Graphical abstract
Keywords: Gossypium hirsutum, Defoliation, Machine picking, Genomic loci, Environmental adaptability
Highlights
-
•
The fundamental phenotypic variations of defoliation traits among 383 cotton accessions were revealed.
-
•
The defoliants had little penalty on yield and fiber quality.
-
•
Two significantly associated loci were dissected, and key candidate genes, GhLRR and GhCYCD3;1, were clarified.
-
•
The combining of two favorable haplotypes improved sensitivity to defoliant.
-
•
The favorable haplotype frequency was generally increased in high latitudes in China for adaptation to the local environment.
Abstract
Introduction
Defoliation by applying defoliants before machine picking is an important agricultural practice that enhances harvesting efficiency and leads to increased raw cotton purity. However, the fundamental characteristics of leaf abscission and the underlying genetic basis in cotton are not clearly understood.
Objectives
In this study, we aimed to (1) reveal the phenotypic variations in cotton leaf abscission, (2) discover the whole-genome differentiation sweeps and genetic loci related to defoliation, (3) identify and verify the functions of key candidate genes associated with defoliation, and (4) explore the relationship between haplotype frequency of loci and environmental adaptability.
Methods
Four defoliation-related traits of 383 re-sequenced Gossypium hirsutum accessions were investigated in four environments. The genome-wide association study (GWAS), linkage disequilibrium (LD) interval genotyping and functional identification were conducted. Finally, the haplotype variation related to environmental adaptability and defoliation traits was revealed.
Results
Our findings revealed the fundamental phenotypic variations of defoliation traits in cotton. We showed that defoliant significantly increased the defoliation rate without incurring yield and fiber quality penalties. The strong correlations between defoliation traits and growth period traits were observed. A genome-wide association study of defoliation traits identified 174 significant SNPs. Two loci (RDR7 on A02 and RDR13 on A13) that significantly associated with the relative defoliation rate were described, and key candidate genes GhLRR and GhCYCD3;1, encoding a leucine-rich repeat (LRR) family protein and D3-type cell cyclin 1 protein respectively, were functional verified by expression pattern analysis and gene silencing. We found that combining of two favorable haplotypes (HapRDR7 and HapRDR13) improved sensitivity to defoliant. The favorable haplotype frequency generally increased in high latitudes in China, enabling adaptation to the local environment.
Conclusion
Our findings lay an important foundation for the potentially broad application of leveraging key genetic loci in breeding machine-pickable cotton.
Introduction
The abscission in plants is the process of shedding excess organs, such as fruits, flowers and leaves [1]. Abscission is triggered when plants are subjected to environmental stimulation or enter a new developmental period. In general, the abscission of plant organs is a superb strategy to save energy needed for life-sustaining functions; for example, plants shed flowers after pollination, senescent leaves, ripened fruit and grain as well as infected or damaged organs. Moreover, controlling abscission is a key agricultural concern; for example, seed shattering before harvest is a major factor limiting the yield of cereal crops [2], while leaf abscission before mechanical harvest benefits the yields of cotton [3], [4] and sugarcane [5], [6], [7].
Abscission takes place in the abscission zone (AZ), a specific region consisting of small and dense cells interconnected by plasmodesmata [8]. Abscission can be roughly divided into four stages: abscission zone development, abscission signal activation, AZ cell separation, and the differentiation of a protective layer [9]. Studies have shown that several genes are involved in the control of abscission zone development; for instance, BLADE-ON-PETIOLE1 (BOP1) and BOP2 function redundantly to control AZ formation [10]. A MADS-box transcription factor, JOINTLESS, is essential for the formation of pedicels AZ in tomato [11], [12]. A main signaling cascade pathway regulating abscission was discovered in Arabidopsis. The small-peptide INFLORESCENCE DEFICIENT in ABSCISSION (IDA) binds and activates a receptor complex consisting of HAESA, HAESA-LIKE2 [13], [14], [15] and SOMATIC EMBRYOGENESIS RECEPTOR KINASES (SERK) [16], [17]. The receptor complex ultimately activates KNOX transcription factors through a MAPK cascade [18], [19]. KNOX induces the transcription of cell wall remodeling- and degrading-related enzymes such as polygalacturonases and xyloglucan endotransglucosylases/hydrolases [20]. Subsequently, the cells separate, and the organs drop.
Cotton represents the main source of natural textile fibers in the world [21]. Upland cotton (Gossypium hirsutum L.) has become the most frequently cultivated species in China and the world because of its high adaptability and yield. In China, machine picking is trending due to its advantages in large-scale planting and labor savings [22]. Machine picking is approximately one-fourth the cost of manual cotton picking. In the past decade, the area of machine-picked cotton that is planted in China has expanded rapidly, and it currently, counts for approximately 50% of all cotton planted area. Cotton-picking machinery and the supporting picking technology are relatively mature; however, there are few cotton varieties suitable for machine picking and with compact plant architecture, hard stems, concentrated boll opening and high sensitivity to defoliants. Even fewer of these varieties show high fiber quality and high yield. Most machine-picked cotton varieties are temporary substitution cottons.
Compared with manual picking, machine picking reduces production costs and increases cotton planting benefits, but the cotton quality is profoundly decreased because of the low defoliation rate before machine picking, which leads to highly impure raw cotton. Thus, applying chemical defoliants prior to machine picking to accelerate leaf abscission in cotton plants is required [23]. This strategy not only enhances the concentrated picking rate and harvest efficiency but also promotes boll opening and reduces the impurity (such as crushed leaves) levels in raw cotton [4]. Whether sensitivity to defoliants is a critical factor for the development of machine-picked cultivars in cotton production. However, due to the lack of large-scale cotton defoliation research involving hundreds of cultivars, it is difficult to provide valuable guidance for breeding machine-picked cotton based on the defoliation properties and the underlying genetic architecture.
At present, research on cotton defoliation is insufficient. Mishra et al. [24] reported that higher expression of cellulose 1 (GhCel1) was associated with cotton leaf abscission. The crosstalk between cytokinin, ethylene and auxin, as well as the crosstalk between ROS (Reactive Oxygen Species) metabolism and photosynthesis both regulated cotton defoliation [25], [3], [4]. In addition, the defoliant application technology has been a focus. However, the fundamental features of the defoliation phenotype and underlying molecular mechanism in cotton are still unclear. Therefore, discovering the phenotypic variations and genetic mechanisms underlying leaf abscission in G. hirsutum will be key to improving defoliation efficiency and breeding machine-picked cotton varieties.
Genome-wide association study (GWAS) is an effective method for large-scale mining of loci or genes associated with targeted phenotypic traits. In recent years, GWAS has been widely used to study cotton yield and quality [21], [26], [27], [28]. In this study, to reveal the fundamental characteristics of leaf abscission, four defoliation-related traits for 383 resequenced G. hirsutum accessions were first investigated in four environments over two years and at three locations. On the basis of GWAS, genome linkage disequilibrium (LD) interval genotyping and functional identification, the genetic basis and candidate genes of associated elite loci for defoliation-related traits were identified in G. hirsutum. Finally, the haplotype variation related to environmental adaptability and defoliation traits was discovered. The results provide new insights into the genetic basis of leaf abscission and serve as a reference supporting increased machine-picked cottonbreeding.
Materials and methods
Plant materials and phenotyping
An elite core panel of upland cotton, including 383 accessions, was used for phenotyping and genotyping in the present study. These accessions were obtained from holdings of the National Mid-term Gene Bank for Cotton at the Institute of Cotton Research, Chinese Academy of Agricultural Sciences. Samples had been originally obtained from diverse geographic origins, such as China, the USA, the former Soviet Union (FSU), Australia, Pakistan, and other countries (Table S1, Fig. S1).
Phenotype characterization experiments were carried out in four eco-environments in three locations over two years. In 2018, experiments were conducted at Alar (40.61°N, 81.33°E) and Kuitun (44.45°N, 84.89°E) in southern and northern Xinjiang, China, respectively. In 2019, 383 accessions were grown at Alar and Anyang (36.07°N, 114.50°E, Henan, China). The field experiments were conducted by using a split plot experimental design with two replications. Cotton varieties were grown in the main plot, and defoliant-treatment experiments were performed in a split plot. Each plot in Alar and Kuitun contained three rows with a row spacing of 76 cm, while in Anyang, each plot contained one row, and the plot spacing was 80 cm. Field cultivation and management were conducted strictly according to local standards.
