Graphical abstract

Keywords: Depression, FZD6, CRISPR/Cas9, Cell proliferation, Wnt/β-catenin
Highlights
-
•
FZD6 expression was significantly reduced in MDD patients.
-
•
Fzd6 knockdown mice presented significant changes in depressive-like behaviors.
-
•
The cell proliferation was decreased in the hippocampus of Fzd6 knockdown mice.
-
•
By applying both in vitro and in vivo models, we demonstrated that the significant role of FZD6 in depression is mainly mediated by Wnt/β-catenin signaling pathway.
Abstract
Introduction
As one of the common psychiatric diseases, depression poses serious threats to human health. Although many genes have been nominated for depression, few of them were investigated in details at the molecular level.
Objectives
To demonstrate Frizzled class receptor 6 (FZD6) functions in depression through disrupting Wnt/β-catenin signal pathway.
Methods
The FZD6 edited cell line and mouse model were generated by using CRISPR/Cas9 technique. The expression of key genes and proteins in Wnt/β-catenin pathway was determined by qRT-PCR and Western blotting, respectively. Animal behavioral tests, including open field test (OFT), elevated plus maze test (EPM), forced swimming test (FST), tail suspension test (TST), and sucrose preference test (SPT), were employed to determine anxiety- and depressive-like behaviors. Immunofluorescent staining was used to assess cell proliferation in the hippocampus of mouse brain.
Results
Among patients with depression, FZD6, one of the receptors of Wnt ligand, was significantly decreased. In CRISPR/Cas9-based FZD6 knockdown cells, we showed that FZD6 plays a significant role in regulating expression of genes involved in Wnt/β-catenin pathway. Subsequently behavioral studies on Fzd6 knockdown mice (with a 5-nucleotide deletion; Fzd6-Δ5) revealed significant changes in depressive symptoms, including increased immobility duration in FST, less preference of sucrose in SPT, reduction of distance traveled in OFT, and decreased time spent in open arms in EPM. Immunofluorescent staining showed decreased cell proliferation in the hippocampus of Fzd6-Δ5 mice with reduced number of Ki67+ and PCNA+ cells.
Moreover, decreased Gsk3β mRNA expression, phosphorylated GSK3β, and cytoplasmic β-catenin in the hippocampus of Fzd6-Δ5 mice provided further evidence supporting the role of Fzd6 in depression.
Conclusion
Together, above findings proved the significant role of FZD6 in depression through its effect on hippocampal cell proliferation and its ability to regulate canonical Wnt/β-catenin pathway.
Introduction
>300 million person suffer from major depressive disorder (MDD) worldwide, which is one of the most commonly diagnosed psychiatric disorders [1]. It contributes significantly to the global burden of diseases and is still increasing annually [2], [3]. By 2030, the World Health Organization predicted that MDD will rank as the first of global burden of disease (GBD) [4]. Considering depression is a complex disease, numerous studies have been conducted so far with the goal of discovering susceptibility genes for the disease and their underlying mechanisms.
It has been recently reported that G protein-coupled receptors (GPCRs) are key regulators for depressive-like behaviors [5]. Frizzled (FZD) proteins belong to GPCRs, which are seven-pass transmembrane cell surface receptors for secreted Wnt proteins, and they are encoded by ten FZD genes in vertebrates [6], [7]. Both Fzd4 and Fzd6 expressed more abundantly in the adult mouse primary brain endothelial cells than any of other eight Fzd genes [8], indicating their essential roles in brain.
There are accumulating evidences showing that the Wnt/β-catenin pathway plays a significant role in the pathophysiology of depression and to be a potential plausible target for antidepressants [9], [10], [11], [12]. FZD6 was reported to participate in Wnt/β-catenin pathway, which was documented in the process of many diseases, such as cancers [13], neural tube defects [14], [15] and brain morphogenesis defect [16]. However, whether and how FZD6 is involved in depression is largely unknown. Voleti et al. showed that virus-mediated inhibition of Fzd6 in the brain resulted in depressive-like behaviors in a rodent model [17]. Very recently, we found a missense single nucleotide polymorphism (SNP) located in FZD6 associated significantly with depressive symptoms [18], but the underlying mechanism of such an association was not addressed.
Considering decreased expression of FZD6 in MDD patients and significantly association of genetic variants in the gene with MDD, our primary purpose was to determine how this gene participates in the pathogenesis of depression at molecular level by using a combination of cellular analysis, animal behavioral tests, morphological analysis, and molecular analysis with in vitro and in vivo models.
Materials and Methods
Expression analysis of FZD6 in patients with depression
To characterize the correlation between depression and expression of FZD6 in human beings, we downloaded two datasets (GSE54562 and GSE92538) from Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/). After extracting FZD6 mRNA expression values from each sample, we examined the expression difference of FZD6 between MDD patients and healthy control subjects by applying unpaired t-test.
Cell culture and transfections
The HEK293T cells and SH-SY5Y neuronal cells were obtained from ATCC (Manassas, VA, USA), and cultured in DMEM medium (Hyclone, Logan, UT, USA) supplied with 10% FBS (GIBCO, Grand Island, NY, USA) and 1% penicillin/streptomycin (GIBCO) at 37 °C with 5% CO2 atmosphere.
CRISPR/Cas9 plasmid with a 2A-Puro co-expression vector pSpCas9(BB)-2A-Puro (PX459) was purchased from Addgene (https://www.addgene.org/). We applied the CRISPR online tool (https://tools.genome-engineering.org/) to design single guide-RNA (sgRNA) aiming exon 4 of FZD6, and the designed sgRNA (i.e., 5′-GAACAAGTCCAAAGAGACAT-3′) was then cloned into PX459 (namely FZD6-Cas9-Puro) as previously described [19], [20], [21]. The FZD6-specific targeting plasmid was transfected into HEK293T or SH-SY5Y cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Cells were selected with puromycin (3 μg/ml) for 96 h one day after transfection, and then subjected to validate the guided target site by Sanger sequencing (Sango Biotech, Shanghai, China).
Animals
All mice (GemPharmatech Co. Ltd, Nanjing, China) were housed under standard conditions (12-hour light–dark cycle) and were provided with free food and water at the Core Animal Facility at Zhejiang University. Mice were deeply anesthetized before sacrifice.
Ethics statement
The Animal Care and Use Committee of the First Affiliated Hospital of Zhejiang University approved the experimental procedures in this study (Approval No. 1093–1).
