Abstract
Targeting the gut–bone axis with probiotics and prebiotics is considered as a promising strategy to reduce the risk of osteoporosis. Gut-derived short chain fatty acids (SCFA) mediate the effects of probiotics on bone via Tregs, but it is not known whether prebiotics act through a similar mechanism. We investigated how 2 different prebiotics, tart cherry (TC) and fructooligosaccharide (FOS), affect bone, and whether Tregs are required for this response. Eight-wk-old C57BL/6 female mice were fed with diets supplemented with 10% w/w TC, FOS, or a control diet (Con; AIN-93M) diet, and they received an isotype control or CD25 Ab to suppress Tregs. The FOS diet increased BMC, density, and trabecular bone volume in the vertebra (~40%) and proximal tibia (~30%) compared to the TC and control diets (Con), irrespective of CD25 treatment. Both prebiotics increased (P < .01) fecal SCFAs, but the response was greater with FOS. To determine how FOS affected bone cells, we examined genes involved in osteoblast and osteoclast differentiation and activity as well as genes expressed by osteocytes. The FOS increased the expression of regulators of osteoblast differentiation (bone morphogenetic protein 2 [Bmp2], Wnt family member 10b [Wnt10b] and Osterix [Osx]) and type 1 collagen). Osteoclasts regulators were unaltered. The FOS also increased the expression of genes associated with osteocytes, including (Phex), matrix extracellular phosphoglycoprotein (Mepe), and dentin matrix acidic phosphoprotein 1 (Dmp-1). However, Sost, the gene that encodes for sclerostin was also increased by FOS as the number and density of osteocytes increased. These findings demonstrate that FOS has a greater effect on the bone mass and structure in young adult female mice than TC and that its influence on osteoblasts and osteocytes is not dependent on Tregs.
Keywords: gut–bone axis, prebiotics, short chain fatty acids, fructooligosaccharide, tart cherry, osteocytes
Graphical Abstract
Graphical Abstract.
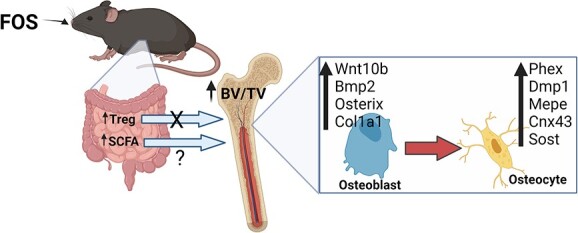
Introduction
Osteoporosis continues to be a growing public health concern in part due to an aging population demographic and poor adherence to currently available treatment options.1-4 One approach to reducing the risk for osteoporosis later in life is to focus on optimizing the bone tissue accrual or peak bone mass. Bone mass attained during the first 2–3 decades of life is recognized as a major determinant of skeletal health and fracture risk.5 In fact, lifetime fracture risk has been reported to decrease by 50% with each SD increase in peak bone mass.6 Lifestyle factors, such as physical activity and diet, are known to enhance bone acquisition and have been estimated to account for 20%–40% of an adult’s peak bone mass.7 Consequently, lifestyle interventions focused on optimizing the peak bone mass have the potential to significantly reduce the incidence of osteoporosis later in life.
The concept of multi-organ communication between the gastrointestinal tract and bone health is not new.8 From early observations that patients with inflammatory bowel diseases were at risk for osteoporosis to discoveries that serotonin produced by duodenal enterochromaffin cells inhibits osteoblast proliferation and activity, our understanding of the physiological links between the gut and bone has continued to evolve.9,10 More recently, the focus has shifted to the connection between the microorganisms residing within the intestine and bone. The gut microbiota can affect intestinal barrier function, gut mucosal immune function as well as the production of secondary metabolites, all of which can have local and distal effects in tissues such as the bone.11-13 Female C57BL/6 gnotobiotic mice (ie, germ-free) exhibit high bone mass in conjunction with a decrease in CD4+ T cells, osteoclast precursors, and pro-inflammatory cytokines (ie, TNF-α and IL-6) within the bone marrow.14 Manipulation of the gut microbiota using probiotics (eg, Lactobacillus and Bifidobacterium) protects against bone loss resulting from estrogen deficiency, periodontitis, glucocorticoids, and aging.15-21 In conjunction with their effects on bone, probiotics expand Treg number and their trafficking from the gut to the bone marrow, downregulate inflammatory cytokines (eg, TNFα, IL-1β, and IL-17A), and increase short chain fatty acids (SCFAs; eg, butyrate, propionate, and acetate), resulting in improved gut barrier integrity.22,23 Moreover, the importance of Treg cells in this response was confirmed when the benefits of probiotics on osteoblasts were abrogated in mice treated with CD25 Ab to suppress their Treg population.23 The connection between probiotic-mediated gut microbial shifts and the positive effects on bone has renewed interest in dietary approaches that could be used to target the gut microbiota for preventing bone loss.
Prebiotics, which are non-digestible compounds that are metabolized by microorganisms in the gut, modulate the composition and activity of the gut microbiota, thus conferring a beneficial physiological effect on the host.24 Some examples are fructooligosaccharides (FOSs), galactooligosaccharides, inulin, and phenolic acids that have a positive effect on BMD and bone strength in animal models.25-28 Prebiotics increase SCFA production, lower luminal pH, and improve calcium and magnesium absorption.25,29 In the past, the effects of prebiotics on bone have been attributed primarily to these mechanisms; however, prebiotics also exhibit anti-inflammatory properties and increase Tregs in the gut.30-33 It is not known whether their effects on bone are mediated through a similar Treg-dependent mechanism as reported with probiotics.
Dietary sources of prebiotics are usually plant-based foods that provide one or more components with prebiotic activity. Montmorency tart cherries (TCs) (Prunus cerasus) are a good source of phenolic acids and FOS, both of which can act as prebiotics and alter the gut microbiota in young healthy adults and animal models.34-37 The bioactive components in TC also have anti-inflammatory, antioxidant, and bone protective activity.38-42 Dietary supplementation with TC prevented bone loss in a rheumatoid arthritis model and restored age-related loss of trabecular and cortical bone.41,42 Postmenopausal women consuming TC juice twice daily exhibited a decrease in the bone resorption marker, tartrate-resistant acid phosphatase type 5b.43 Phenolic acids reduce osteoclastogenesis by inhibiting the receptor activator of nuclear factor κB (RANKL) and promoting osteoblast differentiation via TGF-β signaling in both bone and gut in a Treg cell differentiation–dependent manner.42,44 However, these phenolic acids are relatively poorly absorbed, which suggests that their effects on bone cells may also be mediated via gut-derived metabolites.45,46 With growing interest in the gut microbiota as a target for osteoporosis prevention and treatment, it is important to understand how complex prebiotics, such as TC, affect bone in contrast to a simple fiber such as FOS. Thus, the purpose of this study was to investigate how dietary TC and FOS supplementation affect the bone mass and structure in young adult mice and to determine whether immune modulation of Tregs is required for this response as observed with probiotics.
Materials and methods
Animal care and diet
Eight-wk-old C57BL/6 female mice (n = 96; Taconic Biosciences, Germantown, NY) were acclimated for 2 wk at the environmentally controlled Laboratory Animal Research Facility at Oklahoma State University (OSU) before the initiation of the study. Mice (n = 16/group in 2 reps of n = 8 mice/rep) were then randomized to the following treatment groups in a 3 × 2 factorial design with diet (AIN93M control diet [Con], TC, or short chain FOS) and anti-CD25 Ab (isotype control [−CD25] or CD25 Ab [+CD25]) as factors. The TCs were purchased from Shoreline Fruits at Peterson Farms (Shelby, MI), pitted, freeze-dried, and ground into a powder so that it could be incorporated into the diet at a dose of 10% (w/w). This dose of TC was selected based on previous studies in our lab and others that showed beneficial effects on bone.41,42 The FOS was purchased from NUTRAFLORA, soluble prebiotic fiber was purchased from FB P-95 Ingredion Incorporated, Westchester, IL, and it was also supplemented at a dose of 10% w/w. Throughout the 8-wk study, mice received an intraperitoneal injection of either anti-CD25 Ab (500 μg/mice/injection) or the isotype control Ab (IgG; -CD25) (BioXcell, Lebanon, NH) twice per wk to suppress the CD25+ Treg cells. The diets were adjusted to contain similar carbohydrate, protein, fat, fiber, calcium, and phosphorus as the AIN93-M diet (Con) diet (Supplementary Table 1). Mice had free access to food and RO water throughout the study, and food intake and weekly BWs were monitored.
At the end of 8 wks of treatment, mice were fasted for 3 h, anesthetized with a ketamine/xylazine cocktail (100 mg/10 mg per kg BW), followed by whole-body DXA scans (GE Lunar PixiMus). Mice were then exsanguinated via the carotid artery, and whole blood was collected for quantification of total WBCs and differential counts or were processed for serum assays. Tissues were harvested (ie, small intestine, femurs, tibiae, and spine), cecal contents were collected, and immune cells from the ileum and bone marrow were processed for FACS analyses. All procedures adhered to the guidelines for the ethical care and treatment of animals under the IACUC at OSU.
Body composition and bone densitometry
Immediately prior to necropsy, whole-body DXA scans (GE Medical Systems Lunar, Madison, WI) were performed to determine body composition, and whole-body bone mineral area, BMC, and BMD.
Micro-CT
The tibia and fifth lumbar vertebra were scanned using X-ray micro-CT (μCT40, SCANCO Medical, Switzerland) to quantify the changes in trabecular and cortical bone microarchitecture. Tibia scans were performed at high resolution (2048 × 2048 pixels), and the proximal tibial metaphysis was analyzed by evaluating 150 slices (900 μm) within the volume of interest (VOI). Vertebral samples were analyzed by acquiring images at a resolution of 1024 × 1024 pixels and a VOI of ~170 slices (2.7 mm) between the dorsal and caudal growth plates. Trabecular bone parameters assessed at both sites included the bone volume relative to total volume (BV/TV), trabecular number (TbN), trabecular thickness (TbTh), trabecular separation (TbSp), connective density, and structural model index (SMI). The midshaft of the tibia was also evaluated, analyzing 30 slices (180 μm) within the VOI. In terms of cortical bone, porosity, cortical thickness, cortical area, and medullary area were evaluated. All analyses were performed at a threshold of 350 and a sigma and support of 1.2 and 2, respectively.
