of 3.75 kPa or greater, and diagnostic accuracy was higher for MRE than for serum fibrosis markers (35). Preliminary data suggest that MRE-derived liver stiffness can help predict recurrence risk in patients treated for hepatocellular carcinoma (36).
Future Directions
Integrating MRE and Other Noninvasive Biomarkers
To further improve the performance of noninvasive methods, researchers have investigated algorithmic combinations of MRE-derived stiffness and other noninvasive biomarkers (37). Tests can be performed in a sequential or combined approach.
Sequential approach.—In the sequential approach, one test, often a blood test or a point-of-care test such as FibroScan (Echosens), is performed first. Depending on the results of the initial test, fibrosis may be ruled out, or further assessment, such as MRE, may be performed. This approach essentially functions as a decision tree.
A prototype of this is MEFIB (MRE plus Fibrosis-4 [FIB-4]) (31), designed to rule in or rule out significant fibrosis. FIB-4 is calculated using the following formula: (age [years] × AST [U/L])/(platelets [109/L] × ALT [U/L])1/2, where AST is aspartate aminotransferase (a serum marker of active liver injury) and ALT is alanine aminotransferase. In this approach, FIB-4 is calculated first. If FIB-4 is less than 1.6, fibrosis is ruled out. If FIB-4 is 1.6 or greater, the patient undergoes MRE, where the threshold for fibrosis is a liver stiffness of 3.3 kPa or greater (indicating ≥F2). The combination of FIB-4 value of 1.6 or greater and MRE-derived liver stiffness of 3.3 kPa or greater has higher diagnostic performance than either method alone or other tests, with a positive predictive value of 97.1% to rule in stage F2 or greater, potentially identifying patients eligible for treatment without invasive biopsy (Fig 5) (26,38).
Figure 5:

Example of the MR elastography (MRE) plus Fibrosis-4 (FIB-4) (MEFIB) sequential approach used to rule in or rule out significant fibrosis in a 66-year-old man with clinical suspicion of significant fibrosis. A FIB-4 value of 5.15 (higher than the ≥1.6 threshold) computed from age, aspartate aminotransferase, alanine transaminase, and platelet count justified proceeding to MRE, which revealed a mean stiffness of 5.7 kPa. The axial elastogram represents a stiffness map expressed in kilopascals, on a scale from 0 to 8 kPa, where low stiffness is represented in purple and high stiffness in red, with a confidence mask overlaid (hatching). The combination of a FIB-4 value of 1.6 or greater and an MRE-derived liver stiffness of 3.3 kPa or greater has a high positive predictive value to rule in significant fibrosis (≥F2). A liver biopsy performed in a research setting confirmed cirrhosis (F4) related to metabolic dysfunction–associated steatohepatitis.
Preliminary studies also indicate that MEFIB has excellent negative predictive value for clinical outcomes such as gastrointestinal varices requiring treatment, ascites, and hepatic encephalopathy (39,40). Until now, the application of MEFIB has been simulated using data from research studies in which all participants underwent FIB-4 calculation and MRE, but the prospective performance MEFIB as a decision tree has not been investigated. Other sequential approaches have been proposed, including combining vibration-controlled transient elastography–derived stiffness, controlled attenuation parameter, and AST (41), However these approaches show lower diagnostic performance and have not been systematically investigated. Theoretical advantages of sequential approaches are that they mirror current clinical practice, can be integrated into clinical care pathways, and are cost-effective in that they reduce the number of unnecessary tests.
Combined approach.—In the combined approach, two or more tests are performed simultaneously, with the values from each test integrated into a single equation, rather than following a decision tree. A prototype of this approach is MRI-AST (MAST), designed to rule in or rule out progressed forms of MAFLD defined by high inflammatory activity, that is, metabolic dysfunction–associated steatohepatitis (MASH) activity score of 4 or greater and fibrosis stage of F2 or greater (called fibro-MASH). Individuals with fibro-MASH are potential candidates for pharmacologic treatment, but their identification in clinical practice may be challenging due to the lack of specific symptoms. AST serves as a marker of active liver injury, and an MRI- and serum-based score could potentially help identify these patients. To compute MAST, AST, MRI proton density fat fraction (PDFF), and MRE-derived stiffness are assessed. The values of the three tests are combined using the following formula: MAST = expit(−12.17 + 7.07 log[stiffness] + 0.037 × PDFF + 3.55 log[AST]), where expit(x) = exp(x)/(1 + exp[x]). MAST scores of less than 0.165 and 0.242 or greater are used for ruling out and ruling in fibro-MASH, respectively (Fig 6) (42).
