Abstract
Objective
Storke is a leading cause of death and disability affecting million people worldwide, 80% of which is ischemic stroke (IS). Recently, traditional Chinese medicines (TCMs) have received great attentions in treating IS due to their low poisonous effects and high safety. Buyang Huanwu Decoction (BHD), a famous and classical Chinese prescription, has been used for treating stroke-induced disability for centuries. Yet, its underlying mechanism is still in fancy.
Methods
We first constructed an IS model by middle cerebral artery occlusion (MCAO). Then, a metabonomics study on serum samples was performed using UHPLC-QTOF/MS, followed by multivariate data analysis including principal components analysis (PCA) and orthogonal partial least squares-discriminate analysis (OPLS-DA).
Results
Metabolic profiling of PCA indicated metabolic perturbation caused by MCAO was regulated by BHD back to normal levels, which is in agreement with the neurobehavioral evaluations. In the OPLS-DA, 12 metabolites were screened as potential biomarkers involved in MCAO-induced IS. Three metabolic pathways were recognized as the most relevant pathways, involving one carbon pool by folate, sphingolipid metabolism and inositol phosphate metabolism. BHD significantly reversed the abnormality of 7 metabolites to normal levels.
Conclusions
This is the first study to investigate the effect of BHD on IS at the metabolite level and to reveal the underlying mechanisms of BHD, which is complementary to neurobehavioral evaluation. In a broad sense, the current study brings novel and valuable insights to evaluate efficacy of TCMs, to interpret the action mechanisms, and to provide the theoretical basis for further research on the therapeutic mechanisms in clinical practice.
Keywords: ischemic stroke, Chinese herbal medicines, Buyang Huanwu decoction, serum metabonomics, middle cerebral artery occlusion (MCAO) model, LC-MS
Introduction
Following cardiovascular diseases and cancer, ischemic stroke (IS) is ranked as the third most common causes of death and disability with high incidence and relapse rate, bringing a big burden to the healthcare system and society.1,2 Although IS is attracting more and more attentions, there is still lack of effective therapeutic. The only drug for treating IS approved by U.S. Food and Drug Administration (FDA) is tissue type plasminogen activator, whose clinic application however has been largely limited due to the narrow therapeutic time window and severe side effects.3 It is therefore in great demand to develop therapeutic agents with better efficacy but fewer side-effects.
From the perspective of IS, complex factors are involving in the process of IS, including but not limiting, free radical formation, inflammation, necrosis, and apoptosis, etc.4 Taken this into account, thus, single-component-single-target therapy is not an effective strategy for treating IS.3 This also highlights the necessirity of searching multi-target and multi-pathway therapeutic strategies. In this regards, traditional Chinese Medicines (TCM) that have successfully been used for centuries to treat a wide variety of ailments are attracting more and more attentions.
Buyang Huanwu decoction (BHD) is a represent and classic TCM prescription to treat the sequelae of cerebrovascular events due to qi deficiency and blood stasis (such as stroke), consisted of seven crude herbs, namely Radix Astragali (Huangqi), Radix Angelicae Sinensis (Danggui), Radix Paeoniae Rubra (Chishao), Rhizoma Ligustici (Chuanxiong), Semen Persicae (Taoren), Flos Carthami (Honghua), and Lumbricus (Dilong). From the perspective of TCM theory, BHD can benefit Qi, activate blood circulation and dredge collaterals.5 BHD has been widely used to improve neurological functional function damaged by stroke and stroke-induced disability in China for more than 300 years.5 Modern pharmacological studies also have demonstrated the neuroprotective effects of BHD on ameliorating stroke-induced neurological dysfunctions.6,7 Possible therapeutic mechanisms of BHD might be associated with promoting neural differentiation and proliferation in ischemic penumbra6–8 and upregulating brain derived neurotrophic factor (BDNF) expression in infarct brain.9 However, it is still in great demand to elucidate the underlying mechanisms of BHD in treating IS, which will be of great significance in terms of providing not only a better understanding on TCM, but also a scientific support for promoting worldwide application of the ancient TCM formulae and developing modern drugs/formulae from the ancient ones.
Metabolomics, a key technology of modern systems biology, offers an approach to unveil new insights in understanding the underlying mechanisms of physiological regulations and the networks involved on the level of metabolites.10 The comprehensive and holistic features of metabolomics make it extremely suitable for the evaluation of the efficacy and mechanism of TCM formula which however is not easy to be performed by conventional methods due to the multi-component and multi-target characteristics of TCM.
The main methodologies used for metabolomics are mass spectrometry (MS) and nuclear magnetic resonance (NMR). In MS-based metabolomics, ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS), has been considered as one of the best analytical techniques due to its high analytic speed and sensitivity and high resolution of chromatographic peaks for complex biological samples, which has been widely used in TCM studies.11
Herein, for the first time, by using metabolomics tools, we aimed to clarify the effects and underlying mechanisms of BHD on IS, induced by middle cerebral artery occlusion (MCAO) model. An UPLC-QTOF/MS-based metabolomics approach was established to identify and relatively quantify altered metabolites in response to IS and therapeutic effects of BHD. Further, multivariate data analysis was performed to assess the changes in plasma metabolite levels and identify potential biomarkers for IS. As well, the reversed metabolites and corresponding metabolic pathways that are relevant to BHD intervention were also analyzed. This study could provide a valuable insight into the understanding of pathological changes of IS and therapeutic mechanisms of BHD.
Methods
Information of experimental design and resources
The information regarding the experimental design, statistics, and resources used in this study are attached in the minimum standards of reporting checklist (Supplementary file 1).
Materials and reagents
The seven herbs that were contained in BHD, including Radix Astragali (Huangqi), Radix Angelicae Sinensis (Danggui), Rhizoma Ligustici (Chuanxiong), Lumbricus (Dilong), Radix Paeoniae Rubra (Chishao), Flos Carthami (Honghua), and Semen Persicae (Taoren) were purchased from the Sheng Shi Herb Company of Tianjin (Tianjin, China), and authenticated by Professor Kui-jun Zhao, Department of pharmacy, Beijing Friendship Hospital, Capital Medical University.
