Graphical abstract
Keywords: Cardiovascular diseases, Erectile dysfunction, Lifestyle factors, Mendelian randomization, Metabolic diseases, Mental disorder
Highlights
-
•
Our study explored the causal association between 42 predominant risk factors and erectile dysfunction (ED) under a two-sample MR framework.
-
•
This comprehensive MR study supported the causal role of obesity, type 2 diabetes, basal metabolic rate, poor self-health rating, cigarette and alcohol consumption, insomnia and snoring, depression, hypertension, stroke, ischemic stroke, coronary heart disease, myocardial infarction, heart failure, SHBG and adiponectin in the onset and development of ED. A clear causal link may be beneficial to early identification and target intervention in patients with ED.
-
•
No significant association was detected between lipid levels and ED.
-
•
Genetic predisposition to higher sex hormone-binding globulin (SHBG) levels can decrease the risk of ED.
-
•
Identifying reversible risk factors is the first-line evaluation for ED patients as per the diagnostic work-up of the European Association of Urology Guidelines. This study provides a better understanding of the risk factors of ED and is conducive to early identification and intervention of ED.
Abstract
Introduction
The causal association between modifiable risk factors and erectile dysfunction (ED) remains unclear, which hinders the early identification and intervention of patients with ED. The present study aimed to clarify the causal association between 42 predominant risk factors and ED.
Methods
Univariate Mendelian Randomization (MR), multivariate MR, and mediation MR analyses were used to investigate the causal association between 42 modifiable risk factors and ED. Combined results were pooled from two independent ED genome-wide association studies to verify the findings.
Results
Genetically predicted body mass index (BMI), waist circumference, trunk fat mass, whole body fat mass, poor overall health rating, type 2 diabetes, basal metabolic rate, adiponectin, cigarette consumption, insomnia, snoring, hypertension, stroke, ischemic stroke, coronary heart disease, myocardial infarction, heart failure, and major depressive disorder were found to increase the risk of ED (all P < 0.05). Additionally, genetic liability to higher body fat percentage and alcohol consumption were suggestively associated with an increased risk of ED (P < 0.05 and adjusted P > 0.05). Genetic predisposition to higher sex hormone-binding globulin (SHBG) levels could decrease the risk of ED (P < 0.05). No significant association was detected between lipid levels and ED. Multivariate MR identified type 2 diabetes, basal metabolic rate, cigarette consumption, hypertension, and coronary heart disease as risk factors for ED. The combined results confirmed that waist circumference, whole body fat mass, poor overall health rating, type 2 diabetes, basal metabolic rate, adiponectin, cigarette consumption, snoring, hypertension, ischemic stroke, coronary heart disease, myocardial infarction, heart failure, and major depressive disorder could increase the risk of ED (all P < 0.05), while higher SHBG decreased the risk of ED (P = 0.004). There were suggestive significances of BMI, insomnia, and stroke on ED (P < 0.05 and adjusted P > 0.05).
Conclusion
This comprehensive MR study supported the causal role of obesity, type 2 diabetes, basal metabolic rate, poor self-health rating, cigarette and alcohol consumption, insomnia and snoring, depression, hypertension, stroke, ischemic stroke, coronary heart disease, myocardial infarction, heart failure, SHBG, and adiponectin in the onset and development of ED.
Introduction
Erectile dysfunction (ED) is characterized by inadequate penile erection and unsatisfactory vaginal intercourse [1]. This condition is unlethal but poses a noteworthy challenge to a considerable proportion of males. The prevalence of ED is 52% in Americans aged 40–70 years, 30% in Europeans aged 40–79 years, and 63% in Asians aged 50–80 years [2], [3], [4]. Notably, ED is no longer simply confined to sexual intercourse but aggravates psychological and social disintegration in affected individuals. It was reported that 79.82% and 79.56% of ED patients show comorbidity with anxiety and depression, respectively [5]. Therefore, clarifying the risk factors for ED is required and potentially beneficial for early identification and targeted intervention of risk factors in ED patients.
Previous reports have identified some risk factors in observational studies. Metabolic diseases like dyslipidemia are reportedly associated with ED [6]. In addition, lifestyle factors, including cigarette and alcohol consumption are involved in the onset of ED [7], [8], [9], [10]. Moreover, mental disorders like depression and cardiovascular diseases like coronary heart disease have been linked with ED [5], [11]. However, other observational studies do not support the association between coronary heart disease, sleep disorders, cigarette and alcohol consumption, dyslipidemia and ED [12], [13], [14], [15], [16], [17]. A Mendelian Randomization (MR) study has also indicated a null association between lipid levels and ED [18]. The findings remain controversial in different surveys and need further exploration. It is urgent to clarify whether the modifiable factors play causal roles in the onset and development of ED or are just comorbid conditions.
To address the bias from observational design, we used a new approach, employing MR to investigate the causal impact of modifiable factors on ED. MR uses genetic variants (single nucleotide polymorphisms, SNPs) as instrumental variables (IVs) to replace the exposures (i.e., obesity) and outcomes (i.e., ED) [19]. SNPs are assorted randomly during gestation, and not affected by postnatal factors like lipids levels. Therefore, bias from residual confounding is minimized, and the reverse causality in observational design can be avoided. To date, limited evidences from MR has been used to clarify the causal association between several modifiable factors and ED, thus hindering a full understanding of potential causal risk factors for ED.
Herein we explored the causal association between 42 predominant risk factors and ED under a two-sample MR framework. The results were combined from two independent genome-wide association datasets. A clear causal link may be beneficial to early identification and targeted intervention in patients with ED.
Materials and methods
Ethics statement
All experiments involving animals and humans were conducted according to the ethical policies and procedures approved by the ethics committee of West China Hospital, Sichuan University, China (Approval no. 20220421002). Informed consent was obtained from all subjects in the original genome-wide association studies. For genome-wide association study (GWAS) datasets, ethical review and approval can be accessed in the original studies.
MR design and selection of IVs
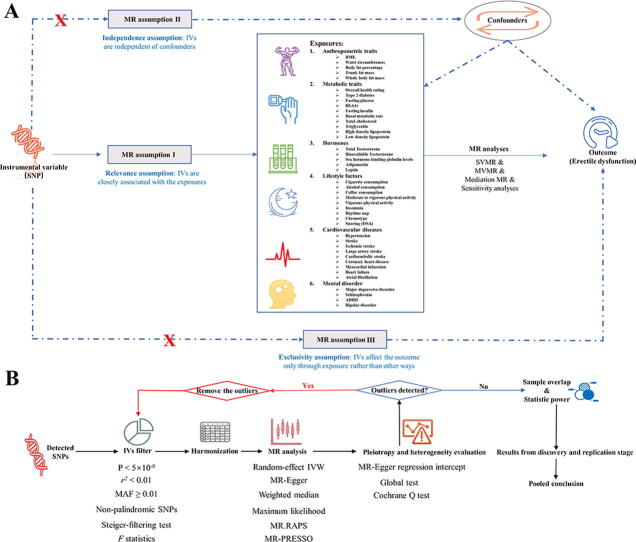
MR has three basic assumptions that assure the reliability of the results, namely relevance, exclusivity, and independence (Fig. 1A). The relevance assumption requires a close association between IVs and exposure. The independence assumption requires that IVs should be independent of confounders. The exclusivity assumption highlights that IVs can only affect the outcomes (i.e., ED) through exposure, and not other pathways. In this study, two ED GWASs were used as outcome datasets. The GWAS dataset from Bovijn J et al. [20] was used as the discovery dataset and the summary statistics from FinnGen (https://r5.finngen.fi/) were used to replicate the findings (Fig. 1A). As reported previously, the results from two GWAS datasets were combined using a fixed-effect model [21], [22].
Fig. 1.