At the boll-opening stage, 1800 mL·hm−2 defoliant mixture (Xinsaili, 50% thidiazuron·ethephon, suspension concentrate) was applied to the defoliant treatment plots. The cotton plants in the control plots were sprayed with water instead of defoliant. The defoliation rate (DR) was calculated using the following formula: DR (%) = (NTL-NRL)/NTL × 100, where NTL represents the number of total mature leaves before defoliant treatment, and NRL represents the number of leaves that remained on Day 10 after defoliant or water treatment. The petiole-breaking strength (PBS) of the top first mature leaf was measured using an FGJ-50 tester (SHIMPO) after defoliant or water treatment. The average value of five plants in a plot represented the phenotypic data for a line in this plot. The relative DR was the ratio of DRT to DRCK; the relative PBS was the ratio of PBST to PBSCK. The values of the best linear unbiased prediction (BLUP) across multiple environments for defoliation-related traits on a per line basis were calculated using the R package ‘lme4’ [29].
After screening defoliant-sensitive and defoliant-insensitive materials according to the defoliation-related traits obtained from the field experiments, the defoliant sensitivity of ten cotton materials (five selected from the defoliant-sensitive group and five from the defoliant-insensitive group) was further characterized in a greenhouse. At the eight-leaf stage, the defoliant thidiazuron (TDZ, 100 mg/L) was sprayed evenly on all the leaves of the cotton plants. The abscission layer formation rate (%) and defoliation rate (%) of each plant were recorded and calculated from the second day to the sixth day after defoliant treatment.
To evaluate the effect of defoliant on cotton yield and quality, yield component traits and fiber quality traits of different treatments were investigated. Yield component traits consisted of boll weight (BW) and lint percentage (LP). BW and LP were calculated after the seed cotton had been weighed and ginned. The fiber quality traits included fiber strength (FS), fiber length (FL), micronaire value (FM), elongation rate (FE) and uniformity (FU). After removing the cotton seeds, fiber quality traits were evaluated using an HVI 9000 in the Cotton Quality Supervision, Inspection and Testing Center, Ministry of Agriculture, Anyang, China. Analysis of variance (ANOVA) of phenotypic data was performed by using the aov function in R software.
Histological experiment of abscission zone
In order to determine the histological difference of abscission zone between defoliant-sensitive accession (BL34) and -insensitive accession (LM28), the histological observations were conducted. The leaf abscission zones of BL34 and LM28 were sampled at 0 h, 24 h, 48 h and 72 h after TDZ (100 mg/L) treatment, and samples were fixed in 70% FAA. They were dehydrated with graded alcohols and embedded in paraffin. After cutting into longitudinal sections and staining with Fancy Red and Solid Green, the histological views were obtained using a light microscope (Nikon Eclipse E100).
Sequencing and genotyping
A panel including 383 accessions was analyzed in this study. Total genomic DNA was extracted from young leaves of each accession seedling by using the CTAB method. Then, 150-bp paired-end sequencing libraries were constructed according to the manufacturer’s specifications. Sequencing was implemented using the Illumina HiSeq sequencing platform at Biomarker Technologies. After filtering low-quality paired reads, a total of ∼3.87 TB of clean sequencing data with an average genome coverage of 14.6 × were generated (Table S1).
The remaining high-quality reads were aligned to the reference genome of G. hirsutum TM-1 [30] using BWA with the MEM algorithm with default parameters [31]. SNP calling on a population scale was performed with SAMtools [32] based on BAM files generated during the alignment process. Finally, 1,076,652 high-quality SNPs with a missing ratio within the population ≤ 20% and minor allele frequency (MAF) ≥ 0.05 were retained for subsequent analysis. The ANNOVAR package [33] was used to obtain SNP annotation information on the basis of the G. hirsutum reference genome.
Population genetic analysis
A total of 1,076,652 high-quality SNPs was subjected to population structure inference by ADMIXTURE v.1.3.0 [34], and bar plots generated based on a given K-value were visualized with R software. A principal component analysis (PCA) was carried out using GCTA [35], and two-dimensional coordinates were plotted for the 383 lines using the ‘ggplot2′ package in R software. An unrooted phylogenetic tree was constructed using FastTreeMP v.21.1.11 [36] with “-gtr -nt” parameters and visualized using FigTree (v.1.4.4). Linkage disequilibrium (LD) decay was analyzed using PopLDdecay v.3.31 [37], and an LD decay plot was generated with a R script.
VCFtools v.0.1.17 [38] software was employed in population evolution analysis, which included the fixation statistic (FST) and nucleotide diversity (θπ) representing population differentiation between subgroups and genetic diversity of one population, respectively. The sliding window size was 20 kb with a 10 kb length step for calculating FST and θπ. The top 5% of the Fst scores for each comparison were considered candidate differentiation regions. Adjacent windows with a length of <500 kb were merged into a single region.
GWAS and haplotype analysis
A genome-wide association study was performed with 1,076,652 high-quality SNPs to identify the associated signals of defoliation-related traits and their BLUP values using efficient mixed-model association expedited (EMMAX) software [39]. The Bonferroni-corrected significance threshold was set to be P = 1/n (where n is the total SNP number). Thus, the significance threshold − log (P value) in the GWAS was ∼6.0. The GWAS results were visualized with Manhattan and quantile–quantile (Q-Q) plots using the “qqman” package in R software [40].
To analyze the haplotypes within this candidate region, we referred to the method described by He et al. [28]. Firstly, the boundary of strong LD, defined as LD coefficient r2 > 0.7, was determined by conducting an LD block analysis using Tassel 5.0. Then the regional population SNPs were extracted and phylogenetic tree was constructed, generating a genotype heatmap according to the tree order and numerical genotype (1 for major and 0 for minor) in R software. The accessions clustered and with same color were then categorized into same haplotype.
The transcriptome
At the eight-leaf stage, the leaf abscission zones of BL34 ( defoliant-sensitive accession) and LM28 (defoliant-insensitive accession) were sampled at 12 h, 24 h, and 48 h after TDZ (100 mg/L) and water treatment. Total RNA was extracted using RNAprep Pure Plant Plus Kit (TIANGEN Biotech Co., Ltd.). The total RNA quality of 36 samples (two genotypes × three time points × two treatments × three biological replicates) was assessed, and cDNA libraries were constructed. Subsequently, these libraries were sequenced using an Illumina NovaSeq platform at Biomarker Technologies, and 150-bp paired-end reads were generated. After trimming the raw data, clean reads were obtained and aligned to the G. hirsutum reference genome [30] using bowtie2 v2.2.4 [41]. Gene expression levels were calculated using RSEM software v.1.3.1 [42].
Gene expression analysis
To assess the expression levels of the candidate genes for defoliation-related traits, abscission zone tissues were collected from defoliant sensitive (BL34) and defoliant insensitive (LM28) genotypes after defoliant application. Total RNA was extracted using a Plant Total RNA Isolation Kit (Sangon Biotech), and cDNA was synthesized in a 20-μl reaction mixture using a PrimeScriptTM RT Reagent Kit with gDNA Eraser Kit (TaKaRa Biomedical Technology Co., Ltd.). Gene-specific primers were designed using Primer-BLAST. The real-time PCR amplification program was run on a Roche LightCycler 480Ⅱ. Gene expression levels were calculated using the 2-ΔΔCT method, and three independent biological replicates with three technical replicates were established for each sample. Histone 3 (AF024716) was used as the internal control for qRT–PCR. All primer sequences used for qRT–PCR are listed in Table S2.
Subcellular localization
The full-length sequence of Gh_A13G042700 was amplified using specific primers designed with the online tool CE Design (https://www.vazyme.com) (Table S2). Subsequently, the sequence was inserted into the transient expression vector pCambia2300 to generate the construct 35S::Gh_A13G042700-GFP. The recombinant plasmid 35S::Gh_A13G042700-GFP and the positive control plasmid 35S::GFP were transferred to Agrobacterium tumefaciens strain LBA4404. Agrobacterium tumefaciens LBA4404 carrying the expression and control vectors was injected into tobacco leaves. A Zeiss LSM 710 confocal microscope (Zeiss, Oberkochen, Germany) was used to observe the fluorescent protein signal at excitation and emission wavelengths of 488 nm and 495–530 nm, respectively.
Virus-induced gene silencing
The approximately 300-bp gene-specific fragment of Gh_A13G042700 was amplified as a template of primers and inserted into the TRV2 vector. TRV2, TRA2:CLA1 and TRV2:Gh_A13G042700 were mixed with TRV1 at 1:1 and transferred into cotton cotyledons through Agrobacterium tumefaciens strain LBA4404. To ensure that the transfer was successful, the transfer was performed in the dark for 48 h. TRV2 and TRA2:CLA1 were used as empty-vector positive controls. The primers used for vector construction are listed in Table S2.