2,3,5-triphenyltetrazolium chloride (TTC) (Nr. 0765) was purchased from Shanghai Sangon Biological Engineering Technology and Services (Shanghai, China). Ultra-pure distilled water was prepared by a Milli-Q purification system.
Preparation and quantity control of BHD
BHD is composed of Huangqi (120 g), Danggui (6 g), Chuanxiong (3 g), Dilong (3 g), Chishao (5 g), Honghua (3 g) and Taoren (3 g), which were soaked in water (18.5 L) for 12 h before extraction. They were then extracted three times with boiling water. Afterwards, the decoction was dried in vacuo (70 °C) and then ground into powder, which was further dissolved in purified water to a decoction concentrations of 1 g/mL.
To assure the quality and warrant the safety and effectiveness of BHD, BHD was analyzed by HPLC (details in Supplementary file 2). In the finger printing analysis, paeoniflorin, hydroxysafflor yellow A, Calycosin-7-O-β-D-glucoside, formononetin and acanthopanosine were identified. The representative HPLC is shown in Fig. S1 in the Supplementary File 2. The conditions of chromatographic analysis were provided in the Supplementary File 2.
Construction of the middle cerebral artery occlusion (MCAO) model
The model of focal cerebral ischemia–reperfusion is established with the suture-occluded method by Longa et al.12 Briefly, after being anesthetized by 10% chloral hydrate (0.35 mg/kg, i.p.), the rats were fixed on the operating table with supine position. After a midline incision was made at the ventral surface of the neck skin, the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were isolated. The CCA and ICA were temporarily clamped with microsurgical clips. At the distal part of the ECA, two closely spaced permanent knots were tied to prevent the backflow of blood and the ECA was cut between the knots. A 50 mm monofilament nylon suture with a rounded tip (final tip diameter of 0.38 ± 0.02 mm) (Beijing Sunbio Biotech Co., Ltd, Beijing, China) was introduced into the arteriotomy hole made between the ECA 18-20 mm, from ECA to block MCA, to achieve cerebral ischemia. After two-hour occlusion, reperfusion was accomplished. The rats in the sham-operation control group underwent the same surgical procedures, but without arterial occlusion.
Experimental animals, grouping and drug administration
30 male Sprague–Dawley rats (200 ± 20 g) were purchased from the Experimental Animal Center of the Chinese Military Medical Sciences Academy (Beijing, China). During the whole experimental procedure, all rats were housed in propylene cages under standard experimental conditions with room temperature at 24 ± 1 °C, relative humidity 45 ± 15% and 12/12-h light–dark cycle. Rats were free access to food and tap water.
After one-week acclimatization, rats were randomly grouped into sham-operation (sham), MCAO model and BHD treated groups, with 10 rats each. Rats in the BHD group were treated with 4 g/kg/d BDH, showing the best effects on IS rats, which was 2 times of adult per day in clinic, while those in the sham and MCAO groups were intrgastrically administered with 0.9% NaCl solution. After 7-day administration, neurobehavioral changes were evaluated. Blood samples were collected from the inferior vena cava. Blood samples were centrifuged at 3,500 rpm at 4 °C for 10 min, and the supernatants were stored at −80 °C until metabolomics analysis.
At the end of the experiment, rats were anesthetized by ethyl carbamate (1.5 g/kg B.W.). This study was carried out in accordance with the principles of the Basel Declaration and recommendations of Health Guide for the Care and Use of Laboratory Animals, the Ethics Committee of Beijing Friendship Hospital, Capital Medical University. The protocol was approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University. We did our maximum efforts to minimize animal suffering and the number of animals necessary for the capture of reliable data.
Neurobehavioral abnormality evaluation
Neurobehavioral abnormality was assessed by means of blinding to the experiment with Longa’s five-point scale as guidance: 0, normal (no-neuro behavioral dysfunction); 1, slight, failure of flexing left forepaw fully; 2, moderate, circling counter clock wisely); 3, severe, leaning to the affected side; 4, very serious, unconsciousness and failure of moving autonomously.12
Measurement of cerebral infracted area and histopathology analysis
The cerebral infracted area was measured referring to a previous study.13 Briefly, 2-mm sections of brains were incubated in TTC at 37 °C for 30 min, and fixed in 4% paraformaldehyde overnight away from light. Dehydrogenase catalyze the reaction of TTC and NADH (β-Nicotinamide adenine dinucleotide), the infracted cells are in gray or white. Accordingly, the infarct area was calculated as the infarction volume/the brain volume × 100%.
Hematoxylin-eosin (HE) staining was applied to evaluate the histopathological abnormalities of brain tissues. Fresh rat brains were fixed and preserved in 10% neutral buffered formalin and then prepared for paraffin sectioning. A series of 4 μm—thick sections was cut, processed for HE staining, and then analyzed under light microscope. Images from different groups were captured using a microscope (Leica DM6000B, Germany). 400 × magnification was used to visualize the images of HE staining.
Preparation of serum samples
250 μL serum samples mixed with 750 μL methanol were transferred into a 1.5 mL polypropylene tube. Then the solution was mixed and allowed to stand for 20 min at 4 °C before use. After the centrifugation at 12,000 rpm for 10 min at 4 °C, the supernatant of samples was transferred to a polypropylene tube. Being filtered through a syringe filter (0.22 μm), the supernatants (4 μL) were injected for UHPLC–MS analysis.
UPLC-MS based metabolomics analysis
Metabolomics analysis was performed using a LCMS-IT-TOF system (Shimadzu, Japan). Separation was carried out on a X Bridge® C18 Column (3.5 μm, 2.1 × 100 mm, Waters) which was kept at 40 °C and at a flow rate of 0.3 mL/min.
Aqueous formic acid (0.1%) (v/v) (A) and 0.1% formic acid in acetonitrile (B) were used as mobile phase. The gradient elution of B was performed as follows: 5% B at 0–3 min, 5%–50% B at 3-5 min, 50% B for 3 min, 50%–70% B at 8–10 min, 70% B for 10 min, 70%–95% B at 20–22 min, 95% B for 3 min, and 95%–5% B at 25–27 min and then maintained at 5% B for 8 min. The injected volume was 5 μL. During analyzing period, all samples were maintained at 4 °C. The eluent was directly introduced to the mass spectrometer. After the injection of 10 samples, the quality control (QC) sample, a pooled sample, followed by a blank was injected in order to ensure the stability and repeatability of the LC-MS systems.