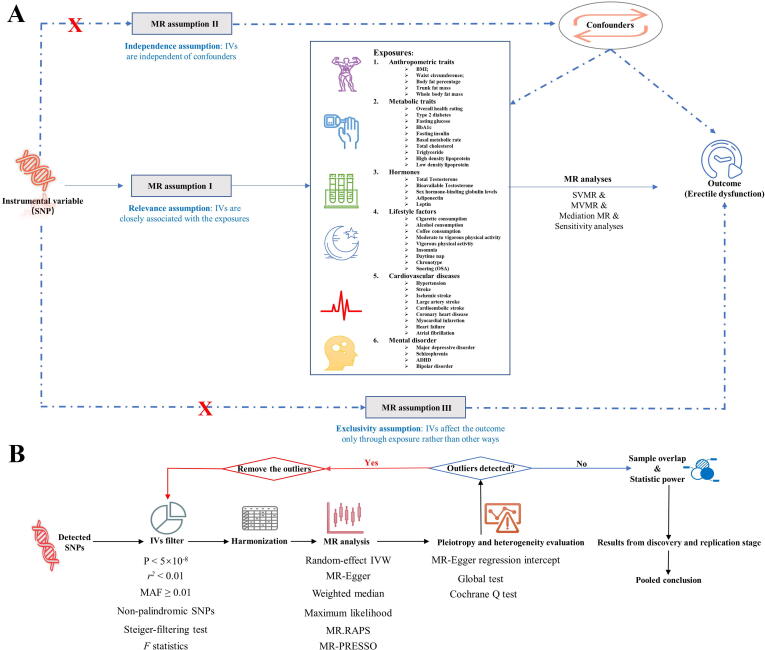
Overview of the study design and analysis strategy. (A): Overview of the study design. Exposures are from six domains including anthropometric traits, metabolic traits, lifestyle factors, hormones, cardiovascular diseases, and mental disorders. The MR framework is based on the three basic MR assumptions. (B): Analysis strategy of MR. Qualified SNPs are filtered as IVs and then subjected to sensitivity analyses and evaluation of heterogeneity and pleiotropy. The biases from sample overlap and statistic power are further evaluated. Results from the discovery and replication stages are combined. MR: Mendelian Randomization; SNP: single nucleotide polymorphism; BMI: body mass index; HbA1C: glycosylated hemoglobin type A1C; SHBG: sex hormone-binding globulin; SVMR: single variable MR; MVMR: multivariate MR.
Summary statistics and IVs from 42 GWAS datasets
The 42 modifiable factors were clustered into six domains as follows: (1) anthropometric traits including body mass index (BMI), waist circumference, body fat percentage, trunk fat mass and whole fat mass; (2) metabolic traits including overall health rating, type 2 diabetes, fasting glucose, glycosylated hemoglobin type A1C (HbA1c), fasting insulin, basal metabolic rate, total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL); (3) hormones including testosterone, bioavailable testosterone and sex hormone-binding globulin (SHBG) levels, adiponectin, and leptin; (4) lifestyle factors including cigarette, alcohol, and coffee consumption, physical activity (moderate to vigorous / vigorous), insomnia, daytime napping, chronotype and snoring; (5) cardiovascular diseases including stroke and its subtypes (ischemic, large artery, and cardioembolic), hypertension, coronary heart disease, myocardial infarction, heart failure, and atrial fibrillation, and (6) mental disorders including major depressive disorder, schizophrenia, attention deficit and hyperactivity disorder (ADHD), and bipolar disorder. The GWAS datasets were from different consortia.
IVs were extracted from the following sources: (1) the largest summary-level statistics that could be publicly downloaded at the time of analysis; (2) Genetic Investigation of ANthropometric Traits consortium (GIANT); (3) Psychiatric Genomics Consortium (PGC); (4) UK biobank (including Neale Lab and MRC-IEU); (5) Global Lipids Genetics Consortium (GLGC); (6) Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC); (7) MEGASTROKE; (8) Heart Failure Molecular Epidemiology for Therapeutic Targets (HERMES); (9) Coronary Artery Disease Genome-Wide Replication and Meta-analysis plus the Coronary Artery Disease Genetics (CARDIoGRAMplusC4D), and (10) GWAS and Sequencing Consortium of Alcohol and Nicotine use (GASCAN). Detailed information regarding the ancestry, consortia, diagnostic criteria, etc., has been described in Supplemental File 1. Of note, GWAS datasets from mixed races were filtered to avoid population architecture bias, and only genetic data from White European individuals were used, except for the dataset from the GLGC. GLGC is the most widely used lipid GWAS dataset with adequate participants, and 90.10% of individuals included are of European descent. Consequently, the bias from GLGC may be minimal.
As shown in Fig. 1B, IVs were extracted from corresponding summary-level statistics to perform single variable MR (SVMR), multivariable MR (MVMR), and mediation MR. In this study, the significance threshold was set at P < 5 × 10−8 to satisfy the relevance assumption. Additionally, SNPs with linkage disequilibrium, r2 < 0.01, at a window size of 10,000 kb were then filtered to confirm independence. Moreover, filtered SNPs were further pruned if they were palindromic or their minor allele frequencies were < 0.01. The MR-Steiger test, which calculated the variance explained by the exposures (i.e., BMI) and outcomes (i.e., ED), was performed to avoid reverse causality. Insignificant results under the test disclosed that these SNPs might affect the outcomes more than the exposure. Therefore, insignificant SNPs were deleted before harmonization. The extracted IVs were displayed in Supplemental Table 1. To reduce bias from weak IVs, we also calculated the F-statistics of SNPs, representing the strength of IVs. According to one previous study, F-statistics can be estimated using the following formula: F-statistics = (Beta/Se)2. Generally, F-statistics < 10 indicates the presence of weak IVs [21].
Summary statistics of ED GWASs
The outcome of GWASs on ED was obtained from two different consortia. The main discovery ED GWAS comprised 6,175 cases and 217,630 controls from three cohorts (UKB, Estonian Genome Center of the University of Tartu [EGCUT], and Partners HealthCare Biobank [PHB]) [20]. The cases were diagnosed according to the code of International Classification of Diseases version 10 (N48.4 and F52.2), medical history (medication and surgery for ED), or self-reporting. All the included individuals were of White ancestry. The METAL software was used for meta-analysis. GWAS data were adjusted for age and principal components (PCs), like population stratification, if necessary. The summary statistics of the second ED GWAS were from FinnGen, with 1,154 cases and 94,024 controls. A total of 16,378,833 SNPs were analyzed using SAIGE (https://github.com/weizhouUMICH). In the regression model, age, 10 PCs, and genotyping batch were included as covariates. Detailed information regarding the endpoint definition can be accessed on the official website (https://risteys.finngen.fi/endpoints/ERECTILE_DYSFUNCTION).
Ethical approval of GWASs included was approved by the corresponding ethics committee. In this MR study, only summary-level statistics were used. No identifiable private information was contained in the GWAS datasets. All the datasets were publicly accessible and could be used without restrictions.
Statistical analyses
As displayed in Fig. 1B, the qualified SNPs were used as IVs and then retrieved from the ED GWAS for harmonization. The Wald ratio was calculated to estimate the effect size of each IV and then combined using the inverse variance weighting (IVW) method. Moreover, five other approaches were employed to verify the findings yielded by the IVW estimator. The five sensitivity methods included MR-Egger, Weighted median, Maximum likelihood, robust adjusted profile score (MR.RAPS), and MR Pleiotropy Residual Sum and Outlier (MR-PRESSO). Weighted median and MR-Egger estimators could produce unbiased causal estimates in the presence of invalid IVs. The Maximum likelihood estimator displays minimal bias in limited sample sizes, which can be ignored biologically. The MR.RAPS estimator has high statistical power and remains consistent when weak and pleiotropic IVs exist. The MR-PRESSO method is a variant of the IVW estimator and can produce consistent estimates by excluding significant pleiotropic outliers. To control for the type I error rate, the Benjamini–Hochberg method was used to adjust for multiple testing. The False Discovery Rate (FDR) threshold was set at 0.05 for significance. The results from two ED GWASs were combined using a fixed-effect model, as reported previously [21], [22].
MVMR and mediation MR are extensions of MR analysis. The IVW model was used to obtain the direct and indirect effects. In MVMR, the genetic association of BMI was adjusted and the indirect effect was obtained by multiplying the results of the two MR analyses. The mediating effect percentage was further calculated using the product of coefficients method [23]. The standard error of indirect effect was estimated using the delta method [24].
Heterogeneity and pleiotropy are two main concerns in MR analyses. In this study, Cochran's Q test was used to detect heterogeneity. In addition, to quantify pleiotropy, the MR-Egger regression intercept and the global test from the MR-PRESSO estimator were employed. If pleiotropic outliers were detected by the global test, the outliers were deleted, and the remaining IVs were reanalyzed.
Given that some of the participants in the discovery ED GWAS were recruited from UKB, we further evaluate the bias from sample overlap using an online tool (https://sb452.shinyapps.io/overlap/). The bias from sample overlap was limited (< 9‰ with the type I error set at 0.05, Table 1), indicating less likelihood of weak IVs bias. We also assessed the statistical power using another online tool (https://cnsgenomics.shinyapps.io/mRnd/).
Table 1.