Protein structures
The 3D structures of the two allele proteins encoded by GhLRR were predicted using I-TASSER [43] and visualized with PyMOL v2.5.0 [44].
Climate data
The average temperature from April to October of 1970 to 2000 was downloaded from https://www.worldclim.org v.2.1 [45] and visualized with QGIS v.3.10.13.
Results
Phenotype analysis revealed fundamental variations in cotton defoliation traits
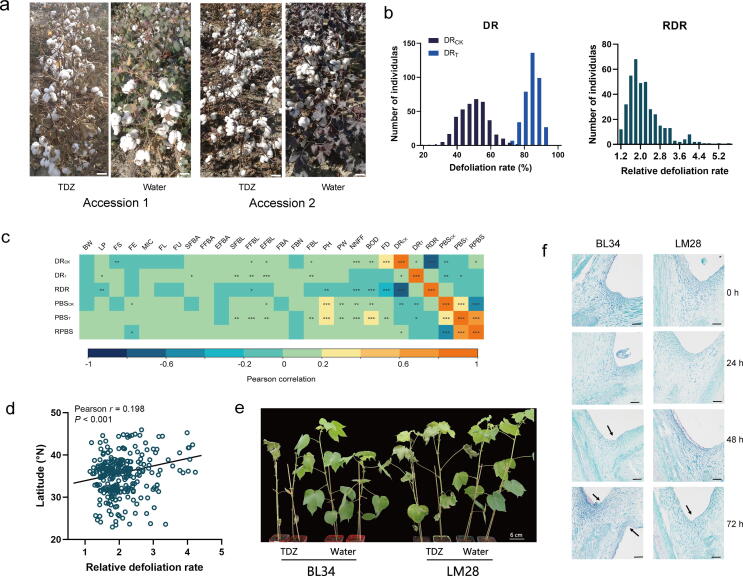
In the present study, a total of 383 diverse G. hirsutum accessions collected worldwide were used for phenotyping and genotyping (Fig. S1, Table S1). The large-scale phenotype characterization of cotton defoliation was determined via experiments performed in four different environments (Fig. 1a). Extensive phenotypic variations in four defoliation-related traits (DR, defoliation rate; RDR, relative defoliation rate; PBS: petiole-breaking strength; RPBS: relative petiole-breaking strength) were determined for 383 accessions (Fig. 1b; Figs. S2 and S3; Table S3). Under normal conditions, the average defoliation rate (DRCK) was 50.33%, ranging from 20.95% to 75.32% across all environments. The average petiole-breaking strength (PBSCK) was 0.68 kg, varying from 0.34 kg to 1.13 kg (Table S3). After using chemical defoliants, the average defoliation rate of the treated plants (DRT) was 83.72%, ranging from 68.42% to 93.67% (Fig. 1b, Table S3), but the PBST varied from 0.12 kg to 0.63 kg with a mean value of 0.33 kg (Table S3). The increased DR and decreased PBS values indicated that the application of defoliant significantly promoted G. hirsutum leaf shedding. The relative defoliation rate (RDR), which directly represents the sensitivity to defoliant, varied greatly among genotypes, ranging from 1.14 to 5.66 (Fig. 1b; Table S3).
Fig. 1.
The fundamental phenotypic variation features of cotton defoliation. (a) Chemical defoliant experiments in the field. Two varieties selected randomly by 14 days after defoliant and water treatment are displayed here. TDZ: Thidiazuron, a chemical defoliant. The scale bar is 5 cm. (b) The frequency distributions of the mean defoliation rate (DR) and relative defoliation rate (RDR) in four environments. (c) The correlation analysis of defoliation traits and yield, fiber quality, plant architecture, and growth period. DR: defoliation rate, RDR: relative DR, PBS: petiole breaking strength, RPBS: relative PBS, BW: boll weight, LP: lint percentage, FL: fiber length, FS: fiber strength, MIC: micronaire, FE: fiber elongation, FU: fiber uniformity, SFBA: second fruit branch angle, FFBA: fifth FBA, EFBA: eighth FBA, SFBL: second fruit branch length, FFBL: fifth FBL, EFBL: eighth FBL, FBN: fruit branch number, PH: plant height, PW: plant width, NNFF: number of nodes of first fruit branch, BOD: boll opening day, FD: flowering day. *, **and *** significant at P < 0.05, P < 0.01 and P < 0.001, respectively. (d) Scatter plot showing the relationship between the relative defoliation rate (RDR) and latitude for the accessions in China. (e) Identification of defoliant sensitivity for BL34 and LM28 in the greenhouse. BL34 and LM28 were identified as defoliant-sensitive and defoliant-insensitive cotton varieties, respectively. These two varieties displayed here were photographed by 6 days after defoliant and water treatment. The scale bar is 6 cm. (f) Longitudinal sections of leaf abscission zone for BL34 and LM28 at 0 h, 24 h, 48 h and 72 h after defoliant treatment. Black arrows indicate the formation of an abscission zone. The scale bar is 100 μm.
To study the effect of defoliant treatment on yield and fiber quality, two fiber yield traits (BW: boll weight and LP: lint percentage) and five fiber quality traits (FL: fiber length, FS: fiber strength, FM: micronaire, FE: fiber elongation rate, and FU: fiber uniformity) were also measured. Multivariate analysis of variance (ANOVA) was carried out to identify the effects of the defoliant (D), genotype (G), environment (E) and their interactions on these traits (Table S4). Significant variations were found in G, E and G × E (P < 0.001). Although the main effect of defoliant on FL and FM was significant, defoliant treatment did not play the predominant role; cotton genotype was the key factor (Table S4). Defoliant treatment reduced the FL only from 28.66 mm to 28.56 mm and increased the FM from 4.54 to 4.57 (Table S3); these changes are unimportant to agricultural production in practice. The results indicated that defoliant treatment exerted no significant effect on yield and a negligible effect on fiber quality. In contrast, the DR and PBS were significantly affected by defoliant, environment and genotype, and genotype playing the dominant role. Furthermore, the ANOVA showed that the G × D interaction exerted a significant effect on DR, indicating that the effect of defoliant treatment differed among varieties.
In addition to yield and fiber quality, 11 plant architecture (PA) traits and three growth period (GP) traits were analyzed on the basis of the correlations between these traits and defoliation-related traits (Fig. 1c, Table S5). Only a few significant correlations between defoliation-related traits and yield traits, as well as fiber quality, were found (Fig. 1c). These results implied that the link between defoliant and yield and fiber quality were very weak, which was in line with the ANOVA results. However, more significant correlations were found between defoliation traits and PA, as well as GP, especially plant height (PH), plant width (PW), four fruit branch length (FBL)-related traits, and three GP traits (Fig. 1c, Table S5). As a whole, the more compact the plant architecture (shorter height and branches and narrower width) and the earlier the maturity are, the more profound the effect of defoliant. This result provided alternative guidance for identifying defoliant-sensitive accessions.
High latitudes germplasms in China may more sensitive to defoliant
Based on the aforementioned analysis, the relative defoliation rate (RDR) was determined as the direct screening index of defoliant-sensitive cotton. The overlapping cotton germplasms among the top 25% candidates based on the RDR in at least three environments were identified as defoliant-sensitive materials, meaning they were candidates for breeding machine-picked cotton, while the bottom 25% candidates based on the RDR in at least three environments were considered to be defoliant-insensitive germplasms. Here, 33 defoliant-sensitive materials and 23 defoliant-insensitive materials were identified, and the average RDR of the defoliant-sensitive germplasms was 3.23, while the average RDR of the defoliant-insensitive germplasms was 1.38 (Table S6). The geographical origins of these defoliant-sensitive and defoliant-insensitive materials were complicated (Table S6). However, we found that accessions from Northwest China (NWC) and North China (NC) in higher latitudes were only included in defoliant-sensitive material group, and accessions from Yellow River region (YER) and Yangtze River region (YZR) of China in lower latitudes were predominantly found among defoliant-insensitive materials (13/23, Table S6). These findings suggested that the germplasms from different latitudes showed distinct defoliant sensitivities. The relative defoliation rate likely increased when the latitude increased, especially for Chinese cotton cultivars (Fig. 1d). The results implied that the germplasms from high latitudes (NC and NWC) showed the potential to be cultivated as machine-picked cotton with highly efficient defoliation. It also shown that the defoliation traits in cotton was significantly affected by the geographical environment.