The ESI source in positive ion modes was applied in MS analysis. The electrospray capillary voltage was 1.57 kV. MS data were collected in the mass range of 100–1,000 Da. Ionization was achieved using electrospray and the electrospray source parameters were fixed as followed: the electrospray capillary voltage was 1.57 kV, mass range ranged from m/z 100 to 1,000, gas temperature was 200 °C, gas flow was 0.8 L/min, ion accumulation time was 30 msec.
Data analysis
UPLC Q-TOF-MS raw data dealing
The UPLC Q-TOF MS raw data were first processed by SHIMADZU LCMS solution workstation software. Afterwards, the peaks were detected and aligned. And then, data were normalized to the summed total ion intensity of each chromatogram. On top of peaks alignment and normalized peak areas, a data matrix was constructed.
Multivariate data (MVD) analysis
Then the data matrix was subjected to SIMCA-P software for multivariate statistical analysis. Both principal component analysis (PCA) and orthogonal projection to latent structures square-discriminate analysis (OPLS-DA) were performed.
Prior to PCA, all variables obtained from data matrix were mean-centered and scaled to pareto variance. PCA was used to present natural interrelation among observations. OPLS-DA was applied to confirm the separation between the control and the model groups, and to screendifferential metabolites contributing to the separation.
The cumulative values of total Y explained variance (R2) and the predicable variation (Q2) were used to evaluate the goodness and the predictive ability of a model, respectively. Meanwhile, in this study, the permutation test with n = 200 was applied to further assess the consistency and performance of the models.
Identification of potential biomarkers
From the OPLS-DA model, the S-plot, the value of variable importance for projection (VIP) and independent sample t test were used to select the potential biomarkers. In S-plots, the spots located at the ends of the plot represent endogenous components which have greater VIP values. And then, variables with a VIP value (VIP ≥1.0) were selected as potential biomarkers that contributed most to the separations of the model group and the control group. Subsequently, t-test was used to test the significance of the differences of these potential metabolites between the control and the model groups, with P < 0.05 as the significance threshold.
For identification, the National Institute of Standards and Technology (NIST) library was performed, where the mass spectrometry information of these potential biomarkers was compared with the information of standard compounds stored in the databases. The identification is on the basis of similarity indices (Match and R. match >700). Also, accurate mass-to-charge ratios were referred to available databases, including HMDB (www.hmdb.ca), Metlin, KEGG (http://www.kegg.jp/kegg/pathway.html), according which the potential biomarkers were identified.
Metabolic pathway construction
Metabolic pathway analysis was performed by MetaboAnalyst 3.0 (www.metaboanalyst.ca/) to reveal disturbed metabolism on the basis of the pathway library of rats (Rattus norvegicus). The values of pathway impact calculated from pathway topology analysis with MetPA above 0.1 were screened out as the potential target pathways.
Additionally, a network diagraph was constructed to visualize the links between metabolites and corresponding pathways on the basis of literature information and the KEGG database.
Statistic analysis
First, Kolmogorov–Smirnov test was applied to evaluate the normality of the data. The data obtained met a normal shape of distribution and therefore t-test was employed for this study.
SPSS was used for independent sample t test between groups. The threshold for significance was P < 0.05.
Results
Characteristics of the HPLC fingerprints of BHD
The chemical profile of BHD was characterized by HPLC. Accordingly, BHD contains a wide range of chemical components, including paeoniflorin, hydroxysafflor yellow A, Calycosin-7-O-beta-D-glucoside, formononetin and acanthopanosine (Fig. S1).
Effects of BHD on the neurobehavioral abnormality and cerebral infraction of MCAO rats
MCAO induced a high neurobehavioral score of 3.34 (± 1.04) while after the treatment of BHD, it decreased to 1.65 (Fig. 1A). The cerebral infraction volume of MCAO group was 28.9 ± 5.8%. BHD significantly reduced the infraction volume of the model rats to 18.0 ± 1.8% (Fig. 1B). These results suggested relieve effects on the MCAO-induced brain damages.
Fig. 1.
Protective effect of Buyang Huanwu decoction (BHD) on ischemia stroke induced by the middle cerebral artery occlusion (MCAO) model. A) Neurobehavioral scores; B) Cerebral infarct volume percentage; C) Pathological abnormalities of brain tissues collected from MCAO model group, sham-operation group and BHD group, manifested from hematoxylin–eosin (HE) staining. Data obtained were expressed as mean ± standard deviation (S.D.), n = 10 *P < 0.05, **P < 0.01 MCAO model vs. BHD treatment, indicating a good therapeutic effect of BHD.
Effects of BHD on pathological damages of brains of MCAO rats
First, significantly histological changes in the brain tissues suggested that the MCAO model has been constructed successfully.
As compared to sham-operation group, rats in MCAO model group showed obviously pathological changes in brain with destructed neuro structure, neuronal loss and poly-nesic sponginess (Fig. 1C). The administration of BHD significantly attenuated the histopathological abnormalities.
Metabolomics profiling of serum samples and multivariate data analysis
Figure 2 is a typical chromatogram acquired in full mode of a serum sample collected from a sham-operation rat. Subsequent data processing with MarkerLynx XS detected 1,264 ions and then were subjected for multivariate data analysis.
Fig. 2.

The LC-MS total ion chromatogram of serum sample collected from sham-operation rats.