Baseline characteristics of included GWASs.
| Trait | Overlap Bias | nSNP | F statistics | R2 | Sample size | Consortium | Descent | Min OR | PMID | Unit |
|---|---|---|---|---|---|---|---|---|---|---|
| Anthropometric traits | ||||||||||
| BMI | <0.001 | 834 | 60.99 | 2.72% | 681275 | GIANT | European | 1.098 | 30124842 | SD |
| Waist circumference | 0.006 | 278 | 49.51 | 1.55% | 336639 | Neale Lab | European | 1.291 | NA | SD |
| Body fat percentage | 0.005 | 553 | 51.31 | 2.38% | 454633 | MRC-IEU | European | 1.235 | NA | SD |
| Trunk fat mass | 0.006 | 632 | 54.09 | 2.80% | 454588 | MRC-IEU | European | 1.217 | NA | SD |
| Whole body fat mass | 0.006 | 630 | 54.08 | 2.82% | 454137 | MRC-IEU | European | 1.216 | NA | SD |
| Metabolic traits | ||||||||||
| Overall health rating | 0.006 | 119 | 39.31 | 0.40% | 460844 | MRC-IEU | European | 1.579 | NA | SD |
| Type 2 diabetes | 0.008 | 127 | 72.24 | 5.73% | 655666 | NA | European | 1.082 | 30054458 | logOR |
| Fasting glucose | <0.001 | 34 | 103.32 | 2.64% | 133010 | MAGIC | European | 1.165 | 22885924 | SD |
| HbA1C | 0.001 | 286 | 52.48 | 11.82% | 46368 | MAGIC | European | 1.105 | 20858683 | SD |
| Fasting insulin | 0.006 | 14 | 50.22 | 0.65% | 108557 | MAGIC | European | 1.331 | 22885924 | SD |
| Basal metabolic rate | 0.002 | 984 | 62.92 | 4.78% | 454874 | MRC-IEU | European | 1.166 | NA | SD |
| Total cholesterol | 0.006 | 113 | 127.32 | 2.48% | 187365 | GLGC | Mixed (90.10% European) | 1.230 | 24097068 | SD |
| Triglycerides | 0.003 | 71 | 149.21 | 1.89% | 177861 | GLGC | Mixed (90.10% European) | 1.264 | 24097068 | SD |
| HDL cholesterol | 0.002 | 118 | 119.76 | 2.89% | 187167 | GLGC | Mixed (90.10% European) | 1.213 | 24097068 | SD |
| LDL cholesterol | 0.003 | 97 | 154.03 | 2.35% | 173082 | GLGC | Mixed (90.10% European) | 1.236 | 24097068 | SD |
| Hormones | ||||||||||
| Total T levels | 0.003 | 216 | 78.71 | 1.18% | 194453 | NA | European | 1.334 | 32042192 | SD |
| Bioavailable T levels | 0.005 | 157 | 73.72 | 1.97% | 178782 | NA | European | 1.258 | 32042192 | SD |
| SHBG | 0.005 | 597 | 100.08 | 5.02% | 180726 | NA | European | 0.840 | 32042192 | SD |
| Adiponectin | 0.004 | 14 | 92.57 | 1.26% | 39883 | ADIPOGen | European | 1.079 | 22479202 | ln(mg/dL) |
| Leptin | 0.003 | 4 | 51.32 | 0.20% | 49909 | NA | European | 1.815 | 32917775 | SD |
| Lifestyle factors | ||||||||||
| Cigarette consumption | 0.005 | 76 | 40.27 | 0.28% | 461066 | MRC-IEU | European | 1.688 | NA | SD |
| Alcohol consumption | 0.007 | 35 | 76.41 | 0.14% | 335394 | GASCAN | European | 1.177 | 30643251 | SD |
| Coffee intake | 0.009 | 40 | 75.01 | 0.27% | 428860 | MRC-IEU | European | 1.701 | NA | SD |
| Physical activity (M to V) | 0.004 | 19 | 34.39 | 0.07% | 377234 | NA | European | 1.169 | 29899525 | SD |
| Physical activity (V) | 0.009 | 7 | 40.81 | 0.05% | 261055 | NA | European | 1.156 | 29899525 | SD |
| Insomnia | 0.007 | 39 | 43.33 | 0.14% | 462341 | MRC-IEU | European | 1.974 | NA | SD |
| Daytime napping | 0.007 | 100 | 48.78 | 0.42% | 452633 | NA | European | 1.346 | 33568662 | logOR |
| Chronotype | 0.006 | 10 | 36.95 | 0.09% | 128266 | UKB | European | 2.215 | 27494321 | SD |
| Snoring | 0.008 | 19 | 37.54 | 0.09% | 314449 | Neale Lab | European | 2.215 | NA | SD |
| Cardiovascular diseases | ||||||||||
| Hypertension | 0.008 | 64 | 46.99 | 0.65% | 463010 | MRC-IEU | European | 1.450 | NA | logOR |
| Stroke | 0.003 | 6 | 38.13 | 0.02% | 446696 | MEGASTROKE | European | 1.394 | 29531354 | logOR |
| Ischemic stroke | 0.008 | 8 | 38.27 | 0.03% | 440328 | MEGASTROKE | European | 1.382 | 29531354 | logOR |
| Large artery stroke | 0.008 | 4 | 37.3 | 0.03% | 150765 | MEGASTROKE | European | 1.270 | 29531354 | logOR |
| Cardioembolic stroke | <0.001 | 4 | 74.24 | 0.02% | 211763 | MEGASTROKE | European | 1.313 | 29531354 | logOR |
| Coronary artery disease | 0.003 | 169 | 69.14 | 1.34% | 296525 | UKB & CARDIoGRAMplusC4D | European | 1.313 | 29212778 | logOR |
| Myocardial infarction | 0.005 | 75 | 65.35 | 2.71% | 471717 | UKB & CARDIoGRAMplusC4D | European | 1.220 | 33532862 | logOR |
| Heart failure | 0.001 | 9 | 41.5 | 0.04% | 977323 | HERMES | European | 1.122 | 31919418 | logOR |
| Atrial fibrillation | 0.003 | 122 | 82.56 | 0.33% | 1030836 | NA | European | 1.633 | 30061737 | logOR |
| Mental disorder | ||||||||||
| Major depressive disorder | 0.004 | 44 | 38.98 | 0.34% | 500199 | PGC | European | 1.296 | 30718901 | logOR |
| Schizophrenia | 0.003 | 89 | 40.9 | 4.42% | 77096 | PGC | European | 1.172 | 25056061 | logOR |
| ADHD | 0.003 | 11 | 34.1 | 0.68% | 55374 | PGC | European | 1.107 | 29325848 | logOR |
| Bipolar disorder | 0.003 | 4 | 34.21 | 0.82% | 16731 | PGC | European | 1.401 | 21926972 | logOR |
GWASs: genome-wide association studies; SNP: single nucleotide polymorphisms; BMI: body mass index; HbA1C: glycosylated hemoglobin type A1C; HDL: high density lipoprotein; LDL: low density lipoprotein; T: testosterone; M to V: moderate to vigorous; ADHD: attention deficit and hyperactivity disorder; PMID: PubMed identifier; SD: standard deviation; OR: odds ratio; GIANT: Genetic Investigation of ANthropometric Traits; MRC-IEU: Medical Research Council Integrative Epidemiology Unit; MAGIC: Meta-Analyses of Glucose and Insulin-related traits Consortium; GLGC: Global Lipids Genetics Consortium; GASCAN: GWAS and Sequencing Consortium of Alcohol and Nicotine use; UKB: UK biobank; CARDIoGRAMplusC4D: Coronary Artery Disease Genome-Wide Replication and Meta-analysis plus the Coronary Artery Disease Genetics; HERMES: Heart Failure Molecular Epidemiology for Therapeutic Targets; PGC: Psychiatric Genomics Consortium; NA: not available.
This study was reported according to the STROBE-MR guidelines (Supplemental file 2). All analyses in this study were performed using R 4.0.2 software (R Foundation for Statistical Computing, Vienna, Austria). In different stages, packages including “TwoSampleMR”, “MR-PRESSO”, “forestplot”, and “MendelianRandomization” were used. P < 0.05 and FDR adjusted P > 0.05 were considered to have suggestive significance.
Results
Baseline characteristics
Forty-two modifiable factors were included and clustered into six domains (Table 1). SNPs used as IVs varied from 4 to 984, and the explained variances ranged from 0.02% to 21.17%. The F statistics of each IV and the exposures were all > 10, indicating less likelihood of weak IVs. The estimated bias from sample overlap fluctuated from < 0.001 to 0.009, suggesting that these findings were less likely to be biased. The minimum ORs in the MR analyses with a statistic power of 80% are presented in Table 1.