Figure 6:
Example of the MRI–aspartate aminotransferase (AST) (MAST) combined approach used to rule in or rule out metabolic dysfunction–associated steatohepatitis (MASH) in a 52-year-old woman with clinical suspicion of MASH. The MAST score is computed from MR elastography (MRE)–derived stiffness, MRI proton density fat fraction (PDFF), and AST. (A) The axial elastogram represents a stiffness map expressed in kilopascals, on a scale from 0 to 8 kPa, where low stiffness is represented in purple and high stiffness in red, with a confidence mask overlaid (hatching). The mean MRE stiffness was 2.7 kPa. (B) MRI proton density fat fraction, determined from the axial MRI scan, was 20.5%, indicating moderate to severe steatosis. With an AST value of 144 U/L, the computed MAST score was 0.332. A value of 0.242 or higher is used to rule in fibro-MASH (MASH activity score of 4 or greater and fibrosis stage of F2 or greater). A liver biopsy performed in a research setting confirmed a MASH activity score of 5 and a fibrosis stage of F2.
Sensitivity and negative predictive value for the rule-out cutoff and specificity and positive predictive value for the rule-in cutoff are 90% or greater (43). The few patients with MAST scores between these two values are considered in the “gray zone,” and further work-up may be required, including potential liver biopsy for definitive diagnosis. Preliminary data indicate that MAST score has higher diagnostic performance and a lower rate of indeterminate results than other noninvasive biomarkers for identifying patients with fibro-MASH (38,44). Other combined approaches have also been proposed. The combination of MRE-derived liver stiffness, MRI proton density fat fraction, and serum markers has shown high discriminatory performance in differentiating MASH from simple steatosis (45), as has the combination of MRE stiffness with MR relaxation values such as T1, although the latter technique is still limited by technical variability and the lack of a reference standard (45,46). A theoretical advantage of the combined approach is that it uses more data and therefore might have higher diagnostic performance than the sequential approach.
Other Diagnostic Pathways
Considering the current armamentarium for noninvasive diagnosis and staging of liver fibrosis, well-defined guidelines are needed to determine which test should be performed. These guidelines should consider patient characteristics, available treatment options, and diagnostic performance. This approach aims to more objectively make the decision of which patients should be evaluated with MRE. For example, one potential approach is to initially evaluate patients with serologic tests or point-of-care elastographic techniques, reserving MRE for technically difficult cases (eg, obese patients) or for patients whose initial results were inconclusive.
The high diagnostic performance of MRE, either alone or in combination with other tests, might render liver biopsy unnecessary. This paradigm shift would allow the management of patients with chronic liver disease to rely on MRE or other noninvasive tests, eliminating the need to translate MRE-derived stiffness values to histologic stages. Instead, stiffness values, individually or in combination, could guide management (21). Moreover, MRE-derived liver stiffness as a continuous variable could inform patient selection and surveillance frequency in hepatocellular carcinoma screening programs. It could also impact the pretest probability of malignancy or hepatocellular carcinoma in imaging-based systems like the Liver Imaging Reporting and Data System (LI-RADS) or indicate a need to adjust the LI-RADS category (eg, some LR-3 findings could be upgraded to LR-4 based on liver stiffness criteria).
Emerging Technologies
Developments on the technical front aim to improve workflow, patient experience, efficiency, image quality, or classification accuracy. Artificial intelligence–based guidance for technologists performing MRE is an unmet need and focus of research. Automated analysis methods can potentially reduce the variability found in manual analysis (47). Free-breathing MRE acquisitions either gated or combined with radial acquisitions improve patient comfort and can improve image quality and performance in critically ill patients and in children (48,49). Flexible passive drivers with better fit on the abdominal wall improve patient comfort (50). The combination of multiple flexible drivers allows coverage of a larger area of the abdomen and stiffness measurements of multiple organs (51).
Multifrequency and three-dimensional (3D) MRE acquisitions have the potential to improve image quality, reproducibility, and disease characterization (34,52). Different 3D MRE–derived parameters for the detection and grading of liver inflammation and its differentiation from early fibrosis have shown promising results. Shi et al (17) distinguished inflammation from early fibrosis using a 3D MRE–derived marker of viscosity in patients with hepatitis B and C virus infections. Khalfallah et al (53) found significant associations between viscoelasticity measured with 3D MRE and histologic features that differentiate MAFLD from MASH. In another study, the combination of 3D MRE parameters (shear stiffness and damping ratio) and proton density fat fraction showed higher performance for diagnosing fibro-MASH than the combination of two-dimensional MRE–derived stiffness and proton density fat fraction (area under the receiver operating characteristic curve = 0.973 vs 0.906, P = .081; net reclassification improvement = 0.28, P < .05) (54). The improved characterization of these additional parameters using 3D MRE techniques may also help differentiate other coexistent processes in liver disease such as congestion from fibrosis in patients with congenital heart disease (55,56).