Creation of Fzd6 knockdown (Fzd6-KD) mouse model with CRISPR/Cas9 technique
Based on C57BL/6J background mice, we created Fzd6-KD mice by CRISPR/Cas9 editing system [22] at GemPharmatech (Nanjing, China). Briefly, the Cas9 mRNA and sgRNA were co-injected into the zygotes of mouse. The sgRNA was designed following above website with the sequence of 5′-CACCAAAATCCAATGTCTCT-3′, which was targeted to exon 4 of Fzd6. Through homologous recombination, the broke double-strand was repaired, leading to generation of Fzd6 knockdown heterozygote mice. The pups were genotyped by PCR followed by Sanger sequencing (Sango Biotech, Shanghai, China). The primers for genotyping and sequencing were forward: 5′-TCTGTGAATGCAGCAAAGTCATGG-3′, reverse: 5′-GTCTCTCTGGGTATCTGAATCGTC-3′. By crossbreeding the heterozygote mice, we obtained both homozygous Fzd6-KD mice and wild-type (WT) mice. Since only five nucleotides were deleted in the Fzd6-KD mice, we named them as Fzd6-Δ5 mice thereafter.
Behavioral tests
The male and female homozygous WT and Fzd6-Δ5 mice (2 ∼ 3-month old) were used for the following tests. Before each behavioral test, mice were adapted to testing room for about 1 h. After finishing behavioral tests, the hippocampus from each mouse brain was collected and stored at −80 °C refrigerator.
Forced swim test (FST)
FST was conducted as reported before [23]. Briefly, each mouse was put into a clear 12 cm diameter cylinder filled with 23–25 °C water (∼16 cm depth) for 6 min. We monitored the behaviors and counted the total immobility during the last 4 min. The animals with no any movement except breathing was defined as immobility.
Tail suspension test (TST)
TST was performed as previously reported [24]. Briefly, the tail of each mouse was stuck with adhesive tape and suspended 50 cm above the floor. The behaviors were recorded for 6 min by a camera from side and counted the total immobility during the last 4 min.
Sucrose preference test (SPT)
The SPT was performed following previously reported protocol [24], [25], [26]. Animal was singly housed and adapted to two bottles filled with water. After 48-hour habituation, all mice were subjected to two bottles filling with either 2% water or sucrose for 48 h. To avoid side preference, we switched the positions of two bottles every 6 h. The amount of consumed sucrose relative to the total amount of consumed fluid during testing phase was used to calculate the sucrose preference.
Open field test (OFT)
Every mouse was put individually into the OFT apparatus (44 × 44 × 44 cm3) facing the wall. The locomotor activity was recorded 10 min with a camera above the apparatus under dim light. The anxiety-like behavior was measured with the total distance travelled, center entries, and time spent in the central zone. After each test, we cleaned the apparatus with 75% ethanol. We used Any-maze software (Stoelting Co., Wood Dale, IL, USA) to analyze the collected data.
Elevated plus maze (EPM)
The EPM apparatus comprised of two closed arms (30 × 5 × 20 cm3) and two open arms (30 × 5 cm2). The maze was placed above the floor at a height of 50 cm. Being faced to one of the open arms, we put every mouse into the middle of the apparatus and allowed to explore the apparatus for 6 min. The track of each animal was recorded with a camera above the apparatus. Total time spent and total distance travelled in each arm were counted. We used Any-maze software (Stoelting) to analyze the collected data.
Serum corticosterone detection
The blood was collected from each animal and centrifuged at 3000 rpm at 4 °C for 15 min. The supernatants were collected and stored at − 80 °C for later use. Serum corticosterone level was measured by ELISA kit (Abcam, ab108821).
RNA extraction and quantitative real-time PCR (qRT-PCR)
The total RNA from cells or hippocampus was isolated with TRIzol reagent (Life Technologies, Grand Island, NY, USA). RNA quantity was determined by NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA). Primers for each gene of interest were designed and synthesized at Sangon Biotech Co., Ltd (Shanghai, China) and are listed in Supplementary Table S1. One μg RNA was used to synthesize the cDNAs with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The qRT-PCR was conducted using Power SYBR Green PCR Master Mix (Applied Biosystems). A total of 10 μl mixture containing 5 μl Master Mix, 2.5 μl sense and antisense primers (the final concentration is 1 μM), as well as 2.5 μl diluted cDNA in a 384-well plate. The PCR was conducted with 7900 HT Fast Real Time PCR system (Applied Biosystems). The relative gene expression was normalized to β-actin for in vivo study and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) for in vitro study using 2-ΔΔCt method [27].
Western blotting
The cells or brain tissues were homogenized by ultrasonic disruptor in RIPA solution (Beyotime Biotechnology, Shanghai, China) containing Phenylmethanesulfonylfluoride (PMSF) protease inhibitors. All samples were centrifuged at 12,000 rpm for 15 min after being incubated on ice for 30 min, and collected the liquid supernatant. Cytoplasmic protein was extracted following nuclear and cytoplasmic extraction instruction (Pierce, Rockford, IL, USA). The protein concentration was measured with the Enhanced BCA Protein Assay Kit (Beyotime Biotechnology). A mixture of protein loading buffer and equal amount of protein was electrophoresed on an 8% SDS-PAGE gel, and they were then electrophoretically transferred to polyvinylidene difluoride (PVDF) membrane (Millipore). After blocking with 5% BSA in TBST for 1 h at room temperature, the membrane was incubated overnight at 4 °C with the following primary antibodies: FZD6, GSK3β, phospho-GSK3β (Ser9) and phospho-β-catenin (Ser675) (1:1000 for all of them, Cell Signaling Technology, Beverly, MA, USA), β-catenin (1:5000, Proteintech, Wuhan, China). Following three times of washing with TBST, immunoblots were incubated with a 1:4000 dilution of HRP-conjugated anti-rabbit secondary antibody (1:5000, Proteintech) for 1 h, followed by detection with Clarity Western ECL substrate (Bio-Rad, Hercules, CA, USA). The total protein expression was normalized to β-actin (1:10000, Abclonal, Wuhan, China). The β-tublin (1:1000, Proteintech) was used as the cytoplasmic loading control, and Lamin B1 (1:2000, Proteintech) was used as the nuclear loading control. We applied ImageJ software (NIH) to quantify the immunoblot results.