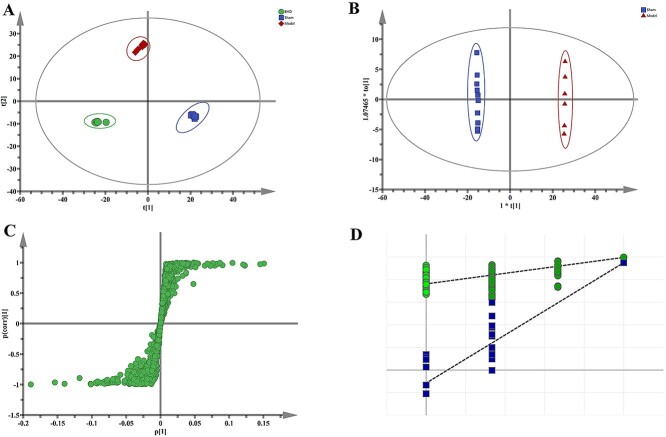
PCA score plot showed a good separation between the sham-operation group, the MCAO group and the BHD treatment group, reflecting the differences of metabolic profiles among the three groups (Fig. 3A). First, the metabolic profiles of MCAO model rats were far from those of control rats, reflecting that MCAO caused significant changes of metabolic profiles. Additionally, along the PC2 axis, difference between the BHD group and the model group was clearly evident, while the metabolic profile of BHD group was close to control group, indicating BHD could regulate the metabolic disturbance induced by MCAO. This is consistent with neurobehavioral assessments (Fig. 1). The statistical parameters of the PCA model were 0.705 and 0.664 for R2 and Q2 respectively, which verified the validation of the PCA model (Table 1).
Fig. 3.
PCA score plot based on UHPLC-MS data of serum samples from sham-operation group (blue), MCAO model group (red), and BHD treatment group (green) (A), the OPLS-DA score plots of serum metabolic profiling between model group and control group (B), and R2, Q2 permutation test that was randomly permuted 200 times (C), as well as the corresponding S-pots (D).
Table 1.
Parameters of PCA and OPLS-DA models.
| Model type | Groups included | Component | R2X | R2Y (cum) | Q2Y (cum) |
|---|---|---|---|---|---|
| PCA | Con, Model & BHD | 2 | 0.705 | - | 0.664 |
| OPLS-DA | Con & Mod (ESI+) | 1 + 0 + 0 | - | 0.664 | 0.990 |
| OPLS-DA | Con & Mod (ESI-) | 1 + 0 + 0 | - | 0.989 | 0.983 |
R2Xcum and R2Ycum represent the cumulative sum of squares (SS) of all the X’s and Y’s explained by all extracted components.
Q2Ycum is an estimate of how well the model predicts the Y’s.
Orthogonal partial least-squares discriminant analysis (OPLS-DA) was used to further validate the separation of the metabolic profiles of different groups (Fig. 3B) and to screen out the potential variables that responsible for the separation (Fig. 3C). The R2X, R2Y values indicated goodness of fit and the Q2 values showed the model had satisfactory goodness of prediction. Meanwhile, a random class permutation test (n = 200) was shown in Fig. 3D. Values of the parameters for evaluating modeling quality were listed in Table 1. The results suggested that models established in this study had good differentiating, fitness and prediction.
Potential biomarkers identification
In the A OPLS-DA model differentiating control group (blue) and MCAO model (red) group was built. The score plot showed a clear separation between the control and the model groups (Fig. 3B), confirming the metabolic differences we observed in PCA analysis.
The corresponding S-plot, VIP values and P values indicate the relative importance of each variable in differentiating control and model groups, and therefore were used for identifying potential biomarkers. Initially, the corresponding S plot (Fig. 3C) was used to extract possible differential variables, where highlighted in red are ions found to be at significantly higher concentrations in control than in MCAO rats while highlighted in blue are ions found to be at significantly higher concentrations in MCAO than in control. Afterwards, the metabolites with VIP values >1.0 were considered to have an above average influence on the classification. Besides, t-test tested the significance of the differences of metabolites, with P values <0.05 as significance threshold. Based upon the precise molecular masses and structural information, discrimination and identification were performed in terms of comparing the feature exact mass with the exact mass of the putative biomarker in the HMDB and METLIN databases.
To this end, a total of 12 variables in serum was potentially regarded as differential metabolites in response to MCAO-induced IS. The retention times, masses of the ions, their percentage increase/decreases, and their significance values were listed in Table 2. Compared with controls, the levels of glutaric acid, cholesterol, sphinganine, and aldosterone in serum were significantly increased, while the levels of inositol, tetrahydrofolic acid, lactrobionic acid, N-acetylaspartic acid, tetradecanedioic acid, decosatrienoic acid, phytosphingosin, L-homoarginine in serum were significantly decreased (Table 2, Fig. 4).
Table 2.
Differential identified metabolites for discrimination between the control group and the model group, and between the model group and the BHD treatment group.
| RT(min) | Metabolite Name |
|---|---|
| Data from the ESI+ mode | |
| 7.12 | Glutaric acid |
| 7.29 | Tetradecanedioic acid |
| 23.13 | Cholesterol |
| 27.04 | Docosatrienoic acid |
| 27.23 | Phytosphingosine |
| 47.04 | Sphinganine |
| Data from the ESI- mode | |
| 17.79 | Inositol |
| 23.70 | Aldosterone |
| 27.10 | L-homoarginine |
| 37.70 | Tetrahydrofolic acid |
| 61.62 | Lactobionic acid |
| 61.67 | N-acetylaspartic acid |
Fig. 4.
Box-plots of relative intensities of 12 potential biomarkers in sham-operation group (sham), MCAO model group (mod) and BHD treatment group (BHD). A) Glutaric acid; B) Tetradecanedioic acid; C) Cholesterol; D) Docosatrienoic acid; E) Phytosphingosine; F) Sphinganine; G) Inositol; H) Aldosterone; I) L-homoarginine; J) Tetrahydrofolic acid; K) Lactobionic acid; L) N-acetylaspartic acid. Values were expressed as mean ± SD. *P < 0.015, **P < 0.01, ***P < 0.001compared with the MCAO model rats.
Effects of BHD on discriminated metabolites in response to MO-induced IS
On the basis of results of MVD analysis, the relative intensities of the predominant changes metabolites were further analyzed by using the box plots (Fig. 4), which demonstrate not only the changes of metabolite levels in IS model group compared to controls, but also the changes in BHD group compared to the model group, as a consequence, reflecting the regulation effects of BHD on serum metabolites.
Of all 12 serum potential biomarkers, the levels of 7 metabolites were versing trend to normal levels with the treatment of BHD. Specifically, BHD decreased the contents of glutaric acid, cholesterol, sphinganine, and aldosterone, while increased the levels of inositol, lactobionic acid and N-acetylaspartic acid, as compared with the MCAO model group (Table 2).
Metabolic pathways analysis
Next, to further reveal the impacts of the potential metabolites on aging and to identify the most relevant pathways associated with aging, MetPA (Metabolomics Pathway Analysis) was constructed based on the identified biomarkers in Table 2.