The Need for Improved Access and Community Validation
Although evidence supports the high diagnostic performance of MRE for liver fibrosis assessment, and the technique has had Food and Drug Administration approval since 2009, several barriers to wider adoption remain. MRE is still mostly performed in academic or tertiary centers, with analysis being performed by expert radiologists, which limits patient access. While community practices are starting to adopt the technology, the validation of MRE results in a community setting requires systematic evaluation. Cost may be a potential barrier to adoption; however, recent data show that MRE can be more cost-effective than US-based vibration-controlled transient elastography in patients with MAFLD (57). Further, a quantitative MRI examination limited to MRE as a standalone test costs less and requires less scanning time than a full abdominal MRI examination. While some abbreviated protocols have been proposed (58), studies to validate the performance of these protocols are still needed.
Conclusion
Considering the current burden of chronic liver disease worldwide, MR elastography (MRE), with very high diagnostic performance in noninvasive staging of liver fibrosis, can better inform treatment options to improve patient care. MRE-derived liver stiffness measurements provide better clinical and prognostic correlation than histologic stages. If needed, guidance from the Society of Abdominal Radiology and the Liver Imaging Reporting and Data System provides simple thresholds for interpretation of MRE-derived stiffness values. Emerging diagnostic techniques that integrate MRE and laboratory tests improve the detection of fibrosis, enhance the classification of disease, and help predict disease progression and treatment response.
Supported by the National Institutes of Health (grants R01 EB17197-10, R01 DK132718-2, and R01 DK136731-1 to S.K.V. and grant R37 EB001981 to S.K.V. and R.L.E.), National Institute of Diabetes and Digestive and Kidney Diseases (grants U01 DK130190, U01 DK061734, R43 DK135225, R01 DK088925, R01 DK106419, and R01 DK110096 to C.B.S.), Food and Drug Administration (grant U01 FD007773 to C.B.S.), Foundation for the National Institutes of Health (grant 20192423 to C.B.S.), U.S. Department of Defense (grant W81XWH1910583-NCE to S.K.V), Canadian Institutes of Health Research Institute of Nutrition, Metabolism and Diabetes (grants #301520 and #273738 to A.T.), and Fonds de Recherche du Québec en Santé and Fondation de l'Association des Radiologistes du Québec (Clinical Research Scholarship–Senior Salary Award FRQS-ARQ #298509 to A.T.).
Disclosures of conflicts of interest: G.M.C. No relevant relationships. B.F. No relevant relationships. P.J.N. No relevant relationships. D.O. No relevant relationships. S.K.V. No relevant relationships. R.L.E. Research contract from Resoundant, royalties paid to institution (Mayo Clinic) from Resoundant, patents owned by institution (Mayo Clinic), and stock or stock options from Resoundant; the Mayo Clinic and R.L.E. have intellectual property rights and a financial interest in MR elastographic technology. C.B.S. Research grants from American College of Radiology, Bayer, Foundation for the National Institutes of Health, General Electric, Gilead, Pfizer, Philips, Siemens, and V Foundation; lab service agreements with OrsoBio, Enanta Pharmaceuticals, Gilead, ICON, Intercept Pharmaceuticals, NuSirt BioPharma, Shire, Synageva, and Takeda; institutional consulting for Bristol Myers Squibb, Exact Sciences, IBM Watson, and Pfizer; personal consulting for Altimmune, Ascelia Pharma, Blade Therapeutics, Boehringer Ingelheim, Epigenomics, and Guerbet; receipt of royalties and/or honoraria from Medscape and Wolters Kluwer; ownership of stock options in Livivos; unpaid advisory board position for Quantix Bio; and chief medical officer for Livivos (unsalaried position with stock options and stock) through June 28, 2023, and currently principal advisor for Livivos (both appointments approved by his university). A.T. Steering committee member for Liver Imaging Reporting and Data System.