Immunofluorescence staining
Mice were deeply anesthetized and subjected to transcardial perfusion with cold saline followed by 4% paraformaldehyde (PFA). Whole brain was collected and post-fixed in 4% PFA for 24 h. Then, samples were given graded dehydration and embedded in paraffin, and immunostaining was conducted with 5 μm sections. Next, sections were deparaffinized, followed by rehydrated and subjected to block with blocking solution containing 0.1% Triton X-100 for permeabilization, and then incubated at 4 °C overnight with anti-Ki67 (1:200, Servicebio, Wuhan, China), anti-PCNA (1:500, Abcam, Cambridge, MA, USA), or anti-NeuN (1:200, Servicebio). After being washed three times in PBS for 5 min, each section was incubated with secondary antibodies conjugated to Alexa Fluor 488 and/or Cy3 (1:400, Servicebio) at room temperature for 1 h. Finally, sections were mounted with antifade mounting media containing DAPI after washing in PBS. Images were scanned by Pannoramic 250FLASH slice scanner (3DHISTECH, Budapest, Hungary). Quantitative analysis was conducted using ImageJ software as a measure of positive cells in the DG region of hippocampus.
Statistical analysis
All data were analyzed with GraphPad Prism 8.0 (San Diego, CA, USA) and are presented as Mean ± SEM. Two-tailed unpaired t-test was applied to perform statistical analysis. p-value < 0.05 was defined as statistically significant difference.
Results
Characterization of FZD6 in depression
To determine whether there existed any expression difference of FZD6 between MDD patients and healthy controls, we compared the expression level of FZD6 between healthy controls and MDD patients in two human expression datasets (GSE54562 and GSE92538). The results showed that the mRNA expression level of FZD6 significantly reduced in MDD patients (p < 0.05; Supplementary Fig. S1A and S1B), indicating FZD6 may play significant roles in depression.
Demonstration of the effects of FZD6 knockdown on Wnt/β-catenin pathway in HEK293T and SH-SY5Y cells
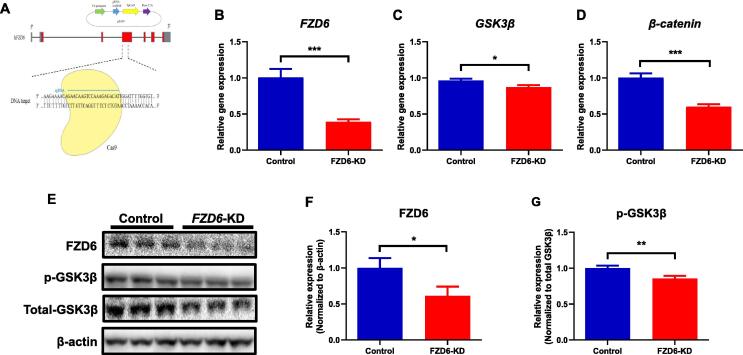
To determine FZD6 roles in depression, we constructed two CRISPR/Cas9-based FZD6-knockdown cell lines (Fig. 1A). As FZD6 is one of the receptors for Wnt proteins involving in Wnt/β-catenin signaling pathway, several key genes within this pathway were measured. In SH-SY5Y neuronal cells, qRT-PCR analysis showed a 61% reduction of FZD6 mRNA expression in FZD6-KD cells compared with controls (p < 0.001; Fig. 1B). Consistent with the mRNA results, we also detected a downregulation at the FZD6 protein level (p < 0.05; Fig. 1F). We then examined the effect of decreased FZD6 expression on the downstream genes in the Wnt/β-catenin pathway. We found that knockdown of FZD6 resulted in reduced GSK3β mRNA expression (p < 0.05; Fig. 1C) and decreased phosphorylation of GSK3β protein expression normalized to the total GSK3β (p < 0.01; Fig. 1G). The β-catenin mRNA expression was also downregulated significantly in FZD6-KD cells (p < 0.001; Fig. 1D), a targeted downstream gene regulated by GSK3β. In addition, most of the other gene expression levels in the Wnt/β-catenin pathway, such as Casein kinase 1 (CK1), Adenomatous polyposis coli (APC), and Dishevelled 3 (DVL3) also showed significant decrease in FZD6-KD cells (p < 0.01) (Supplementary Fig. S2A).
Fig. 1.
Regulatory effects of FZD6 knockdown (FZD6-KD) on Wnt/β-catenin signaling pathway in SH-SY5Y cells. (A) Schematic of FZD6-KD created by CRISPR/Cas9 technology. The sgRNA (blue) was cloned into a Cas9 nuclease (yellow) expression plasmid (PX459), then the Cas9 nuclease was targeted to exon 4 of FZD6 by a 20-nt guide sequence and induced a double-strand break. (B-D)FZD6-KD significantly decreased mRNA expression of (B)FZD6, (C)GSK3β, and (D)β-catenin in Wnt/β-catenin pathway, which were assessed by qRT-PCR in SH-SY5Y cells (n = 3). (E) Representative Western blotting images of FZD6, total GSK3β, and phosphorylation of GSK3β (p-GSK3β) at Ser9 with β-actin as loading control in SH-SY5Y cells. (F, G)FZD6-KD remarkably reduced expression of (F) FZD6 protein and (G) phosphorylation of GSK3β (p-GSK3β) at Ser9 in SH-SY5Y cells (n = 3). Optical densities were normalized to loading control (β-actin), and p-GSK3β was expressed as ratio of p-GSK3β over total-GSK3β. All data are shown as mean ± SEM. Unpaired t-test was used to conduct the statistical analysis. *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Consistently, we also constructed FZD6 knockdown HEK293T cells with CRISPR/Cas9 system. Both the FZD6 mRNA and protein expression were markedly decreased in FZD6-KD HEK293T cells (Supplementary Fig. S3A and S3E). Similarly, as shown in Supplementary Fig. S2B, S3B, and S3C, GSK3β, β-catenin, APC, DVL3, AXIN1, and AXIN2 mRNA expression were markedly decreased in the FZD6-KD cells (p < 0.05). The GSK3β protein phosphorylation (p < 0.05; Supplementary Fig. S3F) was also significantly reduced, but not for the total GSK3β between the WT and FZD6-KD cells (p > 0.05).