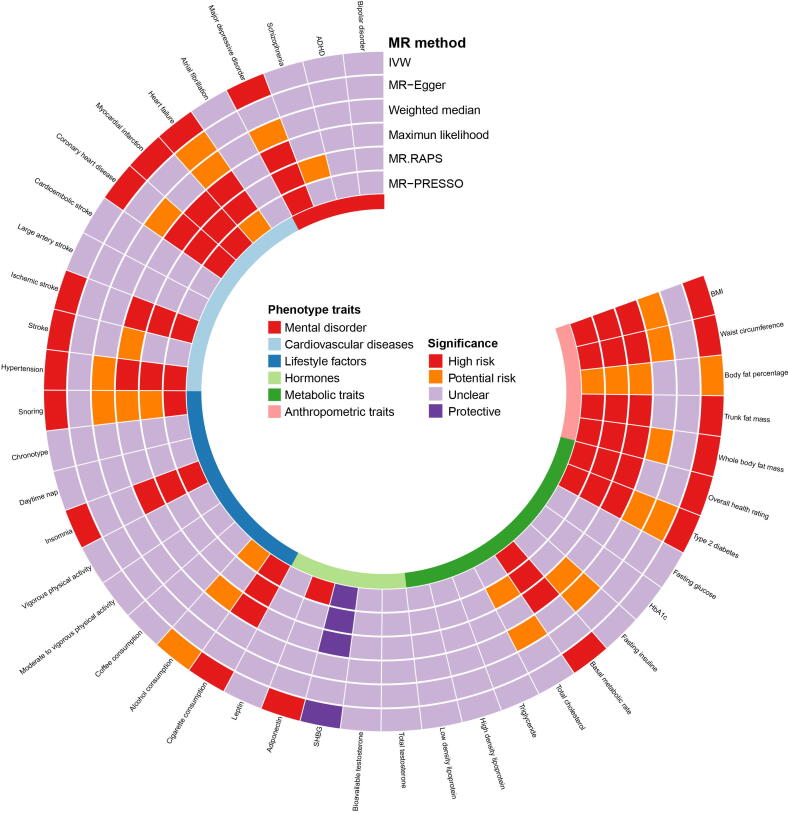
Discovery results of ED
The discovery results of ED are summarized in Fig. 2 and detailed in the subsequent sections (Fig. 3 and Supplemental Table 2). As shown in Supplemental Fig. 1, in the discovery of ED GWAS, no pleiotropic outliers were identified by MR-Egger and MR-PRESSO approaches. The funnel plots for visualizing the heterogeneity are displayed in Supplemental Fig. 2. The scatter plots of the SNP-exposure association against the SNP-ED association are presented in Supplemental Fig. 3.
Fig. 2.
Analysis of 42 predominant risk factors on ED in the discovery ED GWAS. The analysis is performed in the discovery ED GWAS and the results of six approaches including IVW, MR-Egger, Weighted median, Maximum likelihood, MR.RAPS, and MR-PRESSO are summarized here. Detailed statistics are described in Supplemental Table 2 and Fig. 3. ED: erectile dysfunction; BMI: body mass index; SHBG: sex hormone-binding globulin; HbA1C: glycosylated hemoglobin type A1C; GWAS: genome-wide association study; MR: Mendelian Randomization; IVW: inverse variance weighting; MR-PRESSO: MR Pleiotropy Residual Sum and Outlier; MR.RAPS: robust adjusted profile score. High risk: P < 0.05 and FDR < 0.05; Potential risk: P < 0.05 and FDR > 0.05; Unclear: P > 0.05; Protective: P < 0.05 and FDR < 0.05.
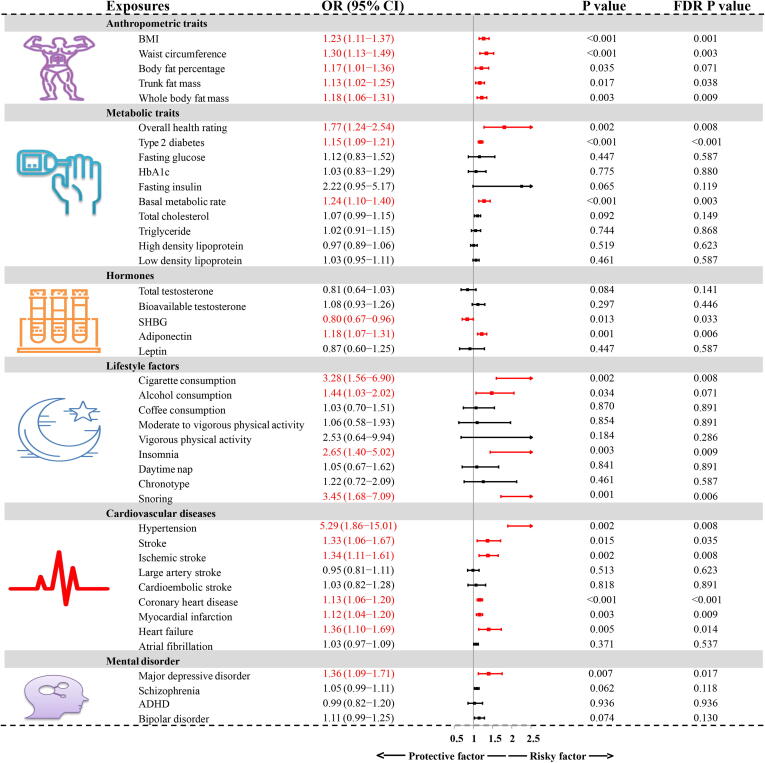
Fig. 3.
Forest plot of IVW estimator results in the discovery ED GWAS. GWAS: genome-wide association study; IVW: inverse variance weighting; BMI: body mass index; ED: erectile dysfunction; SHBG: sex hormone-binding globulin; HbA1C: glycosylated hemoglobin type A1C; OR: odds ratio; CI: confidence interval; FDR: false discovery rate.
Anthropometric traits as risk factors for ED
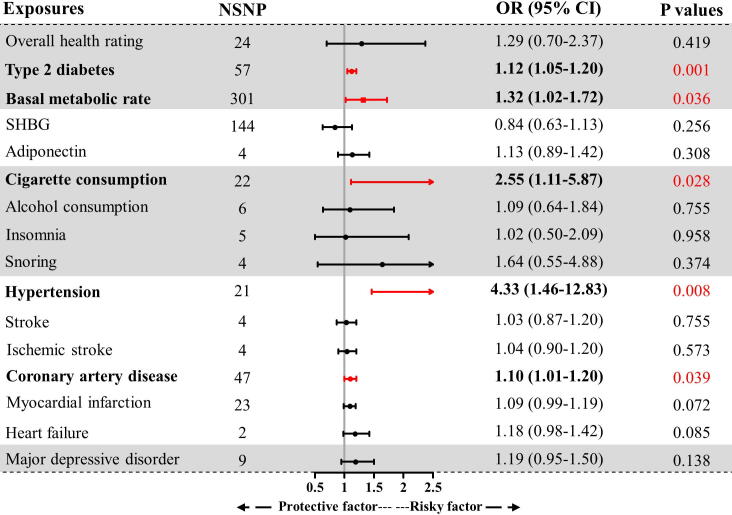
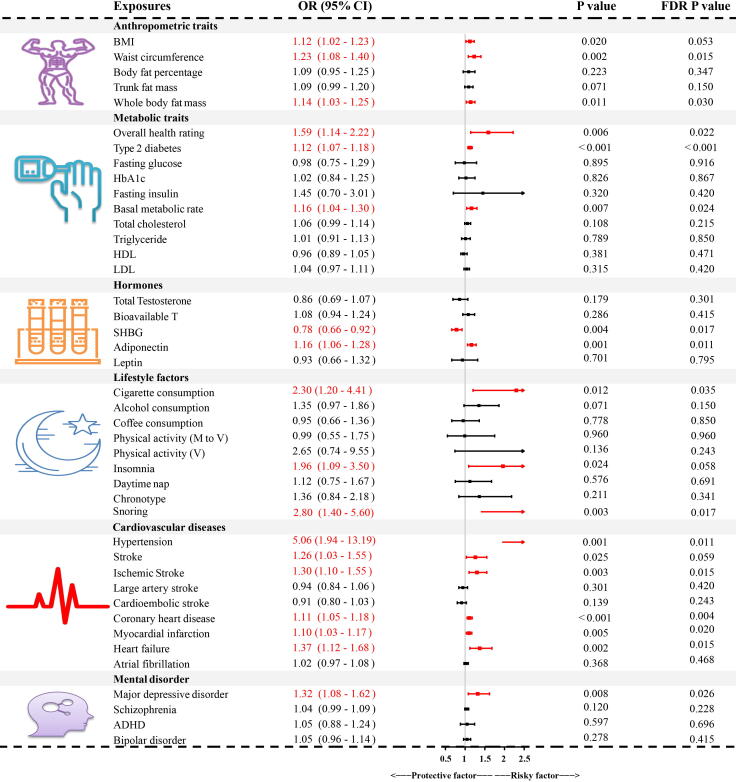
As indicated by the IVW estimator (Fig. 3), the odds of ED increased per 1-SD increase in BMI (OR = 1.23, P < 0.001), waist circumference (OR = 1.30, P < 0.001), trunk fat mass (OR = 1.13, P = 0.017), and whole body fat mass (OR = 1.18, P = 0.003). We also detected a suggestive association between genetically predicted body fat percentage and ED (P = 0.035, FDR = 0.071). Since anthropometric traits were highly correlated with BMI, these traits were not subjected to further MVMR and mediation analyses.