Abbreviations:
- AST
- aspartate aminotransferase
- FIB-4
- Fibrosis-4
- MAFLD
- metabolic dysfunction–associated fatty liver disease
- MASH
- metabolic dysfunction–associated steatohepatitis
- MAST
- MRI-AST
- MRE
- MR elastography
References
- 1. Chi H , Hansen BE , Tang WY , et al . Multiple biopsy passes and the risk of complications of percutaneous liver biopsy . Eur J Gastroenterol Hepatol 2017. ; 29 ( 1 ): 36 – 41 . [DOI] [PubMed] [Google Scholar]
- 2. Bedossa P , Carrat F . Liver biopsy: the best, not the gold standard . J Hepatol 2009. ; 50 ( 1 ): 1 – 3 . [DOI] [PubMed] [Google Scholar]
- 3. Bedossa P , Dargère D , Paradis V . Sampling variability of liver fibrosis in chronic hepatitis C . Hepatology 2003. ; 38 ( 6 ): 1449 – 1457 . [DOI] [PubMed] [Google Scholar]
- 4. Goodman ZD . Grading and staging systems for inflammation and fibrosis in chronic liver diseases . J Hepatol 2007. ; 47 ( 4 ): 598 – 607 . [DOI] [PubMed] [Google Scholar]
- 5. Yeh WC , Li PC , Jeng YM , et al . Elastic modulus measurements of human liver and correlation with pathology . Ultrasound Med Biol 2002. ; 28 ( 4 ): 467 – 474 . [DOI] [PubMed] [Google Scholar]
- 6. Huwart L , Sempoux C , Vicaut E , et al . Magnetic resonance elastography for the noninvasive staging of liver fibrosis . Gastroenterology 2008. ; 135 ( 1 ): 32 – 40 . [DOI] [PubMed] [Google Scholar]
- 7. Tang A , Cloutier G , Szeverenyi NM , Sirlin CB . Ultrasound elastography and MR elastography for assessing liver fibrosis: part 1, principles and techniques . AJR Am J Roentgenol 2015. ; 205 ( 1 ): 22 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh S , Venkatesh SK , Wang Z , et al . Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data . Clin Gastroenterol Hepatol . 2015. ; 13 ( 3 ): 440 – 451 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Venkatesh SK , Ehman RL . Magnetic resonance elastography of liver . Magn Reson Imaging Clin N Am 2014. ; 22 ( 3 ): 433 – 446 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. QIBA profile: magnetic resonance elastography of the liver. Stage 3: technically confirmed profile . Quantitative Imaging Biomarkers Alliance . https://qibawiki.rsna.org/images/5/54/MRE-QIBAProfile-2022-02-14-TECHNICALLY-CONFIRMED.pdf. Published February 14, 2022. Accessed September 24, 2023 .
- 11. Venkatesh SK , Yin M , Ehman RL . Magnetic resonance elastography of liver: clinical applications . J Comput Assist Tomogr 2013. ; 37 ( 6 ): 887 – 896 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serai SD , Obuchowski NA , Venkatesh SK , et al . Repeatability of MR elastography of liver: a meta-analysis . Radiology 2017. ; 285 ( 1 ): 92 – 100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higuchi M , Tamaki N , Kurosaki M , et al . Changes of liver stiffness measured by magnetic resonance elastography during direct-acting antivirals treatment in patients with chronic hepatitis C . J Med Virol 2021. ; 93 ( 6 ): 3744 – 3751 . [DOI] [PubMed] [Google Scholar]
- 14. Hartl J , Denzer U , Ehlken H , et al . Transient elastography in autoimmune hepatitis: timing determines the impact of inflammation and fibrosis . J Hepatol 2016. ; 65 ( 4 ): 769 – 775 . [DOI] [PubMed] [Google Scholar]
- 15. Ichikawa S , Motosugi U , Morisaka H , et al . MRI-based staging of hepatic fibrosis: comparison of intravoxel incoherent motion diffusion-weighted imaging with magnetic resonance elastography . J Magn Reson Imaging 2015. ; 42 ( 1 ): 204 – 210 . [DOI] [PubMed] [Google Scholar]
- 16. Chang W , Lee JM , Yoon JH , et al . Liver fibrosis staging with MR elastography: comparison of diagnostic performance between patients with chronic hepatitis B and those with other etiologic causes . Radiology 2016. ; 280 ( 1 ): 88 – 97 . [DOI] [PubMed] [Google Scholar]
- 17. Shi Y , Qi YF , Lan GY , et al . Three-dimensional MR elastography depicts liver inflammation, fibrosis, and portal hypertension in chronic hepatitis B or C . Radiology 2021. ; 301 ( 1 ): 154 – 162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imajo K , Honda Y , Kobayashi T , et al . Direct comparison of US and MR elastography for staging liver fibrosis in patients with nonalcoholic fatty liver disease . Clin Gastroenterol Hepatol 2020. ; 20 ( 4 ): 908 – 917.e11 . [DOI] [PubMed] [Google Scholar]