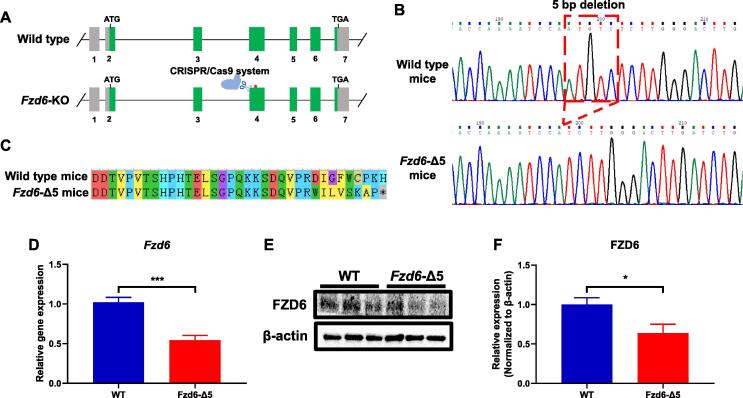
Generation of Fzd6-KD mice
To further investigate FZD6 roles in depression, the Fzd6-KD mouse model was created with the CRISPR/Cas9 system targeting the fourth exon of Fzd6 (Fig. 2A). After crossbreeding between the heterozygote mice, we obtained Fzd6-Δ5 homozygote mice with a five-nucleotide deletion (Fig. 2B), which led to generation of an early stop codon in the amino acid of Fzd6 (Fig. 2C). Subsequent qRT-PCR and Western blotting analyses presented that the level of Fzd6 mRNA reduced by 50% (p < 0.001), and the level of Fzd6 protein decreased by 40% (p < 0.05) in Fzd6-Δ5 mice, respectively (Fig. 2D). These results demonstrated that we have successfully generated a Fzd6-KD mice, which could be used for further functional studies.
-
1.
Anxiety-like behaviors
-
2.
Altered hormones feel
-
3.
Depressive disorder
Fig. 2.
Generation of Fzd6 knockdown (Fzd6-KD) mice by CRISPR/Cas9 system. (A) Schematic of strategy for construction of Fzd6-KD mice via the CRISPR/Cas9 system. The system targeted to the fourth exon of Fzd6. (B) Sequencing chromatogram to identify wild-type and Fzd6 knockdown mice. Dashed red line indicated 5-bp deletion in Fzd6-KD mice, named as Fzd6-Δ5 thereafter. (C) BLAST of amino acid from wild-type (WT) and Fzd6-5 bp deletion (Fzd6-Δ5) mice. The asterisk indicates a stop codon in Fzd6-Δ5 mice. (D) Comparison of Fzd6 mRNA expression in WT and Fzd6-Δ5 mice as detected by qRT-PCR. (E) Representative Western blotting images of FZD6 with β-actin as loading control in WT and Fzd6-Δ5 mice. (F) Quantitative analysis of FZD6 expression in WT and Fzd6-Δ5 mice. All data are shown as mean ± SEM. Unpaired t-test was used to conduct the statistical analysis. *p < 0.05, ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Alterations of depressive-like and anxiety-like behaviors in Fzd6-Δ5 mice
To determine Fzd6 knockdown on depressive-like behaviors in animals, we applied FST, TST and SPT to evaluate despair or anhedonia of mice. Compared with WT mice, Fzd6-Δ5 mice showed increased immobile durations in FST (Fig. 3A; p < 0.05) and a similar trend but at a less extent in TST (Fig. 3B; p = 0.0834). In the SPT, Fzd6-Δ5 mice showed less preference for sucrose (Fig. 3C) compared with WT mice (p < 0.05), suggesting Fzd6 knockdown induced the mice presenting depressive-like behaviors.
Fig. 3.
Effects of FZD6 on depressive-like behaviors in Fzd6-Δ5 mice. The forced swimming test (FST), tail suspension test (TST), and sucrose preference test (SPT) were used to measure depressive-like behaviors in WT and Fzd6-Δ5 mice. (A) The immobility seconds in FST. (B) The immobility seconds in TST. (C) The percentage of sucrose consumption over total fluid consumption in SPT. All behavioral tests were evaluated with number of mice from 7 to 9 per genotype. Data were analyzed using unpaired t-test and are shown as mean ± SEM. *p < 0.05.
To further measure the anxiety along with depression after Fzd6 knockdown, we used OFT (Fig. 4A) and EPM tests (Fig. 4D) to determine anxiety-like behaviors. In the OFT, compared with the WT mice, we found that Fzd6-Δ5 mice took less time in the center area (p < 0.05; Fig. 4B), but there are no markedly changes for the distance travelled were detected between the WT and Fzd6-Δ5 mice (p > 0.05; Fig. 4C). In EPM, compared with the WT mice, the Fzd6-Δ5 mice travelled less distance (Fig. 4E) and took less time (Fig. 4F) in the open arms (p < 0.05). Together, above results indicated that the Fzd6-Δ5 mice exhibited anxiety-like behaviors.
Fig. 4.
Effects of FZD6 on anxiety-like behaviors in Fzd6-Δ5 mice. The open field test (OFT) and elevated plus maze (EPM) test were applied to measure anxiety-like behaviors in WT and Fzd6-Δ5 mice. (A) The representative tracking plot in OFT. Graphs show (B) the time spent in center area and (C) the total distance travelled in OFT. (D) Representative tracking plot in EPM test. Graphs present (E) distance travelled and (F) time spent in open arms in EPM. All behavioral tests were evaluated with number of mice from 7 to 9 per genotype. Data were analyzed using unpaired t-test and are presented as mean ± SEM. *p < 0.05, ns = not significant.
We also found that the serum corticosterone, a major hallmark of stress hormones for depressive disorder [28], was significantly elevated in Fzd6-Δ5 mice compared with the WT mice (p < 0.05; Fig. 4G). This suggests that the Fzd6-Δ5 mice not only presented changed depressive-like and anxiety-like behaviors, but also showed altered hormones level, which is related to depressive disorder.
Alteration of cell proliferation in the hippocampus of Fzd6-Δ5 mice
To measure the cell proliferation in the hippocampus, an important region implicated in depression [29], we used Ki67 and PCNA (Proliferating Cell Nuclear Antigen) as markers to label the proliferating cells in the dentate gyrus (DG) region of hippocampus. As shown in Fig. 5A and 5B, compared to the WT mice, the number of Ki67+ cells and PCNA+ cells were markedly decreased by 40% (p < 0.05; Fig. 5C) and 54% (p < 0.001; Fig. 5D) in the DG of Fzd6-Δ5 mice, respectively, suggesting a significant decrease of proliferating cells in Fzd6-Δ5 mice. However, no significant difference of NeuN+ (mature neuron marker) cells in the DG region was detected between the WT and Fzd6-Δ5 mice (p > 0.05; Fig. 5E). This indicated that Fzd6 knockdown reduced cell proliferation in the hippocampus but had no significant effect on the mature neuron.