Metabolic traits as risk factors for ED
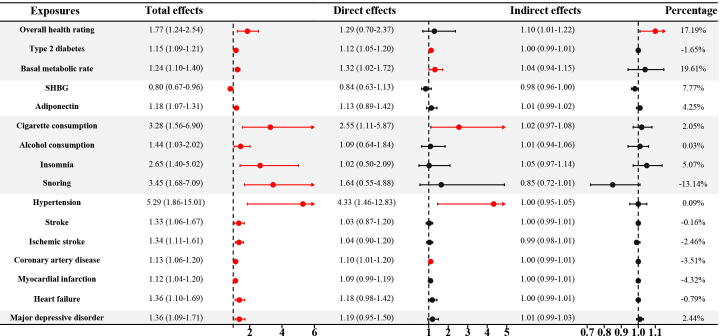
We found that genetic liability to poor overall health rating could increase the risk of ED (OR = 1.77, P = 0.002, Fig. 3); however, the risk was not significant after adjusting for BMI in MVMR (P = 0.419, Fig. 4). The indirect effects mediated by BMI accounted for 17.19% of the total effects (OR = 1.10, P < 0.05, Fig. 5). In addition, patients with type 2 diabetes and a 1-SD increment of basal metabolic rate had an increased risk of ED (OR = 1.15, P < 0.001; OR = 1.24, P < 0.001, respectively). The increased risk was also consistent in MVMR adjustment for BMI (OR = 1.12, P = 0.001; OR = 1.32, P = 0.036, respectively), and the indirect effects of BMI correspondingly accounted for −1.65% and 19.61%. No causal association was noted between other metabolic traits including fasting glucose, fasting insulin, HbA1c, TC, TG, HDL, and LDL levels and ED (all P > 0.05 & FDR > 0.05, Fig. 3).
Fig. 4.
The association between modifiable factors and ED in multivariate MR. All the analyses were performed in the discovery ED GWAS. GWAS: genome-wide association study; MR: Mendelian Randomization; ED: erectile dysfunction; NSNP: number of single nucleotide polymorphisms; SHBG: sex hormone-binding globulin; M to V: moderate to vigorous; ADHD: attention deficit and hyperactivity disorder; OR: odds ratio; CI: confidence interval.
Fig. 5.
Results of mediation MR results in the discovery ED GWAS. GWAS: genome-wide association study; ED: erectile dysfunction; SHBG: sex hormone-binding globulin; OR: odds ratio; CI: confidence interval.
Hormones as risk factors for ED
Among hormones, a 1-SD increment of SHBG was found to decrease the risk of ED (OR = 0.80, P = 0.013), whereas, although the causal effect size and direction of SHBG remained similar in MVMR, the result was insignificant after adjusting for BMI (OR = 0.84, P = 0.256). Further mediation analysis revealed that 7.77% of total direct was mediated by BMI (Fig. 5). In addition, genetically predicted adiponectin was significantly associated with an increased risk of ED (OR = 1.18, P = 0.001). However, this significant association did not exist in MVMR analysis (OR = 1.13, P = 0.308). The insignificance may be attributed to the indirect effect mediated by BMI (4.25% of the total effect). No causal association was detected between total testosterone, bioavailable testosterone, and leptin levels, and ED (all P > 0.05, Fig. 3).
Lifestyle factors as risk factors for ED
As shown in Fig. 3, several lifestyle factors were causally associated with higher risks of ED, including cigarette consumption (OR = 3.28, P = 0.002), insomnia (OR = 2.65, P = 0.003), and snoring (OR = 3.45, P = 0.001). Further MVMR analysis suggested that cigarette consumption was an independent risk factor for ED (OR = 2.55, P = 0.028), whereas alcohol consumption and insomnia were not (OR = 1.02, P = 0.958; OR = 1.64, P = 0.374, respectively). The indirect effects mediated by BMI accounted for 5.07% and −13.14% of the total effects, respectively (Fig. 5). Alcohol consumption was found to be a suggestive risk factor for ED (OR = 1.44, P = 0.034, FDR = 0.071); however, the significant association vanished after adjusting for BMI (P = 0.755). The indirect effect of BMI was minimal (0.03% of the total effect). There was no significant association between coffee consumption, physical activity, daytime napping, chronotype, and ED (all P > 0.05).
Cardiovascular diseases as risk factors for ED
Among cardiovascular diseases, genetically predicted hypertension, stroke, ischemic stroke, coronary heart disease, myocardial infarction, and heart failure were found to increase the risk of ED. The ORs were 5.29 (P = 0.002), 1.33 (P = 0.015), 1.34 (P = 0.002), 1.13 (P < 0.001), 1.12 (P = 0.003), and 1.36 (P = 0.005), for a one-unit increment in log-transformed odds, respectively (Fig. 3). MVMR analyses still supported that hypertension, coronary heart disease, and ED were positively correlated (OR = 4.33, P = 0.008; OR = 1.10, P = 0.039, respectively), whereas stroke, ischemic stroke, myocardial infarction, and heart failure were not (P > 0.05). Notably, the significance for myocardial infarction and heart failure was adjacent to the statistical threshold (P = 0.072 and P = 0.085). Further verification with more samples and higher statistical power is required.
The ORs of indirect effects ranged from 0.99 to 1.00, indicating rather limited influence (Fig. 5). Heterogeneity was detected only for cardioembolic stroke among the cardiovascular diseases (Supplemental Fig. 1). The causal effect of cardioembolic stroke on ED was verified using the random effect IVW estimator (OR = 1.03, P = 0.818). No significant association was found between large artery stroke, cardioembolic stroke, atrial fibrillation, and ED (all P > 0.05).
Mental disorders as risk factors for ED
Among mental disorders, genetically predicted major depressive disorder was found to increase the risk of ED, while other mental disorders, including schizophrenia, ADHD, and bipolar disorder were not. The ORs were 1.36 (P = 0.007), 1.05 (P = 0.062), 0.99 (P = 0.936), and 1.11 (P = 0.074), for a one-unit increment in log-transformed odds, respectively (Fig. 3). However, in the MVMR analysis, the significant association between major depressive disorder and ED vanished (OR = 1.19, P = 0.138, Fig. 4). It was estimated that 2.44% of the total effect was mediated by BMI, which may have led to insignificant results (Fig. 5).
Combined results for ED from meta-analysis
There were 1,154 ED cases among 95,178 participants in the FinnGen dataset and 6,175 ED cases from 223,805 participants in the discovery of ED GWAS. Given the limited sample size in the replication stage, the identified significantly associated risk factors in the discovery stage did not reach the significance threshold (Supplemental Table 3). Therefore, we combined the results from the discovery ED GWAS and FinnGen dataset, using the meta-analysis technique to increase the statistical power (Fig. 6). The combined results confirmed the causal risk role of waist circumference, whole body fat mass, poor overall health rating, type 2 diabetes, basal metabolic rate, adiponectin, cigarette consumption, snoring, hypertension, ischemic stroke, coronary heart disease, myocardial infarction, heart failure, and major depressive disorder (all P < 0.05 and FDR adjusted P < 0.05). Similarly, the protective effect of SHBG was also verified in the combined results (P < 0.05 and FDR adjusted P < 0.05). The results were suggestive of a significant association between BMI, insomnia, stroke, and ED (P < 0.05 and FDR adjusted P > 0.05).