To further investigate the sensitivity to defoliant of the selected germplasms, five defoliant-sensitive germplasms and five defoliant-insensitive germplasms were identified in a greenhouse (Table S7). Overall, the identification of the plants in the greenhouse were in accordance with that in the field experiment. For instance, for the defoliant-sensitive accession Bole 34 (BL34), the abscission zones of all the leaves had formed, and all the leaves had dropped by Day six, but in the defoliant-insensitive accession Lumianyan 28 (LM28), only 76.19% abscission zones were formed and 4.76% leaves were detached (Fig. 1e, Table S7). Histological experiment illustrated that the abscission zone of BL34 formed at 48 h following defoliant treatment, but in LM28, the onset of abscission zone formation was delayed to 72 h (Fig. 1f). Thus, these two accessions were used in further transcriptome analysis.
Genome-wide variation and population structure revealed population divergence
To identify the elite loci related to defoliation traits, we first analyzed the whole-genome variations and population structure of the core collection comprising 383 accessions. A total of 3.87 Tb of high-quality clean data with an average depth of 14.6-fold were generated by resequencing 383 upland cotton accessions (Table S1). Subsequently, a final set of 1,076,652 SNPs (MAF ≥ 0.05, missing rate ≤ 20%) was identified. Statistical analysis of the SNP distribution showed that chromosome A08 carried the largest number of SNPs (154,908) as well as the SNPs at the highest density (1.23 SNP/kb), followed by A06 (126,438 and 1.00 SNP/kb, respectively; Fig. S4, Table S8), which may due to the extensive inversions in these two chromosomes [28], [46].
Among the SNPs identified, 70% (753,151) were found in intergenic regions, and 4.3% were in coding regions, including 18,062 synonymous, 28,218 nonsynonymous, 499 stop-gain and 103 stop-loss SNPs (Table S9). The ratio of nonsynonymous to synonymous SNPs was 1.56, which was similar to that in a previous report (1.62) [27]. Furthermore, 207,168 SNPs located in upstream or downstream regions were annotated.
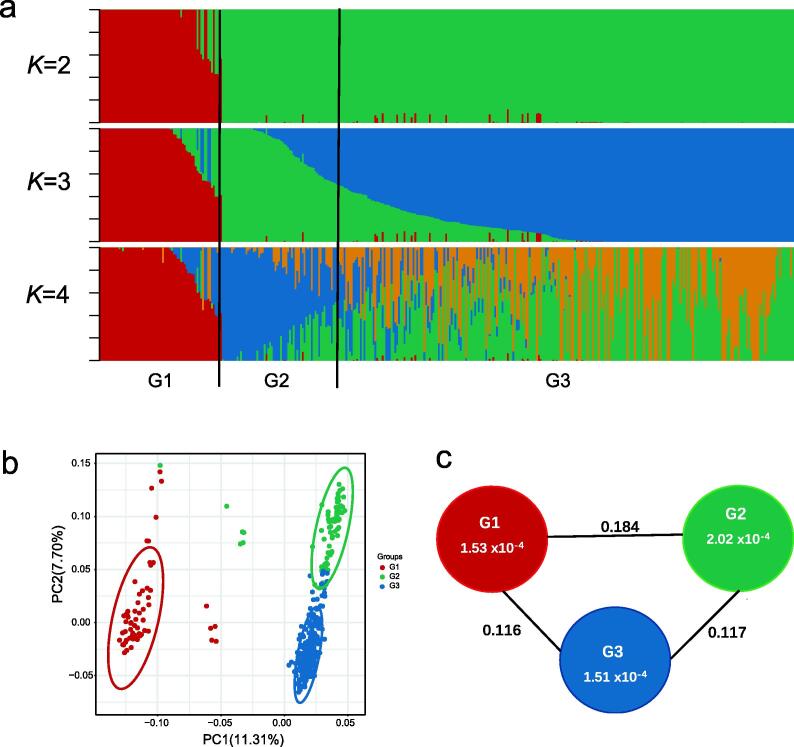
To dissect the population composition within the 383 accessions, a population structure analysis was performed. As shown in Fig. 2a, 383 lines were clustered into three groups when K = 3, namely, Group 1 (G1), G2 and G3. These results were supported by a principal component analysis (PCA, Fig. 2b) and phylogenetic analysis (Fig. S5a). G1 contained 62 varieties, mainly from YZR, while the accessions distributed mainly in NWC and NC were included in G2 (total of 70 accessions); the lines from YER and the United States were primarily assigned to G3 and consisted of 251 samples (Fig. S1 and Table S1).
Fig. 2.
Population structure and population divergence of 383 re-sequenced accessions. (a) Population structure analysis when K = 2, 3 and 4. The x-axis represents the 383 accessions. The accessions in Group 1 (red), Group 2 (green), and Group 3 (blue) are marked by different colors. (b) Principal component analysis of all 383 accessions. The first two components (PC1 and PC2) are shown. The oval circles indicate the levels of the 95% confidence intervals. Colors correspond to the structure grouping; the same color coding is used below. (c) Genetic diversity and population differentiation in the three groups. The values in the circles represent the genetic diversity (θπ) of the groups, and the values between the groups indicate population differentiation (FST). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The genetic differentiation value (FST) across the three groups ranged from 0.116 to 0.184 (Fig. 2c), which was much higher than values for the improved cotton cultivars (0.032–0.049; 0.019–0.067) [27], [28] and implied that a relatively broad genetic divergence among different groups in this study. The average θπ value was 1.51 × 10−4–2.02 × 10−4 across the three groups (Fig. 2c), which was similar to the value reported in a previous study (1.79 × 10−4) [26] but much lower than the value of 1.32 × 10−3 in wild cotton accessions [21], suggesting that the genetic diversity in improved upland cotton had decreased. Linkage disequilibrium (LD) decayed with physical distance between SNPs in all cotton groups (Fig. S5b). The extent of LD for each group was calculated as the chromosomal distance when r2 decreased to half of its maximum value (0.92). LD decay occurred within 819 kb in G3 and increased to 1,113 kb in G1, but both decay values were lower than that in G2 (1701 kb). This finding indicated that the LD of the YZR population (G1) decayed faster with weaker adaptive pressures than that of the YER and USA populations (G3) and that the LD of the NC and NWC populations (G2) decayed the slowest with the greatest pressures. All three decay distance values covered those reported by Fang et al. (1000 kb) [26] but were higher than those reported by Wang et al. (296 kb) [21] and Ma et al. (742.7 kb) [27].
Population differentiation analysis
In consideration of the genomic differences between pairwise subpopulations revealed by the population genetic results, a whole-genome population differentiation approach (FST) was applied to detect differentiation sweeps. However, the signal intensities on A06 and A08 were exceedingly high, obscuring the significant divergent signals (above the thresholds of the top 5%) on the other chromosomes (Fig. S6). Therefore, we conducted the analysis of differentiation sweeps by excluding A06 and/or A08 (Tables S10–S12). The average Fst scores were reduced to 0.075 and 0.080 between G1-G2 and G2-G3, respectively. The average Fst score between G1 and G3 declined sharply to 0.028. Above the dashed horizontal thresholds of the top 5%, 512 sweeps (157.71 Mb), 338 sweeps (161.54 Mb), and 589 sweeps (133.18 Mb) in the comparisons of G1 versus G2, G2 versus G3, and G1 versus G3 were generated (Fig. S6, Tables S10–S12). The statistical analysis of the differentiation sweeps showed that the number of sweeps in the At subgenome was approximately twofold to threefold as high as that in the Dt subgenome, except in G1-G2 (Fig. S7a, c and g), which was consistent with the number of SNPs in the At subgenome, which was approximately 2.71-fold that in the Dt subgenome (Table S8). The longest sweeps and average size of the sweeps for G1-G2, G2-G3 and G1-G3 were found in D03, D06 and A05, respectively (Fig. S7b, d and h; Tables S10–S12). Furthermore, sweeps <20 kb accounted for a substantial portion of all the sweeps (54.3% in G1-G2, 52.37% in G2-G3 and 61.46% in G1-G3), followed by those 200–500 kb in size (Fig. S7c, e and i).
Whole genome identification of defoliation-related loci
A genome-wide association study of four defoliation-related traits was performed across the 383 accessions using 1,076,652 high-quality SNPs. A total of 174 significantly associated SNPs, which were mainly distributed on chromosomes A02, A13, D05 and D07, were identified. Among these significant SNPs, 64 were associated with the defoliation rate (Fig. S8 and S12, Table S13), 79 were associated with the relative defoliation rate (Fig. S9 and S12, Table S14), 18 were associated with the petiole-breaking strength (Fig. S10 and S12, Table S15), and 13 were associated with the relative petiole-breaking strength (Fig. S11 and S12, Table S16).