Proof EPM tets —
OFT, compred ith the WT mice, we fund hat Fzd6-D5 mice
Fig. 5.
Effects of FZD6 on cell proliferation in the dentate gyrus (DG) region of hippocampus of Fzd6-Δ5 mice. (A) The representative immunofluorescent staining images of hippocampus staining with Ki67 (red) in WT and Fzd6-Δ5 mice. Nuclei were counterstained with DAPI (blue). The arrowheads show Ki67 positive cells. Scale bar = 50 μm. (B) The representative immunofluorescent staining images of NeuN (green) and PCNA (red) staining in the hippocampus of WT and Fzd6-Δ5 mice. Nuclei were counterstained with DAPI (blue). Scale bar = 100 μm. Quantification analysis of Ki67 (C), PCNA (D), and NeuN (E) positive cells in the DG region of hippocampus in WT and Fzd6-Δ5 mice. Data were analyzed using unpaired t-test and are presented as mean ± SEM. *p < 0.05, ***p < 0.001, ns = not significant, n = 3 animals per group. PCNA, proliferating cell nuclear antigen. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Regulation of Wnt/β-catenin pathway in hippocampus of Fzd6-Δ5 mice
To further study the molecular mechanisms of FZD6 in depression, the mRNA and protein expressions of GSK3β and β-catenin in Wnt/β-catenin pathway in hippocampus were examined. We found the relative mRNA expression of Gsk3β reduced significantly (p < 0.05; Fig. 6A) in Fzd6-Δ5 mice, but no markedly difference was found in β-catenin mRNA level (Fig. 6B). Further, we found that, compared with the WT mice, phosphorylation of GSK3β was significantly decreased in Fzd6-Δ5 mice normalized to the total GSK3β (p < 0.05; Fig. 6D), but only a decrease trend was detected for phosphorylation of β-catenin in Fzd6-Δ5 mice normalized to the total β-catenin (p = 0.07; Fig. 6E). Given that β-catenin translocate from cytoplasm to nucleus when the Wnt/β-catenin pathway was activated [30], we next determined both the cytoplasmic and nuclear β-catenin levels. As shown in Fig. 6G, we observed lower protein level of β-catenin in the cytoplasmic fraction in Fzd6-Δ5 mice (p < 0.05), but β-catenin protein level in the hippocampal nuclear fraction only showed a decrease trend (p > 0.05; Fig. 6I). All above results clearly demonstrated the role of Fzd6 in depression mainly functions in hippocampus through disrupting the Wnt/β-catenin signaling pathway, especially GSK3β and β-catenin.
Fig. 6.
Effects of FZD6 on Wnt/β-catenin pathway in hippocampus of Fzd6-Δ5 mice. The mRNA relative expression of (A) Gsk3β and (B) β-catenin in hippocampus of WT and Fzd6-Δ5 mice. (C) Representative immunoblot images of total GSK3β and β-catenin, phospho-GSK3β (p-GSK3β) at Ser9, and phospho-β-catenin (p-β-catenin) at Ser675 in hippocampus of Fzd6-Δ5 mice. β-actin was applied as the loading control. Quantitative analysis of (D) p-GSK3β and (E) p-β-catenin expression levels in hippocampus, which were normalized to total GSK3β and total β-catenin, respectively. Representative immunoblot images of β-catenin in (E) hippocampal cytoplasmic fraction and (H) nuclear fraction of WT and Fzd6-Δ5 mice. Quantitative analysis of β-catenin expression in (G) cytoplasm and (I) nucleus of hippocampus normalized to loading control β-tubulin and Lamin B1, respectively. Data were analyzed using unpaired t-test and are presented as mean ± SEM. *p < 0.05, ns = not significant.
Discussion
Here, we first found a significantly decreased expression of FZD6 in MDD patients compared with healthy controls. By generating a gene knockdown animal model, we found that Fzd6 knockdown mice presented depressive-like and anxiety-like behaviors, a direct evidence of the gene function in depression. Moreover, decreased GSK3β and β-catenin mRNA levels, reduced GSK3β phosphorylation, and the imbalance of β-catenin level in cytoplasm and nucleus of hippocampus in Fzd6 knockdown mice provided further evidence for FZD6 roles in depression at mechanistic level.1
So far, several pathways have been documented in the pathogenesis of depression. For example, proinflammatory cytokines were increased in the patients suffering from depression, in which nod-like receptor pyrin containing 3 (NLRP3) and toll-like receptor (TLR) are two known signaling pathways participated in neuroinflammation involving depression [31], [32], [33]www.google.com. Further, the levels of quinolinic acid (QUIN) and kynurenic acid (KYNA), two key metabolites in kynurenine (KYN) pathway, were reported to be abnormal, an indication of the contribution of aberrant metabolic pathway to the pathophysiology of depression [34]. The deficiency of trophic factors is another neurobiological mechanism involved in depression, in which brain derived neurotrophic factor (BDNF) is the main factor related to hippocampal neurogenesis [35]. In addition, the Wnt/β-catenin pathway, nuclear factor kappa-B (NF-κB) pathway, as well as mammalian target of rapamycin (mTOR) pathway were all reported involving in the process of neurogenesis and antidepressant [9], [35], [36]. However, the regulatory role of many abovementioned pathways in depression, especially the Wnt/β-catenin pathway, is largely unknown.
Frizzled receptor family was widely reported to mediate Wnt/β-catenin pathway, which is involving in several diseases including neural tube defects brain morphogenesis defects [37]. Recently, accumulating reports indicated that the FZD family is related to depression. For instance, Calabro and colleagues [38] found that SNP rs352428 in FZD3 was strongly liked to depression, where the person who carries G allele having greater risk of experiencing depressive episode. Li and colleagues [5] reported FZD7 to be a new GPCR regulators involving depression. FZD6 was found to be related to depression according to an observation of altered depressive-like behaviors after Fzd6 knockdown in rats [17]. All these studies strongly demonstrated a significant role of this family in depression [17]. Very recently, we found a SNP rs61753730 located in FZD6 was linked to depression and allele specifically affected the expression of FZD6 [18].