Fig. 6.
Combined results from the discovery and replication ED GWASs. GWASs: genome-wide association studies; BMI: body mass index; ED: erectile dysfunction; SHBG: sex hormone-binding globulin; HbA1C: glycosylated hemoglobin type A1C; M to V: moderate to vigorous; ADHD: attention deficit and hyperactivity disorder; OR: odds ratio; CI: confidence interval; FDR: false discovery rate.
Obvious heterogeneity in trunk fat mass, whole body fat mass, type 2 diabetes, and SHBG were detected (P < 0.05, Supplemental Fig. 1). Pleiotropy of whole body fat mass, type 2 diabetes, and SHBG were also identified (P of global tests < 0.05). We further removed the identified outliers and reanalyzed the FinnGen ED GWAS dataset. The results for whole body fat mass remained unchanged (P = 0.333); however, the association between SHBG and ED were found to be significant (OR = 0.66, P < 0.05, Supplemental Table 3). No outliers were detected by the MR-PRESSO outlier test for type 2 diabetes, and the OR from the MR-Egger approach was deemed the main finding (OR = 1.54, P = 0.009, Supplemental Table 3).
Discussion
This MR study analyzed the largest number of modifiable risk factors for ED using genetic data. Our study substantiates that genetic liability to obesity, several metabolic diseases, poor lifestyle and mental status, cardiovascular dysfunction and elevated adiponectin levels can increase the risk of ED, whereas higher SHBG can decrease the risk of ED. Evidence supporting the causal effects of lipids, leptin, coffee consumption, physical activity, daytime napping, chronotype, atrial fibrillation, ADHD, schizophrenia, and bipolar disorder on ED is insufficient. MVMR and mediation analyses also highlighted the mediating role of BMI in the causal association between the identified risk factors and ED.
Obesity is a major risk factor for many diseases. Observational studies also reveal the risk role of BMI for ED, as further verified by one previous MR study [18], [25]. Our study also supports this supposition. BMI is generally deemed an indicator of general obesity. More detailed causal evidence of central obesity is lacking but is provided by our findings. The combined results identified the causal impact of waist circumference and whole body fat mass on ED. Findings from a cross-sectional survey also highlighted the risk posed by central obesity in ED [26]. However, if central obesity or general obesity plays a more centric role is unclear. The combined results suggest that, BMI is suggestively associated with ED and another indicator, body fat percentage, is not causally associated with ED. Waist circumference could predict sexual symptoms better than BMI in men with hypogonadism and ED as per the report from Yassin AA and his colleagues. [27]. Janiszewski PM also reported that abdominal obesity was associated with ED, independent of BMI [28]. These results may support the core role of central obesity in developing ED. General obesity tends to have better metabolic patterns than central obesity, which may explain the discrepancy [29]. Therefore, the impact of peripheral body fat on ED should be further assessed in future prospective studies.
As for metabolic diseases, a genetic predisposition to type 2 diabetes increased the risk of ED. This corroborates the findings from previous observational studies, MR analysis, and animal models [18], [30], [31]. Endothelial dysfunction, reactive oxygen species, and sex hormone disturbances may be responsible for the adverse effects [32]. To the best of our knowledge, we first report the adverse causal impact of basal metabolic rate on ED with a statistical power of 0.98. According to MVMR analysis, the risk is independent of BMI. Similar risks have also been identified between basal metabolic rate and certain cancers (urinary, genital, respiratory, intrathoracic, lymphoid and hematopoietic tumors) [33]. This may be partly explained by increased oxidative metabolism, leading to an overload of reactive oxygen species and further ED [34]. Of note, there may be potential correlations between exposures that jointly adversely affect ED. For example, lifestyle factors like insomnia may lead to dyslipidemia, obesity and diabetes, further or simultaneously triggering ED. Thus, the evaluation and intervention for modifiable risk factors should be comprehensive.
In this study, no causal associations between lipids levels (TC, TG, HDL, and LDL) and ED were identified. Most of the observational studies support that lipids pose a risk on the onset of ED [35], [36]. However, some cross-sectional studies suggest otherwise. Nikoobakht M et al. reported no difference in plasma TG and HDL levels between ED and non-ED participants, in line with Hyde Z et al, [37], [38]. The discrepancy in these studies may arise from their limited sample sizes and residual confounding factors from observational design. Generally, lipids are well-documented risk factors for cardiovascular health, further triggering ED. However, our data do not support the causal relationship between lipids levels and ED, which seems to be contrary to the widely accepted conclusion. This may be partly attributed to the relatively limited adverse effects from lipids. Dyslipidemia usually takes a long time to induce cardiovascular diseases and metabolic diseases like obesity. The effects from dyslipidemia are generally persistent but not intense, which lead to the insignificance.
Among lifestyle factors, alcohol consumption was suggestively associated with ED. Most but not all observational studies have reported the adverse outcomes of smoking. In a representative umbrella review of 3,971,122 participants, smoking was found to be negatively associated with ED [39]. Conversely, Shiri R et al. [14] recruited 1,442 men aged 50–75 years and found no such significant association during a 5-year follow-up period. An opposite conclusion was also found for the association of alcohol consumption with ED. Most observational studies report a beneficial effect of light to moderate drinking on ED [39], [40]. However, in a cross-sectional study with 3,501 participants, the association was found to be insignificant regardless of the frequency and amount [15]. The residual confounding in the observational design may have led to the contrasting results. It is difficult to overcome the bias from coupled obesity, poor metabolic status, and cardiovascular impairment for alcoholics, subsequently yielding opposite conclusions. Of note, a nonlinear relationship was found between them in some observational studies [41]. Future studies should be performed to investigate the nonlinear causal association using individual data. Accumulating evidence is against the “safe limit” of alcohol intake [42]. Our findings first highlight the risk of cigarette and alcohol consumption using MR, providing reliable causal evidence. Therefore, to prevent ED, we recommend giving up cigarette and alcohol consumption. In line with previous observational studies and MR analyses, we also report a significant association between insomnia, snoring, and ED [43], [44], [45]. Clinicians should recognize and treat sleep disorders in the general population, especially in patients with ED.
Previous studies have reported that ED is an early indicator for subsequent cardiovascular disease like coronary heart disease [46]. The two conditions share common mechanisms, i.e., endothelial dysfunction. In this study, conversely, we found that cardiovascular diseases, including hypertension, stroke, ischemic stroke, coronary heart disease, myocardial infarction, and heart failure could lead to ED. A clearer causal direction is supplemented. Additionally, this study also addresses the contradictions in the relationship between cardiovascular diseases and ED in observational studies. Kałka D et al. enrolled 751 males and found that those with familial coronary heart disease had higher erectile function than those without [47]. In addition, one clinical trial also failed to replicate the significant association between ED and coronary heart disease [48]. The limited sample size and confounding factors may bias the true association. Mechanistically, endothelial dysfunction due to hypertension, central nervous system impairment by stroke, decreased blood perfusion and further hypoxia by reduced cardiac output (coronary heart disease, myocardial infarction, and heart failure) may be responsible [49], [50]. Therefore, it is better to evaluate cardiovascular diseases and ED simultaneously in clinical settings.
Observational studies have frequently reported the close association between mental disorders and ED [5]. However, mental disorders can lead to ED and vice versa. A possible bilateral relationship is noted but the causal direction is unclear. As revealed by previous surveys, our findings support that depression can increase the risk of ED [51]. In addition, contrary to previous studies that found a positive association, we found insufficient evidence supporting the role of schizophrenia, ADHD, and bipolar disorder as risk factors for ED [52], [53], [54]. However, the association approached the statistically significant threshold in the discovery stage and combined results. The insignificant causal association may be canceled out. The sample sizes for the available GWASs of schizophrenia, ADHD, and bipolar disorder were relatively limited, leading to less statistical power. Thus, further investigation should be performed with larger sample sizes to clarify these relationships.
Identifying reversible risk factors is the first-line evaluation for ED patients as per the diagnostic work-up of the European Association of Urology Guidelines [55]. Thus, we scanned the modifiable factors for ED, which could help clinicians propose a strategy to prevent ED based on the results of this study. According to the findings, metabolic diseases including diabetes and obesity and cardiovascular diseases like hypertension require early intervention in the general population. Quitting cigarette and alcohol consumption should be recommended for ED patients by doctors. Good sleep quality may also bring benefits for erectile function. Thereby, medical interventions like hypnotics can be recommended for ED patients. In clinical practice, clinicians should consider these risk factors. Some emerging and non-invasive therapies like nanocomposites and hydrogels may be useful in this process [56], [57], [58].