Interestingly, 27 overlapping GWAS signals were found after comparing with the differentiation sweeps generated by Fst (Fig. S6a-b, Table S17). Notably, 10 and 11 overlapping signals in G1-G2 and G2-G3, respectively, but only 6 in G1-G3 (Table S17), implying a population difference in defoliation-related differentiation loci that was probably driven by different compositions within groups. These overlapping loci, especially the locus of RDR7 on A02, RDR13 on A13 (Table S17), were prioritized for further research.
Haplotypes and candidate genes of the relative defoliation rate locus RDR7 on A02
A significantly associated relative defoliation rate locus (RDR7) was identified on chromosome A02 (Fig. 3a, Table S14). This RDR-associated region was ∼186 kb (from 1.28 Mb to 1.47 Mb) and roughly divided into three LD blocks (Fig. 3b and c). Block 3 showed the highest LD coefficient r2, meaning that the SNPs within this block were closely linked; therefore, we focused on it. The peak SNP and the SNP A02:1,403,053, with a signal slightly lower than the threshold value, were within Block 3. Moreover, the SNP A02:1,403,053 was in the exonic region and was a nonsynonymous SNP with the allele A/G, which changed the encoded amino acid from a histidine residue to an arginine residue at position 153 of Gh_A02G015900 (Fig. 3d). The side chain of the amino acid at the position of the ninth α-helix was also changed, which may affect the enzymatic activity or binding capability of the two haplotypes (HapAA and HapGG, Fig. 3e). The accessions carrying HapRDR7(HapAA) showed a significantly higher relative abscission rate than those carrying Haprdr7 (HapGG, Fig. 3f). Gh_A02G015900 encoded a leucine-rich repeat (LRR) family protein; thus, it was designated GhLRR. However, GhLRR was previously undescribed, and we know little about its expression or function in cotton. Thus, the expression differences of GhLRR in the sensitive variety (BL34) and insensitive variety (LM28) were explored via a transcriptome analysis. The RNA-seq results revealed that the expression of GhLRR remained low at 12 h and 24 h after defoliant (TDZ) treatment in both the BL34 and LM28 accessions, but it increased sharply at 48 h compared with the water control, and an expression difference was evident (Fig. 3g, Table S18). To validate the expression pattern of GhLRR, we performed quantitative PCR (qRT–PCR). The increasing trend of the expression levels at 48 h was consistent with the RNA-seq results, and the expression level of GhLRR in BL34 at 48 h was also lower than that in LM28 (Fig. 3h, Table S19). Overall, these results indicated that GhLRR likely plays an important role in leaf abscission in cotton and that 48 h may be a key time point in which GhLRR responds to defoliants.
Fig. 3.
Identification of haplotypes and candidate genes associated with RDR on A02. (a) Manhattan plots based on GWAS showing the relative defoliation rate (RDR). The red circle denotes the genomic location of the RDR7 locus on chromosome A05. The dashed red line indicates the threshold − log P = 6. (b) Gene models (upper) and local Manhattan plots (lower) in the RDR7 region. The red circle represents the SNP A02:1403053. (c) Local LD heatmap in the RDR7 region. (d) Gene structure of GhLRR. The nonsynonymous SNP at 458 bp is marked by a black line. (e) Protein structure model of GhLRR. Comparing with His153, the Arg153 side chain pattern was changed. The 3D structures of the two allele proteins were predicted using I-TASSER and visualized with PyMOL v2.5.0. (f) Comparison of the relative defoliation rates among different haplotypes of locus RDR7. ****P < 0.0001, significance was evaluated by two-tailed Student’s t test. (g) Gene expression profiles of GhLRR at 24 h, 48 h and 72 h after TDZ and water treatment based on RNA-seq data. TDZ, thidiazuron. BL34 and LM28 represent the sensitive accession and insensitive accession, respectively. (h) Validation of GhLRR gene expression between BL34 and LM28 using qRT-PCR. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Haplotypes and candidate genes of the relative defoliation rate locus RDR13 on A13
The locus of the relative defoliation rate (RDR13) was identified on A13 with an interval length of ∼46 kb within the range of 5.17–5.21 Mb (Fig. 4a; Table S14), and four genes were found in this association region (Fig. 4b). A linkage disequilibrium analysis showed that the 37 SNPs within the candidate interval formed two LD blocks (Fig. 4c). A molecular phylogenetic tree was constructed based on these 37 SNPs, and the association panel was reordered by local clustering. Then, a genotype heatmap was drawn (Fig. 4d). On the basis of the heatmap and LD Block 1, the association population was divided into 2 haplotypes. These haplotypes were further characterized and analyzed, but it was found that there were no significant differences between the two haplotypes in terms of the relative abscission rate. Thus, we focused on LD Block 2, and the genotype of association panel was divided into three haplotypes and designated HapRDR13-1, HapRDR13-2, and Hap RDR13-3 (Fig. 4d). The genotyping of the haplotypes indicated that the relative abscission rate of the HapRDR13-2 varieties was significantly higher than that of other haplotypes at P < 0.001 (Fig. 4e). Thus, HapRDR13-2 was termed HapRDR13, while HapRDR13-1 and Hap RDR13-3 were merged and termed Haprdr13. Gh_A13G042700 was identified as a possible candidate gene on the basis of the annotation of four genes in the candidate interval. Gh_A13G042700 encoded D3-type cell cyclin 1 (CYCD3;1) and was designated GhCYCD3;1. CYCs were induced by thidiazuron to promote the cell cycle during leaf abscission in cotton [25]. In Arabidopsis, CYCD3;1 increased the sensitivity of cells to KNOX gene activity, which in turn promoted cytokinin synthesis and responses [47]. Both the KNOX gene and cytokinin played important roles in organ abscission [3], [48].
Fig. 4.
Identification of haplotypes and the candidate gene associated with RDR on A13. (a) Manhattan plots based on the GWAS showing the relative defoliation rate (RDR). The red circle denotes the genomic location of the RDR13 locus on chromosome A13. The dashed red line indicates the threshold − log P = 6. (b) Gene models (upper) and local Manhattan plots (lower) in the RDR13 region. (c) Local LD heatmap in the RDR13 region. (d) Haplotypes of the RDR13 locus in the 383 panel. Accessions (vertical) are reordered according to clustering based on regional SNPs (horizontal). The genotypes of the accessions are categorized into three haplotypes (HapRDR13_1, HapRDR13_2 and HapRDR13_3). (e) Comparison of the relative defoliation rate among different haplotypes of locus RDR13. ***P < 0.001, ****P < 0.0001. Significance was tested by two-tailed Student’s t test. (f) Gene expression profiles of GhCYCD3:1 at 24 h, 48 h and 72 h after TDZ and water treatment based on RNA-seq data. BL34 and LM28 represent the sensitive accession and insensitive accession, respectively. (g) Validating the gene expression of GhCYCD3:1 between BL34 and LM28 at 24 h, 48 h and 72 h using qRT-PCR. (h) Expression level of GhCYCD3:1 between the haplotype RDR13 and RDR13, empty control and VIGS lines. (i) Defoliation rate comparison between VIGS lines (RDR13 accession and rdr13 accession) at 84 h, 96 h, 108 h and 120 h after TDZ treatment. (i) Defoliation phenotype of VIGS lines for GhCYCD3;1 gene at 120 h after TDZ treatment. TRV:00: empty control; TRV:GhCYCD3;1: VIGS line; TDZ: thidiazuron. The scale bar is 6 cm. (k) Comparison of RDR values of four haplotype combinations (RDR7 + RDR13, RDR7, RDR13, and NA). NA indicates accessions carrying no favorable haplotypes. Significance was tested by two-tailed Student’s t test. (l) Frequencies of the four haplotype combinations in the three groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To validate the function of GhCYCD3;1 during defoliation in cotton, we performed a gene expression analysis on two varieties with different defoliant sensitivities. We found that the expression of this gene was upregulated at 12 h and 48 h after TDZ treatment in both defoliant-sensitive (BL34) and defoliant-insensitive (LM28) accessions, respectively, but in BL34, the increase at 12 h was greater and the decrease at 48 h was lesser than those in LM28 (Fig. 4f, Table S18). The expression pattern of GhCYCD3;1 identified via qRT-PCR was similar to the obtained with RNA-seq data (Fig. 4g, Table S19), namely, the expression level of GhCYCD3;1 in LM28 was lower than that in BL34, but it declined faster. The subcellular localization of GhCYCD3;1 was indicated by green fluorescent signals from GhCYCD3;1-GFP, which were found exclusively in nuclei (Fig. S13), suggesting that GhCYCD3;1 is a nucleus-localized protein. We further demonstrated the function of GhCYCD3;1 through virus-induced gene silencing (VIGS) in two haplotype cotton varieties (HapRDR13 and Haprdr13). To measure silencing efficiency, the gene expression level was measured by qRT-PCR after the albino phenotype appeared, and the gene expression level of the TRV:GhCYCD3;1-silenced strain was significantly lower than that of the TRV:00 (empty vector) and CK strains. Moreover, no significant difference in gene expression levels between CK and TRV:00 was found (Fig. 4h). At the eight- to ten-leaf stage, the defoliation rate of all VIGS materials was calculated after TDZ treatment. The defoliation rate of the TRV:GhCYCD3;1-silenced lines was significantly lower than that of both the TRV:00 in HapRDR13 and Haprdr13 accessions (Figs. 4I, j, S14 and S15), implying that GhCYCD3;1 plays a potential role in regulating cotton defoliation.