It is documented that Fzd6-KD in the hippocampus region of rat brain can induce depressive-like behavior (i.e., decreased sucrose preference) and anxiety-like behaviors (i.e., repressed feeding and presentation in the EPM) [17]. Mice with knockdown expression of the FZD6 ligand Wnt2/Wnt3 also showed decreased immobility duration in TST and FST, as well as reduced sucrose consumption, an indication of involvement of Wnt2/Wnt3 in depressive-like behaviors [39]. To study the role of FZD6 involving depression, here, we generated Fzd6-Δ5 mice with Fzd6 knockdown by employing CRISPR/Cas9 technique and found that the Fzd6-Δ5 mice showed significant depressive-like behaviors, such as increased immobility in FST and less prefer for sucrose. These behavioral alterations further demonstrated that Fzd6 plays a regulation role in depression-related behaviors. Because the hypothalamic pituitary adrenal (HPA) axis and elevated level of corticosterone in serum and different brain regions have been found as major hallmark of depressive disorder [28], we also measured corticosterone level in serum which was significantly elevated in Fzd6-Δ5 mice. MDD patients not only presented the changes of hormones, but also showed disturbances in neurotransmitters in the periphery and brain, including dopamine, glutamate, γ-aminobutyric acid (GABA), and serotonin (5-HT) [40], [41]. Although we did not measure neurotransmitters level in the brain of Fzd6-Δ5 mice by ourselves in this study, a recent study reported that the neurotransmitters level (including BDNF, 5-HT, and NE) were all significantly decreased in Fzd6 mutant mice [42]. These results demonstrated that Fzd6 plays an important role in depression.
FZD6 is the receptor for Wnt involving in Wnt/β-catenin pathway. This pathway is not only important for neuron development for instance neuron proliferation, neuron survival, migration, and differentiation in the brain, but also has been implicated in synaptic plasticity modulation, which is correlated with learning and memory, and emotional disorders including depression [43], [44]. Here, we demonstrated that Fzd6 knockdown significantly altered the cell proliferation with decreased Ki67 and PCNA positive cells. It is well known that GSK3β phosphorylation at Ser9 negatively regulates GSK3β activity and further increasing cytosolic β-catenin level, but GSK3β phosphorylation at Tyr216 positively regulates GSK3β activity further promoting β-catenin degradation [45], [46]. The chronic stress induces depressive-like behaviors, decreases phosphorylation of GSK3β at Ser9 but increases phosphorylation of GSK3β at Tyr216, reduces β-catenin in the hippocampus of rodents [47], [48]. These alterations also were found in the postmortem brains of depressed persons [49]. In the present study, we detected a decreased mRNA expression of GSK3β and β-catenin, as well as decreased phosphorylation of GSK3β in CRISPR/Cas9-based FZD6-KD HEK293T and SH-SY5Y cells. Similarly, in the hippocampus of Fzd6-Δ5 mice, we also detected a decreased mRNA expression of Gsk3β and a reduction of GSK3β phosphorylation. Although no significant change was shown in the β-catenin mRNA expression, we did detect decreased β-catenin level in the cytoplasm of the hippocampus. The reduced β-catenin expression in protein but not in mRNA level may due to the posttranslational modifications such as ubiquitination [50].
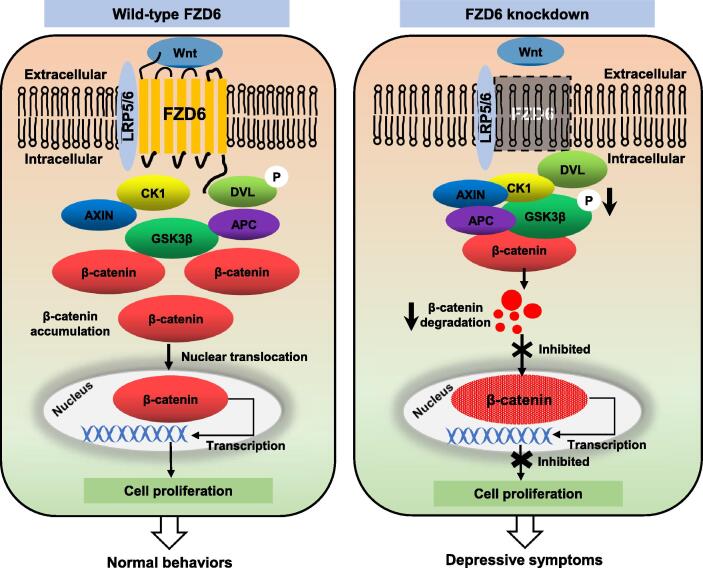
To gain a clear picture on roles of FZD6 in depression, we proposed a working model for its function (see Fig. 7, left). Normally, Wnt protein binds to its ligand FZD6 and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) to activate the canonical Wnt/β-catenin signaling pathway, then recruits the scaffold protein DVL to form a ligand–receptor complex. The activated DVL suppresses the kinase activity of GSK3β and destabilizes a “β-catenin destruction complex” consisted of CK1, AXIN, APC, and GSK3β. Then, β-catenin accumulates in the cytoplasm, which further translocate into the nucleus to promote Wnt target genes transcription and neurons proliferation [30], [51]. There is an increasing evidences indicating that disruption of canonical Wnt/FZD signaling is related to multiple neurodevelopmental and neuropsychiatric disorders [10], [52]. For instance, blocking Dvl2 in the nucleus accumbens of mice makes them more vulnerable to depression [53]. Meanwhile, studies on the antidepressants target CREB led to the discovery of CREB and Wnt/FZD pathway connections [54]. Chronic ECS resulted in enhanced CREB binding to the Fzd6 promoter and greater hippocampal Fzd6 expression as determined by chromatin immunoprecipitation followed by microarray analysis (ChIP-chip) [17]. As a result of FZD6 knockdown (see Fig. 7, right), the binding of Wnt to the receptors is blocked, or at least attenuated, which results in the aberrant inactivation of the Wnt/β-catenin pathway. GSK3β in the β-catenin destruction complex was dephosphorylated at Ser 9 and further phosphorylated in β-catenin, which resulted in a low concentration of β-catenin in cytoplasm. Attributing to the ubiquitination and proteasomal degradation, β-catenin reduction further results in inhibition of β-catenin nuclear translocation and suppression of the cell proliferation, ultimately, it may present depressive symptoms.
Fig. 7.