Flow cytometry
Single-cell suspensions of lymphocytes were prepared from the ileum of the small intestine, the site of some of the most intimate host–microbe–nutrient interactions, based on our previously published study.47 In short, the ileum was dissected and flushed with a mixture of RPMI, 2% FBS, and 1 mM DTT; and Peyer’s patches were removed. The tissue was cut into small pieces and incubated with HBSS with 2 mM EDTA at room temperature to remove epithelial cells, followed by a series of incubations (n = 3) with 0.20 mg/mL collagenase type VIII (Sigma-Aldrich). The cells were collected, resuspended, and filtered through a 70-μm sterile filter before lymphocytes were isolated by density–gradient centrifugation using 40% and 80% Percoll gradients. Cells at the interface of the 2 gradients were collected and washed in complete media (3×).
Bone marrow lymphocytes were harvested by flushing the femur with incomplete DMEM media. The erythrocytes and platelets were lysed using BD Bioscience lysing buffer following the manufacturers guidelines. Next, the cells were centrifuged and resuspended in 2 mL complete DMEM media with 0.5% BSA, and 10 mM EDTA, adjusted to pH 7.4.
Viable cells (2 × 106) from the ileum and bone marrow were first stained with the live/dead stain (BD Biosciences, Franklin Lakes, NJ). Cells were then stained with surface markers (CD3, CD4, CD8, and CD25). Next, the cells (1 × 107 cells/mL) were fixed and permeabilized using the mouse fixation buffer (BD Biosciences), washed, and stained for intracellular markers (FOXP3, IL-17) following the manufacturer’s instructions. Flow cytometry analyses were carried out using BD FACSAria III (BD Biosciences) at the College of Veterinary Medicine Immunopathology Core Laboratory. Data were analyzed with the FlowJo software (Version 10.8).
Fecal SCFA analyses
Fecal samples collected at the end of the study were processed in duplicates for SCFA analyses according to previously published protocol.48 To assess fecal SCFA concentration, samples were freeze-dried and then pulverized into powder. Approximately, 150 mg fecal powder was mixed with 250 μL hydrochloric acid, 45 μL internal standard (1 mM 2-ethyl butyric acid in 12% formic acid), followed by 2 extractions with 1 mL diethyl ether. An aliquot of the organic extract was transferred into glass vials for gas chromatographic analysis using Agilent 6890N GC system with a flame ionizable detector and an automatic liquid sampler (Agilent Technologies, Santa Clara, CA). Sample concentrations were determined using a 5-point calibration curve, with each standard containing the SCFAs, acetic, propionic, butyric, valeric, isovaleric, and isobutyric acids (Sigma-Aldrich).
Serum bone biomarkers
To determine systemic alterations in osteoblast and osteoclast activities induced by treatments, serum indicators of bone formation, N-terminal P1NP, and bone resorption, C-telopeptide of type I collagen (CTX-1) were assessed using commercially available EIA kits (Immunodiagnostic Systems, Inc., Fountain Hills, AZ).
RNA extraction and gene expression analysis
Total RNA was extracted from pulverized tibias (hard tissue only) and colon lamina propria specimens using Trizol (Invitrogen, Rockville, MD). The quality of RNA was confirmed using a Nanodrop Spectrophotometer (Rockland, DE) and gel electrophoresis. After DNase treatment, cDNA was synthesized and qRT-PCR was performed (CFX Opus 384 Real-Time PCR System, Bio Rad, CA) using SYBR green chemistry. Relative gene expression was determined using the 2−ΔΔCt method with target genes normalized to Gapdh for the bone tissues or Hprt for the colon.
Genes of interest in the bone tissue included regulators of osteoblastogenesis, wingless-type MMTV integration site family, member 10b (Wnt10b), runt-related transcription factor 2 (Runx2), bone morphogenetic protein 2 (Bmp2), and osterix (Osx), and indices of osteoblast activity that included bone sialoprotein (Bsp), α-1 type 1 collagen (Col1α1), osteopontin (Opn), and osteocalcin (bone gamma-carboxyglutamate protein 2 [Ocn (Bglap2)]). Gene expression for receptor activator of nuclear factor kappa-Β ligand (Rankl) and osteoprotegerin (Opg), which regulate osteoclastogenesis were also assessed. Additionally, the effects of treatments on genes expressed by osteocytes, connexin 43 (Cx43), phosphate-regulating endopeptidase x-linked (Phex), matrix extracellular phosphoglycoprotein (Mepe), sclerostin (Sost), and dentin matrix acidic phospho protein 1 (Dmp1) were assessed.
In the colon lamina propria, genes encoding for chemokines involved in T cell trafficking (C-X-C motif chemokine receptor 4 [Cxcr4], C-X-C motif chemokine ligand 12 [Cxcl12], C-X-C motif chemokine ligand 10 [Cxcl10], C-C motif chemokine receptor 7 [Ccr7], and vascular cell adhesion molecule 1 [Vcam1]), anti-inflammatory cytokines ([Il-10] and [Il-22], transforming growth factor beta [Tgf-β]), pro-inflammatory cytokines ([Il-6], [Il-17], [Tnf-α], and [Il-23]), and G protein-coupled receptors (G protein-coupled receptor 41 [Gpr41], 43 [Gpr43], and 109a [Gpr109a]) that can bind SCFA were evaluated. Primer sequences are provided in Supplementary Table 2.
Histology for osteocyte density
To assess the abundance of osteocytes, H&E staining was performed on sections of the sixth lumbar vertebra. Specimens were fixed in 10% NBF, decalcified in EDTA prior to processing, and then paraffin-embedded. Five-micrometer longitudinal sections (cephalic–caudal direction) were stained. Total osteocytes were counted within a ROI that included the primary spongiosa and corresponding cortices of the vertebral body and expressed per unit of bone surface (mm2) derived from the micro-CT scans.
Statistical analysis
The SAS software package (Version 9.4, SAS Institute Inc., Cary, NC) was used for all data analyses. Data were first assessed for outliers and normal distribution by the Shapiro–Wilks test. If the assumption for normality was not met, data were log-transformed. Continuous variables were analyzed by 2-way ANOVA using the generalized linear model with CD25 and diet as factors. If data failed to meet the criteria of normal distribution after log transformation, they were analyzed using Friedman’s test. Group comparisons were evaluated using Fischer’s least square means post hoc test when the overall P-value was significant for interactions or main effects. Data are expressed as mean ± SE, and the α was set at 0.05.
Results
Body weight, food intake, body composition, and tissue weight
Over the course of the 8-wk study, there was no effect of CD25 treatment on BW, but there was an effect of diet (Table 1). After the first wk of treatment, the FOS groups had lower body weight (P < .05) compared to the TC and Con diet groups. These differences persisted even though the FOS treated mice gained weight throughout the remainder of the study (data not shown). No differences were noted in the food intake between treatment groups (Table 1). The lower body weight with the FOS treatment occurred in conjunction with a decrease (P < .01) in fat mass and percent fat compared to the groups on the Con and TC groups (Table 1). By contrast, no alterations in lean mass were noted in response to diet, and CD25 treatment did not affect body composition.
Table 1.
Anthropometric, daily food intake, and tissue weight data.
| Con | TC | FOS | P-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| −CD25 | +CD25 | −CD25 | +CD25 | −CD25 | +CD25 | CD25 | Diet | CD25 * diet | |
| Body weights | |||||||||
| Final (g) | 26.48 ± 0.50 | 26.64 ± 0.71 | 26.39 ± 0.66 | 25.06 ± 0.54 | 23.74 ± 0.64#$ | 23.74 ± 0.58#$ | .4559 | .0002 | .4052 |
| Daily food consumption | |||||||||
| Food intake (g/d) | 3.24 ± 0.08 | 3.40 ± 0.16 | 3.40 ± 0.11 | 3.38 ± 0.14 | 3.44 ± 0.08 | 3.42 ± 0.15 | .6676 | .1332 | .9319 |
| Body composition | |||||||||
| Lean mass (g) | 17.19 ± 0.25 | 16.79 ± 0.30 | 16.68 ± 0.26 | 16.92 ± 0.26 | 17.56 ± 0.33 | 17.48 ± 0.26 | .7295 | .0562 | .4634 |
| Fat (g) | 10.09 ± 0.51 | 10.70 ± 0.69 | 10.18 ± 0.71 | 9.63 ± 0.64 | 6.60 ± 0.77#$ | 6.76 ± 0.46#$ | .7673 | <.0001 | .6312 |
| Percent fat (%) | 36.72 ± 1.30 | 38.32 ± 1.65 | 37.11 ± 1.83 | 35.78 ± 1.55 | 26.60 ± 2.34#$ | 27.57 ± 1.38#$ | .8904 | <.0001 | .6377 |
| Tissue wt | |||||||||
| WAT (mg/g) | 45.64 ± 1.66 | 49.55 ± 3.53 | 47.46 ± 2.58 | 50.71 ± 2.90 | 34.48 ± 3.39#$ | 34.77 ± 2.33#$ | .3013 | <.0001 | .8226 |
| Thymus (mg/g) | 2.75 ± 0.18 | 2.92 ± 0.14 | 3.08 ± 0.10 | 2.75 ± 0.12 | 2.75 ± 0.16 | 2.75 ± 0.10 | .6369 | .4946 | .1536 |
| Spleen (mg/g) | 3.34 ± 0.11 | 3.37 ± 0.15 | 3.41 ± 0.14 | 3.5 ± 0.15 | 3.17 ± 0.23 | 3.32 ± 0.17 | .4955 | .3067 | .8772 |
| Uterus (mg/g) | 2.74 ± 0.18 | 2.78 ± 0.23 | 2.67 ± 0.22 | 3.24 ± 0.24 | 3.22 ± 0.61 | 2.66 ± 0.23 | .3398 | .6175 | .3572 |
| Cecum (mg/g) | 2.42 ± 0.10 | 2.72 ± 0.12 | 3.19 ± 0.21# | 3.12 ± 0.24# | 5.74 ± 0.24#$ | 6.06 ± 0.25#$ | .4582 | <.0001 | .2177 |
| Cecal Content (mg) | 351.8 ± 16.6 | 315.3 ± 13.8 | 410.9 ± 18.5 | 374.6 ± 29.9 | 637.3 ± 67.7#$ | 648.3 ± 46.2#$ | .2749 | <.0001 | .2820 |
| Colon length (cm) | 5.86 ± 0.10 | 6.00 ± 0.14 | 5.94 ± 0.13 | 6.12 ± 0.07 | 6.19 ± 0.12 | 6.23 ± 0.10 | .3529 | .1076 | .1572 |
| Tibia length (mm) | 17.29 ± 0.06 | 17.11 ± 0.08 | 17.19 ± 0.08 | 17.05 ± 0.10 | 17.44 ± 0.09#$ | 17.49 ± 0.07#$ | .2357 | .0014 | .4604 |
Data presented as mean + SE. n = 15-16/group. Tissue wt expressed as mg/g of BW. Abbreviations: Control diet = Con; TC diet = TC; FOS diet = FOS; Isotype control Ab = −CD25; CD25 Ab = +CD25.