- 19. Tang A , Dzyubak B , Yin M , et al . MR elastography in nonalcoholic fatty liver disease: inter-center and inter-analysis-method measurement reproducibility and accuracy at 3T . Eur Radiol 2022. ; 32 ( 5 ): 2937 – 2948 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim HJ , Kim B , Yu HJ , et al . Reproducibility of hepatic MR elastography across field strengths, pulse sequences, scan intervals, and readers . Abdom Radiol (NY) 2020. ; 45 ( 1 ): 107 – 115 . [DOI] [PubMed] [Google Scholar]
- 21. Ferraioli G , Barr RG . Interpreting liver stiffness values in clinical practice: is histologic classification necessary for clinical relevance? Radiology 2023. ; 307 ( 1 ): e220553 . [DOI] [PubMed] [Google Scholar]
- 22. Tang A , Cloutier G , Szeverenyi NM , Sirlin CB . Ultrasound elastography and MR elastography for assessing liver fibrosis: part 2, diagnostic performance, confounders, and future directions . AJR Am J Roentgenol 2015. ; 205 ( 1 ): 33 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naganuma H , Ishida H , Uno A , Nagai H , Kuroda H , Ogawa M . Diagnostic problems in two-dimensional shear wave elastography of the liver . World J Radiol 2020. ; 12 ( 5 ): 76 – 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang YN , Fowler KJ , Boehringer AS , et al . Comparative diagnostic performance of ultrasound shear wave elastography and magnetic resonance elastography for classifying fibrosis stage in adults with biopsy-proven nonalcoholic fatty liver disease . Eur Radiol 2022. ; 32 ( 4 ): 2457 – 2469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon JH , Lee JM , Joo I , et al . Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation . Radiology 2014. ; 273 ( 3 ): 772 – 782 . [DOI] [PubMed] [Google Scholar]
- 26. Jung J , Loomba RR , Imajo K , et al . MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis . Gut 2021. ; 70 ( 10 ): 1946 – 1953 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lefebvre T , Wartelle-Bladou C , Wong P , et al . Prospective comparison of transient, point shear wave, and magnetic resonance elastography for staging liver fibrosis . Eur Radiol 2019. ; 29 ( 12 ): 6477 – 6488 . [DOI] [PubMed] [Google Scholar]
- 28. Singh S , Allen AM , Wang Z , Prokop LJ , Murad MH , Loomba R . Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies . Clin Gastroenterol Hepatol 2015. ; 13 ( 4 ): 643 – 654.e9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takamura T , Motosugi U , Ichikawa S , et al . Usefulness of MR elastography for detecting clinical progression of cirrhosis from Child-Pugh class A to B in patients with type C viral hepatitis . J Magn Reson Imaging 2016. ; 44 ( 3 ): 715 – 722 . [DOI] [PubMed] [Google Scholar]
- 30. Gidener T , Dierkhising RA , Mara KC , et al . Change in serial liver stiffness measurement by magnetic resonance elastography and outcomes in NAFLD . Hepatology 2023. ; 77 ( 1 ): 268 – 274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ajmera VH , Liu A , Singh S , et al . Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in nonalcoholic fatty liver disease . Hepatology 2020. ; 71 ( 3 ): 849 – 860 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han MAT , Vipani A , Noureddin N , et al . MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: a multicenter study . Liver Int 2020. ; 40 ( 9 ): 2242 – 2251 . [DOI] [PubMed] [Google Scholar]
- 33. Ichikawa S , Motosugi U , Enomoto N , Onishi H . Magnetic resonance elastography can predict development of hepatocellular carcinoma with longitudinally acquired two-point data . Eur Radiol 2019. ; 29 ( 2 ): 1013 – 1021 . [DOI] [PubMed] [Google Scholar]
- 34. Catania R , Lopes Vendrami C , Bolster BD , Niemzcura R , Borhani AA , Miller FH . Intra-patient comparison of 3D and 2D magnetic resonance elastography techniques for assessment of liver stiffness . Abdom Radiol (NY) 2022. ; 47 ( 3 ): 998 – 1008 . [DOI] [PubMed] [Google Scholar]