The combination of two favorable haplotypes improved sensitivity to defoliant
The effects of combining favorable haplotypes for the RDR trait were evaluated by comparing the RDR values of accessions carrying different combinations of favorable alleles. (Fig. 4k–l). We found that eight accessions carrying two favorable haplotypes (HapRDR7 and HapRDR13) showed higher sensitivity to defoliant than those carrying only one haplotype (HapRDR7 or HapRDR13) or no haplotype (NA, Fig. 4k). Frequency differences in favorable RDR haplotype combinations were found among the three groups. G2 lines carried more RDR-favorable haplotypes than the lines in the other two groups, that carried similar proportions of favorable haplotypes (Fig. 4l). These results lead to new insights into increasing leaf abscission; that is, breeders can transfer the favorable haplotype from G2 lines to other plants to elevate the sensitivity of upland cotton through breeding.
To understand the genotype differences between defoliant-sensitive and defoliant-tolerant germplasms, we selected the top 25% and bottom 25% germplasms based on the mean values of RDR in four environments (Table S20). These accessions were highly representative because most of the sensitive or insensitive lines identified were included (Table S20). In the top 25% of the material, six accessions carried two favorable haplotypes (HapRDR7 and HapRDR13) and showed the highest relative defoliation rate (average RDR 4.15) (Table S21). In the top 25% of the material, 16 accessions carried one favorable haplotype (HapRDR7 or HapRDR13) and showed a relative high defoliation rate (average RDR 3.33) (Table S21). However, only one accession with two favorable haplotypes and 8 accessions with one favorable haplotype were found in the bottom 25% of the material, and their relative defoliation rates were the lowest (average RDRs of 1.66 and 1.48, respectively, Table S21). These results further showed that the varieties with more favorable haplotypes usually showed increased defoliation rates.
Favorable haplotype frequency varied in different cotton-growing areas
The haplotype frequencies of the RDR7 and RDR13 loci related to the relative defoliation rate in different groups and geographic regions were calculated. A higher frequency of favorable haplotypes (carrying HapRDR7 or HapRDR13) was detected in the G2 group (18.18% or 21.21%, Fig. S1, Table S22) and in NC germplasm grown in high latitudes (30.00% and 44.44%, Fig. 5, Table S23), respectively. The haplotype frequencies of both HapRDR7 and HapRDR13 gradually decreased from that of NC to that of YER and further that of YZR until it dropped to zero in SC with the reduction in latitude and the increase in annual average temperature. However, the favorable haplotype frequency of HapRDR7 or HapRDR13 carried by NWC was lower because of the weak adaptive pressure in breeding (Fig. 5, Table S23). The haplotype frequencies of USA and FSU were in the intermediate range. These results imply that these two defoliation-related loci in the germplasm from high latitudes of China (east of 105° east longitude) had typically been subjected to stronger adaptive pressure than the germplasm in low latitudes.
Fig. 5.
Haplotype frequency analysis in different geographic regions. Pie charts represent haplotype frequencies of the RDR7 and RDR13 loci in different geographic populations. The accessions distributed in five major cotton cultivation regions in China are circled and colored. Climate data (average temperature from April to October of 1970–2000) were downloaded from www.worldclim.org v.2.1 [45] and visualized by QGIS v.3.10.13. NC: North of China, NWC: Northwest China, YER: Yellow River region, YZR: Yangtze River region, SC: South of China, FSU: Former Soviet Union, USA: The United States of America. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Discussion
With the increase in labor costs and planting scale, machine harvesting is increasingly being applied in a wide range of crops, such as cotton and sugarcane. Concentrated defoliation by applying defoliant prior to machine harvesting may not only increase harvesting efficiency but also increase crop quality by reducing impurities in grains or fibers. Defoliation before harvest is a promising agricultural practice. Therefore, identifying the genes that regulate defoliation and dissecting they underlying genetic mechanisms to promote leaf shedding through genetic manipulation will be of important practical significance in agricultural production.
In the present study, a pattern of favorable haplotype frequencies for leaf abscission loci was observed; that is, the frequencies of both HapRDR7 and HapRDR13 in accessions from different geographic populations in China, except NWC, gradually decreased from the north to the south (Fig. 5, Table S23). Correspondingly, the annual temperature gradually increased (Fig. S16a), and the annual temperature was significantly negatively correlated with RDR (Fig. S16b). Thus, the climatic conditions in the cotton-growing season, such as the accumulated temperature, may play critical roles in the allele frequencies of cotton defoliation in different cultivation regions (Fig. 1d, Fig. 5). Notably, most NC accessions were specific early-maturity cultivars (SEM) and derived from King cotton, a primitive variety that was introduced to China from the USA. King cotton has been suggested to be the major donor of the early maturity trait in modern Chinese breeding programs [49]. Most NWC accessions are early and medium maturity cultivars (EMM), and some central Asian varieties are dominant in the genetic components of independent breeding varieties in NWC [49]. The favorable haplotype frequencies of the RDR7 and RDR13 loci have been increased due to the very different ecological growing conditions between the NC and areas of origin for King cotton. However, the frequencies were decreased in the NWC accessions because the local environment was similar to that of central Asia. We suggest that a favorable haplotype for defoliation might have originated from King cotton.
The growth period (GP) of cotton is an adaptive trait that is significantly influenced by climatic conditions [50], but we know only the correlation between defoliation-related traits and GP (Fig. 1c, Table S5). Thus, we compared phenotypic variation trends and analyzed the relationship between these trends (Fig. S17). The comparison of different groups showed that the DRCK in both NC and G2 was the lowest (Fig. S17a and S17d), and the RDR trait followed the opposite trend when DRCK was denominator (Fig. S17c and S17f). However, no pattern was found for DRT (Fig. S17b and S17e). The highest frequency of haplotypes in the NC and G2 lines might explain the higher sensitivity to defoliants of the NC and G2 lines (Fig. 5, Fig. S1, Table S22–S23). The variation trends of different regions and subpopulations for the GP trait (BOD, boll open day) were similar to those for the DRCK trait but contrary to those for RDR because GP was significantly positively correlated with DRCK and significantly negatively correlated with RDR (Fig. 1c, Table S5). In addition, most NC materials (7/11) were included in G2, which likely raised the favorable haplotype frequency of the G2 lines and ultimately the sensitivity to defoliants.
The genome-wide differentiation between pairwise subpopulations was evaluated based on the FST value. The lowest population differentiation estimate (FST) was observed between G1 and G3 (0.116, Fig. 2c). The number of overlapping loci identified via GWAS of G1-G3 was approximately one-half that of G1-G2 and G2-G3 (Fig. S6a-6b, Table S10-S12, S17). All of these results indicated obvious differentiation between pairwise subgroups with the significant genomic feature of large divergent regions in A06 and/or A08 (Fig. S6). These genomic regions overlapped with the extensive chromosome inversions of A06 and A08, which affected population differentiation and environmental adaptability to a great extent [28], [46]. As previously mentioned, most NC and NWC materials considered to be SEM and EMM were included in the G2 subgroup. However, the main accessions of G1 were from YZR with late maturity (Fig. S17g) [51], and the accessions from YER and the USA were medium and late maturity (Fig. S17g) and were primarily assigned to G3. Furthermore, the main founder parents of the YER and YZR cultivars, such as Deltapine 15, were introduced from the US cotton belt [50], [51], where the local environment is similar to that of YER and YZR. Early- maturity and special early-maturity cotton has undergone adaptive changes through its breeding history [53]. The medium- and late-maturity cotton mainly in G1 and G3 may have been subjected to weaker adaptive pressures, resulting in faster LD decay in G1 and G3 lines than in G2 lines (Fig. S5b). Thus, the differentiation between subpopulations may be explained by the long period of environmental pressure on adaptation traits, such as GP (Fig. S17a, S17d, S17g, S17h). However, the differentiation between G1 and G2 may be specifically associated with the genomic divergence of both A06 and A08 [28], G1-G3 lines are likely connected to A08, which is the primary reason for the differentiation and extensive adaptation of Deltapine 15-derived cultivars [51], [52], and G2-G3 may be associated with A06, with eco-haplotypes that are favored north of 32.5°north latitude [46].