Indicates main effects of TC or FOS diets vs Con diet (P < 0.05).
Indicates differences between TC vs FOS diet groups (P < 0.05). Bolded p-values indicate statistical signficance.
Relative tissue wt revealed that there was a main diet effect on visceral adiposity (i.e., white adipose tissue or WAT) and the wt of the cecum, but not on the thymus, spleen, and uterus (Table 1). Consistent with the body composition measures, the relative wt of WAT was significantly reduced (P < .01) in FOS animals compared to the Con and TC groups (Table 1). Both sources of prebiotics, the TC and FOS, increased cecal wt (P < .01) compared to mice on the Con diet, which is expected with prebiotics (Table 1). Only FOS treatment increased the wt of cecal contents compared to the Con and TC groups. However, the colon length did not exhibit any main or interaction effects, indicating that the doses of prebiotics used did not elicit a stress response (Table 1).
Whole-body bone density and tibia length
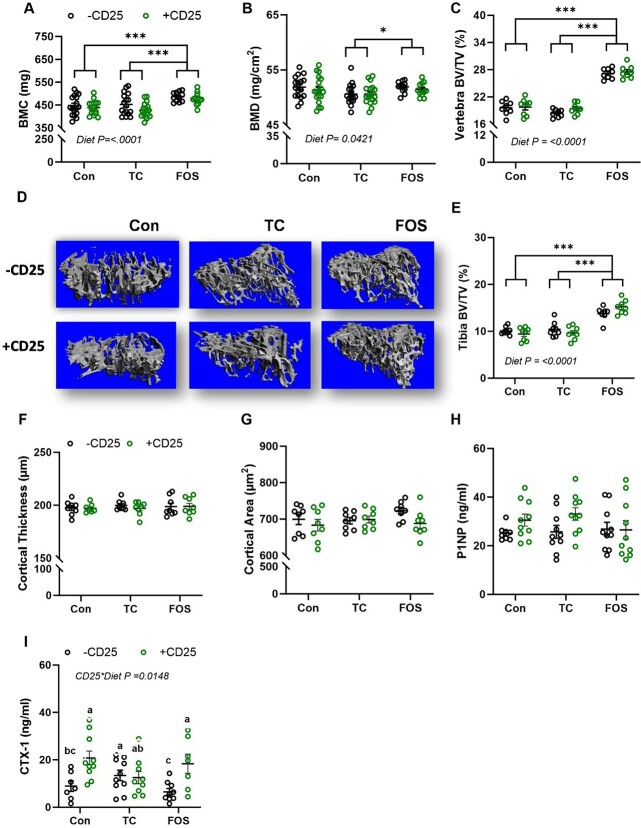
The DXA scans were assessed to determine how whole-body BMC and BMD were altered in response to diet and CD25 alone and in combination. Though there was no interaction effect of CD25 and diet treatment on whole-body BMC and BMD, a significant diet effect was observed (Figure 1A and B). The FOS diet increased whole-body BMC (P < .01) compared with the Con and TC groups (Figure 1A). Furthermore, whole-body BMD was significantly increased (P < .05) in FOS mice relative to TC animals (Figure 1B). No CD25 effect or interaction was noted on tibial length, but FOS fed mice had longer tibia (P < .01) than TC or Con animals (Table 1).
Figure 1.
Bone mass, structural alterations, and biomarkers in mice consuming control diet (Con), TC, or FOS with (+CD25; green) or without CD25 (−CD25; black) Ab: whole-body (A) BMC, (B) BMD, (C) lumbar vertebra trabecular bone volume relative total volume (BV/TV), (D) representative 3D images of the proximal tibia, (E) proximal tibia BV/TV, (F) tibia midshaft cortical thickness, (G) cortical area, (H) serum P1NP, and (I) CTX-1. Data presented as mean + SE. P-values < .05 are statistically different and P-values > .05 are not shown. *** indicates a main effect P < 0.0001, ** indicates a main effect P < 0.01, and * indicates a main effect P < 0.05. Superscript letters show differences between groups with a significant CD25 * diet interaction.
Trabecular and cortical bone microarchitecture
We used micro-CT imaging to evaluate the changes in structural parameters of trabecular and cortical bone of the tibia and lumbar vertebra after 8 wk of CD25 and dietary treatments. There was no interaction or main effect of CD25 Ab on tibial or vertebral trabecular BV, but a main diet effect was observed on trabecular BV/TV at both sites. Within the lumbar vertebral body (Figure 1C) and proximal tibial metaphysis (Figure 1E), trabecular BV/TV was increased (P < .01) in the FOS group compared to the Con and TC groups. Although the FOS-treated groups gained wt throughout the study, their final BW was less than the mice on the Con and TC diets. Therefore, we investigated whether or not there was an influence of BW on the trabecular bone response to diet. The effect of diet on BV/TV in the tibia and vertebra was not altered when the data were expressed per unit of BW (data not shown). Representative 3D images of trabecular bone in the proximal tibia (Figure 1D) and lumbar vertebra (Supplementary Figure 1A) are shown. The increase in trabecular BV/TV with FOS treatment occurred in conjunction with an increase in TbN and TbTh (P < .01) and a decrease in TbSp (P < .01) in the tibia and vertebra compared to the Con and TC groups (Supplementary Table 3). Dietary supplementation with TC did not improve any indices in the tibia in this study, but a decrease in vertebral Tb. Th (P < .01) was noted compared to mice on the Con diet.
Additionally, no interactions of CD25 and diet or main effects of CD25 on trabecular bone SMI and connectivity density occurred; however, there was a main diet effect on these parameters (Supplementary Table 3). Trabecular connectivity density was increased (P < .01) in the FOS-treated group compared to the Con and TC group after 8 wk. There was a decrease (P < .01) in the SMI in both spine and tibia (Supplementary Table 4) with FOS supplementation compared to Con or TC. In contrast, SMI was significantly increased with TC compared to the Con group. SMI provides an insight into the orientation of trabecular struts to understand whether the effect of treatment on bone results in a more rod-like or plate-like structure. FOS supplementation improved the orientation of the trabecular struts to become more plate-like, whereas in this study, the TC treatment resulted in a more rod-like structure. The CD25 Ab treatment did not affect the SMI or connectivity density at either skeletal site.
Cortical bone was evaluated at the tibia mid-diaphysis and representative images are shown (Supplementary Figure 1B). No interactions or main effects of diet and CD25 were observed in cortical thickness and cortical area (Figure 1F and G). However, when the data were expressed per unit of BW, the FOS diet significantly increased both of these cortical bone parameters (data not shown). A main effect of diet was noted on the medullary area with FOS treatment increasing the medullary area (P < .01) compared to the Con and TC diet groups (Supplementary Table 3). No changes in cortical porosity were noted.
Serum bone biomarkers
To assess the systemic biomarkers of bone formation and bone resorption in response to diet and CD25 Ab treatment, P1NP and CTX-1 were evaluated. There was no interaction or main effect of CD25 or diet on the serum bone formation marker, P1NP (Figure 1H). There was a significant interaction (P < .05) on serum CTX-1 with +CD25 increasing CTX-1 in the Con- and FOS-treated groups, but not in the TC group (Figure 1I). Surprisingly, the mice fed the TC diet without CD25 exhibited an increase in CTX-1 (P < .05) compared to mice receiving the –CD25 Ab on the Con and FOS diets.
T lymphocytes in ileum and bone marrow
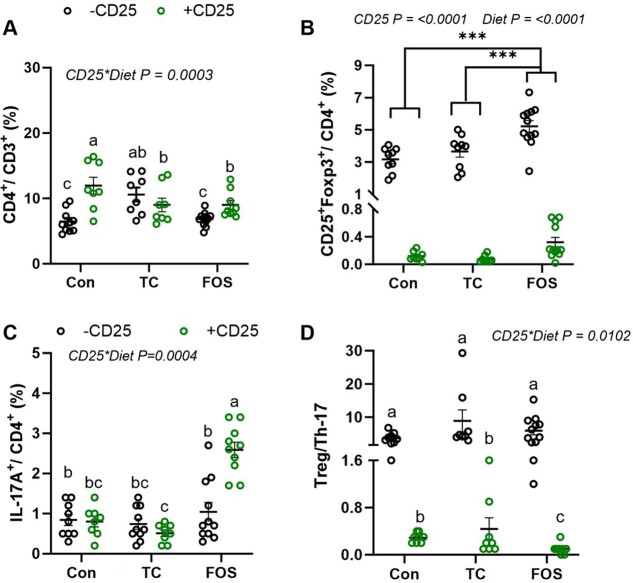
Flow cytometry analysis, performed on lymphocytes from the ileum, revealed significant alterations in the relative abundance of Treg and Th17 cells as well as their absolute counts in response to CD25 and diet. The CD25 treatment increased the percentage of CD3+CD4+ cells in the groups fed with the Con- or FOS-supplemented diets, but not in the mice receiving the TC diet (Figure 2A). Interestingly, the mice receiving the TC diet exhibited a higher percentage of CD4+CD3+ cells, irrespective of CD25 treatment (Figure 2A), but there were no differences in the absolute CD4+CD3+ counts between groups (Supplementary Table 4). The relative abundance of CD4+ CD25+ Foxp3+ Treg cells was suppressed with the CD25 Ab as expected in the lamina propria of the ileum (Figure 2B). Furthermore, a main effect of diet was observed with the FOS diet increasing this Treg population, but not the TC diet compared to the Con (Figure 2B). By contrast, there were no differences in the absolute number Tregs in the FOS compared to the Con group, but the mice consuming the TC (−CD25) had a ~1.9-fold higher number of Tregs compared to the Con diet (Supplementary Table 4). To evaluate how pro-inflammatory Th-17 cells responded to treatments, we assessed the CD4+IL-17A+, Th17 population. Interestingly, FOS increased the percentage of Th17 (P < .01) in the presence of CD25 compared to the Con and TC groups (Figure 2C) as well as the absolute Th17 cell count (Supplementary Table 4). As a result of these alterations in the Treg and Th17 cells, we observed a CD25 × diet interaction with the mice fed the FOS diet and receiving +CD25 Ab, exhibiting the greatest reduction in the Treg:Th17 ratio (Figure 2D).