Proposed model describing role of FZD6 knockdown on disrupting Wnt/β-catenin signaling pathway in depression. Left schema shows physiological process of Wnt signaling in wild-type subjects. Wnt glycoproteins bind to FZD6 and a low-density lipoprotein (LRP)5/6 co-receptor, resulting in activation of Disheveled (DVL). This leads to phosphorylation of GSK3β, resulting in disruption of protein complex consisting of AXIN, adenomatous polyposis coli (APC), casein kinase 1 (CK1), and GSK3β. This causes stabilization of β-catenin and its subsequent translocation to nucleus, which will activate transcription of target genes. Schema on right shows the role of FZD6 in this signaling pathway. Knockdown of FZD6 expression might affect formation of ligand-receptor complex and increase stability of destruction complex. Dephosphorylation of GSK3β leads to degradation of β-catenin accompanied by decreased expression. The process of β-catenin into nucleus is inhibited, expression of downstream genes is restrained, and cell proliferation is also inhibited, this eventually results in depressive symptoms.
Conclusions
In summary, based on our recent study where we found a missense SNP in FZD6 markedly associated with depressive symptom and allele specifically affected the structure and expression of FZD6. In this study, to further investigate the mechanism and functional role of FZD6 in depression, firstly, we found decreased expression of FZD6 in MDD patients. Then, by using various animal behavioral tests on Fzd6 knockdown mice, we demonstrated that Fzd6 was highly related to depressive symptoms. Finally, by applying both in vitro and in vivo approaches, we proved the above effect was caused, at least partly, by altering the expression and phosphorylation of β-catenin and GSK3β in hippocampus of the Wnt/β-catenin signaling pathway, and further affecting cell proliferation of DG region, which contributes to depression occurrence. Taken together, our current findings clearly demonstrate that FZD6 is an important gene for depression. More studies are necessary to conduct to investigate the mechanisms of how FZD6 in the Wnt/β-catenin pathway affects neuronal development such as neurogenesis and contributes to depression.
Compliance with Ethics Requirements
The Animal Care and Use Committee of the First Affiliated Hospital of Zhejiang University approved the experimental procedures in this study (Approval No. 1093-1).
CRediT authorship contribution statement
Haijun Han: Data curation, Visualization, Investigation, Writing – original draft, Funding acquisition. Mengxiang Xu: Data curation, Investigation, Validation, Writing – original draft. Ju Wang: Methodology, Software, Investigation, Writing – original draft. Ming D. Li: Conceptualization, Supervision, Funding acquisition, Investigation, Project administration, Resources, Writing - original draft, Writing - review & editing. Zhongli Yang: Conceptualization, Supervision, Funding acquisition, Investigation, Project administration, Resources, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (82271560), China Precision Medicine Initiative (2016YFC0906300), China National Postdoctoral Program for Innovative Talent (BX20190295), and China Postdoctoral Science Foundation (2020M681891). We also thank the technical support by the Core facilities of Zhejiang University School of Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.06.001.
Fzd6-D5 mice normlized to the totalb-catenin.
Contributor Information
Ming D. Li, Email: ml2km@zju.edu.cn.
Zhongli Yang, Email: zy3p@zju.edu.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.

References
- 1.Charlson F., van Ommeren M., Flaxman A., Cornett J., Whiteford H., Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet. 2019;394(10194):240–248. doi: 10.1016/S0140-6736(19)30934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Üstün T.B., Ayuso-Mateos J.L., Chatterji S., Mathers C., Murray C.J.L. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184(5):386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 3.Steel N., Ford J.A., Newton J.N., Davis A.C.J., Vos T., Naghavi M., et al. Changes in health in the countries of the UK and 150 English Local Authority areas 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10158):1647–1661. doi: 10.1016/S0140-6736(18)32207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhi G.S., Mann J.J. Depression. Lancet. 2018;392(10161):2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 5.Li S., Luo H., Lou R., Tian C., Miao C., Xia L., et al. Multiregional profiling of the brain transmembrane proteome uncovers novel regulators of depression. Sci Adv. 2021;7(30) doi: 10.1126/sciadv.abf0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H.C., Klein P.S. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 2004;5(7):234. doi: 10.1186/gb-2004-5-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji Y.B., et al. Normalization of non-canonical Wnt signalings does not compromise blood-brain barrier protection conferred by upregulating endothelial Wnt/beta-catenin signaling following ischemic stroke. CNS Neurosci Ther. 2021 doi: 10.1111/cns.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tayyab M., Shahi M.H., Farheen S., Mariyath M.P.M., Khanam N., Castresana J.S., et al. Sonic hedgehog, Wnt, and brain-derived neurotrophic factor cell signaling pathway crosstalk: potential therapy for depression. J Neurosci Res. 2018;96(1):53–62. doi: 10.1002/jnr.24104. [DOI] [PubMed] [Google Scholar]
- 10.Oliva C.A., Montecinos-Oliva C., Inestrosa N.C. Wnt Signaling in the Central Nervous System: New Insights in Health and Disease. Prog Mol Biol Transl Sci. 2018;153:81–130. doi: 10.1016/bs.pmbts.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Voleti B., Duman R.S. The roles of neurotrophic factor and Wnt signaling in depression. Clin Pharmacol Ther. 2012;91(2):333–338. doi: 10.1038/clpt.2011.296. [DOI] [PubMed] [Google Scholar]
- 12.Sani G., et al. The wnt pathway in mood disorders. Curr Neuropharmacol. 2012;10(3):239–253. doi: 10.2174/157015912803217279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corda G., Sala A. Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogenesis. 2017;6(7):e364. doi: 10.1038/oncsis.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Marco P., Merello E., Rossi A., Piatelli G., Cama A., Kibar Z., et al. FZD6 is a novel gene for human neural tube defects. Hum Mutat. 2012;33(2):384–390. doi: 10.1002/humu.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi O.-Y., Yang H.-Y., Shen Y.-M., Sun W., Cai C.-Y., Cai C.-Q. Polymorphisms in FZD3 and FZD6 genes and risk of neural tube defects in a northern Han Chinese population. Neurol Sci. 2014;35(11):1701–1706. doi: 10.1007/s10072-014-1815-4. [DOI] [PubMed] [Google Scholar]
- 16.Stuebner S., et al. Fzd3 and Fzd6 deficiency results in a severe midbrain morphogenesis defect. Dev Dyn. 2010;239(1):246–260. doi: 10.1002/dvdy.22127. [DOI] [PubMed] [Google Scholar]