- 35. Tamaki N , Higuchi M , Kurosaki M , et al . Risk assessment of hepatocellular carcinoma development by magnetic resonance elastography in chronic hepatitis C patients who achieved sustained virological responses by direct-acting antivirals . J Viral Hepat 2019. ; 26 ( 7 ): 893 – 899 . [DOI] [PubMed] [Google Scholar]
- 36. Cho HJ , Kim B , Kim HJ , et al . Liver stiffness measured by MR elastography is a predictor of early HCC recurrence after treatment . Eur Radiol 2020. ; 30 ( 8 ): 4182 – 4192 . [DOI] [PubMed] [Google Scholar]
- 37. Boursier J , de Ledinghen V , Zarski JP , et al. ; multicentric groups from SNIFF 32, VINDIAG 7, and ANRS/HC/EP23 FIBROSTAR studies . Comparison of eight diagnostic algorithms for liver fibrosis in hepatitis C: new algorithms are more precise and entirely noninvasive . Hepatology 2012. ; 55 ( 1 ): 58 – 67 . [DOI] [PubMed] [Google Scholar]
- 38. Kim BK , Tamaki N , Imajo K , et al . Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD . J Hepatol 2022. ; 77 ( 6 ): 1482 – 1490 . [DOI] [PubMed] [Google Scholar]
- 39. Ajmera V , Kim BK , Yang K , et al . Liver stiffness on magnetic resonance elastography and the MEFIB index and liver-related outcomes in nonalcoholic fatty liver disease: a systematic review and meta-analysis of individual participants . Gastroenterology 2022. ; 163 ( 4 ): 1079 – 1089.e5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ajmera V , Nguyen K , Tamaki N , Sharpton S , Bettencourt R , Loomba R . Prognostic utility of magnetic resonance elastography and MEFIB index in predicting liver-related outcomes and mortality in individuals at risk of and with nonalcoholic fatty liver disease . Therap Adv Gastroenterol 2022. ; 15 : 17562848221093869 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamaki N , Imajo K , Sharpton S , et al . Magnetic resonance elastography plus Fibrosis-4 versus FibroScan-aspartate aminotransferase in detection of candidates for pharmacological treatment of NASH-related fibrosis . Hepatology 2022. ; 75 ( 3 ): 661 – 672 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshimitsu K , Mitsufuji T , Shinagawa Y , et al . MR elastography of the liver at 3.0 T in diagnosing liver fibrosis grades; preliminary clinical experience . Eur Radiol 2016. ; 26 ( 3 ): 656 – 663 . [DOI] [PubMed] [Google Scholar]
- 43. Cheng YW , Chang YC , Chen YL , Chen RC , Chou CT . Feasibility of measuring spleen stiffness with MR elastography and splenic volume to predict hepatic fibrosis stage . PLoS One 2019. ; 14 ( 5 ): e0217876 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Noureddin M , Truong E , Gornbein JA , et al . MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis . J Hepatol 2022. ; 76 ( 4 ): 781 – 787 . [DOI] [PubMed] [Google Scholar]
- 45. Alsaqal S , Hockings P , Ahlström H , et al . The combination of MR elastography and proton density fat fraction improves diagnosis of nonalcoholic steatohepatitis . J Magn Reson Imaging 2022. ; 56 ( 2 ): 368 – 379 . [DOI] [PubMed] [Google Scholar]
- 46. Hoffman DH , Ayoola A , Nickel D , Han F , Chandarana H , Shanbhogue KP . T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis . Abdom Radiol (NY) 2020. ; 45 ( 3 ): 692 – 700 . [DOI] [PubMed] [Google Scholar]
- 47. Cunha GM , Delgado TI , Middleton MS , et al . Automated CNN-based analysis versus manual analysis for MR elastography in nonalcoholic fatty liver disease: intermethod agreement and fibrosis stage discriminative performance . AJR Am J Roentgenol 2022. ; 219 ( 2 ): 224 – 232 . [DOI] [PubMed] [Google Scholar]
- 48. Morin CE , Dillman JR , Serai SD , Trout AT , Tkach JA , Wang H . Comparison of standard breath-held, free-breathing, and compressed sensing 2D gradient-recalled echo MR elastography techniques for evaluating liver stiffness . AJR Am J Roentgenol 2018. ; 211 ( 6 ): W279 – W287 . [DOI] [PubMed] [Google Scholar]