Figure 2.
The relative abundance of T lymphocytes using FACS in the lamina propria of the ileum after 8 wk of control (con), TC, or FOS diets with (+CD25; green) or without (−CD25; black) CD25 Ab. The percentage of (A) CD4+cells, (B) CD25+Foxp3+ Tregs, (C) Th-17+ cells, and the (D) ratio of Treg to Th-17 cells are shown. Data presented as mean + SE. P-values < .05 are statistically significant and P-values > .05 are not shown. *** indicates main effect P < 0.0001, ** indicates main effect P < 0.01, and * indicates main effect P < 0.05. Superscript letters show differences between groups with a significant CD25 * diet interaction. Groups that do not have the same superscript letter are statistically different from each other (P < 0.05).
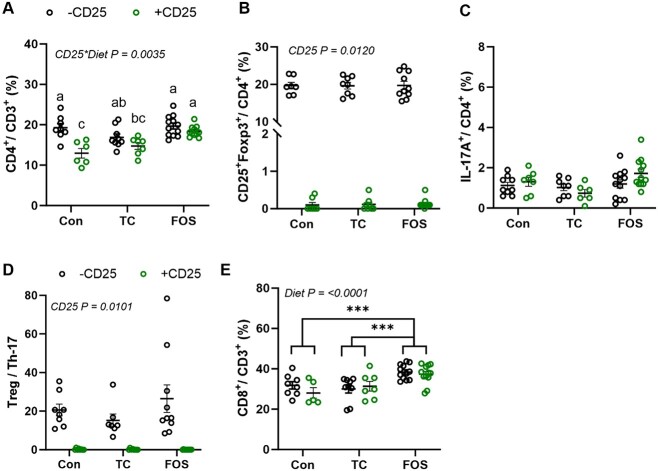
In the bone marrow, the FOS and TC diets in contrast to the Con diet prevented the decrease in the percentage of CD4+ T cells that occurred with CD25 treatment (Figure 3A). Administration of the CD25 Ab effectively reduced the percentage of CD4+ CD25+ Foxp3+ Tregs (P < .05) (Figure 3B) and the absolute counts in the bone marrow (Supplementary Table 4) as expected. Similar to the response observed in the ileum, mice fed the FOS diet without CD25 (−CD25) exhibited an increase in Tregs (~3-fold) (Supplementary Table 4). Neither a main effect of diet nor an interaction was observed in the percentage of CD4+IL-17A+ Th17 population (Figure 3C), but the FOS diet increased the absolute Th17 cell counts (P < .0001). As a result of the FOS-induced increase in both the Treg and Th17 populations, there were no diet effects on the Treg:Th17 ratio and this ratio was only altered (P < .05) by the CD25 treatment (Figure 3D). Due to previous reports23 that the effects of Tregs on Wnt10b were mediated via CD8+ T cells, we examined this population of T cells within the bone marrow. No main effect of CD25 or CD25 × diet effect was noted on the percentage of CD3+CD8+ T cells, but a main effect of FOS supplementation increasing (P < .01) the abundance of this population occurred compared to Con and TC groups (Figure 3E).
Figure 3.
The FACS analysis of bone marrow T lymphocytes after 8 wk of control (Con), TC or FOS diets with (+CD25; green) or without (−CD25; black) CD 25 Ab. The percentage of (A) CD4+cells, (B) CD25+Foxp3+ Tregs, (C) Th-17+ cells, the (D) ratio of Treg to Th-17 cells, and (E) CD8+ cells are shown. Data presented as mean + SE. P-values < .05 are statistically significant and P-values > .05 are not shown. *** indicates a main effect P < 0.0001, ** indicates a main effect P < 0.01, and * indicates a main effect P < 0.05. Superscript letters show differences between groups with a significant CD25 * diet interaction. Groups that do not have the same superscript letter are statistically different from each other (P < 0.05).
Fecal SCFA analysis
The SCFA analysis of the fecal samples revealed main effects of diet, but no effect of CD25 or an interaction (Table 2). The FOS fed mice had higher fecal concentrations of acetic, propionic, and n-butyric acid (P < .01) and a lower fecal concentration of i-butyric acid (P < .01) compared to the mice fed the Con and TC diets (Table 2). The TC also increased acetic, propionic, i-butyric, and n-butyric acid concentrations compared to the Con diet, but to a lesser degree than FOS supplementation. Only the TC fed mice had increased (P < .01) fecal i-valeric acid (Table 2). An interaction was observed in the total SCFA response. The FOS diet significantly increased the total amount of SCFAs, but interestingly, the CD25 Ab suppressed this response in contrast to Con and TC groups (Table 2). This interaction appeared to be driven by the influence of FOS + CD25 on acetic acid, which is the most abundant SCFA.
Table 2.
Fecal short chain fatty acids.
| Con | TC | FOS | P-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| −CD25 | +CD25 | −CD25 | +CD25 | −CD25 | +CD25 | CD25 | Diet | CD25 * diet | |
| Individual fecal SCFA | |||||||||
| Acetic (μmol/g) | 5.93 ± 0.43 | 6.22 ± 0.76 | 8.70 ± 0.33# | 7.67 ± 0.44# | 33.96 ± 4.55#$ | 24.35 ± 3.34#$ | .0813 | <.0001 | .0779 |
| Propionic (μmol/g) | 0.66 ± 0.07 | 0.70 ± 0.08 | 1.00 ± 0.04# | 0.88 ± 0.04# | 5.41 ± 1.24#$ | 3.21 ± 0.50#$ | .3098 | <.0001 | .2867 |
| i-Butyric (μmol/g) | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.12 ± 0.01# | 0.13 ± 0.01# | 0.01 ± 0.01#$ | 0.0 ± 0.0#$ | .1285 | <.0001 | .3425 |
| n-Butyric (μmol/g) | 0.19 ± 0.01 | 0.28 ± 0.06 | 0.51 ± 0.05# | 0.57 ± 0.39# | 5.54 ± 1.61#$ | 4.19 ± 0.88#$ | .3360 | <.0001 | .0679 |
| i-Valeric (μmol/g) | 0.09 ± 0.02 | 0.10 ± 0.02 | 0.16 ± 0.01 | 0.15 ± 0.10 | 0.08 ± 0.03$ | 0.04 ± 0.02$ | .3481 | <.0001 | .4410 |
| n-Valeric (μmol/g) | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.11 ± 0.07 | 0.11 ± 0.01 | 0.13 ± 0.06 | 0.12 ± 0.04 | .3555 | .0805 | .3758 |
| Total SCFA (μmol/g) | 7.00 ± 0.54c | 7.46 ± 0.91c | 8.41 ± 1.45c | 9.36 ± 0.50c | 45.10 ± 6.74a | 34.76 ± 4.03b | .1054 | <.0001 | .0414 |
Data presented as mean + SE. n = 8/group. Abbreviations: Control diet = Con; TC diet = TC; FOS diet = FOS. Isotype control Ab = −CD25; CD25 Ab = +CD25.
Superscript letters note significant interactions (CD25 * diet) and groups that share the same superscript letter are not significantly different from each other. When only main effects were detected for diet, #indicates differences vs Con (P < 0.05). $indicates differences between the TC vs FOS diet groups (P < 0.05). Bolded p-values indicate statistical signficance.
Relative gene expression of key indicators of inflammation and other mediators in gut lamina propria
To further explore the gut–bone connection, we assessed the transcriptional changes in cytokines involved with Th-17 and Treg cell differentiation and activity as well as molecules that regulate T cell trafficking in the lamina propria of the colon. FOS downregulated the relative abundance of pro-inflammatory Il17 (P < .05) and Il23 (P < .01), but it did not alter the Tnfα and Il6 (Table 3). We also assessed the relative abundance of genes encoding for chemokines and adhesion molecules involved in T cell trafficking. We observed a decrease in the ligand, Cxcl12 (P < .01) and its receptor, Cxcr4 (P < .01), as well as the adhesion molecule Vcam1 (P < .01) with FOS treatment, but we observed no changes with Ccr7 and Cxcl10 (Table 3), which is consistent with no sign of inflammation within the colon. Il22, a member of the IL-10 superfamily of cytokines that is involved in the protection of intestinal barrier,49 was upregulated in mice receiving CD25 Ab (Table 3). No changes were observed in anti-inflammatory cytokines Tgfβ and Il10 in response to diet or CD25 treatment. These findings indicate that FOS suppressed the gene expression of inflammatory molecules within the colon.
Table 3.