On the basis of the influence coefficient of the metabolic pathways, a value of the pathway impact higher than 0.1 is considered to be a potential pathway. According to the pathway impact values (x-axis) and P values (y-axis), the most impacted pathways were found. In total, 3 metabolic pathways were considered closely related to IS, including one carbon pool by folate, sphingolipid metabolism and inositol phosphate metabolism, respectively (Fig. 5; Table 3).
Fig. 5.
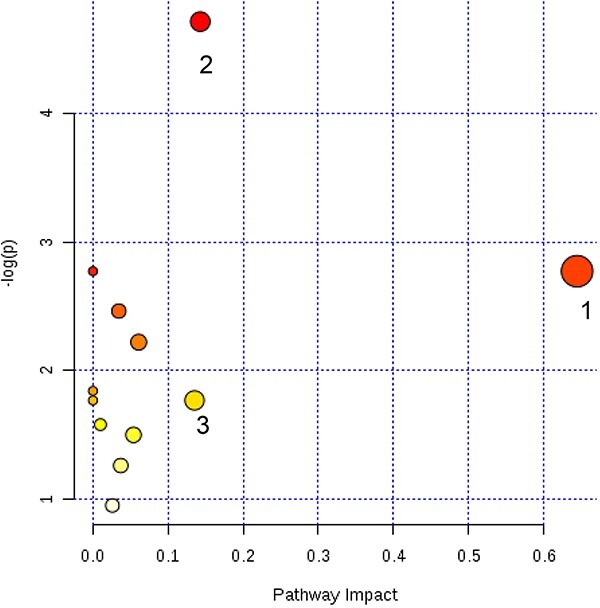
Summary of the altered metabolic pathways with MetPA based on the identified biomarkers in Table 2, as analyzed using MetaboAnalyst 3.0. The size and color of each pathway were set according to pathway impact and P-value, respectively.
Table 3.
Results from the pathway analysis (shown in Fig. 5).
| Pathway name | Total | Hits | P | -Log (p) | FDR | Impact |
|---|---|---|---|---|---|---|
| One carbon pool by folate | 9 | 1 | <0.05 | 2.78 | 1.0 | 0.65 |
| Sphingolipid metabolism | 21 | 2 | <0.01 | 4.72 | 0.72 | 0.14 |
| Inositol phosphate metabolism | 26 | 1 | NS | 1.77 | 1.0 | 0.14 |
FDR, false discovery rate values.
Discussion
The current study is of significance for two concerns. First, from the perspective of diseases, stroke, a heterogeneous multifactorial disorder, is caused by multiple genetic factors, environmental factors, and interactions among these factors. Approximately 85% of stroke cases is IS. Studies have shown that cell necrosis and apoptosis are one of the pathogenesis of stroke. Yet, very few studies have been performed from the perspective of metabolites. This is however of vital importance as finding biomarkers and the corresponding metabolic changes of IS could provide information for a rapid evaluation and an early diagnosis to target therapy.
Another important consideration is the treatment of IS. There has been a lack of effective treatments for stroke, thus far. Concerning the comprehensive features of IS, a combination therapy instead of a mono-therapy, or the incorporation of a multi-target drug has been suggested. Thus, it has given rise to an urgent need to find ideal drugs treating IS. Among others, TCMs are drawing ever-increasing interests as they are highly effective and therapeutic but have few side-effects and low toxicity. Herein, BHD, a famous and classical Chinese prescription, was taken as an instance. It is well documented that BHD along with its component herbs and compounds showed therapeutic effects on neurodegenerative diseases and could protect neurons from damage induced by IS due to a variety of functions they possessing: anti-oxidation, anti-inflammation, anti-apoptosis, and so on. However, taking into account the characteristics of TCMs, i.e. the complexity of chemical constituents in TCMs and their holistic therapeutic efficacy on multiple targets in different pathways, system studies are needed.
On top of these concerns above, it is recommended to apply novel approaches to study the pathogenesis of IS and the therapeutic effects of BHD. Metabolomics, one of important aspects of system biology, provides a comprehensive and simultaneous analysis on metabolite changes occurring in living organisms in response to pathophysiological stimuli and/or genetic modification.10 Of note, metabolomics provides an integrated view of the whole bio-system, which is of particular consistence with the holistic thinking of TCM. Therefore, we performed a serum metabonomics approach coupled with MVD analysis to discover potential biomarkers of IS and to further reveal the underlying mechanisms of BHD from a metabolic point of view.
Specifically, we identified 12 metabolites in serum that may enable the classification of disease status, which therefore were regarded as significant biomarkers of IS, including glutaric acid, tetradecanedioic acid, cholesterol, docosatrienoic acid, phytosphingosine, sphinganine, aldosterone, inositol, L-homoarginine, tetrahydrofolic acid (THFA), N-acetylaspartic acid, lactobionic acid (LA). On the one hand, the levels of the 12 metabolites significantly altered in MCAO model rats as compared to control group. The underlying differences might be significant to the IS caused by MCAO. On the other hand, the levels of 7 metabolites out of 12 were significantly reversed by the treatment of BHD. Such a regulation on the levels and dysfunctions of these metabolites could be one of the underlying mechanisms of therapeutic effects of BHD on IS.
Herein, the metabolic pathway analyzed by MetPA suggested that metabolites identified in serum were significantly important for the efficacy of BHD as follows.
Sphingolipid metabolism abnormality
Sphingolipids like sphinganine and phytosphingosine are a series of cell membrane-derived lipids. It has been reported that sphingolipid activities change after stroke, indicating tight correlation of sphingolipids with stroke.14 Besides that, sphinganine also supplies a basic unit for synthesizing various complex sphingolipids, therefore play an important role in the metabolic process of sphingomyelin.15 Phytosphingosine also play a role in immune-potentiating activity, which can induce cell apoptosis.16 Studies have shown that cell necrosis and apoptosis are one of the pathogenesis of stroke.16 In this study, abnormal levels of phytosphingosine and sphinganine in MCAO model rats possibly induced cell necrosis and apoptosis, which consequently triggers stroke.