- 49. Kafali SG , Armstrong T , Shih SF , et al . Free-breathing radial magnetic resonance elastography of the liver in children at 3 T: a pilot study . Pediatr Radiol 2022. ; 52 ( 7 ): 1314 – 1325 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang K , Manning P , Szeverenyi N , et al . Repeatability and reproducibility of 2D and 3D hepatic MR elastography with rigid and flexible drivers at end-expiration and end-inspiration in healthy volunteers . Abdom Radiol (NY) 2017. ; 42 ( 12 ): 2843 – 2854 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen J , Chen J , Heilman JA , et al . Abdominal MR elastography with multiple driver arrays: performance and repeatability . Abdom Radiol (NY) 2023. ; 48 ( 6 ): 1945 – 1954 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shahryari M , Meyer T , Warmuth C , et al . Reduction of breathing artifacts in multifrequency magnetic resonance elastography of the abdomen . Magn Reson Med 2021. ; 85 ( 4 ): 1962 – 1973 . [DOI] [PubMed] [Google Scholar]
- 53. Khalfallah M , Doblas S , Hammoutene A , et al . Visco-elastic parameters at three-dimensional MR elastography for diagnosing non-alcoholic steatohepatitis and substantial fibrosis in mice . J Magn Reson Imaging 2024. ; 59 ( 1 ): 97 – 107 . [DOI] [PubMed] [Google Scholar]
- 54. Lin H , Qiu S , Yang Y , et al . Three-dimensional magnetic resonance elastography combining proton-density fat fraction precisely identifies metabolic dysfunction-associated steatohepatitis with significant fibrosis . Magn Reson Imaging 2023. ; 104 : 1 – 8 . [DOI] [PubMed] [Google Scholar]
- 55. Acosta Izquierdo L , Rai A , Saprungruang A , et al . Assessment of liver fibrosis using a 3-dimensional high-resolution late gadolinium enhancement sequence in children and adolescents with Fontan circulation . Eur Radiol 2023. ; 33 ( 8 ): 5446 – 5454 . [Published correction appears in Eur Radiol 2023;33(8):5909.] [DOI] [PubMed] [Google Scholar]
- 56. Sofue K , Onoda M , Tsurusaki M , et al . Dual-frequency MR elastography to differentiate between inflammation and fibrosis of the liver: comparison with histopathology . J Magn Reson Imaging 2020. ; 51 ( 4 ): 1053 – 1064 . [DOI] [PubMed] [Google Scholar]
- 57. Sangha K , Chang ST , Cheung R , Deshpande VS . Cost-effectiveness of MRE versus VCTE in staging fibrosis for nonalcoholic fatty liver disease (NAFLD) patients with advanced fibrosis . Hepatology 2023. ; 77 ( 5 ): 1702 – 1711 . [DOI] [PubMed] [Google Scholar]
- 58. Cunha GM , Villela-Nogueira CA , Bergman A , Lobo Lopes FPP . Abbreviated mpMRI protocol for diffuse liver disease: a practical approach for evaluation and follow-up of NAFLD . Abdom Radiol (NY) 2018. ; 43 ( 9 ): 2340 – 2350 . [DOI] [PubMed] [Google Scholar]
- 59. Yin M , Talwalkar JA , Glaser KJ , et al . Assessment of hepatic fibrosis with magnetic resonance elastography . Clin Gastroenterol Hepatol 2007. ; 5 ( 10 ): 1207 – 1213.e2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Choi YR , Lee JM , Yoon JH , Han JK , Choi BI . Comparison of magnetic resonance elastography and gadoxetate disodium-enhanced magnetic resonance imaging for the evaluation of hepatic fibrosis . Invest Radiol 2013. ; 48 ( 8 ): 607 – 613 . [DOI] [PubMed] [Google Scholar]
- 61. Asbach P , Klatt D , Schlosser B , et al . Viscoelasticity-based staging of hepatic fibrosis with multifrequency MR elastography . Radiology 2010. ; 257 ( 1 ): 80 – 86 . [DOI] [PubMed] [Google Scholar]
- 62. Wang Y , Ganger DR , Levitsky J , et al . Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging . AJR Am J Roentgenol 2011. ; 196 ( 3 ): 553 – 561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fu F , Li X , Chen C , et al . Non-invasive assessment of hepatic fibrosis: comparison of MR elastography to transient elastography and intravoxel incoherent motion diffusion-weighted MRI . Abdom Radiol (NY) 2020. ; 45 ( 1 ): 73 – 82 . [DOI] [PubMed] [Google Scholar]
- 64. Schawkat K , Ciritsis A , von Ulmenstein S , et al . Diagnostic accuracy of texture analysis and machine learning for quantification of liver fibrosis in MRI: correlation with MR elastography and histopathology . Eur Radiol 2020. ; 30 ( 8 ): 4675 – 4685 . [DOI] [PubMed] [Google Scholar]
- 65. Li M , Yang H , Liu Y , et al . Comparison of the diagnostic performance of 2D and 3D MR elastography in staging liver fibrosis . Eur Radiol 2021. ; 31 ( 12 ): 9468 – 9478 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sato N , Kenjo A , Nishimagi A , et al . Accuracy comparison of MR elastography and biological markers in detecting liver fibrosis and predicting postoperative ascites . HPB (Oxford) 2021. ; 23 ( 9 ): 1383 – 1391 . [DOI] [PubMed] [Google Scholar]