- 17.Voleti B., Tanis K.Q., Newton S.S., Duman R.S. Analysis of target genes regulated by chronic electroconvulsive therapy reveals role for Fzd6 in depression. Biol Psychiatry. 2012;71(1):51–58. doi: 10.1016/j.biopsych.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han H., Xu M., Wen L., Chen J., Liu Q., Wang J. Identification of a novel functional nonsynonymous single nucleotide polymorphism in Frizzled 6 (FZD6) gene for involvement in depressive symptoms. Front Mol Neurosci. 2022;(15) doi: 10.3389/fnmol.2022.882396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han H., Huang W., Du W., Shen Q., Yang Z., Li M.D., et al. Involvement of Interferon Regulatory Factor 7 in Nicotine's Suppression of Antiviral Immune Responses. J Neuroimmune Pharmacol. 2019;14(4):551–564. doi: 10.1007/s11481-019-09845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J., Liu Q., Fan R., Han H., Yang Z., Cui W., et al. Demonstration of critical role of GRIN3A in nicotine dependence through both genetic association and molecular functional studies. Addict Biol. 2020;25(1):e12718. doi: 10.1111/adb.12718. [DOI] [PubMed] [Google Scholar]
- 22.Shen B., Zhang W., Zhang J., Zhou J., Wang J., Chen L.i., et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat Methods. 2014;11(4):399–402. doi: 10.1038/nmeth.2857. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y., Cui Y., Sang K., Dong Y., Ni Z., Ma S., et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554(7692):317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 24.Shen C.-J., Zheng D.i., Li K.-X., Yang J.-M., Pan H.-Q., Yu X.-D., et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med. 2019;25(2):337–349. doi: 10.1038/s41591-018-0299-9. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhury D., Walsh J.J., Friedman A.K., Juarez B., Ku S.M., Koo J.W., et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493(7433):532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteggia L.M., Luikart B., Barrot M., Theobold D., Malkovska I., Nef S., et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61(2):187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Winer J., Jung C.K.S., Shackel I., Williams P.M. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270(1):41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 28.Stephens M.A., Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34(4):468–483. [PMC free article] [PubMed] [Google Scholar]
- 29.Otte C., et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 30.Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueroa-Hall L.K., Paulus M.P., Savitz J. Toll-Like Receptor Signaling in Depression. Psychoneuroendocrinology. 2020;121 doi: 10.1016/j.psyneuen.2020.104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiepers O.J., Wichers M.C., Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann F.N., Costa A.P., Ghisleni G., Diaz A.P., Rodrigues A.L.S., Peluffo H., et al. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav Immun. 2017;64:367–383. doi: 10.1016/j.bbi.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Ogyu K., Kubo K., Noda Y., Iwata Y., Tsugawa S., Omura Y., et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16–25. doi: 10.1016/j.neubiorev.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Caviedes A., et al. BDNF/NF-kappaB Signaling in the Neurobiology of Depression. Curr Pharm Des. 2017;23(21):3154–3163. doi: 10.2174/1381612823666170111141915. [DOI] [PubMed] [Google Scholar]
- 36.Abelaira H.M., Réus G.Z., Neotti M.V., Quevedo J. The role of mTOR in depression and antidepressant responses. Life Sci. 2014;101(1-2):10–14. doi: 10.1016/j.lfs.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y., Wang W., Zhao C. Frizzled Receptors in Tumors, Focusing on Signaling, Roles, Modulation Mechanisms, and Targeted Therapies. Oncol Res. 2021;28(6):661–674. doi: 10.3727/096504020X16014648664459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabro M., et al. Genetic variants associated with psychotic symptoms across psychiatric disorders. Neurosci Lett. 2020;720 doi: 10.1016/j.neulet.2020.134754. [DOI] [PubMed] [Google Scholar]
- 39.Zhou W.-J., Xu N., Kong L., Sun S.-C., Xu X.-F., Jia M.-Z., et al. The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors. Transl Psychiatry. 2016;6(9):e892. doi: 10.1038/tp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nutt D.J., et al. Consensus statement and research needs: the role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67(Suppl 6):46–49. [PubMed] [Google Scholar]
- 41.Pan J.-X., Xia J.-J., Deng F.-L., Liang W.-W., Wu J., Yin B.-M., et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: a targeted metabolomics study. Transl Psychiatry. 2018;8(1) doi: 10.1038/s41398-018-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan X., et al. Construction of Fzd6(Q152E)mice through CRISPR/Cas9 technology and their reproduction and identification. Mol Biol Rep. 2022;49(10):9575–9584. doi: 10.1007/s11033-022-07848-6. [DOI] [PubMed] [Google Scholar]
- 43.Maguschak K.A., Ressler K.J. The dynamic role of beta-catenin in synaptic plasticity. Neuropharmacology. 2012;62(1):78–88. doi: 10.1016/j.neuropharm.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teo C.H., Soga T., Parhar I.S. Brain Beta-Catenin Signalling During Stress and Depression. Neurosignals. 2018;26(1):31–42. doi: 10.1159/000487764. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q.M., Fiol C.J., DePaoli-Roach A.A., Roach P.J. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994;269(20):14566–14574. [PubMed] [Google Scholar]
- 46.Ren X., et al. Altered Wnt signalling in the teenage suicide brain: focus on glycogen synthase kinase-3beta and beta-catenin. Int J Neuropsychopharmacol. 2013;16(5):945–955. doi: 10.1017/S1461145712001010. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y.C., et al. The effect of citalopram on chronic stress-induced depressive-like behavior in rats through GSK3beta/beta-catenin activation in the medial prefrontal cortex. Brain Res Bull. 2012;88(4):338–344. doi: 10.1016/j.brainresbull.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Garza J.C., et al. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Mol Psychiatry. 2012;17(8):790–808. doi: 10.1038/mp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karege F., et al. Protein levels of beta-catenin and activation state of glycogen synthase kinase-3beta in major depression. A study with postmortem prefrontal cortex. J Affect Disord. 2012;136(1–2):185–188. doi: 10.1016/j.jad.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Gao C., Xiao G., Hu J. Regulation of Wnt/beta-catenin signaling by posttranslational modifications. Cell Biosci. 2014;4(1):13. doi: 10.1186/2045-3701-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okerlund N.D., Cheyette B.N. Synaptic Wnt signaling-a contributor to major psychiatric disorders? J Neurodev Disord. 2011;3(2):162–174. doi: 10.1007/s11689-011-9083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zwamborn R.A.J., et al. Wnt Signaling in the Hippocampus in Relation to Neurogenesis, Neuroplasticity, Stress and Epigenetics. Prog Mol Biol Transl Sci. 2018;158:129–157. doi: 10.1016/bs.pmbts.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson M.B., et al. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 2011;31(25):9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlezon W.A., Jr., Duman R.S., Nestler E.J. The many faces of CREB. Trends Neurosci. 2005;28(8):436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.