Steroid hormone metabolism abnormality
Renin-angiotensin-aldosterone system (RAAS) activation is an important factor in the pathophysiology of cardiovascular disease. Aldosterone (ALD) is a steroid hormone with mineralocorticoid activity. As a component of the RAAS, aldosterone is classically known to play a regulatory role of pathophysiological functions, for instance, in body fluid and electrolyte homeostasis, thus contributing to the development of hypertension.
Not only experimental, but also clinical evidences have demonstrated that aldosterone contributes to the incidence and outcome of stroke.17 For instance, Rocha and Stier Jr18 found that aldosterone had a pernicious impact on cerebrovascular comorbidity. In addition, aldosterone levels are tightly associated with future vascular events, for instance, atherosclerotic process.19 The mechanisms may involve in causing vascular remodeling in terms of increasing media thickness, increasing extracellular matrix proteins, etc.
Herein, the level of ALD was significantly increased in the MCAO model group as compared to the sham-operation group. This is line with a previous study in the IS patients.20 Also, Ivanes et al.21 found the level of aldosterone is strongly and independently associated with mortality and the occurrence of acute ischaemic events.
Influence on nitric oxide (NO) bioactivity
L-Arginine (Arg) is a semi-essential, proteinogenic, functional amino acid in nutrition and health and plays multiple roles in growth, health and disease. The Arg-homologous L-homoarginine (hArg), a non-essential, non-proteinogenic amino acid, is biosynthesized from Arg by the catalytic action of L-arginine: glycine amidinotransferase (AGAT).
Both Arg and hArg serve as substrates for nitric oxide synthases (NOS). Additionally, hArg may also indirectly increase NO production by inhibiting arginase activity. NO, an important pleiotropic gas, possesses numerous biological activities including inhibition of vascular inflammation and platelet aggregation, prevention of adhesion of immune cells and regulation of blood pressure.22
The cardiovascular effect of hArg has been related to NO, a potent endogenous vasodilator. By means of stable-isotope dilution gas chromatography-mass spectrometry, Hanff et al.23 measured all biochemical parameters and found that a strong relation between the Arg/NO pathway and aortic atherosclerosis. Low concentration of hArg in the blood has been established as a cardiovascular risk marker.24 In a 10-year follow-up study on patients with a history of cerebrovascular disease, the hArg level significantly decreased.25 In agreement with this result, we found the hArg level significantly decreased as compared to sham-operation group.
Changes of folic acid cycle
Tetrahydrofolic acid (THFA) is involved in the metabolism of folic acid and in “activation-carbon cycle”, and therefore plays an important role in maintaining the stability of protein and DNA, synthesizing other molecules, and combating oxidative metabolites.
In the current study, we found a lower level of THFA in the MCAO model rats than that of control rats, which is in agreement with a previous study where a significant reduction of THFA level was observed in serum samples collected from IS patients.26 The decrease in THFA level indicates an imbalance in the production of reactive oxygen species, which consequently cause the occurrence of IS.20
Membrane disruption and reconstruction abnormality
Neuronal membranes, rich in polyunsaturated fatty acids, are particularly susceptible to free radical attack. Lipids, especially polyunsaturated fatty acids (PUFAs), are preferential targets for oxidative damage.27 PUFAs are important components of cell membranes, like erythrocyte membranes and neuronal membranes, and can be released from the degradation of glycerophospholipids. Several lipid mediators can be produced by PUFA oxidation, which are all closely related with neuronal pathways involved in neurodegenerative diseases, and also suggests that an interplay among lipids occurs in brain tissue.28 Reactions of PUFAs with oxygen occurred in a living cell may result in defects in membrane function, which as a consequence may cause cell death.29
In this experiment, the level of docosatrienoic acid, an UFA, was significantly decreased in the MCAO-ischemic stroke rats as compared to control rats. Although further studies might be required to clarify the role of UFA in stroke, altering the metabolism of UFA might provide an interesting system to study pharmacological mechanisms of BHD.
Inositol and other compounds are precursors of all membrane phospholipases.30 It could be used for constructing damaged membranes as a self-repair mechanism. The elevated level of inositol in serum in stroke rats suggested an abnormality of re-utilization of inositol, and therefore suggested a dysfunction of the self-repair system.
Abnormality of neuronal and glial integrity
Mitochondrion was believed to be one of the major factories of reactive oxygen species (ROS) during respiratory metabolism. Mitochondrial dysfunction aggravates energy crisis.
N-Acetylaspartate (NAA), an important source of acetyl groups, is considered as a general maker of neuron integrity and viability, involving in metabolism of several neurotransmitters, e.g. aspartate, N-acetyl-aspartyl glutamate.31 NAA has been considered a marker of neuronal integrity as the enzyme responsible for the synthesis of NAA is localized exclusively in neuronal mitochondria membrane.32 NAA can be hydrolyzed to aspartate and acetate by the action of aspartoacylaseII, and the released acetate may be used for lipid synthesis. The increased NAA level in IS rats therefore reflected mitochondrial and neuronal dysfunction caused by MCAO, which was in line with previous results.33 BHD significantly enhanced the content of NAA in brains of MCAO rats, indicating that it could protect neurons and mitochondria from MCAO induced damages, which might be due to its anti-oxidative and neuroprotective effects.
In addition to its function in cell membrane, inositol, a cerebral osmolyte, has also been hypothesized to be a glial marker.34 The significant increase of inositol level in serum samples of MCAO rats indicated a distribution of MCAO in the ionic balance, which consequently caused brain edema. This may be related to the ischemia-induced changes in local osmolality.
Organic acids and cholesterol
The neurotoxicity of glutaric acid has been observed in experimental studies, especially in some autosomal recessive metabolic disorders. The elevated neurotoxicity of glutaric acid is largely due to the deficiency of enzyme glutaryl-CoA dehydrogenase. Further, the enzyme defect leads to secondary damage to central nervous system due to the accumulation of glutaric acid. In the current study, we found a higher concentration of glutaric acid in the MCAO-induced ischemic stroke rats than control rats. Together with other organic acids, e.g. 3-hydoxyglutaric acid and glutarylcarnitine, the accumulation of glutaric acid may lead to neuronal damage, lymphocyte infiltration, elevated concentrations of inflammatory cytokines and nitric oxide, glial proliferation, atrophy of striatal neurons, and neurological dysfunction,35 consequently contributing to the occurrence of stroke.