These GWAS loci and the underlying genes provided potential valuable resources for the genetic development of defoliation traits in cotton. For instance, GhCYCD3;1, which is associated with RDR, was identified in A13 (Fig. 4). CYCD3;1 encodes the D3-type cell cyclin 1 protein, which promotes mitotic cell division and inhibits endoreduplication [54], [55], [56]. In this study, we demonstrated that GhCYCD3;1 may play a positive role in regulating leaf abscission (Fig. 4). CYCD3;1 also functions in increasing the sensitivity of cells to KNOX gene activity, which in turn promotes cytokinin synthesis and responses in Arabidopsis [47]. Recently, reports have shown that the cytokinin signaling pathway participates in defoliation in cotton [3], and KNOX transcription factors regulate abscission zone development [48], [57]. Therefore, we proposed a possible mechanistic link to defoliation, which involves cytokinin, KNOX and CYCD3;1. GhCYCD3;1 promotes the transcriptional regulation of KNOX transcription factors to cytokinin synthesis and response-related genes, and then, the level of endogenous cytokinin is increased, and the process of defoliation is accelerated. GhLRR was another gene found to be associated with RDR (Fig. 3). GhLRR encodes a leucine-rich repeat (LRR) family protein and is involved in signal transduction. Varieties carrying HapRDR7 showed increased sensitivity compared with those carrying Haprdr7. Integrating the allele analysis data, it has been shown suggested that GhLRR may play a crucial role in the abscission signaling pathway [13] and may be useful for increasing defoliation sensitivity during upland cotton breeding.
Conclusions
In this study, four defoliation-related traits in 383 resequenced G. hirsutum accessions were first investigated in four environments over two years at three locations to reveal the fundamental characteristics of leaf abscission. On the basis of a GWAS, genome linkage disequilibrium (LD) interval genotyping and functional identification, the genetic basis and candidate genes of associated elite loci for defoliation-related traits were identified in G. hirsutum. Finally, the haplotype variation for environmental adaptability related to defoliation traits was discovered. The results provide new insights into the genetic basis of leaf abscission and together serve as a reference supporting machine-picked cotton improvement.
Data and materials availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All the genomic sequencing data for genetic structure analysis and GWAS were available from the NGDC BioProject accession number PRJCA010331. The transcriptome sequencing data have been also deposited in NGDC under accession number PRJCA009376.
CRediT authorship contribution statement
Hongge Li: Formal analysis, Investigation, Funding acquisition, Writing - original draft, Writing - review & editing. Xiangru Wang: Formal analysis, Investigation, Writing - original draft. Ning Qin: Formal analysis, Visualization, Investigation. Daowu Hu: Investigation. Yinhua Jia: Investigation. Gaofei Sun: Software, Data curation. Liangrong He: Resources. Hengheng Zhang: Investigation. Panhong Dai: Software. Zhen Peng: Formal analysis. Nianchang Pang: Investigation. Zhaoe Pan: Resources. Xiaomeng Zhang: Visualization. Qiang Dong: Formal analysis. Baojun Chen: Resources. Huiping Gui: Investigation. Baoyin Pang: Resources. Xiling Zhang: Conceptualization. Shoupu He: Supervision, Writing – original draft, Writing – review & editing. Meizhen Song: Conceptualization, Writing – original draft, Writing – review & editing. Xiongming Du: Conceptualization, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate the National Cotton Germplasm Mid-term bank (Anyang) for providing the cotton germplasm seeds. The authors also thank Peng Zhang, Liting Hu, Kang Ruan, Liru Wang, Juming Zhang, and Dingsha Jin, Institute of Cotton Research, China Academy of Agricultural Sciences, for their helps in field experiments. This work was supported by a grant from the National Key Research and Development Program of China (2022YFD1200300), the Crop Germplasm Conservation Program of Ministry of Agriculture in China (19221957) and the Central Public-Interest Scientific Institution Basal Research Fund of Cotton Research Institute, CAAS (1610162023037).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.05.007.
Contributor Information
Xiling Zhang, Email: zhangxiling@caas.cn.
Shoupu He, Email: heshoupu@caas.cn.
Meizhen Song, Email: songmeizhen@caas.cn.
Xiongming Du, Email: dujeffrey8848@hotmail.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Patharkar O.R., Walker J.C. Advances in abscission signaling. J Exp Bot. 2018;69(4):733–740. doi: 10.1093/jxb/erx256. [DOI] [PubMed] [Google Scholar]
- 2.Ji H., Kim S.R., Kim Y.H., Kim H., Eun M.Y., Jin I.D., et al. Inactivation of the CTD phosphatase-like gene OsCPL1 enhances the development of the abscission layer and seed shattering in rice. Plant J. 2010;61(1):96–106. doi: 10.1111/j.1365-313X.2009.04039.x. [DOI] [PubMed] [Google Scholar]
- 3.Xu J., Chen L., Sun H., Wusiman N., Sun W., Li B., et al. Crosstalk between cytokinin and ethylene signaling pathways regulates leaf abscission in cotton in response to chemical defoliants. J Exp Bot. 2019;70(5):1525–1538. doi: 10.1093/jxb/erz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin D., Wang X., Xu Y., Gui H., Zhang H., Dong Q., et al. Chemical defoliant promotes leaf abscission by altering ROS metabolism and photosynthetic efficiency in Gossypium hirsutum. Int J Mol Sci. 2020;21(8):2738. doi: 10.3390/ijms21082738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M., Liang Z., Zeng Y., Jing Y., Wu K., Liang J., et al. De novo analysis of transcriptome reveals genes associated with leaf abscission in sugarcane (Saccharum officinarum L.) BMC Genomics. 2016;17:195. doi: 10.1186/s12864-016-2552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M., Liang Z., He S., Zeng Y., Jing Y., Fang W., et al. Genome-wide identification of leaf abscission associated microRNAs in sugarcane (Saccharum officinarum L.) BMC Genomics. 2017;18(1):754. doi: 10.1186/s12864-017-4053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu S., Wang J., Shang H., Huang Y., Yao W., Chen B., et al. Transcriptomic characterization and potential marker development of contrasting sugarcane cultivars. Sci Rep. 2018;8(1):1683. doi: 10.1038/s41598-018-19832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsson V., Butenko M.A. Abscission in plants. Current Biol. 2018;28(8):R338–R339. doi: 10.1016/j.cub.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 9.Kim J. Four shades of detachment: regulation of floral organ abscission. Plant Signal Behav. 2014;9(11):e976154. doi: 10.4161/15592324.2014.976154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKim S.M., Stenvik G.E., Butenko M.A., Kristiansen W., Cho S.K., Hepworth S.R., et al. The BLADE-ON-PETIOLE genes are essential for abscission zone formation in Arabidopsis. Development. 2008;135(8):1537–1546. doi: 10.1242/dev.012807. [DOI] [PubMed] [Google Scholar]
- 11.Mao L., Begum D., Chuang H.W., Budiman M.A., Szymkowiak E.J., Irish E.E., et al. JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature. 2000;406(6798):910–913. doi: 10.1038/35022611. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y., Nakano T. Development and regulation of pedicel abscission in tomato. Front Plant Sci. 2015;6:442. doi: 10.3389/fpls.2015.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jinn T.L., Stone J.M., Walker J.C. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000;14(1):108–117. [PMC free article] [PubMed] [Google Scholar]