- 67. Lee JE , Lee JM , Lee KB , et al . Noninvasive assessment of hepatic fibrosis in patients with chronic hepatitis B viral infection using magnetic resonance elastography . Korean J Radiol 2014. ; 15 ( 2 ): 210 – 217 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Venkatesh SK , Wang G , Lim SG , Wee A . Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B . Eur Radiol 2014. ; 24 ( 1 ): 70 – 78 . [DOI] [PubMed] [Google Scholar]
- 69. Hennedige TP , Wang G , Leung FP , et al . Magnetic resonance elastography and diffusion weighted imaging in the evaluation of hepatic fibrosis in chronic hepatitis B . Gut Liver 2017. ; 11 ( 3 ): 401 – 408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ichikawa S , Motosugi U , Ichikawa T , et al . Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C . Magn Reson Med Sci 2012. ; 11 ( 4 ): 291 – 297 . [DOI] [PubMed] [Google Scholar]
- 71. Shi Y , Xia F , Li QJ , et al . Magnetic resonance elastography for the evaluation of liver fibrosis in chronic hepatitis B and C by using both gradient-recalled echo and spin-echo echo planar imaging: a prospective study . Am J Gastroenterol 2016. ; 111 ( 6 ): 823 – 833 . [DOI] [PubMed] [Google Scholar]
- 72. Chen J , Talwalkar JA , Yin M , Glaser KJ , Sanderson SO , Ehman RL . Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography . Radiology 2011. ; 259 ( 3 ): 749 – 756 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim D , Kim WR , Talwalkar JA , Kim HJ , Ehman RL . Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography . Radiology 2013. ; 268 ( 2 ): 411 – 419 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Loomba R , Wolfson T , Ang B , et al . Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study . Hepatology 2014. ; 60 ( 6 ): 1920 – 1928 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Imajo K , Kessoku T , Honda Y , et al . Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography . Gastroenterology 2016. ; 150 ( 3 ): 626 – 637.e7 . [DOI] [PubMed] [Google Scholar]
- 76. Loomba R , Cui J , Wolfson T , et al . Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study . Am J Gastroenterol 2016. ; 111 ( 7 ): 986 – 994 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Singh S , Venkatesh SK , Loomba R , et al . Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis . Eur Radiol 2016. ; 26 ( 5 ): 1431 – 1440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park CC , Nguyen P , Hernandez C , et al . Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease . Gastroenterology 2017. ; 152 ( 3 ): 598 – 607.e2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Furlan A , Tublin ME , Yu L , Chopra KB , Lippello A , Behari J . Comparison of 2D shear wave elastography, transient elastography, and MR elastography for the diagnosis of fibrosis in patients with nonalcoholic fatty liver disease . AJR Am J Roentgenol 2020. ; 214 ( 1 ): W20 – W26 . [DOI] [PubMed] [Google Scholar]
- 80. Kim RG , Nguyen P , Bettencourt R , et al . Magnetic resonance elastography identifies fibrosis in adults with alpha-1 antitrypsin deficiency liver disease: a prospective study . Aliment Pharmacol Ther 2016. ; 44 ( 3 ): 287 – 299 . [DOI] [PubMed] [Google Scholar]
- 81. Wang J , Malik N , Yin M , et al . Magnetic resonance elastography is accurate in detecting advanced fibrosis in autoimmune hepatitis . World J Gastroenterol 2017. ; 23 ( 5 ): 859 – 868 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen J , Yin M , Talwalkar JA , et al . Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity . Radiology 2017. ; 283 ( 2 ): 418 – 428 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Trout AT , Sheridan RM , Serai SD , et al . Diagnostic performance of MR elastography for liver fibrosis in children and young adults with a spectrum of liver diseases . Radiology 2018. ; 287 ( 3 ): 824 – 832 . [DOI] [PubMed] [Google Scholar]
- 84. Schwimmer JB , Behling C , Angeles JE , et al . Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease . Hepatology 2017. ; 66 ( 5 ): 1474 – 1485 . [DOI] [PMC free article] [PubMed] [Google Scholar]