This study has some strengths. The major merit is the MR design, allowing for deriving causal inference rather than a correlation. In addition, this is the first MR study that analyzed a large number of modifiable factors. The majority of the predominant risk factors, such as waist circumference, cigarette and alcohol consumption, hormones, cardiovascular diseases, and mental disorders have not been analyzed under the MR framework. Moreover, the investigation was performed in two independent ED GWAS datasets and their combination to improve the statistical power. A rigorous IV selection and multiple sensitivity analyses were considered in the MR analyses, ensuring compliance with three basic assumptions in MR. These endeavors provide reliability to our findings. Moreover, the included participants were primarily of European descent, thus reducing population architecture bias. The sample overlap was very low in the included datasets with high F statistics and low weak IV bias.
However, several limitations should also be noted in this study. Pleiotropy is a main concern in MR analysis. The existence of pleiotropy may violate the basic MR assumptions and further distort the findings. We applied two methods MR-Egger and MR-PRESSO to detect the horizontal pleiotropy. IVs of three exposures showed pleiotropy in the FinnGen dataset, although no pleiotropy was observed in the discovery stage. The corrected results still support the findings in the discovery stage and the combined results, indicating less likelihood of potential biases. Another limitation is the strict restriction on the descent of the included samples, thus reducing the generalizability to other nations. Therefore, the results should be interpreted cautiously and further verification in other ancestries is required. Additionally, this study was performed based on summary-level statistics. We can neither explore the nonlinear relationship between modifiable factors and ED nor that with disease severity. Lastly, although we adopted the largest ED GWASs at present, the relatively low rate of ED cases in the discovery and FinnGen datasets could have led to less statistical power for some exposures. Thus, we combined the results, which could alleviate this deficiency to some extent. Further validation with larger sample size can be considered. In the end, the biological mechanisms linking the identified risk factors to ED were not explored, which should be investigated in future studies.
In conclusion, our comprehensive MR analyses support that 1) genetic predisposition to metabolic diseases including obesity and type 2 diabetes; 2) poor lifestyle including cigarette and alcohol consumption, insomnia and snoring; 3) poor mental status like depression; 4) genetically predicted cardiovascular diseases; and 5) high adiponectin levels can increase the risk of ED; while genetic liability to high SHBG can decrease the risk of ED. This study provides a better understanding of the risk factors of ED and is conducive to the early identification and intervention of these patients.
Declarations:
CRediT authorship contribution statement
Yang Xiong: Conceptualization, Data curation, Formal analysis, Writing – original draft. Fuxun Zhang: Data curation, Writing – original draft. Yangchang Zhang: Data curation, Writing – original draft. Wei Wang: Formal analysis, Writing – review & editing. Yuxin Ran: Writing – review & editing. Changjing Wu: Formal analysis, Writing – review & editing. Shiyu Zhu: Formal analysis, Writing – review & editing. Feng Qin: Conceptualization, Formal analysis, Writing – review & editing. Jiuhong Yuan: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors sincerely thank the authors who shared the original dataset in this study. This work was supported by the Natural Science Foundation of China (No. 81871147 and No. 82071639) and the Sichuan Science and Technology Program (No. 2022YFS0028 and No. 2022YFS0134).
Ethnics approval and consent to participant
Ethical review and approval can be accessed in the original studies. Informed consent was obtained from all subjects in the original genome-wide association studies. In this MR study, only summary-level statistics were used. No identifiable private information was contained in the GWAS datasets.
Consent for publication
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.05.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.NIH Consensus Conference Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 2.Feldman H.A., Goldstein I., Hatzichristou D.G., Krane R.J., McKinlay J.B. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151(1):54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 3.G Corona, DM Lee, G Forti, DB O'connor, M Maggi, TW O'neill et al. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med 2010; 7: 1362–80. [DOI] [PubMed]
- 4.Li M.-K., Garcia L.A., Rosen R. Lower urinary tract symptoms and male sexual dysfunction in Asia: a survey of ageing men from five Asian countries. BJU Int. 2005;96(9):1339–1354. doi: 10.1111/j.1464-410X.2005.05831.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Song Y., Lu Y., Xu Y., Liu L., Liu X. Associations between erectile dysfunction and psychological disorders (depression and anxiety): a cross-sectional study in a Chinese population. Andrologia. 2019;51:e13395. doi: 10.1111/and.13395. [DOI] [PubMed] [Google Scholar]
- 6.Yafi F.A., Jenkins L., Albersen M., Corona G., Isidori A.M., Goldfarb S., et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2(1) doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivaratnam L., Selimin D.S., Abd Ghani S.R., Nawi H.M., Nawi A.M. Behavior-related erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2021;18(1):121–143. doi: 10.1016/j.jsxm.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Bacon C.G., Mittleman M.A., Kawachi I., Giovannucci E., Glasser D.B., Rimm E.B. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–168. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kalejaiye O., Raheem A.A., Moubasher A., Capece M., McNeillis S., Muneer A., et al. Sleep disorders in patients with erectile dysfunction. BJU Int. 2017;120(6):855–860. doi: 10.1111/bju.13961. [DOI] [PubMed] [Google Scholar]
- 10.Pohjantähti-Maaroos H., Palomäki A., Hartikainen J. Erectile dysfunction, physical activity and metabolic syndrome: differences in markers of atherosclerosis. BMC Cardiovasc Disord. 2011;11:36. doi: 10.1186/1471-2261-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solak Y., Akilli H., Kayrak M., Aribas A., Gaipov A., Turk S., et al. Uric acid level and erectile dysfunction in patients with coronary artery disease. J Sex Med. 2014;11(1):165–172. doi: 10.1111/jsm.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian G.-X., Li S., Liu T.-Z., Zeng X.-T., Wei W.-L., Wang X.-H. Association between coronary heart disease and erectile dysfunction in Chinese Han population. Oncotarget. 2017;8(33):55562–55566. doi: 10.18632/oncotarget.15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seftel A.D., Strohl K.P., Loye T.L., Bayard D., Kress J., Netzer N.C. Erectile dysfunction and symptoms of sleep disorders. Sleep. 2002;25:643–647. [PubMed] [Google Scholar]
- 14.Shiri R., Hakama M., Häkkinen J., Tammela T.L.J., Auvinen A., Koskimäki J. Relationship between smoking and erectile dysfunction. Int J Impot Res. 2005;17(2):164–169. doi: 10.1038/sj.ijir.3901280. [DOI] [PubMed] [Google Scholar]
- 15.Cho B.L., Kim Y.S., Choi Y.S., Hong M.H., Seo H.G., Lee S.Y., et al. Prevalence and risk factors for erectile dysfunction in primary care: results of a Korean study. Int J Impot Res. 2003;15:323–328. doi: 10.1038/sj.ijir.3901022. [DOI] [PubMed] [Google Scholar]
- 16.Kadihasanoglu M., Karabay E., Yucetas U., Erkan E., Ozbek E. Relation between monocyte to high-density lipoprotein cholesterol ratio and presence and severity of erectile dysfunction. Aktuelle Urol. 2018;49(03):256–261. doi: 10.1055/s-0042-123163. [DOI] [PubMed] [Google Scholar]
- 17.Ma W.-J., Qin M., Cui T.-W., Zhang X.-P., Ke Z.-H., Pan Z.-K., et al. Relationship between the risk factors of cardiovascular disease by testing biochemical markers and young men with erectile dysfunction: a case-control study. Transl Androl Urol. 2021;10(2):724–733. doi: 10.21037/tau-20-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan C., Jian Z., Gao X., Jin X.i., Wang M., Xiang L., et al. Type 2 diabetes mellitus increases risk of erectile dysfunction independent of obesity and dyslipidemia: a Mendelian randomization study. Andrology. 2022;10(3):518–524. doi: 10.1111/andr.13132. [DOI] [PubMed] [Google Scholar]