- 14.Stenvik G.E., Butenko M.A., Urbanowicz B.R., Rose J.K., Aalen R.B. Overexpression of INFLORESCENCE DEFICIENT IN ABSCISSION activates cell separation in vestigial abscission zones in Arabidopsis. Plant Cell. 2006;18(6):1467–1476. doi: 10.1105/tpc.106.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenvik G.E., Tandstad N.M., Guo Y., Shi C.L., Kristiansen W., Holmgren A., et al. The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in Arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell. 2008;20(7):1805–1817. doi: 10.1105/tpc.108.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng X., Zhou J., Tang J., Li B., de Oliveira M.V.V., Chai J., et al. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Rep. 2016;14(6):1330–1338. doi: 10.1016/j.celrep.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santiago J., Brandt B., Wildhagen M., Hohmann U., Hothorn L.A., Butenko M.A., et al. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. Elife. 2016;5:e15075. doi: 10.7554/eLife.15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho S.K., Larue C.T., Chevalier D., Wang H., Jinn T.L., Zhang S., et al. Regulation of floral organ abscission in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008;105(40):15629–15634. doi: 10.1073/pnas.0805539105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi C.L., Stenvik G.E., Vie A.K., Bones A.M., Pautot V., Proveniers M., et al. Arabidopsis class I KNOTTED-like homeobox proteins act downstream in the IDA-HAE/HSL2 floral abscission signaling pathway. Plant Cell. 2011;23(7):2553–2567. doi: 10.1105/tpc.111.084608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meir S., Philosoph-Hadas S., Riov J., Tucker M.L., Patterson S.E., Roberts J.A. Re-evaluation of the ethylene-dependent and -independent pathways in the regulation of floral and organ abscission. J Exp Bot. 2019;70(5):1461–1467. doi: 10.1093/jxb/erz038. [DOI] [PubMed] [Google Scholar]
- 21.Wang M., Tu L., Yuan D., Zhu D., Shen C., Li J., et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat Genet. 2019;51(2):224–229. doi: 10.1038/s41588-018-0282-x. [DOI] [PubMed] [Google Scholar]
- 22.Chao L., Pan Z., Wang J., Wu Y., Shui G., Aini N., et al. Genetic mapping and analysis of a compact plant architecture and precocious mutant in upland cotton. Plants (Basel) 2022;11(11):1483. doi: 10.3390/plants11111483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGarry R.C., Prewitt S.F., Culpepper S., Eshed Y., Lifschitz E., Ayre B.G. Monopodial and sympodial branching architecture in cotton is differentially regulated by the Gossypium hirsutum SINGLE FLOWER TRUSS and SELF-PRUNING orthologs. New Phytol. 2016;212(1):244–258. doi: 10.1111/nph.14037. [DOI] [PubMed] [Google Scholar]
- 24.Mishra A., Khare S., Trivedi P.K., Nath P. Ethylene induced cotton leaf abscission is associated with higher expression of cellulase (GhCel1) and increased activities of ethylene biosynthesis enzymes in abscission zone. Plant Physiol Biochem. 2008;46(1):54–63. doi: 10.1016/j.plaphy.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Li F., Wu Q., Liao B., Yu K., Huo Y., Meng L., et al. Thidiazuron promotes leaf abscission by regulating the crosstalk complexities between ethylene, auxin, and cytokinin in cotton. Int J Mol Sci. 2022;23(5):2696. doi: 10.3390/ijms23052696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang L., Wang Q., Hu Y., Jia Y., Chen J., Liu B., et al. Genomic analyses in cotton identify signatures of selection and loci associated with fiber quality and yield traits. Nat Genet. 2017;49(7):1089–1098. doi: 10.1038/ng.3887. [DOI] [PubMed] [Google Scholar]
- 27.Ma Z., He S., Wang X., Sun J., Zhang Y., Zhang G., et al. Resequencing a core collection of upland cotton identifies genomic variation and loci influencing fiber quality and yield. Nat Genet. 2018;50(6):803–813. doi: 10.1038/s41588-018-0119-7. [DOI] [PubMed] [Google Scholar]
- 28.He S., Sun G., Geng X., Gong W., Dai P., Jia Y., et al. The genomic basis of geographic differentiation and fiber improvement in cultivated cotton. Nat Genet. 2021;53(6):916–924. doi: 10.1038/s41588-021-00844-9. [DOI] [PubMed] [Google Scholar]
- 29.Bates D., Machler M., Bolker B.M., Walker S.C. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 30.Yang Z., Ge X., Yang Z., Qin W., Sun G., Wang Z., et al. Extensive intraspecific gene order and gene structural variations in upland cotton cultivars. Nat Commun. 2019;10(1):2989. doi: 10.1038/s41467-019-10820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander D.H., Novembre J., Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Lee S.H., Goddard M.E., Visscher P.M. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price M.N., Dehal P.S., Arkin A.P. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26(7):1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C., Dong S.S., Xu J.Y., He W.M., Yang T.L. PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics. 2019;35(10):1786–1788. doi: 10.1093/bioinformatics/bty875. [DOI] [PubMed] [Google Scholar]
- 38.Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang HM, Sul JH, Service SK, Zaitlen NA, Kong SY, Freimer NB, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet 2010; 42(4):348–54. [DOI] [PMC free article] [PubMed]
- 40.Turner SD. qqman: an R package for visualizing GWAS results using QQ and manhattan plots. bioRxiv 2014:005165.
- 41.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J., Zhang Y. I-TASSER server: new development for protein structure and function predictions. Nucl Acids Res. 2015;43(W1):W174–W181. doi: 10.1093/nar/gkv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLano W.L., Lam J.W. PyMOL: a communications tool for computational models. Abstr Pap Am Chem Soc. 2005;230:U1371–U1372. [Google Scholar]
- 45.Fick S.E., Hijmans R.J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol. 2017;37(12):4302–4315. [Google Scholar]
- 46.Dai P., Sun G., Jia Y., Pan Z., Tian Y., Peng Z., et al. Extensive haplotypes are associated with population differentiation and environmental adaptability in Upland cotton (Gossypium hirsutum) Theor Appl Genet. 2020;133(12):3273–3285. doi: 10.1007/s00122-020-03668-z. [DOI] [PubMed] [Google Scholar]
- 47.Scofield S., Dewitte W., Nieuwland J., Murray J.A.H. The Arabidopsis homeobox gene SHOOT MERISTEMLESS has cellular and meristem-organisational roles with differential requirements for cytokinin and CYCD3 activity. Plant J. 2013;75(1):53–66. doi: 10.1111/tpj.12198. [DOI] [PubMed] [Google Scholar]
- 48.Wang X.Q., Xu W.H., Ma L.G., Fu Z.M., Deng X.W., Li J.Y., et al. Requirement of KNAT1/BP for the development of abscission zones in Arabidopsis thaliana. J Integr Plant Biol. 2006;48(1):15–26. [Google Scholar]
- 49.He S., Sun G., Huang L., Yang D., Dai P., Zhou D., et al. Genomic divergence in cotton germplasm related to maturity and heterosis. J Integr Plant Biol. 2019;61(8):929–942. doi: 10.1111/jipb.12723. [DOI] [PubMed] [Google Scholar]
- 50.Wang P., Dong N., Wang M., Sun G., Jia Y., Geng X., et al. Introgression from Gossypium hirsutum is a driver for population divergence and genetic diversity in Gossypium barbadense. Plant J. 2022;110(3):764–780. doi: 10.1111/tpj.15702. [DOI] [PubMed] [Google Scholar]
- 51.He S., Wang P., Zhang Y.M., Dai P., Nazir M.F., Jia Y., et al. Introgression leads to genomic divergence and responsible for important traits in upland cotton. Front Plant Sci. 2020;11:929. doi: 10.3389/fpls.2020.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Nazir M.F., He S., Li H., Pan Z., Sun G., et al. Deltapine 15 contributes to the genomic architecture of modern upland cotton cultivars. Theor Appl Genet. 2022;135(4):1401–1411. doi: 10.1007/s00122-022-04042-x. [DOI] [PubMed] [Google Scholar]
- 53.Li L., Zhang C., Huang J., Liu Q., Wei H., Wang H., et al. Genomic analyses reveal the genetic basis of early maturity and identification of loci and candidate genes in upland cotton (Gossypium hirsutum L.) Plant Biotechnol J. 2021;19(1):109–123. doi: 10.1111/pbi.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riou-Khamlichi C., Huntley R., Jacqmard A., Murray J.A. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999;283(5407):1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- 55.Dewitte W., Riou-Khamlichi C., Scofield S., Healy J.M., Jacqmard A., Kilby N.J., et al. Altered cell cycle distribution, hyperplasia, and inhibited differentiation in Arabidopsis caused by the D-type cyclin CYCD3. Plant Cell. 2003;15(1):79–92. doi: 10.1105/tpc.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dewitte W., Scofield S., Alcasabas A.A., Maughan S.C., Menges M., Braun N., et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci USA. 2007;104(36):14537–14542. doi: 10.1073/pnas.0704166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao M., Li C., Ma X., Xia R., Chen J., Liu X., et al. KNOX protein KNAT1 regulates fruitlet abscission in litchi by repressing ethylene biosynthetic genes. J Exp Bot. 2020;71(14):4069–4082. doi: 10.1093/jxb/eraa162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.