Cholesterol plays an importantly role in the development of arherosclerotic lesions of cerebral arteries. Some studies showed that the increased levels of the total cholesterol, low density lipoprotein (LDL) cholesterol and high density lipoprotein (HDL) cholesterol are found to be associated with the increased risk of IS.36 Previous epidemiological studies also have showed that cholesterol levels are associated with SI. In this regard, high level of cholesterol therefore has been considered as a predictor of IS, and other cardiovascular and cerebrovascular diseases. In the current study, we found the level of total cholesterol increased in the MCAO-induced IS rats. It has been suggested that elevated level of cholesterol could aggravate the formation of atherosclerotic plaques, which eventually triggered the occurrence of IS.37 Therefore, the increase of cholesterol in the MCAO-induced IS rats may directly reflect the pathological state of stroke.
Except for tetradecanedioic acid, docosatrienoic acid, phytosphingosine, L-homoarginine, and tetrahydrofolic acid, BHD significantly reversed the abnormality of the other 7 metabolites to normal-like levels, thereby alleviating sphingolipid metabolism, steroid hormone metabolism, membrane disruption and reconstruction, neuronal and glial integrity and cholesterol bioactivity, and so forth, and suggesting the possible mechanism of the protective effect of BHD on IS.
Conclusion
In this study, a serum metabolomics study based on UHPLC-MS was conducted to evaluate the protective effect of BHD, a famous and classic Chinese prescription, on MCAO -induced IS rats. The results of neurobehavioral abnormality evaluation suggested that BHD could improve the behavioral abnormalities to ameliorate symptoms of ischemic stroke. Further, metabolic profile of the MCAO model rats was significantly different from that of the control rats, while BHD group was more similar to the control group, as shown by pattern recognition with MVD analysis. 12 serum metabolites were found involving in the metabolic differences between MCAO rats and control rats. BHD showed significant regulatory effects on restoring the levels of 7 metabolites to normal levels. This further indicated that the therapeutic effect of BHD on ischemic stroke may involve regulating sphingolipid metabolism, steroid hormone metabolism, membrane disruption and reconstruction, neuronal and glial integrity and so forth. This study points out the huge value for the application of metabolomics on investigating the action mechanism of TCM. Further studies are currently underway to reveal the exact mechanisms of BHD in depth.
Supplementary Material
Contributor Information
Rou-jun Wang, Nanjing University of Chinese Mdicine, 282 Hanzhong Road, Nanjing City, Jiangsu Province, Nanjing 210029, China; Department of Diabetes and Endocrinology, Kunming Municipal Hospital of Traditional Chinese Medicine, No. 2628 Xiangyuan Road, Chenggong District, Kunming 650500, China.
Guang-chao Ma, School of Chemical Science and Engineering, Yunnan University, Wujiaying Street, Chenggong District, Kunming 650500, China.
Shun Yu, Yunnan University of Traditional Chinese Medicine, 1076 Yuhua Road, Chenggong District, Kunming 650500, China.
Mei Zhang, Nanjing University of Chinese Mdicine, 282 Hanzhong Road, Nanjing City, Jiangsu Province, Nanjing 210029, China; Yunnan Institude of Traditional Chinese medicine and materia medical, Lianhua chi, Kumning 650000, China.
Shi-biao Pu, Yunnan University of Traditional Chinese Medicine, 1076 Yuhua Road, Chenggong District, Kunming 650500, China.
Author contributions
Shi-biao Pu, Rou-Jun Wang, and Guang-chao Ma conceived and designed the experiments. Guang-chao Ma performed the experiments. Rou-Jun Wang wrote the manuscript. Mei Zhang conducted literature research on the relevant content, asked questions and participated in the design of these experiments. Shunyu participated in the animal experiments and collated the data.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81760812), Yunnan Province high-level Talent Training Support Program “Yunnan Province Ten Thousand Plan Young Top Talent Special” (No. YNWR-QNBJ-2018-190) and the Yunnan Provincial Science and Technology Department—Applied Basic Research Joint Special Funds of Yunnan University of Traditional Chinese Medicine (No. 2017FF117(-035) and 2018FF001(-021)).
Conflict of interest statement. The authors declare no competing interests.
Data availability
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
All procedures performed in this study were conducted in accordance with the principles outlined in the Declaration of Helsinki and associated guidelines for animal experiments. All institutional and national guidelines for the care and use of laboratory animals were followed. The experiments were approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University in China.