- 19.Emdin C.A., Khera A.V., Kathiresan S. Mendelian randomization. JAMA. 2017;318:1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 20.Bovijn J., Jackson L., Censin J., Chen C.-Y., Laisk T., Laber S., et al. GWAS identifies risk locus for erectile dysfunction and implicates hypothalamic neurobiology and diabetes in etiology. Am J Hum Genet. 2019;104(1):157–163. doi: 10.1016/j.ajhg.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L., Yang H., Li H., He C., Yang L., Lv G. Insights into modifiable risk factors of cholelithiasis:a Mendelian randomization study. Hepatology. 2022;75(4):785–796. doi: 10.1002/hep.32183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.J Xie, H Huang, Z Liu, Y Li, C Yu. The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology 2022. [DOI] [PubMed]
- 23.Carter A.R., Sanderson E., Hammerton G., Richmond R.C., Davey Smith G., Heron J., et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–478. doi: 10.1007/s10654-021-00757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z., Wang L. Methods for evaluating mediation effects: Rationale and comparison. New Trends Psychometrics. 2008;595:604. [Google Scholar]
- 25.X Zhang, B Yang, N Li, Li H. Prevalence and risk factors for erectile dysfunction in Chinese adult males. J Sex Med 2017; 14: 1201–8. [DOI] [PubMed]
- 26.Fillo J., Levcikova M., Ondrusova M., Breza J., Labas P. Importance of different grades of abdominal obesity on testosterone level, erectile dysfunction, and clinical coincidence. Am J Mens Health. 2017;11(2):240–245. doi: 10.1177/1557988316642213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yassin A.A., Nettleship J.E., Salman M., Almehmadi Y. Waist circumference is superior to weight and BMI in predicting sexual symptoms, voiding symptoms and psychosomatic symptoms in men with hypogonadism and erectile dysfunction. Andrologia. 2017;49(4):e12634. doi: 10.1111/and.12634. [DOI] [PubMed] [Google Scholar]
- 28.Janiszewski P.M., Janssen I., Ross R. Abdominal obesity and physical inactivity are associated with erectile dysfunction independent of body mass index. J Sex Med. 2009;6:1990–1998. doi: 10.1111/j.1743-6109.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 29.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616–627. doi: 10.1016/S2213-8587(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 30.Kouidrat Y., Pizzol D., Cosco T., Thompson T., Carnaghi M., Bertoldo A., et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34(9):1185–1192. doi: 10.1111/dme.13403. [DOI] [PubMed] [Google Scholar]
- 31.Musicki B., Hannan J.L., Lagoda G., Bivalacqua T.J., Burnett A.L. Mechanistic link between erectile dysfunction and systemic endothelial dysfunction in type 2 diabetic rats. Andrology. 2016;4(5):977–983. doi: 10.1111/andr.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 33.Ng J.C.M., Schooling C.M. Effect of basal metabolic rate on cancer: a Mendelian randomization study. Front Genet. 2021;12 doi: 10.3389/fgene.2021.735541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatt B.A., Dedousis N., Sipula I.J., O'Doherty R.M. Elevated metabolic rate and skeletal muscle oxidative metabolism contribute to the reduced susceptibility of NF-κB p50 null mice to obesity. Physiol Rep. 2018;6(18):e13836. doi: 10.14814/phy2.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yilmaz M., Karaaslan M., Tonyali S., Celik M., Toprak T., Odabas O. Triglyceride-Glucose Index (TyG) is associated with erectile dysfunction: a cross-sectional study. Andrology. 2021;9(1):238–244. doi: 10.1111/andr.12904. [DOI] [PubMed] [Google Scholar]
- 36.Li L., Yao H., Dai W., Chen Y., Liu H., Ding W., et al. A higher TyG index is related with a higher prevalence of erectile dysfunction in males between the ages 20–70 in the United States, according to a cross-sectional research. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.988257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikoobakht M., Pourkasmaee M., Nasseh H. The relationship between lipid profile and erectile dysfunction. Urol J. 2005;2(1):40–44. [PubMed] [Google Scholar]
- 38.Hyde Z., Flicker L., Hankey G.J., Almeida O.P., McCaul K.A., Chubb S.A.P., et al. Prevalence and predictors of sexual problems in men aged 75–95 years: a population-based study. J Sex Med. 2012;9(2):442–453. doi: 10.1111/j.1743-6109.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 39.Allen MS, Walter EE. Erectile dysfunction: an umbrella review of meta-analyses of risk-factors, treatment, and prevalence outcomes. J Sex Med 2019; 16: 531–41. [DOI] [PubMed]
- 40.Li S., Song J.-M., Zhang K.e., Zhang C.-L. A meta-analysis of erectile dysfunction and alcohol consumption. Urol Int. 2021;105(11-12):969–985. doi: 10.1159/000508171. [DOI] [PubMed] [Google Scholar]
- 41.Wang X.-M., Bai Y.-J., Yang Y.-B., Li J.-H., Tang Y., Han P. Alcohol intake and risk of erectile dysfunction: a dose-response meta-analysis of observational studies. Int J Impot Res. 2018;30(6):342–351. doi: 10.1038/s41443-018-0022-x. [DOI] [PubMed] [Google Scholar]
- 42.Griswold M.G., Fullman N., Hawley C., Arian N., Zimsen S.R.M., Tymeson H.D., et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez K.M., Kohn T.P., Kohn J.R., Sigalos J.T., Kirby E.W., Pickett S.M., et al. Shift work sleep disorder and night shift work significantly impair erectile function. J Sex Med. 2020;17(9):1687–1693. doi: 10.1016/j.jsxm.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong Y., Zhong X., Zhang F., Wang W., Zhang Y., Wu C., et al. Genetic evidence supporting a causal role of snoring in erectile dysfunction. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.896369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong Y., Zhang F.X., Zhang Y.C., Wu C.J., Qin F., Yuan J.H. Genetically predicted insomnia causally increases the risk of erectile dysfunction. Asian J Androl. 2023;25(3):421–425. doi: 10.4103/aja202261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gandaglia G., Briganti A., Jackson G., Kloner R.A., Montorsi F., Montorsi P., et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65(5):968–978. doi: 10.1016/j.eururo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Kałka D., Gebala J., Biernikiewicz M., Mrozek-Szetela A., Rożek-Piechura K., Sobieszczańska M., et al. Erectile dysfunction in men burdened with the familial occurrence of coronary artery disease. J Clin Med. 2021;10(18):4046. doi: 10.3390/jcm10184046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speel T.G.W., van Langen H., Meuleman E.J.H. The risk of coronary heart disease in men with erectile dysfunction. Eur Urol. 2003;44(3):366–371. doi: 10.1016/s0302-2838(03)00304-x. [DOI] [PubMed] [Google Scholar]
- 49.Gallo G., Volpe M., Savoia C. Endothelial dysfunction in hypertension: current concepts and clinical implications. Front Med. 2021;8 doi: 10.3389/fmed.2021.798958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pistoia F., Govoni S., Boselli C. Sex after stroke: a CNS only dysfunction? Pharmacol Res. 2006;54(1):11–18. doi: 10.1016/j.phrs.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Seidman S.N., Roose S.P. The relationship between depression and erectile dysfunction. Curr Psychiatry Rep. 2000;2(3):201–205. doi: 10.1007/s11920-996-0008-0. [DOI] [PubMed] [Google Scholar]
- 52.Üçok A., İncesu C., Aker T., Erkoç Ş. Sexual dysfunction in patients with schizophrenia on antipsychotic medication. Eur Psychiatry. 2007;22(5):328–333. doi: 10.1016/j.eurpsy.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Amani Jabalkandi S., Raisi F., Shahrivar Z., Mohammadi A., Meysamie A., Firoozikhojastefar R., et al. A study on sexual functioning in adults with attention-deficit/hyperactivity disorder. Perspect Psychiatr Care. 2020;56:642–648. doi: 10.1111/ppc.12480. [DOI] [PubMed] [Google Scholar]
- 54.Hou PH, Mao FC, Chang GR, Huang MW, Wang YT, Huang SS. Newly diagnosed bipolar disorder and the subsequent risk of erectile dysfunction: a nationwide cohort study. J Sex Med 2018; 15: 183–91. [DOI] [PubMed]
- 55.Salonia A., Bettocchi C., Boeri L., Capogrosso P., Carvalho J., Cilesiz N.C., et al. European association of urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. 2021;80(3):333–357. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Mahdi M.A., Yousefi S.R., Jasim L.S., Salavati-Niasari M. Green synthesis of DyBa2Fe3O7.988/DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: photocatalytic and antibacterial activities. Int J Hydrogen Energ. 2022;47(31):14319–14330. [Google Scholar]
- 57.Yousefi S.R., Alshamsi H.A., Amiri O., Salavati-Niasari M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J Mol Liq. 2021;337:116405. [Google Scholar]
- 58.Ren Y., Yuan J., Xue Y., Zhang Y., Li S., Liu C., et al. Advanced hydrogels: New expectation for the repair of organic erectile dysfunction. Mater Today Bio. 2023;19:100588. doi: 10.1016/j.mtbio.2023.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.