Colon lamina propria gene expression data.
| Con | TC | FOS | P-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| −CD25 | +CD25 | −CD25 | +CD25 | −CD25 | +CD25 | CD25 | Diet | CD25 * diet | |
| Pro-inflammatory cytokines | |||||||||
| Il17 | 1.00 ± 0.59 | 1.23 ± 0.29 | 0.91 ± 0.24 | 0.62 ± 0.24 | 0.24 ± 0.05#$ | 0.22 ± 0.11#$ | .9217 | .0374 | .6835 |
| Il23 | 1.00 ± 0.19 | 0.89 ± 0.11 | 0.94 ± 0.07 | 1.07 ± 0.20 | 0.26 ± 0.02#$ | 0.58 ± 0.12#$ | .3418 | .0005 | .3192 |
| Tnf-α | 1.00 ± 0.14 | 1.25 ± 0.28 | 0.77 ± 0.16 | 0.88 ± 0.11 | 1.01 ± 0.14 | 1.46 ± 0.39 | .1541 | .1031 | .9370 |
| Il6 | 1.00 ± 0.30 | 1.03 ± 0.26 | 0.93 ± 0.29 | 0.96 ± 0.19 | 0.41 ± 0.12 | 0.80 ± 0.45 | .4196 | .0990 | .9767 |
| Chemokines and adhesion molecule | |||||||||
| Cxcr4 | 1.00 ± 0.16 | 0.87 ± 0.24 | 1.01 ± 0.22 | 0.97 ± 0.22 | 0.53 ± 0.09#$ | 0.39 ± 0.11#$ | .3497 | .0176 | .8498 |
| Cxcl12 | 1.00 ± 0.59 | 0.97 ± 0.20 | 1.22 ± 0.32 | 1.18 ± 0.29 | 0.14 ± 0.03#$ | 0.19 ± 0.09#$ | .9366 | <.0001 | .9052 |
| Vcam1 | 1.00 ± 0.59 | 0.70 ± 0.18 | 1.05 ± 0.29 | 0.62 ± 0.14 | 0.24 ± 0.05#$ | 0.22 ± 0.11#$ | .1178 | .0002 | .7206 |
| Ccr7 | 1.00 ± 0.15 | 0.90 ± 0.22 | 1.09 ± 0.22 | 0.99 ± 0.24 | 0.99 ± 0.27 | 0.93 ± 0.33 | .6685 | .9183 | .9972 |
| Cxcl10 | 1.00 ± 0.20 | 1.09 ± 0.38 | 1.05 ± 0.29 | 0.90 ± 0.11 | 0.73 ± 0.14 | 0.72 ± 0.29 | .7313 | .7313 | .8867 |
| Anti-inflammatory cytokines | |||||||||
| Tgfb | 1.00 ± 0.20 | 0.48 ± 0.29 | 0.86 ± 0.30 | 0.63 ± 0.18 | 0.80 ± 0.32 | 1.27 ± 0.67 | .4797 | .8130 | .4903 |
| Il10 | 1.00 ± 0.36 | 0.48 ± 0.08 | 0.86 ± 0.18 | 0.63 ± 0.11 | 0.80 ± 0.15 | 1.14 ± 0.37 | .1366 | .1366 | .1397 |
| Il22 | 1.00 ± 0.40 | 1.46 ± 0.83 | 0.89 ± 0.50 | 2.45 ± 1.05 | 0.36 ± 0.03 | 6.27 ± 3.21 | .0004 | .5710 | .0512 |
| Receptor for SCFA | |||||||||
| Gpr109a | 1.00 ± 0.24# | 0.63 ± 0.05bc | 0.77 ± 0.12ab | 0.81 ± 0.07ab | 0.40 ± 0.04c | 0.47 ± 0.10c | .2382 | .0003 | .0425 |
| Gpr41 | 1.00 ± 0.09 | 1.35 ± 0.15 | 1.45 ± 0.17 | 1.23 ± 0.16 | 1.37 ± 0.16 | 1.20 ± 0.28 | .9037 | .6134 | .1967 |
| Gpr43 | 1.00 ± 0.11 | 1.12 ± 0.19 | 1.11 ± 0.19 | 0.97 ± 0.11 | 1.00 ± 0.17 | 0.87 ± 0.22 | .7101 | .7324 | .6611 |
Data presented as mean + SE. n = 6/group. Abbreviations: Control diet = Con; TC diet = TC; FOS diet = FOS; Isotype control Ab = - CD25; CD25 Ab = +CD25.
Superscript letters note significant interactions (CD25 * diet) and groups that share the same superscript letter are not significantly different from each other. When only main effects were detected for diet, #indicates differences vs the Con diet (P < 0.05). $indicates differences between the TC vs FOS diet groups (P < 0.05). Bolded p-values indicate statistical signficance.
Additionally, we investigated the gene expression of G-protein-coupled receptors that are known to interact with some of the SCFAs to explore the relationship between the bone mass and structural changes and the SCFAs. The Grp109a (P < .05) gene, which is expressed by epithelial and dendritic cells within the colon, was suppressed by CD25 in the mice on the Con diet. However, the mice consuming the TC- and FOS-supplemented diets did not exhibit a decrease in the expression of Grp109a with CD25 (Table 3). No alterations were noted in the expression of Grp41 and Grp43 genes in response to either CD25 or diet.
Relative gene expression in bone
As improvements in bone tissue were evident in the FOS, but not the TC treated mice, we focused our investigation of the alterations in gene expression in bone tissue (i.e., bone marrow removed) on the FOS and Con diet groups with and without CD25. First, we assessed the regulators of osteoblast differentiation. We observed a significant decrease in the osteoblast differentiation genes, Wnt10b (P < .01) and Bmp2 (P < .05), with +CD25 treatment (Figure 4A and B). Previously, blocking T-reg cells with CD25 Ab was shown to suppress Wnt10b expression in pre-osteoblasts.23 In the current study, FOS increased Wnt10b, Bmp2 (P < .01), and Osx (P < .01) expressions, which promote the differentiation of mesenchymal stem cells to osteoblasts (Figure 4B and C). FOS also increased the relative abundance of Col1α1 (P < .01) indicative of increased osteoblast activity (Figure 4D). No significant effects of CD25 or diet were noted on the gene expression of Bsp, Opn, or Bglap2 (Supplementary Table 5). These findings suggest that FOS upregulates osteogenesis.
Figure 4.
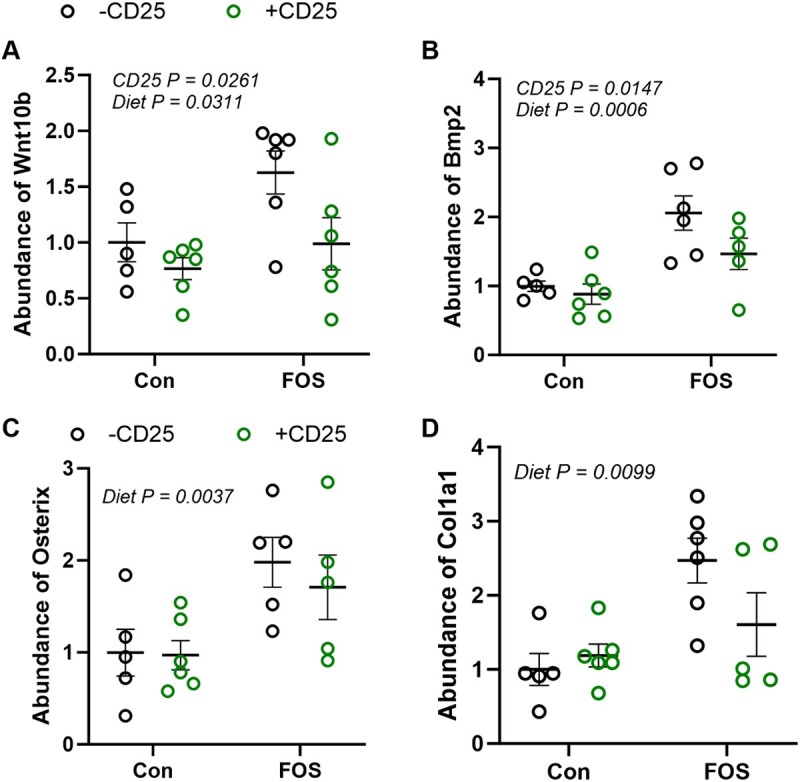
Supplementation with FOS compared to control (Con) diet with (CD + 25; green) or without (−CD25; black) CD25 Ab alters the abundance of mRNA of genes involved in osteoblast differentiation (A) Wnt10b, (B) Bmp2, (C) Osx, and (D) indicator of osteoblast activity Col1a1 over the course of the 8-wkstudy. Data presented as mean + SE. P-values < .05 are considered to be statistically significant. No interactions were observed and P-values for statistically significant main effects are shown.
The RANK–RANKL signaling is an important regulator of osteoclastogenesis and osteolysis. Osteocytes express RANKL and OPG. No alteration was noted in Rankl, Opg, or the Rankl:Opg ratio in response to diet or CD25 alone or their combination in the hard tissue (Supplementary Table 5). This lack of changes suggests that, in young adult animals, osteolysis is not affected by FOS or the abundance of Tregs.
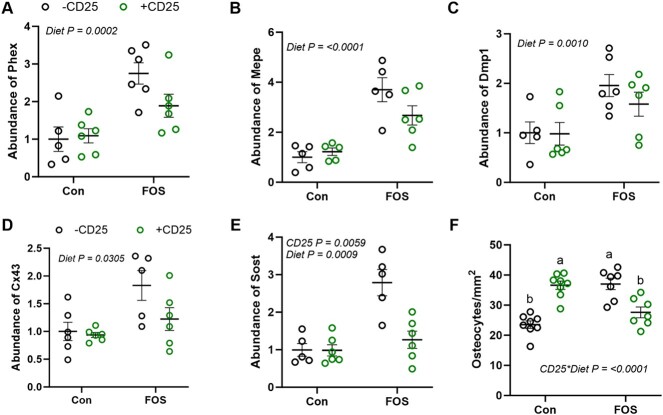
Osteocytes are the most abundant cells within the hard tissue of bone and are responsible for mechanosensing, coordinating the activity of osteoclasts and osteoblasts as well as bone mineralization. Among the highly expressed genes of osteocytes, Phex, Dmp-1, and Mepe play an important role in bone mineralization and mineral homeostasis.50 Although no interaction of diet and CD25 was noted, a main diet effect was observed on these genes. FOS treatment significantly upregulated Phex (P < .01), Mepe (P < .01), and Dmp1 (P < .01) (Figure 5A–C). We also evaluated the expression of the osteocyte gap junction protein, Cx43, which is important for osteocyte communication, cell survival, and the maintenance of bone homeostasis. FOS upregulated Cx43 mRNA (P < .05) compared to Con (Figure 5D). Surprisingly, FOS also increased the expression of the Wnt signaling pathway inhibitor, Sost (P < .01). A main CD25 Ab effect was also observed on Sost gene expression. In the presence of CD25, Sost expression (P < 0.01) was downregulated (Figure 5E). These findings highlight the effects of FOS dietary supplementation on bone are likely mediated through the osteocytes.
Figure 5.