References
- 1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008:371(9624):1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Varghese C, Onuma O, Johnson W, Brainin M, Hacke W, Norrving B. Organizational update: World Health Organization. Stroke. 2017:48(12):e341–e342. [DOI] [PubMed] [Google Scholar]
- 3. Chen HS, Qi SH, Shen JG. One-compound-multi-target: combination prospect of natural compounds with thrombolytic therapy in acute ischemic stroke. Curr Neuropharmacol. 2017:15(1):134–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010:17(3):197–218. [DOI] [PubMed] [Google Scholar]
- 5. Wang HW, Liou KT, Wang YH, Lu CK, Lin YL, Lee IJ, Huang ST, Tsai YH, Cheng YC, Lin HJ, et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J Ethnopharmacol. 2011:138(1):22–33. [DOI] [PubMed] [Google Scholar]
- 6. Cui HJ, Yang AL, Zhou HJ, Wang C, Luo JK, Lin Y, Zong YX, Tang T. Buyang huanwu decoction promotes angiogenesis via vascular endothelial growth factor receptor-2 activation through the PI3K/Akt pathway in a mouse model of intracerebral hemorrhage. BMC Complem Altern M. 2015:15(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao LD, Wang JH, Jin GR, Zhao Y, Zhang HJ. Neuroprotective effect of Buyang Huanwu decoction against focal cerebral ischemia/reperfusion injury in rats-time window and mechanism. J Ethnopharmacol. 2012:140(2):339–344. [DOI] [PubMed] [Google Scholar]
- 8. Sun JH, Gao YM, Yang L, Wang X, Bao LH, Liu WJ. Effects of Buyang Huanwu decoction on neurite outgrowth and differentiation of neuroepithelial stem cells. Chin J Physiol. 2007:50:151–156. [PubMed] [Google Scholar]
- 9. Kong XY, Su XH, Zhu J, Wang JZ, Wan H, Zhong MC, Li L, Lin N. Neuroprotective effect of buyang huanwu decoction on rat ischemic/reperfusion brain damage by promoting migration of neural precursor cells. Rejuvenation Res. 2014:17(3):264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicholson J, Lindon J, Holmes E. Metabonomics: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999:29(11):1181–1189. [DOI] [PubMed] [Google Scholar]
- 11. Wang JH, Byun J, Pennathur S. Analytical approaches to metabolomics and applications to systems biology. Semin Nephrol. 2010:30(5):500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989:20(1):84–91. [DOI] [PubMed] [Google Scholar]
- 13. Wang PR, Wang JS, Yang MH, Kong LY. Neuroprotective effects of Huang-Lian-Jie-Du-decoction on ischemic stroke rats revealed by 1H NMR metabolomics approach. J Pharmaceut Biomed. 2014:88:106–116. [DOI] [PubMed] [Google Scholar]
- 14. Sun N, Keep RF, Hua Y, Xi G. Critical role of the sphingolipid pathway in stroke: a review of current utility and potential therapeutic targets. Transl Stroke Res. 2016:7(5):420–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tidhar R, Sims K, Rosenfeld-Gur E, Shaw W, Futerman AH. A rapid ceramide synthase activity using NBD-sphinganine and solid phase extraction. J Lipid Res. 2015:56(1):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho YJ, Song HS, Bhang SH, Lee S, Kang BG, Lee JC, An J, Cha CI, Nam DH, Kim BS, et al. Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J Neurosci Res. 2012:90(9):1794–1802. [DOI] [PubMed] [Google Scholar]
- 17. Litchfield WR, Anderson BF, Weiss RJ, Lifton RP, Dluhy RG. Intracranial aneurysm and hemorrhagic stroke in glucocorticoid-remediable aldosteronism. Hypertension. 1998: 31(1):445–450. [DOI] [PubMed] [Google Scholar]
- 18. Rocha R, Stier CT Jr. Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab. 2001:12(7):308–314. [DOI] [PubMed] [Google Scholar]
- 19. Rossi G, Boscaro M, Ronconi V, Funder JW. Aldosterone as a cardiovascular risk factor. Trends Endocrinol Metab. 2005:16(3):104–107. [DOI] [PubMed] [Google Scholar]
- 20. Jiang ZT, Sun JB, Liang QL, Cai YF, Li SS, Huang Y, Wang Y, Luo G. A metabonomic approach applied to predict patients with cerebral infarction. Talanta. 2011:84(2):298–304. [DOI] [PubMed] [Google Scholar]
- 21. Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautière K, Collet JP, Beygui F, Hennache B, Ennezat PV, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012:33(2):191–202. [DOI] [PubMed] [Google Scholar]
- 22. Epstein FH, Moncada S, Higgs A. The l-arginine-nitric oxide pathway. New Engl J Med. 1993:329(27):2002–2012. [DOI] [PubMed] [Google Scholar]
- 23. Hanff E, Kayacelebi AA, Yanchev GR, Maassen N, Haghikia A, Tsikas D. Simultaneous stable-isotope dilution GC-MS measurement of homoarginine, guanidinoacetate and their common precursor arginine in plasma and their interrelationships in healthy and diseased humans. Amino Acids. 2016:48(3):721–732. [DOI] [PubMed] [Google Scholar]
- 24. Haghikia A, Yanchev GR, Kayacelebi AA, Hanff E, Bledau N, Widera C, Sonnenschein K, Haghikia A, Weissenborn K, Bauersachs J, et al. The role of L-arginine/L-homoarginine/nitric oxide pathway for aortic distensibility and intima-media thickness in stroke patients. Amino Acids. 2017:49(6):1111–1121. [DOI] [PubMed] [Google Scholar]
- 25. Pilz S, Meinitzer A, Tomaschitz A, Drechsler C, Ritz E, Krane V, Wanner C, Boehm BO, Marz W. Low homoarginine concentration is a novel risk factor for heart disease. Heart. 2011:97(15):1222–1227. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Qin X, Demirtas H, Li JP, Mao GY, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007:369(9576):1876–1882. [DOI] [PubMed] [Google Scholar]
- 27. Niki E. Antioxidants in relation to lipid peroxidation. Chem Phys Lipids. 1987:44(2-4):227–253. [DOI] [PubMed] [Google Scholar]
- 28. Chang CY, Ke DS, Chen JY. Essential fatty acids and human brain. Acta Neurol Taiwanica. 2009:18(4):231–241. [PubMed] [Google Scholar]
- 29. Oberley M. Free radical and diabetes. Free Radic Biol Med. 1988:5(2):113–124. [DOI] [PubMed] [Google Scholar]
- 30. Yang MX, Wang S, Hao FH, Li YJ, Tang HR, Shi XM. NMR analysis of the rat neurochemical changes induced by middle cerebral artery occlusion. Talanta. 2012:88:136–144. [DOI] [PubMed] [Google Scholar]
- 31. Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989:13(1):23–31. [DOI] [PubMed] [Google Scholar]
- 32. Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR Biomed. 2007:20(3):216–237. [DOI] [PubMed] [Google Scholar]
- 33. Demougeot C, Marie C, Giroud M, Beley A. N-Acetylaspartate: a literature review of animal research on brain ischemia. J Neurochem. 2004:90(4):776–783. [DOI] [PubMed] [Google Scholar]
- 34. Czéh B, Michaelis T, Watanabe T, Frahm J, Biurrun G, Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001:98(22):12796–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Funk CB, Prasad AN, Frosk P, Sauer S, Kölker S, Greenber CR. Neuropathological, biochemical and molecular findings in a glutaric acidemia type 1 cohort. Brain. 2005:128(4):711–722. [DOI] [PubMed] [Google Scholar]
- 36. Bowman TS, Sesso HD, Ma J, Kurth T, Kase CS, Stampfer MJ, Gaziano JM. Choleterol and the risk of ischemic stroke. Stroke. 2003:34(12):2930–2934. [DOI] [PubMed] [Google Scholar]
- 37. Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008:134(5):714–717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.