Eight weeks of dietary supplementation with FOS compared to control (Con) diet with (CD +25; green) or without (−CD25; black) the CD25 Ab alters the abundance of genes expressed by osteocytes: (A) Phex, (B) Mepe, (C) Dmp1, (D) Cx43, (E) Sost, and (F) osteocyte number. Data presented as mean + SE. P-values < .05 are considered to be statistically significant. No interactions were observed and P-values for statistically significant main effects are shown.
In attempt to explore factors that could be driving the bone response to FOS, we assessed Gpr109a expression in bone. Only trends were observed with Gpr109a expression. The CD25 tended to suppress (P = .073) and FOS treatment tended to increase (P = .066) the relative abundance of Gpr109a mRNA (data not shown).
Osteocyte density
Due to the unexpected increase in the relative abundance of Sost with FOS in conjunction with the gene expression of Wnt10b, Phex, Dmp1, and Mepe, and improved bone structure, we asked whether the number of osteocytes was altered by the diet. A significant interaction (P < .0001) was observed with FOS and CD25 treatments. The FOS diet and CD25 Ab independently increased the number of osteocytes expressed per unit of bone surface by ~1.6-fold within the vertebral body, but this response was attenuated when the FOS + CD25 were combined (Figure 5F).
Discussion
This study aimed to investigate how 2 different prebiotics, TC, a good source of phenolic acids and FOS, as well as FOS as a single-agent prebiotic, affect bone in the young adult C57BL/6 mouse. Furthermore, we determined whether Tregs were required for the skeletal response. Our findings showed that supplementing the diet with 10% FOS for 8 wk increased whole-body BMC and trabecular bone within the tibial proximal metaphysis and lumbar vertebral body. When these bone parameters were expressed relative to BW, these findings were not altered; however, cortical thickness and area were significantly improved with FOS treatment when the data were expressed relative to BW. By contrast, TC consumption did not improve the trabecular or cortical bone phenotype in a similar manner as our lab and others have reported in previous studies.41,42 Suppression of the CD25+Foxp3+ Treg population using a CD25 Ab did not alter the bone response to the prebiotics demonstrating that the skeletal response to FOS was not Treg-dependent.
Clinical and pre-clinical studies support the benefits of FOS-containing products on bone and mineral metabolism. In adolescent girls51 and healthy adult men,52 FOS supplementation improved the intestinal mineral absorption. Postmenopausal women supplemented with FOS exhibited an increase in calcium uptake53 along with a decrease in the serum bone resorption marker, c-terminal telopeptide of type I collagen.53 Generally, these effects on intestinal calcium uptake have been linked to increases in SCFAs. In addition to promoting increased mineral absorption, supplementation with FOS has been demonstrated to enhance the bone mass in male rats and ovariectomized rats.25,54 Ohta et al.55 demonstrated that 5% FOS prevented bone loss due to sex hormone deficiency and increased cecal beta-glucosidase activity, which facilitates the hydrolysis of glycosides and oligosaccharides. In the current study, FOS supplementation improved trabecular bone in the proximal tibia by 30% and in the vertebral body by 40% compared to the groups consuming the Con diet. However, the dried TC powder utilized in this study did not significantly increase the bone mass or improve the trabecular and cortical bone microarchitecture, and it only induced a modest increase in SCFA. It should be noted that all of the earlier animal studies in our lab utilized a TC powder from the same source over several different harvests, but that source was not available for this study. Despite the similarities in macronutrient, fiber, and total phenolic content of the product used here and the product used in our previous studies (data not shown), the tempered increase in SCFAs that was observed was not enough to yield a positive effect of TC on bone.
In recent studies, supplementing the diets of dextran sodium sulfate–induced colitis mice models with prebiotics, such as galactooligosaccharides, and short-chain and long-chain inulin type fructans was found to increase Treg cells in gut-associated lymphoid tissues.33,56,57 Likewise, in clinical trials, FOS supplementation increased the abundance of dendritic cells expressing IL-10 in biopsy samples from patients with Crohn’s disease. The IL-10 is known to play a crucial role in the proliferation and survival of Treg cells, and this response was attributed to the promotion of beneficial gut bacteria (ie, Bifidobacteria).58,59 Probiotics have been reported to modulate the bone cellular activity via the effects of the SCFA, butyrate, on Treg cells.16,23,60 In particular, Tyagi and colleagues23 reported that young female mice receiving Lactobacillus rhamnosus GG exhibited an increase in the bone marrow Tregs that stimulated CD8+ T cell secretion of the Wnt ligand, Wnt10b, thus, promoting bone formation and mineralization. Moreover, this effect of probiotics on bone was blocked when Tregs were suppressed by treating mice with a CD25 Ab. Similar to probiotics, prebiotics, such as wheat-derived arabinoxylan, pectin, and lactulose, have the capacity to expand the Treg population.31,61,62 Only the oligosaccharide, lactulose, has been shown to upregulate Tregs in the small intestine and decrease TNF-α, IL-6, and RANKL in the bone marrow of ovariectomized mice.31 Others have maintained that Tregs can increase the osteoblast activity by secreting the anti-inflammatory cytokine, IL-10, or activating the TGF-β mediated Smad pathway and Wnt signaling.23,63 Our flow cytometry data showed that FOS, but not TC, increased the percentage of Tregs in the ileum and the absolute number of Tregs in the bone marrow. However, suppression of Tregs utilizing the CD25 Ab did not attenuate the improvements in whole-body BMC and trabecular BV/TV with FOS treatment. These findings suggest that the mechanism through which FOS affects bone in the young adult female mouse differs from that reported with probiotics.23
Another possible mechanism in which FOS could favorably affect bone is by upregulating the production of gut-derived metabolites such as the SCFAs or their receptors. Earlier studies demonstrated that FOS increased the bone density coincident with enhancing the absorption of calcium and magnesium.25,64,65 Improvements in intestinal mineral absorption were linked to a reduction in pH elicited by the increase in SCFA production. Later studies revealed that FOS can also increase SCFA-producing Bifidobacteria, which provide an osteogenic effect.54 Ovariectomized rats dosed with FOS (1.85 g/kg/d) for 12 wk displayed increased trabecular BV/TV, trabecular bone mineral apposition and bone formation rates, and SCFA.54 However, no alterations in bone resorption or calcium absorption were observed. Likewise, in our study, the positive effects of FOS on trabecular bone occurred in conjunction with SCFAs, namely acetate, propionate, and n-butyrate. The wt of the cecum in FOS-treated animals was also increased, which has been linked to increased microbial fermentation activity.66,67 Butyrate and propionate have been reported to shift pre-osteoclast metabolism during their differentiation toward glycolysis, which induces cell stress and prevents osteoclast differentiation.68 These effects were independent of GPR41 and GPR43. Another G-protein-coupled protein receptor, GPR109A, that is expressed in bone tissue, binds to nicotinic acid as well as metabolites such as hippuric acid and butyrate.69,70 In global GPR109-/- mice, the expression of bone resorption markers (ie, Cathepsin K) was reduced and β-catenin was upregulated.69 In our study, we only observed a trend in the upregulation of the Gpr109a gene expression in the bones of our young naive mice with FOS. However, serum CTX-1 and local expressions of Rankl and Opg in bone were not altered by FOS. Whether or not the effects of FOS on bone are mediated by GPR109A signaling warrants further investigation.
Despite the FOS-induced increase in bone mass and trabecular structural properties in conjunction with the up-regulation of Wnt10b, the relative abundance of Sost was also increased. The Sost gene is expressed by osteocytes and encodes for sclerostin, which can inhibit Wnt signaling as a means of regulating osteoblast activity.71 Our findings demonstrate that FOS also upregulated the gene expression of Bmp2 and Osx, which are involved in the differentiation of mesenchymal stem cells. Bmp2 is a member of TGF-β superfamily of proteins that stimulates Runx2. Osx is a downstream target of the Runx2, and it is required for osteoblast formation. Supplementation of the diet with FOS also increased the expression of Col1α1, which is consistent with previous reports of increased osteoblastic differentiation and activity in vitro model and in a zebra fish model.72,73 Other genes expressed by osteocytes, Phex, Dmp-1, Mepe, and Cx-43 were also upregulated in the bone tissue with FOS. The endopeptidase enzyme, Phex, is released by mature osteoblasts or early osteocytes, and it modulates phosphate homeostasis and bone mineralization. Phex represses FGF (FGF23) and prevents the increase in urinary phosphate excretion and maintains 1,25(OH2)D.74 Phex can also directly promote bone formation by regulating osteopontin and bone sialoprotein protein, which are involved in bone mineralization. In this study, we did not observe any transcriptional changes in Opn and Bsp. Our data did reveal an increase in the gene expression of Mepe and Dmp-1 with FOS treatment. Mepe is considered as an inhibitor of the bone crystal formation; however, Phex can complex with MEPE and repress this repressor.75 These alterations in Phex and Mepe in conjunction with the increase in Dmp-1, a regulator of hydroxyapatite nucleation,76 may represent an attempt on the part of the osteocyte to balance bone mineralization. Interestingly, we also observed a FOS-induced increase in Cx43. The CX43 is a gap junction expressed by osteocytes that promotes cell-to-cell communication and cell survival.77 Overexpression of Cx43 prevents age-induced cortical bone loss in animal model,78 whereas deletion or truncation of Cx43 exacerbated cortical and trabecular bone loss by preventing osteocyte apoptosis and osteoclast recruitment.79,80 To examine whether FOS treatment has implications on the density of osteocytes, we quantified the number of osteocytes per unit of bone surface area in the lumbar vertebra. Our data revealed that FOS increased the number and density of osteocytes. However, Con mice treated with CD25 also exhibited an increase in osteocyte density without a corresponding increase in osteocyte gene expression or improvements in bone mass and structural properties. While these data offer some insight into the increase in gene expression with FOS, the implications on bone quality and other functions of the osteocyte such as mechanosensing remain to be answered.
Based on these findings, we can conclude that the prebiotic, FOS, enhances bone mass in young female adult mice; however, Treg cells were not required for this response as has been reported with probiotics.23 Although SCFA likely play a role in the skeletal phenotype with FOS, it is not clear whether they directly affect bone cells or their influence is mediated through indirect mechanisms such as immune cells other than Tregs, calcium homeostasis, or metabolites. Further investigation into their mechanisms of action and the potential role of Gpr109a, especially as it relates to the terminal differentiation of osteoblasts into osteocytes and their survival, is warranted. The TC product utilized in the present study shared similarities with the test product used in prior studies (i.e., macronutrient, total fiber, and total phenolic content) and had positive effects on Th17 cells and fecal SCFAs to some degree, but it did not benefit the bone. Insights may be gleaned regarding the fruit’s bioactive component(s) and mechanism of action with additional metabolomics analyses on samples from this study compared to prior studies that are currently underway. Our data do suggest that supplementing the diet with FOS may improve bone acquisition and peak bone mass, but future studies are needed to determine its effects on the biomechanical properties of bone and whether FOS conveys these benefits in males as well as in the case of aging.
Supplementary Material
Acknowledgments
B.J.S., E.A.L., A.N.F.V., and J.R. conceptualized and acquired funding for the project. P.I., J.A.I., S.E.A, P.A., B.H., J.R., J.A.I., and K.R. participated in formal analysis and data acquisition. P.I., B.J.S., and E.A.L. drafted the manuscript that was reviewed and edited by other authors listed. B.J.S., E.A.L., and S.L.C. were responsible for project administration. The graphic abstract was created with BioRender.com.
Contributor Information
Proapa Islam, Nutritional Sciences Department, Oklahoma State University, Stillwater, OK 74078, USA.
John A Ice, Nutritional Sciences Department, Oklahoma State University, Stillwater, OK 74078, USA.
Sanmi E Alake, Nutritional Sciences Department, Oklahoma State University, Stillwater, OK 74078, USA.
Pelumi Adedigba, Indiana Center for Musculoskeletal Health, Indiana School of Medicine, Indianapolis, IN 46202, USA.
Bethany Hatter, Nutritional Sciences Department, Oklahoma State University, Stillwater, OK 74078, USA.
Kara Robinson, Nutritional Sciences Department, Oklahoma State University, Stillwater, OK 74078, USA.
Stephen L Clarke, Nutritional Sciences Department, Oklahoma State University, Stillwater, OK 74078, USA.
Ashlee N Ford Versypt, Department of Chemical and Biological Engineering, University at Buffalo, Buffalo, NY 14260, USA.
Jerry Ritchey, Veterinary Pathobiology Department, Oklahoma State University, Stillwater, OK 74078, USA.
Edralin A Lucas, Nutritional Sciences Department, Oklahoma State University, Stillwater, OK 74078, USA.
Brenda J Smith, Indiana Center for Musculoskeletal Health, Indiana School of Medicine, Indianapolis, IN 46202, USA; Department of Obstetrics and Gynecology, Indiana School of Medicine, Indianapolis, IN 46202, USA.
Author contributions
Proapa Islam (Data curation, Formal analysis, Investigation, Writing – original draft), John Ice (Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Writing – review & editing), Sanmi Alake (Data curation, Formal analysis, Investigation, Writing – review & editing), Pelumi Adedigba (Data curation, Formal analysis, Investigation, Writing – review & editing), Bethany Hatter (Data curation, Formal analysis, Writing – review & editing), Kara Robinson (Data curation, Formal analysis, Writing – review & editing), Stephen Clarke (Project administration), Ashlee Ford Versypt (Investigation, Methodology, Writing – review & editing), Jerry Ritchey (Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – review & editing), Edralin Lucas (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Writing – review & editing) and Brenda Smith (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Writing – review & editing).
Funding
Research reported in this publication was supported by the National Center for Complementary and Integrative Health and the Office of Dietary Supplements of the National Institutes of Health under Award Numbers (NCCIH) R15AT010725 and (ODS) R15AT010725-S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
None declared.
Data availability
Data related to this project will be made available upon request.
Ethics approval statement
All procedures associated with this project adhered to the guidelines for the ethical care and treatment of animals under the IACUC at OSU.
References
- 1. Nader S, Darvishi N, Bartina Y, et al. Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inderjeeth CA, Inderjeeth KA. Osteoporosis in older people. J Pharm Pract Res. 2021;51(3):265–274. 10.1002/jppr.1743. [DOI] [Google Scholar]
- 3. Chodick G, Moser SS, Goldshtein I. Non-adherence with bisphosphonates among patients with osteoporosis: impact on fracture risk and healthcare cost. Expert Rev Pharmacoeconomics Outcomes Res. 2016;16(3):359–370. 10.1586/14737167.2016.1171145. [DOI] [PubMed] [Google Scholar]
- 4. Fardellone P, Lello S, Cano A, et al. Real-world adherence and persistence with bisphosphonate therapy in postmenopausal women: a systematic review. Clin Ther. 2019;41(8):1576–1588. 10.1016/j.clinthera.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 5.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, Osteoporosis prevention, diagnosis, and therapy. Jama, 2001;285(6):785–795. [Google Scholar]
- 6. Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Publica Mex. 2009;51(Suppl 1):S5–S17. 10.1590/S0036-36342009000700004. [DOI] [PubMed] [Google Scholar]
- 7. Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–1386. 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Short D. Osteomalacia associated with steatorrhoea: report of a case. Glasgow Med J. 1948;29(2):53–56. [PMC free article] [PubMed] [Google Scholar]
- 9. Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–837. 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salvesen HA, Boe J. Osteomalacia in sprue. Acta Medica Scandinavica. 1953;146(4):290–299. 10.1111/j.0954-6820.1953.tb10243.x. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y-C, Greenbaum J, Shen H, Deng HW. Association between gut microbiota and bone health: potential mechanisms and prospective. J Clin Endocrinol Metab. 2017;102(10):3635–3646. 10.1210/jc.2017-00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo S, al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182(2):375–387. 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sjogren K, Engdahl C, Henning P, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–1367. 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Britton RA, Irwin R, Quach D, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–1830. 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohlsson C, Engdahl C, Fåk F, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9(3):e92368. 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Messora MR, Oliveira LFF, Foureaux RC, et al. Probiotic therapy reduces periodontal tissue destruction and improves the intestinal morphology in rats with ligature-induced periodontitis. J Periodontol. 2013;84(12):1818–1826. 10.1902/jop.2013.120644. [DOI] [PubMed] [Google Scholar]
- 18. Garcia V, Knoll LR, Longo M, et al. Effect of the probiotic Saccharomyces cerevisiae on ligature-induced periodontitis in rats. J Periodontal Res. 2016;51(1):26–37. 10.1111/jre.12274. [DOI] [PubMed] [Google Scholar]
- 19. Li P, Ji B, Luo H, Sundh D, Lorentzon M, Nielsen J. One-year supplementation with Lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. NPJ Biofilms Microbiomes. 2022;8(1):84. 10.1038/s41522-022-00348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei M, Hua L, Wang D. The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomised clinical trial. Benefic Microbes. 2016;7(5):631–637. 10.3920/BM2016.0067. [DOI] [PubMed] [Google Scholar]
- 21. Lee CS, Kim BK, Lee IO, Park NH, Kim SH. Prevention of bone loss by using lactobacillus-fermented milk products in a rat model of glucocorticoid-induced secondary osteoporosis. Int Dairy J. 2020;109:104788. 10.1016/j.idairyj.2020.104788. [DOI] [Google Scholar]
- 22. Yu M, Pal S, Paterson CW, et al. Ovariectomy induces bone loss via microbial-dependent trafficking of intestinal TNF+ T cells and Th17 cells. J Clin Invest. 2021;131(4):1–13. 10.1172/JCI143137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tyagi AM, Yu M, Darby TM, et al. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity. 2018;49(6):1116–1131.e7. 10.1016/j.immuni.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weaver CM, Martin BR, Nakatsu CH, et al. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem. 2011;59(12):6501–6510. 10.1021/jf2009777. [DOI] [PubMed] [Google Scholar]
- 25. Takahara S, Morohashi T, Sano T, Ohta A, Yamada S, Sasa R. Fructooligosaccharide consumption enhances femoral bone volume and mineral concentrations in rats. J Nutr. 2000;130(7):1792–1795. 10.1093/jn/130.7.1792. [DOI] [PubMed] [Google Scholar]
- 26. Lobo AR, Colli C, Filisetti TM. Fructooligosaccharides improve bone mass and biomechanical properties in rats. Nutr Res. 2006;26(8):413–420. 10.1016/j.nutres.2006.06.019. [DOI] [Google Scholar]
- 27. Folwarczna J, Zych M, Burczyk J, Trzeciak H, Trzeciak H. Effects of natural phenolic acids on the skeletal system of ovariectomized rats. Planta Med. 2009;75(15):1567–1572. 10.1055/s-0029-1185904. [DOI] [PubMed] [Google Scholar]
- 28. Chen JR, Lazarenko OP, Zhang J, Blackburn ML, Ronis MJJ, Badger TM. Diet-derived phenolic acids regulate osteoblast and adipocyte lineage commitment and differentiation in young mice. J Bone Miner Res. 2014;29(5):1043–1053. 10.1002/jbmr.2034. [DOI] [PubMed] [Google Scholar]
- 29. McCabe L, Britton RA, Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep. 2015;13(6):363–371. 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scholz-Ahrens KE, Schaafsma G, van den Heuvel EGHM, Schrezenmeir J. Effects of prebiotics on mineral metabolism. Am J Clin Nutr. 2001;73(2):459s–464s. 10.1093/ajcn/73.2.459s. [DOI] [PubMed] [Google Scholar]
- 31. Chen X, Zhang Z, Hu Y, et al. Lactulose suppresses osteoclastogenesis and ameliorates estrogen deficiency-induced bone loss in mice. Aging Dis. 2020;11(3):629–641. 10.14336/AD.2019.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bassaganya-Riera J, DiGuardo M, Viladomiu M, et al. Soluble fibers and resistant starch ameliorate disease activity in interleukin-10–deficient mice with inflammatory bowel disease. J Nutr. 2011;141(7):1318–1325. 10.3945/jn.111.139022. [DOI] [PubMed] [Google Scholar]
- 33. Chen K, Chen H, Faas MM, et al. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol Nutr Food Res. 2017;61(8):1601006. 10.1002/mnfr.201601006. [DOI] [PubMed] [Google Scholar]
- 34. Mayta-Apaza AC, Pottgen E, de Bodt J, et al. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J Nutr Biochem. 2018;59:160–172. 10.1016/j.jnutbio.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 35. Cherbut C, Michel C, Lecannu G. The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats. J Nutr. 2003;133(1):21–27. 10.1093/jn/133.1.21. [DOI] [PubMed] [Google Scholar]
- 36. Campos D, Betalleluz-Pallardel I, Chirinos R, Aguilar-Galvez A, Noratto G, Pedreschi R. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012;135(3):1592–1599. 10.1016/j.foodchem.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 37. Alves-Santos AM, Sugizaki CSA, Lima GC, Naves MMV. Prebiotic effect of dietary polyphenols: a systematic review. J Funct Foods. 2020;74:104169. 10.1016/j.jff.2020.104169. [DOI] [Google Scholar]
- 38. Ou B, Bosak KN, Brickner PR, Iezzoni DG, Seymour EM. Processed tart cherry products—comparative phytochemical content, in vitro antioxidant capacity and in vitro anti-inflammatory activity. J Food Sci. 2012;77(5):H105–H112. 10.1111/j.1750-3841.2012.02681.x. [DOI] [PubMed] [Google Scholar]
- 39. Martin KR, Burrell L, Bopp J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: a randomized, crossover pilot study. Food Funct. 2018;9(10):5290–5300. 10.1039/C8FO01492B. [DOI] [PubMed] [Google Scholar]
- 40. Chai SC, Davis K, Zhang Z, Zha L, Kirschner K. Effects of tart cherry juice on biomarkers of inflammation and oxidative stress in older adults. Nutrients. 2019;11(2):228. 10.3390/nu11020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith BJ, Crockett EK, Chongwatpol P, et al. Montmorency tart cherry protects against age-related bone loss in female C57BL/6 mice and demonstrates some anabolic effects. Eur J Nutr. 2018;58(8):3035–3046. 10.1007/s00394-018-1848-1. [DOI] [PubMed] [Google Scholar]
- 42. Moon N, Effiong L, Song L, Gardner T, Soung D. Tart cherry prevents bone loss through inhibition of RANKL in TNF-overexpressing mice. Nutrients. 2018;11(1):1–13. 10.3390/nu11010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dodier T, Anderson KL, Bothwell J, Hermann J, Lucas EA, Smith BJ. U.S. Montmorency tart cherry juice decreases bone resorption in women aged 65–80 years. Nutrients. 2021;13(2):544. 10.3390/nu13020544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Batlle E, Massague J. Transforming growth factor-beta Signaling in immunity and cancer. Immunity. 2019;50(4):924–940. 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith BJ, Hatter B, Washburn K, et al. Dried plum’s polyphenolic compounds and carbohydrates contribute to its osteoprotective effects and exhibit prebiotic activity in estrogen deficient C57BL/6 mice. Nutrients. 2022;14(9):1685. 10.3390/nu14091685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mansoorian B, Combet E, Alkhaldy A, Garcia AL, Edwards CA. Impact of fermentable fibres on the colonic microbiota metabolism of dietary polyphenols rutin and quercetin. Int J Environ Res Public Health. 2019;16(2):292. 10.3390/ijerph16020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ojo BA, O'Hara C, Wu L, et al. Wheat germ supplementation increases lactobacillaceae and promotes an anti-inflammatory gut milieu in C57BL/6 mice fed a high-fat, high-sucrose diet. J Nutr. 2019;149(7):1107–1115. 10.1093/jn/nxz061. [DOI] [PubMed] [Google Scholar]
- 48. Scortichini S, Boarelli MC, Silvi S, Fiorini D. Development and validation of a GC-FID method for the analysis of short chain fatty acids in rat and human faeces and in fermentation fluids. J Chromatogr B. 2020;1143:121972. 10.1016/j.jchromb.2020.121972. [DOI] [PubMed] [Google Scholar]
- 49. Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118(2):534–544. 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020;82(1):485–506. 10.1146/annurev-physiol-021119-034332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van den Heuvel EG, Muijs T, Brouns F, Hendriks HFJ. Short-chain fructo-oligosaccharides improve magnesium absorption in adolescent girls with a low calcium intake. Nutr Res. 2009;29(4):229–237. 10.1016/j.nutres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 52. Coudray C, Bellanger J, Castiglia-Delavaud C, Rémésy C, Vermorel M, Rayssignuier Y. Effect of soluble or partly soluble dietary fibres supplementation on absorption and balance of calcium, magnesium, iron and zinc in healthy young men. Eur J Clin Nutr. 1997;51(6):375–380. 10.1038/sj.ejcn.1600417. [DOI] [PubMed] [Google Scholar]
- 53. Slevin MM, Allsopp PJ, Magee PJ, et al. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J Nutr. 2014;144(3):297–304. 10.3945/jn.113.188144. [DOI] [PubMed] [Google Scholar]
- 54. Porwal K, Pal S, Kulkarni C, et al. A prebiotic, short-chain fructo-oligosaccharides promotes peak bone mass and maintains bone mass in ovariectomized rats by an osteogenic mechanism. Biomed Pharmacother. 2020;129:110448. 10.1016/j.biopha.2020.110448. [DOI] [PubMed] [Google Scholar]
- 55. Ohta A, Sakai K, Takasaki M, et al. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice. J Nutr. 2002;132(7):2048–2054. 10.1093/jn/132.7.2048. [DOI] [PubMed] [Google Scholar]
- 56. Chu H, Tao X, Sun Z, Hao W, Wei X. Galactooligosaccharides protects against DSS-induced murine colitis through regulating intestinal flora and inhibiting NF-κB pathway. Life Sci. 2020;242:117220. 10.1016/j.lfs.2019.117220. [DOI] [PubMed] [Google Scholar]
- 57. Lee K-H, Park M, Ji KY, et al. Bacterial β-(1, 3)-glucan prevents DSS-induced IBD by restoring the reduced population of regulatory T cells. Immunobiology. 2014;219(10):802–812. 10.1016/j.imbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 58. Lindsay JO, Whelan K, Stagg AJ, et al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55(3):348–355. 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benjamin JL, Hedin CRH, Koutsoumpas A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn's disease. Gut. 2011;60(7):923–929. 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- 60. Dar HY, Pal S, Shukla P, et al. Bacillus clausii inhibits bone loss by skewing Treg-Th17 cell equilibrium in postmenopausal osteoporotic mice model. Nutrition. 2018;54:118–128. 10.1016/j.nut.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 61. Chudan S, Ishibashi R, Nishikawa M, et al. Effect of wheat-derived Arabinoxylan on the gut microbiota composition and colonic regulatory T cells. Molecules. 2023;28(7):1–13. 10.3390/molecules28073079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Beukema M, Jermendi É, Oerlemans MMP, et al. The level and distribution of methyl-esters influence the impact of pectin on intestinal T cells, microbiota, and Ahr activation. Carbohydr Polym. 2022;286:119280. 10.1016/j.carbpol.2022.119280. [DOI] [PubMed] [Google Scholar]
- 63. Zhao L, Jiang S, Hantash BM. Transforming growth factor β1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng A. 2010;16(2):725–733. 10.1089/ten.tea.2009.0495. [DOI] [PubMed] [Google Scholar]
- 64. Ohta A, Ohtsuki M, Baba S, Adachi T, Sakata T, Sakaguchi E. Calcium and magnesium absorption from the colon and rectum are increased in rats fed fructooligosaccharides. J Nutr. 1995;125(9):2417–2424. 10.1093/jn/125.9.2417. [DOI] [PubMed] [Google Scholar]
- 65. Ohta A, Ohtsuki M, Hosono A, Adachi T, Hara H, Sakata T. Dietary fructooligosaccharides prevent osteopenia after gastrectomy in rats. J Nutr. 1998;128(1):106–110. 10.1093/jn/128.1.106. [DOI] [PubMed] [Google Scholar]
- 66. Sabater-Molina M, Larqué E, Torrella F, Zamora S. Dietary fructooligosaccharides and potential benefits on health. J Physiol Biochem. 2009;65(3):315–328. 10.1007/BF03180584. [DOI] [PubMed] [Google Scholar]
- 67. Hooshmand S, Juma S, Arjmandi BH. Combination of genistin and fructooligosaccharides prevents bone loss in ovarian hormone deficiency. J Med Food. 2010;13(2):320–325. 10.1089/jmf.2009.0059. [DOI] [PubMed] [Google Scholar]
- 68. Lucas S, Omata Y, Hofmann J, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):1–10. 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen J-R, Lazarenko OP, Blackburn ML. GPR109A gene deletion ameliorates gonadectomy-induced bone loss in mice. Bone. 2022;161:116422. 10.1016/j.bone.2022.116422. [DOI] [PubMed] [Google Scholar]
- 70. Chen J-R, Zhao H, Wankhade UD, et al. GPR109A mediates the effects of hippuric acid on regulating osteoclastogenesis and bone resorption in mice. Commun Biol. 2021;4(1):53. 10.1038/s42003-020-01564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Semënov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 72. Wang C, Zhang D, Zhang M, Jiao Y, Jiang K, Yan C. Structural characterization of a novel oligosaccharide from Achyranthes bidentata and its anti-osteoporosis activities. Ind Crop Prod. 2017;108:458–469. 10.1016/j.indcrop.2017.07.018. [DOI] [Google Scholar]
- 73. Yan C, Zhang S, Wang C, Zhang Q. A fructooligosaccharide from Achyranthes bidentata inhibits osteoporosis by stimulating bone formation. Carbohydr Polym. 2019;210:110–118. 10.1016/j.carbpol.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 74. Barros NM, Hoac B, Neves RL, et al. Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Miner Res. 2013;28(3):688–699. 10.1002/jbmr.1766. [DOI] [PubMed] [Google Scholar]
- 75. Staines KA, MacRae VE, Farquharson C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J Endocrinol. 2012;214(3):241–255. 10.1530/JOE-12-0143. [DOI] [PubMed] [Google Scholar]
- 76. Narayanan K, Ramachandran A, Hao J, et al. Dual functional roles of dentin matrix protein 1: implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem. 2003;278(19):17500–17508. 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 77. Civitelli R. Cell–cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys. 2008;473(2):188–192. 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Davis HM, Aref MW, Aguilar-Perez A, et al. Cx43 overexpression in osteocytes prevents osteocyte apoptosis and preserves cortical bone quality in aging mice. JBMR plus. 2018;2(4):206–216. 10.1002/jbm4.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res. 2008;23(11):1712–1721. 10.1359/jbmr.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bivi N, Condon KW, Allen MR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27(2):374–389. 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data related to this project will be made available upon request.